Abstract
PURPOSE
Pirtobrutinib is a highly selective, noncovalent (reversible) Bruton tyrosine kinase inhibitor (BTKi). We report the safety and efficacy of pirtobrutinib in patients with covalent Bruton tyrosine kinase inhibitor (cBTKi) pretreated mantle-cell lymphoma (MCL), a population with poor prognosis.
METHODS
Patients with cBTKi pretreated relapsed/refractory (R/R) MCL received pirtobrutinib monotherapy in a multicenter phase I/II trial (BRUIN; ClinicalTrials.gov identifier: NCT03740529). Efficacy was assessed in the first 90 consecutively enrolled patients who met criteria for inclusion in the primary efficacy cohort. The primary end point was overall response rate (ORR). Secondary end points included duration of response (DOR) and safety.
RESULTS
The median patient age was 70 years (range, 46-87), the median prior lines of therapy was 3 (range, 1-8), 82.2% had discontinued a prior cBTKi because of disease progression, and 77.8% had intermediate- or high-risk simplified MCL International Prognostic Index score. The ORR was 57.8% (95% CI, 46.9 to 68.1), including 20.0% complete responses (n = 18). At a median follow-up of 12 months, the median DOR was 21.6 months (95% CI, 7.5 to not reached). The 6- and 12-month estimated DOR rates were 73.6% and 57.1%, respectively. In the MCL safety cohort (n = 164), the most common treatment-emergent adverse events (TEAEs) were fatigue (29.9%), diarrhea (21.3%), and dyspnea (16.5%). Grade ≥3 TEAEs of hemorrhage (3.7%) and atrial fibrillation/flutter (1.2%) were less common. Only 3% of patients discontinued pirtobrutinib because of a treatment-related adverse event.
CONCLUSION
Pirtobrutinib is a first-in-class novel noncovalent (reversible) BTKi and the first BTKi of any kind to demonstrate durable efficacy after prior cBTKi therapy in heavily pretreated R/R MCL. Pirtobrutinib was well tolerated with low rates of treatment discontinuation because of toxicity.
INTRODUCTION
Mantle-cell lymphoma (MCL) is an aggressive, rare subtype of B-cell non-Hodgkin lymphoma. Covalent Bruton tyrosine kinase inhibitors (cBTKi) have transformed the therapeutic landscape of multiple B-cell malignancies, including relapsed/refractory (R/R) MCL.1,2 However, the efficacy of cBTKi in this setting is limited by drug resistance or intolerance.3-6 After cBTKi therapy, patients with R/R MCL have historically had very poor outcomes with a median overall survival (OS) <10 months.3,5,7-9 Recent availability of CD19-targeted chimeric antigen receptor (CAR) T-cell therapy for R/R MCL has expanded treatment options, but access is limited, not all patients qualify, and treatment is associated with severe toxicities.10 Therefore, there remains a significant unmet medical need for efficacious, broadly accessible, and well-tolerated therapies for patients with MCL after cBTKi treatment.
CONTEXT
Key Objective
Despite the efficacy of covalent Bruton tyrosine kinase inhibitors (cBTKi) in mantle-cell lymphoma (MCL), resistance invariably develops. Treatment options are then limited, and consequently, patient outcomes are poor with a median overall survival of <10 months. Pirtobrutinib, a highly selective, noncovalent (reversible) Bruton tyrosine kinase inhibitor (BTKi), inhibits both wild-type and C481-mutant Bruton tyrosine kinase (BTK) with equal low nM potency and has favorable oral pharmacology that enables continuous BTK inhibition throughout the daily dosing interval, regardless of intrinsic rate of BTK turnover. Here, we report the safety and efficacy of pirtobrutinib in patients with cBTKi pretreated MCL.
Knowledge Generated
Pirtobrutinib is a first-in-class noncovalent (reversible) BTKi, and the first BTKi of any kind to demonstrate durable efficacy after prior cBTKi therapy in heavily pretreated patients with relapsed/refractory MCL. Pirtobrutinib was well tolerated with low rates of cBTKi-associated adverse events and discontinuation because of drug-related toxicity.
Relevance (J.W. Friedberg)
-
These data directly inform the regulatory approval of pirtobrutinib for patients with MCL, and provide rationale for planned and ongoing phase III studies comparing covalent to non-covalent BTKi in several hematological malignancies.*
*Relevance section written by JCO Editor-in-Chief Jonathan W. Friedberg, MD.
Resistance mechanisms to cBTKi vary by tumor type. In chronic lymphocytic leukemia, Bruton tyrosine kinase (BTK) mutations have been well described, most commonly at the C481 position.11 These mutations prevent irreversible drug binding and confer cross resistance to all cBTKi. In MCL, BTK mutations are uncommon, and mechanisms of resistance are less well understood but may converge on epigenetic or genetic mechanisms that collectively restore BTK signaling.4,12-14 Neoplastic MCL cells may also become more proliferative over time, leading to increased BTK protein turnover and incomplete target inhibition with cBTKi.15 Although three cBTKi (ibrutinib, acalabrutinib, and zanubrutinib) have been approved for the treatment of R/R MCL, data suggest similar efficacy for each of these agents.16-18 Importantly, no cBTKi has demonstrated efficacy after progression when used sequentially after another cBTKi.
Pirtobrutinib, a highly selective, noncovalent (reversible) BTKi, inhibits both wild-type and C481-mutant BTK with equal low nM potency and has favorable oral pharmacology that enables continuous BTK inhibition throughout the once daily dosing interval, regardless of the intrinsic rate of BTK turnover.19 Here, we report the primary efficacy and safety analysis from cBTKi pretreated patients with MCL enrolled in the phase I/II BRUIN trial.
METHODS
Patients
Patients with R/R MCL and other B-cell malignancies, including those who were previously treated with a cBTKi, were eligible for treatment with pirtobrutinib monotherapy in the first-in-human open-label, multicenter, phase I/II BRUIN trial.19 Patient allocation by B-cell malignancy is included in the Data Supplement ([Fig S1], online only). The overall trial design and full eligibility criteria have been previously described19 and are detailed in the protocol (Data Supplement). Eligible patients with MCL were enrolled at 37 sites in eight countries.
The trial Protocol (online only) was approved by the institutional review boards overseeing each site. The trial was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines, and local laws. All patients provided written informed consent. This trial is registered with ClinicalTrials.gov (identifier: NCT03740529).
Trial Design and Treatment
Patients with R/R MCL were treated in either the dose escalation or expansion portion of the trial. The phase I portion explored doses ranging from 25 to 300 mg once daily and the phase II portion utilized the recommended phase II dose of 200 mg once daily. Treatment was administered until disease progression, discontinuation because of toxicity, or patient/physician decision to withdraw. Patients with disease progression were permitted to continue pirtobrutinib treatment if clinical benefit was experienced at the investigator's discretion.
Trial Assessments
The safety cohort included all patients with MCL who were administered at least one dose of pirtobrutinib monotherapy as of the data cutoff date. The primary efficacy cohort included the first 90 patients with MCL consecutively enrolled to either the phase I or II who had measurable disease as assessed per investigator, had received a prior cBTKi-containing regimen, and had no known central nervous system involvement. A data cutoff date of January 31, 2022 was selected to ensure that the vast majority (approximately 90%) of responders in the efficacy cohort would be followed for at least 9 months from date of response onset. Efficacy was also separately assessed in 14 enrolled patients with cBTKi-naïve MCL, who were enrolled in earlier versions of the protocol to both phase I and II portions of the study. Positron emission tomography-computed tomography (PET-CT) scans were used as the primary response assessment modality, when available, with the remainder of patients being assessed by CT scans only.
The primary end point was overall response rate (ORR) as assessed by an independent review committee (IRC). Secondary end points included IRC-assessed best overall response (BOR), duration of response (DOR), progression-free survival (PFS), OS, and safety. Disease response assessments were performed at 8-week intervals in the first year, 12-week intervals in the second year, and then every 6 months. In the MCL cohort, the ORR was assessed according to Lugano 2014 criteria,20 integrating CT measurements with FDG-PET when available.21 DOR was measured from the start date of the first documented response to the earlier of the documentation of progressive disease or death from any cause. PFS was measured from the first dose of pirtobrutinib to the earlier of the documentation of progressive disease or death from any cause. OS was measured from first dose of pirtobrutinib to the date of death from any cause. Frequency, attribution, and severity of treatment-emergent adverse events (TEAEs) were investigator assessed from the first dose of pirtobrutinib and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0 (CTCAE v5.0).
Statistical Analysis
All analyses were conducted according to the statistical analysis plan (SAP) as reviewed by global health authorities (Data Supplement). Under the originally proposed SAP, a sample size of 65 patients in the primary efficacy cohort, also called primary analysis set, was estimated to provide approximately 95% power to have the lower boundary of a two-sided 95% exact binomial CI >20%, if the true ORR is 40%. Ruling out a lower limit of 20% for ORR is considered clinically meaningful for patients with MCL who have discontinued prior cBTKi therapy, as ORRs of approximately 20%-30% were reported in clinical studies testing agents given as monotherapy in patients with cBTKi-naïve advanced MCL (temsirolimus, 22%22; bortezomib, 31%23; lenalidomide, 28%24). The sample size for the primary efficacy cohort was subsequently increased to 90 patients following US regulatory feedback.
Descriptive statistics were used to present patient disposition, demographics, and disease characteristics, BOR, and safety data. ORR was estimated with an exact two-sided 95% CI. The Kaplan-Meier method was used to analyze DOR, PFS, and OS. Analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
Baseline Patient and Disease Characteristics
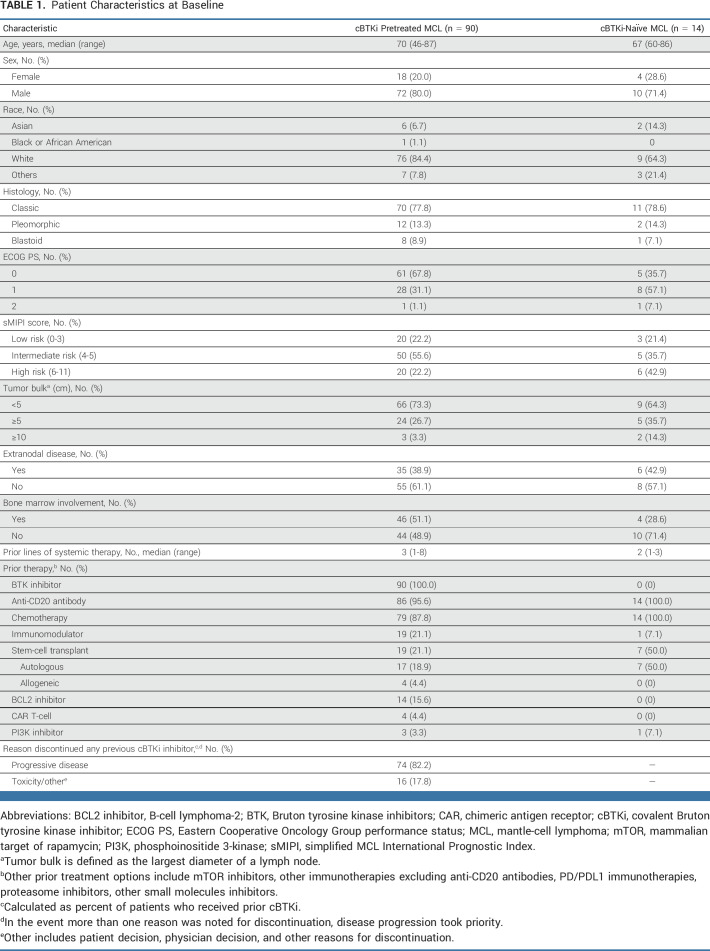
From March 21, 2019, to January 31, 2022, a total of 164 patients with MCL were enrolled and treated with pirtobrutinib, including 90 cBTKi pretreated patients in the primary efficacy cohort and 14 cBTKi-naïve patients (Data Supplement [Fig S1]). The additional 60 patients with MCL not included in the efficacy analyses were either not eligible for the primary efficacy cohort or had insufficient follow-up. PET-CT scans were used in response assessments in 47% (n = 42) of patients in the primary efficacy cohort (n = 90), with the remainder being assessed by CT scans only. Among patients in the primary efficacy cohort, the median age was 70 (range, 46-87) years, the median number of prior lines of therapy was 3 (range, 1-8), and the majority of patients (77.8%, n = 70) had intermediate- or high-risk disease on the basis of the simplified MCL International Prognostic Index score (Table 1). Additional prior therapies included an anti-CD20 antibody (95.6%, n = 86), chemotherapy (87.8%, n = 79), immunomodulatory drugs (21.1%, n = 19), stem-cell transplantation (21.1%, n = 19; autologous [18.9%] or allogeneic [4.4%]), B-cell lymphoma-2 inhibitor (15.6%, n = 14), CAR T-cell therapy (4.4%, n = 4), and phosphoinositide 3-kinase inhibitor (3.3% n = 3). The majority of patients discontinued their prior cBTKi because of disease progression (82.2%, n = 74), followed by toxicity/intolerance (17.8%, n = 16). Six patients (6.7%) received only one prior line of therapy which was a BTKi, 66 (73.3%) patients received one prior cBTKi but also had other types of prior lines of therapy, and 18 patients (20%) received more than one prior cBTKi. Most patients (n = 79, 87.8%) received at least one dose of pirtobrutinib at the recommended phase II dose of 200 mg once daily, with 77 (85.6%) patients receiving 200 mg once daily as starting dose. The median time on treatment was 5.2 months (range, 0.2-33.7). Treatment was discontinued in 72 (80.0%) patients, 49 (54.4%) because of disease progression, 11 (12.2%) because of a TEAE with 3 (3.3%) of these considered to be treatment-related adverse event (AE; weight loss, cholecystitis, and neutrophil count decrease), 3 (3.3%) because of commencement of an alternative therapy, 2 (2.2%) because of withdrawal of consent, 5 (5.6%) because of death, and 2 (2.2%) because of other reasons. Baseline characteristics for the 14 cBTKi-naïve patients with MCL are also provided in Table 1.
TABLE 1.
Patient Characteristics at Baseline
Efficacy
Among the primary efficacy cohort (n = 90), the ORR as determined by IRC was 57.8% (95% CI, 46.9 to 68.1), including 20.0% (n = 18) with complete responses and 37.8% (n = 34) with partial responses (Table 2; Fig 1). The median time-to-response was 1.8 months (IQR, 1.0-7.5). In patients with blastoid histology (n = 8) and pleomorphic histology (n = 12), the ORR was 75.0% (95% CI, 34.9 to 96.8) and 50.0% (95% CI, 21.1 to 78.9), respectively. Two of four patients who received previous CAR T-cell therapy attained disease response, and the ORR among 19 patients who had received previous stem cell transplantation was 57.9% (95% CI, 33.5 to 79.7). The ORR was generally consistent across other prespecified subgroups regardless of demographics, number of prior lines of therapy, or prior therapy (Data Supplement [Fig S2]). Similarly, the ORR to pirtobrutinib was similar in patients previously treated with ibrutinib, acalabrutinib, or zanubrutinib (Data Supplement [Table S1]).
TABLE 2.
Efficacy of Pirtobrutinib in Patients With cBTKi Pre-treated and cBTKi-Naïve MCL
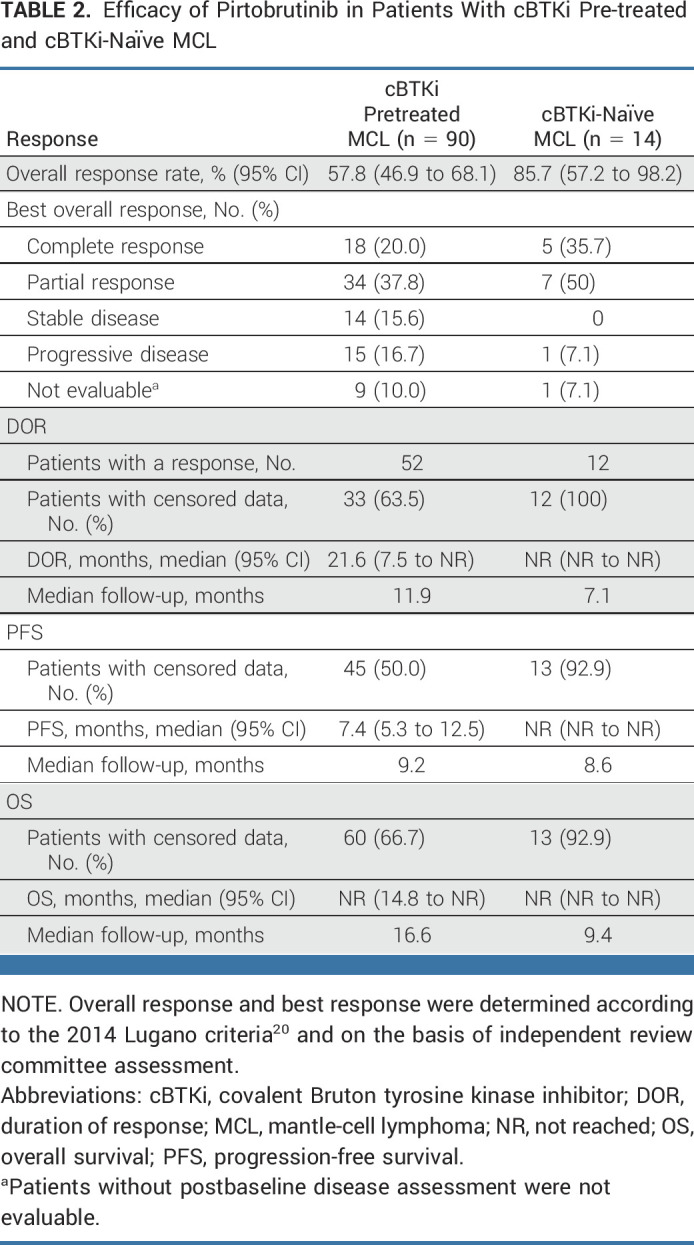
FIG 1.
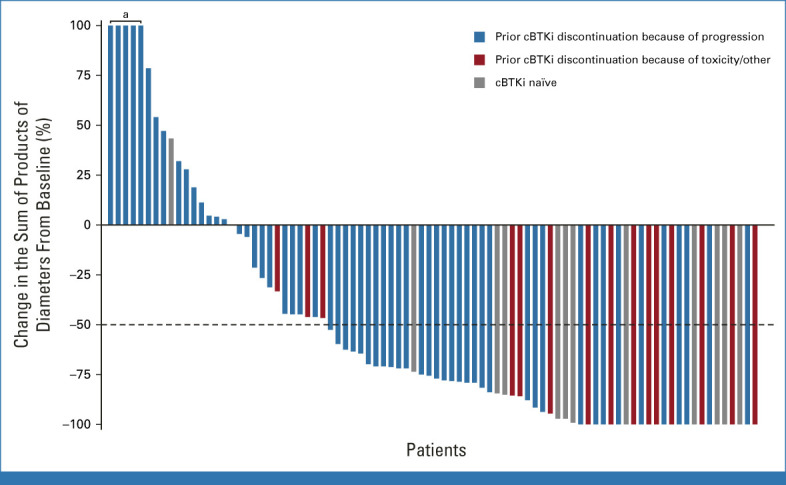
Efficacy of pirtobrutinib in patients with relapsed/refractory mantle-cell lymphoma. Change in tumor burden from baseline was measured by changes in the sum of product of diameters on axial CT images of index lesions. Waterfall plot includes patients with baseline and at least one evaluable postbaseline tumor measurement. Data for 18 patients are not shown in the waterfall plot because of no measurable target lesions identified by CT at baseline, discontinuation before first response assessment, or lack of adequate imaging in follow-up. aIndicates patients with >100% increase in SPD. cBTKi, covalent Bruton tyrosine kinase inhibitor; CT, computed tomography; SPD, sum of product of diameter.
At a median response follow-up of 12 months, the median DOR by IRC among the 52 responders was 21.6 months (95% CI, 7.5 to not reached [NR]; Fig 2A; Table 2). The 6-, 12-, and 18-month estimated DOR rates were 73.6% (95% CI, 58.0 to 84.2), 57.1% (95% CI, 39.3 to 71.5), and 52.4% (95% CI, 33.9 to 67.9), respectively. Among responding patients, 35% of responses were ongoing at the time of data cutoff, with the longest ongoing response observed at 26.2 months. Patients with BTKi as their most recent prior line of therapy (n = 55) had a median DOR of 14.8 months (95% CI, 5.55 to not estimable [NE]) and an ORR of 52.7% (Data Supplement [Table S5]; n = 29). The median PFS by IRC was 7.4 months (95% CI, 5.3 to 12.5; Fig 2B; Table 2). The median OS was NR (95% CI, 14.8 to NR; Fig 2C; Table 2). The 12- and 18-month estimated OS rates were 67.6% (95% CI, 55.7 to 77.0) and 59.3% (95% CI, 46.1 to 70.2), respectively. After treatment with pirtobrutinib, 17 (18.9%) patients went on to receive subsequent CAR T-cell therapy.
FIG 2.
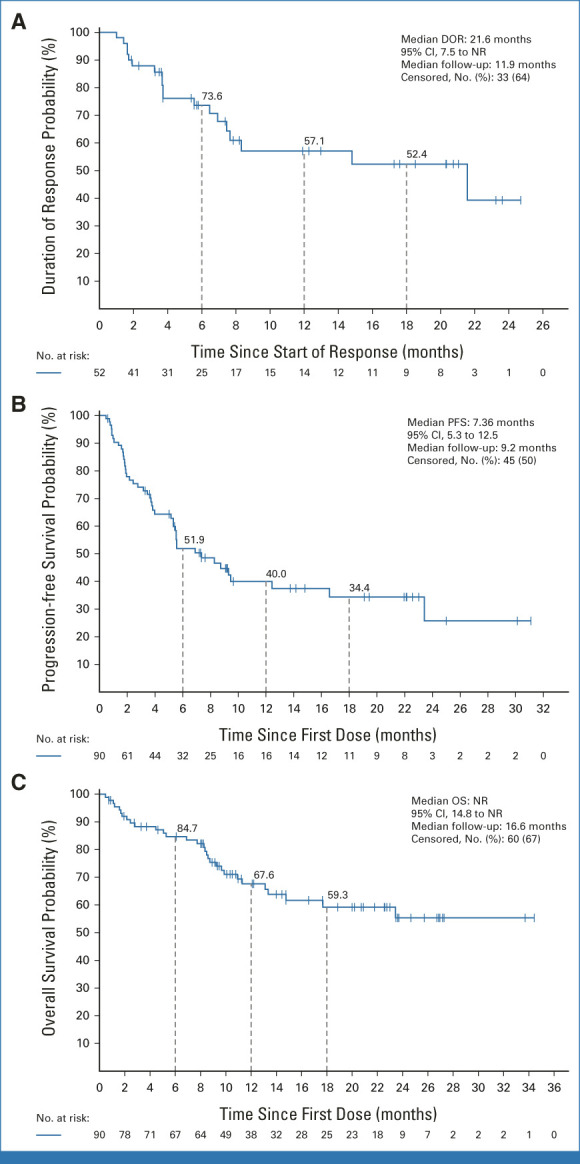
Kaplan-Meier plot of (A) DOR, (B) PFS, and (C) OS in patients with cBTKi pre-treated MCL treated with pirtobrutinib. cBTKi, covalent Bruton tyrosine kinase inhibitor; DOR, duration of response; MCL, mantle-cell lymphoma; NR, not reached; OS, overall survival; PFS, progression-free survival.
Among the 14 cBTKi-naïve patients, the ORR according to IRC was 85.7% (95% CI, 57.2 to 98.2), including 35.7% (5 of 14) with complete response and 50.0% (7 of 14) with partial responses (Table 2; Fig 1). At a median response follow-up of 7.1 months, the median DOR was NR (95% CI, NR to NR; Table 2). The 6 months estimated DOR rate was 100% (95% CI, NR to NR; Data Supplement [Fig S3]). Median PFS and OS had not been reached (Table 2). At 6 months, the PFS and OS rates were both 92.3% (95% CI, 56.6 to 98.9; Data Supplement [Figs S4 and S5]). Patients who discontinued previous cBTKi treatment because of disease progression (n = 74) had an ORR of 50% (n = 37; 95% CI, 38.1 to 61.9), and a median DOR of 14.8 months (95% CI, 5.6 to NE). These patients had a median PFS of 5.5 months (95% CI, 3.7 to 8.3) and a median OS of 23.4 months (95% CI, 10.9 to NE). Patients who discontinued previous BTKi because of toxicity (n = 12) had an ORR of 92% (n = 11), an NE median DOR (95% CI, 7.5 to NE), NE median PFS (95% CI, 9.3 to NE), and an NE median OS (95% CI, 13.3 to NE). These patients had a 12-month DOR of 78.8% (95% CI, 38.1 to 94.3), 12-month PFS of 82.5% (95% CI, 46.1 to 95.3), and a 12-month OS of 91.7% (95% CI, 53.9 to 98.8).
Safety
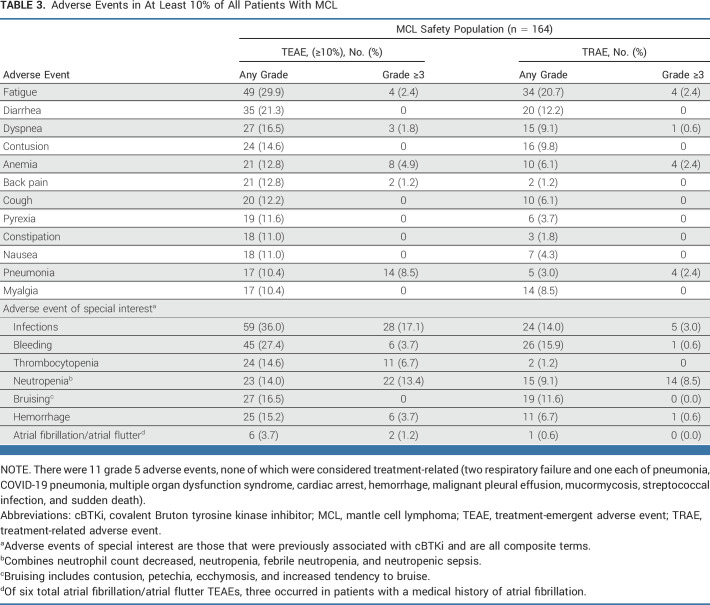
Among the 164 patients with MCL treated with pirtobrutinib as of the data cutoff date, 92.1% had received at least one dose of pirtobrutinib at the recommended phase II dose of 200 mg once daily. The median time on treatment was 4.5 months. The most common TEAEs and AEs of special interest are presented in Table 3. The most common TEAEs, regardless of attribution, were fatigue (29.9%, n = 49), diarrhea (21.3%, n = 35), and dyspnea (16.5%, n = 27). The most frequent grade ≥3 TEAE was infection (17.1%, n = 28).
TABLE 3.
Adverse Events in At Least 10% of All Patients With MCL
Grade ≥3 pirtobrutinib-related AEs were infrequent, with neutropenia (8.5%, n = 14) being the most common. No grade ≥3 TEAEs of hypertension were observed. Atrial fibrillation/flutter was uncommon and seen in 6 (3.7%) patients, three of whom had a history of atrial fibrillation, despite 17 (10.4%) patients enrolled with a medical history of atrial fibrillation/flutter. Only two events of atrial fibrillation/atrial flutter were grade ≥3, neither of which resulted in discontinuation of pirtobrutinib. Low rates of grade ≥3 TEAEs of hemorrhage (3.7%, n = 6) were observed, with no grade 3 or higher bruising events (Table 3). Grade ≥3 TEAEs of infection occurred in 28 (17.1%) patients. The most common infection of any grade observed was pneumonia (10.4%, n = 17) and COVID-19–related pneumonia (3%, n = 5). There were 8 (4.9%) patients with documented COVID-19 disease of any grade. AEs leading to dose interruptions and dose reductions were observed in 42 (25.6%) and 8 (4.9%) patients, respectively. Permanent discontinuations because of TEAEs occurred in 15 (9.1%) patients, the most common being pneumonia (n = 2, 1.2) while no other event accounted for more than one discontinuation (Data Supplement [Table S3]). Permanent discontinuations for drug-related adverse events occurred in 5 (3.0%) patients (Data Supplement [Table S4]). There were 11 grade 5 TEAEs, none of which were considered drug-related by investigators (Table 3), although treatment effect can never be completely ruled out. The safety profile of all patients with MCL (n = 164) was consistent with the overall population comprising all patients treated with pirtobrutinib across B-cell malignancies (N = 725; Data Supplement [Tables S2 and S3]).
DISCUSSION
Pirtobrutinib demonstrated durable efficacy and a favorable safety profile in patients with cBTKi pretreated MCL in the BRUIN phase I/II trial. These data suggest that re-establishing BTK inhibition with pirtobrutinib, a noncovalent BTKi, is an effective and safe approach in patients with MCL who had prior cBTKi treatment. Pirtobrutinib has the potential to meaningfully extend the total period of clinical benefit from BTK inhibition when used sequentially after cBTKi exposure.
The availability of effective and safe therapies for patients with MCL after treatment with cBTKi remains an area of high unmet need. In this trial, patients with MCL and prior cBTKi exposure receiving pirtobrutinib monotherapy achieved a clinically meaningful ORR of 58%, with 57% of responders maintaining response at 12 months. Responses with pirtobrutinib were consistent in patients who discontinued their prior cBTKi because of disease progression or toxicity/other reasons and across most prespecified subgroups including in patients with blastoid/pleomorphic histologies, those who previously received CAR T-cell therapy and stem-cell transplantation, and those who received multiple prior lines of therapy. Notably, similar efficacy was observed in patients previously treated with ibrutinib, acalabrutinib, or zanubrutinib, suggesting that there may not be a unique pattern of BTK-mediated resistance associated with these covalent agents. Importantly, the OS observed 12-month survival rate of 68% appears promising, given the reports in similar cohorts from the literature (median survival <10 months).3,7-9,25
The exact mechanisms by which pirtobrutinib is efficacious in MCL after cBTKi treatment is incompletely understood as BTK mutations are rarely observed in MCL.4,12,13 Pirtobrutinib has favorable pharmacokinetics with high oral bioavailability and a 19-hour half-life, attaining continuous BTK inhibition (>IC90) throughout the dosing interval, regardless of intrinsic rate of BTK turnover. The highly selective nature of pirtobrutinib may also reduce off-target inhibition, thus minimizing adverse events while permitting maximal on-target drug coverage. Finally, other features of pirtobrutinib besides its pharmacologic properties and reversible binding mode may be responsible for its unique clinical profile and preclinical studies are ongoing.
The safety of pirtobrutinib was favorable in patients with R/R MCL and was similar to the larger population of pirtobrutinib-treated patients. This favorable safety profile is consistent with the high selectivity of pirtobrutinib for BTK. Non-BTK–mediated grade 3 or higher adverse events and treatment discontinuation because of drug-related toxicity were both uncommon. Despite allowing patients with a history of prior atrial fibrillation on cBTKi to enroll, atrial fibrillation rates observed here were consistent with that expected in age-matched population controls.17,26 Rates of grade ≥3 infection and bleeding were also low, despite enrolling patients with a history of these events on prior cBTKi. Finally, the frequency of any grade hypertension was low, and no high-grade hypertension was observed, which has been usually reported with cBTKi therapy with longer follow-up.27-29
The approval of CD19-targeted CAR T-cell therapy for R/R MCL has expanded treatment options in this setting; however, delivery of this therapy is often not feasible because of the rapidly progressive kinetics of relapsed MCL30 and is limited to patients with access to tertiary centers, often associated with severe adverse events,10 and commonly requires an effective bridging therapy.31 Other investigational therapies such as bispecific antibodies have shown promising results in the R/R MCL setting, including patients with previous BTKi exposure, although evidence is limited with small sample size and limited follow-up.32 Nemtabrutinib, a noncovalent BTKi, is under investigation for the treatment of R/R B-cell malignancies although data are currently only available for six patients with MCL.33
This trial has some important limitations. As this was a single-arm trial, formal comparison to other available therapies typically used for the treatment of R/R MCL after cBTKi treatment is not possible. Additionally, numerous subgroups have limited patient numbers, resulting in large confidence intervals for response rates. The median DOR, although reached, is not fully mature and may change in response to additional follow-up. Finally, additional follow-up is needed to assess the long term-safety profile of pirtobrutinib.
In summary, pirtobrutinib is the first noncovalent (reversible) BTK inhibitor to demonstrate meaningful response rates and durable efficacy in patients with heavily pretreated MCL who received a prior cBTKi. Pirtobrutinib was well tolerated with low rates of cBTKi-associated adverse events and discontinuation because of drug-related toxicity. Several ongoing clinical trials are evaluating pirtobrutinib in the treatment of B-cell malignancies, including a randomized, global, phase III trial comparing pirtobrutinib with investigator's choice of cBTKi in patients with pretreated BTKi-naïve MCL (BRUIN MCL-321; ClinicalTrials.gov identifier: NCT04662255).34
ACKNOWLEDGMENT
We thank the BRUIN clinical trial participants and their caregivers, without whom this work would not be possible. We also thank the BRUIN trial investigators and study staff. Garreth Lawrence PhD, employee of Eli Lilly and Company, provided medical writing support.
APPENDIX
TABLE A1.
BRUIN Investigators
Michael L. Wang
Honoraria: Janssen Research & Development, Dava Oncology, AstraZeneca, Cahon, Acerta Pharma, BeiGene, Kite, a Gilead company, Moffit Cancer Center, Physicans' Education Resource, Breast-Gynecological International Cancer Society, Pharmacyclics/Janssen, Eastern Virginia Medical School, Leukemia and Lymphoma Society, Medscape, Meeting Minds Experts, OncLive/MJH Life Sciences, Practice Point Communications, Bioinvent, AbbVie, Bantam Pharmaceutical, Bristol Myers Squibb Foundation, Genmab, IDEOlogy Health, MD Education, Merck, Nurix, Oncology Specialty Group, Studio ER Congressi
Consulting or Advisory Role: AstraZeneca, Janssen Research & Development, Bioinvent, Pharmacyclics/Janssen, Kite, a Gilead company, Innocare, Oncternal Therapeutics, Genentech, BeiGene, DTRM, Miltenyi Biomedicine, VelosBio, Deciphera, Lilly, Pepromene, AbbVie, Acerta Pharma, ADC Therapeutics, Amphista Therapeutics, Be Biopharma, Leukemia and Lymphoma Society, Merck, Milken Institute, Parexel
Research Funding: AstraZeneca, Janssen Research & Development, Pharmacyclics, Kite, a Gilead company, Juno Therapeutics, BeiGene, Acerta Pharma, Oncternal Therapeutics, Bioinvent, Loxo, VelosBio, Celgene, Molecular Templates, Lilly, Innocare, Genmab, Genentech, Vincerx Pharma
Travel, Accommodations, Expenses: AstraZeneca, Celgene, Dava Oncology, Kite, a Gilead company, Physicans' Education Resource
Wojciech Jurczak
Consulting or Advisory Role: BeiGene, AstraZeneca, Lilly
Research Funding: Roche, Takeda, Janssen-Cilag, BeiGene, Morphosys, AstraZeneca, Lilly
Pier Luigi Zinzani
Consulting or Advisory Role: Celltrion, Gilead Sciences, Janssen-Cilag, Bristol Myers Squibb, Servier, Sandoz, MSD, Roche, EUSA Pharma, Kyowa Hakko Kirin, Takeda, Secura Bio, TG Therapeutics, Novartis, ADC Therapeutics, Incyte, BeiGene
Speakers' Bureau: MSD, EUSA Pharma, Novartis
Toby A. Eyre
Honoraria: Roche, Gilead Sciences, Janssen Oncology, AbbVie, AstraZeneca/MedImmune, Loxo/Lilly, Incyte, Secura Bio, Autolus Therapeutics
Consulting or Advisory Role: Roche, Kite/Gilead, AbbVie, AstraZeneca/MedImmune, Loxo, BeiGene, Incyte, Secura Bio
Research Funding: AstraZeneca/MedImmune, BeiGene
Chan Y. Cheah
Honoraria: Roche/Genentech, Janssen-Cilag, TG Therapeutics, Loxo/Lilly, AstraZeneca, Bristol Myers Squibb, Gilead Sciences, BeiGene, Novartis
Consulting or Advisory Role: Janssen-Cilag, Roche/Genentech (Inst), TG Therapeutics, Loxo/Lilly, Gilead Sciences, AstraZeneca, Bristol Myers Squibb, Ascentage Pharma, Merck
Research Funding: Roche/Genentech (Inst), Bristol Myers Squibb (Inst), AbbVie (Inst), Merck (Inst)
Travel, Accommodations, Expenses: Roche
Chaitra S. Ujjani
Honoraria: Pharmacyclics, AbbVie, Genentech, Janssen, Incyte, BeiGene, Lilly
Consulting or Advisory Role: AstraZeneca, Epizyme, Atara Biotherapeutics
Research Funding: Pharmacyclics (Inst), AbbVie (Inst), Lilly, AstraZeneca/MedImmune, Adaptive Biotechnologies, Kite, a Gilead company
Youngil Koh
Leadership: GenomeOpinion
Stock and Other Ownership Interests: Curocell
Honoraria: Janssen, Amgen, Novartis, Takeda Korea
Consulting or Advisory Role: Prothena
Koji Izutsu
Honoraria: Takeda, Chugai Pharma, Eisai, Janssen, AbbVie, Novartis, MSD, Dainippon Sumitomo Pharma, Ono Pharmaceutical, Mundipharma, HUYA Bioscience International, AstraZeneca, Bayer, Bristol Myers Squibb, Kyowa Kirin, Fujifilm, Celgene, Daiichi Sankyo, Allergan, SymBio Pharmaceuticals, Pfizer, Meiji Seika Kaisha, Nippon Kayaku, Lilly
Consulting or Advisory Role: Bayer, Celgene, AstraZeneca, Ono Pharmaceutical, Kyowa Kirin, AbbVie, Genmab, Bristol Myers Squibb, Nippon Shinyaku, Mitsubishi Tanabe Pharma
Research Funding: Eisai, Chugai Pharma
James N. Gerson
Research Funding: Loxo
Ian Flinn
Consulting or Advisory Role: AbbVie (Inst), TG Therapeutics (Inst), Kite, a Gilead company (Inst), BeiGene (Inst), Genentech (Inst), Novartis (Inst), Century Therapeutics (Inst), Hutchison MediPharma (Inst), Servier (Inst), Vincerx Pharma (Inst), Genmab (Inst), Innocare (Inst), Myeloid Therapeutics (Inst), Secura Bio (Inst), Xencor (Inst)
Research Funding: Acerta Pharma (Inst), Agios (Inst), Celgene (Inst), Constellation Pharmaceuticals (Inst), Genentech (Inst), Gilead Sciences (Inst), Incyte (Inst), Infinity Pharmaceuticals (Inst), Janssen (Inst), Kite, a Gilead company (Inst), Novartis (Inst), Pharmacyclics (Inst), Portola Pharmaceuticals (Inst), Roche (Inst), TG Therapeutics (Inst), Trillium Therapeutics (Inst), AbbVie (Inst), ArQule (Inst), BeiGene (Inst), Curis (Inst), Forma Therapeutics (Inst), Forty Seven (Inst), Merck (Inst), Pfizer (Inst), Verastem (Inst), AstraZeneca (Inst), Unum Therapeutics (Inst), MorphoSys (Inst), Seagen (Inst), IGM Biosciences (Inst), Loxo (Inst), Rhizen Pharmaceuticals (Inst), Triact Therapeutics (Inst), Bristol Myers Squibb (Inst), CALGB (Inst), CTI (Inst), Fate Therapeutics (Inst), Millennium (Inst), Tessa Therapeutics (Inst), City of Hope (Inst), CALIBR (Inst), Bio-Path Holdings, Inc (Inst), Nurix (Inst), Innocare (Inst), Myeloid Therapeutics (Inst), Epizyme (Inst), Marker Therapeutics (Inst), Step Pharma (Inst), Vincerx Pharma (Inst), 2seventy bio (Inst)
Benoit Tessoulin
Honoraria: Kite/Gilead, AbbVie
Travel, Accommodations, Expenses: Kite/Gilead, AbbVie
Alvaro J. Alencar
Consulting or Advisory Role: Kite, a Gilead company, Amgen, Karyopharm Therapeutics, Seagen, Epizyme, Janssen, BeiGene, Incyte, TG Therapeutics, Genentech, Dr Reddy's Laboratories, Lilly
Research Funding: Loxo
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1171980
Shuo Ma
Consulting or Advisory Role: Genentech/Roche, AbbVie, Pharmacyclics, Janssen Oncology, AstraZeneca, BeiGene, Bristol Myers Squibb/Celgene/Juno, TG Therapeutics
Speakers' Bureau: Pharmacyclics, Janssen Oncology, AstraZeneca, BeiGene, Lilly
Research Funding: Pharmacyclics (Inst), AbbVie (Inst), BeiGene (Inst), Loxo/Lilly (Inst), Juno/Bristol-Myers Sqibb (Inst), AstraZeneca (Inst), IgM Biosciences (Inst)
David Lewis
Consulting or Advisory Role: Janssen Oncology, Kite/Gilead, BeiGene, AbbVie
Travel, Accommodations, Expenses: Kite, a Gilead company
Joanna Rhodes
Honoraria: Dava Oncology, Curio Science, Aptitude Health
Consulting or Advisory Role: AbbVie, Verastem, Genentech, Pharmacyclics, TG Therapeutics, SeaGen, Morphosys, Janssen, BeiGene, Genmab, Epizyme
Research Funding: Loxo/Lilly (Inst), Acerta Pharma (Inst), Pharmacyclics (Inst), Oncternal Therapeutics (Inst), VelosBio (Inst), Epizyme (Inst)
Travel, Accommodations, Expenses: DAVA Pharmaceuticals, Loxo, Curio Science
Krish Patel
Consulting or Advisory Role: AstraZeneca, Genentech, BeiGene, Pharmacyclics, Bristol Myers Squibb/Celgene/Juno, Morphosys, Kite, a Gilead company, TG Therapeutics, Loxo/Lilly, AbbVie, Seagen, Epizyme, ADC Therapeutics, Caribou Biosciences, Xencor, Fate Therapeutics
Speakers' Bureau: AstraZeneca, Bristol Myers Squibb/Celgene, Kite, a Gilead company, TG Therapeutics
Research Funding: AstraZeneca (Inst), Xencor (Inst), Pharmacyclics (Inst), Curis (Inst), Bristol Myers Squibb (Inst), Celgene (Inst), MEI Pharma (Inst), Trillium Therapeutics (Inst), Kite/Gilead (Inst), Roche/Genentech (Inst), Fate Therapeutics (Inst), Takeda (Inst), Epizyme (Inst), Aptevo Therapeutics (Inst), Nurix (Inst), Loxo/Lilly (Inst)
Kami Maddocks
Honoraria: Pharmacyclics, Celgene, Seagen, MorphoSys, BMS, Karyopharm Therapeutics, Kite, a Gilead company, ADC Therapeutics, Genmab, Lilly, Genentech, Epizyme, AstraZeneca/Merck, BeiGene, Incyte, AbbVie
Research Funding: Pharmacyclics, Merck, Bristol Myers Squibb
Nicole Lamanna
Consulting or Advisory Role: Celgene, Genentech, AbbVie, ProNAi, Pharmacyclics, Juno Therapeutics, Roche, Janssen, AstraZeneca, Gilead Sciences, BeiGene, Loxo/Lilly, Bristol Myers Squibb Foundation
Research Funding: Genentech/AbbVie (Inst), AbbVie (Inst), Infinity Pharmaceuticals (Inst), Gilead Sciences (Inst), ProNAi (Inst), Beigene (Inst), AstraZeneca (Inst), Verastem (Inst), Juno Therapeutics (Inst), TG Therapeutics (Inst), Acerta Pharma/AstraZeneca (Inst), Loxo (Inst), Oncternal Therapeutics, Inc (Inst), MingSight (Inst), Octapharm (Inst)
Yucai Wang
Employment: Merck
Stock and Other Ownership Interests: Merck
Honoraria: Kite, a Gilead company (Inst)
Consulting or Advisory Role: Loxo (Inst), Incyte (Inst), Innocare (Inst), TG Therapeutics (Inst), Kite, a Gilead company (Inst), Lilly (Inst), Janssen (Inst), BeiGene (Inst)
Research Funding: InnoCare (Inst), Incyte (Inst), Novartis (Inst), Genentech (Inst), Loxo (Inst), MorphoSys (Inst), Genmab (Inst)
Constantine S. Tam
Honoraria: Janssen-Cilag, AbbVie, Novartis, Beigene, Pharmacyclics, Roche/Genentech, Loxo/Lilly
Consulting or Advisory Role: Janssen, Loxo, Roche, BeiGene, AbbVie
Research Funding: Janssen-Cilag (Inst), AbbVie (Inst), BeiGene (Inst)
Talha Munir
Consulting or Advisory Role: Janssen-Cilag, AstraZeneca, BeiGene, Sobi, Roche, AbbVie, Alexion Pharmaceuticals, Lilly
Speakers' Bureau: AbbVie, Janssen-Cilag, Gilead Sciences, Alexion Pharmaceuticals, AstraZeneca, Sobi
Travel, Accommodations, Expenses: Janssen-Cilag, AbbVie, Alexion Pharmaceuticals, AstraZeneca
Hirokazu Nagai
Honoraria: Janssen, Ono Pharmaceutical, Bristol Myers Squibb, Chugai Pharma, Takeda, Eisai, Mundipharma, AstraZeneca, Novartis, Lilly, Meiji Seika Kaisha, AbbVie, GlaxoSmithKline, Genmab, Sumitomo Pharma Oncology, CSL Behring
Research Funding: Janssen (Inst), Celgene (Inst), AbbVie (Inst), Takeda (Inst), Bristol Myers Squibb (Inst), Ono Pharmaceutical (Inst), Zenyaku Kogyo (Inst), AstraZeneca (Inst), Chugai Pharma (Inst), Solasia Pharma (Inst), Kyowa Kirin (Inst), Nippon Shinyaku (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Genmab (Inst), Lilly Japan (Inst), Regeneron (Inst), Incyte Japan (Inst), HUYA Bioscience International (Inst)
Francisco Hernandez-Ilizaliturri
Consulting or Advisory Role: Seagen, Pharmacyclics, Novartis, Kite/Gilead, Epizyme, Morphosys, BeiGene, AbbVie
Anita Kumar
Stock and Other Ownership Interests: Bridgebio
Consulting or Advisory Role: Celgene, Kite, a Gilead company, AstraZeneca/MedImmune, Janssen, Genentech, Loxo/Lilly
Research Funding: AbbVie/Genentech, Adaptive Biotechnologies, Celgene, Seagen, AstraZeneca/MedImmune, Pharmacyclics, MorphoSys/Incyte, Loxo/Lilly
Timothy S. Fenske
Stock and Other Ownership Interests: Merck
Honoraria: Adaptive Biotechnologies, AstraZeneca, BeiGene, Kite, a Gilead company, MorphoSys, Pharmacyclics, Sanofi, Seagen, Servier, TG Therapeutics
Consulting or Advisory Role: Adaptive Biotechnologies, BeiGene, MorphoSys, Pharmacyclics, Seagen, Servier, TG Therapeutics
Speakers' Bureau: AstraZeneca, BeiGene, Kite, a Gilead company, Sanofi, Seagen, TG Therapeutics
Expert Testimony: Bayer
Travel, Accommodations, Expenses: AstraZeneca, Kite, a Gilead company, Sanofi, Seagen, TG Therapeutics
John F. Seymour
Honoraria: AbbVie, Janssen, Roche, BMS, AstraZeneca, Gilead Sciences, BeiGene
Consulting or Advisory Role: AbbVie, Janssen, Roche, AstraZeneca, BMS, Gilead Sciences, TG Therapeutics, Genor BioPharma, BeiGene
Speakers' Bureau: AbbVie, Roche
Research Funding: AbbVie, Celgene, Janssen, Roche
Patents, Royalties, Other Intellectual Property: Named patent holder for venetolcax dose ramp up and combination treatment. I do not receive any royalties
Expert Testimony: Roche, TG Therapeutics
Travel, Accommodations, Expenses: AbbVie, Roche
Andrew D. Zelenetz
Honoraria: NCCN, Curio Science, OncLive/MJH Life Sciences
Consulting or Advisory Role: Genentech/Roche, Celgene, AstraZeneca, Dava Oncology, BeiGene, MEI Pharma, Kite, a Gilead company, Juno/Celgene/Bristol Myers Squibb, Sandoz, Ono Pharmaceutical
Research Funding: Genentech/Roche, MEI Pharma, BeiGene, AbbVie (Inst)
Travel, Accommodations, Expenses: Kite, a Gilead company, NCCN, BeiGene
Binoj Nair
Employment: Loxo/Lilly
Stock and Other Ownership Interests: Lilly
Donald E. Tsai
Employment: Loxo at Lilly
Stock and Other Ownership Interests: Lilly, TG Therapeutics
Patents, Royalties, Other Intellectual Property: Patent on retinoic acid compounds
Travel, Accommodations, Expenses: Loxo at Lilly
Minna Balbas
Employment: Loxo
Stock and Other Ownership Interests: Lilly, ORIC Pharmaceuticals
Richard A. Walgren
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Patents, Royalties, Other Intellectual Property: Patent applicant/holder, without royalties, for therapeutic applications related to ramucirumab and merestinib (Inst)
Paolo Abada
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Chunxiao Wang
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Junjie Zhao
Employment: Loxo
Stock and Other Ownership Interests: Loxo/Lilly
Anthony R. Mato
Consulting or Advisory Role: TG Therapeutics, AbbVie/Genentech, Pharmacyclics, Adaptive Biotechnologies, Johnson & Johnson, Acerta Pharma/AstraZeneca, Loxo/Lilly, Curio/Vaniam Group, Merck, Bristol Myers Squibb/Pfizer, PerView, DAVA Oncology, BMS, Genmab, AXIS Education, PER
Research Funding: Regeneron, TG Therapeutics, Sunesis Pharmaceuticals, Loxo, AbbVie/Genentech, Pharmacyclics, Adaptive Biotechnologies, Johnson & Johnson, Acerta Pharma/AstraZeneca, DTRM, Genmab, Nurix
Nirav N. Shah
Stock and Other Ownership Interests: Tundra Targeted Therapeutics
Consulting or Advisory Role: Kite, a Gilead company, Loxo/Lilly, TG Therapeutics, Seagen, Incyte, Novartis, Juno/Bristol-Myers Sqibb, Janssen Oncology
Research Funding: Miltenyi Biotec, Loxo/Lilly, Adaptive Biotechnologies
Travel, Accommodations, Expenses: Miltenyi Biotec
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the American Society of Hematology 2022 congress, New Orleans, LA, December 12, 2022.
SUPPORT
Supported by Loxo Oncology Inc, a wholly owned subsidiary of Eli Lilly and Company.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Eli Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
AUTHOR CONTRIBUTIONS
Conception and design: Michael L. Wang, Wojciech Jurczak, Chan Y. Cheah, Donald E. Tsai
Provision of study materials or patients: Wojciech Jurczak, Pier Luigi Zinzani, Toby A. Eyre, Chan Y. Cheah, Youngil Koh, Shuo Ma, David Lewis, Joanna Rhodes, Krish Patel, Nicole Lamanna, Yucai Wang, Talha Munir, Francisco Hernandez-Ilizaliturri, Timothy S. Fenske, John F. Seymour, Andrew D. Zelenetz
Collection and assembly of data: Michael L. Wang, Wojciech Jurczak, Pier Luigi Zinzani, Toby A. Eyre, Chan Y. Cheah, Chaitra S. Ujjani, Youngil Koh, Koji Izutsu, Ian Flinn, Benoit Tessoulin, Alvaro J. Alencar, Shuo Ma, David Lewis, Ewa Lech-Maranda, Joanna Rhodes, Krish Patel, Kami Maddocks, Yucai Wang, Constantine S. Tam, Talha Munir, Hirokazu Nagai, Francisco Hernandez-Ilizaliturri, Timothy S. Fenske, John F. Seymour, Andrew D. Zelenetz, Binoj Nair, Donald E. Tsai, Minna Balbas, Richard A. Walgren, Paolo Abada, Anthony R. Mato, Nirav N. Shah
Data analysis and interpretation: Wojciech Jurczak, Pier Luigi Zinzani, Toby A. Eyre, Chan Y. Cheah, Chaitra S. Ujjani, Youngil Koh, James N. Gerson, Alvaro J. Alencar, Shuo Ma, David Lewis, Krish Patel, Kami Maddocks, Nicole Lamanna, Yucai Wang, Talha Munir, Francisco Hernandez-Ilizaliturri, Anita Kumar, Timothy S. Fenske, John F. Seymour, Andrew D. Zelenetz, Binoj Nair, Minna Balbas, Richard A. Walgren, Paolo Abada, Chunxiao Wang, Junjie Zhao, Anthony R. Mato, Nirav N. Shah
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Pirtobrutinib in Covalent Bruton Tyrosine Kinase Inhibitor Pretreated Mantle-Cell Lymphoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Michael L. Wang
Honoraria: Janssen Research & Development, Dava Oncology, AstraZeneca, Cahon, Acerta Pharma, BeiGene, Kite, a Gilead company, Moffit Cancer Center, Physicans' Education Resource, Breast-Gynecological International Cancer Society, Pharmacyclics/Janssen, Eastern Virginia Medical School, Leukemia and Lymphoma Society, Medscape, Meeting Minds Experts, OncLive/MJH Life Sciences, Practice Point Communications, Bioinvent, AbbVie, Bantam Pharmaceutical, Bristol Myers Squibb Foundation, Genmab, IDEOlogy Health, MD Education, Merck, Nurix, Oncology Specialty Group, Studio ER Congressi
Consulting or Advisory Role: AstraZeneca, Janssen Research & Development, Bioinvent, Pharmacyclics/Janssen, Kite, a Gilead company, Innocare, Oncternal Therapeutics, Genentech, BeiGene, DTRM, Miltenyi Biomedicine, VelosBio, Deciphera, Lilly, Pepromene, AbbVie, Acerta Pharma, ADC Therapeutics, Amphista Therapeutics, Be Biopharma, Leukemia and Lymphoma Society, Merck, Milken Institute, Parexel
Research Funding: AstraZeneca, Janssen Research & Development, Pharmacyclics, Kite, a Gilead company, Juno Therapeutics, BeiGene, Acerta Pharma, Oncternal Therapeutics, Bioinvent, Loxo, VelosBio, Celgene, Molecular Templates, Lilly, Innocare, Genmab, Genentech, Vincerx Pharma
Travel, Accommodations, Expenses: AstraZeneca, Celgene, Dava Oncology, Kite, a Gilead company, Physicans' Education Resource
Wojciech Jurczak
Consulting or Advisory Role: BeiGene, AstraZeneca, Lilly
Research Funding: Roche, Takeda, Janssen-Cilag, BeiGene, Morphosys, AstraZeneca, Lilly
Pier Luigi Zinzani
Consulting or Advisory Role: Celltrion, Gilead Sciences, Janssen-Cilag, Bristol Myers Squibb, Servier, Sandoz, MSD, Roche, EUSA Pharma, Kyowa Hakko Kirin, Takeda, Secura Bio, TG Therapeutics, Novartis, ADC Therapeutics, Incyte, BeiGene
Speakers' Bureau: MSD, EUSA Pharma, Novartis
Toby A. Eyre
Honoraria: Roche, Gilead Sciences, Janssen Oncology, AbbVie, AstraZeneca/MedImmune, Loxo/Lilly, Incyte, Secura Bio, Autolus Therapeutics
Consulting or Advisory Role: Roche, Kite/Gilead, AbbVie, AstraZeneca/MedImmune, Loxo, BeiGene, Incyte, Secura Bio
Research Funding: AstraZeneca/MedImmune, BeiGene
Chan Y. Cheah
Honoraria: Roche/Genentech, Janssen-Cilag, TG Therapeutics, Loxo/Lilly, AstraZeneca, Bristol Myers Squibb, Gilead Sciences, BeiGene, Novartis
Consulting or Advisory Role: Janssen-Cilag, Roche/Genentech (Inst), TG Therapeutics, Loxo/Lilly, Gilead Sciences, AstraZeneca, Bristol Myers Squibb, Ascentage Pharma, Merck
Research Funding: Roche/Genentech (Inst), Bristol Myers Squibb (Inst), AbbVie (Inst), Merck (Inst)
Travel, Accommodations, Expenses: Roche
Chaitra S. Ujjani
Honoraria: Pharmacyclics, AbbVie, Genentech, Janssen, Incyte, BeiGene, Lilly
Consulting or Advisory Role: AstraZeneca, Epizyme, Atara Biotherapeutics
Research Funding: Pharmacyclics (Inst), AbbVie (Inst), Lilly, AstraZeneca/MedImmune, Adaptive Biotechnologies, Kite, a Gilead company
Youngil Koh
Leadership: GenomeOpinion
Stock and Other Ownership Interests: Curocell
Honoraria: Janssen, Amgen, Novartis, Takeda Korea
Consulting or Advisory Role: Prothena
Koji Izutsu
Honoraria: Takeda, Chugai Pharma, Eisai, Janssen, AbbVie, Novartis, MSD, Dainippon Sumitomo Pharma, Ono Pharmaceutical, Mundipharma, HUYA Bioscience International, AstraZeneca, Bayer, Bristol Myers Squibb, Kyowa Kirin, Fujifilm, Celgene, Daiichi Sankyo, Allergan, SymBio Pharmaceuticals, Pfizer, Meiji Seika Kaisha, Nippon Kayaku, Lilly
Consulting or Advisory Role: Bayer, Celgene, AstraZeneca, Ono Pharmaceutical, Kyowa Kirin, AbbVie, Genmab, Bristol Myers Squibb, Nippon Shinyaku, Mitsubishi Tanabe Pharma
Research Funding: Eisai, Chugai Pharma
James N. Gerson
Research Funding: Loxo
Ian Flinn
Consulting or Advisory Role: AbbVie (Inst), TG Therapeutics (Inst), Kite, a Gilead company (Inst), BeiGene (Inst), Genentech (Inst), Novartis (Inst), Century Therapeutics (Inst), Hutchison MediPharma (Inst), Servier (Inst), Vincerx Pharma (Inst), Genmab (Inst), Innocare (Inst), Myeloid Therapeutics (Inst), Secura Bio (Inst), Xencor (Inst)
Research Funding: Acerta Pharma (Inst), Agios (Inst), Celgene (Inst), Constellation Pharmaceuticals (Inst), Genentech (Inst), Gilead Sciences (Inst), Incyte (Inst), Infinity Pharmaceuticals (Inst), Janssen (Inst), Kite, a Gilead company (Inst), Novartis (Inst), Pharmacyclics (Inst), Portola Pharmaceuticals (Inst), Roche (Inst), TG Therapeutics (Inst), Trillium Therapeutics (Inst), AbbVie (Inst), ArQule (Inst), BeiGene (Inst), Curis (Inst), Forma Therapeutics (Inst), Forty Seven (Inst), Merck (Inst), Pfizer (Inst), Verastem (Inst), AstraZeneca (Inst), Unum Therapeutics (Inst), MorphoSys (Inst), Seagen (Inst), IGM Biosciences (Inst), Loxo (Inst), Rhizen Pharmaceuticals (Inst), Triact Therapeutics (Inst), Bristol Myers Squibb (Inst), CALGB (Inst), CTI (Inst), Fate Therapeutics (Inst), Millennium (Inst), Tessa Therapeutics (Inst), City of Hope (Inst), CALIBR (Inst), Bio-Path Holdings, Inc (Inst), Nurix (Inst), Innocare (Inst), Myeloid Therapeutics (Inst), Epizyme (Inst), Marker Therapeutics (Inst), Step Pharma (Inst), Vincerx Pharma (Inst), 2seventy bio (Inst)
Benoit Tessoulin
Honoraria: Kite/Gilead, AbbVie
Travel, Accommodations, Expenses: Kite/Gilead, AbbVie
Alvaro J. Alencar
Consulting or Advisory Role: Kite, a Gilead company, Amgen, Karyopharm Therapeutics, Seagen, Epizyme, Janssen, BeiGene, Incyte, TG Therapeutics, Genentech, Dr Reddy's Laboratories, Lilly
Research Funding: Loxo
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1171980
Shuo Ma
Consulting or Advisory Role: Genentech/Roche, AbbVie, Pharmacyclics, Janssen Oncology, AstraZeneca, BeiGene, Bristol Myers Squibb/Celgene/Juno, TG Therapeutics
Speakers' Bureau: Pharmacyclics, Janssen Oncology, AstraZeneca, BeiGene, Lilly
Research Funding: Pharmacyclics (Inst), AbbVie (Inst), BeiGene (Inst), Loxo/Lilly (Inst), Juno/Bristol-Myers Sqibb (Inst), AstraZeneca (Inst), IgM Biosciences (Inst)
David Lewis
Consulting or Advisory Role: Janssen Oncology, Kite/Gilead, BeiGene, AbbVie
Travel, Accommodations, Expenses: Kite, a Gilead company
Joanna Rhodes
Honoraria: Dava Oncology, Curio Science, Aptitude Health
Consulting or Advisory Role: AbbVie, Verastem, Genentech, Pharmacyclics, TG Therapeutics, SeaGen, Morphosys, Janssen, BeiGene, Genmab, Epizyme
Research Funding: Loxo/Lilly (Inst), Acerta Pharma (Inst), Pharmacyclics (Inst), Oncternal Therapeutics (Inst), VelosBio (Inst), Epizyme (Inst)
Travel, Accommodations, Expenses: DAVA Pharmaceuticals, Loxo, Curio Science
Krish Patel
Consulting or Advisory Role: AstraZeneca, Genentech, BeiGene, Pharmacyclics, Bristol Myers Squibb/Celgene/Juno, Morphosys, Kite, a Gilead company, TG Therapeutics, Loxo/Lilly, AbbVie, Seagen, Epizyme, ADC Therapeutics, Caribou Biosciences, Xencor, Fate Therapeutics
Speakers' Bureau: AstraZeneca, Bristol Myers Squibb/Celgene, Kite, a Gilead company, TG Therapeutics
Research Funding: AstraZeneca (Inst), Xencor (Inst), Pharmacyclics (Inst), Curis (Inst), Bristol Myers Squibb (Inst), Celgene (Inst), MEI Pharma (Inst), Trillium Therapeutics (Inst), Kite/Gilead (Inst), Roche/Genentech (Inst), Fate Therapeutics (Inst), Takeda (Inst), Epizyme (Inst), Aptevo Therapeutics (Inst), Nurix (Inst), Loxo/Lilly (Inst)
Kami Maddocks
Honoraria: Pharmacyclics, Celgene, Seagen, MorphoSys, BMS, Karyopharm Therapeutics, Kite, a Gilead company, ADC Therapeutics, Genmab, Lilly, Genentech, Epizyme, AstraZeneca/Merck, BeiGene, Incyte, AbbVie
Research Funding: Pharmacyclics, Merck, Bristol Myers Squibb
Nicole Lamanna
Consulting or Advisory Role: Celgene, Genentech, AbbVie, ProNAi, Pharmacyclics, Juno Therapeutics, Roche, Janssen, AstraZeneca, Gilead Sciences, BeiGene, Loxo/Lilly, Bristol Myers Squibb Foundation
Research Funding: Genentech/AbbVie (Inst), AbbVie (Inst), Infinity Pharmaceuticals (Inst), Gilead Sciences (Inst), ProNAi (Inst), Beigene (Inst), AstraZeneca (Inst), Verastem (Inst), Juno Therapeutics (Inst), TG Therapeutics (Inst), Acerta Pharma/AstraZeneca (Inst), Loxo (Inst), Oncternal Therapeutics, Inc (Inst), MingSight (Inst), Octapharm (Inst)
Yucai Wang
Employment: Merck
Stock and Other Ownership Interests: Merck
Honoraria: Kite, a Gilead company (Inst)
Consulting or Advisory Role: Loxo (Inst), Incyte (Inst), Innocare (Inst), TG Therapeutics (Inst), Kite, a Gilead company (Inst), Lilly (Inst), Janssen (Inst), BeiGene (Inst)
Research Funding: InnoCare (Inst), Incyte (Inst), Novartis (Inst), Genentech (Inst), Loxo (Inst), MorphoSys (Inst), Genmab (Inst)
Constantine S. Tam
Honoraria: Janssen-Cilag, AbbVie, Novartis, Beigene, Pharmacyclics, Roche/Genentech, Loxo/Lilly
Consulting or Advisory Role: Janssen, Loxo, Roche, BeiGene, AbbVie
Research Funding: Janssen-Cilag (Inst), AbbVie (Inst), BeiGene (Inst)
Talha Munir
Consulting or Advisory Role: Janssen-Cilag, AstraZeneca, BeiGene, Sobi, Roche, AbbVie, Alexion Pharmaceuticals, Lilly
Speakers' Bureau: AbbVie, Janssen-Cilag, Gilead Sciences, Alexion Pharmaceuticals, AstraZeneca, Sobi
Travel, Accommodations, Expenses: Janssen-Cilag, AbbVie, Alexion Pharmaceuticals, AstraZeneca
Hirokazu Nagai
Honoraria: Janssen, Ono Pharmaceutical, Bristol Myers Squibb, Chugai Pharma, Takeda, Eisai, Mundipharma, AstraZeneca, Novartis, Lilly, Meiji Seika Kaisha, AbbVie, GlaxoSmithKline, Genmab, Sumitomo Pharma Oncology, CSL Behring
Research Funding: Janssen (Inst), Celgene (Inst), AbbVie (Inst), Takeda (Inst), Bristol Myers Squibb (Inst), Ono Pharmaceutical (Inst), Zenyaku Kogyo (Inst), AstraZeneca (Inst), Chugai Pharma (Inst), Solasia Pharma (Inst), Kyowa Kirin (Inst), Nippon Shinyaku (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Genmab (Inst), Lilly Japan (Inst), Regeneron (Inst), Incyte Japan (Inst), HUYA Bioscience International (Inst)
Francisco Hernandez-Ilizaliturri
Consulting or Advisory Role: Seagen, Pharmacyclics, Novartis, Kite/Gilead, Epizyme, Morphosys, BeiGene, AbbVie
Anita Kumar
Stock and Other Ownership Interests: Bridgebio
Consulting or Advisory Role: Celgene, Kite, a Gilead company, AstraZeneca/MedImmune, Janssen, Genentech, Loxo/Lilly
Research Funding: AbbVie/Genentech, Adaptive Biotechnologies, Celgene, Seagen, AstraZeneca/MedImmune, Pharmacyclics, MorphoSys/Incyte, Loxo/Lilly
Timothy S. Fenske
Stock and Other Ownership Interests: Merck
Honoraria: Adaptive Biotechnologies, AstraZeneca, BeiGene, Kite, a Gilead company, MorphoSys, Pharmacyclics, Sanofi, Seagen, Servier, TG Therapeutics
Consulting or Advisory Role: Adaptive Biotechnologies, BeiGene, MorphoSys, Pharmacyclics, Seagen, Servier, TG Therapeutics
Speakers' Bureau: AstraZeneca, BeiGene, Kite, a Gilead company, Sanofi, Seagen, TG Therapeutics
Expert Testimony: Bayer
Travel, Accommodations, Expenses: AstraZeneca, Kite, a Gilead company, Sanofi, Seagen, TG Therapeutics
John F. Seymour
Honoraria: AbbVie, Janssen, Roche, BMS, AstraZeneca, Gilead Sciences, BeiGene
Consulting or Advisory Role: AbbVie, Janssen, Roche, AstraZeneca, BMS, Gilead Sciences, TG Therapeutics, Genor BioPharma, BeiGene
Speakers' Bureau: AbbVie, Roche
Research Funding: AbbVie, Celgene, Janssen, Roche
Patents, Royalties, Other Intellectual Property: Named patent holder for venetolcax dose ramp up and combination treatment. I do not receive any royalties
Expert Testimony: Roche, TG Therapeutics
Travel, Accommodations, Expenses: AbbVie, Roche
Andrew D. Zelenetz
Honoraria: NCCN, Curio Science, OncLive/MJH Life Sciences
Consulting or Advisory Role: Genentech/Roche, Celgene, AstraZeneca, Dava Oncology, BeiGene, MEI Pharma, Kite, a Gilead company, Juno/Celgene/Bristol Myers Squibb, Sandoz, Ono Pharmaceutical
Research Funding: Genentech/Roche, MEI Pharma, BeiGene, AbbVie (Inst)
Travel, Accommodations, Expenses: Kite, a Gilead company, NCCN, BeiGene
Binoj Nair
Employment: Loxo/Lilly
Stock and Other Ownership Interests: Lilly
Donald E. Tsai
Employment: Loxo at Lilly
Stock and Other Ownership Interests: Lilly, TG Therapeutics
Patents, Royalties, Other Intellectual Property: Patent on retinoic acid compounds
Travel, Accommodations, Expenses: Loxo at Lilly
Minna Balbas
Employment: Loxo
Stock and Other Ownership Interests: Lilly, ORIC Pharmaceuticals
Richard A. Walgren
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Patents, Royalties, Other Intellectual Property: Patent applicant/holder, without royalties, for therapeutic applications related to ramucirumab and merestinib (Inst)
Paolo Abada
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Chunxiao Wang
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Junjie Zhao
Employment: Loxo
Stock and Other Ownership Interests: Loxo/Lilly
Anthony R. Mato
Consulting or Advisory Role: TG Therapeutics, AbbVie/Genentech, Pharmacyclics, Adaptive Biotechnologies, Johnson & Johnson, Acerta Pharma/AstraZeneca, Loxo/Lilly, Curio/Vaniam Group, Merck, Bristol Myers Squibb/Pfizer, PerView, DAVA Oncology, BMS, Genmab, AXIS Education, PER
Research Funding: Regeneron, TG Therapeutics, Sunesis Pharmaceuticals, Loxo, AbbVie/Genentech, Pharmacyclics, Adaptive Biotechnologies, Johnson & Johnson, Acerta Pharma/AstraZeneca, DTRM, Genmab, Nurix
Nirav N. Shah
Stock and Other Ownership Interests: Tundra Targeted Therapeutics
Consulting or Advisory Role: Kite, a Gilead company, Loxo/Lilly, TG Therapeutics, Seagen, Incyte, Novartis, Juno/Bristol-Myers Sqibb, Janssen Oncology
Research Funding: Miltenyi Biotec, Loxo/Lilly, Adaptive Biotechnologies
Travel, Accommodations, Expenses: Miltenyi Biotec
No other potential conflicts of interest were reported.
REFERENCES
- 1.Owen C, Berinstein NL, Christofides A, et al. : Review of Bruton tyrosine kinase inhibitors for the treatment of relapsed or refractory mantle cell lymphoma. Curr Oncol 26:e233-e240, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seiler T, Dreyling M: Bruton's tyrosine kinase inhibitors in B-cell lymphoma: Current experience and future perspectives. Expert Opin Investig Drugs 26:909-915, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Martin P, Maddocks K, Leonard JP, et al. : Postibrutinib outcomes in patients with mantle cell lymphoma. Blood 127:1559-1563, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Jain P, Kanagal-Shamanna R, Zhang S, et al. : Long-term outcomes and mutation profiling of patients with mantle cell lymphoma (MCL) who discontinued ibrutinib. Br J Haematol 183:578-587, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Epperla N, Hamadani M, Cashen AF, et al. : Predictive factors and outcomes for ibrutinib therapy in relapsed/refractory mantle cell lymphoma-a “real world” study. Hematol Oncol 35:528-535, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Shah B, Zhao X, Silva AS, et al. : Resistance to ibrutinib in B cell malignancies: One size does not fit all. Trends Cancer 4:197-206, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Cheah CY, Chihara D, Romaguera JE, et al. : Patients with mantle cell lymphoma failing ibrutinib are unlikely to respond to salvage chemotherapy and have poor outcomes. Ann Oncol 26:1175-1179, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Rai S, Tanizawa Y, Cai Z, et al. : Outcomes for recurrent mantle cell lymphoma post-ibrutinib therapy: A retrospective cohort study from a Japanese Administrative Database. Adv Ther 39:4792-4807, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hess G, Dreyling M, Oberic L, et al. : Real-world experience among patients with relapsed/refractory mantle cell lymphoma after Bruton tyrosine kinase inhibitor failure in Europe: The SCHOLAR-2 retrospective chart review study. Br J Haematol 10.1111/bjh.18519 [epub ahead of print on October 18, 2022] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M, Munoz J, Goy A, et al. : KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med 382:1331-1342, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma S, Galanina N, Guo A, et al. : Identification of a structurally novel BTK mutation that drives ibrutinib resistance in CLL. Oncotarget 7:68833-68841, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaffer AL, 3rd, Phelan JD, Wang JQ, et al. : Overcoming acquired epigenetic resistance to BTK inhibitors. Blood Cancer Discov 2:630-647, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiron D, Di Liberto M, Martin P, et al. : Cell-cycle reprogramming for PI3K inhibition overrides a relapse-specific C481S BTK mutation revealed by longitudinal functional genomics in mantle cell lymphoma. Cancer Discov 4:1022-1035, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson ER, Nguyen T, Kankanige Y, et al. : Single-cell sequencing demonstrates complex resistance landscape in CLL and MCL treated with BTK and BCL2 inhibitors. Blood Adv 6:503-508, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tiemann M, Schrader C, Klapper W, et al. : Histopathology, cell proliferation indices and clinical outcome in 304 patients with mantle cell lymphoma (MCL): A clinicopathological study from the European MCL Network. Br J Haematol 131:29-38, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Wang M, Rule S, Zinzani PL, et al. : Durable response with single-agent acalabrutinib in patients with relapsed or refractory mantle cell lymphoma. Leukemia 33:2762-2766, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Tam CS, Opat S, Simpson D, et al. : Zanubrutinib for the treatment of relapsed or refractory mantle cell lymphoma. Blood Adv 5:2577-2585, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rule S, Dreyling M, Goy A, et al. : Ibrutinib for the treatment of relapsed/refractory mantle cell lymphoma: Extended 3.5-year follow up from a pooled analysis. Haematologica 104:e211-e214, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mato AR, Shah NN, Jurczak W, et al. : Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): A phase 1/2 study. Lancet 397:892-901, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheson BD, Fisher RI, Barrington SF, et al. : Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol 32:3059-3067, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Heertum RL, Scarimbolo R, Wolodzko JG, et al. : Lugano 2014 criteria for assessing FDG-PET/CT in lymphoma: An operational approach for clinical trials. Drug Des Devel Ther 11:1719-1728, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hess G, Smith SM, Berkenblit A, et al. : Temsirolimus in mantle cell lymphoma and other non-Hodgkin lymphoma subtypes. Semin Oncol 36:S37-S45, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Fisher RI, Bernstein SH, Kahl BS, et al. : Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol 24:4867-4874, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Goy A, Kalayoglu Besisik S, Drach J, et al. : Longer-term follow-up and outcome by tumour cell proliferation rate (Ki-67) in patients with relapsed/refractory mantle cell lymphoma treated with lenalidomide on MCL-001(EMERGE) pivotal trial. Br J Haematol 170:496-503, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eyre TA, Walter HS, Iyengar S, et al. : Efficacy of venetoclax monotherapy in patients with relapsed, refractory mantle cell lymphoma after Bruton tyrosine kinase inhibitor therapy. Haematologica 104:e68-e71, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang ML, Shah NN, Jurczak W, et al. : Efficacy of pirtobrutinib in covalent BTK-inhibitor pre-treated relapsed/refractory mantle cell lymphoma: Additional patients and extended follow-up from the phase 1/2 BRUIN study. Blood 140:9368-9372, 2022. (suppl 1) [Google Scholar]
- 27.Byrd JC, Hillmen P, Ghia P, et al. : Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: Results of the first randomized phase III trial. J Clin Oncol 39:3441-3452, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibrutinib [package insert]. San Francisco, CA, Pharmacyclics, 2018
- 29.Zanubrutinib [package insert]. San Mateo, CA, BeiGene USA Inc, 2019
- 30.Sharman JP, Egyed M, Jurczak W, et al. : Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzumab for treatment-naive chronic lymphocytic leukaemia (ELEVATE-TN): A randomised, controlled, phase 3 trial. Lancet 395:1278-1291, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Reilly MA, Sanderson R, Wilson W, et al. : Brexucabtagene autoleucel for relapsed/refractory mantle cell lymphoma: Real-world outcomes in the United Kingdom. Blood 140 (Suppl 1):7519–7521, 2022
- 32.Jain T, Bar M, Kansagra AJ, et al. : Use of chimeric antigen receptor T cell therapy in clinical Practice for relapsed/refractory aggressive B cell non-Hodgkin lymphoma: An expert panel opinion from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transpl 25:2305-2321, 2019 [DOI] [PubMed] [Google Scholar]
- 33.Woyach JA, Flinn IW, Awan FT, et al. : Preliminary efficacy and safety of MK-1026, a non-covalent inhibitor of wild-type and C481S mutated Bruton tyrosine kinase, in B-cell malignancies: A phase 2 dose expansion study. Blood 138, 2021. (suppl 1; abstr 392) [Google Scholar]
- 34.Eyre TA, Shah NN, Dreyling M, et al. : BRUIN MCL-321: Phase III study of pirtobrutinib versus investigator choice of BTK inhibitor in BTK inhibitor naïve mantle cell lymphoma. Future Oncol 18:3961-3969, 2022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Eli Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.