Abstract
Recent advances in insect genetic engineering offer alternative genetic biocontrol solutions to control populations of pests and disease vectors. While success has been achieved, sex-sorting remains problematic for scaling many genetic biocontrol interventions. Here we describe the development of a sex-sorting technique for female and male selection with a proof-of-concept in D. melanogaster termed SEPARATOR (Sexing Element Produced by Alternative RNA-splicing of A Transgenic Observable Reporter). This approach utilizes dominant fluorescent proteins and differentially spliced introns to ensure sex-specific expression. The system has the potential for adaptability to various insect species and application for high-throughput insect sex-sorting.
Keywords: Sex sorting, Flies, SIT
Introduction
Genetic biocontrol methods are highly effective and sustainable alternatives to traditional insecticide-based approaches for suppressing pest and vector populations1,2. Sterile insect technique (SIT), for example, is a genetic biocontrol technology that has successfully eradicated screwworms in the United States3,4. This technology suppresses populations through the frequent releases of sterile males, as female insects are often responsible for destroying agricultural resources or pathogen transmission5. While SIT has proven to be effective in some species, it requires the release of only sterile males, necessitating the development of technologies to efficiently sort the sexes. This indeed remains a grand challenge for many genetic biocontrol technologies and what is needed is a technology that can be rapidly developed in many species to enable rapid and accurate sex sorting.
Early sex sorting was a labor-intensive process that involved manually sorting individuals by their sexual dimorphisms, severely limiting throughput and scalability. More recent approaches utilize machine learning to automate sex sorting6,7. However, these still rely on natural sexual dimorphisms, making them challenging to develop to other species.
Existing genetic approaches for sex-sorting insects also have limitations that impact their scalability. The classic Genetic Sex Separation (GSS) method involves irradiation-induced translocation of a conditional lethal gene, such as the gene conferring resistance to the insecticide dieldrin (Rdl), to the Y chromosome8. As a result, only males carrying the translocated gene on their Y chromosome can survive when exposed to the insecticide. This GSS method has succeeded in certain insect species, but overall this method is difficult to develop and prone to breakage via meiotic recombination.
A scalable sex-sorting method is essential to ensure cost-effective and scalable genetic technologies are available for the control of a variety of insect species. Incorporating fluorescent sex-specific markers facilitates the visual identification and separation of males and females with a Complex Parametric Analyzer and Sorter (COPAS, Union Biometrica) instrument. The COPAS has been used to sort D. melanogaster embryos and larvae based on fluorescence as well as transgenic Aedes aegypti and Anopheles gambiae larvae9,10. The COPAS instrument was used to fully automate the sorting process, achieving the efficiency, speed, and accuracy needed to scale genetic technologies10.
Here we describe the proof of principle for a sex-sorting technique termed SEPARATOR (Sexing Element Produced by Alternative RNA-splicing of A Transgenic Observable Reporter) that utilizes fluorescent proteins and sex-specific introns of the sex-determination gene transformer (tra) to ensure sex-specific expression. Female-specific expression is achieved by disrupting the coding sequences (CDS) of a fluorescent reporter with female-specific transformer introns (traF). These female-specific introns are only spliced in females, resulting in the restoration of gene function and female-specific fluorescence. In this project, traF from four separate species were tested, including Drosophila melanogaster, Drosophila suzukii, Ceratitis capitata, and Anastrepha ludens. When traF from D. melanogaster, D. suzukii, and C. capitata is inserted into the CDS of fluorescence protein, we achieved 100% female-specific fluorescence. Notably, C. capitata traF permits the selection of females as early as L1 instar larval stage. However, A. ludens traF resulted in dsRed expression in both sexes in D. melanogaster flies, indicating that A. ludens traF is not sex-specific when transcribed in D. melanogaster. This SEPARATOR technique is a valuable method for insect sex-sorting as it (1) exploits highly conserved sex-specific splicing mechanisms, making it widely transferable to different insect species; (2) allows 100% positive selection for either females or males based on fluorescence instead of morphological differences, providing better accuracy; (3) permits sex-sorting during different life stages and as early as L1 instar stage; (4) can be combined with existing genetic control methods and a COPAS machine for precise high-throughput screening.
Results
Development of female-specific expression of the fluorescent protein
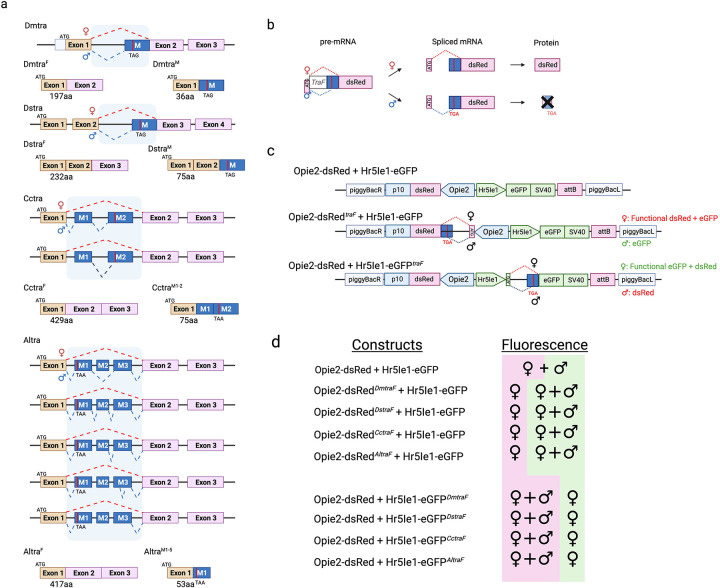
To engineer female-specific expression of a reporter for positive selection of females, we exploited the sex-specific alternative splicing of a conserved sex-determination gene. In D. melanogaster, the transformer (tra) intron between exons 1 and 2 is spliced out in females, resulting in a functional tra protein. In males, alternative tra splicing results in a premature stop codon that terminates the tra protein11. This female-specific alternative splicing mechanism occurs not only in Drosophila but also in Ceratitis and Anastrepha, suggesting that it is highly conserved in Dipterans (Fig. 1a, Fig. s1 & s2)12. Consequently, the traF from D. melanogaster, D. suzukii, C. capitata, or A. ludens were inserted into the fluorescent protein coding sequences to test for female-specific fluorescent protein expression (Fig. 1b). We generated two sets of dual fluorescent marker constructs encoding a fluorescent marker for both sexes and a female-specific fluorescent marker (Fig. 1c). The constructs were cloned into a plasmid containing an attP recombination site and a piggyBac (PB) transposable element. Set 1 constructs have an eGFP fluorescence expressed under a ubiquitous promoter Hr5Ie1 (Hr5Ie1-eGFP) as the selectable marker for the transgene. To promote constitutive expression of female-specific fluorescent proteins, we used another ubiquitous promoter Opie2 to express dsRed and inserted traF immediately downstream of the ATG translational start codon of dsRed (Opie2-ATG-traF-dsRed). Constructs in set 2 have the opposite marker configuration, with traF inserted downstream of the ATG translational start codon of eGFP under promoter Hr5Ie1 (Hr5Ie1-ATG-traF-eGFP) for the female-specific fluorescent expression and Opie2-dsRed as the selectable marker for the transgene. In total, nine constructs were created: a control construct (795G) and eight experimental constructs, with four constructs in each set (Fig. 1d).
Fig. 1.
Sex-sorter cassette in Drosophila. (a) Sex-specific alternative splicing and the resulting protein of the transformer (tra) in D. melanogaster, D. suzukii, C. capitata, and A. ludens. (b) Splicing of the female-specific transformer (TraF) intron should result in functional dsRed protein in females but not in males. (c) Schematic of the sex-sorter constructs engineered and tested in the study. TraF introns from D. melanogaster, D. suzukii, C. capitata, and A. ludens are inserted into the coding sequence of either dsRed or eGFP after the ATG translational start codon. (d) Fluorescence expression of females and males carrying the respective constructs.
DmtraF, DstraF, and CctraF resulted in female-specific fluorescence
The transgene integration site can impact gene expression, so we opted to integrate all nine constructs into the same site through phiC31 attP integration on the second chromosome (BDSC #25709). Nine homozygous transgenic strains were established. Six constructs that harbor DmtraF, DstraF, and CctraF resulted in female-specific fluorescence (795H,I,J,L,M, and N, Fig. 2). These results indicate that inserting the traF into the coding sequence of the fluorescence proteins can result in female-specific fluorescence. However, for constructs harboring AltraF (795K and O), both females and males exhibited the intended female-specific fluorescence (Fig. 2). This result suggests that AltraF is spliced out in both females and males rather than in a female-specific manner.
Fig. 2.
Expression of Opie2-TraF-dsRed or Hr5ie1-TraF-eGFP transgenes in D. melanogaster can be observed in different developmental stages (a) L1-L3 larval stage, (b) pupal stage, (c) adult under white light, RFP and GFP filters.
To validate the alternative splicing variants, adult flies were collected for RT-PCR analysis to obtain the fluorescent protein transcripts. Primers were designed to anneal to the 5’ UTR region at the 3’end of the Opie2 promoter, and the 3’ end of the dsRed sequence (Supplementary Table 1). Multiple bands were obtained from the RT-PCR samples for CctraF males, and both sexes of DmtraF and DstraF (Fig. s3a & b). Sequencing of these bands indicates that CctraF, DmtraF, and DstraF resulted in functional dsRed expression explicitly in females, while AltraF had dsRed expression in both males and females (Fig. s3c). The molecular results obtained from RT-PCR analysis were consistent with our observations in the flies, confirming that CctraF, DmtraF, and DstraF exhibited female-specific splicing in D. melanogaster. This outcome demonstrates the feasibility of this fluorescent sex-sorting approach, as these female-specific splicing events allow for the positive selection of either sex.
Confirmation of sex-specific fluorescence at multiple developmental stages
Next, we evaluated the intensity and sex specificity of the fluorescence over multiple life stages. The six constructs (795H, I, J, L, M, and N) that exhibited female-specific fluorescent expression were evaluated. Female-specific fluorescence was observed as early as in the first instar larvae (L1) life stage in both CctraF transgenic lines: 795H and 795L (Fig 2a, Fig 3, Supplymentary Table 2). Female-specific fluorescence was also observed in the third instar larvae (L3) of DmtraF 795I and CctraF 795L, and the pupal stage for DmtraF 795M and DstraF 795N. Despite the identical introns in the DmtraF 795I and DmtraF 795M and the CctraF 795L and DstraF 795N strains, female-specific fluorescence was detected earlier in strains with the female-specific dsRed marker. This result is presumably due to the deeper tissue penetrance and lower auto-fluorescence of red fluorescent protein (RFP)13. Notably, the intensity of the female-specific fluorescence varies among introns. The CctraF exhibits the highest brightness, followed in order of brightness by DmtraF and DstraF (Fig. 2). This is unexpected as CctraF is an exogenous/non-native intron for D. melanogaster, potentially hindering successful intron recognition and splicing efficiency.
Fig. 3.
Female selection efficiency at different life stages in D. melanogaster for all six sex-sorter cassettes that give female-specific fluorescence and the numbers of scored flies are indicated for each bar.
Assessing the fitness of the sex-sorting strains
Strain fitness is essential for scalability. Fluorescent proteins have documented fitness costs to genetically engineered organisms, but we expected that including traF in their coding sequences would minimally affect the fitness of the sex-sorting strain. We, therefore, compared the egg hatching and larval to adult survival rate of all eight homozygous sex-sorting strains to a control strain (795G) containing fluorescent reporters lacking traF introns. Our finding indicates that there is a significantly higher hatching rate in flies harboring Opie2-ATG-traF-dsRed constructs when compared to 795G control (Fig. 4). This effect could possibly be attributed to the fitness cost associated with dsRed functioning as a tetramer14. With the inclusion of traF introns, the expression of dsRed occurs at a reduced level. As a consequence, the fitness cost is diminished, which, in turn, leads to a higher hatching rate. We observed lower larvae to adult survival only in the CctraF-dsRed 795H strain (p< 0.05, Student’s t-test with equal variance, Fig. 4). These results suggest that the traF intron does not impose substantial fitness costs on the strain, making them suitable for potential large-scale insect population control projects.
Fig. 4.
Fitness cost of all eight sex-sorting cassettes was accessed through two parameters: (a) egg-hatching rate and (b) survival rate to adulthood. Fitness cost was observed in CctraF-dsRed strain in the parameter of survival rate to adulthood (*p<0.05, ***p<0.001, Student’s t-test with equal variance.)
Discussion
In this study, we engineered a novel sex-sorting method, SEPARATOR, that integrates a female-specific intron into the coding sequencing of a fluorescent reporter to generate an efficient female-specific marker. This innovative technique uses splicing mechanisms conserved across dipteran species, and has even been successfully engineered in other dipteran species15. We examined the splicing patterns of four traF introns, DmtraF, DstraF, CctraF, and AltraF, and found that three of them (DmtraF, DstraF, and CctraF) had 100% female-specific splicing. Among the six constructs tested for female-specific fluorescence expression in D. melanogaster, those carrying the CctraF introns (795H and 795L) exhibited expression at the earliest life stage and the brightest expression. These strains can be used for sex-sorting as early as the L1 stage and throughout the entire life cycle. This observation aligns with previous studies demonstrating the functional conservation of CctraF and its applicability in various insect species16–18.
It is worth noting the significant size difference between the traF introns in Drosophilidae species (DmtraF and DstraF) and Tephritidae species (CctraF and AltraF). The traF introns in Drosophilidae species are approximately 200 bp in length, whereas in Tephritidae species, they are approximately ten times longer. Although AltraF is not female-specifically spliced, it is spliced out entirely in both females and males, thus resulting in functional fluorescent expression in both sexes. This result could be attributed to species differences in splicing signals as AltraF is a foreign intron in D. melanogaster. These size differences may also impact intron splicing efficiency and, consequently, the dual sex expression of the AltraF fluorescent reporter. Larger introns, for example, possess more heterogeneous nuclear ribonucleoproteins (hnRNP) and serine/arginine-rich (SR) proteins recognition sites, leading to higher splicing efficiency19,20. However, further testing in Tephritidae species like C. capitata or A. ludens may better elucidate the capabilities of AltraF intron for SEPARATOR systems.
The CctraF introns, on the other hand, generate efficient and easily screenable female-specific fluorescent phenotypes. This efficiency may be due to the simplified splicing of CctraF, which does not depend on the presence of the sex-lethal (sxl) protein required in Drosophila for tra transcript splicing16,21–24. In C. capitata, the tra gene is autoregulated and continuously expressed from the embryo to adulthood16, allowing for the consistent and stable splicing of CctraF. Therefore, CctraF may be a versatile tool for sex-specific expression in a wide range of insect species.
SEPARATOR has numerous advantages over traditional sexing methods. These include its potential multiple species adaptability, positive selection based on dominant fluorescence for both sexes, sex differentiation during early stages of development, genetic stability, and minimal impact on fitness. All of these characteristics improve the potential scalability and timing of sex sorting. The fluorescent sex-sorting cassettes were engineered to be highly transferable to numerous insect species. They utilize a PB transposable element, which has high transgenesis efficiency in many insect species25. The two constitutive baculovirus promoters for fluorescent protein expression, Opie2 and Hr5Ie1, have been shown to facilitate high expression in various insects throughout development26–28. Furthermore, dsRed and eGFP are the two most commonly used fluorescent proteins and can penetrate most insect tissues29. Female-specific alternative splicing of tra intron is also highly conserved across insect species24,30.
The SEPARATOR technology enables the positive selection of females, or males, in all post-embryonic developmental stages. Sex sorting at earlier developmental stages would be advantageous for large-scale insect population control projects, as it would extend the sex sorting timeline, facilitate the release of earlier life stages, and may reduce the costs of large-scale releases31. Our approach simplifies manual sex sorting, but the primary benefit is to large-scale sex-sorting applications. SEPARATOR can be combined with existing genetic control methods and a COPAS machine for precise and high-throughput screening. Fluorescence can also serve as a reliable indicator of undesired genetic events such as loss-of-function (LOF), gain-of-function (GOF), or genetic exchange such as chromosomal translocation and recombination that would result in loss of fluorescence. The GSS used for many current SIT applications rely on reciprocal chromosomal translocations between the Y-chromosome and a region of the autosome containing a selectable marker. These GSS methods have been designed for male-only release programs, where females possess distinct phenotypes or markers that facilitate easy identification and separation32. However, these GSS methods are prone to genetic instability and can be disrupted by meiotic recombination or chromosomal rearrangements. Studies on the multi-generation of GSS lines have revealed that chromosomal recombination can occur at a frequency of approximately 0.07%, leading to the breakdown of the sexing system33. To enhance stability, one potential solution is to generate new translocations with a breakpoint located in close proximity to the sexing genes33.
Although the main emphasis of this work has sex-sorting applications, SEPARATOR can be used to study fly development. Differentiation between female and male adult flies is easily accomplished under the microscope, but accurate larvae sex-sorting can be challenging, particularly when they are embedded within the food medium. The major distinguishing factor between female and male larvae is the presence or absence of gonads, which is not easily visible34. With the CctraF 795H or 795L sex-sorter cassettes, the fluorescent protein markers can be easily observed as early as first instar larvae, even in the food medium and with minimal autofluorescence. The ability to accurately sex-sort larvae at early developmental stages provides a valuable tool for tracking the sex of flies and could potentially facilitate the study of sex-specific developmental processes in flies.
Materials and Methods
Molecular cloning
All genetic constructs were produced utilizing the Gibson enzymatic assembly. The construct 795G was created using a pre-existing plasmid containing piggyBac, attB-docking sites, and an Opie2 promoter regulating dsRed. This plasmid was subsequently linearized with XhoI and NotI enzymes. The Hr5Ie1 promoter, along with eGFP were cloned into the linearized plasmid to make 795G, which serves as the control plasmid. To generate female-specific dsRed (795H-K), the plasmid 795G was linearized with AvrII and BamHI to allow insertion of introns into dsRed. Alternatively, to generate female-specific eGFP (795L-O), 795G was linearized using MluI and BsrGI to insert introns into eGFP. The traF introns from D. melanogaster, D. suzukii, C. capitata, or A. ludens were amplified from their respective genomic DNA using the primers listed in the Supplementary Table 1.
Reverse transcription PCR (RT-PCR) of the female-specific splicing transcripts
To access the splicing transcripts of four traF introns, we screened for female- and male-specific dsRed mRNA. Total RNA of ten virgin females or males from w-, 795G, H, I, J, and K were extracted using the miRNeasy Tissue/Cells Advanced Kits (Qiagen). DNase treatment is done using the TURBO™ DNA-free (Invitrogen), and followed by the cDNA synthesis using the RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific™). The genomic DNA (gDNA) was amplified using primers 795.s2F and 795.s2R, and the cDNA was amplified using primers 795.s3F and 795.s1R (Supplementary Table 1). The gDNA samples were run on 1% TAE agarose gel, and the cDNA samples were run on a 2% TAE agarose gel.
Rearing and fly transgenesis.
Transgenic flies were maintained under standard conditions at 25°C with a 12H/12H light/dark cycle and fed on the Old Bloomington Molasses Recipe. Embryonic injections were performed in the lab following the standard injection protocol. Plasmids diluted to 300–350ng/μL in water were inserted at P{CaryP}attP40 on the 2nd chromosome (Bloomington #25709). Recovered transgenic lines were balanced on the 2nd chromosome using a single chromosome balancer line w1118; CyO/sna[Sco]. Multiple independent lines were obtained for each plasmid and tested for sex-specific fluorescence. We used homozygous transgenic lines containing two copies of the transgene to assess the sex-sorting efficiency. Sex-sorting lines with CctraF introns 795H and 795L are deposited at the Bloomington Drosophila Stock Center (BDSC# pending).
Genetics and sex selection
To assess the fluorescent sex selection efficiency, we crossed ten virgin females to ten males in a fly vial. The parental flies were flipped into a fresh vial every 12hrs, and the numbers of the laid embryos were scored. After hatching, the larvae or pupa were scored and transferred to different vials based on their fluorescent markers. The sex and the fluorescent markers of the adult offsprings were recorded after eclosion. Flies were scored using a Leica M165FC fluorescent stereomicroscope. Images were taken using a View4K camera. Each genetic cross was set up five times using different parental flies.
Fitness estimation
The fitness of the sex-sorting strains is assessed based on two parameters: the rate of egg-hatching (from embryos to larvae) and the rate of adult survival (from larvae to adult). To evaluate the egg-hatching rate, flies are allowed to lay embryos in fly vials for a duration of 24 hrs, and the number of eggs laid in each vial is recorded. After 24 hrs of egg laying, the number of larvae is recorded. To assess the adult survival rate, the number of both female and male adult flies that successfully eclosed is recorded.
Statistical analysis
Statistical analysis was performed in Prism9 by GraphPad Software, LLC. Three to five biological replicates were used to generate statistical means for comparisons.
Supplementary Material
Acknowledgements
This work was funded by the United States Department of Agriculture (USDA) - Animal and Plant Health Inspection Service (APHIS) - Plant Protection and Quarantine (PPQ) (AP19PPQS&T00C237 and AP22PPQS&T00C188) and funding from an NIH award (R01AI151004) awarded to O.S.A. The views, opinions, and/or findings expressed are those of the authors and should not be interpreted as representing the official views or policies of the U.S. government. Figures were created with BioRender.com.
Footnotes
Competing interests
O.S.A is a founder of Agragene, Inc. and Synvect, Inc. with equity interest. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies. All other authors declare no competing interests.
Data availability
Complete sequence maps and plasmids are deposited at Addgene.org (#205481-205489). Transgenic lines 795H and 795L have been made available for order from Bloomington Drosophila stock center. The other transgenic lines are available upon request to O.S.A.
References
- 1.Alphey N. & Bonsall M. B. Genetics-based methods for agricultural insect pest management. Agric. For. Entomol. 20, 131–140 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leftwich P. T. et al. Genetic pest management and the background genetics of release strains. Philos. Trans. R. Soc. Lond. B Biol. Sci. 376, 20190805 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bushland R. C., Lindquist A. W. & Knipling E. F. Eradication of Screw-Worms through Release of Sterilized Males. Science 122, 287–288 (1955). [DOI] [PubMed] [Google Scholar]
- 4.Scott M. J., Concha C. & Welch J. B. Review of research advances in the screwworm eradication program over the past 25 years. Entomologia (2017). [Google Scholar]
- 5.Knipling E. F. Possibilities of Insect Control or Eradication Through the Use of Sexually Sterile Males1. J. Econ. Entomol. 48, 459–462 (1955). [Google Scholar]
- 6.Crawford J. E. et al. Efficient production of male Wolbachia-infected Aedes aegypti mosquitoes enables large-scale suppression of wild populations. Nat. Biotechnol. 38, 482–492 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Zheng X. et al. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature 572, 56–61 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Focks D. A. An improved separator for the developmental stages, sexes, and species of mosquitoes (Diptera: Culicidae). J. Med. Entomol. 17, 567–568 (1980). [DOI] [PubMed] [Google Scholar]
- 9.Furlong E. E., Profitt D. & Scott M. P. Automated sorting of live transgenic embryos. Nat. Biotechnol. 19, 153–156 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Lutrat C. et al. Sex Sorting for Pest Control: It’s Raining Men! Trends Parasitol. 35, 649–662 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Boggs R. T., Gregor P., Idriss S., Belote J. M. & McKeown M. Regulation of sexual differentiation in D. melanogaster via alternative splicing of RNA from the transformer gene. Cell 50, 739–747 (1987). [DOI] [PubMed] [Google Scholar]
- 12.Li F., Vensko S. P. II, Belikoff E. J. & Scott M. J. Conservation and Sex-Specific Splicing of the transformer Gene in the Calliphorids Cochliomyia hominivorax, Cochliomyia macellaria and Lucilia sericata. PLoS One 8, e56303 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shcherbakova D. M., Subach O. M. & Verkhusha V. V. Red Fluorescent Proteins: Advanced Imaging Applications and Future Design. Angew. Chem. Int. Ed Engl. 51, 10724 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matz M. V. et al. Fluorescent proteins from nonbioluminescent Anthozoa species. Nat. Biotechnol. 17, 969–973 (1999). [DOI] [PubMed] [Google Scholar]
- 15.Weng S.-C., Antoshechkin I., Marois E. & Akbari O. S. Efficient Sex Separation by Exploiting Differential Alternative Splicing of a Dominant Marker in Aedes aegypti. bioRxiv 2023.06.16.545348 (2023) doi: 10.1101/2023.06.16.545348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pane A., Salvemini M., Delli Bovi P., Polito C. & Saccone G. The transformer gene in Ceratitis capitata provides a genetic basis for selecting and remembering the sexual fate. Development 129, 3715–3725 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Pane A., De Simone A., Saccone G. & Polito C. Evolutionary conservation of Ceratitis capitata transformer gene function. Genetics 171, 615–624 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perrotta M. M. et al. Female Sex Determination Factors in Ceratitis capitata: Molecular and Structural Basis of TRA and TRA2 Recognition. Insects 14, 605 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanchette M. et al. Genome-wide analysis of alternative pre-mRNA splicing and RNA-binding specificities of the Drosophila hnRNP A/B family members. Mol. Cell 33, 438–449 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaul O. How introns enhance gene expression. Int. J. Biochem. Cell Biol. 91, 145–155 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Inoue K., Hoshijima K., Sakamoto H. & Shimura Y. Binding of the Drosophila sex-lethal gene product to the alternative splice site of transformer primary transcript. Nature 344, 461–463 (1990). [DOI] [PubMed] [Google Scholar]
- 22.Valcárcel J., Singh R., Zamore P. D. & Green M. R. The protein Sex-lethal antagonizes the splicing factor U2AF to regulate alternative splicing of transformer pre-mRNA. Nature 362, 171–175 (1993). [DOI] [PubMed] [Google Scholar]
- 23.Saccone G. et al. The Ceratitis capitata homologue of the Drosophila sex-determining gene sex-lethal is structurally conserved, but not sex-specifically regulated. Development 125, 1495–1500 (1998). [DOI] [PubMed] [Google Scholar]
- 24.Bopp D., Saccone G. & Beye M. Sex determination in insects: variations on a common theme. Sex Dev. 8, 20–28 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Yusa K. piggyBac Transposon. Microbiol Spectr 3. (2015). [DOI] [PubMed]
- 26.Masumoto M., Ohde T., Shiomi K., Yaginuma T. & Niimi T. A Baculovirus immediate-early gene, ie1, promoter drives efficient expression of a transgene in both Drosophila melanogaster and Bombyx mori. PLoS One 7, e49323 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Theilmann D. A. & Stewart S. Molecular analysis of the trans-activating IE-2 gene of Orgyia pseudotsugata multicapsid nuclear polyhedrosis virus. Virology 187, 84–96 (1992). [DOI] [PubMed] [Google Scholar]
- 28.Jarvis D. L., Weinkauf C. & Guarino L. A. Immediate-early baculovirus vectors for foreign gene expression in transformed or infected insect cells. Protein Expr. Purif. 8, 191–203 (1996). [DOI] [PubMed] [Google Scholar]
- 29.Chudakov D. M., Matz M. V., Lukyanov S. & Lukyanov K. A. Fluorescent proteins and their applications in imaging living cells and tissues. Physiol. Rev. 90, 1103–1163 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Verhulst E. C., van de Zande L. & Beukeboom L. W. Insect sex determination: it all evolves around transformer. Curr. Opin. Genet. Dev. 20, 376–383 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Gendron W. et al. Cost-effectiveness of Precision Guided SIT for Control of Anopheles gambiae in the Upper River Region, The Gambia. bioRxiv 2023.07.20.549762 (2023) doi: 10.1101/2023.07.20.549762. [DOI] [Google Scholar]
- 32.Porras M. F., Meza J. S., Rajotte E. G., Bourtzis K. & Cáceres C. Improving the Phenotypic Properties of the Ceratitis capitata (Diptera: Tephritidae) Temperature-Sensitive Lethal Genetic Sexing Strain in Support of Sterile Insect Technique Applications. J. Econ. Entomol. 113, 2688–2694 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franz G. Genetic Sexing Strains in Mediterranean Fruit Fly, an Example for Other Species Amenable to Large-Scale Rearing for the Sterile Insect Technique. in Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management (eds. Dyck V. A., Hendrichs J. & Robinson A. S.) 427–451 (Springer; Netherlands, 2005). [Google Scholar]
- 34.Coutelis J. B., Petzoldt A. G., Spéder P., Suzanne M. & Noselli S. Left-right asymmetry in Drosophila. Semin. Cell Dev. Biol. 19, 252–262 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Complete sequence maps and plasmids are deposited at Addgene.org (#205481-205489). Transgenic lines 795H and 795L have been made available for order from Bloomington Drosophila stock center. The other transgenic lines are available upon request to O.S.A.