Summary:
Optical methods for measuring intracellular ions including Ca2+ revolutionized our understanding of signal transduction. However, these methods are not extensively applied to intact organs due to issues including inner filter effects, motion, and available probes. Mitochondrial Ca2+ is postulated to regulate cell energetics and death; pathways that are best studied in an intact organ. Here, we develop a method to optically measure mitochondrial Ca2+ and demonstrate its validity for mitochondrial Ca2+ and metabolism using hearts from wild-type mice and mice with germline knockout of the mitochondria calcium uniporter (MCU-KO). We previously reported that germline MCU-KO hearts do not show an impaired response to adrenergic stimulation. We find these MCU-KO hearts do not take up Ca2+ consistent with no alternative Ca2+ uptake mechanisms in the absence of MCU. This approach can address the role of mitochondrial Ca2+ to the myriad of functions attributed to alterations in mitochondrial Ca2+.
Keywords: calcium, mitochondria, heart, isoproterenol, spectroscopy
Introduction
The systems approach to studying cellular function has expanded the ability to monitor many aspects of cellular metabolism and ionic composition in intact functioning systems. Optical spectroscopy provides real-time, non-destructive measurement of the redox state of the chromophores associated with the mitochondrial oxidative phosphorylation complexes as well as the cytosolic oxygen tension from myoglobin (Wittenberg and Wittenberg, 1989) in intact tissues. Coupling this method with extrinsic fluorescence probes of the ionic milieu, or other properties of the cell, expands the information content of this system. With regard to the heart and heart mitochondria, recent transmission optical spectroscopy visible light studies (Chess et al., 2013; Femnou et al., 2017; Giles et al., 2018; Kuzmiak-Glancy et al., 2018) have demonstrated the detection of numerous aspects of oxidative phosphorylation, in addition to the monitoring of cytosolic oxygen via myoglobin.
A limitation with studies in the small mouse heart has been the influence of scattering and motion on the optical detection of these chromophores or other extrinsic probes. Recently, it has been demonstrated that by using an integrating sphere to collect the transmitted light from a light source catheter in the cardiac ventricle space, high signal to noise transmission data can be collected with minimal scattering and motion artifacts since all of the transmitted light is sampled by the integrating sphere (Bauer et al., 2019).
The purpose of this study is to develop methods to combine the wealth of information from absorption spectroscopy of the intrinsic chromophores of the heart, or other tissues, with information on the intracellular milieu using exogenous fluorescent probes. There are numerous issues with monitoring fluorescence from intact tissues specifically motion, and the impact of dynamic optical filtering of the data from primary filters on the excitation light and secondary filters on the emitted light (Lakowicz, 2006); these issues are particularly challenging when working with an intact organ in vivo or In vitro. We hypothesized that the transmitted absorption spectra collected alternately with the fluorescence signal could be used to correct for both the dynamic primary and secondary filters on the emission signal, while the integrating sphere can minimize the impact of motion by sampling all of the emitted light. In this study we also introduce and utilize a genetic mouse model with labeling of the outer mitochondria membrane with TOMM20-mNeonGreen to demonstrate matrix targeting of Rhod-2 with our loading conditions. Although cytosolic and sarcoplasmic reticulum (SR) Ca2+ have been measured from the epicardial surface of heart using fluorescent Ca2+ indicators (Aguilar-Sanchez et al., 2019; Wang et al., 2015; Wei et al., 2020), there are currently no methods to measure mitochondrial Ca2+ in a beating perfused heart.
We monitored matrix mitochondrial Ca2+ levels with the exogenously added Ca2+ probe, Rhod-2. Mitochondrial Ca2+ is an important regulator of mitochondrial function and cell death (Murphy and Steenbergen, 2020). The role of mitochondrial Ca2+ and the MCU in regulation of cardiac mitochondrial energetics has been debated (Holmstrom et al., 2015; Kwong et al., 2015; Luongo et al., 2015; Szibor et al., 2020; Wescott et al., 2019). To specifically address this issue, several mouse models with deletion of MCU were developed to test the role of MCU in this process, however, rather than clarifying the role of MCU and mitochondrial Ca2+ in matching energy supply and demand, the studies in the different models of MCU deletion have provided conflicting results (Holmstrom et al., 2015; Kwong et al., 2015; Luongo et al., 2015; Rasmussen et al., 2015). In contrast to deletion in the adult (Kwong et al., 2015; Luongo et al., 2015), when MCU is deleted in germline there was little or no effect on contractility following adrenergic stimulation in the in vivo mouse (Holmstrom et al., 2015). In none of these studies was matrix Ca2+ directly measured in a functioning heart, thus the interpretation of these knockout experiments is complicated. It has been suggested that in additional to MCU there are alternative mitochondrial Ca2+ uptake pathways (Bisbach et al., 2020), providing a possible explanation for the differences.
In this study we combine optical spectroscopy methods in the perfused mouse heart monitoring tissue absorbance, intrinsic mitochondrial and cytoplasmic chromophores along with corrected Rhod-2 fluorescence to measure mitochondrial Ca2+ in control and MCU knockout mice during transient stimulation with a beta agonist to stimulate work to address the role of mitochondrial Ca2+ in matching energy supply and demand.
Results
There are several challenges to measuring mitochondrial Ca2+ in a beating perfused heart. The first challenge is to selectively deliver a Ca2+ sensitive indicator to the mitochondria. A second issue is to correct for inner filter effects and the third obstacle is to overcome motion artifacts without inhibiting contractility.
Targeting a mitochondrial Ca2+ indicator to the mitochondria
The first challenge in measuring mitochondrial Ca2+ was to identify a method to preferentially load a Ca2+ sensitive dye into the mitochondria. Although we considered developing a mouse with a genetically encoded mitochondrial targeted Ca2+ sensitive indicator, these indicators typically have low fluorescent intensity, are often mistargeted and suffer from other limitations (Filippin et al., 2005). We therefore examined whether a Ca2+ sensitive dye such as the acetoxymethyl-ester of Rhod-2 (Rhod-2-AM) could be targeted to the mitochondria. Another advantage is that Rhod-2 fluoresces at long wavelengths which allows better separation from the endogenous chromophores of the heart. A number of studies have reported that Rhod-2-AM preferentially accumulates in the mitochondria (Brandes and Bers, 2002; Liu et al., 2020; Trollinger et al., 1997, 2000), particularly when loading is done at 25 or 30°C. To test whether Rhod-2 loads into the mitochondria, we sought a means of clearly delineating the mitochondria. We developed a mouse in which the mitochondrial membrane protein, TOMM20 (translocator of outer mitochondrial membrane protein 20), was tagged with the mNeonGreen fluorescent protein (Figure 1, Supplemental Figure 6). Figure 1a shows an embryo without (left) and with (right) the tagged TOMM20. The embryo to the right clearly shows mNeonGreen labelling throughout the embryo. Figure 1b shows surface fluorescence of a heart from a TOMM20-mNeonGreen mouse and Figure 1c shows the exposed torso. These data clearly show that the TOMM20-mNeonGreen fluorescent protein is widely expressed throughout the body, suggesting that this mouse line can be a useful tool for delineating mitochondria in a variety of cell types. We utilized this mouse model to identify conditions under which Rhod-2 could be localized to the mitochondria. We loaded an ex-vivo perfused heart with 3.5 μM Rhod2-AM at 30°C for 25 minutes followed by 40 minutes of perfusion at 37°C without Rhod-2-AM to washout uncleaved dye. The heart was sectioned and fluorescence was measured at 580 nm using super resolution stimulated emission depletion (STED) microscopy. As shown in Figure 1d and 1e Rhod-2 fluorescence is localized within the TOMM20 delimited mitochondria. Figure 1f provides the line scan data (see line in Figure 1e) showing the location of Rhod-2 fluorescence and TOMM20-mNeonGreen fluorescence and demonstrates that the Rhod-2 fluorescence is contained within TOMM20 tagged mitochondria. As shown in Supplemental Figure 1, under our loading conditions esterase activity is higher in mitochondrial than in the non-mitochondrial fraction of the cell. Thus, Rhod-2 is targeted to the mitochondria under these loading conditions and can be used to monitor mitochondrial Ca2+.
Figure 1. Development of TOMM20-mNeonGreen tagged mouse model to confirm Rhod-2-AM loading into mitochondria.
Panel a shows fluorescence images of mNeonGreen-tagged TOMM20 in a widefield image of a WT mouse embryo (left) and a TOMM20- mNeonGreen expressing embryo (right). Panel b shows a widefield image of an embryonic mouse heart expressing mNeonGreen TOMM20. Panel c shows an excised torso section showing TOMM20-mNeonGreen expression in skeletal muscle. Scale bar represent 2 mm, 500 μm, and 2 mm for panels a-c respectively. Panel d shows Stimulated Emission Depletion (STED) microscopy image from a slice of an ex vivo mNeonGreen labelled TOMM20 (green) heart loaded with Rhod-2 (red). This image is a slice from a larger six slice z-stack that was taken over an axial range of 0.8 mm2 and deconvolved. The scale bar represents 5 μm. An enlarged image from the box region of interest is shown in panel e, and a line profile of intensities through the dotted line shown in panel f.
Optical monitoring of the beating heart
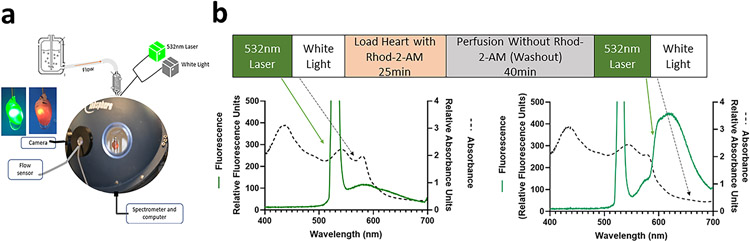
Figure 2a schematically shows the apparatus we adapted for this approach. A light source catheter was placed within the ventricle of the perfused mouse heart situated in an integrating sphere to sample all transmitted light, minimizing motion and scattering effects. In this application, the light source of the catheter was alternated between two modes: first, a white light source, to determine tissue transmission characteristics, follow redox sensitive mitochondria chromophores and cytoplasmic myoglobin and second, a 532 nm laser to excite the mitochondrial Rhod-2 fluorescence. A single rapid scanning spectrometer collected both the white light and fluorescence emission, sequentially, from the tissue.
Figure 2. Method for interleaving fluorescent and absorbance measurements in perfused mouse heart.
Panel a shows the integrating sphere system for light collection from perfused heart. Heart is placed in chamber in center of integrating sphere, and perfusate flows outside of chamber through outflow tubing. Flow rate is collected through the flow sensor and flow box. Heart is monitored by endoscope camera. White light and 532 nm laser sources are connected to optic fiber, inserted into left ventricle of heart. Transmural light is collected through the light guide, then processed through a spectrometer and computer. Panel b shows the protocol for interleaving 532 nm laser and white light before and after Rhod-2-AM loading and wash. Left graph in panel b shows white light absorbance (dashed lines) and fluorescence emission with 532 nm laser (green) of an unloaded perfused mouse heart. Green arrows indicates 532 nm emission traces and black arrows indicate white light transmission traces. Right graph in panel b shows white light absorbance and fluorescence emission of heart following Rhod-2-AM loading and washout.
Correction for inner filter effects
As shown in Figure 2b, we alternated collection of transmural fluorescent emission and white light transmissions from the heart throughout the experimental time course. The left panel shows the fluorescence emission spectrum in green and the black trace shows the white light absorbance, both measured before Rhod-2 loading. The right panel shows the fluorescence emission (green) and white light absorbance (black) measured after Rhod-2 loading and 40 minutes of perfusion without Rhod-2-AM (washout).
Figure 3a shows fluorescence emission of a perfused heart during excitation with 532nm prior to Rhod-2 loading (red), the same heart following Rhod-2 loading and washout of any extracellular or uncleaved Rhod-2-AM (black), and the difference spectrum between the emission spectrum of the heart after and before Rhod-2 loading (blue). For comparison we also show the emission spectrum of pure Rhod-2 in solution (dashed purple line). Consistent with previous studies (Du et al., 2001) these data show that the background fluorescence of the heart is low compared to the increase in fluorescence that occurs with Rhod-2 loading which leads to a ~ 4-fold increase in fluorescence from 550-700 nm. Figure 3a further illustrates the secondary inner filter effect on the Rhod-2 emission by the natural chromophores of the heart; the peak of the Rhod-2 fluorescence in tissue is shifted to the right compared to the fluorescence of Rhod-2 in solution (dashed line). There is a broadened emission spectrum with peaks around 595 nm and 625 nm, red-shifted from the pure Rhod-2 emission spectra with a single peak at 581 nm. As shown in Figure 3b, the white light data show that there is little absorbance of natural chromophores at wavelengths greater than 640 nm, with conditions such as isoproterenol (purple dashed line) or even with more extreme conditions such as 20 minutes of hypoxia (orange dashed line); thus there is little interference above 640 nm in the fluorescent emission spectra from natural chromophores.
Figure 3. Correction for filtering effects.
Panel a shows fluorescence emission spectra with excitation from 532 nm laser of perfused heart before Rhod-2-AM loading (red) and the end of Rhod-2-AM washout (black). The difference spectrum between after and before Rhod-2-AM loading is shown in blue. Fluorescence spectra from Rhod-2-AM in solution taken in cuvette in integrating sphere is shown as purple dashed line. Panel b shows the raw fluorescence spectrum of a Rhod-2 loaded heart (green). Difference absorbance spectra during the hypoxia-inducing conditions of ischemia (orange) and isoproterenol (purple) compared to control are shown. The black box indicates the bandwidth of 650-680 nm in the fluorescence and difference absorbance spectra.
It is important to note that the tissue absorbance at 532 nm is significant and varies with the metabolic state of the tissue. Supplemental Figure 2a shows the change in absorbance at 532 nm in a perfused heart, in the absence of Rhod-2 loading following adrenergic stimulation. This demonstrates that depending on the conditions, the tissue will absorb differing amounts of excitation light, thus altering the excitation of Rhod-2, altering the Rhod-2 emission independent of mitochondria Ca2+. Thus a primary filter correction for the 532 nm excitation laser is required to properly monitor Ca2+ sensitive Rhod-2 fluorescence in this tissue. We corrected the overall Rhod-2 fluorescence for the relative 532 nm transmission in the white light spectrum using equation 1 adapted from the approach described by Lakowicz (Lakowicz, 2006).
The secondary filter of the Rhod-2 emitted light is more complex as seen in Figure 3a. As discussed in the literature, accurate correction for the secondary filter is complicated by geometry and scattering patterns that can be difficult to quantitate. However as Krimer et al (Krimer et al., 2017) demonstrated, the most robust solution is to avoid analysis of spectral regions impacted by a secondary filter. Fortunately, under our loading and collection conditions, Rhod-2 has adequate emission in the 650-680 nm band (black box in Figure 3b) to permit the detection of the Ca2+ sensitive alterations in Rhod-2 outside of the secondary filters of the tissue. Furthermore, as shown in Supplemental Figure 2b in hearts without Rhod-2 loading there is little or no change in emission fluorescence at 650-680 nm following changes in work load.
Taking into account the primary tissue filter at 532 nm, and utilizing emission from 650-680 nm to avoid secondary filter effects, we generated equation 2 to follow calcium sensitive mitochondria Rhod-2 in the intact heart. Equation 2 corrects the collected fluorescence (Fobs) from 650-680 nm for the primary filter effect. The absorbance of the white light at 532 nm was used as ODex instead of the absorbance of the 532 nm laser. This substitution was made because the laser emission at 532 nm was saturating, and we did not want to reduce the intensity of the laser as that would likely reduce signal to noise. As described in the Method section to maximize signal to noise we summed the spectral domain from 650 to 680 nm to calculate changes in Rhod-2 fluorescence. Background fluorescence () taken prior to Rhod-2 loading was subtracted from (Eqn 2).
| (Eqn 1:) |
| (Eqn 2:) |
Analysis of chromophores
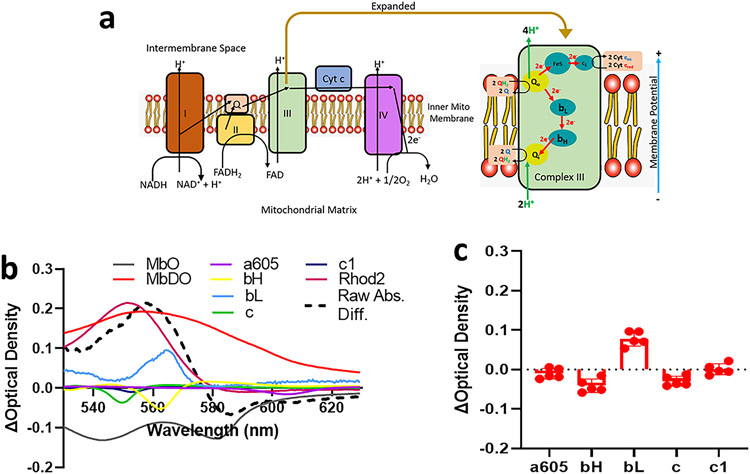
As described, the fiber optic provides the ability to alternate white light with the laser allowing us to measure changes in the cytochromes and cytoplasmic myoglobin with the alternating white light illumination. We analyzed the spectra data as described previously (Bauer et al., 2019; Femnou et al., 2017; Giles et al., 2018). As shown in Figure 4a, cytochromes bH, bL and c1 are all components of complex III, cytochrome a605 is a component of complex IV, and cytochrome c is a mobile carrier of electrons between complex III and IV. Using calibrations and modifications of a previously published program (Femnou et al., 2017), as shown in Figure 4b we deconvoluted the spectral changes due to Rhod-2 (pink) loading and natural chromophores. Figure 4c shows summary data for spectral changes in WT hearts before and after loading. We found that Rhod-2 loading did not alter absorbance of a605, a measure of mitochondrial oxygenation. Rhod-2 loading also had no effect on cytochromes bH, bL, c or c1.
Figure 4. Analysis of cytochromes following Rhod-2-AM loading.
Panel a shows electron handling between cytochromes within the electron transport chain. Panel b shows the difference spectra between Rhod-2-AM washout and baseline deconvoluted using LabView program to fit reference chromophores including myoglobin (oxygenated and deoxygenated), cytochrome oxidase (a605), bH, bL, c, and c1, and Rhod-2. Panel c shows averaged peak difference absorbance for chromophores from wild-type male mice (n=5 biological replicates).
Is there evidence for an alternative mitochondrial Ca2+ uptake mechanism in MCU-KO hearts?
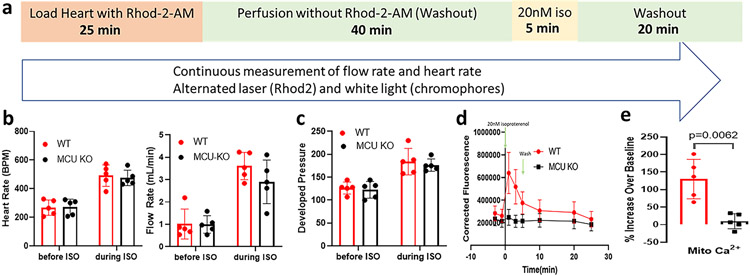
To determine whether this method was suitable for measuring physiological changes in mitochondrial Ca2+ we treated ex vivo wild type hearts with 20 nM isoproterenol using the protocol in Figure 5a. Supplemental Figure 3a shows the raw emission fluorescence summed between 650 and 680 nm as a function of time after isoproterenol treatment (t=0) in a WT heart. Supplemental Figure 3b shows the change in absorbance at 532 nm, measured from the white light as described above and used in Eqn 1 to corrected for primary inner filter effects and Supplemental Figure 3c shows the corrected fluorescence (FCorr) obtained from Eqn 2. The data show a clear increase in Rhod-2 fluorescence consistent with an increase in mitochondrial Ca2+.
Figure 5. Mitochondrial Ca2+ measurements following isoproterenol treatment.
Panel a shows the protocol for the study. Panels b and c show heart rate (HR), flow rate (FR) and left ventricular developed pressure (LVDP) for wild-type (red) and MCU-KO (black) hearts before and during 20 nM isoproterenol treatment. Panel d shows the time course of mitochondrial Ca2+ in wild-type (red circle) and MCU-KO (black square) hearts during and following isoproterenol (ISO) treatment. N=5 biological replicates for each genotype. Panel e shows the percent changes at 1min ISO compared to baseline for mitochondrial Ca2+ in the wild-type and MCU-KO hearts.
An increase in cytosolic Ca2+, which occurs with isoproterenol and leads to increased contractility and work, promotes an increase in mitochondrial Ca2+ presumably via MCU, which is proposed to match ATP generation to increased work (Brandes and Bers, 2002; Glancy and Balaban, 2012). As discussed, mice in which MCU was deleted in the adult showed significant impairment in the contractile response to an adrenergic stimulus (Kwong et al., 2015; Luongo et al., 2015). In contrast, mice with germline deletion of MCU showed only modest impairment in contractility following adrenergic stimulation (Holmstrom et al., 2015). To test whether this lack of impairment in germline MCU-KO mice was due to an alternative Ca2+ uptake mechanism we measured mitochondrial Ca2+ in WT and MCU-KO hearts (n=5 for each genotype) following treatment with 20 nM isoproterenol (see Figure 5a). As shown in Figure 5b and 5c, the WT and MCU-KO hearts exhibited a similar increase in heart rate, flow rate and left ventricular developed pressure (LVDP) confirming our previous results in which we found little or no difference in contractility following adrenergic stimulation in an in vivo model in the germline MCU-KO mice (Holmstrom et al., 2015). Concurrent with the measurement of flow rate and heart rate we measured changes in mitochondrial Ca2+ following isoproterenol addition in WT and MCU-KO hearts. As shown in Figure 5d we observed a 3-4 fold increase in Rhod-2 fluorescence in WT hearts with addition of isoproterenol, but consistent with the lack of MCU we observed no increase in Rhod-2 fluorescence in the MCU-KO hearts. In Figure 5e we compare the peak increase in Ca2+ following isoproterenol in WT and MCU-KO hearts. Taken together these data do not support an alternative Ca2+ entry mechanism into the mitochondria in the MCU-KO hearts, at least during 5 min of isoproterenol stimulation.
Furthermore, the lack of increase in Rhod-2 fluorescence following isoproterenol treatment in the MCU-KO hearts confirms the mitochondrial localization of the Rhod-2 as the increase in heart rate and flow with isoproterenol demonstrate the cytosolic Ca2+ did increase without any detected changes in corrected Rhod-2 fluorescence.
Changes in chromophores following isoproterenol
Isoproterenol caused similar changes in blood flow and heart rate in the WT and MCU-KO hearts, suggesting that in the germline MCU-KO the absence of MCU did not impact the net function of the heart at these workloads. We used optical spectroscopy to characterize the physiological and metabolic response to isoproterenol. The cardiac chromophores permit monitoring of cytosolic oxygenation and steady state redox of the cytochromes providing insight into the reducing equivalent feed into the chain. An increase in mitochondrial Ca2+ has been proposed to enhance electron transport by activating mitochondrial dehydrogenases to increase reducing equivalent delivery as well as other aspects of oxidative phosphorylation (Glancy and Balaban, 2012). One might expect that because the work in WT and KO is essentially the same, but KO hearts do not have matrix Ca2+ activation, that the electron transport chain might become more oxidized and the mitochondrial membrane potential might become more depolarized in the MCU-KO hearts compared to WT hearts after isoproterenol treatment. To investigate this hypothesis, we examined the redox state of the cytochromes during isoproterenol administration.
The most striking feature of the optical spectroscopy time course in Figure 6a was the near immediate increase in deoxymyoglobin and a605 consistent with a transient hypoxia (Giles et al., 2018) in the tissue likely due to a poor balance of work (heart rate) with flow rate (see Supplemental Figure 4). Once flow rate increases a605 returned to control while the heart rate remained elevated. We evaluated different steady state conditions: [1] control before isoproterenol, [2] peak deoxygenation and [3] after recovery of tissue oxygenation but maintained high heart rate and flow rate. Figure 6b shows the deconvolution of a difference spectra between white light at time [2], peak deoxygenation, minus [1], control. Consistent with the time course data in Figure 6a, there is an increase in the optical density of cytochromes a605, consistent with a small demand ischemia induced deoxygenation in the heart at time [2] during isoproterenol treatment compared to [1]. As this deoxygenation would alter redox of the cytochromes we waited until the reduction of cytochrome a605 returned to within 10% of baseline before analyzing changes in the chromophores; this occurred with a similar time course in WT and MCU-KO (3.4 min in WT and 3.2 min in MCU-KO). Furthermore the level of the transient reduction was similar in WT and MCU-KO. The a605, bH, bL, c, and c1 chromophore data were therefore analyzed at time [3], when a605 returned to baseline. As shown in Figure 6c, comparing times [1] and [3] there were no significant differences in any of the cytochromes in either the WT or MCU-KO hearts. The two b hemes of complex III (see Figure 4a), high (cyt bH) and low (cyt bL), can provide an estimate of the mitochondrial membrane potential (ΔΨm) under some conditions (see Figure 4c) (Kim et al., 2012); no differences in mitochondrial membrane potential was observed between WT or MCU-KO with isoproterenol treatment.
Figure 6. Analysis of chromophores during isoproterenol treatment.
Panel a shows the time course of peak absorbance of cytochrome a605 (purple) during isoproterenol treatment and washout. Cytochrome changes were analyzed at timepoints labeled 1 (control), 2 (transient hypoxia during isoproterenol treatment), and 3 (return from hypoxia). Panels b and c show the chromophores fit using LabView showing the difference spectra between times 2 vs 1, and 3 vs 1, respectively. Panel d shows the average peak difference absorbance values from chromophores in wild-type (red) and MCU-KO (black) taken before isoproterenol treatment (time 1) and after transient hypoxia time (time 3). N=5 biological replicates for each genotype.
Ru360 blocks Isoproterenol induced mitochondrial Ca2+ uptake
To further examine the role of MCU in regulating mitochondrial Ca2+ following isoproterenol, we examine whether Ru360, an inhibitor of MCU would alter mitochondrial Ca2+ uptake in WT hearts. WT hearts were loaded with Rhod-2-AM as described followed by washout perfusion for 40 minutes, with addition of Ru360 during the last 10 minutes. Figure 7a, shows changes in chromophores of heart following Ru360 treatment, which were similar to changes found in control (non-treated) hearts. Ru360 does not induce deoxygenation or significantly change state of mitochondria compared to control conditions. Isoproterenol (20 nM) was added in the presence of Ru360 and Rhod-2 fluorescence was monitored. As shown in Figure 7b, following addition of isoproterenol, heart rate increased similarly in the presence and absence of Ru360. This is similar to what was observed by Unitt et al (Unitt et al., 1989). In the absence of Ru360, isoproterenol caused a 2-3 fold increase in mitochondrial Ca2+; however addition of Ru360 significantly blunted the isoproterenol induced rise in mitochondrial Ca2+ (figure 7b, c). We also analyzed the chromophore change before and after isoproterenol addition and found no differences in chromophore redox state between control and Ru360 treated hearts.
Figure 7. Ru360 blocks isoproterenol mediated mitochondrial Ca2+ uptake.
Wild-type Langendorff hearts were treated with 2.5 ug/mL Ru360 for 10min prior to 20 nM isoproterenol treatment (n=4 biological replicates for each group). Panel a shows changes in absorbance of chromophores in heart during Ru360 treatment compared to before Ru360 (blue) compared to control hearts (red). Panel b shows heart rate during experimental protocol for control (left bars) and Ru360 treated hearts (right bars). Black bars represent heart rate for control period before Ru360 and ISO treatment. Blue bar represents heart rate during Ru360 treatment. Red bars represent heart rate during ISO treatment. Panel c shows the time course of Rhod-2 fluorescence for Ru360 treated (blue) and control (red) hearts during experimental protocol, where 20nM isoproterenol was administered at 0 min. Error bars represent SEM. Panel d shows percent increase in mitochondrial Ca2+ at 1 min ISO treatment compared to the period before ISO treatment. Panel e shows the average peak difference absorbance values from chromophores in wild-type (red) and Ru360 treated hearts (black) taken before isoproterenol treatment and after transient hypoxia.
Discussion
Using a combination of transmission spectroscopy and fluorescence we quantitatively monitored a fluorescent probe of free Ca2+ in the mitochondria matrix of a beating heart. The transmission spectroscopy data provided corrections for inner filter effects of the tissue on the fluorescence emission while simultaneously reporting the cytosolic oxygenation and mitochondria redox state. To accomplish this task, we interleave transmission absorption with fluorescent measurements using an optical catheter in the ventricular cavity and an integrating sphere light collection system. The integrating sphere light collection system minimized tissue motion effects allowing us to make measurements without inhibiting contractility with agents such as blebbistatin. This study demonstrates that without proper correction for inner filter effects the interpretation of fluorescent data from the intact heart, or other intact biological systems in vitro or In vivo, would be erroneous (see Supplemental Figure 5). This approach should be applicable to any exogenous or genetically programed fluorescent probe for following cellular events in complex intact biological systems.
Matrix Ca2+ is believed to play a major role in the normal and compromised physiology of the heart as well as other organs. We demonstrated that the fluorescent Ca2+ probe, Rhod-2, can be localized to the intact heart mitochondria revealed using STED microscopy in combination with a genetically inserted marker of the outer mitochondria membrane, TOMM20-mNeoGreen, to delineate the mitochondria borders. In addition to the spatial localization, functionally the lack of a Rhod-2 fluorescence increase in the MCU-KO hearts with beta-adrenergic stimulation despite the increase in contractility consistent with an increase in cytosolic Ca2+ (Figure 5d) confirms the targeting of Rhod-2 to the mitochondria with minimal cytosolic contribution. We suggest that the localization of Rhod-2 to the mitochondria matrix is due to several factors. The lipid soluble Rhod-2 AM has a weak positive charge that will drive the probe into the highly polarized mitochondria matrix. The trapping of this probe is dependent on esterases to cleave the Rhod-2 AM ester generating a highly charged membrane impermeable and Ca2+ binding negative compound, Rhod-2. The esterase activity is much higher in the mitochondria matrix than cytosol (supplemental Fig 1) such that if the conditions are appropriate the majority of the probe can traverse the cytosol in the AM form to be taken up and then trapped in the mitochondria matrix esterases. If conditions, including high temperature or high [Rhod-2 AM], where significant cytosolic esterase cleavage occurs, the high dynamic range in cytosolic [Ca2+] in comparison to the mitochondria matrix may result in the cytosolic signal dominating the Rhod-2 emission as observed by several investigators. Rhod-2 has been used to measure cytosolic Ca2+ (Aguilar-Sanchez et al., 2019; Mejia-Alvarez et al., 2003) and in most of these studies Rhod-2 AM is loaded at high concentrations that may contribute to cytosolic loading that would dominate the Rhod-2 emission as discussed above. In this study we used low concentrations of Rhod-2-AM at low temperature that apparently permitted the higher esterase activity of the mitochondria matrix (Supplemental Figure 1) to trap the vast majority of Rhod-2 when compared to the cytosol.
We demonstrate that this method has sufficient sensitivity to measure a physiological increase in mitochondrial Ca2+ as occurs following adrenergic stimulation in wild type mouse hearts. We further show that hearts from mice lacking MCU do not show an increase in mitochondrial Ca2+ following addition of isoproterenol. Two critical hypotheses were tested with this approach. It has been suggested that in additional to MCU there are alternative mitochondrial Ca2+ uptake pathways (Bisbach et al., 2020). We clearly demonstrate that in the absence of MCU there is no Ca2+ uptake following adrenergic stimulation. This approach was also used to test whether an increase in mitochondria Ca2+ is required for the balance of mitochondria energy conversion with work load (Brandes and Bers, 2002; Denton and McCormack, 1980; Glancy and Balaban, 2012; Murphy and Steenbergen, 2020). Despite the lack of increase in matrix Ca2+ in the MCU-KO, the MCU-KO and WT hearts exhibited a similar increases in contractility and heart rate following addition of isoproterenol. This physiological result is consistent with data of Unitt et al (Unitt et al., 1989), who treated perfused rat hearts with isoproterenol in the presence and absence of ruthenium red (RR) an inhibitor of MCU and found no physiological effect. However, no data on matrix Ca2+ has been collected in the intact heart to confirm the lack of matrix Ca2+ in this or any studies. Our data revealed that no change in matrix Ca2+ was required for a normal increase in contractility, perfusion and mitochondrial redox state with isoproterenol. These data suggest that Ca2+ matrix activation of mitochondrial metabolism, including dehydrogenases, is not mandatory for the support of contractility generated in these studies. Indeed, the mitochondrial metabolic response was apparently identical in the presence and absence of MCU and matrix Ca2+ suggesting that other factors dominate the activation of oxidative phosphorylation with isoproterenol under these conditions. Interestingly, Unitt et al used 31P NMR, and found that addition of isoproterenol in the absence of RR, led to a transient decrease in phosphocreatine and a transient increase in ADP and Pi, but no change in ATP and the values all returned to control levels within one minute. Using our optical transmission data we demonstrated that the initial impact of isoproterenol is a transient demand hypoxia (see Figure 6) followed by a return to normoxia after a few minutes. More likely the transient 31P NMR measures was a result of this transient hypoxia with isoproterenol as revealed by the simultaneous monitoring of tissue oxygenation and mitochondria redox state not done in previous studies. The added information on an organ level system provided by transmission and fluorescence spectroscopy improves the ability to properly interpret physiological or pharmacological perturbations in complex biological systems.
In summary, we report a method to interleave measurement of fluorescent indicator to measure mitochondrial Ca2+ and absorbance to measure changes in mitochondrial cytochromes. We demonstrate that following adrenergic stimulation that mitochondrial Ca2+ increases in WT but not in MCU-KO hearts. The method describe herein can be applied to other fluorescent indicators.
Limitations
We provide a method for measuring mitochondrial Ca2+ in a beating, ex-vivo mouse heart and demonstrate that germline MCU-KO and wild-type RU360 treated mitochondria lack alternative Ca2+ uptake pathways under beta-adrenergic stimulation. Due to the nature of the optical instruments used, white and fluorescent light collection were alternated, resulting in individual Ca2+ measurements approximately every minute. Our method does not collect continuous mitochondria Ca2+ measurements and has a time resolution of approximately one minute. Thus, short, transient changes in Ca2+ or beat-to-beat changes are challenging to observe with this method.
STAR Methods
Resource Availability
Lead contact
Further information and requests should be directed to the lead contact, Dr. Elizabeth Murphy (murphy1@nih.gov).
Materials availability
Mouse lines generated in this study are available upon request.
Data and code availability
All data reported in this paper will be shared by the lead contact upon request. This paper does not report original code. Any additional information or requests for data reported in paper is available from the lead contact.
Experimental Model and Subject details
Mammalian Cell Lines
HEK-293T female cells were cultured in complete D-MEM medium containing 10% FBS supplemented with 0.1 mM MEM Non-Essential Amino Acids, 1 mM sodium pyruvate, 2 mM L-glutamine, and 50 U/mL Penicillin-Streptomycin. Cells were placed in 37°C incubator with a humidified atmosphere of 5% CO2 in air and subcultured until 80-90% confluence.
Mouse Lines
TOMM-20 transgenic mice were generated on the C57BL6/N background. MCU KO and WT littermate mice were on a mixed C57BL/6 and CD1 background as described (Pan et al., 2013). All animal studies were performed in a manner consistent with the recommendations established by the Guide for the Care and Use of Laboratory Animals (National Institutes of Health), and all animal protocols were approved by the National Heart, Lung and Blood Institute’s Animal Care and Use Committee. Mice were male and between 12 to 21 weeks of age.
Method Details
Generation of transgenic DNA construct
It has been previously shown that fusing mNeonGreen to the TOMM20 gene can target the mNeonGreen signal to the mitochondrial outer membrane in cultured cells (Shaner et al., 2013). To ubiquitously express this fusion protein in transgenic mice, we obtained the mNeonGreen-TOMM20-N-10 plasmid (http://www.allelebiotech.com/mneongreen/) from Allele Biotechnology. It was used as a template for PCR amplification of the fusion gene using a forward primer (AAAGGAGGTACCCACCATGGTGGGTCGGAACAG). which contained an added KpnI restriction site (underlined), and a reverse primer (ACAACTTCTAGACTTGTACAG CTC GTCCATGC), which contained an added XbaI restriction site (underlined). The PCR was carried out with an initial denaturation at 94°C for 2 min, then 30 cycles of 94°C for 30 seconds, 60°C for 30 seconds and 72°C for 60 seconds and a final extension at 72°C for 7 min. After digesting the PCR fragment with KpnI and XbaI, it was then cloned into the pSF-CAG-Kan vector (Sigma-Aldrich, Cat# OGS505-5UG), which had been digested with the same restriction enzymes. To test the completed DNA construct (Supplemental Figure 6a), we transfected it into HEK293T cells, and the punctate fluorescent image pattern suggested that the mNeonGreen was tagged to mitochondria as expected (Supplemental Figure 6b & c).
Microinjection and generation of the TOMM20-mNeonGreen transgenic mice
For generating transgenic mice, the CAG-TOMM20-mNeonGreen transgenic fragment, containing the CAG promoter, TOMM20-mNeonGreen fusion gene, and SV40 polyadenylation signal, was released from the plasmid vector by digesting with AsiSI and PacI. After separation from the cloning vector on a 0.8% agarose gel, the 3.9 Kb transgenic band was cut out from the gel and purified using a gel extraction kit. The purified transgenic DNA fragment was dissolved in 10 mM Tris (pH7.5), 0.1 mM EDTA, and 100 mM NaCl at the concentration of 2 μg/ml, and microinjected into the pronuclei of fertilized eggs collected from B6D2F1/J mice (The Jackson Laboratory, Stock No. 100006). Injected zygotes were cultured in M16 medium (Millipore Sigma Cat#MR-016-D) overnight in a humidified incubator with 5% CO2 at 37°C. In the next morning, those embryos that had reached 2-cell stage of development were implanted into the oviducts of surrogate mothers that had mated with vasectomized males.
Mice born to the surrogate mothers were genotyped by tail biopsies. Briefly, tail tip DNA was extracted using Promega’s Wizard Genomic DNA Extraction kit following supplier provided protocol. These DNA samples were used as templates for detecting the presence of the transgene using PCR primers ATGACCAACTCGCTGACC (forward) and CACTCCTTGAAGTTGAGC (reverse). The PCRs were carried out with an initial denaturation at 95°C for 1 min, and followed by 35 cycles of 95°C for 15 seconds, 60°C for 30 seconds and 72°C for 10 seconds and a final extension at 72°C for 7 min. Among the 47 offspring born to the foster mothers, two contained the intact transgene (Extended data Fig 7d). One of these two founder mice, designated #6775, was expanded and backcrossed to C57BL/6N strains for seven generations.
Langendorff perfused mouse heart
Experiments were conducted on male mitochondrial calcium uniporter knockout mice (MCU-KO) and their wild-type (WT) littermates. Mice were anesthetized using sodium pentobarbitol (50 mg/kg) by intraperitoneal injections. Heparin (50 USP) was also administered by intraperitoneal injection. Hearts were isolated and placed in ice-cold Krebs-Heinseleit (KH) buffer (25 mM NaHCO3, 120 mM NaCl, 11 mM glucose, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, and 1.75 mM CaCl2). The aorta was cannulated on a 26 gauge needle and retrograde perfused on a Langendorff perfusion system. All hearts were held at a constant pressure of 100 cm of water and perfused with KH buffer gassed with 95% O2 and 5% CO2. Ru360 was added as indicated to inhibit MCU.
Flow rate (FR) from the heart was constantly measured as effluent flow passed through a 1PXN flow-through senior coupled to a TS410 Flow Module (Transonic). Heart rate was calculated from the sinusoidal flow rate curve (LabChart, ADInstruments). In non-optical experiments, the left atrial appendage was excised and a water-filled latex balloon was inserted into the LV cavity through the mitral valve to measure hemodynamic parameters using (LabChart ADInstruments).
Rhod-2-AM Loading
Hearts were perfused for 15 minutes to allow stabilization followed by baseline (pre-loading) measurement of white light and fluorescence emission spectra. Following stabilization and baseline measurement the heart was loaded with the Ca2+ sensitive dye Rhod-2 in the cell-permeant acetoxymethyl ester (AM) form (Cayman Chemical). Rhod-2-AM was dissolved in dimethyl sulfoxide (DMSO) to create a stock solution with a final concentration of 8.9 mM. Aliquots of this solution were frozen at −20°C. Prior to loading into hearts, the Rhod-2-AM stock solution was dissolved in oxygenated, KH buffer at 37°C to a final concentration of 3.5 μM. During loading the temperature of the water jacketed heart was lowered to 30°C to enhance loading into the mitochondria. Hearts were perfused with the KH Rhod-2-AM solution for 25 minutes in the dark at 30°C, followed by perfusion for 40 minutes with KH buffer at 37°C to washout out uncleaved dye.
Optical Measurements
The left atrial appendage was excised and an optic fiber (Polymicro Molex) was inserted into the left ventricular (LV) cavity through the mitral valve. The optical set-up was modified from that previously described (Bauer et al., 2019). Briefly, the perfused heart was placed in an optically transparent, water jacketed glass chamber at 37°C. The chamber was then lowered into a 6-inch diameter integrating sphere with Spectraflect reflectance coating. A white light source (Mightex) and a 532 nm laser (Ocean Optics) were connected via a switch to the optic fiber in the LV cavity to transmit light through the myocardium. A light guide was inserted into the bottom of the sphere to carried light that passed through the heart tissue to a spectrophotometer (Wasatch Photonics).
Isoproterenol treatment
Following the 40 min of Rhod-2-AM washout period, isoproterenol was administered to the perfused heart via a syringe pump (Harvard Apparatus) for five minutes. Isoproterenol (Sigma) was dissolved in oxygenated KH buffer to a final concentration of 2 mM, which was pumped into the perfusion line at 1% the flow rate to achieve a final concentration of 20 nM isoproterenol. White light and 532 nm laser were alternated during the 5 min stimulation period. Then, isoproterenol was washed out for 25 min while the white light and 532 nm laser were alternated.
Widefield and Stimulated Emission Depletion (STED) Imaging
Large area fluorescence imaging of mNeonGreen expressed in mouse embryos and excised tissues were conducted using a Leica MZFLIII fluorescence stereo microscope (Leica Microsystems, Inc., Wetzlar, Germany), a 1x lens, an Olympus DP72 CCD camera (Olympus Microscope Corporation, Tokyo, Japan), a pixel format of 1360 x 1024 pixels, variable optical zoom, and a 50W mercury fluorescence excitation lamp. In all cases mNeonGreen fluorescence was imaged using a 470/40nm excitation and 515 LP emission filter. Camera exposure times were set to 1.7 and 0.8s for embryos and excised tissues respectively.
To determine the location of Rhod-2-AM loading, TOMM20-mNeonGreen transgenic mice were loaded with Rhod-2-AM at 30°C for 25 minutes. The temperature was then raised to 37°C and the hearts were perfused for an additional 40 minutes to washout uncleaved dye. The fresh hearts were immediately sliced and imaged. Fluorescence images of freshly excised strips of TOMM20-mNeonGreen transgenic mouse heart loaded with the fluorophore Rhod-2-AM were acquired using a Leica SP8 3X STED microscope, a white-light laser for fluorescence excitation (470-670 nm), a Leica HyD SMD time-gated PMT, and a Leica 100x (1.4 NA) STED White objective (Leica Microsystems, Inc., Wetzlar, Germany). STED Images of Rhod-2 and mNeonGreen were taken using 561 nm and 488 nm excitation, emission band-passes of 570-700 nm and 495-530 nm respectively, a pinhole size of 0.7 A.U., a scan speed of 600 Hz., and a pixel format of 1024 x 1024. STED depletion of Rhod-2 was conducted using 775 nm pulsed laser depletion (20% power with ≈ 85mW at the back aperture of the objective) in the XY dimension, and time-gating on the HyD SMD PMT to a range of 0.7 to 6.5 ns, and optical zoom to produce pixel sizes of 26-30 nm. STED imaging of mNeonGreen was similarly conducted using continuous wave depletion in the XY dimension at 592 nm (2.4% power with ≈ 16 mW at the back aperture of the objective), time-gating range on the HyD SMD PMT to a range of 1.0 to 6.5 ns. All images for both colors were acquired with a 2-frame line average and a 2-frame line average combined with 2-frame integration respectively. The program Huygens Professional (version 19.1, Scientific Volume Imaging, Hilversum, The Netherlands) was used to deconvolve STED images. All deconvolution was based on idealized point spread functions, using the classic maximum likelihood estimation (CMLE) deconvolution algorithm.
Optical absorption spectroscopy
Spectral data was collected from hearts via a fiber optic light guide (Thor Labs, 0.39 NA) and transmitted to a cooled, rapid-scanning spectrometer. White light absorbance spectra were fit using reference spectra from active chromophores in the cardiac mitochondria, including oxygenated (MbO) and deoxygenated (MbDO) myoglobin, complex IV (a605), complex III (cyt bH, bL, and c1), cyt c, and I0 as described (Bauer et al., 2019). Spectra were analyzed during steady-state periods using an average of 30 spectra (30 seconds) and corrected for changes in pathlength of light through the heart tissue following isoproterenol treatment, chromophores were analyzed from 530-630 nm.
Quantification of Rhod-2 fluorescent emission
Raw fluorescent emission was collected from the heart tissue. The maximum emission wavelength of Rhod-2 loaded in cardiac tissue was 630nm, which is right-shifted from the known peak emission of 581 nm for Rhod-2 in a pure solution (Figure 3A). A correction for the inner filter effect modified from Lakowicz (Lakowicz, 2006) was applied to raw fluorescent spectra.
Wavelengths 650-680 nm were chosen to calculate Ca2+ bound Rhod-2 fluorescent emission as tissue fluorescence does not interfere at these wavelength and thus at these wavelengths we do not need to correct for the secondary filter effect following Krimer, et al (Krimer et al., 2017). As there are minimal changes in absorbance from 650-680 nm in the Rhod-2 loaded mouse heart, there is a negligible secondary filter effect on the Ca2+ bound Rhod-2 fluorescence emission. Fluorescence emission from 650-680 nm was analyzed at steady-state periods using an average of 30 spectra blocks (30 seconds). A532 values were obtained in the 30 second time period immediately following the fluorescence emission collection. Background fluorescent emission from the hearts before Rhod-2-AM loading was corrected for primary filter effects. Ca2+ bound Rhod-2 fluorescent emission values were corrected for this background fluorescence.
Statistical Analysis
Data are presented as mean and standard error measurement. Students two-tailed t-test were used to compare differences between 2 groups. P<0.05 was considered significant. Goodness-of-fit statistics for the spectral fitting were established using the LabView weighted mean square error in the General Linear Fit function.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Rhod-2 AM | Cayman Chemical | CAS: 145037-81-6 |
| (−)-Isoproterenol hydrochloride | Sigma | CAS: 5984-95-2 |
| Ru360 | Sigma | CAS: 557440 |
| Experimental models: Cell lines | ||
| Human: HEK293T | ATTC | https://www.atcc.org/products/all/crl-3216.aspx#generalinformation |
| Experimental models: Organisms/strains | ||
| Mouse: MCU-KO line | Pan, et al 2013 | N/A |
| Mouse: TOMM20-mNeonGreen | This paper | N/A |
| Mouse: B6D2F1/J | The Jackson Laboratory | Stock No. 100006 |
| Mouse: C57BL/6N | Taconic Farms | https://www.taconic.com/mouse-model/black-6-b6ntac |
| Recombinant DNA | ||
| Plasmid: mNeonGreen-TOMM20-N-10 | Allele Biotechnology | https://reagents.allelebiotech.com/mneongreen-1/ |
| Vector: pSF-CAG-Kan | Sigma-Aldrich | Cat# OGS505-5UG |
| Oligonucleotides | ||
| Primer: AAAGGAGGTACCCACCATGGTGGGTCGGAACAG (Forward) | Integrated DNA Technologies | N/A |
| Primer: ACAACTTCTAGACTTGTACAGCTC GTCCATGC (Reverse) | Integrated DNA Technologies | N/A |
| Critical commercial assays | ||
| Wizard Genomic DNA Extraction kit | Promega | Cat# A1120 |
| Software and algorithms | ||
| LabVIEW Spectroscopy Program | This paper | N/A |
| Huygens Professional | Scientific Volume Imaging | Version 19.1; https://svi.nl/Huygens-Professional |
| LabChart | ADInstruments | Version 8.0; https://www.adinstruments.com/products/labchart/versions-and-licenses |
Acknowledgments:
The research was funded by the NHLBI intramural program. We thank Lanelle Edwards and Raul Covian for the helpful suggestions.
Inclusion and Diversity:
One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science. While citing references scientifically relevant for this work, we also actively worked to promote gender balance in our reference list.
Footnotes
Declaration of Interests: The authors declare no competing interests.
References:
- Aguilar-Sanchez Y, Rodriguez de Yurre A, Argenziano M, Escobar AL, and Ramos-Franco J (2019). Transmural Autonomic Regulation of Cardiac Contractility at the Intact Heart Level. Front Physiol 10, 773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer TM, Giles AV, Sun J, Femnou A, Covian R, Murphy E, and Balaban RS (2019). Perfused murine heart optical transmission spectroscopy using optical catheter and integrating sphere: Effects of ischemia/reperfusion. Anal Biochem 586, 113443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisbach CM, Hutto RA, Poria D, Cleghorn WM, Abbas F, Vinberg F, Kefalov VJ, Hurley JB, and Brockerhoff SE (2020). Mitochondrial Calcium Uniporter (MCU) deficiency reveals an alternate path for Ca(2+) uptake in photoreceptor mitochondria. Sci Rep 10, 16041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes R, and Bers DM (2002). Simultaneous measurements of mitochondrial NADH and Ca(2+) during increased work in intact rat heart trabeculae. Biophys J 83, 587–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess DJ, Billings E, Covian R, Glancy B, French S, Taylor J, de BH, Murphy E, and Balaban RS (2013). Optical spectroscopy in turbid media using an integrating sphere: mitochondrial chromophore analysis during metabolic transitions. Anal Biochem. 439, 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton RM, and McCormack JG (1980). The role of calcium in the regulation of mitochondrial metabolism. Biochem Soc Trans 8, 266–268. [DOI] [PubMed] [Google Scholar]
- Du C, MacGowan GA, Farkas DL, and Koretsky AP (2001). Calcium measurements in perfused mouse heart: quantitating fluorescence and absorbance of Rhod-2 by application of photon migration theory. Biophys J 80, 549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femnou AN, Kuzmiak-Glancy S, Covian R, Giles AV, Kay MW, and Balaban RS (2017). Intracardiac light catheter for rapid scanning transmural absorbance spectroscopy of perfused myocardium: measurement of myoglobin oxygenation and mitochondria redox state. Am J Physiol Heart Circ Physiol 313, H1199–H1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippin L, Abad MC, Gastaldello S, Magalhaes PJ, Sandona D, and Pozzan T (2005). Improved strategies for the delivery of GFP-based Ca2+ sensors into the mitochondrial matrix. Cell Calcium 37, 129–136. [DOI] [PubMed] [Google Scholar]
- Giles AV, Sun J, Femnou AN, Kuzmiak-Glancy S, Taylor JL, Covian R, Murphy E, and Balaban RS (2018). Paradoxical arteriole constriction compromises cytosolic and mitochondrial oxygen delivery in the isolated saline-perfused heart. Am J Physiol Heart Circ Physiol 315, H1791–H1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glancy B, and Balaban RS (2012). Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry 51, 2959–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmstrom KM, Pan X, Liu JC, Menazza S, Liu J, Nguyen TT, Pan H, Parks RJ, Anderson S, Noguchi A, et al. (2015). Assessment of cardiac function in mice lacking the mitochondrial calcium uniporter. J Mol Cell Cardiol 85, 178–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobsis PD, Rothstein EC, and Balaban RS (2007). Limited utility of acetoxymethyl (AM)-based intracellular delivery systems, in vivo: interference by extracellular esterases. J Microsc 226, 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Ripple MO, and Springett R (2012). Measurement of the mitochondrial membrane potential and pH gradient from the redox poise of the hemes of the bc1 complex. Biophysical journal 102, 1194–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimer NI, Rodrigues D, Rodriguez HB, and Mirenda M (2017). Steady-State Fluorescence of Highly Absorbing Samples in Transmission Geometry: A Simplified Quantitative Approach Considering Reabsorption Events. Anal Chem 89, 640–647. [DOI] [PubMed] [Google Scholar]
- Kuzmiak-Glancy S, Covian R, Femnou AN, Glancy B, Jaimes R 3rd, Wengrowski AM, Garrott K, French SA, Balaban RS, and Kay MW (2018). Cardiac performance is limited by oxygen delivery to the mitochondria in the crystalloid-perfused working heart. Am J Physiol Heart Circ Physiol 314, H704–H715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong JQ, Lu X, Correll RN, Schwanekamp JA, Vagnozzi RJ, Sargent MA, York AJ, Zhang J, Bers DM, and Molkentin JD (2015). The Mitochondrial Calcium Uniporter Selectively Matches Metabolic Output to Acute Contractile Stress in the Heart. Cell Rep 12, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz J. (2006). Principles of Fluorescence Spectroscopy. (Berlin, Germany: Springer US,). [Google Scholar]
- Liu J, Syder N, Ghorashi N, Willingham T, Parks RJ, Sun J, Fergusson M, Liu J, Holmstrom KM, Menazza S, et al. (2020). EMRE is essential for mitochondrial calcium uniporter activity in a mouse model. JCI Insight In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luongo TS, Lambert JP, Yuan A, Zhang X, Gross P, Song J, Shanmughapriya S, Gao E, Jain M, Houser SR, et al. (2015). The Mitochondrial Calcium Uniporter Matches Energetic Supply with Cardiac Workload during Stress and Modulates Permeability Transition. Cell Rep 12, 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E, and Steenbergen C (2020). Regulation of Mitochondrial Ca(2+) Uptake. Annu Rev Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Liu J, Nguyen T, Liu C, Sun J, Teng Y, Fergusson MM, Rovira II, Allen M, Springer DA, et al. (2013). The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol 15, 1464–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen TP, Wu Y, Joiner ML, Koval OM, Wilson NR, Luczak ED, Wang Q, Chen B, Gao Z, Zhu Z, et al. (2015). Inhibition of MCU forces extramitochondrial adaptations governing physiological and pathological stress responses in heart. Proc Natl Acad Sci U S A 112, 9129–9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner NC, Lambert GG, Chammas A, Ni Y, Cranfill PJ, Baird MA, Sell BR, Allen JR, Day RN, Israelsson M, et al. (2013). A bright monomeric green fluorescent protein derived from Branchiostoma lanceolatum. Nat Methods 10, 407–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szibor M, Gizatullina Z, Gainutdinov T, Endres T, Debska-Vielhaber G, Kunz M, Karavasili N, Hallmann K, Schreiber F, Bamberger A, et al. (2020). Cytosolic, but not matrix, calcium is essential for adjustment of mitochondrial pyruvate supply. J Biol Chem 295, 4383–4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trollinger DR, Cascio WE, and Lemasters JJ (1997). Selective loading of Rhod 2 into mitochondria shows mitochondrial Ca2+ transients during the contractile cycle in adult rabbit cardiac myocytes. Biochem Biophys Res Commun 236, 738–742. [DOI] [PubMed] [Google Scholar]
- Trollinger DR, Cascio WE, and Lemasters JJ (2000). Mitochondrial calcium transients in adult rabbit cardiac myocytes: inhibition by ruthenium red and artifacts caused by lysosomal loading of Ca(2+)-indicating fluorophores. Biophys J 79, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unitt JF, McCormack JG, Reid D, MacLachlan LK, and England PJ (1989). Direct evidence for a role of intramitochondrial Ca2+ in the regulation of oxidative phosphorylation in the stimulated rat heart. Studies using 31P n.m.r. and ruthenium red. Biochem J 262, 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, De Jesus NM, and Ripplinger CM (2015). Optical Mapping of Intra-Sarcoplasmic Reticulum Ca2+ and Transmembrane Potential in the Langendorff-perfused Rabbit Heart. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Yao J, Belke D, Guo W, Zhong X, Sun B, Wang R, Estillore JP, Alexander V, Benitez R, et al. (2020). Ca(2+)-CaM Dependent Inactivation of RyR2 Underlies Ca(2+) Alternans in Intact Heart. Circ Res. [DOI] [PubMed] [Google Scholar]
- Wescott AP, Kao JPY, Lederer WJ, and Boyman L (2019). Voltage-energized Calcium-sensitive ATP Production by Mitochondria. Nat Metab 1, 975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg BA, and Wittenberg JB (1989). Transport of oxygen in musle. Anu.Rev.Physiol 51, 857–878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request. This paper does not report original code. Any additional information or requests for data reported in paper is available from the lead contact.