Documenting the journey to establish inpatient care for small and sick newborns in Ethiopia, India, Malawi, and Rwanda, the authors showcase the remarkable progress and share lessons with stakeholders in other countries who aim to do the same.
Key Findings
-
Each country’s unique journey to establish inpatient care and roll out service delivery for small and sick newborns shows the diversity of actions and actors required to scale a new practice across different geographies:
In Ethiopia, care was implemented in a stepwise expansion from a newborn care corner to community-based newborn care to neonatal intensive care units.
In India, a model newborn care unit was an exemplar for national scale.
In Malawi, a hub-and-spoke model used the introduction of continuous positive airway pressure as a grounding point.
In Rwanda, integrated small and sick newborn care was initiated at the district level in the eastern region of the country, far from the capital city.
Key Implications
National stakeholders should document country-level strategies and innovation related to the establishment of small and sick newborn care, thereby giving voice to lived country experience.
Program managers can use the learnings from various countries to establish or strengthen small and sick newborn care service delivery.
ABSTRACT
Background:
Limited information is available about the approaches used and lessons learned from low- and middle-income countries that have implemented inpatient services for small and sick newborns. We developed descriptive case studies to compare the journeys to establish inpatient newborn care across Ethiopia, India, Malawi, and Rwanda.
Methods:
A total of 57 interviews with stakeholders in Ethiopia (n=12), India (n=12), Malawi (n=16), and Rwanda (n=17) informed the case studies. Our heuristic data analysis followed a deductive organizing framework approach. We informed our data analysis via targeted literature searches to uncover details related to key events. We used the NEST360 Theory of Change for facility-based care, which reflects the World Health Organization (WHO) Health Systems Framework as a starting point and added, as necessary, in an edit processing format until data saturation was achieved.
Findings:
Results highlight the strategies and innovation used to establish small and sick newborn care by health system building block and by country. We conducted a gap analysis of implementation of WHO Standards for Improving Facility-Based Care. The journeys to establish inpatient newborn care across the 4 countries are similar in terms of trajectory yet unique in their implementation. Unifying themes include leadership and governance at national level to consolidate and coordinate action to improve newborn quality of care, investment to build staff skills on data collection and use, and institutionalization of regular neonatal data reviews to identify gaps and propose relevant strategies.
Conclusion:
Efforts to establish and scale inpatient care for small and sick newborns in Ethiopia, India, Malawi, and Rwanda over the last decade have led to remarkable success. These country examples can inspire more nascent initiatives that other low- and middle-income countries may undertake. Documentation should give voice to lived country experience, not all of which is fully captured in existing, peer-reviewed published literature.
BACKGROUND
Over the past 3 decades, significant improvements in newborn care have occurred. Since 2000, global neonatal mortality has virtually halved, from 4 million newborn deaths annually to slightly more than 2 million deaths in 2021.1 These improvements have been achieved through a variety of factors: increased leadership, focus, and funding; strengthened programmatic realignments and integration of newborn health into traditional reproductive, maternal, and child health programs; concerted research and measurement initiatives; numerous technological innovations; and heightened evidence-based advocacy efforts. Both the Lancet Neonatal Survival2 and Every Newborn series3 provided the evidence base to raise awareness of the need to prioritize newborn mortality and morbidity. These heightened evidence-based advocacy efforts, particularly the Every Newborn Action Plan (ENAP),4 created common goals, targets, and metrics and, most importantly, fostered adoption and ownership of newborn health at national and subnational levels. In their entirety, these initiatives5–7 offered an innovative vision for inpatient newborn care. However, there is a paucity of in-depth practical guidance and information about the approaches used and lessons learned in low- and middle-income countries (LMICs) as they designed and implemented their inpatient newborn services for small and sick newborns.8
Several countries are making concerted efforts to strengthen inpatient newborn care units for small and sick newborns. Complementary efforts at the international level include the creation of guidance, such as the recently released World Health Organization (WHO) Standards for Improving the Quality of Care for Small and Sick Newborns in Health Facilities.9 At the country level, the descriptions of the approaches taken and evaluation of their success have not been comprehensively documented, analyzed, and communicated. This case study describes holistically and chronologically the journey undertaken in 4 countries (Ethiopia, India, Malawi, and Rwanda) to establish and scale inpatient newborn care uniquely from the country perspective.
Several countries are making concerted efforts to strengthen inpatient small and sick newborn care, but their approaches have not been comprehensively documented.
METHODS
Case Study Approach
We articulate a purely country-focused perspective to describe how inpatient care for small and sick newborns was established. This work does not attempt to assess how well small and sick newborn care services are now performing in these countries, but rather it showcases how these services came to be.
We developed descriptive, multiple case studies to identify and compare the journeys to establishing inpatient newborn care across these countries. The funding agency purposively selected these 4 geographies to capture countries with some advanced experiences in establishing and scaling services for small and sick newborns. Data collection included a scoping review of published and gray literature (including key newborn care guidelines and clinical protocols) to document national and subnational policies and scale-up strategies, in-person or virtual in-depth interviews with key newborn stakeholders using semistructured standardized questionnaires, follow-up email correspondence, and data validation webinars with national stakeholders in each country.
To start this case study process, we sought people who were the core drivers for establishing small and sick newborn care in each country, regardless of their institutional affiliation. Some of these individuals had been or were currently affiliated with the ministry of health at national or subnational levels at the time of the interview. Our main recruitment criterion was that the key informant had been integrally involved in the process of establishing small and sick newborn care over the last decade and, thereby, had been part of a nucleus of stakeholders in each country that drove implementation of small and sick newborn care programming.
Our sampling and recruitment methods included criterion sampling to identify published and gray literature for the scoping review, intensity sampling to identify an initial core set of key informants who could provide in-depth information about the establishment of small and sick newborn care in each country, and respondent-driven sampling to identify additional key stakeholders that could fill in specific gaps in the overall case study. We recruited key informants through personalized email and telephone contact. Stakeholders gave their verbal consent to be interviewed after they were informed about the purpose and description of the study.
Our data analysis was heuristic and followed a deductive organizing framework approach. We informed our data analysis via targeted literature searches to uncover details related to key events. We used the NEST360 Theory of Change for facility-based care, which reflects the WHO Health Systems Building Blocks Framework as a starting point and added, as necessary, in an edit processing format until data saturation was achieved. Saturation refers to the point during data analysis at which incoming data points (interviews) produce little or no new useful information relative to the objective of our case study. The WHO Health Systems Framework10 can identify specific entry points to improve the implementation of newborn interventions at critical health system building blocks: leadership and governance, financing, workforce, information systems, and essential medical commodities and was adapted by NEST360 to include family-centered care and by our team to include service delivery (Figure 1).11 This framework was also adapted by the ENAP to assess bottlenecks to implementing critical newborn care interventions and packages.12
FIGURE 1.
WHO Health System Building Blocks Used as Organizing Principle for Case Studies on Small and Sick Newborn Care in 4 Countries12
Abbreviation: WHO, World Health Organization.
Credit: © NEST360/UNICEF Implementation Toolkit for Small and Sick Newborn Care.
Ethical Approval
The Research Determination Committee at PATH reviewed this case study protocol and determined that it is not human subjects research.
FINDINGS
A total of 57 interviews with key stakeholders in Ethiopia (n=12), India (n=12), Malawi (n=16), and Rwanda (n=17) informed the case studies. At the time of the interviews, stakeholders were working as health care providers or program planners from the ministry of health at various levels, nongovernmental organization implementing partners, professional associations, or private facilities. In this overview publication, we focus on strategies used, innovations implemented, and approaches used to roll out service delivery.
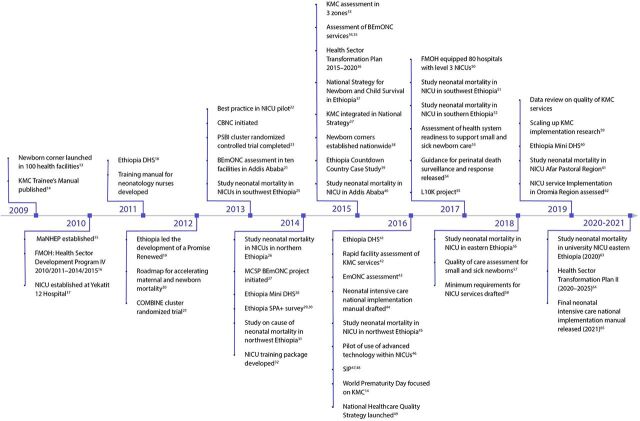
We created an event timeline that shows the significant activities that were undertaken over the last decade to establish a system of care for small and sick newborns in Ethiopia (Figure 2),13–65 India (Figure 3),66–115 Malawi (Figure 4),116–163 and Rwanda (Figure 5).164–215 The timeline tracks each country’s efforts, which range from the inception of planning for care for small and sick newborns through activities to operationalize and scale inpatient care.
FIGURE 2.
Ethiopia Timeline Showing Significant Activities Undertaken to Establish Small and Sick Newborn Care, 2009–2021
Abbreviations: BEmONC; basic emergency obstretric and newborn care; CBNC, community-based newborn care; DHS, Demographic and Health Survey; EmONC; emergency obstetric and newborn care; FMOH, Federal Ministry of Health; KMC, kangaroo mother care; L10K, Last 10 Kilometers; MaNHEP, Maternal and Newborn Health Ethiopia Partnership; MCSP, Maternal and Child Survival Program; NICU, newborn intensive care unit; PSBI, possible severe bacterial infections; SIP, Study of Illness in Preterms; SPA+, Service Provision Assessment Plus.
FIGURE 3.
India Timeline Showing Significant Activities Undertaken to Establish Small and Sick Newborn Care, 2005–2021
Abbreviations: HBNC, home-based newborn care; HMBCON, Human Milk Bank Conference; F-IMNCI; Facility Based Integrated Management of Neonatal and Childhood Illness; JSY, Janani Suraksha Yojana; NSSK, Navajat Shishu Surkisha Karyakram; RBSK, Rashtriya Bal Swasthya Karyakam; RMNCH+, reproductive, maternal, newborn and child health plus; SAANS, Social Action and Awareness to Neutralize Pneumonia; SNCU, special newborn care unit.
FIGURE 4.
Malawi Timeline Showing Significant Activities Undertaken to Establish Small and Sick Newborn Care, 2010–2021
Abbreviations: BCC, behavior change communication; COIN, Care for the Infant and Newborn; CPAP, continuous positive air pressure; ENAP, Every Newborn Action Plan; IMCHA, Innovating for Maternal and Child Health; KMC, kangaroo mother care; MNH, maternal and newborn health; MOH, Ministry of Health; PDSR, perinatal death surveillance and response; SNL, Saving Newborn Lives.
FIGURE 5.
Rwanda Timeline Showing Significant Activities Undertaken to Establish Small and Sick Newborn Care, 2009–2020
Abbreviations: ABC, All Babies Count; CHP, community health program; COINN, Council of Internal Neonatal Nurses; DHS, Demographic and Health Survey; HB-MNCHP, home-based maternal and neonatal health care package; KMC, kangaroo mother care; MCH, maternal and child health; MPDSR, maternal and perinatal death surveillance and response; MNCAH, maternal, newborn, child, and adolescent health; MNCH, maternal, newborn, and child health; MOH, Ministry of Health; PHIT, Population Health Implementation and Training; TSAM, Training Support and Access Model.
Strategies Used to Establish and Scale Small and Sick Newborn Care
Strategies used to establish and scale small and sick newborn care varied by country (Table 1).216–221 Overall, the broad approaches used were fairly similar across countries, although each strategy has its unique permutations. Overall, good governance, commitment, and leadership at the national level helped drive improvements in care. Building the capacity of a specialized workforce, measuring neonatal-specific indicators, and adapting existing infrastructure were used as strategies to support small and sick newborn service delivery across all countries.
TABLE 1.
Strategies to Establish Small and Sick Newborn Care, by Health System Building Block and Country
| Health System Building Block | Ethiopia | India | Malawi | Rwanda |
|---|---|---|---|---|
| Leadership and governance | Improve ownership, leadership, and accountability through Ministry of Health-led partnerships and coordination platforms for newborn and child survival.16,17 | Coordinate systematic implementation of small and sick newborn activities led by national (financial and technical guidance), state, and district health departments (planning and implementation) using national FBNC guidelines and training modules.81 | Oversight of small and sick newborn care led by newborn focal point at the Ministry of Health.4,134 | Government of Rwanda provides strong leadership and accountability. |
| Human resources | Accelerate health workforce development through in-service training, mentorship, and continuing professional development of mid-level health workforce. | Develop health workforce with the necessary skills to provide the appropriate level of care through specialized training courses for FBNC.77 | Build capacity among available personnel to create a pool of specialized health workers to deliver inpatient care and are retained within the setting by embedding neonatal care in preservice training and specialized courses decisions to scale up care to prevent neonatal mortality.140 | Build capacity of specialized workforce to deliver small and sick newborn care through a learning collaborative model where doctors, nurses, community health workers, and political leaders share data and take appropriate decisions to scale up care to prevent neonatal mortality.165,180,181 |
| Information systems | Include neonatal care indicators in the HMIS to ensure data use for informed decision-making. | Monitor measurable indicators to inform health policy and programs on newborn health. | Capture all neonatal-related indicators within the health management information system. | Integrate data collection, monitoring, and use at all levels of care and building capacity of those using data to make life-changing decisions for improving the quality of small and sick newborn care services. |
| Infrastructure | Standardize and build appropriate infrastructure for NICU and newborn care at hospitals and health centers, respectively. | Establish nationwide network of NBCCs at every point of childbirth, NBSUs at first referral units, and SNCUs at district hospitals.71,84 | Advocate that all hospitals to be built and those being renovated should have a purpose-built neonatal unit included in it. | Adapt existing infrastructure for small and sick newborn care according to available budgets. |
| Health system financing | Subsidize the cost of care in public health facilities and implement community-based health insurance schemes.216 | Support states with financial resources in 60:40 (national/state) sharing ratio using annual program implementation plans to develop budgets for FBNC. | Ensure that funding for small and sick newborn care is allocated at the district and facility levels. | Fully fund small and sick newborn care services through universal health coverage. |
| Medical supplies and devices | Ensure availability of essential medical supplies and devices for NICU through an Integrated Pharmaceutical Logistic System. | Encourage collaboration of neonatologists, engineers, and entrepreneurs to produce and supply high-quality neonatal equipment of several high-volume categories at affordable cost.217–221 | Maintain adequate inventory of equipment and supplies for management of small and sick newborns in the central medical stores.121,128 | Provide centralized guidance around the small and sick newborn package of care and related supply/equipment needs. |
| Service delivery | Apply the life course continuum of care to gradually expand access to small and sick newborn care at facilities and referrals from the community through the community-based newborn care approach. | Provide no-cost, quality newborn care services at public health facilities along with introductions of various other schemes (like JSY, JSSK, PMSMA, SUMAN initiative) to reduce out-of-pocket expenditures and wage loss for parents.67,108 | Introduce services for small and sick newborn care at district hospitals in a stepwise manner—focusing first on KMC, then CPAP, and finally integrated care.121,127,131,136,145,157 | Test a model of neonatal care in limited sites and then scaling successful results at national level.164,182,183 |
| Family-centered care | Communicate and counsel both mother and husband for informed decision. | Introduce a national policy to integrate family-centered care in all SNCUs.104,105 | Care for the mother-baby dyad as a unit.155,161,162 | Create an enabling environment for family members to become involved in provision of newborn care. |
Abbreviations: CPAP, continuous positive airway pressure; FBNC, facility-based newborn care; HMIS, health management information system; JSSK, Janani Shishu Suraksha Karyakaram; JSY, Janani Suraksha Yojana; KMC, kangaroo mother care; NBCC, newborn care corner; NBSU, newborn stabilization unit; NICU, neonatal intensive care unit; PMSMA, Pradhan Mantri Surakshit Matritva Abhiyan; SNCU, special newborn care unit; SUMAN, Surakshit Matritva Aashwasan.
Across all 4 countries, building the capacity of a specialized workforce, measuring neonatal-specific indicators, and adapting existing infrastructure were used as strategies to support small and sick newborn service delivery.
Workforce training was a key strategic priority in Malawi, where the longstanding practice of nursing rotation was addressed by (1) identifying that no policy for nursing rotation was in place, (2) creating staffing rotations and transfer schedules that allowed for continuity of small and sick newborn care by trained specialized staff, (3) avoiding rotation of health personnel who had been trained in neonatal care from neonatal facilities to non-neonatal facilities, and (4) building a case with district health management teams to freeze rotations of personnel experienced and trained in care for small and sick newborns and to designate staff for neonatal units. In Ethiopia, a quality improvement approach developed by the Ethiopian Pediatric Society, in collaboration with the Institute for Healthcare Improvement, that pairs education with external mentorship to support facility-based quality improvement was tested.222 In Rwanda, the Human Resources for Health program focused on building capacity among pediatricians and neonatal nurses. In India, training consisted of a 4-day training for medical officers and staff nurses posted in district hospital special newborn care units (SNCUs) followed by a 2-week observership in an SNCU collaborative center or a medical college hospital with a level-3 neonatology unit.
Information systems were strengthened to capture and use neonatal data to improve quality of care. For example, Malawi introduced an online system to track maternal and neonatal outcomes (MATSURV). Also, the Ethiopian Neonatal Network, in collaboration with the Vermont Oxford Network, was created to use data captured on REDCap (Research Electronic Data Capture) to improve the quality of neonatal care through supportive supervision and mentorship and periodic review meetings at national and subnational levels.223
Efforts to upgrade infrastructure appeared to follow the standardized guidelines developed in India for SNCUs (i.e., 12 beds per 3,000 deliveries and 4 beds for additional 1,000 deliveries, with approximately 50 square feet per bed for patient care areas and 50 square feet for ancillary care). For example, Ethiopia has adapted its neonatal intensive care unit (NICU) layout from the floor plan currently used in India.
Strategies to finance inpatient small and sick newborn care were distinct. The Ethiopian strategy was to subsidize the cost of care in public health facilities and implement community-based health insurance schemes.224 In India, financial resources were provided to states in a 60:40 (national/state) sharing ratio using annual program implementation plans to develop budgets for facility-based newborn care. In Malawi, the focus was on advocacy for subnational budget allocation. In Rwanda, care was fully funded through universal health coverage.
Ensuring availability of essential medical supplies and equipment by improving supply chain logistics was a priority. Malawi introduced security measures, such as an electronic tracker system for equipment used in the small and sick newborn care units. In addition, priority was given to negotiating service contracts for regular equipment maintenance and extended warranties with equipment manufacturers when possible. India encouraged collaboration of neonatologists, engineers, and entrepreneurs to produce and supply high-quality neonatal equipment of several high-volume categories at affordable cost.225 Appropriate technology was also developed collaboratively in Malawi (e.g., Pumani bubble continuous positive airway pressure [bCPAP]) and Rwanda (e.g., Dreamwarmer non-electric infant warmer).
Strategies to expand service delivery to the district level differed substantially by country. In Ethiopia, the life course continuum of care was applied to gradually expand access to small and sick newborn care at facilities, along with bolstering referrals through community-based newborn care. The Indian strategy to expand care included provision of no-cost, quality newborn care services at public health facilities, along with other schemes to reduce out-of-pocket expenditures and wage loss for parents. SNCUs were operationalized at district levels, and care was extended to the subdistrict levels by establishing newborn stabilization units at the first referral units and community health centers. Malawi introduced services for small and sick newborn care at district hospitals in a stepwise manner—focusing first on kangaroo mother care (KMC), then CPAP, and finally, integrated care. Conversely, in Rwanda, a model of small and sick newborn care was developed and tested at the district hospital level in the country’s more remote, rural eastern region and then transferred back to the tertiary level in the capital and nationally.
Innovations Used to Support the Establishment of Small and Sick Newborn Care
To ensure feasible adaptation to local health systems, country stakeholders innovated a myriad of solutions to support the establishment of small and sick newborn care (Table 2).226–228
TABLE 2.
Innovations Used to Support the Establishment of Small and Sick Newborn Care, by Health System Building Block and Country
| Health System Building Block | Ethiopia | India | Malawi | Rwanda |
|---|---|---|---|---|
| Leadership and governance | Using the National Newborn and Child Survival technical working group and stakeholder coordination meetings to harmonize plans, avoid duplication of efforts and mobilize resources to strengthen newborn and child survival interventions. | Identify high-priority districts, high-focused districts, and aspirational districts by the Ministry of Health and Family Welfare for focused interventions based on maternal and newborn health indicators to address equity gaps. | Newborn focal person to create a platform for discussing and planning for small and sick newborn care at both the national (Ministry of Health) and district (facility and ward) levels. | High-level government leads and owns activities to ensure programmatic sustainability. |
| Human resources | Task-shift small and sick newborn inpatient NICU care from doctors to trained nurses.16,226 | National and regional collaborative centers that provide leadership and technical guidance to ensure uniform, high-quality introduction and implementation of facility-based newborn care. | Implement the COIN curriculum in preservice training for all health providers (including clinical officers) that do not receive directed training on small and sick newborn care. | Develop new cadres and staffing models, including partnership with Rwanda Pediatric Association for a continuous mentorship model. |
| Information systems | Integrate perinatal and maternal death surveillance and response into the national public health emergency management system.54 | The SNCU online portal, a real-time data monitoring system, records vital information on the performance of SNCUs in the country as well as the long-term outcomes of discharged neonates; these data are used for guiding policy and initiating action for improving perinatal care. | A robust information system using the NEST360 platform and facility quality improvement dashboard to summarize outcomes of clinical care, enabling stakeholders to improve the quality of service. | Use electronic data management systems like electronic medical records, RapidSMS-MCH,176,227 and RapidPro228 to improve data management system (in general) and use of SMS-based system to notify mothers and newborns with danger signs in the community who need urgent care. |
| Infrastructure | Establish a newborn corner in all labor and delivery wards and a NICU at hospitals for comprehensive small and sick newborn care.13,36 | Situate comprehensive lactation management centers close to SNCUs to ensure access to mothers’ own milk or donor human milk for admitted infants. | Create a maternity and neonatal unit in a dedicated building that is separate from other clinical services. | Bring the neonatal unit close to the obstetrics unit to avoid transfer delay and newborn hypothermia. |
| Health system financing | Enhance financial risk protection to access essential health services free of charge through mechanisms such fee waiver and exemption programs. | Direct benefit transfer scheme transfers cash entitlements directly to Aadhaar-seeded bank accounts of all eligible beneficiaries, such as Accredited Social Health Activists and contractual staff to further reduce delays in payments and corruption practices. | Leverage the advocacy skills of civil society and learnings from the family planning sector, which has secured budget line items and higher levels of funding in Malawi. | Establish community-based health insurance so services are accessible to all. |
| Medical supplies and devices | A web-based medical equipment management information system to support efficient use and proper management. | Train existing cold chain handlers to repair and maintain equipment in the SNCU. This approach was developed in Maharashtra state and then was replicated in other states. | Standardize the specifications of equipment that facilities can purchase or receive as donations to facilitate maintenance; make sure spare parts are provided and assess 7% of device cost for servicing. | An innovative neonatal transport and the DreamWarmer are 2 examples of innovation cocreated in Rwanda. |
| Service delivery | Health extension workers provide community-based newborn care that includes identifying newborn danger signs, treatment, and referral linkages to health center and hospital levels. | LaQshya certification of health facilities to improve quality of care during delivery and immediate postpartum period, focusing on enhancing satisfaction of beneficiaries, positive birthing experiences, and providing respectful maternity care to all pregnant women attending public health facilities. | Village health clinics that are staffed by health surveillance assistants who are able to identify and refer small and sick newborns. | Use community health workers for early identification of pregnant women, support for antenatal care visits, postnatal and follow-up; creation of center of excellence for NICUs in provinces. |
| Family-centered care |
Family counseling tool in local language for health extension workers/health workers to counsel caregivers through the continuum of care at community and health facility levels. This tool provides an avenue to raise awareness among families about the importance of linkage to inpatient services for small and sick newborns. |
Mother-neonatal intensive care unit where the mother’s bed is placed by the infant’s warmer in the NICU to support zero separation between mother and infant and maternal involvement in taking care of her own baby under guidance of staff. The mother-neonatal intensive care unit is a collaborative effort between the neonatology and obstetrics departments in each facility. | Keep mother and baby together as much as possible when in a facility. |
Establishing a policy to encourage the involvement of husbands in labor, delivery, and newborn care. A “companion of choice” is included in the current newborn protocol. Curtains for privacy are provided for each family. |
Abbreviations: COIN; Care of the Infant and Newborn; NICU, neonatal intensive care unit; SMS, short message service; SNCU, special newborn care unit.
Overall, incorporating a specific focal person for newborn care and a supporting institutional structure, such as a technical working group, were used for coordination. Innovative staffing models recognized the need for a dedicated cadre of trained NICU staff and supported task-shifting care to trained nurses. To support task-shifting, neonatal care training packages were developed, notably the Care of the Infant and Newborn140 course implemented in Malawi. In Rwanda, after the genocide, alliances were formed with professional associations (e.g., Rwanda Pediatric Association and the Royal College of Paediatrics and Child Health) to meet human resource needs in facilities, such as trained medical staff that included pediatricians and neonatologists.
Innovative staffing models recognized the need for a dedicated cadre of trained NICU staff and supported task-shifting care to trained nurses.
Across all countries, information systems were upgraded to manage data in an electronic and/or interactive platform, and perinatal and maternal death surveillance and response systems were implemented. Infrastructure innovation ranged from moving the neonatal and obstetrics units closer together to constructing newborn corners and comprehensive lactation management centers. New health financing schemes emerged, such as community-based health insurance and fee waivers that improved access to care and direct benefit transfer schemes that supported the retention of skilled staff. New ways to manage the efficient and proper use of medical supplies and devices were developed, including a web-based medical equipment management system in Ethiopia and training existing cold chain handlers to repair and maintain equipment in the SNCU in India. Provision of services at the community level was enhanced to support the referral of high-risk pregnancies and the management of small and sick newborns once they are discharged from the facility. Family-centered care was incorporated into the existing health system through policy directives that encouraged the involvement of husbands in labor, delivery, and newborn care and that implemented “no separation of mother and baby,” such as in the mother-NICU.101
Distinct Approaches to Roll-Out of Small and Sick Newborn Care Services
We identified 4 distinct approaches to the roll-out of small and sick newborn care services in each country: (1) Ethiopia used a stepwise expansion from a newborn care corner to community-based newborn care to NICU, (2) India used a model newborn care unit as an exemplar for national scale, (3) Malawi used a hub-and-spoke model initiated through KMC and using the introduction of CPAP as a grounding point, and (4) Rwanda introduced small and sick newborn care through a pilot at the district level in a peripheral region as a basis for national roll-out.
In Malawi, a hub-and-spoke model (i.e., piloting at a major urban center and referral hospital and then expanding into select regional locations, eventually reaching across all districts in the country) was used for the roll-out of small and sick newborn care, using the introduction of CPAP as a foundation.
Services for small and sick newborns rolled out over time, beginning around 1999 when KMC was introduced at Zomba Central Hospital, until today. In 2004, KMC was included in the national policy, and in 2005, national guidelines229 and a training manual for KMC230 were released (Figure 6). Meanwhile, in 2006, Rice University partnered with Queen Elizabeth Central Hospital in Blantyre to help solve the problem of severe respiratory failure in newborns by designing a low-cost bCPAP device. In 2009, just 10 years from the initial introduction, KMC national guidelines were revised,231 and the intervention had been scaled to all district hospitals. In 2012, the community-based maternal newborn package of care was introduced to further support KMC. In 2014, an evaluation of Malawi’s progress toward the Millennium Development Goal 4 highlighted a renewed need to focus on neonatal health. The Ministry of Health realized that neonates received care in inappropriate places and that KMC was not being delivered effectively for unstable small and sick newborns. An initial pilot of bCPAP took place at Queen Elizabeth Central Hospital in 2014. In 2015, the ENAP for Malawi was created to ensure that functional newborn units exist in all districts (Figure 7). In 2016, UNICEF provided support to the Ministry of Health to establish standalone newborn care points in at least 10 health facilities. WHO supported 11 districts to develop NICUs, and bCPAP was scaled to all district hospitals. In 2017, a family-led care model—which positioned caregivers and families as active participants in the care of preterm and low birth weight babies in the health facility and at home—was piloted in Balaka District, and, by 2020, district-level inpatient care facilities for small and sick newborns were scaled fully in the country (Figure 8).
FIGURE 6.
Scaling Up Kangaroo Mother Care in Malawi, 1999–2009
Abbreviations: KMC, kangaroo mother care; QECH, Queen Elizabeth Central Hospital.
FIGURE 7.
Scaling Up bCPAP in Malawi, 2012–2021
Abbreviations: bCPAP, bubble continuous positive airway pressure; CHAM, Christian Health Association of Malawi; MOH, Ministry of Health; QECH, Queen Elizabeth Central Hospital.
FIGURE 8.
Scaling Up Neonatal Care in Malawi, 2016–2020
Abbreviations: MOH, Ministry of Health; NICU, newborn intensive care unit.
In Rwanda, integrated small and sick newborn care was initiated at the district level in the eastern region, far from the capital city. Beginning around 2007, services for small and sick newborns rolled out over time. Services initially focused on KMC, which was introduced as a pilot in 2007 in Muhima District Hospital in Kigali that was established that year as a center of excellence for KMC. From 2007 to 2010, KMC was scaled to 8 district-level facilities. In 2014, KMC was scaled in an additional 15 district hospitals.195 In 2009, a more comprehensive approach to small and sick newborn care was piloted in Rwamagana District Hospital. Using the lessons learned from the pilot effort, an integrated neonatal care protocol and standards were developed and implemented in 2 other district hospitals. Based on this experience, the neonatal care protocol and standards were revised and released in 2014 as the National Neonatal Care Protocol, second edition. This effort effectively expanded the reach of small and sick newborn care services into all district hospitals in the country. In 2019, the National Neonatal Care Protocol, third edition, provided updated guidelines for small and sick newborns (Figure 9 and Figure 10).
FIGURE 9.
Scaling Up Kangaroo Mother Care in Rwanda, 2007–2014
Abbreviations: CHUK, Centre Hospitalier Universitaire de Kigali; KMC, kangaroo mother care.
FIGURE 10.
Scaling Up Small and Sick Newborn Care in Rwanda, 2009–2014
In India, district-level services for small and sick newborns rolled out over time, beginning in 2003. The feasibility of establishing and operating a district-level SNCU was demonstrated in Purulia district of West Bengal in 2003. The Government of India adapted the Purulia district model for a national model by operationalizing SNCUs at district levels and extending the care of sick newborns to the subdistrict levels by establishing newborn stabilization units at the first referral units and community health centers. In addition, a dedicated space (newborn care corner) was ensured at all delivery points for strengthening essential newborn care and resuscitation. In 2008–2009, UNICEF supported pilot implementation of the SNCU in 12 districts (Faridabad, Shivpuri, Guna, Vaisad, Latur, Medak, Lakshadweep, Krishnagiri, Raichur, Warangal, Nandurbar, Purulia). This experience demonstrated the viability of expanding this small and sick newborn care model in India, and the model was included in the National Rural Health Mission. Next, UNICEF, the Indian Academy of Pediatrics, National Neonatology Forum, and other professional stakeholders worked together to implement model newborn care units in Mayurbhanj in Odisha, Guna, and Shivpuri in Madhya Pradesh, Andaman, and Nicobar. The Government of India created operational guidelines,81 which included set up, costing, and steps for establishing newborn care facilities, and developed and launched the facility-based newborn care package and implementation plan in 2011 (Figure 11). By 2020, facility-based newborn care was scaled across the country.
FIGURE 11.
Scaling Up Facility-Based Newborn Care in India, 2003–2020
Abbreviation: SNCU, special newborn care unit.
Source: Gupta G. The NICU story of India: pilot to national scale in 10 years. https://youtu.be/WdXHsC-cks4
In Ethiopia, services for small and sick newborns rolled out over time, beginning around 1996 when KMC was introduced at Black Lion Hospital. Over the next decade, services for small and sick newborn care were limited to specialized tertiary facilities where the quality of care was marginal. In 2003, the Ethiopia Federal Ministry of Health (FMOH) began implementing the community health extension program, primarily through health extension workers, to improve access to health services in rural and remote regions. In a corollary effort to expand and strengthen care for small and sick newborns, KMC was included as an annex in the first edition of the Standard Treatment Guidelines for district hospitals.13 In 2005, a study on facility-based KMC showed that facility-based KMC was feasible and acceptable to mothers and that survival of preterm low birth weight infants was better than the conventional method of care.232 In 2006, Integrated Management of Newborn and Childhood Illnesses launched and included KMC for preterm or low birth weight babies, neonatal resuscitation, infection prevention, and advanced life support in referral hospitals. In 2009, the FMOH, UNICEF, and the Ethiopian Pediatric Society piloted a newborn care corner—which consisted of a hard table, attached overhead heat and light sources, drawer to store essential supplies, self-inflating ambu bag, and face masks in different sizes—that was easy to disassemble and reassemble for cleaning and disinfection in 100 health facilities (Figure 12).233 Based on positive results, the newborn care corner was rolled out to the majority of health centers and hospitals beginning in 2010.
FIGURE 12.

Initial Roll-Out of Newborn Care Corners to 100 Health Facilities in Ethiopia, 2009–2020
Source: Nigussie and Worku.13
In 2012, an expanded focus on community-based newborn care within the health extension program was phased in (Figure 13). Also in 2012, the FMOH and UNICEF constructed a neonatology unit, the first of its kind in Ethiopia, at the Yekatit 12 Hospital, a public referral hospital in Addis Ababa. The NICU at Yekatit 12 Hospital also became a center of excellence in 2012, and NICU services were expanded to an additional 8 facilities. In 2013, NICU services expanded to another 27 facilities. Then, in 2014, based on this experience, the FMOH developed a training package for NICU services at district hospitals and a NICU implementation guideline in 2016. Also in 2016, the use of advanced technology—2 radiant warmers (General Electric Lullaby warmer), a bCPAP device, 2 high-performance phototherapy devices (General Electric Lullaby), a neonatal monitor (General Electric Carescape V100), resuscitation support laryngoscope, and thermal support to transfer neonates from the labor and delivery ward to the NICU—within NICUs was piloted.42 In 2018, the FMOH drafted minimum requirements for NICU services that serve as a reference (e.g., human resource and equipment readiness) for hospital management teams when they establish a NICU and guide procurement of NICU equipment at the national level, as well as capacity-building at all levels. As of 2019, more than 353 hospitals were functional in the country, of which 196 (56%) provided NICU services. Throughout this period, KMC and NICU services have been recognized as high-impact interventions for low birth weight and preterm infants and integrated into policy and guidance documents, most notably the Newborn and Child Survival Strategy 2015–2020, Health Sector Transformation Plan I and II, and National Healthcare Quality Strategy.
FIGURE 13.
Phased Scale-Up of Community-Based Newborn Care in Ethiopia, 2013–2014
Abbreviation: CBNC, community-based newborn care.
We worked with country stakeholders to assess qualitatively the status of implementation of the recently released 2020 WHO Standards for Improving the Quality of Care for Small and Sick Newborn in Health Facilities9 (Table 3).
TABLE 3.
Gap Analysis of Implementation of 2020 WHO Standards for Improving the Quality of Care of Small and Sick Newborns in Health Facilities, by Country
| WHO Standard | Rwanda | Malawi | Ethiopia | India |
|---|---|---|---|---|
| 1.22 NEW: Small and sick newborns are assessed for surfactant deficiency, and surfactant replacement therapy is administered to preterm newborns within the first 2 hours of birth according to WHO guidelines. | X | X | X | |
| 1.23 NEW: Small and sick newborns at risk of bronchopulmonary dysplasia are assessed, investigated, and managed as per standard guidelines. | X | X | X | |
| 1.25 NEW: Small and sick newborn who cannot tolerate enteral feeding or for whom enteral feeding is contraindicated are provided with parenteral nutrition in correct amounts and composition according to standard guidelines. | X | |||
| 1.27 NEW: All very low birthweight newborns are given vitamin D, calcium phosphorus, and iron supplements, according to WHO guidelines. | X | |||
| 1.32 NEW: Small and sick newborns at risk of necrotizing enterocolitis are assessed and managed according to WHO guidelines. | X | |||
| 1.33 NEW: Small and sick newborns at risk of retinopathy of prematurity are appropriately identified, screened, and treated. | X | X | ||
| 1.34 NEW: Small and sick newborns at risk of intraventricular hemorrhage are assessed and managed according to standard guidelines. | X | |||
| 1.40 All small and sick newborns are assessed routinely for pain or symptoms of distress and receive appropriate management according to WHO guidelines. | X | X | ||
| 3.3 For every newborn referred or counter-referred within or between health facilities, there is appropriate information exchange and feedback to relevant health care staff. | X | |||
| 3.5 NEW: Newborn transfer services provide safe, efficient transfer to and from referral neonatal care by experienced, qualified personnel, preferably specialist transport teams, in specialist transport vehicles. | X | X | ||
| 4.4 NEW: Carers of small and sick newborns and staff understand the importance of nurturing interaction with the newborn, recognize and respect the newborn’s behavior and cues, and include them in care decisions. | X | |||
| 4.6 NEW: In humanitarian and fragile settings, including outbreak and pandemic situations, special consideration is given to the specific psychosocial and practical needs of small and sick newborns and their carers. | X | X | X | X |
| 5.4 All newborns are protected from any physical or mental violence, injury, abuse, neglect or any other form of maltreatment. | X | |||
| 5.6 NEW: All newborns who die and all stillbirths have their death registered. | X |
Abbreviation: WHO, World Health Organization.
Across all countries, 24 gap areas were identified, with 15 (62.5%) of those relating to completely new standards for care. Gaps in implementation were observed across all countries, with Ethiopia and Malawi reporting relatively more gaps than Rwanda and India. Similarly, gaps in care for small and sick newborns in fragile or humanitarian settings were noted in all countries. All countries except Rwanda noted facing challenges currently in clinical care, such as administration of surfactant replacement therapy and management of bronchopulmonary dysplasia. All countries except India noted lacking equipment designed specifically for the medical care and developmental and emotional support of small and sick newborns; adequate space for KMC; family-centered care; privacy for mothers to express breast milk; and facilities for hygiene, cooking, and laundry. Countries are working to close these gaps in varying ways. For example, in Malawi, a review of guidelines for retinopathy of prematurity is currently in process in collaboration with ophthalmologists to update the current national standard of care small and sick newborns.
Gaps in implementation were observed across all countries, with Ethiopia and Malawi reporting relatively more gaps than Rwanda and India.
As demonstrated, efforts in each country are uniquely distinct. However, across all 4 countries, stakeholders prioritized a similar set of critical actions to support the establishment of small and sick newborn care (Table 4).234,235
TABLE 4.
Critical Actions to Support the Establishment of Small and Sick Newborn Care as Prioritized by Stakeholders, by Health System Building Block
| Health System Building Block | Actions |
|---|---|
| Leadership and governance |
|
| Human resources |
|
| Information systems |
|
| Infrastructure |
|
| Health system financing |
|
| Medical supplies and devices |
|
| Service delivery |
|
| Family-centered care |
|
Abbreviations: KMC, kangaroo mother care; NICU, neonatal intensive care unit.
LESSONS LEARNED
Literature that documents the holistic systems approach to the establishment of inpatient newborn care in LMICs is sparse. Details from the country perspective on what it takes to establish, scale up, and strengthen inpatient newborn care services are critical to guiding future implementation, with learnings that incorporate historical information about key moments and timelines, champions, barriers and enablers, strategies, and key advice to other countries embarking on this journey.
Our in-depth case studies attempt to fill this gap by providing a contextualized narrative of how inpatient newborn care was established and scaled from the perspective of key stakeholders who were key drivers of the implementation of small and sick newborn care in each country. We used the NEST360 Theory of Change for facility-based care, which reflects the WHO health system building blocks to frame the case study to align with learning resources12 gathered by global stakeholders around the implementation of care for small and sick newborns.
Efforts to establish and scale inpatient care for small and sick newborns in Ethiopia, India, Malawi, and Rwanda over the last decade have led to remarkable success. Each country devised locally relevant, specific strategies to change their “business as usual” practices and improve care for small and sick newborns. Unifying themes about how this has been addressed at the country level are apparent. These include the importance of leadership and governance at the national level to consolidate and coordinate action related to improvements in quality of care for newborns. In this regard, countries employed national coordination committees and a newborn focal person situated within the ministry of health. This collaborative team, led by the ministry of health, created a national strategy and championed it at national and subnational levels.
Dissemination and utilization of data about newborns, such as the ENAP target-setting effort, raised awareness and focus on the newborn and spurred dialogue and action by country stakeholders. This is a marked shift from 20–25 years ago when data on newborns, especially small and sick newborns in LMICs, were virtually nonexistent. The ENAP was an important catalyst for countries to consolidate their action plans for newborn health, and its impact was particularly evident in India and Malawi, where stakeholders created country-specific ENAPs.133,236 Each country invested in and built skills of staff on data collection and data use and institutionalized regular neonatal data reviews to identify gaps and propose relevant strategies. Electronic data dashboards or real-time data monitoring systems facilitated the use of data for decision-making and program improvement.
Adaptation of the existing infrastructure was crucial to establishing quality care for small and sick newborns. Inpatient units were reorganized or constructed to allocate sufficient space in line with established guidance for very sick babies, isolated infectious babies, KMC babies, and the provision of human milk. Although it was not always available at the country level, comprehensive guidance on infrastructure layout to enable family-centered care is essential. Development of this type of guidance can benefit from the engagement of multidisciplinary teams (i.e., hospital planners, clinicians, public health experts, civil and biomedical engineers, and subnational management).
All countries advocated for continuous budget allocations to support provision of quality neonatal care at all levels, with specific focus on district and facility levels. However, the specifics of how each country conducted budget advocacy differed. Donor dependency was noted as a hurdle in some countries, and donor funding was used in different ways. For example, in Ethiopia, national goals define the use of donor funds. However, in Malawi, changes in personnel/corruption allowed donors to identify their own agenda. Health financing schemes, such as community health insurance, helped to remove barriers to accessing health services and, at the same time, transform health-seeking behavior.
Standardizing required neonatal supplies for inclusion into routine supply chain requirements was employed universally. Successfully implementing family-centered care is most advanced in India, which serves as a model of care for other countries. India is also at the forefront of involving the private sector to care for small and sick newborns in a coordinated manner.
It is challenging to capture all the components required to create a functional intervention at scale. Although documentation around efforts to introduce and scale a new intervention exist, they are not always kept in the same central location and do not always illustrate the entire picture. Those working to advance components of small and sick newborn care do not always have the opportunity to review the entirety of complementary efforts that are advancing progress simultaneously. Keeping documentation in a centralized location and using that information to create event timelines that showcase overall country-level effort could be a best practice for documenting the journey. This type of documentation is important because it (1) celebrates successes at the country level, (2) can serve as an example to other countries, and (3) helps the country to assess the status and identify gaps that still need to be addressed. In general, implementing small and sick newborn care is evolving rapidly at the country level. Not infrequently, content specific to small and sick newborns in policy documents reflects only a partial alignment with WHO standards, which may not depict the larger scale of actual implementation and practice.
The case study approach we used to document the journey to scale helps to illustrate the remarkable progress that is being made to improve the quality of small and sick newborn care at the country level. This reporting approach balances the paucity of gray literature and documentation and literature published in peer-reviewed journals with lived experience from stakeholders who were intimately involved in the process. An additional output based on these lessons includes a virtual multimedia experience where any person can “walk through” an existing unit in Malawi that provides care for small and sick newborns.237 This virtual experience offers a realistic glimpse into the newborn care unit and its practices, and health care providers comment on how to successfully establish and scale the care of small and sick newborns via a pop-up video feature.
The case study approach we used to document the journey to scale helps to illustrate the remarkable progress that is being made to improve the quality of small and sick newborn care at the country level.
Building on the numerous lessons234 across these 4 case studies, the following next steps to strengthen and transform inpatient newborn care globally should be prioritized.
Identify cost drivers and determine cost effectiveness of best practices to inform future, sustainable, and scalable implementation.
Build country-level capacity to document local innovations, approaches, and strategies and to disseminate learnings for informing future investments.
Transform newborn care experiences through a human-centered approach, exploring documenting and linking both facility-based and family-level experiences to improve systems for providing quality neonatal care.
Further document the scalability of successful programs for new settings to learn and adopt.
Limitations
Limitations of the case study approach relate primarily to its subjective nature and heavy reliance on qualitative inquiry methods. The case study approach aimed to conduct an exploratory investigation to gather facts about the scale-up of newborn care. It is conceivable that unconscious bias was introduced through the evaluation of what data were included in the case study. To mitigate this, we standardized data collection methods across all countries, using the NEST360 Theory of Change for facility-based care, which reflects the WHO Health Systems Framework as the organizing principle and triangulating data from multiple sources. We continued to interview key stakeholders until data saturation was apparent and did not bind the upper limit of our sample size in any way. Furthermore, data validation sessions were held in each country to ensure that all relevant data points and the country narrative rang authentic, accurate, and true with all country stakeholders. In line with the case study approach, we collected data from a relatively limited number of participants with highly pertinent experience, which may have provided a skewed view of the journey to scale undertaken in each country. Finally, we did not attempt to report on the quality of care being implemented in each county or collect data on programmatic outcomes, which conforms to our study objective of documenting how small and sick newborn care was established in each country.
We also note the lack of standardized reporting for programmatic case studies. For example, the Equator Network238 includes multiple guidelines for reporting clinical case studies; however, none for case studies of this nature. As country case studies are increasingly being used, it will be important to standardize criteria for reporting country experience in a case study format.
CONCLUSIONS
Around 2010, in Ethiopia, India, Malawi, and Rwanda, services for small and sick newborns were available primarily in tertiary care centers. Over the next decade, constellations of events created momentum to establish inpatient care services at the district level in these countries. In Ethiopia, more than half of the over 353 hospitals in the country provide NICU services. In Malawi, district-level inpatient care facilities for small and sick newborns were scaled fully in the country, with NICUs scaled in all 28 districts. In Rwanda, integrated small and sick newborn care has been established in all 42 district hospitals. In India, the number of SNCUs has increased from 263 to 895 over 10 years, exemplifying the level of scale of these services.
The journeys toward establishing inpatient newborn care in Ethiopia, India, Malawi, and Rwanda over the last decade are similar in terms of trajectory yet unique in implementation. Each country devised locally relevant, specific strategies and innovations that addressed identified gaps in programming and health systems. Four distinct approaches to the roll-out of service delivery were identified. The examples of these countries can inspire other more nascent initiatives. From the global perspective, there has not been a uniform funding approach; more effective coordination may be beneficial. Country-level strategies and innovation related to the establishment of small and sick newborn care require greater documentation and recognition. Documentation should give voice to lived country experience, not all of which is fully captured in existing, peer-reviewed published literature.
Acknowledgments
The authors acknowledge and appreciate the contributions of the small and sick newborn care stakeholder experts, as well as the helpful guidance from our Program Officer at the Bill & Melinda Gates Foundation, Dr. Hema Magge.
Peer Reviewed
First published online: August 7, 2023.
Cite this article as: Coffey PS, Israel-Ballard K, Meyer L, et al. The journey toward establishing inpatient care for small and sick newborns in Ethiopia, India, Malawi, and Rwanda. Glob Health Sci Pract. 2023;11(4):e2200510. https://doi.org/10.9745/GHSP-D-22-00510
Funding
This work was supported by the Bill & Melinda Gates Foundation.
Author contributions
PSC conceptualized the case studies, analyzed the data, contributed to interpretation of the results, and drafted the initial manuscript. CE and KIB conceptualized the case studies, contributed to interpretation of the results, and edited the article. KM and LM collected and analyzed secondary data. All other authors collected and analyzed primary data and contributed to interpretation of the results. All authors read and approved the final article.
Competing interests
None declared.
REFERENCES
- 1.United Nations Inter-Agency Group for Child Mortality Estimation. Levels and Trends in Child Mortality. 2021 Report. UNICEF; 2021. Accessed June 22, 2023. https://data.unicef.org/resources/levels-and-trends-in-child-mortality/ [Google Scholar]
- 2.Neonatal Survival Series. Lancet; 2005. Accessed June 22, 2023. https://www.thelancet.com/series/neonatal-survival [Google Scholar]
- 3.Every Newborn Series. Lancet; 2014. Accessed June 22, 2023. https://www.thelancet.com/series/everynewborn [Google Scholar]
- 4.World Health Organization (WHO); UNICEF. Every Newborn: An Action Plan to End Preventable Deaths. WHO; 2014. Accessed June 22, 2023. https://www.who.int/publications/i/item/9789241507448 [Google Scholar]
- 5.World Health Organization (WHO). WHO Recommendations on Newborn Health: Guidelines Approved by the WHO Guidelines Review Committee. WHO; 2017. Accessed June 22, 2023. https://www.who.int/publications/i/item/WHO-MCA-17.07 [Google Scholar]
- 6.World Health Organization (WHO). WHO Recommendations on Postnatal Care of the Mother and Newborn. WHO; 2013. Accessed June 22, 2023. https://www.who.int/publications/i/item/9789241506649 [PubMed] [Google Scholar]
- 7.World Health Organization (WHO); UNICEF. Survive and Thrive: Transforming Care for Every Small and Sick Newborn. WHO; 2019. Accessed June 22, 2023. https://www.unicef.org/media/58076/file [Google Scholar]
- 8.Engmann CM, Khan S, Moyer CA, Coffey PS, Bhutta ZA. Transformative innovations in reproductive, maternal, newborn, and child health over the next 20 years. PLoS Med. 2016;13(3):e1001969. 10.1371/journal.pmed.1001969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization (WHO). Standards for Improving the Quality of Care for Small and Sick Newborns in Health Facilities. WHO; 2020. Accessed June 22, 2023. https://www.who.int/publications/i/item/9789240010765 [Google Scholar]
- 10.World Health Organization (WHO). Everybody’s Business: Strengthening Health Systems to Improve Health Outcomes: WHO’s Framework for Action. WHO; 2007. Accessed June 22, 2023. https://apps.who.int/iris/handle/10665/43918 [Google Scholar]
- 11.Implementation toolkit: Small and sick newborn care. NEST 360. Accessed June 22, 2023. https://www.newborntoolkit.org/
- 12.Dickson KE, Simen-Kapeu A, Kinney MV, et al. Every Newborn: health-systems bottlenecks and strategies to accelerate scale-up in countries. Lancet. 2014;384(9941):438–454. 10.1016/S0140-6736(14)60582-1. [DOI] [PubMed] [Google Scholar]
- 13.Nigussie AK, Worku B. Newborn care corner: a simplified approach to providing optimal newborn care immediately after birth. Ethiop Med J. 2019;3. Accessed June 22, 2023. https://emjema.org/index.php/EMJ/article/view/1392/550 [Google Scholar]
- 14.Save the Children. Kangaroo Mother Care in Ethiopia. Save the Children; 2017. Accessed June 22, 2023. https://www.healthynewbornnetwork.org/hnn-content/uploads/Ethiopia-KAP-Summary-Sheet.pdf [Google Scholar]
- 15.Sibley LM, Amare Y, Abebe ST, Belew ML, Shiffra K, Barry D. Appropriateness and timeliness of care-seeking for complications of pregnancy and childbirth in rural Ethiopia: a case study of the Maternal and Newborn Health in Ethiopia Partnership. J Health Popul Nutr. 2017;36(Suppl 1):50. 10.1186/s41043-017-0120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Federal Democratic Republic of Ethiopia. Federal Ministry of Health (FMOH). Health Sector Development Program IV 2010/11–2014/15. FMOH; 2010. Accessed June 22, 2023. https://www.healthynewbornnetwork.org/hnn-content/uploads/HSDP-IV-Final-Draft-October-2010-2.pdf [Google Scholar]
- 17.UNICEF USA. Investing in Survival: Enhancing the Neonatal Intensive Care Unit of Yekatit 12 Hospital. UNICEF USA; 2013. Accessed June 22, 2023. https://www.unicefusa.org/sites/default/files/Ethiopia%20Report.pdf [Google Scholar]
- 18.Central Statistical Agency (CSA); ICF International. Ethiopia Demographic and Health Survey 2011. CSA/ICF; 2012. Accessed June 22, 2023. https://dhsprogram.com/publications/publication-fr255-dhs-final-reports.cfm [Google Scholar]
- 19.A Promise Renewed. Every Woman Every Child. Accessed June 22, 2023. https://www.everywomaneverychild.org/commitment/a-promise-renewed
- 20.Federal Democratic Republic of Ethiopia. Federal Ministry of Health (FMOH). Road Map for Accelerating the Reduction of Maternal and Newborn Morbidity and Mortality in Ethiopia, 2012–2015. FMOH; 2012. Accessed June 22, 2023. http://repository.iifphc.org/handle/123456789/1327 [Google Scholar]
- 21.Mathewos B, Owen H, Sitrin D, et al. Community-Based Interventions for Newborns in Ethiopia (COMBINE): cost-effectiveness analysis. Health Policy Plan. 2017;32(Suppl 1):i21–i32. 10.1093/heapol/czx054. [DOI] [PubMed] [Google Scholar]
- 22.VSO Ethiopia. Commitment to Saving Innocent Lives: Best Practice in Neonatal Intensive Care Units. VSO Ethiopia; 2014. Accessed June 22, 2023. https://www.vso.nl/app/uploads/2019/09/2017-Ethiopia-NICU-report.pdf [Google Scholar]
- 23.Degefie Hailegebriel T, Mulligan B, Cousens S, et al. Effect on neonatal mortality of newborn infection management at health posts when referral is not possible: a cluster-randomized trial in rural Ethiopia. Glob Health Sci Pract. 2017;5(2):202–216. 10.9745/ghsp-d-16-00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirkuzie AH, Sisay MM, Reta AT, Bedane MM. Current evidence on basic emergency obstetric and newborn care services in Addis Ababa, Ethiopia; a cross sectional study. BMC Pregnancy Childbirth. 2014;14:354. 10.1186/1471-2393-14-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Debelew GT, Afework MF, Yalew AW. Determinants and causes of neonatal mortality in Jimma Zone, southwest Ethiopia: a multilevel analysis of prospective follow up study. PLoS One. 2014;9(9):e107184. 10.1371/journal.pone.0107184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mengesha HG, Sahle BW. Cause of neonatal deaths in northern Ethiopia: a prospective cohort study. BMC Public Health. 2017;17(1):62. 10.1186/s12889-016-3979-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maternal and Child Survival Program (MCSP). Ethiopia Basic Emergency Obstetric and Newborn Care: EOP Summary and Results. MCSP: 2019. Accessed June 22, 2023. https://www.mcsprogram.org/wp-content/uploads/2019/12/Ethiopia.pdf [Google Scholar]
- 28.Central Statistical Agency (CSA). Ethiopia Mini Demographic and Health Survey 2014. CSA: 2014. Accessed June 22, 2023. http://www.statsethiopia.gov.et/wp-content/uploads/2019/06/Ethiopia-Demographic-Health-Survey-Report-2014.pdf [Google Scholar]
- 29.Ethiopian Public Health Institute (EPHI); Federal Ministry of Health; ICF International. Ethiopia Service Provision Assessment Plus Survey 2014. EPHI; 2014. Accessed June 22, 2023. http://repository.iifphc.org/handle/123456789/1072 [Google Scholar]
- 30.Zemedu T, Defar A, Bekele A, et al. Maternal and newborn health service provision in Ethiopia – SPA+. In: Special Bulletin 17th Annual Review Meeting 2015. Federal Democratic Republic of Ethiopia Ministry of Health; 2020:57–58. Accessed June 22, 2023. 10.13140/RG.2.2.10522.59840 [DOI] [Google Scholar]
- 31.Kokeb M, Desta T. Institution based prospective cross-sectional study on patterns of neonatal morbidity at Gondar University hospital neonatal unit, north-west Ethiopia. Ethiop J Health Sci. 2016;26(1):73–79. 10.4314/ejhs.v26i1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Federal Democratic Republic of Ethiopia. Federal Ministry of Health (FMOH). Neonatal Intensive Care Unit (NICU) Training Participants’ Manual. FMOH; 2014. Accessed June 22, 2023. http://ndl.ethernet.edu.et/bitstream/123456789/78888/2/NICU%20Nurses%20Training%20Participants%20Manual%20August%202014.pdf [Google Scholar]
- 33.Mathewos B, Sitrin D, Valsankar B, et al. Delivery of Kangaroo Mother Care in Ethiopian Facilities: Results from a Rapid Assessment in Three Zones. Maternal and Child Survival Program; 2015. Accessed June 22, 2023. As referenced in https://www.mcsprogram.org/wp-content/uploads/2018/06/Ethiopia-KAP-Summary-Sheet.pdf [Google Scholar]
- 34.JSI Research and Training Institute, Inc. Last Ten Kilometers Project. Measuring Implementation Strength of Basic Emergency Obstetric and Newborn Care in 134 Health Centers of Amhara, Oromia, SNNP and Tigray Regions of Ethiopia. JSI/Last Ten Kilometers Project; 2015. Accessed June 22, 2023. https://l10k.jsi.com/Resources/Docs/implementation-strength-of-bemonc.pdf [Google Scholar]
- 35.Tiruneh GT, Karim AM, Avan BI, et al. The effect of implementation strength of basic emergency obstetric and newborn care (BEmONC) on facility deliveries and the met need for BEmONC at the primary health care level in Ethiopia. BMC Pregnancy Childbirth. 2018;18(123). 10.1186/s12884-018-1751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Federal Democratic Republic of Ethiopia. Federal Ministry of Health (FMOH). Health Sector Transformation Plan, 2015/16–2019/20. FMOH; 2015. Accessed June 22, 2023. https://faolex.fao.org/docs/pdf/eth208347.pdf [Google Scholar]
- 37.Federal Democratic Republic of Ethiopia. Federal Ministry of Health (FMOH). Maternal and Child Health Directorate. National Strategy for Newborn and Child Survival in Ethiopia 2015/16-2019/20. FMOH; 2015. Accessed June 22, 2023. https://www.healthynewbornnetwork.org/hnn-content/uploads/nationalstrategy-for-newborn-and-child-survival-in-ethiopia-201516-201920.pdf [Google Scholar]
- 38.World Health Organization (WHO). Success Factors for Women’s and Children’s Health. WHO; 2015. Accessed June 22, 2023. https://fctc.who.int/publications/i/item/success-factors-for-women-s-and-children-s-health [Google Scholar]
- 39.Ethiopian Public Health Institute (EPHI); Federal Democratic Republic of Ethiopia. Federal Ministry of Health; UNICEF. Countdown to a Healthier Ethiopia: Building on Successes to Accelerate Newborn Survival. EPHI; 2015. Accessed June 22, 2023. http://www.countdown2015mnch.org/documents/EthiopiaBrief.pdf [Google Scholar]
- 40.Tekleab AM, Amaru GM, Tefera YA. Reasons for admission and neonatal outcome in the neonatal care unit of a tertiary care hospital in Addis Ababa: a prospective study. Res Rep Neonatol. 2016;6(2016):17–23. 10.2147/RRN.S95455 [DOI] [Google Scholar]
- 41.Central Statistical Agency (CSA); ICF. Ethiopia Demographic and Health Survey 2016. CSA/ICF; 2016. Accessed June 22, 2023. https://dhsprogram.com/publications/publication-fr328-dhs-final-reports.cfm [Google Scholar]
- 42.Save the Children Ethiopia. Rapid Health Facility Assessment on Service Availability and Delivery of Care to Premature and/or Low Birth Weight Babies. Save the Children Ethiopia; 2015. Accessed June 22, 2023. https://www.healthynewbornnetwork.org/hnn-content/uploads/KMC_Facilities_Brief_.pdf [Google Scholar]
- 43.Ethiopian Public Health Institute (EPHI); Federal Democratic Republic of Ethiopia. Federal Ministry of Health; Averting Maternal Death and Disability (AMDD) – Columbia University. Ethiopian Emergency Obstetric and Newborn Care (EmONC) Assessment 2016. EPHI; 2017. Accessed June 22, 2023. http://repository.iifphc.org/handle/123456789/472 [Google Scholar]
- 44.Federal Democratic Republic of Ethiopia. Federal Ministry of Health (FMOH). Neonatal Intensive Care Unit (NICU) Implementation Guide (draft version). FMOH; 2016. Accessed June 22, 2023. http://repository.iifphc.org/bitstream/handle/123456789/708/NICU%20Implementation%20Guide%20Print%20Version%20.pdf [Google Scholar]
- 45.Demisse AG, Alemu F, Gizaw MA, Tigabu Z. Patterns of admission and factors associated with neonatal mortality among neonates admitted to the neonatal intensive care unit of University of Gondar Hospital, Northwest Ethiopia. Pediatric Health Med Ther. 2017;8:57–64. 10.2147/phmt.s130309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Worku B, Kidane L, Abera K, Kumar A, Egeli P, Kidolezi Y. Improving neonatal health outcomes in Ethiopia through an innovative and sustainable healthcare model. Ethiopia J Pediatrics Child Health. 2016;12(2). Accessed June 22, 2023. https://ejpch.net/index.php/ejpch/article/view/92 [Google Scholar]
- 47.Muhe LM, McClure EM, Nigussie AK, et al. Major causes of death in preterm infants in selected hospitals in Ethiopia (SIP): a prospective, cross-sectional, observational study. Lancet Glob Health. 2019;7(8):e1130–e1138. 10.1016/s2214-109x(19)30220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kinnery MV, Rhoda NR. Understanding the causes of preterm birth: solutions depend on context. Lancet Glob Health. 2019;7(8):e1000–e1001. 10.1016/s2214-109x(19)30281-5. [DOI] [PubMed] [Google Scholar]
- 49.Federal Democratic Republic of Ethiopia. Federal Ministry of Health (FMOH). Ethiopian National Healthcare Quality Strategy, 2016–2020. FMOH; 2015. Accessed June 22, 2023. http://repository.iifphc.org/bitstream/handle/123456789/268/Ethiopian%20National%20Health%20Care%20Quality%20Strategy.pdf [Google Scholar]
- 50.Engida E. Giving Premature Babies a Chance for a Healthy Start to Life. UNICEF Ethiopia; 2021. Accessed June 22, 2023. https://www.unicef.org/ethiopia/stories/giving-premature-babies-chance-healthy-start-life [Google Scholar]
- 51.Seid SS, Ibro SA, Ahmed AA, et al. Causes and factors associated with neonatal mortality in neonatal intensive care unit (NICU) of Jimma University medical center, Jimma, south west Ethiopia. Pediatric Health Med Ther. 2019;10(2019):39–48. 10.2147/phmt.s197280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orsido TT, Asseffa NA, Berheto TM. Predictors of neonatal mortality in neonatal intensive care unit at referral hospital in southern Ethiopia: a retrospective cohort study. BMC Pregnancy Childbirth. 2019;19(1):1–9. 10.1186/s12884-019-2227-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Usman AK, Wolka E, Tadesse Y, et al. Health system readiness to support facilities for care of preterm, low birth weight, and sick newborns in Ethiopia: a qualitative assessment. BMC Health Serv Res. 2019;19(860). 10.1186/s12913-019-4672-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ethiopian Public Health Institute (EPHI). National Technical Guidance for Maternal and Perinatal Death Surveillance and Response. EPHI; 2017. Accessed July 17, 2023. https://web.archive.org/web/20221017143321/https://ephi.gov.et/images/pictures/National-Maternal-and-Perinatal--Death-Surveillance-and-Response-guidance-2017.pdf [Google Scholar]
- 55.JSI Research and Training Institute, Inc. Clinical Skills Laboratories for Continuous Professional Development: Cultivating a Culture of Self-Directed, Simulation-Based Learning. JSI; 2020. Accessed June 22, 2023. https://publications.jsi.com/JSIInternet/Inc/Common/_download_pub.cfm?id=24404&lid=3 [Google Scholar]
- 56.Desalew A, Sintayehu Y, Teferi N, et al. Cause and predictors of neonatal mortality among neonates admitted to neonatal intensive care units of public hospitals in eastern Ethiopia: a facility-based prospective follow-up study. BMC Pediatrics. 2020;20(160). 10.1186/s12887-020-02051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jebessa S, Litch JA, Senturia K, et al. Qualitative assessment of the quality of care for preterm, low birth weight, and sick newborns in Ethiopia. Health Serv Insights. 2021;14:11786329211025150. 10.1177/11786329211025150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Federal Democratic Republic of Ethiopia. Federal Ministry of Health (FMOH). Maternal and Child Health Directorate. Classification of Facilities for Newborn Services and the Minimum Requirements in Ethiopian Setup Neonatal Care Services in Ethiopia (draft version). FMOH; 2018. [Google Scholar]
- 59.Mony PK, Tadele H, Gobezayehu AG, et al. Scaling up kangaroo mother care in Ethiopia and India: a multisite implementation research study. BMJ Glob Health. 2021;6:e005905. 10.1136/bmjgh-2021-005905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ethiopian Public Health Institute (EPHI); ICF. Ethiopia Mini Demographic and Health Survey 2019: Final Report. EPHI/ICF; 2021. Accessed June 22, 2023. https://dhsprogram.com/publications/publication-FR363-DHS-Final-Reports.cfm [Google Scholar]
- 61.Woday Tadesse A, Mekuria Negussie Y, Aychiluhm SB. Neonatal mortality and its associated factors among neonates admitted at public hospitals, pastoral region, Ethiopia: a health facility based study. PLoS One. 2021;16(3):e0242481. 10.1371/journal.pone.0242481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oromia Regional Health Bureau. Assessment on Neonatal Intensive Care Unit (NICU) Service Implementation in Selected 14 Hospitals in Oromia Region, Ethiopia. Oromia Regional Health Bureau; 2019. [Google Scholar]
- 63.Eyeberu A, Shore H, Getachew T, Atnafe G, Dheresa M. Neonatal mortality among neonates admitted to NICU of Hiwot Fana specialized university hospital, eastern Ethiopia, 2020: a cross-sectional study design. BMC Pediatrics. 2021;21(1):125. 10.1186/s12887-021-02598-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Federal Democratic Republic of Ethiopia. Federal Ministry of Health (FMOH). Health Sector Transformation Plan II, 2020/21–2025/26. FMOH; 2021. Accessed June 22, 2023. https://www.familyplanning2020.org/sites/default/files/HSTP-II.pdf [Google Scholar]
- 65.Federal Democratic Republic of Ethiopia. Federal Ministry of Health (FMOH). Neonatal Intensive Care Unit (NICU) Management Protocol (Updated). FMOH; 2021. [Google Scholar]
- 66.Government of India. Ministry of Health and Family Welfare (MOHFW). National Rural Health Mission – Framework for Implementation (2005–2012). MOHFW; 2005. Accessed June 22, 2023. https://nhm.gov.in/WriteReadData/l892s/nrhm-framework-latest.pdf [Google Scholar]
- 67.Janani Suraksha Yojana. National Health Mission. Accessed June 22, 2023. https://nhm.gov.in/index1.php?lang=1&level=3&lid=309&sublinkid=841
- 68.Bang AT, Bang RA, Reddy HM. Home-based neonatal care: summary and applications of the field trial in rural Gadchiroli, India (1993 to 2003). J Perinatol. 2005;25:S108–S122. 10.1038/sj.jp.7211278. [DOI] [PubMed] [Google Scholar]
- 69.Gogia S, Sachdev HP. Home-based neonatal care by community health workers for preventing mortality in neonates in low- and middle-income countries: a systematic review. J Perinatol. 2016;36(Suppl 1):S55–S73. 10.1038/jp.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cambridge Economic Policy Associates; India Development Foundation. Evaluation of the Norway India Partnership Initiative for Maternal and Child Health. Cambridge Economic Policy Associates; 2013. Accessed June 22, 2023. https://www.docslides.com/slides/evaluation-of-the-norway-india.html [Google Scholar]
- 71.National Health Mission. Best Practices - Facility Based Newborn Care Database. National Health Mission. Accessed July 17, 2023. https://web.archive.org/web/20220719062836/https://www.nhmmp.gov.in/WebContent/BestPractice/Best%20Practices%20-%20Facility%20Based%20Newborn%20Care%20Data%20Base.pdf
- 72.Bansal CP, Gupta S. The past half century of Indian Academy of Pediatrics. Indian Pediatr. 2012;50:39–48. Accessed June 22, 2023. https://indianpediatrics.net/jan2013/jan-39-48.htm#:∼:text=Indian%20Academy%20of%20Pediatrics%20was,India%20and%20Indian%20Pediatric%20Society [DOI] [PubMed] [Google Scholar]
- 73.Government of India. Ministry of Health and Family Welfare (MOHFW). Navjaat Shishu Suraksha Karyakaram. MOHFW; 2009. Accessed June 22, 2023. https://nhm.gov.in/images/pdf/programmes/child-health/guidelines/NSSK/NSSK-Resource-Manual.pdf [Google Scholar]
- 74.Government of India. Ministry of Health and Family Welfare (MOHFW). Facility Based IMNCI (F-IMNCI) Participants Manual. MOHFW; 2009. Accessed June 22, 2023. https://www.wbhealth.gov.in/uploaded_files/FIMNCI/FIMNCI_Participants_Manual.pdf [Google Scholar]
- 75.Government of India. Ministry of Health and Family Welfare (MOHFW). Facility Based Integrated Management of Neonatal and Childhood Illness (F-IMNCI) Chart Booklet. MOHFW; 2009. Accessed June 22, 2023. https://www.wbhealth.gov.in/uploaded_files/FIMNCI/chart_booklet_FIMNCI.pdf [Google Scholar]
- 76.National Neonatology Forum. Evidence Based Clinical Practice Guidelines. Chandika Press Pvt. Ltd.; 2010. Accessed June 22, 2023. http://my.ipsolutionz.com/IAP-neo-chap/uploads/acd-corner/nnf_guidelines-2011.pdf [Google Scholar]
- 77.Neonatology achievements. King Edward Memorial Hospital. Accessed June 22, 2023. https://www.kem.edu/achievements-neonatology/
- 78.Sudan P, Jhalani M, Gurnani V, et al. Family Participatory Care in India – A Gateway to Nurturing Small and Sick Newborns. World Health Organization; 2018. Accessed June 22, 2023. http://nurturing-care.org/resources/nurturing-care-case-study-india.pdf [Google Scholar]
- 79.Kumar H, Bhat A, Alwadhi V, et al. An assessment of implementation of family participatory care in special newborn care units in three states of India. Indian Pediatr. 2021;58:349–353. 10.1007/s13312-021-2194-6. [DOI] [PubMed] [Google Scholar]
- 80.Janani Shishu Suraksha Karyakram. National Health Mission. Accessed June 23, 2023. https://nhm.gov.in/index1.php?lang=1&level=3&sublinkid=842&lid=308
- 81.Government of India. Ministry of Health and Family Welfare (MOHFW). Facility Based Newborn Care Operational Guide. MOHFW; 2011. Accessed June 23, 2023. https://www.aspirationaldistricts.in/wp-content/uploads/2019/02/Facility-Based-Newborn-Care-Operational-Guide-FBNC.pdf [Google Scholar]
- 82.Government of India. Ministry of Health and Family Welfare (MOHFW). Home Based Newborn Care Operational Guidelines. MOHFW; 2011. Accessed June 23, 2023. https://nhsrcindia.org/sites/default/files/2021-03/Revised%20HBNC%20Operational%20Guidelines%202014%20English.pdf [Google Scholar]
- 83.Government of India. Ministry of Health and Family Welfare (MOHFW). Operational Guidelines on Facility Based Management of Children with Severe Acute Malnutrition. MOHFW; 2011. Accessed June 22, 2023. https://rajswasthya.nic.in/MTC%20Guideline-%20MOHFW.pdf [Google Scholar]
- 84.Government of India. Ministry of Health and Family Welfare (MOHFW); Save the Children. Two-Year Progress of Special Newborn Care Units in India: A Brief Report. MOHFW; 2014. Accessed June 22, 2023. https://nhm.gov.in/images/pdf/programmes/child-health/annual-report/Two_Year_Progress_of_SNCUs-A_Brief_Report_(2011-12_&_2012-13).pdf [Google Scholar]
- 85.National Health Mission. Accessed June 22, 2023. https://nhm.gov.in/index4.php?lang=1&level=0&linkid=445&lid=38
- 86.National Call to Action - Child Survival and Development Summit - Reaffirming Child Survival and Development in India. Press release. Government of India. Ministry of Health and Family Welfare; February 7, 2013. Accessed June 22, 2023. https://pib.gov.in/newsite/PrintRelease.aspx?relid=92074 [Google Scholar]
- 87.RMNCH+A. National Health Portal. Accessed June 22, 2023. https://nhm.gov.in/index1.php?lang=1&level=1&sublinkid=794&lid=168
- 88.Government of India. Ministry of Health and Family Welfare (MOHFW). Use of Antenatal Corticosteroids in Preterm Labour. MOHFW; 2014. Accessed June 22, 2023. http://nhm.gov.in/images/pdf/programmes/child-health/guidelines/Operational_Guidelines-Use_of_Antenatal_Corticosteroids_in_Preterm_Labour.pdf [Google Scholar]
- 89.About us. Rashtriya Bal Swasthya Karyakram. Accessed June 22. 2023. https://rbsk.gov.in/RBSKLive/AboutUs.aspx
- 90.Public Health Foundation of India; All India Institute of Medical Sciences; Save the Children. State of India’s Newborns (SOIN) 2014 – A Report. Public Health Foundation of India/All India Institute of Medical Sciences/Save the Children; 2014. Accessed June 22, 2023. https://www.newbornwhocc.org/pdf/SOIN_PRINTED%2014-9-2014.pdf [Google Scholar]
- 91.Government of India. Ministry of Health and Family Welfare (MOHFW). India Newborn Action Plan. MOHFW; 2014. Accessed June 22, 2023. https://nhm.gov.in/images/pdf/programmes/inap-final.pdf [Google Scholar]
- 92.Government of India. Ministry of Health and Family Welfare (MOHFW). Operational Guidelines: Use of Gentamicin by ANMs for Management of Sepsis in Young Infants Under Specific Situations. MOHFW; 2014. Accessed June 22, 2023. https://www.healthynewbornnetwork.org/resource/operational-guidelines-use-gentamicin-anms-management-sepsis-young-infants-specific-situations/ [Google Scholar]
- 93.Government of India. Ministry of Health and Family Welfare (MOHFW). Kangaroo Mother Care & Optimal Feeding of Low Birth Weight Infants. MOHFW; 2014. Accessed June 22, 2023. https://www.nhm.gov.in/images/pdf/programmes/child-health/guidelines/Operational_Guidelines-KMC_&_Optimal_feeding_of_Low_Birth_Weight_Infants.pdf [Google Scholar]
- 94.Government of India. Ministry of Health and Family Welfare (MOHFW). Strengthening Facility Based Paediatric Care: Operating Guidelines for Planning & Implementation in District Hospitals. MOHFW; 2015. Accessed June 22, 2023. https://nhm.gov.in/images/pdf/programmes/child-health/guidelines/Strenghtening_Facility_Based_Paediatric_Care-Operational_Guidelines.pdf [Google Scholar]
- 95.First International Human Milk Banking Conference; Pune, India; April 10–11, 2015. Accessed June 22, 2023. https://medical.dpu.edu.in/Humanmilkbank/Documents/1st-International-Human-Milk-Banking-Conference.pdf
- 96.Government of India. Ministry of Health & Family Welfare (MOHFW). National Health Mission. Quarterly NHM MIS Report. MOHFW; 2015. Accessed June 22, 2023. http://nhm.gov.in/index4.php?lang=1&level=0&linkid=457&lid=686 [Google Scholar]
- 97.MAA (Mothers’ Absolute Affection) Programme for Promotion of Breastfeeding and IYCF. National Health Portal. Accessed June 22, 2023. https://nhm.gov.in/index1.php?lang=1&level=4&sublinkid=1413&lid=330
- 98.Government of India. Ministry of Health and Family Welfare (MOHFW). National Health Mission. Operational Guidelines for Establishing Sentinel Stillbirth Surveillance System. MOHFW; 2016. Accessed June 22, 2023. https://www.nhm.gov.in/images/pdf/programmes/RBSK/Operational_Guidelines/Operational_Guidelines_for_establishing_Sentinel_Stillbirth_Surveillance_System.pdf [Google Scholar]
- 99.Government of India. Ministry of Health and Family Welfare (MOHFW). Operational Guidelines for Establishing Hospital Based Birth Defect Sentinel Surveillance System. MOHFW; 2016. Accessed June 22, 2023. http://www.nhm.gov.in/images/pdf/programmes/RBSK/Operational_Guidelines/Operational_Guidelines_Hospital_based_Birth_Defect_Sentinel_Surveillance_System.pdf [Google Scholar]
- 100.Government of India. Ministry of Health and Family Welfare (MOHFW). Guidelines for Standardization of Labor Rooms at Delivery Points. MOHFW; 2016. Accessed June 22, 2023. https://nhm.gov.in/images/pdf/programmes/maternal-health/guidelines/Labor_Room%20Guideline.pdf [Google Scholar]
- 101.Chellani H, Mittal P, Arya S. Mother-neonatal intensive care unit (M-NICU): a novel concept in newborn care. Indian Pediatr. 2018;55(12):1035–1036. Accessed June 22, 2023. https://www.indianpediatrics.net/dec2018/dec-1035-1036.htm [PubMed] [Google Scholar]
- 102.Government of India. Ministry of Health and Family Welfare (MOHFW), National Health Mission. LaQshya: Labour Room Quality Improvement Initiative. MOHFW; 2017. Accessed June 22, 2023. https://nhm.gov.in/New_Updates_2018/NHM_Components/RMNCH_MH_Guidelines/LaQshya-Guidelines.pdf [Google Scholar]
- 103.Government of India. Ministry of Health and Family Welfare (MOHFW); Rashtriya Bal Swasthya Karyakram. Guidelines for Universal Eye Screening in Newborns Including Retinopathy of Prematurity. MOHFW; 2017. Accessed June 22, 2023. https://www.nhm.gov.in/images/pdf/programmes/RBSK/Resource_Documents/Revised_ROP_Guidelines-Web_Optimized.pdf [Google Scholar]
- 104.Government of India. Ministry of Health and Family Welfare (MOHFW). Family Participatory Care for Improving Newborn Health. MOHFW; 2017. Accessed June 22, 2023. http://nhm.gov.in/images/pdf/programmes/child-health/guidelines/Family_Participatory_Care_for_Improving_Newborn_Health-Operational_guideline.pdf [Google Scholar]
- 105.Government of India. Ministry of Health and Family Welfare (MOHFW). National Guidelines on Lactation Management Centres in Public Health Facilities. MOHFW; 2017. Accessed June 22, 2023. https://nhm.gov.in/images/pdf/programmes/IYCF/National_Guidelines_Lactation_Management_Centres.pdf [Google Scholar]
- 106.Government of India. Ministry of Health and Family Welfare (MOHFW), Ministry of Women and Child Development. Home Based Care for Young Child (HBYC): Strengthening of Health & Nutrition Through Home Visits. Operational Guidelines. MOHFW; 2018. Accessed June 22, 2023. http://www.nhm.gov.in/images/pdf/programmes/RBSK/Operational_Guidelines/HBYC_Guidelines.pdf [Google Scholar]
- 107.Poshan Abhiyaan. Government of India. Accessed June 22, 2023. https://www.mygov.in/campaigns/poshanabhiyaan2021/
- 108.Naib P, Gautam K, Mishra G, Goel H. Utilization of Neonatal Care Packages under PMJAY- Findings from Preliminary Analysis. Working Paper No. 2. National Health Authority; 2019. Accessed July 17, 2023. https://web.archive.org/web/20210917195152/https://pmjay.gov.in/sites/default/files/2021-05/Working%20paper%20002-%20Utilization%20of%20Neo-natal%20Care%20Packages.pdf [Google Scholar]
- 109.e-Integrated Management of Neonatal and Childhood Illnesses Mobile Application (IMNCI). Government of India. Accessed June 22, 2023. https://innovate.mygov.in/innovation/e-imnci/
- 110.Social Awareness and Actions to Neutralize Pneumonia Successfully (SAANS). National Health Mission. Accessed June 22, 2023. https://nhm.gov.in/index4.php?lang=1&level=0&linkid=502&lid=789#:∼:text=SAANS%20Campaign%20is%20rolled%20out,behaviours%20among%20parents%20and%20caregivers
- 111.National Health Mission. Surakshit Matritva Aashwasan (Suman): Standard Operational Guidelines. National Health Mission; 2019. Accessed June 22, 2023. https://nhm.gov.in/New_Updates_2018/NHM_Components/RMNCHA/MH/Guidelines/SUMAN%20Guideline%202020%20Web%20Version.pdf [Google Scholar]
- 112.Government of India. Ministry of Health and Family Welfare (MOHFW). Annual Report 2020–21. MOHFW; 2021. Accessed June 22, 2023. https://main.mohfw.gov.in/sites/default/files/Annual%20Report%202020-21%20English.pdf [Google Scholar]
- 113.Kumar H, Khanna R, Alwadhi V, et al. Catalytic support for improving clinical care in special newborn care units (SNCU) through composite SNCU quality of care index (SQCI). Indian Pediatr. 2021;58:338–344. Accessed June 22, 2023. https://indianpediatrics.net/apr2021/apr-338-344.htm [PubMed] [Google Scholar]
- 114.Liverpool School of Tropical Medicine (LSTM). Evaluation of UNICEF’s Support to the Facility Based Newborn Care (FBNC) Programme in India. LSTM; 2019. Accessed June 22, 2023. https://gdc.unicef.org/media/5116/download [Google Scholar]
- 115.Government of India. Ministry of Health and Family Welfare (MOHFW). Navjaat Shishu Suraksha Karyakaram 2020: Resuscitation and Essential Newborn Care Resource Manual. MOHFW; 2020. Accessed June 22, 2023. https://nhm.gov.in/images/pdf/programmes/child-health/guidelines/NSSK/NSSK-Resource-Manual.pdf [Google Scholar]
- 116.Save the Children. Invisible Lives: Save the Children’s Dr. Joy Lawn on Saving Newborn Lives. Save the Children; 2010. Accessed June 22, 2023. https://www.youtube.com/watch?v=oWebc7NXvMk&t=2s [Google Scholar]
- 117.Save the Children. Annex A. Ekwendeni Agogo Training Manual. Save the Children; 2007. Accessed June 22, 2023. https://www.healthynewbornnetwork.org/hnn-content/uploads/Ekwendeni-Agogo-Approach_Training-Manual_Annex-A.pdf [Google Scholar]
- 118.Save the Children. The Ekwendeni Agogo Approach: Grandparents as Agents of Change for Newborn Survival. Save the Children; 2010. Accessed June 22, 2023. https://www.healthynewbornnetwork.org/resource/the-ekwendeni-agogo-approach-grandparents-as-agents-of-change-for-newborn-survival/ [Google Scholar]
- 119.The Health Foundation. Improving Maternal and Newborn Health in Malawi. The Health Foundation; 2013. Accessed June 22, 2023. https://www.health.org.uk/publications/improving-maternal-and-newborn-health-in-malawi [Google Scholar]
- 120.March of Dimes; Partnership for Maternal, Newborn, & Child Health; Save the Children; World Health Organization (WHO). Born Too Soon: The Global Action Report on Preterm Birth. WHO; 2012. Accessed June 22, 2023. https://www.who.int/publications/i/item/9789241503433 [Google Scholar]
- 121.Bergh AM, Banda L, Lipato T, Ngwira G, Luhanga R, Ligowe R. Evaluation of Kangaroo Mother Care Services in Malawi. Maternal and Child Health Integrated Program; 2012. Accessed June 22, 2023. https://www.mchip.net/sites/default/files/Malawi%20KMC%20Report.PDF [Google Scholar]
- 122.Save the Children. Born Too Soon: Kangaroo Mother Care Saves Preterm Babies. Save the Children; 2012. Accessed June 22, 2023. https://vimeo.com/41435895 [Google Scholar]
- 123.Republic of Malawi. Ministry of Health (MOH). Road Map for Accelerating the Reduction of Maternal and Neonatal Morbidity and Mortality in Malawi. MOH; 2012. Accessed June 22, 2023. https://www.healthynewbornnetwork.org/hnn-content/uploads/Malawi-Roadmap-for-Reducing-MN-mortality-2012.pdf [Google Scholar]
- 124.Bar-Zeev N, Gladstone M, Kungwimba E, Ngwira B, Dube Q, Bar-Zeev S. Situational Analysis of Newborn Health in Malawi 2013. Reproductive Health Unit Malawi Ministry of Health/Save the Children/SSDI/UNICEF/WHO; 2013. Accessed July 13, 2023. https://www.scribd.com/document/300019739/Malawi-s-Newborns-Report-2013 [Google Scholar]
- 125.International Neonatal Nursing Excellence Award – Netsayi Gowero. Healthy Newborn Network. September 6, 2013. Accessed June 22, 2023. https://www.healthynewbornnetwork.org/resource/international-neonatal-nursing-excellence-award-netsayi-gowero/
- 126.Asibon A, Lufesi N, Choudhury A, et al. Using a peer mentorship approach improved the use of neonatal continuous positive airway pressure and related outcomes in Malawi. Acta Paediatr. 2020;109(4):705–710. 10.1111/apa.15025. [DOI] [PubMed] [Google Scholar]
- 127.Kawaza K, Machen HE, Brown J, et al. Efficacy of a low-cost bubble CPAP system in treatment of respiratory distress in a neonatal ward in Malawi. Malawi Med J. 2016;28(3):131–137. [PMC free article] [PubMed] [Google Scholar]
- 128.Chavula K, Likomwa D, Valsangkar B, et al. Readiness of hospitals to provide Kangaroo Mother Care (KMC) and documentation of KMC service delivery: analysis of Malawi 2014 Emergency Obstetric and Newborn Care (EmONC) survey data. J Glob Health. 2017;7(2):020802. 10.7189/jogh.07.020802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.International Confederation of Midwives. 10,000 Happy Birthdays: Partnerships. Laerdal Global Health; 2014. Accessed June 22, 2023. https://www.youtube.com/watch?v=sjpkrXONQnM [Google Scholar]
- 130.Zimba E, Kinney MV, Kachale F, et al. Newborn survival in Malawi: a decade of change and future implications. Health Policy Plan. 2012;27(Suppl 3):iii88–iii103. 10.1093/heapol/czs043. [DOI] [PubMed] [Google Scholar]
- 131.William Marsh Rice University. Low-Cost Respiratory Support: Reducing Early Neonatal Death in Rural Malawi. Saving Lives at Birth; 2019. Accessed June 22, 2023. https://savinglivesatbirth.net/low-cost-respiratory-support-reducing-early-neonatal-death-in-rural-malawi/ [Google Scholar]
- 132.Oden M. Improving care for babies in low-resource settings through essential newborn health technologies. Healthy Newborn Network. October 3, 2014. Accessed June 22, 2023. https://www.healthynewbornnetwork.org/blog/improving-care-for-babies-in-low-resource-settings-through-essential-newborn-health-technologies/ [Google Scholar]
- 133.Republic of Malawi. Ministry of Health (MOH). Every Newborn Action Plan: An Action Plan to End Preventable Neonatal Deaths in Malawi. MOH; 2013. Accessed June 22, 2023. https://www.healthynewbornnetwork.org/hnn-content/uploads/Malawi-Newborn-Action-Plan-Feb-2014.pdf [Google Scholar]
- 134.Dube Q. Strengthening and sustaining KMC effective coverage – lessons from Malawi. Presented at: Saving Newborn Lives Legacy E-Talks, October 13, 2020; virtual. Accessed June 22, 2023. https://www.healthynewbornnetwork.org/hnn-content/uploads/Q.Dube-KMC-scale-up-SNL-Legacy-series.pdf
- 135.Smith H, Asfaw AG, Aung KM, et al. Implementing the WHO integrated tool to assess quality of care for mothers, newborns and children: results and lessons learnt from five districts in Malawi. BMC Pregnancy Childbirth. 2017;17:271. 10.1186/s12884-017-1461-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Integrating a neonatal healthcare package for Malawi (IMCHA). International Development Research Centre. Accessed June 22, 2023. https://www.idrc.ca/en/project/integrating-neonatal-healthcare-package-malawi-imcha
- 137.Hundalani SG, Richards-Kortum R, Oden M, Kawaza K, Gest A, Molyneux E. Development and validation of a simple algorithm for initiation of CPAP in neonates with respiratory distress in Malawi. Arch Dis Child Fetal Neonatal Ed. 2015;100(4):F332–F336. 10.1136/archdischild-2014-308082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Crehan C, Colbourn T, Heys M, Molyneux E. Evaluation of ‘TRY’: an algorithm for neonatal continuous positive airways pressure in low-income settings. Arch Dis Child. 2018;103(8):732–738. 10.1136/archdischild-2017-313867. [DOI] [PubMed] [Google Scholar]
- 139.Save the Children Malawi. Development of a National Routine Reporting System for Kangaroo Mother Care (KMC) Services in Malawian Health Facilities. Save the Children, 2015. Accessed June 22, 2023. https://www.healthynewbornnetwork.org/hnn-content/uploads/KMC-Register-Brief-and-Forms-Final-2015.10.09-web.pdf [Google Scholar]
- 140.Republic of Malawi. Ministry of Health (MOH). Care of the Infant and Newborn in Malawi—The COIN Course (Participant’s Manual). MOH; 2016. Accessed June 22, 2023. https://research-repository.st-andrews.ac.uk/bitstream/handle/10023/12839/COIN.2017_participants_manual.pdf [Google Scholar]
- 141.Banda G, Guenther T, Chavula K,et al. Khanda Ndi Mphatso: applying social and behaviour change communication to newborn health in Malawi. J Dev Comm. 2018;29(1):111–136. Accessed June 22, 2023. https://resourcecentre.savethechildren.net/document/khanda-ndi-mphatso-applying-social-and-behavior-change-communication-newborn-health-malawi [Google Scholar]
- 142.Save the Children. ‘Khanda Ndi Mphatso’: Sustaining and Expanding Activities to Shift Social Norms and Care Practices for Preterm and Low Birthweight Babies. Save the Children Malawi; 2020. Accessed June 22, 2023. https://www.healthynewbornnetwork.org/hnn-content/uploads/SBCC-Brief_Web.pdf [Google Scholar]
- 143.Chavula K, Guenther T, Valsangkar B, et al. Improving skin-to-skin practice for babies in Kangaroo Mother Care in Malawi through the use of a customized baby wrap: a randomized control trial. PLoS One. 2020;15(3):e0229720. 10.1371/journal.pone.0229720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Carns J, Kawaza K, Liaghati-Mobarhan S, et al. Neonatal CPAP for respiratory distress across Malawi and mortality. Pediatrics. 2019;144(4):e20190668. 10.1542/peds.2019-0668. [DOI] [PubMed] [Google Scholar]
- 145.Kawaza K, Kinshella MW, Hiwa T, et al. Assessing quality of newborn care at district facilities in Malawi. BMC Health Serv Res. 2020;20(1):227. 10.1186/s12913-020-5065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Balakasi E. Government launches quality management policy for health. SMASH—Social and Mass Media Hub. 2018. [Google Scholar]
- 147.Quality of Care Network. Quality, equity, dignity: a network for improving quality of care for maternal, newborn, and child health. Malawi. Poster presented at Network for Improving Quality of Care for Maternal, Newborn and Child Health; December 2017. Accessed June 22, 2023. https://www.qualityofcarenetwork.org/sites/default/files/2019-07/2250%20MCA%2010%20poster-Malawi%20press%20ready%20141217_0.pdf
- 148.Save the Children Malawi. Kangaroo Mother Care in Malawi. Save the Children; 2017. Accessed June 22, 2023. https://cdn1.sph.harvard.edu/wp-content/uploads/sites/2413/2017/11/Malawi-KAP-Summary-Sheet.pdf [Google Scholar]
- 149.Quality of Care in Malawi. Quality of Care Network. Accessed June 22, 2023. https://www.qualityofcarenetwork.org/country-data/malawi
- 150.Carns J, Kawaza K, Liaghati-Mobarhan S, et al. Neonatal CPAP for respiratory distress across Malawi and mortality. Pediatrics. 2019;144(4):e20190668. 10.1542/peds.2019-0668. [DOI] [PubMed] [Google Scholar]
- 151.Torres M. Report From Kangaroo Mother Care Acceleration Partnership Community of Practice Meeting, October 24–26, 2017; Blantyre, Malawi.
- 152.Nyondo-Mipando AL, Woo Kinshella ML, Bohne C, et al. Barriers and enablers of implementing bubble continuous positive airway pressure (CPAP): perspectives of health professionals in Malawi. PLoS One. 2020;15(2):e0228915. 10.1371/journal.pone.0228915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cairns J, Kawaza K, Quinn MK, et al. Impact of hypothermia on implementation of CPAP for neonatal respiratory distress syndrome in a low-resource setting. PLoS One. 2018;13(3):e0194144. 10.1371/journal.pone.0194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Republic of Malawi. Ministry of Health (MOH). Perinatal Death Surveillance & Response: Malawi National Guidelines. MOH: 2019. [Google Scholar]
- 155.Mhango P, Chipeta E, Muula AS, et al. Implementing the family-led care model for preterm and low birth weight newborns in Malawi: experience of healthcare workers. Afr J Prim Health Care Fam Med. 2020;12(1):1–11. 10.4102/phcfm.v12i1.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.World Health Organization (WHO). Standards of Care to Improve Maternal and Newborn Quality of Care in Facilities. WHO; 2016. Accessed June 22, 2023. https://www.who.int/docs/default-source/mca-documents/advisory-groups/quality-of-care/standards-for-improving-quality-of-maternal-and-newborn-care-in-health-facilities.pdf [Google Scholar]
- 157.NEST360. Accessed June 22, 2023. https://nest360.org/
- 158.Kinshella MLW, Salimu S, Chiwaya B, et al. ‘So sometimes, it looks like it’s a neglected ward’: health worker perspectives on implementing kangaroo mother care in southern Malawi. PLoS One. 2020;15(12):e0243770. 10.1371/journal.pone.0243770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nyondo-Mipando LA, Kinshella MLW, Salimu S, et al. “It brought hope and peace in my heart:” caregivers perceptions on kangaroo mother care services in Malawi. BMC Pediatr. 2020;20(1):541. 10.1186/s12887-020-02443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kinshella MLW, Salimu S, Hiwa T, et al. Scaling up newborn care technologies from tertiary- to secondary-level hospitals in Malawi: an implementation case study of health professional perspectives on bubble CPAP. Implement Sci Commun. 2020;1:100. 10.1186/s43058-020-00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Respectful maternity care charter. White Ribbon Alliance. Accessed June 22, 2023. https://whiteribbonalliance.org/resources/rmc-charter
- 162.Hacker HP, Ateva E, Jolivet RR, Al-makaleh B, Shaver T, Sacks E. Global research priorities for understanding and improving respectful care for newborns: a modified Delphi study. Glob Health Sci Pract. 2022;10(1):e2100292. 10.9745/ghsp-d-21-00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.WHO Immediate KMC Study Group. Immediate “kangaroo mother care” and survival of infants with low birth weight. N Engl J Med. 2021;384:2028–2038. 10.1056/nejmoa2026486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kayinamura AM, Serubibi Y, Kakoma JB. Benefits of a neonatology service in a rural district hospital: case study of Rwamagana District hospital, Eastern Province of Rwanda. Rwanda Medical J. 2010;68(4):38–40. Accessed June 22, 2023. http://www.bioline.org.br/pdf?rw10008 [Google Scholar]
- 165.Drobac PC, Basinga P, Condo J, et al. Comprehensive and integrated district health systems strengthening: the Rwanda Population Health Implementation and Training (PHIT) Partnership. BMC Health Serv Res. 2013;13(Suppl 2):S5. 10.1186/1472-6963-13-s2-s5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Beck L. Newborn babies' health in Rwanda: evolution of factors associated with neonatal mortality trends. Article in French. Santé Publique. 2009;2(2):159–172. 10.3917/spub.092.0159. [DOI] [PubMed] [Google Scholar]
- 167.National Institute of Statistics of Rwanda (NISR); Republic of Rwanda. Ministry of Health (MOH); ICF International. Rwanda Demographic and Health Survey 2010. NISR/MOH/ICF International; 2012. Accessed June 22, 2023. https://dhsprogram.com/pubs/pdf/FR259/FR259.pdf [Google Scholar]
- 168.Institute for Health Metrics and Evaluation (IHME). GBD Profile: Rwanda. IHME; 2010. Accessed July 17, 2023. https://web.archive.org/web/20181222164016/http://www.healthdata.org/sites/default/files/files/country_profiles/GBD/ihme_gbd_country_report_rwanda.pdf [Google Scholar]
- 169.Kayinamura AM. Introduction and expansion of KMC program. Presented at: International Conference on Kangaroo Mother Care; November 17–19; 2014; Kigali, Rwanda.
- 170.U.S. Agency for International Development (USAID); Maternal and Child Health Integrated Program (MCHIP); Save the Children. Summary of Rwanda Country Experience – Implementing PNC Home Visits. USAID/MCHIP/Save the Children; 2014. Accessed June 22, 2023. https://www.healthynewbornnetwork.org/hnn-content/uploads/Country-Report-PNC-Home-Visits-Rwanda.pdf [Google Scholar]
- 171.Tuyisenge G, Hategeka C, Luginaah I, Cechetto DF, Rulisa S. ‘I cannot say no when a pregnant woman needs my support to get to the health centre’: involvement of community health workers in Rwanda’s maternal health. BMC Health Serv Res. 2020;20(1):524. 10.1186/s12913-020-05405-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tuyisenge G, Crooks VA, Berry NS. Facilitating equitable community-level access to maternal health services: exploring the experiences of Rwanda’s community health workers. Int J Equity Health. 2019;18(1):181. 10.1186/s12939-019-1065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tuyisenge G, Hategeka C, Kasine Y, Luginaah I, Cechetto D, Rulisa S. Mothers’ perceptions and experiences of using maternal health-care services in Rwanda. Women Health. 2019;59(1):68–84. 10.1080/03630242.2018.1434591. [DOI] [PubMed] [Google Scholar]
- 174.Tuyisenge G, Hategeka C, Luginaah I, Babenko-Mould Y, Cechetto D, Rulisa S. Continuing professional development in maternal health care: barriers to applying new knowledge and skills in the Hospitals of Rwanda. Matern Child Health J. 2018;22(8):1200–1207. 10.1007/s10995-018-2505-2. [DOI] [PubMed] [Google Scholar]
- 175.Save the Children. Kangaroo Mother Care in Rwanda. Save the Children; 2017. Accessed June 22, 2023. https://cdn1.sph.harvard.edu/wp-content/uploads/sites/2413/2017/11/Rwanda-KAP-Summary-Sheet.pdf [Google Scholar]
- 176.Ngabo F, Nguimfack J, Nwaigwe F, et al. Designing and implementing an innovative SMS-based alert system (RapidSMS-MCH) to monitor pregnancy and reduce maternal and child deaths in Rwanda. Pan Afr Med J. 2012;13:31. [PMC free article] [PubMed] [Google Scholar]
- 177.Maternal and Child Survival Program (MCSP). Scaling Up Practice Improvement for Labor Management and Immediate Newborn Care in Rwanda. MCSP; 2018. Accessed June 22, 2023. https://www.mcsprogram.org/resource/scaling-up-practice-improvement-for-labor-management-and-immediate-newborn-care-in-rwanda/ [Google Scholar]
- 178.A promise renewed: the call to action. Every Woman Every Child. Accessed June 22, 2023. https://www.everywomaneverychild.org/commitment/a-promise-renewed/
- 179.Bergh AM, Sayinzoga F, Mukarugwiro B, et al. Evaluation of Kangaroo Mother Care Services in Rwanda. Rwanda Ministry of Health; 2012. Accessed June 22, 2023. https://mchip.net/sites/default/files/Rwanda%20KMC%20Evaluation%20Report_0.pdf [Google Scholar]
- 180.Binagwaho A, Kyamanywa P, Farmer PE, et al. The human resources for health program in Rwanda-new partnership. N Engl J Med. 2013;369(21):2054–2059. 10.1056/nejmsr1302176. [DOI] [PubMed] [Google Scholar]
- 181.Ntigurirwa P, Mellor K, Langer D, et al. A health partnership to reduce neonatal mortality in four hospitals in Rwanda. Global Health. 2017;13(1):28. 10.1186/s12992-017-0252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hansen A, Magge H, Labrecque M, et al. The development and implementation of a newborn medicine program in resource-limited setting. Public Health Action. 2015;5(1):17–22. 10.5588/pha.14.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nyishime M, Borg R, Ingabire W, et al. A retrospective study of neonatal case management and outcomes in rural Rwanda post implementation of a national neonatal care package for sick and small infants. BMC Pediatr. 2018;18:353. 10.1186/s12887-018-1334-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Republic of Rwanda. Ministry of Health (MOH). Neonatology Clinical Treatment Guidelines. MOH; 2012. Accessed July 17, 2023. https://docplayer.net/17476998-Neonatology-clinical-treatment-guidelines-republic-of-rwanda-ministry-of-health-september-2012-p-o-box-84-kigali-www-moh-gov.html [Google Scholar]
- 185.Khurmi MS, Sayinzoga F, Berhe A, et al. Newborn survival case study in Rwanda - bottleneck analysis and projections in key maternal and child mortality rates using lives saved tool (LiST). Int J MCH AIDS. 2017;6(2):93–108. 10.21106/ijma.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Technical report on bottlenecks analysis for newborn care. Presented at: Mostej Hotel, November 13–15, 2013; Gisenyi, Rubavu, Rwanda.
- 187.Wilmot E, Yotebieng M, Norris A, Ngabo F. Missed opportunities in neonatal deaths in Rwanda: applying the three delays model in a cross-sectional analysis of neonatal death. Matern Child Health J. 2017;21(5):1121–1129. 10.1007/s10995-016-2210-y. [DOI] [PubMed] [Google Scholar]
- 188.Musafili A, Persson LÅ, Baribwira C, Påfs J, Mulindwa PA, Essén B. Case review of perinatal deaths at hospitals in Kigali, Rwanda: perinatal audit with application of a three-delays analysis. BMC Pregnancy Childbirth. 2017;17(1):85. 10.1186/s12884-017-1269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hategeka C, Shoveller J, Tuyisenge L, Lynd LD. Assessing process of paediatric care in a resource-limited setting: a cross-sectional audit of district hospitals in Rwanda. Paediatr Int Child Health. 2018;38(2):137–145. 10.1080/20469047.2017.1303017. [DOI] [PubMed] [Google Scholar]
- 190.Republic of Rwanda. Ministry of Health (MOH); Rwanda Biomedical Centre (RBC). Maternal, Newborn, and Child Health Strategic Plan 2013–2018. MOH; 2012. Accessed June 22, 2023. https://rbc.gov.rw/fileadmin/user_upload/maternal_newborn_and_child_health_national_strategy_july_2013-_june_2018.pdf [Google Scholar]
- 191.Magge H, Chilengi R, Jackson EF, et al. Tackling the hard problems: implementation experience and lessons learned in newborn health from the African Health Initiative. BMC Health Serv Res. 2017;17(Suppl 3):829. 10.1186/s12913-017-2659-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Werdenberg J, Biziyaremye F, Nyishime M, et al. Successful implementation of a combined learning collaborative and mentoring intervention to improve neonatal quality of care in rural Rwanda. BMC Health Serv Res. 2018;18:941. 10.1186/s12913-018-3752-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Binagwaho A, Scott KW, Dushime T, et al. Creating a pathway for public hospital accreditation in Rwanda: progress, challenges and lessons learned. Int J Qual Health Care. 2020;32(1):76–79. 10.1093/intqhc/mzz063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Republic of Rwanda. Ministry of Health (MOH). National Neonatal Care Protocol. 2nd ed. MOH; 2014. [Google Scholar]
- 195.Kayinamura M. Early outcomes of preterm babies hospitalized in kangaroo mother care units in Rwanda. Presented at: 10th International Conference on Kangaroo Mother Care; November 17–19, 2014; Kigali, Rwanda. Accessed June 22, 2023. https://fundacioncanguro.co/wp-content/uploads/2017/10/KMC-DAY213.-Early-outcomes-PPT-Assumpta-Mwali-Rwanda.pdf
- 196.Paradis J. The 10th International Conference on Kangaroo Mother Care: hope for all babies. HNN Blog. November 17, 2014. Accessed July 17, 2023. http://www.healthynewbornnetwork.org/blog/the-10th-international-conference-on-kangaroo-mother-care-hope-for-all-babies/
- 197.National Institute of Statistics of Rwanda (NISR); Republic of Rwanda. Ministry of Health (MOH); ICF International. Rwanda Demographic and Health Survey 2014–15. NISR/MOH/ICF International; 2015. Accessed June 22, 2023. http://www.statistics.gov.rw/publication/demographic-and-health-survey-20142015-final-report [Google Scholar]
- 198.Magge H. The all babies count initiative . Presented at: Global Maternal Newborn Health Conference 2015; October 21, 2015; Mexico City, Mexico.
- 199.Rwanda Governance Board (RGB). Rwanda Community Health Workers Programme: 1995–2015. 20 Years of Building Healthier Communities. RGB; 2017. Accessed June 22, 2023. https://www.rgb.rw/fileadmin/user_upload/RGB/Publications/HOME_GROWN_SOLUTIONS/Rwanda_Community_Healt_Workers_Programme_2017.pdf [Google Scholar]
- 200.Condo J, Mugeni C, Naughton B, et al. Rwanda’s evolving community health worker system: a qualitative assessment of client and provider perspectives. Hum Resour Health. 2014;12:71. 10.1186/1478-4491-12-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.FUAPI-project: Follow-up Assessment of Preterm Infants in the District of Huye, Rwanda. 2015.
- 202.Republic of Rwanda. Ministry of Health (MOH). Maternal and Child Health Division. National Postnatal Care Guideline for Mother and Newborn. MOH; 2016. [Google Scholar]
- 203.Rwanda Pediatric Association; Royal College of Paediatrics and Child Health. Neonatal Care Capacity-Building and Mentoring at District Hospitals in Rwanda. Rwanda Pediatric Association/Royal College of Paediatrics and Child Health; 2016. [Google Scholar]
- 204.Meeting Summary KMC Acceleration Partnership Community of Practice 1st Africa Regional Meeting, December 15-17, 2016. Kigali, Rwanda.
- 205.Tayebwa E, Sayinzoga F, Umunyana J, et al. Assessing implementation of maternal and perinatal death surveillance and response in Rwanda. Int J Environ Res Public Health. 2020; 17(12):4376. 10.3390/ijerph17124376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ngabonzima A, Kenyon C, Hategeka C, et al. Developing and implementing a novel mentorship model (4+ 1) for maternal, newborn and child health in Rwanda. BMC Health Serv Res. 2020;20(1):924. 10.1186/s12913-020-05789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Nahimana E, May L, Gadgil A, et al. A low cost, re-usable, electricity-free infant warmer: evaluation of safety, effectiveness and feasibility. Public Health Action. 2018;8(4):211–217. 10.5588/pha.18.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.May L, Nshimyiryo A, Kubwimana M, et al. Performance of a nonelectric infant warmer in Rwandan health centers. Glob Pediatr Health. 2019;6:1–10. 10.1177/2333794x19884820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Rwanda Paediatric Associations held their 3rd scientific conference. Press release. Rwanda Ministry of Health. September 4, 2018. Accessed June 22, 2023. https://moh.prod.risa.rw/news-detail/rwanda-paediatric-associations-held-their-3rd-scientific-conference
- 210.Maternal and Child Survival Program (MCSP). Situation Analysis of Inpatient Care of Newborns and Young Infants: Rwanda. MCSP; 2019. Accessed June 22, 2023. https://www.mcsprogram.org/resource/situation-analysis-of-inpatient-care-of-newborns-and-young-infants-rwanda/ [Google Scholar]
- 211.Council of International Neonatal Nurses; Global Engagement Institute. Africa Conference on Neonatal Nursing. University of Rwanda, Kigali, Rwanda; October 5–6, 2018.
- 212.Every Preemie – SCALE. Situational Analysis of Inpatient Care of Newborns and Young Infants: Highlights of Findings for Ghana, Rwanda, Tanzania, and Uganda. Every Preemie – SCALE; 2019. Accessed June 22, 2023. https://www.everypreemie.org/wp-content/uploads/2019/10/Multicountry_Analysis_10.4.2019.pdf [Google Scholar]
- 213.Republic of Rwanda. Ministry of Health (MOH). National Neonatal Care Protocol. 3rd ed. MOH; 2019. Accessed July 17, 2023. https://path.ent.box.com/s/w1zwpeh4ahealcvgpuythz9kh6by4q84 [Google Scholar]
- 214.Uwamariya J, Maimpaka C, May L, et al. Safety and effectiveness of a non-electric infant warmer for hypothermia in Rwanda: a cluster-randomized stepped-wedge trial. EClinicalMedicine. 2021;34:1–9. 10.1016/j.eclinm.2021.100842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ngabonzima A, Kenyon C, Hategeka C, et al. Developing and implementing a novel mentorship model (4+ 1) for maternal, newborn and child health in Rwanda. BMC Health Serv Res. 2020. Oct 7;20(1):924. 10.1186/s12913-020-05789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Abt Associates. Ethiopia’s Community-Based Health Insurance: A Step on the Road to Universal Health Coverage. Abt Associates; 2015. Accessed June 22, 2023. https://pdf.usaid.gov/pdf_docs/PA00KDXT.pdf [Google Scholar]
- 217.Saans. InnAccel. Accessed June 22, 2023. https://innaccel.com/products/saans
- 218.Jangir N, Deval N, Kumar B, Olson, Kishore V, Shetty S. A multi-powered neonatal CPAP device. Poster presented at: WHO Global Forum on Medical Devices; Visakhapatnam, India; December 13–15, 2018. Accessed June 22, 2023. https://apps.who.int/iris/bitstream/handle/10665/312154/WHO-MVP-EMP-2019.04-eng.pdf
- 219.Embrace Warmer. Phoenix Medical Systems. Accessed June 22, 2023. https://www.phoenixmedicalsystems.com/infant-care/embrace-warmer
- 220.Neobreathe. Phoenix Medical Systems. Accessed June 22, 2023. https://www.phoenixmedicalsystems.com/infant-care/neobreathe
- 221.Nimbalkar SM, Shah BV, Amin AA, et al. Comparing positive pressure ventilation efficacy of a novel foot operated resuscitator with self-inflating bag and mask in a manikin model. BMJ Innov. 2020;6:48–54. 10.1136/bmjinnov-2018-000309 [DOI] [Google Scholar]
- 222.Patterson J, Worku B, Jones D, Clary A, Ramaswamy R, Bose C. Ethiopian Pediatric Society quality improvement initiative: a pragmatic approach to facility-based quality improvement in low-resource settings. BMJ Open Qual. 2021;10(1):e000927. 10.1136/bmjoq-2020-000927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Global health neonatal quality improvement database. Vermont Oxford Network. Accessed June 22, 2023. https://public.vtoxford.org/data-and-reports/global-neonatal-database/
- 224.Abt Associates. Ethiopia’s Community-based Health Insurance: A Step on the Road to Universal Health Coverage. Abt Associates; 2015. Accessed June 22, 2023. https://pdf.usaid.gov/pdf_docs/PA00KDXT.pdf [Google Scholar]
- 225.Sashi Kumar V, Paul VK, Sathasivam K. Innovating affordable neonatal care equipment for use at scale. J Perinatol. 2016;36(Suppl 3):S32–S36. 10.1038/jp.2016.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Gary RA, Rogers M, Demisse L, Hayes R, McCauley L. The Emory-Addis Ababa PhD in Nursing Program: a sustainable model for strengthening nursing research capacity in Ethiopia. J Prof Nurs. 2020;36(6):531–537. 10.1016/j.profnurs.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 227.Ruton H, Musabyimana A, Gaju E, et al. The impact of an mHealth monitoring system on heatlh care utilization by mothers and children: an evaluation nursing routine health information in Rwanda. Health Policy Plan. 2018;33(8):920–927. 10.1093/heapol/czy066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.RapidPro. Accessed June 22, 2023. https://community.rapidpro.io/
- 229.Republic of Malawi. Ministry of Health (MOH). Malawi National Guidelines for Kangaroo Mother Care. MOH; 2005. [Google Scholar]
- 230.Save the Children. Kangaroo Mother Care Training Manual. Save the Children; 2005. Accessed June 22, 2023. https://www.healthynewbornnetwork.org/resource/malawi-kangaroo-mother-care-training-manual/ [Google Scholar]
- 231.Republic of Malawi. Ministry of Health (MOH). Malawi National Guidelines for Kangaroo Mother Care. MOH; 2009. Accessed June 22, 2023. https://www.medbox.org/document/malawi-national-guidelines-for-kangaroo-mother-care#GO [Google Scholar]
- 232.Worku B, Kassie A. Kangaroo mother care: a randomized controlled trial on effectiveness of early kangaroo mother care for the low birthweight infants in Addis Ababa, Ethiopia. J Trop Pediatr. 2005;51(2):93–97. 10.1093/tropej/fmh085. [DOI] [PubMed] [Google Scholar]
- 233.Kassie A, Worku B. Newborn care corner: a simplified approach to providing optimal newborn care immediately after birth. Ethiopian Med J. 2019;(Suppl 3):193–196. https://emjema.org/index.php/EMJ/article/view/1392/550 [Google Scholar]
- 234.NeoLENS case studies. PATH. Accessed June 22, 2023. https://www.path.org/programs/maternal-newborn-child-health-and-nutrition/neolens-case-studies/
- 235.PATH. NeoLENS Project Global Overview: Lessons Learned in Establishing Inpatient Newborn Care. PATH; 2022. Accessed June 22, 2023. https://www.path.org/resources/neolens-project-global-overview-lessons-learned-establishing-inpatient-newborn-care/ [Google Scholar]
- 236.Government of India. Ministry of Health and Family Welfare (MOHFW). INAP: India Newborn Action Plan. MOHFW; 2014. Accessed June 22, 2023. https://nhm.gov.in/images/pdf/programmes/inap-final.pdf [Google Scholar]
- 237.NeoLENS virtual experience. PATH. Accessed June 23, 2023. https://www.path.org/programs/maternal-newborn-child-health-and-nutrition/neolens-virtual-experience/
- 238.Search for reporting guidelines. Equator Network. Accessed June 23, 2023. https://www.equator-network.org/reporting-guidelines/