Abstract
More than 40% of individuals infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have experienced persistent or relapsing multi-systemic symptoms months after the onset of coronavirus disease 2019 (COVID-19). This post-COVID-19 condition (PCC) has debilitating effects on the daily life of patients and encompasses a broad spectrum of neurological and neuropsychiatric symptoms including olfactory and gustative impairment, difficulty with concentration and short-term memory, sleep disorders and depression. Animal models have been instrumental to understand acute COVID-19 and validate prophylactic and therapeutic interventions. Similarly, studies post-viral clearance in hamsters, mice and nonhuman primates inoculated with SARS-CoV-2 have been useful to unveil some of the aspects of PCC. Transcriptomic alterations in the central nervous system, persistent activation of immune cells and impaired hippocampal neurogenesis seem to have a critical role in the neurological manifestations observed in animal models infected with SARS-CoV-2. Interestingly, the proinflammatory transcriptomic profile observed in the central nervous system of SARS-CoV-2-inoculated mice partially overlaps with the pathological changes that affect microglia in humans during Alzheimer’s disease and aging, suggesting shared mechanisms between these conditions. None of the currently available animal models fully replicates PCC in humans; therefore, multiple models, together with the fine-tuning of experimental conditions, will probably be needed to understand the mechanisms of PCC neurological symptoms. Moreover, given that the intrinsic characteristics of the new variants of concern and the immunological status of individuals might influence PCC manifestations, more studies are needed to explore the role of these factors and their combinations in PCC, adding further complexity to the design of experimental models.
Subject terms: Central nervous system infections, Viral infection
In this Perspective, the authors summarize the current knowledge on the post-COVID-19 condition, focusing on the neurological manifestations, and discuss the applicability of existing animal models to recapitulate human condition.
Main
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly pathogenic betacoronavirus that emerged in China in late 2019; its rapid spread across the world resulted in the coronavirus disease 2019 (COVID-19) pandemic1,2. SARS-CoV-2 shares its genomic organization with other betacoronaviruses, encoding four structural proteins (Spike, Envelope, Membrane and Nucleoprotein), and several nonstructural proteins. The structural proteins of SARS-CoV-2 share more than 90% amino acid identity with SARS-CoV, except for the Spike (S) protein, which displays an insertion of a polybasic furin cleavage site at the junction between the S1 and S2 subunits of the receptor-binding domain (RBD) of SARS-CoV-2 (ref. 3). Antibodies against the RBD have been detected in the majority of patients with COVID-19, often with neutralizing activity, prompting the early development of RBD and S-based vaccines4.
Since the early phases of the pandemic, it became evident that the novel disease was a multi-systemic disorder. Besides the respiratory outcome, patients affected by COVID-19 may suffer from extrapulmonary manifestations, including hematological, cardiovascular, gastrointestinal, dermatological, endocrine, ophthalmological and neurological symptoms. Initially, it was unclear whether all these clinical–pathological features resulted from a widespread replication of the virus itself or a consequence of the virus-induced inflammatory and immunological responses5–7. Nowadays, both hypotheses are considered valid8–10.
Owing to the virtually ubiquitous expression of angiotensin-converting enzyme 2 (ACE2), the main cell receptor for SARS-CoV-2 that is expressed on endothelial and smooth muscle cells, it was suspected that the multi-organ effects of SARS-CoV-2 infection were directly linked to viral presence and replication11. However, the mechanisms underlying SARS-CoV-2 multi-organ spread are still unclear because viremia is not a usual outcome of the infection and has been detected in very few severe cases12–14. As of May 2023, only one severe case with high and persistent SARS-CoV-2 viremia leading to meningitis, probably facilitated by blood–brain barrier (BBB) injury, has been well documented15.
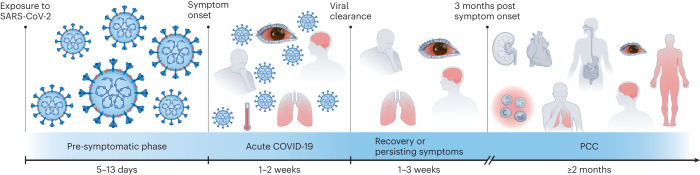
As soon as the very first wave of COVID-19 in 2020 waned, it was observed that a proportion of patients were experiencing symptoms weeks or months after viral clearance. The most common post-acute symptoms reported were shortness of breath, fatigue, pain, increased heart rate, anosmia–dysgeusia, headache and cognitive and neurological impairment, which can considerably impact daily activities and quality of life16–18. This condition initially named ‘long-COVID’, and now known as ‘post-COVID-19 condition’ (PCC), was clinically defined by a Delphi consensus process in 2022 as the presence of symptoms in individuals with a probable or confirmed SARS-CoV-2 infection, 3 months from the onset of COVID-19. Such symptoms may occur after recovery from acute COVID-19 or persist from the initial disease and may fluctuate or relapse over time. Such a definition might be different for children19. Differences between cohorts reported in the literature also suggest the existence of two populations: (1) patients with symptoms that persist after the resolution of a severe acute infection, and (2) patients with symptoms that develop only in the post-acute phase of the disease, usually after mild COVID-19 (ref. 20). PCC is also referred to as post-acute sequelae of COVID-19. A timeline of acute COVID-19 and PCC is represented in Fig. 1.
Fig. 1. Timeline of acute COVID-19 and PCC in humans.
The timings stated in each section of the figure indicate the duration of the corresponding phase of the disease. After exposure to SARS-CoV-2, a pre-symptomatic phase characterized by viral replication and early viral shedding precedes the onset of symptoms. The most common symptoms of acute COVID-19 are fever, cough, headache, dyspnea and fatigue; during this phase, the virus is still replicating, and the patient is contagious. In the weeks immediately after viral clearance, symptoms can either remit or persist. The common consensus for PCC is the persistence or new onset of symptoms three months after the beginning of the acute phase, which lasts for at least two months1,19,95–97. Adapted from ref. 82, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).
PCC has a debilitating effect on the daily life of patients, reducing their ability to work or engage in social and physical activities. Given the hundreds of millions of individuals who have become infected with SARS-CoV-2, the social implications of the long-term consequences of COVID-19 are of great relevance and add to the health burden caused by the pandemic. The societal and public health impact of PCC is even more concerning given that PCC has also been linked to an increased incidence of noncommunicable diseases. However, the mechanisms of PCC pathogenesis are far from being satisfactorily unveiled, leaving patients suffering from this condition without proper treatment.
Animal models have been instrumental in the understanding of the acute phase of SARS-CoV-2 infection; the development of adequate models allowing the long-term follow-up of convalescent animals will be useful to better understand PCC pathology and to design counteracting measures. Among the several species used as animal models of SARS-CoV-2 infection21, some have proven to be suitable for studying various aspects of PCC. However, due to the differences in lifespan and viral kinetics between humans and most laboratory animals, translating the PCC concept to other species can be challenging. In general, it is considered that all animal studies extending beyond viral clearance and displaying multi-organ damage could provide important information about the long-term effects of SARS-CoV-2 infection and their mechanisms. Golden Syrian hamsters (GSH), mice (including transgenic or transduced with human ACE2 (hACE2)) and nonhuman primates (NHP) have all been used for the study of the long-term neurological sequelae of SARS-CoV-2 infection.
This Perspective aims to summarize the current knowledge on PCC, with a particular focus on its neurological manifestations, and to discuss the applicability of existing animal models to recapitulate some of the symptoms observed in patients with PCC.
Potential mechanisms of PCC
It is estimated that, worldwide, 43% of patients who had SARS-CoV-2 infection have experienced PCC, with regional prevalence ranging from 11.5% to 75.0% (refs. 22–26). A fraction of patients who survived severe acute respiratory syndrome (SARS, caused by SARS-CoV) and Middle East respiratory syndrome (MERS, caused by MERS-CoV) also experienced psychological sequelae and chronic fatigue after the resolution of the infection, but PCC seems to be more pleiotropic than post-SARS and post-MERS syndromes27,28. Whether this is actually the case, or whether this assumption is skewed by the higher number of people infected by SARS-CoV-2, remains to be determined. Factors such as the severity of acute COVID-19, as well as comorbidities or specific demographic characteristics (respiratory disease, body mass index, older age and Black or Asian ethnicity), have been associated with symptom persistence and intensity29. Nonetheless, PCC has been reported even in patients who had mild to moderate acute disease30. Interestingly, PCC seems to be more frequently associated with the female gender, while studies have shown that severe acute disease is more prevalent in male patients30–33.
The mechanisms probably contributing to the pathogenesis of PCC fall under five main categories (Fig. 2): (1) persistence of the whole virus or viral fragments; (2) prolonged inflammation and immunological aberrations; (3) virally induced autoimmunity; (4) alterations of the renin–angiotensin system (RAS); and/or (5) microbiome dysbiosis29,34–36. In addition to the aforementioned mechanisms, post-intensive care syndrome (a condition of impaired physical, cognitive and psychiatric state, irrespective of the critical illness suffered by the patient) is also thought to contribute to PCC in patients with severe COVID-19 who had required intensive care unit hospitalization29,37. Similar to acute COVID-19, PCC encompasses extrapulmonary symptoms such as hematological and immune-related, cardiovascular, renal, gastrointestinal and hepatobiliary, endocrine, neurological, ophthalmological and dermatological disorders summarized in Table 1 (refs. 29,34,35,38). In this Perspective, we will focus solely on the neurological symptoms of PCC.
Fig. 2. Putative mechanisms of PCC.
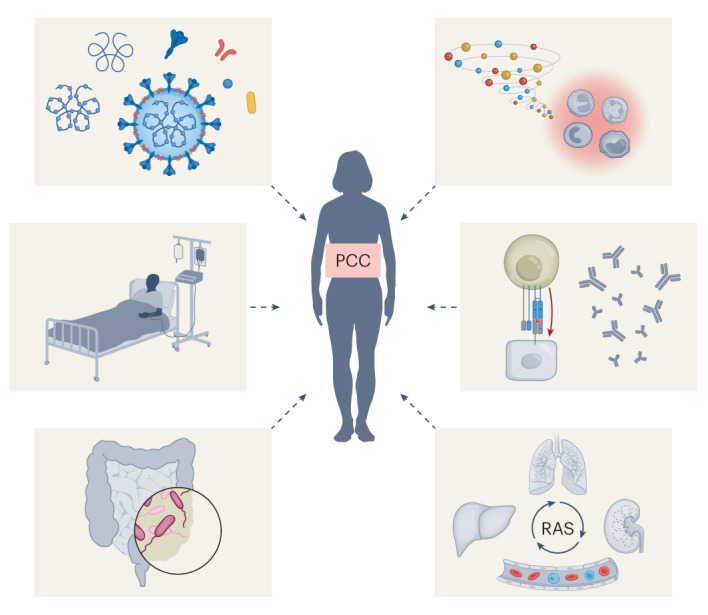
From top left in clockwise order: persistence of the whole virus or viral fragments; prolonged inflammation and immunological aberrations; virally induced autoimmunity; alterations of the RAS; microbiome dysbiosis; post-intensive care syndrome.
Table 1.
Most common manifestations of both acute and persistent COVID-19 in humans
| Organ or system | Lesions and symptoms |
|---|---|
| Respiratory |
• Dyspnea • Decreased exercise capacity • Chronic cough • Exacerbation of asthma |
| Cardiovascular |
• Palpitation • Chest pain and tightness • Arrhythmias • Postural orthostatic tachycardia • Orthostatic hypotension • Vasovagal syncope • Myocarditis and/or pericarditis • Myocardial ischemia • Ventricular dysfunction • Venous thromboembolic events |
| Cerebrovascular | • Ischemic stroke |
| Renal |
• Impaired renal function • Severe acute kidney injury • Microhematuria |
| Endocrine |
• New onset or worsening of existing diabetes mellitus • Glycemic abnormalities • Diabetic ketoacidosis • Hormonal abnormalities • Subacute thyroiditis • Autoimmune thyroiditis • Bone demineralization • Lipid abnormalities • Hot and cold sensation |
| Gastrointestinal and hepatobiliary |
• Abdominal pain • Nausea • Loss of appetite • Altered bowel motility • Irritable bowel syndrome • Dysphagia • Hepatitis |
| Dermatological |
• Hair loss • Skin rash • Nail alterations • Papulo-squamous eruptions (pernio- or chilblain-like) |
| Musculoskeletal |
• Myalgia • Arthralgia • Asthenia |
| Neuropsychiatric |
• Migraine-like headache • Olfactory and gustatory impairments • Difficulties with concentration and short-term memory • Dizziness • Imbalance • Encephalitis • Myelopathy • Neuropathy • Depressive symptoms • Anxiety • Insomnia and sleep disorders • Mood disorders • Post-traumatic stress disorder |
| Miscellaneous |
• Fever • Hearing loss • Tinnitus • Red eyes • Sore throat • Light and sound sensitivity • Blurry vision • Excessive bruising |
| Multi-system inflammatory syndrome in children (MIS-C) |
• Fever • Abdominal pain • Vomiting • Diarrhea • Skin rash • Mucocutaneous lesions • Hypotension • Cardiovascular and neurological symptoms |
Neurological symptoms of PCC
Approximately one-third of patients with acute COVID-19 report neurological symptoms including headache, dizziness, anosmia, ageusia, anorexia, myalgia and fatigue; more severe manifestations such as stroke, encephalopathy (with or without encephalitis) and Guillain–Barré syndrome have also been described5,39. Considering the broad spectrum of neurological manifestations of PCC, different mechanisms may be involved in the pathogenesis of specific disorders. ‘Brain fog’, which is one of the most common self-reported symptoms in both acute and post-acute COVID-19, describes a feeling of being mentally slow (bradyphrenia), with confusion, memory loss and difficulty concentrating40. This phenomenon is very similar to the so-called chemo-brain, often experienced by patients with cancer during or after chemotherapy; this condition also known as cancer-related or chemotherapy-related cognitive impairment is believed to be caused by neuroinflammation41,42.
So far, evidence of SARS-CoV-2 infection in the central nervous system (CNS) is limited to post-mortem samples from severe cases; CNS SARS-CoV-2 infection is also supported by transcriptomic data reporting ACE2 expression in both neuronal and nonneuronal cells of the human brain43,44. Although not confirmed in humans or experimental animal models following SARS-CoV-2 natural infection, studies have shown that the soluble monomeric S1 subunit of the Spike protein can cross the mouse BBB in vivo after both intravenous and intranasal administration, and spread to different areas of the brain45. Given that coronavirus S1 subunits are often shed from the virions by the action of host proteases located on cell surface, it cannot be ruled out that soluble SARS-CoV-2 Spike S1 triggers inflammation and tissue damage, even in the absence of intact viral particles.
On the other hand, the presence of the virus itself has seldom been demonstrated in the cerebrospinal fluid (CSF) of patients; an altered CSF cytokine profile (with increased levels of IL-6, IL-8 and IL-10) has also been reported in one case of SARS-CoV-2-associated encephalitis46–48. Furthermore, a sizable single-cell transcriptomic study analyzing the brains of patients who succumbed to COVID-19 did not find any molecular traces of SARS-CoV-2 in the brain, but did identify the upregulation of inflammation-related genes (predominantly in astrocytes), antiviral defense markers (interferon-induced transmembrane protein 3 among others) and T cell infiltration49. The transcriptomic perturbations identified in the study, in particular those suggesting compromised neurotransmission and information processing, share features with pathological cell states of neurodegenerative disease. Moreover, Alzheimer’s-like features such as excessive levels of reactive oxygen species (ROS), activation of the TGF-β pathway and increased tau phosphorylation have been found in the brains of these patients. It is interesting to note that olfactory dysfunctions are also common symptoms of early Alzheimer’s and Parkinson’s diseases, further suggesting a shared mechanism between PCC and neurodegenerative diseases50,51. Table 2 compares the hallmark neurological symptoms and the associated lesions and study findings between PCC and these neurodegenerative diseases.
Table 2.
Shared neurological symptoms and lesions between PCC, Parkinson’s and Alzheimer’s diseases
| Condition | |||
|---|---|---|---|
| PCC | Parkinson’s disease | Alzheimer’s disease | |
| Symptoms | |||
| Headache | ✓ | – | – |
| Dizziness | ✓ | – | – |
| Anosmia | ✓ | ✓ | ✓ |
| Ageusia | ✓ | – | – |
| Anorexia | ✓ | – | – |
| Myalgia | ✓ | ✓ | – |
| Fatigue | ✓ | – | – |
| Bradyphrenia | ✓ | – | ✓ |
| Confusion | ✓ | – | ✓ |
| Memory loss | ✓ | – | ✓ |
| Impaired concentration | ✓ | – | ✓ |
| Sleep disorders | ✓ | ✓ | – |
| Depression | ✓ | ✓ | – |
| Lesions and study findings | |||
| Altered CSF cytokine profile | ✓ | ✓ | ✓ |
| Microglia activation | ✓ | – | ✓ |
| Astrocyte activation | ✓ | – | ✓ |
| T cell infiltration | ✓ | – | – |
| Excessive ROS | ✓ | ✓ | ✓ |
| Activated TGF-β pathway | ✓ | – | ✓ |
| Tau hyper-phosphorylation | ✓ | – | ✓ |
| α-Synuclein hyper-phosphorylation | ✓ | ✓ | ✓ |
Animal models for the study of PCC
In this Perspective, we considered all studies that followed animals for more than 14 days post-inoculation (dpi) as ‘long-term studies’.
GSH
The GSH (Mesocricetus auratus) is a valuable small animal model for the study of SARS-CoV-2 infection, because the GSH is naturally susceptible to infection52. A study has reported multi-organ damage with infiltration of inflammatory cells and necrosis in upper and lower airways, secondary lymphoid organs, the digestive tract, kidneys, adrenal gland and ovaries in this model 5–7 dpi with the SARS-CoV-2/WH-09/human/2020/CHN isolate. These lesions were very mild in nonrespiratory tissues and could still be present by 18 dpi. In addition, different levels of viral genomic RNA (detected by quantitative PCR with reverse transcription (RT–qPCR) and in situ hybridization) and/or S and N proteins (detected by immunohistochemistry) were detectable up to 7 dpi in nasopharynges, trachea, lungs, kidneys, testis, vesicular gland, prostate, adrenal gland, spleen and lymph nodes53. Similarly, another study showed that microglial activation and accumulation of hyperphosphorylated tau and α-synuclein persisted in the brain of SARS-CoV-2-inoculated hamsters after viral clearance (14 dpi), in the olfactory bulb (OB) and in the cortical neurons, respectively, without any evidence of neuroinvasion (as shown by a lack of detectable S protein by immunohistochemistry), which suggests these pathological features contribute to the long-lasting neurological signs of SARS-CoV-2 infection54.
Additionally, a long-term study compared the systemic effects of SARS-CoV-2 (USA-WA1/2020 isolate) with those of influenza A virus (IAV) pandemic strain H1N1 (A/California/04/2009 isolate) in GSH for up to 31 dpi (a timepoint when infectious particles for either infection were no longer detectable in any of the investigated tissues)55. This study found that the histological alterations induced by SARS-CoV-2 in the lungs (peribronchiolar metaplasia and monocyte and neutrophil infiltration in the alveolar space) and kidneys (areas of tubular atrophy and presence of proteinaceous fluid in the interstitial space) were more severe than those induced by IAV and persisted for a longer period after the resolution of infection. Moreover, the transcriptomic analysis of the olfactory tissue and of different areas of the CNS revealed that, while both viruses caused a similar pattern of perturbations in the CNS that persisted for over a month, SARS-CoV-2 induced a unique profile in the olfactory tissue of GSH. Thirty-one days after SARS-CoV-2 infection, genes associated with microglial activation and macrophage infiltration (these increases in gene expression were confirmed by immunostaining), T cell recruitment and activation, type I interferon and chemokine response were upregulated, while genes involved in sensory perception and olfactory capabilities were downregulated. At 26 dpi, SARS-CoV-2-infected GSH also showed a reduction in burying activity when subjected to the marble-burying assay compared with IAV-infected and noninfected hamsters, demonstrating a change in this spontaneous rodent behavior following SARS-CoV-2 infection55. Interestingly, injury and chronic inflammation of the OB have been previously linked to neurobehavioral disorders because OB function can impact sensory, emotional and cognitive processes given the proximity of the OB to the limbic system responsible for such reactions56. Therefore, the behavioral changes observed in SARS-CoV-2-infected GSH suggest that long-term OB inflammation causes neurodegenerative changes that are compatible with PCC neurological symptoms. This observation is in line with evidence of gray matter loss in the limbic and olfactory cortical areas of patients who have recovered from COVID-19, even with mild acute manifestations55,57.
In addition, SARS-CoV-2 infection in GSH has been linked to mechanical hypersensitivity during both the acute and post-acute phases (28 dpi) of SARS-CoV-2 infection, which resembles the somatosensory abnormalities observed in patients with PCC. This prolonged hypersensitivity coincided with the differential expression of several genes associated with neuro-oncological and neurodegenerative disorders (including glioblastoma, Alzheimer’s and Parkinson’s diseases and neurilemmoma) compared with noninfected hamsters. The same transcriptomic analysis predicted the contribution of macrophages and Schwann cells, already known to be involved in neuropathic pain and hypersensitivity, to the SARS-CoV-2-induced proinflammatory state. Interestingly, these results were obtained long after viral clearance, suggesting that the presence of viral transcript (but not infectious particles) and type I interferon response in the peripheral nervous system (dorsal root ganglia and spinal cord) during the first 24 h post-inoculation was sufficient to induce persistent maladaptive neuronal responses58.
Mice
Given the substantial differences in amino acid sequences between human and mouse ACE2, the latter is not a functional receptor for the ancestral SARS-CoV-2 strain. hACE2-transgenic (hACE2-tg) mice, transient hACE2 expression and virus adaptation to the mouse are among the strategies employed to circumvent this limitation21. In addition, different humanized mice have been used as models of PCC, with a particular focus on the lungs and the respiratory tract. MISTRG6 humanized mice, reconstituted with human immune cells and transiently expressing hACE2 via an adeno-associated vector (AAV-hACE2), have been used to reproduce the human innate and adaptive responses to SARS-CoV-2 infection. Results from this model have suggested that human immune cells contribute to the pathological inflammatory response in the lungs and to viral RNA persistence, which was detected until 35 dpi, either as a result of direct infection of ACE2-expressing myeloid cells or as a residue of phagocyted infected epithelial cells59. Similar results have been obtained in DRAGA (HLA-A2.HLA-DR4.Rag1KO.IL2RγcKO.NOD) humanized mice, which when infused with human hematopoietic stem cells from cord blood reconstitute both a functional human immune system and hACE2-expressing human epithelial and endothelial cells in the lung and upper respiratory airways. Inoculated animals were followed for 25 days, which revealed mild to severe lung pathology with inflammatory infiltrate and alveolar damage until the end of the study60.
A longer-term study monitored neuroinflammation up to 7 weeks post-infection (wpi) with SARS-CoV-2 (isolate USA-WA1/2020) in wild-type CD1 mice transduced with AAV-hACE2 in the trachea and lungs by intratracheal injection61. This model simulates a mild respiratory infection with no to moderate lung pathology and no viral neuroinvasion. Increased protein levels of proinflammatory cytokines (IFNγ, IL-6, TNF, CXCL10, CCL7, CCL2, CCL11, BAFF and GMCSF) were observed both in the serum and CSF of infected mice as early as 7 dpi. Interestingly, CSF levels of C–C motif chemokine 11 (CCL11), known to limit neurogenesis and contribute to cognitive impairment, further increased until at least 7 wpi, while they normalized in the serum at the same timepoint. This increase in CCL11 CSF levels was associated with increased microglial activation in the subcortical white matter (as indicated by increased expression of allograft inflammatory factor 1 (AIF1; also known as IBA1) marker in CD68+ cells), which persisted during the entire study period. Moreover, transcriptomic analysis at 7 dpi and 7 wpi revealed an increase in the abundance of a chemokine-expressing subpopulation of the microglia as well as an altered profile in the homeostatic microglia subpopulation, with upregulation of genes associated with cytotoxicity, inflammation, antigen processing and presentation. Interestingly, this gene signature partially overlaps with the expression profiles of pathological cell states such as disease-associated microglia observed in Alzheimer’s disease, and white-matter-associated microglia observed in aging. SARS-CoV-2-infected mice also exhibited a persistent decrease in neurogenesis in the hippocampus and a decrease in myelinated axon density during the entire study period, as well as a decrease in mature oligodendrocytes (ASPA+ CC1+ population) at 7 dpi, which recovered by 7 wpi. The magnitude of myelin loss was strikingly similar to that observed after chemotherapy exposure, confirming the similarity between ‘COVID-19 brain fog’ and ‘chemo fog’61.
Studies using a mouse-adapted SARS-CoV-2 strain (MA10) have reported productive infection with severe lung injury and mortality in standard laboratory mice62,63. Although no virus was detected in the brain of intranasally infected animals, this model showed an upregulation of proinflammatory cytokine messenger RNAs, altered BBB permeability and an increase in IBA1+ microglial cells in the cortical region of 1-year-old Rag–/– mice (a mouse model lacking mature lymphocytes). Histological analysis of aged Rag–/– brain samples revealed prominent lymphocyte perivascular cuffing, indicating inflammation of blood vessels at 30-days post inoculation64. Long-term studies using the mouse adapted MA10 strain in immunocompetent mice are needed to confirm these findings and to determine the extent of brain damage and neurological impairment caused by SARS-CoV-2.
NHPs
NHPs have been successfully used for the study of human brain disorders, viral infections and respiratory diseases, taking advantage of their phylogenetic proximity and anatomical and physiological similarity to humans65–67. Over the past 3 years, NHPs have been of great interest in the study of both acute and post-acute SARS-CoV-2 infection21,68.
A study following rhesus macaques and African green monkeys for 28 days after infection with the SARS-CoV-2 USA-WA1/2020 virus strain revealed neuroinflammation, as well as brain hypoxia, microhemorrhages and lesions similar to autopsy findings from patients who died after SARS-CoV-2 infection69. Neuroinflammation signatures, determined by the expression of the pan-microglial marker IBA1 and glial fibrillary acidic protein (GFAP), were greater in infected animals than in mock-infected control animals, and revealed more pronounced astrocytic hypertrophy in these animals. In addition, the inoculated animals in this study displayed neuronal morphological changes in the cerebellum and the brainstem, such as degenerated Purkinje neurons exhibiting cellular blebs and cytoplasmic vacuoles, and adjacent pyknotic glial cells with condensed nuclei. These lesions were found concomitantly with cleaved caspase 3 staining, indicating that apoptosis was one of the mechanisms underlying such neuronal degeneration. Very low levels of viral S RNA were detected in the brain of some of the infected animals; S expression was always limited to vascular endothelial cells, with no involvement of parenchymal cells. The virus was not detected in the CSF of infected NHPs, which might suggest hematological dissemination of the virus to the brain. Interestingly, hypoxic–ischemic injuries, with areas of intense positivity for the hypoxia-inducible factor 1α (HIF1α) were detected within and around blood vessels in the brain of infected animals69. Given that the expression of this protein can be upregulated by proinflammatory cytokines such as TNF — a factor abundant in the serum of patients with severe COVID-19 — and by the SARS-CoV-2 protein translated from ORF3 (ref. 10), at least in vitro, hypoxia could be both a direct and an indirect effect of SARS-CoV-2 infection. It is worth mentioning that signs of hypoxia were also detected in animals that did not develop severe respiratory disease, suggesting that it could be one of the causes of neurological disorder even after mild COVID-19, as observed in patients70,71.
The advanced age (13–21 years) of the NHPs used for this study might have acted as a confounding factor: all morphological changes and inflammatory features were also observed in mock-infected animals, although at a lower level. It is therefore probable that the infection contributed to the worsening of pre-existing, age-related lesions rather than being their triggering cause. Moreover, none of the 12 infected animals displayed clinical signs of neurological disorders, except for one female African green monkey that was found recumbent and marginally responsive to stimuli at 8 dpi (ref. 69).
Animal models with CNS involvement during the acute phase of COVID-19
Considering the small number of models currently available for the study of the long-term neurological manifestations of SARS-CoV-2 infection, it is worth considering all preclinical studies that have shown either neuroinvasion or neuroinflammation during the acute phase of the infection.
In one study, infectious viral particles were isolated from different areas of the brain of SARS-CoV-2-infected hamsters 4 dpi, indicating that the virus can reach and productively replicate in the CNS of this animal model. Viral particles were also detected in the same animals' olfactory neuroepithelium, along with infiltrating myeloid cells and upregulated expression of proinflammatory markers72.
On the other hand, K18-hACE2-tg mice — mice carrying the hACE2 sequence under the control of the cytokeratin-18 (K18) gene promoter — are susceptible to SARS-CoV-2 infection, develop severe lesions including massive brain invasion, neuronal death and neuroinflammation, and succumb by 5–7 dpi when given a dose of either 103 TCID50 SARS-CoV-2 or 105 plaque forming unit intranasally73–75. This model has been successfully used for testing vaccines and antivirals but does not properly replicate human COVID-19. However, a short-term study in 2022 has now shown that the administration of aerosolized SARS-CoV-2 to K18-hACE2 mice leads to efficient respiratory infection and anosmia without fatal neuroinvasion, even at the same dose76. Similarly, a study showed that K18-hACE2 mice intranasally inoculated with 103 plaque forming unit of the BA.1 (Omicron) variant develop mild respiratory pathology without neuroinvasion up to 7 dpi (ref. 77). Notwithstanding the differences in ACE2 expression and in the severity of SARS-CoV-2-induced disease between K18-hACE2 mice and humans, it is possible to achieve a nonfatal disease via a fine modulation of the experimental inoculation (that is, use of a sublethal dose or a less pathological variant, and use of a more natural administration route such as aerosolization), which might extend the survival of the infected animals sufficiently to allow the study of long-term consequences of SARS-CoV-2 infection78.
Less extensively used than the K18-hACE2 mice, hACE2 knock-in (KI) mice are considered a more physiologically relevant model, because the expression of the human receptor is under the control of the endogenous promoter of mACE2, thereby preventing ectopic expression. One short-term study using hACE2-KI mice has shown very low levels of SARS-CoV-2 RNA in the brain and OB of some of the inoculated mice at 7 dpi (ref. 79). The presence of replicating particles and the long-term effect of viral presence in the brain have not been investigated in these animals.
An early study on rhesus macaques infected with SARS-CoV-2 (isolate KMS1/2020) also showed neuroinvasion. Viral RNA was detected by RT–qPCR in the brain and spinal cord of the NHPs on day 9 post-intranasal infection, while it was detected as soon as 2 dpi and during the entire study period in the lungs and trachea. The animals in the study had transient viremia, suggesting systemic spread of the virus through the bloodstream. Histological analysis of the CNS also revealed hyperemia and edema in some of the samples80, but those findings might not be specific to SARS-CoV-2 infection.
Interestingly, ferrets (Mustela putorius furo) are susceptible to SARS-CoV-2 infection, but they develop only minor clinical signs even in the presence of mild histological lesions in the lower respiratory tract. In a study aimed at determining the effect of age in SARS-CoV-2-induced pathology and immunity, viral RNA was detected in extra respiratory tissues including the brain of both young and adult animals up to 5 dpi, but the production of infectious virus was not demonstrated81. Moreover, no evidence of neurological clinical signs was observed.
Conclusion and future perspectives
Currently available animal models for SARS-CoV-2 infection should be considered useful tools for understanding the long-term consequences of SARS-CoV-2 infection82 (Table 3). In particular, studies in hamster and mouse models have confirmed that some of the neurological manifestations of PCC share molecular mechanisms with neurodegenerative disease characterized by neuroinflammation and proteinopathy53,54,61. Promising results have also been obtained from long-term studies in NHPs, which share similar infection kinetics with humans. However, data on neurological signs in NHPs following SARS-CoV-2 infection are still scarce, and more studies are needed to determine the actual usefulness of this model69. On the other hand, ferrets seem not to be an adequate model for the study of the long-term consequences of SARS-CoV-2 infection, because they do not develop any clinical signs, and active viral replication has not been clearly demonstrated in lung samples81. Given that none of the models presented in this Perspective seems to comprehensively phenocopy PCC in humans, it is reasonable to foresee that multiple animal models will be necessary to unveil the several aspects of this multi-systemic condition.
Table 3.
List of potential PCC animal models
| Model | Similarities to human disease | Differences with the human disease | Neurological manifestations and laboratory findings | Technical considerations | References |
|---|---|---|---|---|---|
| GSH |
• Naturally susceptible to SARS-CoV-2 infection • Multi-organ damage • No evidence of neuroinvasion • Spontaneous viral clearance |
• Rapid humoral neutralizing immune response • Severe disease manifestations are limited by quick spontaneous viral clearance |
• Accumulation of hyper -phosphorylated tau and α-synuclein in cortical neurons • Transcriptomic perturbations in the CNS • Behavioral changes |
• Easy handling in the laboratory • Lack of hamster-specific research tools for the study of immune activation |
21,52–55,72,86 |
| Wild-type laboratory mice | • Susceptible to the Alpha, Beta, and Omicron SARS-CoV-2 variants with mild disease course | • Not susceptible to SARS-CoV-2 ancestral strain infection | • Neuroinflammation (observed with SARS-CoV-2 mouse adapted strain; studies limited to the acute phase) |
• Easy handling in the laboratory • Wide availability of research tools |
21,64,84–86 |
| K18-hACE2-tg mice | • Susceptible to SARS-CoV-2 infection (multiple variants tested) |
• Massive neuroinvasion and neuroinflammation • Lethal infection without viral clearance by 7 dpi |
• Nonsuppurative meningoencephalitis • Diffuse astrogliosis and microgliosis • Apathy, depression, trembling and seizures |
• Easy handling in the laboratory • Wide availability of research tools |
21,43,73,74,76,78 |
| hACE2-KI mice | • Susceptible to SARS-CoV-2 infection | • Minimal lung pathology |
• Low amounts of viral RNA occasionally detected in brain samples • Studies limited to the acute phase |
• Easy handling in the laboratory • Wide availability of research tools |
78,94 |
| AAV-hACE2 transduced mice (multiple strains) |
• Mild to moderate respiratory disease • No neuroinvasion |
• Targeted expression of hACE2 determines which organs and tissues are infected by SARS-CoV-2 • Transient hACE2 expression, dependent on the turnover rate of the transduced cells |
• Increased CSF levels of CCL11 • Increased microglial activation in the subcortical white matter • Decreased neurogenesis in the hippocampus • Myelin loss |
• Variability in results might depend on the AAV batch. • If used in different mouse strains (such as different humanized mice), the model allows for the study of specific pathways or immune cell types |
59,61 |
| NHPs |
• Naturally susceptible to SARS-CoV-2 infection • Neuroinflammation observed without evidence of neuroinvasion • Spontaneous viral clearance |
• Different NHP species display a heterogeneous spectrum of COVID-19 symptoms |
• Neuroinflammation • Brain hypoxia • Neuronal morphological changes in cerebellum and brainstem |
• Ethical limitations • Handling in the laboratory may be difficult depending on the species of choice |
68,69,80 |
AAV, adeno-associated virus.
When focusing on the neurological manifestations of PCC, it is important to consider that productive SARS-CoV-2 infection of the CNS might not be necessary for the development of cognitive and behavioral signs, because these manifestations might be triggered by inflammatory signals originating in other tissues. This is particularly relevant for K18-hACE2-tg mice, one of the most used small animal models for SARS-CoV-2 infection so far, which develop massive lethal neuroinvasion upon intranasal SARS-CoV-2 inoculation; this limitation has been overcome by the use of an aerosolized inoculum instead of direct intranasal inoculation73,74,76.
All studies published so far have analyzed the long-term consequences of SARS-CoV-2 infections in naïve animals using the ancestral strain. However, after the first wave of the COVID-19 pandemic, several variants of concern (VOCs) have appeared with different characteristics (such as transmissibility, replication abilities and lesion severity)83 that may influence the intensity and/or duration of possible PCC manifestations. In this regard, it is worth mentioning that the B.1.1.7 (Alpha), B.1.351 (Beta) and BA.1.1 (Omicron) variants can infect wild-type mice causing a mild disease without neurological signs during the acute phase84–86. This points to the possibility of developing mouse models to study the infection by specific VOCs of SARS-CoV-2, which would be extremely helpful for characterizing the long-term consequences of SARS-CoV-2 in people infected after the first wave of the pandemic. The use of such models might help to dissect the molecular pathways associated with different PCC symptoms when they are observed specifically with certain VOCs.
Moreover, the global COVID-19 vaccine rollout has reduced the percentage of the immunologically naïve population. An interesting point to consider is that PCC occurs also after vaccine breakthrough infections; whether PCC would then substantially differ from the condition observed in nonvaccinated naïve patients remains a question to be answered87. All these factors might limit the translatability of the currently available animal models to the entire affected population and call for the development of models exploring the influence of different vaccination status and VOCs on PCC; however, such models might require longer follow-up time and increase the complexity of the studies. Translating the neurological and cognitive symptoms experienced by patients with COVID-19 and PCC into objective and measurable outcomes for animal studies is a necessary step, which adds another level of complexity to the preclinical studies on PCC.
A practical aspect to be considered when carrying out long-term in vivo studies of SARS-CoV-2 infection is the requirement for biosafety level 3 animal facilities and adequately trained personnel. Pseudoviruses have been used to develop in vivo models of infection by highly pathogenic agents including SARS-CoV-2 (refs. 88–90), but to our knowledge, this technology has not been used yet to study the long-term effect of COVID-19. Pseudoviruses are a promising alternative to the use of infectious virus, which would also circumvent the biosafety level 3 requirements.
The heterogeneity of PCC manifestations observed in patients and the finding that PCC is apparently independent of the severity of the prior acute infection makes it unlikely that a single animal model will be able to fully recapitulate the human disease. This problem could be overcome by challenging the animals with a range of viral doses to induce a wider spectrum of clinical signs. In addition, animal models and humans do not always perfectly match in terms of genetic background, lifespan and aging processes, among other factors, which might affect responses to SARS-CoV-2. As already pointed out by other authors, a useful strategy to experimentally reproduce such variability would be to introduce systematic heterogeneity in the study design91.
Given that none of the currently available animal models seems to fully replicate PCC, it seems sensible to use multiple models to study different aspects of this condition. In particular, we think that GSHs are suitable for profiling neuroinflammation signature after SARS-CoV-2 inoculation, while mouse models would be the best tool to test sublethal doses of SARS-CoV-2 and less virulent VOCs as a mean to extend animal survival and observe the potential long-term effects of the infection.
Acknowledgements
The authors acknowledge the funding from the European Commission EPIVINF (HORIZON-HLTH-2021-DISEASE-04, no. 101057548) and CaixaHealth EPIVIRCO (HR22-00681) projects.
Author contributions
C.U., J.V.-A. and J.S. conceptualized the article; C.U. and L.M. performed the literature research; C.U. conceptualized the figures; C.U. wrote the draft of the manuscript; L.M., C.B., J.V.-A. and J.S. critically reviewed the manuscript. All authors contributed to and approved the final version of the manuscript.
Peer review
Peer review information
Lab Animal thanks Jacob Raber and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Júlia Vergara-Alert, Joaquim Segalés.
References
- 1.Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19 - 11 March 2020 (World Health Organization, 2020).
- 3.Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2020;19:141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore, J. P. & Klasse, P. J. COVID-19 vaccines: ‘warp speed’ needs mind melds, not warped minds. J. Virol. 94, e01083-20 (2020). [DOI] [PMC free article] [PubMed]
- 5.Gupta A, et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 6.Johnson KD, et al. Pulmonary and extra-pulmonary clinical manifestations of COVID-19. Front. Med. 2020;7:526. doi: 10.3389/fmed.2020.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song W-C, FitzGerald GA. COVID-19, microangiopathy, hemostatic activation, and complement. J. Clin. Invest. 2020;130:3950–3953. doi: 10.1172/JCI140183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stein SR, et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature. 2022;612:758–763. doi: 10.1038/s41586-022-05542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torge D, Bernardi S, Arcangeli M, Bianchi S. Histopathological features of SARS-CoV-2 in extrapulmonary organ infection: a systematic review of literature. Pathogens. 2022;11:867. doi: 10.3390/pathogens11080867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian M, et al. HIF-1α promotes SARS-CoV-2 infection and aggravates inflammatory responses to COVID-19. Signal Transduct. Target. Ther. 2021;6:308. doi: 10.1038/s41392-021-00726-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamming I, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, et al. Detection of SARS-CoV-2 in different types of clinical specimens. J. Am. Med. Assoc. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bwire GM, Majigo MV, Njiro BJ, Mawazo A. Detection profile of SARS-CoV-2 using RT-PCR in different types of clinical specimens: a systematic review and meta-analysis. J. Med. Virol. 2021;93:719–725. doi: 10.1002/jmv.26349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy-Vallejo E, et al. SARS-CoV-2 viremia precedes an IL6 response in severe COVID-19 patients: results of a longitudinal prospective cohort. Front. Med. 2022;9:855639. doi: 10.3389/fmed.2022.855639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Abramo A, et al. The first case of meningitis associated to SARS-Coronavirus-2 BA.2 variant infection with persistent viremia. Int. J. Infect. Dis. 2022;124:38–40. doi: 10.1016/j.ijid.2022.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantovani A, et al. Long Covid: where we stand and challenges ahead. Cell Death Differ. 2022;29:1891–1900. doi: 10.1038/s41418-022-01052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Oliveira JF, et al. Persistent symptoms, quality of life and risk factors in long COVID: a cross-sectional study of hospitalized patients in Brazil. Int. J. Infect. Dis. 2022;122:1044–1051. doi: 10.1016/j.ijid.2022.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wurz A, et al. ‘I feel like my body is broken’: exploring the experiences of people living with long COVID. Qual. Life Res. 2022;31:3339–3354. doi: 10.1007/s11136-022-03176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022;22:e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahmer T, et al. Severity, predictors and clinical correlates of post-COVID syndrome (PCS) in Germany: a prospective, multi-centre, population-based cohort study. EClinicalMedicine. 2022;51:101549. doi: 10.1016/j.eclinm.2022.101549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muñoz-Fontela C, et al. Animal models for COVID-19. Nature. 2020;586:509–515. doi: 10.1038/s41586-020-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen C, et al. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J. Infect. Dis. 2022;226:1593–1607. doi: 10.1093/infdis/jiac136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreno-Pérez O, et al. Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J. Infect. 2021;82:378–383. doi: 10.1016/j.jinf.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang C, et al. 6-Month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2023;401:e21–e33. doi: 10.1016/S0140-6736(23)00810-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballering AV, van Zon SKR, olde Hartman TC, Rosmalen JGM. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet. 2022;400:452–461. doi: 10.1016/S0140-6736(22)01214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perlis RH, et al. Prevalence and correlates of Long COVID symptoms among US adults. JAMA Netw. Open. 2022;5:e2238804. doi: 10.1001/jamanetworkopen.2022.38804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lam MH-B, et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch. Intern. Med. 2009;169:2142–2147. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- 28.Lee SH, et al. Depression as a mediator of chronic fatigue and post-traumatic stress symptoms in Middle East respiratory syndrome survivors. Psychiatry Investig. 2019;16:59–64. doi: 10.30773/pi.2018.10.22.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nalbandian A, et al. Post-acute COVID-19 syndrome. Nat. Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blomberg B, et al. Long COVID in a prospective cohort of home-isolated patients. Nat. Med. 2021;27:1607–1613. doi: 10.1038/s41591-021-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sudre CH, et al. Attributes and predictors of long COVID. Nat. Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fumagalli C, et al. Factors associated with persistence of symptoms 1 year after COVID-19: a longitudinal, prospective phone-based interview follow-up cohort study. Eur. J. Intern. Med. 2022;97:36–41. doi: 10.1016/j.ejim.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dorjee K, Kim H, Bonomo E, Dolma R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: A comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PLoS ONE. 2020;15:e0243191. doi: 10.1371/journal.pone.0243191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehandru S, Merad M. Pathological sequelae of long-haul COVID. Nat. Immunol. 2022;23:194–202. doi: 10.1038/s41590-021-01104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramakrishnan RK, Kashour T, Hamid Q, Halwani R, Tleyjeh IM. Unraveling the mystery surrounding post-acute sequelae of COVID-19. Front. Immunol. 2021;12:686029. doi: 10.3389/fimmu.2021.686029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gang J, Wang H, Xue X, Zhang S. Microbiota and COVID-19: long-term and complex influencing factors. Front. Microbiol. 2022;13:963488. doi: 10.3389/fmicb.2022.963488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inoue S, et al. Post-intensive care syndrome: its pathophysiology, prevention, and future directions. Acute Med. Surg. 2019;6:233–246. doi: 10.1002/ams2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID-19. Science. 2022;375:1122–1127. doi: 10.1126/science.abm8108. [DOI] [PubMed] [Google Scholar]
- 39.Xu E, Xie Y, Al-Aly Z. Long-term neurologic outcomes of COVID-19. Nat. Med. 2022;28:2406–2415. doi: 10.1038/s41591-022-02001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asadi-Pooya AA, et al. Long COVID syndrome-associated brain fog. J. Med. Virol. 2022;94:979–984. doi: 10.1002/jmv.27404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao, V. et al. in Advances in Cancer Research (eds Gewirtz, D. A. & Fisher, P. B.) vol. 155 29–76 (Academic Press, 2022).
- 42.Van Dyk K, Ganz PA. The inflammation complication: new evidence in cancer-related cognitive impairment. Brain. Behav. Immun. 2019;81:6–7. doi: 10.1016/j.bbi.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 43.Song E, et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021;218:e20202135. doi: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen R, et al. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in the human and mouse brains. Front. Neurol. 2020;11:573095. doi: 10.3389/fneur.2020.573095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rhea EM, et al. The S1 protein of SARS-CoV-2 crosses the blood–brain barrier in mice. Nat. Neurosci. 2021;24:368–378. doi: 10.1038/s41593-020-00771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al Saiegh F, et al. Status of SARS-CoV-2 in cerebrospinal fluid of patients with COVID-19 and stroke. J. Neurol. Neurosurg. Psychiatry. 2020;91:846–848. doi: 10.1136/jnnp-2020-323522. [DOI] [PubMed] [Google Scholar]
- 47.Benameur K, et al. Encephalopathy and encephalitis associated with cerebrospinal fluid cytokine alterations and coronavirus disease, Atlanta, Georgia, USA, 2020. Emerg. Infect. Dis. 2020;26:2016–2021. doi: 10.3201/eid2609.202122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang YH, Jiang D, Huang JT. SARS-CoV-2 detected in cerebrospinal fluid by PCR in a case of COVID-19 encephalitis. Brain. Behav. Immun. 2020;87:149. doi: 10.1016/j.bbi.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang AC, et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature. 2021;595:565–571. doi: 10.1038/s41586-021-03710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reiken S, et al. Alzheimer’s-like signaling in brains of COVID-19 patients. Alzheimers Dement. J. Alzheimers Assoc. 2022;18:955–965. doi: 10.1002/alz.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kay LM. COVID-19 and olfactory dysfunction: a looming wave of dementia? J. Neurophysiol. 2022;128:436–444. doi: 10.1152/jn.00255.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan JFW, et al. Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19) in a Golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin. Infect. Dis. 2020;71:2428–2446. doi: 10.1093/cid/ciaa644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song Z, et al. SARS-CoV-2 causes a systemically multiple organs damages and dissemination in hamsters. Front. Microbiol. 2021;11:618891. doi: 10.3389/fmicb.2020.618891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Käufer C, et al. Microgliosis and neuronal proteinopathy in brain persist beyond viral clearance in SARS-CoV-2 hamster model. EBioMedicine. 2022;79:103999–103999. doi: 10.1016/j.ebiom.2022.103999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frere JJ, et al. SARS-CoV-2 infection in hamsters and humans results in lasting and unique systemic perturbations post recovery. Sci. Transl Med. 2022;14:eabq3059. doi: 10.1126/scitranslmed.abq3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hellweg R, Zueger M, Fink K, Hörtnagl H, Gass P. Olfactory bulbectomy in mice leads to increased BDNF levels and decreased serotonin turnover in depression-related brain areas. Neurobiol. Dis. 2007;25:1–7. doi: 10.1016/j.nbd.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 57.Douaud G, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604:697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Serafini RA, et al. SARS-CoV-2 airway infection results in the development of somatosensory abnormalities in a hamster model. Sci. Signal. 2023;16:eade4984. doi: 10.1126/scisignal.ade4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sefik E, et al. A humanized mouse model of chronic COVID-19. Nat. Biotechnol. 2021;40:906–920. doi: 10.1038/s41587-021-01155-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brumeanu T-D, et al. Human-Immune-System (HIS) humanized mouse model (DRAGA: HLA-A2.HLA-DR4.Rag1KO.IL-2RγcKO.NOD) for COVID-19. Hum. Vaccines Immunother. 2022;18:2048622. doi: 10.1080/21645515.2022.2048622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fernández-Castañeda A, et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell. 2022;185:2452–2468.e16. doi: 10.1016/j.cell.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gu H, et al. Adaptation of SARS-CoV-2 in BALB/c mice for testing vaccine efficacy. Science. 2020;369:1603–1607. doi: 10.1126/science.abc4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leist SR, et al. A mouse-adapted SARS-CoV-2 induces acute lung injury and mortality in standard laboratory mice. Cell. 2020;183:1070–1085.e12. doi: 10.1016/j.cell.2020.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amruta N, et al. Mouse adapted SARS-CoV-2 (MA10) viral infection induces neuroinflammation in standard laboratory mice. Viruses. 2022;15:114. doi: 10.3390/v15010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buffalo EA, Movshon JA, Wurtz RH. From basic brain research to treating human brain disorders. Proc. Natl Acad. Sci. USA. 2019;116:26167–26172. doi: 10.1073/pnas.1919895116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Estes JD, Wong SW, Brenchley JM. Nonhuman primate models of human viral infections. Nat. Rev. Immunol. 2018;18:390–404. doi: 10.1038/s41577-018-0005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller LA, Royer CM, Pinkerton KE, Schelegle ES. Nonhuman primate models of respiratory disease: past, present, and future. ILAR J. 2017;58:269–280. doi: 10.1093/ilar/ilx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Böszörményi KP, et al. The post-acute phase of SARS-CoV-2 infection in two macaque species is associated with signs of ongoing virus replication and pathology in pulmonary and extrapulmonary tissues. Viruses. 2021;13:1673. doi: 10.3390/v13081673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rutkai I, et al. Neuropathology and virus in brain of SARS-CoV-2 infected non-human primates. Nat. Commun. 2022;13:1745–1745. doi: 10.1038/s41467-022-29440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Østergaard L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: Consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol. Rep. 2021;9:e14726. doi: 10.14814/phy2.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brouqui P, et al. Asymptomatic hypoxia in COVID-19 is associated with poor outcome. Int. J. Infect. Dis. 2021;102:233–238. doi: 10.1016/j.ijid.2020.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Melo GD, et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci. Transl. Med. 2021;13:eabf8396. doi: 10.1126/scitranslmed.abf8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moreau GB, et al. Evaluation of K18- hACE2 mice as a model of SARS-CoV-2 infection. Am. J. Trop. Med. Hyg. 2020;103:1215–1219. doi: 10.4269/ajtmh.20-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vidal E, et al. Chronological brain lesions after SARS-CoV-2 infection in hACE2-transgenic mice. Vet. Pathol. 2022;59:613–626. doi: 10.1177/03009858211066841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Villadiego J, et al. Full protection from SARS-CoV-2 brain infection and damage in susceptible transgenic mice conferred by MVA-CoV2-S vaccine candidate. Nat. Neurosci. 2023;26:226–238. doi: 10.1038/s41593-022-01242-y. [DOI] [PubMed] [Google Scholar]
- 76.Fumagalli V, et al. Administration of aerosolized SARS-CoV-2 to K18-hACE2 mice uncouples respiratory infection from fatal neuroinvasion. Sci. Immunol. 2022;7:eabl9929. doi: 10.1126/sciimmunol.abl9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seehusen F, et al. Neuroinvasion and neurotropism by SARS-CoV-2 variants in the K18-hACE2 mouse. Viruses. 2022;14:1020. doi: 10.3390/v14051020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dong W, et al. The K18-Human ACE2 transgenic mouse model recapitulates non-severe and severe COVID-19 in response to an infectious dose of the SARS-CoV-2 virus. J. Virol. 2022;96:e00964–21. doi: 10.1128/JVI.00964-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Winkler ES, et al. SARS-CoV-2 causes lung infection without severe disease in human ACE2 knock-in mice. J. Virol. 2022;96:e0151121. doi: 10.1128/JVI.01511-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng H, et al. Virulence and pathogenesis of SARS-CoV-2 infection in rhesus macaques: a nonhuman primate model of COVID-19 progression. PLoS Pathog. 2020;16:e1008949. doi: 10.1371/journal.ppat.1008949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van de Ven K, et al. Pathology and immunity after SARS-CoV-2 infection in male ferrets is affected by age and inoculation route. Front. Immunol. 2021;12:750229. doi: 10.3389/fimmu.2021.750229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jansen EB, et al. After the virus has cleared-Can preclinical models be employed for Long COVID research? PLoS Pathog. 2022;18:e1010741. doi: 10.1371/journal.ppat.1010741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mahilkar S, et al. SARS-CoV-2 variants: impact on biological and clinical outcome. Front. Med. 2022;9:409–424. doi: 10.3389/fmed.2022.995960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shuai H, et al. Emerging SARS-CoV-2 variants expand species tropism to murines. eBioMedicine. 2021;73:103643. doi: 10.1016/j.ebiom.2021.103643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tarrés-Freixas F, et al. Heterogeneous infectivity and pathogenesis of SARS-CoV-2 variants beta, delta and omicron in transgenic K18-hACE2 and wildtype mice. Front. Microbiol. 2022;13:840757. doi: 10.3389/fmicb.2022.840757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Halfmann PJ, et al. SARS-CoV-2 Omicron virus causes attenuated disease in mice and hamsters. Nature. 2022;603:687–692. doi: 10.1038/s41586-022-04441-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 2022;28:1461–1467. doi: 10.1038/s41591-022-01840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tseng S-H, et al. A novel pseudovirus‐based mouse model of SARS-CoV-2 infection to test COVID-19 interventions. J. Biomed. Sci. 2021;28:34. doi: 10.1186/s12929-021-00729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nie J, et al. Nipah pseudovirus system enables evaluation of vaccines in vitro and in vivo using non-BSL-4 facilities. Emerg. Microbes Infect. 2019;8:272–281. doi: 10.1080/22221751.2019.1571871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Q, Tang K, Zhang X, Chen P, Guo Y. Establishment of pseudovirus infection mouse models for in vivo pharmacodynamics evaluation of filovirus entry inhibitors. Acta Pharm. Sin. B. 2018;8:200–208. doi: 10.1016/j.apsb.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Voelkl B, et al. Reproducibility of animal research in light of biological variation. Nat. Rev. Neurosci. 2020;21:384–393. doi: 10.1038/s41583-020-0313-3. [DOI] [PubMed] [Google Scholar]
- 92.Su M, Nizamutdinov D, Liu H, Huang JH. Recent mechanisms of neurodegeneration and photobiomodulation in the context of Alzheimer’s disease. Int. J. Mol. Sci. 2023;24:9272. doi: 10.3390/ijms24119272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021;397:2284–2303. doi: 10.1016/S0140-6736(21)00218-X. [DOI] [PubMed] [Google Scholar]
- 94.Zhou B, et al. SARS-CoV-2 spike D614G change enhances replication and transmission. Nature. 2021;592:122–127. doi: 10.1038/s41586-021-03361-1. [DOI] [PubMed] [Google Scholar]
- 95.Alene M, et al. Serial interval and incubation period of COVID-19: a systematic review and meta-analysis. BMC Infect. Dis. 2021;21:257. doi: 10.1186/s12879-021-05950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.He F, Deng Y, Li W. Coronavirus disease 2019: what we know? J. Med. Virol. 2020;92:719–725. doi: 10.1002/jmv.25766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]