Abstract
Purpose
This study aims to develop and evaluate a cost-effective, user-friendly multiplex quantitative real-time polymerase chain reaction (qPCR) method for detecting multiple tick-borne pathogens associated with human and veterinary diseases.
Methods
In silico PCR was performed to design and evaluate primer sequences reported for amplifying Rickettsia spp., Borrelia spp., and Ehrlichia spp. Single and multiplex qPCR assays were then standardized to detect individual pathogens and multiple pathogens in a single reaction. Positive controls were generated to determine the dynamic range of the methods. In the validation phase, a total of 800 samples were screened for the presence of tick-borne pathogens.
Results
Identification in a single qPCR reaction (multiplex) of Ehrlichia spp., and Borrelia spp. with a limit of detection of 10 copies and Rickettsia spp. with 100 copies, a PCR efficiency (E) of 90–100% and a coefficient of correlation (R2) of 0.998–0.996 for all pathogens.
Conclusion
The ability to detect three significant pathogens (Ehrlichia spp., Rickettsia spp., and Borrelia spp.) in a single qPCR reaction offers a significant advantage in the field of molecular diagnostics for tick-borne diseases. This advancement has a profound impact on public health as it facilitates the selection of appropriate treatment protocols, thereby reducing complications associated with disease progression. The streamlined approach provided by this method simplifies the diagnostic process and enables timely intervention, ultimately improving patient outcomes and mitigating the potential risks associated with untreated or misdiagnosed tick-borne infections.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11686-023-00702-0.
Keywords: Tick-borne pathogens, Multiplex PCR, SYBR Green, PCR efficiency, Diagnostics
Ticks, belonging to two primary families, namely Argasidae (soft ticks) and Ixodidae (hard ticks), are ectoparasites that infest a wide range of terrestrial vertebrates, including certain amphibians and birds [1]. With approximately 867 tick species identified globally, spanning regions such as Asia [2], the Caribbean [3], Europe [4], Africa [5], South America [6], and Australia [7], nearly 10% of these species serve as vectors for an extensive array of pathogens. These pathogens are responsible for a diverse spectrum of diseases affecting both domestic animals and humans [8]. In the United States, tick-borne diseases exhibit a significant prevalence, with approximately 50,865 reported cases annually [9]. Among the bacterial diseases transmitted by ticks, the Borreliaceae, Ehrlichiaceae, and Rickettsiaceae families are of utmost concern [10]. Borrelia burgdorferi infection in humans is the leading cause of Lyme disease, however, in some cases, it has been associated with Borrelia mayonii and Borrelia miyamotoi, which are also found in black-legged tick (Ixodes scapularis) [11, 12]. Ehrlichiosis is a bacterial infection in humans caused by various species of Ehrlichia especially E. chaffeensis, E. ewingii and Anaplasma (formerly Ehrlichia) phagocytophilum which are primarily transmitted through the bite of infected ticks, such as the lone star tick (Amblyomma americanum) [13]. Different diseases related to the infection with rickettsia such as Rocky Mountain spotted fever (RMSF) are transmitted by the American dog tick (Dermacentor variabilis), Rocky Mountain wood tick (Dermacentor andersoni), and the brown dog tick (Rhipicephalus sanguineous) in the U.S [14]. Various diagnostic tools are available for diagnosing tick-borne diseases. These include serological tests such as ELISA, Western blot, and Indirect fluorescent antibody (IFA). It is important to note that these methods may exhibit limitations such as low sensitivity, potential cross-reactivity, or longer turnaround time, as seen in the case of bacterial culture. [15]. In contrast, molecular methods offer enhanced specificity, sensitivity, and rapidity for diagnostic test; in this sense, specific primers are generated to analyze conserved regions of DNA in genes like 16S rRNA and ompA of the target pathogen [16]. In this study, we have devised a novel approach using quantitative PCR (qPCR) to detect Borrelia, Ehrlichia, and Rickettsia pathogens by amplifying and detecting specific conserved regions of each organism. The development of a user-friendly diagnostic tool is imperative for strengthening disease prevention and control programs, as well as for providing precise and timely diagnosis crucial for effective treatment. For this purpose, we carefully selected genes and primer sequences that exhibit a close association with the respective bacterial families of concern. These primers were meticulously chosen and subjected to rigorous testing, focusing on conserved regions of the 16S rRNA gene for Ehrlichia spp. and Borrelia spp., and the ompA gene for Rickettsia spp. (See Table 1).
Table 1.
General characteristics of the primers designed to amplify each target
| Species | Target gene | Sequence of primers (5′-3′) * | Amplicon size (bp) | Reference |
|---|---|---|---|---|
| Borrelia spp. | 16S rRNA |
F-GCTGTAAACGATGCACACTTGGT R-GGCGGCACACTTAACACGTTAG |
69 | [28] |
| Ehrlichia spp. | 16S rRNA |
F-TCGCTATTAGATGAGCCTACGT R-GAGTCTGGACCGTATCTCAGT |
124 | [29] |
| Rickettsia spp. | OmpA |
F-GGAGCGAATGCTGCACTAAT R-GTTCCGTTAATGGCAGCATCT |
116 | [23] |
*All gene-specific primers synthetized and provided by T4 OLIGO® (Irapuato, Mex)
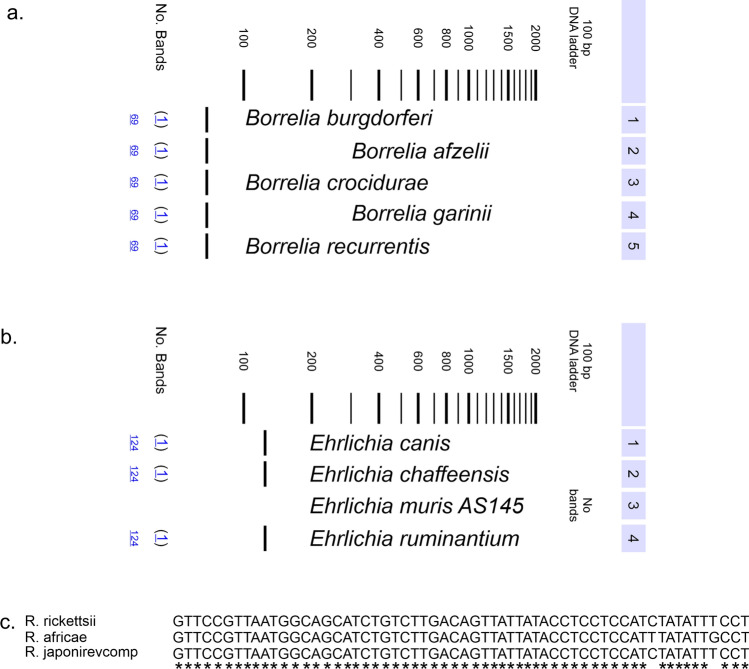
To confirm the cross-species amplification potential of selected primers, sequence homology analysis was performed using the Multiple Sequence Comparison by Log-Expectation (Muscle) tool from European Molecular Biology Laboratory (EMBL) and sequences from National Center for Biotechnology Information (NCBI) obtained via Basic Local Alignment Search Tool (BLAST) (Fig 1).
Fig. 1.
In silico analysis of primer sequences. Example of the in silico qPCR outcome format for a couple of primers targeting A Borrelia spp. B Ehrlichia. and C Rickettsia spp. genome sequences. Example of the in silico alignments of genome sequences in a conserved region of D Borrelia spp. E Ehrlichia spp. Using MUSCLE and F Rickettsia spp. Using BLAST
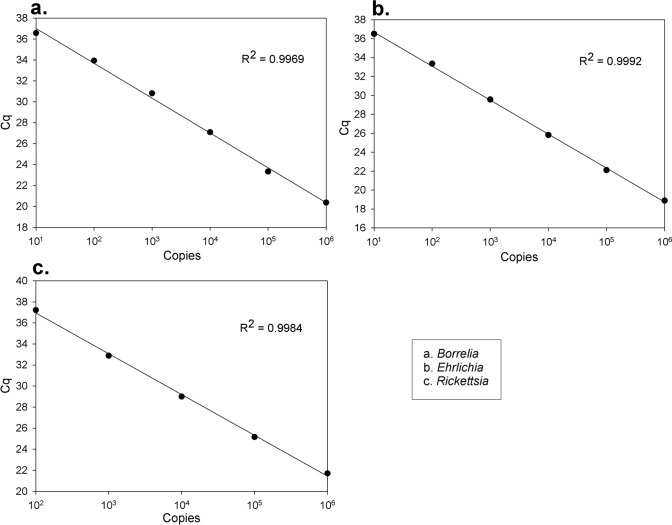
Positive qPCR controls for each target fragment were generated by cloning them into the pGEM-T Easy vector (Promega-Madison, U.S.A) and validated through sequencing. The detection limit [14] analysis involved constructing a standard curve comprising seven known concentration standards (STD) achieved through 1:10 serial dilution from the most concentrated STD (1,000,000 copies) to the most diluted STD (10 copies and one copy). The efficiency of the qPCR (E) and coefficient of correlation (R2) closely approached the desired values for a limit of detection of 10 copies STD, with E = 90.33% and R2 = 0.998 for Ehrlichia spp, E = 99.73% and R2 = 0.995 for Borrelia spp, and E = 90% and R2 = 0.99 for Rickettsia spp. (Fig2). Co-infection controls were generated by all the combinations possible of positive controls and following the same parameters of STD preparation and evaluation the 1000 copies. STD results were E = 112 to 75.3% and R2 > 0.9 (see supplementary Information S1).
Fig. 2.
The detection limit of the target fragments by qPCR was analyzed for Borrelia spp. A, Ehrlichia spp. B and Rickettsia spp. C. The standard curves were analyzed using the SigmaPlot 11.0 software; the amplification efficiency was 90–100%, while the R2 was 0.99 on average for all the pathogens
To validate the multiplex qPCR method developed, 800 samples of DNA isolated from blood extracted from dogs (n = 400) and ticks (n = 400) were screened (Table 2). The sample status (positive or negative) was unknown prior to this test (see supplementary information S2).
Table 2.
Summary of tick-borne pathogens screening in blood from dogs and tick samples
| Pathogen | Positive DNA samples | Negative DNA samples | Samples screened | |||
|---|---|---|---|---|---|---|
| Dog (n = 61) | Tick (n = 254) | Dog (n = 339) | Tick (n = 146) | Dog (n = 400) | Tick (n = 400) | |
| Borrelia, n (%) | 14 (5) | 21 (6) | 286 (95) | 329 (94) | 300 (75) | 350 (88) |
| Ehrlichia, n (%) | 28 (7) | 226 (57) | 372 (93) | 174 (43) | 400 (100) | 400 (100) |
| Rickettsia, n (%) | 19 (6) | 9 (3) | 281 (94) | 291 (97) | 300 (75) | 300 (75) |
The Ehrlichiaceae family exhibited the highest prevalence among the analyzed samples, with 57% (226) in tick samples and 7% (28) in dog samples. Screening the samples using qPCR revealed that 5% of the tested samples were positive for at least one pathogen. Since these pathogens can co-infect the same host, it was necessary to create “co-infected” samples by mixing positive samples of different pathogens and standardize the qPCR to distinguish between them based on their characteristic signals. Notably, we successfully detected the “co-infection” of Rickettsia spp and Borrelia spp, making this assay the first multiplex SYBR-based test for detecting two out of three major tick-borne pathogens of human concern (refer to supplementary information S3). Details on the selection of study groups, bioethics, sampling, sample transportation, and DNA extraction can be found in supplementary information S2.
Collection and distribution of the blood samples obtained (dogs and ticks) are shown in Fig. 3.
Fig. 3.
The 800 samples per collection center distribution shows that 50% of the samples were obtained in Mexican private clinics from Nuevo León State. The lowest proportion of samples was obtained in municipal kennels; the lowest percentage was Escobedo
Compared to other detection methods like TaqMan assays, our Ehrlichia spp detection sensitivity of ten copies was considerably good. TaqMan assays typically report detection limits of 5–50 copies [17–19]. Similarly, our Borrelia spp pathogen detection limit of ten copies aligns with the results of Saidac et al., who combined SYBR and TaqMan detection to achieve a quantification limit of 1 copy due to the low spirochete count [20]. Shan et al. achieved a detection limit of up to six copies in human DNA samples using TaqMan assays [21]. Our Rickettsia genus detection limits were tenfold below those of other studies, which detected at least five copies of the pathogen. Differences in assay type or amplicon size used in various studies may account for this variation [22, 23]. Our assay is less specific than the multiplex TaqMan assays which detect a lower number of copies, however, it remains having sensitivity and specificity above the gold standard ELISA or IFA technique, as the specificity of the proposed method remains above 99% for the pathogens evaluated [24, 25].
The intentional co-infection detected in this study involved mixing positive samples of Rickettsia and Borrelia genera in a single qPCR reaction. While no previous reports exist on this specific co-infection and its impact on the host, there have been reports of co-infections involving different Rickettsia spp., which can influence the transmissibility and disease dynamics caused by these pathogens [26]. Given the close phylogenetic relationship observed between Ehrlichia chaffeensis and Anaplasma phagocytophilum, our Ehrlichia primer set exhibits potential utility in a diagnostic PCR test for Ehrlichiosis caused by either of these two pathogens. In the validation results, Ehrlichia showed a higher prevalence compared to the other two pathogens in both tick and dog samples. This can be attributed to the strong affinity between E. canis and dogs. Notably, samples obtained from kennels yielded a higher number of positive samples, possibly due to the ease of transmission and the compromised health conditions typically found in such environments. While Rickettsia and Borrelia species were detected in smaller numbers, it is crucial to recognize that they remain significant health concerns, causing subclinical symptoms in infected dogs and in humans, these pathogens are responsible for several conditions including Rocky Mountain spotted fever and Lyme disease [4].
In this study, we have successfully developed the first effective and easy-to-use diagnostic tool using SYBR-based PCR technology, which despite being less specific, is still very sensitive and less expensive than TaqMan technology [27]. This tool enables the detection of three tick-borne pathogen families that are of significant clinical importance. By targeting conserved regions of the 16S rRNA and ompA genes, which are crucial in understanding the pathologies affecting both animals and humans, we have achieved the identification of multiple pathogens through a multiplex qPCR reaction. These findings serve as a foundation for future analyses, aiming to expand the scope of targets within the same diagnostic tool. The streamlined approach provided by this method simplifies the diagnostic process and facilitates timely intervention. By promptly identifying tick-borne infections, we can improve patient outcomes and mitigate the potential risks associated with untreated or misdiagnosed cases. The development of this diagnostic tool represents a significant advancement in the field, and we are optimistic about its potential to enhance diagnostic accuracy and improve patient care.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contributions
Conceived or designed study, MLMF, SACC, and MECL; performed research, MLMF, SACC., IAM, JLA; analyzed data, MECL., IGV., SVR., IPRS.; wrote the paper, SACC., MECL., FEMM, MLMF. All authors have read and agreed to the published version of the manuscript.
Funding
No external funding sources were applied. The edition and publishing costs were covered by Molecular Medicine Laboratory from Academic Unit of Human Medicine and Health Sciences, Universidad Autónoma de Zacatecas Francisco García Salinas.
Data availability
All data supporting the reported results are included in the manuscript. Additional information regarding data that support the findings of this study will be available from the corresponding author upon reasonable request.
Declarations
Conflict of Interest
The authors declare that they have no competing interests.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Footnotes
The original online version of this article was revised: The original version of this article, published on 02 August 2023, unfortunately contained a mistake.
In this article ref. 28 was incorrect and should have been “Tsao JI, Wootton T, Bunikis J, Luna MG, Fish D, Barbour AG (2004) An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. PNAS 101(52):18159–18164. 10.1073/pnas.0405763102”.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sergio Andres Cardenas-Cadena and Maria Eugenia Castañeda-Lopez have contributed equally to this work.
Change history
3/21/2024
A Correction to this paper has been published: 10.1007/s11686-023-00785-9
References
- 1.Llòria I, Llàcer MT. Garrapatas parásitos animals. Farmacia Prof. 2002;16(5):73–77. [Google Scholar]
- 2.Ikadai H, Tanaka H, Shibahara N, Matsuu A, Uechi M, Itoh N, Oshiro S, Kudo N, Igarashi I, Oyamada T. Molecular evidence of infections with Babesia gibsoni parasites in Japan and evaluation of the diagnostic potential of a loop-mediated isothermal amplification method. J Clin Microbiol. 2004;42(6):2465–2469. doi: 10.1128/JCM.42.6.2465-2469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly PJ, Xu C, Lucas H, Loftis A, Abete J, Zeoli F, Stevens A, Jaegersen K, Ackerson K, Gessner A, Kaltenboeck B, Wang C. Ehrlichiosis, babesiosis, anaplasmosis and hepatozoonosis in dogs from St. Kitts. West Indies PloS one. 2013;8(1):e53450. doi: 10.1371/journal.pone.0053450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw SE, Day MJ, Birtles RJ, Breitschwerdt EB. Tick-borne infectious diseases of dogs. Trends Parasitol. 2001;17(2):74–80. doi: 10.1016/s1471-4922(00)01856-0. [DOI] [PubMed] [Google Scholar]
- 5.Williams BM, Berentsen A, Shock BC, Teixiera M, Dunbar MR, Becker MS, Yabsley MJ. Prevalence and diversity of Babesia, Hepatozoon, Ehrlichia, and Bartonella in wild and domestic carnivores from Zambia. Africa Parasitol Res. 2014;113(3):911–918. doi: 10.1007/s00436-013-3722-7. [DOI] [PubMed] [Google Scholar]
- 6.Dantas-Torres F, Chomel BB, Otranto D. Ticks and tick-borne diseases: a one health perspective. Trends Parasitol. 2012;28(10):437–446. doi: 10.1016/j.pt.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Hii SF, Kopp SR, Thompson MF, O'Leary CA, Rees RL, Traub RJ. Canine vector-borne disease pathogens in dogs from south-east queensland and north-east northern territory. Aust Vet J. 2012;90(4):130–135. doi: 10.1111/j.1751-0813.2012.00898.x. [DOI] [PubMed] [Google Scholar]
- 8.Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129:S3–14. doi: 10.1017/s0031182004005967. [DOI] [PubMed] [Google Scholar]
- 9.Prevention CfDCa (2021) Tickborne Disease Surveillance Data Summary. U.S. Department of Health & Human Services. https://www.cdc.gov/ticks/data-summary/index.html. Accessed 11 April 2022
- 10.Nicholson WL, Allen KE, McQuiston JH, Breitschwerdt EB, Little SE. The increasing recognition of rickettsial pathogens in dogs and people. Trends Parasitol. 2010;26(4):205–212. doi: 10.1016/j.pt.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Krause PJ, Fish D, Narasimhan S, Barbour AG. Borrelia miyamotoi infection in nature and in humans. Clinical Microbiol Infect. 2015;21(7):631–639. doi: 10.1016/j.cmi.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dolan MC, Breuner NE, Hojgaard A, Boegler KA, Hoxmeier JC, Replogle AJ, Eisen L. Transmission of the lyme disease spirochete borrelia mayonii in relation to duration of attachment by nymphal ixodes scapularis (Acari: Ixodidae) J Med Entomol. 2017;54(5):1360–1364. doi: 10.1093/jme/tjx089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganguly S, Mukhopadhayay SK. Tick-borne ehrlichiosis infection in human beings. J Vector Borne Dis. 2008;45(4):273–280. [PubMed] [Google Scholar]
- 14.Breitschwerdt EB, Hegarty BC, Maggi RG, Lantos PM, Aslett DM, Bradley JM. Rickettsia rickettsii transmission by a lone star tick North Carolina. Emerg Infect Dis. 2011;17(5):873–875. doi: 10.3201/eid1705.101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melnicakova J, Derdakova M, Barak I. A system to simultaneously detect tick-borne pathogens based on the variability of the 16S ribosomal genes. Parasit Vectors. 2013;6:269. doi: 10.1186/1756-3305-6-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brouqui P, Bacellar F, Baranton G, Birtles RJ, Bjoersdorff A, Blanco JR, Caruso G, Cinco M, Fournier PE, Francavilla E, Jensenius M, Kazar J, Laferl H, Lakos A, Lotric Furlan S, Maurin M, Oteo JA, Parola P, Perez-Eid C, Peter O, Postic D, Raoult D, Tellez A, Tselentis Y, Wilske B, Escmid Study Group on Coxiella, European Network for Surveillance of Tick-Borne D Guidelines for the diagnosis of tick-borne bacterial diseases in Europe. Clin Microbiol Infect. 2004;10(12):1108–1132. doi: 10.1111/j.1469-0691.2004.01019.x. [DOI] [PubMed] [Google Scholar]
- 17.Allerdice MEJ, Pritt BS, Sloan LM, Paddock CD, Karpathy SE. A real-time PCR assay for detection of the Ehrlichia muris-like agent, a newly recognized pathogen of humans in the upper Midwestern United States. Ticks Tick-Borne Dis. 2016;7(1):146–149. doi: 10.1016/j.ttbdis.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doyle CK, Labruna MB, Breitschwerdt EB, Tang YW, Corstvet RE, Hegarty BC, Bloch KC, Li P, Walker DH, McBride JW. Detection of medically important Ehrlichia by quantitative multicolor TaqMan real-time polymerase chain reaction of the dsb gene. J Mol Diagn. 2005;7(4):504–510. doi: 10.1016/S1525-1578(10)60581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peleg O, Baneth G, Eyal O, Inbar J, Harrus S. Multiplex real-time qPCR for the detection of Ehrlichia canis and Babesia canis vogeli. Vet Parasitol. 2010;173(3–4):292–299. doi: 10.1016/j.vetpar.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 20.Saidac DS, Marras SA, Parveen N. Detection and quantification of Lyme spirochetes using sensitive and specific molecular beacon probes. BMC Microbiol. 2009;9:43. doi: 10.1186/1471-2180-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shan J, Jia Y, Teulieres L, Patel F, Clokie MRJ. Targeting multicopy prophage genes for the Increased detection of Borrelia burgdorferi Sensu Lato (s.l.), the causative agents of lyme disease, in blood. Front Microbiol. 2021;12:651217. doi: 10.3389/fmicb.2021.651217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato CY, Chung IH, Robinson LK, Austin AL, Dasch GA, Massung RF. Assessment of real-time PCR assay for detection of Rickettsia spp. and Rickettsia rickettsii in banked clinical samples. J Clin Microbiol. 2013;51(1):314–317. doi: 10.1128/JCM.01723-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eremeeva ME, Dasch GA, Silverman DJ. Evaluation of a PCR assay for quantitation of Rickettsia rickettsii and closely related spotted fever group rickettsiae. J Clin Microbiol. 2003;41(12):5466–5472. doi: 10.1128/JCM.41.12.5466-5472.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maggi RG, Birkenheuer AJ, Hegarty BC, Bradley JM, Levy MG, Breitschwerdt EB. Comparison of serological and molecular panels for diagnosis of vector-borne diseases in dogs. Parasit Vectors. 2014;7:127. doi: 10.1186/1756-3305-7-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schotthoefer AM, Meece JK, Ivacic LC, Bertz PD, Zhang K, Weiler T, Uphoff TS, Fritsche TR. Comparison of a real-time PCR method with serology and blood smear analysis for diagnosis of human anaplasmosis: importance of infection time course for optimal test utilization. J Clin Microbiol. 2013;51(7):2147–2153. doi: 10.1128/JCM.00347-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jönsson J (2016) Identification of the tick-borne pathogens Anaplasma phagocytophilum, Neoehrlichia mikurensis and Rickettsia in Swedish ticks: investigation of transovarial transmission and co-infection.
- 27.Tajadini M, Panjehpour M, Javanmard SH. Comparison of SYBR Green and TaqMan methods in quantitative real-time polymerase chain reaction analysis of four adenosine receptor subtypes. Adv Biomed Res. 2014;3:85. doi: 10.4103/2277-9175.127998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsao JI, Wootton T, Bunikis J, Luna MG, Fish D, Barbour AG. An ecological approach to preventing human infection: Vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. PNAS. 2004;101(52):18159–18164. doi: 10.1073/pnas.0405763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudoler N, Baneth G, Eyal O, van Straten M, Harrus S. Evaluation of an attenuated strain of Ehrlichia canis as a vaccine for canine monocytic ehrlichiosis. Vaccine. 2012;31(1):226–233. doi: 10.1016/j.vaccine.2012.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the reported results are included in the manuscript. Additional information regarding data that support the findings of this study will be available from the corresponding author upon reasonable request.