Abstract
Evidence is accumulating that perturbed postnatal development of the gut microbiome contributes to childhood malnutrition1–4. Designing effective microbiome-directed therapeutic foods to repair these perturbations requires knowledge about how food components interact with the microbiome to alter its expressed functions. Here we use biospecimens from a randomized, controlled trial of a microbiome-directed complementary food prototype (MDCF-2) that produced superior rates of weight gain compared to a conventional ready-to-use supplementary food (RUSF) in 12–18-month-old Bangladeshi children with moderate acute malnutrition (MAM)4. We reconstructed 1000 bacterial genomes (metagenome-assembled genomes, MAGs) present in their fecal microbiomes, identified 75 whose abundances were positively associated with weight gain (change in weight-for-length Z score, WLZ), characterized gene expression changes in these MAGs as a function of treatment type and WLZ response, and used mass spectrometry to quantify carbohydrate structures in MDCF-2 and feces. The results reveal treatment-induced changes in expression of carbohydrate metabolic pathways in WLZ-associated MAGs. Comparing participants consuming MDCF-2 versus RUSF, and MDCF-2-treated children in the upper versus lower quartiles of WLZ responses revealed that two Prevotella copri MAGs positively associated with WLZ were principal contributors to MDCF-2-induced expression of metabolic pathways involved in utilization of its component glycans. Moreover, the predicted specificities of carbohydrate active enzymes expressed by polysaccharide utilization loci (PULs) in these two MAGs correlate with the (i) in vitro growth of Bangladeshi P. copri strains, possessing differing degrees of PUL and overall genomic content similarity to these MAGs, cultured in defined medium containing different purified glycans representative of those in MDCF-2, and (ii) levels of carbohydrate structures identified in feces from clinical trial participants. In the accompanying paper5, we use a gnotobiotic mouse model colonized with age- and WLZ-associated bacterial taxa cultured from this study population, and fed diets resembling those consumed by study participants, to directly test the relationship between P. copri, MDCF-2 glycan metabolism, host ponderal growth responses, and intestinal gene expression and metabolism. The ability to identify bioactive glycan structures in MDCFs that are metabolized by growth-associated bacterial taxa will help guide recommendations about use of this MDCF for children with acute malnutrition representing different geographic locales and ages, as well as enable development of bioequivalent, or more efficacious, formulations composed of culturally acceptable and affordable ingredients.
Keywords: human gut microbiome development and repair, nutritional status, therapeutic foods, postnatal human development, metagenome-assembled genomes (MAGs), in silico metabolic reconstructions, carbohydrate utilization by bacteria, glycan structure, Prevotella copri
The global health challenge of childhood undernutrition is great; in 2020, an estimated 149 million children under five years of age were stunted (low height-for-age) while 45 million exhibited wasting (low weight-for-length, WLZ)6. Undernutrition and its long-term sequelae are the leading causes of morbidity and mortality in this age range. Sequelae include persistent impairments in linear growth, immune and metabolic functions, and neurodevelopment - all of which have proven to be largely resistant to current interventions7. Although food insecurity is not the sole driver of undernutrition8, the profound disruption of economies and food systems by the COVID-19 pandemic has greatly exacerbated this global health challenge9.
Studies of healthy members of birth cohorts living in several countries have identified shared features of gut microbial community assembly – a process that is largely completed by the end of the second postnatal year10,11. Children with moderate or severe acute malnutrition (MAM or SAM) have impaired ponderal growth (wasting). Their microbial community development is perturbed, resulting in microbiota configurations that resemble those of chronologically younger children10. The metabolic maturation of malnourished children is also compromised compared to their healthy peers12. Colonization of gnotobiotic mice with fecal microbiota samples collected from healthy children or from chronologically age-matched children with acute malnutrition disclosed that microbial communities from the latter transmitted impaired weight gain and altered bone growth phenotypes, plus produced immune and metabolic abnormalities1,2,13.
We have used gnotobiotic mouse and piglet models to design MDCF formulations for repairing the microbial communities of children with MAM. MAM is defined as having a WLZ score that is 2–3 standard deviations below the median of a multi-national cohort of age-matched healthy children. In a 3-month randomized controlled feeding study of 12–18-month-old Bangladeshi children with MAM, we demonstrated that a lead formulation (MDCF-2) produced significant improvement in the rate of weight gain (β-WLZ) compared to a conventional RUSF that was not designed to alter the gut microbiota4. The superior effect of MDCF-2 on β-WLZ occurred even though its caloric density is 15% lower than RUSF. Plasma proteomic analyses revealed 70 proteins whose levels had statistically significant positive correlations with the change in WLZ, including mediators of musculoskeletal growth and neurodevelopment. These proteins were increased to a significantly greater degree in MDCF-2-treated children compared to those receiving RUSF. Levels of several proteins involved in immunoinflammatory processes were negatively correlated with WLZ and significantly reduced by MDCF-2 treatment4. Sequencing PCR amplicons generated from bacterial 16S rRNA genes present in fecal biospecimens disclosed 23 bacterial taxa that were significantly associated with WLZ; 21 were positively associated while two were negatively associated. The abundances of the positively associated taxa increased to a significantly greater degree with MDCF-2 compared to RUSF treatment4.
In this study, we reconstruct the genomes of bacteria present in the gut communities of participants in the completed trial, identify metabolic pathways that are differentially expressed in response to MDCF-2 in MAGs that are positively associated with WLZ, and determine how their differential expression relates to the processing of components of MDCF-2 and ponderal growth responses. The results highlight the remarkable strain specificity of microbiome responses and point to two Prevotella copri strains as key mediators of MDCF-2 glycan metabolism and host ponderal responses. In the accompanying study, we directly test these relationships using a gnotobiotic mouse model.
Results
Reconstructing bacterial genomes associated with ponderal growth responses
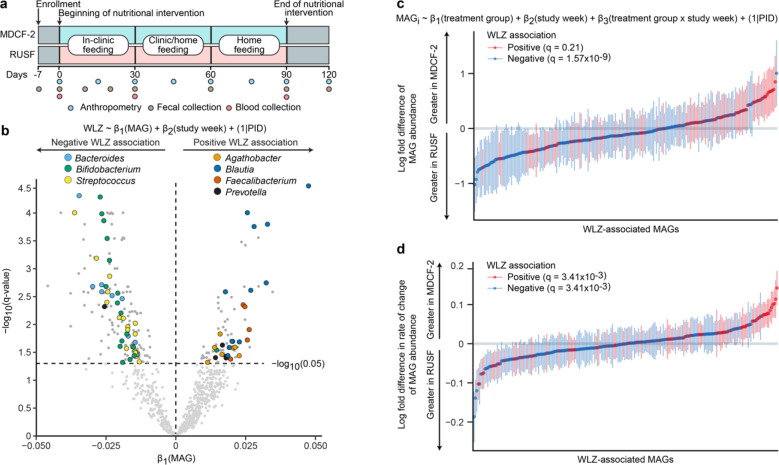
Fig. 1a summarizes the design of the randomized, controlled feeding study of children with MAM, aged 15.4±2.0 months (mean±SD) at enrollment. These children lived in an impoverished urban area (Mirpur) located in Dhaka, Bangladesh. The 3-month intervention involved twice-daily dietary supplementation with either MDCF-2 or RUSF (2 × 25g servings, providing ~220–250 kcal/day)4. A total of 59 children in each treatment group completed the intervention and a one month follow-up; fecal samples were collected every 10 days during the first month and every four weeks thereafter. There were no statistically significant differences in the amount of nutritional supplement consumed between children receiving MDCF-2 versus RUSF, no differences in the proportion of children who satisfied World Health Organization requirements for minimum meal frequency or minimum acceptable diet, and no differences in the amount of breast milk consumed between the two treatment groups4.
Fig. 1. Identification of ponderal growth (WLZ)-associated MAGs.
(a) Study design. (b) Volcano plot indicating the results of linear mixed effects modeling of the relationship between MAG abundance and WLZ scores for all trial participants, irrespective of treatment. Bacterial genera that are prevalent in the list of MAGs significantly associated with WLZ are colored by their taxonomic classification. Abbreviation: PID, participant identifier. (c,d) Results of gene set enrichment analysis (GSEA) of WLZ-associated MAGs ranked by the magnitude of their difference in abundance (panel c) or by their change in ‘abundance over time’ (panel d) in response to MDCF-2 versus RUSF treatment. Plotted values indicate the mean log2-fold difference (±SEM) in each model coefficient between the two treatment groups. The statistical significance of enrichment (q-value, GSEA) of MAGs that are positively or negatively associated with WLZ is shown.
To reconstruct the genomes of bacterial taxa present in the gut microbiomes of study participants, we isolated DNA from all fecal samples (n=942; 7–8 samples/participant) and performed short-read shotgun sequencing. DNA recovered from fecal biospecimens collected at t=0 and 3 months from the subset of participants comprising the upper quartile of the ponderal growth response to MDCF-2 (n=15)4 were subjected to additional long-read sequencing. We assembled pooled shotgun sequencing data from each participant’s fecal samples (short-read only, or short- plus long-reads when available) and aggregated contigs into metagenome-assembled genomes (MAGs) (Extended Data Fig. 1a; Supplementary Methods; Supplementary Discussion). The resulting set of 1,000 high-quality MAGs (defined as ≥90% complete and ≤5% contaminated based on marker gene analysis; Supplementary Table 1a) represented 65.6±8.0% and 66.2±7.9% of all quality controlled, paired-end shotgun reads generated from all 942 fecal DNA samples analyzed in the MDCF-2 and RUSF treatment groups, respectively [2.3±1.4×107 150 nt paired-end reads/sample (mean±SD); Supplementary Table 2a]. Taxonomy was assigned to MAGs using a consensus approach that included marker gene and kmer-based classification together with the Genome Taxonomy Database (GTDB; Supplementary Table 1a)14. Abundances were calculated for each MAG in the 707 fecal samples that spanned the beginning of treatment through the 1-month post-intervention time point and for which matching anthropometric measurements from children had been collected. A total of 837 MAGs satisfied our abundance and prevalence thresholds (see Methods and Supplementary Table 2b). We then employed linear mixed effects models to identify 222 MAGs whose abundances were significantly associated with WLZ [β1(MAG), q<0.05, Fig. 1b] over the 90-day course of the intervention and 30-day follow-up (see Supplementary Table 3 for the 75 positively associated and 147 negatively associated MAGs). MAGs that were significantly positively associated with WLZ were predominantly members of the genera Agathobacter, Blautia, Faecalibacterium and Prevotella, while members of Bacteroides, Bifidobacterium and Streptococcus were prevalent among MAGs negatively associated with WLZ (Fig. 1b, Extended Data Fig. 2, Supplementary Table 3).
Changes in MAG abundances were subsequently modeled as a function of treatment group, study week, and the interaction between treatment group and study week, controlling for repeated measurements taken from the same individual (see equation in Fig. 1c and Methods). The ‘treatment group’ coefficient describes the mean difference in abundance of a given MAG between the MDCF-2 and RUSF groups over the course of the intervention (Fig. 1c), while the interaction coefficient in the equation describes the difference in the rate of change in abundance of a given MAG (Fig. 1d). Restricting this analysis to the time of initiation of treatment did not reveal any statistically significant differences in MAG abundances between the two groups (q>0.05, one linear model per MAG; Supplementary Table 2b). Expanding the analysis to include all time points from initiation to end of treatment disclosed that although no individual MAG abundances were significantly associated with MDCF-2 or RUSF consumption, MAGs whose abundances increased faster in the MDCF-2 compared to the RUSF group were significantly enriched for those positively associated with WLZ [q=3.41×10−3, gene set enrichment analysis (GSEA); Fig. 1d]. In contrast, MAGs with a higher mean abundance as well as those that increased more rapidly in RUSF-treated children were significantly enriched for those negatively associated with WLZ (q=1.57×10−9 and q=3.41×10−3, respectively; GSEA) (Fig. 1c,d; Supplementary Table 4).
We utilized a ‘subsystems’ approach adapted from the SEED genome annotation platform15,16 to identify genes that comprise metabolic pathways represented in WLZ-associated MAGs. To do so, genes were aligned to a reference collection of 2,856 human gut bacterial genomes that had been subjected to in silico reconstructions of metabolic pathways reflecting major nutrient biosynthetic and degradative capabilities in mcSEED, a microbial community-centered implementation of SEED17. We employed this reference collection and procedures described in Extended Data Fig. 3 and Supplementary Methods to assign putative functions to a subset of 199,334 proteins in the 1,000 MAGs (Supplementary Table 5); these proteins, which represented 1,308 non-redundant functions, formed the basis for predicting which of 106 metabolic pathways were present or absent in each MAG. This effort generated a set of inferred metabolic phenotypes for each MAG (Supplementary Tables 6 and 7). GSEA disclosed multiple metabolic pathways involved in carbohydrate utilization that were significantly enriched in WLZ-associated MAGs (q<0.05), and in MAGs ranked by their changes in abundance in response to MDCF-2 compared to RUSF treatment. While other non-carbohydrate pathways were also identified using this approach (e.g., those involved in aspects of amino acid and bile acid metabolism), pathways involved in carbohydrate utilization predominated (P = 0.006, Fisher’s test; Extended Data Fig. 4; Supplementary Table 8).
Carbohydrate composition of MDCF-2 and RUSF
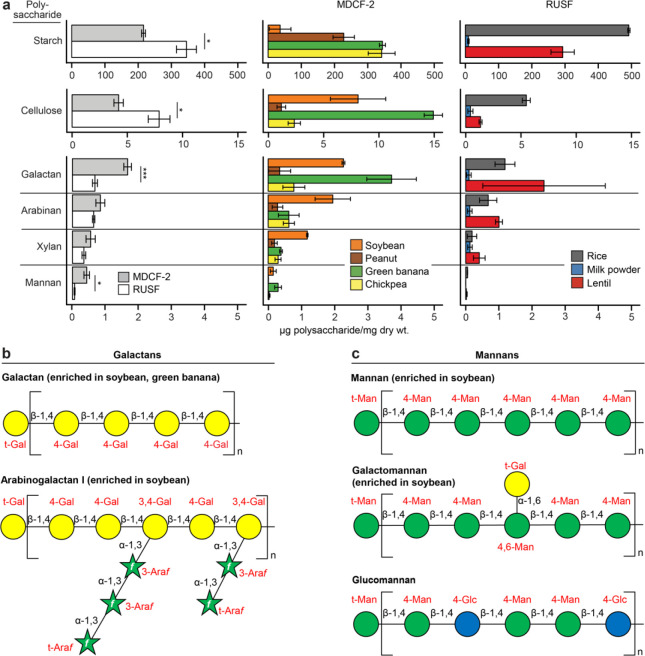
Prior to analyzing the transcriptional responses of MAGs to each nutritional intervention, we characterized the carbohydrates present in MDCF-2 and RUSF, as well as their constituent ingredients [chickpea flour, soybean flour, peanut paste and mashed green banana pulp in the case of MDCF-2; rice, lentil and milk powder in the case of RUSF (Supplementary Table 9a)]. Ultrahigh-performance liquid chromatography-triple quadrupole mass spectrometry (UHPLC-QqQ-MS) was used to quantify 14 monosaccharides and 49 unique glycosidic linkages. Polysaccharide content was defined using a procedure in which polysaccharides were chemically cleaved into oligosaccharides, after which the structures of these liberated oligosaccharides were then used to characterize and quantify their ‘parent’ polysaccharide18.
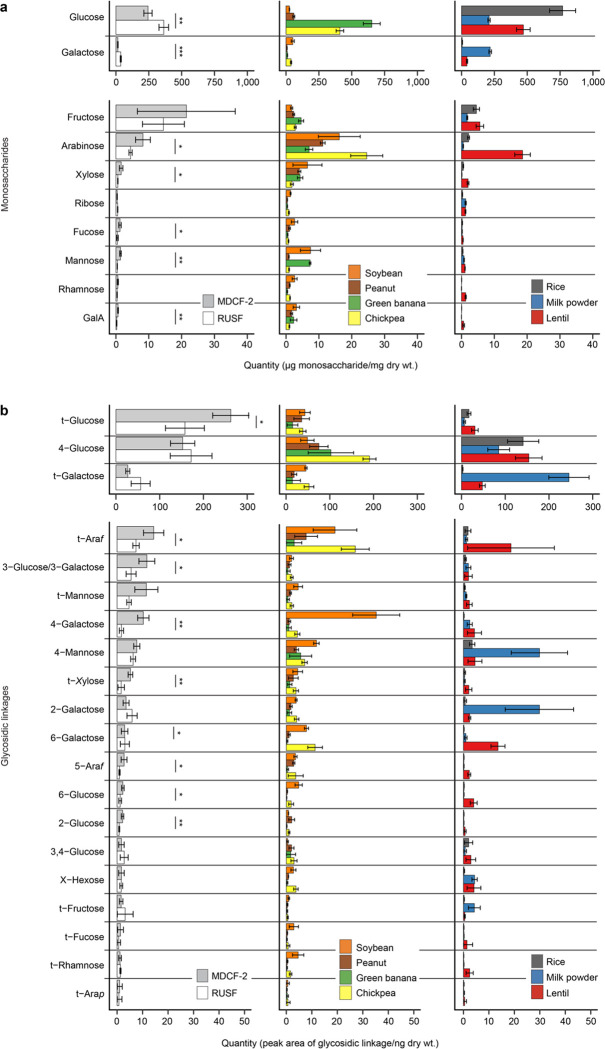
The results revealed that L-arabinose, D-xylose, L-fucose, D-mannose, and D-galacturonic acid (GalA) are significantly more abundant in MDCF-2 (P<0.05; t-test), as are 14 linkages, eight of which contain these monosaccharides (Extended Data Fig. 5a,b; Supplementary Table 9b,c,e,f). Integrating the quantitative polysaccharide and glycoside linkage data allowed us to conclude that MDCF-2 contains a significantly greater abundance of galactans and mannans than RUSF (P<0.05; t-test), while RUSF contains significantly more starch and cellulose (P<0.05; t-test) (Fig. 2a; Supplementary Table 9d,g). Galactans are represented in MDCF-2 as unbranched β-1,4-linked galactan as well as arabinogalactan I (Fig. 2b). Mannans are present as unbranched β-1,4-linked mannan (β-mannan), galactomannan and glucomannan (Fig. 2c). Arabinan is abundant in both formulations, although the representation of arabinose and glycosidic linkages containing arabinose is significantly greater in MDCF-2 than in RUSF (see Extended Data Fig. 5a,b and Supplementary Table 9e,f for results of statistical tests). Arabinan in MDCF-2 is largely derived from its soybean, banana, and chickpea components, while in RUSF this polysaccharide originates from rice and lentil (Fig. 2a). Arabinans in both formulations share a predominant 1,5-linked-L-arabinofuranose (Araf) backbone. Soybean arabinans are characterized by diverse side chains composed of 1,2- and 1,3-linked-L-Araf connected by 1,2,3-, 1,2,5-, and 1,3,5-L-Araf branch points, while chickpea, lentil, and banana arabinans primarily contain 1,3-linked side chains from 1,3,5-L-Araf branch points (Extended Data Fig. 5)19.
Fig. 2. Principal polysaccharides in MDCF-2, RUSF and their component food ingredients.
(a) Mean values ± SD are plotted. *, P<0.05; ***, P<0.001 (t-test). (b,c) Structures of galactans (panel b) and mannans (panel c) in MDCF-2.
MDCF-2 effects on WLZ-associated MAG gene expression
Microbial RNA-Seq was performed using RNA isolated from fecal samples collected from all study participants just prior to initiation of treatment, and after 1- and 3-months of treatment (n=350 samples). Transcripts were then quantified by mapping reads from each sample to MAGs. The resulting counts tables were filtered based on the abundance and prevalence of MAGs in the full set of all fecal samples. These filtering steps were designed to exclude MAGs with minimal contributions to the meta-transcriptome from subsequent differential expression analysis (exclusion criteria were benchmarked against a simulated meta-transcriptomic dataset using the approach described in Supplementary Methods).
We employed principal components analysis (PCA) to determine baseline differences in overall (DNA-based) MAG abundance profiles, or the abundance of MAG-derived RNAs in the expressed meta-transcriptomes, between the treatment groups, and to subsequently identify microbes that were principal drivers of shifts during treatment. Fig 3a plots (i) the percent variance explained by the first principal component (PC) in analyses of 837 MAGs in fecal samples collected across all time points from all study participants and (ii) taxa enriched (q<0.05; GSEA) along the first principal component of the MAG abundance and meta-transcriptome datasets (Fig. 3a; see Extended Data Fig. 6 for details of analyses of additional PCs). There were no statistically significant differences in microbiome or meta-transcriptome configuration between groups prior to treatment, or between the MDCF-2 and RUSF groups at each study week (P>0.1; PERMANOVA). Analysis of MAG contributions to each PCA highlights the remarkable enrichment of Prevotella spp. transcripts, and to a lesser extent, Bifidobacterium spp. transcripts, along the principal axis of variation (PC1) of the RNA-based PCA, and to a much lesser degree, the enrichment of these organisms along PC1 of the DNA-based MAG abundance PCA (Supplementary Table 10).
Fig. 3. Principal taxonomic features and expressed functions of MDCF-2 and RUSF-treated fecal microbiomes.
(a) Significant enrichment of taxa (q<0.1; GSEA) along the first principal component (PC1) of MAG abundance or transcript abundance. Abbreviation: NES; Normalized enrichment score. (b) Carbohydrate utilization pathways significantly enriched (q<0.1; GSEA) by treatment group (β1, circles) or the interaction of treatment group and study week (β3, squares). Right subpanel: Each point represents a MAG transcript assigned to each of the indicated functional pathways (rows), ranked by the direction and statistical significance of their differential expression in MDCF-2 versus RUSF treated participants (defined as the direction of the fold-change × −log10(P-value)). Transcripts are colored by their MAGs of origin. Larger, black outlined circles indicate leading edge transcripts assigned to the pathway described at the left of the panel. (c) Carbohydrate utilization pathways significantly enriched (q<0.1; GSEA) in upper vs lower WLZ quartile responders (β1, diamonds), or the interaction of WLZ response quartile and study week (β3, triangles) (see linear mixed effects model). Right subpanel: Transcripts assigned to each functional pathway. The coloring and outlining of circles have identical meaning as in panel b. The enrichment of glucuronate and galacturonate pathways was driven by the same transcripts; hence, these pathways were considered as a single unit. See Supplementary Tables 10–14 for supporting information.
We subsequently focused on transcripts expressed by the 222 MAGs whose abundances were significantly associated with WLZ. Transcripts were ranked by their response to MDCF-2 versus RUSF treatment or by their response over time (negative binomial generalized linear model; see equation in Fig. 3b). GSEA was then performed to identify metabolic pathways enriched in these ranked transcripts. The analysis revealed an MDCF-2-associated pattern of gene expression characterized by significant enrichment (q<0.1; GSEA) of three metabolic pathways related to carbohydrate utilization [α-arabinooligosaccharide (aAOS), arabinose and fucose; Fig. 3b], three pathways related to de novo amino acid synthesis (arginine, glutamine, and lysine biosynthesis), and one pathway for de novo vitamin synthesis (folate; Supplementary Tables 11 and 12). In contrast, none of the 106 metabolic pathways exhibited statistically significant enrichment in their expression in children who received RUSF.
We next investigated which MAGs were responsible for the observed enrichment of expressed pathways. To do so, we turned to ‘leading edge transcripts,’ a term defined by GSEA as those transcripts responsible for enrichment of a given pathway (Methods). Among positively WLZ-associated MAGs, two belonging to P. copri (MAG Bg0018 and MAG Bg0019) were the source of 11 of the 14 leading-edge transcripts related to aAOS utilization (Supplementary Table 12) – a pathway whose expression was significantly elevated in children treated with MDCF-2 compared to RUSF (Fig. 3b). Of the 11 P. copri MAGs in our dataset, these two were the only MAGs assigned to this species whose abundances were significantly positively correlated with WLZ. Both MAGs are members of a P. copri clade (Clade ‘A’) that is broadly distributed geographically20,21 (Extended Data Fig. 7a; see Extended Data Fig. 7b for the predicted carbohydrate utilization pathways represented in all 51 MAGs assigned to the genus Prevotella that were identified in our 1,000 MAG dataset).
Although P. copri MAGs were the greatest source of leading-edge transcripts related to arabinose and aAOS utilization, other MAGs in the microbiome display expression responses consistent with their participation in metabolizing MDCF-2 glycans (or their breakdown products); these include MAGs that are negatively correlated with WLZ. For example, leading-edge transcripts assigned to aAOS, arabinose and fucose utilization also arose from MAGs assigned to Bifidobacterium longum subsp. longum (Bg0006), Bifidobacterium longum subsp. suis (Bg0001), Bifidobacterium breve (Bg0010; Bg0014), Bifidobacterium sp. (Bg0070), and Ruminococcus gnavus (Bg0067) (Supplementary Table 12). Features of the metabolism of these glycans in Bifidobacterium and Ruminococcus MAGs are distinct from those expressed by the P. copri MAGs. For example, B. longum subsp. longum MAG Bg0006 encodes an extracellular exo-α-1,3-arabinofuranosidase (BlArafA) that belongs to glycoside hydrolase (GH) family 43_22; this enzyme cleaves terminal 1,3-linked-L-Araf residues present at the ends of branched arabinans and arabinogalactans, two abundant glycans found in MDCF-2 (Fig. 2b; Extended Data Fig. 5c)22,23. In contrast, P. copri possesses an endo-α-1,5-L-arabinanase that cleaves interior α-1,5-L-Araf linkages, generating aAOS. Integrating these predictions suggests a complex set of interactions between primary arabinan degraders like P. copri and members of B. longum, such as Bg0001 and Bg0006, that are capable of metabolizing products of arabinan degradation (see Extended Data Fig. 8 for reconstructions of carbohydrate utilization pathways in Bifidobacterium MAGs). We cannot discern whether the arabinose available to Bifidobacterium is derived from free arabinose or the breakdown products of arabinan polysaccharides. It is important to consider that in these 12- to 18-month-old children with MAM, responses to MDCF-2 are occurring in the context of the underlying co-development of their microbial community and host biology, during the period of transition from exclusive milk feeding to a fully weaned state. A MAG defined as positively associated with WLZ by linear modeling is an organism whose fitness (abundance) increases as WLZ increases. Our studies in healthy 1- to 24-month-old children living in Mirpur have documented how B. longum and other members of Bifidobacterium decrease in absolute abundance during the period of complementary feeding24. For the negatively WLZ-associated Bifidobacterium MAGs described above, the levels of consumption of MDCF-2 metabolic products during the period of complementary feeding may not be sufficient to overcome a more dominant effect exerted on their abundance/fitness by the state of community-host co-development. Moreover, the metabolic capacities of B. longum including, as well as beyond, those related to processing of MDCF-2 glycans, may influence host growth despite B. longum being naturally depleted over developmental time.
Based on these observations, we sought further evidence that the two P. copri MAGs are related to the magnitude of ponderal growth responses and to levels of fecal glycan structures generated from MDCF-2 metabolism.
Bacterial carbohydrate utilization pathways and clinical responses
PUL conservation in P. copri MAGs
As noted above, the primary outcome measure of the clinical trial was the rate of change of WLZ over the 3-month intervention. We stratified participants receiving MDCF-2 into WLZ-response quartiles4 and focused our analysis on (i) children in the upper and lower WLZ quartiles (n=15/group) and (ii) transcripts expressed by the 222 MAGs whose abundances were significantly associated with WLZ. We tested for enrichment of carbohydrate utilization pathways in transcripts rank-ordered by the strength and direction of their relationship with WLZ response quartile or, in a separate analysis, the interaction between WLZ response quartile and study week; we then performed GSEA to identify enriched pathways (Supplementary Tables 13 and 14). Eight carbohydrate utilization pathways were significantly enriched in transcripts differentially expressed in upper compared to lower WLZ quartile responders. One of these pathways (fructooligosaccharides utilization), plus three other pathways that are involved in arabinose, β-glucoside, and xylooligosaccharide utilization, were enriched in transcripts with a positive ‘WLZ quartile × study week’ interaction coefficient (β3), suggesting that the extent of the difference in expression of these pathways increases over the course of treatment (Fig. 3c, Supplementary Table 14a; see Supplementary Table 14b for enrichment of expressed vitamin and amino acid biosynthetic pathways related to WLZ-response quartile).
Remarkably, over half of the leading-edge transcripts (67/99; 68%) from the eight, upper WLZ quartile enriched carbohydrate utilization pathways were expressed by P. copri MAGs Bg0018 and Bg0019. Moreover, these two MAGs contributed no leading-edge transcripts to lower WLZ-response quartile enriched pathways.
P. copri is a member of the phylum Bacteroidota. Members of this phylum contain syntenic sets of genes known as polysaccharide utilization loci (PULs) that mediate detection, import and metabolism of a specific glycan or set of glycans25. To further define how expressed genomic features distinguish the capacity of MAGs Bg0018 and Bg0019 to respond to MDCF-2, we identified PULs in these MAGs and compared them to PULs present in the nine other P. copri MAGs in this study. These two WLZ-associated P. copri MAGs share (i) seven PULs we designated as conserved (i.e., pairwise comparisons of ORFs satisfy the requirements that their protein products have >90% amino acid identity and are organized in an identical way within the respective genomes) plus (ii) three PULs designated as present but ‘structurally distinct’ (i.e., a given PUL is present in the genomes being compared but component CAZymes or SusC/SusD proteins are missing or fragmented in a way likely to impact their function, or where extra ORFs are present; see Supplementary Methods). The representation of these 10 PULs varied among the other nine P. copri MAGs which span three of the four principal clades of this organism (Fig. 4a; Supplementary Table 15). Strikingly, the degree of genomic conservation of these PULs is significantly associated with the strength of WLZ association for each of the 11 P. copri MAGs in our MAG dataset across both treatment groups [Pearson r between Euclidean distance from Bg0019 PUL profile and β1(MAG) = −0.79 (P = 0.0035); Fig. 4b, see Supplementary Table 3 for WLZ associations]. Five of the seven highly conserved PULs are related to utilization of mannan and galactan – glycans that are significantly more abundant in MDCF-2 than RUSF. Expression of three of these seven PULs, as well as two of the conserved but structurally distinct PULs, is also related to the enrichment of transcripts in carbohydrate utilization pathways that distinguish upper from lower WLZ-quartile responders (‘WLZ-response quartile’ or ‘WLZ quartile × study week’ terms in Fig. 3c). PULs that generate these leading-edge transcripts are predicted to metabolize β-glucan, glucomannan, β-mannan, xylan, pectin/pectic galactan and arabinogalactan (see Fig. 4a for which of these 10 PULs contribute differentially expressed transcripts, plus Supplementary Table 15).
Fig. 4. Conservation and expression of Polysaccharide Utilization Loci (PULs) in P. copri MAGs and isolates.
(a) PUL conservation in P. copri MAGs identified in study participants (blue font) and in P. copri isolates cultured from the fecal microbiota of Bangladeshi children (red font). The phylogenetic tree (left) indicates the relatedness of P. copri MAGs and isolates as determined by a marker gene-based phylogenetic analysis (see Methods and Extended Data Fig. 7). P. copri clade designations are indicated by colored vertical bars. The β1(WLZ) coefficient for each P. copri MAG is indicated on the right of the panel; significant associations (q<0.05) are bolded. The color-coded matrix in the center indicates the extent of conservation of PULs in Bg0019 and Bg0018 versus the other P. copri MAGs and cultured isolates. The known or predicted polysaccharide substrates of these PULs are noted. Since MAG Bg0019 displayed a stronger association with WLZ than the other significantly WLZ-associated P. copri MAG Bg0018, it was used as a reference for the comparative PUL analysis. The number of leading-edge differentially expressed PUL transcripts in MAG Bg0018 and Bg0019 are shown within the colored cells; they were identified based on analysis of the fecal meta-transcriptomes of MDCF-2 versus RUSF treated participants, and/or from MDCF-2 treated participants in the upper- versus lower-WLZ quartiles (see Supplementary Table 15 for PUL numbering convention and for annotations of leading-edge transcripts). (b) Relationship between PUL conservation in the 11 P. copri MAGs identified in study participants and the strength of each MAG’s association with WLZ. (c,d) In vitro growth assays for five P. copri isolates in defined medium supplemented with individual purified glycans representative of those in MDCF-2. Panel c shows growth curves for P. copri BgF5_2, the isolate whose PUL profile is most similar to MAGs Bg0019/Bg0018. Data represent mean OD600 measurements with standard deviations indicated as grey ribbons (n=3 replicates/condition). Panel d summarizes PUL conservation as well as the growth rates for each of the five P. copri strains tested (panel c and Extended Data Fig. 9a). Each row represents a given strain. Each colored box represents PULs in that organism predicted to use the carbohydrate tested. PULs are noted as ‘functionally conserved’ (black, bold font), ‘structurally distinct’ but functionally similar (black, not bolded) or ‘not conserved’ (grey) according to the scheme shown in panel a. The color intensity surrounding each box indicates the mean maximum growth rate for each isolate in the presence of each glycan.
A comparative analysis of MAGs Bg0018 and Bg0019 and 22 reference P. copri genomes in PULDB26 indicated that one of the highly conserved PULs (PUL7) contains a bimodular GH26|GH5_4 β-glycanase with 52% amino acid sequence identity to an enzyme known to cleave β-glucan, β-mannan, xylan, arabinoxylan, glucomannan, and xyloglucan (Fig. 4a, Supplementary Table 15)27,28. The gene encoding this multifunctional enzyme did not satisfy our criteria for statistically significant differential expression between MDCF-2 and RUSF treatment, nor between upper versus lower quartile WLZ-responders. However, it was consistently expressed across these conditions/comparisons (Supplementary Table 15) and its enzymatic product is expected to contribute to the utilization of a broad range of plant glycans, including those represented in MDCF-2.
Together, these results highlight both the versatility in carbohydrate metabolic capabilities of these two WLZ-associated P. copri MAGs, as well as the specificity of their treatment-inducible metabolic pathways for carbohydrates prominently represented in MDCF-2.
Effects of different carbohydrates on in vitro growth of cultured representatives of MAGs Bg0018 and Bg0019
To contextualize our observations regarding conserved polysaccharide degradation features of P. copri MAGs, we engaged in an extensive effort to culture and characterize representatives of these MAGs from fecal samples obtained from participants in this clinical trial, plus a previous, shorter duration pilot study of MDCF prototypes3. Based on this effort, we selected a set of six P. copri isolates that represented diverse repertoires of conserved PULs as well as a range of phylogenetic distances from the WLZ-associated MAGs Bg0018 and Bg0019 (Fig. 4a; Supplementary Table 1b). Strains BgD5_2 and BgF5_2 are highly related phylogenetically to each other and to MAGs Bg0018 and Bg0019. Notably, they possess 9 of the 10 conserved PULs in these MAGs (see Supplementary Table 7c and Supplementary Table 15b for more details of functional conservation between the genomes of these and the other cultured P. copri strains and MAGs). Based on the substrate predictions for each conserved PUL, the measured glycan components of MDCF-2, and the variation in conservation of these PULs across our P. copri MAGs and isolates, we selected eight candidate glycan substrates for in vitro screening: sugar beet arabinan, wheat arabinoxylan, barley β-glucan, potato galactan, carob galactomannan, soybean rhamnogalacturonan, tamarind xyloglucan, and beechwood xylan (Supplementary Table 16a). Chondroitin sulfate was included in the panel as a negative control given its resistance to degradation by P. copri29. Each cultured isolate was grown in a defined medium containing 1% (w/v) of each glycan as the sole carbon source, and growth was determined by tracking optical density over time (Fig. 4c, Extended Data Fig. 9a). Strain BgD5_2 displayed poor and inconsistent growth in this medium compared to BgF5_2, even when glucose was used as the sole carbon source; therefore, the BgD5_2 isolate was not included in these in vitro experiments. The results underscore the broad glycan utilization capabilities of the P. copri isolates but also highlight their distinct preferences for individual glycans. Fig. 4c,d, Extended Data Fig. 9 and Supplementary Table 16b–d demonstrate how their growth phenotypes are aligned with their PUL repertoires, the known and predicted substrate specificities of the carbohydrate-active enzymes (CAZymes) encoded by their PULs, and the results of mass spectrometry-based quantification of their consumption of monosaccharide components of the tested glycans. Isolates whose PUL profiles matched the two WLZ-associated MAGs most closely (BgF5_2, Bg2C6, Bg2H3) displayed the strongest preference for glycan substrates that were enriched in and/or unique to MDCF-2 relative to RUSF, including arabinans (arabinan, arabinoxylan) and galactans/mannans (galactan, galactomannan) (Fig. 2; Extended Data Fig. 5). Notably, strain BgF5_2 displayed growth preferences for arabinoxylan and galactan; whereas all other strains favored arabinan over arabinoxylan. Together, these results support predictions of the capacities of the two WLZ-associated MAGs to utilize MDCF-2 glycans; they also indicate that BgF5_2 could be considered as a cultured representative of Bg0018 and Bg0019 given its similar glycan utilization preferences/capacities to those predicted for these two MAGs.
Fecal glycosidic linkage levels, WLZ responses and PUL expression
The same fecal samples collected at the 0- and 3-month time points from participants in the upper and lower WLZ quartiles in the MDCF-2 treatment group that had been used for the DNA- and RNA-level analyses were subjected to UHPLC-QqQ-MS-based quantitation of 49 glycosidic linkages. These linkages were measured after their liberation by in vitro hydrolysis of fecal glycans (Supplementary Table 17). We used linear mixed effects modeling to compare the changes in levels of fecal glycosidic linkages from baseline/pre-intervention to the treatment endpoint (3 months) as a function of WLZ response quartile. The results demonstrated that with treatment, levels of 14 linkages increased significantly more (q<0.05) in members of the upper compared to the lower WLZ response quartile. None of the 49 linkages increased significantly more in children who were in the lower compared to upper WLZ response quartile (Fig. 5a, Extended Data Fig. 10a, Supplementary Table 18a). All 14 glycosidic linkages elevated in upper quartile responders are represented in MDCF-2 [e.g., 4,6-mannose, which is predicted to be a product of soybean galactomannan cleavage by endo-1,4-β-mannosidases encoded by PUL7 and PUL8 present in the two WLZ-associated MAGs (Fig. 5d, Supplementary Table 9c); also see Fig. 5a for the likely polysaccharide sources of these 14 linkages in MDCF-2].
Fig. 5. Treatment-responsive glycosidic linkages and corresponding polysaccharide sources and structures, cleavage sites, and predicted products of CAZyme activity.
(a) Significant changes in fecal glycosidic linkage levels (q<0.05) over time in upper compared to lower WLZ quartile responders. Likely polysaccharide sources for each of the 14 glycosidic linkages are noted in the middle column (see Extended Data Fig. 5d). PULs present in P. copri MAGs Bg0018 and Bg0019 with known or predicted cleavage activity for the listed polysaccharide sources are noted in the right subpanel. (b,d) Structures of the MDCF-2 polysaccharides branched arabinan (panel b) and galactomannan (panel d), plus glycan fragments and their constituent glycosidic linkages predicted to be liberated by conserved P. copri MAGs Bg0019 and Bg0018 PULs (see Fig. 4a for results of PUL conservation analysis). Linkages highlighted with arrows are putative sites of cleavage by P. copri CAZymes based on their known or predicted enzyme activities; enzymes are labeled by their CAZyme module or modules predicted to perform the cleavage. The size of these arrows (large versus small) denotes the relative likelihood (high versus low, respectively) of glycosidic linkage cleavage by these CAZymes, considering steric hindrance at glycan branch points. The right subpanels illustrate the expression of PUL genes in MDCF-2 treated, upper vs lower WLZ quartile responders (only PUL genes with mcSEED or CAZy annotations are shown). (c,e) Predicted activities and expression of P. copri PUL CAZymes. Panel c depicts CAZymes assigned to PUL17b, including the GH51 family CAZyme (blue) expected to cleave α-1,2- and α-1,3-linked arabinofuranose (Araf) side chains and the GH43 family CAZymes (brown), including GH43_4 and GH43_5 subfamilies predicted to cleave α-1,5-Araf-linked backbone of branched arabinan, yielding products containing t-Araf, 2-Araf, 2,3 Araf and 5-Araf linkages. Panel e depicts CAZymes assigned to PUL7, including GH26 and GH5_4 CAZymes (magenta) predicted to cleave β-1,4 linked mannose residues of galactomannan, yielding products containing 4,6-mannose, the most significantly differentially abundant linkage in the upper quartile WLZ responders (see panel a).
Levels of glycosidic linkages in feces reflect a complex dynamic that includes, but is not necessarily limited to, the substrate specificities of the CAZymes encoded and expressed by PULs in primary consumers of available polysaccharides, the levels of host consumption of MDCF-2 and components of their ‘background’ diets, and the degree to which the initial products of polysaccharide degradation can be further processed by community members. These points are illustrated by the following observations. First, the presence of the 14 glycosidic linkages in feces can be explained in part by the specificity of CAZymes encoded and expressed by PULs conserved between P. copri MAGs Bg0018 and Bg0019. Fig. 5b–e, Extended Data Fig. 10b and Extended Data Fig. 11 describe which of their PULs are predicted to generate glycan fragments containing these linkages – predictions that are supported by the in vitro data generated from the cultured representative of the two MAGs. For example, t-Araf, 5-Araf, 2-Araf, and 2,3-Araf are components of polysaccharides (arabinan, arabinoxylan and arabinogalactan type I/II) present in soybean, chickpea, peanut and banana. CAZymes encoded by P. copri Bg0019 PULs 4, 7, 8, 16 and 17b have substrate specificities that allow them to cleave accessible linkages in these polysaccharides (see Fig. 5b,c; Extended Data Fig. 10 and Extended Data Fig. 11). Some of the products of these cleavage events are likely resistant to further degradation. The exo-α-1,2/1,3-L-arabinofuranosidase and endo-α-1,5-L-arabinanase activities encoded by PUL17b (Fig. 5b) are predicted to remove successive residues from the 1,2 and 1,3-linked-L-Araf chains of branched arabinan and hydrolyze the 1,5-linked-L-Araf backbone from this polysaccharide, yielding an enzyme-resistant product containing t-Araf, 5-Araf, 2-Araf, and 2,3-Araf linkages. Second, CAZyme transcripts assigned to PULs 4, 7, 8, 16 and 17b were detectable in the fecal meta-transcriptomes of all but one of the 30 participants assigned to the upper or lower WLZ responder quartiles. Levels of expression of the majority of these CAZymes genes were modestly elevated in upper compared to lower WLZ-quartile responders over the course of treatment, although the difference did not reach our threshold cutoff for statistical significance (q<0.05). These transcripts include the GH51 encoded by PUL17b plus the GH26, GH26|GH5_4, GH130 and carbohydrate esterase family 7 (CE7) transcripts from PUL7; see Extended Data Fig. 10b. Third, while intake of MDCF-2 was not significantly different between the upper and lower WLZ quartile participants (P>0.05; linear mixed effects model), data from a food frequency questionnaire (FFQ) administered at the time of each fecal sampling disclosed a positive correlation between consumption of legumes and nuts and the levels of t-Araf, 5-Araf, 2,3-Araf, t-GalA, and 2,4,6-Glucose (Supplementary Table 18b). Consumption of these foods was also the most discriminatory response between upper compared to lower WLZ quartile responders (Supplementary Table 18c). These observations suggest that children consuming more of the classes of complementary food ingredients present in MDCF-2 may also exhibit enhanced growth responses.
The confounding effects of background diet and the role of P. copri in processing MDCF-2 glycans can be directly tested in gnotobiotic mice colonized with a defined community of cultured representatives of WLZ-associated MAGs. One such gnotobiotic model is described in our companion study where mice were colonized with a defined consortium of age- and WLZ-associated Bangladeshi bacterial strains, with or without P. copri isolates that captured key features of the carbohydrate metabolic apparatus present in Bg0018 and Bg0019 (such as BgF5_2/BgD5_2). Analyses revealed that these P. copri strains were the principal mediators of MDCF-2 glycan degradation in vivo and that the combination of the presence of P. copri and a MDCF-2 diet was associated with promotion of ponderal growth and had marked effects on multiple aspects of metabolism in intestinal epithelial cell lineages5.
Discussion
The current study illustrates an approach for characterizing the gut microbiome targets and structure-function relationships of a therapeutic food - in this case, MDCF-2. MDCF-2 produced significantly greater weight gain during a 3-month, randomized controlled study of 12- to 18-month-old Bangladeshi children with moderate acute malnutrition compared to a conventional, more calorically dense RUSF. We have focused on metagenome-assembled genomes (MAGs), specifically (i) treatment-induced changes in expression of carbohydrate metabolic pathways in MAGs whose abundances were significantly associated with weight gain (WLZ), and (ii) mass spectrometric analysis of the metabolism of glycans present in the two food formulations. Quantifying monosaccharides, glycosidic linkages and polysaccharides present in MDCF-2, RUSF and their component ingredients disclosed that MDCF-2 contains more galactans and mannans (e.g., galactan, arabinogalactan I, galactomannan, β-mannan, glucomannan). Two types of comparisons were performed of the transcriptional responses of MAGs that were significantly associated with WLZ: one involved study participants who had consumed MDCF-2 versus RUSF, and the other focused on MDCF-2 treated children in the upper versus lower quartiles of WLZ response. The results revealed that two P. copri MAGs, both positively associated with WLZ, were the principal contributors to MDCF-2-induced expression of metabolic pathways involved in the utilization of its component glycans.
UHPLC-QqQ-MS was able to identify statistically significant changes in glycan composition in feces from children consuming a therapeutic food, even in the face of complex and varied background diets. Intriguingly, although intake of MDCF-2 did not differ between children in the upper and lower quartiles of clinical (WLZ) response, children in the upper quartile trended toward diets containing more legumes and nuts than those in the lower quartile. The “legumes and nuts” food group includes major components of MDCF-2. We postulate that MDCF-2 ‘kick-starts’ a microbiome response that includes changes in the fitness and expressed metabolic functions of key growth-associated bacterial strains, such as P. copri. Background diet can further modify this response, as evidenced by the higher levels of microbial metabolic products of legume/nut-associated glycans in the feces of children displaying upper quartile WLZ responses. This observation also suggests that further optimization of the dose of MDCF-2 may be possible; in our study, it was administered as a dietary supplement designed to provide ~20% of the childrens’ daily energy requirements. More detailed, quantitative assessments of food consumption during future clinical studies of MDCF-2 could serve to not only facilitate design of improved formulations/doses but also to inform future recommendations regarding complementary feeding practices - recommendations that recognize the important role of the gut microbiome in the healthy growth of children.
One definition of ‘microbiome repair’ in malnourished children is a rebalancing of the representation and expressed functions of beneficial organisms so that it assumes a configuration more conducive to healthy microbiome-host co-development. Linking dietary glycans and microbial metabolism in this fashion provides a starting point for culture-based initiatives designed to retrieve isolates of these ‘effector’ taxa for use as potential probiotic agents, or if combined with key nutrients that they covet, synbiotic formulations for repairing perturbed microbiomes that are insufficiently responsive to food-based interventions alone.
Much remains to be discovered about how MDCF-2 treatment is related to weight gain/healthy growth. For example, further work is needed to clarify whether the mediators of P. copri effects on the host arise from direct products of its metabolism of MDCF-2 glycans, or whether products of other metabolic pathways in P. copri, whose activities are regulated by biotransformation of MDCF-2 glycans, are involved. Additionally, the contributions of metabolites from other community members to these effects are unknown. Furthermore, the observed relationships between strains of P. copri and MDCF-2 glycans does not exclude the contribution of other macro- or micronutrients to the superiority of MDCF-2 over RUSF on weight gain in our study. Direct tests of the role played by organisms such as P. copri in mediating microbial community and host responses to components of microbiome-targeted therapeutic foods can come from additional clinical studies of probiotic or synbiotic formulations consisting of strains closely related to WLZ-associated MAGs, such as P. copri BgF5_2, administered in conjunction with MDCF-2 or with its glycan components. Another approach involves ‘reverse translation’ experiments of the type illustrated in our companion study to this report5. This companion study uses (i) a gnotobiotic mouse model colonized with defined collections of cultured, WLZ-associated gut bacterial taxa with or without P. copri, (ii) single nucleus RNA-Seq and microbial RNA-Seq and (iii) UHPLC-QqQ-MS to characterize the contributions of P. copri to post-weaning weight gain, processing of MDCF-2 glycans, and regulation of intestinal gene expression and metabolism.
Methods
Collection and handling of biospecimens obtained from participants in the randomized controlled clinical study of the efficacy of MDCF-2
The human study entitled ‘Community-based Clinical Trial With Microbiota-Directed Complementary Foods (MDCFs) Made of Locally Available Food Ingredients for the Management of Children With Primary Moderate Acute Malnutrition (MAM)’, was approved by the Ethical Review Committee at the icddr,b (Protocol PR-18073; ClinicalTrials.gov identifier: NCT04015999)4. Informed consent was obtained for all participants. The objective of the study was to determine whether twice daily, controlled administration of a locally produced, microbiota-directed complementary food (MDCF-23,4) for 3 months to children with MAM provided superior improvements in weight gain, microbiota repair, and improvements in the levels of key plasma biomarkers/mediators of healthy growth, compared to a commonly used rice- and lentil-based ready-to-use supplementary food (RUSF) formulation.
A total of 124 male and female children with MAM (WLZ −2 to −3) between 12- and 18-months-old who satisfied the inclusion criteria were enrolled, with 62 children randomly assigned to each treatment group using the permuted block randomization method. Children in each treatment group were fed their assigned dietary supplement (MDCF-2 or RUSF) twice daily at a study center for the first month, once daily at a study center and once daily at home for the second month, and twice daily at home for the third month. Mothers were otherwise encouraged to practice their customary breast- and complementary-feeding practices. At the end of the intervention period, children returned to their normal feeding routine with continued intensive monitoring for one additional month. Fifty-nine participants in each treatment group completed the 3-month intervention and 1-month post-treatment follow-up4.
To minimize the risk of degradation of fecal DNA/RNA, fecal samples were collected within 20 minutes of their production and immediately transferred to liquid nitrogen-charged vapor shippers for transport to a −80 °C freezer at the study center. Specimens were shipped to Washington University on dry ice where they were stored at −80 °C in a dedicated repository with approval from the Washington University Human Research Protection Office.
Defining the relationship between MAG abundances and WLZ
Procedures used for shotgun sequencing of fecal DNA and preprocessing of the resulting reads, plus MAG assembly and quantitation are described in Supplementary Methods.
Linear mixed effects models were used to relate the abundances of MAGs identified in each trial participant to WLZ using the formula:
Our data normalization strategies prior to linear modeling did not include a consideration of MAG assembly length. Therefore, we analyzed the TPM (reads per kilobase per million) output of kallisto (v0.43.0) by applying a filter requiring each MAG’s abundance to be >5 TPM in >40% of the 707 fecal samples collected at time points where anthropometry was also measured. This filtering approach yielded 837 MAGs. We then returned to the unfiltered count output from kallisto, performed a variance stabilizing transformation [VST, DESeq230 (v1.34.0)] to control for heteroskedasticity, and filtered the dataset to the same 837 MAGs. We subsequently fit linear mixed effects models to the transformed abundances of each MAG across all 707 fecal samples (lme431, v1.1–27.1; lmerTest32, v3.1–3). We used ANOVA to determine the statistical significance of the fixed effects in our model – specifically, the relationship between MAG abundance and WLZ. ‘WLZ-associated MAGs’ were defined as those having P-values adjusted for false discovery rate (q-values) <0.05.
Determining the effects of MDCF-2 supplementation on the abundances of WLZ-associated MAGs
We employed dream33 (variancePartition R package, v1.24.0) an empirical Bayesian linear mixed effects modeling framework, to model MAG abundance as a function of treatment group, study week and their interaction, controlling for the repeated measurements taken from each study participant with a random effect term for participant. The equation used to quantify the effects of treatment on MAG abundance took the form:
The ‘treatment group’ coefficient β1 indicates whether MDCF-2 produced changes in the mean abundance of a given MAG relative to RUSF over the 3-month intervention, while the ‘treatment group × study week’ interaction coefficient β3 indicates whether MDCF-2 affected the rate of change of a given MAG more so than RUSF (i.e., was a MAG increasing or decreasing more rapidly in the microbiomes of participants in the MDCF-2 versus the RUSF treatment group?). Each coefficient for each MAG abundance analysis is described by an associated t-statistic - a standardized measure, based on standard error, of a given coefficient’s deviation from zero which can be used to calculate a P-value and infer the significance of the effect of a given coefficient on the dependent variable. The t-statistics produced by this method can also be used as a ranking factor for input to GSEA. For this analysis, gene sets were defined as groups of MAGs that were either significantly positively (n=75) or significantly negatively (n=147) associated with WLZ. This analysis was conducted for both the ‘treatment group’ (β1) coefficient and the ‘treatment group × study week’ interaction (β3) coefficient. Statistical significance is reported as q-values after adjustment for false-discovery rate (Benjamini-Hochberg method).
Microbial RNA-Seq analysis of MAG gene expression
For RNA extraction, approximately 50 mg of a fecal sample, collected from each participant at the baseline, 1-month and 3-month time points, was pulverized under liquid nitrogen with a mortar and pestle and aliquoted into 2 mL cryotubes. A 3.97 mm steel ball and 250 μL of 0.1 mm zirconia/silica beads were subsequently added to each sample tube, together with 500 μL of a mixture of phenol:chloroform:isoamyl alcohol (25:24:1, pH 7.8–8.2), 210 μL of 20% SDS, and 500 μL of 2X Qiagen buffer A (200 mM NaCl, 200 mM Trizma base, 20 mM EDTA). After a 1-minute treatment in a bead beater (Biospec Minibeadbeater-96), samples were centrifuged at 3,220 × g for 4 minutes at 4 °C. One hundred microliters of the resulting aqueous phase were transferred by a liquid handling robot (Tecan) to a deep 96-well plate along with 70 μL of isopropanol and 10 μL of 3M NaOAc, pH 5.5. The solution was mixed by pipetting 10 times. The crude DNA/RNA mixture was incubated at −20 °C for 1 hour and then centrifugated at 3,220 × g at 4 °C for 15 minutes before removing the supernatant to yield nucleic acid-rich pellets. A Biomek FX robot was used to add 300 μL Qiagen Buffer RLT to the pellets and to resuspend the RNA/DNA by pipetting up and down 50 times. A 400 μL aliquot was transferred from each well to an Qiagen AllPrep 96 DNA plate, which was centrifuged at 3,220 × g for 1 minute at room temperature. The RNA flow-through was purified as described in the AllPrep 96 protocol. cDNA libraries were prepared from extracted RNA using an Illumina Total RNA Prep with Ribo-Zero Plus and dual unique indexes. Libraries were balanced, pooled, and sequenced in two runs of an Illumina NovaSeq using S4 flow cells.
As an initial pre-processing step, raw reads were aggregated by sample across the two NovaSeq runs, resulting in a total of 5.0×107±4.7×106 paired-end 150 nt reads per sample (mean±SD). Adapter sequences and low-quality bases were removed from raw reads (Trim Galore34, v0.6.4), and pairs of trimmed reads were filtered out if either one of the paired reads was less than 100 nt long. Pre- and post-trimmed sequence quality and adapter contamination were assessed using FastQC35 (v0.11.7). Filtered reads were pseudoaligned to the set of 1,000 annotated, dereplicated high quality MAGs to quantify transcripts with kallisto36. Reads that pseudoaligned to rRNA genes were excluded, leaving an average of 7.1×106±3.9×106 bacterial mRNA reads (mean±SD) per sample. Counts tables were further filtered to retain only transcripts that pseudoaligned to the 837 MAGs that passed the abundance and prevalence thresholds described above. To minimize inconsistently quantified counts related to low-abundance MAGs, we assigned a transcript count of zero, on a per-sample basis, to any MAG with a DNA abundance <0.5 TPM in that sample.
Differential expression analysis (edgeR37, v3.32.1) was conducted using the following steps: (i) transcript filtering for presence/absence and prevalence; (ii) library size normalization using TMM (trimmed mean of M-values); (iii) estimating per-gene count dispersions; and (iv) testing for differentially expressed genes. Transcripts were first filtered using edgeR default parameters, followed by a parameter sweep of transcript abundance and prevalence threshold combinations. Based on this analysis, transcripts with ≥ 5 counts per million mapped reads (CPM) in ≥ 35% of samples were retained for differential expression analysis. The transcripts that passed this filtering were normalized using a TMM-based scaling factor. We next estimated negative binomial dispersions and fit trended per-gene dispersions (using the power method) to negative binomial generalized linear models. These models were used to characterize (i) the effect of treatment group and study week among all participants and (ii) the effect of WLZ quartile and study week among MDCF-2 participants in the upper and lower quartiles of WLZ response using the following model formulae:
From these models, we identified genes that exhibited significant differential expression using the quasi-likelihood F-test (edgeR, function glmQLFTest) which accounts for the uncertainty in estimating the dispersion for each gene.
For subsequent functional metabolic pathway enrichment analyses, we (i) ordered transcripts assigned to WLZ-associated MAGs based on a ranking metric calculated as the direction of the fold-change × −log10(P-value) for a given differential expression analysis, (ii) defined gene sets as groups of these ranked transcripts assigned to the same metabolic pathway, and (iii) performed GSEA (fgsea38, v3.14). This set of analyses allowed us to identify differentially expressed metabolic pathways comprised of ≥10 genes over time (i) between treatment groups, (ii) between WLZ response quartiles or (iii) as a function of interacting terms in the linear mixed effect models (treatment group × study week; WLZ response quartile × study week). Enrichment results were considered statistically significant if they exhibited q-values<0.1 after controlling for false-discovery rate (Benjamini-Hochberg method).
For targeted transcriptional analyses of the CAZymes encoded by P. copri MAGs Bg0018 and Bg0019, we employed dream33 in R with no additional filtering, and the formula above relating transcripts to WLZ response quartile, study week, and the interaction of both terms, with the addition of a random effect for participant.
Principal Components Analysis
Principal Components Analysis (PCA) was performed on VST-transformed DNA or transcript counts for the 837 MAGs passing the filter described in the section entitled ‘Defining the relationship between MAG abundances and WLZ’ above. The PCA performed on transcript abundances encompassed 27,518 genes expressed by these MAGs at thresholds for levels and prevalence that are described in the section entitled ‘Microbial RNA-Seq analysis of MAG gene expression’ above. PCA was performed in R using the ‘prcomp’ function, with each data type centered but not scaled since the dataset was already VST-normalized. The functions ‘get_eigenvalues,’ ‘get_pca_ind,’ and ‘get_pca_var’ from the factoextra39 (v1.0.7) package were utilized to extract (i) the variance explained by each principal component, (ii) the coordinates for each sample along principal components, and (iii) the contributions of each variable to principal components 1–3. We used the ‘adonis2’ function within the vegan40 library (v2.5–7) to test for the statistical significance of differences in the microbiome (MAGs) or meta-transcriptome between the two treatment groups at baseline or over time.
LC-MS analyses of carbohydrates present in MDCF-2, RUSF, their component ingredients, fecal specimens and culture medium.
Sample preparation for glycan structure analysis
Frozen samples of MDCF-2, RUSF, their respective ingredients, and fecal biospecimens were ground with a mortar and pestle while submerged in liquid nitrogen. A 50 mg aliquot of each homogenized sample was lyophilized to dryness. Lyophilized samples were shipped to the Department of Chemistry at the University of California, Davis. On receipt, samples were pulverized to a fine powder using 2 mm stainless steel beads (for foods) or 2 mm glass beads (for feces). A 10 mg/mL stock solution of each sample was prepared in Nanopure water. All stock solutions were again bead homogenized, incubated at 100 °C for 1 h, bead homogenized again, and stored at −20 °C until further analysis.
Monosaccharide composition analysis
Methods were adapted from previous publications41,42. For analyses of food ingredients and fecal biospecimens, 10 μL aliquots were withdrawn from homogenized stock solutions and transferred to a 96-well plate containing 2 mL wells. For analyses of monocultures of P. copri strains grown in the presence of different purified polysaccharides, microplates were withdrawn from anaerobic chamber at the conclusion of the incubation and centrifuged (5,000 × g for 5 minutes). The resulting supernatants were removed and immediately frozen at −80 °C.
Each sample was subjected to acid hydrolysis (4 M trifluoroacetic acid for 1 h at 121 °C) followed by addition of 855 μL of ice-cold Nanopure water. Hydrolyzed samples, plus an external calibration standard comprised of 14 monosaccharides with known concentrations (0.001–100 μg/mL each) were derivatized with 0.2 M 1-phenyl-3-methyl-5-pyrazolone (PMP) in methanol plus 28% NH4OH for 30 minutes at 70 °C. The derivatized glycosides were fully dried by vacuum centrifugation, reconstituted in Nanopure water (Thermo Fischer Scientific), and excess PMP was extracted with chloroform. A 1 μL aliquot of the aqueous layer was injected into an Agilent 1290 Infinity II ultrahigh-performance liquid chromatography (UHPLC) system, separated using a 2-minute isocratic elution on a C18 column (Poroshell HPH, 2.1 × 50 mm, 1.9 μm particle size, Agilent Technologies), and analyzed using an Agilent 6495A triple quadrupole mass spectrometer (QqQ-MS) operated in dynamic multiple reaction monitoring (dMRM) mode. Monosaccharides in the food and fecal samples were identified and quantified by comparison to the external calibration curve.
Glycosidic linkage analysis
Methods were adapted from a previous publication with modifications43,44. Under an argon atmosphere, a 5 μL aliquot from each homogenized stock solution of a sample was permethylated in a 200 μL reaction that contained 5 μL saturated NaOH and 40 μL iodomethane in 150 μL of DMSO. Permethylated glycosides were extracted with dichloromethane, and the extract was dried by vacuum centrifugation. The extracted glycosides were subjected to acid hydrolysis (4 M trifluoroacetic acid for 2 h at 100) followed by vacuum centrifugation to dryness. Samples were then derivatized with PMP as described above for monosaccharide analysis, followed by another vacuum centrifugation to complete dryness. Methylated monosaccharides were then reconstituted with 100 μL of 70% methanol in water. A 1 μL aliquot of the aqueous layer was injected into an Agilent 1290 Infinity II UHPLC system, separated using a 16-minute gradient elution on a C18 column (ZORBAX RRHD Eclipse Plus, 2.1 × 150 mm, 1.8 μm particle size, Agilent Technologies), and analyzed using an Agilent 6495A QqQ-MS operated in multiple reaction monitoring (MRM) mode. A standard pool of oligosaccharides and a reference MRM library were used to identify and quantify glycosidic linkages in all samples.
Fenton’s initiation toward defined oligosaccharide groups (FITDOG) polysaccharide analysis
Methods were adapted from previous publications18,45. To separate endogenous oligosaccharides from the background food matrix, polysaccharides were precipitated with 80% aqueous ethanol. Dried precipitates were reconstituted, homogenized, and 10 mg/mL stock solutions were prepared. The FITDOG reaction was carried out using a 100 μL aliquot of the 10 mg/mL resuspended food pellet and 900 μL of reaction buffer (44 mM sodium acetate, 1.5% H2O2, 73 μM Fe2(SO4)3(H2O)5). The reaction mixture was incubated at 100 °C for 45 minutes, quenched with 500 μL 2 M NaOH, and then neutralized with 61 μL of glacial acetic acid. The resulting oligosaccharides were then reduced to their corresponding alditols with sodium borohydride (NaBH4) to prevent anomerization during chromatographic separation. For the reduction of oligosaccharides, a 400 μL aliquot of the reaction mixture was incubated with 400 μL 1 M NaBH4 at 65 °C for 60 minutes. Oligosaccharide products were then enriched using C18 and porous graphitized carbon (PGC) 96-well solid-phase extraction plates. For the C18 enrichment, cartridges were primed with 2 × 250 μL acetonitrile (ACN) and then 5 × 250 μL water washes prior to loading the reduced sample. Cartridge effluent was collected and subjected to subsequent PGC clean-up. PGC cartridges were primed with 400 μL water, 400 μL 80% ACN/0.1% (v/v) trifluoroacetic acid (TFA), and then 400 μL water prior to loading the C18 effluent. Washing was performed with 8 × 400 μL water, and the oligosaccharides were eluted with 40% ACN/0.05% (v/v) TFA and then dried using a vacuum centrifugal dryer. Oligosaccharides were reconstituted with 100 μL Nanopure water and a 10 μL aliquot was injected into the HPLC-Q-TOF instrument. Separation was carried out using an Agilent 1260 Infinity II HPLC with a PGC column (Hypercarb, 1 × 150 mm, 5 μm particle size, Thermo Scientific) coupled to an Agilent 6530 Accurate-Mass Q-TOF mass spectrometer. Specific HPLC, electrospray source, and MS acquisition parameters are described in greater detail in previous publications18,45. Oligosaccharide identification was based on MS/MS fragmentation and retention time (RT) compared to reacted polysaccharide standards (amylose, cellulose, mannan, galactan, linear arabinan, and xylan). Food polysaccharides were quantified using an external calibration curve that included the three most abundant oligosaccharides from each parent polysaccharide as the quantifier species.
Statistical analysis of carbohydrate composition
We analyzed the abundances of glycosidic linkages over time and between WLZ-response quartiles using linear mixed effects models (lme431, lmerTest32 packages in R) of the following form:
Linkages displaying a significant interaction (q <0.05) between WLZ response quartile and study week (β3 coefficient) were identified.
Statistical Information
Statistical analyses were conducted using the approaches described in Methods and the figure legends. Samples sizes are indicated along with each statistic test. All relevant statistical tests are two-tailed unless otherwise specified. All measurements were collected from distinct samples. Technical replicates were not collected and analyzed unless otherwise noted.
Extended Data
Extended Data Fig. 1. Bioinformatic workflow for MAG assembly, refinement, and quantitation.
(a) Pipeline for MAG assembly from short-read only or short-read plus long-read shotgun sequencing data. Steps are indicated on the left while the bioinformatic tools employed to accomplish each step are described within each box. (b) Comparison of MAG assembly summary statistics derived from CheckM (completeness, contamination) or Quast (number, length and N50 of contigs) for 82 high-quality MAGs obtained from short- plus long-read hybrid assemblies versus 918 high-quality MAGs from short-read only assembly methods. Boxplots show the median, first and third quartiles; whiskers extend to the largest value no further than 1.5 × the interquartile range. ***, P < 0.001 (Wilcoxon test).
Extended Data Fig. 2. Distribution of WLZ-associated MAGs across taxonomic groups.

Left subpanel, density plot showing WLZ-associated MAGs tabulated based on their genus-level classification. β1 refers to the coefficient in the linear mixed effects model presented at the bottom of the figure. Genera containing >3 significantly WLZ-associated MAGs are shown. Right subpanel, number of statistically significant WLZ-associated MAGs assigned to each genus depicted in the left subpanel.
Extended Data Fig. 3. Bioinformatics pipeline for subsystems-based annotation and prediction of functional capabilities (metabolic phenotypes) of MAGs.
The flow diagram shows the input data (2,856 reference genomes, 80 curated metabolic subsystems and 1,000 target MAGs) and the main computational steps performed. The pipeline produces two major outputs: (i) a complete set of functionally annotated proteins contributing to 80 reconstructed metabolic subsystems identified in the collection of 1,000 MAGs (annotation results are detailed in Supplementary Table 5 and Supplementary Table 6); and (ii) a Binary Phenotype Matrix (BPM) reflecting the inferred presence or absence of 106 functional metabolic pathways in each of the 1,000 MAGs shown in Supplementary Table 7a.
Extended Data Fig. 4. Enrichment of metabolic pathways in WLZ- and treatment-associated MAGs.
MAGs were ranked by their WLZ association (negative to positive) or treatment association (RUSF-associated to MDCF-2-associated) and GSEA was employed to determine overrepresentation of pathways in MAGs at the extremes of each ranked list. The results (Normalized Enrichment Score, NES) only include pathways that display a statistically significant enrichment (q<0.05, GSEA) in both the WLZ-associated MAG and treatment-associated MAG analyses. For carbohydrate utilization pathways, disaccharides and oligosaccharides are indicated with an asterisk.
Extended Data Fig. 5. LC-MS analysis of glycans present in MDCF-2, RUSF and their component ingredients.
(a,b) Analysis of monosaccharides (panel a) and glycosidic linkages (panel b) liberated by hydrolysis of glycans present in MDCF-2 and RUSF, and in the food ingredients used to formulate them. Mean±SD are plotted. *, P<0.05, **, P<0.01 (t-test). (c) Structures of glycans enriched in components of MDCF-2 or RUSF. (d) Measured glycosidic linkages in MDCF-2 and RUSF and their likely polysaccharide sources. Sources were inferred based on analysis of literature as well as polysaccharide standards. ‘†’ refers to instances where literature reports were not available and commercial polysaccharide preparations were used for direct experimental validation.
Extended Data Fig. 6. Principal components analysis of transcript and MAG abundances in fecal specimens.
(a,b) Percent variance explained by the top 10 principal components of a PCA analysis including abundance of MAGs (panel a) at the 0, 2, 4, 8, 12, and 16 week time points in Fig. 1a, or transcripts (panel b) at 0, 4, and 12 weeks. (c) Significantly enriched taxa (q<0.05, GSEA) along the first three principal components (PC1-PC3) of the fecal microbiome or meta-transcriptome PCA.
Extended Data Fig. 7. Phylogenetic tree and representation of mcSEED carbohydrate utilization pathways in Prevotella MAGs.
(a) Unrooted, marker gene-based phylogenetic tree of 51 Prevotella MAGs from this study, plus 1,049 P. copri genomes and MAGs previously assigned to each of four clades20. Pink stars denote the two WLZ-associated P. copri MAGs. The nine remaining P. copri MAGs from this study are highlighted by the green pentagons. The 40 Prevotella MAGs not classified as P. copri, based on an average branch length >0.5 from all 1,049 reference P. copri isolates, are grouped together and depicted as a yellow triangle. (b) mcSEED carbohydrate utilization pathways in 51 Prevotella MAGs from the current study. MAGs are hierarchically clustered based on the predicted presence (red) or absence (white) of these pathways.
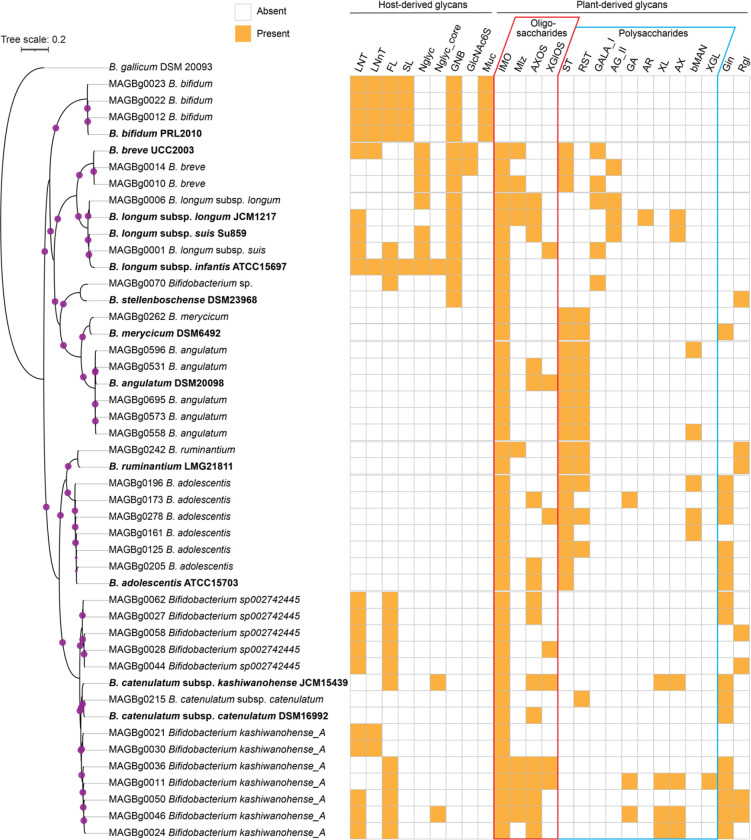
Extended Data Fig. 8. Phylogenetic tree and inferred carbohydrate utilization phenotypes of Bifidobacterium MAGs.
The phylogenetic tree indicates the relatedness of 34 Bifidobacterium MAGs and 14 reference genomes, as determined by sequence similarity among 142 core genes. The size of the pink circles in the dendrogram correspond to bootstrap support for the nodes (out of 100 bootstraps). Type stains used for taxonomic assignments and phenotypic comparisons are bolded. The matrix describes the presence (orange) or absence (white) of 25 predicted carbohydrate utilization phenotypes encompassing host- and plant-derived glycans. LNT, lacto-N-tetraose; LNnT, lacto-N-neotetraose; FL, 2- and 3-fucosyllactose; SL, 3- and 6-sialyllactose; Nglyc, N-glycans; Nglyc_core, N-glycan core (Fucα1–6GlcNAcβ1-Asn); GNB, galacto-N-biose; GlcNAc6S, N-acetylglucosamine-6-sulfate; Muc, mucin O-glycans; IMO, isomaltooligosaccharides and panose; Mlz, melezitose; AXOS, arabinoxylooligosaccharides; XGlOS, xyloglucan oligosaccharides; ST, starch and glycogen; RST, resistant starch; GALA_I, type I galactan and arabinogalactan; AGII, type II galactan and arabinogalactan; GA, gum arabic; AR, arabinan; XL, xylan; AX, arabinoxylan; bMAN, β-mannan; XGL, xyloglucan; Gin, ginsenosides; Rgl, rhamnoglycosides.
Extended Data Fig. 9. Growth of Bangladeshi P. copri strains in defined medium supplemented with purified polysaccharides.
(a) Growth curves in defined medium containing individual purified polysaccharides similar to those that are abundant in or unique to MDCF-2 compared to RUSF. Curves describe mean values ± sd for OD600 measurements (n=3 replicates/growth condition). (b) Growth of P. copri strains versus a PUL-based prediction of their growth phenotypes. Growth is expressed as ‘+’ or ‘−’ for each of the triplicate cultures according to whether a threshold OD600 > 0.25 was attained. The color key expresses ‘prediction’ as the fraction of possible cleavage sites in each polysaccharide that are known or predicted to be targeted by the PUL-associated CAZymes of a given strain. (c) CAZymes present in the PULs of each strain and their predicted activities against glycosidic linkages in each purified polysaccharide. Linkages in a polysaccharide that are predicted to be targeted by PUL-associated CAZymes are labeled ‘a,b…y’ in both panels b and c. All CAZyme family assignments for a given enzymatic activity are shown; the family that displays this activity most commonly is noted in bold font. (d) Heatmaps representing bacterial consumption of monosaccharides present in the different polysaccharides. UHPLC-QqQ-MS-based monosaccharide analysis was performed using defined medium harvested from monocultures of the P. copri stains. Control incubations did not contain added bacteria. Cells in each matrix show the mean difference (at the end of the 189 hour-long incubation) between the concentration of each monosaccharide in three technical replicates of each strain/polysaccharide combination compared to the corresponding uninoculated control.
Extended Data Fig. 10. Changes in levels of fecal glycosidic linkages and expression of P. copri CAZyme genes after MDCF-2 treatment.
(a) Boxplot of changes in the levels of fecal glycosidic linkages relative to initiation of treatment among upper and lower WLZ quartile responders. Levels of these 14 linkages increased to a significantly greater extent over time in the upper vs lower WLZ response quartiles (Model: linkage abundance ~ WLZ-response quartile × study week + (1|PID)). Boxplots indicate the median, first and third quartiles; whiskers extend to the largest value no further than 1.5 × the interquartile range. (b) The β3 coefficient for the interaction of WLZ-response quartile and study week is shown for CAZymes in consensus PULs in Bg0018 and Bg0019. Predicted PUL substrates and potential glycosidic linkages in each of these substrates are shown on the right. Glycosidic linkages whose abundances were significantly different in fecal samples from the upper versus lower WLZ quartile responders are highlighted in bold font (see Fig. 5a).
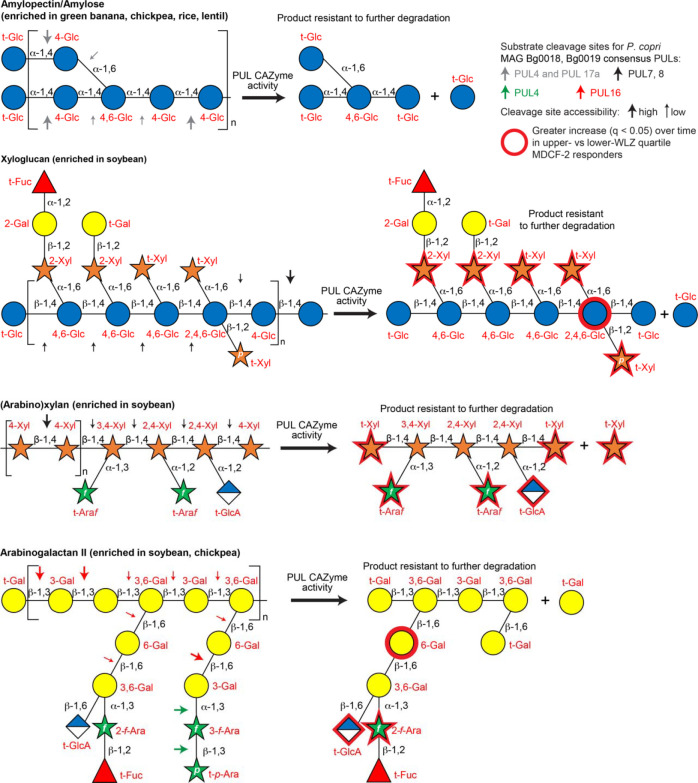
Extended Data Fig. 11. Polysaccharide structures, cleavage sites, and predicted products of CAZyme activity.
Glycosidic linkages highlighted with arrows are those predicted as sites of cleavage by CAZymes expressed by the set of PULs, described in Fig. 4a, that are present in P. copri MAG Bg0019 and/or Bg0018. Consensus PUL numbers are listed except in the case of Bg0019 PUL3, which is not represented in Bg0018 (see Supplementary Table 15). The size of the arrows (large versus small) denotes the relative likelihood (high versus low, respectively) of cleavage of glycosidic linkages by P. copri CAZymes when considering steric hindrance at branch points.
Extended Data Fig. 12. Validation of the MAG assembly pipeline.
(a) Bioinformatic workflow for comparing the fidelity of MAG assembly from alignment-based (bowtie2) versus pseudoalignment-based (kallisto) contig quantitation. (b) A detailed description of the workflow is described in panel a. Each box includes a summary of the computational task plus the name of the program and, where relevant, included the command used to complete the task (in parentheses). Color of the text in parentheses: brown, default code from kallisto; purple, default code from bowtie2; blue, custom script written to achieve tasks described; black, default code used for programs to assemble MAGs and dereplicate contigs. Boxes with a black outline and the thick black arrows emanating from them indicate that binned contigs were used as input to AMBER for MAG assembly comparisons across methods. (c) Boxplot describing summary statistics (completeness and purity) for MAG assembly approaches. Boxplots indicate the median, first and third quartiles; whiskers extend to the largest value no further than 1.5 × the interquartile range. (d) Number of MAGs, obtained from each assembly and quantitation strategy, that are distributed across two quality metrics (completeness, contamination). The number of ‘gold standard’ MAGs (theoretical maximum) in the simulated metagenomic dataset is indicated by a horizontal dashed line. *, P<0.05; **, P<0.01; ***, P < 0.001 (Wilcoxon test for panel c; Fisher’s exact test for panel d).
Supplementary Material
Acknowledgements:
We thank Martin Meier, Justin Serugo, Su Deng, Kazi Ahsan, Jessica Hoisington-Lopez, and MariaLynn Crosby for superb technical assistance, members of the Genome Technology Access Core at Washington University School of Medicine for Illumina NovaSeq-based generation of fecal DNA shotgun sequencing and microbial RNA-Seq datasets, plus Eric Martin and Brian Koebbe for high-performance computing support. This work was supported by the Bill & Melinda Gates Foundation, the National Institutes of Health (DK30292) and the Washington University-Centene Personalized Medicine Initiative.
Footnotes
Competing Interests: A.O. and D.R. are co-founders of Phenobiome Inc., a company pursuing development of computational tools for predictive phenotype profiling of microbial communities. C.B.L. is a co-founder of Infinant Health, interVenn Bio, and BCD Bioscience - companies involved in the characterization of glycans and developing carbohydrate applications for human health.
Supplementary information is available for this paper.
References
- 1.Smith M. I. et al. , Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science 339, 548–554 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanton L. V. et al. , Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 351, aad3311 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gehrig J. L., Venkatesh S., Chang H.-W. et al. , Effects of microbiota-directed foods in gnotobiotic animals and undernourished children. Science 365, eaau4732 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen R. Y., Mostafa I., Hibberd M. C. et al. , A microbiota-directed food intervention for undernourished children. N. Engl. J. Med. 384, 1517–1528 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y., Chang H.-W. Lee, E. M. et al. , Prevotella copri-linked effects of a therapeutic food for malnutrition. bioRxiv doi: 10.1101/2023.08.11.553030 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.United Nations Children’s Fund. United Nations Children’s Fund (UNICEF), World Health Organization, International Bank for Reconstruction and Development/The World Bank. Levels and trends in child malnutrition: key findings of the 2021 edition of the joint child malnutrition estimates. New York: (2021). [Google Scholar]
- 7.Victoria C. G. et al. , Revisiting maternal and child undernutrition in low-income and middle-income countries: variable progress towards an unfinished agenda. Lancet 397, 1388–1399 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heidkamp R. A. et al. , Mobilising evidence, data, and resources to achieve global maternal and child undernutrition targets and the sustainable development goals: an agenda for action. Lancet 397, 1400–1418 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Roberton T. et al. , Early estimated of the indirect effects of COVID-19 pandemic on maternal and child mortality in low-income and middle-income countries; a modelling study. Lancet Global Health 8, e901–e908 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subramanian S. et al. , Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 510, 417–421 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raman A. S. et al. , A sparse covarying unit that describes healthy and impaired human gut microbiota development. Science 365, eaau4735 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giallourou N. et al. , Metabolic maturation in the first 2 years of life in resource-constrained settings and its association with postnatal growths. Sci. Adv. 6, eaay5969 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kau A. L. et al. , Functional characterization of IgA-targeted bacteria taxa from undernourished Malawian children that produce diet-dependent enteropathy. Science Transl. Med. 7, 276ra24 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaumeil P. A., Mussig A. J., Hugenholtz P., Parks D. H., GTDB-Tk: a toolkit to classify genomes with the Genome Taxonomy Database. Bioinformatics 36, 1925–1927 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Overbeek R. et al. , The subsystems approach to genome annotation and its use in the project to annotate 1000 genomes. Nucleic Acids Res. 33, 5691–702 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aziz R. K. et al. , SEED servers: high-performance access to the SEED genomes, annotations, and metabolic models. PLOS One 7, e48053 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodionov D. A. et al. , Micronutrient requirements and sharing capabilities of the human gut microbiome. Frontiers Microbiol. 10, 1316 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amicucci M. J. et al. , A nonenzymatic method for cleaving polysaccharides to yield oligosaccharides for structural analysis. Nat. Commun. 11, 3963 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aspinall G. O., Cottrell I. W., Polysaccharides of soybeans. VI. Neutral polysaccharides from cotyledon meal. Can. J. Chem. 49, 1019–1022 (1971). [Google Scholar]
- 20.Tett A. et al. , The Prevotella copri complex comprises four distinct clades underrepresented in Westernized populations Cell Host and Microbe 26, 666–679 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vatanen et al. , A distinct clade of Bifidobacterium longum in the gut of Bangladeshi children thrives during weaning, Cell 185, 4280–4297 (2022). [DOI] [PubMed] [Google Scholar]
- 22.Fujita K. et al. , Degradative enzymes for type II arabinogalactan side chains in Bifidobacterium longum subsp. longum. Appl. Microbiol. Biotechnol. 103, 1299–1310 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Komeno M., Hayamizu H., Fujita K., and Ashida H., Two Novel α-l-Arabinofuranosidases from Bifidobacterium longum subsp. longum belonging to glycoside hydrolase family 43 cooperatively degrade arabinan. Appl. Environ. Microbiol. 85, e02582 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barratt M. J. et al. Bifidobacterium longum subsp. infantis strains for treating severe acute malnutrition in Bangladeshi infants. Science Trans. Med., 14, 640 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terrapon N., Lombard V., Gilbert H. J., and Henrissat B., Automatic prediction of polysaccharide utilization loci in Bacteroidetes species. Bioinformatics 31, 647–655 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Terrapon N. et al. , PULDB: the expanded database of Polysaccharide Utilization Loci. Nucleic Acids Res. 46, D677–D683. (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palackal N. et al. , A multifunctional hybrid glycosyl hydrolase discovered in an uncultured microbial consortium from ruminant gut. Appl. Microbiol. Biot. 74, 113–124 (2007). [DOI] [PubMed] [Google Scholar]
- 28.McGregor N. et al. , Structure-function analysis of a mixed-linkage β-Glucanase/Xyloglucanase from the key ruminal Bacteroidetes Prevotella bryantii B14. J. Biol. Chem. 291, 1175–1197 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.