Abstract
Contour-clamped homogeneous electric field pulsed-field gel electrophoresis (CHEF-PFGE) was used to compare Wisconsin isolates of Escherichia coli O157:H7, including 39 isolates from a 1994 day care center outbreak, 28 isolates from 18 individuals from the surrounding geographic area with sporadic cases occurring during the 3 months before the outbreak, and 3 isolates, collected in 1995, from patients with hemolytic-uremic syndrome (HUS) who were from eastern Wisconsin counties other than those inhabited by the day care center and sporadic-case individuals. The technique of CHEF-PFGE using XbaI identified seven highly related restriction endonuclease digestion profiles (REDPs) (93 to 98% similarity) among the 39 day care center isolates and nine XbaI REDPs (63 to 93% similarity) among the 28 isolates from sporadic-case individuals, including REDP 33, which was exhibited by both day care and sporadic-case isolates. PFGE analyses of sequential E. coli O157:H7 isolates from symptomatic day care center attendees revealed that the REDPs of 25 isolates from eight patients were indistinguishable whereas the REDPs of 2 of 6 isolates from two patients differed slightly (93 to 95% similarity). The REDPs of the three isolates from 1995 HUS patients were 78 to 83% similar, with REDP 26 being exhibited by one HUS-associated isolate and an isolate from one day care attendee who did not develop HUS. The genes for both Shiga toxins I and II (stx1 and stx2, respectively) were detected in all but one isolate (sporadic case), and Shiga toxin production by the day care center isolates was not significantly different from that of the other isolates, including the three HUS-associated isolates. Analyses of E. coli O157:H7 isolates from both the day care center outbreak and sporadic cases by CHEF-PFGE permitted us to define the REDP variability of an outbreak and geographic region and demonstrated that the day care center outbreak and a HUS case in 1995 were caused by E. coli O157:H7 strains endemic to eastern Wisconsin.
Escherichia coli O157:H7 is now recognized as an important cause of hemorrhagic colitis and hemolytic-uremic syndrome (HUS) worldwide (16, 17, 36, 37). Bovine food products, particularly ground beef, have been implicated in the majority of foodborne outbreaks, but a diversity of foods, as well as water and person-to-person transmission, have also been linked with outbreaks (9, 12, 23, 29, 46, 49, 50). Person-to-person transmission of E. coli O157:H7 is well documented in day care and extended-care facilities (1, 7, 8, 14, 28, 31, 39, 52), most likely because of the low infectious dose of this pathogen and the increased susceptibility of the individuals in these facilities (16, 17).
A number of subtyping methods have been employed for epidemiological and evolutionary studies of E. coli, including serotype O157:H7 (3, 6, 11, 18, 40, 51). Of these subtyping methods, pulsed-field gel electrophoresis (PFGE) is reportedly the most discriminatory method for subtyping E. coli O157:H7 (3, 48). An enigma associated with PFGE subtyping is the interpretation of minor variations, such as the presence or absence of a single fragment, in the restriction endonuclease digestion profiles (REDPs) of the strains. Minor deviations in REDP can occur as a result of mutations, insertions, and/or deletions within the genome or the gain or loss of plasmids and phages (22, 33, 48). Tenover et al. (48) established criteria for relating empirical differences in REDP to genetic changes and epidemiological relevance. Statistical methods to establish similarity indices among the REDPs of isolates are another way of linking isolates with an index strain (13, 34). Additional investigations of the variation in REDPs of isolates from an outbreak and sporadic-case individuals are needed to support or refine criteria for the application of molecular subtyping techniques to epidemiological investigations.
This study was conducted to determine the genomic variability of E. coli O157:H7 isolates associated with a day care center outbreak and sporadic cases in southeastern Wisconsin. In addition, data on duration of fecal shedding among symptomatic children and the production of Shiga toxins (ST) by O157:H7 isolates from patients with or without HUS were obtained.
(Portions of this work were presented at the 96th General Meeting of the American Society for Microbiology, New Orleans, La., 19 to 23 May 1996 [27].)
MATERIALS AND METHODS
Day care center outbreak and sporadic cases.
Beginning on 25 May 1994, a cluster of sporadic E. coli O157:H7 cases was identified within three contiguous counties (Kenosha, Milwaukee, and Waukesha) in southeastern Wisconsin. During the period 20 to 31 July 1994, 43 of 196 children from a single day care center in Kenosha County were reported to have symptoms of diarrhea and abdominal cramps. Thirty-nine (91%) of the symptomatic children from the day care center had laboratory-confirmed cases of E. coli O157:H7. A case of infection was defined by bloody diarrhea, or at least three loose stools in a 24-h period, and a stool specimen that was positive for E. coli O157:H7. None of the day care center attendees or individuals with sporadic cases developed HUS.
Stool specimen kits were provided for day care center attendees and any family member(s) that experienced diarrhea in the week preceding 28 July 1994. Stool specimens were submitted to the local health department laboratories for routine bacterial and parasitic testing and for an initial screening for E. coli O157:H7 using MacConkey sorbitol medium (Difco Laboratories, Detroit, Mich.). Sorbitol-negative colonies were forwarded to the state Laboratory of Hygiene (Madison, Wis.) for O157 and H7 agglutination tests. Isolates confirmed as O157:H7 were then sent to the Food Research Institute of the University of Wisconsin for further analyses.
Bacterial strains.
A total of 67 E. coli O157:H7 isolates were obtained, including 39 from the day care center outbreak and 28 from individuals with sporadic cases. The sporadic cases occurred during the 3 months preceding the day care center outbreak and were in the same geographic region as the day care center. Additionally, O157:H7 isolates from three individuals with HUS in three different counties in eastern Wisconsin were obtained in 1995. These individuals included a 9-year-old female (strain 854) who resided in a county 150 miles from the day care center and reported onset of symptoms on 22 May 1995; a 19-year-old female (strain 853), with onset of symptoms on 1 June 1995, who had worked at a fast-food restaurant located 120 miles from the day care center; and a 7-year-old male (strain 856) who had visited a farm in a county about 50 miles from the day care center within a week before onset of symptoms on 11 October 1995. These three HUS cases were considered sporadic since no common linkages in time or exposure among the three patients were identified. Isolates were restreaked onto MacConkey sorbitol agar (Difco) to check for purity and stored at −70°C in nutrient broth (Difco) containing 10% glycerol.
PFGE.
The PFGE technique of contour-clamped homogeneous electric field (CHEF) electrophoresis was conducted as previously described (18). Genomic DNA in agarose plugs was digested with XbaI (Promega Corp., Madison, Wis.) as recommended by the manufacturer. The resulting macrorestriction fragments were separated by CHEF-PFGE, using a CHEF-DRII apparatus (Bio-Rad Laboratories, Richmond, Calif.) at 200 V and 17°C for 21 h with switch times ranging from 1 to 40 s. Lambda concatamers (New England Biolabs, Inc., Beverly, Mass.) were used as DNA size standards.
Calculation of similarity indices.
The presence or absence of restriction fragments for each strain was entered into ELBAMAP as binary scores. The ELBAMAP program was used to calculate the Dice similarity indices among strains as described by Brosch et al. (13).
Detection of ST genes.
The ST genes (stx1 and stx2) were detected by Southern blot hybridization (41). The DNA from each strain was extracted and digested with EcoRI (Promega). After electrophoresis, the DNA was transferred to a nylon membrane. Two 20-bp oligonucleotide probes (20, 26) (National Biosciences, Plymouth, Minn.) were labeled with digoxigenin (Genius 6 tailing kit; Boehringer Mannheim, Indianapolis, Ind.). Hybridized probe was detected by following the manufacturer’s instructions (Genius 3; Boehringer Mannheim).
Protein concentrations.
The protein contents of E. coli cell extracts were determined by using the Micro BCA Protein Assay Reagent according to the manufacturer’s instructions (Pierce, Rockford, Ill.). Briefly, 50-μl samples of each cell extract were added separately to wells of a microtiter plate, and the plates were incubated for 45 min at 60°C. The standard curve was generated by using bovine serum albumin (Sigma Chemical Co., St. Louis, Mo.). The absorbance at 560 nm (A560) was measured (MR 600 microplate reader; Dynatech Laboratories, Inc., Alexandria, Va.).
ELISA for ST.
Strains of E. coli O157:H7 were grown in Trypticase soy broth (Difco) for 19 h at 35°C with shaking (150 rpm). Cells were pelleted by centrifugation (13,800 × g, 10 min) and washed twice with 0.2 M phosphate buffer (pH 6.5). The resulting pellets were resuspended in phosphate buffer containing 0.1 mg of polymyxin B/ml and incubated for 30 min at 37°C (4). Cells and debris were pelleted by centrifugation, and the supernatants were filtered through 0.22-μm-pore-size filters (Gelman Science, Ann Arbor, Mich.). Culture supernatants and polymyxin extracts were tested for quantities of STI and STII by using protein G (Pharmacia, Uppsala, Sweden)-purified ascites fluid from hybridomas ATCC CRL 1794 and ATCC CRL 1907 (32, 44), respectively, in an enzyme-linked immunosorbent assay (ELISA) (5). Globotriacylceramide (Gb3; Matreya, Inc., Pleasant Gap, Pa.) (20 mg/ml, 100 μl/well) was used to coat the wells of a MicroTest III flexible assay plate (Falcon, Oxnard, Calif.). Peroxidase-conjugated goat anti-mouse antibody (Sigma) was used as a secondary antibody with K-Blue (ELISA Technologies, Lexington, Ky.) substrate for horseradish peroxidase. After 15 min of incubation at room temperature, the reaction was stopped with 2.5 N H2SO4, and the contents of the wells were transferred to a new microtitration plate for determination of the dual end absorbance at 450 to 650 nm (THERMOmax microtiter plate reader; Molecular Devices Co., Menlo Park, Calif.).
Partially purified STI and STII were used to establish standard curves. The toxin concentrations of the purified preparations were determined by densitometry (Kendrick Laboratories, Madison, Wis.) in a nondenaturing polyacrylamide gel (gradient, 4 to 20%; Bio-Rad). Four trials were done for each standard curve.
Statistical analyses.
The data were processed by using the Statistical Analysis System (SAS) (version 6.1; SAS Institute Inc., Cary, N.C.). The standard curve followed a sigmoidal distribution, and a four-parameter logistical model (35) was used: OD = A + {(B − A)/[1 + exp(log concentration − C)/D]}, where OD is the optical density, A is the asymptote at concentration μ, B is the asymptote at concentration 0, C is the inflection point, and D is a scale parameter tied to the slope of the curve near the inflection point. When the ELISA absorbance values were equivalent to background values, an arbitrary value 0.01 OD units higher than the negative control (background) was entered for statistical comparisons. Analysis of variance was conducted with SAS. Log values of the estimated toxin concentrations were used to ensure constant variance. An analysis of least significant differences was used for multiple-comparison tests of toxin concentration.
RESULTS
Day care center outbreak.
Epidemiological data for 20 cases associated with the Kenosha County day care center outbreak are shown in Table 1. Onset of diarrhea among children in the six rooms occurred between 20 and 31 July 1994 and ceased 1 to 23 days later. The children (8 males and 12 females) ranged in age from 11 to 62 months. Five of the children were prescribed antibiotics for their illness. E. coli O157:H7 was isolated from patient stool samples 2 to 39 days after onset of diarrhea. Stool specimens from 10 patients continued to be positive for 5 to 23 days (mean, 15.1 days; median, 11.5 days), even after diarrhea ceased. Two negative stool specimens were required by the day care facility’s administration for readmission to the day care center. Among the 20 cases shown in Table 1, six individuals had one or more positive stool specimens following the first negative stool sample and required multiple samples before two sequential negative stool specimens were obtained (data not shown). This process resulted in 59 total stool specimens from 18 patients, and 39 yielded O157:H7 isolates. Isolates from two individuals were unavailable for further analysis. Only a single isolate from a positive stool specimen was retained for CHEF-PFGE analyses. The status of the day care center employees with regard to infection by E. coli O157:H7 was unknown because they did not submit fecal samples for testing.
TABLE 1.
Epidemiological data for E. coli O157:H7 isolates from the day care center outbreak, Kenosha County, Wis., July to August 1994
| Patient no. | Age (mo) | Sexa | Day care room | Date of onset of diarrhea (mo/day) | Duration of diarrhea (days) | Date symptoms ceased (mo/day) | Dates antibiotics administered (mo/day) | Minimum shedding time (days)b | No. of isolates evaluatedc | Isolate REDPd |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 38 | M | 4 | 7/20 | 14 | 8/2 | Ne | 19 | 2 | 33 |
| 2 | 31 | M | 3 | 7/20 | 21 | 8/9 | N | 10 | 1 | 33 |
| 3 | 62 | F | 6 | 7/22 | NAf | NA | N | 1 | 1 | 33 |
| 4 | 22 | F | 2 | 7/22 | NA | NA | N | 39 | 4 | 33 |
| 5 | 21 | M | 2 | 7/22 | 6 | 7/27 | 7/27–8/5 | 26 | 2 | 33 |
| 6 | 18 | M | 2 | 7/23 | 7 | 7/29 | N | 11 | 1 | 50 |
| 7 | 41 | M | 4 | 7/23 | 3 | 7/24 | N | 18 | 2 | 26, 48 |
| 8 | 23 | M | 2 | 7/23 | NA | NA | N | 11 | 1 | 33 |
| 9 | 38 | F | 4 | 7/24 | NA | NA | N | 36 | 5 | 51 |
| 10 | 35 | M | 3 | 7/24 | 2 | 7/25 | 7/25–8/3 | 25 | 3 | 33 |
| 11 | 35 | F | 3 | 7/25 | 10 | 8/3 | N | 5 | NDg | |
| 12 | 32 | F | 3 | 7/26 | 7 | 8/1 | N | 23 | 3 | 33 |
| 13 | 51 | F | 5 | 7/26 | NA | NA | 7/27–8/5 | 8 | ND | |
| 14 | 15 | F | 2 | 7/27 | 2 | 7/28 | N | 11 | 1 | 33 |
| 15 | 44 | F | 4 | 7/27 | 2 | NA | N | 2 | 1 | 33 |
| 16 | 11 | F | 1 | 7/28 | 24 | 8/21 | 8/21–8/25 | 25 | 4 | 33, 49 |
| 17 | 28 | F | 3 | 7/28 | 2 | 7/29 | N | 15 | 2 | 51 |
| 18 | 38 | F | 4 | 7/29 | 2 | 7/30 | N | 12 | 4 | 33 |
| 19h | 35 | M | 3 | 7/31 | 3 | 8/2 | N | 2 | 1 | 47 |
| 20h | 35 | F | 3 | 7/31 | 3 | 8/2 | 8/8–8/12 | 2 | 1 | 33 |
M, male; F, female.
Number of days between onset of diarrhea and last E. coli O157:H7-positive fecal sample.
Number of E. coli O157:H7 isolates obtained from sequential fecal samples.
A single designation indicates that all isolates displayed the same REDP.
N, no antibiotics administered.
NA, information not available.
ND, not determined; the case was culture confirmed, but the isolate was not available for further analysis.
Siblings.
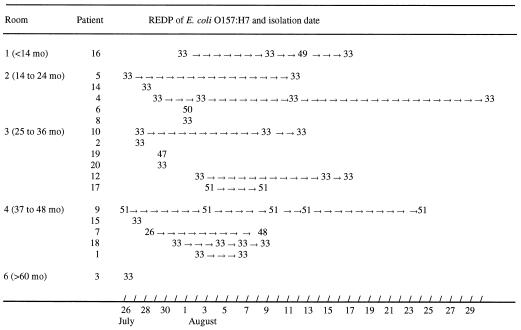
The 39 O157:H7 isolates typed by using XbaI and CHEF-PFGE displayed seven different REDPs. A single profile, REDP 33, was identified from 13 cases (72%), with REDP 51 being displayed by isolates from two children (11%) and REDP 26, 47, 48, 49, and 50 being displayed by isolates from individual children (5.5%). The predominant strain (REDP 33) was isolated from children in five of the six rooms of the day care facility (Table 1; Fig. 1). The REDPs of multiple isolates from eight children were the same throughout the period of shedding. For example, four isolates recovered from patient 4 over 39 days were all REDP 33, and five isolates from patient 9 recovered over 36 days were all REDP 51. The REDPs of isolates from two children differed (patient 7, REDP 26 and 48; patient 16, REDP 33 and 49) but were highly similar (93% similarity).
FIG. 1.
The duration of shedding and REDPs of isolates from children in different rooms (age groups) of the day care center.
Sporadic cases.
A total of 28 E. coli O157:H7 isolates were obtained from 18 individuals with sporadic cases during the period May to July 1994 (Table 2). The infected individuals (10 males and 8 females) were from 1 to 78 years of age. The sporadic cases occurred in three counties in southeastern Wisconsin in the 3 months preceding the day care center outbreak. The E. coli O157:H7 isolates displayed nine XbaI REDPs, including the predominant pattern (REDP 33) exhibited by most of the isolates from the day care center outbreak. Isolates from 2 of the 18 (11%) individuals with sporadic cases displayed REDP 33, whereas 13 of 18 (72%) individuals from the day care center displayed this REDP. The predominant REDPs of sporadic-case isolates were REDP 40 (17%), 43 (17%), and 41 (28%). The REDPs of isolates from one family pair (patient 24, REDP 40; patient 33, REDP 42) differed and were only 63% similar. However, other cases involving families (patients 31 and 32, REDP 41; patients 34, 35, and 38, REDP 43) involved O157:H7 strains with the same REDP. The REDPs of sequential isolates from patient 30 differed but were highly similar (98% similarity).
TABLE 2.
Epidemiological data for E. coli O157:H7 clinical isolates from patients in southeastern Wisconsin, May to July 1994 (sporadic cases)
| Patient no. | Age (yr) | Sexa | Specimen collection date (mo/day) | County of residence | No. of isolates evaluatedb | Isolate REDPc |
|---|---|---|---|---|---|---|
| 21 | 15 | M | 5/26 | Milwaukee | 1 | 33 |
| 22 | Ud | M | 6/4 | Milwaukee | 1 | 46 |
| 23 | 42 | M | 6/6 | Kenosha | 2 | 40 |
| 24e | 45 | F | 6/6 | Kenosha | 2 | 40 |
| 25 | 78 | F | 6/9 | Kenosha | 2 | 40 |
| 26 | 17 | M | 6/10 | Waukesha | 1 | 39 |
| 27 | 40 | F | 6/13 | Kenosha | 2 | 41 |
| 28 | 3 | M | 6/17 | Milwaukee | 1 | 43 |
| 29 | 2 | M | 6/23 | Waukesha | 1 | 41 |
| 30 | 53 | F | 6/24 | Kenosha | 2 | 41, 45 |
| 31f | 3 | M | 7/5 | Kenosha | 2 | 41 |
| 32f | 4 | M | 7/6 | Kenosha | 2 | 41 |
| 33e | 2 | F | 7/9 | Kenosha | 3 | 42 |
| 34g | 5 | M | 7/11 | Milwaukee | 1 | 43 |
| 35g | 7 | F | 7/11 | Milwaukee | 1 | 43 |
| 36 | 13 | F | 7/12 | Kenosha | 2 | 44 |
| 37 | 9 | M | 7/15 | Waukesha | 1 | 33 |
| 38g | 1 | F | 7/17 | Milwaukee | 1 | 43 |
M, male; F, female.
Number of E. coli O157:H7 isolates obtained from patient.
A single designation indicates that all isolates displayed indistinguishable REDPs.
U, unknown.
Family pair member.
Family pair member.
Family group member.
REDP similarity.
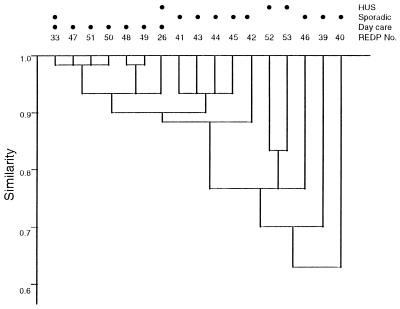
XbaI and CHEF-PFGE analyses of the 67 E. coli O157:H7 isolates from the day care center outbreak and sporadic-case individuals identified a total of 15 REDPs, each of which displayed 19 to 23 fragments ranging from ca. <48.5 to 580 kb in length (Fig. 2). All strains displayed similar numbers and migration profiles of fragments in the size range of ca. <48 kb and 200 to 300 kb, including a pronounced doublet at ca. 290 kb. Other restriction enzymes were not evaluated since XbaI is most discriminatory for analyzing isolates of E. coli O157:H7 (18). Although seven different REDPs were identified among the isolates from the day care center patients, the REDPs were 93 to 98% similar by the Dice similarity index (Fig. 3). In comparison, the REDPs of isolates recovered from individuals with sporadic cases were 63 to 93% similar. The 15 REDPs were compared to the XbaI REDPs of three isolates from the 1995 HUS patients in eastern Wisconsin. The isolates from individuals with HUS were 78 to 83% similar to each other, and the REDP of one HUS isolate was identical to the REDP of an isolate from a day care center attendee (REDP 26). The data from analyses of this limited number of HUS strains indicate that the REDPs of HUS isolates are not appreciably different from those of non-HUS isolates.
FIG. 2.
Diagram, generated by ELBAMAP, of the 17 E. coli O157:H7 XbaI REDPs identified among strains isolated from day care center attendees, individuals with sporadic cases, and HUS patients from Wisconsin. λ, lambda concatamers (sizes are shown to the left).
FIG. 3.
The XbaI REDP relatedness of isolates of E. coli O157:H7 from day care center attendees, individuals with sporadic cases, and HUS patients. The Dice REDP similarities were subjected to cluster analysis as unweighted matched-pair groups. Horizontal lines represent the degree of similarity among isolates or groups connected by the lines. Closed circles designate the source(s) of the isolates whose REDPs were displayed.
Presence of stx1 and stx2.
Hybridization of EcoRI digests of an E. coli O157:H7 isolate from the day care center outbreak and individuals with sporadic cases representing each of the 15 XbaI REDPs with oligonucleotide probes for stx1 and stx2 (20) demonstrated that both genes were present in all of the isolates except strain 551, which did not hybridize with either of the stx probes. With the exception of strain 526, common EcoRI fragments hybridized with the stx1 and stx2 oligonucleotide probes among the positive strains (data not shown).
ST production.
The quantities of STI and STII produced by a strain representative of each of the 15 XbaI E. coli O157:H7 REDP were determined by ELISA (Table 3). The levels of STI and STII produced by the day care center isolates ranged from 6.46 to 16.38 and from 397.38 to 826.39 ng/mg of protein, respectively. In comparison, the toxin levels from sporadic-case isolates ranged from 6.49 to 29.54 ng/mg of protein for STI and <30.46 to 760.00 ng/mg of protein for STII. Three isolates from sporadic-case individuals (strains 526, 535, and 551) produced significantly less STII (P < 0.05) than the other isolates. In contrast, strain 529 (sporadic case) produced significantly more STI (P < 0.05) than the other strains. The quantity of STI and STII produced by isolates from patients with HUS ranged from 6.46 to 14.28 and from 463.62 to 904.97 ng/mg of protein, respectively, which was not significantly different from the toxin levels for other E. coli O157:H7 isolates, with the exception of the strains previously noted. There was no apparent association between REDP and the level of ST produced. For example, strain 535 (REDP 44) was highly related (>90% similar) to strains 528 (REDP 43) and 541 (REDP 45), but strain 535 produced significantly less STII (Table 3). However, strain 526, which produced only 30.46 ± 12.46 ng of STII/mg of protein (mean ± standard deviation), had a REDP that was only 60% similar to that of the other strains (Fig. 3) and carried the stx2 gene on an EcoRI fragment different from that of the other strains (data not shown). The absence of STI and STII production by strain 551 was due to the absence of stx1 and stx2 sequences, which were most likely lost during subculture (21, 22, 36).
TABLE 3.
Quantities of STI and STII produced by E. coli O157:H7 strains from sporadic, day care, and HUS cases, by REDP
| Group and strain no. | REDPa | Concn (ng/mg of protein) ofb:
|
|
|---|---|---|---|
| STI | STII | ||
| Day carec | |||
| 583 | 33 | 16.38 ± 4.46 | 467.08 ± 14.90 |
| 579 | 47 | 12.51 ± 2.84 | 637.12 ± 62.85 |
| 729 | 48 | 8.11 ± 2.26 | 552.03 ± 103.55 |
| 723 | 49 | 9.92 ± 2.14 | 455.17 ± 58.82 |
| 589 | 50 | 15.20 ± 2.70 | 397.38 ± 57.41 |
| 585 | 51 | 14.44 ± 4.14 | 444.82 ± 76.09 |
| Sporadicd | |||
| 523 | 39 | 6.49 ± 0.30 | 684.59 ± 165.07 |
| 526 | 40 | 6.93 ± 0.05 | *30.46 ± 12.46 |
| 529 | 41 | *29.54 ± 4.35 | 616.12 ± 55.07 |
| 533 | 42 | 12.64 ± 1.69 | 534.67 ± 54.50 |
| 528 | 43 | 6.70 ± 0.11 | 760.00 ± 27.36 |
| 535 | 44 | 7.06 ± 0.24 | *224.87 ± 59.56 |
| 541 | 45 | 16.89 ± 3.40 | 627.89 ± 73.20 |
| 551e | 46 | *6.29 | *10.16 |
| HUSf | |||
| 856 | 26 | 6.46 ± 0.21 | 826.39 ± 141.40 |
| 853 | 52 | 14.28 ± 1.97 | 463.62 ± 42.54 |
| 854 | 53 | 7.12 ± 0.08 | 904.97 ± 66.07 |
See Fig. 1.
As determined by ELISA, in both cell extracts and culture supernatants. Values are averages ± standard deviations for data from three trials. A standard curve was generated with STI and STII (n = 4). Values preceded by an asterisk are significantly different (P < 0.05).
Isolates from day care center outbreak in 1994. The isolate from patient 7 (Table 1) that displayed REDP 26 was not tested (see strain 856).
Isolates from individuals with sporadic cases in 1994. The isolates from patients 21 and 37 (Table 2) that displayed REDP 33 were not tested (see strain 583).
Negative for stx1 and stx2.
Isolates from patients with HUS (1995).
DISCUSSION
Person-to-person transmission continues to be a significant mode of E. coli O157:H7 transmission, particularly in day care and long-term care centers (17, 45), in part because of the susceptible populations in these facilities. In the Kenosha County day care center outbreak, none of the children developed HUS, and 65% of the affected individuals were children 2 years of age or younger. Studies of previous day care center outbreaks have shown an incidence of HUS ranging from 4 to 17% (median, 7.5%) (1, 7, 16, 36–38, 52). The highest incidence of HUS is in children under 5 years of age, particularly those between the ages of 1 and 2 years (16, 38, 43). One possible explanation for the absence of HUS among the day care center cases in this report is that the E. coli O157:H7 strain produced low levels of ST, which plays a key role in HUS (16). In addition, there is little quantitative data on the levels of STI and STII produced by E. coli O157:H7 isolates from hemorrhagic colitis and HUS cases.
The duration of fecal shedding of E. coli O157:H7 observed in the Kenosha County day care outbreak ranged from 2 to 39 days (median, 13 days), which is comparable to the median duration of shedding reported in other day care center outbreaks (7, 22, 42, 52). It is noteworthy that the median duration of shedding in a day care center in Germany was also 13 days (range, 2 to 62 days) for children with diarrhea and hemorrhagic colitis but was 21 days for cases with HUS (22). The administration of antibiotics to children during the Kenosha County day care center outbreak did not influence the duration of shedding; such treatment should be discouraged, since there is some evidence that this practice is associated with progression to HUS (16, 47). Additionally, shedding of viable organisms continued past the date when diarrhea ceased, and this should be taken into consideration when establishing criteria for readmission of children into day care. Cohorting of returning children that were previously symptomatic into a single room of the day care facility until two negative stools are obtained is a reasonable policy (2). Reentry of previously symptomatic or stool-positive children into their originally assigned room should be dependent on submission of two consecutive negative stool samples (2, 45). If a single negative stool specimen had been required for the Kenosha County day care outbreak, approximately one-third of the previously symptomatic children would have returned to the center when they were still shedding E. coli O157:H7, potentially exposing other susceptible children and staff members. Moreover, asymptomatic children and day care center personnel should also be tested, because they may also be infected and shedding this pathogen despite the absence of symptoms (36). However, control measures should not be limited to testing of stool specimens (10, 45), because shedding of E. coli O157:H7 is intermittent and is difficult to detect in the later stages of infection (24, 36, 45, 47).
The epidemic curve of illness stratified by age group (room) suggests that the mode of transmission was person to person between rooms and within rooms rather than from a point source. A food history questionnaire did not implicate a common food source, but the children attending the day care center ate the same snacks and lunches. There were eight families with two or more siblings attending the day care facility. Among these families, four had no symptomatic day care center attendees, both siblings of one family were positive, and only one of two or three siblings in the other three families were infected. This reinforces the probability that in-home or transportation-based settings did not play a role in this outbreak.
Prior to the day care center outbreak, a cluster of sporadic cases of E. coli O157:H7 was identified in three contiguous counties in southeastern Wisconsin. The main REDP displayed by day care center isolates was exhibited by isolates from two individuals with sporadic cases, including one Waukesha County case occurring five days prior to the onset of the day care facility outbreak. The proximity of Waukesha and Kenosha counties and the isolation of the index strain before the day care center outbreak support the hypothesis that the day care facility outbreak resulted from the introduction of a serotype O157:H7 strain that was circulating within this community. Furthermore, the isolation of an O157:H7 strain that displayed REDP 26 in 1995 from an individual with HUS (approximately 100 miles from Kenosha County), indistinguishable from an isolate from a day care attendee, demonstrates the persistence of a strain in a region. The circulation of an endemic strain(s) within a region is further supported by the isolation of E. coli O157:H7 that displayed either REDP 26 or 33 from heifer cows on two southern Wisconsin dairy farms approximately 100 and 200 miles from Kenosha County (15). It is important to note that REDP 26 and 33 are 97% similar. Thus, E. coli O157:H7 isolates from cows and humans in this region display REDP 33 or a highly related REDP. It would be of interest to survey retail foods originating from this region for E. coli O157:H7 to determine if the isolates recovered from food display REDPs related to those of the isolates examined in this study. Although contaminated food was not implicated in the day care center outbreak, person-to-person spread likely contributed to the dissemination of E. coli O157:H7.
In contrast to the day care facility isolates, which were ≥93% similar, the nine REDPs displayed by isolates recovered from the 18 sporadic-case individuals were between 63 and 93% similar. The greater variation in the REDPs of sporadic-case isolates was expected, since these REDPs were displayed by isolates from separate cases in three counties, rather than from within a single county like the outbreak isolates. Although there was not a predominant strain, REDP 41 was the most common pattern, and it was displayed by isolates from five individuals with sporadic cases, four in Kenosha County and one in Waukesha County. Also, REDP 41 is related to REDP 33 (90% similarity). In contrast, three Kenosha County cases yielded isolates displaying REDP 40, which was only 63% similar to other sporadic-case and day care center strains. This strain was likely a recent introduction to eastern Wisconsin; this theory is supported by the isolation of a strain with REDP 40 from one family member but not the other (REDP 42), and the REDPs differed greatly (ca. 63% similarity), suggesting that two sources of infection affected this family. The other two families identified among sporadic cases had isolates with indistinguishable REDPs.
For the day care facility outbreak, in 8 of 10 cases in which sequential isolates were obtained, a single REDP was recovered. It is possible that additional individuals shed strains with slightly different REDPs, because only a single isolate from each positive sample was retained for CHEF-PFGE analysis. The instability of stx (21, 22, 36) could account for minor differences in REDP, but the stx genes were detected in all of the isolates but one. Thus, the stx genes were not responsible for the minor changes in REDP noted in sequential isolates. Karch et al. (22) described the appearance of different but similar REDP as “clonal turnover” which results from mutations and rearrangements within the genome or the gain or loss of plasmids. The high degree of similarity between the REDPs of isolates from two day care attendees in which two REDPs were identified suggests that a genetic change occurred in the infecting strain, rather than an infection by a second strain.
The ability to associate isolates with an outbreak has traditionally relied on epidemiological association and, if possible, microbiological substantiation. Molecular subtyping methods have been used for epidemiological studies to verify the relatedness of isolates. Among the various methods used to subtype E. coli O157:H7, PFGE is highly discriminatory (3). The optimization and application of PFGE for molecular subtyping have necessitated the development of guidelines for interpreting results in an epidemiologically relevant manner. As one approach, Tenover et al. (48) proposed that strains be grouped as indistinguishable, closely related, possibly related, or different based on the number of restriction fragment differences when compared with the outbreak strain. As a complement to the aforementioned subjective approach, other investigators have used mathematical methods to better quantify intrinsic biological and perhaps technical REDP variability for grouping strains (13, 19, 25, 34). As outlined above, we utilized both approaches for grouping strains and complementing both epidemiological and microbiological data. The occurrence of sporadic cases of E. coli O157:H7 in the same area as the day care center outbreak enabled us to identify REDP 33 as the predominant day care strain among sporadic cases, and it provided an opportunity to compare outbreak isolates with sporadic-case isolates from the same area. The REDPs of the day care facility isolates differed by one to three bands, which corresponds to a Dice similarity index of ≥93%, and the most closely related sporadic-case isolates, excluding REDP 33 (the day care outbreak strain), were 90% similar to day care center isolates (Fig. 3). Thus, a Dice similarity index of ≥93% for XbaI REDP of E. coli O157:H7 isolates appears to be a suitable threshold value for including an isolate(s) as part of an outbreak and is close to the value Krause et al. (25) suggested (≥95% similarity) for linking E. coli O157:H7 isolates. In a study of several gram-negative and gram-positive bacteria, isolates that displayed REDPs that were ≥85% similar (no more than three band differences in REDP) were considered related (19). Since the Dice similarity index is influenced by the total number of bands in the REDP, the value for including an isolate as part of an outbreak will vary with the bacterial species and the restriction enzyme used. Establishment of REDP databases and analyses of other outbreaks are needed to refine criteria for interpretation of REDPs and to link isolates with an outbreak.
The XbaI REDPs of O157:H7 strains recovered from individuals with HUS were not highly related (78 to 83% similar), and they did not contain fragment(s) that could be associated with HUS. The absence of HUS among children involved in the day care center outbreak was unusual because 4 to 17% of children under 5 years of age that are infected with E. coli O157:H7 typically develop HUS (16, 31, 43). One possible explanation for the absence of HUS in the day care facility outbreak was that the O157:H7 outbreak strain produced low levels of ST. However, ST production by isolates from the day care center outbreak was not significantly different (P < 0.05) from that of isolates from individuals with sporadic cases or HUS, but some sporadic-case isolates produced more STI and some produced less STII (Table 3). Thus, ST production by the strain(s) involved in the day care center outbreak was not attenuated, and the absence of HUS is more likely due to host or other strain factors (16, 30).
These data further highlight the discriminatory power of CHEF-PFGE and the utility of molecular subtyping of isolates from outbreaks as well as from individuals with sporadic cases. The establishment of REDP databases will be valuable in the identification of endemic strains and refinement of criteria for REDP interpretation. To this end, additional isolates of E. coli O157:H7 from retail foods are needed for databases.
ACKNOWLEDGMENTS
We are grateful to Roger Johnson, Health Canada, for providing purified Shiga toxins I and II.
This project was supported in part by the College of Agricultural and Life Sciences, University of Wisconsin—Madison, and by contributions to the Food Research Institute.
REFERENCES
- 1.Allaby M A K, Mayon-White R. Escherichia coli O157: outbreak in a day nursery. CDR Rev PHLS. 1995;5:R4–R6. [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics. Escherichia coli diarrhea. In: Peter G, editor. Red book: report of the Committee on Infectious Diseases. 24th ed. Elk Grove Village, Ill: American Academy of Pediatrics; 1997. p. 82. [Google Scholar]
- 3.Arbeit R D, Arthus M, Dunn R, Kim C, Selander R K, Goldstein R. Resolution of recent evolutionary divergence among Escherichia coli from related lineages: the application of pulsed field electrophoresis to molecular epidemiology. J Infect Dis. 1990;161:230–235. doi: 10.1093/infdis/161.2.230. [DOI] [PubMed] [Google Scholar]
- 4.Ashkenazi S, Cleary T G. Rapid method to detect Shiga toxin and Shiga-like toxin I based on binding to globotriosyl ceramide (Gb3), their natural receptor. J Clin Microbiol. 1989;27:1145–1150. doi: 10.1128/jcm.27.6.1145-1150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashkenazi S, Cleary T G. A method for detecting Shiga toxin and Shiga-like toxin-I in pure and mixed culture. J Med Microbiol. 1990;32:255–261. doi: 10.1099/00222615-32-4-255. [DOI] [PubMed] [Google Scholar]
- 6.Barrett T J, Lior H, Green J H, Khakhria R, Wells J G, Bell B P, Greene K D, Lewis J, Griffin P M. Laboratory investigation of a multistate food-borne outbreak of Escherichia coli O157:H7 by using pulsed-field gel electrophoresis and phage typing. J Clin Microbiol. 1994;32:3013–3017. doi: 10.1128/jcm.32.12.3013-3017.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belongia E A, Osterholm M T, Soler J T, Ammend D A, Braun J E, MacDonald K L. Transmission of Escherichia coli O157:H7 infection in Minnesota child day-care facilities. JAMA. 1993;269:883–888. [PubMed] [Google Scholar]
- 8.Bender J B, Hedberg C W, Besser J M, Boxrud D J, MacDonald K L, Osterholm M T. Surveillance for Escherichia coli O157:H7 infections in Minnesota by molecular subtyping. N Engl J Med. 1997;337:388–394. doi: 10.1056/NEJM199708073370604. [DOI] [PubMed] [Google Scholar]
- 9.Besser R E, Lett S M, Weber J T, Doyle M P, Barrett T J, Wells J G, Griffin P M. An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157:H7 in fresh-pressed apple cider. JAMA. 1993;269:2217–2220. [PubMed] [Google Scholar]
- 10.Black R E, Dykes A C, Anderson K E, Wells J G, Sinclair S P, Gary G W, Jr, Hatch M H, Gangarosa E J. Handwashing to prevent diarrhea in day-care centers. Am J Epidemiol. 1981;113:445–451. doi: 10.1093/oxfordjournals.aje.a113112. [DOI] [PubMed] [Google Scholar]
- 11.Böhm H, Karch H. DNA fingerprinting of Escherichia coli O157:H7 strains by pulsed-field gel electrophoresis. J Clin Microbiol. 1992;30:2169–2172. doi: 10.1128/jcm.30.8.2169-2172.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brewster D H, Brown M I, Robertson D, Houghton G L, Bimson J, Sharp J C M. An outbreak of Escherichia coli O157 associated with a children’s paddling pool. Epidemiol Infect. 1994;112:441–447. doi: 10.1017/s0950268800051141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brosch R, Chen J, Luchansky J B. Pulsed-field fingerprinting of listeriae: identification of genomic divisions for Listeria monocytogenes and their correlation with serovar. Appl Environ Microbiol. 1994;60:2584–2592. doi: 10.1128/aem.60.7.2584-2592.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter A O, Borczyc A A, Carlson J A K, Harvey B, Hockin J C, Karmali M A, Chandrasekar Krishnan C B, Korn D A, Lior H. A severe outbreak of Escherichia coli O157:H7-associated hemorrhagic colitis in a nursing home. N Engl J Med. 1987;317:1496–1500. doi: 10.1056/NEJM198712103172403. [DOI] [PubMed] [Google Scholar]
- 15.Faith N G, Shere J A, Brosch R, Arnold K W, Ansay S E, Lee M-S, Luchansky J B, Kaspar C W. Prevalence and clonal nature of Escherichia coli O157:H7 on dairy farms in Wisconsin. Appl Environ Microbiol. 1996;62:1519–1525. doi: 10.1128/aem.62.5.1519-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffin P M. Escherichia coli O157:H7 and other enterohemorrhagic Escherichia coli. In: Blaser M J, Smith P D, Ravidin J I, Greenberg H B, Guerrant R L, editors. Infections of the gastrointestinal tract. New York, N.Y: Raven Press; 1995. pp. 739–761. [Google Scholar]
- 17.Griffin P M, Tauxe R V. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 18.Harsono K D, Kaspar C W, Luchansky J B. Comparison and genomic sizing of Escherichia coli O157:H7 isolates by pulsed-field gel electrophoresis. Appl Environ Microbiol. 1993;59:3141–3144. doi: 10.1128/aem.59.9.3141-3144.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartstein A I, Chetchotisakd P, Phelps C L, LeMonte A M. Typing of sequential bacterial isolates by pulsed-field gel electrophoresis. Diagn Microbiol Infect Dis. 1995;22:309–314. doi: 10.1016/0732-8893(95)00139-8. [DOI] [PubMed] [Google Scholar]
- 20.Karch H, Meyer T. Evaluation of oligonucleotide probes for identification of Shiga-like-toxin-producing Escherichia coli. J Clin Microbiol. 1989;27:1180–1186. doi: 10.1128/jcm.27.6.1180-1186.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karch H, Meyer T, Rüssmann H, Heesemann J. Frequent loss of Shiga-like toxin genes in clinical isolates of Escherichia coli upon subcultivation. Infect Immun. 1992;60:3464–3467. doi: 10.1128/iai.60.8.3464-3467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karch H, Rüssman H, Schmidt H, Schwarzkopf A, Heesemann J. Long-term shedding and clonal turnover of enterohemorrhagic Escherichia coli O157 in diarrheal diseases. J Clin Microbiol. 1995;33:1602–1605. doi: 10.1128/jcm.33.6.1602-1605.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keene W E, Sazie E, Kok J, Rice D H, Hancock D D, Balan V K, Zhoa T, Doyle M P. An outbreak of Escherichia coli O157:H7 infections traced to jerky made from deer meat. JAMA. 1997;277:1229–1231. doi: 10.1001/jama.1997.03540390059036. [DOI] [PubMed] [Google Scholar]
- 24.Kehl K S, Proctor M, Mellen J, Havens P. Abstracts of the 95th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1995. Culture for E. coli O157:H7 and testing for Shiga-like toxin during an outbreak of diarrhea in a daycare center, abstr. C177; p. 31. [Google Scholar]
- 25.Krause U, Thomson-Carter F M, Pennington T H. Molecular epidemiology of Escherichia coli O157:H7 by pulsed-field gel electrophoresis and comparison with that by bacteriophage typing. J Clin Microbiol. 1996;34:959–961. doi: 10.1128/jcm.34.4.959-961.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee M-S, Kaspar C W, Brosch R, Shere J, Luchansky J B. Genomic analysis using pulsed-field gel electrophoresis of Escherichia coli O157:H7 isolated from dairy calves during the United States national dairy heifer evaluation project (1991–1992) Vet Microbiol. 1996;48:223–230. doi: 10.1016/0378-1135(95)00135-2. [DOI] [PubMed] [Google Scholar]
- 27.Lee M-S, Proctor M, Gouveia S, Luchansky J B, Kaspar C W. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Genomic comparisons of Escherichia coli O157:H7 isolated from Wisconsin during 1994, abstr. C-354; p. 64. [Google Scholar]
- 28.Lerman Y, Cohen D, Gluck A, Ohad E, Sechter I. A cluster of cases of Escherichia coli O157 in a day-care center in a communal settlement (kibbutz) in Israel. J Clin Microbiol. 1992;30:520–521. doi: 10.1128/jcm.30.2.520-521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludwig K, Ruder H, Bitzan M, Zimmermann S, Karch H. Outbreak of Escherichia coli O157:H7 infection in a large family. Eur J Clin Microbiol Infect Dis. 1997;16:238–241. doi: 10.1007/BF01709588. [DOI] [PubMed] [Google Scholar]
- 30.Paton A W, Voss E, Manning P A, Paton J C. Shiga toxin-producing Escherichia coli isolates from cases of human disease show enhanced adherence to intestinal epithelial (Henle 407) cells. Infect Immun. 1997;65:3799–3805. doi: 10.1128/iai.65.9.3799-3805.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavia A T, Nichols C R, Green D P, Tauxe R V, Mottice S, Greene K D, Wells J G, Siegler R L, Brewer E D, Hannon D, Blake P A. Hemolytic-uremic syndrome during an outbreak of Escherichia coli O157:H7 infections in institutions for mentally retarded persons: clinical and epidemiological observations. J Pediatr. 1990;116:544–551. doi: 10.1016/s0022-3476(05)81600-2. [DOI] [PubMed] [Google Scholar]
- 32.Perera L P, Marques L R M, O’Brien A D. Isolation and characterization of monoclonal antibodies to Shiga-like toxin II of enterohemorrhagic Escherichia coli and use of the monoclonal antibodies in a colony enzyme-linked immunosorbent assay. J Clin Microbiol. 1988;26:2127–2131. doi: 10.1128/jcm.26.10.2127-2131.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prevost G, Jaulhac B, Piemont Y. DNA fingerprinting by pulsed-field gel electrophoresis is more effective than ribotyping in distinguishing among methicillin-resistant Staphylococcus aureus isolates. J Clin Microbiol. 1992;30:967–973. doi: 10.1128/jcm.30.4.967-973.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Proctor M E, Brosch R, Mellen J W, Garrett L A, Kaspar C W, Luchansky J B. Use of pulsed-field gel electrophoresis to link sporadic cases of invasive listeriosis with recalled chocolate milk. Appl Environ Microbiol. 1995;61:3177–3179. doi: 10.1128/aem.61.8.3177-3179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ratkowsky D A. Handbook of nonlinear regression models. New York, N.Y: Marcel Dekker; 1990. p. 46. [Google Scholar]
- 36.Reida P, Wolff M, Pöhls H-W, Kuhlmann W, Lehmacher A, Aleksi S, Karch H, Bockemühl J. An outbreak due to enterohemorrhagic Escherichia coli O157:H7 in a child day care centre characterized by person-to-person transmission and environmental contamination. Zentralbl Bakteriol. 1994;281:534–543. doi: 10.1016/s0934-8840(11)80342-7. [DOI] [PubMed] [Google Scholar]
- 37.Rowe P C, Orrbine E, Lior H, Wells G A, McLaine P N CPKDRC coinvestigators. A prospective study of exposure to verotoxin-producing Escherichia coli among Canadian children with haemolytic uraemic syndrome. Epidemiol Infect. 1993;110:1–17. doi: 10.1017/s0950268800050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rowe P C, Orrbine E, Wells G A, McLaine P N. Epidemiology of hemolytic syndrome in Canadian children from 1986 to 1988. J Pediatr. 1991;119:218–224. doi: 10.1016/s0022-3476(05)80730-9. [DOI] [PubMed] [Google Scholar]
- 39.Ryan C A, Tauxe R V, Hosek G W, Wells J G, Stoesz P A, McFadden H W, Smith P W, Wright G F, Blake P A. Escherichia coli O157:H7 diarrhea in a nursing home: clinical, epidemiological and pathological findings. J Infect Dis. 1986;154:631–638. doi: 10.1093/infdis/154.4.631. [DOI] [PubMed] [Google Scholar]
- 40.Samadpour M. Molecular epidemiology of Escherichia coli O157:H7 by restriction fragment length polymorphism using Shiga-like toxin genes. J Clin Microbiol. 1995;33:2150–2154. doi: 10.1128/jcm.33.8.2150-2154.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 42.Shah S, Hoffman R, Shillam P, Wilson B. Prolonged fecal shedding of Escherichia coli O157:H7 during an outbreak in a day care center. Clin Infect Dis. 1996;23:835–836. doi: 10.1093/clinids/23.4.835. [DOI] [PubMed] [Google Scholar]
- 43.Spika J S, Parsons J E, Nordenberg D, Wells J C, Gunn R A, Blake P A. Hemolytic uremic syndrome and diarrhea associated with Escherichia coli O157:H7 in a day care center. J Pediatr. 1986;109:287–291. doi: 10.1016/s0022-3476(86)80386-9. [DOI] [PubMed] [Google Scholar]
- 44.Strockbine N A, Marques L R M, Holmes R K, O’Brien A D. Characterization of monoclonal antibodies against Shiga-like toxin from Escherichia coli. Infect Immun. 1985;50:695–700. doi: 10.1128/iai.50.3.695-700.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swerdlow D L, Griffin P M. Duration of faecal shedding of Escherichia coli O157:H7 among children in day-care centers. Lancet. 1997;349:745–746. doi: 10.1016/S0140-6736(05)60196-1. [DOI] [PubMed] [Google Scholar]
- 46.Swerdlow D L, Woodruff B A, Brady R C, Griffin P M, Tippen S, Donnell H D, Geldreich E, Payne B J, Meyer A, Wells J G, Greene K D, Bright M, Bean N H, Blake P A. A waterborne outbreak in Missouri of Escherichia coli O157:H7 associated with bloody diarrhea and death. Ann Intern Med. 1992;117:812–818. doi: 10.7326/0003-4819-117-10-812. [DOI] [PubMed] [Google Scholar]
- 47.Tarr P I. Escherichia coli O157:H7: clinical, diagnostic, and epidemiological aspects of human infection. Clin Infect Dis. 1995;20:1–10. doi: 10.1093/clinids/20.1.1. [DOI] [PubMed] [Google Scholar]
- 48.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tilden J, Jr, Young W, McNamara A M, Custer C, Boesel B, Lambert-Fair M A, Majkowski J, Vugia D, Werner S B, Hollingsworth J, Morris J G. A new route of transmission for Escherichia coli O157:H7: infection from dry fermented salami. Am J Public Health. 1996;86:1142–1145. doi: 10.2105/ajph.86.8_pt_1.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weagant S D, Bryant J L, Bark D H. Survival of Escherichia coli O157:H7 in mayonnaise-based sauces at room and refrigerated temperatures. J Food Prot. 1994;57:629–631. doi: 10.4315/0362-028X-57.7.629. [DOI] [PubMed] [Google Scholar]
- 51.Whittam T S, Wolfe M L, Wachsmuth I K, Ørskov F, Ørskov I, Wilson R A. Clonal relationships among Escherichia coli strains that cause hemorrhagic colitis and infantile diarrhea. Infect Immun. 1993;61:1619–1629. doi: 10.1128/iai.61.5.1619-1629.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams L D, Hamilton P S, Wilson B W, Estock M D. An outbreak of Escherichia coli O157:H7 involving long term shedding and person-to-person transmission in a child care center. Environ Health. 1997;May:9–14. [Google Scholar]