Abstract
Upon infection, transcriptional shifts in both a host bacterium and its invading phage determine host and viral fitness. The xenobiotic response element (XRE) family of transcription factors (TFs), which are commonly encoded by bacteria and phages, regulate diverse features of bacterial cell physiology and impact phage infection dynamics. Through a pangenome analysis of Caulobacter species isolated from soil and aquatic ecosystems, we uncovered an apparent radiation of a paralogous XRE TF gene cluster, several of which have established functions in the regulation of holdfast adhesin development and biofilm formation in C. crescentus. We further discovered related XRE TFs across the class Alphaproteobacteria and its phages, including the φCbK Caulophage, suggesting that members of this gene cluster impact host-phage interactions. Here we show that that a closely related group of XRE proteins, encoded by both C. crescentus and φCbK, can form heteromeric associations and control the transcription of a common gene set, influencing processes including holdfast development and the production of φCbK virions. The φCbK XRE paralog, tgrL, is highly expressed at the earliest stages of infection and can directly repress transcription of hfiA, a potent holdfast inhibitor, and gafYZ, a transcriptional activator of prophage-like gene transfer agents (GTAs) encoded on the C. crescentus chromosome. XRE proteins encoded from the C. crescentus chromosome also directly repress gafYZ transcription, revealing a functionally redundant set of host regulators that may protect against spurious production of GTA particles and inadvertent cell lysis. Deleting host XRE transcription factors reduced φCbK burst size, while overexpressing these genes or φCbK tgrL rescued this burst defect. We conclude that an XRE TF gene cluster, shared by C. crescentus and φCbK, plays an important role in adhesion regulation under phage-free conditions, and influences host-phage dynamics during infection.
Introduction
Surface attachment is a highly regulated process that provides fitness advantages for bacteria in a variety of environmental contexts [4–6]. Our recent studies of surface adherence in the freshwater isolate, Caulobacter crescentus, have revealed an elaborate network of transcription factors (TFs) that control development of the cell envelope-anchored adhesin known as the holdfast (Figure 1) [2, 3, 7, 8]. Among the direct regulators of holdfast development are proteins classified as xenobiotic response element-family (XRE) TFs, which are widely distributed in bacteria, archaea, bacteriophage, and plasmids [9]. XRE proteins stand out as one of the most abundant classes of TFs in the bacterial kingdom, with thousands of entries in the InterPro database [10]. These proteins are known to regulate diverse metabolic processes and environmental responses [11–13], including biofilm development [14, 15], oxidative stress resistance [16], phase variation and pigment production [17]. However, the most well-studied members of this TF family are likely the Cro and CI repressors of bacteriophage λ and 434 [18]. These temperate phages can switch between a lysogenic state, in which the phage integrates into the host chromosome as a prophage, and a lytic state, in which the phage replicate and lyse their hosts. Cro and CI regulate the bistable genetic switch that controls the lysogenic-lytic decision circuit [19–22].
Figure 1. A network of two-component regulators and XRE-family transcription factors control Caulobacter holdfast development.
A) Phase contrast micrograph of C. crescentus CB15 cultivated on peptone-yeast extract (PYE) medium, provides an example of unipolar polysaccharide adhesins in Alphaproteobacteria (630x magnification). The C. crescentus adhesin, known as the holdfast, is stained with wheat germ agglutinin conjugated to Alexa594 dye (green arrows). The holdfast is present at the tip of the stalk in a fraction of pre-divisional cells when cultivated in PYE. Scale bar = 1 μm. B) Schematic of a regulatory network that regulates transcription of the holdfast inhibitor, hfiA. Network includes the essential cell cycle regulators CtrA and GcrA [1–3]. A set of interacting sensor histidine kinases (LovK-SkaH-SpdS) activate the DNA-binding response regulator, SpdR. Dashed lines indicate post-transcriptional regulation and solid lines indicate transcriptional regulation. Black arrows indicate activation and red bar-ended lines indicate repression.
To develop a more complete understanding of C. crescentus XRE-family holdfast regulators and to investigate their relationship with other XRE-family TFs in the genus, we constructed a Caulobacter pangenome (Figure 2), which revealed hundreds of XRE proteins that grouped into dozens of distinct gene clusters. The largest cluster of XRE-family TFs – and the third largest gene family overall – includes known C. crescentus holdfast regulators RtrA and RtrB [2]. Some species encode as many as seven paralogs in this gene cluster, which suggests it has undergone a process of radiation in Caulobacter. We expanded our genome analysis beyond Caulobacter and identified members of this large XRE family across Alphaproteobacteria and in a diverse array of bacteriophages that infect Alphaproteobacteria including viruses of Agrobacterium, Sinorhizobium, Brevundimonas, and Caulobacter.
Figure 2. Conservation of XRE-family adhesion regulators in Caulobacter.
A) Pangenome constructed from whole-genome sequences of 19 Caulobacter species isolated from freshwater or soil. Internal dendrogram based on shared presence and absence of gene clusters generated using the Markov Cluster (MCL) algorithm (mcl-inflation=3; min-occurrence=2); gene clusters organized based on Euclidian distance metric and Ward linkage. Black bars in the internal 19 circles show presence of gene clusters; gray bars indicate absence of gene clusters. Number of genomes that contribute to a gene cluster (from 2–19), number of genes in a gene cluster (from 2–82), and maximum number of paralogs in any single species (from 1–7) are plotted on the outer 3 circles. Genomes are organized by average nucleotide identity (ANI). Dendrogram based on ANI shown above the red heatmap. Single-copy core genes (SCG), i.e. genes present in one copy in all species, are marked in dark green. Multi-copy core genes, i.e. genes present in all species and in more than one copy in at least one species, are marked with a dotted line outside the circle. Gene clusters containing previously reported holdfast regulators rtrA, rtrB, rtrC, and staR, and newly-discovered XRE-family transcriptional regulators, cdxA (CCNA_00049) and cdxB (CCNA_02755) are marked with black arrows.
The proliferation of XRE gene clusters within Caulobacter, coupled with their presence across varied Alphaproteobacteria phage, prompted us to explore the function of both host and phage XRE genes in the infection cycle of φCbK, a virulent Caulobacter phage. Our data reveal that a collection of C. crescentus XRE proteins, along with the closely related XRE TF φCbK gp216 – hereafter named tgrL – have correlated DNA binding profiles. These TFs regulate a common set of genes, including a post-translational inhibitor of holdfast development (hfiA) [1] and a prophage-like gene transfer agent (GTA) cluster, which is recognized for its ability to package genomic DNA and initiate cell lysis [23]. Deletion of selected host XRE TFs reduced the burst size of φCbK, and this burst size defect could be rescued by expressing φCbK tgrL from the chromosome. A time series transcriptomic analysis showed that φCbK tgrL is highly expressed during the earliest stages of infection, during which it primarily functions to inhibit host transcription. The temporal pattern of φCbK tgrL transcription aligns with its function as an “early” viral gene that supports infection. This study illuminates the functions of a related group of XRE-family TFs that are present in both Caulobacter and φCbK, demonstrating that these regulators can influence host adhesin development and govern phage infection.
Results
Discovery of putative XRE-family adhesion regulators in Caulobacter and its bacteriophage
To assess the conservation and relatedness of known adhesion regulators within the Caulobacter genus, we constructed a Caulobacter pangenome comprising 19 species isolated from a diverse range of soil and aquatic ecosystems (Figure 2; Table S1). Considering the importance of XRE-family transcription factors (TFs) in the regulation of Caulobacter holdfast development and adhesion, we primarily focused our analysis on this gene class. We identified a total of 413 XRE domain-containing proteins across the 19 species, which grouped into 57 distinct gene clusters (GCs) (Table S1). GC_0003, the third largest gene cluster in the pangenome, contained 74 distinct XRE-family TFs, with species encoding up to seven paralogs. Known holdfast regulators RtrA and RtrB [2] and an uncharacterized C. crescentus TF encoded by gene locus CCNA_00049 were contained in GC_0003 (Figure 2 and 3A). Cluster GC_0408 was among the single-copy core genes found in all species and contained StaR, a known regulator of C. crescentus stalk development [24] and a repressor of hfiA transcription [1] (Figure 2 and 3A). GC_2778 was limited to the C. vibrioides and C. segnis clades and includes the C. crescentus XRE TF gene, CCNA_02755 (Figure 2 and 3A), while homologs of the hfiA transcriptional repressor, rtrC [3], were present in one or two copies in select Caulobacter species (GC_2397) (Figure 2 and 3A). Relative protein sequence identity of the nineteen HTH_XRE domain proteins in C. crescentus is presented in Figure 3B.
Figure 3. Pangenome summary of holdfast regulators.
A) Number of paralogs in each gene cluster (mcl-inflation = 3). C. crescentus members of GC_0003: RtrA, RtrB, CdxA (CCNA_00049); GC_0408: StaR; GC_2778: CdxB (CCNA_02755); GC_2397: RtrC. B) Percent amino acid identity of the full set of XRE-family transcription factors (HTH_XRE; Conserved Domain Database accession - cd00093) of C. crescentus NA1000 based on pairwise sequence alignment. Numbers indicate the corresponding locus ID (CCNA_XXXXX).
The genomic regions surrounding rtrA, rtrB, CCNA_02755, and staR exhibit synteny homology across the Caulobacter genus (Figure S1) while CCNA_00049 and its adjacent genomic region are conserved across the class Alphaproteobacteria (Figure S2), suggesting that CCNA_00049 is likely the ancestral XRE-family regulator in Alphaproteobacteria [25]. Published RNA-seq and ChIP-seq data on the RtrC adhesion regulator indicate that it directly activates CCNA_00049 and CCNA_02755 transcription [3] (Figure S3A–D). Consistent with this result, rtrC overexpression enhanced CCNA_00049 and CCNA_02755 expression by 4.8- and 1.8-fold, respectively in a transcriptional reporter assay (Figure S3B and S3D). We hereafter refer to CCNA_00049 and CCNA_02755 as cdxA and cdxB, for RtrC-dependent XRE family transcriptional regulators, respectively. We conclude that cdxA and cdxB are XRE-family TFs that function within the recently defined RtrC adhesion regulon [3].
Given the broad conservation cdxA in Alphaproteobacteria, well-described mechanisms of gene exchange between bacteria and their viruses [26, 27], and the established role of XRE-family TFs in bacteriophage biology, we further searched for cdxA-related genes in Alphaproteobacterial phage genomes. This effort identified cdxA-related genes in φCbK-like Caulophage, Agrobacterium phage, Sinorhizobium phage, and Brevundimonas phage (Figure S4) suggesting these viral regulatory proteins related to Caulobacter adhesion factors impact host processes during phage infection.
C. crescentus and φCbK XRE proteins repress hfiA expression and promote holdfast production
RtrA, RtrB, and StaR directly repress transcription of the hfiA holdfast inhibitor, and thereby promote holdfast development [1, 2]. Given the sequence similarity of CdxA and CdxB to these proteins (Figure 2–3), we postulated that they would also repress hfiA transcription and promote holdfast development. We further predicted that that the CdxA-related regulator from Caulophage φCbK, Gp216, would regulate Caulobacter holdfast development. To test these predictions, we overexpressed C. crescentus and φCbK XRE TFs, and monitored hfiA expression using a fluorescent transcriptional reporter. As expected, overexpression of rtrA and rtrB significantly reduced transcription from the hfiA promoter (PhfiA) (Figure 4A). Similarly, overexpression of cdxA, cdxB, or φCbK gp216 reduced PhfiA reporter signal by 85%, 52%, and 18%, respectively (Figure 4A). Consistent with the observed decrease in hfiA transcription, inducing expression of rtrA, rtrB, cdxA, cdxB, or φCbK gp216 led to a significant increase in cells with holdfasts (Figure 4B). Thus, CdxA and CdxB, like RtrA and RtrB, can promote holdfast development. Additionally, our results indicate that φCbK Gp216, a Caulophage XRE-family regulator, can modulate host holdfast development. We hereafter refer to φCbK gp216 as φCbK tgrL for transcription factor involved in host gene regulation encoded by lytic phage φCbK.
Figure 4. C. crescentus and φCbK XRE regulators repress hfiA transcription and promote holdfast development.
A) hfiA expression using a PhfiA-mNeonGreen transcriptional reporter. Fluorescence was measured in a wild type background containing either the empty vector (EV), rtrA, rtrB, cdxA, cdxB, or φCbK tgrL overexpression (++) vectors. Fluorescence was normalized to cell density. B) Percentage of cells with stained holdfast. Stained holdfasts were quantified by fluorescence microscopy in a wild type background containing either the empty vector (EV), rtrA, rtrB, cdxA, cdxB, or φCbK tgrL overexpression (++) vectors. Cells were grown in M2-xylose defined medium. Data are the mean and error bars are standard deviation of at least three biological replicates. Statistical significance was determined by one-way ANOVA followed by Dunnett’s multiple comparison (p-value ≤ 0.01,**).
Functional overlap of C. crescentus and φCbK XRE Transcription Factors
To define the DNA binding profiles of C. crescentus and φCbK XRE TFs, we conducted chromatin immunoprecipitation sequencing (ChIP-seq) with 3x-FLAG-tagged variants of each protein (Table S2). Consistent with previous work [2], our analysis revealed a significant enrichment of RtrA and RtrB within the hfiA promoter region (Figure 5A). ChIP-seq peaks for CdxA, CdxB, and φCbK TgrL exhibited similar enrichment within this promoter (Figure 5A). We comprehensively examined the overlap of ChIP-seq peak summits (+/−50 bp around the peak) across all the datasets for each protein and uncovered considerable binding site overlap: 59% of RtrA (649 out of 1094), 86% of RtrB (468 out of 544), 72% of CdxA (584 out of 809), and 94% of CdxB (191 out of 202) peaks overlapped with at least one other XRE binding site (Figure 5B). Remarkably, 99% of peak summits for the φCbK XRE protein TgrL (135 out of 136) overlapped with the summits from one of the other Caulobacter XRE proteins (Figure 5B).
Figure 5. C. crescentus and φCbK XRE regulators have redundant DNA binding repertoires.
A) XRE proteins bind the hfiA promoter in vivo. ChIP-seq profile from pulldowns of 3xFLAG-tagged XRE proteins are shown. Lines indicate the fold-enrichment from pulldowns compared to an input control. Genomic position and relative position of genes are indicated. Data are in 25 bp bins and are the mean of three biological replicates. B) XRE proteins bind overlapping sites on the C. crescentus chromosome. Venn diagram showing the number of ChIP-seq peaks (100 bp centered on summit) that occupy overlapping regions as identified by ChIPpeakAnno [77]. C) DNA sequence motif enriched in indicated XRE ChIP-seq peaks as identified by XSTREME [76].
To identify putative binding motifs for these XRE TFs, we analyzed the sequences from the peak summits using the XSTREME tool from the MEME suite [28], and identified similar centralized motifs across all five datasets (Figure 5C). We conclude that that the C. crescentus and the φCbK TgrL TFs display largely overlapping binding profiles when overexpressed.
Temporal Pattern of φCbK Gene Expression During Infection: tgrL is an Early Gene
Data presented thus far indicate that φCbK tgrL is functionally related to XRE-family adhesion regulators when expression is induced in C. crescentus cells, though it was not known if tgrL is expressed during φCbK infection. To test this, we conducted time series RNA-sequencing (RNA-seq) measurements on mRNA isolated from infected wild type C. crescentus to define the timing and pattern of φCbK gene expression during infection.
By 15 minutes post infection, φCbK mRNA accounted for 14% of total detected transcripts; this fraction rose to 39% by 90 minutes (Figure 6A). Analysis of φCbK gene expression by hierarchical clustering revealed six distinct temporal expression patterns, which we label as early, constitutive, early-middle, middle, late-middle, and late (Figure S5A–G). Approximately 41% of φCbK genes reached their peak expression by 15 minutes and then gradually decreased, fitting the early category (Figure S5B). Constitutive genes were maximally expressed by 15 minutes and maintained this level throughout the infection (Figure S5C). Early-middle and late-middle genes peaked at 30- and 45-minutes post-infection respectively, before diminishing (Figure S5D and S5F), while middle genes achieved their highest expression between 30 and 60 minutes (Figure S5E). Late genes steadily rose to maximum levels between 60- and 90-minutes post-infection (Figure S5G). The complete φCbK temporal expression profile is presented in Table S3.
Figure 6. Temporal expression profile of φCbK transcription during infection of C. crescentus.
A) Relative φCbK transcript levels over an infection time course as measured by RNA-seq. Percentage of reads from RNA-seq mapped to the φCbK genome compared to the C. crescentus host genome. B) φCbK tgrL is transcribed at the earliest stages infection. Normalized transcript levels (RPKM) for φCbK tgrL from RNA-seq were plotted over the course of an infection. C) Relative expression of φCbK genes during an infection. Transcript levels for each φCbK gene were plotted and relative values were calculated by normalizing transcript levels at a time point to the maximum transcript levels for that gene over the infection time course. Columns correspond to φCbK genes and genes are placed in the order that they appear on the phage chromosome. Genomic modules for structural, lysis, and DNA replication genes as indicated by [82] are marked below the heatmap. The position of φCbK tgrL is indicated by a vertical line and labeled. A-C) Wild type cells were infected during logarithmic growth at 10 multiplicity of infection (MOI). Samples for t = 0 min were harvested prior to phage addition. Data are the mean and error bars represent the standard deviation of three biological replicates.
φCbK tgrL was highly expressed and clearly clustered into the early group, peaking at 15 minutes post-infection and declining thereafter (Figure 6B–C). Steady-state transcript levels of φCbK tgrL were 6 to 28 times higher than its host homologs, rtrA, rtrB, cdxA, and cdxB by 15 minutes. Overall, temporal patterns of bacteriophage gene expression were as expected. For example, DNA replication genes within the φCbK genome were expressed early in infection, while structural and lysis genes were expressed in the middle and late stages of infection (Figure 5C; Table S3).
Regulation of Transcription by the φCbK XRE Protein, tgrL
To define the impact of φCbK tgrL on transcription of C. crescentus genes, we used multiple approaches. Overexpressing φCbK tgrL in a quadruple deletion background (ΔrtrA ΔrtrB ΔcdxA ΔcdxB) that allowed us to determine a TgrL regulon that was not confounded by expression of closely related host XRE TFs. Transcript levels of 440 genes changed significantly when φCbK tgrL was overexpressed (Figure 7). Direct targets of this early φCbK TF were defined as genes that a) showed significant differential regulation upon φCbK tgrL overexpression (|fold-change| ≥ 1.5 and FDR p-value ≤ 0.0001), and b) had a TgrL ChIP-seq peak present within an associated promoter (Table S4). In total, 99 regulated genes were defined as direct TgrL targets. Among these, 93% (92 out of 99) were repressed (Figure 7) including the holdfast inhibitor, hfiA, which decreased by a factor of approximately six. These results provide evidence that φCbK tgrL can act as a direct regulator of C. crescentus gene expression, including many genes that have direct binding sites for chromosomally-encoded XRE adhesion regulators (Table S4).
Figure 7. CbK TgrL regulates C. crescentus gene expression.
RNA-seq analysis of genes significantly regulated upon induction of φCbK tgrL expression. Volcano plot displays log2(fold-change) and −log10(FDR p-value) for all C. crescentus genes, comparing φCbK tgrL expression from a plasmid to that from an empty vector (EV). Experiment was conducted in a genetic background lacking the host XRE genes: ΔrtrA ΔrtrB ΔcdxA ΔcdxB background. Gray dots indicate genes for which expression does not change significantly, blue dots indicate genes without a TgrL ChIP-seq peak in an associated promoter, and pink dots indicate genes with a φCbK TgrL ChIP-seq in an associated promoter. Vertical red lines mark boundaries for ±1.5-fold-change; horizontal red line marks 0.0001 FDR p-value. Points represent the mean of three biological replicates.
We further analyzed our RNA-seq infection time course data to define changes in host transcription, focusing on genes that were classified as direct targets of φCbK TgrL. Transcription of 337 C. crescentus genes changed significantly relative to pre-infection at one or more time points, and differentially expressed genes clustered into distinct temporal groups. The largest cluster (Group A) contained 120 genes that had maximum transcript levels prior to infection, which decreased following infection. Multiple group A genes contained φCbK TgrL ChIP-seq peaks within their promoter regions. This cohort includes a set of GIY-YIG nuclease genes (CCNA_00744 and CCNA_1405); the cdzAB transporter genes implicated in contact-dependent toxin transport; an operon encompassing the lodAB-like L-amino acid oxidase genes (CCNA_00592–00589); a pair of hypothetical protein genes (CCNA_00593–00594); and the operon CCNA_02815–02816, encoding an ice nucleation protein and a hypothetical protein. Though not likely regulated by φCbK TgrL, the transcription of a subset of oxidative stress defense genes including the catalase-peroxidase katG (CCNA_03138), alkyl hydroperoxide reductase subunits C and F (CCNA_03012 and CCNA_03013, respectively), and an AhpD-family hydroperoxidase (CCNA_03812), were markedly repressed post-infection (Table S3) suggesting that φCbK infection represses oxidative stress response. The impact of this response on host and/or phage fitness is uncertain.
Caulobacter and φCbK XRE proteins repress cell lysis activators
To better understand the functions of both host and phage XRE TFs, we looked for genes that had common XRE TF ChIP-seq peaks in their promoter regions. Notably, we identified ChIP-seq peaks for RtrA, RtrB, CdxA, CdxB, and φCbK TgrL directly upstream of the gafYZ operon (Figure 8A). Expression of gafYZ induces expression of prophage-like gene transfer agents (GTA) that package genomic DNA fragments from C. crescentus and trigger cell lysis [23]. Induction of host cell lysis prior to the completion of a phage infection cycle would be deleterious for phage fitness, and we hypothesized that φCbK TgrL represses GTA expression. To test this hypothesis, we measured gafYZ transcriptional reporter activity in a wild type background and in a quadruple deletion background (ΔrtrA ΔrtrB ΔcdxA ΔcdxB), which lacks the related host XRE TFs. Deletion of the four host XRE TFs resulted in a 2.3-fold increase in gafYZ transcription (Figure 8B). Expressing rtrA, rtrB, cdxA, cdxB, or φCbK tgrL in the quadruple deletion background reduced gafYZ transcription by 34–91% (Figure 8B).
Figure 8. C. crescentus and φCbK XRE regulators repress gafYZ and gene transfer agent (GTA) production.
A) XRE proteins bind the gafYZ promoter in vivo. ChIP-seq profile from pulldowns of 3xFLAG-tagged protein are shown. Lines show fold-enrichment from pulldowns compared to an input control. Genomic position and relative position of genes are indicated. Data are in 25 bp bins and are the mean of three biological replicates. B) gafYZ expression using a PgafYZ-mNeonGreen reporter. Fluorescence was measured and normalized to cell density. Data are the mean and error bars are the standard deviation of three biological replicates (black dots). Statistical significance was determined by one-way ANOVA followed by Šídák's multiple comparisons test (p-value ≤ 0.0001,****). C) XRE proteins repress GTA production. Total DNA was purified, separated by gel electrophoresis, and imaged. GTA-associated DNA resolved at ~8 kb. Image is a representative gel from at least 3 biological replicates. B-C) Experiments were performed with wild type (WT) or strains harboring in-frame deletions (Δ) in rtrA, rtrB, cdxA, cdxB, gafYZ, and/or rogA. Δxre indicates the ΔrtrA ΔrtrB ΔcdxA ΔcdxB background. Strains contained either an empty vector (EV), rtrA, rtrB, cdxA, cdxB, or φCbK tgrL overexpression (++) vectors. Strains were grown to stationary phase in complex medium (PYE).
Prior research [23] has demonstrated that deleting rogA, a strong repressor of gafYZ, stimulates the production of GTAs during the stationary phase and results in packaging of chromosomal DNA into 8 kb fragments. To assess production of GTA-associated DNA fragments in the quadruple deletion, we extracted DNA from stationary phase cultures and performed gel electrophoresis. As expected, we observed an 8 kb GTA-associated DNA band in ΔrogA, but not in a ΔrogA ΔgafYZ mutant (Figure 8C). However, increased transcription of gafYZ in the quadruple deletion was not sufficient to trigger the production of GTA fragments. Although we failed to detect a GTA-associated DNA band in the quadruple deletion, expression of either rtrA, rtrB, cdxA, cdxB, or φCbK tgrL was sufficient to ablate production of the 8 kb GTA band in a ΔrogA strain (Figure 8C). These observations support a model in which related XRE TFs from both C. crescentus and φCbK can repress GTA production.
Caulobacter and φCbK XRE transcription factors promote phage infection
A transposon-based genetic screen previously revealed that resistance to φCbK phage infection was increased in mutants harboring insertions in cdxB, which indicated a role for cdxB in supporting phage infection [29]. Given the functional overlap of cdxB with other XRE proteins described in this study, we postulated that both host (rtrA, rtrB, cdxA, cdxB) and φCbK (φCbK tgrL) XRE TFs are genetic factors that support φCbK infection. To test this, we assessed viral burst size in the C. crescentus quadruple deletion strain (ΔrtrA ΔrtrB ΔcdxA ΔcdxB). Deletion of these four paralogous host XRE TFs reduced viral burst size by 46% relative to wild type after 180 min (Figure 9) while expression of either cdxA, cdxB, or φCbK tgrL in the quadruple deletion background significantly increased the burst size (Figure 9). Collectively, these data show that XRE TFs from both the host and virus can support φCbK phage infection.
Figure 9. C. crescentus and φCbK XRE regulators support phage infection.

φCbK burst size in mutant backgrounds. Plaque forming units (PFU) per infected cell (i.e. burst size) was plotted for either wild type or mutants with in-frame deletions (Δ) of rtrA, rtrB, cdxA, and cdxB. Δxre indicates the ΔrtrA ΔrtrB ΔcdxA ΔcdxB background. Strains contained either an empty vector (EV), or rtrA, rtrB, cdxA, cdxB, or φCbK tgrL overexpression (++) vectors. Strains were infected with φCbK at 0.01 multiplicity of infection (MOI) in logarithmic growth phase in complex medium (PYE). Data bars represent the mean and error bars are the standard deviation of at least three biological replicates (black dots). Statistical significance was determined by one-way ANOVA followed by Dunnett’s multiple comparison (p-value ≤ 0.05,*).
Caulobacter and φCbK XRE transcription factors can form heteromers
Transcription factors within the XRE family are known to form homomeric structures, such as homodimers or homotetramers [30–33]. Given the structural similarity among RtrA, RtrB, CdxA, CdxB, StaR, and φCbK TgrL XREs, we postulated that these proteins could form heteromeric interactions, in addition to homomeric associations. To test this hypothesis, we employed a bacterial two-hybrid (BTH) assay, wherein each XRE was fused to either the T18 or T25 fragments of adenylate cyclase. As expected, co-expression of any XRE gene with itself resulted in increased β-galactosidase activity, demonstrating that the five C. crescentus XRE proteins and the phage XRE protein form homomeric structures (Figure 10). We then co-expressed different XRE proteins and discovered that RtrA, RtrB, CdxA, and CdxB interacted in a two-hybrid assay (Figure 10). StaR and φCbK TgrL interacted with CdxA and CdxB, but not with RtrA or RtrB (Figure 10 and Figure S6). In fact, interaction of φCbK TgrL with itself was as strong as its interactions with CdxA and CdxB in this assay. The ability of φCbK TgrL to interact with both CdxA and CdxB points toward a possible mechanism in which this phage protein can impact host gene expression directly or indirectly by interacting with host transcription factors.
Figure 10. C. crescentus and φCbK XRE regulator interactions in a two-hybrid assay.
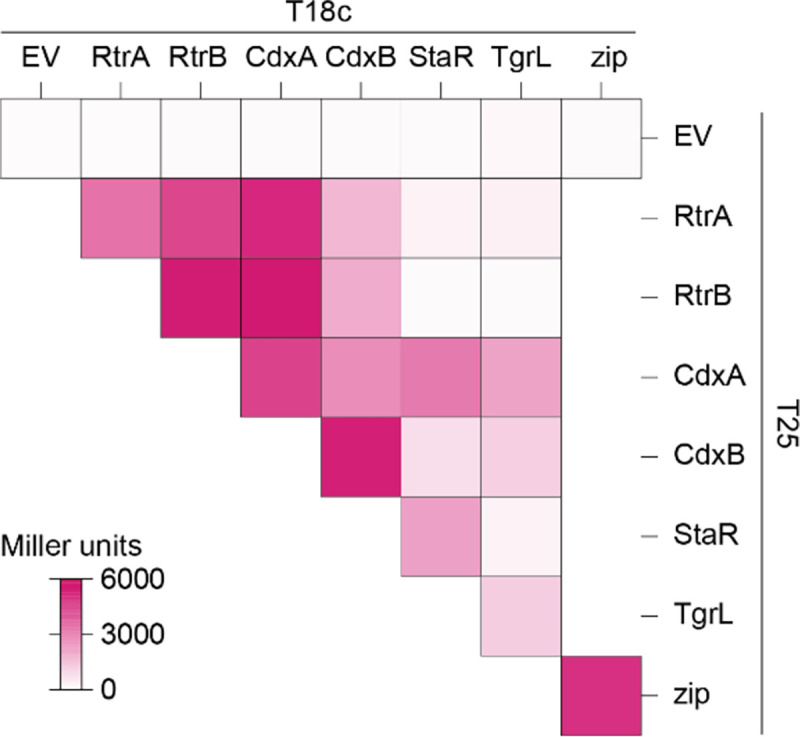
Heatmap summarizing interactions between RtrA, RtrB, CdxA, CdxB, StaR, and φCbK TgrL based on bacterial two-hybrid (BTH) assays. Proteins were fused to split adenylate cyclase fragments (T18c and T25) and co-expressed in E. coli. Interactions between the fused proteins reconstitutes adenylate cyclase, promoting expression of a lacZ reporter. Empty vector (EV) are the negative control and Zip is the positive control. β-galactosidase activity was measured for each pairing, and Miller units were calculated. Data are the mean of at least three biological replicates. See Figure S6 for statistical analysis.
Discussion
A pangenome analysis of the Caulobacter genus illuminated a probable evolutionary radiation of a group of XRE-family transcription factors (XRE TFs) that includes several genes previously implicated in the regulation of cell adhesion. Study of this group of XRE TFs in C. crescentus and of a closely related gene encoded by Caulophage φCbK (tgrL) has illuminated new functional roles for this gene class in Caulobacter-virus interactions. Expression of φCbK tgrL is highly induced in the early stages of infection of host cells, i.e. tgrL is an “early” gene. The transcriptional regulon and functional properties of this viral transcription factor overlap its host-encoded homologs. These TFs not only govern cell adhesion processes, but also repress transcription of a regulator associated with the gene transfer agent (GTA) locus, which produces bacteriophage-like particles that encapsulate host DNA. Our data further provide evidence that both host and φCbK XRE TFs play an important role in promoting φCbK infection. Specifically, deletion of host XRE TFs led to a reduction in viral burst size, while expression of host or bacteriophage XRE TFs rescued this burst size defect.
An ancestral XRE TF and its function across genera
The gene neighborhoods of the XRE TFs studied here are highly conserved in Caulobacter. Synteny homology of cdxA (C. crescentus gene locus: CCNA_00049) is particularly notable as its neighborhood is conserved across the class Alphaproteobacteria (Figure S2) [25], suggesting it is the ancestral XRE TF in C. crescentus. Orthologs of this gene have been studied in several genera and demonstrated to regulate diverse processes. For example, mutation of the Azorhizobium caulinodans cdxA homolog, praR, resulted in abnormal root nodule development in its plant host and reduced nitrogen fixation during symbiosis [25, 34]. Anomalous nodulation of praR mutants was attributed to increased expression of the reb locus, known to control the formation of R-bodies [25], which can modulate interactions between bacteria and eukaryotic cells [35]. The disruption of praR in Rhizobium leguminosarum bv. viciae 3841 resulted in enhanced adhesin production, which is congruent with adhesion phenotypes that we have reported in C. crescentus. Increased adhesin production in the Rhizobium system is a consequence of the interaction of the PraR repressor with CinS, an anti-repressor [36] that facilitates synchronization of biofilm regulation with population density [36, 37] and improves fitness in the root nodule [37]. Notably, R. leguminosarum encodes a praR paralog, RL0149, that is repressed by PraR but does not impact biofilm formation [37]. However, RL0149 mutants face a competitive disadvantage in the root nodule, providing evidence for functional diversification of these XRE TF paralogs in this species [38].
Homologs of XRE TFs studied here were also identified in several Alphaproteobacterial phages, including Caulobacter phage φCbK, Brevundimonas phage vB_BgoS-Bajun, Sinorhizobium phage PBC5, and Agrobacterium phage Atu_ph08 (Figure S4). We provide evidence that the XRE homologs from C. crescentus and its phage, φCbK, support phage infection. It is unclear whether this role in phage infection is conserved in other Alphaproteobacteria and their respective phages. Considering that cdxA and its orthologs can regulate diverse processes in Alphaproteobacteria, it is possible that the regulatory functions of phage XRE homologs vary from host to host.
Caulobacter and phage XRE proteins add to an expanding group of holdfast regulators
The process of surface attachment in C. crescentus, which is mediated by the adhesin known as the holdfast, is a permanent event and is therefore subject to intensive transcriptional and post-transcriptional regulation. The small protein HfiA plays a crucial role in regulating holdfast synthesis by binding and inhibiting HfsJ, a membrane-associated glycosyltransferase that is essential for holdfast production [1]. A complex array of regulatory factors coordinately control hfiA transcription [1–3, 39, 40] including an assortment of transcription factors that are activated by the stationary phase response regulator, SpdR [2]
In this study, we report two new XRE family transcription factors, CdxA and CdxB. These proteins function within the C. crescentus adhesion control system to repress hfiA and enhance holdfast synthesis (Figure S3A–D and Figure 4A–B), raising the total number of direct hfiA regulators to eight. The advantage of the highly redundant, and seemingly baroque, regulation of hfiA certainly piques curiosity. A possible explanation could be that regulatory redundancy buffers the cell against transient environmental fluctuations that impact holdfast development. Alternatively, this array of transcription factors could allow for a multi-dimensional response to varying environmental cues that impact hfiA transcription. Although the SpdR-RtrC signaling axis is known to activate the quartet of XRE genes studied here (rtrA, rtrB, cdxA, and cdxB), it remains uncertain whether other regulatory systems or signals influence their expression or activity. If secondary mechanisms - such as protein-protein interactions, chemical modifications, or metabolite binding - further regulate the XRE proteins, they could introduce an added layer of complexity to the adhesion decision circuit.
Intriguingly, we discovered that a related Caulophage XRE gene, φCbK tgrL, can directly repress hfiA transcription and enhance holdfast formation (Figure 4 and Figure 10). This raises questions about potential advantages of surface adherence regulation by Caulophage. For example, might phage promote holdfast formation under select conditions to facilitate spread of phage progeny in surface-associated biofilms? Given that the infection strategy of φCbK requires the presence of flagella and pili on the cell surface, which only exist in swarmer and pre-divisional cells [41–43], such a tactic could offer competitive benefits. Alternatively, possible phage-induced modulation of hfiA during infection might not directly boost phage fitness and could merely be an incidental consequence of the phage harnessing host XRE TFs to regulate other processes. Indeed, we did not observe significant regulation of hfiA in C. crescentus cells infected with φCbK under our experimental conditions (Table S3).
Functional redundancy and heteromeric interactions
Transcription factors with shared ancestry often bind similar DNA motifs due to highly conserved DNA-binding domains. In line with this, we found that 59–99% of binding sites identified in the RtrA, RtrB, CdxA, CdxB, and φCbK TgrL ChIP-seq datasets exhibit overlap with each other (Figure 5B). Functional redundancy can allow for the evolution of ecoparalogs, in which paralogous proteins perform the same function but are expressed under different environmental conditions [44]. For the C. crescentus XRE TFs studied here, all four are controlled by the same transcriptional regulatory pathway. From this we infer that these genes are typically expressed under similar conditions (Figure S3A–D) [2, 3, 45] though we cannot exclude the possibility that additional transcriptional or post-transcriptional mechanisms modulate their expression or activities.
Paralogs can also undergo subfunctionalization, where the function of the original gene is partitioned, or neofunctionalization, in which a completely new function evolves. [46]. The ChIP-seq data revealed that a portion of peaks (41% for rtrA, 14% for rtrB, 28% for cdxA, and 6% for cdxB) are unique, suggesting a degree of functional specialization among the C. crescentus XRE paralogs (Figure 5B). RtrA, RtrB, CdxA, and CdxB, and φCbK TgrL can form both homomers and heteromers in a two-hybrid assay (Figure 10), and it is possible that these proteins bind to different sites depending on the levels of their interaction partners. Gene expression regulation often leverages heteromerization. For instance, the Escherichia coli response regulator RcsB is known to form direct interactions with a variety of TFs to control disparate gene expression processes [47–49]. In C. crescentus the paralogous zinc-finger transcription factors, MucR1 and MucR2, known for their roles in cell cycle control, can form heterodimers [50]. Finally, in Streptomyces venezuelae, BldM homodimers control the activation of early developmental genes, whereas heterodimers of BldM and WhiI regulate a group of late-stage developmental genes [51]. These dimers display distinct DNA binding patterns: BldM homodimers bind palindromic sequences with two BldM half-sites, whereas BldM-WhiI heterodimers bind non-palindromic sequences featuring one half-site each for BldM and WhiI [51].
MEME analysis of each XRE TFs from C. crescentus in our study revealed a prevalent motif with one highly conserved half-site and a second, weakly conserved half-site (Figure 5C). Considering the sequence identity between the DNA binding domains of the XRE proteins (64 – 80% identity), it is plausible that each exhibit subtle differences in DNA binding preference. The weakly conserved second half-site could stem from mixed signals of homomers and heteromers in the ChIP-seq data. The formation of heteromers might not only alter binding site specificity but could also influence the regulatory functions of these XRE TFs.
Caulobacter and phage XRE repress GTA production
Expression of a prophage-like gene transfer agent (GTA) in C. crescentus results in packaging of genomic DNA into phage-like particles that ultimately lead to cellular lysis [23]. To prevent genome packaging and untimely lysis, the expression of the GTA gene cluster is tightly regulated: transcription of GTA genes is activated by GafYZ while RogA represses gafYZ expression [23]. Our study provides evidence that RtrA, RtrB, CdxA, and CdxB directly repress the transcription of gafYZ (Figure 8A–B), though deletion of these four XRE genes did not result in increased GTA-related DNA production (Figure 8C). Therefore, RogA is likely the dominant inhibitor of gafYZ, while the Rtr and Cdx proteins may function to modulate gafYZ expression under particular environmental conditions. C. crescentus GTA synthesis has only been reported in stationary phase cultures, which indicates that there are intra- or extracellular cues present in stationary phase that enhance the expression of gafYZ through an unknown mechanism [23]. rtrA and rtrB may contribute to stationary phase control of gafYZ as expression of these genes increases during stationary phase as a consequence of increased activity of the stationary phase response regulator, SpdR [3, 45].
We have further discovered that φCbK TgrL can directly repress gafYZ expression and inhibit GTA production (Figure 8B–C). Many bacteria are equipped with abortive infection (Abi) modules that detect phage infection and prompt cell death before replication is complete. This mechanism effectively prevents viral spread within the population [52]. While a phage-sensing module hasn’t been identified for the gafYZ module, it is possible that the production of GTA and subsequent cell lysis during phage replication could stem the spread of infection, much like Abi. We propose that φCbK synthesizes φCbK TgrL in part to ensure strong repression of gafYZ transcription, thereby blocking GTA production. Our infection time course data align with this hypothesis, as the levels of φCbK tgrL transcripts peak at the earliest stages of infection. Despite an observed increase in φCbK tgrL expression, we did not observe changes in the levels of gafYZ transcripts in our experiment. This could be due to several factors. First, under the conditions tested, the host repressor proteins (RogA, RtrA, RtrB, CdxA, and CdxB) may already maximally inhibit gafYZ expression. Alternatively, φCbK TgrL could repress gafYZ expression at time points earlier than we have measured. While a phage sensing module tied to GTA activation has not been identified, gafYZ expression might be triggered during infection when φCbK tgrL is absent. Phage strains lacking φCbK tgrL would enable tests of this model.
φCbK infection and XRE transcription factors
Transposon insertions in cdxB increased host resistance to the φCbK phage [29]. This is consistent with our results showing that deleting four XRE family TFs, including cdxB, reduced the phage burst size and supports our conclusion that these TFs support phage replication (Figure 9). Ectopic expression of φCbK tgrL, cdxA, or cdxB was sufficient to restore burst size to wild type levels in a quadruple deletion background (ΔrtrA ΔrtrB ΔcdxA ΔcdxB). Phage infections conducted in this study used a wild type φCbK phage, which expresses φCbK TgrL at high levels in the early stages of infection. Accordingly, the phage resistance phenotype of the quadruple deletion strain may be more pronounced if one were to infect C. crescentus with a φCbK strain lacking tgrL.
The mechanisms by which XRE TFs from C. crescentus and φCbK enhance φCbK infection remain undefined. One hypothesis is that these proteins repress transcription of phage defense systems. For example, numerous toxin-antitoxin (TA) systems are known to function as phage defense genes [53] and we observed that XRE TFs bind to the promoters of several TA genes, including CCNA_03255 (PemK-like toxin) and CCNA_03983 (HicA-like toxin). Consistent with this, induced expression of φCbK tgrL decreased expression of these toxins by approximately a factor of 10 (Table S4). φCbK TgrL also repressed the transcription of CCNA_00744 and CCNA_01405 (Table S4), which encode GIY-YIG nuclease proteins that are typically found in restriction modification systems and homing endonucleases [54, 55]. Recent studies have shown that a GIY-YIG-based system can protect E. coli from T4 phage infection [56] while a chimeric GIY-YIG protein provides the ICP1 phage with immunity against parasitic mobile genetic elements during infection of Vibrio cholerae [57]. It is therefore possible that φCbK tgrL-mediated repression of GIY-YIG genes provides an advantage to the phage during the infection process.
Alternatively, XRE TFs may enhance production of bacteriophage by controlling the transcription of φCbK genes, as observed in other host-phage systems. For example, the expression of both early and late φ29 genes is downregulated by spo0A, a master regulator of Bacillus subtilis sporulation [58]. Through this strategy, φ29 harnesses the host’s sensory systems to delay its own development under sub-optimal conditions and to package its DNA into the endospore [58]. In C. crescentus, the primary cell cycle response regulator, CtrA, is reported to control the expression of φCbK genes, which possibly aligns phage development with cell cycle progression [59]. Additional data connect environmental regulatory systems of C. crescentus to bacteriophage infection. Specifically, transposon insertions in the sensor histidine kinase, skaH, enhance survival during φCbK infection [29]. SkaH directly interacts with the LovK and SpdS sensor histidine kinases to regulate SpdR [2], which activates the expression of XRE TFs studied here. The φCbK infection phenotype of skaH insertional mutants could therefore be linked to XRE expression. The SpdS-SpdR system is thought to sense changes in cellular redox state and electron transport chain flux [60–63] while LovK can function as both a photosensor and a redox sensor [64, 65]. Signaling through the SpdS-SkaH-LovK system, which has a known impact on C. crescentus cell adhesion [2], may also impact interactions between C. crescentus and its bacteriophage.
Materials and Methods
Caulobacter pangenome analysis
Nineteen Caulobacter genomes were analyzed in Anvi’o v7.1 [66] using the Snakemake pangenomics workflow [67]. Briefly, genome sequences NZ_CP073078, NZ_CP049199, NZ_CP048815, NZ_CP033875, NZ_CP026100, NZ_CP024201, NZ_CP013002, NZ_CP082923, NC_014100, CP096040, NC_01196, NC_002696, NC_010338, NZ_APMP01000001, NZ_CP023313, NZ_CP023314, NZ_CP023315, NZ_PEGF01000001, NZ_PEGH01000001 were retrieved from NCBI Genbank, reformatted using anvi-script-reformat-fasta and added to an anvi’o contigs database using anvi-gen-contigs-database. Open reading frames were identified using Prodigal [68], and predicted genes were functionally annotated with COG terms [69] and KEGG-KoFams terms [70], and annotation terms were added to the contigs database. Average nucleotide identity (ANI) was calculated using PyANI [71] with anvi-compute-genome-similarity. Gene clusters were identified using mcl [72] and the pangenome was generated using anvi-pan-genome with flags –mcl-inflation 3 and –min-occurrence 2. Pangenome summary data are presented in Table S1.
Bioinformatic analysis
Sequences for proteins from the C. crescentus NA1000 genome (GenBank accession number CP001340) that contained a HTH_XRE domain (cd00093) were extracted. Multiple sequence alignment was performed with Geneious Prime (version 2023.1.2) using Clustal Omega (version 1.2.2) with 1 iteration. Percent identity for pairwise alignments were calculated and plotted. For genomic neighborhood analysis, sequences were analyzed with the webFLaGs server (https://server.atkinson-lab.com/webflags) using the default parameters [73]. For Figure S1, protein sequences were retrieved using the protein accession numbers and associated GCF assembly IDs for proteins from bins GC_0003, GC_0408, and GC_2778 in the pangenome analysis. For Figure S2, protein sequences were retrieved using the protein accession numbers for a sampling of cdxA homologs modified from [25]) and the associated GCF assembly IDs. For Figure S4, to identify CdxA homologs in phage, we performed a search with PSI-BLAST against the non-redundant protein sequence database (limited to Viruses - taxid:10239). Sequences of phage proteins with e-value < 1×10−18 were extracted. CdxA homologs for hosts associated with the phage were extracted in the same manner. Sequences were aligned with Geneious Prime (version 2023.1.2) using Clustal Omega (version 1.2.2) with 100 iterations. A phylogenetic tree was constructed with the Geneious Tree Builder (genetic distance model: Jukes-Cantor; tree build model: Neighbor-joining; resampling method: bootstrap; number of iterations: 1000).
Strain growth conditions
Escherichia coli was grown in Lysogeny broth (LB) or LB agar (1.5% w/v) at 37°C [74]. Medium was supplemented with the following antibiotics when necessary: kanamycin 50 μg ml−1, chloramphenicol 20 μg ml−1, oxytetracycline 12 μg ml−1, and carbenicillin 100 μg ml−1.
Caulobacter crescentus was grown in peptone-yeast extract (PYE) broth (0.2% (w/v) peptone, 0.1% (w/v) yeast extract, 1 mM MgSO4, 0.5 mM CaCl2), PYE agar (1.5% w/v), or M2 defined medium supplemented with xylose (0.15% w/v) as the carbon source (M2X) [75] at 30°C. Solid medium was supplemented with the following antibiotics where necessary: kanamycin 25 μg ml−1, chloramphenicol 1 μg ml−1, and oxytetracycline 2 μg ml−1. Liquid medium was supplemented with the following antibiotics where necessary: chloramphenicol 1 μg ml−1, and oxytetracycline 2 μg ml−1.
Plasmid and strain construction
Plasmids were cloned using standard molecular biology techniques and the primers listed in Table S5. For overexpression constructs, inserts were cloned into pPTM057, which integrates at the xylose locus and contain a cumate-inducible (PQ5) promoter [3]. For reporter constructs, inserts were cloned into pPTM056, which replicates in C. crescentus. Plasmids were transformed into C. crescentus by either electroporation or triparental mating [75]. Transformants generated by electroporation were selected on PYE agar supplemented with the appropriate antibiotic. Strains constructed by triparental mating were selected on PYE agar supplemented with the appropriate antibiotic and nalidixic acid to counterselect against E. coli. Gene deletions and allele replacements were constructed using a standard two-step recombination/counter-selection method, using sacB as the counterselection marker. Briefly, pNPTS138-derived plasmids were transformed into C. crescentus and primary integrants were selected on PYE/kanamycin plates. Primary integrants were incubated overnight in PYE broth without selection. Cultures were plated on PYE agar plates supplemented with 3% (w/v) sucrose to select for recombinants that had lost the plasmid. Mutants were confirmed by PCR amplification of the gene of interest from sucrose resistant, kanamycin sensitive clones.
Analysis of transcription using fluorescent fusions
Strains were incubated in triplicate at 30°C overnight in PYE broth supplemented with 1 μg ml−1 chloramphenicol and 50 μM cumate. Overnight cultures were diluted in the appropriate broth supplemented with 1 μg ml−1 chloramphenicol and 50 μM cumate to 0.01 OD660 for the cdxA and cdxB reporters or 0.05 OD660 for gafYZ reporters. Diluted cultures were incubated at 30°C for 24 hours. For hfiA reporters, strains were inoculated in triplicate in M2X supplemented with 1 μg ml−1 chloramphenicol and 50 μM cumate and grown overnight at 30°C. Strains were subcultured and grown at 30°C for 7 hours. Cultures were diluted to 0.0001 – 0.005 OD660 and incubated at 30°C until reaching 0.05 – 0.1 OD660. For measurements, 200 μl culture was transferred to a black Costar® 96 well plate with clear bottom (Corning). Absorbance at 660 nm and fluorescence (excitation = 497 ± 10 nm; emission = 523 ± 10 nm) were measured in a Tecan Spark 20M plate reader. Fluorescence was normalized to absorbance. Statistical analysis was carried out in GraphPad 9.3.1.
Holdfast imaging and quantification
Strains were inoculated in triplicate in M2X supplemented with 50 μM cumate and grown overnight at 30°C. Strains were subcultured in M2X supplemented with 50 μM cumate and grown for 7 hours at 30°C. Cultures were diluted to 0.0002 – 0.0053 OD660 and incubated at 30°C until reaching 0.05 – 0.1 OD660. Alexa594-conjugated wheat germ agglutinin (WGA) (ThermoFisher) was added to the cultures with a final concentration of 2.5 μg ml−1. Cultures were shaken at 30°C for 10 min at 200 rpm. Then, 1.5 ml culture was centrifuged at 12,000 × g for 2 min, supernatant was removed, and pellets were resuspended in 35 μl M2X. Cells were spotted on 1% (w/v) agarose pads in H2O and imaged with a Leica DMI6000 B microscope. WGA staining was visualized with Leica TXR ET (No. 11504207, EX: 540–580, DC: 595, EM: 607–683) filter. Cells with and without holdfasts were enumerated using the image analysis suite, FIJI. Statistical analysis was carried out in GraphPad 9.3.1.
Chromatin immunoprecipitation sequencing (ChIP-seq)
Strains were incubated in triplicate at 30°C overnight in 10 ml PYE supplemented with 2 μg ml−1 oxytetracycline when appropriate. Then, 5 ml overnight culture was diluted into 46 ml PYE supplemented with 2 μg ml−1 oxytetracycline when appropriate and grown at 30°C for 2 hours. Cumate was added to a final concentration of 50 μM and cultures were grown at 30°C for 6 hours. Cultures were crosslinked with 1% (w/v) formaldehyde for 10 min, then crosslinking was quenched by addition of 125 mM glycine for 5 min. Cells were centrifuged at 7196 × g for 5 min at 4°C, supernatant was removed, and pellets were washed in 25 ml 1x cold PBS pH 7.5 three times. Pellets were resuspended in 1 ml [10 mM Tris pH 8 at 4°C, 1 mM EDTA, protease inhibitor tablet, 1 mg ml−1 lysozyme] and incubated at 37°C for 30 min. Sodium dodecyl sulfate (SDS) was added to a final concentration of 0.1% (w/v) and DNA was sheared to 300–500 bp fragments by sonication for 10 cycles (20 sec on/off). Debris was centrifuged at 15,000 × g for 10 min at 4°C, supernatant was transferred, and Triton X-100 was added to a final concentration of 1% (v/v). Samples were pre-cleared through incubation with 30 μl SureBeads™ Protein A magnetic beads for 30 min at room temp. Supernatant was transferred and 5% lysate was removed for use as input DNA.
For pulldown, 100 μl Pierce™ anti-FLAG magnetic agarose beads (25% slurry) were equilibrated overnight at 4°C in binding buffer [10 mM Tris pH 8 at 4°C, 1 mM EDTA, 0.1% (w/v) SDS, 1% (v/v) Triton X-100] supplemented with 1% (w/v) bovine serum albumin (BSA). Pre-equilibrated beads were washed four times in binding buffer, then incubated with the remaining lysate for 3 hours at room temperature. Beads were washed with low-salt buffer [50 mM HEPES pH 7.5, 1% (v/v) Triton X-100, 150 mM NaCl], high-salt buffer [50 mM HEPES pH 7.5, 1% (v/v) Triton X-100, 500 mM NaCl], and LiCl buffer [10 mM Tris pH 8 at 4°C, 1 mM EDTA, 1% (w/v) Triton X-100, 0.5% (v/v) IGEPAL® CA-630, 150 mM LiCl]. To elute protein-DNA complexes, beads were incubated for 30 min at room temperature with 100 μl elution buffer [10 mM Tris pH 8 at 4°C, 1 mM EDTA, 1% (w/v) SDS, 100 ng μl−1 3xFLAG peptide] twice. Elutions were supplemented with NaCl and RNase A to a final concentration of 300 mM and 100 μg ml−1, respectively, and incubated at 37°C for 30 min. Then, samples were supplemented with Proteinase K to a final concentration of 200 μg ml−1 and incubate overnight at 65°C to reverse crosslinks. Input and elutions were purified with the Zymo ChIP DNA Clean & Concentrator™ kit and libraries were prepared and sequenced at the Microbial Genome Sequencing Center (Pittsburgh, PA). Raw chromatin immunoprecipitation sequencing data are available in the NCBI GEO database under series accession GSE241057.
ChIP-seq analysis
Paired-end reads were mapped to the C. crescentus NA1000 reference genome (GenBank accession number CP001340) with CLC Genomics Workbench 20 (Qiagen), ignoring non-specific matches. Peak calling was performed with the Genrich tool (https://github.com/jsh58/Genrich) on Galaxy; peaks are presented in Table. Briefly, PCR duplicates were removed from mapped reads, replicates were pooled, input reads were used as the control dataset, and peak were called using the default peak calling option [Maximum q-value: 0.05, Minimum area under the curve (AUC): 20, Minimum peak length: 0, Maximum distance between significant sites: 100]. For subsequent analysis, ChIP-seq peaks with q-value ≤ 0.001 were used. ChIPpeakAnno was used to determine the number of peaks that overlapped between the different XRE ChIP-seq datasets. Peaks were designated as 100 bp bins centered on the peak summit as identified by Genrich. Peaks were considered overlapping if at least 1 base pair overlapped.
XRE motif discovery
For motif discovery, DNA sequences of ChIP-seq peaks from each dataset were submitted to the XSTREME module of MEME suite [76]. ChIP-seq peaks were designated as 100 bp bins centered on the summit coordinate identified by Genrich. For XSTREME parameters, the background model was constructed from shuffled input sequences (Markov Model Order: 2). The expected motif site distribution was set to ‘zero or one occurrence per sequence’. Motifs length was designated as between 6 and 15 bp. The top MEME hit was designated as the enriched sequence motif.
RNA preparation, sequencing, and analysis
Strains were incubated in triplicate at 30°C overnight in 2 ml PYE supplemented with 50 μM cumate. Then, 1 ml overnight was diluted into 10 ml PYE supplemented with 50 μM cumate and grown at 30°C for 4 hours (~0.18 – 0.26 OD660). 8 ml culture was pelleted at 15,000 × g for 1 min at 4°C, supernatant was removed, pellets were resuspended in 1 ml TRIzol, and stored at −80°C. For φCbK infection time course RNA-seq, strains were incubated in triplicate at 30°C overnight in 10 ml PYE. Then 2.5 ml overnight was diluted into 50 ml PYE and incubated at 30°C for 4 hours (~0.18 – 0.2 OD660), then diluted to 0.1 OD660. For 0 min time point, 5 ml culture was pelleted at 15,000 × g for 1 min at 4°C, supernatant was removed, pellets were resuspended in 1 ml TRIzol, and stored at −80°C. Then, 3.9 × 1010 PFU φCbK (10 MOI) was added to cultures and cultures were incubated at 30°C for 90 min while shaking. At 15, 30, 45, 60, 75, and 90 min, 5 ml culture was pelleted at 15,000 × g for 1 min at 4°C, supernatant was removed, pellets were resuspended in 1 ml TRIzol, and stored at −80°C. Samples were heated at 65°C for 10 min and 200 μl chloroform was added. Samples were vortexed for 15 sec, then incubated at room temperature for 5 min. Samples were pelleted at 17,000 × g for 15 min at 4°C, then the aqueous phase was transferred to a fresh tube. An equivalent volume 100% isopropanol was added to each sample, then mixed by inversion, and stored at −80°C to precipitation nucleic acids. Thawed samples were then pelleted at 17,000 × g for 30 min at 4°C and supernatant was removed. Samples were washed in 1 ml cold 70% ethanol, then vortexed briefly. Samples were then pelleted at 17,000 × g for 5 min at 4°C, supernatant was removed, and pellets were air dried for 10 min. Pellets were resuspended in 100 μl RNase-free water and incubated at 60°C for 10 min. Samples were treated with TURBO DNase and cleaned up with RNeasy Mini Kit (Qiagen).
Library preparation and sequencing was performed by SeqCenter with Illumina Stranded RNA library preparation and RiboZero Plus rRNA depletion (Pittsburgh, PA). Reads were mapped to the C. crescentus NA1000 reference genome (GenBank accession CP001340) or φCbK reference genome (GenBank accession JX100813.1) using CLC Genomics Workbench (Qiagen). Differential gene expression was determined with CLC Genomics Workbench RNA-seq Analysis Tool. For φCbK tgrL overexpression, differential gene expression was designated as genes that significantly changed compared to the empty vector control (|fold-change| ≥ 1.5 and FDR p-value ≤ 0.0001). For φCbK infection time course, differential expression of C. crescentus genes was designated as genes that significantly changed in at least one time point compared to the 0 minute sample (uninfected) (|fold-change| ≥ 2 and FDR p-value ≥ 0.0001). For differential expression of φCbK genes during infection, fold-change was determined by comparison to the 15 minute sample. Raw sequencing data are available in the NCBI GEO database under series accession GSE241057.
To determine direct targets of TgrL, ChIPpeakAnno was used to identify promoters that overlapped with TgrL ChIP-seq peaks [77]. Promoters were designated as −300 to +100 bp around transcription start sites (TSS) (modified from [78]). For genes/operons that did not have an annotated TSS, the +1 residue of the first gene in the operon was designated as the TSS. Features were considered overlapping if at least 1 base pair overlapped. To cluster RNA-seq infection time course data, RPKM for each gene and time point was averaged and normalized by calculating the percent expression compared to maximum RPKM of the gene in the time course. Genes were then hierarchically clustered (clustering method: average linkage; similarity metric: uncentered correlation) using Cluster 3 [79] and viewed with Java TreeView [80].
Bacterial two-hybrid assay
The previously described bacterial two-hybrid system was utilized [81]. Plasmids containing fusions to T25 or T18c domains of adenylate cyclase were co-transformed into chemically competent BTH101 E. coli through heat shock at 42°C. Transformants were selected on LB agar supplemented with 50 μg ml−1 kanamycin, 100 μg ml−1 carbenicillin, 0.5 mM IPTG, and 40 μg ml−1 X-gal. Strains were inoculated into 2 ml LB broth supplemented 30 μg ml−1 kanamycin, 100 μg ml−1 carbenicillin, and 0.5 mM IPTG, then shaken at 30°C overnight. Overnight cultures were diluted to 0.05 OD600 and 5 μl diluted culture was spotted onto LB agar supplemented with 50 μg ml−1 kanamycin, 100 μg ml−1 carbenicillin, 0.5 mM IPTG, and 40 μg ml−1 X-gal. Plates were imaged after growth at 30°C for 24 hours, followed by a 5 hour incubation at 4°C. For liquid culture assays, strains were inoculated into 2 ml LB broth supplemented 30 μg ml−1 kanamycin, 100 μg ml−1 carbenicillin, and 0.5 mM IPTG, then shaken overnight at 30°C. Then, 200 μl overnight culture was diluted into 2 ml LB broth supplemented 30 μg ml−1 kanamycin, 100 μg ml−1 carbenicillin, and 0.5 mM IPTG, grown at 30°C for 2 hours. OD600 was measured and 100 μl culture was permeabilized with 100 μl chloroform. Then, 600 μl Z-buffer and 200 μl 4 mg ml−1 ONPG was added to the reactions. Color development was stopped through the addition of 1 ml 1 M Na2CO3, OD420 was measured, and Miller units were calculated.
Phage infection assay
Strains were incubated at 30°C overnight in PYE supplemented with 50 μM cumate. Overnight cultures were diluted 1/10 in PYE supplemented with 50 μM cumate and incubated at 30°C for 4 hours. Cultures were diluted to 0.2 OD660 in 1 ml PYE supplemented with 50 μM cumate and 1.3 × 106 φCbK phage were added (0.01 MOI). Phage were allowed to adsorb for 15 min at 30°C while shaking, diluted 1/1000 in PYE supplemented with 50 μM cumate, then shaken at 30°C for 180 min. For plating, 5 ml molten PYE top agar (0.3% (w/v) agar), 1 ml CB15 xylX::pPTM057 (0.2 OD660), and 150 μl 30% (w/v) xylose were mixed and poured over a PYE plate. To enumerate the number of total phage (free + infected phage), 100 μl sample was plated on PYE top agar, then incubated at 30°C overnight. To determine the number of free phage, samples were treated with chloroform. Samples were diluted where appropriate, then 100 μl was plated on PYE top agar and incubated at 30°C overnight. Burst size was calculated as (Total PFU/ml t=180 − Free PFU/ml t=0)/(Total PFU/ml t=0 − Free PFU/ml t=0).
Genomic DNA isolation
Strains were incubated at 30°C overnight in PYE supplemented with 50 μM cumate. Overnight cultures were diluted to 0.05 OD660 in PYE supplemented with 50 μM cumate and incubated at 30°C for 24 hours. Cultures were pelleted at 12,000 × g for 1 min, supernatant was removed, pellets were washed with 0.5 ml H2O, and then resuspended in 100 μl TE buffer (10 mM Tris pH 8.0, 1 mM EDTA). 500 μl GES lysis solution (5.08 M guanidium thiocyanate, 0.1 M EDTA, 0.5% (w/v) sarkosyl) was added, samples were vortexed, then heated at 60°C for 15 min. 250 μl 7.5 M cold ammonium acetate was added, samples were vortexed for 15 sec, and incubated on ice for 10 min. 500 μl chloroform was added and samples were vortexed for 15 sec. Samples were pelleted at 12,000 × g for 10 min, then the aqueous phase was transferred to a fresh Eppendorf tube. 324 μl cold isopropanol (0.54 volumes) was added, samples were mixed by inversion, and incubated at room temperature for 15 min. Samples were pelleted at 12,000 × g for 3 min, supernatant was removed, and pellets were washed with 700 μl 70% (v/v) ethanol. Pellets were air dried for 10 min, then resuspended in 100 μl TE buffer. DNA concentrations were measured by NanoDrop. Samples were diluted to 400 ng/μl and 20 μl diluted sample was run on a 1% (w/v) agarose gel. Gels were imaged on a ChemiDoc MP with 605/50 filter and UV trans illumination.
Supplementary Material
Figure 11. XRE Transcription Factor Network in regulation of Caulobacter and φCbK gene expression.
Schematic of XRE transcription factor (TF) network (top) and φCbK infection schematic (bottom). The sensor histidine kinases LovK, SkaH, and SpdS physically interact and promotes SpdR activity [2]. SpdR activates rtrC expression [3], and RtrC and SpdR activate expression of XRE TF paralogs (rtrA, rtrB, cdxA, and cdxB) [2, 3]. XRE TFs repress holdfast and gene transfer agent (GTA) regulators, hfiA and gafYZ, respectively. φCbK tgrL is highly expressed during early infection of C. crescentus and regulates expression of C. crescentus genes. Dashed lines indicate post-transcriptional regulation and solid lines indicate transcriptional regulation. Black arrows indicate activation and red bar-ended lines indicate repression. Pink phage indicates φCbK phage, grey cells indicate C. crescentus, and cells outlined in dashed lines indicates lysed cells.
Acknowledgements
This work was supported by the National Institute of General Medical Science of the National Institutes of Health under award number R35 GM131762 to S.C. and F32 GM141017 to M.M. We thank members of the Crosson lab, as well as Tung Le and Emma Banks for valuable feedback and discussions throughout the course of this study.
References
- 1.Fiebig A., et al. , A cell cycle and nutritional checkpoint controlling bacterial surface adhesion. PLoS Genet, 2014. 10(1): p. e1004101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reyes Ruiz L.M., Fiebig A., and Crosson S., Regulation of bacterial surface attachment by a network of sensory transduction proteins. PLoS Genet, 2019. 15(5): p. e1008022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLaughlin M., et al. , A cryptic transcription factor regulates Caulobacter adhesin development. PLoS Genet, 2022. 18(10): p. e1010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moons P., Michiels C.W., and Aertsen A., Bacterial interactions in biofilms. Crit Rev Microbiol, 2009. 35(3): p. 157–68. [DOI] [PubMed] [Google Scholar]
- 5.Figueiredo A.M.S., et al. , The role of biofilms in persistent infections and factors involved in ica-independent biofilm development and gene regulation in Staphylococcus aureus. Crit Rev Microbiol, 2017. 43(5): p. 602–620. [DOI] [PubMed] [Google Scholar]
- 6.Dufrene Y.F. and Persat A., Mechanomicrobiology: how bacteria sense and respond to forces. Nat Rev Microbiol, 2020. 18(4): p. 227–240. [DOI] [PubMed] [Google Scholar]
- 7.Hershey D.M., Fiebig A., and Crosson S., A Genome-Wide Analysis of Adhesion in Caulobacter crescentus Identifies New Regulatory and Biosynthetic Components for Holdfast Assembly. mBio, 2019. 10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hershey D.M., Fiebig A., and Crosson S., Flagellar Perturbations Activate Adhesion through Two Distinct Pathways in Caulobacter crescentus. mBio, 2021. 12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santos C.L., et al. , A phylogenomic analysis of bacterial helix-turn-helix transcription factors. FEMS Microbiol Rev, 2009. 33(2): p. 411–29. [DOI] [PubMed] [Google Scholar]
- 10.Blum M., et al. , The InterPro protein families and domains database: 20 years on. Nucleic Acids Res, 2021. 49(D1): p. D344–D354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter M.S. and Alber B.E., Transcriptional Regulation by the Short-Chain Fatty Acyl Coenzyme A Regulator (ScfR) PccR Controls Propionyl Coenzyme A Assimilation by Rhodobacter sphaeroides. J Bacteriol, 2015. 197(19): p. 3048–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerstmeir R., et al. , RamB, a novel transcriptional regulator of genes involved in acetate metabolism of Corynebacterium glutamicum. J Bacteriol, 2004. 186(9): p. 2798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto K., Nakano M., and Ishihama A., Regulatory role of transcription factor SutR (YdcN) in sulfur utilization in Escherichia coli. Microbiology (Reading), 2015. 161(Pt 1): p. 99–111. [DOI] [PubMed] [Google Scholar]
- 14.Chu F., et al. , Targets of the master regulator of biofilm formation in Bacillus subtilis. Mol Microbiol, 2006. 59(4): p. 1216–28. [DOI] [PubMed] [Google Scholar]
- 15.Kearns D.B., et al. , A master regulator for biofilm formation by Bacillus subtilis. Mol Microbiol, 2005. 55(3): p. 739–49. [DOI] [PubMed] [Google Scholar]
- 16.Hu Y., et al. , The XRE Family Transcriptional Regulator SrtR in Streptococcus suis Is Involved in Oxidant Tolerance and Virulence. Front Cell Infect Microbiol, 2018. 8: p. 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furusawa G., et al. , Global analysis of phase variation in Myxococcus xanthus. Mol Microbiol, 2011. 81(3): p. 784–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ptashne M., A Genetic Switch: Phage Lambda Revisited. 2004: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 19.Schubert R.A., et al. , Cro’s role in the CI Cro bistable switch is critical for lambda’s transition from lysogeny to lytic development. Genes Dev, 2007. 21(19): p. 2461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ptashne M., Lambda’s switch: lessons from a module swap. Curr Biol, 2006. 16(12): p. R459–62. [DOI] [PubMed] [Google Scholar]
- 21.Svenningsen S.L., et al. , On the role of Cro in lambda prophage induction. Proc Natl Acad Sci U S A, 2005. 102(12): p. 4465–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ptashne M., et al. , How the lambda repressor and cro work. Cell, 1980. 19(1): p. 1–11. [DOI] [PubMed] [Google Scholar]
- 23.Gozzi K., et al. , Prophage-like gene transfer agents promote Caulobacter crescentus survival and DNA repair during stationary phase. PLoS Biol, 2022. 20(11): p. e3001790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biondi E.G., et al. , A phosphorelay system controls stalk biogenesis during cell cycle progression in Caulobacter crescentus. Mol Microbiol, 2006. 59(2): p. 386–401. [DOI] [PubMed] [Google Scholar]
- 25.Akiba N., et al. , phrR-like gene praR of Azorhizobium caulinodans ORS571 is essential for symbiosis with Sesbania rostrata and is involved in expression of reb genes. Appl Environ Microbiol, 2010. 76(11): p. 3475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puxty R.J. and Millard A.D., Functional ecology of bacteriophages in the environment. Curr Opin Microbiol, 2023. 71: p. 102245. [DOI] [PubMed] [Google Scholar]
- 27.Lawrence J.G., Gene transfer in bacteria: speciation without species? Theor Popul Biol, 2002. 61(4): p. 449–60. [DOI] [PubMed] [Google Scholar]
- 28.Bailey T.L., STREME: Accurate and versatile sequence motif discovery. Bioinformatics, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christen M., et al. , Quantitative Selection Analysis of Bacteriophage phiCbK Susceptibility in Caulobacter crescentus. J Mol Biol, 2016. 428(2 Pt B): p. 419–30. [DOI] [PubMed] [Google Scholar]
- 30.Si M., et al. , MsrR is a thiol-based oxidation-sensing regulator of the XRE family that modulates C. glutamicum oxidative stress resistance. Microb Cell Fact, 2020. 19(1): p. 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu H., et al. , Structure and DNA damage-dependent derepression mechanism for the XRE family member DG-DdrO. Nucleic Acids Res, 2019. 47(18): p. 9925–9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott D.J., et al. , Quaternary re-arrangement analysed by spectral enhancement: the interaction of a sporulation repressor with its antagonist. J Mol Biol, 1999. 293(5): p. 997–1004. [DOI] [PubMed] [Google Scholar]
- 33.Lewis R.J., et al. , Crystallisation of the Bacillus subtilis sporulation inhibitor SinR, complexed with its antagonist, SinI. FEBS Lett, 1996. 378(1): p. 98–100. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki S., et al. , Rhizobial factors required for stem nodule maturation and maintenance in Sesbania rostrata-Azorhizobium caulinodans ORS571 symbiosis. Appl Environ Microbiol, 2007. 73(20): p. 6650–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuoka J.I., et al. , Stringent Expression Control of Pathogenic R-body Production in Legume Symbiont Azorhizobium caulinodans. mBio, 2017. 8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frederix M., et al. , Co-ordination of quorum-sensing regulation in Rhizobium leguminosarum by induction of an anti-repressor. Mol Microbiol, 2011. 81(4): p. 994–1007. [DOI] [PubMed] [Google Scholar]
- 37.Frederix M., et al. , Mutation of praR in Rhizobium leguminosarum enhances root biofilms, improving nodulation competitiveness by increased expression of attachment proteins. Mol Microbiol, 2014. 93(3): p. 464–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wheatley R.M., et al. , Lifestyle adaptations of Rhizobium from rhizosphere to symbiosis. Proc Natl Acad Sci U S A, 2020. 117(38): p. 23823–23834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berne C., et al. , Feedback regulation of Caulobacter crescentus holdfast synthesis by flagellum assembly via the holdfast inhibitor HfiA. Mol Microbiol, 2018. 110(2): p. 219–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lori C., et al. , A Single-Domain Response Regulator Functions as an Integrating Hub To Coordinate General Stress Response and Development in Alphaproteobacteria. mBio, 2018. 9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guerrero-Ferreira R.C., et al. , Alternative mechanism for bacteriophage adsorption to the motile bacterium Caulobacter crescentus. Proc Natl Acad Sci U S A, 2011. 108(24): p. 9963–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agabian-Keshishian N. and Shapiro L., Bacterial differentiation and phage infection. Virology, 1971. 44(1): p. 46–53. [DOI] [PubMed] [Google Scholar]
- 43.Lagenaur C., Farmer S., and Agabian N., Adsorption properties of stage-specific Caulobacter phage phiCbK. Virology, 1977. 77(1): p. 401–7. [DOI] [PubMed] [Google Scholar]
- 44.Sanchez-Perez G., et al. , Adapting to environmental changes using specialized paralogs. Trends Genet, 2008. 24(4): p. 154–8. [DOI] [PubMed] [Google Scholar]
- 45.da Silva C.A., et al. , Transcriptomic analysis of the stationary phase response regulator SpdR in Caulobacter crescentus. BMC Microbiol, 2016. 16: p. 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Birchler J.A. and Yang H., The multiple fates of gene duplications: Deletion, hypofunctionalization, subfunctionalization, neofunctionalization, dosage balance constraints, and neutral variation. Plant Cell, 2022. 34(7): p. 2466–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wehland M. and Bernhard F., The RcsAB box. Characterization of a new operator essential for the regulation of exopolysaccharide biosynthesis in enteric bacteria. J Biol Chem, 2000. 275(10): p. 7013–20. [DOI] [PubMed] [Google Scholar]
- 48.Venkatesh G.R., et al. , BglJ-RcsB heterodimers relieve repression of the Escherichia coli bgl operon by H-NS. J Bacteriol, 2010. 192(24): p. 6456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castanie-Cornet M.P., et al. , Acid stress response in Escherichia coli: mechanism of regulation of gadA transcription by RcsB and GadE. Nucleic Acids Res, 2010. 38(11): p. 3546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fumeaux C., et al. , Cell cycle transition from S-phase to G1 in Caulobacter is mediated by ancestral virulence regulators. Nat Commun, 2014. 5: p. 4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Bassam M.M., et al. , Response regulator heterodimer formation controls a key stage in Streptomyces development. PLoS Genet, 2014. 10(8): p. e1004554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopatina A., Tal N., and Sorek R., Abortive Infection: Bacterial Suicide as an Antiviral Immune Strategy. Annu Rev Virol, 2020. 7(1): p. 371–384. [DOI] [PubMed] [Google Scholar]
- 53.LeRoux M. and Laub M.T., Toxin-Antitoxin Systems as Phage Defense Elements. Annu Rev Microbiol, 2022. 76: p. 21–43. [DOI] [PubMed] [Google Scholar]
- 54.Ibryashkina E.M., et al. , Type II restriction endonuclease R.Eco29kI is a member of the GIY-YIG nuclease superfamily. BMC Struct Biol, 2007. 7: p. 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kowalski J.C., et al. , Configuration of the catalytic GIY-YIG domain of intron endonuclease I-TevI: coincidence of computational and molecular findings. Nucleic Acids Res, 1999. 27(10): p. 2115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vassallo C.N., et al. , A functional selection reveals previously undetected anti-phage defence systems in the E. coli pangenome. Nat Microbiol, 2022. 7(10): p. 1568–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barth Z.K., Nguyen M.H., and Seed K.D., A chimeric nuclease substitutes a phage CRISPR-Cas system to provide sequence-specific immunity against subviral parasites. Elife, 2021. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meijer W.J., et al. , Molecular basis for the exploitation of spore formation as survival mechanism by virulent phage phi29. EMBO J, 2005. 24(20): p. 3647–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mascolo E., et al. , The transcriptional regulator CtrA controls gene expression in Alphaproteobacteria phages: Evidence for a lytic deferment pathway. Front Microbiol, 2022. 13: p. 918015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swem L.R., et al. , Signal transduction by the global regulator RegB is mediated by a redox-active cysteine. EMBO J, 2003. 22(18): p. 4699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu J., et al. , RegB kinase activity is repressed by oxidative formation of cysteine sulfenic acid. J Biol Chem, 2013. 288(7): p. 4755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu J. and Bauer C.E., RegB kinase activity is controlled in part by monitoring the ratio of oxidized to reduced ubiquinones in the ubiquinone pool. mBio, 2010. 1(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Swem L.R., et al. , Identification of a ubiquinone-binding site that affects autophosphorylation of the sensor kinase RegB. J Biol Chem, 2006. 281(10): p. 6768–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Purcell E.B., et al. , A photosensory two-component system regulates bacterial cell attachment. Proc Natl Acad Sci U S A, 2007. 104(46): p. 18241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Purcell E.B., et al. , An analysis of the solution structure and signaling mechanism of LovK, a sensor histidine kinase integrating light and redox signals. Biochemistry, 2010. 49(31): p. 6761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eren A.M., et al. , Community-led, integrated, reproducible multi-omics with anvi’o. Nat Microbiol, 2021. 6(1): p. 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shaiber A., et al. , Functional and genetic markers of niche partitioning among enigmatic members of the human oral microbiome. Genome Biol, 2020. 21(1): p. 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hyatt D., et al. , Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics, 2010. 11: p. 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abel S., et al. , Bi-modal distribution of the second messenger c-di-GMP controls cell fate and asymmetry during the caulobacter cell cycle. PLoS Genet, 2013. 9(9): p. e1003744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kanehisa M., Sato Y., and Morishima K., BlastKOALA and GhostKOALA: KEGG Tools for Functional Characterization of Genome and Metagenome Sequences. J Mol Biol, 2016. 428(4): p. 726–731. [DOI] [PubMed] [Google Scholar]
- 71.Pritchard L., et al. , Genomics and taxonomy in diagnostics for food security: soft-rotting enterobacterial plant pathogens. Analytical Methods, 2016. 8(1): p. 12–24. [Google Scholar]
- 72.van Dongen S. and Abreu-Goodger C., Using MCL to extract clusters from networks. Methods Mol Biol, 2012. 804: p. 281–95. [DOI] [PubMed] [Google Scholar]
- 73.Saha C.K., et al. , FlaGs and webFlaGs: discovering novel biology through the analysis of gene neighbourhood conservation. Bioinformatics, 2021. 37(9): p. 1312–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maniatis T., Fritsch E.F., and Sambrook J., Molecular cloning : a laboratory manual. 1982, Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory. x, 545 p. [Google Scholar]
- 75.Ely B., Genetics of Caulobacter crescentus. Methods Enzymol, 1991. 204: p. 372–84. [DOI] [PubMed] [Google Scholar]
- 76.Grant C.E. and Bailey T.L., XSTREME: Comprehensive motif analysis of biological sequence datasets. bioRxiv, 2021. [Google Scholar]
- 77.Zhu L.J., et al. , ChIPpeakAnno: a Bioconductor package to annotate ChIP-seq and ChIP-chip data. BMC Bioinformatics, 2010. 11: p. 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bharmal M.H., Aretakis J.R., and Schrader J.M., An Improved Caulobacter crescentus Operon Annotation Based on Transcriptome Data. Microbiol Resour Announc, 2020. 9(44). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Hoon M.J., et al. , Open source clustering software. Bioinformatics, 2004. 20(9): p. 1453–4. [DOI] [PubMed] [Google Scholar]
- 80.Saldanha A.J., Java Treeview--extensible visualization of microarray data. Bioinformatics, 2004. 20(17): p. 3246–8. [DOI] [PubMed] [Google Scholar]
- 81.Karimova G., Ullmann A., and Ladant D., A bacterial two-hybrid system that exploits a cAMP signaling cascade in Escherichia coli. Methods Enzymol, 2000. 328: p. 59–73. [DOI] [PubMed] [Google Scholar]
- 82.Gill J.J., et al. , The Caulobacter crescentus phage phiCbK: genomics of a canonical phage. BMC Genomics, 2012. 13: p. 542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.











