Abstract
Bacteria use a diverse range of carbohydrates to generate a profusion of glycans, with amino sugars such as N-acetylglucosamine (GlcNAc) being prevalent in the cell wall and in many exopolysaccharides. The primary substrate for GlcNAc-containing glycans, UDP-GlcNAc, is the product of the bacterial hexosamine pathway, and a key target for bacterial metabolic glycan engineering. Using the strategy of expressing NahK, to circumvent the hexosamine pathway, it is possible to directly feed the analogue of GlcNAc, N-azidoacetylglucosamine (GlcNAz), for metabolic labelling in E. coli. The cytosolic production of UDP-GlcNAz was confirmed using fluorescence assisted polyacrylamide gel electrophoresis. The key question of where GlcNAz is incorporated, was interrogated by analyzing potential sites including: peptidoglycan (PGN), the biofilm-related exopolysaccharide poly-β-1,6-N-acetylglucosamine (PNAG), lipopolysaccharide (LPS) and the enterobacterial common antigen (ECA). The highest levels of incorporation were observed in PGN with lower levels in PNAG and no observable incorporation in LPS or ECA. The promiscuity of the PNAG synthase (PgaCD) towards UDP-GlcNAz in vitro and lack of undecaprenyl-pyrophosphoryl-GlcNAz intermediates generated in vivo confirmed the incorporation preferences. The results of this work will guide the future development of carbohydrate-based probes and metabolic engineering strategies.
Graphical Abstract
INTRODUCTION
The envelopes of bacterial cells contain glycans essential for viability, where they maintain cellular integrity, while other glycans are relevant to human health and disease through their roles as virulence factors, and diagnostic markers. Metabolic labelling is a tool for studying bacterial glycobiology in which chemical probes are incorporated into glycan structures.1 In general, a functionalized analog of a specific monosaccharide is fed to bacteria which then generates the activated nucleotide sugar via endogenous processing. Recognition of the unnatural metabolite as a glycosyltransferase substrate leads to installation in a target glycan.
In bacteria, there are two general strategies for metabolic glycan labelling. First, using an uncommon monosaccharide, or a monosaccharide in a dedicated glycan synthesis pathway, allows for incorporation into a narrow range of target glycans. For example, the prokaryotic sugar bacillosamine is found in the glycoproteins of certain species, allowing selective labelling of these organisms in a diverse microbial community.2 Analogues of rare deoxy amino l-sugars such as N-acetylrhamnosamine and N-acetylquinovosamine can also be tracked to their glycan destinations to discriminate between bacterial species and/or strains.3 Additionally, synthetic precursors for sialic,4 pseudaminic,5,6 and legionaminic acids7,8 have been shown to incorporate into their respective glycoconjugates despite requiring several enzymatic steps to generate the nucleotide sugar donors. For a more detailed discussion on chemical reporters for bacterial glycans, the reader is directed to a recent review.9
Often the desired transformations to yield a target nucleotide sugar analogue in cellulo are not present. To circumvent this challenge, pathway engineering in Escherichia coli has enabled labelling with fucose analogues by replacing the native GDP-fucose pathway with a salvage pathway from Bacterioides fragilis.10 Metabolic engineering has also been applied to the installation of N-acetylmuramic acid (MurNAc) derivatives in peptidoglycan assembly using a recycling pathway from Pseudomonas putida.11
In contrast to targeting a specific glycan for labelling, it is possible to choose a monosaccharide that is central to many glycans and leverage the importance of the metabolite to obtain information on a range of pathways. This approach requires additional validation due to the number of possible labeled structures that can be formed. Analogues of GlcNAc are an interesting choice as a metabolite to explore multiple glycan pathways, as GlcNAc is an efficient food source12 and is also involved in the assembly of peptidoglycan, lipopolysaccharide, exopolysaccharides and capsular polysaccharides. A classic chemical reporter for GlcNAc is the C2-modified N-azidoacetylglucosamine probe GlcNAz13, first used to characterize the O-GlcNAc modification in eukaryotic cells14. However, later studies reported that UDP-GlcNAz was also epimerized to UDP-GalNAz and incorporated into O-linked glycans, emphasizing the importance of characterizing the fate of monosaccharides in glycan labelling.15,16 Additionally, studies in other eukaryotes have demonstrated variability in the usage of GlcNAz, suggesting that metabolic flux or transferase selectivity’s vary sufficiently to alter the distribution of incorporation.17,18 In bacteria, GlcNAz has been shown to be used by Helicobacter pylori to glycosylate flagellin,19 and in Staphylococcus aureus cell surface azides were detectable through azide-clickable fluorophores with GlcNAz feeding.20 These studies demonstrate the viability of targeting bacterial glycoconjugates with GlcNAz, but raise questions about into which glycans GlcNAz is incorporated.
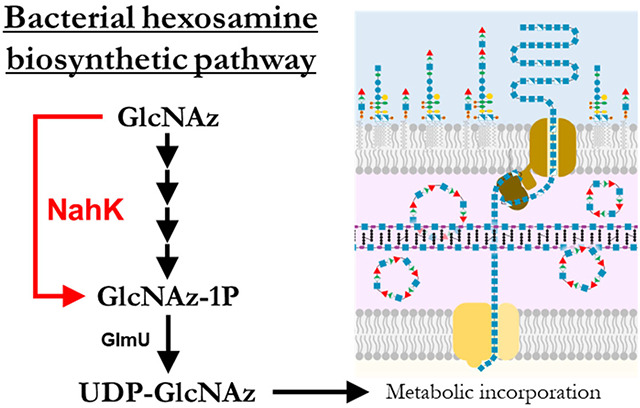
To label glycans with GlcNAz, the key metabolite UDP-GlcNAz must be generated intracellularly. It is not possible to feed GlcNAz to E. coli as the GlcNAc salvage pathway involves de-N-acetylation of the 6-phospho intermediate (GlcNAc-1P) by NagA (Figure 1a). One strategy to circumvent this challenge that was successful in lactic acid bacteria is to feed the acetylated 1-phosphate species which presumably is cell permeable and is deacetylated in cellulo before entering the HBP pathway.21 However, many bacteria, including E. coli, are deficient in the non-specific esterase activity necessary to reveal the active probe, limiting the scope of this strategy.22 In an alternative approach modified pathways have been introduced into E. coli; for example an engineered glycosyltransferase OleD facilitated the formation of unnatural nucleotide sugars from aryl-glycosides.23 Using 2-chloro-4-nitrophenyl GlcNAz in E. coli expressing OleD, the authors were able to demonstrate the in situ production of UDP-GlcNAz and subsequent incorporation of GlcNAz into peptidoglycan.24 In another approach we have used the promiscuous Bifidobacterial enzyme NahK to generate a set of C6-modified UDP-GlcNAc derivatives in cellulo as glycosyltransferase inhibitors.25 NahK is a GlcNAc 1-kinase with a high tolerance for synthetic and modified substrates including GlcNAz,26,27 and has been used in chemoenzymatic syntheses of various 1-phosphosugars.28 It was hypothesized that GlcNAz could be transformed to UDP-GlcNAz in E. coli with endogenous expression of recombinant NahK, and subsequently incorporated into the profusion of amino sugar-containing bacterial glycans.
Figure 1.
A) Hexosamine biosynthetic pathway (in black). GlcNAc and GlcN are imported or recycled from peptidoglycan. The fate of metabolites is decided by the relative activities of NagB and GlmS. Shown in red is the alternative pathway provided by the GlcNAc 1-kinase NahK, allowing GlcNAc to be directly phosphorylated to GlcNAc-1P.
B) Confocal laser scanning microscopy. E. coli BW25113 pBAD(nahK) cells were grown (4 hrs at 37 °C) with GlcNAz (1 mM) or AzAcOH (5 mM). Cells were incubated with DBCO-Cy5 (10 μM; 1 hr at 37 °C) and counterstained with DAPI before widefield (TL BF). Experiment was performed twice with similar results. Scale bar is 1 μM.
C) GlcNAz incorporation into whole cells. E. coli BW25113 pBAD(nahK) were grown (4 hrs at 37 °C) with azide species: GlcNAz (GN; 1 mM) or AzAcOH (AA; 5 mM). Cells were incubated with DBCO-Cy5 (10 μM; 1 hr at 37 °C) and measured in bulk for retained Cy5 fluorescence after washing; values are normalized to the number of cells per sample. Error bars represent ±SD of 3 biological replicates; significance was determined using unpaired t-tests. **P ≤ 0.002.
Here we sought to define the use of UDP-GlcNAz in E. coli K12 based on the well studied glycan biosynthetic machinery which produce the enterobacterial common antigen (ECA), lipopolysaccharide (LPS), and poly-N-acetylglucosamine (PNAG). We find the NahK system produces UDP-GlcNAz in situ, that GlcNAz is readily incorporated into peptidoglycan, that incorporation into ECA and LPS cannot be detected, and that small amounts are incorporated into PNAG. Discerning the incorporation targets of UDP-GlcNAz in E. coli at a molecular level validates the strategy of using sugar 1-kinases to achieve metabolic carbohydrate engineering and as a tool to follow glycan assembly in E. coli.
RESULTS
In vivo incorporation
The incorporation of GlcNAz into E. coli BW25113 cells via the alternative hexosamine salvage pathway introduced by the expression of the hexosamine-1-kinase (NahK) from Bifidobacterium longum JCM1217 using a pBAD expression system was evaluated25. Cells expressed either wild-type NahK (NahKWT) or an inactive mutant (NahKmut) as a negative control for NahK activity. The incorporation of azides into the bacteria was visualized through strain-promoted alkyne-azide cycloaddition (SPAAC) with dibenzocyclooctyne-Cy5 dye (DBCO-Cy5) by confocal microscopy. Cy5 labelling of E. coli is confined mainly to the cell periphery and is both GlcNAz- and NahK-specific; bacteria expressing the inactive NahKmut do not display any pronounced peripheral staining (Figure 1B). To confirm that a potential product of GlcNAz catabolism, 2-azidoacetic acid (AzAcOH), was not the source of azide labelling, AzAcOH was added to the cultures, but no Cy5 labelling was observed, suggesting that no significant transfer of 2-azidoacyl groups from GlcNAz to the cell surface is occurring. To complement the microscopy results, bulk cell fluorescence analysis was carried out and gave similar NahK and GlcNAz-dependent labelling (Figure 1C, Figure S1). The degree of labelling was dependent on the GlcNAz concentration (Figure S2) however at higher concentrations decreases in cell growth were observed (Figure S3). Microscopy of labelled cells grown at high GlcNAz (>1 mM) concentrations revealed unusual cell morphology consistent with disrupted peptidoglycan biosynthesis (data not shown).
Nucleotide sugar detection
The whole cell measurements demonstrated successful incorporation of azide species in a NahK-dependent fashion. It was expected that this was occurring via the hexosamine processing pathway, where the product of NahK, GlcNAz-1-phosphate (GlcNAz-1P), would be converted by GlmU to the central metabolite UDP-GlcNAz for subsequent glycan synthesis (Figure 1a). To support this hypothesis, GlcNAz-fed cells were assayed for the presence of UDP-GlcNAz. The initial attempt to identify the unnatural metabolite involved a previously described ion-pair reverse phase HPLC experiment with tetrabutylammonium phosphate as the ion-pairing reagent.25 However, UDP-GlcNAz has an unexpectedly long retention time using these conditions (Figure S4) leading to peak broadening, and using higher mobile phase concentrations to reduce elution time led to rapid column degradation when running metabolite extracts (data not shown).
Gel electrophoresis was seen as a promising alternative method as it has been applied to the analysis of carbohydrates including nucleotide sugars such as UDP-GlcNAc.29 Two fluorophores were investigated for visualization of the GlcNAz derivatives by PAGE in an approach similar to fluorescence assisted carbohydrate electrophoresis (FACE). Labelling with DBCO-Cy3; negatively charged, or DBCO-TAMRA; neutral charge, imparts different electrophoretic mobilities to the GlcNAz derivatives in the PAGE analysis (Figure S5). Both fluorophore conjugates readily resolved GlcNAz, GlcNAz-1P and UDP-GlcNAz, however TAMRA labelling gave moderately better results due to less background fluorescence entering the gel and only negatively-charged species migrating (Figure 2A). Additional confirmation that visualized bands represent phosphorylated GlcNAz derivatives comes through acid-catalyzed hydrolysis of the sugar-phosphate bond, converting the phosphorylated species to tagged GlcNAz.
Figure 2.
A) GlcNAz metabolic processing. GlcNAz imported into the bacterial cytosolic space is phosphorylated by the recombinant NahK kinase to generate GlcNAz-1P, which is then recognized by the uridyltransferase domain of the essential enzyme GlmU leading to in cellulo production of UDP-GlcNAz.
B) FACE gel of metabolite extract. Nucleotide sugar extracts of GlcNAz-fed E. coli BW25113 pBAD(nahK) cells (2-3 hrs at 37 °C; 1 mM GlcNAz) were selectively heated with acid to hydrolyze sugar phosphates (Acid), incubated with DBCO-TAMRA to tag azides (10 μM; 16 hrs at 37 °C), and spiked with UDP-GlcNAz migration standard (Spiked). FACE gel was run with migration standards of tagged azide species and imaged for TAMRA fluorescence. Note, TAMRA is neutral at gel running pH and only negatively charged species migrate. Arrows indicate migration distances of various tagged azide species.
Crude metabolite extracts of mid-log phase E. coli cells were collected and selectively treated with acid before incubation with DBCO-Cy3 or TAMRA. Lanes with extracts obtained from GlcNAz-fed cells show a faint band with similar migration to the UDP-GlcNAz standard lane (Figure 2B, Figure S6). This band overlaps a sample spiked with UDP-GlcNAz and is absent in cell extracts that lack GlcNAc-1-kinase activity (NahKmut lanes). In samples pretreated with acid to hydrolyze glycosidic phosphate bonds the UDP-GlcNAz band is lost, with the accompanying emergence of a tagged-GlcNAz band seen in FACE gels using Cy3 labelling (Figure S6). Other unidentified signals are also present in these analyses. Most notably, a band corresponding to a species with a similar migration to tagged AzAcOH appears in all cell extract samples, even those not exposed to any azide containing species. This band likely represents multiple species and may contain AzAcOH or other azide derivatives such as GlcNAz-6P, as well as non-azide species that react with DBCO. In an effort to reduce the complexity of gel lanes, methoxypolyethylene glycol azide (PEG-Az) was added to gel samples following tagging with dye, to sequester any unreacted dye or other azide-reactive components of the sample and retard their migration through the gel (Figure S7). No major deconvolution was achieved through this strategy, demonstrating the excess dye is likely reacting through non-specific pathways with other sample components and generating unwanted side products30.
Peptidoglycan incorporation
Given the cytosolic UDP-GlcNAz generation, and the clear azide-dependent labelling in E. coli, the key question was into which glycans are the azides incorporated? A major destination for GlcNAc in bacteria is the peptidoglycan; both monosaccharides in the alternating GlcNAc(β1→4)-N-acetylmuramic acid (MurNAc) backbone are derived from GlcNAc (Figure S8). This repeating unit is generated as a lipid-linked intermediate (Lipid II) on the cytosolic leaflet of the cell membrane before being flipped and transferred to the nascent peptidoglycan chain.31 As has been previously shown in OleD-expressing E. coli, UDP-GlcNAz is used by MurG to generate Lipid II.24
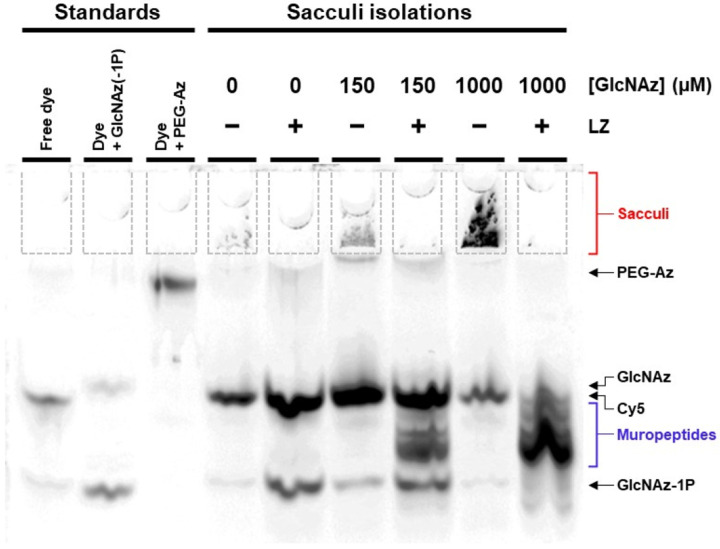
To confirm the presence of GlcNAz in PG through the NahK system, sacculi were isolated from GlcNAz-treated bacteria possessing NahK activity at mid-late log phase.32 Peptidoglycan samples, both as whole sacculi and lysozyme treated samples, were labelled with DBCO-Cy5 and visualized on polyacrylamide FACE gels (Figure 3, Figure S9).
Figure 3.
FACE gel of peptidoglycan sacculi. Isolated sacculi (marked in red) from GlcNAz-fed E. coli BW25113 pBAD(nahK) cells (6 hrs at 37 °C) were selectively treated with lysozyme (LZ; 40 mg/mL; 16 hrs at 37 °C) to generate muropeptides (marked in blue) and incubated with DBCO-Cy5 to tag azides (10 μM; 1 hr at 37 °C). FACE gel was run with migration standards of various tagged azide species (GlcNAz(−1P) standard was generated as an incomplete NahK reaction with GlcNAz) and imaged for Cy5 fluorescence. Arrows indicate migration distances of various tagged azide species. Sample wells are outlined in dashed lines for ease of viewing.
In non-hydrolyzed samples, the large insoluble sacculi do not migrate into the gel and remain in the sample wells, where they can be seen as a fluorescent mass in samples derived from GlcNAz-fed cells. Lysozyme specifically cleaves the PGN glycan backbone, producing a series of smaller soluble fragments, which appear as a ladderlike pattern of bands running above the standard GlcNAz-1P. The soluble samples were analyzed by ESI-MS but no peaks corresponding to azide modifications were observed, likely indicating low levels of GlcNAz incorporation, however the identity of the PG fragments could be clearly observed.
Wzx/Wzy-dependent glycans
In the chosen model Gram-negative system, both the enterobacterial common antigen (ECA) and lipopolysaccharide (LPS) can be found. In the trisaccharide repeating unit of ECA, 2 of the 3 sugars originate from UDP-GlcNAc; N-acetylmannosaminuronic acid (ManNAcA) and GlcNAc (Figure S8).33 LPS is built up from a phosphoglycolipid core, lipid A, composed of a GlcN disaccharide pair which is extensively modified with acyl chains (Figure S8). Additionally, the outer core in K12 strains is non-stoichiometrically modified with a terminal GlcNAc34,35; E. coli K12 has no elaborate O-antigen due to the loss of a rhamnosyltransferase and therefore possesses rough-type LPS.36 The transfer of GlcNAc to ECA and to the terminal sugar in the LPS outer core proceeds via undecaprenyl-pyrophosphoryl-GlcNAc (also known as bactoprenol-pyrophosphoryl-GlcNAc, GlcNAc-BPP) formed by WecA (also known as Rfe). GlcNAc-BPP initiates the biosynthesis of the repeating units of both ECA and the E. coli K12 O-antigen (Figure 4a). The lipid-linked subunits are further modified by other glycosyltransferases and flipped from the cytoplasmic to the periplasmic leaflet of the inner membrane, where they are polymerized by the Wzx/Wzy machinery and transferred to the final carrier lipids: core oligosaccharide (OS) to make lipooligosaccharide (LOS) or OS-linked ECA (ECALPS), with ECA repeats also used to generate cyclical glycans (ECACYC) or installed on diacylglycerol (ECAPG) through a phosphodiester linkage.33
Figure 4.
A) Biosynthesis of Wzx/Wzy-dependent glycans. The transferase WecA generates the shared lipid-linked GlcNAc precursor in the synthesis of both ECA and O-antigen using bactoprenol (BP). In E. coli K12 strains the rhamnosyltransferase WbbL is not expressed leading to truncation of O-antigen production, but the ligase WaaL is capable of transferring a single GlcNAc onto the Lipid A-core acceptor (in grey).
B, C) RP-LC-MS SIM of BPP-linked intermediates. Lysates from ECA knockout strains of E. coli MG1655 pBAD(nahK) grown with GlcNAz were analyzed by LC-MS, monitoring (B) BPP-GlcNAc and (C) BPP-GlcNAz.
The lipid-linked structures of LPS and certain forms of ECA allow a shared isolation and characterization to be conducted.37 To investigate GlcNAz incorporation into either structure, E. coli BW25113 cells were cultured with GlcNAz, harvested and dried in vacuo in preparation for phenol-chloroform-pentanes (PCP) extraction of ECA and rough-type LPS (Figure S10).38 An additional K12 strain, AB113 rfe::Tn10 is deficient in ECA production and terminal GlcNAc installation onto core OS, and was used as a negative control for glycan biosynthesis.39 The resulting extracts were labelled with DBCO-Cy5 and analyzed via SDS-PAGE (Figure S11). Comparing the fluorescence imaging of the gels and the silver-stained signals, there is no evidence of any DBCO-Cy5 labelling, suggesting that UDP-GlcNAz is not tolerated as a substrate for glycan incorporation, or that GlcNAz-containing glycans are exceedingly low abundance.
To determine which step in the pathway failed to use the GlcNAz derivative, the BPP conjugates were analyzed in a ΔwecG strain which is known to accumulated GlcNAc-BPP.40 Overnight cultures were lysed and analyzed by reverse phase high performance liquid chromatography–mass spectrometry (RP-LC-MS) in selective ion monitoring (SIM) mode for either GlcNAc-BPP (m/z=1128.7) or GlcNAz-BPP (m/z=1156.8) (Figure 4.). Additional controls display unmodified bactoprenol (BP) acceptor (m/z=845.7) (Figure S12). These experiments clearly showed the expected presence of GlcNAc-BPP but failed to show ions consistent with GlcNAz-BPP in the presence of NahK and GlcNAz, agreeing with WecA failing to generate GlcNAz-BPP in this system.
Biofilm exopolysaccharide
Another likely site of GlcNAz incorporation is the exopolysaccharide poly-β-1,6-N-acetylglucosamine (PNAG). This polysaccharide is produced by a variety of distantly related microorganisms,41 and is best known for its role as a matrix polymer in bacterial biofilms.42 PNAG is partially deacetylated (Figure S8), giving it an overall positive charge which is thought to aid in its role as a biofilm matrix component by promoting cell-to-cell and cell-to-surface contacts.43
In E. coli and other Gram-negative organisms PNAG is produced on the cytosolic side of the cell membrane by the glycosyltransferase complex PgaCD from UDP-GlcNAc which polymerizes and translocates the growing chain across the membrane.44 A related enzyme complex exists in Gram-positive organisms.45 The nascent PNAG chain is then partially-deacetylated before being exported to the extracellular environment to form the biofilm matrix. C6-modified UDP-GlcNAc analogues are incorporated into PNAG and lead to truncation of the polymer, however the specificity of the synthases for the acetamido moiety has not been evaluated.25
To ascertain whether GlcNAz could be incorporated into cell-produced PNAG, a whole cell approach with a ΔcsrA PNAG-overproducing strain of E. coli was used.46 GlcNAz and NahK activity did not appear to affect the development of static biofilms as analyzed by crystal violet biomass staining (Figure S13). After growth of E. coli MG1655 csrA::kanB strains in liquid culture, PNAG was extracted using treatment with concentrated ammonium bicarbonate which was found to readily solubilize the PNAG. Previously high salt concentrations have been found to solubilize PNAG, and we found the volatile salt worked similarly47 (Figure S14). Successful PNAG isolation was confirmed through a dot blot assay using a lectin-peroxidase conjugate (WGA-HRP) with limited signal observed from strains that do not overproduce PNAG or upon treatment with PNAG-specific hydrolase Dispersin B (DspB).25 Extract from an E. coli strain that does not overproduce exopolysaccharide fails to generate blot-based luminescence, while the positive signal of other extracts is mostly eradicated upon treatment with the PNAG-specific hydrolase Dispersin B (DspB)48; this is not the case upon treatment with the non-specific protease Proteinase K (ProtK) (Figure S15). Analyzing the extracted material via SDS-PAGE showed protein contamination, including abundant membrane protein OmpC (Figure S16),49 necessitating a protease treatment to deconvolute PNAG staining on polyacrylamide gels.47
Stationary cell cultures of E. coli MG1655 csrA::kanB grown with GlcNAz were washed, the PNAG was extracted and analyzed via dot blot (Figure 5a). Upon seeing no significant difference in PNAG extracts between ΔcsrA cell cultures in the presence of GlcNAz, samples were proteolyzed, selectively treated with DspB, and labelled with DBCO-Cy5 before PAGE analysis (Figure 5b,c). The amount of PNAG material stained via Coomassie Brilliant Blue is seen to vary extraction-to-extraction however bulk quantification of PNAG amounts between strains was not seen to vary. The sole sample displaying fluorescent signal is the extract derived from cell cultures fed with GlcNAz and expressing active NahK, and hydrolysis with DspB appears to increase the electrophoretic mobility of this signal, suggesting the smaller molecular weight oligosaccharides are being generated.
Figure 5.
A) PNAG extract dot blot. PNAG extracts from E. coli pBAD(nahK) cultures (2 days at rt) were spotted on nitrocellulose membranes and probed with WGA-HRP. Select samples were pretreated with the PNAG-specific hydrolase DspB (5 μM; 4 hrs at 37 °C), or the broad-spectrum protease ProtK (1 mg/mL; 4 hrs at 37 °C). Overproducing strains of PNAG (ΔcsrA) generate more signal than the wildtype control strain.
B, C) PNAG extract PAGE. Deproteinized PNAG extracts from GlcNAz-fed E. coli pBAD(nahK) cultures (2 days at rt) were labelled with azide-reactive DBCO-Cy5 (10 μM; 1 hr at rt) and analyzed by gel electrophoresis using (B) Cy5 fluorescence and (C) Coomassie stain. Select samples were pretreated with the PNAG-specific hydrolase DspB; effect is apparent as a disappearance of the slow-migrating species (marked in green) and generation of a higher-migration species (marked in orange).
D) Cell-associated PNAG assay. GlcNAz-fed E. coli csrA::kanB pBAD(nahK) cells (4 hrs at 37 °C) were labelled with DBCO-Cy5 to tag azides (10 μM; 1 hr at rt), and incubated with PNAG-binder GFP-DspBE184Q. Cells were washed with buffer and DspB enzyme to hydrolyze PNAG. Data plotted represents the GFP and Cy5 signals in the DspB-solubilized buffer fraction. GFP values are normalized to the fluorescence of the initial labelling solution; Cy5 values are relative to a blank sample without added dye. Error bars represent ±SD of 3 biological replicates; significance was determined using unpaired t-tests. **P ≤ 0.0037, ns = not significant.
As further evidence for the incorporation of GlcNAz into PNAG, a previously-developed assay for quantifying cell-surface PNAG levels was adapted to measure GlcNAz incorporation levels (Figure 5d).50 Briefly, cells at mid-log were harvested and labelled with both DBCO-Cy5 and a GFP-tagged PNAG-binding probe. Sequential washes in 0.22 μm spin filter microcentrifuge tubes then allow for enzymatic treatments with total retention of cells and analysis of the resulting solution. Treatment with DspB will hydrolyze cell-surface PNAG, resulting in the release of both Cy5 and GFP fluorescence into the filtrate; the former an indicator of GlcNAz incorporation, the latter a measure of total PNAG amount. All samples displayed similar levels of PNAG production, but the cells expressing active NahK enzyme also showed high Cy5 fluorescent signal when fed GlcNAz during culturing (Figure S17).
Following detection of fluorophore-conjugated PNAG species, attempts were made to obtain mass spectral support for incorporation. However in the DspB treated samples only unmodified PNAG oligomers were observed (Figure S18).
In vitro PNAG incorporation
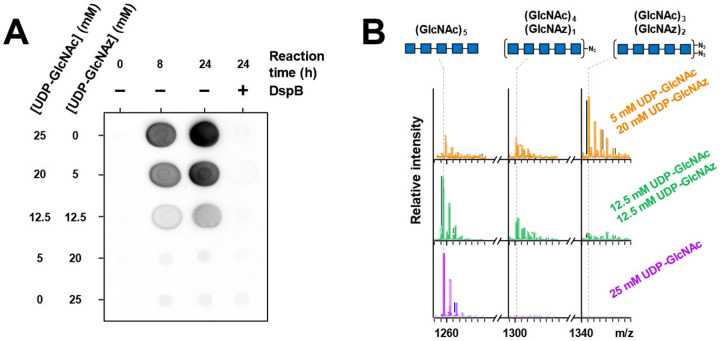
To shed light on low incorporation levels of GlcNAz into PNAG, an in vitro PNAG biosynthesis system with the E. coli synthase complex was explored. Using a previously described membrane preparation25 of the E. coli PgaCD synthase complex,51 UDP-GlcNAz was tested as a substrate for in vitro polymerization. The unnatural nucleotide sugar was chemoenzymatically synthesized from GlcNAz following a previously reported protocol,28 and PNAG product formation in reaction mixture aliquots was visualized using dot blots (Figure 6a).
Figure 6.
A) PgaCD polymerase reaction. Using varying ratios of UDP-GlcNAc and -GlcNAz as sugar donors, reaction mixtures incubated with PgaCD membrane preparations (24 hrs at 37 °C) were spotted on nitrocellulose membranes. Reaction endpoints were also selectively treated with DspB to hydrolyze PNAG (5 μM; 4 hrs at 37 °C), thereby resulting in an apparent loss of product. WGA-HRP was used for visualization of reaction progress.
B) ESI-MS of PgaCD reaction. PgaCD reaction mixtures were digested with DspB (5 <M; 90 min at 37 °C). Sodiated pentasaccharide ions [M+Na]+ containing 0, 1, or 2 GlcNAz units are displayed (see SI for full spectra of each reaction). Peak intensities are relative to the highest intensity signal in each respective spectrum.
The signal from a 24-hour reaction is completely eradicated upon treatment with the DspB hydrolase, confirming the PNAG origin of the luminescent signal. Extending the reaction to further timepoints did not give an increase in product (data not shown). When present as the sole sugar nucleotide, UDP-GlcNAz does not appear to be a competent substrate. Samples with combinations of UDP-GlcNAc and UDP-GlcNAz show product formation, however UDP-GlcNAz appears to inhibit product formation. The binding selectivity of WGA for GlcNAz is not known but it is expected that the lectin would continue to give a faithful measurement of PNAG containing some GlcNAz, as WGA is capable of recognizing single units of terminal or internal GlcNAc in a polymer.52 The inhibitory behaviour of UDP-GlcNAz indicates it is a poor substrate and potentially weak inhibitor of PgaCD (Figure S19).
To detect the potential low levels of incorporation of GlcNAz into PNAG, reaction mixtures were partially hydrolyzed with DspB to obtain soluble oligosaccharides prior to fractionation on a graphitized carbon column. Digested samples were submitted directly for ESI-MS analysis. Mass spectral signals consistent with oligosaccharides containing GlcNAz were observed confirming that UDP-GlcNAz is a poor but tolerated substrate of PgaCD (Figure 6b, Figure S20). As the UDP-GlcNAz:UDP-GlcNAc ratio of the reaction was increased, the number of GlcNAz units present per oligosaccharide is also seen to increase in a concentration-dependent manner, however a clear preference for UDP-GlcNAc can be observed by the limited GlcNAz incorporation at equivalent donor concentrations. (Figure S21, Figure S22, Figure S23, Figure S24, Figure S25).
DISCUSSION
GlcNAz in the hexosamine pathway
The route of GlcNAz uptake to the cytosol in E. coli is a subject of ongoing studies, but the amount available for conversion to GlcNAz-1P by NahK must be sufficient to allow for competition with endogenous GlcNAc-1P production even at lower feeding concentrations (~250 μM). Cytosolic GlcNAz could be undergoing processing by the GlcNAc-6-kinase NagK and deacetylase NagA via the native HBP pathway, leading to production of GlcN-1P and AzAcOH, however AzAcOH is unlikely to be a major contributor to the degree of cell-envelope azide incorporation, as control experiments with AzAcOH did not lead to azide labelling. Additionally, the structure of NagA shows a constrained binding pocket for the acetamido group, making it unlikely that GlcNAz-6P would be accepted as a substrate.53 Conversely the co-crystal structure of GlcNAc bound to a NagK ortholog does appear to permit bulkier substituents at the C2-position suggesting GlcNAz-6P maybe produced.54
GlcNAz in bacterial labelling
A series of strategies have been developed to label the peptidoglycan of bacteria and the NahK/GlcNAz combination described here offers a useful complementary method. MurNAc can be conveniently labeled in PG using MurNAc derivatives11 and unnatural d-amino acids55 can be used to label the peptide side chains. Here we show that GlcNAz derivatives have a strong preference for labelling the peptidoglycan over other GlcNAc containing glycans in E.coli K-12. The use of NahK expressing cells provides an alternative means to achieve sufficient UDP-GlcNAz concentrations in the cytosol to the previously reported use of glycosyl transferase OleD in combination with a phenolic GlcNAz glycoside.24 Unlike the feeding strategy using the free sugar, the production of UDP-GlcNAz from the glycoside also liberates the corresponding nitrophenol during OleD reaction, potentially leading to buildup of a toxic compound.56,57 Additionally, the turnover of GlcNAz-labelled peptidoglycan likely differs between OleD or NahK-expressing bacteria, as the peptidoglycan fragments released by the lytic transglycosylases are imported and metabolized to GlcNAc.58 Thus, GlcNAz could be recycled to UDP-GlcNAz in the presence of NahK, and be reincorporated. In contrast, no pathway is available in the OleD system to recycle cytosolic GlcNAz.
UDP-GlcNAz was also incorporated into PNAG by E. coli albeit at low levels due to the preference of PgaCD for GlcNAc over GlcNAz. Interestingly, in vitro reactions without added UDP-GlcNAc with UDP-GlcNAz as the soul substrate for PgaCD, showed mass spectra corresponding to oligosaccharides with a mix of both GlcNAc and GlcNAz. This may be due to residual UDP-GlcNAc or PNAG oligomers carried over in the enzyme purification. The latter explanation is consistent with slow release of bound-PNAG oligomers, as has been previously suggested in inhibition studies using chain terminating inhibitors.25 Furthermore, there were no oligomers observed that contain exclusively GlcNAz suggesting UDP-GlcNAz is unable to initiate the polymer synthesis. The in vitro experiments with PgaCD using defined nucleotide sugar concentrations give informative bounds on the likely cytosolic ratio of UDP-GlcNAc to UDP-GlcNAz. In vitro, it was challenging to detect GlcNAz incorporated PNAG by ESI-MS at donor ratios below 1:1, and no incorporation was detected in vivo suggesting the concentration of UDP-GlcNAz must be significantly below that of UDP-GlcNAc. In practical terms the low level of UDP-GlcNAz incorporation into PNAG, and the small amounts of PNAG produced by E. coli in the absence of biofilm formation suggest that for most applications this incorporation will not be significant.
The substrate specificity of WecA has not previously been investigated despite the central role this enzyme plays in the synthesis of Wzx/Wzy-dependent glycans ECA and O-antigen in many bacterial strains. The lack of recognition of GlcNAz suggests WecA has a narrow substrate scope. It is tempting to speculate that the specificity may be due to efforts by the bacteria to avoid toxicity that may occur if unusable lipid linked intermediates accumulate diminishing the pool of undecaprenol.59 It is important to note that in other bacterial strains GlcNAc is introduced into the O-antigen repeat at positions other than the reducing terminus, for example E.coli O3 O-antigen contains GlcNAc within the repeat.60 The substrate specificity of the glycosyltransferases introducing these GlcNAc residues will need to be determined before one can rule out incorporation of GlcNAz into O-antigen in these strains.
Other GlcNAc modifications
E. coli uses UDP-GlcNAc as the starting metabolite to generate UDP-ManNAcA. The epimerase WecB first catalyzes the conversion to UDP-ManNAc, which is used by the dehydrogenase WecC to make UDP-ManNAcA. This sugar nucleotide is then used in the biosynthesis of ECA. This would provide a route for UDP-GlcNAz to be incorporated in ECA as ManNAzA, avoiding the need for WecA transferase activity. Previous literature however suggest that the unnatural structure would again run into substrate tolerance issues, as a homologue of WecB in Neisseria meningitidis was found to be unable to epimerize the C2 position of UDP-GlcNAz.61 Other monosaccharides derived from GlcNAc are possible labelling targets, for example GalNAz potentially derived by an epimerase could lead to incorporation into O-antigens containing GalNAc such as E. coli O23A.60 With the knowledge that WecA does not accept GlcNAz it would be possible to interrogate the incorporation of GlcNAz into these strains.
CONCLUSION
Through engineering an alternate metabolic route in the bacterial hexosamine biosynthetic pathway, the utilization of the well-known GlcNAc metabolic probe GlcNAz has been characterized in an E. coli K12 strain, the most widely used laboratory strain of bacteria. In addition to validating the tolerance of peptidoglycan assembly machinery towards GlcNAz as a substrate, the PNAG biosynthetic complex was also found to be capable of using the unnatural sugar for labelling PNAG. A new gentle extraction method for PNAG was also described. Furthermore, the intolerance of WecA towards GlcNAz demonstrates that modification at the C2 position is not tolerated, informing future development of carbohydrate-based metabolic probes or antimicrobial compounds destined for ECA or O-antigen. The ability to generate unnatural UDP-GlcNAz in cellulo starting from the free sugar could be used in other organisms, with the same well-conserved HBP, or used to investigate other amino sugar-containing glycans. Furthermore, this strategy could be used to develop functional polysaccharides for use in biotechnology applications.
Supplementary Material
ACKNOWLEDGEMENTS
Many thanks to the lab of Dr. Chris Whitfield for the gift of an E. coli rfe::Tn10 strain, Matthew Jorgenson University of Arkansas Medical Sciences for the ECA knockout strains of E. coli and to Dr. Matt Forbes and Chung Woo Fung of the Advanced Instrumentation for Molecular Structure (AIMS) mass spectrometry facility for helpful advice regarding sample analysis.
FUNDING
This work was supported in part by a Natural Sciences and Engineering Research Council (NSERC) Grant (to M.N.). National Institutes of Health (NIH) Grant R01GM123251 (to J.M.T) Canadian Institutes of Health Research (CIHR) Grant FDN154327 (to P.L.H.). This work has also been supported by Canada Graduate Scholarships from NSERC (to A.S.S. and Z.A.M.), the Ontario Graduate Scholarship Program (A.E.), and the Hospital for Sick Children Foundation Student Scholarship Program (to A.S.S.).
Footnotes
SUPPORTING INFORMATION
Additional experimental details, materials, and methods are included in the SI.
REFERENCES
- (1).Dube D. H.; Bertozzi C. R. Metabolic Oligosaccharide Engineering as a Tool for Glycobiology. Curr. Opin. Chem. Biol. 2003, 7 (5), 616–625. 10.1016/j.cbpa.2003.08.006. [DOI] [PubMed] [Google Scholar]
- (2).Clark E. L.; Emmadi M.; Krupp K. L.; Podilapu A. R.; Helble J. D.; Kulkarni S. S.; Dube D. H. Development of Rare Bacterial Monosaccharide Analogs for Metabolic Glycan Labeling in Pathogenic Bacteria. ACS Chem. Biol. 2016, 11 (12), 3365–3373. 10.1021/acschembio.6b00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Luong P.; Ghosh A.; Moulton K. D.; Kulkarni S. S.; Dube D. H. Synthesis and Application of Rare Deoxy Amino l -Sugar Analogues to Probe Glycans in Pathogenic Bacteria. ACS Infect. Dis. 2022, 8 (4), 889–900. 10.1021/acsinfecdis.2c00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Rigolot V.; Rossez Y.; Biot C.; Lion C. A Bioorthogonal Chemistry Approach to Detect the K1 Polysialic Acid Capsule in Escherichia Coli. RSC Chem. Biol. 2022, 4 (2), 173–183. 10.1039/d2cb00219a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Liu F.; Aubry A. J.; Schoenhofen I. C.; Logan S. M.; Tanner M. E. The Engineering of Bacteria. Bearing Azido-Pseudaminic Acid-Modified Flagella. ChemBioChem 2009, 10 (8), 1317–1320. 10.1002/cbic.200900018. [DOI] [PubMed] [Google Scholar]
- (6).Andolina G.; Wei R.; Liu H.; Zhang Q.; Yang X.; Cao H.; Chen S.; Yan A.; Li X. D.; Li X. Metabolic Labeling of Pseudaminic Acid-Containing Glycans on Bacterial Surfaces. ACS Chem. Biol. 2018, 13 (10), 3030–3037. 10.1021/acschembio.8b00822. [DOI] [PubMed] [Google Scholar]
- (7).Pons J. M.; Dumont A.; Sautejeau G.; Fugier E.; Baron A.; Dukan S.; Vauzeilles B. Identification of Living Legionella Pneumophila Using Species-Specific Metabolic Lipopolysaccharide Labeling. Angew. Chemie - Int. Ed. 2014, 53 (5), 1275–1278. 10.1002/anie.201309072. [DOI] [PubMed] [Google Scholar]
- (8).Meng X.; Boons G. J.; Wösten M. M. S. M.; Wennekes T. Metabolic Labeling of Legionaminic Acid in Flagellin Glycosylation of Campylobacter Jejuni Identifies Maf4 as a Putative Legionaminyl Transferase. Angew. Chemie - Int. Ed. 2021, 60 (47), 24811–24816. 10.1002/anie.202107181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Banahene N.; Kavunja H. W.; Swarts B. M. Chemical Reporters for Bacterial Glycans: Development and Applications. Chem. Rev. 2022, 122 (3), 3336–3413. 10.1021/acs.chemrev.1c00729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Yi W.; Liu X.; Li Y.; Li J.; Xia C.; Zhou G.; Zhang W.; Zhao W.; Chen X.; Wang P. G. Remodeling Bacterial Polysaccharides by Metabolic Pathway Engineering. Proc. Natl. Acad. Sci. 2009, 106 (11), 4207–4212. 10.1073/pnas.0812432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Liang H.; DeMeester K. E.; Hou C. W.; Parent M. A.; Caplan J. L.; Grimes C. L. Metabolic Labelling of the Carbohydrate Core in Bacterial Peptidoglycan and Its Applications. Nat. Commun. 2017, 8. 10.1038/ncomms15015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Plumbridge J.; Vimr E. Convergent Pathways for Utilization of the Amino Sugars N- Acetylglucosamine, N-Acetylmannosamine, and N-Acetylneuraminic Acid by Escherichia Coli. J. Bacteriol. 1999, 181 (1), 47–54. 10.1128/jb.181.1.47-54.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Lauehlin S. T.; Bertozzi C. R. Metabolic Labeling of Glycans with Azido Sugars and Subsequent Glycan-Profiling and Visualization via Staudinger Ligation. Nat. Protoc. 2007, 2 (11), 2930–2944. 10.1038/nprot.2007.422. [DOI] [PubMed] [Google Scholar]
- (14).Vocadlo D. J.; Hang H. C.; Kim E. J.; Hanover J. A.; Bertozzi C. R. A Chemical Approach for Identifying O-GlcNAc-Modified Proteins in Cells. Proc. Natl. Acad. Sci. U. S. A. 2003, 100 (16), 9116–9121. 10.1073/pnas.1632821100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Boyce M.; Carrico I. S.; Ganguli A. S.; Yu S. H.; Hangauer M. J.; Hubbard S. C.; Kohler J. J.; Bertozzi C. R. Metabolic Cross-Talk Allows Labeling of O-Linked β-N- Acetylglucosamine-Modified Proteins via the N-Acetylgalactosamine Salvage Pathway. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (8), 3141–3146. 10.1073/pnas.1010045108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Zaro B. W.; Yang Y. Y.; Hang H. C.; Pratt M. R. Chemical Reporters for Fluorescent Detection and Identification of O-GlcNAc-Modified Proteins Reveal Glycosylation of the Ubiquitin Ligase NEDD4-1. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (20), 8146–8151. 10.1073/pnas.1102458108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Breidenbach M. A.; Gallagher J. E. G.; King D. S.; Smart B. P.; Wu P.; Bertozzi C. R. Targeted Metabolic Labeling of Yeast N-Glycans with Unnatural Sugars. Proc. Natl. Acad. Sci. U. S. A. 2010, 107 (9), 3988–3993. 10.1073/pnas.0911247107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Zhu Y.; Wu J.; Chen X. Metabolic Labeling and Imaging of N-Linked Glycans in Arabidopsis Thaliana . Angew. Chemie 2016, 128 (32), 9447–9451. 10.1002/ange.201603032. [DOI] [PubMed] [Google Scholar]
- (19).Koenigs M. B.; Richardson E. A.; Dube D. H. Metabolic Profiling of Helicobacter Pylori Glycosylation. Mol. Biosyst. 2009, 5 (9), 909–912. 10.1039/b902178g. [DOI] [PubMed] [Google Scholar]
- (20).Memmel E.; Homann A.; Oelschlaeger T. A.; Seibel J. Metabolic Glycoengineering of Staphylococcus Aureus Reduces Its Adherence to Human T24 Bladder Carcinoma Cells. Chem. Commun. 2013, 49 (66), 7301–7303. 10.1039/c3cc43424a. [DOI] [PubMed] [Google Scholar]
- (21).Sadamoto R.; Matsubayashi T.; Shimizu M.; Ueda T.; Koshida S.; Koda T.; Nishimura S. I. Bacterial Surface Engineering Utilizing Glucosamine Phosphate Derivatives as Cell Wall Precursor Surrogates. Chem. - A Eur. J. 2008, 14 (33), 10192–10195. 10.1002/chem.200801734. [DOI] [PubMed] [Google Scholar]
- (22).Antonczak A. K.; Simova Z.; Tippmann E. M. A Critical Examination of Escherichia Coli Esterase Activity. J. Biol. Chem. 2009, 284 (42), 28795–28800. 10.1074/jbc.M109.027409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Gantt R. W.; Peltier-Pain P.; Singh S.; Zhou M.; Thorson J. S. Broadening the Scope of Glycosyltransferase-Catalyzed Sugar Nucleotide Synthesis. Proc. Natl. Acad. Sci. U. S. A. 2013, 110 (19), 7648–7653. 10.1073/pnas.1220220110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Xu Y.; Hernández-Rocamora V. M.; Lorent J. H.; Cox R.; Wang X.; Bao X.; Stel M.; Vos G.; van den Bos R. M.; Pieters R. J.; et al. Metabolic Labeling of the Bacterial Peptidoglycan by Functionalized Glucosamine. iScience 2022, 25 (8), 104753. 10.1016/j.isci.2022.104753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Morrison Z. A.; Eddenden A.; Subramanian A. S.; Howell P. L.; Nitz M. Termination of Poly- N-Acetylglucosamine (PNAG) Polymerization with N-Acetylglucosamine Analogues. ACS Chem. Biol. 2021. 10.1021/acschembio.1c00855. [DOI] [PubMed] [Google Scholar]
- (26).Cai L.; Guan W.; Kitaoka M.; Shen J.; Xia C.; Chen W.; Wang P. G. A Chemoenzymatic Route to N-Acetylglucosamine-1-Phosphate Analogues: Substrate Specificity Investigations of N-Acetylhexosamine 1-Kinase. Chem. Commun. 2009, No. 20, 2944–2946. 10.1039/b904853g. [DOI] [PubMed] [Google Scholar]
- (27).Li Y.; Yu H.; Chen Y.; Lau K.; Cai L.; Cao H.; Tiwari V. K.; Qu J.; Thon V.; Wang P. G.; et al. Substrate Promiscuity of N-Acetylhexosamine 1-Kinases. Molecules 2011, 16 (8), 6396–6407. 10.3390/molecules16086396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Morrison Z. A.; Nitz M. Synthesis of C6-Substituted UDP-GlcNAc Derivatives. Carbohydr. Res. 2020, 495 (May), 108071. 10.1016/j.carres.2020.108071. [DOI] [PubMed] [Google Scholar]
- (29).Barnes J.; Tian L.; Loftis J.; Hiznay J.; Comhair S.; Lauer M.; Dweik R. Isolation and Analysis of Sugar Nucleotides Using Solid Phase Extraction and Fluorophore Assisted Carbohydrate Electrophoresis. MethodsX 2016, 3, 251–260. 10.1016/j.mex.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Van Geel R.; Pruijn G. J. M.; Van Delft F. L.; Boelens W. C. Preventing Thiol-Yne Addition Improves the Specificity of Strain-Promoted Azide-Alkyne Cycloaddition. Bioconjug. Chem. 2012, 23 (3), 392–398. 10.1021/bc200365k. [DOI] [PubMed] [Google Scholar]
- (31).Egan A. J. F.; Errington J.; Vollmer W. Regulation of Peptidoglycan Synthesis and Remodelling. Nat. Rev. Microbiol. 2020, 18 (8), 446–460. 10.1038/s41579-020-0366-3. [DOI] [PubMed] [Google Scholar]
- (32).Schaub R.; Dillard J. Digestion of Peptidoglycan and Analysis of Soluble Fragments. BIO-PROTOCOL 2017, 7 (15). 10.21769/bioprotoc.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Rai A. K.; Mitchell A. M. Enterobacterial Common Antigen: Synthesis and Function of an Enigmatic Molecule. MBio 2020, 11 (4), 1–19. 10.1128/mBio.01914-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Feldman M. F.; Marolda C. L.; Monteiro M. A.; Perry M. B.; Parodi A. J.; Valvano M. A. The Activity of a Putative Polyisoprenol-Linked Sugar Translocase (Wzx) Involved in Escherichia Coli O Antigen Assembly Is Independent of the Chemical Structure of the O Repeat. J. Biol. Chem. 1999, 274 (49), 35129–35138. 10.1074/jbc.274.49.35129. [DOI] [PubMed] [Google Scholar]
- (35).Müller-Loennies S.; Lindner B.; Brade H. Structural Analysis of Oligosaccharides from Lipopolysaccharide (LPS) of Escherichia Coli K12 Strain W3100 Reveals a Link between Inner and Outer Core LPS Biosynthesis. J. Biol. Chem. 2003, 278 (36), 34090–34101. 10.1074/jbc.M303985200. [DOI] [PubMed] [Google Scholar]
- (36).Liu D.; Reeves P. R. Escherichia Coli K12 Regains Its O Antigen. Microbiology 1994, 140 (1), 49–57. 10.1099/13500872-140-1-49. [DOI] [PubMed] [Google Scholar]
- (37).Gozdziewicz T. K.; Maciejewska A.; Tsybulska A.; Lugowski C.; Lukasiewicz J. A New Look at the Enterobacterial Common Antigen Forms Obtained during Rough Lipopolysaccharides Purification. Int. J. Mol. Sci. 2021, 22 (2), 1–16. 10.3390/ijms22020701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Helander I. M.; Hurme R.; Haikara A.; Moran A. P. Separation and Characterization of Two Chemically Distinct Lipopolysaccharides in Two Pectinatus Species. J. Bacteriol. 1992, 174 (10), 3348–3354. 10.1128/jb.174.10.3348-3354.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Meier-Dieter U.; Starman R.; Barr K.; Mayer H.; Rick P. D. Biosynthesis of Enterobacterial Common Antigen in Escherichia Coli. Biochemical Characterization of Tn10 Insertion Mutants Defective in Enterobacterial Common Antigen Synthesis. J. Biol. Chem. 1990, 265 (23), 13490–13497. 10.1016/s0021-9258(18)77373-0. [DOI] [PubMed] [Google Scholar]
- (40).Eade C. R.; Wallen T. W.; Gates C. E.; Oliverio C. L.; Scarbrough B. A.; Reid A. J.; Jorgenson M. A.; Young K. D.; Troutman J. M. Making the Enterobacterial Common Antigen Glycan and Measuring Its Substrate Sequestration. ACS Chem. Biol. 2021. 10.1021/acschembio.0c00983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Cywes-Bentley C.; Skurnik D.; Zaidi T.; Roux D.; DeOliveira R. B.; Garrett W. S.; Lu X.; O’Malley J.; Kinzel K.; Zaidi T.; et al. Antibody to a Conserved Antigenic Target Is Protective against Diverse Prokaryotic and Eukaryotic Pathogens. Proc. Natl. Acad. Sci. 2013, 110 (24), E2209–E2218. 10.1073/pnas.1303573110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Wang X.; Preston J. F. I.; Romeo T. The PgaABCD Locus of Escherichia Coli Promotes the Synthesis of a Polysaccharide Adhesin Required for Biofilm Formation. J. Bacteriol. 2004, 186 (9), 2724–2734. 10.1128/JB.186.9.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Little D. J.; Li G.; Ing C.; DiFrancesco B. R.; Bamford N. C.; Robinson H.; Nitz M.; Pomes R.; Howell P. L. Modification and Periplasmic Translocation of the Biofilm Exopolysaccharide Poly- −1,6-N-Acetyl-D-Glucosamine. Proc. Natl. Acad. Sci. 2014, 111 (30), 11013–11018. 10.1073/pnas.1406388111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Whitney J. C.; Howell P. L. Synthase-Dependent Exopolysaccharide Secretion in Gram-Negative Bacteria. Trends Microbiol. 2013, 21 (2), 63–72. 10.1016/j.tim.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Heilmann C.; Schweitzer O.; Gerke C.; Vanittanakom N.; Mack D.; Götz F. Molecular Basis of Intercellular Adhesion in the Biofilm-Forming Staphylococcus Epidermidis. Mol. Microbiol. 1996, 20 (5), 1083–1091. 10.1111/j.1365-2958.1996.tb02548.x. [DOI] [PubMed] [Google Scholar]
- (46).Jackson D. W.; Suzuki K.; Oakford L.; Simecka J. W.; Hart M. E.; Romeo T. Biofilm Formation and Dispersal under the Influence of the Global Regulator CsrA of Escherichia Coli. J. Bacteriol. 2002, 184 (1), 290–301. 10.1128/JB.184.1.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Chiba A.; Sugimoto S.; Sato F.; Hori S.; Mizunoe Y. A Refined Technique for Extraction of Extracellular Matrices from Bacterial Biofilms and Its Applicability. Microb. Biotechnol. 2015, 8 (3), 392–403. 10.1111/1751-7915.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Ramasubbu N.; Thomas L. M.; Ragunath C.; Kaplan J. B. Structural Analysis of Dispersin B, a Biofilm-Releasing Glycoside Hydrolase from the Periodontopathogen Actinobacillus Actinomycetemcomitans. J. Mol. Biol. 2005, 349 (3), 475–486. 10.1016/j.jmb.2005.03.082. [DOI] [PubMed] [Google Scholar]
- (49).Molloy M. P.; Herbert B. R.; Slade M. B.; Rabilloud T.; Nouwens A. S.; Williams K. L.; Gooley A. A. Proteomic Analysis of the Escherichia Coli Outer Membrane. Eur. J. Biochem. 2000, 267 (10), 2871–2881. 10.1046/j.1432-1327.2000.01296.x. [DOI] [PubMed] [Google Scholar]
- (50).Eddenden A.; Nitz M. Applications of an Inactive Dispersin B Probe to Monitor Biofilm Polysaccharide Production. In Methods in Enzymology; 2022; pp 209–231. 10.1016/bs.mie.2021.11.006. [DOI] [PubMed] [Google Scholar]
- (51).Itoh Y.; Rice J. D.; Goller C.; Pannuri A.; Taylor J.; Meisner J.; Beveridge T. J.; Preston J. F.; Romeo T. Roles of PgaABCD Genes in Synthesis, Modification, and Export of the Escherichia Coli Biofilm Adhesin Poly-β-1,6-N-Acetyl-D-Glucosamine. J. Bacteriol. 2008, 190 (10), 3670–3680. 10.1128/JB.01920-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Kristiarisen A.; Nysaser Å.; Grasdalen H.; Vårum K. M. Quantitative Studies of the Binding of Wheat Germ Agglutinin (WGA) to Chitin-Oligosaccharides and Partially N-Acetylated Chitosans Suggest Inequivalence of Binding Sites. Carbohydr. Polym. 1999, 38 (1), 23–32. 10.1016/s0144-8617(98)00106-4. [DOI] [Google Scholar]
- (53).Hall R. S.; Brown S.; Fedorov A. A.; Fedorov E. V.; Xu C.; Babbitt P. C.; Almo S. C.; Raushel F. M. Structural Diversity within the Mononuclear and Binuclear Active Sites of N-Acetyl-D-Glucosamine-6-Phosphate Deacetylase. Biochemistry 2007, 46 (27), 7953–7962. 10.1021/bi700544c. [DOI] [PubMed] [Google Scholar]
- (54).Roy S.; Vivoli Vega M.; Ames J. R.; Britten N.; Kent A.; Evans K.; Isupov M. N.; Harmer N. J. Structure and Function of N-Acetylglucosamine Kinase Illuminates the Catalytic Mechanism of ROK Kinases. BioRxiV 2021. [Google Scholar]
- (55).Kuru E.; Radkov A.; Meng X.; Egan A.; Alvarez L.; Dowson A.; Booher G.; Breukink E.; Roper D. I.; Cava F.; et al. Mechanisms of Incorporation for D -Amino Acid Probes That Target Peptidoglycan Biosynthesis. ACS Chem. Biol. 2019, 14 (12), 2745–2756. 10.1021/acschembio.9b00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Biagi G. L.; Gandolfi O.; Guerra M. C.; Barbare A. M.; Cantelli-Forti G. Rm Values of Phenols. Their Relationship with Log P Values and Activity. J. Med. Chem. 1975, 18 (9), 868–873. 10.1021/jm00243a002. [DOI] [PubMed] [Google Scholar]
- (57).Liu C.; Yong D.; Yu D.; Dong S. Cell-Based Biosensor for Measurement of Phenol and Nitrophenols Toxicity. Talanta 2011, 84 (3), 766–770. 10.1016/j.talanta.2011.02.006. [DOI] [PubMed] [Google Scholar]
- (58).Goodell E. W. Recycling of Murein by Escherichia Coli. J. Bacteriol. 1985, 163 (1), 305–310. 10.1128/jb.163.1.305-310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Jorgenson M. A.; Kannan S.; Laubacher M. E.; Young K. D. Dead-End Intermediates in the Enterobacterial Common Antigen Pathway Induce Morphological Defects in Escherichia Coli by Competing for Undecaprenyl Phosphate. Mol. Microbiol. 2016, 100 (1), 1–14. 10.1111/mmi.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Liu B.; Furevi A.; Perepelov A. V.; Guo X.; Cao H.; Wang Q.; Reeves P. R.; Knirel Y. A.; Wang L.; Widmalm G. Structure and Genetics of Escherichia Coli O Antigens. FEMS Microbiol. Rev. 2020, 44 (6), 655–683. 10.1093/femsre/fuz028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Zhang L.; Muthana M. M.; Yu H.; McArthur J. B.; Qu J.; Chen X. Characterizing Non-Hydrolyzing Neisseria Meningitidis Serogroup A UDP-N-Acetylglucosamine (UDP-GlcNAc) 2-Epimerase Using UDP-N-Acetylmannosamine (UDP-ManNAc) and Derivatives. Carbohydr. Res. 2016, 419, 18–28. 10.1016/j.carres.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Varki A.; Cummings R. D.; Aebi M.; Packer N. H.; Seeberger P. H.; Esko J. D.; Stanley P.; Hart G.; Darvill A.; Kinoshita T.; et al. Symbol Nomenclature for Graphical Representations of Glycans. Glycobiology 2015, 25 (12), 1323–1324. 10.1093/glycob/cwv091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Neelamegham S.; Aoki-Kinoshita K.; Bolton E.; Frank M.; Lisacek F.; Lütteke T.; O’Boyle N.; Packer N. H.; Stanley P.; Toukach P.; et al. Updates to the Symbol Nomenclature for Glycans Guidelines. Glycobiology 2019, 29 (9), 620–624. 10.1093/glycob/cwz045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Wang S.; Breslawec A. P.; Alvarez E.; Tyrlik M.; Li C.; Poulin M. B. Differential Recognition of Deacetylated PNAG Oligosaccharides by a Biofilm Degrading Glycosidase. ACS Chem. Biol. 2019, 14, 1998–2005. 10.1021/acschembio.9b00467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.