Abstract
PURPOSE
To determine whether addition of external beam radiation therapy (EBRT) to brachytherapy (BT) (COMBO) compared with BT alone would improve 5-year freedom from progression (FFP) in intermediate-risk prostate cancer.
METHODS
Men with prostate cancer stage cT1c-T2bN0M0, Gleason Score (GS) 2-6 and prostate-specific antigen (PSA) 10-20 or GS 7, and PSA < 10 were eligible. The COMBO arm was EBRT (45 Gy in 25 fractions) to prostate and seminal vesicles followed by BT prostate boost (110 Gy if 125-Iodine, 100 Gy if 103-Pd). BT arm was delivered to prostate only (145 Gy if 125-Iodine, 125 Gy if 103-Pd). The primary end point was FFP: PSA failure (American Society for Therapeutic Radiology and Oncology [ASTRO] or Phoenix definitions), local failure, distant failure, or death.
RESULTS
Five hundred eighty-eight men were randomly assigned; 579 were eligible: 287 and 292 in COMBO and BT arms, respectively. The median age was 67 years; 89.1% had PSA < 10 ng/mL, 89.1% had GS 7, and 66.7% had T1 disease. There were no differences in FFP. The 5-year FFP-ASTRO was 85.6% (95% CI, 81.4 to 89.7) with COMBO compared with 82.7% (95% CI, 78.3 to 87.1) with BT (odds ratio [OR], 0.80; 95% CI, 0.51 to 1.26; Greenwood T P = .18). The 5-year FFP-Phoenix was 88.0% (95% CI, 84.2 to 91.9) with COMBO compared with 85.5% (95% CI, 81.3 to 89.6) with BT (OR, 0.80; 95% CI, 0.49 to 1.30; Greenwood T P = .19). There were no differences in the rates of genitourinary (GU) or GI acute toxicities. The 5-year cumulative incidence for late GU/GI grade 2+ toxicity is 42.8% (95% CI, 37.0 to 48.6) for COMBO compared with 25.8% (95% CI, 20.9 to 31.0) for BT (P < .0001). The 5-year cumulative incidence for late GU/GI grade 3+ toxicity is 8.2% (95% CI, 5.4 to 11.8) compared with 3.8% (95% CI, 2.0 to 6.5; P = .006).
CONCLUSION
Compared with BT, COMBO did not improve FFP for prostate cancer but caused greater toxicity. BT alone can be considered as a standard treatment for men with intermediate-risk prostate cancer.
Phase 3 NRG 0232 trial shows no benefit to adding EBRT to brachytherapy in localized prostate cancer.
INTRODUCTION
Prostate brachytherapy (BT) is a curative option for men with localized prostate cancer. Most early BT series included predominantly low-risk patients with some clinicians favoring the addition of external beam radiation therapy (EBRT) to BT (COMBO) for patients with adverse clinical risk features. It was felt that the addition of external radiation would more effectively treat subclinical extraprostatic extension, seminal vesicle invasion, or regional lymph node metastases compared with BT alone.1-4 In 1999, the American Brachytherapy Society recommended BT monotherapy for low-risk cancers, COMBO for high-risk cancers, and individualized decision making for patients with intermediate-risk cancers.5 A Patterns of Care survey of BT practitioners in the United States demonstrated a majority favoring BT monotherapy for most patients with intermediate-risk disease; however, as risk factors increased, a larger proportion of them would include EBRT.6
CONTEXT
Key Objective
Does combined external beam radiation therapy (EBRT) and brachytherapy (BT) result in better freedom from progression (FFP) compared with BT alone in patients with intermediate-risk prostate cancer?
Knowledge Generated
There was no improvement in FFP when EBRT was added to BT for men with intermediate-risk prostate cancer. There is more toxicity associated with the combination. BT alone can be considered a standard treatment option for patients with intermediate-risk prostate cancer.
Relevance (M.A. Carducci)
-
This important study conducted through the National Clinical Trials Network establishes level 1 evidence for BT as a standard for intermediate-risk prostate cancer.*
*Relevance section written by JCO Associate Editor Michael A. Carducci, MD, FACP, FASCO.
In 2002, the Radiation Therapy Oncology Group (RTOG, now NRG Oncology) launched a phase III trial to determine if the addition of EBRT to BT for patients with intermediate-risk prostate cancer would improve outcomes, using freedom from progression (FFP) as the primary end point. Secondary end points included biochemical failure (BF), disease-specific mortality (DSM), local progression (LP), distant metastases (DM), survival, toxicity, and quality of life (reported separately).
METHODS
Trial Design and Participants
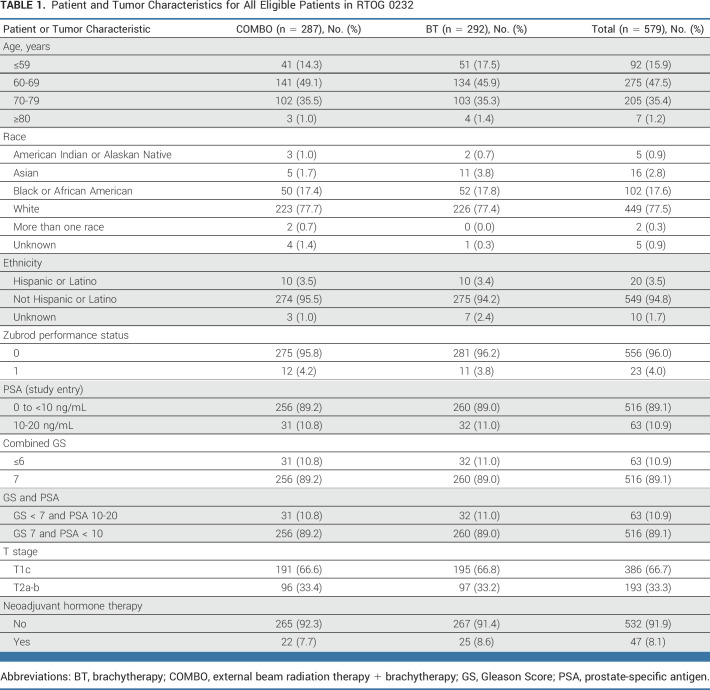
This randomized clinical trial compared COMBO with BT alone. Men with histologically confirmed prostate cancer, Zubrod performance scale of 0-1, clinical stage T1c-T2b with either a Gleason score (GS) of <7 and prostate-specific antigen (PSA) of 10-20 ng/mL or GS of 7, and PSA of <10 ng/mL were eligible. Neoadjuvant androgen deprivation therapy was allowed if started 2-6 months before registration. Patients had minimal urinary voiding symptoms as measured by the American Urological Association obstructive symptom score <15 and a prostate gland volume of ≤60 mL. Before patient enrollment, evaluation included history and physical and a serum PSA (<60 days before registration). The International Society of Urological Pathology (ISUP) now recognizes distinct behaviors of cancers with predominant Gleason patterns 3, 4, or 5. On the basis of institutional pathologist Gleason scoring, post hoc assignments of Gleason grade groups (GG) were done (3 + 3 = GG1, 3 + 4 = GG2, and 4 + 3 = GG3).
Participants were recruited from the members of NRG Oncology after institutional review board approval at each center. All participants provided written informed consent before registration and were to receive protocol-specified care and follow-up at a member site.
Random Assignment
Participants were stratified by clinical stage (T1c v T2a-T2b), GS (≤6 v 7), PSA at diagnosis (<10 v 10-20 ng/mL), and neoadjuvant hormone therapy use (no v yes) and then randomly assigned centrally 1:1 COMBO versus BT alone. A random assignment scheme described by Zelen,7 using key of two, was used to balance patient factors other than institution.
Treatment
Allowable EBRT techniques included two-dimensional (2D) radiation techniques (such as four field box), three-dimensional (3D) radiation therapy (RT), or intensity-modulated radiation therapy (IMRT). The clinical target volume (CTV) included the prostate and seminal vesicles. A planning target volume (PTV) margin of at least 1 cm was required for 2D or 3D techniques and 0.5-1.0 cm for IMRT. At a minimum, weekly verification of treatment localization was required. The minimum dose to 98% of the PTV was 45 Gy; the minimum dose encompassing the CTV was 45 Gy. The maximum dose to all but the hottest 2% of the PTV was <48.1 Gy (no variation), <49.5 Gy (minor variation), or >49.5 Gy (major variation). No normal tissue constraints were defined by the Protocol (online only). Treatments were delivered at 1.8 Gy per fraction, five times per week.
Transperineal BT was performed 2-4 weeks after completion of EBRT (COMBO) or within 4 weeks of study entry (BT). The BT CTV was the prostate determined at a transrectal ultrasound planning volume study. A PTV margin of 2-3 mm was added anteriorly and laterally, 5 mm superiorly and inferiorly, and no margin posteriorly. An evaluation target volume was the prostate gland defined on a postimplant computed tomography (CT) scan performed 3-5 weeks following the implant. The prescription doses for I-125 were 145 Gy and 110 Gy for monotherapy and boost implants, respectively. For Pd-103, these were 125 Gy and 100 Gy, respectively. The prescription minimum peripheral dose was intended to be delivered to the CTV and was the reference dose for the implant.
All participating centers had treatment techniques reviewed and credentialed by the Radiological Physics Center and Image-Guided Therapy QA Center (now the Imaging and Radiation Oncology Core).
Patient Assessment and End Points
Patients were seen weekly during their EBRT (COMBO); 3-5 weeks postimplant; then 4, 6, 9, and 12 months post-treatment start for year 1; every 6 months for 4 years; and then annually. Following treatment, patients underwent interval history, physical examination with assessment of specific genitourinary (GU) and GI morbidity, and PSA at each visit. Acute (≤180 days of treatment start) and late RT toxicities (>180 days of treatment start) were graded using the National Cancer Institute common toxicity criteria v2.0 and the RTOG/European Organisation for Research and Treatment of Cancer Late Radiation Morbidity Scoring Scheme, respectively.
Failure for the primary end point, FFP-American Society for Therapeutic Radiology and Oncology (ASTRO), was defined as the first occurrence of an ASTRO BF,8 clinical failure (LP or DM), or death due to any cause (overall survival [OS]). FFP-Phoenix used the same FFP definition but substituted the Phoenix definition (nadir PSA + 2 ng/mL) of BF.9 In the case of PSA failure, it was recommended that the site of failure be ascertained before instituting further therapy, including bone scan and pelvic CT. Rebiopsy of the prostate was recommended to determine local failure in the event of clinical LP or BF. Clinical criteria for LP included progression (increase in palpable abnormality) at any time, failure of regression of the palpable tumor by 2 years, or redevelopment of a palpable abnormality after complete disappearance of previous abnormalities. DM was determined by radiographic criteria and/or tissue confirmation. DSM failures included death due to prostate cancer or complications of treatment or death associated with (1) clinical progression after initiation of salvage therapy, (2) a rise in the PSA on at least two consecutive occasions that occurred during or after salvage therapy, or (3) disease progression in the absence of any antitumor therapy. All end points were measured from the date of random assignment to the date of first failure or last follow-up for censored patients.
Statistical Methods
The sample size was based on the hypothesis that the COMBO treatment would improve 5-year FFP by at least 10% compared with BT. Assuming a 5-year FFP of 80% for the BT arm, with 90% statistical power, one-sided α = .025, using a two-sample test of proportions, and five interim analyses, 532 patients were required. Allowing for ineligible/lack of data ≤10%, the targeted sample size was 586 patients. Efficacy testing used Haybittle-Peto,10,11 for interims at α = .001 and the final analysis at α = .02 to preserve an overall α = .025. Futility was tested by reversing the null and alternative hypotheses at α = .0001. Patients were analyzed per random assignment.
For the primary end point of FFP-ASTRO (and for FFP-Phoenix), 5-year FFP rates were estimated using the Kaplan-Meier12 method and treatment arms compared with Greenwood T test statistic using standard errors estimated by the Greenwood method. Odds ratios (ORs) and multivariable analyses (MVA) for 5-year FFP were analyzed using logistic regression. Additionally, the full distributions of FFP were also compared between treatment arms using the log-rank test,13 and corresponding hazard ratio (HR) and MVA were done using Cox proportional hazards regression model.14 In all univariate and MVA, treatment was coded such that an OR or HR < 1 indicates a decrease in the odds/hazard of having a failure for the COMBO arm.
The Kaplan-Meier12 approach was used to estimate OS treatment arms compared with the log-rank test,13 and corresponding HR and MVA were done using Cox proportional hazards regression model.14 Cumulative incidence15 was used to estimate ASTRO-BF/Phoenix-BF/LP/DM/DSM; treatment arms were compared using the log-rank test; and the cause-specific hazard rate16 (the instantaneous rate of cause-specific mortality in the presence of competing failure types as a function of time) was used to obtain HR. These secondary end points were all tested at a one-sided α = .025.
Logistic regression was used to evaluate differences between treatment arms for acute grade 2+ and grade 3+ toxicity. Time to late grade 2+ and grade 3+ toxicities were estimated using the cumulative incidence method,13 and treatment arms were compared using the log-rank test11 at a one-sided α = .025. A multivariable Cox12 regression model was used to compare the treatment differences of time to late toxicity. Age (<70 v ≥70), race (White v Black or African American/other), T stage (T1c v T2a-b), Gleason/PSA groups (GS < 7/PSA 10-20 ng/mL v GS7/PSA < 10 ng/mL), and previous neoadjuvant hormonal therapy (no v yes) were adjusted for in all MVA. Data collection was terminated on December 22, 2022. Analyses were based on the data received at NRG Oncology Statistical and Data Management Center through the termination date and performed using SAS statistical software (version 9.4; SAS Institute Inc, Cary, NC).
RESULTS
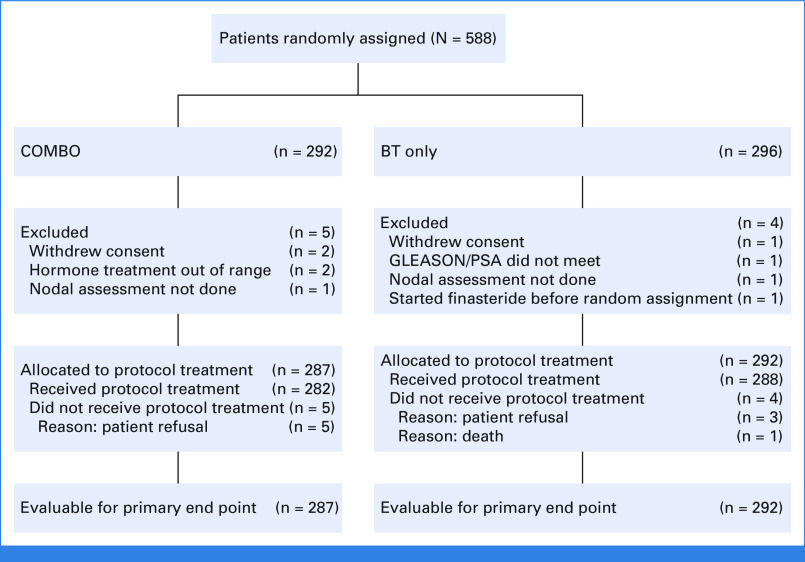
This study opened on June 11, 2003, and closed on February 8, 2012, with 588 patients randomly assigned. Nine patients were ineligible/withdrew consent with exclusion reasons given in Figure 1. Patient and tumor characteristics are summarized in Table 1. The median (min-max) age is 67 years (40-84). Most patients had study entry PSA < 10 ng/mL (89.1%) and no neoadjuvant hormone therapy (91.9%). For the COMBO arm, the EBRT modality distribution was 3D—120 (42%), IMRT—158 (55%), and no RT—9 (3%). Although it was allowed, no patients were treated with 2D techniques. Protocol treatment compliance was reviewed for all cases. The COMBO and BT arms were scored per protocol/acceptable variation for tumor volume contouring, tumor volume dose volume analysis, and organs at risk contouring, respectively, as follows: 93.4% and 93.8%, 96.2% and 97.6%, and 95.5% and 97.6%. The BT dose/volume data were similar between the treatment arms, with the exception of a higher mean 100% of prescription dose (Gy) to the rectum for the BT arm (see Data Supplement [Table S7, online only]).
FIG 1.
CONSORT diagram. BT, brachytherapy; COMBO, external beam radiation therapy + brachytherapy; PSA, prostate-specific antigen.
TABLE 1.
Patient and Tumor Characteristics for All Eligible Patients in RTOG 0232
Interim Analyses
No efficacy/futility boundaries were crossed for the FFP primary end point at any of the first four interim analyses. At the fifth interim analysis, although the futility boundary was not crossed, the 5-year FFP rates for the COMBO arm and the full distribution FFP curve were below the BT arm (5-year FFP: 84.5% for COMBO and 85.6% for BT), and the NRG Data Monitoring Committee recommended reporting the trial.
Outcomes
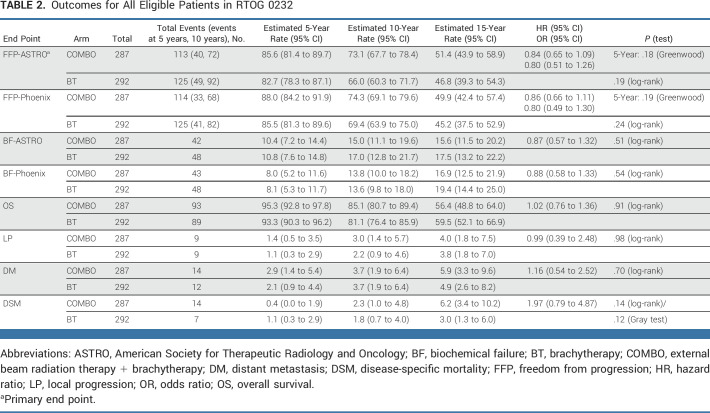
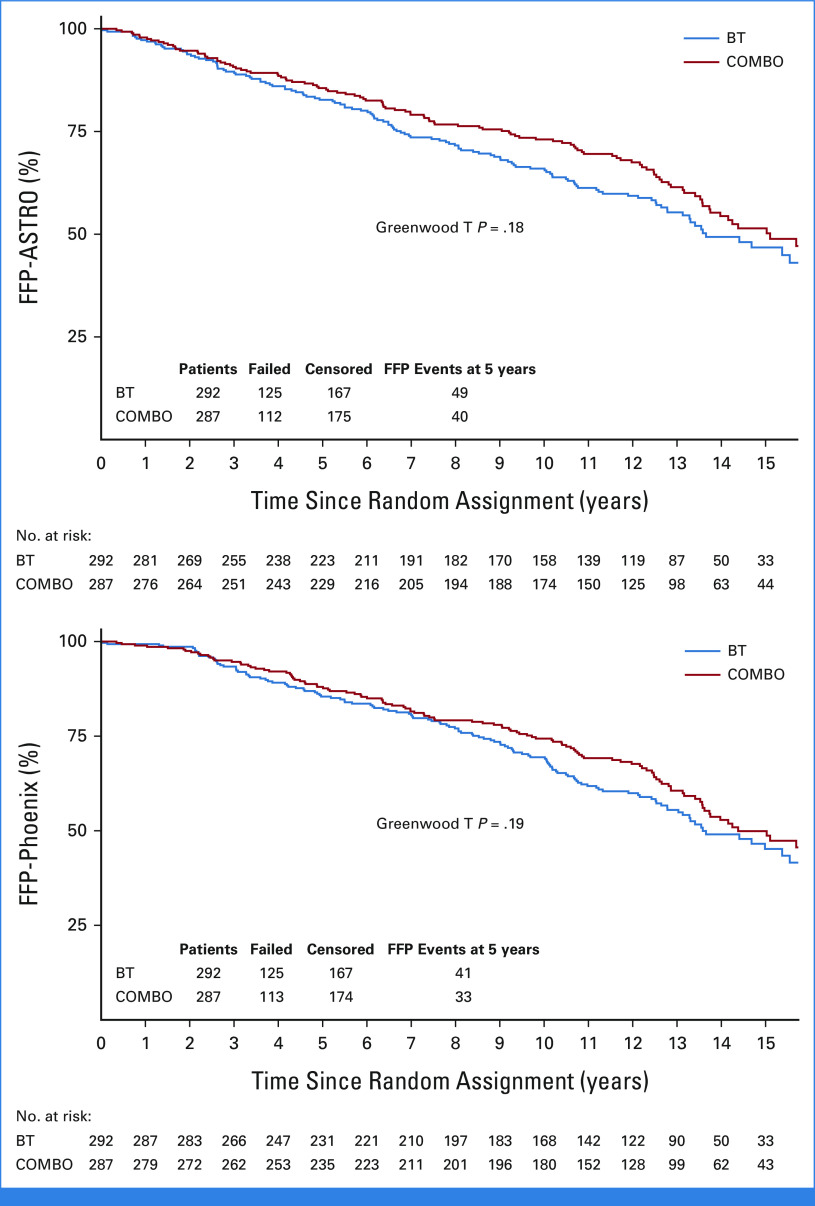
On the basis of the updated data reported here, the median (min-max) follow-up for all patients was 12.1 (0.02-18.2) years. The primary end point of 5-year FFP-ASTRO was 85.6% (95% CI, 81.4 to 89.7) with COMBO compared with 82.7% (95% CI, 78.3 to 87.1) with BT (OR, 0.80 [95% CI, 0.51 to 1.26]; HR, 0.84 [95% CI, 0.65 to 1.09]; Greenwood T P = .18; Table 2, Fig 2, Data Supplement [Fig S1]). Censoring for this end point was minimal, with the same percent on each treatment arm. The 5-year FFP-Phoenix was 88.0% (95% CI, 84.2 to 91.9) with COMBO compared with 85.5% (95% CI, 81.3 to 89.6) with BT (OR, 0.80 [95% CI, 0.49 to 1.30]; HR, 0.86; 95% CI, 0.66 to 1.11; Greenwood T P = .19; Table 2, Fig 2, Data Supplement [Fig S1]). There were no statistically significant differences between arms for 5-year FFP by either ASTRO or Phoenix criteria on multivariable logistic regression analysis (Data Supplement [Table s1]). The 5-year incidence of BF-ASTRO was 10.4% (95% CI, 7.2 to 14.4) with COMBO compared with 10.8% (95% CI, 7.6 to 14.8) with BT (HR, 0.87; 0.57 to 1.32; P = .51; Table 2, Data Supplement [Fig S2]). The 5-year incidence of BF-Phoenix was 8.0% (95% CI, 5.2 to 11.6) with COMBO compared with 8.1% (95% CI, 5.3 to 11.7) with BT (HR, 0.88; 0.58 to 1.33; P = .54; Table 2, Data Supplement [Fig S2]). The median PSA at 5 years for both groups was 0.1 ng/mL (Data Supplement [Table S2]). On MVA, neither D90% nor V100% of the BT was significantly associated with BF by either the ASTRO or the Phoenix definition.
TABLE 2.
Outcomes for All Eligible Patients in RTOG 0232
FIG 2.
FFP (ASTRO) and FFP (Phoenix). ASTRO, American Society for Therapeutic Radiology and Oncology; BT, brachytherapy; COMBO, external beam radiation therapy + brachytherapy; FFP, freedom from progression.
There were 155 deaths, and the 5-year OS was 95.3% (95% CI, 92.8 to 97.8) with COMBO compared with 93.3% (95% CI, 90.3 to 96.2) with BT (HR, 1.02; 95% CI, 0.76 to 1.36; P = .91; Table 2). There were no significant differences in time to LP, DM, nor DSM (Table 2, Data Supplement [Figs S3 and S4]). Details for all efficacy end points, including 10- and 15-year estimates, are presented in Table 2. Even with longer follow-up, no significant differences in these end points were identified.
A post hoc comparison of outcomes by the 2016 ISUP grading criteria was conducted according to study random assignment for patients with GSs 3 + 4 (GG2, n = 413) or 4 + 3 (GG3, n = 103). Distributions of patient/tumor characteristics and treatment assignment were similar across GS groups (Data Supplement [Table S3]). There were few (n = 63) patients with GG1 to compare outcomes. There were no statistically significant differences in the 5-year FFP-ASTRO by treatment in GG2 or GG3 patients. In GG2 patients, the 5-year FFP-ASTRO was 89.4% (95% CI, 85.1 to 93.7) with COMBO compared with 85.3% (95% CI, 80.5 to 90.2) with BT alone (OR, 0.68; 95% CI, 0.37 to 1.23; 5-year Greenwood T P = .11; Data Supplement [Fig S5]). In patients with GG3, the 5-year FFP-ASTRO was 77.0% (95% CI, 65.0 to 89.0) with COMBO compared with 77.3% (95% CI, 65.4 to 89.2) with BT alone (OR, 1.03; 0.40 to 2.63; 5-year Greenwood T P = .51; Data Supplement [Fig S5]).
Adverse Events
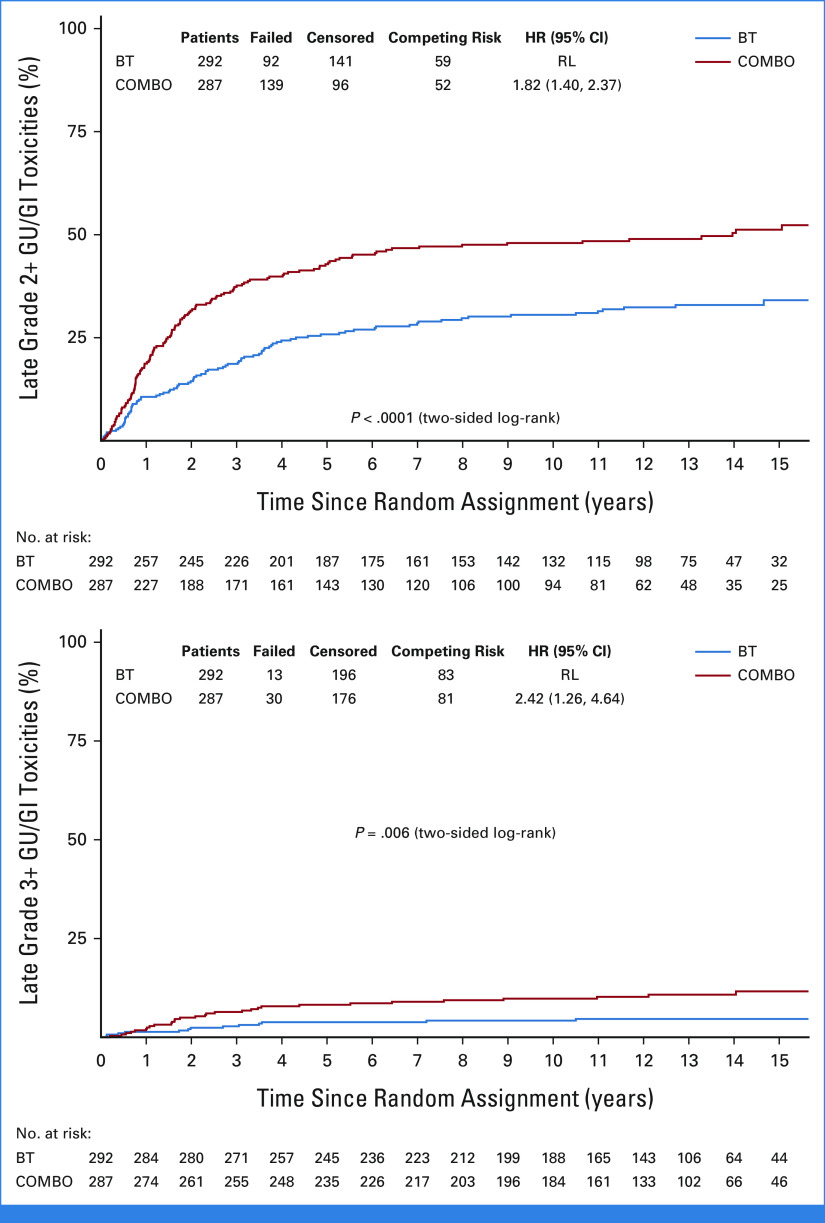
There were no significant differences in frequency of acute GI, GU, combined GI/GU, or overall toxicities (Data Supplement [Table S4]). There were significantly higher rates of late toxicities with COMBO (Data Supplement [Tables S5 and S6]). For time to late grade 2+ or 3+ GU/GI toxicities, the COMBO arm has statistically significantly higher toxicity than the BT-only arm (Fig 3). The 5- and 10-year cumulative incidence for late grade 2+ GU/GI was 42.8% (95% CI, 37.0 to 48.6) and 48.0% (95% CI, 41.9 to 53.8) for COMBO arm compared with 25.8% (95% CI, 20.9 to 31.0) and 30.6% (95% CI, 25.2 to 36.0) for the BT arm (log-rank P < .0001, Fig 3). The 5- and 10-year cumulative incidence for late grade 3+ GU/GI was 8.2% (95% CI, 5.4 to 11.8) and 9.8% (95% CI, 6.6 to 13.7) for COMBO arm compared with 3.8% (95% CI, 2.0 to 6.5) and 4.2% (95% CI, 2.3 to 7.0) for the BT arm (log-rank P = .006, Fig 3). On MVA, patients on the COMBO arm were 2.4 times more likely to have a late grade 3+ GU/GI toxicity than patients on the BT arm after adjusting for age, previous hormones, Gleason/PSA groupings, and T stage (HR, 2.41; 95% CI, 1.26 to 4.63; P = .008; Data Supplement [Table S6]). GU late effects were more common than GI late effects, but in both circumstances, grade 2+ effects were significantly worse in the COMBO arm (Data Supplement [Figs S6 and S7]). Further investigation into the COMBO arm, late grade 2+ GU/GI toxicity by RT modality showed 5- and 10-year cumulative incidence of 54.5% (95% CI, 44.9 to 63.1) and 58.1% (95% CI, 48.5 to 66.5) for IMRT and 34.9% (95% CI, 27.5 to 42.4) and 41.4% (95% CI, 33.5 to 49.1) for 3D conformal external beam radiation therapy. On MVA, patients receiving IMRT were associated with higher incidence of late grade 2+ GU/GI toxicity (HR, 1.56 [95% CI, 1.11 to 2.19]; P = .01). Neither D90% nor V100% of the BT was significantly associated with time to any late grade 2+ or any late grade 3+ toxicity. Likewise, BT V150% was not significantly associated with time to late grade 2+ GU toxicity. There were few late grade 3+ GU toxicities to perform a MVA. Finally, BT V100% and V100 Gy to the rectum were not significantly associated with time to late grade 2+ GI toxicity. There were few late grade 3+ GI toxicities to perform a MVA.
FIG 3.
Time to late GU/GI toxicities. BT, brachytherapy; COMBO, external beam radiation therapy + brachytherapy; GU, genitourinary; HR, hazard ratio; RL, reference level.
DISCUSSION
It has been theorized that LP of prostate cancer leads to subsequent development of DM and worse survival.17-19 Several approaches to intensify local RT by dose escalation using various EBRT techniques or by a combination of EBRT techniques with a BT boost have been tested.20-22 The results of this trial demonstrate that COMBO does not improve outcomes for men with intermediate-risk prostate cancer. Not only was the primary end point of an improvement in FFP not met but also the secondary end points of LP, DM, and disease-specific mortality were unaffected.
At least three prospective studies in higher risk prostate cancer have suggested that COMBO improved local tumor control and better disease-free survival over EBRT alone.21-23 In two of the trials, BT was delivered with high dose rate techniques, and in one trial, it was delivered with permanent low dose rate seed implants. The patient population of these three trials included men with higher risk disease than NRG/RTOG 0232. In these circumstances, the benefits of wide field RT would include the regions of subclinical disease not encompassed by the narrow radiation treatment volumes of BT implants. Two of those trials also included androgen deprivation therapy ranging from 6 to 36 months for patients with intermediate- to high-risk disease. So, while COMBO improved results over EBRT alone, a similar benefit was not established with COMBO over BT in this trial's intermediate-risk population.
While acute side effects were not different between the treatment arms in this study, there were considerably more late effects from the COMBO compared with BT. Both urinary and bowel late grade 2+ effects were more common with the COMBO. There were more grade 3+ GU late effects but similar rates of grade 3+ GI late effects. This finding is similar to that reported in the ASCENDE-RT trial with a greater degree of late toxicities seen with COMBO compared with EBRT alone.21
Intermediate-risk prostate cancer represents a heterogeneous group of patients with variable risks of seminal vesicle invasion, extraprostatic extension, or lymph node metastases. Patients with a predominant Gleason pattern 4, at least 50% biopsy cores involved, or multiple intermediate-risk factors (clinical stage T2b–c, PSA 10-20, or GS 7) have worse cancer control outcomes.24 The ISUP now recognizes that GS 7 consists of two distinct prognostic groups: grade group 2 (GG2, formerly 3 + 4) and grade group 3 (GG3, formerly 4 + 3).25 Furthermore, the National Comprehensive Cancer Network categorizes patients with GG2 as favorable and GG3 as unfavorable intermediate risk, respectively.26 For NRG/RTOG 0232, outcomes were compared by study random assignment for each group and did not find outcomes by study arm impacted. This could be due to the fact that centralized pathology review was not conducted, percent positive core tissue information was not available, or other unaccounted risk factors were not considered. Increasingly, additional prognostic features, such as tumor genomic classification, are being used to better stratify patient risk.27 Ancillary analyses on the basis of these types of prognostic stratification may be pursued.
The EBRT CTV for this trial was limited to the prostate and seminal vesicles. In patients with unfavorable intermediate risk, their risk of lymph node metastases increases as the number of unfavorable features increases.28 Emerging data support the use of elective lymph node irradiation when patients have a significant risk of lymph node involvement.29-31 Failure to cover the pelvic lymph nodes in patients with occult nodal metastases may have contributed to a lack of benefit with EBRT.
The patient population studied represents a heterogenous group of patients with risks of pelvic lymph node involvement ranging from <5% to as high as 30%. Modern risk categorization and patient selection now incorporate ISUP Gleason grade group, the volume of cancer on core biopsies, multiparametric magnetic resonance imaging, and genomic classification. These factors play a role in patient prognosis and could influence outcome by study random assignment. Future studies should include these features for the determination of study eligibility and/or stratification. Some EBRT (no normal tissue constraints and lack of image guidance) allowed in this trial may not reflect contemporary methods and may have contributed to differences in toxicity.
In conclusion, the addition of EBRT to BT for the intermediate-risk population of patients enrolled on this trial did not improve any of the cancer control outcomes. The COMBO was associated with higher rates of late grade 2+ GU and GI toxicity and late grade 3+ GU toxicity. BT alone can be considered a standard of care for men with intermediate-risk prostate cancer.
Jeff M. Michalski
Open Payments Link: https://openpaymentsdata.cms.gov/physician/221723
Bradley R. Prestidge
Consulting or Advisory Role: 2nd.MD
Mahul Amin
Employment: Laborp of America
Leadership: Kidney Cancer Association
Stock and Other Ownership Interests: CORE Science Solutions, CellMax Life, Precipio, Karkinos Health, Morphle, Pathpresenter, IBEX Medical Analytics
Honoraria: Genomic Health
Consulting or Advisory Role: Urogen pharma, Advanced Clinical
Juanita M. Crook
Consulting or Advisory Role: Concure Oncology Breast Microseed, Tersera, Astellas Pharma, Tolmar
Charles N. Catton
Honoraria: AbbVie, Bayer, Tersera, Knight Therapeutics
Adam Raben
Honoraria: Bristol Myers Squibb
Consulting or Advisory Role: Castle Biosciences
Speakers' Bureau: Bristol Myers Squibb
Travel, Accommodations, Expenses: Bristol Myers Squibb
Walter Bosch
Patents, Royalties, Other Intellectual Property: Licensing of Radiation Oncology workflow management software developed at Washington University
David C. Beyer
Stock and Other Ownership Interests: Videra Surgical
Honoraria: Videra Surgical
Steven J. Frank
Leadership: C4 Imaging, National Comprehensive Cancer Network
Stock and Other Ownership Interests: C4 Imaging
Honoraria: IBA
Consulting or Advisory Role: IBA
Research Funding: Hitachi, Affirmed Pharma
Patents, Royalties, Other Intellectual Property: I have developed patents at the UT MD Anderson Cancer Center. These patents have been licensed to C4 Imaging
Travel, Accommodations, Expenses: National Comprehensive Cancer Network, Boston Scientific
Michael A. Papagikos
Stock and Other Ownership Interests: Pfizer, Bayer, CVS Health, HCA Healthcare, Johnson & Johnson/Janssen, Novartis, UnitedHealthcare
Other Relationship: Boston Scientific
Open Payments Link: https://openpaymentsdata.cms.gov/physician/310147
Mack Roach
Honoraria: Merck, Debioscience, UniBanco Brasil, Pfizer, NYU Long Island School of Medicine/NYU Langone Health, Janssen Oncology
Consulting or Advisory Role: Accuray, Merck, Myovant Sciences
Patents, Royalties, Other Intellectual Property: UptoDate (ongoing)
Travel, Accommodations, Expenses: UniBanco Brasil
Uncompensated Relationships: Janssen Oncology
Howard M. Sandler
Consulting or Advisory Role: Janssen
Other Relationship: Caribou Publishing
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the American Society for Radiation Oncology, Boston, MA, September 26, 2016.
SUPPORT
Supported by Grant Nos. UG1CA189867 (NCORP), U10CA180868 (NRG Oncology Operations), U10CA180822 (NRG Oncology SDMC), and U24CA180803 (IROC) from the National Cancer Institute (NCI).
CLINICAL TRIAL INFORMATION
NRG/RTOG 0232 NCT00063882
DATA SHARING STATEMENT
Per NCI requirements, the data from this article will be submitted to the National Cancer Institute NCTN/NCORP data archive (https://nctn-data-archive.nci.nih.gov/) no later than 6 months after publication. After the required NCI reviews are completed, it will be released and available in the data archive for data sharing proposals.
AUTHOR CONTRIBUTIONS
Conception and design: Jeff M. Michalski, Bradley R. Prestidge, Martin G. Sanda, Mahul Amin, William S. Bice, Seth A. Rosenthal, Mack Roach, Howard M. Sandler
Administrative support: Jennifer Moughan
Provision of study materials or patients: Jeff M. Michalski, Bradley R. Prestidge, William S. Bice, Geoffrey S. Ibbott, Juanita M. Crook, Charles N. Catton, Adam Raben, David C. Beyer, Michael A. Papagikos, Mack Roach
Collection and assembly of data: Jeff M. Michalski, Kathryn A. Winter, Bradley R. Prestidge, William S. Bice, Geoffrey S. Ibbott, Juanita M. Crook, Charles N. Catton, Walter Bosch, David C. Beyer, Michael A. Papagikos, Seth A. Rosenthal, H. Joseph Barthold, Mack Roach, Jennifer Moughan
Data analysis and interpretation: Jeff M. Michalski, Kathryn A. Winter, Bradley R. Prestidge, William S. Bice, Hiram A. Gay, Charles N. Catton, Adam Raben, Walter Bosch, David C. Beyer, Steven J. Frank, Seth A. Rosenthal, Mack Roach, Jennifer Moughan
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Effect of Brachytherapy With External Beam Radiation Therapy Versus Brachytherapy Alone for Intermediate-Risk Prostate Cancer: NRG Oncology RTOG 0232 Randomized Clinical Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jeff M. Michalski
Open Payments Link: https://openpaymentsdata.cms.gov/physician/221723
Bradley R. Prestidge
Consulting or Advisory Role: 2nd.MD
Mahul Amin
Employment: Laborp of America
Leadership: Kidney Cancer Association
Stock and Other Ownership Interests: CORE Science Solutions, CellMax Life, Precipio, Karkinos Health, Morphle, Pathpresenter, IBEX Medical Analytics
Honoraria: Genomic Health
Consulting or Advisory Role: Urogen pharma, Advanced Clinical
Juanita M. Crook
Consulting or Advisory Role: Concure Oncology Breast Microseed, Tersera, Astellas Pharma, Tolmar
Charles N. Catton
Honoraria: AbbVie, Bayer, Tersera, Knight Therapeutics
Adam Raben
Honoraria: Bristol Myers Squibb
Consulting or Advisory Role: Castle Biosciences
Speakers' Bureau: Bristol Myers Squibb
Travel, Accommodations, Expenses: Bristol Myers Squibb
Walter Bosch
Patents, Royalties, Other Intellectual Property: Licensing of Radiation Oncology workflow management software developed at Washington University
David C. Beyer
Stock and Other Ownership Interests: Videra Surgical
Honoraria: Videra Surgical
Steven J. Frank
Leadership: C4 Imaging, National Comprehensive Cancer Network
Stock and Other Ownership Interests: C4 Imaging
Honoraria: IBA
Consulting or Advisory Role: IBA
Research Funding: Hitachi, Affirmed Pharma
Patents, Royalties, Other Intellectual Property: I have developed patents at the UT MD Anderson Cancer Center. These patents have been licensed to C4 Imaging
Travel, Accommodations, Expenses: National Comprehensive Cancer Network, Boston Scientific
Michael A. Papagikos
Stock and Other Ownership Interests: Pfizer, Bayer, CVS Health, HCA Healthcare, Johnson & Johnson/Janssen, Novartis, UnitedHealthcare
Other Relationship: Boston Scientific
Open Payments Link: https://openpaymentsdata.cms.gov/physician/310147
Mack Roach
Honoraria: Merck, Debioscience, UniBanco Brasil, Pfizer, NYU Long Island School of Medicine/NYU Langone Health, Janssen Oncology
Consulting or Advisory Role: Accuray, Merck, Myovant Sciences
Patents, Royalties, Other Intellectual Property: UptoDate (ongoing)
Travel, Accommodations, Expenses: UniBanco Brasil
Uncompensated Relationships: Janssen Oncology
Howard M. Sandler
Consulting or Advisory Role: Janssen
Other Relationship: Caribou Publishing
No other potential conflicts of interest were reported.
REFERENCES
- 1.Critz FA, Tarlton RS, Holladay DA: Prostate specific antigen-monitored combination radiotherapy for patients with prostate cancer. I-125 implant followed by external-beam radiation. Cancer 75:2383-2391, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Ragde H, Elgamal AA, Snow PB, et al. : Ten-year disease free survival after transperineal sonography-guided iodine-125 brachytherapy with or without 45-gray external beam irradiation in the treatment of patients with clinically localized, low to high Gleason grade prostate carcinoma. Cancer 83:989-1001, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Blasko JC, Grimm PD, Sylvester JE, et al. : Palladium-103 brachytherapy for prostate carcinoma. Int J Radiat Oncol Biol Phys 46:839-850, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Merrick GS, Butler WM, Galbreath RW, et al. : Five-year biochemical outcome following permanent interstitial brachytherapy for clinical T1-T3 prostate cancer. Int J Radiat Oncol Biol Phys 51:41-48, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Nag S, Beyer D, Friedland J, et al. : American brachytherapy society (ABS) recommendations for transperineal permanent brachytherapy of prostate cancer. Int J Radiat Oncol Biol Phys 44:789-799, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Frank SJ, Grimm PD, Sylvester JE, et al. : Interstitial implant alone or in combination with external beam radiation therapy for intermediate-risk prostate cancer: A survey of practice patterns in the United States. Brachytherapy 6:2-8, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Zelen M: The randomization and stratification of patients to clinical trials. J Chronic Dis 27:365-375, 1974 [DOI] [PubMed] [Google Scholar]
- 8.Cox JD: Consensus statement: Guidelines for PSA following radiation therapy. American Society for Therapeutic Radiology and Oncology Consensus Panel. Int J Radiat Oncol Biol Phys 37:1035-1041, 1997 [PubMed] [Google Scholar]
- 9.Roach M III, Hanks G, Thames H Jr, et al. : Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 65:965-974, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Haybittle JL: Repeated assessment of results in clinical trials of cancer treatment. Br J Radiol 44:793-797, 1971 [DOI] [PubMed] [Google Scholar]
- 11.Peto R, Pike MC, Armitage P, et al. : Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer 34:585-612, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan E, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 13.Mantel N: Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 50:163-170, 1966 [PubMed] [Google Scholar]
- 14.Cox DR: Regression models and life tables. J R Stat Soc 34:187-202, 1972 [Google Scholar]
- 15.Kalbfleisch JD, Prentice RL: The Statistical Analysis of Failure Time Data. New York, NY, John Wiley and Sons, 1980, pp 167-169 [Google Scholar]
- 16.Gaynor J, Feuer EJ, Tan CC, et al. : On the use of cause specific failure and conditional failure probabilities: Examples from clinical oncology data. J Am Stat Assoc 88:400-409, 1993 [Google Scholar]
- 17.Coen JJ, Zietman AL, Thakral H, et al. : Radical radiation for localized prostate cancer: Local persistence of disease results in a late wave of metastases. J Clin Oncol 20:3199-3205, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Zagars GK, von Eschenbach AC, Ayala AG, et al. : The influence of local control on metastatic dissemination of prostate cancer treated by external beam megavoltage radiation therapy. Cancer 68:2370-2377, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Fuks Z, Leibel SA, Wallner KE, et al. : The effect of local control on metastatic dissemination in carcinoma of the prostate: Long-term results in patients treated with 1251 implantation. Int J Radiat Oncol Biol Phys 21:537-547, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Michalski JM, Moughan J, Purdy J, et al. : Effect of standard vs dose-escalated radiation therapy for patients with intermediate-risk prostate cancer: The NRG oncology RTOG 0126 randomized clinical trial. JAMA Oncol 4:e180039, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris WJ, Tyldesley S, Rodda S, et al. : Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE-RT trial): An analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys 98:275-285, 2017 [DOI] [PubMed] [Google Scholar]
- 22.Hoskin PJ, Rojas AM, Bownes PJ, et al. : Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol 103:217-222, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Sathya JR, Davis IR, Julian JA, et al. : Randomized trial comparing iridium implant plus external-beam radiation therapy with external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J Clin Oncol 23:1192-1199, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Zumsteg ZS, Zelefsky MJ: Improved survival with surgery in prostate cancer patients without medical comorbidity: A self-fulfilling prophecy? Eur Urol 64:381-383, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Epstein JI, Zelefsky MJ, Sjoberg DD, et al. : A contemporary prostate cancer grading system: A validated alternative to the Gleason score. Eur Urol 69:428-435, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohler JL, Armstrong AJ, Bahnson RR, et al. : Prostate cancer, version 1.2016. J Natl Compr Cancer Netw 14:19-30, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Spratt DE, Zhang J, Santiago-Jimenez M, et al. : Development and validation of a novel integrated clinical-genomic risk group classification for localized prostate cancer. J Clin Oncol 36:581-590, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zumsteg ZS, Chen Z, Howard LE, et al. : Number of unfavorable intermediate-risk factors predicts pathologic upstaging and prostate cancer-specific mortality following radical prostatectomy: Results from the SEARCH database. Prostate 77:154-163, 2017 [DOI] [PubMed] [Google Scholar]
- 29.Tharmalingam H, Tsang Y, Choudhury A, et al. : External beam radiation therapy (EBRT) and high-dose-rate (HDR) brachytherapy for intermediate and high-risk prostate cancer: The impact of EBRT volume. Int J Radiat Oncol Biol Phys 106:525-533, 2020 [DOI] [PubMed] [Google Scholar]
- 30.Murthy V, Maitre P, Kannan S, et al. : Prostate-only versus whole-pelvic radiation therapy in high-risk and very high-risk prostate cancer (POP-RT): Outcomes from phase III randomized controlled trial. J Clin Oncol 39:1234-1242, 2021 [DOI] [PubMed] [Google Scholar]
- 31.Roach M III, DeSilvio M, Valicenti R, et al. : Whole-pelvis, “mini-pelvis,” or prostate-only external beam radiotherapy after neoadjuvant and concurrent hormonal therapy in patients treated in the Radiation Therapy Oncology Group 9413 trial. Int J Radiat Oncol Biol Phys 66:647-653, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Per NCI requirements, the data from this article will be submitted to the National Cancer Institute NCTN/NCORP data archive (https://nctn-data-archive.nci.nih.gov/) no later than 6 months after publication. After the required NCI reviews are completed, it will be released and available in the data archive for data sharing proposals.