Abstract
Little is known about access of rare disease carriers to health care. To increase this knowledge, the Pan American Hereditary Ataxia Network (PAHAN) conducted an exploratory survey about care for hereditary ataxias in American continents and the Caribbean. A questionnaire was sent to health professionals about the hereditary ataxias identified; access to care; and local teaching and research. The number of ataxics under current care per 100,000 inhabitants was subtracted from the expected overall prevalence of 6/100,000, to estimate the prevalence of uncovered ataxic patients. Local Human Development Indexes (HDI) were used to measure socio-economic factors. Twenty-six sites participated. Twelve sites had very high, 13 had high, and one site had medium HDI. Participants reported on 2239 and 602 patients with spinocerebellar ataxias and recessive forms under current care. The number of patients under current care per inhabitants varied between 0.14 and 12/100,000. The estimated prevalence of uncovered ataxic patients was inversely proportional to HDIs (rho = 0.665, p = 0.003). Access to diagnosis, pre-symptomatic tests, and rehabilitation were associated with HDIs. More and better molecular diagnostic tools, protocols and guidelines, and professional training for ataxia care were the top priorities common to all respondents. Evidence of inequalities was confirmed. Lower HDIs were associated with high potential numbers of uncovered ataxic subjects, and with lack of molecular diagnosis, pre-symptomatic testing, and rehabilitation. More and better diagnostic tools, guidelines, and professional training were priorities to all sites. PAHAN consortium might help with the last two tasks.
Keywords: Inherited ataxias, Access to health care, American continents, The Caribbean
Introduction
Access to health care is a complex concept that usually means the timely use of personal health services to achieve the best health outcomes. Access to health care has been envisaged in different ways by societies in the American continents and the Caribbean (ACC), with different outcomes. At least four aspects are required to guarantee its achievement: coverage, services, timeliness, and workforce [1].
Coverage is heterogeneous among ACC sites. Several Latin American (LA) countries and Canada offer publicly funded health care. The right of access is set down in rules applying to the whole population contributing to the fund—by taxes—and therefore receiving benefits from it. In contrast, in countries as diverse as the United States of America (USA), Chile, and Peru, health systems are private, where citizens need to buy their health insurance. If services are available and an adequate supply of services is at hand, then the opportunity to obtain health care is for real. Timeliness is the ability to provide health care when the need is recognized. Finally, by workforce, we refer to the availability of capable, qualified, and culturally competent health workers [2].
The diversity of this scenario is particularly unknown in the case of rare diseases such as neurogenetic disorders in general and particularly in the case of hereditary ataxias [3]. Rare diseases are those with a prevalence of less than 5 per 10,000 [3]. Unlike several other rare disorders, ataxias stand out for presenting usually a clear phenotype that is easy to recognize by health professionals with basic training in neurology. Therefore, hereditary ataxias can be taken as an example of a rare disease in which the diagnostic delay will be due less to limited knowledge about these conditions among clinicians than to difficulties in accessing health care. Hereditary ataxias can also serve as a model for this kind of survey, because there are already numerous health-care sites where ataxia information has been recorded.
There is an increasing perception that transnational super-structures would help sharing knowledge and care coordination for patients affected by rare neurological diseases [4]. The Pan American Hereditary Ataxia Network (PAHAN) brought together a group of researchers with the aim to enable networks and multilateral studies dedicated to the ataxia field, to promote schools on neurogenetics, and to help the readiness of the ataxic populations from the ACC for clinical trials to come [5]. But data on the number of ataxics diagnosed and followed up as well as their access to health care remain unclear. This might be in line with the fact that populations from ACC suffer from historical inequities that hamper their access to health care.
To help find specific problems and potential solutions, PAHAN sent a survey to several health workers and researchers known to treat ataxic subjects. The survey aimed to raise general preliminary data on hereditary ataxias already identified in ACC; the access of ataxic individuals to health care, from diagnosis to counseling, predictive testing, and rehabilitation; and the researchers and local lines of investigation related to ataxia. We report the results obtained so far.
Methods
Population and Procedures
Invitations to participate in the survey were sent by emails to 72 health professionals known to follow-up hereditary ataxia patients from Argentina, Bolivia, Brazil, Canada, Chile, Colombia, Cuba, Ecuador, Mexico, Peru, Uruguay, the USA, and Venezuela and also through the National Ataxia Foundation (NAF) newsletter. Messages contained the links to the survey, written either in English, Spanish, or Portuguese. The recipients were also asked to freely share the survey with other professionals.
The questionnaire is presented in Supplemental Material 1. In summary, the survey presented 13 questions related to the three aims described above. These questions were:
The number of subjects and families with ataxia under the informant’s current care (the prevalent cases)
The number of subjects and families with ataxias followed during the last 20 years by the informant
The specific diagnoses in the two scenarios (current and cumulative in the last 20 years), of spinocerebellar ataxias types 1 (SCA1); 2 (SCA2); 3, also known as Machado-Joseph disease (SCA3/MJD); 6 (SCA6); 7 (SCA7); 8 (SCA8); 10 (SCA10); 12 (SCA12); and 17 (SCA17); dentatorubral-pallidoluysian atrophy (DRPLA); Friedreich ataxia (FRDA); ataxia-telangiectasia (AT); ataxia with oculomotor apraxia types 1 (AOA1), 2 (AOA2), 3 (AOA3), and 4 (AOA4); autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS) and cerebellar ataxia with neuropathy and vestibular areflexia syndrome (CANVAS)
Whether the health-care funding of the informant’s institution was public or private
On access of local ataxic patients to different alternatives for molecular diagnosis
On access to various complementary tests that are usually listed in diagnostic protocols for hereditary ataxias
On existence of a clinical protocol for ataxias with therapeutic guidelines in the informant’s service
On local access to genetic counseling and pre-symptomatic testing
On access to rehabilitation services
About the appropriateness or limitation of such care locally
On local supervision of health sciences students dedicated to the study of ataxias
About the existence of clinical and experimental research projects in progress, locally.
Finally, we ended up asking the informant to prioritize the next 10 items in relation to local development needs: more doctors, genetic counseling (GC) professionals, rehabilitation professionals, access to molecular tests, access to MRI, clinical protocols and therapeutic guidelines, infrastructure for clinical care, infrastructure for rehabilitation, funding for research, and professional training.
Considering that we were unaware of the way that local investigators stored their information, we raised questions of interest using different timelines and measurement units. We asked for numbers of patients or numbers of families; and we asked both prevalent data and cumulative ataxic data. The type of data most spontaneously stored by local investigators would be the most leveraged in the present report of the results. Answering or not each question was fully eligible for participants: respondents could even submit a totally empty form. This was open research in which one of the objects under study were the respondents themselves, and mandatory items could leave participants out of the survey.
Since there was no direct—through interviews or examinations—or indirect study of patients, through medical records or databases, the study did not require ethical approval or consent forms. On the other hand, all study participants were health-care professionals. At the end of the survey, they participated in the analyses. All of them participate in the list of authors and therefore give their consent to be identified.
Analysis
Data from each participant center was related to a geographic region (called “region” from now on) and population. Private centers were related to local cities; public centers were related to states/departments/provinces, or less frequently to countries. When reports from independent institutions were obtained for the same region, the numbers were added and presented as the total per region.
The population of each geographic region was obtained from the local national census [6–15]. The number of ataxic patients under current care per 100,000 inhabitants of each region was then estimated. The global average (95% CI) prevalence of dominant and recessive hereditary ataxias were estimated by a former meta-analysis as being 2.7/100,000 (1.5–4.0/100,000) and 3.3/100,000 (1.8–4.9/100,000), respectively [16]. An overall expected prevalence was assigned as a standard for the present study and was equivalent to the amount of the two general averages of the meta-analysis, or 6/100,000 inhabitants.
In order to assess how close each site would be to cover the ataxic patients of its region, the number of local ataxic patients under current care/100,000 was subtracted from the overall average prevalence of inherited ataxias. Negative numbers predicted the existence of a large number of uncovered/undiagnosed patients, and positive numbers or numbers close to zero predicted a reasonable diagnostic coverage. The number obtained by this subtraction was an estimation of the prevalence of uncovered ataxic patients per region.
The Human Development Index (HDI) of the city, state, or country was used as an independent variable to measure socio-economic risk factors [17, 18]. In brief, HDI is a measure of average achievement in key three dimensions of human development. The health dimension is measured by life expectancy at birth. The education dimension is assessed by means of years of schooling for adults aged 25 years and more and expected years of schooling for children of school entering age. Finally, the standard of living dimension is measured by gross national income per capita. The three scores are then aggregated into HDI using geometric mean.
This study had an exploratory character. Continuous variables were not normally distributed, and Mann–Whitney, Kruskal–Wallis, and Spearman rank correlation tests were used, for a p < 0.05. Analyses were performed using PASW statistics version 18.
Results
Emails inviting health professionals to participate in the survey were sent between July 26 and October 28, 2021. Twenty-eight respondents agreed to participate, from 26 different sites (Supplemental material 2, Table S1). All participants were included through individual direct emails sent to 72 known professionals. Queries were sent back to some respondents and clarifications improved the records.
Table 1 summarizes the general data and general responses obtained per initial questions of the survey. As expected, numbers were heterogeneous, as each center or professional replied according to her/his wish and according to the way their local records were obtained and stored.
Table 1.
General description of answers obtained in the survey
| Characteristics | Number of cases | |
|---|---|---|
| Responses in | English | 18/28 * |
| Spanish | 7/28 * | |
| Portuguese | 3/28 * | |
| Respondents were | Neurologists | 19/28 * |
| Clinical geneticists | 4/28 * | |
| Neuropediatricians | 1/28 * | |
| Professionals related to molecular diagnoses | 3/28 * | |
| Other | 1/28 * | |
| Answers were about | Public services | 20/26 |
| Private health services | 6/26 | |
| Geographic origin | Argentina | 1/26 |
| Bolivia | 1/26 | |
| Brazil | 11/26 | |
| Canada | 2/26 | |
| Chile | 3/26 | |
| Cuba | 1/26 | |
| Mexico | 3/26 | |
| USA | 2/26 | |
| Uruguay | 1/26 | |
| Peru | 1/26 | |
| Answers about the total number of ataxic patients under current care | 25/26 | |
| Answers about the total number of ataxic families under current care | 19/26 | |
| Answers about specific diagnoses per subject in the last 20 years | 12/26 | |
| Answers about specific diagnoses per family in the last 20 years | 10/26 | |
Number of respondents. The other items comprised the number of sites
Total Number of Ataxia Patients and Families
Twenty-six sites reported on their number of ataxia patients and/or families. The question with most responses from the survey was “How many patients with ataxia are you currently seeing?” In total, 2841 patients were reported to be under current care: 2239 with spinocerebellar ataxias and 602 with confirmed or potential recessive forms. Figure 1 describes the answers obtained.
Fig. 1.
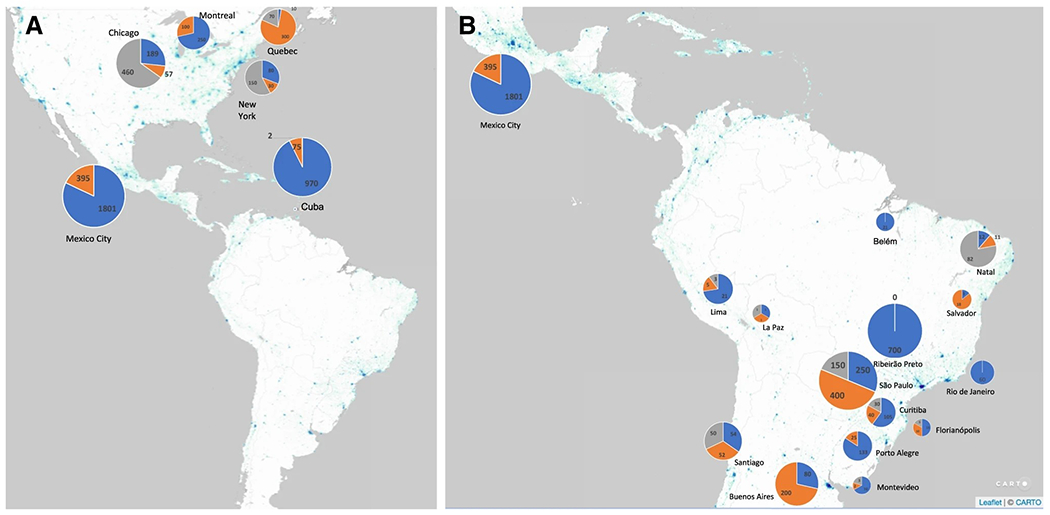
Number of ataxia patients under current care, according to the information given by the participants of the survey. Dominant forms are in blue, recessive forms in orange, and non-genetic forms in gray. A Participant sites from North America. B Participant sites from South America. Data was presented as per city
The numbers of ataxic patients under care were then standardized as per 100,000 inhabitants per region (Table 2). These values were subtracted from the overall average prevalence of inherited ataxias, obtained elsewhere [16], to assess how close each region would be to cover their ataxic patients. The deltas (the difference between the prevalent number of subjects under care and the estimated prevalence of ataxia) obtained correlated well with HDI of the regions (rho = 0.665, p < 0.003; Fig. 2).
Table 2.
The absolute and the estimated number per 100,000 inhabitants of ataxic patients under current care, per region informed
| Country | Region under analysis | HDI | Public or private health services | Number of ataxic patients under current care | Population | Number of ataxic patients under care/inhabitants | ||
|---|---|---|---|---|---|---|---|---|
| SCAs | Autosomal recessive | Total | ||||||
| Argentina | Buenos Aires Province | 0.845 | Public health | 80 | 200 | 280 | 15,625,084 | 1.8/100,000 |
| Chile | Santiago City* | 0.851 | Private health | 54 | 52 | 108 | 5,700,000 | 1.9/100,000 |
| Cuba | Cuba | 0.783 | Public health | 970 | 75 | 2 | 11,330,000 | 9.2/100,000 |
| Uruguay | Montevideo City | 0.817 | Private health | 10 | 2 | 12 | 1,319,108 | 0.9/100,000 |
| Brazil | Rio Grande do Sul state | 0.787 | Public health | 133 | 25 | 158 | 10,860,000 | 1.5/100,000 |
| Santa Catarina state | 0.808 | Public health | 15 | 10 | 25 | 6,091,000 | 0.4/100,000 | |
| Paraná State | 0.792 | Public health | 105 | 40 | 145 | 11,080,000 | 1.3/100,000 | |
| São Paulo State * | 0.826 | Public health | 1180 | 680 | 1860 | 44,035,304 | 4.2/100,000 | |
| Rio de Janeiro State | 0.796 | Public health | 60 | - | 60 | 15,772,000 | 0.4/100,000 | |
| Bahia State | 0.714 | Private health | 3 | 18 | 21 | 14,561,000 | 0.14/100,000 | |
| Rio Grande do Norte state | 0.731 | Public health | 12 | 11 | 23 | 3,168,133 | 0.7/100,000 | |
| Pará state | 0.698 | Public health | 21 | 0 | 21 | 7,136,000 | 0.3/100,000 | |
| Bolivia | La Paz Department | 0.718 | Public health | 1 | 1 | 2 | 2,706,359 | 0.07/100,000 |
| Peru | Lima Province (metropolitan) | 0.777 | Public health | 21 | 5 | 26 | 9,752,000 | 0.3/100,000 |
| Mexico | Mexico City State* | 0.779 | Public health | 1801 | 395 | 2196 | 16,992,418 | 12/100,000 |
| USA | New York City | 0.944 | Public health | 80 | 30 | 110 | 8,804,190 | 1.2/100,000 |
| Chicago | 0.934 | Private health | 189 | 57 | 246 | 2,746,388 | 8.9/100,000 | |
| Canada | Quebec Province * | 0.929 | Public health | 260 | 400 | 660 | 8,500,000 | 7.8/100,000 |
Independent reports (from independent institutions) were obtained for the region under analysis. The numbers were added and presented as the total per region
Fig. 2.
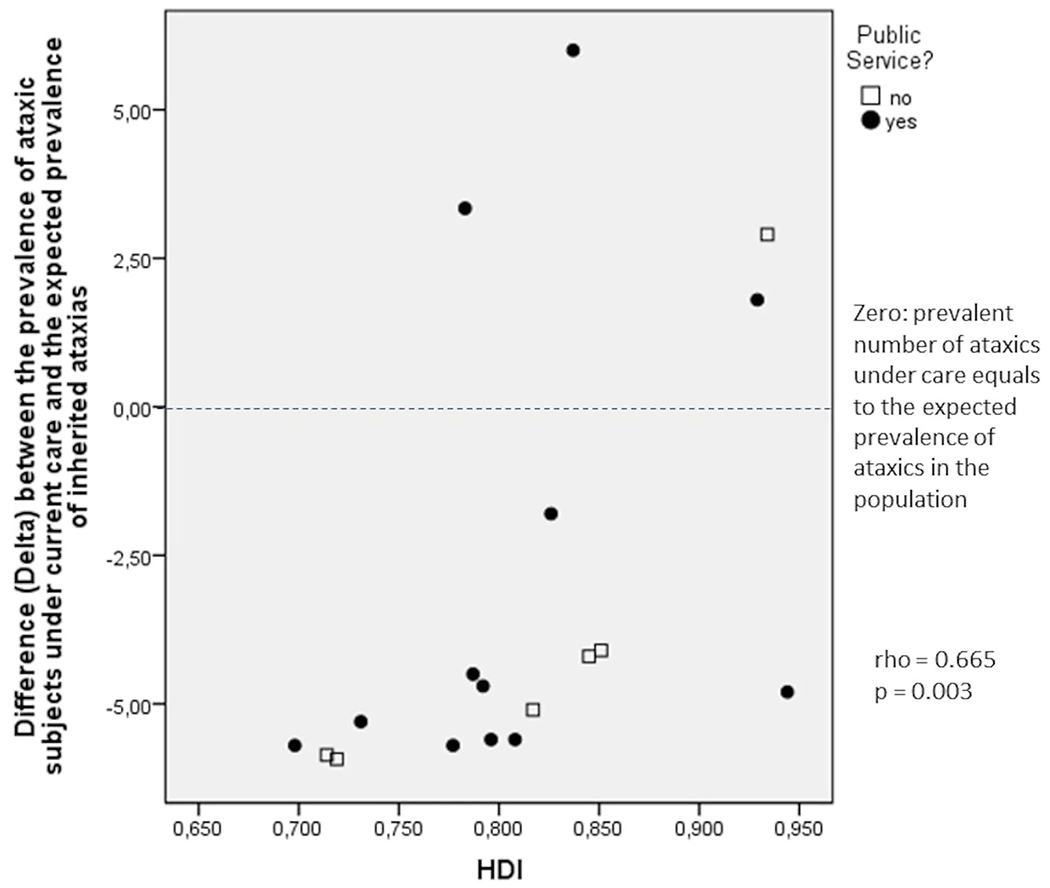
Correlation between Human Development Index (HDI) of each region and the difference (Delta) between the number of ataxic subjects under current care/100,000 local inhabitants and the mean global prevalence of inherited ataxias, 6/100,000 inhabitants
Proportion of Diagnosis According to the Site of Origin
Thirteen centers reported their total number of inherited ataxias diagnosed in the last 20 years. The unit of measure varied across centers: 9 centers reported about the number of patients and the number of families, 3 centers about the number of patients only, and one center about the number of families only. Data are presented in Supplemental Material 2—Tables S2 and S3—and in Fig. 3.
Fig. 3.
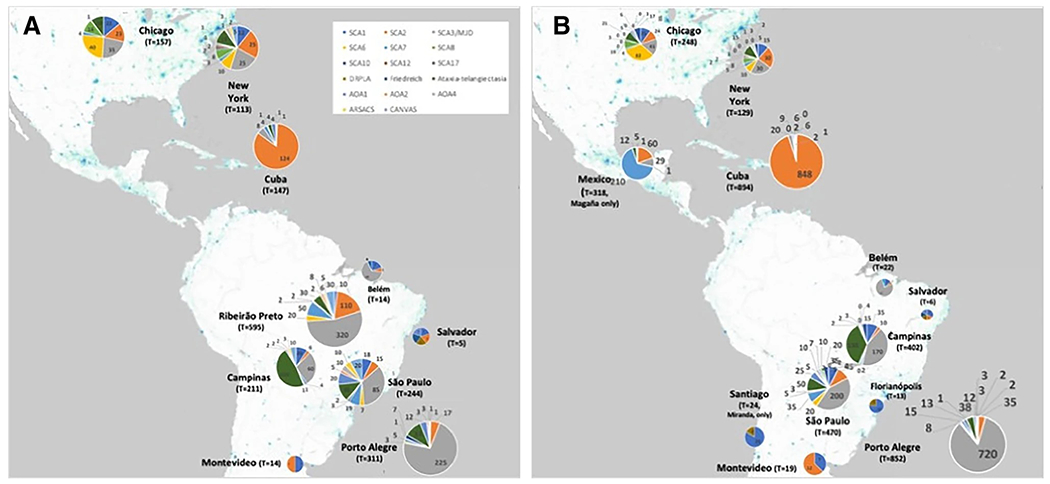
Cumulative numbers of specific diagnoses done in the last 20 years, according to the information given by the participants of the survey. A Data obtained on the number of ataxia families. B Data obtained on the number of ataxia subjects
The specific diagnoses investigated are described in the “Methods” section. The open question on “other forms of inherited ataxias” was filled out by respondents that mentioned the existence of ataxic cases diagnosed with SCA11, SCA15, SCA19, SCA26, SCA34, SCA42, autosomal recessive cerebellar ataxias types 1 (ARCA1) and 3 (ARCA3), ataxia with vitamin E deficiency (AVED), Gerstmann-Straussler-Scheinker disease, spastic paraplegia type 7 (SPG7), and ataxias related to mutations in the genes of the RNA polymerase III subunits A (PolR3A) and B (PolR3B), patatin-like phospholipase domain containing 6 (PNPLA6), and sterile alpha motif domain containing 9 like (SAMD9L). Of note was the report of 20 SPG7, 6 AVED, 3 SCA19, and 2 Gerstmann-Straussler-Scheinker ataxic individuals across these countries—these entities being detected in two sites each.
Access of the Local Population to Health Care in Participant Sites
Several questions aimed at addressing the access to health care in the diverse regions whose health-care providers participated in this survey. The variables that picked up differences between diverse HDIs were the availability of technologies needed for the molecular diagnosis, of pre-symptomatic testing programs, and of rehabilitation approaches, including physical, speech, and occupational therapies (Table 3).
Table 3.
Access to health-care per region, informed by the health-care providers that participated in this survey
| Local availability of | Sites/regions with | HDI Median (95% CI) | p |
|---|---|---|---|
| Clinical diagnosis and follow-up visits | Adequate availability | 0.826 (0.798–0.876) | 0.218* |
| Limited availability | 0.797 (0.752–0.829) | ||
| Clinical protocol for ataxias with therapeutic guidelines | Evidence-based protocol built after public consultation by the country’s health authority | 0.777 (0.700–0.829) | 0.127** |
| Local protocols | 0.829 (0.801–0.863) | ||
| No protocols | 0.821 (0.755–0.855) | ||
| Laboratory work-up | Adequate availability | 0.837 (0.817–0.916) | 0.001* |
| Limited availability | 0.791 (0.756–0.807) | ||
| Technologies needed for the molecular diagnosis of inherited ataxias: (1) panels for expanded repeat ataxias; (2) panels for point mutations, frameshift, etc.; (3) NGS panels; (4) WES; and (5) WGS | At least four techniques | 0.832 (0.798–0.861) | 0.045 * |
| Three or less techniques available | 0.783 (0.696–0.834) | ||
| Genetic counseling | Adequate availability | 0.838 (0.803–0.879) | 0.071* |
| Limited availability | 0.787 (0.744–0.824) | ||
| Pre-symptomatic testing program | Available | 0.839 (0.799–0.874) | 0.019* |
| Not available | 0.784 (0.742–0.812) | ||
| Physical, speech, occupational therapies | Adequate availability | 0.836 (0.797–0.872) | 0.025* |
| Limited availability | 0.782 (0.741–0.810) |
Mann–Whitney U test;
Kruskal–Wallis test; NGS stands for next-generation sequencing; WES stands for whole-exome sequencing; WGS stands for whole-genome sequencing
Local Education and Research in Ataxia
Twelve out of 28 respondents (42%) informed they were supervising undergraduate (11 respondents), MSc (12), PhD (8), or post-doctoral (6) students in the field of hereditary ataxias.
Seventeen sites out of 26 (65%) reported they were performing clinical studies on hereditary ataxias: six sites were focusing on SCA1, 12 on SCA2, 13 on SCA3/MJD, six on SCA6, seven on SCA7, one on SCA8, six on SCA10, six on FRDA, three on AT/AOA, four on ARSACS, and four on CANVAS. Private funding was supporting 24% of these studies.
Five sites reported they were performing experimental studies, being one in SCA1, two in SCA3/MJD, one in SCA6, one in SCA10, three in other SCAs, one in FRDA, and one in CANVAS; 10% of these studies were supported by private funding.
Local Demands and Priorities
To analyze the responses on the priorities to improve the health care of ataxic patients in different region, respondents were divided into two groups according to HDI tiers: 11 participants with very high (0.8–1.0) and eight participants with high (0.7–0.79) or medium HDIs (0.55–0.70). The number one priority chosen by both groups was the access to more and better molecular diagnostic tests. Both groups also included among the top four priorities the need for protocols and guidelines and for professional training for ataxia care (Supplemental material 2—Table S4).
Discussion
As far as we are aware, this was the first survey carried out on the health-care status of ataxic people in the ACC.
Thirty-nine percent of the emails sent by the PAHAN group to professionals were replied, representing all participants of this survey. Most respondents came from Latin America; 11 (42%) of them were Brazilians. In contrast, NAF lists 20 ataxologist clinics in the USA and one in Canada [19]. Although this picture limits the generalizability of our ACC patient and family numbers, it is likely that the correlations described below would not change much if US participation in the survey increased. The peculiar national profile of the participants in the survey may be due to the chances of greater confidence and identification with the study authors, most of whom were also from LA. It may also demonstrate the greater need for support from LA professionals, who are more ready to seek help from networks of researchers. Most respondents were neurologists, work in public health services, and have a clear academic profile. The interpretation of the answers must take these profiles into account.
The nature of the participating sites portrayed the health organization of their home countries. Several participants work in public health services, which usually receive patients referred from basic health units located in large territories, such as states or provinces. This is the model of the Brazilian “Sistema Único de Saúde” (SUS), for instance [20, 21]. In contrast, health care of ataxics is a mostly private service in Argentina, Chile, and the USA. Private services usually cover the urban population where they live. Based on this, we defined that the region of population coverage of private services would be their cities, and that of public services would be their province/state.
Most sites were aware of their approximate total number of ataxic patients under current care. Although this allowed us to estimate how many ataxic patients were under their care per 100,000 inhabitants (Table 2), we cannot assure that all services and physicians who follow ataxics have sent their data about the regions included. So, it is worth stressing that the prevalence rates of ataxic under care may have been underestimated eventually. Conversely, the estimated number of ataxic people who are not covered by these health services might have been overestimated. Despite the uncertainty about these estimated (or minimum) prevalences of patients without care, we believe that the association between them and HDI is genuine (Fig. 2). This finding was certainly expected and supports, to some extent, the objectivity of some other results of this survey.
Data relating to the diagnoses of specific ataxias are useful to raise initial hypotheses about distinct geographic profiles, as previously done for some other conditions in LA, such as multiple system atrophy [22]. And it is relevant to emphasize that our data is preliminar. These results should be seen as exploratory and associated with recent reports on recessive ataxias [23] and on founder effects, the latter also from the PAHAN group [24]. More reliable data should be collected in a standardized manner in the following investigations.
Most data obtained pointed to great inequities in access to health care. Figure 2 shows that many ataxics are not being evaluated or diagnosed in places with lower HDI. There is no previous information to compare with our results. Recently, a study reported a direct correlation between monthly income and frequency of clinic visits of patients with neurogenetic disorders in Sri Lanka. Their results and data presented here clearly support the truism that “poverty is a major cause of ill health and a barrier to accessing healthcare when needed” (Samaranayake et al. 2020). Although both sets of results are predictable, proper reports are highly relevant, aiming to assess the issue and establish a baseline from which the effect of specific public policies could be measured.
Curiously, however, just one physician out of 28 participants reported that his site has no access to any molecular diagnosis. The remaining 27 respondents came from sites with high technological capacities, which influences their point of view. Although the need for high-tech molecular diagnosis, pre-symptomatic testing program, and rehabilitation seemed to be the most important demand for sites with low HDI, as shown in Table 3, this was not always mirrored by the responses to the last question (priorities). For instance, although pre-symptomatic testing programs and rehabilitation are lacking in sites with HDI lower than 0.800 (Table 3), these same sites considered that GC and rehabilitation professionals were placed at seven out of ten priorities of needs (Supplemental Material 2 Table S4).
Latin America had a particular representation in this survey. If 6:100,000 is the average prevalence of inherited ataxias worldwide [16], then among the 569 million LA inhabitants, we can estimate 34,140 persons with ataxia—17 times the number of ataxics under current care reported by the LA participants. At the same time, the fact that inherited ataxias are rare implies that only large centers will have the opportunity to provide access to the best possible healthcare for ataxic persons [4]. Therefore, better regional health networks are needed on the continent.
Listing priorities is one way to start proposing solutions. The average order of priorities obtained from responses from eleven sites with high HDI and eight sites with medium or low HDI confirmed some previous insights. All sites have chosen as their number one priority the provision of more and better molecular diagnostic tools. This information is of great importance for health managers in these countries, and eventually for the implementation of national diagnostic networks.
Finally, we wish to point to the fact that the need for protocols and guidelines and for professional training in ataxia care was among the top four priorities identified by all sites, regardless of their economic status. In fact, both tasks require personal interaction with experts and scientists in the field and, in a way, modest financial investment. Furthermore, both tasks can be embraced by consortiums such as PAHAN. If health professionals embark on a journey of closeness and collective work, and if the health authorities of each country work with effective measures, maybe we will be able to correct the health inequities that are so serious in our continents and offer the care that all people deserve.
Supplementary Material
Acknowledgements
We are grateful to the National Ataxia Foundation (NAF) and the Ataxia Global Initiative (AGI) (https://ataxia-global-initiative.net/projects/pahan-survey/) for supporting the dissemination of this survey. We thank Dr. Paulo Ribeiro Nóbrega for helping with data from Ceará, and the Cuban Network of Hereditary Ataxias for its contribution to the survey. GVF, MLSP, and LBJ were supported by CNPq, Brazil.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s12311-022-01442-z.
Ethics Approval This investigation did not require ethical approval.
Data Availability
The data that support findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Gulliford M, Figueroa-Munoz J, Morgan M, Hughes D, Gibson B, Beech R, Hudson M. What does “access to health care” mean? J Health Serv Res Policy. 2002;7(3):186–8. [DOI] [PubMed] [Google Scholar]
- 2.Access to Health Care. Content last reviewed June 2018. Agency for Healthcare Research and Quality, Rockville, MD. https://www.ahrq.gov/research/findings/nhqrdr/chartbooks/access/access.html Accessed November 12, 2021. [Google Scholar]
- 3.The LN. Rare neurological diseases: a united approach is needed. Lancet Neurol. 2011;10:109. [DOI] [PubMed] [Google Scholar]
- 4.Reinhard C, Bachoud-Lévi AC, Bäumer T, et al. The European Reference Network for Rare Neurological Diseases. Front Neurol. 2021;11:616569. 10.3389/fneur.2020.616569 (Published 2021 Jan 14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Pan-American Hereditary Ataxia Network. Content last reviewed November 2019. https://pan-american-hereditary-ataxia-network-pahan7.webnode.com/) Accessed October 15, 2021.
- 6.“Censo 2010 Argentina”. www.censo2010.indec.gov.ar/. Archived from the original on 8 October 2015. Retrieved 20 October 2015. Accessed November 1, 2021
- 7.INE. “Chile, proyecciones de población al 30 de junio (1990–2020): Región Metropolitana de Santiago”. Archived from the original (XLS) on 12 June 2009. Retrieved 23 December2007. Accessed November 1, 2021
- 8.“Censos 2011 Montevideo”. INE. 2012. Archived from the original on 11 November 2012. Retrieved 3 September2012. Accessed November 1, 2021
- 9.«Resumen del Balance Demográfico» (PDF). Cuba. Consultado em 15 de julho de 2017. Arquivado do original (PDF) em 31 de julho de 2017 [Google Scholar]
- 10.Sistema IBGE de Recuperação Automática – SIDRA (PDF)(in Portuguese). Brazil: IBGE. 2008. ISBN 978–85–240–3919–5. Retrieved August 24, 2014. [Google Scholar]
- 11.https://www.ine.gob.bo/ INE: Instituto Nacional de Estadística, Bolívia. Accessed November 1, 2021. [Google Scholar]
- 12.https://www.inei.gob.pe/estadisticas/indice-tematico/poblacion-y-vivienda/ PERÚ Instituto Nacional de Estadística e Informática. Accessed November 1, 2021. [Google Scholar]
- 13.https://www.inegi.org.mx/ Instituto Nacional de Estadística y Geografía (INEGI), Mexico. Accessed November 1, 2021. [Google Scholar]
- 14.https://www.census.gov/ United States Census Bureau. Accessed November 1, 2021. [Google Scholar]
- 15.https://www12.statcan.gc.ca/census-recensement/index-eng.cfm Census Program, Canada. Accessed November 1, 2021. [Google Scholar]
- 16.Ruano L, Melo C, Silva MC, Coutinho P. The global epidemiology of hereditary ataxia and spastic paraplegia: a systematic review of prevalence studies. Neuroepidemiology. 2014;42(3):174–83. [DOI] [PubMed] [Google Scholar]
- 17.Human Development Index (HDI) | Human Development Reports. hdr.undp.org. United Nations Development Programme. Archived from the original on 28 January 2017. Retrieved 15 December 2020. [Google Scholar]
- 18.Human Development Index (HDI). United Nations Development Programme. Retrieved August 5, 2013. https://www.ataxia.org/neurologists-and-specialty-clinics/, www.ataxia.org. National Ataxia Foundation. Retrieved on March 31, 2022 [Google Scholar]
- 19.National Ataxia Foundation. https://www.ataxia.org/neurologists-and-specialty-clinics/, www.ataxia.org. Retrieved on March 31, 2002.
- 20.Cornwall A, Shankland A. Engaging citizens: lessons from building Brazil’s national health system. Soc Sci Med. 2008;66(10):2173–84. 10.1016/j.socscimed.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 21.Menicucci TM. [The history of the public health reform in Brazil and of the Sistema Único de Saúde: changes, continuities, and the current agenda]. Hist Cienc Saude Manguinhos. Jan-Mar 2014;21(1):77–92. [DOI] [PubMed] [Google Scholar]
- 22.Gatto E, Rodríguez-Violante M, Cosentino C, Chana-Cuevas P, Miranda M, Gallin E, Etcheverry JL, Nuñez Y, Parisi V, Persi G, Vecchi C, Sanguinetti A, Alleva A, Aparcana J, Torres L, Litvan I. Pan-American Consortium of Multiple System Atrophy (PANMSA). A Pan-American multicentre cohort study of multiple system atrophy. J Parkinsons Dis. 2014;4(4):693–8. 10.3233/JPD-140434. [DOI] [PubMed] [Google Scholar]
- 23.Gama MTD, Braga-Neto P, Rangel DM, Godeiro C, Alencar R, Embiruçu EK, Cornejo-Olivas M, Sarapura-Castro EH, Awad PSChaná-Cuevas P, Kauffman M, Quiroga SR, Jardim LB, Graça FF, França MC, Marques W, Teive HAG, Barsottini OGP, Pedroso JLP, Synofzik M. Autosomal recessive cerebellar ataxias in South America: a multi-center study of 1,338 patients. Mov Disord. 10.1002/mds.29046 [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez-Labrada R, Martins AC, Magaña JJ, Vazquez-Mojena Y, Medrano-Montero J, Fernandez-Ruíz J, Cisneros B, Teive H, McFarland KN, Saraiva-Pereira ML, Cerecedo-Zapata CM, Gomez CM, Ashizawa T, Velázquez-Pérez L, Jardim LB. Pan American Hereditary Ataxia Network. Founder effects of spinocerebellar ataxias in the American continents and the caribbean cerebellum. 2020;19(3):446–58. 10.1007/s12311-020-01109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support findings of this study are available from the corresponding author upon reasonable request.


