Abstract
Benefits of physical activity for older adults’ mood and cognitive functioning have been widely supported; however, it is not clear whether risk factors for cardiovascular disease (CVD) affects the relationships of physical activity with these health outcomes among diverse older adults. This study investigated the impact of CVD risk factors on the relationships among physical activity, mood, and cognitive functioning in a diverse sample of 62 adults age 45 and older who completed self-report physical activity and mood questionnaires, and measures of verbal and visual working memory. Results suggest middle-aged to older adults with low CVD risk are more likely to benefit from physical activity. Specifically, we found that higher physical activity was associated with better attention and verbal working memory at lower CVD risk, but with worse attention and verbal working memory at higher CVD risk levels. Thus, higher CVD risk might limit the effectiveness of exercise interventions for mood and cognitive functioning. Future studies are needed to further clarify individual differences that impact the relationships among physical activity, CVD risk, and cognitive outcomes.
Keywords: depression, attention, working memory, older adults, exercise, heart disease
Physical activity (i.e., intentional movement that expends energy) and exercise (i.e., structured physical activity intended to improve or maintain physical fitness) have a variety of health benefits, including improved mood and cognitive functioning (Kvam et al., 2016; Mammen & Faulkner, 2013; Singh-Manoux et al., 2005). Meta-analyses have demonstrated physical activity effectively reduces depressive symptoms, even for symptoms in the non-clinical range (Rebar et al., 2015). Observational and intervention studies have linked physical fitness, higher levels of physical activity, and participation in structured exercise regimens with improved cognitive performance, especially executive functions, in sedentary older adults (Colcombe & Kramer, 2003; Sofi et al., 2011). Although the benefits of physical activity occur regardless of age, they are particularly relevant for older adults, who are vulnerable to clinically significant subthreshold depressive symptoms (Alexopoulos, 2005) and to age-related decline in executive functions and other cognitive abilities (MacPherson et al., 2002; Wilson et al., 2010), particularly cognitive functions that rely heavily on the frontal lobes in the brain (Greenwood, 2000; West, 1996).
Previous studies have shown the benefits of physical activity for adults with vascular risk factors or cardiovascular disease (CVD) (Aizenstein et al., 2016; Jefferson et al., 2015). In patients with mild-to-moderate chronic heart failure, cardiac rehabilitation, which traditionally includes an exercise component, increases exercise capacity and reduces subsequent hospital admissions because of chronic heart failure (Davies et al., 2010). Several other studies showed exercise benefits mood and cognitive functioning in populations with CVD (Brunt et al., 2019; Frith & Loprinzi, 2017; Kruk & Nowicki, 2016; Smith et al., 2007; Vercambre et al., 2011; Yohannes et al., 2010) and improves vascular health in people with risk factors for CVD such as hypertension, hyperlipidemia, obesity, and metabolic syndrome (Church, 2011; Dimeo et al., 2012; Kelly, 2010). For instance, one study investigated the relationship between physical activity and cognitive decline in middle-aged to older women who had at least three vascular risk factors and found that regular physical activity was associated with better cognitive function (Vercambre et al., 2011). Mood disorders and cognitive impairment have been linked to vascular disease; thus, the mood and cognitive benefits of physical activity may be directly tied to changes in vascular function (Gujral et al., 2017; Maass et al., 2015). This suggests that physical activity is a potential alternative intervention or adjunctive intervention for various vascular conditions that not only benefits vascular health but may also improve mood and cognitive functioning in turn. If vascular changes indeed contribute to depressed mood and cognitive decline, then it follows that people with higher CVD burden might benefit more from physical activity than people with low CVD burden.
Given racial disparities in CVD (Graham, 2015) and CVD risk factors (Howard et al., 2017), Black adults might represent one group that particularly benefits from physical activity interventions. Consistent with that possibility, depressive symptoms are more strongly associated with CVD risk factors in Black compared to White adults (Boyle et al., 2007; Lewis et al., 2009), and a longitudinal study found elevated depressive symptoms increased the risk of mortality from CVD in Black, but not White, participants (Lewis et al., 2011). There is also some evidence that CVD risk factors are more strongly associated with dysregulated mood for Black older adults relative to White older adults, although this has not always been observed (see Bogoian & Dotson(Bogoian & Dotson, 2021) for a review). Similarly, there is evidence that CVD risk factors are more strongly linked to cognitive impairment in Black compared to White older adults (Rotblatt et al., 2021; Zahodne et al., 2015).
Despite the clear need, there is a dearth of literature investigating the relationship between physical activity, mood, and cognitive function in diverse samples, similar to what we see across aging, neuroscience, and other fields of science (Brewster et al., 2019; Dotson & Duarte, 2020). To address these gaps in the literature, the current study investigated the hypothesized impact of CVD risk on the relationships of physical activity with cognitive functioning and mood in a predominately Black sample. We predicted higher amounts of self-reported physical activity would be associated with better mood and higher scores on measures of attention and working memory. Given previous research showing that people with CVD experienced more benefits from exercise than people without CVD (Bakker et al., 2021; Jeong et al., 2019), we hypothesized the benefits of physical activity for mood and cognitive function would be stronger in participants with higher CVD risk burden than in those with lower CVD risk burden.
Materials and Methods
Participants
Secondary data analyses were performed on baseline data from a multidisciplinary intervention study that sought to promote healthy aging among diverse middle-aged to older adults (Ellis Gardner et al., 2006; Moore et al., 2008). Participants were aged 45 and older and were recruited from either a local community center or a Louisiana housing facility for seniors with low or fixed incomes. Participants were eligible for the parent study if they consented to participate in a 16-week physical activity and nutrition intervention, and if they were free of neurological impairment and contraindications for exercise (e.g., recent myocardial infarction, unstable angina, or acute systemic infection). The intervention data were not included in the current analyses. All participants provided written informed consent to participate in the study, consistent with the guidelines of the local Institutional Review Board. Data from 94 participants were collected. Sixty-two participants had complete demographic, medical, cognitive, and questionnaire data necessary for our analyses. Participant characteristics are summarized in Table 1. The average age of the included participants is 65.9 (SD = 10.0) years. The sample is 78.1% female and 68.8% Black. Nearly three-quarts of the participants had completed the equivalent of high school or greater, and 79% of participants reported an income below $40,000 per year.
Table 1.
Sample characteristics
| Total Sample (N = 62) |
Low-Income Housing (N = 28) |
Community Center (N = 34) |
Black Sample (N = 44) |
|
|---|---|---|---|---|
| Age (years) | 65.9 ± 10.0 | 65.9 ± 9.5 | 65.9 ± 10.5 | 65.8 ± 10.3 |
| Sex (% female) | 50 (80.6%) | 20 (71.4%) | 30 (88.2%) | 36 (81.8%) |
| Race (% Black) | 44 (71.0%) | 12 (42.9%) | 32 (94.1%) | - |
| CVD risk | 4.9 ± 1.3 | 4.6 ± 1.5 | 5.1 ± 1.1 | 5.2 ± 1.0 |
| Education | ||||
| 7th – 9th grade | 8 (12.9%) | 7 (25.0%) | 1 (2.9%) | 5 (11.4%) |
| 10 – 11th grade | 8 (12.9%) | 3 (10.7%) | 5 (14.7%) | 6 (13.6%) |
| High school diploma/GED | 16 (25.8%) | 8 (28.6%) | 8 (23.5%) | 11 (25.0%) |
| Post high school, business, or trade school | 3 (4.8%) | 1 (3.6%) | 2 (5.9%) | 2 (4.5%) |
| Some college | 13 (21.0%) | 6 (21.4%) | 7 (20.6%) | 9 (20.5%) |
| 4-year degree | 5 (8.1%) | 1 (3.6%) | 4 (11.8%) | 4 (9.1%) |
| Some graduate | 2 (3.2%) | - | 2 (5.9%) | 2 (4.5%) |
| Graduate degree | 7 (11.3%) | 2 (7.1%) | 5 (14.7%) | 5 (11.4%) |
| Income (per year) | ||||
| ≤ $5,000 | 3 (4.8%) | 3 (10.7%) | - | - |
| $5,001-$10,000 | 25 (40.3%) | 19 (67.9%) | 6 (17.6%) | 17 (38.6%) |
| $10,001-$20,000 | 10 (16.1%) | 5 (17.9%) | 5 (14.7%) | 5 (11.4%) |
| $20,001-$30,000 | 5 (8.1%) | - | 5 (14.7%) | 4 (9.1%) |
| $30,001-$40,000 | 6 (9.7%) | - | 6 (17.6%) | 6 (13.6%) |
| $40,001-$50,000 | 2 (3.2%) | - | 2 (5.9%) | 2 (4.5%) |
| $70,001-$80,000 | 1 (1.6%) | - | 1 (2.9%) | 1 (2.3%) |
| $90,001-$100,000 | 1 (1.6%) | - | 1 (2.9%) | 1 (2.3%) |
| Chose not to answer | 5 (8.1%) | - | 5 (14.7%) | 5 (11.4%) |
| Did not know | 4 (6.5%) | 1 (3.6%) | 3 (8.8%) | 3 (6.8%) |
| Mental Component Summary | 53.8 ± 11.1 | 52.1 ± 12.8 | 55.1 ± 9.6 | 55.1 ± 9.5 |
| Longest Digit Span Forward | 5.5 ± 1.2 | 5.3 ± 1.1 | 5.6 ± 1.2 | 5.6 ± 1.2 |
| Longest Digit Span Backward | 3.4 ± 1.0 | 3.5 ± 1.2 | 3.4 ± 0.8 | 3.2 ± 1.0 |
| Longest Size Judgment Span | 3.8 ± 0.9 | 3.5 ± 0.8 | 4.1 ± 0.9 | 4.0 ± 0.9 |
| CHAMPS Total Activity (caloric expenditure per week) | 4843.0 ± 2811.3 | 4057.5 ± 2936.5 | 5489.9 ± 2568.9 | 4897.3 ± 2737.4 |
| CHAMPS Moderate-to-Vigorous Activity (caloric expenditure per week) | 2242.4 ± 2032.0 | 1439.4 ± 1661.5 | 2903.7 ± 2092.0 | 2410.3 ± 2075.1 |
Note. CVD = cardiovascular disease. GED = Test of General Educational Development. CHAMPS = Community Healthy Activities Model Program for Seniors. The low-income housing sample, community center sample, and Black sample are all subsets of the total sample.
Measures
Physical activity
In the parent study, physical activity was assessed at baseline using the CHAMPS Physical activity Questionnaire for Older Adults(Stewart et al., 2001). The CHAMPS is a self-report measure that assesses the weekly frequency and duration of lifestyle physical activities (e.g., gardening, housework) and structured exercise (e.g., walking, running, swimming, yoga) over the past four weeks, with a reported intraclass correlation coefficient of .66 for all activities and .67 for moderate activities and greater (Stewart et al., 2001). The CHAMPS has been validated in a variety of groups, including older adults, Black adults, and people with low income (Glynn et al., 2020; Hekler et al., 2012; Resnicow et al., 2003). Internal consistency reliability was acceptable for the current sample (Cronbach’s α = 0.76). Analyses for the current study used the CHAMPS total caloric expenditure per week score as a continuous variable. This score represents each participant’s average weekly expenditure over the four weeks preceding their testing session. The CHAMPS developers assigned a metabolic weight based to each activity in the questionnaire based on its intensity. Caloric expenditure for each person is calculated based on the metabolic weights and the individual’s body weight based on the formula: (kcal/minute = METs * 3.5 * [body weight in kg/200]).
Mood
Mood was assessed using the Mental Component Summary score from the 36-item Short Form Health Survey (SF-36) (Ware Jr, 2000). Although the SF-36 was not specifically developed to assess depression, it is often used as a screening measure for depression and anxiety (Matcham et al., 2016; Pfoh et al., 2016). In support of its use as a depression screener, Walsh and colleagues suggested measures from general health surveys may be vital screening tools for depressive symptoms, especially when the study involves multiple health-related measures (Walsh et al., 2006). The Mental Component Summary score is a composite score based on the items in the Vitality, Social Functioning, Role-Emotional, and Mental Health scales of the SF-36. Scores range from 0 to 100, with higher scores indicating better mood. A score of 42 or lower on the Mental Component Summary has a specificity of 80.6% and sensitivity of 73.7% for diagnosis of depression (Ware et al., 1993). The internal consistency reliability coefficient of the Mental Component Summary is 0.88 (Ferguson et al., 2002).
Cognition
Assessment of cognitive functioning focused on the Digit Span Forward (attention) and Digit Span Backward (verbal working memory) subtests from the Wechsler Adult Intelligence Scale (Wechsler, 1955) and the Size Judgment Span (visuospatial working memory) (Cherry et al., 2007) test because of the negative effects of mood disturbance and positive effects of physical activity on cognitive domains such as attention and working memory (Bherer et al., 2013; Morimoto et al., 2015). The maximum span for each test (raw score) was used in statistical analyses. Separate analyses were conducted for each of these measures rather than calculating a composite score to better capture the differential relationships that physical activity and cardiovascular risk burden may have on different cognitive domains.
Cardiovascular Risk Factors
Cardiovascular risk burden was calculated by summing self-reported diagnoses of the following seven conditions: any history of diabetes, heart problems, high blood pressure, hyperlipidemia, obesity, or stroke, or smoking history in the past six months. Cardiovascular risk was entered as a continuous measure, which could range from 0 to 7, in statistical analyses.
Data Analysis
Statistical analyses were conducted using R Version 3.6.3 (R Core Team, 2020). Multiple regression analyses were performed with CHAMPS total physical activity, the CVD risk variable, and their interaction as the independent variables and with cognitive (Digit Span Forward, Digit Span Backward and Size Judgment Span) and mood (Mental Component Summary) measures as the outcome variables in separate models. Based on their known association with cognitive functioning, mood, or both, initial covariates included race, age, education level, income, and sex. Education level, income, and sex were not significant in any of the models and were therefore removed to achieve the most parsimonious models (see Supplementary Table 1 for results of the fully adjusted models). For each model, data points with high leverage, distance, and influence were assessed to determine whether they should be removed. Two points that had absolute values for leverage and distance greater than .5 or 2.5, respectively, were removed. An alpha ≤ 0.05 was considered significant.
Two sets of planned follow-up analyses were conducted. First, the primary analyses were repeated using only the subset of Black participants, who comprised nearly three-fourths of the sample, to examine the relationships specific to this population given the dearth of physical activity literature focused on Black individuals. The second set of analyses stratified the data by recruitment location (community center and a low-income housing facility) to address the possibility of confounding factors, such as disability, that might systematically differ between settings. The stratified analysis allows to us to estimate the potential impact of setting on the results without adding an additional variable to our primary analyses, which is important given our fairly small sample size.
Results
Primary Analyses
Results for the primary analyses are summarized in Figure 1. There were no significant main effects of physical activity or CVD risk on mood symptoms, attention, or working memory in the total sample, but analyses revealed significant CVD risk by physical activity interactions for attention (β = −0.36731, p = 0.005) and verbal working memory (β = −0.35265, p = 0.005). Higher physical activity was associated with better attention and verbal working memory at lower levels of CVD, but with worse attention and verbal working memory at higher CVD risk levels. This is contrary to our hypothesis that the benefits of physical activity would be greater for individuals with higher CVD risk levels. There were no significant interaction effects of physical activity and CVD risk on mood symptoms or visual working memory, although the interaction approached statistical significance for visual working memory (p = 0.061).
Figure 1.
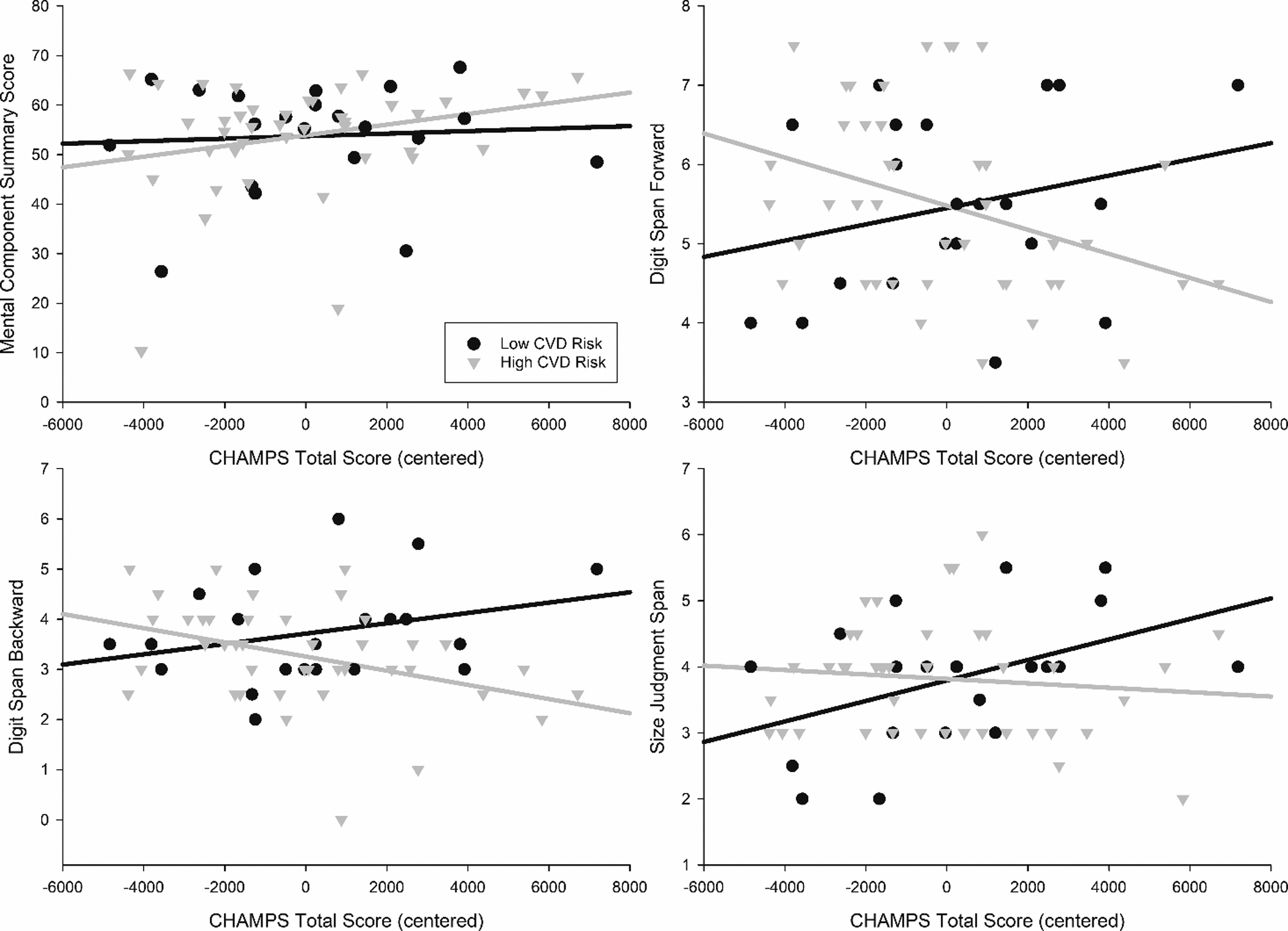
Total physical activity on the CHAMPS by mood (Mental Component Summary) and cognitive functioning (Digit Span Forward, Digit span Backward, and Size Judgment Span). CVD groups are for graphing purposes only and are based on a mean for each subgroup. Values for physical activity and CVD risk were mean centered.
Planned Follow-up Analyses
When the primary analyses were repeated with only Black participants, higher self-reported physical activity was associated with worse attention (β = −0.41139, p = 0.029). There was also a significant CVD risk by physical activity interaction for visual working memory (β = −0.46728, p = 0.015). The direction of this relationship was similar to that observed for attention and verbal working memory in the total sample, such that, for Black participants, higher physical activity was associated with better visual working memory at low, but not high, CVD risk levels. There was no main effect of CVD risk and there were no other significant interaction effects of physical activity and CVD risk on mood symptoms, attention, or verbal working memory.
In the analyses stratified by recruitment setting, the results for participants living in the low-income housing facility mirrored the results in the total sample: There was a CVD risk by physical activity interaction for attention, β = −0.66490, p < .001, and an interaction, reflecting the same pattern, approached significance for verbal working memory, β = −0.36200, p = .05. In community center participants, higher physical activity was associated with worse attention (β = −0.56084, p = 0.017) but better mood (β = 0.47810, p = 0.050).
Discussion
The present study investigated whether physical activity was related to mood and cognitive functioning, and whether risk for CVD impacted these relationships in a predominantly Black sample of middle-aged and older adults. Contrary to our hypotheses, our primary analyses did not replicate previous findings showing associations of higher physical activity level with better mood and cognitive functioning (Colcombe & Kramer, 2003; Kvam et al., 2016; Mammen & Faulkner, 2013; Rebar et al., 2015; Singh-Manoux et al., 2005; Sofi et al., 2011). In partial support of our hypotheses, cardiovascular risk burden significantly impacted the relationship between physical activity and cognitive function in the domains of attention and verbal working memory in our primary analyses. Yet, contrary to our hypothesis, the benefits of physical activity were only apparent at lower risk for CVD, while higher CVD risk was associated with no benefit or even a negative relationship.
The lack of main effects of physical activity on mood or cognitive functioning in the total sample is not consistent with most of the previous literature indicating that physical activity is associated with better mood and cognitive functioning (Barnes et al., 2003; Mammen & Faulkner, 2013). However, findings in the literature are mixed, and there are other studies that have not found such a relationship in middle-aged to older adult populations (Fukukawa et al., 2004; Wassink-Vossen et al., 2014). For example, a large study of 1,151 community-dwelling adults (2004) found that physical activity, as measured by pedometer, was associated with decreased depressive symptoms at two-year follow-up in older (age 65 to 79) but not middle-aged (age 40–64) adults. In the Lifestyle Interventions and Independence for Elders Pilot study (Matthews et al., 2011), a 12-month physical activity intervention did not reduce depressive symptoms in adults aged 70 to 89. However, a follow-up study in the same sample showed benefits for men, but not for women (Dotson et al., 2016). In particular, men, but not women, showed decreases in somatic symptoms of depression, but not total depressive symptoms, after the intervention. These previous also suggest the possibility that the present results might have differed by age, sex, or type of depressive symptoms; however, the sample size and mood measure limited our ability to examine this possibility.
These previous studies with null results all focused on walking as a measure of physical activity, and their respective authors suggested that more intense physical activity may have been required to impact depressive symptoms. Similarly, other studies have suggested that the benefits of physical activity on mood and cognitive functioning may be “dose-dependent,” indicating that significant or stronger relationships may only be observed at more intense levels of physical activity (Hu et al., 2019; Kirk-Sanchez & McGough, 2014; Vidoni et al., 2015). However, it should be noted that in the current study, higher total physical activity regardless of intensity was associated with better mood in the follow-up analysis limited to the community center sample. As shown in Table 1, this subsample had a higher level of physical activity than the total sample or the other subgroups, suggesting perhaps that a threshold level of physical activity might be required for a mood benefit.
In partial support of our second hypothesis, cardiovascular risk burden significantly impacted the relationship between physical activity and cognitive function in the domains of attention and verbal working memory. Yet, contrary our hypothesis, the benefits of physical activity were only apparent in people at lower risk for CVD, while higher CVD risk was associated with no benefit or even a negative relationship. Similar patterns were observed for attention in the low-income housing facility subsample and for visual working memory when analyses were limited to Black participants.
There is little research examining the effects of cumulative vascular risk burden on the relationship between physical activity and health outcomes. However, a number of studies, including a recent meta-analysis (Brunt et al., 2019), suggest physical activity is associated with preservation of cognitive functioning in individuals with or at risk for CVD. Because some studies suggest that people with vascular burden benefit from physical activity (Alosco et al., 2014), it is unclear why this was not the case in the current study. There is evidence that strenuous physical activity can actually exacerbate CVD (Armstrong et al., 2015; Williams & Thompson, 2014). However, the level of physical activity in the current sample was not particularly high, so this would not fully explain our results. Furthermore, co-morbid conditions in individuals at higher health risk may limit their ability to obtain the benefits from physical activity compared to those at lower health risk (Zakari et al., 2018). Treatments that involve physical activity interventions to improve mood or cognitive functioning may need to involve approaches that aim to reduce functional and medical barriers that may prevent individuals from benefiting from exercise. Additional research investigating the optimal amount and intensity of physical activity in populations at risk for CVD with depression and cognitive concerns is also necessary to avoid potential iatrogenic effects of exercise.
Overall, results did not confirm our hypothesis that within a predominately Black sample, the individuals who are most at risk for CVD would benefit more from physical activity due to their vulnerability to vascular-related mood and cognitive disruption. Rather, our results are consistent with an alternative hypothesis, that the protective effects of vascular health allow middle aged to older adults to benefit from physical activity, while CVD risk factors limit potential benefits. In line with this hypothesis, an investigation of factors related to cognitive improvement following antidepressant treatment in depression Barch et al. (2012) found that higher vascular risk significantly predicted less improvement in working memory and executive function. More work is needed to determine the neurobiological, lifestyle, and other factors that impact the interrelationships among physical activity, CVD risk, cognitive functioning, and mood.
The literature on physical activity and exercise, like many areas of science, is limited by a lack of diversity in most study samples. This study is based on a primarily Black sample, and it focuses on CVD risk, which is salient in the Black community given racial disparities in vascular disease. Moreover, the focus on the impact of CVD risk on the antidepressant and physical activity-related cognitive enhancement is unique. However, there are limitations to this study, including the cross-sectional design, the relatively small sample size, and the relatively restricted range in mood and cognitive scores in this sample. Additionally, physical activity and risk factors for CVD were obtained through self-report, which is less reliable and less accurate compared to objective data (Bassett Jr, 2000; Loprinzi, 2013). We also do not have information about whether or not CVD risk factors were well controlled by medication or other interventions. These limitations of our information about CVD risk factors for the current sample could explain why we did not observe a main effect of CVD risk on cognitive functioning or mood. Future longitudinal research with larger sample sizes that includes a range of mood and cognitive scores and objective physical activity measures may give more insight on the impact of CVD risk on the mood and cognitive effects of physical activity in diverse adults.
Finally, it should be noted that this study included a high number of participants considered to be of lower socioeconomic status. These individuals may have had limited means to access resources for engaging in physical activity. Murray and colleagues (2012) found that individuals with higher socioeconomic status reported more exercise and stronger intentions to exercise, which may be explained by reduced barriers to exercise. Factors such as socioeconomic status and potential disability in older adults, and especially in Black older adults, likely play a role in physical activity levels. This important consideration should be addressed in future studies to increase the real-world impact of this line of research.
Acknowledgments
The parent project for this study was supported by two grants from the Faculty Research Grant Program that was sponsored by the Office of Research & Graduate Studies (now the Office of Research & Economic Development) at Louisiana State University. At the time the project was funded and executed, Drs. RE and RHW were affiliated with the Department of Kinesiology at Louisiana State University. VMD is supported by the National Institutes of Health (AG054046-04).
Footnotes
Declaration of Interest
VMD is Founder and President of CerebroFit, LLC.
References
- Aizenstein HJ, Baskys A, Boldrini M, Butters MA, Diniz BS, Jaiswal MK, Jellinger KA, Kruglov LS, Meshandin IA, & Mijajlovic MD (2016). Vascular depression consensus report–A critical update. BMC Medicine, 14(1), 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS (2005). Depression in the elderly. The Lancet, 365(9475), 1961–1970. [DOI] [PubMed] [Google Scholar]
- Alosco ML, Spitznagel MB, Cohen R, Raz N, Sweet LH, Josephson R, Hughes J, Rosneck J, & Gunstad J (2014). Decreased physical activity predicts cognitive dysfunction and reduced cerebral blood flow in heart failure. Journal of the neurological sciences, 339(1–2), 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong ME, Green J, Reeves GK, Beral V, & Cairns BJ (2015). Frequent physical activity may not reduce vascular disease risk as much as moderate activity: large prospective study of women in the United Kingdom. Circulation, 131(8), 721–729. [DOI] [PubMed] [Google Scholar]
- Bakker EA, Lee DC, Hopman MTE, Oymans EJ, Watson PM, Thompson PD, Thijssen DHJ, & Eijsvogels TMH (2021). Dose-response association between moderate to vigorous physical activity and incident morbidity and mortality for individuals with a different cardiovascular health status: A cohort study among 142,493 adults from the Netherlands. PLoS Med, 18(12), e1003845. 10.1371/journal.pmed.1003845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, D’Angelo G, Pieper C, Wilkins CH, Welsh-Bohmer K, Taylor W, Garcia KS, Gersing K, Doraiswamy PM, & Sheline YI (2012). Cognitive improvement following treatment in late-life depression: Relationship to vascular risk and age of onset. The American Journal of Geriatric Psychiatry, 20(8), 682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, Satariano WA, & Tager IB (2003). A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. Journal of the American Geriatrics Society, 51(4), 459–465. [DOI] [PubMed] [Google Scholar]
- Bassett DR Jr (2000). Validity and reliability issues in objective monitoring of physical activity. Research quarterly for exercise and sport, 71(sup2), 30–36. [DOI] [PubMed] [Google Scholar]
- Bherer L, Erickson KI, & Liu-Ambrose T (2013). A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. Journal of aging research, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoian HR, & Dotson VM (2021). Vascular depression in Black Americans: a systematic review of the construct and its cognitive, functional, and psychosocial correlates. The Clinical Neuropsychologist, 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle SH, Surwit RS, Georgiades A, Brummett BH, Helms MJ, Williams RB, & Barefoot JC (2007). Depressive symptoms, race, and glucose concentrations: The role of cortisol as mediator. Diabetes Care, 30(10), 2484–2488. [DOI] [PubMed] [Google Scholar]
- Brewster P, Barnes L, Haan M, Johnson JK, Manly JJ, Nápoles AM, Whitmer RA, Carvajal-Carmona L, Early D, & Farias S (2019). Progress and future challenges in aging and diversity research in the United States. Alzheimer’s & Dementia, 15(7), 995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt A, Albines D, & Hopkins-Rosseel D (2019). The effectiveness of exercise on cognitive performance in individuals with known vascular disease: a systematic review. Journal of clinical medicine, 8(3), 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry KE, Elliott EM, & Reese CM (2007). Age and individual differences in working memory: the size judgment span task. J Gen Psychol, 134(1), 43–65. 10.3200/GENP.134.1.43-65 [DOI] [PubMed] [Google Scholar]
- Church T (2011). Exercise in obesity, metabolic syndrome, and diabetes. Progress in Cardiovascular Diseases, 53(6), 412–418. [DOI] [PubMed] [Google Scholar]
- Colcombe S, & Kramer AF (2003). Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science, 14(2), 125–130. [DOI] [PubMed] [Google Scholar]
- Davies EJ, Moxham T, Rees K, Singh S, Coats AJ, Ebrahim S, Lough F, & Taylor RS (2010). Exercise training for systolic heart failure: Cochrane systematic review and meta-analysis. European journal of heart failure, 12(7), 706–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimeo F, Pagonas N, Seibert F, Arndt R, Zidek W, & Westhoff TH (2012). Aerobic exercise reduces blood pressure in resistant hypertension. Hypertension, 60(3), 653–658. [DOI] [PubMed] [Google Scholar]
- Dotson VM, & Duarte A (2020). The importance of diversity in cognitive neuroscience. Annals of the New York Academy of Sciences, 1464(1), 181–191. [DOI] [PubMed] [Google Scholar]
- Dotson VM, Hsu F-C, Langaee TY, McDonough CW, King AC, Cohen RA, Newman AB, Kritchevsky SB, Myers V, & Manini TM (2016). Genetic moderators of the impact of physical activity on depressive symptoms. The Journal of frailty & aging, 5(1), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis Gardner R, Allen PD, Cherry KE, Monroe PA, O’Neil CE, & Wood RH (2006). Baseline Results of an Interdisciplinary Effort to Promote Healthy Aging among Culturally Diverse Adults. Medicine & Science in Sports & Exercise, 38(5), S305. [Google Scholar]
- Ferguson RJ, Robinson AB, & Splaine M (2002). Use of the reliable change index to evaluate clinical significance in SF-36 outcomes. Quality of Life Research, 11(6), 509–516. [DOI] [PubMed] [Google Scholar]
- Frith E, & Loprinzi PD (2017). Physical activity and cognitive function among older adults with hypertension. Journal of hypertension, 35(6), 1271–1275. [DOI] [PubMed] [Google Scholar]
- Fukukawa Y, Nakashima C, Tsuboi S, Kozakai R, Doyo W, Niino N, Ando F, & Shimokata H (2004). Age differences in the effect of physical activity on depressive symptoms. Psychology and aging, 19(2), 346. [DOI] [PubMed] [Google Scholar]
- Glynn NW, Meinhardt AJ, LaSorda KR, Graves JL, Gmelin T, Gerger AM, Caserotti P, & Boudreau RM (2020). An Optimal Self-Report Physical Activity Measure for Older Adults: Does Physical Function Matter? Journal of aging and physical activity, 1(aop), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham G (2015). Disparities in cardiovascular disease risk in the United States. Current cardiology reviews, 11(3), 238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM (2000). The frontal aging hypothesis evaluated. J Int Neuropsychol Soc, 6(6), 705–726. 10.1017/s1355617700666092 [DOI] [PubMed] [Google Scholar]
- Gujral S, Aizenstein H, Reynolds CF 3rd, Butters MA, & Erickson KI (2017). Exercise effects on depression: Possible neural mechanisms. General Hospital Psychiatry, 49, 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekler EB, Buman MP, Haskell WL, Conway TL, Cain KL, Sallis JF, Saelens BE, Frank LD, Kerr J, & King AC (2012). Reliability and validity of CHAMPS self-reported sedentary-to-vigorous intensity physical activity in older adults. Journal of Physical Activity and Health, 9(2), 225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard G, Safford MM, Moy CS, Howard VJ, Kleindorfer DO, Unverzagt FW, Soliman EZ, Flaherty ML, McClure LA, & Lackland DT (2017). Racial differences in the incidence of cardiovascular risk factors in older black and white adults. Journal of the American Geriatrics Society, 65(1), 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Smith L, Imm KR, Jackson SE, & Yang L (2019). Physical activity modifies the association between depression and cognitive function in older adults. Journal of Affective Disorders, 246, 800–805. [DOI] [PubMed] [Google Scholar]
- Jefferson AL, Hohman TJ, Liu D, Haj-Hassan S, Gifford KA, Benson EM, Skinner JS, Lu Z, Sparling J, Sumner EC, Bell S, & Ruberg FL (2015). Adverse vascular risk is related to cognitive decline in older adults. Journal of Alzheimer’s disease : JAD, 44(4), 1361–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SW, Kim SH, Kang SH, Kim HJ, Yoon CH, Youn TJ, & Chae IH (2019). Mortality reduction with physical activity in patients with and without cardiovascular disease. Eur Heart J, 40(43), 3547–3555. 10.1093/eurheartj/ehz564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RB (2010). Diet and exercise in the management of hyperlipidemia. American Family Physician, 81(9), 1097–1102. [PubMed] [Google Scholar]
- Kirk-Sanchez NJ, & McGough EL (2014). Physical exercise and cognitive performance in the elderly: current perspectives. Clinical interventions in aging, 9, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruk P, & Nowicki M (2016). Effects of regular physical activity on pain, anxiety, and depression in patients with treatment-resistant arterial hypertension. Family Medicine & Primary Care Review (3), 268–273. [Google Scholar]
- Kvam S, Kleppe CL, Nordhus IH, & Hovland A (2016). Exercise as a treatment for depression: A meta-analysis. Journal of Affective Disorders, 202, 67–86. [DOI] [PubMed] [Google Scholar]
- Lewis TT, Everson-Rose SA, Colvin A, Matthews K, Bromberger JT, & Sutton-Tyrrell K (2009). The interactive effects of race and depressive symptoms on calcification in African-American and white women. Psychosomatic Medicine, 71(2), 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TT, Guo H, Lunos S, Mendes de Leon CF, Skarupski KA, Evans DA, & Everson-Rose SA (2011). Depressive symptoms and cardiovascular mortality in older black and white adults: Evidence for a differential association by race. Circulation: Cardiovascular Quality and Outcomes, 4(3), 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loprinzi PD (2013). Objectively measured light and moderate-to-vigorous physical activity is associated with lower depression levels among older US adults. Aging & mental health, 17(7), 801–805. [DOI] [PubMed] [Google Scholar]
- Maass A, Düzel S, Goerke M, Becke A, Sobieray U, Neumann K, Lövdén M, Lindenberger U, Bäckman L, & Braun-Dullaeus R (2015). Vascular hippocampal plasticity after aerobic exercise in older adults. Molecular psychiatry, 20(5), 585–593. [DOI] [PubMed] [Google Scholar]
- MacPherson SE, Phillips LH, & Della Sala S (2002). Age, executive function and social decision making: a dorsolateral prefrontal theory of cognitive aging. Psychology and aging, 17(4), 598. [PubMed] [Google Scholar]
- Mammen G, & Faulkner G (2013). Physical activity and the prevention of depression: A systematic review of prospective studies. American Journal of Preventive Medicine, 45(5), 649–657. [DOI] [PubMed] [Google Scholar]
- Matcham F, Norton S, Steer S, & Hotopf M (2016). Usefulness of the SF-36 Health Survey in screening for depressive and anxiety disorders in rheumatoid arthritis. BMC musculoskeletal disorders, 17(1), 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews MM, Hsu FC, Walkup MP, Barry LC, Patel KV, & Blair SN (2011). Depressive symptoms and physical performance in the lifestyle interventions and independence for elders pilot study. Journal of the american geriatrics society, 59(3), 495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DS, Ellis Gardner R, Allen PD, Monroe PA, Cherry KE, O’Neil CE, & Wood RH (2008). Construct validation of physical activity surveys in culturally diverse older adults: A comparison of four commonly used questionnaires. Research Quarterly for Exercise and Sport, 79(1), 42–50. [DOI] [PubMed] [Google Scholar]
- Morimoto SS, Kanellopoulos D, Manning KJ, & Alexopoulos GS (2015). Diagnosis and treatment of depression and cognitive impairment in late life. Annals of the New York Academy of Sciences, 1345(1), 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray TC, Rodgers WM, & Fraser SN (2012). Exploring the relationship between socioeconomic status, control beliefs and exercise behavior: a multiple mediator model. Journal of behavioral medicine, 35(1), 63–73. [DOI] [PubMed] [Google Scholar]
- Pfoh ER, Chan KS, Dinglas VD, Cuthbertson BH, Elliott D, Porter R, Bienvenu OJ, Hopkins RO, & Needham DM (2016). The SF-36 offers a strong measure of mental health symptoms in survivors of acute respiratory failure. A tri-national analysis. Annals of the American Thoracic Society, 13(8), 1343–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2020). R: A language and environment for statistical computing. In R Foundation for Statistical Computing. [Google Scholar]
- Rebar AL, Stanton R, Geard D, Short C, Duncan MJ, & Vandelanotte C (2015). A meta-meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychology Review, 9(3), 366–378. [DOI] [PubMed] [Google Scholar]
- Resnicow K, McCarty F, Blissett D, Wang T, Heitzler C, & Lee RE (2003). Validity of a modified CHAMPS physical activity questionnaire among African-Americans. Medicine & Science in Sports & Exercise. [DOI] [PubMed] [Google Scholar]
- Rotblatt LJ, Aiken-Morgan AT, Marsiske M, Horgas AL, & Thomas KR (2021). Do Associations Between Vascular Risk and Mild Cognitive Impairment Vary by Race? Journal of Aging and Health, 0898264320984357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A, Hillsdon M, Brunner E, & Marmot M (2005). Effects of physical activity on cognitive functioning in middle age: evidence from the Whitehall II prospective cohort study. American journal of public health, 95(12), 2252–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Babyak MA, Georgiades A, Hinderliter A, & Sherwood A (2007). Effects of exercise and weight loss on depressive symptoms among men and women with hypertension. Journal of Psychosomatic Research, 63(5), 463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofi F, Valecchi D, Bacci D, Abbate R, Gensini GF, Casini A, & Macchi C (2011). Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. Journal of internal medicine, 269(1), 107–117. [DOI] [PubMed] [Google Scholar]
- Stewart AL, MILLS KM, King AC, Haskell WL, Gillis D, & Ritter PL (2001). CHAMPS physical activity questionnaire for older adults: Outcomes for interventions. Medicine & Science in Sports & Exercise, 33(7), 1126–1141. [DOI] [PubMed] [Google Scholar]
- Vercambre M-N, Grodstein F, Manson JE, Stampfer MJ, & Kang JH (2011). Physical activity and cognition in women with vascular conditions. Archives of Internal Medicine, 171(14), 1244–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidoni ED, Johnson DK, Morris JK, Van Sciver A, Greer CS, Billinger SA, Donnelly JE, & Burns JM (2015). Dose-response of aerobic exercise on cognition: a community-based, pilot randomized controlled trial. PloS one, 10(7), e0131647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh TL, Homa K, Hanscom B, Lurie J, Sepulveda MG, & Abdu W (2006). Screening for depressive symptoms in patients with chronic spinal pain using the SF-36 Health Survey. The Spine Journal, 6(3), 316–320. [DOI] [PubMed] [Google Scholar]
- Ware JE, Snow KK, Kosinski M, & Gandek B (1993). SF-36 health survey : manual and interpretation guide. Health Institute, New England Medical Center. [Google Scholar]
- Ware JE Jr (2000). SF-36 health survey update. Spine, 25(24), 3130–3139. [DOI] [PubMed] [Google Scholar]
- Wassink-Vossen S, Collard RM, Voshaar RCO, Comijs HC, de Vocht HM, & Naarding P (2014). Physical (in) activity and depression in older people. Journal of affective disorders, 161, 65–72. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1955). Wechsler adult intelligence scale (WAIS). Journal of Consulting Psychology, 19(4), 319–320. [Google Scholar]
- West RL (1996). An application of prefrontal cortex function theory to cognitive aging. Psychol Bull, 120(2), 272–292. 10.1037/0033-2909.120.2.272 [DOI] [PubMed] [Google Scholar]
- Williams PT, & Thompson PD (2014). Increased cardiovascular disease mortality associated with excessive exercise in heart attack survivors. Mayo Clinic Proceedings, [DOI] [PubMed] [Google Scholar]
- Wilson R, Leurgans S, Boyle P, Schneider J, & Bennett D (2010). Neurodegenerative basis of age-related cognitive decline. Neurology, 75(12), 1070–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohannes AM, Doherty P, Bundy C, & Yalfani A (2010). The long-term benefits of cardiac rehabilitation on depression, anxiety, physical activity and quality of life. Journal of Clinical Nursing, 19(19–20), 2806–2813. [DOI] [PubMed] [Google Scholar]
- Zahodne LB, Manly JJ, Narkhede A, Griffith EY, DeCarli C, Schupf NS, Mayeux R, & Brickman AM (2015). Structural MRI predictors of late-life cognition differ across African Americans, Hispanics, and Whites. Current Alzheimer Research, 12(7), 632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakari M, Alsahly M, Koch LG, Britton SL, Katwa LC, & Lust RM (2018). Are There Limitations to Exercise Benefits in Peripheral Arterial Disease? Frontiers in Cardiovascular Medicine, 5(173). [DOI] [PMC free article] [PubMed] [Google Scholar]


