Abstract
Background:
Nerve transection is the most common form of peripheral nerve injury. Treatment of peripheral nerve injury has primarily focused on stabilization and mechanical cues to guide extension of the regenerating growth cone across the site of transection. The authors investigated the effects of a peripheral nerve matrix (PNM) hydrogel on recovery after nerve transection.
Methods:
The authors used rodent models to determine the effect of PNM on axon extension, electrophysiologic nerve conduction, force generation, and neuromuscular junction formation after nerve transection and repair. The authors complemented this work with in vivo and in vitro fluorescence-activated cell sorting and immunohistochemistry approaches to determine the effects of PNM on critical cell populations early after repair.
Results:
Extension of axons from the proximal stump and overall green fluorescent protein–positive axon volume within the regenerative bridge were increased in the presence of PNM compared with an empty conduit (P < 0.005) 21 days after repair. PNM increased electrophysiologic conduction (compound muscle action potential amplitude) across the repair site (P < 0.05) and neuromuscular junction formation (P = 0.04) 56 days after repair. PNM produced a shift in macrophage phenotype in vitro and in vivo (P < 0.05) and promoted regeneration in a murine model used to characterize the early immune response to PNM (P < 0.05).
Conclusion:
PNM, delivered by subepineural injection, promoted recovery after nerve transection with immediate repair, supporting a beneficial macrophage response, axon extension, and downstream remodeling using a range of clinically relevant outcome measures.
Clinical Relevance Statement:
This article describes an approach for subepineural injection at the site of nerve coaptation to modulate the response to injury and improve outcomes.
Peripheral nerve injuries (PNI) caused by laceration, compression, or stretch traumas, or iatrogenic injuries, such as those caused by tumor resections, have severe and wide-ranging effects on quality of life, productivity, and interpersonal relationships. PNI in the upper extremities can prevent patients from performing basic daily activities; facial nerve injuries can impede vocalization and are associated with social stigma and withdrawal. Despite the large volume of research in peripheral nerve repair and regeneration and advances made in microsurgical techniques, functional outcomes after repair of severe injuries are often disappointing, and full functional recovery is seldom achieved.1,2 For these reasons, clinically translatable solutions that demonstrate improvements in functional outcomes are of great interest and potential in the area of nerve repair.
The majority of work to improve outcomes after PNI has focused on mechanical stabilization and coaptation support for the nerve repair site, such as those provided by wraps3–5 and glues.6–8 Conduits have been frequently used to guide nerve growth and prevent other complications associated with the repair site.9–11 The most commonly used experimental model for PNI is the critical length gap injury, which reflects the most severe, and least recoverable, of nerve injuries. The approaches developed for experimental nerve gap injuries have focused on the addition of nerve-specific growth factors12–17 or cell-specific transfer to the injury site18,19 to attempt to improve axon extension or Schwann cell (SC) migration. Many of these approaches have used mechanical cues to guide the regenerative bridge forming between the transected proximal and distal nerve stumps and diminish the invasion of surrounding fibrous tissue into the growth cone, or the extension of axons outside the regenerating bridge.20–27
We previously described the use of an injectable hydrogel for peripheral nerve repair composed of decellularized nerve tissue, which was formulated into gel upon reaching physiologic pH and temperature. Early studies in a nerve gap model in rats indicated that this peripheral nerve matrix (PNM) hydrogel was an effective scaffold for early SC and macrophage growth in vivo, shifting macrophage phenotype, promoting macrophage and SC migration, and resulting in improvement gait downstream.28 Here, we investigate the ability of PNM to support enhanced functional reinnervation in transection and repair (coaptation) as well as a short gap model in rats and mice. We performed this work because simple transection is the most common type of peripheral nerve injury. An in-depth characterization of the macrophage populations associated with the early host response to PNM also was performed.
METHODS
Experimental Design
A series of experiments was performed in rats and mice to investigate mechanistic and translational aspects associated with the use of PNM in nerve repair. We first used a noncritical nerve gap (8 mm) in a rat model with green fluorescent protein (GFP)–expressing axons (Thy1-GFP) to assess the ability of PNM to support early (21 days) axon extension. The Thy1-GFP rat strain was then used in a transection and primary repair (coaptation) model to assess the ability of PNM to promote improvements in functional recovery (56 days after injury), assessed by electrophysiology, force generation, and neuromuscular junction (NMJ) formation. A short nerve gap (3 mm) in a mouse model was used to investigate the early (5 days) host macrophage response to PNM. Results were assessed by fluorescence-activated cell sorting (FACS) (5 days), gene expression assay (NanoString Technologies, Seattle, WA) (5 days), and immunolabeling (10 days). Retrograde labeling distal to the site of repair was used to assess reinnervation (84 days) in the short (3 mm) nerve gap mouse model.
Ethics Statement
Animal studies were performed in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals, the National Institutes of Health Guide for Care and Use of Laboratory Animals, and federal and state regulations. They were approved by the Cornell University Institutional Animal Care and Use Committee.
Generation of PNM
A xenogenic peripheral nerve–derived PNM was produced from porcine sciatic nerves, which were frozen, decellularized, digested, lyophilized, and reconstituted at 20 mg/mL, as previously described.28
Evaluation of Recovery after Transection and Repair
We evaluated the translational potential of PNM using Sprague-Dawley rats expressing GFP in their axons under the Thy-1 promoter29,30 to determine effects of PNM on axon extension, electrophysiology, NMJ formation, and peak tetanic force. In all experiments, rats were anesthetized with 5% isoflurane and maintained under anesthesia with 1% to 2% isoflurane. Analgesia was provided by Meloxicam (2 mg/kg) preoperatively and 24 hours postoperatively. All surgical procedures were performed by the same surgeon (J.C.).
Determination of Axon Extension
To determine axon extension, a 10-mm silicone conduit was placed between the proximal and distal stumps of the transected sciatic nerve, and each was sutured 1 mm into the conduit with single 10-0 sutures to create an 8-mm defect. The conduits were either filled with PNM or left empty (n = 6 to 8/group). The muscle and skin were reapposed using 6-0 Ethilon sutures. A total of 21 days after repair, the animals were anesthetized and killed (using pentobarbital 250 mg/mL, 4 mL/kg), and the regenerative nerve bridge was harvested. Bridges were placed in a well with phosphate-buffered saline and imaged using confocal microscopy (Zeiss LSM510). Axonal outgrowth from the distal stump was quantified by evaluating GFP signal extending through contiguous regions of interest (ROIs) at 250-µm intervals from the distal end of the proximal stump using BitPlane (Imaris) and calculating GFP+ volumes for each ROI. Differences between groups were determined by evaluating GFP+ volume and the interaction between dose and contiguous ROI in a mixed-effect model.
Determination of Electrophysiologic Conduction, NMJ Formation, and Peak Tetanic Force
Functional recovery was assessed in a transection and primary repair model mimicking a common clinical injury with immediate repair to assess the response to PNM using a range of clinically relevant outcome measures. Investigators were blinded to experimental group for the collection of all outcome measures.
Immediately after repair of the transection with two 10-0 Ethilon sutures, 25 μL of PNM hydrogel was injected using a 30-g needle subepineurally into the distal stump immediately after repair in the PNM group. Animals were followed up for 4 or 8 weeks (n = 8 group/time point), and recovery was assessed using electrophysiology, NMJ formation, and peak tetanic force.
Electrophysiologic function was assessed by measurement of compound motor action potential (CMAP) at the gastrocnemius muscle and tibialis anterior. With the animal under anesthesia and before euthanasia, the sciatic nerve was isolated; two insulated steel electrodes (28 g) were hooked around the sciatic nerve proximal to the injury; and a bipolar concentric recording electrode (Neuroline Concentric 25 × 0.30 mm; Ambu, Inc. Columbia, MD) was inserted into the midbody of the gastrocnemius muscle. Supramaximal stimulation was determined and CMAP recorded in triplicate.
Peak tetanic force was determined using a standardized approach to optimize muscle length and then determine peak tetanic force.31,32 Three recordings were made with a 7-minute rest between observations to allow recovery [Model 305B Dual-Mode Lever Arm System (Aurora Scientific, Inc., Aurora, Ontario, Canada) and LabVIEW].
After euthanasia was performed, NMJ formation was assessed in the extensor digitorum longus (EDL) muscle by determining the number of terminal axons (Thy1-GFP; green) innervating postsynaptic acetylcholine receptors, labeled with rhodamine-ɑ-bungarotoxin conjugated to Alexa Fluor-594 (red), as previously described.30 Four equidistant z-series (stack) were taken across the muscle sample to acquire images of a minimum of 100 motor end plates using a confocal microscope (Zeiss LSM510). Innervation was determined by extension of a GFP-expressing axon to a positively labeled motor end plate. The proportion of innervated NMJ was standardized to the untreated contralateral (fully innervated) limb.
Assessment of the Early Host Response in a Mouse Model
In Vivo Assessment of the Host Response
A short (3 mm) nerve gap C57BL/6J mouse model was used for assessment of the early host response to PNM. Mice were anesthetized with 3% isoflurane and maintained under anesthesia. Analgesia was provided by subcutaneous meloxicam (4 mg/kg) injection preoperatively and for 24 hours after surgery. Proximal and distal nerve stumps of the transected sciatic nerve were then aligned and sutured 1 mm into 5-mm inert silicone conduits with 10–0 suture to create a short gap defect (3 mm) as previously described.33,34 The animals were divided into two groups and the conduit was filled with PNM (15 mg/mL) or left empty (control) (n = 6/group).
Gene Expression of FACS-Sorted Macrophages
Five days after injury, the mice were euthanized, the conduits were harvested, and the tissue bridge was digested to form a single-cell suspension for FACS, as previously described.33,34 Macrophages were sorted as all viable single cells that were F4/80+, CD14+, CD11b+, and CD16/32+. SCs were sorted by exclusion of other expected cell types, as previously described and validated.33,34
Custom panels (NanoString Technologies) specific for genes associated with SC and macrophage lineage, phenotype, and function and previously validated34 were used. Samples were analyzed at the Molecular Biology Core Facility at Geisel School of Medicine at Dartmouth (Hanover, NH). NanoString nSolver 4.0 analysis software was used to process raw data, as previously described.34
Immunolabeling
Immunolabeling was used to quantify the effects of PNM on macrophage phenotype in the early phases of nerve regeneration (n = 5/group, 10 days after injury). At euthanasia, the tissue bridge was mounted and fixed in modified Zamboni solution overnight. Samples were then embedded and sectioned at 4 μm. Antigen retrieval was performed in Tris EDTA buffer (pH 9). Samples were blocked using 10% serum plus 2x casein, followed by application of primary antibodies to either M1 (CD68 and CCR7) or M2 (CD68 and CD206) macrophage markers on consecutive slides. Slides were then then washed with phosphate-buffered saline with Tween 20 for 5 minutes, twice, followed by application of appropriate secondary antibodies. Slides were then washed and coverslipped with mounting media containing DAPI (4′,6-diamidino-2-phenylindole) (Vectashield; Vector Laboratories, Newark, CA). For negative controls, the primary antibodies were replaced with species-specific isotype immunoglobulin G at equivalent concentrations. Two high-power fields were imaged (Aperio Scanscope) and assessed on each edge of the proximal stump to establish the relative proportion of M1 and M2 macrophages. Macrophages were counted using ImageJ.
Retrograde Labeling
Motor neuron regeneration was evaluated 84 days after injury in a third group of mice (n = 6/group) by retrograde labeling to quantify regeneration across a 3-mm gap using tissue clearing (RetroDISCO) and imaging as previously described.35
Statistical Analysis
Treatment groups were concealed from all investigators during tissue harvests, collection of outcome measures, and image and data analysis. Continuous outcome measures were assessed using t test or analysis of variance with Tukey post hoc tests. All data were analyzed using JMP 12 (SAS Institute, Inc., Cary, NC). Significance was set as P < 0.05 throughout. Data were represented graphically using GraphPad Prism 6 (GraphPad software, Inc).
RESULTS
Axon Elongation and Functional Recovery
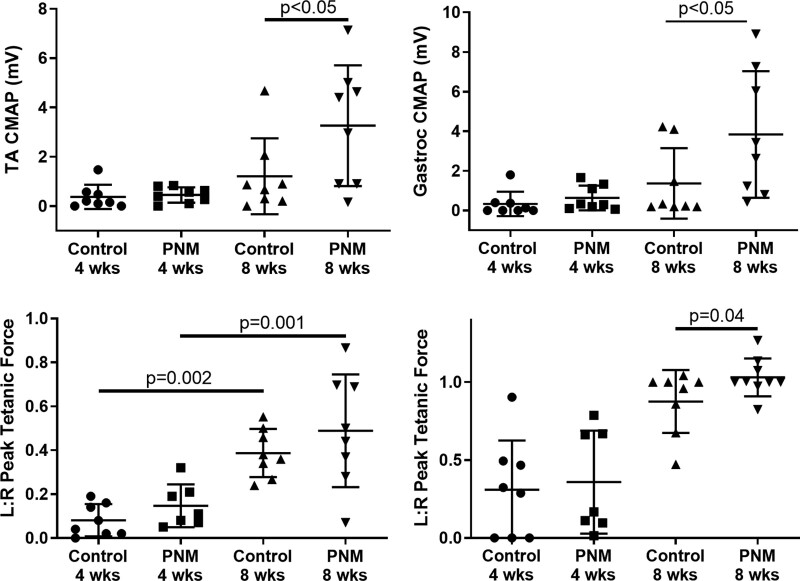
We used an 8-mm noncritical defect within a conduit (Fig. 1) to allow clear quantification of axon extension by confocal microscopy. Extension of axons from the proximal stump and overall GFP+ axon volume within the regenerative bridge were increased in the presence of PNM compared with an empty conduit (P < 0.005; Fig. 1). These GFP+ axon data were obtained 21 days after injury to assess the early response to PNM. We then used a transection and repair (coaptation) model and later time point (56 days) to mimic a common clinical injury with immediate repair and to assess the response to PNM using a range of clinically relevant outcome measures. [See Figure, Supplemental Digital Content 1, which shows augmentation of nerve repair with PNM. Sciatic transection and coaptation with two 9/0 Ethilon sutures in Thy-1 GFP rats is shown (left). A total of 25 µL of 15 mg/mL PNM was injected subepineurally using a 30-g needle or no injection (coaptation only control); n = 8/group (second from left). Excess PNM was removed before muscle (second from right and right). Black bar on retractor is 1 mm, http://links.lww.com/PRS/G52.] We identified increased electrophysiologic conduction, demonstrated by increased CMAP amplitude, across the repair site to both flexor (tibialis anterior) and extensor (gastrocnemius) muscle groups at 56 days after repair (P < 0.05; Fig. 2, above). Normalized peak tetanic force within the gastrocnemius muscle demonstrated expected significant increases in peak tetanic force over time (P < 0.002) but no significant differences between groups (Fig. 2, below, left). Examination of high-powered fields of EDL muscles identified a significant increase in the proportion of innervated NMJ within the EDL muscle in the PNM-treated group (Fig. 2, below, right; P = 0.04). Increased terminal spouting was noted in injured and recovered animals compared with normally innervated contralateral limbs.
Fig. 1.
PNM promotes axon extension across a noncritical gap and extension of GFP+ axons across a noncritical 8-mm sciatic gap defect. Extension of axons from the proximal stump (third row, left and right) and overall GFP+ axon volume within the regenerative bridge (first row and second row) were increased in the presence of PNM compared with an empty conduit (P < 0.005). A noncritical sciatic nerve defect was created in Thy1-GFP rats using a 10-mm inert silicone conduit and nerve stump sutures 1 mm into the conduit to create an 8-mm defect. n = 7/group; *P < 0.05. White line indicates distal end of proximal stump. Scale bar, 250 μm.
Fig. 2.
PNM promotes electrophysiologic recovery and the formation of neuromuscular junction. CMAP after transection and coaptation was increased in both tibialis anterior (TA) (above, left) and gastrocnemius muscle (above, right) groups in the PNM group at 8 weeks compared with coaptation only (P < 0.05). Gastrocnemius peak tetanic force increased over time but was not different between groups (below, left). Proportion of innervated NMJ was increased in the PNM group at 8 weeks (P = 0.04; one-sided t test) (below, right).
Assessment of the Early Host Response in a Mouse Model
We used a short gap model in the mouse to assess the early host response to PNM using a previously validated FACS approach.33,34 Examination of gene expression using NanoString analysis demonstrated decreased expression of genes associated with inflammation and scarring [collagens 1a1 and 1a2 and the receptor for fibroblastic growth factor (Fgfr1)] with increased expression of M2-associated genes in the PNM-treated group compared with the empty conduit controls (Fig. 3, above, left). Little effect on SC gene expression was observed (Fig. 3, above, right). Immunohistochemistry on murine regenerative nerve bridges obtained 10 days after repair confirmed a shift in phenotype with reduced accumulation of CD68+CCR7+ (M1) macrophages in the PNM-treated animals (Fig. 3, center, left; P < 0.01). No differences were observed in the CD68+CD206+ (M2) macrophage population (Fig. 3, center, right), resulting in an increased M2:M1 ratio (Fig. 3, below, left; P = 0.02), which has previously been shown to be associated with improved repair.28,36
Fig. 3.
PNM shifts macrophage phenotype in vivo and promotes recovery without affecting Schwann cell gene expression. In vivo macrophage (above, left) but not Schwann cell (above, right) gene expression is altered by PNM 5 days after repair. Immunohistochemistry 10 days after repair demonstrates reduced accumulation of CD68+CCR7+ macrophages within a specific region of interest in the regenerative nerve bridge (P < 0.01) (center, left). No differences were observed in the number of M2 (CD68+CD206+) macrophages (center, right), resulting in an increased regenerative index (M2:M1 ratio; P = 0.02) (below, left). PNM increases the number of retrograde labeled motor neurons after repair (n = 6 mice/group; P = 0.017) (below, right).
Assessment of Reinnervation in a Mouse Model
To determine the ability of PNM to promote regeneration in mice as well as in rats, and verify the relevance of the host response findings, we used retrograde labeling to determine the number of motor neurons crossing the repair site and reaching their target. This approach confirmed that PNM significantly increased the number of axons crossing the site of transection and repair in a second species (Fig. 3, below, right; P = 0.017).
These results were compared with the effects of PNM on macrophage polarization in vitro using a previously validated NanoString panel of 90 genes related to macrophage and SC phenotype and function.33,34 PNM-stimulated macrophages [M(PNM)] clustered independently from M(lipopolysaccharide + interferon-γ) and M(interleukin-4) groups and were most similar to M(−) macrophages, with little effect of PNM concentration on gene expression. Examining individual gene expression, M(PNM) had increased expression of genes encoding the angiogenesis-related growth factor PDGFb, receptor MAC2, M2 marker Arg1, and Marco, which also demonstrates increased expression after long-term interleukin-10 exposure,37 and downregulation of genes encoding proinflammatory cytokines, IL12 and Il1β; CCL24, which promotes chemotaxis of M1 macrophages38; and CXCL10, which is induced by interferon-γ. [See Figure, Supplemental Digital Content 2, which shows PNM-stimulated bone marrow–derived macrophages are distinct from but most similar to unstimulated macrophages (M−) with minimal effect of PNM concentration. Hierarchical clustering (left) and volcano plot (right) of gene expression using NanoString panel for murine bone marrow–derived macrophages are shown, http://links.lww.com/PRS/G53.]
DISCUSSION
This study demonstrates that a PNM hydrogel derived from a xenogenic source can be readily deployed subepineurally at the site of nerve transection and repair or into a conduit spanning a noncritical gap (3 to 8 mm) to augment existing repair techniques. PNM was found to promote axon extension at an early time point (21 days) and these beneficial effects were correlated with improvements in multiple histologic and functional outcomes at later time points in both rats and mice. Following deployment into the site of a nerve transection and repair, PNM resulted in increased electrophysiologic conduction (CMAP amplitude). Increases in the proportion of innervated NMJs also were observed after a transection repair augmented with PNM. Together these data suggest that PNM produces an increase in the numbers of axons crossing the site of repair early in the remodeling process and that this leads to an increased number of functional NMJs downstream. The retrograde labeling data in mice confirm this finding in a second species using a distinct technique, which adds validity to the translational potential for PNM.
Throughout this study, we used a range of outcome measures designed to reflect the early cellular responses to PNM, through mid-stage responses such as axon extension, to late-stage functional responses such as electrophysiology, NMJ formation, and force generation. We combined immunohistochemistry and gene expression techniques to evaluate the macrophage and SC responses because those cell types are critical for repair. We used electrophysiology and peak tetanic force generation to accelerate the use of this approach to a clinic setting because these are widely used clinical outcome measures. We used NMJ formation in GFP animals to confirm these results.
To investigate the process by which PNM mediates these improvements in recovery after injury, we investigated the early tissue remodeling response to PNM. We evaluated macrophages because these cells, the major cell type in the first few days after nerve injury, remove axonal segments and myelin debris and secrete vascular endothelial growth factor A, which promotes development of the polarized microvasculature that directs SC migration within the regenerative bridge.39–42 These early events are the precursor to formation of the bands of Bunger, axon elongation, and eventual functional recovery. It has been shown that regeneration does not occur if macrophages are excluded from the repair site40,43,44 and a number of studies have demonstrated that certain subsets of macrophages, including anti-inflammatory subsets, are beneficial to nerve repair.28,37,45
We used a broad panel of 90 genes associated with macrophage activation and previously validated against RNA sequencing data34 to characterize precisely the macrophage response to PNM while avoiding overemphasis on a small number of markers to describe a complex spectrum of macrophage activation.46,47 We demonstrate a general reduction of genes associated with inflammation and scarring and an increase in genes associated with nerve regeneration following exposure to PNM. These findings were confirmed by immunohistochemistry, with a decrease in CCR7+ (M1) macrophages and a consequent increase in the M2:M1 ratio, which is associated with nerve regeneration36 and also previously identified by Prest et al.28 Together with the absence of an activated SC response, these findings suggest that injection of PNM supports alteration of macrophage phenotype early in the period after injury, resulting in enhanced axon extension and leading to an opportunity for improved functional recovery in patients with peripheral nerve injury.
It should be noted that, although PNM-treated bone marrow–derived macrophages expressed multiple M2-like genes, the phenotype following exposure to PNM was distinct from both macrophages exposed to M(lipopolysaccharide + interferon) and M(interleukin-4). In particular, PNM-exposed macrophages exhibited an increase in genes associated with angiogenesis. Such shifts in macrophage phenotype have been observed in a wide variety of studies of decellularized tissue–based scaffold materials in both solid and hydrogel forms across organ systems in both preclinical and clinical settings including nerve, spinal cord, and brain.48–52 The mechanisms by which PNM elicits an alteration in macrophage phenotype are largely unknown. These may include structural or mechanical cues, interaction of cells with the inherent ligands that result from isolation from mammalian tissues, and release of bioactive factors, among others.53
One limitation of the study is the absence of axon counts as an outcome measure in the rat transection and repair studies. Having established the effects of PNM on axon extension in a short noncritical gap in Thy1-GFP animals, we focused in later experiments on more functional outcome measures such as electrophysiology, NMJ formation, and force generation.
Here we used a xenogeneic (porcine) source for the development of a scaffold to be implanted into rodents. In a previous study, we examined implantation of a canine-derived material in a rodent model and also saw improved outcomes, albeit in a different injury modality.28
Our work and that of others has demonstrated that the use of xenogeneic tissues is both safe and effective, and not associated with a rejection-type response.54,55 Porcine tissues, such as those used in the current study, also represent a widely available source that allows for traceability and quality control.56–58
The early effects on macrophage migration and gene expression that we demonstrate in the presence of PNM are distinct from the majority of attempts to improve recovery after PNI, which have tended to focus on events occurring relatively late in the response to injury. This includes the addition of nerve-specific growth factors12–17 or cell-specific transfer to the injury site18,19 to attempt to improve axon extension or SC migration. Other approaches have used mechanical cues to guide the regenerative bridge forming between the transected proximal and distal stumps and diminish invasion of surrounding fibrous tissue into the growth cone or extension of axons outside the regenerating bridge.20–27
The current study suggests that the application of PNM supports a beneficial early macrophage response leading to enhanced axonal outgrowth with significant improvements in downstream functional outcomes.
DISCLOSURE
Drs. Cheetham, Brown, and Soletti are cofounders of a company (Renerva LLC) that manufactures a peripheral nerve matrix hydrogel. The remaining authors have nothing to disclose.
DISCLAIMER
The views, opinions, and/or findings contained in this research/presentation/publication are those of the authors and do not necessarily reflect the views of the U.S. Department of Defense and should not be construed as an official U.S. Department of Defense or U.S. Army position, policy, or decision unless so designated by other documentation. No official endorsement should be made. Reference herein to any specific commercial products, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the U.S. government.
ACKNOWLEDGMENTS
This project was made possible in part by a Medical Technology Enterprise Consortium research grant that was awarded and administered by the U.S. Army Medical Research & Development Command and the Joint Program Committee (JPC-8) at Fort Detrick, MD, under contract number MTEC‐18‐05‐Peripheral Nerve‐0025. Support was also provided by the National Institute of Deafness and Communication Disorders at the National Institutes of Health (NIDCD DC017171).
Supplementary Material
Footnotes
Presented at the American Society for Peripheral Nerve 2020 Annual Meeting, in Fort Lauderdale, Florida, January 10 through 12, 2020.
Disclosure statements are at the end of this article, following the correspondence information.
Related digital media are available in the full-text version of the article on www.PRSJournal.com.
REFERENCES
- 1.Nichols CM, Brenner MJ, Fox IK, et al. Effects of motor versus sensory nerve grafts on peripheral nerve regeneration. Exp Neurol. 2004;190:347–355. [DOI] [PubMed] [Google Scholar]
- 2.Mackinnon SE, Doolabh VB, Novak CB, Trulock EP. Clinical outcome following nerve allograft transplantation. Plast Reconstr Surg. 2001;107:1419–1429. [DOI] [PubMed] [Google Scholar]
- 3.Lee JY, Parisi TJ, Friedrich PF, Bishop AT, Shin AY. Does the addition of a nerve wrap to a motor nerve repair affect motor outcomes? Microsurgery 2014;34:562–567. [DOI] [PubMed] [Google Scholar]
- 4.Zhu X, Wei H, Zhu H. Nerve wrap after end-to-end and tension-free neurorrhaphy attenuates neuropathic pain: A prospective study based on cohorts of digit replantation. Sci Rep. 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papatheodorou LK, Williams BG, Sotereanos DG. Preliminary results of recurrent cubital tunnel syndrome treated with neurolysis and porcine extracellular matrix nerve wrap. J Hand Surg Am. 2015;40:987–992. [DOI] [PubMed] [Google Scholar]
- 6.Qiu L, Qi See AA, Steele TWJ, Kam King NK. Bioadhesives in neurosurgery: A review. J Neurosurg. 2019:1–11. [DOI] [PubMed] [Google Scholar]
- 7.Childe JR, Regal S, Schimoler P, Kharlamov A, Miller MC, Tang P. Fibrin glue increases the tensile strength of conduit-assisted primary digital nerve repair. Hand 2018;13:45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sameem M, Wood TJ, Bain JR. A systematic review on the use of fibrin glue for peripheral nerve repair. Plast Reconstr Surg. 2011;127:2381–2390. [DOI] [PubMed] [Google Scholar]
- 9.Yang M, Rawson JL, Zhang EW, Arnold PB, Lineaweaver W, Zhang F. Comparisons of outcomes from repair of median nerve and ulnar nerve defect with nerve graft and tubulization: A meta-analysis. J Reconstr Microsurg. 2011;27:451–460. [DOI] [PubMed] [Google Scholar]
- 10.Herman ZJ, Ilyas AM. Sensory outcomes in digital nerve repair techniques: An updated meta-analysis and systematic review. Hand 2020;15:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narayan SK, Arumugam M, Chittoria R. Outcome of human peripheral nerve repair interventions using conduits: A systematic review. J Neurol Sci. 2019;396:18–24. [DOI] [PubMed] [Google Scholar]
- 12.Hoben G, Yan Y, Iyer N, et al. Comparison of acellular nerve allograft modification with Schwann cells or VEGF. Hand (N Y). 2015;10:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanje M, Skottner A, Sjöberg J, Lundborg G. Insulin-like growth factor I (IGF-I) stimulates regeneration of the rat sciatic nerve. Brain Res. 1989;486:396–398. [DOI] [PubMed] [Google Scholar]
- 14.Kemp SWP, Webb AA, Dhaliwal S, Syed S, Walsh SK, Midha R. Dose and duration of nerve growth factor (NGF) administration determine the extent of behavioral recovery following peripheral nerve injury in the rat. Exp Neurol. 2011;229:460–470. [DOI] [PubMed] [Google Scholar]
- 15.Marquardt LM, Ee X, Iyer N, et al. Finely tuned temporal and spatial delivery of GDNF promotes enhanced nerve regeneration in a long nerve defect model. Tissue Eng Part A. 2015;21:2852–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marquardt LM, Sakiyama-Elbert SE. GDNF preconditioning can overcome Schwann cell phenotypic memory. Exp Neurol. 2015;265:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sulaiman W, Dreesen T, Nguyen D. Single local application of TGF-β promotes a proregenerative state throughout a chronically injured nerve. Neurosurgery 2018;82:894–902. [DOI] [PubMed] [Google Scholar]
- 18.Kemp SWP, Walsh SK, Midha R. Growth factor and stem cell enhanced conduits in peripheral nerve regeneration and repair. Neurol Res. 2008;30:1030–1038. [DOI] [PubMed] [Google Scholar]
- 19.Shakhbazau A, Kawasoe J, a HS, et al. Early regenerative effects of NGF-transduced Schwann cells in peripheral nerve repair. Mol Cell Neurosci. 2012;50:103–112. [DOI] [PubMed] [Google Scholar]
- 20.Chaw JR, Liu HW, Shih YC, Huang CC. New designed nerve conduits with a porous ionic cross-linked alginate/chitisan structure for nerve regeneration. BioMed Mater Eng. 2015;26:S95–102. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh SC, Tang CM, Huang WT, et al. Comparison between two different methods of immobilizing NGF in poly(DL-lactic acid-co-glycolic acid) conduit for peripheral nerve regeneration by EDC/NHS/MES and genipin. J Biomed Mater Res A. 2011;99A:576–585. [DOI] [PubMed] [Google Scholar]
- 22.Pfister LA, Papaloïzos M, Merkle HP, Gander B. Nerve conduits and growth factor delivery in peripheral nerve repair. J Peripher Nerv Syst. 2007;12:65–82. [DOI] [PubMed] [Google Scholar]
- 23.Reid AJ, de Luca AC, Faroni A, et al. Long term peripheral nerve regeneration using a novel PCL nerve conduit. Neurosci Lett. 2013;544:125–130. [DOI] [PubMed] [Google Scholar]
- 24.Shintani K, Uemura T, Yokoi T, Onode E, Okada M, Nakamura H. Protective effect of biodegradable nerve conduit against peripheral nerve adhesion after neurolysis. J Hand Surg Am. 2017;42:S11–S12. [DOI] [PubMed] [Google Scholar]
- 25.Stocco E, Barbon S, Lora L, et al. Partially oxidized polyvinyl alcohol conduit for peripheral nerve regeneration. Sci Rep. 2018;8:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki Y, Tanihara M, Ohnishi K, Suzuki K, Endo K, Nishimura Y. Cat peripheral nerve regeneration across 50 mm gap repaired with a novel nerve guide composed of freeze-dried alginate gel. Neurosci Lett. 1999;259:75–78. [DOI] [PubMed] [Google Scholar]
- 27.Tao J, Hu Y, Wang S, et al. A 3D-engineered porous conduit for peripheral nerve repair. Sci Rep. 2017;7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prest TA, Yeager E, LoPresti ST, et al. Nerve-specific, xenogeneic extracellular matrix hydrogel promotes recovery following peripheral nerve injury. J Biomed Mater Res A. 2018;106:450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kemp SWP, Phua PD, Stanoulis KN, et al. Functional recovery following peripheral nerve injury in the transgenic Thy1-GFP rat. J Peripher Nerv Syst. 2013;18:220–231. [DOI] [PubMed] [Google Scholar]
- 30.Moore AM, Borschel GH, Santosa KA, et al. A transgenic rat expressing green fluorescent protein (GFP) in peripheral nerves provides a new hindlimb model for the study of nerve injury and regeneration. J Neurosci Methods 2012;204:19–27. [DOI] [PubMed] [Google Scholar]
- 31.Barton ER, Lynch G. Measuring isometric force of isolated mouse muscles in vitro. 2008(Id):1–14. Published July 31, 2008 (reviewed June 23, 2019). Available at: https://treat-nmd.org/wp-content/uploads/2016/08/cmd-DMD_M.1.2.002.pdf
- 32.Willand MP, Chiang CD, Zhang JJ, Kemp SWP, Borschel GH, Gordon T. Daily electrical muscle stimulation enhances functional recovery following nerve transection and repair in rats. Neurorehabil Neural Repair 2015;29:690–700. [DOI] [PubMed] [Google Scholar]
- 33.Tomlinson JE, Golshadi M, Donahue CJ, Dong L, Cheetham J. Evaluation of two methods to isolate Schwann cells from murine sciatic nerve. J Neurosci Methods 2020;331:108483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomlinson JE, Žygelytė E, Grenier JK, Edwards MG, Cheetham J. Temporal changes in macrophage phenotype after peripheral nerve injury. J Neuroinflammation 2018;15:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Žygelytė E, Bernard ME, Tomlinson JE, et al. RetroDISCO: Clearing technique to improve quantification of retrograde labeled motor neurons of intact mouse spinal cords. J Neurosci Methods 2016;271:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mokarram N, Dymanus K, Srinivasan A, et al. Immunoengineering nerve repair. Proc Natl Acad Sci U S A. 2017;114:E5077–E5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park-Min KH, Antoniv TT, Ivashkiv LB. Regulation of macrophage phenotype by long-term exposure to IL-10. Immunobiology 2005;210:77–86. [DOI] [PubMed] [Google Scholar]
- 38.Xuan W, Qu Q, Zheng B, Xiong S, Fan G-H. The chemotaxis of M1 and M2 macrophages is regulated by different chemokines. J Leukoc Biol. 2015;97:61–69. [DOI] [PubMed] [Google Scholar]
- 39.Mueller M, Leonhard C, Wacker K, et al. Macrophage response to peripheral nerve injury: The quantitative contribution of resident and hematogenous macrophages. Lab Investig. 2003;83:175–185. [DOI] [PubMed] [Google Scholar]
- 40.Chen P, Piao X, Bonaldo P. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol. 2015. [DOI] [PubMed] [Google Scholar]
- 41.Stratton JA, Holmes A, Rosin NL, et al. Macrophages regulate Schwann cell maturation after nerve injury. Cell Rep. 2018;24:2561–2572.e6. [DOI] [PubMed] [Google Scholar]
- 42.Cattin AL, Burden JJ, Van Emmenis L, et al. Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell 2015;162:1127–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dahlin LB. Prevention of macrophage invasion impairs regeneration in nerve grafts. Brain Res. 1995;679:274–280. [DOI] [PubMed] [Google Scholar]
- 44.Liu P, Peng J, Han GH, et al. Role of macrophages in peripheral nerve injury and repair. Neural Regen Res. 2019;14:1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perry VH, Brown MC, Gordon S. The macrophage response to central and peripheral nerve injury: A possible role for macrophages in regeneration. J Exp Med. 1987;165:1218–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014;6:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014;41:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hong JY, Seo Y, Davaa G, Kim H-W, Kim SH, Hyun JK. Decellularized brain matrix enhances macrophage polarization and functional improvements in rat spinal cord injury. Acta Biomater. 2020;101:357–371. [DOI] [PubMed] [Google Scholar]
- 49.Dziki JL, Huleihel L, Scarritt ME, Badylak SF. Extracellular matrix bioscaffolds as immunomodulatory biomaterials. Tissue Eng Part A. 2017;23:1152–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huleihel L, Dziki JL, Bartolacci JG, et al. Macrophage phenotype in response to ECM bioscaffolds. Semin Immunol. 2017;29:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghuman H, Mauney C, Donnelly J, Massensini AR, Badylak SF, Modo M. Biodegradation of ECM hydrogel promotes endogenous brain tissue restoration in a rat model of stroke. Acta Biomater. 2018;80:66–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mehrban N, Molina CP, Quijano LM, et al. Host macrophage response to injectable hydrogels derived from ECM and α-helical peptides. Acta Biomater. 2020;111:141–152. [DOI] [PubMed] [Google Scholar]
- 53.Brown BN, Badylak SF. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl Res. 2014;163:268–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Badylak SF. Decellularized allogeneic and xenogeneic tissue as a bioscaffold for regenerative medicine: Factors that influence the host response. Ann Biomed Eng. 2014;42:1517–1527. [DOI] [PubMed] [Google Scholar]
- 55.Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang RM, Johnson TD, He J, et al. Humanized mouse model for assessing the human immune response to xenogeneic and allogeneic decellularized biomaterials. Biomaterials 2017;129:98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson TD, Dequach JA, Gaetani R, et al. Human versus porcine tissue sourcing for an injectable myocardial matrix hydrogel. Biomater Sci. 2014;2:735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson TD, Hill RC, Dzieciatkowska M, et al. Quantification of decellularized human myocardial matrix: A comparison of six patients. Proteomics Clin Appl. 2016;10:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.