Summary:
Lupus nephritis (LN) is a life-threatening manifestation of systemic lupus erythematosus (SLE) and is more common in children than adults. The epidemiology and management of childhood-onset SLE (cSLE) have changed over time, prompting the need to reassess expected outcomes. The purpose of this study is to use the Childhood Arthritis and Rheumatology Research Alliance (CARRA) prospective registry to validate historical principles of LN in a contemporary, real-world cohort. After an extensive literature review, six principles of LN in cSLE were identified. The CARRA registry was queried to evaluate these principles in determining the rate of LN in cSLE, median time from cSLE diagnosis to LN, short-term renal outcomes, and frequency of rituximab as an induction therapy. Of the 677 cSLE patients in the CARRA registry, 32% had documented LN. Decline in kidney function was more common in Black cSLE patients than non-Black patients (p=0.04). Black race was associated with worse short-term renal outcomes. In short-term follow up, most children with LN had unchanged or improved kidney function, and end stage kidney disease (ESKD) was rare. Ongoing follow-up of cSLE patients in the CARRA registry will be necessary to evaluate long-term outcomes to inform risk, management, and prognosis of LN in cSLE.
Keywords: pediatric lupus nephritis, lupus nephritis, pediatric rheumatology, childhood onset lupus
BACKGROUND
Systemic lupus erythematosus (SLE) is a multisystem, chronic autoimmune disease that is more aggressive with onset in childhood (<18 years) compared to adulthood, including higher rates of life-threatening organ involvement.1–4 The prevalence of childhood-onset SLE (cSLE) is 9.73 per 100,000 children, and approximately one in five patients with SLE presents prior to age 18. The range of this prevalence rate varies by gender and race.5 Lupus nephritis (LN) is a well-established manifestation of SLE with a high rate of morbidity and mortality.6 Thirty-six to 55% of patients with cSLE develop LN and this frequently occurs shortly after the diagnosis of SLE.2–3,5,7 Most of this data comes from single center studies. In a Canadian cohort, 82% of patients with LN developed it within one year of cSLE diagnosis,7 while in a Pakistani LN cSLE cohort, 92% of children with LN developed it within two years of diagnosis.9
LN in cSLE has high risk for organ damage and poor outcomes. Historically, 22–50% of children with LN develop end stage kidney disease (ESKD). While prognosis has improved over time, there continues to be significant long-term morbidity and mortality.10–11 A 22% mortality rate within 5 years of developing ESKD has been reported.12 In a study of pediatric LN patients from 1980 to 2010, Black race was associated with higher rates of ESKD.13 Estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2, the presence of hypertension, and nephrotic-range proteinuria at presentation of LN are associated with worse kidney outcomes.13 Early identification and treatment of LN is critical given that sustained renal remission is associated with lower rates of chronic kidney disease (CKD) progression, ESKD, and overall mortality.13–14
Utilization of registry data is helpful in assessing the broad phenotypic range of cSLE. The Childhood Arthritis and Rheumatology Research Alliance (CARRA) is a collaborative pediatric rheumatology research organization consisting of centers in the United States and Canada. The CARRA Legacy Lupus Registry (2010–2014) has been used to characterize the phenotype of a pediatric cohort with membranous LN and associated kidney outcomes; it has also been used to retrospectively compare the treatment responsiveness of membranous plus proliferative LN versus isolated proliferative LN.15–16 In March 2017, CARRA launched a new prospective cSLE registry of patients from the United States and Canada.
With advancement in treatment, the natural history of cSLE and LN continue to evolve. Recent studies have recognized that updating the “rules” of rheumatic disease in current cohorts of patients is important to keep data accurate and salient.17 LN has recently been named a research priority by both CARRA and the Lupus Foundation of America.18 Since CARRA currently has a robust active cohort of cSLE patients, our aim was to identify traditional principles related to the diagnosis, treatment, and surveillance of LN in childhood and determine if they remain valid in this contemporary multi-center cohort.
METHODS
A literature review was conducted by searching for studies of LN in cSLE. Search terms included “pediatric lupus nephritis,” “pediatric SLE and nephritis,” and “lupus nephritis in children” in the PubMed database. Through this literature search, fifteen studies were identified from 1992 to 2019. From there, six principles were identified of LN in cSLE. Selection of principles was based on descriptive data and feasibility for the principles to be examined within the CARRA registry. They were:
LN develops within one year of diagnosis in 82% of cSLE patients; 92% develop LN within two years of diagnosis7,9
Membranous (WHO and/or ISN/RPS class V) LN more often presents with proteinuria than does proliferative LN (WHO and/or ISN/RPS class III or IV)6
Short-term kidney outcomes are worse in Black vs non-Black patients6
Short-term kidney outcomes are worse in patients who present with eGFR <60mL/min/1.73 m2 and/or moderate proteinuria (urine protein: creatinine ratio (UPC) > 1 mg/mg)6
Rituximab is used as a steroid-sparing agent for induction in proliferative LN7–8
The CARRA registry, which is a contemporary, prospective, multi-center registry, was queried for all patients with cSLE. This registry began enrolling patients in March 2017. Inclusion criteria required a diagnosis of SLE or LN within 24 months of enrollment, diagnosis prior to the age of 19 years, and subject (and/or parent/legal guardian when required) able to provide written informed consent and willing to comply with study procedures.19 The criteria used to define SLE for the CARRA registry are either 4 of 11 American College of Rheumatology (ACR) criteria for SLE (1997) or 3 of 11 ACR criteria inclusive of biopsy-proven LN (1997). These criteria could manifest simultaneously or over time.20–21
The following were queried from the CARRA registry in January 2020: demographics (age, sex, self-reported race and ethnicity), the occurrence of LN in cSLE, time from diagnosis to LN, LN classification by kidney biopsy, short-term kidney outcomes, and instances of rituximab as induction therapy and additional therapies received. LN diagnosis was determined by kidney biopsy result. Classification was recorded as either WHO or ISN/RPS classification in the CARRA registry.22–23
For analysis of renal outcomes, the eGFR (calculated by the modified Schwartz equation) was separated into three states of CKD. eGFR states are classified as 1 to 3, with an increase in state correlating to a decline in kidney function: eGFR state 1 (>60 ml/min/1.73m2, CKD stage 1–2), state 2 (30–60 ml/min/1.73m2, CKD stage 3), state 3 (<30 ml/min/1.73m2, CKD stage 4–5). Short-term renal outcomes were assessed by change in eGFR as determined by comparing the initial eGFR to the most recent. Occurrences of ESKD, transplant, and dialysis were recorded. LN remission was defined, by ACR Renal Disease Subcommittee, as a serum creatinine within the normal range for age, urine protein: creatinine (UPC) ratio < 0.2mg/mg, and urine red blood cells <5/high powered field. Data variables were recorded for the initial registry visit and subsequent follow-up visits in six-month intervals.24
Statistical analysis.
Summary statistics were used to describe the population and determine the frequency of LN, the time to LN, frequency of rituximab usage, and short-term outcomes. The analysis was done using R version 3.6.3 (R Core Team, 2020).25 This study was deemed exempt from Institutional Board Review at the National Institutes of Health.
RESULTS
Enrolled participants.
A total of 677 patients with cSLE were enrolled in the registry with mean disease duration of one year at enrollment. The median age of participants was 15 years, and female: male ratio was 5:1. Approximately one-third of participants identified as White and 25% identified as Hispanic. Roughly one-quarter of participants identified as Black, and 12% of patients identified as Asian. Median follow-up was 9.3 months. Of the 677 participants, 50% have been followed for at least one year and 12% have been followed for at least two years.
Principle 1: Frequency of LN.
Of the 677 patients with cSLE-related conditions in the CARRA registry, 216 (32%) were documented to have LN, with no data for two of the patients.
Principle 2: Rate of Development of LN.
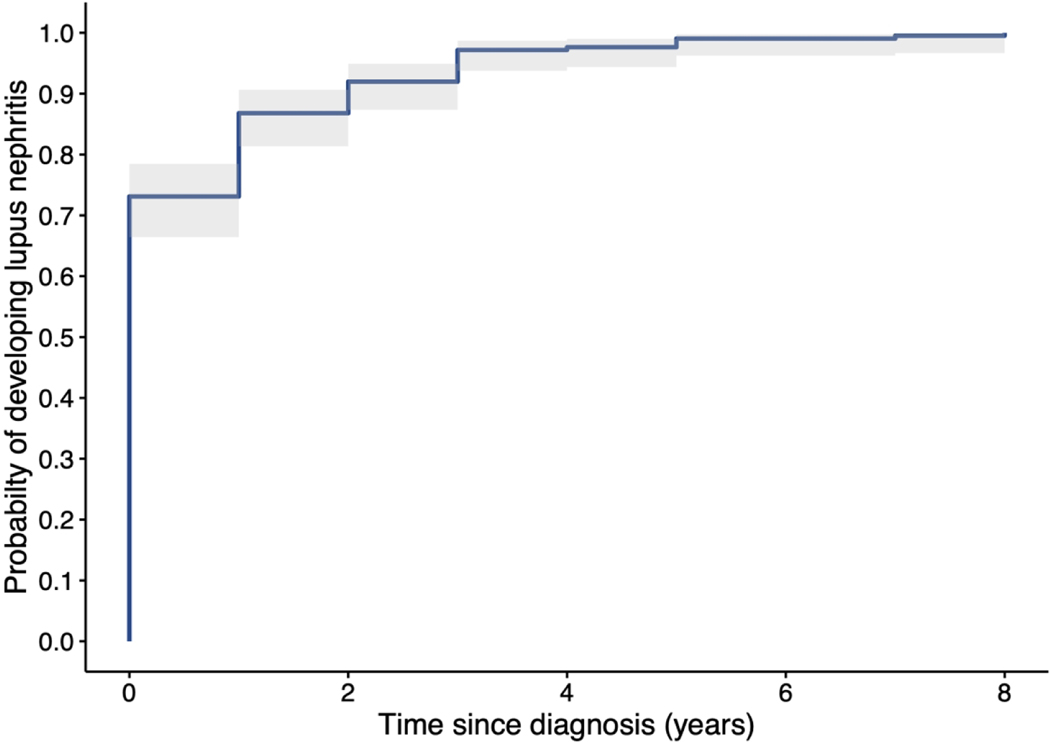
Of the 216 patients with LN, 93% of the patients who were diagnosed with LN received that diagnosis within two calendar years of their cSLE diagnosis, and 158 patients (73.1%) were diagnosed with LN during the same calendar year as their cSLE diagnosis. During the calendar year following their cSLE diagnosis, 29 (13.4%) were diagnosed with LN, totaling 88.4% diagnosed within one calendar year of cSLE diagnosis. Ten patients (4.6%) were diagnosed with LN two years after and 19 patients (8.8%) were diagnosed three or more years after the year in which they were diagnosed with SLE (Figure 1).
Figure 1.
Kaplan-Meier depiction of the relationship, in years, between cSLE diagnosis and LN diagnosis. These provide the best statistical estimate of all 216 subjects going forward in time without censoring (none were lost to follow up).
Principle 3: Proteinuria and LN Class.
Of the 216 LN patients, 140 (64.8%) had pure class III or IV LN, and another 22 (10.2%) had mixed class III/V or IV/V (Table 2). Thirty patients (13.9%) had pure class V LN, while a total of 52 (24.1%) had biopsies consistent with class V LN. Many patients did not have a UPC reported within 30 days of their baseline visit. When proteinuria data were available, a little over half (52.2%) of the class III/IV patients had UPC ≥0.5mg/mg, whereas 60% of patients with class V LN alone met the UPC threshold of ≥0.5mg/mg. There were ten patients with class III/IV + V on biopsy and 80% had a UPC ≥0.5mg/mg. A greater proportion of patients with membranous LN had moderate proteinuria, in comparison to those with proliferative LN, but it was not statistically significant (p=0.71).
Table 2.
Quantification of proteinuria in patients by LN class at initial visit.
| Class III/IV only (N=140) | Class III/IV + V (N=22) | Class V only (N=30) | Other* (N=24) | Overall (N=216) | |
|---|---|---|---|---|---|
| UPC in last 30 days | |||||
| < 0.5 mg/mg | 32 (22.9%) | 2 (9.1%) | 6 (20.0%) | 7 (29.2%) | 47 (21.8%) |
| >= 0.5 mg/mg | 35 (25.0%) | 8 (36.4%) | 9 (30.0%) | 3 (12.5%) | 55 (25.5%) |
| Missing | 73 (52.1%) | 12 (54.5%) | 15 (50.0%) | 14 (58.3%) | 114 (52.8%) |
Principle 4: Racial disparities in renal outcomes.
Black LN patients had worse renal outcomes compared to non-Black patients. Black LN patients had a four-fold increased rate of Stage 3, 4, or 5 CKD. Overall there was no significant difference in achievement of remission (50% of Black patients, compared to 50% of non-Black patients; p-value = 0.11).
Principle 5: Renal recovery.
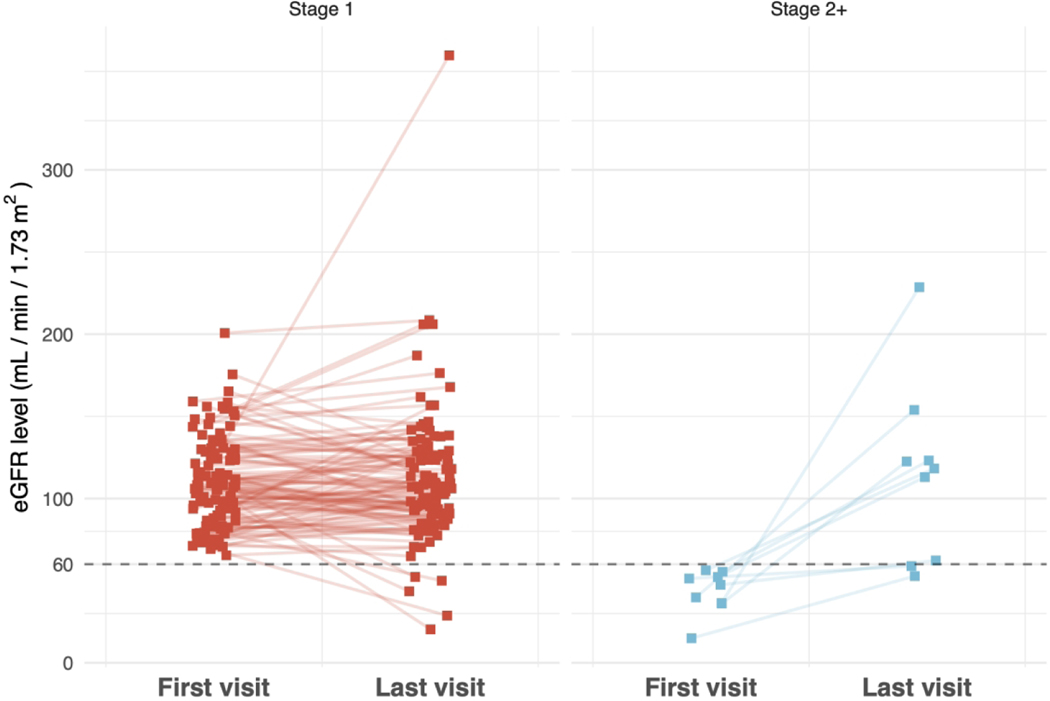
For patients who had eGFR >60 ml/min/1.73 m2 at diagnosis (n=38), 13 (34.2%) achieved remission. In patients with reduced kidney function (eGFR 30–60) at diagnosis (n=8), one (12.5%) achieved remission; neither of the two patients with eGFR <30 at diagnosis achieved remission, and these differences were not statistically significant (p= 0.45). For patients with multiple recorded eGFRs, of those with an initial eGFR >60 (n=114), 4.4% progressed to stage 3–5 CKD (Figure 2). Of the nine patients with an initial eGFR <60, 77.8% recovered kidney function, but 22.2% continued to have an eGFR <60 (p=0.08) (Table 3). Of the total cohort of 216, nine subjects were reported to have ESKD. Three subjects were reported to be on dialysis. There were no kidney transplants reported in the cohort.
Figure 2.
eGFR change between first registry and most recent visit for patients with initial stage 1/2 CKD (State 1 eGFR >60, N=114) and those with initial stage 3–5 CKD (State 2 or higher eGFR <60, N=9).
Table 3.
Changes in eGFR* State (Initial vs Most Recent Visit); N=123
| Most Recent | ||||
|---|---|---|---|---|
| Initial | State 1 | State 2 | State 3 | |
| State 1 | 109 (95.6%) | 3 (2.6%) | 2 (1.8%) | |
| State 2 | 7 (87.5%) | 1 (12.5%) | 0 (0.0%) | |
| State 3 | 0 (0.0%) | 1 (100.0%) | 0 (0.0%) | |
eGFR= estimated glomerular filtration rate (mL/min/1.73m2) based on modified Schwartz equation25
Principle 6: Use of rituximab.
Rituximab was administered in 55 (25.5%) LN patients and in 55 (12%) cSLE patients without LN (p<0.0001). There were no statistical differences in rituximab use based on LN class or patient demographics such as age, race, ethnicity, or sex. Patients treated with rituximab were often prescribed additional immunosuppressant medications, including mycophenolate in either of its formulations (concurrently used in 87.3% of patients on rituximab, compared to 78.9% of patients not on rituximab) and cyclophosphamide (concurrently used in 67.3% of rituximab patients, compared to 16.2% of patients not on rituximab) (Table 4).
Table 4.
Top 10 medications concurrently used by cSLE LN patients with and without rituximab treatment
| Medication | No Rituximab (N=161) | Treatment with Rituximab (N=55) |
|---|---|---|
| Hydroxychloroquine | 155 (96.3%) | 53 (96.4%) |
| Mycophenolate Mofetil | 127 (78.9%) | 48 (87.3%) |
| Cyclophosphamide | 26 (16.2%) | 37 (67.3%) |
| Aspirin | 13 (8.1%) | 9 (16.4%) |
| Mycophenolic Acid | 18 (11.8%) | 7 (12.7%) |
| Angiotensin Converting Enzyme Inhibitor |
20 (12.4%) | 7 (12.7%) |
| Azathioprine | 18 (11.2%) | 7 (12.7%) |
| Lisinopril | 14 (8.7%) | 7 (12.7%) |
| Vitamin D | 47 (29.2%) | 7 (12.7%) |
| Immunoglobulin | 1 (0.6%) | 6 (10.9%) |
| Tacrolimus | 7 (4.4%) | 6 (10.9%) |
DISCUSSION
Prior studies have shown that 36 to 55% of children with SLE develop nephritis.5,7–8 In our contemporary multi-center cohort of cSLE patients, we found 32% of the patients with cSLE have LN. The CARRA Registry enrolls patients from the United States and Canada, and the rates of LN found in this study are consistent with previous smaller studies in these populations. An epidemiologic study of United States patients enrolled in Medicaid found a 37% prevalence rate of LN in cSLE patients.5 A large, single center study from Canada reported the rate of LN in cSLE to be 55%.7 This rate is similar to a multi-center study performed in the United Kingdom that found 37% of cSLE patients have LN at initial presentation.27 Our study supports the previous literature that LN occurs in about one-third of cSLE patients.
The wide variation in the reported rates of LN in children may be due to study methodology, cohort size, or racial or ethnic composition of the populations studied. While some reports have not included racially and ethnically diverse populations, our study consisted of a diverse group of patients throughout North America. Since the cSLE patients in the CARRA registry were mostly Black (30%), White (26%) or Asian (11%), our reported rate of LN is more reflective of the actual diverse cSLE population.
Prior studies have reported the majority of children who develop LN do so within one year of diagnosis. 5,7–8 In our study, 73% of patients were diagnosed with LN in the same year as their cSLE diagnosis, and an additional 13% in the year following cSLE diagnosis (12–24 months following diagnosis). Of cSLE patients in the CARRA registry who developed LN, 93% did within two years of cSLE diagnosis, which is higher than historically reported in North America. This earlier onset of LN could be related to a better understanding of risk factors for developing LN, earlier diagnosis of cSLE, and practice changes, such as a lower threshold to perform a percutaneous kidney biopsy to establish the diagnosis.6,28 This study highlights the importance of routine screening for kidney involvement in newly diagnosed cSLE and prompt referral to pediatric nephrology when kidney disease is suspected.
In our study, 75% of patients had proliferative LN, inclusive of mixed LN class. The remaining patients had mesangioproliferative or non-proliferative LN classes. We evaluated whether membranous (class V) LN more often presents with proteinuria than proliferative LN (class III or IV) as previously reported.12,29 Prior literature indicates that up to 80% of class V LN patients present with nephrotic syndrome in comparison to 30% and 50% in class III and class IV LN, respectively.29 Unfortunately, nephrotic range proteinuria could not be assessed in the CARRA registry because proteinuria data for most subjects was limited to a categorical variable as <0.5 mg/mg or >=0.5 mg/mg at their initial registry visit, and 77% of patients did not have a numerical UPC recorded. We found a greater proportion of patients with membranous LN had proteinuria, in comparison to those with proliferative LN; although this was not statistically significant. Our observation is consistent with prior studies, but also demonstrates the limitations of conventional urine testing in differentiating between disease activity and kidney damage/scarring. Additionally, we found the proportion of those with moderate proteinuria was highest in patients with mixed class III/V and class IV/V. The etiology of this difference requires further investigation in a larger cohort.
In the United States, studies have shown that Black patients with SLE, regardless of age at diagnosis, more frequently have kidney involvement, higher disease activity, and higher risk for progression to ESKD.30–34 Research is still ongoing to determine whether outcome disparities are due to more aggressive disease, genetic disposition, socio-economic causes, unequal access to health care, or a combination of these factors.30 In this study, kidney function from baseline to the latest follow-up and achievement of remission were assessed as short-term outcomes. Based on eGFR, the vast majority of patients, remained in their initial CKD stage.
Compared to Black patients, most non-Black patients entered the registry with good kidney function, which persisted at last follow-up visit. For Black patients, however, 9% progressed from CKD stage 1/2 to stage 3/4, which indicated a worsening in kidney function. The results show that eGFR decline is more frequent among Black patients in the CARRA registry than non-Black patients (p-value = 0.04). The data suggest that this previous principle still holds true, and merits greater attention to preserving kidney function in Black patients.
With accelerated progression of CKD to ESKD, there is a higher associated morbidity due to dialysis and transplant-related complications and a heightened risk of mortality. A study evaluating 5-year outcomes in cSLE patients with ESKD from 1995–2006 reported 49% of the patients were listed for renal transplant, 33% received transplant, and 22% died. It was also found that Black cSLE LN patients had almost double the mortality rate of White cSLE LN patients.12 This stark difference in mortality by race is not unique to SLE. There is a lack of health care equity in the United States, which has been highlighted very recently by the morbidity and mortality rate differences by race in the COVID-19 pandemic.35 One reason for these differences is the rate of access to care. The 2018 Health Insurance Coverage Report for the United States identified lower rates of health insurance in the Black and Hispanic populations in both adults and children.36 In cSLE, access to continuous care is crucial for monitoring of disease activity, determining response to therapy, preventing interruptions in medication compliance, and prompt escalation of therapy to prevent further morbidity as well as mortality.
Hagelberg and colleagues followed a cohort of 67 pediatric LN patients over 11 years and found 9% developed ESKD.37 In our study, only 1.4% progressed to ESKD, with none receiving a kidney transplant during the follow up period. This low rate of ESKD in the CARRA registry is likely primarily due to the limited longitudinal data (≤3 years) in the CARRA registry at the time of analysis, but contemporary data on long-term renal survival in cSLE complicated by LN remains an important priority. It should be noted that this is one of the first studies evaluating outcomes in cSLE LN since the addition of mycophenolate mofetil into the management of LN. Perhaps this will have an impact on the progression to ESKD, but this will require more long-term follow up data to study.
A main goal for SLE therapy, especially those with kidney involvement, is to achieve disease remission. For this study, of the 149 patients where response to therapy could be assessed, 54.4% had active kidney disease at study entry. When stratified by race, more non-Black patients achieved remission than Black patients (81% vs 53%, respectively). This is similar to previous data that have shown that White patients were almost twice as likely to go into disease remission with induction therapy for SLE than Black patients.38
In the CARRA cohort, cSLE patients with initially decreased renal function had a good chance of achieving recovery to normal renal function at follow up. cSLE patients with initially decreased eGFR state 2 had a good chance of recovery with 87.5% showing improvement in their kidney function. Sustained renal remission for five years has been correlated to a better prognosis and lower rates of mortality, ESKD, and kidney flare.14 The rates of remission in the CARRA cohort are similar to the 23% remission rate reported in a cohort of cSLE patients in Tennessee after 12 months of follow-up.39 Hugle et al. reported a higher remission rate of 90% in pure membranous LN in children over a four-year period.40 The difference in rates are likely secondary to the exclusion of proliferative LN in their study and differing length of follow-up.
Our findings also differ from previous data reported by Wu et al., which showed a baseline GFR <60 mL/min/1.73m2 had a nearly four-fold higher risk of reaching ESKD or death. This difference in rate of ESKD could be because that study enrolled patients for a longer duration of time (January 1999 to December 2011) than the CARRA registry, and Wu et al. specifically studied children with proliferative LN lesions.41 The vast majority (96%) of subjects in the CARRA cohort with eGFR state 1 (>60 ml/min/1.73m2) at diagnosis maintained normal kidney function above this threshold during their treatment course.
Assessment of proteinuria is a core marker of LN disease activity to screen for new LN in patients diagnosed with SLE and to assess response to treatment. The degree of proteinuria at the time of LN diagnosis and its duration have been independently associated with chronic kidney damage. Patients with persistent proteinuria following induction therapy despite normalization of serum creatinine and urinary sediment are more likely to have irreversible damage that may accelerate the development of CKD over time.42 Therefore, resolution of proteinuria (UPC < 0.2 mg/mg), as would be seen in complete remission of disease or at least partial remission (≥50% reduction in proteinuria with a goal UPC <0.5mg/mg), is desired.43 Unfortunately, the majority of subjects in the CARRA cohort have missing data on proteinuria in the first two years after LN diagnosis; thus, we were limited in our analysis.
Rituximab was reported to be utilized in a quarter of patients with LN, which was higher than cSLE patients without LN. The potential efficacy of rituximab in LN was suggested by several retrospective studies demonstrating efficacy in refractory LN.44–45 However, the randomized controlled LUNAR trial failed to show any additive effect of rituximab beyond corticosteroids and mycophenolate mofetil (MMF) in combination for proliferative LN.46 In our study, 87% were treated with MMF, which is substantially higher than previous studies reporting 47% of cSLE LN patients initially treated with MMF; this could suggest a practice trend away from the use of cyclophosphamide.44 Previous studies have shown that response to rituximab varies based on class of LN. In the case of rituximab for refractory LN, the complete or partial response criteria were met by 87% of patients with LN class III, 76% with class IV, and 67% with class V, respectively.44 However, we did not intend to evaluate response to rituximab. Although we found no difference in usage of rituximab by LN class, analysis was limited by the small sample size of LN patients being treated with rituximab (n=55). B-cell depleting therapies, such as rituximab, have a promising biologic mechanism in LN. The combination of belimumab and rituximab to improve remission efficacy in SLE is currently under investigation, as are newer B cell depleting agents such as obinutuzumab.47–48
Limitations
This study utilizes the CARRA registry, which is a cohort of recently diagnosed patients that began enrolling patients in March 2017. While approximately 50% of the cohort had been followed for at least one year, only 12% had been followed for at least two years. As the registry matures, further, richer studies will be possible and will be able to assess long term renal outcomes in patients with pediatric lupus nephritis. Moreover, the importance of additional features of lupus nephritis, such as control of hypertension 49 could be examined in cSLE.
In the de-identified data set, event dates of SLE and LN diagnosis, kidney transplant, and onset of chronic dialysis were obtained only for calendar year. Therefore, we are limited in our ability to evaluate long-term outcomes of cSLE patients with LN at this time. Severe outcomes, such as ESKD, occurrence of kidney transplantation, and mortality, are likely to occur more frequently in this cohort as time progresses. In addition, there were missing data points, with almost one-third of the patients not having enough data (i.e. urine protein and creatinine) to assess for remission state. For those with UPC data, nephrotic-range proteinuria could not be assessed in the CARRA registry because the UPC is recorded as a numerical and/or a categorical variable as < 0.5 mg/mg versus ≥ 0.5 mg/mg. Seventy-seven percent of patients did not have a numerical UPC recorded at their initial registry visit. Similarly, nephrotic syndrome also could not be assessed because albumin and edema are not recorded in the CARRA registry. The registry data entry is an iterative process, and after this assessment many impactful changes in data collection and quality control have been implemented. These changes will allow for a more comprehensive analysis in future studies.
CONCLUSION
The field of pediatric rheumatology is rapidly evolving. Large, multi-center registries allow for a diverse representation of patients as well as larger sample sizes. In this multi-center registry, we found that cSLE patients continue to have high rates of LN, and the onset of LN is most likely to occur in the first two years of SLE diagnosis. Black cSLE patients have worse short-term renal outcomes that have been shown in adult studies to be valid surrogates for longer-term outcomes such as ESKD and mortality.
The current CARRA registry is a relatively new multi-center, diverse cohort. As it continues to enroll patients, further studies can be done to assess the short- and long-term outcomes of LN in cSLE. Additionally, as the number of registry subjects and duration of follow-up increase, assessment of response to therapy, across all cSLE patients and subgroups can be studied. Longitudinal data will allow for better understanding of therapeutic approaches and prognostic information.
Table 1.
CARRA SLE Registry Participant Demographics.
| Overall (N=677) | |
|---|---|
| Age at enrollment (years) | |
| Median [Min, Max] | 15.0 [2.00, 20.0] |
| Sex | |
| Female | 563 (83.2%) |
| Male | 114 (16.8%) |
| Race | |
| White | 205 (30.3%) |
| Black | 178 (26.3%) |
| Asian | 80 (11.8%) |
| Mixed | 30 (4.4%) |
| Other | 26 (3.8%) |
| Missing | 158 (23.3%) |
| Ethnicity | |
| Hispanic | 172 (25.4%) |
| Non-Hispanic | 505 (74.6%) |
| Time since diagnosis (years) | |
| Median [Min, Max] | 1.00 [0, 13.0] |
ACKNOWLEDGEMENTS
This work could not have been accomplished without the aid of the following organizations: The National Institutes of Health’s National Institute of Arthritis and Musculoskeletal and Skin Diseases Intramural Research Program & the Arthritis Foundation. We would also like to thank all participants and hospital sites that recruited patients for the CARRA Registry. The authors thank the following CARRA Registry site principal investigators, sub- investigators and research coordinators:
N. Abel, K. Abulaban, A. Adams, M. Adams, R. Agbayani, J. Aiello, S. Akoghlanian, C. Alejandro, E. Allenspach, R. Alperin, M. Alpizar, G. Amarilyo, W. Ambler, E. Anderson, S. Ardoin, S. Armendariz, E. Baker, I. Balboni, S. Balevic, L. Ballenger, S. Ballinger, N. Balmuri, F. Barbar-Smiley, L. Barillas-Arias, M. Basiaga, K. Baszis, M. Becker, H. Bell-Brunson, E. Beltz, H. Benham, S. Benseler, W. Bernal, T. Beukelman, T. Bigley, B. Binstadt, C. Black, M. Blakley, J. Bohnsack, J. Boland, A. Boneparth, S. Bowman, C. Bracaglia, E. Brooks, M. Brothers, A. Brown, H. Brunner, M. Buckley, M. Buckley, H. Bukulmez, D. Bullock, B. Cameron, S. Canna, L. Cannon, P. Carper, V. Cartwright, E. Cassidy, L. Cerracchio, E. Chalom, J. Chang, A. Chang-Hoftman, V. Chauhan, P. Chira, T. Chinn, K. Chundru, H. Clairman, D. Co, A. Confair, H. Conlon, R. Connor, A. Cooper, J. Cooper, S. Cooper, C. Correll, R. Corvalan, D. Costanzo, R. Cron, L. Curiel-Duran, T. Curington, M. Curry, A. Dalrymple, A. Davis, C. Davis, C. Davis, T. Davis, F. De Benedetti, D. De Ranieri, J. Dean, F. Dedeoglu, M. DeGuzman, N. Delnay, V. Dempsey, E. DeSantis, T. Dickson, J. Dingle, B. Donaldson, E. Dorsey, S. Dover, J. Dowling, J. Drew, K. Driest, Q. Du, K. Duarte, D. Durkee, E. Duverger, J. Dvergsten, A. Eberhard, M. Eckert, K. Ede, B. Edelheit, C. Edens, C. Edens, Y. Edgerly, M. Elder, B. Ervin, S. Fadrhonc, C. Failing, D. Fair, M. Falcon, L. Favier, S. Federici, B. Feldman, J. Fennell, I. Ferguson, P. Ferguson, B. Ferreira, R. Ferrucho, K. Fields, T. Finkel, M. Fitzgerald, C. Fleming, O. Flynn, L. Fogel, E. Fox, M. Fox, L. Franco, M. Freeman, K. Fritz, S. Froese, R. Fuhlbrigge, J. Fuller, N. George, K. Gerhold, D. Gerstbacher, M. Gilbert, M. Gillispie-Taylor, E. Giverc, C. Godiwala, I. Goh, H. Goheer, D. Goldsmith, E. Gotschlich, A. Gotte, B. Gottlieb, C. Gracia, T. Graham, S. Grevich, T. Griffin, J. Griswold, A. Grom, M. Guevara, P. Guittar, M. Guzman, M. Hager, T. Hahn, O. Halyabar, E. Hammelev, M. Hance, A. Hanson, L. Harel, S. Haro, J. Harris, O. Harry, E. Hartigan, J. Hausmann, A. Hay, K. Hayward, J. Heiart, K. Hekl, L. Henderson, M. Henrickson, A. Hersh, K. Hickey, P. Hill, S. Hillyer, L. Hiraki, M. Hiskey, P. Hobday, C. Hoffart, M. Holland, M. Hollander, S. Hong, M. Horwitz, J. Hsu, A. Huber, J. Huggins, J. Hui-Yuen, C. Hung, J. Huntington, A. Huttenlocher, M. Ibarra, L. Imundo, C. Inman, A. Insalaco, A. Jackson, S. Jackson, K. James, G. Janow, J. Jaquith, S. Jared, N. Johnson, J. Jones, J. Jones, J. Jones, K. Jones, S. Jones, S. Joshi, L. Jung, C. Justice, A. Justiniano, N. Karan, K. Kaufman, A. Kemp, E. Kessler, U. Khalsa, B. Kienzle, S. Kim, Y. Kimura, D. Kingsbury, M. Kitcharoensakkul, T. Klausmeier, K. Klein, M. Klein-Gitelman, B. Kompelien, A. Kosikowski, L. Kovalick, J. Kracker, S. Kramer, C. Kremer, J. Lai, J. Lam, B. Lang, S. Lapidus, B. Lapin, A. Lasky, D. Latham, E. Lawson, R. Laxer, P. Lee, P. Lee, T. Lee, L. Lentini, M. Lerman, D. Levy, S. Li, S. Lieberman, L. Lim, C. Lin, N. Ling, M. Lingis, M. Lo, D. Lovell, D. Lowman, N. Luca, S. Lvovich, C. Madison, J. Madison, S. Magni Manzoni, B. Malla, J. Maller, M. Malloy, M. Mannion, C. Manos, L.. Marques, A. Martyniuk, T. Mason, S. Mathus, L. McAllister, K. McCarthy, K. McConnell, E. McCormick, D. McCurdy, P. McCurdy Stokes, S. McGuire, I. McHale, A. McMonagle, C. McMullen-Jackson, E. Meidan, E. Mellins, E. Mendoza, R. Mercado, A. Merritt, L. Michalowski, P. Miettunen, M. Miller, D. Milojevic, E. Mirizio, E. Misajon, M. Mitchell, R. Modica, S. Mohan, K. Moore, L. Moorthy, S. Morgan, E. Morgan Dewitt, C. Moss, T. Moussa, V. Mruk, A. Murphy, E. Muscal, R. Nadler, B. Nahal, K. Nanda, N. Nasah, L. Nassi, S. Nativ, M. Natter, J. Neely, B. Nelson, L. Newhall, L. Ng, J. Nicholas, R. Nicolai, P. Nigrovic, J. Nocton, B. Nolan, E. Oberle, B. Obispo, B. O’Brien, T. O’Brien, O. Okeke, M. Oliver, J. Olson, K. O’Neil, K. Onel, A. Orandi, M. Orlando, S. Osei-Onomah, R. Oz, E. Pagano, A. Paller, N. Pan, S. Panupattanapong, M. Pardeo, J. Paredes, A. Parsons, J. Patel, K. Pentakota, P. Pepmueller, T. Pfeiffer, K. Phillippi, D. Pires Marafon, K. Phillippi, L. Ponder, R. Pooni, S. Prahalad, S. Pratt, S. Protopapas, B. Puplava, J. Quach, M. Quinlan-Waters, C. Rabinovich, S. Radhakrishna, J. Rafko, J. Raisian, A. Rakestraw, C. Ramirez, E. Ramsay, S. Ramsey, R. Randell, A. Reed, A. Reed, A. Reed, H. Reid, K. Remmel, A. Repp, A. Reyes, A. Richmond, M. Riebschleger, S. Ringold, M. Riordan, M. Riskalla, M. Ritter, R. RivasChacon, A. Robinson, E. Rodela, M. Rodriquez, K. Rojas, T. Ronis, M. Rosenkranz, B. Rosolowski, H. Rothermel, D. Rothman, E. Roth-Wojcicki, K. Rouster - Stevens, T. Rubinstein, N. Ruth, N. Saad, S. Sabbagh, E. Sacco, R. Sadun, C. Sandborg, A. Sanni, L. Santiago, A. Sarkissian, S. Savani, L. Scalzi, L. Schanberg, S. Scharnhorst, K. Schikler, A. Schlefman, H. Schmeling, K. Schmidt, E. Schmitt, R. Schneider, K. Schollaert-Fitch, G. Schulert, T. Seay, C. Seper, J. Shalen, R. Sheets, A. Shelly, S. Shenoi, K. Shergill, J. Shirley, M. Shishov, C. Shivers, E. Silverman, N. Singer, V. Sivaraman, J. Sletten, A. Smith, C. Smith, J. Smith, J. Smith, E. Smitherman, J. Soep, M. Son, S. Spence, L. Spiegel, J. Spitznagle, R. Sran, H. Srinivasalu, H. Stapp, K. Steigerwald, Y. Sterba Rakovchik, S. Stern, A. Stevens, B. Stevens, R. Stevenson, K. Stewart, C. Stingl, J. Stokes, M. Stoll, E. Stringer, S. Sule, J. Sumner, R. Sundel, M. Sutter, R. Syed, G. Syverson, A. Szymanski, S. Taber, R. Tal, A. Tambralli, A. Taneja, T. Tanner, S. Tapani, G. Tarshish, S. Tarvin, L. Tate, A. Taxter, J. Taylor, M. Terry, M. Tesher, A. Thatayatikom, B. Thomas, K. Tiffany, T. Ting, A. Tipp, D. Toib, K. Torok, C. Toruner, H. Tory, M. Toth, S. Tse, V. Tubwell, M. Twilt, S. Uriguen, T. Valcarcel, H. Van Mater, L. Vannoy, C. Varghese, N. Vasquez, K. Vazzana, R. Vehe, K. Veiga, J. Velez, J. Verbsky, G. Vilar, N. Volpe, E. von Scheven, S. Vora, J. Wagner, L. Wagner-Weiner, D. Wahezi, H. Waite, J. Walker, H. Walters, T. Wampler Muskardin, L. Waqar, M. Waterfield, M. Watson, A. Watts, P. Weiser, J. Weiss, P. Weiss, E. Wershba, A. White, C. Williams, A. Wise, J. Woo, L. Woolnough, T. Wright, E. Wu, A. Yalcindag, M. Yee, E. Yen, R. Yeung, K. Yomogida, Q. Yu, R. Zapata, A. Zartoshti, A. Zeft, R. Zeft, Y. Zhang, Y. Zhao, A. Zhu, C. Zic.
We would like to thank Brian Brown, NIH Library Editing Service, Dr M. Ward, and Dr. R Colbert for reviewing the manuscript.
FUNDING
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr. Vazzana and Dr. Lewandowski are supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health. This work was supported by the Lupus Foundation of America.
Footnotes
DECLARATION OF CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCES
- 1.Kamphuis S and Silverman ED. Prevalence and burden of pediatric-onset systemic lupus erythematosus. Nat Rev Rheumatol 2010; 6: 538–546. 2010/08/05. DOI: 10.1038/nrrheum.2010.121. [DOI] [PubMed] [Google Scholar]
- 2.Tarr T, Derfalvi B, Gyori N, et al. Similarities and differences between pediatric and adult patients with systemic lupus erythematosus. Lupus 2015; 24: 796–803. 2014/12/18. DOI: 10.1177/0961203314563817. [DOI] [PubMed] [Google Scholar]
- 3.Watson L, Leone V, Pilkington C, et al. Disease activity, severity, and damage in the UK Juvenile-Onset Systemic Lupus Erythematosus Cohort. Arthritis Rheum 2012; 64: 2356–2365. 2012/02/02. DOI: 10.1002/art.34410. [DOI] [PubMed] [Google Scholar]
- 4.Sule SD, Moodalbail DG, Burnham J, et al. Predictors of kidney disease in a cohort of pediatric patients with lupus. Lupus 2015; 24: 862–868. 2015/02/15. DOI: 10.1177/0961203315570162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiraki LT, Feldman CH, Liu J, et al. Prevalence, incidence, and demographics of systemic lupus erythematosus and lupus nephritis from 2000 to 2004 among children in the US Medicaid beneficiary population. Arthritis Rheum 2012; 64: 2669–2676. 2012/08/01. DOI: 10.1002/art.34472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wenderfer SE and Eldin KW. Lupus Nephritis. Pediatr Clin North Am 2019; 66: 87–99. 2018/11/21. DOI: 10.1016/j.pcl.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Hiraki LT, Benseler SM, Tyrrell PN, et al. Clinical and laboratory characteristics and longterm outcome of pediatric systemic lupus erythematosus: a longitudinal study. J Pediatr 2008; 152: 550–556. 2008/03/19. DOI: 10.1016/j.jpeds.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Font J, Cervera R, Espinosa G, et al. Systemic lupus erythematosus (SLE) in childhood: analysis of clinical and immunological findings in 34 patients and comparison with SLE characteristics in adults. Ann Rheum Dis 1998; 57: 456–459. 1998/11/03. DOI: 10.1136/ard.57.8.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hafeez F, Tarar AM and Saleem R. Lupus nephritis in children. J Coll Physicians Surg Pak 2008; 18: 17–21. 2008/05/03. DOI: 01.2008/JCPSP.1721. [PubMed] [Google Scholar]
- 10.Baqi N, Moazami S, Singh A, et al. Lupus nephritis in children: a longitudinal study of prognostic factors and therapy. J Am Soc Nephrol 1996; 7: 924–929. 1996/06/01. [DOI] [PubMed] [Google Scholar]
- 11.McCurdy DK, Lehman TJ, Bernstein B, et al. Lupus nephritis: prognostic factors in children. Pediatrics 1992; 89: 240–246. 1992/02/01. [PubMed] [Google Scholar]
- 12.Hiraki LT, Lu B, Alexander SR, et al. End-stage renal disease due to lupus nephritis among children in the US, 1995–2006. Arthritis Rheum 2011; 63: 1988–1997. 2011/03/30. DOI: 10.1002/art.30350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pereira T, Abitbol CL, Seeherunvong W, et al. Three decades of progress in treating childhood-onset lupus nephritis. Clin J Am Soc Nephrol 2011; 6: 2192–2199. 2011/07/30. DOI: 10.2215/CJN.00910111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pakchotanon R, Gladman DD, Su J, et al. Sustained complete renal remission is a predictor of reduced mortality, chronic kidney disease and end-stage renal disease in lupus nephritis. Lupus 2018; 27: 468–474. 2017/09/01. DOI: 10.1177/0961203317726376. [DOI] [PubMed] [Google Scholar]
- 15.Boneparth A, Wenderfer SE, Moorthy LN, et al. Clinical characteristics of children with membranous lupus nephritis: the Childhood Arthritis and Rheumatology Research Alliance Legacy Registry. Lupus 2017; 26: 299–306. 2016/08/12. DOI: 10.1177/0961203316662720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boneparth A, Ilowite NT and Investigators CR. Comparison of renal response parameters for juvenile membranous plus proliferative lupus nephritis versus isolated proliferative lupus nephritis: a cross-sectional analysis of the CARRA Registry. Lupus 2014; 23: 898–904. 2014/04/15. DOI: 10.1177/0961203314531841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang JH, Ward MM, Rucker AN, et al. Ankylosing spondylitis: patterns of radiographic involvement--a re-examination of accepted principles in a cohort of 769 patients. Radiology 2011; 258: 192–198. 2010/10/26. DOI: 10.1148/radiol.10100426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ardoin SP, Daly RP, Merzoug L, et al. Research priorities in childhood-onset lupus: results of a multidisciplinary prioritization exercise. Pediatr Rheumatol Online J 2019; 17: 32. 2019/07/03. DOI: 10.1186/s12969-019-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Observational Study of Pediatric Rheumatic Diseases: The CARRA Registry. 2014.
- 20.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40: 1725. 1997/10/27. DOI: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 21.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982; 25: 1271–1277. 1982/11/01. DOI: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 22.Zappitelli M, Duffy C, Bernard C, et al. Clinicopathological study of the WHO classification in childhood lupus nephritis. Pediatr Nephrol 2004; 19: 503–510. 2004/03/17. DOI: 10.1007/s00467-004-1419-y. [DOI] [PubMed] [Google Scholar]
- 23.Bajema IM, Wilhelmus S, Alpers CE, et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int 2018; 93: 789–796. 2018/02/21. DOI: 10.1016/j.kint.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 24.Renal Disease Subcommittee of the American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Response Criteria. The American College of Rheumatology Response Criteria for Proliferative and Membranous Renal Disease in Systemic Lupus Erythematosus Clinical Trials. Arthritis Rheum. 2006. 54:421–532. [DOI] [PubMed] [Google Scholar]
- 25.R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing V, Austria. URL http://www.R-project.org/ [Google Scholar]
- 26.Schwartz GJ and Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol 2009; 4: 1832–1843. 2009/10/13. DOI: 10.2215/CJN.01640309. [DOI] [PubMed] [Google Scholar]
- 27.Smith EMD, Yin P, Jorgensen AL, et al. Clinical predictors of active LN development in children - evidence from the UK JSLE Cohort Study. Lupus 2018; 27: 2020–2028. 2018/09/25. DOI: 10.1177/0961203318801526. [DOI] [PubMed] [Google Scholar]
- 28.Ishimori S, Kaito H, Shima Y, et al. Clinicopathological characteristics and renal outcomes of childhood-onset lupus nephritis with acute kidney injury: A multicenter study. Mod Rheumatol 2019; 29: 970–976. 2018/10/06. DOI: 10.1080/14397595.2018.1532861. [DOI] [PubMed] [Google Scholar]
- 29.Oliva-Damaso N, Payan J, Oliva-Damaso E, et al. Lupus Podocytopathy: An Overview. Adv Chronic Kidney Dis 2019; 26: 369–375. 2019/11/18. DOI: 10.1053/j.ackd.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Contreras G, Lenz O, Pardo V, et al. Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int 2006; 69: 1846–1851. 2006/04/07. DOI: 10.1038/sj.ki.5000243. [DOI] [PubMed] [Google Scholar]
- 31.Feldman CH, Hiraki LT, Liu J, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000–2004. Arthritis Rheum 2013; 65: 753–763. 2012/12/04. DOI: 10.1002/art.37795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drenkard C and Lim SS. Update on lupus epidemiology: advancing health disparities research through the study of minority populations. Curr Opin Rheumatol 2019; 31: 689–696. 2019/08/23. DOI: 10.1097/BOR.0000000000000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoover PJ and Costenbader KH. Insights into the epidemiology and management of lupus nephritis from the US rheumatologist’s perspective. Kidney Int 2016; 90: 487–492. 2016/06/28. DOI: 10.1016/j.kint.2016.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiraki LT, Benseler SM, Tyrrell PN, et al. Ethnic differences in pediatric systemic lupus erythematosus. J Rheumatol 2009; 36: 2539–2546. 2009/10/17. DOI: 10.3899/jrheum.081141. [DOI] [PubMed] [Google Scholar]
- 35.Evans MK. Health Equity - Are We Finally on the Edge of a New Frontier? N Engl J Med 2020; 383: 997–999. 2020/09/10. DOI: 10.1056/NEJMp2005944. [DOI] [PubMed] [Google Scholar]
- 36.Berchick ER BJ, Upton RD. Health insurance coverage in the United States. 2018. Current population reports. In: Commerce Do, (ed.). census.gov2019. [Google Scholar]
- 37.Hagelberg S, Lee Y, Bargman J, et al. Longterm followup of childhood lupus nephritis. J Rheumatol 2002; 29: 2635–2642. 2002/12/05. [PubMed] [Google Scholar]
- 38.Korbet SM, Schwartz MM, Evans J, et al. Severe lupus nephritis: racial differences in presentation and outcome. J Am Soc Nephrol 2007; 18: 244–254. 2006/12/15. DOI: 10.1681/ASN.2006090992. [DOI] [PubMed] [Google Scholar]
- 39.Lau KK, Jones DP, Hastings MC, et al. Short-term outcomes of severe lupus nephritis in a cohort of predominantly African-American children. Pediatr Nephrol 2006; 21: 655–662. 2006/03/30. DOI: 10.1007/s00467-006-0060-3. [DOI] [PubMed] [Google Scholar]
- 40.Hugle B, Silverman ED, Tyrrell PN, et al. Presentation and outcome of paediatric membranous non-proliferative lupus nephritis. Pediatr Nephrol 2015; 30: 113–121. 2014/08/01. DOI: 10.1007/s00467-014-2908-2. [DOI] [PubMed] [Google Scholar]
- 41.Wu JY, Yeh KW and Huang JL. Early predictors of outcomes in pediatric lupus nephritis: focus on proliferative lesions. Semin Arthritis Rheum 2014; 43: 513–520. 2013/08/27. DOI: 10.1016/j.semarthrit.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Mak A, Mok CC, Chu WP, et al. Renal damage in systemic lupus erythematosus: a comparative analysis of different age groups. Lupus 2007; 16: 28–34. 2007/02/08. DOI: 10.1177/0961203306074469. [DOI] [PubMed] [Google Scholar]
- 43.Mina R, von Scheven E, Ardoin SP, et al. Consensus treatment plans for induction therapy of newly diagnosed proliferative lupus nephritis in juvenile systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2012; 64: 375–383. 2011/12/14. DOI: 10.1002/acr.21558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weidenbusch M, Rommele C, Schrottle A, et al. Beyond the LUNAR trial. Efficacy of rituximab in refractory lupus nephritis. Nephrol Dial Transplant 2013; 28: 106–111. 2012/07/06. DOI: 10.1093/ndt/gfs285. [DOI] [PubMed] [Google Scholar]
- 45.Davies RJ, Sangle SR, Jordan NP, et al. Rituximab in the treatment of resistant lupus nephritis: therapy failure in rapidly progressive crescentic lupus nephritis. Lupus 2013; 22: 574–582. 2013/05/02. DOI: 10.1177/0961203313483376. [DOI] [PubMed] [Google Scholar]
- 46.Rovin BH, Furie R, Latinis K, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum 2012; 64: 1215–1226. 2012/01/11. DOI: 10.1002/art.34359. [DOI] [PubMed] [Google Scholar]
- 47.Carter LM, Isenberg DA and Ehrenstein MR. Elevated serum BAFF levels are associated with rising anti-double-stranded DNA antibody levels and disease flare following B cell depletion therapy in systemic lupus erythematosus. Arthritis Rheum 2013; 65: 2672–2679. 2013/07/11. DOI: 10.1002/art.38074. [DOI] [PubMed] [Google Scholar]
- 48.Reddy V, Klein C, Isenberg DA, et al. Obinutuzumab induces superior B-cell cytotoxicity to rituximab in rheumatoid arthritis and systemic lupus erythematosus patient samples. Rheumatology (Oxford) 2017; 56: 1227–1237. 2017/04/14. DOI: 10.1093/rheumatology/kex067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aydin POA, Shan J, Brunner HI, et al. Blood pressure control over time in childhood-onset systemic lupus erythematous. Lupus. 2018; 27: 657–664. [DOI] [PubMed] [Google Scholar]