Abstract
Background
The mechanisms underlying alcohol-induced breast carcinogenesis are not fully understood but may involve hormonal changes.
Methods
We investigated cross-sectional associations between self-reported alcohol intake and serum or plasma concentrations of oestradiol, oestrone, progesterone (in pre-menopausal women only), testosterone, androstenedione, DHEAS (dehydroepiandrosterone sulphate) and SHBG (sex hormone binding globulin) in 45 431 pre-menopausal and 173 476 post-menopausal women. We performed multivariable linear regression separately for UK Biobank, EPIC (European Prospective Investigation into Cancer and Nutrition) and EHBCCG (Endogenous Hormones and Breast Cancer Collaborative Group), and meta-analysed the results. For testosterone and SHBG, we also conducted two-sample Mendelian Randomization (MR) and colocalisation using the ADH1B (Alcohol Dehydrogenase 1B) variant (rs1229984).
Results
Alcohol intake was positively, though weakly, associated with all hormones (except progesterone in pre-menopausal women), with increments in concentrations per 10 g/day increment in alcohol intake ranging from 1.7% for luteal oestradiol to 6.6% for post-menopausal DHEAS. There was an inverse association of alcohol with SHBG in post-menopausal women but a small positive association in pre-menopausal women. MR identified positive associations of alcohol intake with total testosterone (difference per 10 g/day increment: 4.1%; 95% CI: 0.6%, 7.6%) and free testosterone (7.8%; 4.1%, 11.5%), and an inverse association with SHBG (−8.1%; −11.3%, −4.9%). Colocalisation suggested a shared causal locus at ADH1B between alcohol intake and higher free testosterone and lower SHBG (PP4: 0.81 and 0.97 respectively).
Conclusions
Alcohol intake was associated with small increases in sex hormone concentrations, including bioavailable fractions, which may contribute to its effect on breast cancer risk.
Keywords: alcohol drinking, sex hormones, oestrogens, androgens, breast cancer
Background
Alcoholic beverages are commonly consumed in many populations and are known to be causally associated with increased risk of several diseases including breast cancer [1, 2]. The mechanisms underlying alcohol-induced carcinogenesis are not fully understood; the mutagenic alcohol metabolite acetaldehyde may be the causal factor for some cancers such as those of the upper gastro-intestinal tract, but the effect on breast cancer may involve hormonal changes.
Earlier intervention studies have reported an acute increase in serum/plasma concentrations of oestrogens and/or androgens within hours after intake of alcohol [3–8] in pre- and/or post-menopausal women, although others found no significant effect [9–11]. Other intervention studies have also found an increase in sex hormone concentrations after daily intake of alcohol for two to three months [12–15]. Similarly, more recent cross-sectional observational studies have associated habitual alcohol intake with high sex hormone concentrations as well as differences in sex hormone binding globulin (SHBG), a glycoprotein that binds to oestrogens and androgens [16–18].
In the study reported here, we combined data from 14 cohort studies and conducted cross-sectional analyses to provide the most comprehensive evidence to date on the associations of usual alcohol intake with serum or plasma concentrations of oestradiol, oestrone, testosterone, androstenedione, dehydroepiandrosterone sulphate (DHEAS) and SHBG in pre- and post-menopausal women, and with progesterone in premenopausal women only. To examine the potential causal associations with testosterone and SHBG, we also conducted two-sample Mendelian Randomization (MR) and colocalisation analyses.
Methods
Observational analyses
Data from the UK Biobank, EPIC (European Prospective Investigation into Cancer and Nutrition) and 12 other studies included in the EHBCCG (Endogenous Hormones and Breast Cancer Collaborative Group) consortium were used.
UK Biobank
This is a prospective cohort study involving about 500 000 adults, including over 270 000 women, aged 40–69 years when recruited between 2006 and 2010. At the initial assessment visit, usual alcohol intake was assessed using a touchscreen questionnaire, and blood samples were collected from which serum was prepared and concentrations of hormones and SHBG were measured using chemiluminescent immunoassays. The current analysis included pre-menopausal women, who reported they had not had their menopause (i.e., periods had not stopped), and were younger than 50 years of age, and post-menopausal women, who reported they had gone through menopause, or were 55 years or older, or reported a bilateral oophorectomy; those who had a prior history of cancer ((except for non-melanoma skin cancer) or reported currently using hormone therapy (hormone replacement therapy (HRT) and/or oral contraceptives (OCs)) were excluded. Detailed information on the study design and methodology [19], calculation of alcohol intake in grams per day [18] and the assay data [20] has been reported elsewhere.
EPIC
This is a prospective cohort study involving about 520 000 adults, including over 360 000 women, aged 25–70 years when recruited from 23 centres across 10 European countries between 1992 and 2000. Diet, including usual alcohol intake, was measured by country-specific questionnaires that were validated against reference measurements based on twelve 24-hour diet recall interviews [21]. Blood samples were collected from about 74% of the participants. The current analysis included pre- and post-menopausal women from nested case-control studies on breast, ovarian, endometrial, cervical, liver and thyroid cancer risk for whom serum (in most of these studies) or plasma concentrations of sex hormones and SHBG were measured. Both pre-cases (women who were cancer-free at the time of blood collection but were subsequently diagnosed with the cancer of interest during follow-up) and controls were included, except for the liver cancer study where only controls were included. Participants were categorised as pre-menopausal if they reported regular menstrual cycles over the 12 months prior to blood collection or were younger than 42 years at recruitment, and as post-menopausal if they reported having had no menses over the past 12 months, were older than 55 years, or reported a bilateral oophorectomy. Women who reported currently using hormone therapy (HRT and/or OCs) were excluded, as well as those from Greece (due to a restriction concerning information governance). Detailed information on the study design and methodology [22], calculation of alcohol intake in grams per day and the assay data [23] has been reported elsewhere.
The EPIC study data for breast cancer were included in the EHBCCG but the EPIC data were analysed separately here because, since the publication of the collaborative analyses, more nested case-control studies of other cancer sites have been conducted and hormone assay data are now available for a larger sample of women.
EHBCCG:
Of the seven prospective studies of pre-menopausal women included in the collaborative analysis,[16] three with available information on usual alcohol intake were included: Nurses’ Health Study II (NHS-II), USA; New York University Women’s Health Study (NYU WHS), USA; and the Study of Hormones and Diet in the Etiology of Breast Tumors (ORDET), Italy. Of the 18 studies of post-menopausal women, 11 were included: Cancer Prevention Study-II Nutrition Cohort (CPS-II Nutrition Cohort), USA; Malmö/Umeå, Sweden; the Melbourne Collaborative Cohort Study (MCCS), Australia; the Multiethnic Cohort (MEC), USA; Nurses’ Health Study (NHS I), USA; NYU WHS, USA; ORDET, Italy; Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial cohort (PLCO), USA; Study of Osteoporotic Fractures (SOF), USA; United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS), UK; and the Women’s Health Initiative, Observational Study (WHI-OS), USA. Women who reported currently using hormone therapy (HRT and/or OCs) were excluded.
Details of the individual studies included in this analysis are presented in the Supplementary Materials. These include: references for component studies within EPIC and EHBCCG (Supplementary Table S1), number of women who contributed to each hormone analysis (Supplementary Table S2), measurement of usual alcohol intake (Supplementary Table S3), and blood sample (serum vs. plasma), type of assay and coeffcients of variation for the measured hormones and SHBG (Supplementary Table S4). In all studies, concentrations of free oestradiol and testosterone were calculated from those of total oestradiol and testosterone respectively and of SHBG, assuming that the binding of these hormones to serum SHBG and albumin follows the law of mass action [24]. As albumin concentration was not measured in EPIC and EHBCCG, it was assumed to be constant at 40 g/L [25].
Statistical analysis
Analyses were undertaken separately for pre- and post-menopausal women in UK Biobank, EPIC and EHBCCG. STATA 17 (StataCorp, College Station, Texas) was used for all analyses.
Hormone concentrations were logarithmically transformed. In pre-menopausal women, concentrations were standardised for phase of the menstrual cycle (early follicular, late follicular, mid-cycle, early luteal, mid-luteal and late luteal) with residuals from the mean for each cycle phase. The cycle phase was determined using forward dating (UK Biobank [18]), or both forward and backward dating with the latter used where possible (EPIC [26] and EHBCCG [16]).
For each study, hormone concentrations and 95% confidence intervals (CIs) per 10 g/day (approximately one standard drink/day) increment in alcohol intake were estimated using multivariable linear regression models, adjusting for individual component studies (EPIC and EHBCCG), case-control status (EPIC and EHBCCG), age at blood collection (in 2-year categories for pre-menopausal women and 5-year categories for post-menopausal women), previous alcohol use among non-current drinkers (UK Biobank and EPIC), smoking (never, former, current), body mass index (BMI) (< 22.5 kg/m2, 22.5–24.9 kg/m2, 25–27.4 kg/m2, 27.5–29.9 kg/m2, 30–34.9 kg/m2, ≥ 35 kg/m2), number of full-term pregnancies (0, 1, 2, 3, 4+), past use of hormone therapy (HRT and/or OCs; yes/no), age at menopause (in 3-year categories; post-menopausal women only) and menopausal type (natural, surgical; post-menopausal women only). The study-specific results were then pooled using fixed-effect meta-analysis. Potential differences in the estimates by menopausal status were assessed using the Chi-square test for heterogeneity.
In pre-menopausal women, subgroup analyses were undertaken for total oestradiol, oestrone, progesterone and total testosterone by phase of the menstrual cycle (follicular, mid-cycle and luteal). In both pre- and post-menopausal women, subgroup analyses were undertaken for total oestradiol, oestrone and total testosterone by type of the assay used (direct, extraction and mass spectrometry); the individual studies that contributed to each assay type are presented in Supplementary Table S5. Sensitivity analyses were undertaken by restricting the sample to those who reported alcohol intake of < 15 g/day, to those who reported intake of < 30 g/day (i.e. excluding heavy drinkers), and also to those whose blood samples were collected during an ovulatory cycle (progesterone concentrations measured in the mid-luteal phase ≥ 12.72 nmol/L (~ 400 ng/dL) [27].
MR and colocalisation analyses
Data on alcohol intake
A genetic instrument in the ADH1B (Alcohol Dehydrogenase 1B) gene (rs1229984) for self-reported alcohol intake (number of drinks per week) was extracted from a GWAS (genome-wide association study) meta-analysis undertaken by the GWAS and Sequencing Consortium of Alcohol and Nicotine Use (GSCAN) [28]. This variant was used due to its highly biologically plausible association with alcohol intake [29]. The minor A allele of this variant increases the activity of ADH1B that oxidises ethanol to acetaldehyde, resulting in unpleasant reactions and limiting further drinking [30]. While this polymorphism is less common in people of white European ancestry with a frequency of < 5% (cf. 90% in East Asians), it is nonetheless a strong genetic predictor of alcohol intake in this population [30]. Estimates were available per one SD (approximately 9 drinks/week) increment in alcohol intake and extracted from the GWAS meta-analysis excluding the UK Biobank (n = 226 223) to avoid sample overlap between the GWAS for alcohol intake and that for hormone concentrations. The ADH1B variant explains 0.19% of the variance in alcohol intake.
Data on testosterone and SHBG:
Summary statistics for the association of rs1229984 with SD increments in the concentrations of hormones and SHBG were obtained from a publicly available GWAS of all women, irrespective of menopausal status, from the UK Biobank, extracted from the OpenGWAS platform [31] (dataset used for total testosterone: ieu-b-4864 involving 199 569 women; free testosterone: ieu-b-4869 involving 180 386 women; and SHBG: ieu-b-4870 involving 214 989 women). Data on oestradiol were available but were not used due to the potential limitations related to measurement of this hormone in the UK Biobank (see details in the Discussion); data on the other sex hormones were not available.
MR analyses
MR assesses the associations between exposure(s) and outcome(s) using genetic variants associated with the exposure of interest as instrumental variables. A Wald ratio was calculated using the “TwoSampleMR” [32] package in R. To be able to present the MR results in a way which is directly comparable to the observational results, assuming that one standard drink contains 10 g of alcohol, the β estimates generated from the Wald ratio (per one SD increment in alcohol intake) were converted to the estimates per 10 g/day increment. The results were then multiplied by 0.341 (assuming that, for a normal distribution, one SD is 34.1% of the range) to convert the difference in hormone concentrations from units expressed as SD to percentages.
Colocalisation analyses:
Colocalisation assesses the probability that two traits are affected by the same genetic variants at a given locus. Using the ADH1B variant, colocalisation analyses were conducted to identify the presence of a shared causal locus between alcohol intake and concentrations of testosterone and SHBG where a conventionally significant association was observed in MR analyses. The “coloc” package [33] in R was used to estimate the posterior probability for two traits sharing the same causal variant (PP4) in a 150 kb LD (linkage disequilibrium) window centred on rs1229984, with PP4 > 0.70 corresponding to strong evidence of colocalisation [34]. Priors chosen were: p1 = 10−3, p2 = 10−4, and p12 = 10−5, or approximately a 75% prior belief that a signal will only be observed in the GSCAN GWAS and < 0.01% prior belief in favour of colocalisation between the two traits at a given locus [35].
Results
Observational analyses
In total, 45 431 pre-menopausal (39 188 in UK Biobank, 2343 in EPIC and 3900 in EHBCCG) and 173 476 post-menopausal (160 363 in UK Biobank, 4371 in EPIC and 8742 in EHBCCG) women were included in this analysis. Table 1 presents characteristics of the study participants.
Table 1.
Participant characteristics
| Pre-menopausal women | Post-menopausal women | |||||
|---|---|---|---|---|---|---|
| UK Biobank (n = 39188) | EPIC (n = 2343) | EHBCCG excluding EPIC (n = 3900) | UK Biobank (n = 160363) | EPIC (n = 4371) | EHBCCG excluding EPIC (n = 8742) | |
| Age at recruitment (years), mean (SD) | 44.7 (2.7) | 42.8 (4.3) | 43.3 (4.5) | 60.5 (5.3) | 60.0 (5.4) | 62.5 (7.0) |
| Cases, % | 40.5 | 30.6 | 42.6 | 35.2 | ||
| Usual alcohol intake (grams/day), median (IQR) | 6.9 (13.3) | 2.9 (11.5) | 2.0 (8.0) | 5.7 (12.9) | 2.8 (10.6) | 1.0 (7.0) |
| Usual alcohol intake (grams/day), mean (SD) | 10.8 (12.9) | 7.9 (11.7) | 6.5 (11.7) | 9.4 (11.4) | 7.6 (11.2) | 5.6 (10.6) |
| Current smoker, % | 11.1 | 23.6 | 14.4 | 8.0 | 17.6 | 9.8 |
| Body mass index (kg/m2), mean (SD) | 26.3 (5.3) | 24.7 (4.2) | 24.7 (4.9) | 27.3 (5.1) | 26.5 (4.6) | 26.7 (5.0) |
| Nulliparous, % | 26.8 | 16.7 | 22.5 | 15.4 | 13.9 | 12.6 |
| Past use of hormones, % | 87.7 | 66.7 | 28.6 | 86.5 | 45.5 | 32.4 |
| Age at menopause (years), mean (SD) | 49.5 (5.7) | 49.4 (4.6) | 48.8 (5.2) | |||
| Natural menopause, % | 84.0 | 96.7 | 68.1 | |||
| Total oestradiol (pmol/L), median (IQR) | 346.1 (365.4) | 269.6 (230.3) | 414.8 (289.4) | 73.47 (63.31) | 33.04 (41.66) | |
| Calculated free oestradiol (pmol/L), median (IQR) | 4.05 (4.20) | 3.66 (3.18) | 5.10 (3.46) | 1.10 (1.12) | 0.54 (0.64) | |
| Oestrone (pmol/L), median (IQR) | 312.7 (236.2) | 307.0 (166.4) | 138.34 (79.44) | 88.76 (72.65) | ||
| Progesterone (nmol/L), median (IQR)a | 3.72 (5.48) | 42.77 (38.10) | ||||
| Total testosterone (nmol/L), median (IQR) | 1.12 (0.71) | 1.28 (0.99) | 0.90 (0.52) | 0.85 (0.74) | 1.15 (0.86) | 0.80 (0.62) |
| Calculated free testosterone (pmol/L), median (IQR) | 12.53 (10.32) | 16.38 (16.59) | 10.70 (7.85) | 10.50 (10.78) | 17.18 (16.32) | 11.13 (9.87) |
| Androstenedione (nmol/L), median (IQR) | 4.41 (3.24) | 4.02 (2.49) | 2.88 (2.27) | 2.19 (1.70) | ||
| DHEAS (nmol/L), median (IQR) | 3314.9 (2365.9) | 2794.0 (2206.7) | 1917.8 (1705.9) | 1951.0 (1990.0) | ||
| SHBG (nmol/L), median (IQR) | 62.91 (38.02) | 51.97 (36.68) | 58.51 (38.00) | 53.52 (33.59) | 40.71 (31.90) | 47.71 (31.70) |
SD = Standard deviation
IQR = Interquartile range
DHEAS = Dehydroepiandrosterone sulphate
SHBG = Sex hormone binding globulin
Luteal phase progesterone measured in EHBCCG excluding EPIC
Oestrogens:
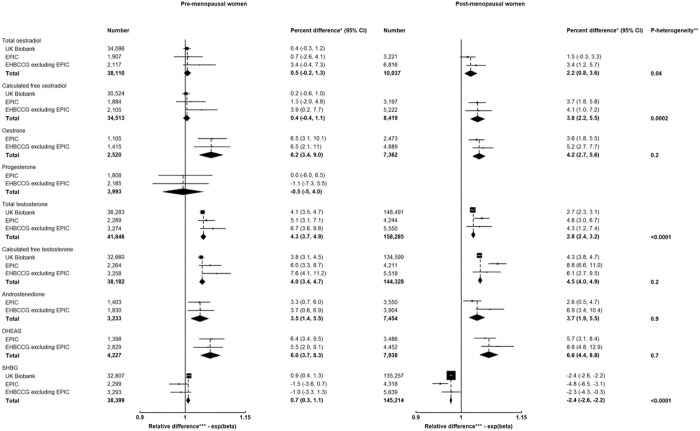
Alcohol intake was positively associated with concentrations of total and calculated free oestradiol in post-menopausal women but not in pre-menopausal women (pheterogeneity by menopausal status = 0.04 for total oestradiol and 0.0002 for calculated free oestradiol). The concentrations were 2.2% (95% CI: 0.8%, 3.6%) and 3.8% (2.2%, 5.5%) higher, respectively, per 10 g/day increment in alcohol intake (approximately one drink/day) in post-menopausal women (Fig. 1).
Figure 1. Associations of usual alcohol intake (per 10 g/day increment) with hormones and SHBG in pre- and post-menopausal women.
EPIC = European Prospective Investigation into Cancer and Nutrition; EHBCCG = Endogenous Hormones and Breast Cancer Collaborative Group
DHEAS = Dehydroepiandrosterone sulphate; SHBG = Sex hormone binding globulin
* Percent difference in concentrations of hormones and SHBG per 10 g/day increment in usual alcohol intake
** p-value for heterogeneity by menopausal status
*** Relative difference in concentrations of hormones and SHBG per 10 g/day increment in usual alcohol intake
Alcohol intake was positively associated with oestrone concentration in both pre- and post-menopausal women (Fig. 1). The concentrations were 6.2% (3.4%, 9.0%) and 4.2% (2.7%, 5.6%) higher, respectively, in pre- and post-menopausal women per 10 g/day increment in alcohol intake.
Progesterone
Alcohol intake was not associated with progesterone concentration in pre-menopausal women (Fig. 1). No data were available for post-menopausal women.
Androgens
Alcohol intake was positively associated with testosterone concentrations in both pre- and post-menopausal women (Fig. 1). Per 10 g/day increment in alcohol intake, the concentrations of total testosterone were 4.3% (3.7%, 4.9%) and 2.8% (2.4%, 3.2%) higher, respectively, in pre- and post-menopausal women, and those of calculated free testosterone were 4.0% (3.4%, 4.7%) and 4.5% (4.0%, 4.9%) higher, respectively. The associations for total testosterone were larger in pre-menopausal women (pheterogeneity<0.0001).
Similarly, alcohol intake was positively associated with concentrations of androstenedione and DHEAS in both pre- and post-menopausal women (Fig. 1). Per 10 g/day increment in alcohol intake, the concentrations of androstenedione were 3.5% (1.4%, 5.5%) and 3.7% (1.9%, 5.5%) higher, and those of DHEAS were 6.0% (3.7%, 8.3%) and 6.6% (4.4%, 8.8%) higher, respectively, in pre- and post-menopausal women. There were no differences in the associations by menopausal status.
SHBG
Alcohol intake was positively associated with SHBG concentration in pre-menopausal women but inversely associated in post-menopausal women (pheterogeneity<0.0001; Fig. 1); the concentration was 0.7% (0.3%, 1.1%) higher in pre-menopausal women but was 2.4% (2.2%, 2.6%) lower in post-menopausal women per 10 g/day increment in alcohol intake.
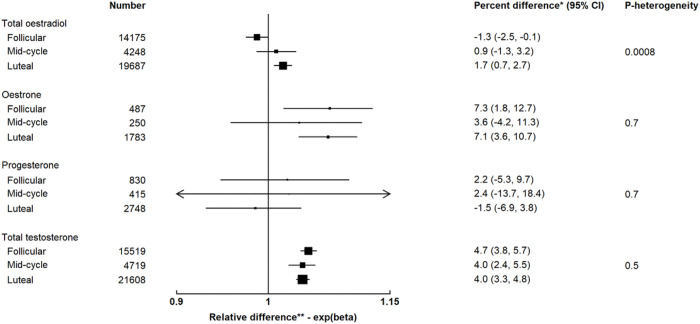
Associations by phase of the menstrual cycle in pre-menopausal women
Alcohol intake was inversely associated with total oestradiol (−1.3%; −2.5%, −0.1%) in the follicular phase but positively associated (1.7%; 0.7%, 2.7%) in the luteal phase (pheterogeneity by cycle phase=0.0008; Fig. 2). The associations for oestrone, progesterone and total testosterone did not differ by cycle phase.
Figure 2. Associations of usual alcohol intake (per 10 g/day increment) with hormones and SHBG by phase of the menstrual cycle in pre-menopausal women.
* Percent difference in concentrations of hormones and SHBG per 10 g/day increment in usual alcohol intake
** Relative difference in concentrations of hormones and SHBG per 10 g/day increment in usual alcohol intake
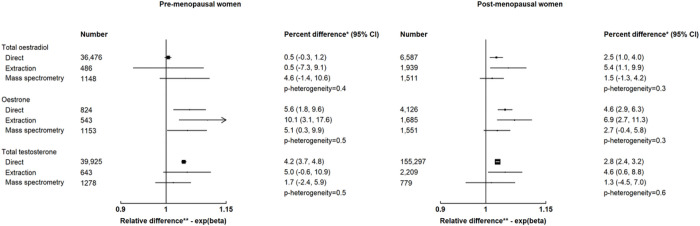
Associations by assay type
There were no differences in the associations by assay type for total oestradiol, oestrone and total testosterone (Fig. 3).
Figure 3. Associations of usual alcohol intake (per 10 g/day increment) with hormones and SHBG by assay type.
EPIC = European Prospective Investigation into Cancer and Nutrition; EHBCCG = Endogenous Hormones and Breast Cancer Collaborative Group
DHEAS = Dehydroepiandrosterone sulphate; SHBG = Sex hormone binding globulin
* Percent difference in concentrations of hormones and SHBG per 10 g/day increment in usual alcohol intake
** Relative difference in concentrations of hormones and SHBG per 10 g/day increment in usual alcohol intake
Sensitivity analyses
The associations did not differ substantially when restricted to those who reported usual alcohol intake of < 15 g/day (Supplementary Figure S1), to those who reported intake of < 30 g/day (data not shown), or to samples collected during ovulatory cycles (data not shown).
MR and colocalisation analyses
Effect estimates for the association of rs1229984 with alcohol intake and with concentrations of testosterone and SHBG are presented in Supplementary Table S6.
In MR analyses, a 10 g/day increment in genetically predicted alcohol intake was associated with higher concentrations of total testosterone (4.1%; 0.6%, 7.6%) and free testosterone (7.8%; 4.1%, 11.5%), and lower concentration of SHBG (−8.1%; −11.3%, −4.9%) (Table 2). Colocalisation analyses showed strong evidence in favour of a shared causal locus between alcohol intake and free testosterone (PP4 = 0.81) and SHBG (PP4 = 0.97) at ADH1B (Table 3, Supplementary Figure S2). MR results by menopausal status were not available.
Table 2.
Mendelian randomization estimates, instrumented by rs1229984, for usual alcohol intake (per 10 g/day increment) with hormones and SHBG in women
| Percent difference (95%CI) | P | |
|---|---|---|
| Total testosterone | 4.1 (0.6, 7.6) | 0.02 |
| Free testosterone | 7.8 (4.1, 11.5) | 0.00003 |
| SHBG | −8.1 (−11.3, −4.9) | 0.000001 |
SHBG = Sex hormone binding globulin
Table 3.
Posterior probabilities from colocalisation analyses for rs1229984
| PP0 | PP1 | PP2 | PP3 | PP4 | |
|---|---|---|---|---|---|
| Total testosterone | 1.68E-58 | 0.910 | 1.08E-60 | 0.006 | 0.084 |
| Free testosterone | 3.46E-59 | 0.188 | 7.34E-61 | 0.004 | 0.808 |
| SHBG | 5.57E-60 | 0.030 | 2.56E-61 | 0.001 | 0.968 |
SHBG = Sex hormone binding globulin
PP0 = Posterior probability for hypothesis 0 (H0): no association with either trait (alcohol intake or testosterone/SHBG concentration)
PP1 = Posterior probability for H1: association with trait 1 (alcohol intake), not with trait 2 (testosterone/SHBG concentration)
PP2 = Posterior probability for H2: association with trait 2 (testosterone/SHBG concentration), not with trait 1 (alcohol intake)
PP3 = Posterior probability for H3: association with both traits (alcohol intake and testosterone/SHBG concentration), two distinct SNPs
PP4 = Posterior probability for H4: association with both traits (alcohol intake and testosterone/SHBG concentration), one shared SNP
Discussion
In this meta-analysis involving over 45 000 pre-menopausal and 173 000 post-menopausal women, we found positive associations of alcohol intake with concentrations of sex hormones. We also found an inverse association with SHBG in post-menopausal women and some evidence of a small positive association in pre-menopausal women. The genetic analyses supported potential causal associations of alcohol intake with higher free testosterone and lower SHBG.
Oestrogens
Alcohol may influence oestrogen concentrations by altering its metabolism and clearance [3], or by affecting aromatisation of androgens to oestrogens.[36] Earlier intervention studies reported an increase in concentrations of oestradiol and/or oestrone after alcohol intake in both pre- [3, 12] and post-menopausal women [13, 14], although some found a positive association only in those on hormone therapy [5, 6], or no significant effect (possibly due to small sample sizes) [9–11, 15].
Our observational analyses showed positive associations of alcohol with oestrone in both pre- and post-menopausal women and with oestradiol in post-menopausal women. Although the overall association with oestradiol in pre-menopausal women was not significant, we found a weak inverse association in the follicular phase and a weak positive association in the luteal phase. In contrast, in an earlier cross-over trial, daily alcohol intake for three consecutive menstrual cycles significantly increased plasma concentrations of ovulatory oestradiol but not follicular or luteal oestradiol [12].
The less conclusive findings observed for oestradiol in pre-menopausal women may be related to the challenges in measuring this hormone reliably; measurement based on a single serum sample may not reflect its long-term average as the hormone level varies substantially across the menstrual cycle. We standardised oestradiol concentrations for phase of the menstrual cycle in the observational analyses, but this may not be sufficient to account for all the variation [37]. Moreover, the studies included in the meta-analysis variably used forward or backward dating to define cycle phase when blood was collected. The positive association of alcohol with oestradiol in post-menopausal women was also of small magnitude, probably because the oestradiol concentration is low in this group and could be below or close to the lower limit of detection of some of the assays used, which is likely to have reduced statistical power; however, we found no differences in the association by assay type.
Progesterone
Alcohol might influence progesterone concentration by altering its metabolism in the liver [9, 10], but the results from previous intervention studies have been mixed [9, 10, 12]. We found no association in pre-menopausal women overall as well as across three cycle phases, although our ability to detect any association may have been limited due to measurement errors associated with variations in the hormone level throughout the menstrual cycle.
Androgens
Alcohol may influence androgen concentrations by altering their secretion from the ovaries and/or adrenal glands, or their metabolism in the liver [38]. Previous intervention studies reported an acute elevation in concentrations of one or more androgens after alcohol intake in both pre- [4, 7, 8] and post-menopausal women [14, 15], although others found no significant effect in pre-menopausal women possibly due to small sample sizes [9–12].
In this meta-analysis, we found positive associations of alcohol with testosterone, androstenedione and DHEAS in both pre- and post-menopausal women. The association with testosterone seemed to be of greater magnitude in pre-menopausal women even after restricting to those with intake of < 15 g/day, which might be due to biological differences or possibly due to differences in the accuracy of self-reported alcohol intake by menopausal status. The associations with androstenedione and DHEAS did not differ by menopausal status.
In the MR analyses, genetically predicted alcohol intake was positively associated with testosterone concentrations, with a larger effect on free testosterone compared to total testosterone. We observed strong colocalisation for alcohol intake at the ADH1B locus with free but not total testosterone. This raises the question as to whether or not alcohol has a direct causal effect on testosterone concentration, because the strong association with free testosterone could be related to the inverse association of alcohol intake with SHBG concentrations as discussed below.
SHBG
Alcohol may influence SHBG concentrations by affecting hormonal balance [39], cytokine levels [40], hepatic synthesis/release or blood clearance [41, 42]. An earlier intervention study in pre-menopausal women showed a slight increase in SHBG concentration particularly in the mid-luteal phase [12] whereas another study of post-menopausal women found a decrease in concentration after 8–12 weeks of daily alcohol intake [13]; however, the results in both studies were not significant possibly due to small sample sizes.
Similarly in this meta-analysis, we found an inverse association of alcohol intake with SHBG in post-menopausal women and some evidence of a small positive association in pre-menopausal women; the latter was driven by the results from UK Biobank with no association in the other datasets, therefore this observation should be interpreted cautiously. The MR and colocalisation analyses at the ADH1B locus identified an inverse association, which is consistent with our observational findings because the GWAS for SHBG was conducted in the UK Biobank where the majority of women were post-menopausal. As SHBG binds testosterone to a greater degree than oestradiol, any reduction in SHBG caused by alcohol would be expected to have a bigger effect in increasing the bioavailable fraction of testosterone than oestradiol as observed in our analyses.
Hormones and alcohol-induced breast carcinogenesis
Alcohol has been associated with an increased risk of several cancers, including female breast cancer [43, 44]. In the Million Women Study, with over 68 000 cases, there was a 12% increase in risk per 10 g/day increment in alcohol intake [44]. Our findings confirming the positive associations of alcohol intake with sex hormones, particularly their bioavailable fractions, support a probable role of sex hormones in alcohol-induced breast carcinogenesis. Given the published evidence supporting the effects of alcohol on both ER (oestrogen receptor) positive and negative breast cancer [45], it is possible that hormones interplay with acetaldehyde and other suggested mechanisms [46–48] influencing cancer risk.
Strengths and limitations
To our knowledge, this is the largest study on this topic. Our meta-analysis involved over 45 000 pre-menopausal and 173 000 post-menopausal women, enabling us to undertake important subgroup analyses by menopausal status, cycle phase in pre-menopausal women and assay type. We additionally conducted MR and colocalisation analyses to support the observational results where possible.
Our main exposure, alcohol intake, was self-reported. While self-reported measures of alcohol intake may have reasonable levels of reliability and validity [49], underreporting is common particularly among those with very high intake [50], which could lead to overestimation of the magnitude of associations of reported alcohol intake with circulating hormones. The potential limitations related to oestradiol measurement have been discussed above; we have therefore not undertaken genetic analyses for this hormone. We used the female-specific genetic instruments for testosterone and SHBG, but were not able to undertake analyses separately for pre- and post-menopausal women. The genetic instruments for other important hormones included in the meta-analysis, such as progesterone, DHEAS and androstenedione were not publicly available. Finally, the study samples comprised mainly women of white European ancestry (e.g., approximately 95% in UK Biobank), limiting the generalisability of the results to other populations.
Conclusions
Our meta-analysis confirmed positive associations of alcohol intake with sex hormones, including the more bioavailable fractions. There was also an inverse association with SHBG in post-menopausal women and some evidence of a small positive association in pre-menopausal women. Genetic analyses supported potential causal overall associations with higher free testosterone and lower SHBG. These associations are likely to contribute to the effect of alcohol on breast cancer risk.
Acknowledgements
The authors express sincere appreciation to all participants, investigators and staff from each study for their contributions that made this research possible.
Funding
STT is supported by the Girdlers’ New Zealand Health Research Council Fellowship (grant number 19/031). This research is also supported by Cancer Research UK (grant numbers C570/A16491 and C8221/A29017).
The American Cancer Society funds the creation, maintenance, and updating of the CPS-II cohort. MEC is partially supported by grant U01 CA164973 from the US National Cancer Institute. NHS is supported by grants U01 CA186107 and R01 CA49449 from the US National Cancer Institute; NHSII is supported by grants U01 CA176726 and R01 CA67262 from the US National Cancer Institute. PLCO was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics and by contracts from the Division of Cancer Prevention, National Cancer Institute, National Institutes of Health. The UKCTOCS trial was funded by Medical Research Council (G9901012 and G0801228), CRUK (C1479/A2884), and the UK Department of Health, with additional support from The Eve Appeal. UM received salary support through MRC core funding (MR_UU_12023). TER is supported in part by the Breast Cancer Research Foundation (BCRF-22-140).
Abbreviations
- ADH1B
Alcohol dehydrogenase 1B
- BMI
Body mass index
- CPS-II
Cancer Prevention Study-II
- DHEAS
Dehydroepiandrosterone sulphate
- EHBCCG
Endogenous Hormones and Breast Cancer Collaborative Group
- EPIC
European Prospective Investigation into Cancer and Nutrition
- ER
Oestrogen receptor
- GSCAN
GWAS and Sequencing Consortium of Alcohol and Nicotine Use
- GWAS
Genome-wide association study
- HRT
Hormone replacement therapy
- LD
Linkage disequilibrium
- MCCS
Melbourne Collaborative Cohort Study
- MEC
Multiethnic Cohort
- MR
Mendelian randomization
- NHS-I
Nurses’ Health Study I
- NHS-II
Nurses’ Health Study II
- NYU WHS
New York University Women’s Health Study
- OC
Oral contraceptives
- ORDET
Study of Hormones and Diet in the Etiology of Breast Tumors
- PLCO
Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial
- SD
Standard deviation
- SHBG
Sex hormone binding globulin
- SOF
Study of Osteoporotic Fractures
- UKCTOCS
United Kingdom Collaborative Trial of Ovarian Cancer Screening
- WHI-OS
Women’s Health Initiative, Observational Study
Footnotes
Declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
UK Biobank is an open access resource, and has been approved by the North West Multi-Centre Research Ethics Committee (approval number 16/NW/0274). This research was undertaken under project reference number 3248 (approved August 2013). EPIC has been approved by the Ethical Committee of the International Agency for Research on Cancer (IARC) and local ethical committees pertaining to EPIC Centres. Studies involved in EHBCCG have been approved by relevant ethical committees. The current analyses did not need further ethics approval. Informed consent was obtained from all individual participants included in each study.
Consent for publication
Not applicable
IARC disclaimer: Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/World Health Organization.
Contributor Information
Sandar Tin Tin, University of Oxford.
Karl Smith-Byrne, University of Oxford.
Pietro Ferrari, International Agency for Research on Cancer (IARC/WHO).
Sabina Rinaldi, International Agency for Research on Cancer (IARC/WHO).
Marjorie L McCullough, American Cancer Society.
Lauren R Teras, American Cancer Society.
Jonas Manjer, Skåne University Hospital Malmö, Lund University.
Graham Giles, Cancer Council Victoria.
Loïc Le Marchand, University of Hawai’i Cancer Center, University of Hawai’i.
Christopher A Haiman, University of Southern California.
Lynne R Wilkens, University of Hawai’i Cancer Center, University of Hawai’i.
Yu Chen, New York University Grossman School of Medicine.
Sue Hankinson, University of Massachusetts.
Shelley Tworoger, Moffitt Cancer Center.
A Heather Eliassen, Harvard T.H. Chan School of Public Health.
Walter C Willett, Harvard T.H. Chan School of Public Health.
Regina G Ziegler, National Cancer Institute.
Barbara J Fuhrman, University of Pittsburgh.
Sabina Sieri, Fondazione IRCCS Istituto Nazionale dei Tumori.
Claudia Agnoli, Fondazione IRCCS Istituto Nazionale dei Tumori.
Jane Cauley, University of Pittsburgh.
Usha Menon, University College London.
Evangelia Ounia Fourkala, University College London.
Thomas E Rohan, Albert Einstein College of Medicine.
Rudolf Kaaks, German Cancer Research Center, DKFZ.
Gillian K Reeves, University of Oxford.
Timothy J Key, University of Oxford.
Availability of data and materials
UK Biobank is an open access resource, and the study website https://www.ukbiobank.ac.uk/ has information on available data and access procedures. For EPIC, details of data access are at https://epic.iarc.fr/access/index.php. For EHBCCG, the principal investigators of each contributing study are responsible for access to the data. Full GWAS summary statistics from GSCAN are available at https://conservancy.umn.edu/handle/11299/201564. The instruments for concentrations of testosterone and SHBG were extracted from the OpenGWAS platform https://gwas.mrcieu.ac.uk/.
References
- 1.Griswold MG, Fullman N, Hawley C, Arian N, Zimsen SRM, Tymeson HD, et al. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392:1015–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 96. Alcohol Consumption and Ethyl Carbamate. Lyon (France): International Agency for Research on Cancer; 2010. [PMC free article] [PubMed] [Google Scholar]
- 3.Mendelson JH, Lukas SE, Mello NK, Amass L, Ellingboe J, Skupny A. Acute alcohol effects on plasma estradiol levels in women. Psychopharmacol. 1988;94:464–467. [DOI] [PubMed] [Google Scholar]
- 4.Eriksson CJP, Fukunaga T, Lindman R. Sex hormone response to alcohol. Nature. 1994;369:711–711. [DOI] [PubMed] [Google Scholar]
- 5.Ginsburg ES, Mello NK, Mendelson JH, Barbieri RL, Teoh SK, Rothman M, et al. Effects of alcohol ingestion on estrogens in postmenopausal women. JAMA. 1996;276:1747–1751. [DOI] [PubMed] [Google Scholar]
- 6.Sarkola T, Mäkisalo H, Fukunaga T, Eriksson CJP. Acute effect of alcohol on estradiol, estrone, progesterone, prolactin, cortisol, and luteinizing hormone in premenopausal women. Alcohol Clin Exp Res. 1999;23:976–982. [PubMed] [Google Scholar]
- 7.Sarkola T, Fukunaga T, Mäkisalo H, Peter Eriksson CJ. Acute effect of alcohol on androgens in premenopausal women. Alcohol Alcohol. 2000;35:84–90. [DOI] [PubMed] [Google Scholar]
- 8.Sarkola T, Adlercreutz H, Heinonen S, von der Pahlen B, Eriksson CJP. The role of the liver in the acute effect of alcohol on androgens in women. J Clin Endocrinol Metab. 2001;86:1981–1985. [DOI] [PubMed] [Google Scholar]
- 9.McNamee B, Grant J, Ratcliffe J, Ratcliffe W, Oliver J. Lack of effect of alcohol on pituitary-gonadal hormones in women. Br J Addict Alcohol Other Drugs. 1979;74:316–317. [DOI] [PubMed] [Google Scholar]
- 10.Välimäki M, Härkönen M, Ylikahri R. Acute effects of alcohol on female sex hormones. Alcohol Clin Exp Res. 1983;7:289–293. [DOI] [PubMed] [Google Scholar]
- 11.Becker U, Gluud C, Bennett P, Micic S, Svenstrup B, Winkler K, et al. Effect of alcohol and glucose infusion on pituitary-gonadal hormones in normal females. Drug Alcohol Depend. 1988;22:141–149. [DOI] [PubMed] [Google Scholar]
- 12.Reichman ME, Judd JT, Longcope C, Schatzkin A, Clevidence BA, Nair PP, et al. Effects of alcohol consumption on plasma and urinary hormone concentrations in premenopausal women. J Natl Cancer Inst. 1993;85:722–727. [DOI] [PubMed] [Google Scholar]
- 13.Dorgan JF, Baer DJ, Albert PS, Judd JT, Brown ED, Corle DK, et al. Serum hormones and the alcohol-breast cancer association in postmenopausal women. J Natl Cancer Inst. 2001;93:710–715. [DOI] [PubMed] [Google Scholar]
- 14.Mahabir S, Baer DJ, Johnson LL, Dorgan JF, Campbell W, Brown E, et al. The effects of moderate alcohol supplementation on estrone sulfate and DHEAS in postmenopausal women in a controlled feeding study. Nutr J. 2004;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sierksma A, Sarkola T, Eriksson CJP, van der Gaag MS, Grobbee DE, Hendriks HFJ. Effect of moderate alcohol consumption on plasma dehydroepiandrosterone sulfate, testosterone, and estradiol levels in middle-aged men and postmenopausal women: a diet-controlled intervention sttudy. Alcohol Clin Exp Res. 2004;28:780–785. [DOI] [PubMed] [Google Scholar]
- 16.Endogenous Hormones and Breast Cancer Collaborative Group; Key TJ, Appleby PN, Reeves GK, Travis RC, Alberg AJ, Barricarte A, et al. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 2013;14:1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Endogenous Hormones and Breast Cancer Collaborative Group; Key TJ, Appleby PN, Reeves GK, Roddam AW, Helzlsouer KJ, Alberg AJ, et al. Circulating sex hormones and breast cancer risk factors in postmenopausal women: reanalysis of 13 studies. Br J Cancer. 2011;105:709–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tin Tin S, Key TJ, Reeves GK. Alcohol intake and endogenous hormones in pre- and postmenopausal women: findings from the UK Biobank. Cancer Epidemiol Biomarkers Prev. 2021;30:2294–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen N, Sudlow C, Downey P, Peakman T, Danesh J, Elliott P, et al. UK Biobank: Current status and what it means for epidemiology. Health Policy Technol. 2012;1:123–126. [Google Scholar]
- 20.Allen N, Arnold M, Parish S, Hill M, Sheard S, Callen H, et al. Approaches to minimising the epidemiological impact of sources of systematic and random variation that may affect biochemistry assay data in UK Biobank. Wellcome Open Res. 2021;5:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaaks R, Slimani N, Riboli E. Pilot phase studies on the accuracy of dietary intake measurements in the EPIC project: overall evaluation of results. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol. 1997;26:S26–36. [DOI] [PubMed] [Google Scholar]
- 22.Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5:1113–1124. [DOI] [PubMed] [Google Scholar]
- 23.Rinaldi S, Peeters PHM, Bezemer ID, Dossus L, Biessy C, Sacerdote C, et al. Relationship of alcohol intake and sex steroid concentrations in blood in pre- and post-menopausal women: the European Prospective Investigation into Cancer and Nutrition. Cancer Causes Control. 2006;17:1033–1043. [DOI] [PubMed] [Google Scholar]
- 24.Bartsch W. Interrelationships between sex hormone-binding globulin and testosterone, 5α-dihydrotestosterone and oestradiol- 7β in blood of normal men. Maturitas. 1980;2:109–118. [DOI] [PubMed] [Google Scholar]
- 25.Södergård R, Bäckström T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810. [DOI] [PubMed] [Google Scholar]
- 26.Kaaks R, Berrino F, Key T, Rinaldi S, Dossus L, Biessy C, et al. Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC). J Natl Cancer Inst. 2005;97:755–765. [DOI] [PubMed] [Google Scholar]
- 27.Hirko KA, Spiegelman D, Willett WC, Hankinson SE, Eliassen AH. Alcohol consumption in relation to plasma sex hormones, prolactin, and sex hormone–binding globulin in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2014;23:2943–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019;51:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swerdlow DI, Kuchenbaecker KB, Shah S, Sofat R, Holmes MV, White J, et al. Selecting instruments for Mendelian randomization in the wake of genome-wide association studies. Int J Epidemiol. 2016;45:1600–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edenberg HJ, Foroud T. Genetics and alcoholism. Nat Rev Gastroenterol Hepatol. 2013;10:487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MRC Integrative Epidemiology Unit (IEU). IEU Open GWAS Project. https://gwas.mrcieu.ac.uk/. Accessed 12 Jun 2023.
- 32.Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018;7:e34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallace C. Eliciting priors and relaxing the single causal variant assumption in colocalisation analyses. PLoS Genet. 2020;16:e1008720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foley CN, Staley JR, Breen PG, Sun BB, Kirk PDW, Burgess S, et al. A fast and efficient colocalization algorithm for identifying shared genetic risk factors across multiple traits. Nat Commun. 2021;12:764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopes KdP, Snijders GJL, Humphrey J, Allan A, Sneeboer MAM, Navarro E, et al. Genetic analysis of the human microglial transcriptome across brain regions, aging and disease pathologies. Nat Genet. 2022;54:4–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purohit V. Can alcohol promote aromatization of androgens to estrogens? A review. Alcohol. 2000;22:123–127. [DOI] [PubMed] [Google Scholar]
- 37.Missmer SA, Spiegelman D, Bertone-Johnson ER, Barbieri RL, Pollak MN, Hankinson SE. Reproducibility of plasma steroid hormones, prolactin, and insulin-like growth factor levels among premenopausal women over a 2- to 3-year period. Cancer Epidemiol Biomarkers Prev. 2006;15:972–978. [DOI] [PubMed] [Google Scholar]
- 38.Dorgan JF, Reichman ME, Judd JT, Brown C, Longcope C, Schatzkin A, et al. The relation of reported alcohol ingestion to plasma levels of estrogens and androgens in premenopausal women (Maryland, United States). Cancer Causes Control. 1994;5:53–60. [DOI] [PubMed] [Google Scholar]
- 39.Selby C. Sex hormone binding globulin: origin, function and clinical significance. Ann Clin Biochem. 1990;27:532–541. [DOI] [PubMed] [Google Scholar]
- 40.Simó R, Sáez-López C, Barbosa-Desongles A, Hernández C, Selva DM. Novel insights in SHBG regulation and clinical implications. Trends Endocrinol Metab. 2015;26:376–383. [DOI] [PubMed] [Google Scholar]
- 41.Achur RN, Freeman WM, Vrana KE. Circulating cytokines as biomarkers of alcohol abuse and alcoholism. J Neuroimmune Pharmacol. 2010;5:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iturriaga H, Lioi X, Valladares L. Sex hormone binding globulin in non-cirrhotic alcoholic patients during early withdrawal and after longer abstinence. Alcohol Alcohol. 1999;34:903–909. [DOI] [PubMed] [Google Scholar]
- 43.Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of alcoholic beverages. Lancet Oncol. 2007;8:292–293. [DOI] [PubMed] [Google Scholar]
- 44.Allen NE, Beral V, Casabonne D, Kan SW, Reeves GK, Brown A, et al. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst. 2009;101:296–305. [DOI] [PubMed] [Google Scholar]
- 45.Jung S, Wang M, Anderson K, Baglietto L, Bergkvist L, Bernstein L, et al. Alcohol consumption and breast cancer risk by estrogen receptor status: in a pooled analysis of 20 studies. Int J Epidemiol. 2016;45:916–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. 2007;7:599–612.. [DOI] [PubMed] [Google Scholar]
- 47.Ratna A, Mandrekar P. Alcohol and cancer: mechanisms and therapies. Biomolecules. 2017;7:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eriksson CJ, Fukunaga T, Sarkola T, Lindholm H, Ahola L. Estrogen-related acetaldehyde elevation in women during alcohol intoxication. Alcohol Clin Exp Res. 1996;20:1192–1195. [DOI] [PubMed] [Google Scholar]
- 49.Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: state of the science and challenges for research. Addiction. 2003;98:1–12. [DOI] [PubMed] [Google Scholar]
- 50.Boniface S, Kneale J, Shelton N. Drinking pattern is more strongly associated with under-reporting of alcohol consumption than socio-demographic factors: evidence from a mixed-methods study. BMC Public Health. 2014;14:1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
UK Biobank is an open access resource, and the study website https://www.ukbiobank.ac.uk/ has information on available data and access procedures. For EPIC, details of data access are at https://epic.iarc.fr/access/index.php. For EHBCCG, the principal investigators of each contributing study are responsible for access to the data. Full GWAS summary statistics from GSCAN are available at https://conservancy.umn.edu/handle/11299/201564. The instruments for concentrations of testosterone and SHBG were extracted from the OpenGWAS platform https://gwas.mrcieu.ac.uk/.