Abstract
Compact chromatin is closely linked with gene silencing in part by sterically masking access to promoters, inhibiting transcription factor binding and preventing polymerase from efficiently transcribing a gene. Here, we propose a broader view: chromatin compaction can be both a cause and a consequence of the histone modification state, and this tight bidirectional interaction can underpin bistable transcriptional states. To test this theory, we developed a mathematical model for the dynamics of the HMR locus in S. cerevisiae, that incorporates activating histone modifications, silencing proteins and a dynamic, acetylation-dependent, three-dimensional locus size. Chromatin compaction enhances silencer protein binding, which in turn feeds back to remove activating histone modifications, leading to further compaction. The bistable output of the model was in good agreement with prior quantitative data, including switching rates from expressed to silent states, and vice versa, and protein binding levels within the locus. We then tested the model by predicting changes in switching rates as the genetic length of the locus was increased, which were then experimentally verified. This bidirectional feedback between chromatin compaction and the histone modification state may be an important regulatory mechanism at many loci.
Although the genome sequence underpins much of the biology of an organism, cells with the same genotype can nevertheless have very different phenotypes, mediated through differential regulation of gene expression. Gene expression is in turn modulated by the multifaceted characteristics of chromatin.
For almost a century, chromatin has been broadly divided in euchromatin, open and generally associated with active genes, and heterochromatin, compact and silent (Passarge, 1979). Coherent with this vision, the three-dimensional arrangement of chromatin in the vicinity of a gene and the post translational modification (PTMs) of the nearby nucleosomes are thought to be major determinants of the strength of transcription. Nevertheless, the relationship between the three-dimensional structure and chromatin modifications has not been elucidated in most cases. In fact, there is lively debate regarding which is the actual transcriptional regulator: whether it is the local density of chromatin (Ou et al., 2017), histone PTMs present in the locus (Allshire & Madhani, 2018), or the identity of the DNA or nucleosome-bound proteins (Kueng et al., 2013).
A common view is that if chromatin is highly compacted, the DNA in this region will be inaccessible and transcriptionally silent (Klemm et al., 2019; Ou et al., 2017). Conversely, if the chromatin is less densely packed, then transcription factors can bind, which will be followed by RNA polymerases (Preissl et al., 2022). However, accessibility and transcription are not always correlated (DeVeale et al., 2022; Kiani et al., 2022).
Key regulators of chromatin compaction are reader-writer enzymes that bind to nucleosomes only if certain histones PTMs are present and which catalyse the spread of that same histone PTM to nearby nucleosomes. For example, PRC2 is activated by H3K27me3 to add further H3K27me3 modifications nearby in a read-write feedback, driving chromatin compaction in vitro (Grau et al., 2021). Other reader-writer enzymes have also been suggested to be involved in chromatin compaction, such as the SIR complex in budding yeast (Ruault et al., 2021). However, there are alternative regulators of chromatin compaction. Significant evidence points towards acetylation of histone tails, especially H4, as controlling the physical conformation of chromatin (Collepardo-Guevara et al., 2015; Robinson et al., 2008).
These concepts are often presented in a sequential manner, where upstream effectors and downstream consequences are clearly distinguished. Here, however, we elucidated a more nuanced description, where the extent of chromatin compaction is controlled by the absence of histone PTMs, but where the absence of histone PTMs is itself influenced by the extent of chromatin compaction. Indeed, many reader-writer enzymes dimerise (e.g., PRC2) and bind to more than one nucleosome at the same time, which makes them very sensitive to the local physical configuration of chromatin. A sufficiently compacted locus would increase the binding of these enzymes and would thereby further influence the histone marks that the enzymes spread or remove. As the presence or absence of histone marks can modulate chromatin conformation, the physical state of the chromatin environment and the histone modification state are inextricably linked in a mutually reinforcing manner.
In this work, we developed this hypothesis via a mathematical model for the S. cerevisiae silenced mating-type locus HMR. This locus is constitutively silenced in budding yeast by the action of the Silent Information Regulator (SIR) proteins, which form the SIR complex. The binding of the SIR complex to the locus causes two major changes in the chromatin: deacetylation of H4K16 (H4K16 is mostly acetylated in yeast) and loss of H3K79 methylation (Rusche et al., 2003). Furthermore, HMR is flanked by two silencers (E and I), which are regulatory sites that recruit Sir proteins and which are essential for heterochromatin formation at the locus, as their deletion leads to loss of silencing. The genomic regions outside the locus and next to the silencers, including a tRNA gene, act as insulators, preventing the spread of heterochromatin beyond the boundaries of the locus (Donze et al., 1999). Moreover, silencing at HMR does not depend on the identity of the gene encoded by the locus, allowing empirical observation of the transcriptional state of the locus by substituting reporter genes for those genes usually resident at HMR.
Under certain conditions that weaken the action of the silencers, the silencing of the locus is rendered bistable, switching between silenced and expressed states. The transcriptional state can be inherited over multiple generations, depending on the strength of silencers, making it an ideal system to study the inheritance of epigenetic information to daughter cells. Capturing its dynamics quantitatively requires an accurate mathematical model, with detailed experimental data available as a strict benchmark for our theory. Here, model outcomes for the HMR locus were successfully tested against both prior experimental data and the results of new experiments specifically designed to test our hypothesis. Overall, our model provided a faithful representation of the biochemical processes involved in epigenetic silencing, enabling us to develop a coherent theory for the feedback between the histone PTM state and chromatin conformation in three-dimensional space.
Previous modelling studies have focused either on a coarse-grained description of large-scale chromatin or on a detailed picture of the regulation at a particular locus. In the coarse-grained description a similar bidirectional interaction between epigenetic state and chromatin folding is considered, but the molecular mechanisms modelled are often abstract and with limited detail, making quantitative comparison with experimental data difficult (Jost & Vaillant, 2018; Michieletto et al., 2018; Michieletto et al., 2016; Owen et al., 2022; Pease et al., 2021; Sandholtz et al., 2020). In parallel, single-locus studies have provided much insight into the regulation of certain genetic regions, capturing their dynamics quantitatively, but they have not incorporated this link between chromatin and the histone PTM state (Angel et al., 2011; Berry et al., 2017; Nickels et al., 2021; Sneppen & Dodd, 2015). In this work, we drew inspiration from both approaches to produce a model that included this important link and, at the same time, kept a detailed description of the molecular mechanisms regulating HMR heterochromatin formation, enabling an extensive quantitative comparison with experimental data. Indeed, the HMR locus is one of a small number of loci from any organism where sufficient detailed molecular data exists to make such an in-depth comparison.
Results
Underlying processes and timescales
Intensive study of the budding yeast HMR locus has identified proteins, enzymes, histone PTMs and regulatory sites involved in silencing/activation (Kueng et al., 2013; Rusche et al., 2003). Sas2 acetylates H4K16 and Dot1 methylates H3K79, especially if the H3 histone substrate is bound to an H4 histone carrying the H4K16ac mark (Valencia-Sánchez et al., 2021). These two PTMs are associated with active loci. With respect to silencing, the silencers delimiting the locus have significant affinity for proteins such as ORC and Rap1 that recruit Sir1 which, in turn, recruits the rest of the SIR complex. If the nucleosome is unmodified, the SIR complex (Sir2-3-4) can bind to it, but in presence of the H4K16ac or H3K79me marks, the Sir complex affinity is greatly reduced (Rusche et al., 2003). Spreading of the SIR complex is then mediated by the read-write action of Sir2, a histone deacetylase whose preferred substrate for the enzymatic reaction are histones acetylated at H4K16, thus increasing the number of binding sites for other Sir proteins by means of its catalytic activity. Notably, binding of the SIR complex to nucleosomes occurs in a highly cooperative manner as its affinity increases roughly 50-fold when it binds to two unmodified nucleosomes (Behrouzi et al., 2016). Importantly, sir1Δ strains are bistable with respect to its transcriptional state at HMR and are able to maintain such a state for multiple generations (Pillus & Rine, 1989). Thus, in a sir1Δ mutant, HMR is found either in an active state (with high levels of acetylation) or in a silenced state (with low levels of H4K16ac and H3K79me3, but with high levels of the SIR complex bound), switching between the two typically on a timescale of tens of generations (Saxton & Rine, 2022).
Given the variety of processes involved in the silencing of the locus, from protein binding to DNA replication, estimating the timescales for these dynamical processes is important for a comprehensive and quantitative understanding of silencing. The fastest process is Sir protein binding and unbinding, on the order of tens of seconds (Behrouzi et al., 2016). Acetylation and deacetylation of histone H4 tails occurs at an intermediate timescale, around 10 minutes (Waterborg, 2001). Finally, since there is no known demethylase in yeast for H3K79, the only way to lower the number of H3K79me3 marked histones is by dilution at replication (Goodnight & Rine, 2020). Hence, the timescale of H3K79 methylation is on the order of the duration of the cell cycle, i.e., approximately 2 hours. The model we developed, described in the next section, reflects these experimental findings.
Modelling histone PTM dynamics
Here we summarise the model constituents and fundamental dynamics (depicted schematically in Fig. 1A; for full details and parameter values, see SI Appendix, Sections 1 and 2).
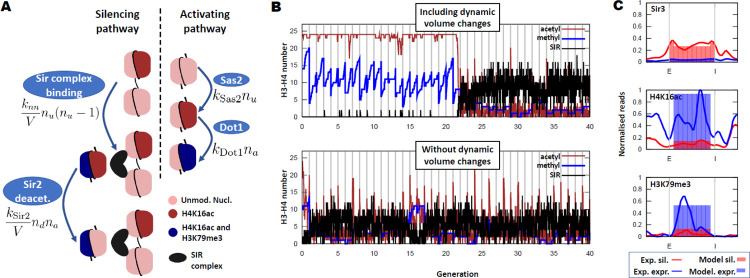
Figure 1. A model with dynamic volume changes can reproduce the bistable dynamics of HMR in the sir1Δ mutant.
A) Schematic of the model. The SIR complex binds by dimerisation to two unmodified H3–H4 pairs and Sir2 deacetylates H4 histones (third nucleosome in the silencing pathway, left column). Sas2 acetylates H4 histones and Dot1 methylates acetylated H3–H4 histone pairs. knm is the reaction constant for the SIR complex binding to two unmodified nucleosomes, nd is the number of H3–H4 histone pairs dimerised by the SIR complex, and ksir2 the reaction constant for Sir2 deacetylation of H4K16. The rest of the symbols are defined in the main text. B) Example output of a simulation for the sir1Δ mutant. The full model (including volume changes) shows a robust bistable behaviour that persists over generations (similar to the phenotype found in experiments), while the same model with a fixed locus volume is neither bistable nor robust. C) The occupancy fraction of Sir proteins and histone PTMs are compared to experimental ChIP profiles for each of the stable states of the model (silenced [sil.] and expressed [expr.]). Experimental data obtained from Ref. (Saxton & Rine, 2022). ChIP profiles were normalised to their highest peak across the locus, in order to enable quantitative comparison with occupancy fraction.
Given that a nucleosome is formed by a histone octamer (two pairs of H2A-H2B histones and another two pairs of H3–H4 histones), we modelled the locus as a set of H3–H4 pairs which can be in five states: Unmodified (U), Unmodified and Sir bound (Ub), Acetylated (H4K16ac, A), Methylated (H3K79me3, M) and Acetylated and Methylated (AM). We assumed that Sir proteins can bind only to unmodified H3–H4 pairs by a dimerisation process, binding two H3–H4 histone pairs at a time, given that individually they have a very low affinity (Behrouzi et al., 2016). To reflect a compact and dynamic arrangement of the locus, the model does not include the relative positions of the nucleosomes, it treats the locus as well-mixed, where contacts between any two H3–H4 pairs are all equally likely. For simplicity, and given the insulator role of the boundaries of the HMR locus (Donze et al., 1999), we considered the locus isolated from the rest of the genome and we do not model the rest of the chromosome or any other genetic region.
The two silencers were modelled as preferred binding sites for the Sir complex, which can be thought of as H3–H4 pairs fixed in the U state but with a higher affinity for Sir proteins. Sir proteins can also dimerise between the two silencers or between a silencer and any unmodified H3–H4 pair. In a one-dimensional representation of the locus, a silencer would stand at each end of the locus and the nucleosomes would be stacked in between, implying that it would be easier for Sir proteins to dimerise between nearest neighbours. Nevertheless, this one-dimensional configuration of nucleosomes is not an accurate depiction of reality and the locus is likely to adopt a more dynamic and disordered configuration (Farr et al., 2021). To reflect this dynamic configuration, we allowed Sir proteins to dimerise between any pair of nucleosome and silencer; but to account for the longer relative length (in the one-dimensional picture) between silencers, and between silencers and nucleosomes (on average), we weighted the dimerization probabilities by a factor determined by the genetic length between them. Given that the average distance between any two nucleosomes in the locus is roughly 1/3 of the length of the locus, and that the average distance between a silencer and any nucleosome is 1/2 of the length of the locus, a weight of 2/3 was applied to the nucleosome-silencer binding constant. Analogously, a factor of 1/3 was applied to the silencer-silencer binding constant (see SI Appendix Section 1). Therefore, we accounted for longer genetic distances while still allowing for long-range binding, approximately reflecting the dynamic and folded architecture of the locus (Valenzuela et al., 2008). In cells with weakened silencers, we reduced the effective binding constant of the Sir complex to silencers, e.g., in the sir1Δ strain we reduced the binding affinity of silencers to almost that of nucleosomes.
The dynamics of the histone PTMs were modelled mathematically with a reaction rate proportional to the substrate amount, i.e., the rate of H4K16 acetylation by Sas2 is ksas2nu, where nu is the number of unmodified (and not SIR-bound) H3–H4 pairs in the locus at any point in time and ksas2 is a constant reflecting the activity and nuclear concentration of Sas2. Similarly, Dot1 methylation of H3K79 is given by the rate kDot1nac, since the most efficient substrate of Dot1 are acetylated H3–H4 pairs (Valencia-Sánchez et al., 2021). The Sir2 deacetylation rate depends on the product of the amount of SIR complex at the locus and the acetylated H3–H4 pairs (see Fig. 1A for the mathematical form). We also considered that Sas2 and Sir2 can acetylate and deacetylate (respectively) H3–H4 pairs methylated at H3K79, with analogously constructed reaction rates. The acetylation and methylation processes comprise the activating pathway, while Sir complex binding and Sir2 deacetylation are defined as the silencing pathway (see Fig. 1A for a diagram of these PTMs and their rates). Finally, at the end of the cell cycle, replication was modelled with every nucleosome having a 50% probability of being replaced by one which is fully acetylated and unmethylated, since, in S. cerevisiae, most of the H4 histones in the nucleoplasm are acetylated (Shia et al., 2005). The 50% probability of replacing a nucleosome at replication is supported experimentally in budding yeast (Schlissel & Rine, 2019).
Crucially, Sir complex binding to histones and Sir2 deacetylation, the two steps in the silencing pathway, rely on contacts between regions within the locus. Contacts within the locus will depend on the three-dimensional locus configuration, which, in a first approximation, could be modelled by quantifying the three-dimensional volume occupied by the locus: large if the locus is open (with few contacts within the locus), or small if the locus is in a compact configuration (allowing many more contacts). In terms of polymer physics, we estimated the volume occupied by the locus, V, as proportional to , where Rg is the radius of gyration of the locus (Rubinstein et al., 2003). Therefore, the overall volume of the locus V modulates the rates of Sir binding and Sir2 deacetylation by changing the frequency of contacts between nucleosomes, see Fig. 1A. Moreover, there is evidence that H4K16 acetylation is critical in determining the physical conformation of chromatin (Collepardo-Guevara et al., 2015; Robinson et al., 2008), due to H4 tail-DNA contacts [according to molecular dynamics simulations (Farr et al., 2021) and in vitro experiments (Wang et al., 2013)]. Thus, drawing inspiration from polymer physics we modelled the varying volume of the locus with the following scaling:
| [1] |
where nub is the number of unmodified and SIR-bound H3–H4 pairs, and nm that of methylated H3–H4 pairs and nu + nub + nm is the total number of H3–H4 pairs that are not acetylated at H4K16. This scaling arises as a limiting case of the Flory picture for real polymers in a poor solvent (Rubinstein et al., 2003), when the coil is compact and the entropic contribution of the chain to the free energy can be neglected (see SI Appendix, Section 3). Thus, the overall locus volume is reduced in loci with many unmodified H4 tails while it expands if the levels of H4K16ac are high. Consistent with this picture, there are frequent contacts between the two silencers and the nucleosomes in the wild type, when the locus is fully silenced, but these contacts disappear in a sir3Δ mutant, where silencing is abolished (Valenzuela et al., 2008). Chromatin compaction has also been observed upon addition of catalytically active Sir proteins to chromatin in vitro presumably due to deacetylation (Johnson et al., 2009). Overall, these acetylation-dependent dynamics provide the link between the physical conformation of the locus and the histone PTMs.
The model was then implemented with a stochastic simulation algorithm (Gillespie, 1977). Importantly, when trying to recapitulate the features of the bistable sir1Δ mutant with the model we found that, if we assumed the volume was fixed and did not depend on the chromatin state, the bistability and robustness of the model became compromised. In Fig. 1B, both simulations were initialised in a highly expressed state (high acetylation and methylation) but, in the absence of dynamic volume changes, the active state was not stable. Generation of bistable behaviour required acetylation-dependent volume changes, arising from a fundamental difference between the processes that activate the locus and those that silence it. In the model, the silencing processes (Sir binding and Sir2 deacetylation) depend on contacts between nucleosomes within the locus, but the activating processes do not. As a result, an expanded locus would favour activation, whereas a compact one would promote silencing. The system could therefore become locked in a compact and silenced locus by the interaction between enzymes promoting loss of activating histone PTMs and the compact volume which enhances the binding of these enzymes. Stochastic silencing loss was rare but did occur (roughly every 100 generations) and when this happened the locus now locked itself into an expressed state, where the locus had expanded due to the higher acetylation making it difficult for the Sir complex to bind and reverse the changes. These two stable states were highly polarised, one of them having high Sir protein binding and low H4K16ac and H3K79me3, while the expressed state showed little protein binding and high levels of acetylation and methylation.
We also found quantitative agreement between the amount of Sir binding and histone PTMs in each of the two states (silenced and expressed), see Fig. 1C. In the simulations the locus is considered expressed if, at the end of the cell cycle but before DNA replication, half or more of the H3–H4 pairs are either acetylated or methylated, a reasonable threshold given recent experimental data (Wu et al., 2021). However, due to the bistable nature of the model, varying this threshold moderately did not produce significant changes to the model output. A quantitative comparison was then made with data from Ref. (Saxton & Rine, 2022), by renormalising the ChIP profiles to the highest peak, in an attempt to estimate the maximum occupancy, with good results. Note that the model did not include details of the position of the nucleosomes or any sequence-dependent effect other than the silencers, so the model results were not able to recapitulate the fine-scale spatial structure of chromatin. Overall, we found that the model effectively captured the known behaviour of the HMR locus.
Switching rates in the HMR loci with different genetic lengths
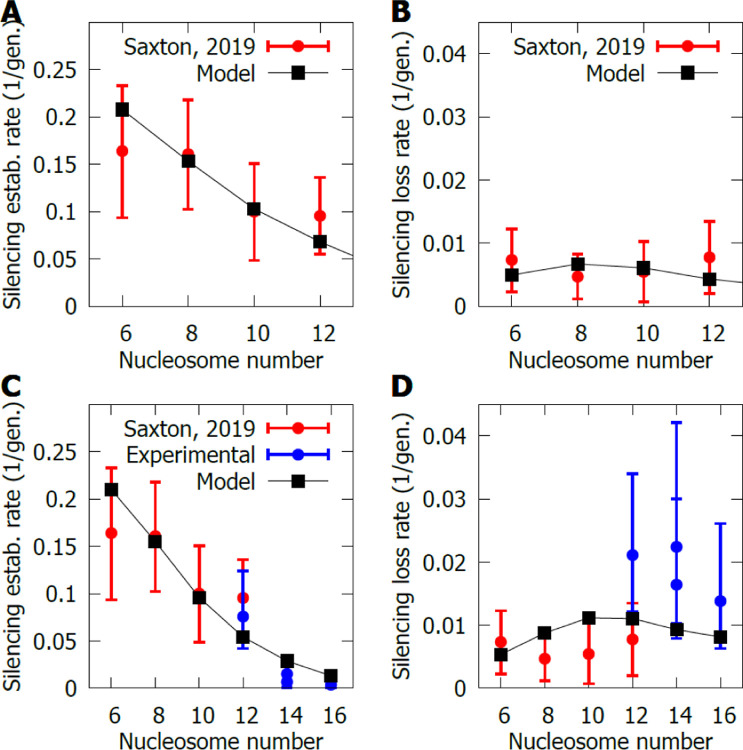
Previously, two of us published the results of an experiment in which the genetic length of the HMR locus, and thus the number of nucleosomes between the silencers, was varied and the silencing establishment/loss switching rates were measured. If epigenetic memory of the transcriptional state of the locus is transferred to daughter cells by nucleosome inheritance, having fewer nucleosomes in the locus should make inheritance less stable, as there would be more variability in the fraction of nucleosomes inherited. However, this is not what was observed in Ref. (Saxton & Rine, 2019). There was no change in silencing loss rates and an increase, although statistically not significant, in the silencing establishment rates as the number of nucleosomes was reduced. Recapitulating these data is a strict test of the model, since it requires a fine balance between the volume of the locus, the number of nucleosomes and the scaling of the reaction rates locus wide.
With appropriate parameters, the model could recover the results obtained in that experiment, see Fig. 2A and B, if we assumed that the fluorescent protein measurements in the experiments are delayed by a few hours with respect to the chromatin state, due to subsequent transcription and translation. Consequently, in the statistics of the model, we did not consider switching events that are reversed in less than two generations, as such events would not be visible experimentally via a fluorescent protein assay. With these considerations, the model produced an important prediction: the silencing loss rate as a function of the number of nucleosomes in the locus remains approximately constant, while the silencing establishment was reduced substantially as the genetic locus size increases. It is worth noting that silencing loss rates are around 1%, a low rate that is enabled in the model by dynamic volume changes.
Figure 2. Switching rates as a function of nucleosome number.
A,B) Silencing establishment and silencing loss rates (respectively) in a sir1Δ background for loci with different nucleosome number (different genetic length). The results from the model are shown in black and, in red, the experimental data from Ref. (Saxton & Rine, 2019). C,D) Switching rates shown as in A and B, but shown for a wider range of genetic lengths, by combining data from Ref. (Saxton & Rine, 2019), in red, with new experimental data obtained specifically for this study (blue). The labelling for panel D is the same as for panel C. There are two datapoints for the 14 nucleosome strains, as two different strains were created by introducing the same DNA into two different locations at the locus. The results of the model are shown in black, but the parameters have been modified slightly with respect to the results in A and B, to better capture the new data. For details regarding the parameters, see SI Appendix, Section 1.6. The error bars represent the 95% confidence interval.
There are two effects at play when reducing the number of nucleosomes between the silencers in the simulations. First, since we still have two silencers, their effect on the remaining nucleosomes is stronger (the number of nucleosomes per silencer decreases), making the locus more prone to silencing. Second, as pointed out above, there is a decrease in the reliability of inheritance for smaller loci. In the case of the silencing loss rate, these effects counteracted each other to a certain extent: as the locus size was reduced, the silencers became more effective (lowering the silencing loss rate), but inheritance less faithful (increasing the loss rate). Crucially, for the case of silencing establishment, both effects reinforced this rate as we decreased the nucleosome number, rather than cancelling out, leading to qualitatively different behaviour.
The model predicted a continuation of this trend for larger loci, an important result which we therefore tested experimentally, since any such trend identified in earlier experiments was not statistically significant. Strains with 14 and 16 nucleosomes in the HMR locus were created by inserting intergenic DNA found at HML into the endogenous HMR-GFP locus. We tested other sources of intragenic DNA with similar results regarding the overall fraction of silenced cells (see SI Appendix, Supplementary Figure 1), since every mutant with an increased number of nucleosomes at the locus showed a higher degree of fluorescent protein expression than for the wild-type length (12 nucleosomes), as expected from the model.
To test the model predictions, we experimentally measured the switching rates for these strains and, in agreement with the model, the silencing establishment rate decreased substantially (Fig. 2C), a decrease that was statistically significant (see SI Appendix, Supplementary Figure 2). In fact, the decrease in the silencing establishment rate was even more marked than initially predicted by the model, which forced us to modify certain parameters. Since the effect of silencers is partially responsible for the trend in the silencing establishment, giving more importance to silencers in the model (with respect to nucleosomes) should increase the steepness of the trend. By increasing the parameter that controls the affinity of the SIR complex to silencers and decreasing that for histones, the model was able to better capture the 20-fold drop in the experimental establishment rate over the 6 to 16 nucleosome range (Fig. 2C, for details on the parameters used in Fig. 2C and D and the rest of the paper see SI Appendix, Section 1.6, and for the results with the original parameter set see SI Appendix, Supplementary Figure 3).
With respect to the silencing loss rate, the model predicted limited changes with both parameter sets (Fig. 2B and D). The experiments confirmed this as the silencing loss rate did not change significantly when increasing the genetic length of the locus (Fig. 2D and see SI Appendix, Supplementary Figure 2). Overall, there did not seem to be a trend in the silencing loss rate since there were no statistically significant changes within the two experimental datasets. Nevertheless, there was a substantial dispersion between the results obtained in this work and those of Ref. (Saxton & Rine, 2019) for reasons that were unclear, although this ~2-fold dispersion remains very small compared to the 20-fold variation in the establishment rate. Altogether, the model predictions for silencing establishment/loss rates as a function of locus size were well supported by our new experimental data.
Chromatin binding
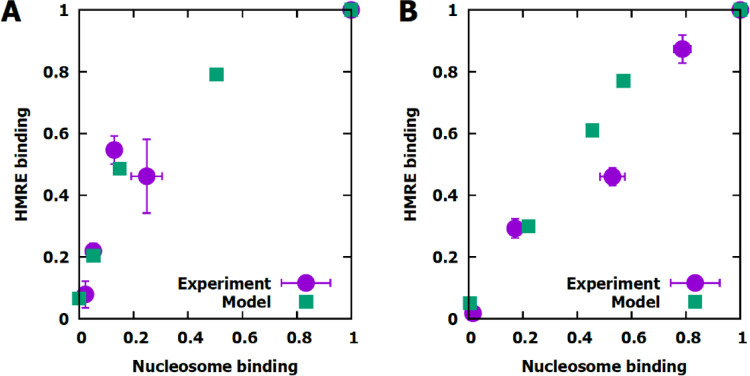
As argued above, silencing at the HMR locus occurs in a highly cooperative manner, due to the SIR complex binding and H4K16ac feedback. Traditionally the process had been viewed as nucleation of the Sir proteins starting at the silencers and subsequent spreading towards the rest of the locus. In contrast, in Ref. (Saxton & Rine, 2022), two of us found that the extent of Sir binding on nucleosomes also has an effect on the occupancy levels of the silencers, making cooperativity between silencers and nucleosomes a two-way interaction.
The model has no sequence dependency other than the silencers’ position and considers every nucleosome to be able to equally interact with any other. Thus, the extent to which such a model could capture the observed cooperativity between genetic features in the locus was unclear. By reanalysing ChIP data for SIR complex binding in different histone mutants, an overall nucleosome occupancy value was obtained, which could be compared to the outcome of the model. Fig. 3A depicts the Area Under the Curve (AUC) for the E silencer as a function of the AUC of the nucleosomes across the locus for the different histone mutants, with both quantities normalised to the wild-type. This analysis revealed a good accordance between the experimental data and the model. The histone and Sir mutants were chosen to reduce the spreading on nucleosomes but not directly alter the binding to silencers. These included H4K16Q (an acetyl mimic), H3K79L (a methyl mimic), Sir3-bahΔ (Sir complex whose nucleosome-binding domain has been deleted) and sir2-N245 (catalytically inactive for H4K16ac deacetylation). For details on the modelling of these mutant strains, see SI Appendix, Section 4. In agreement with the mutual cooperativity picture between silencers and nucleosomes, as the Sir binding to nucleosomes decreased, so did the E silencer binding, both in the model and the experiments. Note the non-linear cooperativity, with more than half of the drop in E silencer binding taking place when the nucleosome Sir binding is less than 20% of the wild-type value.
Figure 3. Binding of the SIR complex to the E silencer and to nucleosomes.
A) Binding at the E silencer (HMRE) as a function of binding at nucleosomes for different histone mutants. Experimental data from (Saxton & Rine, 2022) is shown in purple circles and modelling, in green squares. The data (modelling and experimental) is normalised to the wild-type, shown in the top-right hand corner. The mutant strains are, from right to left, H3K79L, H4K16Q, sir3-bahΔ and sir2-N345. B) Same data as A but for a Sir4 titration. The experimental data (strain with estradiol-inducible promoter) is normalised to the population with the highest SIR complex binding in the titration (data from Ref. (Saxton & Rine, 2022)) while the modelling output has been normalised to the wild-type Sir concentration in simulations. Experimental error bars correspond to standard deviation (independent in each direction) from two different replicates.
According to the model, the cooperativity signature seen in these experiments resulted from the way the SIR complex binds to the locus (dimerisation) and the deacetylation activity of Sir2. As the amount of Sir binding at the nucleosomes decreases, so does the number of unmodified H3–H4 pairs (due to lower deacetylation rates). This results in a lower binding of the SIR complex to the E silencer, as it requires two binding sites, one of them being the E silencer and the other one being either the I silencer or the depleted unmodified H3–H4 pair pool. This argument highlights the importance of the silencer-nucleosome cooperativity and explains why most of the drop in E silencer binding occurs when nucleosome Sir binding is low: the E silencer only needs one unmodified H3–H4 pair to dimerise with, hence until the number of unmodified H3–H4 pairs becomes very low the E silencer does not feel much of the effect.
In a similar manner, we show the E silencer binding against nucleosome binding in a Sir4 titration, using an estradiol-inducible promoter to tune the concentration (data from Ref. (Saxton & Rine, 2022), see Fig. 3B). This time the relationship was roughly linear in both simulations and experiments, since we were varying the overall SIR complex concentration in solution, which directly affected silencers and nucleosomes equally.
Taken together, the analysis shown in Fig. 3 implied that the minimal mathematical model used for chromatin, which represents short-lived locus-wide contacts, was adequate and could capture the observed cooperativity between silencers and nucleosomes. In addition, the model shed light onto the origin of the silencer-nucleosome cooperativity, establishing as a main cause the availability of unmodified H3–H4 pairs with which the silencer can dimerise.
Switching rates in Sir4 titrations
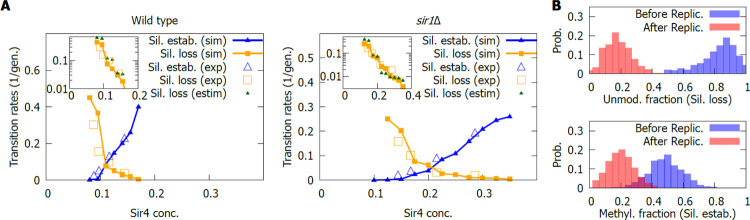
In addition to the ChIP profiles used in Fig. 3B, switching rates were obtained at different estradiol concentrations (Saxton & Rine, 2022), thus modulating the Sir4 concentration and the switching properties of the locus. To compare the results from experiments with that of the model, we measured the switching rates in the simulation when varying the Sir complex concentration. However, the titrated Sir4 and native promoter Sir4 switching rates were different even at approximately the wild-type concentration, possibly due to noise introduced by the inducible promoter. To keep the modelling within a common framework, we therefore multiplied the theoretical switching rates by the ratio between the switching rates from the estradiol-inducible promoter and the native promoter. In addition, a linear conversion was made to estimate the SIR complex concentration in the simulations from the estradiol concentration in experiments (for details see SI Appendix, Section 5). After this linear transformation, there was good agreement between model and experiments (see Fig. 4A).
Figure 4. Switching rates in a Sir4 titration.
A) Silencing establishment and silencing loss rates in in the wild-type (left) and in a sir1Δ background (right). Insets show the silencing loss rates, in logarithmic scale, including an estimate (estim.) based on the inheritance probabilities at chromosome replication, see Eq. [2]. The experimental data was obtained from Ref. (Saxton & Rine, 2022) and was normalised to match switching rates from the native promoter, to keep modelling within a common framework. B) Histone PTMs before (blue) and after (red) replication. Top: Fraction of unmodified H3–H4 pairs before and after the replication event at the beginning of the generation where silencing loss occurs. Bottom: Fraction of methylated H3–H4 pairs before and after the replication event at the beginning of the generation where silencing establishment takes place. Results in panel B shown for a sir1Δ mutant at wild-type concentration.
As expected, both in the wild-type SIR+ and sir1Δ mutant, experiments and modelling agreed on a decrease in the silencing loss rate and increase of the establishment rate as the Sir4 concentration rises. Importantly, the region of bistability of the wild-type silencers is at low Sir concentration, while for the sir1Δ strain, it is at higher concentrations.
More unexpected was the way in which the switching rates vary with Sir concentration. The model predicted a roughly exponential trend for the decrease of the loss rate (see insets in Fig. 4A), consistent with the experimental data. However, this is not the case for the establishment rate (see insets in SI Appendix, Supplementary Figure 4), both in the model and the experimental data, hinting at an asymmetry in the way the switching occurs.
One intuitive reason behind this asymmetry could be the effect of replication. In the simulations, when a cell was silenced at HMR, most H3–H4 pairs at HMR were unmodified and many of them were substituted by acetylated pairs at replication, while in a cell lacking silencing, acetylated and methylated histones were substituted by only acetylated ones. Thus, to obtain insights into the contribution of replication dynamics on the switching of transcriptional states, the distribution of unmodified histones before and after replication was obtained (shown in Fig. 4B, top), only for cases where HMR in the daughter cell became expressed after it was not in the mother cell, i.e., silencing was lost. On average, only half the unmodified H3–H4 pairs should have been lost. However, in cases where the silencing was lost after replication, substantially more than half were lost in most cases, implying a critical role for replication in the loss of HMR silencing.
Therefore, one could speculate that most of the stochastic contribution to the silencing loss stems from an atypically large loss of unmodified nucleosomes at replication and not from the dynamics of the system after replication. In that case, one could set a threshold for the maximum number of unmodified H3–H4 pairs a locus can have in order to lose silencing in the following generation. If, after replication, the locus possessed more unmodified histone pairs than the threshold it would remain silenced, but it would otherwise become expressed. The following approximation to the silencing loss rate could then be obtained, given the knowledge of the distribution of unmodified H3–H4 pairs in the silenced state before replication Psil(nu) and the threshold M:
| [2] |
Where Ploss is the silencing loss probability per cell and generation and Bin(i, nu, p = 0.5) is the binomial probability distribution of i unmodified H3–H4 pairs remaining after replication, given that there were nu before, and that the probability p of losing each of them is 50%. The sum over i implies that, in this approximation, any locus with a number of unmodified H3–H4 pairs between 0 and M will become expressed. The sum over nu accounts for the probabilities that the locus has a different number nu of unmodified H3–H4 pairs and, thus, it would require a different number of successful removals of unmodified histones to become activated. The threshold T was obtained from the average of the probability distribution after replication for cells that will become expressed (red probability distribution in Fig. 4B, top).
This estimate for the silencing loss probability involved several approximations and assumptions: that the stochastic contribution stems exclusively from replication and not from any subsequent histone PTM dynamics and that histone pair removal at replication is independent of each other (a condition that explains the appearance of the binomial distribution in the estimate). As can be seen from the inset in Fig. 4A, these assumptions led to an estimate that was often off by a ~50% with respect to the full model, but did set correctly the order of magnitude of the silencing loss rate and recapitulated the exponential trend. Therefore, the value of this estimate is more qualitative than quantitative: it suggested that the main contribution to the silencing loss rate was a rare replication event, where more unmodified histones than average fail to be inherited, but where other factors also have an effect in the silencing loss rate, which explains why the quantitative values are not accurate. Intuitively, the exponential trend was due to replication-dependent nucleosome removal being an independent event for each nucleosome, which is reflected in an exponential factor from the binomial distribution if the threshold scales linearly with the Sir4 concentration. In fact, the threshold did not decrease in an exactly linear manner with the concentration, but this deviation was partially corrected by the combinatorial prefactor in the binomial distribution.
When this approach was repeated with the silencing establishment rate, the resulting estimate failed to reproduce the outcomes of the model (see SI Appendix, Supplementary Fig. 4). This result implied that, for the silencing establishment, the dynamics of histone PTMs during the rest of the cell cycle are a major source of stochasticity. This could also be seen from the distribution of methylated histones before and after replication (as methylation is the sole activating mark that can be lost at replication), in cases where silencing will be established in the next generation. In Fig. 4B (bottom), these distributions are shown, and the average methylation loss is roughly half of the total amount (the expected average), suggesting that silencing establishment was not very dependent on loss of methylation at replication.
Overall, the model was able to recapitulate the observed switching rates at different Sir concentrations and identified two different potential mechanisms for the transitions: a mechanism based on an anomalously large loss of unmodified histones at replication for the silencing loss, and a stochastic fluctuation in the histone PTM kinetics for the silencing establishment. In addition, the model predicted an exponential trend for the silencing loss that was consistent with the experimental data.
Bistability of silencing in different mutation backgrounds
Another useful aspect of the Sir protein titrations is the ability to look for bistable behaviour for concentrations that would otherwise not be experimentally accessible. Some Sir4 titrations for mutants affecting silencer strength were reported in Ref. (Saxton & Rine, 2022), leading to the conclusion that mutants in which silencers are made weaker are bistable at certain Sir4 concentrations, but even strong-silencer strains, such as the wild-type, become bistable with low enough Sir4 concentrations. Silencer strength was modulated by removing proteins that bind exclusively to silencers (such as Sir1) or by removing binding sites for scaffolding proteins at the silencers (such as the Rap1 binding site, Rap1 b.s.).
The model recapitulated the fact that bistability is enhanced in a different range of Sir concentrations in each strain, depending on the strength of the silencers. By lowering the concentration of Sir proteins, we obtained loci that would transition from a fully expressed state (mostly acetylated) when the Sir concentration is low, to a fully silenced one for high Sir concentrations, passing through an intermediate concentration region where the system can transition back and forth between active and silenced states (see SI Appendix, Supplementary Figure 5). We quantified the bistability of the model for each silencer strength mutant at various concentrations using the bimodality coefficient (BC), defined in Ref. (Institute, 2012). The BC is a function of the skewness and the kurtosis of the distribution (of acetylated histones, in this case), is positive and bounded from above by 1 (for details on the implementation, see SI Appendix, Section 6). Typically, values of the BC larger than ~0.55 are considered to correspond to bimodal distributions, which is an indicator of two stable states. The model predicted a peak in the BC for all mutants considered, but the location of the peak shifted towards smaller Sir4 concentrations for stronger silencers, see Fig. 5, red triangles.
Figure 5. Bistability in a Sir4 titration.
Bimodality coefficient (BC) for the wild-type and two silencer strength mutants, at different Sir4 concentrations. Experimental BC in blue (circles), theoretical BC in red (triangles). Experimental data from Ref. (Saxton & Rine, 2022).
We then sought to compare the theoretical predictions with the experimental data, by computing the BC from the distribution of GFP intensity (in logarithmic scale, as reported in (Saxton & Rine, 2022)) obtained from experiments. The comparison between the experimental and the theoretical BCs, for different silencer strength mutants and Sir4 concentrations, is shown in Fig. 5.
While the comparison was not quantitatively precise, the overall trends and location of the peaks were captured by the model, showing that stronger silencers tend to have a peak in bistability at very low Sir4 concentrations, but weaker silencers can broaden the bistability window and shift it towards higher Sir complex concentrations. It is remarkable that the sir1Δ strain could sustain a high bistability degree over a large concentration window, both in experiments and in the model, as opposed to rap1 b.s.Δ or the wild-type that displayed a more pronounced peak at mid or low concentrations.
Reasons for the imperfect quantitative agreement may include using the same methodology (except for the logarithmic scale in the fluorescence assays) for experimental and synthetic data (which have different sources of noise) or the metric being heavily impacted by very rare events which were either not seen in experiments or overestimated by the model. Finally, given that the output of the model was at the level of chromatin modifications, but the output of experiments was fluorescent protein levels, another reason for a reduced quantitative agreement could be that transient fluctuations at the chromatin level need not translate to fluctuations at the protein level, yielding a lower experimental BC.
Discussion
In the present work we have developed the hypothesis of dynamic changes in the three-dimensional conformation of the locus substantially affecting the removal of histone modifications, thus bidirectionally linking chromatin compaction and histone PTMs. Deacetylation of H4K16 compacts the locus, which promotes Sir binding and further deacetylation via read-write activity of Sir2 with H4K16ac. Hence, we favoured a picture whereby compaction is both cause and consequence of the histone PTM state of the locus: it is consequence as the chromatin conformation resulting from a silenced, unacetylated locus is compact, and it is cause since compact chromatin increases the rates of enzymes, such as the Sir2 deacetylase, that require contacts within the locus for their activity.
Not only is this an attractive hypothesis, but it is also solidly grounded on the current biochemical data. Recent studies found that cooperativity of Sir proteins is limited to a dimerisation process (Behrouzi et al., 2016), suggesting that Sir binding alone cannot explain the highly cooperative behaviour observed in the dynamics of HMR. Indeed, previous modelling studies had captured the bistable behaviour of the HML locus (Sneppen & Dodd, 2015), but only by requiring much more cooperative protein binding dynamics than is observed. Thus, we identified another source of cooperativity, linking the acetylation of H4K16 and the activity of Sir2 to considerable changes in chromatin compaction, which have been observed in vitro (Johnson et al., 2009). Despite its relatively simple form, the model captured and helped elucidate the nature of the cooperativity in the HMR locus, which is a hallmark of epigenetic silencing. From this, we concluded that the experimental data were consistent with a mechanism of locus-wide cooperativity that is dominated by the dimerization behaviour of the Sir complex, often between unmodified nucleosomes and silencers far away in genetic distance, but close in the physical distance. Long distance interactions such as these ones had been reported before (Valenzuela et al., 2008), but the extent to which they contributed to the overall cooperativity in the locus had remained unclear.
Another key finding was the asymmetry in the behaviour of silencing loss and establishment rates when subjected to changes in Sir4 concentration. According to the model, these two events had different origins; the silencing loss probability was mostly controlled by the stochasticity of nucleosome replacement at replication, while for silencing establishment the stochastic histone PTM dynamics played a major role.
Moreover, the switching rates at a variety of locus lengths were well captured and new behaviour predicted by the model was experimentally verified, highlighting the importance of size and physical space in genetic regulation. Two metrics were especially relevant: the one-dimensional genetic length between the silencers and the three-dimensional conformation of chromatin. If the genetic length was reduced, then the silencers would have a greater influence on the remaining (fewer) nucleosomes. In addition, a compact locus in three-dimensional space would enhance Sir binding and Sir2 deacetylation (which depend on intra-locus contacts), but a locus in an expanded conformation would allow other PTMs to take over (H4K16ac and H3K79me3, in this case). Thus, the model successfully integrated one and three-dimensional effects into the dynamics of the locus, capturing crucial features of the locus and making predictions that were experimentally verified. This goes beyond previous theoretical work focused on explaining the interplay between epigenetic marks and chromatin folding (Michieletto et al., 2016; Nickels et al., 2021; Owen et al., 2022; Sandholtz et al., 2020) by providing detailed mechanistic insights and quantitative predictions that can be experimentally tested.
Overall, we have found that the dynamic compaction and decompaction of the locus underpins the bistability of the locus in silencer strength mutants and maintains a robust and heritable transcriptional state across tens to hundreds of generations. However, it remains to be explored how robust it is with respect to cell-cycle dependent processes other than DNA replication, such as genome-wide acetylation changes (Wilkins et al., 2014). Another limitation of this work was the isolated description of the HMR locus in the model: placing the locus in the context of the chromosome and comparing the regulation of the locus with that of other (possibly active) genes would yield valuable insights (Nickels & Sneppen, 2023). Finally, if more detailed measurements of the conformation of the locus were available, a more ambitious physical modelling could be attempted (Farr et al., 2021), which would most likely improve the predictive power of the model.
Taken together, in this study we have proposed and developed a role for chromatin compaction, in which compaction is tightly associated with histone modifications, influencing each other as part of a unified regulatory mechanism. This broader view complements the classical view of chromatin compaction as downstream of histone PTMs and regulatory circuits, by merely blocking transcription sterically. Our model and data, as well as previous literature, allow us to paint a more complete picture of the epigenetic regulation of the HMR locus in budding yeast, underscoring the importance of chromatin conformation and the physical dimension of the problem. We expect that the concepts developed here, such as the two-way feedback interaction between acetylation and chromatin compaction, may be relevant in many other gene regulatory circuits.
Materials and Methods
Model and implementation
A full description of the model, including all the details and parameter values can be found in the SI Appendix, Sections 1, 2 and 4. The implementation of the model, as well as the data generated for the figures in this paper, can be found in https://github.com/AMovillaMiangolarra/Conformation_Acetylation_Feedback_2023. The statistics shown in the figures of this work correspond to simulations sampled over 105 generations.
Experimental methods
Strain construction.
Strains and oligonucleotides used in this study are listed in Table S1 in the SI Appendix. Insertions of DNA into HMRα were achieved using CRISPR/Cas9 technology (Lee et al., 2015). To do this, sgRNAs that spanned the precise insertion sites were generated. Additionally, repair templates containing DNA from HML, K.lac Leu2, or K.lac Ura3 were amplified with flanking homology to HMRα, such that recombination of these templates with HMRα would insert DNA within the sgRNA cut site. Co-transformation of a Cas9/sgRNA plasmid with an appropriate repair template produced cells that constitutively cut the sgRNA-targeted site and thus remain arrested; these cells would only grow into a colony if a mutation, such as insertion of the desired repair template, disrupted the sgRNA-targeted site. All mutations were confirmed by sequencing.
Flow cytometry.
To allow bistable populations of cells to reach equilibrium prior to flow cytometry, cells were grown at log-phase for 24 hours. More specifically, three separate colonies (representing three technical replicates) of a given strain were used to inoculate three different cultures in YPD liquid media, which were grown to saturation at 30°C overnight. These cultures were then serially back-diluted in YPD liquid media and maintained at log-phase at 30°C over 24 hours. After 24 hours of log-phase growth, cells were pelleted, resuspended in 100 μL of 4% paraformaldehyde, fixed for 15 minutes at room temperature, pelleted, and then resuspended in 150 μL of 1x PBS solution.
Flow cytometry was performed with an LSR Fortessa (BD Biosciences) with a FITC filter for Green Fluorescent Protein (GFP). At least 30,000 cells were analyzed per replicate. FlowJo software (BD Biosciences) was utilized to analyze flow cytometry data. All cells in the experiment were gated identically.
Microscopy.
Prior to microscopy, cells were grown in YPD overnight at 30°C, and then to log-phase in the same conditions. 3 μL of 0.5 OD cells were then spotted onto CSM agar plates. Once the spots were dry, a sterile spatula was used to cut out a small square that encompassed the cell spots, which was then removed and inverted onto a 35 mm glass bottom dish (Thermo Scientific 150682). Cells were then imaged using a Zeiss ZA inverted fluorescence microscope with a Prime 95B sCMOS camera (Teledyne Photometrics), Plan-Apochromat 63x/1.40 objective (Zeiss), MS-2000 XYZ automated stage (Applied Scientific Instrumentation), and MicroManager imaging software (Open Imaging).
To generate timelapse images, samples were kept at 30°C and humidified by a P-set 2000 Heading Incubation Insert (PeCon). Brightfield and fluorescence images were collected for 16 fields of view per sample, every 20 mins for 10 hrs. A single Z-slice was acquired for each field of view at each timepoint. Images were analyzed with ImageJ (NIH). Silencing loss and silencing establishment events were counted manually with a single-blind approach.
Supplementary Material
Significance:
Chromatin is the complex formed by proteins, including histones, and DNA to form chromosomes. Specific chromatin structures and states are thought to be key factors regulating transcription. A common view proposes that histone modifications activate or inhibit transcription either via specific activation or inhibition of RNA polymerase binding/elongation at a locus, or by expanding/compacting the locus, thereby modulating its accessibility to many macromolecules. In this work, we elucidated a broader hypothesis that chromatin compaction may both inhibit transcription, and feedback via silencing proteins to remove histone modifications that further control chromatin compaction and correlate with gene activity. We developed a model incorporating these ideas and showed that it explains quantitative experimental data for a silent locus in budding yeast.
Acknowledgements
We thank all members of the Howard group for discussions. We also acknowledge financial support from BBSRC Institute Strategic Programme GEN (BB/P013511/1) to MH and a grant from the National Institutes of Health (R35GM139488) to JR.
References
- Allshire R. C., & Madhani H. D. (2018). Ten principles of heterochromatin formation and function. Nature Reviews Molecular Cell Biology, 19(4), 229–244. 10.1038/nrm.2017.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel A., Song J., Dean C., & Howard M. (2011). A Polycomb-based switch underlying quantitative epigenetic memory. Nature, 476(7358), 105–108. [DOI] [PubMed] [Google Scholar]
- Behrouzi R., Lu C., Currie M. A., Jih G., Iglesias N., & Moazed D. (2016). Heterochromatin assembly by interrupted Sir3 bridges across neighboring nucleosomes. Elife, 5, e17556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry S., Dean C., & Howard M. (2017). Slow chromatin dynamics allow polycomb target genes to filter fluctuations in transcription factor activity. Cell systems, 4(4), 445–457. e448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collepardo-Guevara R., Portella G., Vendruscolo M., Frenkel D., Schlick T., & Orozco M. (2015). Chromatin unfolding by epigenetic modifications explained by dramatic impairment of internucleosome interactions: a multiscale computational study. Journal of the American Chemical Society, 137(32), 10205–10215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVeale B., Liu L., Boileau R., Swindlehurst-Chan J., Marsh B., Freimer J. W., Abate A., & Blelloch R. (2022). G1/S restriction point coordinates phasic gene expression and cell differentiation. Nature communications, 13(1), 3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donze D., Adams C. R., Rine J., & Kamakaka R. T. (1999). The boundaries of the silenced HMR domain in Saccharomyces cerevisiae. Genes & development, 13(6), 698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr S. E., Woods E. J., Joseph J. A., Garaizar A., & Collepardo-Guevara R. (2021). Nucleosome plasticity is a critical element of chromatin liquid--liquid phase separation and multivalent nucleosome interactions. Nature communications, 12(1), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D. T. (1977). Exact stochastic simulation of coupled chemical reactions. The journal of physical chemistry, 81(25), 2340–2361. [Google Scholar]
- Goodnight D., & Rine J. (2020). S-phase-independent silencing establishment in Saccharomyces cerevisiae. Elife, 9, e58910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau D., Zhang Y., Lee C.-H., Valencia-Sánchez M., Zhang J., Wang M., Holder M., Svetlov V., Tan D., Nudler E., Reinberg D., Walz T., & Armache K.-J. (2021). Structures of monomeric and dimeric PRC2:EZH1 reveal flexible modules involved in chromatin compaction. Nature communications, 12(1), 714. 10.1038/s41467-020-20775-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute, S. A. S. (2012). SAS/STAT 12.1 User’s Guide. SAS Institute. https://books.google.co.uk/books?id=vLJLQEro0YMC [Google Scholar]
- Johnson A., Li G., Sikorski T. W., Buratowski S., Woodcock C. L., & Moazed D. (2009). Reconstitution of Heterochromatin-Dependent Transcriptional Gene Silencing. Molecular Cell, 35(6), 769–781. 10.1016/j.molcel.2009.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost D., & Vaillant C. (2018). Epigenomics in 3D: importance of long-range spreading and specific interactions in epigenomic maintenance. Nucleic acids research, 46(5), 2252–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani K., Sanford E. M., Goyal Y., & Raj A. (2022). Changes in chromatin accessibility are not concordant with transcriptional changes for single-factor perturbations. Molecular Systems Biology, 18(9), e10979. 10.15252/msb.202210979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm S. L., Shipony Z., & Greenleaf W. J. (2019). Chromatin accessibility and the regulatory epigenome. Nature Reviews Genetics, 20(4), 207–220. 10.1038/s41576-018-0089-8 [DOI] [PubMed] [Google Scholar]
- Kueng S., Oppikofer M., & Gasser S. M. (2013). SIR proteins and the assembly of silent chromatin in budding yeast. Annual Review of Genetics, 47, 275–306. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Grav L. M., Lewis N. E., & Faustrup Kildegaard H. (2015). CRISPR/Cas9-mediated genome engineering of CHO cell factories: Application and perspectives. Biotechnology Journal, 10(7), 979–994. 10.1002/biot.201500082 [DOI] [PubMed] [Google Scholar]
- Michieletto D., Chiang M., Coli D., Papantonis A., Orlandini E., Cook P. R., & Marenduzzo D. (2018). Shaping epigenetic memory via genomic bookmarking. Nucleic acids research, 46(1), 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michieletto D., Orlandini E., & Marenduzzo D. (2016). Polymer model with Epigenetic Recoloring Reveals a Pathway for the de novo Establishment and 3D Organization of Chromatin Domains. Phys. Rev. X, 6, 041047. 10.1103/PhysRevX.6.041047 [DOI] [Google Scholar]
- Nickels J. F., Edwards A. K., Charlton S. J., Mortensen A. M., Hougaard S. C. L., Trusina A., Sneppen K., & Thon G. (2021). Establishment of heterochromatin in domain-size-dependent bursts. Proceedings of the National Academy of Sciences, 118(15), e2022887118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickels J. F., & Sneppen K. (2023). Theoretical analysis of confinement mechanisms for epigenetic modifications of nucleosomes. bioRxiv, 2022.2012.2020.521095. 10.1101/2022.12.20.521095 [DOI] [Google Scholar]
- Ou H. D., Phan S., Deerinck T. J., Thor A., Ellisman M. H., & O’Shea C. C. (2017). ChromEMT: Visualizing 3D chromatin structure and compaction in interphase and mitotic cells. Science, 357(6349), eaag0025. 10.1126/science.aag0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen J. A., Osmanović D., & Mirny L. A. (2022). Design principles of 3D epigenetic memory systems. bioRxiv, 2022.2009. 2024.509332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarge E. (1979). Emil Heitz and the concept of heterochromatin: longitudinal chromosome differentiation was recognized fifty years ago. American journal of human genetics, 31(2), 106. [PMC free article] [PubMed] [Google Scholar]
- Pease N. A., Nguyen P. H. B., Woodworth M. A., Ng K. K. H., Irwin B., Vaughan J. C., & Kueh H. Y. (2021). Tunable, division-independent control of gene activation timing by a polycomb switch. Cell Reports, 34(12), 108888. 10.1016/j.celrep.2021.108888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillus L., & Rine J. (1989). Epigenetic inheritance of transcriptional states in S. cerevisiae. Cell, 59(4), 637–647. 10.1016/0092-8674(89)90009-3 [DOI] [PubMed] [Google Scholar]
- Preissl S., Gaulton K. J., & Ren B. (2022). Characterizing cis-regulatory elements using single-cell epigenomics. Nature Reviews Genetics, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P. J. J., An W., Routh A., Martino F., Chapman L., Roeder R. G., & Rhodes D. (2008). 30 nm Chromatin Fibre Decompaction Requires both H4–K16 Acetylation and Linker Histone Eviction. Journal of Molecular Biology, 381(4), 816–825. 10.1016/j.jmb.2008.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruault M., Scolari V. F., Lazar-Stefanita L., Hocher A., Loïodice I., Koszul R., & Taddei A. (2021). Sir3 mediates long-range chromosome interactions in budding yeast. Genome research, 31(3), 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein M., Colby R. H., & Others. (2003). Polymer physics (Vol. 23). Oxford university press; New York. [Google Scholar]
- Rusche L. N., Kirchmaier A. L., & Rine J. (2003). The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Annual review of biochemistry, 72(1), 481–516. [DOI] [PubMed] [Google Scholar]
- Sandholtz S. H., MacPherson Q., & Spakowitz A. J. (2020). Physical modeling of the heritability and maintenance of epigenetic modifications. Proceedings of the National Academy of Sciences, 117(34), 20423–20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton D. S., & Rine J. (2019). Epigenetic memory independent of symmetric histone inheritance. Elife, 8, e51421. 10.7554/eLife.51421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton D. S., & Rine J. (2022). Distinct silencer states generate epigenetic states of heterochromatin. Molecular Cell, 82(19), 3566–3579. [DOI] [PubMed] [Google Scholar]
- Schlissel G., & Rine J. (2019). The nucleosome core particle remembers its position through DNA replication and RNA transcription. Proceedings of the National Academy of Sciences, 116(41), 20605–20611. 10.1073/pnas.1911943116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shia W.-J., Osada S., Florens L., Swanson S. K., Washburn M. P., & Workman J. L. (2005). Characterization of the Yeast Trimeric-SAS Acetyltransferase Complex. Journal of Biological Chemistry, 280(12), 11987–11994. 10.1074/jbc.M500276200 [DOI] [PubMed] [Google Scholar]
- Sneppen K., & Dodd I. B. (2015). Cooperative stabilization of the SIR complex provides robust epigenetic memory in a model of SIR silencing in Saccharomyces cerevisiae. Epigenetics, 10(4), 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia-Sánchez M. I., De Ioannes P., Wang M., Truong D. M., Lee R., Armache J.-P., Boeke J. D., & Armache K.J. (2021). Regulation of the Dot1 histone H3K79 methyltransferase by histone H4K16 acetylation. Science, 371(6527), eabc6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela L., Dhillon N., Dubey R. N., Gartenberg M. R., & Kamakaka R. T. (2008). Long-range communication between the silencers of HMR. Molecular and cellular biology, 28(6), 1924–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Li G., Altaf M., Lu C., Currie M. A., Johnson A., & Moazed D. (2013). Heterochromatin protein Sir3 induces contacts between the amino terminus of histone H4 and nucleosomal DNA. Proceedings of the National Academy of Sciences, 110(21), 8495–8500. 10.1073/pnas.1300126110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterborg J. H. (2001). Dynamics of Histone Acetylation in Saccharomyces cerevisiae. Biochemistry, 40(8), 2599–2605. 10.1021/bi002480c [DOI] [PubMed] [Google Scholar]
- Wilkins B. J., Rall N. A., Ostwal Y., Kruitwagen T., Hiragami-Hamada K., Winkler M., Barral Y., Fischle W., & Neumann H. (2014). A Cascade of Histone Modifications Induces Chromatin Condensation in Mitosis. Science, 343(6166), 77–80. 10.1126/science.1244508 [DOI] [PubMed] [Google Scholar]
- Wu K., Dhillon N., Du K., & Kamakaka R. T. (2021). Measuring the buffering capacity of gene silencing in Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences, 118(49), e2111841118. 10.1073/pnas.2111841118 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.