Abstract
Newly acquired memory traces have been thought to become stable and resistant to interruption after they are stored in long-term memory. However, according to a recent research drugs such as beta-adrenergic receptor antagonists enable memories to be updated and rewritten when administered during consolidation and reconsolidation. Propranolol is a widely used beta-adrenergic receptor antagonist that disrupts the consolidation and reconsolidation processes of memory formation as it inhibits protein synthesis in the central nervous system. This review aims to discuss the memory impairing effect of the systemic and intracerebral administration of propranolol during the consolidation and reconsolidation processes associated with different learning tasks. In doing so, this review will help elucidate the effects of propranolol on different stages of memory formation. Since learning and maladaptive memories underpin some of the most common psychological disorders, such as phobias, post-traumatic stress disorder, addiction, drug-seeking behavior, and so on, a thorough understanding of propranolol’s memory-impairing effect has significant clinical value and the potential to help people suffering from these disorders.
Keywords: Memory, propranolol, consolidation, reconsolidation, rodent
INTRODUCTION
Until recently, it was assumed that learned information remained stable after being transferred to long-term memory; however, recent research has revealed that memory formation can be disrupted during the consolidation and reconsolidation processes (1). It has been shown that beta-adrenergic receptors are involved in the process of memory formation, and inhibiting beta-adrenergic receptor activation stands out as a new method in the treatment of psychological disorders arising from maladaptive memories such as post-traumatic stress disorder (PTSD). Therefore, interventions regarding the process of memory formation have been studied in detail in preclinical studies because of their therapeutic potential (1,2).
Memories are in a labile state until they are stabilized via consolidation (3). Consolidation is a time-dependent process that occurs as a result of structural and chemical changes in the nervous system, in which recently learned experiences are stored in long-term memory (4). Many studies have shown that when consolidated memories are retrieved, they become unstable and undergo a protein synthesis-dependent process known as reconsolidation (5). The reconsolidation process can take anywhere from 10 minutes to 6 hours (4).
It has been suggested that noradrenergic signaling increases the strength of memories, especially the ones that have emotional values (5). The activity of beta-adrenergic receptors, which is one of the components of noradrenergic signaling, has been associated with long-term forms of synaptic plasticity (6). In parallel, pharmacological agonism of beta-adrenergic receptors after learning increases memory consolidation, and its antagonism prevents the formation of long-term memory (6,7). Beta-adrenergic signaling also plays an essential role in the memory reconsolidation. Beta-adrenergic receptor agonism during the reconsolidation process prevents the extinction of fear memories (6). Beta-adrenergic receptor antagonism, however, has an impairing effect on long-term memory by preventing the reconsolidation process (7). Research on beta-adrenergic signaling suggest that this pathway is involved in the reconsolidation of reactivated memories.
Highlights
Beta-adrenergic receptors are involved in memory acquisition
Memories are in a labile state during consolidation and reconsolidation phases
Pharmacological intervention can disrupt the memory formation
Propranolol is found to impair memory acquisition for various learning tasks in rodents
Impairing memory acquisition could be a therapeutic tool for some psychological disorders
Propranolol, a non-selective, competitive beta-adrenergic receptor antagonist, is commonly used to inhibit memory formation processes in animals and humans. As well as its peripheral effect in the cardiovascular and pulmonary systems, propranolol can also pass the blood-brain barrier and inhibit the protein synthesis in the central nervous system (5).
After the acquisition of new memories, systemic administration or infusion of propranolol into the nervous system has been found to impair the memory consolidation process in rodents (8). It has also been found that propranolol administered immediately after the reactivation of the consolidated memory disrupts the reconsolidation process in many different learning tasks (1). However, the role of beta-adrenergic signaling in memory consolidation and reconsolidation processes has not been observed in all the studies (9,10). Some studies have found that the systemic administration of propranolol disrupts the reconsolidation process but has no effect on the consolidation (5). In a different study, propranolol administration during reconsolidation did not result in observable memory impairment (9).
There are many animal studies examining the effects that of beta-adrenergic receptor antagonist propranolol has on the consolidation and reconsolidation processes of memory formation in various learning tasks. We aim to review current literature and guide future clinical studies by analyzing the effectiveness, importance, and therapeutic potential of propranolol have on psychological disorders. This review will examine related studies in literature without any bias and it will try to discover the reported findings.
METHOD
The PRISMA Statement, which provides a detailed guideline for writing meta-analyses and systematic reviews, was used to conduct the systematic review. The electronic systematic literature search was performed on the following online databases: APA, PubMed, and Web of Science. In addition to the database search, the references of relevant studies were checked, and suitable papers were included in the review. While the screening was being done, the following keywords were used: “propranolol AND consolidation AND mice”, “propranolol AND reconsolidation AND mice”, “propranolol AND consolidation AND rats”, “propranolol AND reconsolidation AND rats”. The studies in which propranolol was used to impair processes of memory formation were included in the review. The search was conducted in April of 2022. A total of 191 studies were obtained in the PubMed (184 results), Web of Science (0 results), APA (7 results) databases and 1 result was obtained from a different source. During the database search, 35 studies were discovered to be duplicated. Following the removal of duplicates, titles and abstracts were reviewed for inclusion criteria. When that wasn’t enough, full texts were read.
The inclusion criteria were as follows: i) administration of propranolol during consolidation or reconsolidation, ii) the use of a rat or a mouse as a subject, iii) the use of behavioral tests to investigate the effect of the propranolol treatment, and iv) intraperitoneal or intracerebral administration of propranolol. Review and meta-analysis, reports, studies written in a language other than English, clinical studies are excluded from the scope of this review. After considering both the inclusion and exclusion criteria, a total of 36 studies were deemed relevant for the review (Figure 1). Animal model studies for each outcome of interest are reviewed below, and are summarized in Table 1.
Figure 1.
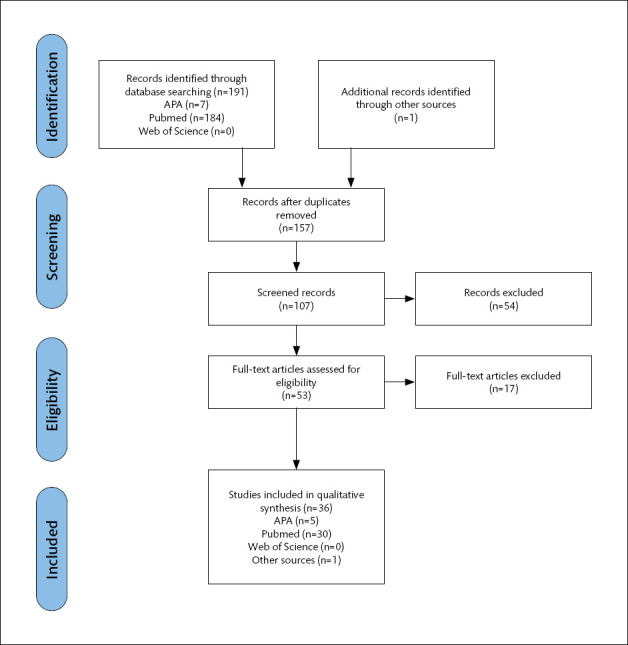
PRISMA flow diagram.
Table 1.
The effect of propranolol on memory consolidation and reconsolidation processes in various learning tasks
| Learning Task | Paper | Strain | Administration | Dose | Consolidation | Reconsolidation |
|---|---|---|---|---|---|---|
| Contextual fear conditioning | ||||||
| 12 | Sprague-Dawley rats | Intracerebral infusion (CA1) | 5 μg | 5 μg dose caused a reduction in freezing behavior in the context paired with shock. | N/A | |
| 8 | Sprague-Dawley rats | Systemic administration | 2 mg/kg 5 mg/kg 10 mg/kg | Doses of 2 mg/kg and 5 mg/kg caused a reduction in freezing behavior in the context paired with shock. This effect was not observed at 10 mg/kg. | N/A | |
| Intracerebral infusion (Dorsal hippocampus) | 1.25 μg 5 μg 10 μg 15 μg | Doses of 1.25 μg, 5 μg, and 10 μg caused a reduction in freezing behavior in the context paired with shock. This effect was not observed at the 15 μg. | N/A | |||
| 13 | NMRI mice | Systemic administration | 0.1 mg/kg | 0.1 mg/kg dose caused a reduction in freezing behavior in the context paired with shock. | N/A | |
| 14 | Wistar rats | Systemic administration | 5 mg/kg | N/A | The 5 mg/kg dose caused a moderate reduction in freezing behavior in the shock-paired context that persisted over three test sessions. | |
| 2 | Wistar rats | Systemic administration | 10 mg/kg | N/A | A dose of 10 mg/kg resulted in permanent impairment of both recent and distant fear memory. No reinstatement was observed after a weak recall shock, and a reduction in freezing behavior continued to be observed in the context paired with the shock. | |
| 15 | CD1 mice | Systemic administration | 10 mg/kg | N/A | While the 10 mg/kg dose caused impairment in fear memory in the group not exposed to early life stressors, this effect was not seen in animals exposed to pre– or postnatal stress. | |
| Cued fear conditioning | ||||||
| 5 | Sprague-Dawley rats | Systemic administration | 10 mg/kg | The 10 mg/kg dose had no effect on the freezing behavior to the sound paired with shock, and the animals continued to show freezing response | A dose of 10 μg impaired fear memory, resulting in a decrease in the freezing behavior to the sound paired with shock. | |
| Intracerebral infusion (Lateral and basal nuclei of amygdala) | 2.5 μg | The 2.5 μg dose had no effect on the freezing behavior to the sound paired with shock, and the animals continued to show freezing response | A dose of 2.5 μg impaired fear memory, resulting in a reduction in the freezing behavior to sound paired with shock. | |||
| 18 | Sprague-Dawley rats | Intracerebral infusion (Lateral nucleus of amygdala) | 1.25 μg | N/A | The 1.25 μg dose blocked the fear memory-enhancing effect of the beta-adrenergic receptor agonist isoproterenol infused into the lateral nucleus of the amygdala. | |
| 7 | Long-Evans rats | Systemic administration | 10 mg/kg | N/A | The 10 mg/kg dose after memory reactivation by context exposure resulted in the selective disruption of contextual fear conditioning with no effect on cued fear conditioning, whereas injection of propranolol following reactivation by the reminder cue (tone) caused impairment in contextual fear conditioning and also in cued fear conditioning | |
| 16 | Sprague-Dawley rats | Intracerebral infusion (Lateral nucleus of amygdala) | 0.1 μg 1 μg | When doses of 0.1 μg and 1 μg were given after conditioning, there was no effect on both short-term (after 3 hours) and long-term (after 48 hours) fear memory, and the animals continued to show a conditioned response to sound paired with shock. | N/A | |
| 19 | C57BL/6J mice | Systemic administration | 10 mg/kg | N/A | When a dose of 10 mg/kg was administered before the auditory fear memory reminder, it caused impairments in fear memory, resulting in a decrease in freezing behavior to the sound paired with shock. This effect was not observed when administered after the reminder. | |
| 20 | Sprague-Dawley rats | Systemic administration | 10 mg/kg | N/A | The 10 mg/kg dose had no effect on fear memory as animals continued to show freezing response to the sound paired with shock. | |
| 10 | Lister-Hooded rats | Systemic administration | 10 mg/kg | N/A | The 10 mg/kg dose had no effect on freezing behavior to the shock-paired sound and the animals continued to conditioned response. | |
| Morris water maze | ||||||
| 21 | Sprague-Dawley rats | Systemic administration | 10 mg/kg | The 10 mg/kg had no effect on spatial memory, and no significant difference was observed between experimental and control animals in terms of time spent in the target quadrant. | N/A | |
| 24 | Sprague-Dawley rats | Systemic administration | 5 mg/kg | The 5 mg/kg dose impaired spatial memory, and animals treated with propranolol spent less time in the target quadrant than the control group. | N/A | |
| 23 | Long-Evans rats | Systemic administration | 10 mg/kg 20 mg/kg | Both doses of 10 mg/kg and 20 mg/kg did not impair spatial memory as the animals learned where the platform was located. | N/A | |
| 22 | Long-Evans rats | Systemic administration | 20 mg/kg 40 mg/kg | Both doses of 20 mg/kg and 40 mg/kg did not impair spatial memory as the animals learned the location of the platform. | N/A | |
| 26 | ICR mice | Systemic administration | 2 mg/kg 4 mg/kg 8 mg/kg 12 mg/kg | All doses did not impair spatial memory as the animals learned the location of the platform. | N/A | |
| 1 | CD1 mice | Systemic administration | 10 mg/kg | The 10 mg/kg dose did not impair spatial memory in both the high (19°C) and low (23°C) aversive versions of the task. | The 10 mg/kg dose did not impair spatial memory in both the high (19°C) and low (23°C) aversive versions of the task. | |
| Passive avoidance test | ||||||
| 29 | Sprague-Dawley rats | Intracerebral infusion (Amygdala) | 8.5 nM 17 nM 34 nM | The 34 nM dose but not 8.5 nM and 17 nM, caused impairments in passive avoidance memory and animals continued to enter the shock-paired dark compartment. | N/A | |
| 27 | Sprague-Dawley rats | Systemic administration | 10 mg/kg | The animals given a dose of 10 mg/kg entered the shock-paired dark compartment earlier with their front two paws compared to the control group. However, no difference was observed in the percentage of animals entering the dark compartment, the latency to enter the dark compartment with four paws, and the total time spent in the dark compartment. | The animals given a dose of 10 mg/kg entered the shock-paired dark compartment earlier with their front two paws compared to the control group. In addition, the percentage of animals entering the dark compartment was higher in the propranolol group. | |
| 7 | Long-Evans rats | Systemic administration | 10 mg/kg | N/A | No memory impairing effect on passive avoidance memory was observed at the dose of 10 mg/kg. Although different intensities of shock (0.9 mA, 0.6 mA, 0.25 mA) were employed as US, no difference was found between the propranolol group and the controls. in the latency of animals entering the dark compartment with four paws | |
| 28 | Long-Evans rats | Systemic administration | 10 mg/kg | While the 10 mg/kg dose impaired the memory consolidation process when administered immediately after exposure to high stress (passive avoidance exercise + swimming stress), no memory-impairing effect was observed when administered after mild stress (only passive avoidance exercise) version of the task. | N/A | |
| 1 | CD1 mice | Systemic administration | 10 mg/kg | The 10 mg/kg dose did not impair passive avoidance memory, and no significant difference was observed between the experimental and control groups in the latency to enter and the percentage of animals entering the dark compartment. | The 10 mg/kg dose impaired passive avoidance memory, and a significant difference was observed between the experimental and control groups in the latency to enter and the percentage of animals entering the dark compartment. | |
| Conditioned taste aversion | ||||||
| 30 | Wistar rats | Intracerebral infusion (Central and basolateral nuclei of amygdala) | 20 μg | When a 20 μg dose was infused into the central nucleus, it impaired conditioned taste aversion memory, but no disruptive effects were observed in the basolateral nucleus. | N/A | |
| 31 | Wistar rats | Intracerebral infusion (Basolateral nucleus of amygdala and insular cortex) | 1 μg 2.5 μg | Infusion of 1 μg dose into the basolateral amygdala and 2.5 μg into the insular cortex did not impair the consolidation of conditioned taste aversion as the animals continued to consume the LiCl-paired solution. | N/A | |
| 33 | Sprague-Dawley rats | Intracerebral infusion (Medial prefrontal cortex) | 5 μg | Infusion of 5 μg dose impaired both short-term (4 hours) and long-term memory (24 hours) of conditional taste aversion induced by LiCl-saccharine pairing. | N/A | |
| 32 | Wistar rats | Intracerebral infusion (Amygdala) | 5 μg | Infusion of 5 μg dose did not impaired short-term (4.5 hours) memory but caused disruption in long-term memory (71 hours) of conditional taste aversion induced by LiCl-saccharine pairing. | N/A | |
| 1 | CD1 mice | Systemic administration | 10 mg/kg | The 10 mg/kg dose had no impairing effect of conditioned taste aversion memory induced by LiCl-saccharine pairing as the animals continued to consume the saccharine solution. | The 10 mg/kg dose impaired conditioned taste aversion memory induced by LiCl-saccharine pairing and the animals display suppressed consumption of saccharine solution. | |
| Object recognition | ||||||
| 35 | C57BL/6J mice | Systemic administration | 10 mg/kg | N/A | The 10 mg/kg dose impaired object recognition memory and the animals did not spend more time exploring the new object. | |
| 36 | CD1 mice | Systemic administration | 10 mg/kg | At a dose of 10 mg/kg, propranolol impaired object recognition memory in the high emotional arousal condition tested after 24 hours and 96 hours, while it had no effect in the low emotional arousal version of the task. | N/A | |
| 1 | CD1 mice | Systemic administration | 10 mg/kg | While the dose of 10 mg/kg impaired object recognition memory, it had no effect on the aquatic version of the task. | While the dose of 10 mg/kg impaired object recognition memory, it had no effect in the aquatic version of the task. | |
| Conditioned place preference | ||||||
| 38 | Sprague-Dawley rats | Systemic administration | 10 mg/kg | N/A | The 10 mg/kg dose impaired place preference memory, and there was no difference between the time the propranolol-treated animals spent in the cocaine-paired compartment and the NaCl-paired compartment. | |
| 39 | Sprague-Dawley rats | Systemic administration | 10 mg/kg | N/A | 10 mg/kg dose of chronic post-test propranolol injections did not reinstate of the animal’s preference for the drug-paired side. In contrast, acute post-retrieval propranolol injection failed to blunt subsequent cocaine reinstatement of the memory. | |
| 3 | Long-Evans rats | Systemic administration | 10 mg/kg | N/A | The dose of 10 mg/kg impaired the retention of the cocaine place preference when administered 20 minutes before the retention test (3). The place preference memory did not return when the animals were tested after 14 days in spontaneous recovery and in cue-induced reinstatement | |
| Drug memory | ||||||
| 40 | Sprague-Dawley rats | Systemic administration | 10 mg/kg | 10 mg/kg dose administered during the consolidation process impeded drug memory as the animals showed an impairment of locomotor sensitization. | N/A | |
| 41 | Sprague-Dawley rats | Systemic administration | 10 mg/kg | N/A | The 10 mg dose administered following the memory reminder 24 hours after extinction learning disrupted the reconsolidation of the drug memory and prevented the cue-induced reinstatement | |
| Context-induced sucrose seeking | ||||||
| 42 | Wistar rats | Systemic administration | 10 mg/kg | N/A | The 10 mg/kg dose impaired context-induced sucrose seeking behaviour (tested 24 hours after training) of animals trained to self-administer sucrose as nose poking behavior was reduced in animals given propranolol after a 20-minute reminder. | |
| Olfactory fear conditioning | ||||||
| 43 | Wistar rats | Systemic administration | 5 mg/kg 10 mg/kg | 10 mg/kg dose administered after the shock-coffee odor pairing disrupted the memory consolidation, as evidenced by the decrease in the conditioned response in the experimental animals. | N/A | |
| Spatial procedural memory | ||||||
| 44 | Albino rats | Systemic administration | 10 mg/kg 20 mg/kg | 10 mg/kg and 20 mg/kg doses did not disrupt the consolidation process as animals continued to prefer the arms that were associated with food reward. | N/A | |
kg: kilogram; LiCl: lithium chloride; mA: milliampere; mg: milligram; NaCl: sodium chloride; nM: nanomolar; μg: microgram.
Contextual Fear Conditioning
Contextual fear conditioning (CFC) involves exposing rodents to an unconditioned stimulus (US) that causes fear (usually shock) in a novel environment. When the animal is re-exposed to the US-paired environment (the conditioned stimulus; CS), it shows a conditioned response (CR) such as freezing (11). Freezing is defined as “the absence of movement other than breathing” (2).
The effects of propranolol administration on fear memory were investigated during the consolidation and reconsolidation of CFC learning. . In Sprague Dawley rats, it was observed that long-term memory was impaired when propranolol (5 μg/ μL) was infused into the CA1 region 5 minutes after ICD, whereas no impairing effect on fear memory was observed when infused 6 hours later (12). In another study, where propranolol was administered systemically at different doses (2 mg/kg, 5 mg/kg, and 10 mg/kg) and infused into the dorsal hippocampus during the consolidation of CFC memory, it disrupted the fear memory (8). The disruptive effect of systemic propranolol (0.1 mg/kg) injection on the consolidation process of CFC learning was repeated in NMRI mice (13).
A study examining the effect of propranolol on the reconsolidation of CFC memory showed that when propranolol (5 mg/kg) was administered after memory reactivation, it caused a modest impairment in the CFC learning of Wistar rats that persisted over three test sessions (14). The same study showed that the reminder shock reversed the memory-impairing effect of the corticosterone (reinstatement); however, in the propranolol group, memory impairment was permanent after the reminder, and the CR did not return (14). These findings are interpreted as evidence that corticosterone reduces subsequent retrieval of CFC memory via facilitation of memory extinction, meanwhile, propranolol-induced amnesia is the result of the blocking of the reconsolidation process. It was also found that propranolol caused a permanent impairment in both recent and remote fear memory in Wistar rats (2). The findings of Abrari et al. (2008) (14) was further amplified in this study as no reinstatement effect was observed in the propranolol treated (10 mg/kg) group after a weak reminder shock (2).
In one study, CD1 mice with pre– or post-natal stress were tested in fear conditioning paradigm after receiving propranolol (10 mg/kg) injections during the memory reconsolidation process. Results of the study showed that the injection of propranolol after memory reactivation greatly reduced the fear memory, but this effect was not observed when mice were exposed to pre– or post-natal stress (15). These findings highlight the impact of early life experiences on the etiology of fear conditioning and are critical to the understanding of therapeutic interventions that target memory formation processes.
Cued Fear Conditioning
A neutral stimulus (sound, light, etc.) is paired with a fear-inducing stimulus (shock) during cued fear conditioning, and later, the freezing response that the animals display to the US-paired cue is analyzed as the CR (16). A study examining the systemic (10 mg/kg) and the intracerebral (2.5 μg/μL) administration of propranolol into the lateral and basal nuclei of the amygdala during the consolidation and reconsolidation processes of auditory fear conditioning in Sprague-Dawley rats found that both the systemic and the intra-amygdala infusions of propranolol blocked the reconsolidation but not the consolidation of fear memories. The disruptive effect on fear memory continued to be seen when animals were tested on days 2, 9, and 16 (5). Another study group also found no disruptive effect on both short-term (after 3 hours) and long-term (after 48 hours) fear memory in Sprague-Dawley rats when propranolol (0.1 μg and 1 μg) was infused into the lateral nucleus of the amygdala during consolidation. The results of mentioned studies contradict with the other studies, which found memory impairing effect of propranolol on the consolidation of CFC, however, these conflicting findings could be due the different expression levels of beta-adrenergic receptors in the responsible brain regions – involved in contextual and cued fear conditioning (17). Another study testing the propranolol (10 mg/kg) administration in the reconsolidation process of auditory fear conditioning in Long Evans rats found that the propranolol injection after memory reactivation by context exposure resulted in the selective disruption of CFC with no effect on cued fear conditioning, whereas the injection of propranolol following reactivation by the reminder cue (tone) caused impairments in both CFC and cued fear conditioning (7). It has been found that single intra-amygdala infusion of beta-adrenergic receptor agonist isoproterenol after the retrieval of a well-consolidated memory enhanced fear memory; however, propranolol infusion into the same region following isoproterenol blocked the memory-enhancing effects of the drug in Sprague-Dawley rats (18).
In another study with C57BL/6J mice, fear learning was associated with proliferation of the dendritic branches in the basolateral amygdala and increased phospho-Erk (p-ERK) signaling in the same region (19). In this study, when propranolol was administered before the auditory fear memory reminder, in addition to causing impairments in fear memory, it prevented the increase in the p-ERK signal (19). However, in a recent study with Lister-Hooded rats, although the duration of the exposures to the CS reminder and the shock intensities were manipulated; the memory-impairing effect of propranolol in the reconsolidation process was not repeated, and propranolol administered at a dose of 10 mg/kg did not cause any impairments in fear memory as animals continued to display CR to the sound paired with shocks (20). It was argued that the lack of memory impairing effect of propranolol is because the process might have failed to induce memory destabilization (20).
Morris Water Maze
The Morris Water Maze (MWM) is a spatial learning task that is based on the animals’ ability to use visual cues in an environment in order to locate an escape platform hidden below the water surface (21). Animals learn and use the spatial cues during training, and memory acquisition is assessed in the retention trial by measuring the time spent in the target quadrant where the platform was previously located (21).
In a study with Sprague-Dawley rats investigating the effect of chronic propranolol administration during the consolidation of spatial memory, it was found that there were no impairments in the retention of memory when propranolol was given at a dose of 10 mg/kg before training trials (21). In two different studies with male Long-Evans rats, propranolol was administered at the doses of 20 mg/kg and 40 mg/kg but it had no disruptive effect on the consolidation of MWM learning as the animals learned the location of the platform (22,23). However, in another study with Sprague-Dawley rats, systemic administration of a low dose of propranolol (5 mg/kg) impaired spatial memory consolidation in the MWM and the propranolol-treated animals spent less time in the target quadrant than the control group during the retention trial conducted 24 hours after the last training session (24). The conflicting results obtained in these studies may be caused by the acute or the chronic administration of propranolol. In studies where no memory impairing effect was observed (22,23), propranolol was administered chronically since the MWM training consisted of multiple sessions; while in the experiment of Cahill et al. (2000), MWM training was performed in a single session and propranolol was administered acutely (24). While memory is stabilized into the long-term memory via consolidation after the initial learning, repetitive training sessions may cause memory to be reconsolidated (25). Therefore, both consolidation and reconsolidation processes of memory formation should be disrupted in procedures that require multiple training sessions (25). The reason for these contradictory results in propranolol studies may be due to the role of different neurotransmitter systems and receptors involved in the consolidation and reconsolidation processes of memory formation. However, in ICR mice, acute administration of propranolol at the doses of 2, 4, 8 or 12 mg/kg did not cause any impairments on the consolidation of spatial memory (26). Another study with CD1 mice showed that no significant difference was observed between propranolol-treated animals and the controls in terms of time spent in the target quadrant, and propranolol injections had no disruptive effect on spatial memory consolidation and reconsolidation processes (1). The same study investigated whether propranolol’s amnestic effect is related to the aversiveness of the learning tasks by changing the water temperature in MWM and discovered no memory impairment in both high (19°C) and low aversive (23°C) tasks. (1). Therefore, it has been suggested that the efficacy of propranolol administration is not related to the averseness of the task (1).
Passive Avoidance
Passive avoidance is a type of classical conditioning in which animals associate a novel context with an aversive stimulus (27). In the training sessions, an animal is placed in the light compartment of a two-compartment setup and receives a foot-shock when it enters naturally preferred dark compartment. After the training is completed, the animal learns to avoid the compartment where it received the foot-shock. Avoidance learning is usually tested after 24 hours by measuring the latency to place two paws into the dark compartment (7). Acquisition of the task is evidenced as the animals avoid entering the normally preferred dark compartment (28).
Conflicting results were obtained in studies examining the memory impairing effect of propranolol on the consolidation and reconsolidation of passive avoidance learning (7,27). One study with Sprague-Dawley rats found that compared to sodium chloride (NaCl) injected animals, propranolol treated animals (10 mg/kg) during consolidation (5 minutes after the initial learning) entered the dark compartment that was paired with a 0.25 milliamperes (mA) shock earlier with their front two paws (27). However, no difference was observed between the groups in the number of animals entering the dark compartment and the total time spent in it (27). In the same study, propranolol or NaCl was administered 5 minutes after the memory reactivation to evaluate the effect of propranolol on the reconsolidation process (27). During the propranolol administration in reconsolidation, a significant difference was observed between experimental and control groups in the latency to place the two front paws and the number of animals that enter the dark compartment (27). In another study with Long Evans rats by Przybyslawski et al. (1999), it was observed that propranolol administered systemically at a dose of 10 mg/kg during the memory reconsolidation did not yield any disruptive effect on passive avoidance memory (7,27). Although different shock intensities (0.9 mA, 0.6 mA, 0.25 mA) were utilized, no difference was found between the propranolol and the NaCl groups in terms of the latency to enter the dark compartment (7). In another study with Long Evans rats, the effect of beta-adrenergic receptor blockade on memory modulation under varied levels of stress is investigated (28). It was found that propranolol (10 mg/kg) disrupted the memory consolidation process when administered after exposure to intense (passive avoidance practice + swimming stress) but not mild stress (only passive avoidance practice) tasks. The mentioned findings demonstrate that the effect of beta-adrenergic-receptor blockade on memory consolidation in the passive avoidance paradigm is dependent upon the intensity of stress, which has led to the suggestion that endogenous adrenergic receptor activity has a memory-enhancing function under stressful conditions (28). Parallel to this suggestion, studies show that increasing beta-adrenergic system activation during reconsolidation causes fear memory to be strengthened and resistant to extinction in other learning paradigms (18). However, Schneider et al. (2011) found the memory impairing effect of propranolol in low-stress learning tasks, but not in high-stress. Also, Schneider et al. (2011) states that the passive avoidance test as a mild– stress task, while Villain et al. (2016) describes it as a high-stress task (1,28). In conclusion, no amnestic effect of propranolol was observed in the consolidation of the normal version of the passive avoidance test in both of the studies. Schneider et al. (2011) only found the memory-impairing effect of propranolol during the consolidation of the high-stress version of the passive avoidance learning (28), but Villain et al. (2016) observed an impairing effect during the reconsolidation process (1). In another study with Sprague-Dawley rats, propranolol (5, 8, 17, and 34 nanomolar) infusion into the amygdala immediately after the passive avoidance training impaired long-term memory as the rats continued to enter the dark compartment paired with shocks (29). In another study with CD1 mice, when propranolol was administered during the passive avoidance memory consolidation, it did not cause any significant difference in the latency to enter the dark compartment and it also had no effect on the percentage of animals entering the dark compartment; however, when it was administered during reconsolidation, the propranolol treated animals entered the dark compartment earlier than controls (1). The observation, suggesting that memory impairing effect is specific to an actively retrieved memory, and that no memory impairment was seen without memory reactivation is suggested as evidence that the amnestic effect of propranolol is due to the disruption in the memory reconsolidation process (1).
Conditioned Taste Aversion
Conditioned taste aversion is a special form of classical conditioning observed in mice, rats, and many other species (30,31). In this learning task, a novel taste (usually a solution, such as sucrose or saccharin) is paired with nausea induced by an administration of a drug (usually lithium chloride; LiCl) (32). As a result, animals develop a taste aversion evidenced by a decreased level of fluid consumption associated with malaise (1).
Studies investigating the memory impairing effect of propranolol administration on taste aversion are focused on the consolidation process (30,31). Propranolol infusion (20 μg/ μL) into the central nucleus but not basolateral nucleus of the amygdala after the conditioning result in in impairments in taste aversion memory induced by saccharin-LiCl pairing. In another study, propranolol infusion into the basolateral amygdala (1 μg/0.2 μL) or insular cortex (2.5 μg/0.5 μL) did not result in the disruption of memory consolidation and the Wistar rats continued to show aversion to the saccharin solution paired with LiCl (31). These two studies replicate the finding that beta-adrenergic receptor activity in the basolateral amygdala is not involved in the taste aversion learning. In another study, propranolol (5 μg/μL) infusion into the medial prefrontal cortex of Sprague-Dawley rats during the consolidation of the LiCl-saccharine pairing had a memory impairing effect on both short– and long-term memory (33). In an in-vivo microdialysis study, glutamate and norepinephrine levels were increased in the amygdala of Wistar rats 45 minutes after the taste aversion acquisition (32). This increase indicated that glutamatergic and adrenergic signaling in the amygdala is involved in the integration of taste aversion, and in parallel, propranolol (5 μg/ μL) infusion to the same region after conditioning had no effect on short-term memory, but impaired long-term memory formation (32). In another study investigating the effect of systemic propranolol administration on both consolidation and reconsolidation processes in conditioned taste aversion paradigm, propranolol (10 mg/kg) had an impairing effect only during the reconsolidation phase in CD1 mice (1).
In conclusion, studies based on propranolol antagonism have shown that the beta-adrenergic receptor activity in the central nucleus of the amygdala and medial prefrontal cortex, but neither the basolateral amygdala nor the insular cortex are involved in the consolidation process of conditioned taste aversion learning.
Object Recognition Test
Rodents tend to interact more with novel objects compared to the ones that are already familiar (34). Object recognition test made use of this natural tendency of animals to study learning and memory related processes. In object recognition task, animals are allowed to explore surrounding objects after being placed in an apparatus (34). Then one of the objects is replaced with a novel one and then the animal is tested by measuring the time spent around the new object. Learning is evidenced as the animals spend more time around the novel object (34). In a study with C57BL/6J mice, it has been found that systemic administration of propranolol (10 mg/kg) impaired object recognition memory reconsolidation, and no difference was observed at the time of the engagement with new and familiar objects (35). Propranolol (10 mg/kg) treatment in high or low emotional/motivational arousal states had varied effects on the object recognition memory in another experiment using CD1 mice. (36). While propranolol disrupted the consolidation process of object recognition memory in the case of high emotional arousal, it did not have any memory impairing effect in the task involving low emotional arousal (36). These findings further support the role of beta-adrenergic receptor activity in the consolidation process of high emotional arousal memory tasks (36). However, in another study with CD1 mice, propranolol administration did not impair the memory consolidation and reconsolidation processes in the high-stress version of the object recognition memory task (the aquatic object recognition test), but a disruptive effect was observed in the low-stress version (normal object recognition test) (1). Although these results indicate that the memory impairing effect of propranolol is not limited to the associative learning tasks with high stress/emotional arousal, the results of the study by Liu et al. (2015) contradict this situation (1,35).
Conditioned Place Preference
Conditioned place preference is another learning paradigm that involves the pairing of a novel context with reward-like stimulus (37). A study testing the effect of systemic administration of propranolol (10 mg/kg) on conditioned place preference reconsolidation with Sprague-Dawley rats found that the animals entered and spent more time in the compartment paired with cocaine rather than NaCl (38). However, when the animals were injected with propranolol following the memory reactivation, no difference was found between the time spent in the cocaine and NaCl-paired compartments (38). In another study evaluating the dose of 10 mg/kg on Long-Evans rats, propranolol administration impaired the retention of the cocaine-paired place preference when administered 20 minutes before the retention test (3). The place preference memory did not return when the animals were tested after 14 days of spontaneous recovery and in cue-induced reinstatement (3). It is suggested that propranolol, given before the first retention test, disrupted the memory reconsolidation process (3). In a study evaluating the effect of acute and chronic administration of propranolol (10 mg/kg) on place preference memory in Sprague-Dawley rats, it was found that the animals that received chronic post-test propranolol injections did not reinstate their preference for the drug-paired side. In contrast, acute post-retrieval propranolol injection failed to blunt subsequent cocaine reinstatement of the memory. This data interpreted as chronic injections of propranolol decrease the conditioned preference by interfering with the reconsolidation of the memory rather than enhancing new extinction learning (39).
Drug Memory
In a study with Sprague-Dawley rats, examining the post-training antagonism of beta-adrenergic receptors in the single-trial cocaine-induced sensitization, it was found that propranolol (10 mg/kg) administered during the consolidation process impeded drug memory and the animals showed an impairment in locomotor sensitization (40). In a recent study, the impact of propranolol administration at a dose of 10 mg/kg on Sprague-Dawley rats’ memory for drugs and behavior in search of heroin was assessed. (41). Rats trained to self-administer heroin in operant cages were then repeatedly exposed to the context for 10 days in which conditioning took place, resulting in extinction learning (41). Propranolol, when administered 24 hours after after the extinction learning, disrupted the reconsolidation of the drug memory and prevented cue-induced reinstatement (41). However, this effect was not observed when NaCl was administered following the memory reminder, when no memory reminder was given before propranolol treatment, or propranolol was administered 6 hours after the memory reminder (41). These findings indicate that propranolol injection impairs drug memory and heroin-seeking behavior by impairing memory reconsolidation (41).
Other Behavioral Tests
A study investigated the effects of propranolol administration during the reconsolidation of reward-related memories in Wistar rats. The animals were trained to self-administer sucrose in operant cages, and after a long post-learning interval (3 weeks), propranolol (10 mg/kg) administration preceded the exposure to the sucrose-paired environment for 0, 10, and 20 minutes as a memory reminder (42). Context-induced sucrose seeking was tested 24 hours after the drug injection. The nose poking behavior was found to be reduced in animals given propranolol after a 20-minute reminder (42). A study with Wistar rats examined the effects of two different doses of propranolol injections (10 mg/kg and 5 mg/kg) on the olfactory fear conditioning paradigm and found that the higher dose of propranolol administered after the shock-coffee odor pairing disrupted memory consolidation, as evidenced by the decrease in the conditioned response in the experimental animals (43). Another study examined the effect of propranolol administration on spatial procedural memory in albino rats by injecting 10 mg/kg and 20 mg/kg of propranolol after the animals found the rewards placed in 4 arms of the 8-arm maze. In this study, propranolol did not disrupt the consolidation process, as animals continued to prefer the arms that were associated with food rewards (44).
DISCUSSION
The memory impairing effect of beta-adrenergic receptor antagonist propranolol on memory consolidation and reconsolidation of various learning tasks is reviewed. The effect of propranolol can be analyzed based on whether the propranolol was administered during the different processes of memory formation, namely consolidation and reconsolidation, the averseness degree of the learning task, and acute or chronic administration.
Consolidation and Reconsolidation
In most of the studies evaluating the effect of propranolol on the consolidation and reconsolidation processes in a single experiment, propranolol caused impairment in memory tasks when administered only during the reconsolidation process. These studies demonstrate that that propranolol only impinges upon reconsolidation processes, not consolidation processes, in passive avoidance (1,27), conditioned taste aversion, and auditory fear conditioning tasks (1). In studies evaluating only consolidation or reconsolidation processes, it has been shown that propranolol has no memory-impairing effects during the consolidation of cued fear conditioning (16), spatial memory (1,21–23,26), passive avoidance (1), conditioned taste aversion (1,31), object recognition (37), and spatial procedural memory (44). Propranolol was found to impair contextual fear conditioning (2,14,15), cued fear conditioning (7,18,19), object recognition (35), conditioned place preference (3,38,39), drug memory (41), and context-induced sucrose seeking in reconsolidation studies (42).
It is also possible that different injection times (before or after conditioning) could be the reason for the lack of memory impairment observed when propranolol is administered during consolidation. It has also been suggested that beta-adrenergic receptors are involved in the later phases of the memory consolidation process which supports this notion (5). However, Przybyslawski et al. (1999) refute this argument by finding no memory impairments when propranolol is administered 5 minutes, 2 hours and 5 hours after the initial learning (27). It also has been suggested that during consolidation, there is an intense noradrenergic signaling compared to reconsolidation, and the propranolol doses used in the consolidation experiments are insufficient to block the adrenergic signaling (5). If that is the case, 10 mg/kg dose can effectively disrupt the memory reconsolidation, however, the same dose could be ineffective to impair memory consolidation. However, in two different studies, high doses of propranolol (20 mg/kg and 40 mg/kg) were used during the consolidation but no memory impairing effect was found (22,23). It was also suggested that propranolol may have increased extinction learning rather than causing impairments in memory reconsolidation (5). In parallel, studies show that adrenergic receptor activity is involved in the extinction of some learning tasks such as conditioned taste aversion (45). However, because extinction learning does not occur after a single CS exposure, and repeated and prolonged exposures are required for extinction learning to occur, this explanation is debatable (4). Additionally, studies show that systemic propranolol administration impairs extinction learning rather than enhancing it (46). These results suggest that beta-adrenergic receptor activation is solely engaged in memory reconsolidation and not consolidation. However, in contrast to these null findings, some studies have found that the administration of propranolol during the consolidation disrupted the memory formation in contextual fear conditioning (13,12), spatial memory (24), passive avoidance (28,29), conditioned taste aversion (30–33), drug memory (40) and olfactory fear conditioning (43). Additionally, some studies have also found no memory impairing effect of propranolol during the reconsolidation process of cued fear conditioning (10,20), spatial memory (1), and passive avoidance (7). One of the reasons for the lack of amnestic effect during the reconsolidation process may be related to the procedural choices that may have failed to induce memory destabilization (41). Supporting this explanation, a study has found memory impairments in animals when propranolol was administered only after a 20-minute reminder exposure but not a 0 or a 10-minute reminder (42). This finding proves that 10-minute exposure, or non-exposure to a memory reminder does not destabilize the memory, suggesting that the duration of exposure has a huge impact. This observation has led us to suggest that in vivo studies targeting memory formation should be supported by in vitro methods. Accordingly, in one study in which the propranolol injection did not impair memory reconsolidation; it was demonstrated that the memory reminder used in the procedure was not sufficient to induce destabilization as shown by the expression levels of proteins associated with memory reconsolidation (10).
Aversiveness of the Learning Tasks
Many studies in which the impairing effects of propranolol on various memory formation processes were observed, highly aversive tasks such as passive avoidance, fear conditioning or taste aversion were used f (5,27,33). These findings led to the suggestion that the efficacy of propranolol administration is dependent upon the averseness of the learning task and that the propranolol causes impairments in high but not moderate or low aversive learning. Supporting this notion, one study has found that propranolol disrupts memory formation when administered immediately after a high stress (passive avoidance practice + swimming stress) learning paradigm, but this effect was not observed in the mild stress (only passive avoidance exercise) version of the same task (28). However, in another study, propranolol administration did not yield any impairments in spatial memory in both high and low stress conditions (1). While propranolol disrupted both the consolidation and reconsolidation processes of object recognition memory, which was defined as a low aversive task in the same study, it did not impair the memory formation processes of the high aversive version of the same task. (1). These findings contradict with the explanation that propranolol is only effective in memories of high-emotional arousal states, and show that the efficacy of propranolol administration is not associated with the averseness of the task.
Acute or Chronic Administration
Another explanation regarding the contradicting results obtained in propranolol studies may be the methodological differences in administration for some learning tasks. While newly learned information is consolidated into long-term memory after the initial training, repeated cycles of the training cause reactivation of the memory which might induce the reconsolidation process (25). Therefore, the drug must have memory impairing effect on both the consolidation and the reconsolidation processes in experiments with multiple training sessions (25). Since different neurotransmitter systems and receptors might be involved during the consolidation and the reconsolidation phases, some of the contradicting results could be explained on the basis of chronic or acute administration. Animals that were trained in the MWM with multiple sessions, in which they were exposed to chronic propranolol administration, propranolol did not cause impairments on spatial memory (21,23). However, in the experiment of Cahill et al. (2000) propranolol impaired the memory when administered acutely (24). On the contrary, another study has found that systemic propranolol administration after a single training session of spatial memory did not cause any disruption in the memory consolidation process (26). Additionally, in a study evaluating the effect of acute and chronic propranolol administration on the drug-related memory consolidation, chronic propranolol injections resulted in the disruption of memory formation, while this effect was not observed in animals that received acute injections (41). These conflicting results rule out the possibility that propranolol efficacy is dependent on whether it is administered acutely or chronically.
CONCLUSION
Although studies investigating the amnestic effects of propranolol on the consolidation and reconsolidation phases of memory produced inconsistent results, more consistent results were obtained when propranolol was administered during the reconsolidation phase. However, considering the studies that did not find any effect of propranolol on memory reconsolidation, one can argue that in vivo studies targeting memory formation should be supported by in vitro methods to elucidate the mechanisms behind the behavioral findings. As another factor, intracerebral administration of propranolol produces more reliable and reproducible results compared to systemic injection, but these studies have a disadvantage since they are clinically less relevant for the time being.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept- SK, ÇFİ; Design- SK, ÇFİ; Supervision- SK, ÇFİ; Resource- (-); Materials- (-); Data Collection and/or Processing- (-); Analysis and/or Interpretation- SK, ÇFİ; Literature Search- SK, ÇFİ; Writing- SK, ÇFİ; Critical Reviews- SK, ÇFİ.
Conflict of Interest: The authors declared that there is no conflict of interest.
Financial Disclosure: No financial support was received for this study.
REFERENCES
- 1.Villain H, Benkahoul A, Drougard A, Lafragette M, Muzotte E, Pech S, et al. Effects of propranolol, a ?-noradrenergic antagonist, on memory consolidation and reconsolidation in mice. Front Behav Neurosci. 2016;10:49. doi: 10.3389/fnbeh.2016.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taherian F, Vafaei AA, Vaezi GH, Eskandarian S, Kashef A, Rashidy-Pour A. Propranolol-induced impairment of contextual fear memory reconsolidation in rats:a similar effect on weak and strong recent and remote memories. Basic Clin Neurosci. 2014;5:231–239. https: //www.ncbi.nlm.nih.gov/pmc/articles/PMC4202546/ [PMC free article] [PubMed] [Google Scholar]
- 3.Otis JM, Mueller D. Inhibition of ?-adrenergic receptors induces a persistent deficit in retrieval of a cocaine-associated memory providing protection against reinstatement. Neuropsychopharmacology. 2011;36:1912–1920. doi: 10.1038/npp.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 5.Dębiec J, Ledoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 6.André MA, Wolf OT, Manahan-Vaughan D. Beta-adrenergic receptors support attention to extinction learning that occurs in the absence, but not the presence, of a context change. Front Behav Neurosci. 2015;9:125. doi: 10.3389/fnbeh.2015.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muravieva EV, Alberini CM. Limited efficacy of propranolol on the reconsolidation of fear memories. Learn Mem. 2010;17:306–313. doi: 10.1101/lm.1794710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu R-T, Liu X-H, Shi Y-W, Wang X-G, Xue L, Zhao H. Propranolol can induce PTSD-like memory impairments in rats. Brain Behav. 2018;8:e00905. doi: 10.1002/brb3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox WR, Faliagkas L, Besseling A, van der Loo RJ, Spijker S, Kindt M, et al. Interfering with contextual fear memories by post-reactivation administration of propranolol in mice:a series of null findings. Front Behav Neurosci. 2021;16:893572. doi: 10.3389/fnbeh.2022.893572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rotondo F, Biddle K, Chen J, Ferencik J, D'Esneval M, Milton AL. Lack of effect of propranolol on the reconsolidation of conditioned fear memory due to a failure to engage memory destabilisation. Neuroscience. 2022;480:9–18. doi: 10.1016/j.neuroscience.2021.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Wehner JM, Radcliffe RA. Cued and contextual fear conditioning in mice. Curr Protoc Neurosci. 2004;8:8–5C. doi: 10.1002/0471142301.ns0805cs27. [DOI] [PubMed] [Google Scholar]
- 12.Ji J-Z, Wang X-M, Li B-M. Deficit in long-term contextual fear memory induced by blockade of beta-adrenoceptors in hippocampal CA1 region. Eur J Neurosci. 2003;17:1947–1952. doi: 10.1046/j.1460-9568.2003.02620.x. [DOI] [PubMed] [Google Scholar]
- 13.Nasehi M, Soltanpour R, Ebrahimi-Ghiri M, Zarrabian S, Zarrindast M-R. Interference effects of transcranial direct current stimulation over the right frontal cortex and adrenergic system on conditioned fear. Psychopharmacology (Berl) 2017;234:3407–3416. doi: 10.1007/s00213-017-4722-6. [DOI] [PubMed] [Google Scholar]
- 14.Abrari K, Rashidy-Pour A, Semnanian S, Fathollahi Y. Administration of corticosterone after memory reactivation disrupts subsequent retrieval of a contextual conditioned fear memory:dependence upon training intensity. Neurobiol Learn Mem. 2008;89:178–184. doi: 10.1016/j.nlm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Villain H, Benkahoul A, Birmes P, Ferry B, Roullet P. Influence of early stress on memory reconsolidation:implications for post-traumatic stress disorder treatment. PloS One. 2018;13:e0191563. doi: 10.1371/journal.pone.0191563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bush DEA, Caparosa EM, Gekker A, Ledoux J. Beta-adrenergic receptors in the lateral nucleus of the amygdala contribute to the acquisition but not the consolidation of auditory fear conditioning. Fron Behav Neurosci. 2010;4:154. doi: 10.3389/fnbeh.2010.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 18.Dębiec J, Bush DEA, LeDoux JE. Noradrenergic enhancement of reconsolidation in the amygdala impairs extinction of conditioned fear in rats-a possible mechanism for the persistence of traumatic memories in PTSD. Depress Anxiety. 2011;28:186–193. doi: 10.1002/da.20803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vetere G, Piserchia V, Borreca A, Novembre G, Aceti M, Ammassari-Teule M. Reactivating fear memory under propranolol resets pre-trauma levels of dendritic spines in basolateral amygdala but not dorsal hippocampus neurons. Front Behav Neurosci. 2013;7:211. doi: 10.3389/fnbeh.2013.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luyten L, Schnell AE, Schroyens N, Beckers T. Lack of drug-induced post-retrieval amnesia for auditory fear memories in rats. BMC Biol. 2021;19:1–15. doi: 10.1186/s12915-021-00957-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Decker MW, Gill TM, McGaugh JL. Concurrent muscarinic and ?-adrenergic blockade in rats impairs place-learning in a water maze and retention of inhibitory avoidance. Brain Res. 1990;513:81–85. doi: 10.1016/0006-8993(90)91091-t. [DOI] [PubMed] [Google Scholar]
- 22.Kenton L, Boon F, Cain D. Combined but not individual administration of ?-adrenergic and serotonergic antagonists impairs water maze acquisition in the rat. Neuropsychopharmacol. 2008;33:1298–1311. doi: 10.1038/sj.npp.1301518. [DOI] [PubMed] [Google Scholar]
- 23.Saber AJ, Cain DP. Combined beta-adrenergic and cholinergic antagonism produces behavioral and cognitive impairments in the water maze:implications for Alzheimer disease and pharmacotherapy with beta-adrenergic antagonists. Neuropsychopharmacology. 2003;28:1247–1256. doi: 10.1038/sj.npp.1300163. [DOI] [PubMed] [Google Scholar]
- 24.Cahill L, Pham CA, Setlow B. Impaired memory consolidation in rats produced with beta-adrenergic blockade. Neurobiol Learn Mem. 2000;74:259–266. doi: 10.1006/nlme.1999.3950. [DOI] [PubMed] [Google Scholar]
- 25.Alberini CM. The role of reconsolidation and the dynamic process of long-term memory formation and storage. Front Behav Neurosci. 2011;5:12. doi: 10.3389/fnbeh.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Czech DA, Nielson KA, Laubmeier KK. Chronic propranolol induces deficits in retention but not acquisition performance in the water maze in mice. Neurobiol Learn Mem. 2000;74:17–26. doi: 10.1006/nlme.1999.3944. [DOI] [PubMed] [Google Scholar]
- 27.Przybyslawski J, Roullet P, Sara SJ. Attenuation of emotional and nonemotional memories after their reactivation:role of beta adrenergic receptors. J Neurosci. 1999;19:6623–6628. doi: 10.1523/JNEUROSCI.19-15-06623.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider AM, Simson PE, Atapattu RK, Kirby LG. Stress-dependent impairment of passive-avoidance memory by propranolol or naloxone. Pharmacol Biochem Behav. 2011;98:539–543. doi: 10.1016/j.pbb.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallagher M, Kapp B, Musty R, Driscoll P. Memory formation:evidence for a specific neurochemical system in the amygdala. Science. 1977;198:423–425. doi: 10.1126/science.20664. [DOI] [PubMed] [Google Scholar]
- 30.Bahar A, Samuel A, Hazvi S, Dudai Y. The amygdalar circuit that acquires taste aversion memory differs from the circuit that extinguishes it. Eur J Neurosci. 2003;17:1527–1530. doi: 10.1046/j.1460-9568.2003.02551.x. [DOI] [PubMed] [Google Scholar]
- 31.Miranda MI, Rodriguez-Garcia G, Reyes-López JV, Ferry B, Ferreira G. Differential effects of ?-adrenergic receptor blockade in basolateral amygdala or insular cortex on incidental and associative taste learning. Neurobiol Learn Mem. 2008;90:54–61. doi: 10.1016/j.nlm.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Guzmán-Ramos K, Osorio-Gómez D, Moreno-Castilla P, Bermúdez-Rattoni F. Post-acquisition release of glutamate and norepinephrine in the amygdala is involved in taste-aversion memory consolidation. Learn Mem. 2012;231:238. doi: 10.1101/lm.024703.111. [DOI] [PubMed] [Google Scholar]
- 33.Reyes-López J, Nuñez-Jaramillo L, Morán-Guel E, Miranda MI. Differential effects of ?-adrenergic receptor blockade in the medial prefrontal cortex during aversive and incidental taste memory formation. Neuroscience. 2010;169:195–202. doi: 10.1016/j.neuroscience.2010.04.054. [DOI] [PubMed] [Google Scholar]
- 34.Lueptow LM. Novel object recognition test for the investigation of learning and memory in mice. J Vis Exp. 2017;(126):55718. doi: 10.3791/55718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X, Ma L, Li HH, Huang B, Li YX, Tao YZ, et al. ?-Arrestin-biased signaling mediates memory reconsolidation. Proc Natl Acad Sci U S A. 2015;112:4483–4488. doi: 10.1073/pnas.1421758112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conversi D, Cruciani F, Accoto A, Cabib S. Positive emotional arousal increases duration of memory traces:Different role of dopamine D1 receptor and ?-adrenoceptor activation. Pharmacol Biochem Behav. 2014;122:158–163. doi: 10.1016/j.pbb.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 37.McKendrick G, Graziane NM. Drug-induced conditioned place preference and its practical use in substance use disorder research. Front Behav Neurosci. 2020;14:582147. doi: 10.3389/fnbeh.2020.582147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernardi RE, Lattal KM, Berger SP. Postretrieval propranolol disrupts a cocaine conditioned place preference. Neuroreport. 2006;17:1443–1447. doi: 10.1097/01.wnr.0000233098.20655.26. [DOI] [PubMed] [Google Scholar]
- 39.Fricks-Gleason AN, Marshall JF. Post-retrieval beta-adrenergic receptor blockade:effects on extinction and reconsolidation of cocaine-cue memories. Learn Mem. 2008;15:643–648. doi: 10.1101/lm.1054608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernardi RE, Lattal KM. Post-conditioning propranolol disrupts cocaine sensitization. Pharmacol Biochem Behav. 2012;102:515–519. doi: 10.1016/j.pbb.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L, Huang S, Yang C, Wu F, Zheng Q, Yan H, et al. Blockade of ?-adrenergic receptors by propranolol disrupts reconsolidation of drug memory and attenuates heroin seeking. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.686845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diergaarde L, Schoffelmeer AN, De Vries TJ. ?-adrenoceptor mediated inhibition of long-term reward-related memory reconsolidation. Behav Brain Res. 2006;170:333–336. doi: 10.1016/j.bbr.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 43.Kroon JA V, Carobrez AP. Olfactory fear conditioning paradigm in rats:Effects of midazolam, propranolol or scopolamine. Neurobiol Learn Mem. 2009;91:32–40. doi: 10.1016/j.nlm.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Beatty WW, Rush JR. Spatial working memory in rats:Effects of monoaminergic antagonists. Pharmacol Biochem Behav. 1983;18:7–12. doi: 10.1016/0091-3057(83)90242-3. [DOI] [PubMed] [Google Scholar]
- 45.Berman DE, Dudai Y. Memory extinction, learning anew, and learning the new:dissociations in the molecular machinery of learning in cortex. Science (New York, N. Y.) 2001;291:2417–2419. doi: 10.1126/science.1058165. [DOI] [PubMed] [Google Scholar]
- 46.Cain CK, Blouin AM, Barad M. Adrenergic transmission facilitates extinction of conditional fear in mice. Learn Mem. 2004;11:179–187. doi: 10.1101/lm.71504. [DOI] [PMC free article] [PubMed] [Google Scholar]


