Abstract
Biolistic delivery of biomolecular cargoes to plants with micron-scale projectiles is a well-established technique in plant biotechnology. However, the relatively large micron-scale biolistic projectiles can result in tissue damage, low regeneration efficiency, and create difficulties for the biolistic transformation of isomorphic small cells or subcellular target organelles (i.e., mitochondria and plastids). As an alternative to micron-sized carriers, nanomaterials provide a promising approach for biomolecule delivery to plants. While most studies exploring nanoscale biolistic carriers have been carried out in animal cells and tissues, which lack a cell wall, we can nonetheless extrapolate their utility for nanobiolistic delivery of biomolecules in plant targets. Specifically, nanobiolistics has shown promising results for use in animal systems, in which nanoscale projectiles yield lower levels of cell and tissue damage while maintaining similar transformation efficiencies as their micron-scale counterparts. In this chapter, we specifically discuss biolistic delivery of nanoparticles for plant genetic transformation purposes and identify the figures of merit requiring optimization for broad-scale implementation of nanobiolistics in plant genetic transformations.
Keywords: Biolistics, Nanobiolistics, Plant transformation, Agriculture, Bionanotechnology, Nanoparticles, Gold nanoparticles, Mesoporous silica nanoparticles (MSNs), Carbon nanotubes (CNTs)
1. Introduction
Nanomaterials are traditionally defined as materials with at least one dimension measuring under 100 nm, whereby the small size can confer unique physical, chemical, and biological properties to the material compared to its bulk counterpart [1]. Because of their unique and tunable properties, nanomaterials have enabled numerous novel applications in the fields of energy and electronics [2, 3], medicine and healthcare [4, 5], biotechnology [6], and agriculture [7]. Specifically, nanomaterials have engendered the field of bionanotechnology—the intersection of biology and nanotechnology in which nanotechnology is applied to fields such as medicine, molecular biology, synthetic biology, biochemistry, and agriculture. Several subfields of bionanotechnology, such as nanomedicine, have leveraged nanomaterials for the development of drug delivery systems that can deliver drugs to specific cell types, thus lowering overall drug dose and side-effects. Similarly, and more recently, nanomaterials have begun to advance plant science and agriculture through the usage of engineered nanoparticles that serve as nanocarriers, containing herbicides, fertilizers, chemicals, or genes, and can be targeted to specific plant sites prior to releasing their content [8]. Additionally, several studies report that certain nanoparticles can facilitate plant growth and overall plant health [9–11].
The nanomaterials most commonly used in biotechnology and medicine can be grouped into several categories: metal nanoparticles, lipids and liposomes, polymer-based nanoparticles, and silica-and carbon-based nanoparticles (Fig. 1a). Gold (Au) and magnetic iron oxide nanoparticles (MIONPs) are the two most routinely used metal nanoparticles for gene, protein, and drug delivery applications. MIONPs are advantageous for delivery due to their unique magnetic properties that allow cell sorting, magnetic guidance for targeted delivery, and tumor thermotherapy [12, 13]. Many studies report successful delivery of drugs and genes into animal cells via MIONPs that have multifunctional coatings, which allow for targeted and controlled cargo release into cells [14–17]. Gold nanoparticles are another class of nanomaterials that are highly preferred for biomolecule and drug delivery due to their ability to bind a wide range of organic and inorganic molecules, their low toxicity, and their strong and tunable optical absorption [18]. Recently, many well-defined hybrid gold/drug nanoparticles have been delivered to cells for targeted therapy and controlled release of payloads [19–21]. Additionally, Au metal nanoparticles are heavily used for nucleic acid and protein delivery into animal and plant systems, have been shown to protect DNA from enzymatic degradation, and enable targeted DNA cargo release with glutathione treatment [22, 23].
Fig. 1.
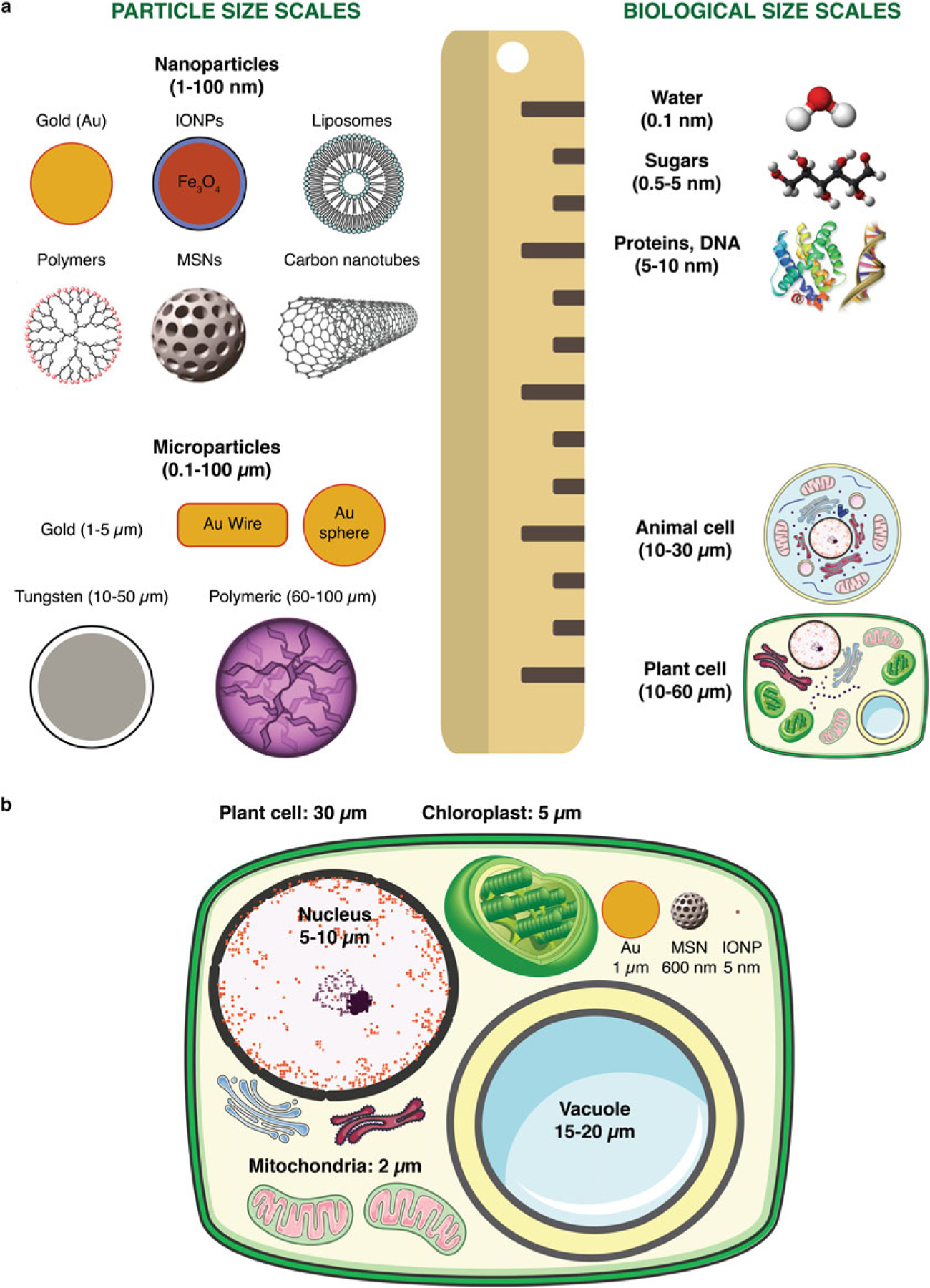
(a) Schematic of nanoparticle and microparticle sizes relative to biological matter, particularly the plant cell, which is the target of biolistic transformation. (b) Comparison of micro- and nanoparticles within a plant cell and plant cell organelles (drawn approximately to scale)
Lipids and liposomes (spherical vesicles formed by at least one lipid bilayer) can be efficient delivery vehicles of nucleic acids and drugs for both therapeutic and research applications. Specifically, cationic liposomes are among the most extensively studied nonviral delivery vehicles because their positive charge at physiological pH can be exploited to self-assemble with negatively charged nucleic acids and drugs, thus creating nano-sized complexes called lipoplexes [24]. Lipoplexes enable efficient delivery of many different cargo types (drugs [25], nucleic acids [26], proteins [27], and ribonucleoproteins (RNPs) [28]) specifically and in a stimuli-sensitive manner through multifunctional coatings that target lipoplexes to specific cell types and facilitate their internalization. Furthermore, lipoplexes can protect their cargoes from immune recognition, increase their longevity, and enable in vivo imaging of complexes [29].
Polymer-based nanoparticles can be divided into two subgroups: naturally-derived biopolymers and synthetic branched polymers called dendrimers. Naturally-derived biopolymers are advantageous for intracellular cargo delivery due to their biocompatibility, biodegradability, and low immunogenicity. Polymer-based nanoparticles are mostly utilized in tissue engineering applications where the goal is to achieve controlled delivery of a payload to cells, and polymer nanoparticles can also co-deliver therapeutic factors that enhance the efficacy of cell integration for tissue engineering [30]. Even though dendrimers can possess some degree of cytotoxicity in vivo depending on their size and structure, the first reported dendrimer polyamidoamine (PAMAM) remains widely utilized and many studies have shown successful delivery of drugs, plasmid DNA, and small interfering RNA (siRNA) for cancer therapy in animals [31] and delivery of genes to grass cells [32] using nano-sized dendrimer structures.
Mesoporous silica nanoparticles (MSNs) enable controlled release cargo delivery and co-delivery of genes and drugs both in animal [33] and plant systems [34]. MSNs enable retention of genetic cargo until its desired release site by loading MSNs with a gene vector and its chemical inducer, and by capping the MSN ends with gold. Following biolistic delivery of MSNs, the gold can be chemically uncapped to provide controlled release of DNA and the inducer to initiate gene expression. Carbon nanotubes (CNTs) are another nanoparticle system that has shown promise for drug and gene delivery into animals and plants [35] due to several advantageous CNT features: high aspect ratio, high tensile strength, a large surface area, and biocompatibility [36]. CNTs have been used to deliver drugs [37–39], proteins [40, 41], and genes [42–44] to animal cells and tissues. Furthermore, when bound to CNTs, biomolecules are shown to be protected from enzymatic and metabolic degradation [45], exhibiting superior biostability compared to free biomolecules. Moreover, single-walled carbon nanotubes (SWCNTs) have strong intrinsic near-infrared (nIR) fluorescence [46, 47] within the 1000–1300 nm tissue-transparency optical window, thus enabling tracking of cargo–nanoparticle complexes in vivo. Under certain surface chemistries, CNTs have also recently been shown to traverse extracted chloroplast [48] and plasma membranes [49], and deliver genetic cargoes (DNA, RNA) into mature plants with high efficiency and without any toxicity [50, 51, 101].
In the broader context of bionanotechnology, nanomaterials have been intensively explored and utilized for animal genetic transformations as described above; however, their use in plant systems remains understudied. The limited scope of nanotechnology for plant genetic transformation is likely due to the presence of the rigid and multilayered plant cell wall that limits internalization of most particles into plant cells. The internalization challenge caused by the plant cell wall is usually overcome by using a mechanical aid to penetrate the cell wall, such as the use of a gene gun in biolistic plant transformations. In traditional biolistic delivery, micron-sized gold particles are loaded with genetic cargo (oligo-nucleotides, nucleic acids, proteins, RNPs, etc.) and accelerated into the plant cells for plant genetic transformations. However, nano-sized delivery vehicles, due to their small size and tunable chemical properties, can enable the translocation of biological barriers that are otherwise not possible by micron-sized vehicles (Fig. 1b) [52]. This enables applications of such nanocarriers for plant chloroplast transformation, which is challenging to achieve with micron-sized vehicles due to the similarly sized chloroplast organelle that limits the number of micron-sized vehicles that can be taken up into the organelle without damage or organelle rupture [53]. Notably, pH-responsive nanomaterials have been used for selective delivery of genetic material to chloroplasts [54]. Nanomaterials offer the additional advantage of protecting the genetic cargo from degradation until the cargo reaches its final intracellular destination, thus increasing the genetic transformation efficiency [45].
Nanomaterials can be made to fall below the plant cell wall size exclusion limit of ~5–20 nm, and thus their tunable size could enable passive (biolistic-free) traversal of biological barriers such as the cell wall and membranes [50, 102]. In the context of prior work leveraging nanoparticles for plant biology, it is worthwhile to note that the 100 nm smallest dimension size boundary for the classical definition of nanomaterials is largely arbitrary and not necessarily the size scale at which all materials acquire new properties arising from size confinement effects. Recent studies suggest nanomaterial properties are tunable along a continuum of size scales and have called for new criteria for defining nanomaterials, such as a volume specific surface area (VSSA > 60 m2/cm3) [55]. Regardless of definition, particularly for plant transformation applications requiring internalization into the cell through the cell wall and lipid membrane, nanomaterial carrier size is one of the most important factors that determine whether particles internalize passively or will require assistance from biolistics.
Nanoparticles are increasingly used as delivery vehicles for applications in plant science; therefore, it is essential to understand plant-nanomaterial interactions. In particular, it is important to understand how nanomaterials interact with plants, if there could be adverse or toxic effects, or if nanomaterials could affect endogenous plant biology. Several research groups have begun studying these plant–nanomaterial interactions [7, 56–59]. For instance, an early study revealed that silica nanoparticles do not adversely affect the wheat seed germination, emergence, or growth of seedlings [60]. Another follow-up study determined the effects of functionalized and non-functionalized SWCNTs on root elongation of six different crop species: cabbage, carrot, cucumber, lettuce, onion, and tomato. Results showed that both types of CNTs enhanced root elongation in onion and cucumber, cabbage and carrot were not affected by either form of nanotubes, root elongation in lettuce was inhibited with functionalized CNTs, and tomato was found to be most sensitive for CNTs with significant root length reduction [61]. It was also demonstrated by another study that SWCNTs enhanced germination of rice and zucchini seeds and did not show adverse effects on root elongation [62]. A few studies reported the uptake, translocation, and specific localization of magnetic iron oxide nanoparticles in pumpkin plants, with no observable toxicity on plant growth [63]. Despite progress in assessing plant-nanomaterial interactions, it is worthwhile to note that interactions will be specific to plant species and tissue type, plant age, nanoparticle type, nanoparticle surface chemistry, and other parameters, and that more research in this multiparameter space will be necessary to fully understand nanomaterial interactions with plants.
Nanomaterial-based delivery vehicles show promise to bring unique advantages to plant genetic transformations given the success in animal studies over the past few decades. Nanomaterials can provide controlled, target-specific, and stimuli-responsive release of genetic cargoes into plant cells and can also be targeted to subcellular locations such as plastids. In this chapter, we discuss the synthesis and characterization of the few instances of nanoparticles used for nanobiolistics, information gained from animal nanobiolistic studies, and compare nanobiolistic to microbiolistic delivery.
2. Discussion
2.1. Synthesis and Characterization of Nanoparticles for Nanobiolistics
The most commonly used nanoparticles for nanobiolistic delivery are mesoporous silica nanoparticles (MSNs) and gold nanoparticles (AuNPs). MSNs contain a highly porous structure that permits internal loading of biomolecules—such as DNA, RNA, and proteins—and subsequent biolistic delivery to animal and plant tissues. AuNPs are chemically identical to the standard 0.6 μm gold projectile traditionally used in biolistic delivery; however, their smaller size offers several advantages. The remainder of this section will focus on the synthesis and characterization of these two common nanoparticles used in plant biolistics, and examples from the literature of their successful application in animal and plant transformations.
2.1.1. Synthesis of Mesoporous Silica Nanoparticles
MSN synthesis proceeds by a modified Stöber process, which relies on alkyl silicate polymerization in the presence of an ionic surfactant to produce porous spherical particles [64]. The most commonly used alkyl silicate precursor and surfactant are tetraethyl orthosilicate (TEOS) and cetyltrimethylammonium bromide (CTAB), respectively. Alkyl silicates undergo sequential hydrolysis in acidic conditions and condensation in basic conditions to produce 3-dimensional siloxane networks. Ionic surfactants form micelles with positively charged surfaces that associate with silicate molecules and provide a template around which the polymer network is formed. The surfactant is then removed by calcination, dialysis, or solvent extraction, resulting in a siloxane mesostructure with particle diameters on the order of tens to hundreds of nanometers and pores on the order of several nanometers. Precise control over particle diameter is achieved by tuning the reaction pH and precursor-surfactant ratio, whereas pore diameter can be tuned by changing the surfactant chain length, adding organic swelling agents, or by using block copolymer cotemplates [65]. Furthermore, functionalized MSNs can be synthesized by chemically modifying the alkyl silicate precursor prior to polymerization. For example, Slowing et al. synthesized fluorescent-labeled MSNs through the coupling of fluorescein isothiocyanate (FITC) to an aminosilane APTMS, which then underwent co-condensation with TEOS to form FITC-MSNs, allowing cellular internalization to be monitored by fluorescence microscopy [66]. Additionally, co-condensation with a mercaptosilane generates thiolated MSNs [67] that are useful for downstream functionalization such as bioconjugation or pore-capping for improved delivery efficiency. For example, Lai et al. synthesized pore-capped MSNs through disulfide linkage with cadmium sulfide (CdS) nanoparticles, allowing for controlled release of small molecules upon the introduction of a disulfide-reducing agent [68].
2.1.2. Synthesis of Gold Nanoparticles
AuNP synthesis typically proceeds in situ by Au(III) reduction from chloroauric acid (HAuCl4) precursor in the presence of a stabilizing agent to form colloidal Au. The major synthetic routes for spherical AuNPs are the Turkevich–Frens method [69], where surface-adsorbed citrate acts as a stabilizer, and the Brust–Schriffin method [70], where covalently bonded thiols act as a stabilizer. Many advancements have been made to these standard protocols, allowing control over particle diameter through tuning of the Au–stabilizer ratio or use of alternate reducing and stabilizing agents [71]. The Brust–Schriffin synthesis is preferred for downstream bioconjugation applications as thiolated surface groups are amenable to a wide variety of biocompatible linker chemistries. Lastly, cylindrical gold nanorods may be synthesized electrochemically by deposition onto polycarbonate or alumina pore templates or in the presence of rod-inducing surfactants [72].
2.1.3. Nanoparticle Characterization
Many techniques have been developed to measure critical parameters of MSNs and AuNPs such as size, morphology, dispersity, colloidal stability, and porosity. Zeta potential (ZP), or the electric potential at the interfacial double layer, is a convenient and well-established indicator of colloidal stability that is simple and inexpensive to measure. Commonly accepted values for ZP are >±30 mV and <±10 mV for stable and unstable colloids, respectively, although in rare cases, ZP alone is not indicative of stability [73]. Nanoparticle size and polydispersity are typically measured through dynamic light scattering (DLS) and UV-vis spectroscopy which, like ZP, have been adopted by the field as a quick tabletop measurement of nanoparticle properties. In general, spectroscopic methods are widely used in nanoparticle research as characteristic changes to scattering patterns and absorption spectra are observed upon functionalization and cargo loading. In the case of MSNs, X-ray diffraction patterns are an indicator of pore structure, and N2 adsorption isotherms are employed to measure pore dimensions. Lastly, high-resolution microscopic techniques such as transmission electron microscopy (TEM) are used for direct observation of nanoparticle morphology and size.
2.2. Testing Nanobiolistics in Animals
Although the focus of this book chapter centers on biolistic DNA delivery in plants, there is still a very limited body of plant nanobiolistics literature. Initially developed as a method of particle delivery for use in plants [74], the gene gun system has since been adapted for and more broadly applied in animal systems. While animal cells lack the cell wall present in plant cells which biolistic delivery is often needed to traverse, biolistics is nevertheless useful for bio-cargo delivery to certain types of animal cells and tissues. Notably, the earliest use of nanoparticles for biolistic particle delivery was reported by Roizenblatt et al. in 2006 for the delivery of calcium indicator-loaded nanoparticles to mice retinal whole mounts, where the nanobiolistic protocol resulted in significantly less damage to cultured retinal progenitor cells than an analogous protocol with micron-sized particles [75]. Nanobiolistics in animal systems can thus provide valuable insights regarding the nanoparticle parameters important for delivery in plants, particularly as several studies in retinal cells, human embryonic kidney (HEK) cells, and mouse brains have run comparisons between micron-sized and nanometer-sized biolistic particles (Table 1). These studies have shown that nanoparticle usage results in significantly less damage than micron-sized particles, while successfully transfecting cells or tissue at comparable efficiencies [75–77]. However, analogous nanobiolistic transformation efficiencies reported in plant systems are limited in number, and the effect of biolistic particle size, composition, biolistic pressure, and surface chemistry on important metrics such as loading capacity, penetration depth, cell viability, and transfection efficiency remain to be determined for nanobiolistics in plants.
Table 1.
Summary of nanobiolistics based studies in the animal systems
| Nanoparticle | Delivery metrics | |||||||
|---|---|---|---|---|---|---|---|---|
| Type | Size | Cargo | Target tissue/cell | Delivery pressure (psi) | Depth penetration | Cell damage | Transfection efficiency | Reference |
| Tungsten | 1.3 μm | – | Retinal progenitor cells | 75 | – | 12% | – | [77] |
| – | Retinal progenitor cells | 150 | – | 25% | – | |||
| Silver | 80 nm | – | Retinal progenitor cells | 250 | – | 2% | – | |
| Gold | 1 μm | EYFP DNA | HEK | 50 | – | 63.7 ± 3.5 μma | 27 ± 4% | [77] |
| 40 nm | EYFP DNA | HEK | 50 | – | 21.7 ± 1.3 μma | 25 ± 4% | ||
| 1 μm | NS | Mouse ear tissue | 75 | 50 ± 11 μm | 22 ± 3% | – | ||
| 40 nm | NS | Mouse ear tissue | 75 | 31 ± 6 μm | 9 ± 2% | – | ||
| Gold | 1 μm | EYFP DNA | Organotypic brain slices (OTPS) | 50 | – | 47% | 23% | [76] |
| 40 nm | EYFP DNA | OTPS | 50 | – | Negligible | 33% | ||
NS: not specified
Cell and tissue damage quantified using diameter of zone of dead cells dislodged by bombardment
In addition to Roizenblatt et al., O’Brien and Lummis also used micron-sized and nano-sized particles, conducting a comparative analysis of transfection efficiency, depth penetration, and tissue damage [77]. Quantifying transfection efficiency in HEK cells with a reporter plasmid and depth penetration studies of mouse ear tissue showed no significant difference using either sized particle in terms of transformation efficiency. The viability of cells from mouse ear samples was surveyed using DAPI staining, showing significantly less damage with nanoparticles. Decreased cell damage as an advantage of nanoparticles over micron-sized particles is supported by Arsenault and O’Brien’s later work on transforming organotypic brain slices from mice [76]. An LDH assay, a Terminal deoxynucleotide transferase dUTP nick end labeling (TUNEL) assay and propidium iodide (PI) was used to analyze cell survival, leading to the conclusion that nanoparticles in biolistics reduce cell and tissue damage.
Though not strictly within the canonical size range of “nanoparticles,” Lee et al. have developed two forms of polymeric particles for use in biolistic delivery [78, 79]. Chitosan (CS) and poly-g-glutamic acid (g-PGA) nanoparticle were prepared using an ionicgelation method. Particles of sizes from 150 to 250 nm were created by adjusting the ratio of CS to g-PGA and used to deliver GFP-encoding plasmids for transdermal delivery in mice. Another formulation utilized a core–shell design, where 250 nm particles were composed of a poly(d,l-lactic-co-glycolic acid) (PLGA) core and glycol chitosan (GC) shell. The particles were loaded with GFP plasmids and targeted at Langerhans cells present in the mice epidermis. Both nanoparticles are believed to release DNA cargo in a pH-responsive manner. Huang et al. have also utilized CS polymeric nanoparticles sized 150–270 nm for low-pressure biolistic delivery of GFP reporter genes, a plasmid encoding β-galactosidase genes, and a Japanese encephalitis virus DNA vaccine to mice via transdermal bombardment [80]. The successful use of these formulations for biolistics supports the viability of nanobiolistics as an alternative to traditional biolistics and showcases the possibilities of chemical and physical modifications to particles in biolistic delivery for increased transfection efficacy.
The field of nanobiolistics has created considerable excitement for genetic transformation, drug delivery, and tissue imaging due to its potential to cause less cellular damage, target more cell types (including difficult-to-transfect cells due to cell/organelle size or penetration depth), and increased loading capacity. Applying nanometer-sized particles in biolistic delivery to animal systems has demonstrated that nanoparticles result in lower levels of cell and tissue damage while maintaining similar transformation efficiency levels, a result likely to be replicable in plants as well. Certainly, there is still a dearth of information regarding the effect of using nanoparticles in place of microparticles on different cell and tissue types. More studies of the effect of particle size on delivery efficacy need to be conducted to create a set of heuristics where nanobiolistics can be accurately compared to already well-developed microbiolistics protocols, particularly for use in plants.
2.3. Emerging Studies of Nanobiolistics in Plants
Over the past several decades, numerous plant species have been successfully genetically transformed and recently genetically engineered via biolistics using micron-sized gold particles. These plant species include but are not limited to maize [81, 82], wheat [83, 84], rice [85, 86], tomato [87], barley [88] and soybean [89]. However, many of these studies also revealed that biolistics using micron-sized gold particles have some limitations and disadvantages that can be improved by using nano-sized particles.
First, the transformed cell survival rate is low, possibly because of the damage caused by accelerated microparticles [90, 91]. Second, studies have shown that genetic transformation of plant cells via biolistics using micron-sized particles resulted in multiple gene insertions into the host plant genome rather than the often-desired single-gene integration [92], which is suspected to cause unstable gene expression in transformed plants [93]. Third, due to the co-transfer of large fragments of the vector backbone DNA and transgene rearrangements during the integration process, there is a risk of obtaining transgenic plants expressing undesired genes, such as antibiotic resistance genes [94, 95]. Lastly, it is difficult to transform small cells and subcellular organelles (e.g., plastids and mitochondria) by microbiolistics due to the size of particles being comparable to the target size. It is thought that nanoparticles delivered via biolistics can address these limitations given their small size, less target impact, and highly controlled, specific and tunable cargo loading and releasing abilities.
Although most of the thorough characterization for nanobiolistic parameters and comparisons with traditional microbiolistics has been undertaken in animal systems, there has been an emergence of work in nanobiolistics in plant systems (Table 2). The first example of nanobiolistics in plant transformation was published in 2007 by the Wang group, where surface-precipitated DNA and pore-loaded small molecules were co-delivered by Au-capped MSNs with particle and pore diameters of 100–200 and 2 nm, respectively [34]. This study found that MSNs alone were not effective for biolistic delivery of GFP plasmid, probably owing to their very low mass density. However, MSNs became effective when pore-capped with 10–15 nm AuNPs linked by amide coupling, probably due to the larger hybrid particle density, wherein gold caps were released in the presence of dithiothreitol in regeneration media [34]. Notably, this study demonstrated biolistic co-delivery of an inducible-GFP plasmid and β-estradiol, a chemical inducer, to Nicotiana benthamiana cotyledons validating the Au-MSN platform as a useful tool for delivery of DNA and small molecules [34].
Table 2.
Summary of nanobiolistic transformation studies in plants
| Species/tissue | NP type | Cargo | Loading capacity (w/w) | Delivery pressure (psi) | Target distance (cm) | Efficiency | Reference |
|---|---|---|---|---|---|---|---|
| N. tabacum plantlets | Au-capped MSNs (2.3 nm pores) | GFP plasmid and inducer | 0.1 DNA:MSN | 650 | 10 | 35.6 ± 12.8 GFP+ foci per plantlet | [34] |
| A. cepa epidermis | Au-capped MSNs (10 nm pores) | GFP or FITC-BSA protein, mCherry plasmid | 0.625 BSA:MSN 0.15 GFP:MSN 0.01 DNA:MSN | 1350 | 6 | 8% (GFP) and 28% (BSA) protein released | [98] |
| Z. mays embryos | Au-plated MSNs (10 nm pores) | Cre protein | 0.03 Cre:MSN | 650 | 6 | 24 events out of 120 embryos | [98] |
| O. sativa embryogenic calli | AuNPs (50, 200, 600, 1000 nm) | cry plasmid with hgh selectable marker | Not reported | 1100 | 5 | 5 events out of 12 putative lines | [99] |
| A. cepa epidermis | Au nanorods (NRs, 25 × 73 nm) | mCherry plasmid | 0.1 DNA: NRs | 1350 | 4 | 77% of bombarded cells | [97] |
The Wang group has also used the Au-MSN system for delivery of pore-loaded proteins by bombardment with 600 nm Au-MSNs with large pore diameters (10 nm) [96]. Active GFP and FITC-BSA were successfully delivered by bombardment to onion epidermal cells, tobacco leaves, and teosinte leaves [96]. This study is a good proof of concept that few-hundred nanometer Au-MSNs with 10 nm pores are suitable vehicles for delivery of proteins with hydrodynamic radii of several nanometers. A parameter optimization study with Au nanorods (NRs) and Au-capped-MSNs revealed that 2 times bombardment at higher pressures (1350 or 1550 psi) and smaller target distances (4 cm) greatly improve transient expression efficiencies compared to lower pressures (650 psi) and longer target distances (10 cm) in tobacco leaves and maize immature embryos [97]. Furthermore, with respect to optimizing particle formulation, it was found that Au-plating of MSNs improves particle mass density and expression efficiency more so than Au-capping and that CaCl2/spermidine DNA precipitation greatly improves the adsorption of DNA cargo onto the MSN surface [97]. The Wang’s group then employed the Au-plated MSN system to deliver protein cargo for genome editing by Cre–Lox recombination in maize, thereby demonstrating that the nanobiolistic approach can be used for the production of precisely modified nontransgenic plants by DNA-free methods [98].
Systematic and direct comparisons of nanobiolistic and microbiolistic delivery in plants are sparse, but a few studies do exist. Wang and colleagues found that DNA-coated Au-MSN (100–200 nm MSNs, 10–15 nm AuNPs) bombardment produced 32 ± 11 GFP-fluorescent foci per cotyledon, while the standard 0.6 μm AuNP bombardment produced 73 ± 24 GFP-fluorescent foci per cotyledon [34]. Mortazavi and Zohrabi found that plasmid delivery to rice embryogenic calli by bombardment with 50, 100, 600, and 1000 nm AuNPs resulted in similar levels of transgene integration across all carrier sizes [99]. Okuzawi et al. found that 300 nm gold particles were more effective than 600 nm gold particles and slightly less effective than 70 nm gold particles for tobacco plastid transformation; while these data lack statistical significance, they support the claim that smaller AuNPs are amenable for the transformation of subcellular organelles [100]. While these limited studies suggest that nanoparticles do not always necessarily provide an advantage in plant transformation efficiency, they do however demonstrate certain unique advantages of nanobiolistics over the traditional microbiolistics: plastid transformation and co-delivery/controlled release of DNA, proteins, and small molecules in whole plant cells.
3. Conclusions
Biolistic delivery is a powerful and popular biotechnology tool that has advanced drug, gene, and protein delivery into both animal and plant systems. Traditional carriers of biolistic delivery are micron-sized particles (most commonly gold, tungsten, or polymeric particles) and have been shown to cause variable levels of tissue/cell damage. Furthermore, micron-sized particles are difficult to implement for the transformation of small cells or subcellular targets such as mitochondria and plastids. Nanoparticles as cargo delivery platforms with biolistics—nanobiolistics—can offer unique advantages over micron-sized particles: decreased damage, ability to transform smaller targets, tunable physical and chemical properties, controlled and targeted release of cargo, and protection of the cargo from cellular metabolism. Orthogonally, nanoparticles below the plant cell wall size exclusion limit may offer additional opportunities for passive (non-biolistic) cell entry.
Recent nanobiolistics studies with animal cells have demonstrated that nanoparticle-mediated transformations result in lower levels of cell and tissue damage while maintaining similar transformation efficiency; a feature of nanobiolistics that may be achievable in plant systems in the future studies. However, a systematic investigation of parameters affecting transformation efficiency and tissue damage as a function of nanoparticle properties (size, shape, charge, stiffness, etc.) is necessary to optimize nanobiolistics for routine use in plant systems. Based on the remarkable progress of nanobiolistics in animal tissues, this newly emerging nanobiolistics technology, once optimized, could similarly advance the field of plant genetic transformations.
Acknowledgments
The authors acknowledge support from a Burroughs Wellcome Fund Career Award at the Scientific Interface (CASI), a Beckman Foundation Young Investigator Award, a USDA AFRI award, a grant from the Gordon and Betty Moore Foundation, a USDA NIFA award, an NIH MIRA award, support from the Chan-Zuckerberg foundation, and an FFAR New Innovator Award (to M.P.L). F.J.C is supported by an NSF Graduate Research Fellowship, N.S.G is supported by a FFAR Fellowship, and G.S.D. is supported by a Schlumberger Foundation Faculty for the Future Fellowship.
References
- 1.Subcommittee N (2007) The national nanotechnology initiative. Nanotechnology. 10.4135/9781412972093.n338 [DOI] [Google Scholar]
- 2.Johlin E, Al-Obeidi A, Nogay G, Stuckelberger M, Buonassisi T, Grossman JC (2016) Nanohole structuring for improved performance of hydrogenated Amorphous silicon photovoltaics. ACS Appl Mater Interfaces 8:15169–15176 [DOI] [PubMed] [Google Scholar]
- 3.Lee YM, Lee D, Kim J, Park H, Kim WJ (2015) RPM peptide conjugated bioreducible polyethylenimine targeting invasive colon cancer. J Control Release 205:172–180 [DOI] [PubMed] [Google Scholar]
- 4.LaVan DA, McGuire T, Langer R (2003) Small-scale systems for in vivo drug delivery. Nat Biotechnol 21:1184–1191 [DOI] [PubMed] [Google Scholar]
- 5.Cavalcanti A, Shirinzadeh B, Freitas RA Jr, Hogg T (2007) Nanorobot architecture for medical target identification. Nanotechnology 19:15103 [Google Scholar]
- 6.Zadegan RM, Norton ML (2012) Structural DNA nanotechnology: from design to applications. Int J Mol Sci 13:7149–7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghormade V, Deshpande MV, Paknikar KM (2011) Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol Adv 29:792–803 [DOI] [PubMed] [Google Scholar]
- 8.Thangavelu RM, Gunasekaran D, Jesse MI, Riyaz MSU, Sundarajan D, Krishnan K (2018) Nanobiotechnology approach using plant rooting hormone synthesized silver nanoparticle as “nanobullets” for the dynamic applications in horticulture – an in vitro and ex vitro study. Arab J Chem 11:48–61 [Google Scholar]
- 9.Barrena R, Casals E, Colón J, Font X, Sánchez A, Puntes V (2009) Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 75:850–857 [DOI] [PubMed] [Google Scholar]
- 10.Arora S, Sharma P, Kumar S, Nayan R, Khanna PK, Zaidi MGH (2012) Gold-nanoparticle induced enhancement in growth and seed yield of Brassica juncea. Plant Growth Regul 66:303–310 [Google Scholar]
- 11.Salama HMH (2012) Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L.). Int Res J Biotechnol 3:190–197 [Google Scholar]
- 12.Tian H, Chen J, Chen X (2013) Nanoparticles for gene delivery. Small 9:2034–2044 [DOI] [PubMed] [Google Scholar]
- 13.Demirer GS, Okur AC, Kizilel S (2015) Synthesis and design of biologically inspired biocompatible iron oxide nanoparticles for biomedical applications. J Mater Chem B 3:7831–7849 [DOI] [PubMed] [Google Scholar]
- 14.Laurent S, Saei AA, Behzadi S, Panahifar A, Mahmoudi M (2014) Superparamagnetic iron oxide nanoparticles for delivery of therapeutic agents: opportunities and challenges. Expert Opin Drug Deliv 11:1449–1470 [DOI] [PubMed] [Google Scholar]
- 15.Nazli C, Demirer GS, Yar Y, Acar HY, Kizilel S (2014) Targeted delivery of doxorubicin into tumor cells via MMP-sensitive PEG hydrogel-coated magnetic iron oxide nanoparticles (MIONPs). Colloids Surfaces B Biointerfaces 122:674–683 [DOI] [PubMed] [Google Scholar]
- 16.Mu Q, Jeon M, Hsiao M-H, Patton VK, Wang K, Press OW, Zhang M (2015) Stable and efficient paclitaxel nanoparticles for targeted glioblastoma therapy. Adv Healthc Mater 4:1236–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin S, Leach JC, Ye K (2009) Nanoparticle-mediated gene delivery. In: Foote RS, Lee JW (eds) Micro and nano technologies in bioanalysis: methods and protocols. Humana, Totowa, NJ, pp 547–557 [Google Scholar]
- 18.Pissuwan D, Niidome T, Cortie MB (2011) The forthcoming applications of gold nanoparticles in drug and gene delivery systems. J Control Release 149:65–71 [DOI] [PubMed] [Google Scholar]
- 19.Gu Y-J, Cheng J, Lin C-C, Lam YW, Cheng SH, Wong W-T (2009) Nuclear penetration of surface functionalized gold nanoparticles. Toxicol Appl Pharmacol 237:196–204 [DOI] [PubMed] [Google Scholar]
- 20.Gibson JD, Khanal BP, Zubarev ER (2007) Paclitaxel-functionalized gold nanoparticles. J Am Chem Soc 129:11653–11661 [DOI] [PubMed] [Google Scholar]
- 21.Shiotani A, Mori T, Niidome T, Niidome Y, Katayama Y (2007) Stable incorporation of gold nanorods into N-isopropylacrylamide hydrogels and their rapid shrinkage induced by near-infrared laser irradiation. Langmuir 23:4012–4018 [DOI] [PubMed] [Google Scholar]
- 22.Han G, Martin CT, Rotello VM (2006) Stability of gold nanoparticle-bound DNA toward biological, physical, and chemical agents. Chem Biol Drug Des 67:78–82 [DOI] [PubMed] [Google Scholar]
- 23.Han G, Chari NS, Verma A, Hong R, Martin CT, Rotello VM (2005) Controlled recovery of the transcription of nanoparticle-bound DNA by intracellular concentrations of glutathione. Bioconjug Chem 16:1356–1359 [DOI] [PubMed] [Google Scholar]
- 24.Pezzoli D, Kajaste-Rudnitski A, Chiesa R, Candiani G (2013) Lipid-based nanoparticles as nonviral gene delivery vectors. In: Bergese P, Hamad-Schifferli K (eds) Nanomaterial interfaces in biology: methods and protocols. Humana, Totowa, NJ, pp 269–279 [DOI] [PubMed] [Google Scholar]
- 25.Blanco E, Shen H, Ferrari M (2015) Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol 33:941–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hui SW, Langner M, Zhao Y-L, Ross P, Hurley E, Chan K (1996) The role of helper lipids in cationic liposome-mediated gene transfer. Biophys J 71:590–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatin B, Mével M, Devallière J, Dallet L, Haudebourg T, Peuziat P, Colombani T, Berchel M, Lambert O, Edelman A, Pitard B (2015) Liposome-based formulation for intracellular delivery of functional proteins. Mol Ther Nucleic Acids 4:e244. [DOI] [PubMed] [Google Scholar]
- 28.Wang M, Zuris JA, Meng F, Rees H, Sun S, Deng P, Han Y, Gao X, Pouli D, Wu Q, Georgakoudi I, Liu DR, Xu Q (2016) Efficient delivery of genome-editing proteins using bioreducible lipid nanoparticles. Proc Natl Acad Sci U S A 113:2868–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torchilin VP (2014) Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov 13:813–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nitta KS, Numata K (2013) Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering. Int J Mol Sci 14:1629–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Liang H, Liu J, Wang Z (2018) Poly (amidoamine) (PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy. Int J Pharm 546:215–225 [DOI] [PubMed] [Google Scholar]
- 32.Pasupathy K, Lin S, Hu Q, Luo H, Ke PC (2008) Direct plant gene delivery with a poly (amidoamine) dendrimer. Biotechnol J 3:1078–1082 [DOI] [PubMed] [Google Scholar]
- 33.Hartono SB, Phuoc NT, Yu M, Jia Z, Monteiro MJ, Qiao S, Yu C (2014) Functionalized large pore mesoporous silica nanoparticles for gene delivery featuring controlled release and co-delivery. J Mater Chem B 2:718–726 [DOI] [PubMed] [Google Scholar]
- 34.Torney F, Trewyn BG, Lin VSY, Wang K (2007) Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat Nanotechnol 2:295–300 [DOI] [PubMed] [Google Scholar]
- 35.Karimi M, Solati N, Ghasemi A, Estiar MA, Hashemkhani M, Kiani P, Mohamed E, Saeidi A, Taheri M, Avci P, Aref AR, Amiri M, Baniasadi F, Hamblin MR (2015) Carbon nanotubes part II: a remarkable carrier for drug and gene delivery. Expert Opin Drug Deliv 12:1089–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demirer GS, Landry MP (2017) Delivering genes to plants. Chem Eng Prog 113:40–45 [Google Scholar]
- 37.Bhirde AA, Patel V, Gavard J, Zhang G, Sousa AA, Masedunskas A, Leapman RD, Weigert R, Gutkind JS, Rusling JF (2009) Targeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery. ACS Nano 3:307–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heister E, Neves V, Tîlmaciu C, Lipert K, Beltrán VS, Coley HM, Silva SRP, McFadden J (2009) Triple functionalisation of single-walled carbon nanotubes with doxorubicin, a monoclonal antibody, and a fluorescent marker for targeted cancer therapy. Carbon 47:2152–2160 [Google Scholar]
- 39.Liu Z, Chen K, Davis C, Sherlock S, Cao Q, Chen X, Dai H (2008) Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res 68:6652–6660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi Kam NW, Jessop TC, Wender PA, Dai H (2004) Nanotube molecular transporters: internalization of carbon nanotube–protein conjugates into mammalian cells. J Am Chem Soc 126:6850–6851 [DOI] [PubMed] [Google Scholar]
- 41.Kam NWS, Liu Z, Dai H (2006) Carbon nanotubes as intracellular transporters for proteins and DNA: an investigation of the uptake mechanism and pathway. Angew Chem 118:591–595 [DOI] [PubMed] [Google Scholar]
- 42.Dong H, Ding L, Yan F, Ji H, Ju H (2011) The use of polyethylenimine-grafted graphene nanoribbon for cellular delivery of locked nucleic acid modified molecular beacon for recognition of microRNA. Biomaterials 32:3875–3882 [DOI] [PubMed] [Google Scholar]
- 43.Qin W, Yang K, Tang H, Tan L, Xie Q, Ma M, Zhang Y, Yao S (2011) Improved GFP gene transfection mediated by polyamidoamine dendrimer-functionalized multi-walled carbon nanotubes with high biocompatibility. Colloids Surfaces B Biointerfaces 84:206–213 [DOI] [PubMed] [Google Scholar]
- 44.Karmakar A, Bratton SM, Dervishi E, Ghosh A, Mahmood M, Xu Y, Saeed LM, Mustafa T, Casciano D, Radominska-Pandya- A, Biris AS (2011) Ethylenediamine functionalized-single-walled nanotube (f-SWNT)-assisted in vitro delivery of the oncogene suppressor p53 gene to breast cancer MCF-7 cells. Int J Nanomedicine 6:1045–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Y, Phillips JA, Liu H, Yang R, Tan W (2008) Carbon nanotubes protect DNA strands during cellular delivery. ACS Nano 2:2023–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng M, Jagota A, Semke ED, Diner BA, McLean RS, Lustig SR, Richardson RE, Tassi NG (2003) DNA-assisted dispersion and separation of carbon nanotubes. Nat Mater 2:338–342 [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Koleilat GI, Liu P, Jiménez-Osés G, Lai YC, Vosgueritchian M, Fang Y, Park S, Houk KN, Bao Z (2014) High-yield sorting of small-diameter carbon nanotubes for solar cells and transistors. ACS Nano 8:2609–2617 [DOI] [PubMed] [Google Scholar]
- 48.Wong MH, Misra RP, Giraldo JP, Kwak SY, Son Y, Landry MP, Swan JW, Blankschtein D, Strano MS (2016) Lipid exchange envelope penetration (LEEP) of nanoparticles for plant engineering: a universal localization mechanism. Nano Lett 16:1161–1172 [DOI] [PubMed] [Google Scholar]
- 49.Giraldo JP, Landry MP, Faltermeier SM, McNicholas TP, Iverson NM, Boghossian AA, Reuel NF, Hilmer AJ, Sen F, Brew JA, Strano MS (2014) Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat Mater 13:400–408 [DOI] [PubMed] [Google Scholar]
- 50.Demirer GS, Zhang H, Matos J, Goh NS, Cunningham FJ, Sung Y, Chang R, Aditham AJ, Chio L, Cho MJ, Staskawicz B, Landry MP (2018) High aspect ratio nanomaterials enable delivery of functional genetic material without DNA integration in mature plants. Nat Nanotechnol 14:456–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cunningham FJ, Goh NS, Demirer GS, Matos JL, Landry MP (2018) Nanoparticle-mediated delivery towards advancing plant genetic engineering. Trends Biotechnol 36:882–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang P, Lombi E, Zhao F-JJ, Kopittke PM (2016) Nanotechnology: a new opportunity in plant sciences. Trends Plant Sci 21:699–712 [DOI] [PubMed] [Google Scholar]
- 53.Sidorov VA, Kasten D, Pang S, Hajdukiewicz PT, Staub JM, Nehra NS (1999) Stable chloroplast transformation in potato: use of green fluorescent protein as a plastid marker. Plant J 19:209–216 [DOI] [PubMed] [Google Scholar]
- 54.Kwak S-Y, Lew TTS, Sweeney CJ, Koman VB, Wong MH, Bohmert-Tatarev K, Snell KD, Seo JS, Chua NH, Strano MS (2016) Chloroplast-selective gene delivery and expression in planta using chitosancomplexed single-walled carbon nanotube carriers. Nat Nanotechnol 14:447–455 [DOI] [PubMed] [Google Scholar]
- 55.Kreyling WG, Semmler-Behnke M, Chaudhry Q (2010) A complementary definition of nanomaterial. Nano Today 5:165–168 [Google Scholar]
- 56.Nair R, Varghese SH, Nair BG, Maekawa T, Yoshida Y, Kumar DS (2010) Nanoparticulate material delivery to plants. Plant Sci 179:154–163 [Google Scholar]
- 57.Parisi C, Vigani M, Rodríguez-Cerezo E (2015) Agricultural nanotechnologies: what are the current possibilities? Nano Today 10:124–127 [Google Scholar]
- 58.Miralles P, Church TL, Harris AT (2012) Toxicity, uptake, and translocation of engineered nanomaterials in vascular plants. Environ Sci Technol 46:9224–9239 [DOI] [PubMed] [Google Scholar]
- 59.Pérez-de-Luque A (2017) Interaction of nanomaterials with plants: what do we need for real applications in agriculture? Front Environ Sci 5:12 [Google Scholar]
- 60.Zhang F, Wang R, Xiao Q, Wang Y, Zhang J (2006) Effects of slow/controlled-release fertilizer cemented and coated by nano-materials on biology. II Effects of slow/controlled-release fertilizer cemented and coated by nano-materials on plants. Nanoscience 11:18–26 [Google Scholar]
- 61.Cañas JE, Long M, Nations S, Vadan R, Dai L, Luo M, Ambikapathi R, Lee EH, Olszyk D (2008) Effects of functionalized and nonfunctionalized single-walled carbon nanotubes on root elongation of select crop species. Environ Toxicol Chem 27:1922–1931 [DOI] [PubMed] [Google Scholar]
- 62.Stampoulis D, Sinha SK, White JC (2009) Assay-dependent phytotoxicity of nanoparticles to plants. Environ Sci Technol 43:9473–9479 [DOI] [PubMed] [Google Scholar]
- 63.Zhu H, Han J, Xiao JQ, Jin Y (2008) Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. J Environ Monit 10:713–717 [DOI] [PubMed] [Google Scholar]
- 64.Grün M, Lauer I, Unger KK (1997) The synthesis of micrometer- and submicrometer-size spheres of ordered mesoporous oxide MCM-41. Adv Mater 9:254–257 [Google Scholar]
- 65.Wu S-H, Mou C-Y, Lin H-P (2013) Synthesis of mesoporous silica nanoparticles. Chem Soc Rev 42:3862–3875 [DOI] [PubMed] [Google Scholar]
- 66.Slowing I, Trewyn BG, Lin VS (2006) Effect of surface functionalization of MCM-41-type mesoporous silica nanoparticles on the endocytosis by human cancer cells. J Am Chem Soc 128:14792–14793 [DOI] [PubMed] [Google Scholar]
- 67.Lim MH, Blanford CF, Stein A (1998) Synthesis of ordered microporous silicates with organosulfur surface groups and their applications as solid acid catalysts. Chem Mater 102:467–470 [Google Scholar]
- 68.Lai CY, Trewyn BG, Jeftinija DM, Jeftinija K, Xu S, Jeftinija S, Lin VS (2003) A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. J Am Chem Soc 125:4451–4459 [DOI] [PubMed] [Google Scholar]
- 69.Frens G (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci 241:20–22 [Google Scholar]
- 70.Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R (1994) Synthesis of thiolderivatised gold nanoparticles in a two-phase liquid–liquid system. J Chem Soc Chem Commun 7:801–802 [Google Scholar]
- 71.Zhao P, Li N, Astruc D (2013) State of the art in gold nanoparticle synthesis. Coord Chem Rev 257:638–665 [Google Scholar]
- 72.Pérez-Juste J, Pastoriza-Santos I, Liz-Marzán LM, Mulvaney P (2005) Gold nanorods: synthesis, characterization and applications. Coord Chem Rev 249:1870–1901 [Google Scholar]
- 73.Bhattacharjee S (2016) DLS and zeta potential – what they are and what they are not? J Control Release 235:337–351 [DOI] [PubMed] [Google Scholar]
- 74.Klein TM, Wolf ED, Wu R, Sanford JC (1987) High-velocity microprojectiles for delivering nucleic acids into living cells. Nature 327:70–73 [PubMed] [Google Scholar]
- 75.Roizenblatt R, Weiland JD, Carcieri S, Qiu G, Behrend M, Humayun MS, Chow RH (2006) Nanobiolistic delivery of indicators to the living mouse retina. J Neurosci Methods 153:154–161 [DOI] [PubMed] [Google Scholar]
- 76.Arsenault J, O’Brien JA (2013) Optimized heterologous transfection of viable adult organotypic brain slices using an enhanced gene gun. BMC Res Notes 6:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Brien JA, Lummis SC (2011) Nanobiolistics: a method of biolistic transfection of cells and tissues using a gene gun with novel nanometer-sized projectiles. BMC Biotechnol 11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee P-W, Peng S-F, Su C-J, Mi FL, Chen HL, Wei MC, Lin HJ, Sung HW (2008) The use of biodegradable polymeric nanoparticles in combination with a low-pressure gene gun for transdermal DNA delivery. Biomaterials 29:742–751 [DOI] [PubMed] [Google Scholar]
- 79.Lee P-W, Hsu S-H, Tsai J-S, Chen FR, Huang PJ, Ke CJ, Liao ZX, Hsiao CW, Lin HJ, Sung HW (2010) Multifunctional core-shell polymeric nanoparticles for transdermal DNA delivery and epidermal Langerhans cells tracking. Biomaterials 31:2425–2434 [DOI] [PubMed] [Google Scholar]
- 80.Huang HN, Li TL, Chan YL, Chen CL, Wu CJ (2009) Transdermal immunization with low-pressure-gene-gun mediated chitosan-based DNA vaccines against Japanese encephalitis virus. Biomaterials 30:6017–6025 [DOI] [PubMed] [Google Scholar]
- 81.Raji JA, Frame B, Little D, Santoso TJ, Wang K (2018) Agrobacterium- and biolistic-mediated transformation of maize b104 inbred. In: Lagrimini LM (ed) Maize: methods and protocols. Springer, New York, NY, pp 15–40 [DOI] [PubMed] [Google Scholar]
- 82.Svitashev S, Schwartz C, Lenderts B, Young JK, Mark Cigan A (2016) Genome editing in maize directed by CRISPR-Cas9 ribonucleo-protein complexes. Nat Commun 7:13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ismagul A, Yang N, Maltseva E, Iskakova G, Mazonka I, Skiba Y, Bi H, Eliby S, Jatayev S, Shavrukov Y, Borisjuk N, Langridge P (2018) A biolistic method for high-throughput production of transgenic wheat plants with single gene insertions. BMC Plant Biol 18:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Y, Cheng X, Shan Q, Zhang Y, Liu J, Gao C, Qiu JL (2014) Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat Biotechnol 32:947–951 [DOI] [PubMed] [Google Scholar]
- 85.Kumari M, Rai AK, Devanna BN, Singh PK, Kapoor R, Rajashekara H, Prakash G, Sharma V, Sharma TR (2017) Co-transformation mediated stacking of blast resistance genes Pi54 and Pi54rh in rice provides broad spectrum resistance against Magnaporthe oryzae. Plant Cell Rep 36:1747–1755 [DOI] [PubMed] [Google Scholar]
- 86.Srivastava V, Underwood JL, Zhao S (2017) Dual-targeting by CRISPR/Cas9 for precise excision of transgenes from rice genome. Plant Cell Tissue Organ Cult 129:153–160 [Google Scholar]
- 87.Chaithra N, Gowda RPH, Guleria N (2015) Transformation of tomato with Cry2ax1 by biolistic gun method for fruit borer resistance. Int J Agric Environ Biotechnol 8:795–803 [Google Scholar]
- 88.Kumar N, Galli M, Ordon J, Stuttmann J, Kogel KH, Imani J (2018) Further analysis of barley MORC1 using a highly efficient RNA-guided Cas9 gene-editing system. Plant Biotechnol J 16:1892–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jacobs TB, LaFayette PR, Schmitz RJ, Parrott WA (2015) Targeted genome modifications in soybean with CRISPR/Cas9. BMC Biotechnol 15:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rafsanjani MS, Alvari A, Samim M, Hejazi MA, Abdin MZ (2012) Application of novel nanotechnology strategies in plant biotrans-formation: a contemporary overview. Recent Pat Biotechnol 6:69–79 [DOI] [PubMed] [Google Scholar]
- 91.Zalewski W, Orczyk W, Gasparis S, Nadolska-Orczyk A (2012) HvCKX2 gene silencing by biolistic or Agrobacterium-mediated transformation in barley leads to different phenotypes. BMC Plant Biol 12:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alok A, Sharma S, Kumar J, Verma S, Sood H (2017) Engineering in plant genome using Agrobacterium: progress and future. In: Kalia VC, Saini AK (eds) Metabolic engineering for bioactive compounds: Strategies and processes. Springer, Singapore, pp 91–111 [Google Scholar]
- 93.Anand A, Trick HN, Gill BS, Muthukrishnan S (2003) Stable transgene expression and random gene silencing in wheat. Plant Biotechnol J 1:241–251 [DOI] [PubMed] [Google Scholar]
- 94.Kohli A, Leech M, Vain P, Laurie DA, Christou P (1998) Transgene organization in rice engineered through direct DNA transfer supports a two-phase integration mechanism mediated by the establishment of integration hot spots. Proc Natl Acad Sci U S A 95:7203–7208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tassy C, Partier A, Beckert M, Feuillet C, Barret P (2014) Biolistic transformation of wheat: increased production of plants with simple insertions and heritable transgene expression. Plant Cell Tissue Organ Cult 119:171–181 [Google Scholar]
- 96.Martin-Ortigosa S, Valenstein JS, Lin VS-Y, Trewyn BG, Wang K (2012) Gold functionalized mesoporous silica nanoparticle mediated protein and DNA codelivery to plant cells via the biolistic method. Adv Funct Mater 22:3576–3582 [Google Scholar]
- 97.Martin-Ortigosa S, Valenstein JS, Sun W, Moeller L, Fang N, Trewyn BG, Lin VS, Wang K (2012) Parameters affecting the efficient delivery of mesoporous silica nanoparticle materials and gold nanorods into plant tissues by the biolistic method. Small 8:413–422 [DOI] [PubMed] [Google Scholar]
- 98.Martin-Ortigosa S, Peterson DJ, Valenstein JS, Lin VS, Trewyn BG, Lyznik LA, Wang K (2014) Mesoporous silica nanoparticle-mediated intracellular cre protein delivery for maize genome editing via loxP site excision. Plant Physiol 164:537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mortazavi SE, Zohrabi Z (2018) Biolistic co-transformation of rice using gold nanoparticles. Iran Agric Res 37:75–82 [Google Scholar]
- 100.Okuzaki A, Kida S, Watanabe J, Hirasawa I, Tabei Y (2013) Efficient plastid transformation in tobacco using small gold particles (0.07–0.3 μm). Plant Biotechnol 30:65–72 [Google Scholar]
- 101.Demirer GS, Zhang H, Goh NS, Pinals RL, Chang R, Landry MP (2019). Carbon Nano-carriers Deliver siRNA to Intact Plant Cells for Efficient Gene Knockdown. bioRxiv, 564427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang H, Demirer GS, Zhang H, Ye T, Goh NS, Aditham AJ, Cunningham FJ, Fan C, Landry MP (2019) DNA nanostructures coordinate gene silencing in mature plants. Proc Natl Acad Sci 116(15):7543–7548 [DOI] [PMC free article] [PubMed] [Google Scholar]


