Abstract
Recombinant hemagglutinin (H) protein of the measles virus (MV) was produced in mammalian cells with a high-yield expression system based on the Semliki Forest virus replicon. Crude membrane preparations of H protein-transfected BHK-21 cells were used to coat microtiter plates to measure specific immunoglobulin G antibodies in 228 serologically defined serum samples mainly from measles late-convalescent adults. The titers by the enzyme-linked immunosorbent assay for the H protein (H-ELISA) closely correlated with neutralization test (NT) titers (R2 = 0.66), hemagglutination inhibition test (HI) titers (R2 = 0.64), with the titers from a certified commercial ELISA based on whole MV-infected cells (MV-ELISA; R2 = 0.45). The correlations described above were better than those of the commercial MV-ELISA titers with the NT (R2 = 0.52) or HI (R2 = 0.48) titers. By using the 2nd International Standard for anti-measles serum, the detection level of the assay corresponds to 215 mIU/ml for undiluted serum, which corresponds to the estimated threshold for protective immunity. The specificity, accuracy, and positive predictive value were, in general, better for the H-ELISA than for a commercial MV-ELISA, independent of whether HI, NT, or HI and NT were used as “gold standards.” In contrast, the H-ELISA proved to be slightly less sensitive than the MV-ELISA (sensitivities, 98.6 versus 99.5%, respectively; P was not significant). The assays did not differ significantly in the number of serum samples with positive HI and NT results (n = 212) which measured false negative (H-ELISA, 2 of 212 [0.94%]; MV-ELISA, 1 of 212 [0.47%]), but the H-ELISA detected significantly more measles-susceptible individuals than the MV-ELISA (10 of 11 versus 3 of 11, respectively; P < 0.05) among the individuals whose sera had negative HI and NT results. Our data demonstrate that the H-protein preparation that we describe could be a cost-effective alternative to current whole-virus-based ELISAs for surveillance for immunity to measles and that such an assay could be more efficient in detecting susceptibility to measles. Furthermore, unlike whole MV-based antigens, H-protein would also be suitable for use in the development of a simple field test for the diagnosis of measles.
Immunity against measles involves a cellular response and a humoral response (4, 5, 13, 43–45). It is well known that passive antibodies protect an individual against measles (1, 28), and protection was also obtained after passive transfer of cellular immunity, at least in an animal model (38). Monitoring of immunity to measles relies solely on the detection of specific antibodies, but it is not clear to what extent antibody titers reflect protective cellular immunity. Past experience with enzyme-linked immunosorbent assays (ELISAs) based on whole measles virus (MV) suggests that antibodies directed against whole virus are a reliable measure of measles immunity (8, 15, 35, 50). Assays for immunity based on recombinant proteins may offer a number of advantages over whole-virus-based ELISAs. Such advantages include simplified production, improved standardization, and enhanced stability. An inexpensive and simple diagnostic test as an alternative to an MV-infected cell- or whole-virus-based ELISA is also required to monitor measles immunity as part of eradication programs (22). Such assays could rely on the detection of antibodies directed against selected MV proteins (48, 49). Antibodies against the nucleoprotein were found to correlate with total MV antibodies (27). Whether antibodies specific for other proteins also correlate with total MV antibodies and with immunity has not been demonstrated with a panel of human sera. Most functional antibodies are directed against the hemagglutinin (H) protein: they neutralize MV in vitro and provide protection against MV in vivo (9, 10, 14, 17, 20, 21, 32, 46). Therefore, H protein-specific immunoglobulin G (IgG) antibodies are considered to be most important in determining immunity to MV (6).
The MV H protein has been expressed in a number of expression systems including baculovirus (40, 47), vaccinia virus (14, 41, 51), canarypox virus (42), adenovirus (3), and other (19) systems. Expression of this glycoprotein in procaryote or lower eucaryote systems should result in glycosylation different from that in MV. Proper glycosylation has been found to be important for the processing, the functional integrity, and the antigenicity of this protein (23–25). For proper posttranslational modification, this glycoprotein should therefore be expressed in mammalian cells. However, in most mammalian systems the yield is low (19). We analyze here whether the H protein expressed in a high-yield mammalian expression system based on the Semliki Forest virus replicon (29, 30) is suitable for monitoring measles immunity.
(This work was done by Fabienne Bouche in partial fulfillment of her doctoral thesis.)
MATERIALS AND METHODS
Serum panel.
Sera were obtained from 217 consecutive outpatients over the age of 25 years at the Laboratoire National de Santé who underwent venipuncture for measles-unrelated reasons in December 1995 and January 1996. The volunteers consisted of 87 males (age range, 25 to 80 years) and 130 females (age range, 25 to 92 years). It can be assumed that the vast majority of the persons in this age bracket had measles during their childhoods because they were born at a time (1905 to 1970) when immunity was mostly acquired by early natural infection. In addition, 11 negative serum samples were obtained from seven unvaccinated 15-month-old children, two adolescents, and two adults.
The hemagglutination inhibition test (HI) and neutralization test (NT) titers of the test sera were determined as described before (26). Titers are expressed as log2 dilutions, with values of ≤1:24 being negative. Anti-MV antibody levels were measured by using a certified commercial ELISA based on MV-infected simian cells, following the supplier’s instructions.
The recombinant MV H protein.
Three overlapping cDNA fragments of the MV H protein were obtained by reverse transcription-PCR from total RNA of virus-infected Vero cells. After appropriate digestions, the full-length 1.9-kb cDNA was reconstituted from these fragments into the BamHI cloning site of the pUC-18 vector (pUC-MVHrev), which was then transferred into the BamHI site of the pSFV1 plasmid (pSFV1-MVH; unpublished data). As described by Liljeström and Garoff (30), SpeI-linearized plasmids pSFV1-MVH and pSFV3-lacZ (as a negative control) (29) were transcribed in vitro, and the resulting RNA transcripts were transfected into BHK-21 cells by electroporation. BHK-21 cells transfected with the recombinant SFV1 RNA of the H protein are referred to as BHK-H; control cells transfected with SFV1-β-galactosidase RNA are called BHK-gal. High levels of expression were confirmed by flow cytometry (unpublished data).
Preparation of total membrane fraction.
At 24 h posttransfection, the cells were mechanically disrupted in hypotonic solution (10 mM Tris-HCl [pH 7.6], 0.5 mM MgCl2). In brief, nuclei and large debris were sedimented at 500 × g (5 min, 4°C). Total membranes were sedimented from the supernatants by centrifugation at 110,000 × g (45 min, 4°C). After resuspension in phosphate-buffered saline–0.5% Nonidet P-40, the pellet containing insoluble debris and total membrane were centrifuged again at 14,000 × g (15 min, 4°C) to remove insoluble particles. The supernatant containing the total membrane fraction was stored at 4°C in 0.05% azide. The protein concentration was determined by the method of Bradford (8a).
H-ELISA.
Serum-specific IgG levels were measured by using an ELISA based on the recombinant MV H protein (H-ELISA). Each well of Maxisorp microtiter plates (NUNC, Roskilde, Denmark) was coated with 50 μl of 2 μg of total protein from the above membrane preparation per ml in 0.1 M bicarbonate buffer (pH 9.6). After washing, the plates were blocked with 1% bovine serum albumin in Tris-buffered (15 mM) saline. Fifty microliters of serum diluted 1:300 in Tris-buffered saline containing 0.1% Tween 20 and 1% bovine serum albumin (dilution buffer) was incubated for 90 min at room temperature. Alkaline phosphatase-conjugated goat anti-human IgG (1:700; Southern Biotechnology Associates) and the corresponding substrate were used to measure antibody binding.
The optical density (OD) was measured after 90 min at 405 nm. Data are expressed as net milli-OD (mOD) by computing for each serum sample the mOD of BHK-H − the mOD of BHK-gal. The BHK-gal background levels were 302 ± 68 mOD units. In these cells β-galactosidase is produced as a soluble, cytosolic, irrelevant protein. All serum samples were tested in two independent experiments. The thresholds for positivity and negativity were defined on the basis of the values measured for sera negative by both HI and NT. By using the 2nd International Standard for anti-measles serum obtained from the National Institute for Biological Standards and Control (Hertfordshire, United Kingdom) (16), the threshold for positivity defined here (120 mOD) corresponds to 215 mIU/ml for undiluted serum (data not shown).
Statistics.
Data were analyzed with Sigmastat software (Jandel Scientific, Erkrath, Germany). The coefficient of determination (R2) was obtained by regression analysis. The characteristics of the assays (see Table 1) were calculated according to the definitions of Bland (7).
TABLE 1.
Performance characteristics of the H-ELISA, MV-ELISA, and whole-virus-based ELISAsa
| Assay (cohort) | Specificity (%) | Sensitivity (%) | Accuracy (%) | Prevalence (%) | Positive predictive value (%) | Negative predictive value (%) | Undefined sera (%) |
|---|---|---|---|---|---|---|---|
| H-ELISA (this studya) | 100 | 98.6 | 98.7 | 95.1 | 100 | 78.6 | 0.9 |
| MV-ELISA (this studya) | 45.5 | 99.5 | 96.9 | 95.1 | 97.2 | 83.3 | 0.9 |
| MV-ELISA (other studiesb) | 80.0–100 | 99.1–100 | 94.7–99.2 | 73.5–87.5 | 93.3–100 | 94.0–100 | 0–0.4 |
“Gold standards,” HI and NT.
Data are for undefined populations (8, 12) and high school children before an outbreak (34). For maximal comparability, the values were recalculated after adding the undefined sera to the negative sera by using the same algorithms (7). The minimal and maximal values obtained from the three previous studies (8, 12, 34) are presented.
RESULTS
Detection of MV-specific IgG.
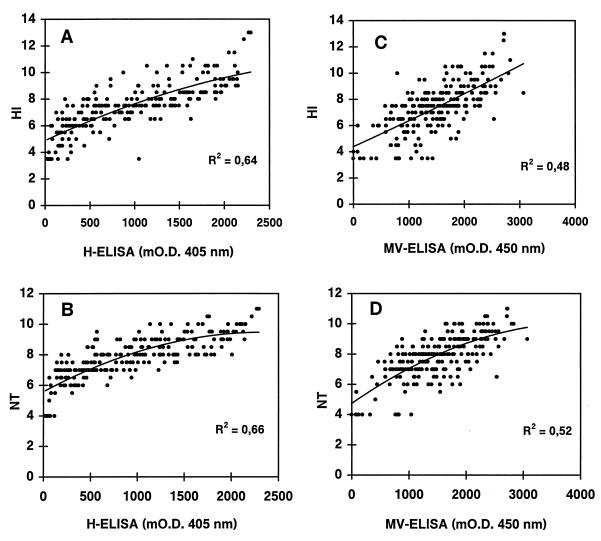
The ability of our H-ELISA to detect MV-H-specific human IgG was tested with a panel of serologically characterized sera obtained from measles late-convalescent adults. The comparison of the recombinant H-ELISA with the commercial MV-ELISA with all 228 serum samples gave an R2 value of 0.45 (data not shown). Figure 1 shows the correlation of the titers obtained by the recombinant H-ELISA and of a certified commercial MV-ELISA with HI and NT titers. The better correlations with HI (R2 = 0.64 versus 0.48) and NT (R2 = 0.66 versus 0.52) titers were found with the recombinant H-protein assay. Readout values of H-ELISA after 60 or 90 min had identical R2 values. The R2 values for the H-ELISA presented above correspond to the R2 for these sera obtained by NT and HI (R2 = 0.67).
FIG. 1.
Correlation between the H-ELISA (A and B) or the MV-ELISA (C and D) with HI (A and C) and NT (B and D) titers (expressed as log2). Each point corresponds to one test serum sample (n = 228 measles late-convalescent donors). The R2 values were calculated on the basis of the best polynomial trendline (order 2).
Analysis of false-positive and false-negative sera.
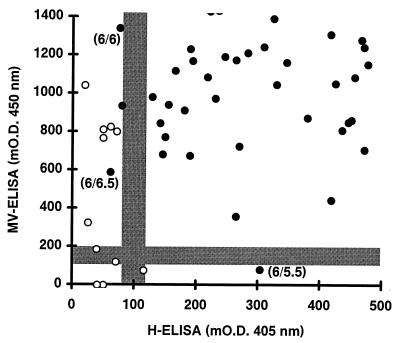
Negativity and positivity were defined on the basis of the mODs (mean ± standard deviation [SD] = 54 ± 26) for the sera negative by both HI and NT (titers, ≤1:24). Sera with mODs of >120 (mean + 2.5 SD) and mODs of <80 (mean + 1 SD) were considered positive and negative, respectively, with 80 to 120 mOD units being a gray zone in which sera were undefined. The corresponding values for the commercial MV-ELISA are defined by the supplier as <100 and >200 mOD units. By these criteria, significantly more sera negative by both HI and NT were false positive by the MV-ELISA (6 of 11) than by the H-ELISA (0 of 11; P < 0.05; Fig. 2). Figure 2 also shows that among sera positive by both HI and NT the H-ELISA detected as many false-negative sera as the MV-ELISA (2 of 212 versus 1 of 212; P was not significant). The HI and NT values for the false-negative sera were low (≤1:26.5; cf. Fig. 2).
FIG. 2.
Comparison of H-ELISA and MV-ELISA values for sera which were negative by both HI and NT (open symbols; n = 11) or by both HI and NT positive (closed symbols; n = 212). Values below and above the gray zone (H-ELISA, 80 to 120 mOD units; MV-ELISA, 100 to 200 mOD units) are considered negative and positive, respectively. Values inside the gray zone are undefined. HI and NT titers (expressed as log2 dilutions) for false-negative sera are given in parentheses as HI titer/NT titer. The x and y axes are truncated at 500 and 1,400 mOD so that not all of the 212 serum samples are represented.
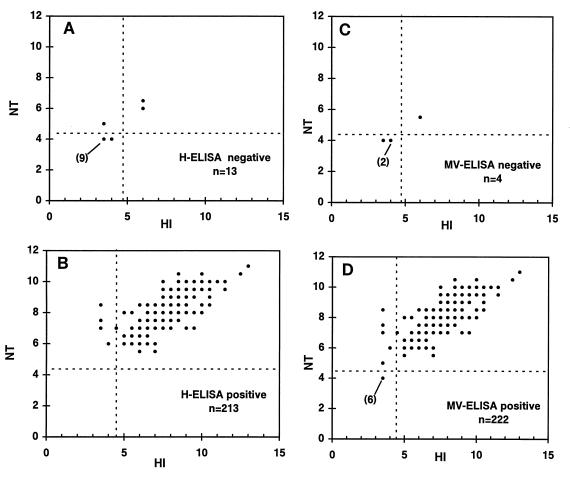
Since HI is thought to be less sensitive than NT (2, 34, 50), the analysis based on the titers obtained by HI and NT described above potentially excludes weakly positive sera. Figure 3 presents the HI and NT values for sera positive or negative by each one of the two ELISAs. Of the sera which were negative by H-ELISA, 10 of 13 were negative by both HI and NT (Fig. 3A). All sera which were positive by H-ELISA were also positive by NT, with 4 of 213 serum samples being HI negative and NT positive and 209 of 213 serum samples being both HI and NT positive (Fig. 3B). Four serum samples were negative by the MV-ELISA; three of these were negative by both HI and NT (Fig. 3C). Among the sera positive by MV-ELISA, 6 of 222 serum samples were negative by both HI and NT, an additional 5 serum samples were HI negative and NT positive, and all others (i.e., 211 of 222) were positive by both HI and NT (Fig. 3D). A total of 209 of 213 (98.1%) and 211 of 222 (95.0%) of the serum samples which were seropositive by the H-ELISA or the MV-ELISA were positive by both HI and NT and 10 of 13 (76.9%) and 3 of 4 (75.0%) of the serum samples which were negative by the H-ELISA or the MV-ELISA were negative by both HI and NT.
FIG. 3.
HI and NT titers (expressed as log2 dilutions) for sera negative by H-ELISA (A) or MV-ELISA (C) and sera positive by H-ELISA (B) or MV-ELISA (D). Numbers in parentheses are the numbers of serum samples with overlapping values.
Performance of the H-ELISA.
The different performance parameters of the H-ELISA were compared with those of whole-virus-based ELISAs from this study and from the literature (8, 12, 34). For a valid comparison, all values were recalculated with the same algorithms by adding undefined sera to the sera negative by ELISA (7). The results of the latter computation are presented in Table 1. The performance of the H-ELISA compared favorably with those of the whole-virus ELISAs both when results for the same or different cohorts were considered. Results were similar whether undefined sera were excluded or added to the negative sera.
DISCUSSION
Different serological assays measure diverse subsets of MV antibodies, and it is not clear which subset reflects the cellular immune response best. Whole MV-based ELISAs detect a broad range of immunoglobulins, irrespective of their functional activities (8, 15, 35, 50). Functional assays, such as HI and NT, are therefore considered to provide a better estimate of the immune status. Hemagglutination-inhibition and neutralization antibodies are mainly H protein specific (21, 31). Our study demonstrates for a large cohort of human serum samples that antibody levels measured by an ELISA based on the recombinant H protein expressed in a mammalian system correlate considerably better with HI and NT titers than those measured by conventional whole-virus-based ELISAs, indicating that the H-ELISA measures predominantly functional antibodies. Since hemagglutination-inhibition and neutralization antibodies are strongly associated with in vivo protection (11, 21), our results also suggest that the H-ELISA may be more appropriate than MV-ELISAs for measuring immunity to measles.
Sera negative by both HI and NT and positive by both HI and NT served to find the thresholds for positivity (>120 mOD units) and negativity (<80 mOD units) of the H-ELISA. Since HI is normally less sensitive than NT (2, 34, 50) (Fig. 3), negativity by both HI and NT more rigorously defines negative individuals than negativity by NT alone (12, 34). Titration of the 2nd International Standard for anti-measles serum obtained from the National Institute for Biological Standards and Control (16) confirmed that the threshold for positivity given above corresponds to a protective antibody level: 120 mOD units corresponds to 215 mIU/ml (data not shown); different investigators (18, 33, 37) have considered concentrations of >200 (corresponding to 114 mOD units) to >255 mIU/ml to correspond to protective immunity. Under the assumption that the antibody specificities of test and standard sera are similar, this may suggest that individuals who are seropositive by the H-ELISA have protective immunity.
On the basis of the thresholds presented above and the results presented in Fig. 2, it is concluded that a positive serum sample has a chance of 209 in 212 (98.6%; with 1 serum sample being undefined) or 211 in 212 (99.5%) of testing positive and a negative serum has a chance of 10 in 11 (90.9%; with 1 negative serum sample being undefined) or 3 in 11 (27.3%; with 2 serum sample being undefined) of testing negative by H-ELISA or MV-ELISA, respectively. The assays did not differ significantly in the numbers of false-negative sera (for the H-ELISA, 2 of 212 [0.94%]; for the MV-ELISA, 1 of 212 [0.47%]). In contrast, significantly more serum samples had false-positive results by the MV-ELISA than by the H-ELISA (6 of 11 versus 0 of 11, respectively; P < 0.05).
Although the number of negative serum samples was limited, the performance characteristics of the H-ELISA were determined (Table 1). The specificity, accuracy, and positive predictive value were generally better for the H-ELISA than for the MV-ELISA, independent of whether HI, NT, or HI and NT was used as the “gold standard.” In contrast, the H-ELISA proved to be slightly less sensitive than the MV-ELISA (98.6 versus 99.5% [99.6%], according to the supplier). The H-ELISA was also better by all parameters than HI (data not shown) (12, 34).
When undefined sera were included the MV-ELISA detected as negative only 5 serum samples among the 11 serum samples negative by both HI and NT. Although in larger seronegative cohorts between 58 and 100% of the serum samples were found to be negative by the same or different whole MV-based (fully optimized) commercial ELISAs (12, 34, 37), our data seem to suggest that the H-ELISA may be more efficient for identifying seronegative individuals.
Five of the six serum samples which were false positive by MV-ELISA were from 15 month-old, unvaccinated children. Discrepancies between ELISA and NT or HI measurements have been reported for those with maternally acquired antibodies (36, 37, 39). The results presented above indicate that these antibodies do not seem to interfere with our H-ELISA.
Measurements of specific MV IgG antibodies should, most importantly, predict susceptibility to measles infection. In any given cohort, only a few individuals will be seronegative for measles. Among these, false-positive donors are at risk of disease and can support viral circulation. Identification of such false-positive sera would require the retesting of most serum samples (by a different assay). In contrast, individuals who test false negative have no enhanced risk and are epidemiologically irrelevant. Also, rare false-negative sera could, in principle, be retested (by another assay) or such individuals could simply be vaccinated or revaccinated. For these reasons, false-negative results can be better tolerated than false-positive results, which did not occur by our assay.
The global measles eradication efforts of the World Health Organization require an inexpensive and standardized source of antigen for surveillance of measles immunity, in particular, for the detection of seronegativity. Our data demonstrate that the H-protein preparation derived from the Semliki Forest virus expression system could be a simple alternative to current whole-virus-based ELISAs for surveillance of measles immunity and that such an assay could be more efficient in detecting susceptibility to measles. More importantly, however, unlike for antigens based on MV-infected cells, the properties of the H-protein antigen such as its stability are more compatible with the development of a simple field test for the diagnosis of measles, which is another requirement of eradication programs.
ACKNOWLEDGMENTS
We thank the volunteers for providing blood and N. H. C. Brons for technical help.
F.B. was supported by a fellowship from the Ministère de l’Education Nationale. This study was also supported by a grant from the Centre de Recherche Public-Santé, Luxembourg (CRP93/08).
REFERENCES
- 1.Albrecht P, Ennis F A, Saltzman E J, Krugman S. Persistence of maternal antibody in infants beyond 12 months: mechanism of measles vaccine failure. J Pediatr. 1977;91:715–718. doi: 10.1016/s0022-3476(77)81021-4. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht P, Hermann K, Burns G R. Role of virus strain in conventional and enhanced measles plaque neutralization test. J Virol Methods. 1981;3:251–260. doi: 10.1016/0166-0934(81)90062-8. [DOI] [PubMed] [Google Scholar]
- 3.Alkhatib G, Briedis D J. High-level eucaryotic in vivo expression of biologically active measles virus hemagglutinin by using an adenovirus type 5 helper-free vector system. J Virol. 1988;62:2718–2727. doi: 10.1128/jvi.62.8.2718-2727.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bankamp B, Brinckmann U G, Reich A, Niewiesk S, ter Meulen V, Liebert U G. Measles virus nucleocapsid protein protects rats from encephalitis. J Virol. 1991;65:1695–1700. doi: 10.1128/jvi.65.4.1695-1700.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellini W J, McFalin D E, Silver G D, Mingioli E S, McFarland H F. Immune reactivity of purified hemagglutinin of measles virus. Infect Immun. 1981;32:1051–1057. doi: 10.1128/iai.32.3.1051-1057.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black F L. Measles active and passive immunity in a worldwide perspective. Prog Med Virol. 1989;36:1–33. [PubMed] [Google Scholar]
- 7.Bland M. An introduction to medical statistics. 2nd ed. Oxford, United Kingdom: Oxford University Press; 1995. pp. 265–276. [Google Scholar]
- 8.Boteler W L, Luipersbeck P M, Fuccillo D A, O’Beirne A J. Enzyme-linked immunosorbent assay for detection of measles antibody. J Clin Microbiol. 1983;17:814–818. doi: 10.1128/jcm.17.5.814-818.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 9.Brinckmann U G, Bankamp B, Reich A, ter Meulen V, Liebert U G. Efficacy of individual measles virus structural proteins in the protection of rats from measles encephalitis. J Gen Virol. 1991;72:2491–2500. doi: 10.1099/0022-1317-72-10-2491. [DOI] [PubMed] [Google Scholar]
- 10.Cardoso A I, Blixenkrone-Moller M, Fayolle J, Liu M, Buckland R, Wild T F. Immunization with plasmid DNA encoding for the measles virus hemagglutinin and nucleoprotein leads to humoral and cell-mediated immunity. Virology. 1996;225:293–299. doi: 10.1006/viro.1996.0603. [DOI] [PubMed] [Google Scholar]
- 11.Chen R T, Markowitz L E, Albrecht P, et al. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;162:1036–1042. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- 12.Cremer N E, Cossen C K, Shell G, Diggs J, Gallo D, Schmidt N J. Enzyme immunoassay versus plaque neutralization and other methods for determination of immune status to measles and varicella-zoster viruses and versus complement fixation for serodiagnosis of infections with those viruses. J Clin Microbiol. 1985;21:869–874. doi: 10.1128/jcm.21.6.869-874.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhib-Jalbut S, Jacobson S. Cytotoxic T cells in paramyxovirus infections of humans. Curr Top Microbiol Immunol. 1994;189:109–121. doi: 10.1007/978-3-642-78530-6_7. [DOI] [PubMed] [Google Scholar]
- 14.Drillien R, Spehner D, Kirn A, Giraudon P, Buckland R, Wild F, Lecocq J P. Protection of mice from fatal measles encephalitis by vaccination with vaccinia virus recombinants encoding either the hemagglutinin or the fusion protein. Proc Natl Acad Sci USA. 1988;85:1252–1256. doi: 10.1073/pnas.85.4.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erdman D D, Anderson L J, Adams D R, Stewart J A, Markowitz L E, Bellini W J. Evaluation of monoclonal antibody-based capture enzyme immunoassays for detection of specific antibodies to measles virus. J Clin Microbiol. 1991;29:1466–1471. doi: 10.1128/jcm.29.7.1466-1471.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forsey T, Heath A B, Minor P D. The 1st international standard for anti-measles serum. Biologicals. 1991;19:237–241. doi: 10.1016/1045-1056(91)90042-i. [DOI] [PubMed] [Google Scholar]
- 17.Fournier P, Brons N H C, Berbers G A M, Wiesmueller K H, Fleckenstein B T, Schneider F, Jung G, Muller C P. Antibodies to a new linear site at the topographic or functional interface between the hemagglutinin and fusion protein protect against measles encephalitis. J Gen Virol. 1997;78:1295–1302. doi: 10.1099/0022-1317-78-6-1295. [DOI] [PubMed] [Google Scholar]
- 18.Garenne M, Leroy O, Beau J P, Sene I. High-titer measles virus vaccines: protection evaluation. In: Kurstak E, editor. Measles and poliomyelitis: vaccines, immunization, and control. Vienna, Austria: Springer-Verlag; 1993. pp. 119–131. [Google Scholar]
- 19.Gerald C, Buckland R, Barker R, Freeman G, Wild T F. Measles virus haemagglutinin gene: cloning, complete nucleotide sequence analysis and expression in COS cells. J Gen Virol. 1986;67:2695–2703. doi: 10.1099/0022-1317-67-12-2695. [DOI] [PubMed] [Google Scholar]
- 20.Giraudon P, Wild T F. Monoclonal antibodies against measles virus. J Gen Virol. 1981;54:325–332. doi: 10.1099/0022-1317-54-2-325. [DOI] [PubMed] [Google Scholar]
- 21.Giraudon P, Wild T F. Correlation between epitopes on hemagglutinin of measles virus and biological activities: passive protection by monoclonal antibodies is related to their hemagglutination inhibiting activity. Virology. 1985;144:46–58. doi: 10.1016/0042-6822(85)90303-4. [DOI] [PubMed] [Google Scholar]
- 22.Hinman A R, Orenstein W A. Is measles eradicable? In: Kurstak E, editor. Measles and Poliomyelitis: vaccines, immunization, and control. Vienna, Austria: Springer-Verlag; 1993. pp. 33–61. [Google Scholar]
- 23.Hu A, Kovamees J, Norrby E. Intracellular processing and antigenic maturation of measles virus hemagglutinin protein. Arch Virol. 1994;136:239–253. doi: 10.1007/BF01321055. [DOI] [PubMed] [Google Scholar]
- 24.Hu A, Cattaneo R, Schwartz S, Norrby E. Role of N-linked oligosaccharide chains in the processing and antigenicity of measles virus haemagglutinin protein. J Gen Virol. 1994;75:1043–1052. doi: 10.1099/0022-1317-75-5-1043. [DOI] [PubMed] [Google Scholar]
- 25.Hu A, Cathomen T, Cattaneo R, Norrby E. Influence of N-linked oligosaccharide chains on the processing, cell surface expression and function of the measles virus fusion protein. J Gen Virol. 1995;76:705–710. doi: 10.1099/0022-1317-76-3-705. [DOI] [PubMed] [Google Scholar]
- 26.Huiss S, Damien B, Schneider F, Muller C P. Characteristics of asymptomatic secondary immune responses to measles virus in late convalescent donors. Clin Exp Immunol. 1997;109:416–420. doi: 10.1046/j.1365-2249.1997.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hummel K B, Erdman D D, Heath J, Bellini W J. Baculovirus expression of the nucleoprotein gene of measles virus and utility of the recombinant protein in diagnostic enzyme immunoassays. J Clin Microbiol. 1992;30:2874–2880. doi: 10.1128/jcm.30.11.2874-2880.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janeway C A. Use of concentrated human serum gamma globulin in the prevention and attenuation of measles. Bull N Y Acad Med. 1949;21:202–222. [PMC free article] [PubMed] [Google Scholar]
- 29.Liljeström P, Garoff H. A new generation of animal cell expression vectors based on the Semliki forest virus replicon. BioTechnology. 1991;9:1356–1361. doi: 10.1038/nbt1291-1356. [DOI] [PubMed] [Google Scholar]
- 30.Liljeström P, Garoff H. Expression of proteins using Semliki Forest virus vectors. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Smith J A, Seidman J G, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley Interscience; 1995. pp. 16.20.1–16.20.16. [Google Scholar]
- 31.Malvoisin E, Wild F. Contribution of measles virus fusion protein in protective immunity: anti-F monoclonal antibodies neutralize virus infectivity and protect mice against challenge. J Virol. 1990;64:5160–5162. doi: 10.1128/jvi.64.10.5160-5162.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McFarlin D, Bellini W J, Mingioli E S, Behar T N, Trudgett A. Monospecific antibody to the haemagglutinin of measles. J Gen Virol. 1980;48:425–429. doi: 10.1099/0022-1317-48-2-425. [DOI] [PubMed] [Google Scholar]
- 33.Miller E, Hill A, Morgan-Capner P, Forsey T, Rush M. Antibodies to measles, mumps and rubella in UK children 4 years after vaccination with different MMR vaccines. Vaccine. 1995;13:799–802. doi: 10.1016/0264-410x(94)00086-3. [DOI] [PubMed] [Google Scholar]
- 34.Neumann P W, Weber J M, Jessamine A G, O’Shaughnessy M V. Comparison of measles antihemolysin test, enzyme-linked immunosorbent assay, and hemagglutination inhibition test with neutralization test for determination of immune status. J Clin Microbiol. 1985;22:296–298. doi: 10.1128/jcm.22.2.296-298.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ozanne G, d’Halewyn M A. Performance and reliability of the Enzygnost measles enzyme-linked immunosorbent assay for detection of measles virus-specific immunoglobulin M antibody during a large measles epidemic. J Clin Microbiol. 1992;30:564–569. doi: 10.1128/jcm.30.3.564-569.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ratnam S, Chandra R, Gadag V. Maternal measles and rubella antibody levels and serologic response in infants immunized with MMR II vaccine at 12 months of age. J Infect Dis. 1993;168:1596–1598. doi: 10.1093/infdis/168.6.1596-a. [DOI] [PubMed] [Google Scholar]
- 37.Ratnam S, Gadag V, West R, Burris J, Oates E, Stead F, Bouilianne N. Comparison of commercial enzyme immunoassay kits with plaque reduction neutralization test for detection of measles virus antibody. J Clin Microbiol. 1995;33:811–815. doi: 10.1128/jcm.33.4.811-815.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reich A, Erlwein O, Niewiesk S, ter Meulen V, Liebert U G. CD4+ T cells control measles virus infection of the central nervous system. Immunology. 1992;76:185–191. [PMC free article] [PubMed] [Google Scholar]
- 39.Sabin A B, Arechiga A F, de Castro F J, et al. Successful immunization of infants with and without maternal antibody by aerosolized measles vaccine II. Vaccine comparisons and evidence for multiple antibody response. JAMA. 1984;251:2363–2371. [PubMed] [Google Scholar]
- 40.Takehara K, Hashimoto H, Ri T, Mori T, Yoshimura M. Characterization of baculovirus-expressed hemagglutinin and fusion glycoproteins of the attenuated measles virus strain AIK-C. Virus Res. 1992;26:167–175. doi: 10.1016/0168-1702(92)90155-3. [DOI] [PubMed] [Google Scholar]
- 41.Taylor J, Pincus S, Tartaglia J, Richardson C, Alkhatib G, Briedis D, Appel M, Norton E, Paoletti E. Vaccinia virus recombinants expressing either the measles virus fusion or hemagglutinin glycoprotein protect dogs against canine distemper virus challenge. J Virol. 1991;65:4263–4274. doi: 10.1128/jvi.65.8.4263-4274.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor J, Weinberg R, Tartaglia J, Richardson C, Alkhatib G, Briedis D, Appel M, Norton E, Paoletti E. Nonreplicating viral vectors as potential vaccines: recombinant canarypox virus expressing measles virus fusion (F) and hemagglutinin (HA) glycoproteins. Virology. 1992;187:321–328. doi: 10.1016/0042-6822(92)90321-f. [DOI] [PubMed] [Google Scholar]
- 43.Uytdehaag F G C M, van Binnendijk R S, Kenter M J H, Osterhaus A D M E. Cytotoxic T lymphocyte responses against measles virus. Curr Top Microbiol Immunol. 1994;189:151–167. doi: 10.1007/978-3-642-78530-6_9. [DOI] [PubMed] [Google Scholar]
- 44.Van Binnendijk R S, Van Baalen C A, Poelen M C, de Vries P, Boes J, Cerundolo V, Osterhaus A D, Uytdehaag F G. Measles virus transmembrane fusion protein synthesized de novo or presented in immunostimulating complexes is endogenously processed for HLA class I- and class II-restricted cytotoxic T cell recognition. J Exp Med. 1992;176:119–128. doi: 10.1084/jem.176.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Binnendijk R S, Versteeg-van Oosten J P, Poelen M C, Brugghe H F, Hoogerhout P, Osterhaus A D, Uytdehaag F G. Human HLA class I- and HLA class II-restricted cloned cytotoxic T lymphocytes identify a cluster of epitopes on the measles virus fusion protein. J Virol. 1993;67:2276–2284. doi: 10.1128/jvi.67.4.2276-2284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varsanyi T M, Morein B, Love A, Norrby E. Protection against lethal measles virus infection in mice by immune-stimulating complexes containing the hemagglutinin or fusion protein. J Virol. 1987;61:3896–3901. doi: 10.1128/jvi.61.12.3896-3901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vialard J, Lalumiere M, Vernet T, Briedis D, Alkhatib G, Henning D, Levin D, Richardson C. Synthesis of the membrane fusion and hemagglutinin proteins of measles virus, using a novel baculovirus vector containing the β-galactosidase gene. J Virol. 1990;64:37–50. doi: 10.1128/jvi.64.1.37-50.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warnes A, Fooks A R, Stephenson J R. Production of measles nucleoprotein in different expression systems and its use as a diagnostic reagent. J Virol Methods. 1994;49:257–268. doi: 10.1016/0166-0934(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 49.Warnes A, Fooks A R, Dowsett A B, Wilkinson G W G, Stephenson J R. Expression of the measles virus nucleoprotein gene in Escherichia coli and assembly of nucleocapsid-like structures. Gene. 1995;160:173–178. doi: 10.1016/0378-1119(95)00227-w. [DOI] [PubMed] [Google Scholar]
- 50.Weigle K A, Murphy M D, Brunell P A. Enzyme-linked immunosorbent assay for evaluation of immunity to measles virus. J Clin Microbiol. 1984;19:376–379. doi: 10.1128/jcm.19.3.376-379.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wild T F, Bernard A, Spehner D, Drillien R. Construction of vaccinia virus recombinants expressing several measles virus proteins and analysis of their efficacy in vaccination of mice. J Gen Virol. 1992;73:359–367. doi: 10.1099/0022-1317-73-2-359. [DOI] [PubMed] [Google Scholar]