Abstract
OBJECTIVES:
We analyzed whether patients with the International Classification of Diseases, 10th Edition (ICD-10) discharge diagnosis code for sepsis are different in regard to demographics and outcome variables when comparing those with sepsis only to those also diagnosed with COVID-19 or those with a COVID-19 diagnosis alone.
DESIGN:
Retrospective cohort study.
SETTING:
Nine hospitals in an academic health system.
PATIENTS:
Patients with a final ICD-10 discharge diagnostic code for sepsis only, a diagnosis of COVID-19-only, or a final sepsis ICD-10 discharge code + a diagnosis of COVID-19 admitted to the hospital were analyzed for demographic and outcome differences between the cohorts.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
A total of 11,395 patients met inclusion criteria: 6,945 patients (60.9%) were ICD-10 sepsis code only, 3,294 patients (28.9%) were COVID-19 diagnosis-only, and 1,153 patients (10.1%) were sepsis ICD-10 code + COVID-19 diagnosis. Comparing sepsis ICD-10 code + COVID-19 diagnosis patients to sepsis ICD-10 code only and COVID-19 diagnosis-only patients, the sepsis ICD-10 code + COVID-19 diagnosis patients were: older (69 [58–78] vs 67 [56–77] vs 64 [51–76] yr), less likely to be female (40.3% vs 46.7% vs 49.5%), more frequently admitted to the ICU (59.3% [684/1,153] vs 54.9% [1,810/3,297] vs 15% [1,042/6,945]), more frequently required ventilatory support (39.3% [453/1,153] vs 31.8% [1,049/3,297] vs 6.0% [417/6,945]), had longer median hospital length of stay (9 [5,16] vs 5 [3,8] vs 7. [4,13] d), and were more likely to die in the hospital (39.2% [452/1,153] vs 22.3% [735/3,297] vs 6.4% [444/6,945]).
CONCLUSIONS:
During the COVID-19 pandemic the sickest cohort of patients was those receiving an explicit ICD-10 code of sepsis + a COVID-19 diagnosis. A significant percentage of COVID-19 diagnosis-only patients appear to have been under-coded as they received a level of critical care (ICU admission; intubation) suggestive of the presence of acute organ dysfunction during their admission.
Keywords: coding, COVID-19, organ dysfunction, outcomes, sepsis, viral sepsis
KEY POINTS
Question: In this study, patients with a final International Classification of Diseases, 10th Edition (ICD-10) discharge diagnostic code for sepsis only, a diagnosis of COVID-19-only, or a sepsis ICD-10 code + a COVID-19 diagnosis admitted to the hospital were analyzed for outcome differences between cohorts.
Findings: We found that the sickest cohort of patients was those receiving a final discharge diagnostic code of sepsis + a COVID-19 diagnosis. A small but significant percentage of COVID-19 diagnosis-only patients appear to have been under-coded as they received a level of critical care (ICU admission; intubation) suggestive of the presence of acute organ dysfunction and many received a secondary ICD-10 code for a specific acute organ dysfunction.
Meanings: These findings have implications for optimal surveillance, detection, and management of severe COVID-19 patients.
Sepsis, defined as life-threatening acute organ dysfunction (AOD) caused by a dysregulated host response to infection, is common and deadly (1), with approximately 2,000,000 cases annually in the United States and in-hospital mortality between 15 and 20% (2). Early detection, risk stratification, and treatment are key to improving outcomes and reversing AOD (3–7). Since the first International Sepsis Definitions were published in 1992, the pathogens causing infections leading to sepsis have included bacteria, viruses, fungi, and parasites (8).
Despite this shared pathophysiologic rationale, there has been resistance from many clinicians to consider severe viral infections characterized by life-threatening AOD as “viral sepsis.” Time to appropriate antimicrobials has been central to optimal treatment strategies for sepsis and part of the controversy about viral sepsis stems from the lack of an analog in treating severe viral infections, which undermines the concept of “a time-sensitive disease (9–12).” This ongoing lack of consensus became particularly clear during the early months of the severe acute respiratory syndrome coronavirus 2 pandemic. For example, Li et al (13) hypothesized “that a process called viral sepsis is crucial to the disease mechanism of COVID-19,” and includes T cell exhaustion, cytokine-driven inflammation, coagulation and fibrinolysis, and multiple organ failure (14–16). However, guidelines for the management of COVID-19 issued by The Surviving Sepsis Campaign make no mention of viral sepsis (17), which may limit consensus on best practices for COVID-19 management (18). A meta-analysis found a pooled estimate of the prevalence of sepsis in COVID-19 cohorts in the ICU of 77.9% and on general wards of 33.3% (19).
The primary objective of this study was to understand differences, including demographics, comorbidities, clinical profiles, and hospital outcomes between patients receiving an International Classification of Diseases, 10th Edition (ICD-10) discharge code for severe sepsis or septic shock only, a COVID-19 diagnosis-only, or an ICD-10 discharge code for sepsis + a COVID-19 diagnosis. We hypothesized that patients with a sepsis ICD-10 code + a COVID-19 diagnosis would have higher disease severity and in-hospital mortality than those with a sepsis ICD-10 code only or a COVID-19 diagnosis-only. Our primary outcome measure was in-hospital mortality. Secondary outcomes included potential under-coding in the COVID-19-only cohort (evidenced by ICU admission or need for mechanical ventilation during the hospital admission), presence of AOD, inpatient length of stay (LOS), ICU LOS, and discharge to home.
Study Design and Methods
This is a retrospective cohort study that used inpatient Electronic Medical Record (EMR) data from nine hospitals in an academic health system with patients admitted to the hospital between March 12, 2020, and April 18, 2021, and was approved by and performed in accordance with the ethical standards set by Thomas Jefferson University (TJU)’s institutional review board (approval date, August 0, 2021; number 20E.793) in expedited review with waiver of informed consent and performed in accordance with the Helsinki Declaration of 1975. Using ICD-10 discharge codes for sepsis and a diagnosis of COVID-19, we extracted all COVID-19 and sepsis patients during the study period and grouped them into three nonoverlapping groups: sepsis ICD-10 code only; COVID-19 diagnosis-only; and a sepsis ICD-10 code + a COVID-19 diagnosis. Sepsis patients were defined by the explicit discharge ICD-10 diagnostic codes of R65.20 (severe sepsis without septic shock) or R65.21 (severe sepsis with septic shock). COVID-19 positivity was defined by the TJU health system-wide definition of COVID-19 infection, which was defined by a COVID task force at the start of the pandemic and revised after the COVID-19 ICD-10 code became available: 1) positive COVID-19 test within 21 days of or during the hospital admission, 2) confirmed COVID-19 infection documented in the EMR within 21 days of or during the hospital admission, or 3) ICD-10 U07.1 (COVID-19) code listed in the discharge diagnoses (starting October 1, 2020). We also collected infection codes (Supplemental eTable 1, http://links.lww.com/CCX/B240) and AOD codes (Supplemental eTable 2, http://links.lww.com/CCX/B240) for all patients. Patient demographics, inpatient encounter information, discharge diagnoses, laboratory results, and clinical findings were collected, reviewed for clinical relevance and plausibility, and processed for analysis. Using patient discharge diagnosis data, we collected each patient’s Charlson comorbidity conditions and calculated their Charlson Comorbidity Index (CCI) based on the study by Quan et al (20) modification of the CCI using ICD-10 codes. The EMR generates clinical flow sheets for bedside patient care. These flow sheets include vital signs, laboratory results, comorbidities, medications, allergies, and demographic information. The data on the clinical flow sheets automatically generate clinical severity scores including the Modified Early Warning Score (MEWS), modified Sequential Organ Failure Assessment (mSOFA) score, and the LACE (Length of Stay, Acute Admission [Yes/No], Charlson Comorbidity Index, and number of ED visits in past 6 months [excluding the current one]) score.
Descriptive statistics were calculated for all study measures. We compared patient outcomes, demographic characteristics, comorbidities, laboratory result values, and clinical findings across the three patient groups. All laboratory results are the median value of all the values available during the hospital stay. We also extracted 30 clinical findings from the clinical flow sheets and compared them across the three cohorts. Continuous variables were calculated as medians and compared using the Student t test. We assessed for extreme outliers and used an F test to test equality of the two groups’ variance. If equal variance was not rejected, the pooled t test was used for mean difference testing; if equal variance was rejected, the Satterthwaite t test was used. p values of less than 0.05 were considered statistically significant. Categorical variables were calculated as percentages and compared by using the Chi-square test. Assumptions for the use of Chi-square testing including independence and mutual exclusiveness were met. Using in-hospital mortality and discharge to home as separate outcome variables, we estimated multivariable logistic regression models using backward variable selection with a p value of 0.05 for each patient group. This was an exploratory correlation analysis to identify any significant risk factors being related to the outcomes in each cohort and identify any differences across the three cohorts. The covariate variables included patient demographics, encounter information, admission vital signs, and comorbidities. C-statistics or receiver operating characteristic curves were used to evaluate the potential predictive value of risk factors for in-hospital mortality or discharge to home. No imputation was performed for the multivariate logistic models. The majority of variables were coded as 0 or 1; for example, if a patient had had a myocardial infarction, this was coded 1; if they had not, it was coded as 0. For each multivariable logistic model, we reported our results in terms of estimated odds ratios (OR), associated p values, and C-statistics from each cohort. All data processing and statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Categories
During the study period 130,528 patients were admitted to the nine hospitals and we identified a total of 11,395 hospitalized patients who met inclusion criteria. Of these, 3,297 patients (28.9%) had a sepsis ICD-10 code only; 6,945 patients had a COVID-19 diagnosis-only (61%); and 1,153 patients had a sepsis ICD-10 code + a COVID-19 diagnosis (10.1%).
Demographics and Admission Information
The median age of the sepsis ICD-10 code + a COVID-19 diagnosis patients was 69 years (58–78), which was significantly older than the sepsis ICD-10 code only (67 yr [56–77]; p = 0.0016) or COVID-19 diagnosis-only (64 yr [51–76]; p < 0.0001) patients (Table 1). A lower percentage of patients in the sepsis ICD-10 code + a COVID-19 diagnosis cohort were female (40.3% [465/1,153]) than in the sepsis-only ICD-10 code or COVID-19 diagnosis-only cohorts (46.7 [1,539/3,297], p = 0.0002 vs 49.5% [3,435/6,945], p < 0.0001). The vast majority of patients were admitted through the emergency department (ED): 98.9% [1,140/1,153] in the sepsis ICD-10 code + a COVID-19 diagnosis cohort, which was higher than the sepsis ICD-10 code only and the COVID-19 diagnosis-only cohorts (94.3% [3,109/3,297], p < 0.0001 vs 93.5% [6,492/6,945]; p < 0.0001).
TABLE 1.
Patient Demographic and Encounter Profile Comparison
| Variable | Cohort A: Sepsis-Only (n = 3,297)a | Cohort B: COVID-19-only (n = 6,945) | Cohort C: Sepsis + COVID-19 (n = 1,153) |
|---|---|---|---|
| n (%) | |||
| Age, yr, median (interquartile range) | 67 (56.0–77.0) | 64 (51.0–76.0) | 69 (58.0–78.0) |
| Female | 1,539 (46.7%) | 3,435 (49.5%) | 465 (40.3%) |
| Marital status—married | 1,200 (36.4%) | 2,596 (37.4%) | 465 (40.3%) |
| Marital status—single | 1,131 (34.3%) | 2,436 (35.1%) | 346 (30.0%) |
| Marital status—widowed | 471 (14.3%) | 960 (13.8%) | 175 (15.2%) |
| Marital status—divorced | 268 (8.1%) | 462 (6.7%) | 87 (7.5%) |
| Race—White | 2,215 (67.2%) | 3,680 (53.0%) | 638 (55.3%) |
| Race—African American | 785 (23.8%) | 2,152 (31.0%) | 329 (28.5%) |
| Race—Hispanic | 108 (3.3%) | 491 (7.1%) | 56 (4.9%) |
| Race—Asian | 126 (3.8%) | 485 (7.0%) | 108 (9.4%) |
| Advanced directive—yes | 375 (11.4%) | 433 (6.2%) | 117 (10.1%) |
| 10 ≤ Patient age < 20 | 9 (0.3%) | 44 (0.6%) | 0 (0.0%) |
| 20 ≤ Patient age < 30 | 83 (2.5%) | 289 (4.2%) | 17 (1.5%) |
| 30 ≤ Patient age < 40 | 201 (6.1%) | 536 (7.7%) | 52 (4.5%) |
| 40 ≤ Patient age < 50 | 255 (7.7%) | 704 (10.1%) | 85 (7.4%) |
| 50 ≤ Patient age < 60 | 493 (15.0%) | 1,171 (16.9%) | 155 (13.4%) |
| 60 ≤ Patient age < 70 | 794 (24.1%) | 1,490 (21.5%) | 285 (24.7%) |
| 70 ≤ Patient age < 80 | 802 (24.3%) | 1,389 (20.0%) | 313 (27.1%) |
| 80 ≤ Patient age < 90 | 480 (14.6%) | 964 (13.9%) | 187 (16.2%) |
| Patient age ≥ 90 | 179 (5.4%) | 356 (5.1%) | 59 (5.1%) |
| Admission type—emergency | 3,109 (94.3%) | 6,492 (93.5%) | 1,140 (98.9%) |
| Medicare | 1,373 (41.6%) | 2,209 (31.8%) | 455 (39.5%) |
| Medicare—managed care | 785 (23.8%) | 1,563 (22.5%) | 278 (24.1%) |
| Managed care | 428 (13.0%) | 1,563 (22.5%) | 198 (17.2%) |
| Medicaid—managed care | 536 (16.3%) | 1,070 (15.4%) | 141 (12.2%) |
| Medicaid | 86 (2.6%) | 233 (3.4%) | 44 (3.8%) |
n = number of patients.
Student t test was used for age. χ2 was used in all other measures.
Comorbidities
The median CCI in the sepsis ICD-10 code-only cohort was 2 (1–4), which was significantly higher than the sepsis ICD-10 code + a COVID-19 diagnosis cohort (2 [1–3]; p < 0.0001) or the COVID-19 diagnosis-only cohort (1 [0–2]; p < 0.0001) (Table 2). Patients in the sepsis-only cohort had significantly higher percentages of all 17 Charlson comorbidities than patients in the COVID-19 diagnosis-only cohort; in comparison, differences between the sepsis ICD-10 code only and sepsis ICD-10 code + a COVID-19 diagnosis cohorts varied from comorbidity to comorbidity (Supplemental eTable 3, http://links.lww.com/CCX/B240).
TABLE 2.
Clinical Variables and Patient Outcomes Comparison
| Outcome Measure | Cohort A: Sepsis-Only (n = 3,297)a | Cohort B: COVID-19-Only (n = 6,945) | Cohort C: Sepsis + COVID-19 (n = 1,153) | Compare Cohort A vs. B | Compare Cohort A vs C | Compare Cohort B vs C |
|---|---|---|---|---|---|---|
| n (%) | p | |||||
| In-hospital mortality | 735 (22.3%) | 444 (6.4%) | 452 (39.2%) | < 0.0001 | < 0.0001 | < 0.0001 |
| Admitted to ICU | 1,810 (54.9%) | 1,042 (15.0%) | 684 (59.3%) | < 0.0001 | 0.0092 | < 0.0001 |
| Ventilation | 1,049 (31.8%) | 417 (6.0%) | 453 (39.3%) | < 0.0001 | < 0.0001 | < 0.0001 |
| Home discharge | 607 (18.4%) | 3,514 (50.6%) | 254 (22.0%) | < 0.0001 | 0.0074 | < 0.0001 |
| ICD-10 Code for infection | 1,405 (42.6%) | 514 (7.4%) | 232 (20.1%) | < 0.0001 | < 0.0001 | < 0.0001 |
| ICD-10 Code for acute organ dysfunction | 3,109 (94.3%) | 514 (7.4%) | 1,131 (98.1%) | < 0.0001 | < 0.0001 | < 0.0001 |
| IP length of stay, days | 7 (4–13)b | 5 (3–8)b | 9 (5–16)b | < 0.0001 | < 0.0001 | < 0.0001 |
| ICU length of stay, days | 3 (1–5)b | 2 (1–6)b | 4 (1–10) | 0.4324 | < 0.0001 | < 0.0001 |
| Charlson Comorbidity Index, n (mean)c | 2 (1–4)b | 1 (0–2)b | 2 (1–3)b | < 0.0001 | < 0.0001 | < 0.0001 |
| Admission SBP, mm Hg | 120 (101–141)b | 132 (117–148)b | 127 (110–143)b | < 0.0001 | < 0.0001 | < 0.0001 |
| Admission diastolic blood pressure, mm Hg | 66 (56–79)b | 73 (64–83)b | 70 (60–81)b | < 0.0001 | < 0.0001 | < 0.0001 |
| Admission SBP < 120 mm Hg | 1,651 (50.1%) | 2,027 (29.2%) | 454 (39.4%) | < 0.0001 | < 0.0001 | < 0.0001 |
| Admission BMI, kg/m2 | 26.8 (22.7–32.8)b | 28.8 (24.8–34.3)b | 28.4 (24.0–34.3)b | < 0.0001 | < 0.0001 | 0.2654 |
| Admission BMI < 25 | 1,402 (42.5%) | 2,097 (30.2%) | 366 (31.7%) | < 0.0001 | < 0.0001 | 0.2897 |
| Admission BMI ≥ 35 | 590 (17.9%) | 1,519 (21.9%) | 245 (21.2%) | < 0.0001 | 0.012 | 0.6351 |
IP = In-Patient, SBP = systolic blood pressure.
n = number of patients.
Median and interquartile range.
Charlson Comorbidity Index (CCI), which measures the number of comorbidity conditions that a patient has (see eTable 2, http://links.lww.com/CCX/B240, for details of individual comorbidities).
χ2 was used for IP mortality, ventilation, admitted to ICU, home discharge, and BMI. Student t test was used for CCI, IP length of stay and ICU length of stay.
Clinical and Administrative Data
The median admission BMI ranged from 26.8 [22.7–32.8] kg/m2 for the sepsis-only cohort to 28.4 [24.0–34.3] kg/m2 for the sepsis ICD-10 code + a COVID-19 diagnosis and 28.8 [24.8–34.3] kg/m2 for COVID-19 diagnosis-only cohorts (Table 2). A low BMI (< 25 kg/m2) was most common in the sepsis ICD-10 code only cohort (42.5% [1,402/3,297]) compared to the COVID-19-only diagnosis or sepsis ICD-10 code + a COVID-19 diagnosis cohorts (30.2% [2,097/6,945] vs 31.7% [366/1,153]); a high BMI (≥ 35 kg/m2) was more common in the COVID-19 diagnosis-only (21.9% [1,519/6,945]) and sepsis ICD-10 code + a COVID-19 diagnosis (21.2% [245.1,153]) cohorts compared with the sepsis-only ICD-10 code (17.9% [590/3,297]) cohort. The median admission systolic and diastolic blood pressures in the COVID-19 diagnosis-only cohort were significantly higher than those in sepsis ICD-10 code + a COVID-19 diagnosis cohort, which in turn were significantly higher than those in sepsis ICD-10 code only cohort.
We extracted and evaluated 59 laboratory results across the three cohorts (Supplemental eTable 4, http://links.lww.com/CCX/B240); the median values of the 19 most clinically relevant are reported (Table 3). The most clinically relevant differences in laboratory values included (comparing sepsis ICD-10 code only vs COVID-19 diagnosis-only vs sepsis ICD-10 code + a COVID-19 diagnosis cohort): blood urea nitrogen: 26.4 vs 19.0 vs 32.1 mg/dL, creatinine: 1.2 vs 0.9 vs 1.2 mg/dL, WBC: 11.9 vs 7.2 vs 10.1 × 103, and lactate: 2.2 vs 1.5 vs 1.9 mmol/L.
TABLE 3.
Comparison of Laboratory Results by Patient Type
| Laboratory Measure | Cohort A: Sepsis-Only (n = 3,297)a | Cohort B: COVID-19-Only (n = 6,945) | Cohort C: Sepsis+ COVID-19 (n = 1,153) |
|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | |
| Carbon dioxide, mmol/L | 23 (20 to 25) | 24 (22 to 26) | 23 (22 to 25) |
| Anion gap | 11 (9 to 14) | 11 (10 to 13) | 12 (10 to 14) |
| Blood urea nitrogen, mg/dL | 26 (17 to 43) | 19 (13 to 29) | 32 (20 to 50) |
| Creatinine, mg/dL | 1.2 (0.8 to 2.0) | 0.9 (0.7 to 1.3) | 1.2 (0.9 to 2.0) |
| Estimated glomerular filtration rate, non-African American | 51.5 (31.6 to 59.9) | 59.9 (50.0 to 60.0) | 52 (33.4 to 59.9) |
| Estimated glomerular filtration rate, African American | 55.8 (37.7 to 60.0) | 60 (56.0 to 60.0) | 56.3 (39.3 to 60.0) |
| Glucose, mg/dL | 127 (109 to 164) | 124 (106 to 162) | 151 (121 to 197) |
| WBC count, ×103 | 11.9 (8.7 to 15.7) | 7.2 (5.4 to 9.7) | 10.1 (7.5 to 14.1) |
| Hemoglobin, g/dL | 9.7 (8.4 to 11.5) | 12.1 (10.5 to 13.4) | 11.3 (9.3 to 12.9) |
| Hematocrit, % | 30.3 (26.5 to 35.4) | 36.8 (32.2 to 40.3) | 34.6 (29.2 to 39.3) |
| Platelet, ×103 | 221 (150 to 306) | 227 (173 to 293) | 224 (163 to 289) |
| Lactate, mmol/L | 2.2 (1.6 to 3.2) | 1.5 (1.2 to 1.9) | 1.9 (1.5 to 2.9) |
| C-reactive protein, mg/dL | 12 (5.9 to 19.7) | 5.1 (2.2 to 9.2) | 9.4 (5.5 to 15.4) |
| d-dimer, ng/mL | 990 (12.4 to 3,308.0) | 192.5 (1.5 to 490.5) | 200.3 (2.3 to 1,139.3) |
| International Normalized Ratio | 1.3 (1.2 to 1.6) | 1.2 (1.1 to 1.3) | 1.2 (1.1 to 1.4) |
| Ferritin, ng/mL | 396 (172.0 to 912.0) | 447.9 (202.0 to 910.5) | 730 (335.0 to 1,482.0) |
| Bicarbonate, mmol/L | 21.5 (18.0 to 25.1) | 24.3 (21.5 to 28.0) | 23.8 (20.7 to 27.5) |
| Base excess, mmol/L | 1 (–3.9 to 3.8) | 1.6 (–1.1 to 4.4) | 1.2 (–2.4 to 4.3) |
| Base deficit, mmol/L | 5.4 (2.9 to 9.1) | 3 (1.6 to 5.5) | 4 (2.3 to 6.0) |
n = number of patients.
We also extracted 30 clinical findings from the clinical flow sheets and compared them across the three cohorts. The 10 most clinically relevant variables are presented (Table 4) and include (comparing sepsis-only vs COVID-19 diagnosis-only vs sepsis ICD-10 code + a COVID-19 diagnosis): mean arterial pressure: 83 (77–91) vs. 90 (84–97) versus 86 (80–92) mm Hg, Glasgow Coma Scale (GCS): 14 (11–15) versus 15 (14–15) versus 13 (10–15), MEWS: 4.6 (3.1–6.8) versus 3.2 (2.1–4.7) versus 6.1 (4.3–7.9), mSOFA: 3 (1–6) versus 1 (0–2) versus 4 (2–6), and LACE: 9 (7–11) versus 8 (7–10) versus 9 (8–11).
TABLE 4.
Comparison of Clinical Findings from Flowsheet by Patient Type
| Finding Measure (Units) | Cohort A: Sepsis-Only (n = 3,297)a | Cohort B: COVID-19-Only (n = 6,945) | Cohort C: Sepsis + COVID-19 (n = 1,153) |
|---|---|---|---|
| Median (Interquartile range) | |||
| Mean arterial pressure (mm Hg) | 83 (77–91) | 90 (84, 97) | 86 (80–92) |
| LACE Scoreb | 9 (7–11) | 8 (7–10) | 9 (8–11) |
| LACE+ Scorec | 63.8 (52.3–72.5) | 57.6 (42.7–69.4) | 60.9 (47.5–70.9) |
| Modified Early Warning Score | 4.6 (3.1–6.8) | 3.2 (2.1–4.7) | 6.1 (4.3–7.9) |
| Glasgow Coma Scale (3–15) | 14 (11–15) | 15 (14–15) | 13 (10–15) |
| Pain rating at rest (0–10) | 1 (0–4) | 0 (0–2) | 0 (0.0–1) |
| Modified Sequential (Sepsis) Organ Failure Assessment Score | 3.1 (1.3–5.5) | 1 (0.2–2.4) | 3.7 (1.7–6.3) |
| Braden Score | 14.6 (12.0–18.0) | 19.7 (16.3–21.0) | 14.3 (12.0–18.7) |
n = number of patients.
LACE Score = LACE Index for Readmission, LACE = Length of Stay; Acute (Emergent) Admission; Charlson Comorbidity Index; Number of Emergency Department visits within 6 months (not including the current one).
LACE + Score = modified LACE Index for readmission.
Student t test was used for all clinical finding measures.
Outcomes
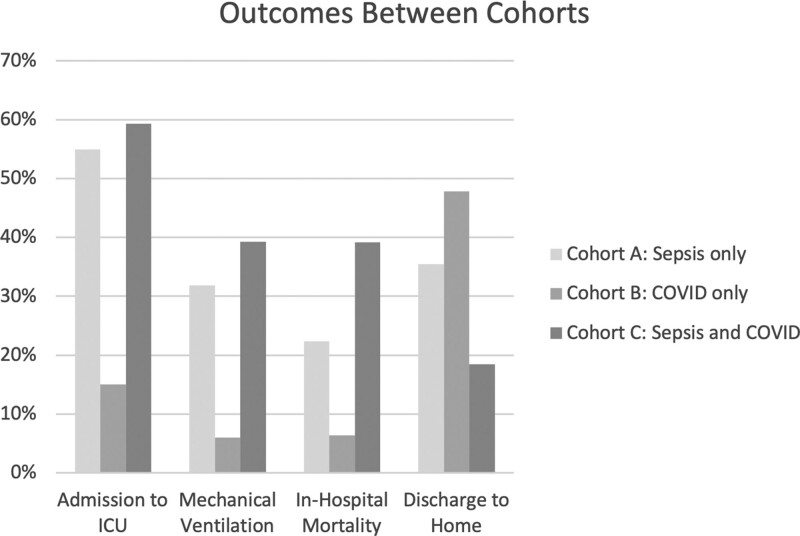
During the study period in-hospital mortality was 3.52% (4,596/130,528) for all patients admitted to the nine hospitals. In the study cohort 1,631 patients died, for an in-hospital mortality rate of 14.3%, 31.0% (3,536/11,395) were admitted to the ICU, 16.8% (1,919/11,395) required mechanical ventilation, mean inpatient LOS was 8.9 days, and 34.8% (3,966/11,395) were discharged to home. In-hospital mortality was 39.2% (452/1,153) for the sepsis ICD-10 code + a COVID-19 diagnosis cohort, which was 75% higher than the mortality rate for sepsis ICD-10 code only patients (22.3% [735/3,297]) and more than six times higher than for a COVID-19 diagnosis-only patients (6.4% [444/6,945]) (Table 2 and Fig. 1). ICU admission in the sepsis ICD-10 code + a COVID-19 diagnosis cohort was significantly higher than in the sepsis ICD-10 code only cohort (59.3% [684/1,153] vs 54.9% [1,810/3,297]) and both significantly exceeded the 15% [1.042/6,945] ICU admission rate of COVID-19 diagnosis-only cohort. Assignment of a specific infection ICD-10 code was significantly more common in the sepsis ICD-10 only code patients than in the sepsis ICD-10 code + COVID-19 diagnosis patients and was significantly more common in both when compared to the COVID-19 diagnosis-only patients (42.6% [1,405/3,297] vs 20.1% [232/1,153] vs 7.4% [514/6,945]; p < 0.0001). Further, assignment of a specific AOD ICD-10 code was significantly more common in the sepsis ICD-10 only and sepsis ICD-10 code + COVID-19 diagnosis patients compared with the COVID-19 diagnosis-only patients (94.3% [3,109/3,297] vs 98.1% [1,131/1,153] vs 7.4% [514/6,945]; p < 0.0001). Mechanical ventilation in the sepsis ICD-10 code + COVID-19 diagnosis cohort was significantly higher than in the sepsis ICD-10 code only cohort (39.3% [453/1,153] vs 31.8% [1.049/3,297]), and both significantly exceeded the 6% [417/6,945] of the COVID-19 diagnosis-only cohort. Median inpatient LOS was longer in the sepsis ICD-10 code + COVID-19 diagnosis cohort than the sepsis ICD-10 code only and COVID-19 diagnosis-only cohorts (9 [5–16] vs 5 [3–8] vs 7. [4–13] d). Median ICU LOS in the sepsis ICD-10 code + COVID-19 diagnosis cohort was longer than in the sepsis ICD-10 code only and COVID-19 diagnosis-only cohorts (4 [1–10] vs 2 [1,6] vs 3 [1–5] d). In the two COVID-19 cohorts combined, 21.3% required ICU level care, COVID-19 diagnosis-only patients had the highest rate of home discharge compared with sepsis ICD-10 code + COVID-19 diagnosis patients and sepsis ICD-10 code only patients (50.6% [3,514/6,945] vs 22% [254/1,153] vs 18.4% [607/3,297]). The 15% admission rate to the ICU in the COVID-19 diagnosis-only group suggests that approximately 1,050 patients were under-coded and potentially met criteria for an explicit sepsis code.
Figure 1.
Outcome variables between cohorts.
Multivariable Logistic Regression Analysis
In-Hospital Mortality
The results of the multivariable logistic regression indicate that age, comorbidities and vital signs correlated with in-hospital mortality across the three cohorts (Supplemental eTable 5, http://links.lww.com/CCX/B240). When compared to younger age groups for sepsis ICD-10 code only patients, less than 50-year-old patients for the COVID-19 diagnosis-only cohort, and less than 40-year-old patients for the sepsis ICD-10 code + a COVID-19 diagnosis cohort, the OR of in-hospital mortality increased significantly for each ten-year increase in age. Female gender correlated with survival (OR 0.8, p = 0.015) in the COVID-19 diagnosis-only cohort but was not significant in the other two cohorts. African American race was correlated with survival in the COVID-19 diagnosis-only and sepsis ICD-10 code + COVID-19 diagnosis cohorts (OR 0.7, p = 0.016 and OR 0.7, p = 0.043, respectively), but was not significant for the sepsis-only cohort. Compared to patient admission BMI less than or equal to 25, higher BMI patients had lower OR of mortality in the sepsis ICD-10 code-only cohort. In contrast, sepsis + COVID-19 diagnosis patients with a BMI greater than or equal to 35 had a significantly higher OR of mortality when compared to those with lower BMIs (OR 1.7, p = 0.002).
Discharge to Home
Older age groups were negatively correlated with being discharged to home across the three cohorts and the likelihood decreased as age increased (Supplemental eTable 6, http://links.lww.com/CCX/B240]. Being married, Asian race, and having a higher BMI positively correlated with discharge to home for all three cohorts. Compared to White patients, African American patients were more likely to be discharged to home in the COVID-19 diagnosis-only and sepsis ICD-10 code + COVID-19 diagnosis cohorts as were patients of Hispanic race in the COVID-19 diagnosis-only cohort.
DISCUSSION
We found significant differences between patients assigned a sepsis-only ICD-10 code at hospital discharge versus those also diagnosed with COVID-19 versus those diagnosed only with COVID-19 in terms of demographics, comorbidities, hospital care, coding, and outcomes. Patients with a sepsis ICD-10 code + a COVID-19 diagnosis had the highest disease severity and highest in-hospital mortality and patients with only a diagnosis of COVID-19 had the lowest disease severity and best outcomes. This leads to the conclusion that the involvement of sepsis in COVID-19 infection is associated with a sicker patient population with worse outcomes and is reflected in charting for disease severity that is sufficient to lead to a discharge diagnosis of sepsis accompanying a diagnosis of COVID-19.
However, within the COVID-19 diagnosis-only cohort there existed a subset of patients experiencing a viral-induced acute multi-organ dysfunction very similar to the patients in the sepsis ICD-10 code + a COVID-19 diagnosis cohort as identified by ICU admission (15%) and intubation (6.2%). In our two COVID-19 cohorts, 21.3% required ICU-level care, which is consistent with the findings of Wiersinga et al, who reported that 20% of hospitalized COVID-19 patients require ICU care (21). These findings present an opportunity to more accurately code patients experiencing virally-induced immune system dysregulation and associated AOD. More accurate classification would lead to more accurate description of the epidemiology, burden of disease, and costs associated with the COVID-19 pandemic. Guidelines for what defines a sepsis patient need to be amended to account for the increasing prevalence of viral-induced sepsis (13).
A disconnect between clinical severity and ICD codes assigned to a specific hospitalization episode has been reported before. In a cohort of sepsis patients, Whittaker et al (22) demonstrated that there were shortcomings in documentation and under-coding such that only 20.5% of patients with severe sepsis and 49.5% of patients with septic shock received a severe sepsis-specific ICD code. Factors associated with receiving the proper codes included older age, higher initial lactate level, and ICU admission. This disconnect has also been demonstrated in COVID-19 patients. Using chart review, the study by Shappell et al (23) found that a COVID-19 ICD-10 discharge diagnostic code had only a fair positive predictive value (74%) for an acute COVID-19 infection as the primary or secondary reason for admission. Similarly, we found that patients with sepsis ICD-10 code + COVID-19 diagnosis had lower systolic blood pressure, higher initial lactate level, increased ICU admission, and higher baseline disease severity (GCS, MEWS scores) when compared to COVID-19 diagnosis-only patients.
There were significant granular differences inpatient demographics and comorbidities across the three cohorts, including age, diabetes, and obesity. The sepsis ICD-10 code only and sepsis ICD-10 code + COVID-19 diagnosis classifications were more likely to occur in older age groups and older age was an important risk factor for in-hospital mortality, findings supported by published analyses of sepsis and COVID-19 literature reviews (24, 25). Diabetes was significantly associated with mortality in COVID-19 diagnosis-only patients and decreased discharge to home in both sepsis ICD-10 only and COVID-19 diagnosis-only patients, which may reflect an increased susceptibility to and severity of sepsis in diabetic patients (26, 27). Finally, morbid obesity (BMI > 35) was significantly associated with mortality in the sepsis ICD-10 code + a COVID-19 diagnosis (OR, 1.7) cohort only. A meta-analysis of COVID-19 studies found that patients with obesity were at a significantly increased risk of COVID-19 infection and severe disease (28); in addition, an elevated BMI increases a patient’s susceptibility to severe COVID-19, subsequent sepsis, and mortality (29).
We also found that sepsis ICD-10 code-only patients had more comorbid conditions than the sepsis ICD-10 code + COVID-19 diagnosis patients, which may suggest that many deaths in septic patients are due to underlying comorbidities (e.g., heart failure, cancer) rather than sepsis itself (30). In contrast, the opposite may be true for sepsis + COVID-19 diagnosis patients who had fewer comorbidities: their mortality may be due primarily to the severity of their COVID-19.
This study has several limitations. First, we did not review the clinical notes for any patients including COVID-19 diagnosis-only patients, and do not know if sufficient documentation of sepsis and AOD was recorded to justify ICD-10 codes for severe sepsis or septic shock. Second, because our study was performed in a single health system, it is unclear whether our findings are generalizable to other health systems, with different resources and different COVID-19 case definitions. However, we included data from nine diverse hospitals, ranging from smaller community hospitals to large tertiary academic hospitals so the presented data reflect a large percentage of U.S. hospital sepsis and COVID-19 admissions. Third, since we did not perform manual chart reviews, we do not know if COVID-19 was a primary or secondary factor in a patient’s admission or an incidental finding (23). However, the study period spans the first and second waves of the COVID-19 pandemic when primary COVID-19 admissions increased significantly. Fourth, we did not extract individual chart-level data investigating bacterial coinfection or secondary infection and are not able to report their specific contribution to disease severity in our sepsis ICD-10 code + COVID-19 diagnosis cohort. Fifth, we used an adjudicated institutional definition of COVID-19 diagnosis because the COVID-19 ICD-10 code was not available until October 1, 2020, and our study period started on March 12, 2020, when the first COVID-19-positive patients were seen at our hospitals. There is a limitation to this approach as it is unclear how variable the assignment of the different ICD-10 codes is depending on the primary etiology of infection. However, this was the most inclusive approach to answer our research question—what similarities exist between the sepsis ICD-10 only, the COVID-19 diagnosis-only, and the sepsis ICD-10 + COVID-19 diagnosis cohorts? Finally, because a large number of comparisons were made between groups and variables within groups in the article, statistical results are susceptible to the problem of multiple comparisons, with some results being statistically significant simply because of the volume of calculations performed. However, we limited our primary and secondary outcomes to a few central questions and these results are not hampered by the multiple comparisons problem; also, we only reported p values for these central analyses.
Interpretation
During the first year of the COVID-19 pandemic, the sickest cohort of patients was those receiving A small but significant percentage of COVID-19 diagnosis-only patients appear to have been under-coded as they received a level of critical care (ICU admission; intubation; high mSOFA score) suggestive of the presence of AOD. Correct categorization of these patients would more objectively describe the burden of disease associated with the COVID-19 pandemic and help clinicians and hospitals with disaster preparedness.
Supplementary Material
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
This study was approved in expedited review with waiver of informed consent by the Thomas Jefferson University institutional review board.
Dr. Gaieski: hypothesis generation, research plan, data analysis, writing first draft, and editing. Dr. Tsukuda was involved in the research plan, data analysis, and editing. Dr. Maddox was involved in research plan, data analysis, writing first draft, and editing. Dr. Li was involved in hypothesis generation, research plan, programming, data collection, data analysis, writing first draft, and editing.
A de-identified data set will be made available to researchers after institutional review board approval at their institution and Thomas Jefferson University’s institutional review board.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
REFERENCES
- 1.Singer M, Deutschman CS, Seymour CW, et al. : The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaieski DF, Edwards JM, Kallan MJ, et al. : Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 2013; 41:1167–1174 [DOI] [PubMed] [Google Scholar]
- 3.Rivers E, Nyugen B, Havstad S, et al. : Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–1377 [DOI] [PubMed] [Google Scholar]
- 4.The ProCESS Investigators: A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014; 370:1683–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The ARISE Investigators and the ANZICS Clinical Trials Group: Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014; 371:1496–1506 [DOI] [PubMed] [Google Scholar]
- 6.The ProMISe Trial Investigators: Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 2015; 372:1301–1311 [DOI] [PubMed] [Google Scholar]
- 7.Seymour CW, Gesten F, Prescott HC, et al. : Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med 2017; 376:2235–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bone RC, Balk RA, Cerra FB, et al. : Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101:1644–1655 [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Schneider AM, Mehta A, et al. : SARS-CoV-2 viremia is associated with distinct proteomic pathways and predicts COVID-19 outcomes. J Clin Invest 2021; 131:e148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar A, Roberts D, Wood KE, et al. : Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–1596 [DOI] [PubMed] [Google Scholar]
- 11.Gaieski DF, Mikkelsen ME, Band RA, et al. : Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med 2010; 38:1045–1053 [DOI] [PubMed] [Google Scholar]
- 12.Puskarich MA, Trzeciak S, Shapiro NI, et al. ; Emergency Medicine Shock Research Network (EMSHOCKNET): Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med 2011; 39:2066–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Liu L, Zhang D, et al. : SARS-CoV-2 and viral sepsis: Observations and hypotheses. Lancet 2020; 395:1517–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zafer MM, El-Mahallawy HA, Ashour HM: Severe COVID-19 and sepsis: Immune pathogenesis and laboratory markers. Microorganisms 2021; 9:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.López-Collazo E, Avendaño-Ortiz J, Quirós AM, et al. : Immune response and COVID-19: A mirror image of Sepsis. Int J Biol Sci 2020; 16:2479–2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouck EG, Denorme F, Holle LA, et al. : COVID-19 and sepsis are associated with different abnormalities in plasma procoagulant and fibrinolytic activity. Arterioscler Thromb Vasc Biol 2021; 41:401–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alhazzani W, Møller MH, Arabi YM, et al. : Surviving sepsis campaign: Guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med 2020; 46:854–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ochieng’ Olwal C, Nganyewo NN, Tapela K, et al. : Parallels in sepsis and COVID-19 conditions: Implications for managing severe COVID-19. Front Immunol 2021; 12:602848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karakike E, Giamarellos-Bourboulis EJ, Kyprianou M, et al. : Coronavirus disease 2019 as cause of viral sepsis: A systematic review and meta-analysis. Crit Care Med 2021; 49:2042–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quan H, Li B, Couris CM, et al. : Updating and validating the Charlson Comorbidity Index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidem 2011; 173:676–682 [DOI] [PubMed] [Google Scholar]
- 21.Wiersinga WJ, Rhodes A, Cheng AC, et al. : Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA 20202020; 324:782–793 [DOI] [PubMed] [Google Scholar]
- 22.Whittaker SA, Mikkelsen ME, Gaieski DF, et al. : Severe sepsis cohorts derived from claims-based strategies appear to be biased toward a more severely ill patient population. Crit Care Med 2013; 41:945–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shappell CN, Klompas M, Chan C, et al. : Impact of changing case definitions for coronavirus disease 2019 (COVID-19) hospitalization on pandemic metrics. Infect Control Hosp Epidemiol 2023:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neumann-Podczaska A, Al-Saad SR, Karbowski LM, et al. : COVID 19—clinical picture in the elderly population: A qualitative systematic review. Aging Dis 2020; 11:988–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasa P, Juneja D, Singh O: Severe sepsis and septic shock in the elderly: An overview. World J Crit Care Med 2012; 1:23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh AK, Gupta R, Ghosh A, et al. : Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr 2020; 14:303–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuetz P, Castro P, Shapiro NI: Diabetes and sepsis: Preclinical findings and clinical relevance. Diabetes Care 2011; 34:771–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Popkin BM, Du S, Green WD, et al. : Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes Rev 2020; 21:e13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ponsford MJ, Gkatzionis A, Walke VM, et al. : Cardiometabolic traits, sepsis, and severe COVID-19: A mendelian randomization investigation. Circulation 2020; 142:1791–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhee C, Jones TM, Hamad Y, et al. ; Centers for Disease Control and Prevention (CDC): Prevention Epicenters program (2019) prevalence, underlying causes, and preventability of sepsis-associated mortality in US Acute Care Hospitals. JAMA Netw Open 7571; 2:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.