Abstract
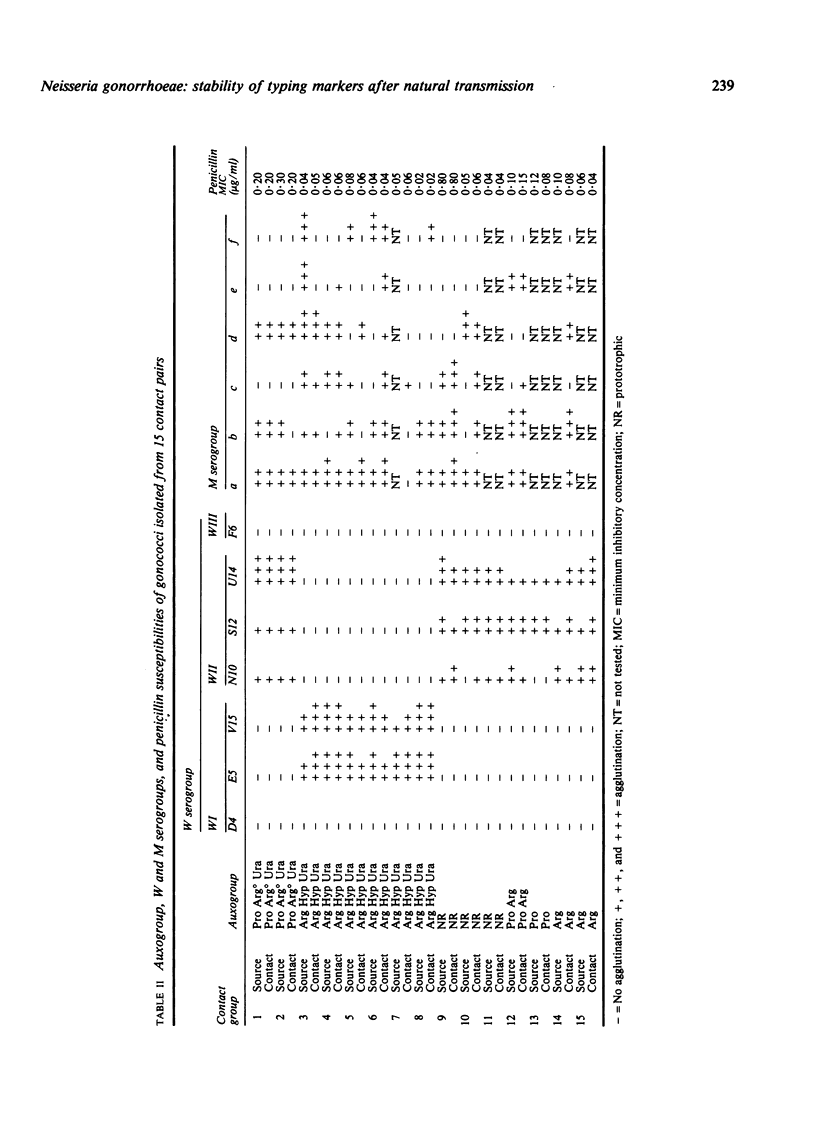
The gonococcal isolates from 15 contact pairs and three large contact groups were examined using various methods to assess the stability of different typing markers. With the exception of one contact group which showed variable proline requirements, the auxotypes were stable during natural transmission. Serogrouping using the coagglutination method to detect W and M antigens was undertaken. The lipopolysaccharide M antigens were readily lost and gained during transmission whereas the protein W antigens represented stable markers and are thus useful for epidemiological studies.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apicella M. A. Serogrouping of Neisseria gonorrhoeae: identification of four immunologically distinct acidic polysaccharides. J Infect Dis. 1976 Oct;134(4):377–383. doi: 10.1093/infdis/134.4.377. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M., Hildebrandt J. F. Antigen-specific serotyping of Neisseria gonorrhoeae: characterization based upon principal outer membrane protein. Infect Immun. 1981 Jun;32(3):985–994. doi: 10.1128/iai.32.3.985-994.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carifo K., Catlin B. W. Neisseria gonorrhoeae auxotyping: differentiation of clinical isolates based on growth responses on chemically defined media. Appl Microbiol. 1973 Sep;26(3):223–230. doi: 10.1128/am.26.3.223-230.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973 Aug;128(2):178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- Geizer I. Studies on serotyping of Neisseria gonorrhoeae. Zentralbl Bakteriol Orig A. 1975 Jul;232(2-3):213–220. [PubMed] [Google Scholar]
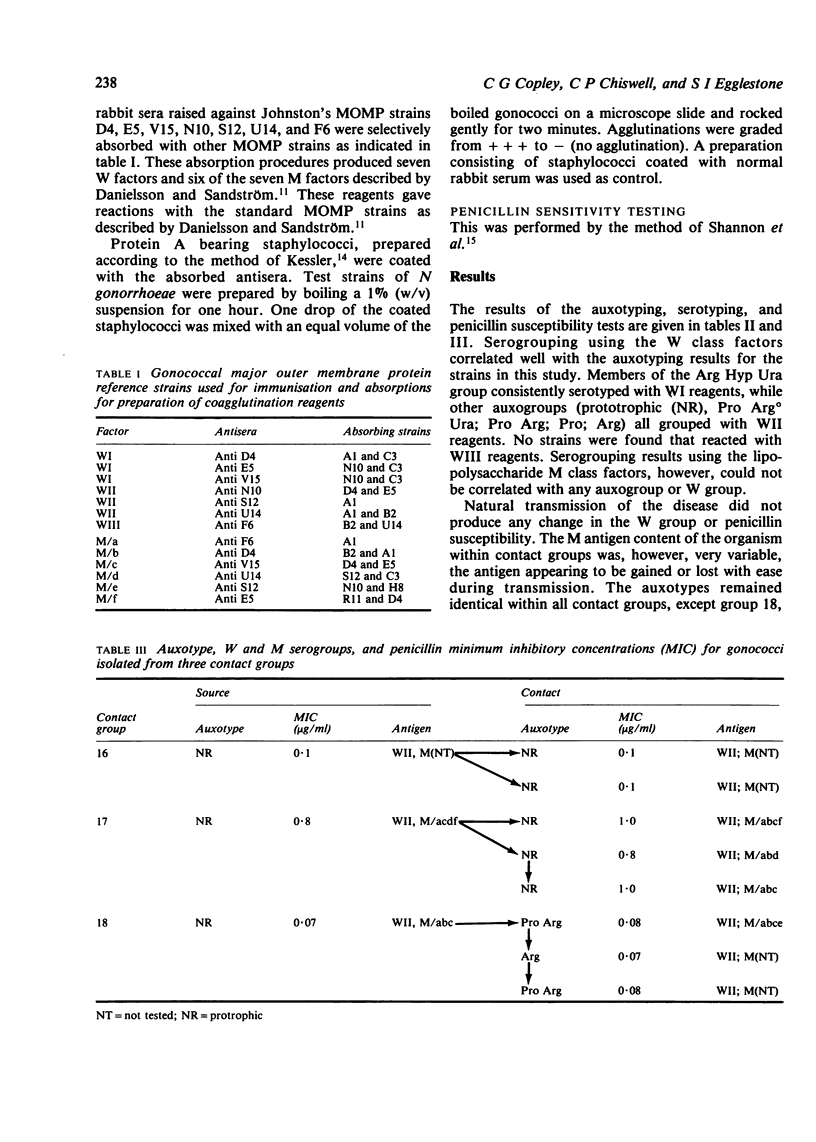
- Johnston K. H., Holmes K. K., Gotschlich E. C. The serological classification of Neisseria gonorrhoeae. I. Isolation of the outer membrane complex responsible for serotypic specificity. J Exp Med. 1976 Apr 1;143(4):741–758. doi: 10.1084/jem.143.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Knapp J. S., Holmes K. K. Disseminated gonococcal infections caused by Neisseria gonorrhoeae with unique nutritional requirements. J Infect Dis. 1975 Aug;132(2):204–208. doi: 10.1093/infdis/132.2.204. [DOI] [PubMed] [Google Scholar]
- Knapp J. S., Thornsberry C., Schoolnik G. A., Wiesner P. J., Homes K. K. Phenotypic and epidemiologic correlates of auxotype in Neisseria gonorrhoeae. J Infect Dis. 1978 Aug;138(2):160–165. doi: 10.1093/infdis/138.2.160. [DOI] [PubMed] [Google Scholar]
- Maeland J. A., Kristoffersen T., Hofstad T. Immunochemical investigations on neisseria gonorrhoeae endotoxin. 2. Serological multispecificity and other properties of phenol-water preparations. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(2):233–238. [PubMed] [Google Scholar]
- Perry M. B., Daoust V. The lipopolysaccharides of Neisseria gonorrhoeae colony types 1 and 4. Can J Biochem. 1975 May;53(5):623–629. doi: 10.1139/o75-084. [DOI] [PubMed] [Google Scholar]
- Sandstrom E. G., Knapp J. S., Buchanan T. B. Serology of Neisseria gonorrhoeae: W-antigen serogrouping by coagglutination and protein I serotyping by enzyme-linked immunosorbent assay both detect protein I antigens. Infect Immun. 1982 Jan;35(1):229–239. doi: 10.1128/iai.35.1.229-239.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandström E., Danielsson D. Serology of Neisseria gonorrhoeae. Classification by co-agglutination. Acta Pathol Microbiol Scand B. 1980 Feb;88(1):27–38. doi: 10.1111/j.1699-0463.1980.tb02600.x. [DOI] [PubMed] [Google Scholar]
- Shannon R., Hedges A. J., Edwards R. J. Distribution of levels of penicillin resistance among freshly isolated strains of N. gonorrhoeae. Application of a novel sensitivity assay. Br J Vener Dis. 1975 Aug;51(4):246–250. doi: 10.1136/sti.51.4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart I. O., Hendry A. T. Association between the auxogroup of Neisseria gonorrhoeae and the minimal inhibitory concentration of penicillin. Sex Transm Dis. 1979 Oct-Dec;6(4):247–252. doi: 10.1097/00007435-197910000-00004. [DOI] [PubMed] [Google Scholar]
- Wang S. P., Holmes K. K., Knapp J. S., Ott S., Kyzer D. D. Immunologic classification of Neisseria gonorrhoeae with micro-immunofluorescence. J Immunol. 1977 Sep;119(3):795–803. [PubMed] [Google Scholar]


