Abstract
Background
Intra-abdominal hypertension and abdominal compartment syndrome are common with clinically significant consequences. We investigated the pathophysiological effects of raised IAP as part of a more extensive exploratory animal study. The study design included both pneumoperitoneum and mechanical intestinal obstruction models.
Methods
Forty-nine female swine were divided into six groups: a control group (Cr; n = 5), three pneumoperitoneum groups with IAPs of 20mmHg (Pn20; n = 10), 30mmHg (Pn30; n = 10), 40mmHg (Pn40; n = 10), and two mechanical intestinal occlusion groups with IAPs of 20mmHg (MIO20; n = 9) and 30mmHg (MIO30; n = 5).
Results
There were significant changes (p<0.05) noted in all organ systems, most notably systolic blood pressure (SBP) (p<0.001), cardiac index (CI) (p = 0.003), stroke volume index (SVI) (p<0.001), mean pulmonary airway pressure (MPP) (p<0.001), compliance (p<0.001), pO2 (p = 0.003), bicarbonate (p = 0.041), hemoglobin (p = 0.012), lipase (p = 0.041), total bilirubin (p = 0.041), gastric pH (p<0.001), calculated glomerular filtration rate (GFR) (p<0.001), and urine output (p<0.001). SVV increased progressively as the IAP increased with no obvious changes in intravascular volume status. There were no significant differences between the models regarding their impact on cardiovascular, respiratory, renal and gastrointestinal systems. However, significant differences were noted between the two models at 30mmHg, with MIO30 showing worse metabolic and hematological parameters, and Pn30 and Pn40 showing a more rapid rise in creatinine.
Conclusions
This study identified and quantified the impact of intra-abdominal hypertension at different pressures on several organ systems and highlighted the significance of even short-lived elevations. Two models of intra-abdominal pressure were used, with a mechanical obstruction model showing more rapid changes in metabolic and haematological changes. These may represent different underlying cellular and vascular pathophysiological processes, but this remains unclear.
Introduction
Intra-abdominal hypertension (IAH) is present in 25–30% of critically ill patients on admission and causes profound systemic physiological derangement [1–10]. The consequences include renal dysfunction, prolonged intensive care and hospital stays, increased morbidity, multi-organ failure, and mortality [11]. Increased pressure within the intra-abdominal compartment reduces abdominal perfusion pressure (calculated as mean arterial pressure minus intra-abdominal pressure (IAP)), [12] thereby impairing perfusion to the intra- and extra-abdominal organs and, in particular, watershed areas such as the intestinal mucosa. Venous compression increases venous pressure resulting in congestion, intestinal edema, and gastrointestinal bacterial translocation [13].
The interaction between different organ systems within neighbouring anatomical compartments has been described as polycompartment syndrome [14, 15]. It is for this reason we have studied multiple organ systems together. This study includes two models of raised intraabdominal pressure, a pneumoperitoneum and a mechanical intestinal obstruction model [16]. We have previously demonstrated that intestinal vascular occlusion may differ between a pneumoperitoneum versus a mechanically obstructed model [16]. This may affect organ systems differently. Lactate increased substantially in a mechanically obstructed model which may reflect increased anaerobic metabolism because of imbalances in cardiorespiratory dynamics. This may in turn promote an inflammatory state but has yet to be proven, and probably requires a longer period of sustained elevated IAP.
The primary aim was to study the systemic effects of raised IAP on various organ systems. We used both the pneumoperitoneum and mechanical obstruction models to generate elevated intra-abdominal pressure. Data from all pigs, regardless of the model used, were included in the analysis for the primary outcome. We felt it important to study these effects across various IAP levels, as this has not been well documented previously. The secondary aim was to describe the effects between the two different models. We hypothesised that there would be no conceivable difference between the two models because of the relatively short duration of raised IAP.
Methods
Regulatory issues
The study was performed in accordance with the recommendations in the Royal Decree 1201/2005 of 10 October 2005 on the protection of animals used for experimentation and other scientific purposes. All experimental protocols were approved by the Committee on the Ethics of Animal Experiments of Minimally Invasive Surgery Centre Jesús Usón and by the Council of Agriculture and Rural Development of the Regional Government of Extremadura (No. ES100370001499), Spain.
Animal study population
Forty-nine white female pigs (24.1 kg; range 17.3–33 kg) were fasted for 24 hours before receiving premedication with intramuscular atropine (0.04 mg/kg), diazepam (0.4 mg/kg) and ketamine (10 mg/kg). Induction and anesthesia were the same as described previously by Correa-Martin et al. [16]. The animals were pre-oxygenated with fractional inspired oxygen of 1.0 (fresh gas flow of 3–5 l/min), before propofol 1% (3 mg/kg) was administered. They were intubated, mechanically ventilated, and anesthesia was maintained with isoflurane (MAC of 1.25). Intravenous 0.9% sodium chloride fluid (2 ml/kg/h) and a remifentanil infusion (0.3 mcg/kg/min) for analgesia was provided intraoperatively. The animals were euthanised using potassium chloride (1–2 mmol/kg) on completion of the study, as per the American Veterinary Medical Association Panel on Euthanasia guidelines.
Study design
The swine were consecutively allocated into six groups: a single control group (Cr; n = 5), three pneumoperitoneum groups with IAPs of 20 mmHg (Pn20; n = 10), 30 mmHg (Pn30; n = 10), and 40 mmHg (Pn40; n = 10), and two mechanical intestinal occlusion groups with IAPs of 20 mmHg (MIO20; n = 9) and 30 mmHg (MIO30; n = 5). Each group was studied for three and five hours, except the MIO30 that was only studied for 3 hours. A laparoscopic insufflation technique was used for the pneumoperitoneum model. The mechanical intestinal obstruction model, which was previously published, was achieved by placing a laparoscopic suture at the ileocaecal valve and infusing 0.9% saline into the bowel (16).
All the swine were positioned supine. IAP was measured using three methods simultaneously, with the direct intraperitoneal measurement technique used as the method to achieve the IAP target [16]. Gastric and bladder pressures were measured using the same methods as those described for humans in the WSACS consensus guidelines [16–18]. Multiple physiological parameters, together with blood samples, were measured every 30 minutes. Once IAP was stabilised, measurements were initiated and designated as T1. The control group did not have any intervention to increase IAP and received the same anesthetic and 30-minute physiological measurements as the experimental groups.
Data collection
IAP was measured simultaneously at 30 minutes intervals in each pig using the three methods (i.e., transperitoneal [TP], transvesical [TV], and transgastric [TG]). The direct TP technique was considered the gold standard as it was a direct measure of IAP. A Jackson-Pratt catheter was inserted laparoscopically into the abdominal cavity and placed on the liver to perform TP IAP measurements [16]. A Foley catheter in the bladder and urine drainage bag were used for TV IAP measurements together with a manual manometer system (Holtech Medical, Charlottenlund, Denmark). An endoscopically placed gastric balloon-tipped catheter was connected to an electronic pressure transducer (Spiegelberg, Hamburg, Germany) to measure TG IAP. Transgastric measurements were graphically recorded in real time. The results of these comparisons have been previously reported [19].
Physiological signs and laboratory tests were collected every 30 minutes. Cardiovascular parameters measured included heart rate, blood pressure, cardiac output parameters (cardiac output, pulse pressure variation (PPV), stroke volume variation (SVV), assessed with continuous electrocardiogram, pulse oximetry (with hemodynamic E-modulus PRESTN anesthesia monitor S/5TM General Electric Datex-Ohmeda®), invasive arterial blood pressure measurement and pulse contour cardiac output (PiCCO, Getinge, Sölna, Sweden) device. Respiratory parameters measured included respiratory rate, arterial oxygen saturation (SpO2), partial pressure of carbon dioxide (pCO2), mean pulmonary artery pressure (MPP), and ventilation parameters (tidal volumes (Vt), compliance, plateau pressure (PP), minute volume). These measurements came from the anesthetic monitor, ventilator, and arterial blood gases. Ventilation was provided with an FiO2 of 1.0, a tidal volume of 10-15ml/kg, positive end-expiratory pressure of 2cmH2O, at a rate set (14 breaths/minute) to obtain an end-expiratory CO2 partial pressure of 35-40mmHg. Metabolic and hematological parameters from blood samples included pH, base excess, bicarbonate, lactate, APTT, INR, and platelets. Gastrointestinal parameters from blood samples included ALT, ALP, GGT, LDH, total bilirubin, and lipase. An endoscopically inserted monitor connected to the anesthesia monitor (S/5TM General Electric Datex-Ohmeda, Helsinki, Finland) measured continuous gastric mucosal pH via tonometry. Renal function was assessed via a calculated glomerular filtration, urine output, and blood samples for urea and creatinine.
Statistical analysis
We performed a statistical power analysis for sample size estimation from data analysing hemodynamic parameters from previous pig studies with an alpha of 0.05 and power equal to 0.80 [20–22]. With an alpha of 0.05 and power of 80%, the projected sample size needed with this effect size for the primary endpoints was 4 (Appendix A in S2 File supplementary electronic media). The proposed sample size, both in groups and combined by model, was adequate for the objectives of this study.
Categorical variables were described as proportions (%) and compared using Chi-square or Fisher exact test. Continuous variables are presented as means with standard deviations when normally distributed, and as medians with interquartile ranges when non-normal distribution occurred. Normality was evaluated with the Kolmogorov-Smirnov test. One-way ANOVA or Kruskal-Wallis tests were used to compare continuous variables as appropriate.
Physiological parameters were clustered into six groups: cardiovascular, respiratory, metabolic, hematological, gastrointestinal, and renal. These parameters were considered dependent variables. The interdependencies between the physiological parameters were explored for completeness. A linear mixed model was used to estimate changes in the physiological parameters over time, with measurements every 30 minutes. The mixed model accounted for the dependencies over time and the imbalanced nature of the data with a random intercept for the pig-specific averages and a random slope for the pig-specific changes over time. Pig-specific variances were also allowed. Not all combinations of treatment and pressure were present in the dataset. Therefore, specific comparisons were created to analyse the appropriate subgroups of observations. Various variables were highly correlated. Some were on an interval scale, while others were binary.
As a result, a combination of Pearson, polyserial, and polychoric correlations was performed. Data evolution over time was plotted to represent the relationship between pressure, IAP model, and duration. To assist in interpretation, all data were rescaled by a factor of 10. Rank 10 refers to subtracting each time point by ten so that ten becomes zero, and five becomes minus five. The advantage is that the value of the intercept can be interpreted. This value is the expected value if all predictors in the model were zero, and this is now true for a time equal to ten.
A linear mixed model was used to analyse the physiological parameters to explain the observed scores while incorporating changes over time. We used the Shaffer p-value correction for multiple comparisons. All analyses were performed using R version 3.5.3 (R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/).
This is a secondary, but more complete analysis, of a study previously published by the same group (16).
Results
The pathophysiological effects of elevated intra-abdominal pressure on various organ systems are shown in the following tables: cardiovascular (Table 1), respiratory (Table 2), metabolic and hematological (Table 3), gastrointestinal (Table 4), and renal systems (Table 5).
Table 1. Cardiovascular parameters regardless of IAP model presented as median values with interquartile ranges and significance.
| Control | IAP 20mmHg | IAP 30mmHg | IAP 40mmHg | p-value | |
|---|---|---|---|---|---|
| n = 5 | n = 19 | n = 15 | n = 10 | ||
| Heart rate (beats/min) | 104 (14) | 92 (41.0) | 113.0 (42.5) | 117 (58.5) | <0.001 |
| Systolic BP (mmHg) | 86.0 (17.0) | 57.0 (20.0) | 61.0 (15.0) | 64.0 (16.0) | <0.007 |
| Cardiac index (L/min/m 2 ) | 4.2 (1.3) | 2.03 (0.86) | 2.28 (0.83) | 2.31 (0.82) | <0.001 |
| SVI (mL/m 2 ) | 39.0 (13.2) | 21.0 (6.8) | 20.0 (6.0) | 17.0 (9.5) | <0.001 |
| PPV (%) | 17.0 (5.8) | 26.0 (7.0) | 25.0 (7.0) | 28.0 (3.0) | <0.001 |
| SVV (%) | 19.5 (7.0) | 26.0 (8.0) | 30.0 (7.0) | 30.0 (5.0) | <0.001 |
BP = blood pressure; SVI = stroke volume index; PPV = pulse pressure variation; SVV = stroke volume variation
Table 2. Respiratory parameters regardless of IAP model, presented as median values with interquartile ranges and significance.
| Control | IAP 20mmHg | IAP 30mmHg | IAP 40mmHg | p-value | |
|---|---|---|---|---|---|
| n = 5 | n = 19 | n = 15 | n = 10 | ||
| MPP (mmHg) | 5.0 (0.0) | 6.0 (0.0) | 7.0 (1.0) | 8.0 (1.8) | <0.001 |
| Compliance (mL/cmH 2 0) | 18.0 (2.5) | 8.4 (1.9) | 6.3 (2.0) | 6.5 (1.4) | <0.001 |
| pO2 (mmHg) | 355.5 (70.1) | 407.0 (113.0) | 489.0 (187.3) | 371.0 (83.0) | 0.003 |
| VTE (ml) | 290.0 (75.0) | 210.0 (50.0) | 240.0 (50.0) | 275.0 (40.0) | <0.002 |
| pCO2 (mmHg) | 42.2 (5.0) | 47.8 (17.3) | 46.1 (11.5) | 37.0 (11.6) | 0.091 |
| Plateau pressure (cmH 2 0) | 18.0 (3.0) | 27.0 (6.0) | 34.0 (6.8) | 43.0 (10.0) | <0.001 |
| Driving pressure (cmH 2 0) | 17.0 (3.0) | 26.0 (5.0) | 32.0 (7.0) | 42.0 (11.0) | <0.001 |
MPP = mean pulmonary airway pressure; pO2 = arterial partial pressure of oxygen; VTE = expiratory minute volume; pCO2 = arterial partial pressure of carbon dioxide
Table 3. Metabolic and hematological parameters regardless of IAP model, presented as median values with interquartile ranges and significance.
| Control | IAP 20mmHg | IAP 30mmHg | IAP 40mmHg | p-value | |
|---|---|---|---|---|---|
| n = 5 | n = 19 | n = 15 | n = 10 | ||
| Base excess (mmol/L) | 4.0 (3.0) | 2.6 (7.0) | -1.0 (8.3) | -6.0 (8.5) | 0.088 |
| Bicarbonate (mmol/L) | 28.5 (4.5) | 27.7 (7.1) | 24.7 (7.3) | 20.1 (8.6) | 0.038 |
| Haemoglobin (g/dL) | 7.6 (0.8) | 8.6 (0.8) | 9.3 (1.5) | 10.0 (1.5) | 0.021 |
| Platelets (x10 9 /L) | 412.0 (140.0) | 429.0 (144.0) | 436.0 (248.8) | 397.0 (98.5) | <0.001 |
| APTT (sec) | 14.3 (6.2) | 24.0 (40.0) | 29.0 (46.4) | 55.0 (65.3) | 0.124 |
| INR | 0.8 (0.2) | 1.2 (0.21) | 1.14 (0.30) | 1.07 (0.24) | 0.652 |
APTT = activated partial thromboplastin time; INR = international normalised ratio
Table 4. Gastrointestinal parameters regardless of IAP model, presented as median values with interquartile ranges and significance.
| Control | IAP 20mmHg | IAP 30mmHg | IAP 40mmHg | p-value | |
|---|---|---|---|---|---|
| n = 5 | n = 19 | n = 15 | n = 10 | ||
| LDH (U/L) | 1046.0 (153.5) | 903.0 (406.0) | 1100.0 (338.0) | 1032.0 (349.0) | 0.834 |
| Lipase (U/L) | 3.6 (0.8) | 6.5 (1.9) | 8.7 (5.95) | 8.5 (4.5) | 0.032 |
| ALT (IU/L) | 38.5 (7.8) | 30.0 (10.3) | 37.5 (17.4) | 27.0 (13.5) | 0.001 |
| GGT (IU/L) | 30.0 (13.5) | 30.0 (10.5) | 34.0 (16.0) | 35.0 (12.0) | 0.798 |
| ALP (U/L) | 368.5 (217.5) | 339.0 (138.0) | 321.0 (93.0) | 383.9 (163.8) | 0.023 |
| Total bilirubin (mg/dL) | 0.3 (0.2) | 0.15 (0.14) | 0.30 (0.18) | 0.42 (0.38) | 0.037 |
| Gastric tonometry (pH) | 7.24 (0.06) | 7.13 (0.14) | 6.91 (0.28) | 6.68 (0.37) | <0.001 |
| pCO2 gap (mmHg) | 3.00 (1.36) | 11.20 (12.72) | 11.40 (17.84) | 25.0 (10.0) | <0.001 |
LDH = lactate dehydrogenase; ALT = alanine aminotransferase; GGT = Gamma-glutamyl transferase; ALP = alkaline phosphatase
Table 5. Renal parameters regardless of IAP model, presented as median values with interquartile ranges and significance.
| Control | IAP 20mmHg | IAP 30mmHg | IAP 40mmHg | p-value | |
|---|---|---|---|---|---|
| n = 5 | n = 19 | n = 15 | n = 10 | ||
| Calculated GF (mmHg) | 59.0 (17.5) | 10.0 (24.0) | -14.5 (18.5) | -34.0 (15.0) | <0.001 |
| Urine output (ml/kg/hr) | 2.8 (1.0) | 0.39 (0.95) | 0.36 (0.15) | 0.23 (0.18) | <0.001 |
| Urea (mg/dL) | 20.7 (9.4) | 24.5 (11.1) | 25.1 (9.1) | 26.3 (9.2) | 0.349 |
| Creatinine (mg/dL) | 2.3 (0.6) | 2.69 (1.14) | 2.73 (0.79) | 2.33 (0.44) | 0.283 |
GF = glomerular filtration
1. Cardiovascular system
Blood pressure
There was a decrease in both systolic and diastolic pressure as IAP increased and as time progressed. The most significant changes occurred at the highest pressures. A strong intra-class correlation was present.
For systolic blood pressure, the downward evolution occurred in all models but was more substantial at higher pressures. Diastolic pressure changes and relationships were similar to systolic pressure changes, only less significant (S3 Fig in S2 File).
Cardiac output parameters
IAH lowered the cardiac output and stroke volume index in all models (S4 Fig in S2 File). The most significant changes occurred in the MIO models.
Stroke volume index decreased significantly (61.1%), with the greatest changes seen as the pressure increased. Stroke volume variation (SVV) and pulse pressure variation (PPV) increased (100.9% and 93.5% respectively, p<0.001) across all groups and in both models compared to the control group.
2. Respiratory system
Mean pulmonary airway pressure (MPP)
MPP increased (62.5%, p<0.001) across all group, with the highest pressures corresponding to the highest IAP. There were almost no differences for MPP between the groups except for Pn30 and Pn40, which differed significantly (p<0.001) (S7 Fig in S2 File). There seemed to be an inverse relationship between MPP and weight.
Compliance
Dynamic compliance [calculated as tidal volume divided by (plateau pressure minus PEEP)] was reduced (60.2%, p<0.001) in all IAH models, with moderate intra-class correlation (S8 Fig in S2 File).
Partial pressure of oxygen (pO2)
The partial pressure of oxygen differed in all the IAH models compared to the control. A strong intra-class correlation was present.
Expiratory minute volume (MVE)
Expiratory minute volume was decreased (33.6%, p<0.002) for all models compared to the control group (S10 Fig in S2 File).
Partial pressure of carbon dioxide (pCO2)
None of the comparisons suggested any difference in pC02 readings. Lower pCO2 readings were seen in heavier pigs (S11 Fig in S2 File).
Plateau pressure (PP) and driving pressure
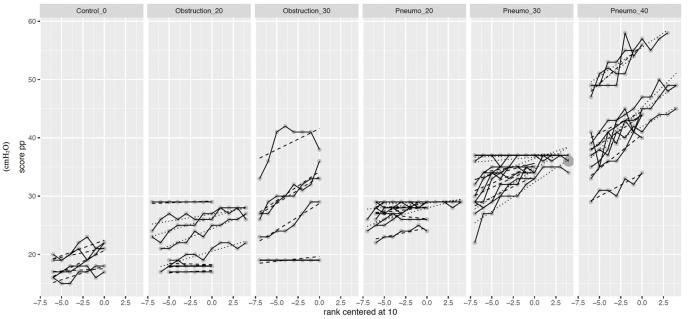
Driving pressure and PP (125.6%, p<0.001) increased with IAP, with the highest values being reached in the P40 and MIO30 groups (Fig 1). Especially noticeable were some extreme increases in the MIO30 and Pn40 groups (p<0.001).
Fig 1. Scatter plot of plateau pressure variables.
3. Metabolic and hematological systems
Base excess (BE)
The base excess decreased (102.1%, p = 0.088) in nearly all models compared to the control group (S14 Fig in S2 File). The Pn20 group differed the least when compared to the control.
Bicarbonate (HCO3)
Bicarbonate responded similarly to base excess, with readings decreasing (12.5%, p = 0.038) more rapidly in all groups when compared to the control group (except in the Pn20 group) (S15 Fig in S2 File).
Hematology
Hematological parameters mainly showed a positive correlation in both models and at all pressures. Platelet levels revealed an increasing trend from lower IAPs to the higher IAPs in both models (25.7% increase regardless of model) and were significant (p<0.001). Activated partial thromboplastin time (APTT) had an upwards trend (517.3% increase regardless of model, p = 0.124) and was significant in the MIO group. The international normalised ratio (INR) was relatively consistent across both models and at all pressures.
4. Gastrointestinal system
Lactate dehydrogenase (LDH)
LDH increased (14.4% increase, p = 0.834) as IAP increased, however, the changes were not significant. The Pn20 model increased more rapidly than in the control group (S17 Fig in S2 File). There were outliers in each group. The pigs with the highest LDH showed higher mortality.
Lipase
The increase in lipase (147.3%, p = 0.032) was seen across all groups, with the higher values corresponding to higher IAP. Some pigs in the PN30 and Pn40 groups showed the greatest increases in lipase.
Liver tests
Alanine aminotransferase (ALT) increased over the 20mmHg and 30mmHg groups, regardless of the pressures. ALT unexpectantly decreased in the PN40 group.
Gamma-glutamyl transferase (GGT) decreased (6.9% decrease, p = 0.798) and showed a difference in evolution between Pn20 and Pn30. There was also a difference between the control and each of the 20mmHg conditions (p = 0.038 and 0.044 respectively). There were significant differences observed for all groups at rank 10 (p<0.001).
Alkaline phosphatase (ALP) and total bilirubin (totBil) both showed gradual, yet significant changes (31.2% decrease, p = 0.023 and 50% increase, p = 0.037, respectively) as IAP rose. There were significant differences observed for all groups at rank 10 for ALP (p<0.001).
Gastric tonometry
There was a significant and progressive decrease in pH for gastric tonometry as the intra-abdominal pressure increased. The decline was this most marked when the IAP reached 40mmHg (a 4% reduction). The pCO2 gap (a surrogate marker for cardiac output) increased significantly as the IAP rose.
5. Renal system
Calculated glomerular filtration
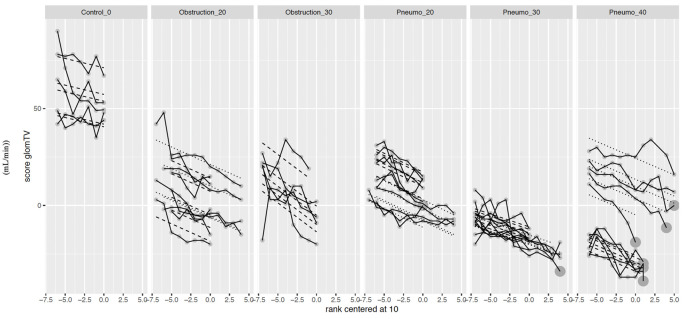
Glomerular filtration was calculated using the formula: filtration gradient = MAP–(2 x IAP). IAP measures were obtained transvesically, transperitoneally, and transgastrically. As time progressed, there was a downward trend for all groups (104.6%, p<0.001). The most significant changes were seen with the highest pressures (Fig 2). A strong intra-class correlation was noted.
Fig 2. Scatter plot of calculated glomerular filtration variables.
Urine output
Urine output was significantly greater in the control group when compared to each of the groups. Urine output decreased with the increasing IAP (S20 Fig in S2 File). At worst, this reduction was 74.6% (p<0.001).
Urea
Urea increased (20.1%, p = 0.349) in the IAH models compared to the control (S21 Fig in S2 File). Moderate intra-class correlation was identified.
Creatinine
Creatinine increased (5.7%, p = 0.283) as time progressed in all IAH models compared to the control group (S22 Fig in S2 File). There was a strong intra-class correlation.
Secondary objectives (refer to supplementary data)
The comparison between the pneumoperitoneum model and the mechanical obstruction model did not show any significant differences in the cardiovascular or respiratory parameters measured. The changes were at times more rapid when the abdominal pressures were higher. There were minor differences observed between groups.
Metabolic parameters showed a decreased base excess across both models. The Pn20 group differed the least when compared to the control. Bicarbonate decreased in both models but was more marked in the MIO model compared to the PN group at 20mmHg (S15 Fig in S2 File). Haematological changes were consistent across the models and groups, however, the MIO30 group showed greater changes in INR, platelets and APTT.
The gastrointestinal system revealed unexpected results. The bilirubin and ALT decreased or only marginally increased in both 20mmHg models. Bilirubin rose the most when pressures increased, especially at 40mmHg. There were no significant differences between models for GGT and ALP.
The evolution of increase for LDH was greater in the Pn compared to the MIO models (S17 Fig in S2 File).
The opposite occurred for lipase, where lipase increased across both models and at all pressures (S18 Fig in S2 File). The evolution of increase for lipase and ALT were strongest in the MIO30 group.
Renal parameters reflected similar trends independent of the model of intra-abdominal pressure used. The creatinine increased more sharply in the pneumoperitoneum groups, especially Pn30 and Pn40 (S22 Fig in S2 File).
Discussion
Several previous studies aimed to investigate aspects of the pathophysiological consequences of IAP. This study found worsening cardiorespiratory function and a predictable effect on renal function. The metabolic, hematological, and gastrointestinal effects are significant for some, but not all, parameters studied. This may be a result of the short duration of the implemented models, with insufficient time to reach significance. Alternatively, it may represent more complicated pathophysiology or protective mechanisms in early IAH. Metabolic changes showed a positive correlation in the Pn model, but a negative correlation in the MIO group. The reason for this is uncertain but may represent different underlying pathophysiology related to the effects of mechanical obstruction. The results are discussed according to organ system.
Cardiovascular system
The cardiovascular changes observed were as expected following the rise in IAP with a subsequent increased afterload, a decreased preload, and thereby decreased cardiac output. Additionally, as the stroke volume is determined by a combination of preload, contractility, afterload, and heart rate, there were significant increases in heart rate to compensate for a reduced stroke volume (heart rate increased by 40.2% regardless of model). This was particularly noticeable when IAP was 30mmHg or more.
An important finding was the relationship between elevated intra-abdominal pressure and markers of fluid responsiveness. SVV and PPV have been helpful in volume status assessment; however, the impact of intra-abdominal pressure appears to make these measurements difficult to interpret [21, 23]. Previous research has published conflicting results. Deloya et al. found an IAP greater than 15mmHg to cause significant changes in SVV despite the subjects being normovolaemic.
Contrary to this, Jacques et al. concluded that SVV was still helpful in the face of elevated IAP, but different thresholds may apply. These two previous studies were pig models; however, Liu et al. examined the same question in patients undergoing laparoscopic cholecystectomy [23]. The findings showed SVV increased progressively as the IAP was raised with no changes in volume status. In our study, the pigs were presumed to have been kept euvolemic with a constant infusion of IV crystalloids and only insensible losses. Despite this, IAP lowered the cardiac output in all models and increased PPV and SVV across all groups and in both models. This supports previous findings of the difficulty in using cardiovascular markers of volume status that use respiratory variation in subjects with elevated IAP. Measurement of IAP should be considered when using SVV or PPV, and for the utility of SVV should be determined in patients with IAP.
Interestingly, the systolic BP and cardiac index were closer to the control group values in the animals with higher IAP (40mmHg) versus lower IAP (20mmHg). Theoretically, these haemodynamic findings may be the consequence of the mechanical and neurohormonal responses to extremely elevated intra-abdominal pressure. These are related to inferior vena cava compression, aortic compression, decreased splanchnic blood flow, decreased renal blood flow, and diaphragmatic displacement. The overall result of these effects includes increased right atrial pressure, increased systemic vascular resistance, countered by a possible decrease in cardiac output. However, a slightly elevated systolic BP may be seen with a higher cardiac index at higher IAP if the effects on SVR and RAP are greater than the effects on cardiac output. Another explanation might be the mathematical coupling between high levels of IAP and AP due to the mechanical transmission of pressures from one compartment to another as is seen in the polycompartment syndrome.
Respiratory system
The pressure exerted on the diaphragm by high IAP causes decreased tidal volumes, compliance, functional residual capacity, and increased respiratory and airway pressures [24–29]. Previous animal and human studies have demonstrated a non-uniform decrease in lung volume and functional residual capacity with abdominal distension and elevated IAP. Most studies are conducted over hours, and hypothetically, we expect these findings to worsen as time progresses. Both chest wall and lung compliance are affected. The progressive decrease in compliance across both models and at each IAH pressure, with significant negative correlation, was in keeping with previous studies [26, 28, 30]. MPP and plateau pressure) measurements increased with a significant positive correlation in most groups, the exception being the MIO20 group. IAH appears to increase both inspiratory airway and pleural pressures, and thus does not seem to affect transpulmonary pressures significantly.
The pO2 decreased in the Pn40 model, but the apparent increase in pO2 in the other models requires further exploration. Ventilation-perfusion relationships have been studied in animals and humans [27, 31–33]. There appears to be a redistribution of blood flow from atelectatic and dependent areas of the lung when IAP is raised. This causes a redistribution of blood flow to improve the ventilation-perfusion mismatch. This compensatory mechanism may account for the increases in pO2 in some groups. These changes may occur rapidly, but how and when it is overwhelmed requires further research. The partial pressure of carbon dioxide responded as predicted.
PEEP was not adjusted during the study despite increasing IAP. This was done to assess the effects of the rising IAP. Previous animal studies have investigated the effects of PEEP and demonstrated an improvement in end-expiratory lung volumes, shunt fraction, dead space, and oxygenation when matching PEEP levels with IAP. This study did not intend to describe the effects of PEEP [27, 28, 34]. The importance of recognising increased IAP and its potential influence on the chest wall and lung compliance, tidal volumes, and functional residual capacity, should be a focus of ventilation teaching. Incorporation of IAP measurement into ventilator setting decision pathways should be considered.
Metabolic and hematological systems
Interestingly, the metabolic findings showed varying results between the models, but overall, values decreased in nearly all models compared to the control group. These changes were relatively rapid for base excess and serum bicarbonate, and highlight the importance of early identification of IAH.
The increasing platelet trend was unexpected. Mean platelet volume (MPV) has been studied in response to elevated IAP in patients undergoing laparoscopic cholecystectomy as a model for IAH [35–41]. Interestingly, there was a significant increase in MPV on insufflation and a decrease with deflation, confirming a platelet response to elevated IAP. There are several theories about platelet response to elevated IAP. MPV has previously been linked as a marker of inflammation to several different pathologies, including septicemia, cardiac, respiratory and intra-abdominal pathologies [42–44]. The platelet response may be driven by the secretion of free oxygen radicals and inflammatory mediators in response to poor abdominal perfusion. This in turn promotes the production of platelets through the stimulation of megakaryocytes [45]. Other theories suggest hormonal action on megakaryocytes, the retention of small platelets in ischaemic organs, and platelet swelling in response to thrombopoietin [46–48]. However, the increase in platelets over a relatively short time (maximum 5 hours), despite the long average lifespan of platelets (8–10 days), suggests a relatively rapid process that requires further investigation.
Gastrointestinal system
In this study, GGT, alkaline phosphatase, and LDH all showed progressive change as the IAP increased in both models. There was a progressive increase in the lipase measurements, most marked in the Pn and MIO30 groups, with corresponding statistical significance. Total bilirubin increased significantly between the control group and IAP 40mmHg.
The pCO2 (pCO2 gap = PcvCO2 –PaCO2) has previously been identified as a surrogate marker for cardiac output and used in various settings [49–53]. A pCO2 gap >6mmHg suggests a persistent shock state. All groups of raised IAP had a pCO2 gap of >6mmHg and rose significantly with the IAP increase.
Renal system
Renal dysfunction occurs with elevated intra-abdominal pressure because of venous congestion, increased left ventricular afterload and decreased renal perfusion, and a systemic inflammatory response [54–57]. The compression of renal veins will result in venous congestion and a decrease in glomerular filtration with an increase in renin release. Diminished renal perfusion and salt and water retention may contribute to a vicious cycle of fluid accumulation and increasing IAP [58, 59].
Creatinine, urea, glomerular filtration and urine output all demonstrated changes that were expected as a consequence of the pathophysiology described above. The lack of statistically significant changes, for all but glomerular filtration, may be due to the short duration of the raised IAP.
Comparison between raised IAP models
The lack of differences between the two models in the cardiorespiratory parameters may be expected when both models had the same IAP, and the inflammatory responses may not have had enough time to influence contractility through inflammatory mediated negative inotropy.
The metabolic effects of IAP appear to be influenced by the mechanism of intra-abdominal hypertension [16]. The explanation may be linked to the impact on bowel mucosa and underlying tissue that was ligated as part of the model (like that which occurs in bowel obstruction). This may result in a perfusion deficit, as shown by the decrease in pHi.
Lipase appears to be a useful marker to monitor, regardless of the model of IAH. In comparison, total bilirubin increased significantly between the control group and IAP 40mmHg but did not reveal any trends or consistent statistically significant correlation between the two models.
Glomerular filtration was shown to be a useful marker of renal decline and showed a statistically significant correlation in both models and at all pressures (Pn20 vs. MIO20 and Pn30 vs. MIO30).
Limitations
Although care was taken to control and standardise all interventions, there may have been a difference in each animal’s underlying physiology. While this was a relatively large animal study, there were only five or fewer animals in each group. Due to funding limitations, one group had four, which still satisfied our sample size requirements. The implications of this include a limited ability to identify statistically significant changes, especially when parameters are measured over only a few hours. Set pressures were used versus gradually increasing pressures over time which may have been closer to clinical situations. However, we chose this design to define the size of the groups, as pigs may not have survived raised pressures for long periods, thus missing out on observing what happens at 30mmHg and 40mmHg. We did not measure readings beyond 5 hours, and there was not a 40mmHg intervention for the mechanical obstruction group. Consequently, changes that take longer to manifest, such as elevation of creatinine and urea, would not be identified. The effect of PEEP could not be removed completely because a PEEP of zero was not possible on the anaesthetic machine used. Unfortunately, lactate readings, global end-diastolic volume, and extravascular lung water could not be reported because of incomplete data.
Conclusion
This study identified and quantified the impact of intra-abdominal hypertension at different pressures on several organ systems and highlighted the significance of even short-lived elevations. Two models of intra-abdominal pressure were used, with a mechanical obstruction model showing more rapid changes in metabolic and haematological changes. These may represent different underlying cellular and vascular pathophysiological processes, but this remains unclear.
Supporting information
(PDF)
(PDF)
The file contains S1-S22 Figs, S1, S2 Tables, and Appendix A.
(PDF)
Acknowledgments
We would like to acknowledge Wilfried Cools from the Interfaculty Center Data processing and Statistics at the Vrije Universiteit Brussel (VUB), for his statistical analysis contribution.
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
This work was supported by one grant from Extremadura Regional Government through the Plan Regional de Investigación de Extremadura, Spain (PRI09A161 to Minimally Invasive Surgery Center Jesus Usón) to FSM and GCE. The funder had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript. The funders of the grant did not play any role in the study.
References
- 1.Arabadzhiev G, Tzaneva V, Peeva K. Intra-abdominal hypertension in the ICU–a prospective epidemiological study. Clujul Med. 2015;88(2):188–95. doi: 10.15386/cjmed-455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smit M, Koopman B, Dieperink W, et al. Intra-abdominal hypertension and abdominal compartment syndrome in patients admitted to the ICU. Ann. Intensive Care 10, 130 (2020). doi: 10.1186/s13613-020-00746-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holodinsky JK, Roberts DJ, Ball CG, Blaser AR, Starkopf J, Zygun DA, et al. Risk factors for intra-abdominal hypertension and abdominal compartment syndrome among adult intensive care unit patients: a systematic review and meta-analysis. Critical care. 2013;17(5): R249. doi: 10.1186/cc13075 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reintam Blaser A, Regli A, De Keulenaer B, Kimball E, Starkopf L, Davis W, et al., The Incidence, Risk Factors, and Outcomes of Intra-Abdominal (IROI) Study Investigators. Incidence, Risk Factors, and Outcomes of Intra-Abdominal Hypertension in Critically Ill Patients—A Prospective Multicenter Study (IROI Study). Critical Care Medicine 47(4):p 535–542, April 2019. doi: 10.1097/CCM.0000000000003623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horoz O, Yildizdas D, Asilioglu N, Kendirli T, Erkek N, Anil A, et al. The prevalence of and factors associated with intra-abdominal hypertension on admission day in critically ill pediatric patients: A multicenter study. J Crit Care. 2015;30(3):584–8. [DOI] [PubMed] [Google Scholar]
- 6.Kim I, Prowle J, Baldwin I, Bellomo R. Incidence, risk factors and outcome associations of intra-abdominal hypertension in critically ill patients. Anaesth Intensive Care. 2012;40(1):79–89. doi: 10.1177/0310057X1204000107 [DOI] [PubMed] [Google Scholar]
- 7.Muturi A, Ndaguatha P, Ojuka D, et al. Prevalence and predictors of intra-abdominal hypertension and compartment syndrome in surgical patients in critical care units at Kenyatta National Hospital. BMC Emerg Med 17, 10 (2016). doi: 10.1186/s12873-017-0120-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murtaza G, Pal K, Jajja M, Nawaz Z, Koondhar R, Nasim S. Intra-abdominal hypertension; incidence, prevalence and outcomes in a mixed intensive care unit: Prospective cohort study. Int J Surg. 2015;Jul(19):67–71. [DOI] [PubMed] [Google Scholar]
- 9.Santa-Teresa P, Munoz J, Montero I, Zurita M, Tomey M, Alvarez-Sala L, et al. Incidence and prognosis of intra-abdominal hypertension in critically ill medical patients: a prospective epidemiological study. Annals of intensive care. 2012. Jul 5;2 Suppl 1:S3. doi: 10.1186/2110-5820-2-S1-S3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Liu D, Tang H, Sun S, Ai S, Yang W, et al. Prevalence and diagnosis rate of intra-abdominal hypertension in critically ill adult patients: A single-center cross-sectional study. Chin J Traumatol. 2015;18(6):352–6. doi: 10.1016/j.cjtee.2015.11.015 [DOI] [PubMed] [Google Scholar]
- 11.Vidal MG, Ruiz Weisser J, Gonzalez F, Toro MA, Loudet C, Balasini C, et al. Incidence and clinical effects of intra-abdominal hypertension in critically ill patients. Critical care medicine. 2008. Jun;36(6):1823–31. doi: 10.1097/CCM.0b013e31817c7a4d . [DOI] [PubMed] [Google Scholar]
- 12.Nagappan R, Ernest D, Whitfield A. Recognition and management of intra-abdominal hypertension and abdominal compartment syndrome. Critical care and resuscitation: journal of the Australasian Academy of Critical Care Medicine. 2005. Dec;7(4):298–302. . [PubMed] [Google Scholar]
- 13.Wauters J, Claus P, Brosens N, McLaughlin M, Malbrain M, Wilmer A. Pathophysiology of renal hemodynamics and renal cortical microcirculation in a porcine model of elevated intra-abdominal pressure. The Journal of trauma. 2009. Mar;66(3):713–9. doi: 10.1097/TA.0b013e31817c5594 . [DOI] [PubMed] [Google Scholar]
- 14.Malbrain M, Roberts D, Sugrue M, De Keulenaer B, Ivatury R, Pelosi P, et al. The polycompartment syndrome: A concise state-of-the-art review. Anaesthesiology intensive therapy. 2014;46(5):433–50. doi: 10.5603/AIT.2014.0064 [DOI] [PubMed] [Google Scholar]
- 15.Malbrain M, Peeters Y, Wise R. The neglected role of abdominal compliance in organ-organ interactions. Critical care. 2016;20(67):1–10. doi: 10.1186/s13054-016-1220-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Correa-Martin L, Parraga E, Sanchez-Margallo FM, Latorre R, Lopez-Albors O, Wise R, et al. Mechanical Intestinal Obstruction in a Porcine Model: Effects of Intra-Abdominal Hypertension. A Preliminary Study. PloS one. 2016;11(2):e0148058. doi: 10.1371/journal.pone.0148058 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malbrain ML. Different techniques to measure intra-abdominal pressure (IAP): time for a critical re-appraisal. Intensive care medicine. 2004. Mar;30(3):357–71. doi: 10.1007/s00134-003-2107-2 . [DOI] [PubMed] [Google Scholar]
- 18.Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive care medicine. 2013. Jul;39(7):1190–206. doi: 10.1007/s00134-013-2906-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wise RD, Rodseth RN, Correa-Martin L, Sanchez Margallo F M, Becker P, Castellanos G et al. Correlation between different methods of intra-abdominal pressure monitoring in varying intra-abdominal hypertension models. South. Afr. j. crit. care (Online) [Internet]. 2017. July [cited 2022 Aug 19]; 33 (1): 15–18. doi: http%3A//dx.doi.org/10.7196/SAJCC.2017.v33i1.327 [Google Scholar]
- 20.Renner J, Gruenewald M, Quaden R, Hanss R, Meybohm P, Steinfath M, et al. Influence of increased intra-abdominal pressure on fluid responsiveness predicted by pulse pressure variation and stroke volume variation in a porcine model. Crit Care Med. 2009. Feb;37(2):650–8. doi: 10.1097/CCM.0b013e3181959864 . [DOI] [PubMed] [Google Scholar]
- 21.Jacques D, Bendjelid K, Duperret S, Colling J, Piriou V, Viale JP. Pulse pressure variation and stroke volume variation during increased intra-abdominal pressure: an experimental study. Crit Care. 2011;15(1): R33. doi: 10.1186/cc9980 Epub 2011 Jan 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu F, Zhu S, Ji Q, Li W, Liu J. The impact of intra-abdominal pressure on the stroke volume variation and plethysmographic variability index in patients undergoing laparoscopic cholecystectomy. Biosci Trends. 2015. Apr;9(2):129–33. doi: 10.5582/bst.2015.01029 . [DOI] [PubMed] [Google Scholar]
- 23.Deloya TE, Poblano MM, Hernández LD, et al. Modification of stroke volume variation with increased intra-abdominal pressure in normovolemic porcine model. Med Crit. 2013;27(1):25–32. [Google Scholar]
- 24.Quintel M, Pelosi P, Caironi P, Meinhardt JP, Luecke T, Herrmann P, et al. An increase of abdominal pressure increases pulmonary edema in oleic acid-induced lung injury. Am J Respir Crit Care Med. 2004. Feb 15;169(4):534–41. doi: 10.1164/rccm.200209-1060OC Epub 2003 Dec 11. . [DOI] [PubMed] [Google Scholar]
- 25.Ranieri VM, Brienza N, Santostasi S, Puntillo F, Mascia L, Vitale N, et al. Impairment of lung and chest wall mechanics in patients with acute respiratory distress syndrome: role of abdominal distension. Am J Respir Crit Care Med. 1997. Oct;156(4 Pt 1):1082–91. doi: 10.1164/ajrccm.156.4.97-01052 . [DOI] [PubMed] [Google Scholar]
- 26.Mutoh T, Lamm WJ, Embree LJ, Hildebrandt J, Albert RK. Abdominal distension alters regional pleural pressures and chest wall mechanics in pigs in vivo. J Appl Physiol (1985). 1991. Jun;70(6):2611–8. doi: 10.1152/jappl.1991.70.6.2611 . [DOI] [PubMed] [Google Scholar]
- 27.Regli A, Hockings LE, Musk GC, Roberts B, Noffsinger B, Singh B, et al. Commonly applied positive end-expiratory pressures do not prevent functional residual capacity decline in the setting of intra-abdominal hypertension: a pig model. Crit Care. 2010;14(4): R128. doi: 10.1186/cc9095 Epub 2010 Jul 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Regli A, Chakera J, De Keulenaer BL, Roberts B, Noffsinger B, Singh B, et al. Matching positive end-expiratory pressure to intra-abdominal pressure prevents end-expiratory lung volume decline in a pig model of intra-abdominal hypertension. Crit Care Med. 2012. Jun;40(6):1879–86. doi: 10.1097/CCM.0b013e31824e0e80 . [DOI] [PubMed] [Google Scholar]
- 29.Mutoh T, Lamm WJ, Embree LJ, Hildebrandt J, Albert RK. Volume infusion produces abdominal distension, lung compression, and chest wall stiffening in pigs. J Appl Physiol (1985). 1992. Feb;72(2):575–82. doi: 10.1152/jappl.1992.72.2.575 . [DOI] [PubMed] [Google Scholar]
- 30.Wauters J, Claus P, Brosens N, McLaughlin M, Hermans G, Malbrain M, et al. Relationship between Abdominal Pressure, Pulmonary Compliance, and Cardiac Preload in a Porcine Model. Crit Care Res Pract. 2012;2012:763181. doi: 10.1155/2012/763181 Epub 2012 Feb 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strang CM, Fredén F, Maripuu E, Hachenberg T, Hedenstierna G. Ventilation-perfusion distributions and gas exchange during carbon dioxide-pneumoperitoneum in a porcine model. Br J Anaesth. 2010. Nov;105(5):691–7. doi: 10.1093/bja/aeq211 Epub 2010 Aug 6. . [DOI] [PubMed] [Google Scholar]
- 32.Andersson L, Lagerstrand L, Thörne A, Sollevi A, Brodin LA, Odeberg-Wernerman S. Effect of CO2 pneumoperitoneum on ventilation-perfusion relationships during laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 2002. May;46(5):552–60. doi: 10.1034/j.1399-6576.2002.460513.x . [DOI] [PubMed] [Google Scholar]
- 33.Regli A., Pelosi P. & Malbrain M.L.N.G. Ventilation in patients with intra-abdominal hypertension: what every critical care physician needs to know. Ann. Intensive Care 9, 52 (2019). doi: 10.1186/s13613-019-0522-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Regli A, Mahendran R, Fysh ET, Roberts B, Noffsinger B, De Keulenaer BL, et al. Matching positive end-expiratory pressure to intra-abdominal pressure improves oxygenation in a porcine sick lung model of intra-abdominal hypertension. Crit Care. 2012. Oct 26;16(5): R208. doi: 10.1186/cc11840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Celep RB, Kahramanca Ş, Özsoy M, Azili C, Çetinkünar S, Hasdemir AO, et al. Effects of intraabdominal pressure on mean platelet volume during laparoscopic cholecystectomy. Turk J Med Sci. 2014;44(3):360–4. doi: 10.3906/sag-1304-23 . [DOI] [PubMed] [Google Scholar]
- 36.Kisacik B, Tufan A, Kalyoncu U, Karadag O, Akdogan A, Ozturk MA, et al. Mean platelet volume (MPV) as an inflammatory marker in ankylosing spondylitis and rheumatoid arthritis. Joint Bone Spine. 2008. May;75(3):291–4. doi: 10.1016/j.jbspin.2007.06.016 Epub 2008 Apr 9. . [DOI] [PubMed] [Google Scholar]
- 37.Yüksel O, Helvaci K, Başar O, Köklü S, Caner S, Helvaci N, et al. An overlooked indicator of disease activity in ulcerative colitis: mean platelet volume. Platelets. 2009. Jun;20(4):277–81. doi: 10.1080/09537100902856781 . [DOI] [PubMed] [Google Scholar]
- 38.Köşüş N, Köşüş A, Turhan N. Mean platelet volume as a marker of future cardiovascular disease risk in pregnant women with impaired fasting glucose and impaired glucose tolerance. Turk J Med Sci 2012; 42: 245–51. [Google Scholar]
- 39.Karaman H, Karakükcü Ç, Karaman A, Kayman T, Yalçın S, Taşdemir EA, et al. Mean platelet volume as a fibrosis marker in patients with chronic hepatitis C. Turk J Med Sci 2013; 43: 39–45. [Google Scholar]
- 40.Ozdemir O, Soylu M, Alyan O, Geyik B, Demir AD, Aras D, et al. Association between mean platelet volume and autonomic nervous system functions: Increased mean platelet volume reflects sympathetic overactivity. Exp Clin Cardiol. 2004. Winter;9(4):243–7. [PMC free article] [PubMed] [Google Scholar]
- 41.Bilici S, Sekmenli T, Göksu M, Melek M, Avci V. Mean platelet volume in diagnosis of acute appendicitis in children. Afr Health Sci. 2011. Sep;11(3):427–32. doi: 10.1177/1076029610364520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karaman K, Bostanci EB, Aksoy E, Kurt M, Celep B, Ulas M, et al. The predictive value of mean platelet volume in differential diagnosis of non-functional pancreatic neuroendocrine tumors from pancreatic adenocarcinomas. Eur J Intern Med. 2011. Dec;22(6):e95–8. doi: 10.1016/j.ejim.2011.04.005 Epub 2011 Jun 12. . [DOI] [PubMed] [Google Scholar]
- 43.Slavka G, Perkmann T, Haslacher H, Greisenegger S, Marsik C, Wagner OF, et al. Mean platelet volume may represent a predictive parameter for overall vascular mortality and ischemic heart disease. Arterioscler Thromb Vasc Biol. 2011. May;31(5):1215–8. doi: 10.1161/ATVBAHA.110.221788 Epub 2011 Feb 17. . [DOI] [PubMed] [Google Scholar]
- 44.Van der Lelie J, Von dem Borne AK. Increased mean platelet volume in septicaemia. J Clin Pathol. 1983. Jun;36(6):693–6. doi: 10.1136/jcp.36.6.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson CB, Eaton KA, Princiotta SM, Rushin CA, Valeri CR. Size dependent platelet subpopulations: relationship of platelet volume to ultrastructure, enzymatic activity, and function. Br J Haematol. 1982. Mar;50(3):509–19. doi: 10.1111/j.1365-2141.1982.tb01947.x . [DOI] [PubMed] [Google Scholar]
- 46.Boos CJ, Lip GY. Assessment of mean platelet volume in coronary artery disease—what does it mean? Thromb Res. 2007;120(1):11–3. doi: 10.1016/j.thromres.2006.09.002 Epub 2006 Oct 12. . [DOI] [PubMed] [Google Scholar]
- 47.Martin JF, Bath PM, Burr ML. Influence of platelet size on outcome after myocardial infarction. Lancet. 1991. Dec 7;338(8780):1409–11. doi: 10.1016/0140-6736(91)92719-i . [DOI] [PubMed] [Google Scholar]
- 48.Thomas PR. Platelet size and venous disease. Lancet. 1992. Jan 25;339(8787):250–1. doi: 10.1016/0140-6736(92)90060-g . [DOI] [PubMed] [Google Scholar]
- 49.Mallat J, Pepy F, Lemyze M, Gasan G, Vangrunderbeeck N, Tronchon L, et al. Central venous-to-arterial carbon dioxide partial pressure difference in early resuscitation from septic shock: a prospective observational study. Eur J Anaesthesiol. 2014. Jul;31(7):371–80. doi: 10.1097/EJA.0000000000000064 . [DOI] [PubMed] [Google Scholar]
- 50.Mesquida J, Saludes P, Gruartmoner G, Espinal C, Torrents E, Baigorri F, Artigas A. Central venous-to-arterial carbon dioxide difference combined with arterial-to-venous oxygen content difference is associated with lactate evolution in the hemodynamic resuscitation process in early septic shock. Crit Care. 2015. Mar 28;19(1):126. doi: 10.1186/s13054-015-0858-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vallée F, Vallet B, Mathe O, Parraguette J, Mari A, Silva S, et al. Central venous-to-arterial carbon dioxide difference: an additional target for goal-directed therapy in septic shock? Intensive Care Med. 2008. Dec;34(12):2218–25. doi: 10.1007/s00134-008-1199-0 Epub 2008 Jul 8. . [DOI] [PubMed] [Google Scholar]
- 52.Vallet B, Pinsky MR, Cecconi M. Resuscitation of patients with septic shock: please "mind the gap"! Intensive Care Med. 2013. Sep;39(9):1653–5. doi: 10.1007/s00134-013-2998-5 Epub 2013 Jun 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vallet B, Teboul JL, Cain S, Curtis S. Venoarterial CO2 difference during regional ischemic or hypoxic hypoxia. J Appl Physiol (1985). 2000. Oct;89(4):1317–21. doi: 10.1152/jappl.2000.89.4.1317 . [DOI] [PubMed] [Google Scholar]
- 54.De Waele JJ, De Laet I, Kirkpatrick AW, Hoste E. Intra-abdominal Hypertension and Abdominal Compartment Syndrome. Am J Kidney Dis. 2011. Jan;57(1):159–69. doi: 10.1053/j.ajkd.2010.08.034 . [DOI] [PubMed] [Google Scholar]
- 55.Smit M, Koopman B, Dieperink W, Hulscher JBF, Hofker HS, van Meurs M, et al. Intra-abdominal hypertension and abdominal compartment syndrome in patients admitted to the ICU. Ann Intensive Care. 2020. Oct 1;10(1):130. doi: 10.1186/s13613-020-00746-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mohmand H, Goldfarb S. Renal dysfunction associated with intra-abdominal hypertension and the abdominal compartment syndrome. J Am Soc Nephrol. 2011. Apr;22(4):615–21. doi: 10.1681/ASN.2010121222 Epub 2011 Feb 10. . [DOI] [PubMed] [Google Scholar]
- 57.Patel DM, Connor MJ Jr. Intra-Abdominal Hypertension and Abdominal Compartment Syndrome: An Underappreciated Cause of Acute Kidney Injury. Adv Chronic Kidney Dis. 2016. May;23(3):160–6. doi: 10.1053/j.ackd.2016.03.002 . [DOI] [PubMed] [Google Scholar]
- 58.Van Regenmortel N., Moers L., Langer T. et al. Fluid-induced harm in the hospital: look beyond volume and start considering sodium. From physiology towards recommendations for daily practice in hospitalized adults. Ann. Intensive Care 11, 79 (2021). doi: 10.1186/s13613-021-00851-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malbrain M.L.N.G., Langer T., Annane D. et al. Intravenous fluid therapy in the perioperative and critical care setting: Executive summary of the International Fluid Academy (IFA). Ann. Intensive Care 10, 64 (2020). doi: 10.1186/s13613-020-00679-3 [DOI] [PMC free article] [PubMed] [Google Scholar]