Abstract
Fascioliasis causes high economic losses in livestock and underlies public health problems in rural areas, mainly of low-income countries. The increasing animal infection rates in Bangladesh were assessed, by focusing on host species, different parts of the country, and rDNA sequences. Fasciolid flukes were collected from buffaloes, cattle, goats and sheep from many localities to assess prevalences and intensities of infection. The nuclear rDNA internal transcribed spacer (ITS) region including ITS-1 and ITS-2 spacers was analyzed by direct sequencing and cloning, given the detection of intermediate phenotypic forms in Bangladesh. The 35.4% prevalence in goats and 55.5% in buffaloes are the highest recorded in these animals in Bangladesh. In cattle (29.3%) and sheep (26.8%) prevalences are also high for these species. These prevalences are very high when compared to lowlands at similar latitudes in neighboring India. The high prevalences and intensities appear in western Bangladesh where cross-border importation of animals from India occur. The combined haplotype CH3A of Fasciola gigantica widely found in all livestock species throughout Bangladesh fits its historical connections with the western Grand Trunk Road and the eastern Tea-Horse Road. The “pure” F. hepatica sequences only in clones from specimens showing heterozygotic positions indicate recent hybridization events with local “pure” F. gigantica, since concerted evolution did not yet have sufficient time to homogenize the rDNA operon. The detection of up to six different sequences coexisting in the cloned specimens evidences crossbreeding between hybrid parents, indicating repeated, superimposed and rapidly evolving hybridization events. The high proportion of hybrids highlights an increasing animal infection trend and human infection risk, and the need for control measures, mainly concerning goats in household farming management. ITS-1 and ITS-2 markers prove to be useful for detecting recent hybrid fasciolids. The introduction of a Fasciola species with imported livestock into a highly prevalent area of the other Fasciola species may lead to a high nucleotide variation in the species-differing positions in the extremely conserved fasciolid spacers. Results suggest that, in ancient times, frequent crossbreeding inside the same Fasciola species gave rise to the very peculiar characteristics of the present-day nuclear genome of both fasciolids.
Keywords: Fasciola gigantica and F. hepatica, Livestock, Prevalences and intensities, Nuclear rDNA internal transcribed spacer region, ITS-1 and ITS-2, Sequencing and cloning, Heterozygotic sequence complexity, Crossbreeding hybrids, Animal importation, Bangladesh
Graphical abstract
Highlights
-
•
Assessment of the increasing fascioliasis infection rates in livestock in Bangladesh.
-
•
Complete sequencing and cloning of the nuclear rDNA ITS-1 and ITS-2 spacers.
-
•
F. hepatica sequences in clones indicate recent hybridization with local F. gigantica.
-
•
Hybrid proportion highlights an increasing animal infection trend and human risk.
-
•
Consequences of livestock importation on the conserved fasciolid rDNA spacers.
1. Background
Two liver fluke species of the genus Fasciola cause the zoonotic trematodiasis called fascioliasis. This disease is of great veterinary importance due to the high economic losses in livestock husbandry [1] and has been demonstrated to underlie public health problems in many rural areas, mainly in low-income countries of different continents. The World Health Organization has therefore included this disease among the group of foodborne trematodiases within the Neglected Tropical Diseases (NTDs) to which priority should be given [2,3]. This is based on its pathogenicity and the increasing emergence of this disease apparent in the 1990 and 2000 decades. Recent studies have shown that infection may affect the sufferer very early in life [4] and that severe clinical pictures also develop during the long chronic phase and not only in the short initial acute phase [5,6].
Both climate change, among which the global warming phenomenon but also rainfall-rate changes, and other anthropogenic effects, including man-made environmental modifications such as irrigation projects, human and animal movements, and livestock importation/exportation, are driving forces for the potential emergence of fascioliasis in many areas, both in developed and developing countries [7,8]. Numerous studies have analyzed climate change impacts on animal fascioliasis, and others have also demonstrated the influence of both climate and anthropogenic modifications of the milieu on human fascioliasis [9,10].
Fasciola hepatica originated in cooler areas and mountainous foothills of the Asian Near East in the latest Miocene to Early Pliocene, around 6.0 to 4.0 mya and perhaps shortly afterwards. Fasciola gigantica originated in warm lowlands of southeastern Africa in the mid-Miocene, around 13.5 mya and later spread northward to the same Asian Near East through the eastern Mediterranean Levantine Corridor [11]. Thus, both fasciolids were present in the Fertile Crescent and took advantage of human-guided movements of domesticated ruminants, equines and camelids for subsequent further spread throughout the Neolithic and up to the present day, F. hepatica expanding worldwide and F. gigantica eastward into Asia [8]. The preference for warmer lowlands allowed F. gigantica to spread along all southern latitudes of Asia up to South East Asia and Pacific islands. On the contrary, multidisciplinary analyses indicate that F. hepatica did not expand throughout these southern Asian warm lowlands in the past, because of the development temperature thresholds of this fasciolid [8].
From the public health point of view, F. gigantica was traditionally only given secondary importance, but its higher pathogenicity [12], and the increasing number of reports of human infection are leading to a reconsideration of the scenario in Africa [[13], [14], [15]] and Asia [8,16].
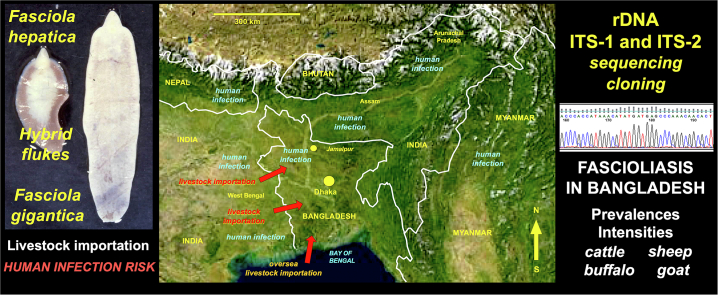
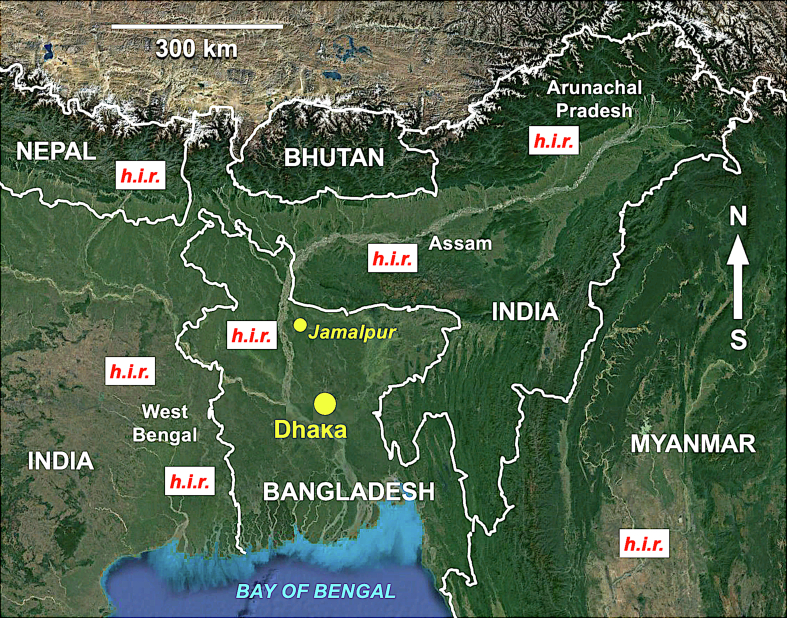
In Asia, the very wide south-central region eastward from Afghanistan has become a focus for studies on fascioliasis after recent reports of human infection. In Bangladesh, there have only been two human fascioliasis reports, one of a 22-year-old Bangladeshi women diagnosed at a London hospital [17] and another of a 35-year-old house-wife from Jamalpur [18]. This contrasts, however, with the number of human infection cases reported in zones of other countries neighboring Bangladesh, such as India including West Bengal [19,20], Assam [21] and Arunachal Pradesh [19], Nepal [[22], [23], [24]] and Myanmar [25] (Fig. 1). This scenario suggests the entire region of northward from the Bay of Bengal and southward from the Himalayan chain to be a hot spot of fascioliasis with human infection risk throughout very high-density populated areas.
Fig. 1.
Geographic map of Bangladesh and neighboring countries, showing zones where human infection by fasciolid flukes have been reported. h.i.r. = human infection reported (h.i.r. labels noted in zones of reports except in Myanmar). Background from composed satellite map of Asia orthographic projection by NASA (full resolution of 1887 × 1962 pixels; public domain) via Wikimedia Commons.
The present study had two objectives in agreement with the One-Health focus recently prioritized by WHO for the strategic assessment and control of neglected tropical diseases, mainly those with zoonotic characteristics [26]. First, the purpose was to assess the distribution of fasciolid liver flukes infecting the main livestock species (buffaloes, cattle, goats and sheep) throughout the whole of Bangladesh by including animals from the four different agro-ecological zones, i.e. coastal, hilly, Barindh steppe, and floodplains, to verify the apparently increasing rates, in which parts of the country, which animal species, and in which types of zones this might be occurring. Second, the study has focused on the genetic characterization of the liver flukes collected, by means of DNA sequencing and cloning in the way to assess the potential reasons for the aforementioned increasing problem in Bangladesh.
A previous phenotypic assessment of fasciolid flukes from Bangladesh demonstrated the existence of intermediate forms between F. gigantica and F. hepatica by means of CIAS morphometry analyses [27]. Therefore, the nuclear ribosomal DNA (rDNA) internal transcribed spacers (ITS-1 and ITS-2) were used as molecular markers for sequencing purposes, because the nuclear rDNA has been verified to agree with fluke adult morphology whereas the mitochondrial DNA does not always do so, potentially indicating hybrid origins of introgression [28]. This is the first time that the complete sequences of both rDNA ITSs have been used for fasciolid fluke assessment in Bangladesh.
2. Material and methods
2.1. Liver fluke materials
A total of 2295 adult flukes of fasciolids were collected directly from the livers of the livestock species studied. Rubber-coated forceps were used to avoid any structural damage to the flukes. Details of the specimens of livestock species analyzed and their geographical origins of localities, coordinates and agro-ecological zones are given in Table 1 and Fig. 2.
Table 1.
Agro-ecological zones, coordinates and altitudes of the localities in Bangladesh from where livestock specimens were analyzed for fasciolid fluke collection.
| No. in map⁎ | Localities (Administrative Units) | Agro-eco-logical zones | Latitude | Longitude | Altitude (m) |
|---|---|---|---|---|---|
| 1 | Chittagong/Cox's Bazar/Chakaria | Coastal | 21°45′41.36″N | 92°04′33.45″E | 11.21 |
| 2 | Barisal /Bhola/Bhola Sadar | Coastal | 22°41′12.48″N | 90°38′30.76″E | 7.88 |
| 3 | Chittagong/Rangamati/Rangamati Sadar | Hilly | 22°39′28.51″N | 92°10′24.54″E | 45.45 |
| 4 | Sylhet/Sylhet/Sylhet Sadar | Hilly | 24°54′23.77″N | 91°50′50.29″E | 21.21 |
| 5 | Rajshahi/Naogaon/Patnitola | Barindh steppe | 25°02′40.55″N | 88°45′19.68″E | 24.24 |
| 6 | Rajshahi/Naogaon/Shapahar | Barindh steppe | 25°07′30.31″N | 88°35′24.50″E | 44.85 |
| 7 | Rajshahi/Naogaon/Naogaon Sadar | Barindh steppe | 24°48′39.24″N | 88°56′37.04″E | 20.61 |
| 8 | Rajshahi/Pabna/Ishwardi | Floodplains | 24°07′12.43″N | 89°04′03.56″E | 18.48 |
| 9 | Dhaka/Mymensingh/Ishwarganj | Floodplains | 24°41′10.20″N | 90°35′36.88″E | 16.67 |
| 10 | Rangpur/Lalmonirhat/Lalmonirhat Sadar | Floodplains | 25°58′26.15″N | 89°17′02.26″E | 37.58 |
| 11 | Dhaka/Mymensingh/Mymensingh Sadar | Floodplains | 24°44′04.60″N | 90°25′43.80″E | 20.00 |
| 12 | Rajshahi/Bogra/Dhunat | Floodplains | 24°41′29.12″N | 89°32′10.33″E | 16.36 |
| 13 | Khulna/Jhenaidah/Shailkupa | Floodplains | 23°40′45.47″N | 89°14′48.28″E | 13.03 |
| 14 | Khulna/Jhenaidah/ Jhenaidah Sadar |
Floodplains | 23°32′43.15″N | 89°10′28.16″E | 14.24 |
For numbers of localities see map in Fig. 2.
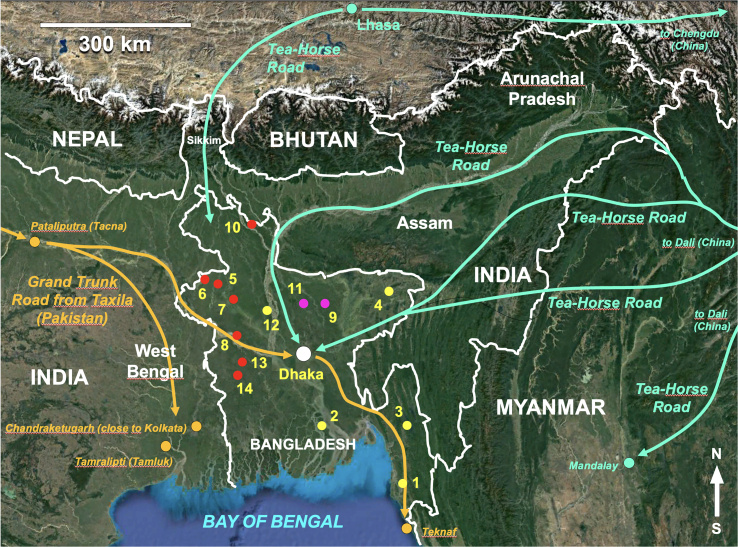
Fig. 2.
Geographic map of Bangladesh, showing the sites where the hosts of the liver flukes analyzed were collected. Coordinates and altitudes of each site are indicated in Table 1. 1) Chakaria; 2) Bhola Sadar; 3) Rangamati Sadar; 4) Sylhet Sadar; 5) Patnitola; 6) Shapahar; 7) Naogaon Sadar; 8) Ishwardi; 9) Ishwarganj; 10) Lalmonirhat Sadar; 11) Mymensingh Sadar; 12) Dhunat; 13) Shailkupa; 14) Jhenaidah Sadar. Yellow dots = rural localities where only rDNA ITS region sequences of the combined haplotype Fg-CH3A of “pure” F. gigantica were found; red dots = rural localities where heterozygotic sequences of the rDNA ITS region were found; pink dots = localities surrounding Mymensingh city where heterozygotic sequences of the rDNA ITS region were also found; pumpkin lines = routes from the West followed by F. gigantica along the Grand Trunk Road; green lines = routes from the North and the East followed by F. gigantica along the Tea-Horse Road (for more details about the Grand Trunk Road and the Tea-Horse Road, see [8]). Background from composed satellite map of Asia orthographic projection by NASA (full resolution of 1887 × 1962 pixels; public domain) via Wikimedia Commons. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Bangladesh has an area of 147,570 km2 and is bordered on the west, north, and east by a 4095-km land frontier with India and, in the southeast, by a short land and water frontier (193 km) with Burma (Myanmar). It is a low-lying riverine country vulnerable to flood and drought, located in South Asia with a largely marshy jungle coastline of 710 km on the northern littoral of the Bay of Bengal. The country has a tropical monsoon climate characterized by heavy seasonal rainfall, high temperatures, and high humidity with mild winters from October to March, and hot, humid summers from March to June.
2.2. Fasciolid specimens and DNA markers for molecular characterization
A total of 83 adult flukes were used for DNA sequencing purposes, including specimens infecting buffaloes (n = 6), cattle (n = 29), goats (n = 42) and sheep (n = 6). Fasciolid specimens were washed extensively in physiological saline (0.85% NaCl) to remove blood and bile and finally preserved in ethanol 70% until DNA extraction.
The sequence of the complete nuclear ribosomal DNA (rDNA) internal transcribed spacer region, including the spacers ITS-1 and ITS-2 and the 5.8S gene, which separate these was selected to characterize the flukes. These two ITS markers have already proved to be useful for the genetic characterization of Fasciola species and strains at local and regional levels as well as in worldwide analyses, including the assessment of the routes of spread [28].
2.3. Extraction, amplification and sequencing of DNA
For DNA extraction, a small part of the anterior body region of each fasciolid was individually processed using the phenol-chloroform extraction and ethanol precipitation method as previously described [[29], [30], [31]]. Materials were suspended in 400 μl of lysis buffer (10 mM Tris-HCl, pH 8.0, 100 mM EDTA, 100 mM NaCl, 1% sodium dodecyl sulfate SDS) containing 500 μg/ml Proteinase K (Promega, Madison, WI, USA). The digestion was performed for 2 h at 55 °C, including shaking every 15 min. Methods previously outlined were followed concerning the procedure steps [32]. The phenol-chloroform extraction and ethanol precipitation method was applied for total DNA isolation. Each pellet was dried and resuspended in 30 μl sterile TE buffer (pH 8.0), and subsequently this suspension was stored at −20 °C until needed.
The selected DNA marker was amplified by PCR for each liver fluke individual. Forward and reverse primers were designed in the regions flanking the rRNA genes 18S and 28S for the subsequent amplification of the complete ITS-1, 5.8S, ITS-2 region [29,33].
For the PCR amplification, the Biotools DNA polymerase® (Biotools B&M Labs. S.A., Madrid, Spain) was used in a Verity-96-well Thermal Cycler (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA USA). The program for this rDNA ITS region comprised one cycle of 2 min at 94 °C, 35 cycles of 1 min at 93 °C, 1 min at 55 °C and 1 min at 72 °C each, preceded by 2 min at 72 °C, and followed by a final cooling at 4 °C.
For the purification of the PCR product, the Ultra Clean™ PCR Clean-up DNA Purification System (MoBio, Solana Beach, CA, USA) was used following the manufacturer's protocol and eluted in 50 μl of 10 mM TE buffer (pH 7.6). The final DNA concentration (in μg/ml) and the absorbance at 260/280 nm were determined in an Eppendorf BioPhotometer (Hamburg, Germany).
Each PCR product was sequenced, for a definitive haplotype characterization, on both strands by the dideoxy chain-termination method performed with the Taq dye-terminator chemistry kit on an Applied Biosystems 3730xl DNA Analyzer (Applied Biosystems, Foster City, CA, USA). The PCR primers were used as sequencing primers.
2.4. Sequence analyses
The software Sequencher v. 5.4.6 (Gene Codes Co. MI, USA) was used to edit and assemble the sequences. The electropherograms (ABI format) of the rDNA intergenic sequence were thoroughly reviewed, especially in the positions that differentiate between F. hepatica and F. gigantica, using FinchTV v. 1.5 (Geospiza, Inc., Seattle, WA, USA; http://www.geospiza.com) and ClustalW to align them by means of default parameters in MEGA X [34].
Corresponding penalties for gaps were included in pairwise and multiple alignments. All changes, comprising transitions (ts), transversions (tv) and insertions/deletions (indels), were considered as character states in MEGA X. By means of the ALTER web server [35], the sequences aligned were collapsed to haplotypes, counting gaps as differences. Closely related sequences were searched by utilizing the BLASTN programme from the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov/BLAST). Comparative analyses were performed with available rDNA ITS-1 and ITS-2 sequences of F. gigantica, F. hepatica and Fasciola spp. downloaded from the GenBank and also with the fasciolid haplotype collection of the Valencia WHO Collaborating Center.
To define Fasciola species and “pure” haplotypes, a total of 50 sequences were used for comparison purposes, including 20 sequences of “pure” F. hepatica from Europe, the Americas and Algeria representing the haplotypes Fh1A (GenBank Accession No. MG569980), Fh2A (MG569978, MG569981), Fh3A (MK212150) and Fh1/2A (OQ064782); and 30 sequences of “pure” F. gigantica from Africa representing the haplotypes Fg1A (AJ853848), Fg2A (ON661090), and Fg-HtzA (ON661091). The term “pure” is here used to refer to haplotypes of fasciolid specimens appearing within the intraspecific variability shown by populations of a Fasciola species where the other Fasciola species is absent, as described and proposed previously [28]. These haplotypes are almost always homozygotes, but in a very few specimens a heterozygote may be found (i.e., presenting a double peak in a given position indicating the existence of two different nucleotides in that position depending on the rDNA operon copy).
2.5. Cloning
Cloning procedures were applied to PCR products from specimens whose electropherograms obtained by direct sequencing showed unexpected double peaks in relevant positions. The amplification products from a subsample of 6 specimens with apparent heterozygotic sequences and 1 control of “pure” F. gigantica, according to our DNA markers, were cloned with pGEM-T Easy Vector System I (Promega, Madison, WI) and introduced in Escherichia coli DH5α competent cells, to confirm the identity of the heterozygous sequences. After the growth of colonies, standard PCR of 8 different colonies per sample was performed and individually sequenced. DNA sequencing and sequence analyses of the clones were performed as described above.
2.6. DNA haplotype nomenclature
The terminology to identify the haplotype (H) of the two aforementioned DNA markers follows the previously proposed combined haplotyping (CH) nomenclature [28,36]. According to this nomenclature, ITS-2 haplotypes are defined by numbers, and ITS-1 haplotypes by capital letters. In combined haplotypes, the ITS-2 code is noted before that of the ITS-1 because of the evolutionary usefulness of ITS-2 for species differentiation. Worth mentioning is that haplotype codes are only definitive when the sequences are complete, i.e. full-length sequences. When dealing with fragments or incomplete sequences, haplotype codes are considered only provisional.
2.7. New sequences deposited in DNA repositories
Sequences of pure and hybrid haplotypes of fasciolids from Bangladesh obtained in this study have been deposited in the GenBank Data Library under Accession Nos. OQ064778- OQ064781.
3. Results
3.1. Fascioliasis infection rates
A total of 2352 fasciolid adult flukes were collected from 115 infected livestock specimens analyzed, including: (i) 10 infected from 18 buffaloes dissected (prevalence of 55.5%) showing an infection intensity of 20–61 flukes/animal (mean 38.0 flukes/animal); (ii) 36 infected from 123 cattle dissected (prevalence of 29.3%) showing an infection intensity of 7–103 flukes/animal (mean 28.3 flukes/animal); (iii) 58 infected from 164 goats dissected (prevalence of 35.4%) showing an infection intensity of 8–89 flukes/animal (mean 16.6 flukes/animal); and (iv) 11 infected from 41 sheep dissected (prevalence of 26.8%) showing an infection intensity of 3–31 flukes/animal (mean 11.7 flukes/animal).
The distribution of prevalences and intensities according to the localities, sex and age of the hosts for buffaloes and cattle are noted in Table 2 and those of goats and sheep in Table 3.
Table 2.
Prevalences and intensities of fasciolid flukes collected from buffaloes and cattle in Bangladesh.
| No. in map⁎ | Localities (Administrative Units) | Buffaloes |
Cattle |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. hosts analyzed | Sex i.h. |
Age i.h. (years) |
No. i.h. (preva-lence %) |
Total No. flukes collected | Intensity range (mean) | No. hosts analyzed | Sex i.h. |
Age i.h. (years) |
No. i.h. (preva-lence %) |
Total No. flukes collected | Intensity range (mean) | ||
| 1 | Chittagong/Cox's Bazar/ Chakaria | 5 | 1 M | 6 y | 1 (20%) | 121 | – | 5 | 1 F | 5 y | 1 (20.0%) | 23 | – |
| 2 | Barisal /Bhola/Bhola Sadar | – | – | – | – | – | – | 18 | 3 M, 4 F | 3–6 y | 7 (38.8%) | 307 | 26–67, (43.8) |
| 3 | Chittagong/Rangamati/Rangamati Sadar | – | – | – | – | – | – | 11 | 1 M, 1 F | 3–5 y | 2 (18.1%) | 28 | 7–21 (14.0) |
| 4 | Sylhet/Sylhet/Sylhet Sadar | 2 | 2 M | 3–4 y | 2 (100%) | 42 | 20–22 (21.0) |
4 | 1 M | 5 y | 1 (25.0%) | 13 | – |
| 5 | Rajshahi/Naogaon/Patnitola | – | – | – | – | – | – | 6 | 1 M | 6 y | 1 (16.6%) | 8 | – |
| 6 | Rajshahi/Naogaon/Shapahar | 4 | 1 M | 5 y | 1 (25%) | 82 | – | 7 | 1 M | 6 y | 1 (14.3%) | 13 | – |
| 7 | Rajshahi/Naogaon/Naogaon Sadar | – | – | – | – | – | – | – | – | – | – | – | – |
| 8 | Rajshahi/Pabna/Ishwardi | 6 | 4 M, 1 F | 3–7 y | 5 (83.3%) | 224 | 27–61 (44.88) |
13 | 2 M, 2 F | 3–6 y | 4 (30.7%) | 27 | 4–12 (6.75) |
| 9 | Dhaka/Mymensingh/Ishwarganj | – | – | – | – | – | – | – | – | – | – | – | – |
| 10 | Rangpur/Lalmonirhat/Lalmonirhat Sadar | – | – | – | – | – | – | 5 | 1 M | 1.5 y | 1 (20.0%) | 12 | – |
| 11 | Dhaka/Mymensingh/Mymensingh Sadar | – | – | – | – | – | – | 40 | 6 M, 4 F | 1.5–4 y | 10 (25.0%) | 44 | 10–31 (16.4) |
| 12 | Rajshahi/Bogra/Dhunat | – | – | – | – | – | – | – | – | – | – | – | – |
| 13 | Khulna/Jhenaidah/Shailkupa | 1 | 1 M | 5 y | 1 (100%) | 3 | – | 6 | 1 M, 1 F | 4–5 y | 2 (33.3%) | 43 | 9–34, (21.5) |
| 14 | Khulna/Jhenaidah/Jhenaidah Sadar | – | – | – | – | – | – | 18 | 4 M, 2 F | 2–6 y | 6 (33.3%) | 267 | 7–103 (44.5) |
| TOTAL | 18 | 9 M, 1 F | 3–7 y | 10 (55.5%) | 472 | 20–61 (38.0) |
123 | 21 M, 14 F | 1.5–6 y | 36 (29.3%) | 785 | 7–103 (28.3) | |
i.h. = infected hosts.
For numbers of localities see map in Fig. 2.
Table 3.
Prevalences and intensities of fasciolid flukes collected from goats and sheep in Bangladesh.
| No. in map⁎ | Localities (Administrative Units) | Goats |
Sheep |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. hosts analyzed | Sex i.h. |
Age i.h. (years) |
No. i.h. (preva-lence %) |
Total No. flukes collected | Intensity range (mean) | No. hosts analyzed | Sex i.h. |
Age i.h. (years) |
No. i.h. (preva-lence %) |
Total No. flukes collected | Intensity range (mean) | ||
| 1 | Chittagong/Cox's Bazar/ Chakaria | 6 | 1 M | 2 y | 1 (16.6%) | 9 | – | – | – | – | – | – | – |
| 2 | Barisal /Bhola/Bhola Sadar | – | – | – | – | – | – | – | – | – | – | – | – |
| 3 | Chittagong/Rangamati/Rangamati Sadar | – | – | – | – | – | – | – | – | – | – | – | – |
| 4 | Sylhet/Sylhet/Sylhet Sadar | 9 | 1 M, 2 F | 1.5–4 y | 3 (33.3%) | 62 | 11–27 (20.7) | – | – | – | – | – | – |
| 5 | Rajshahi/Naogaon/Patnitola | 5 | 1 M | 2 y | 1 (20.0%) | 11 | – | 5 | 1 M | 2 y | 1 (20%) | 3 | |
| 6 | Rajshahi/Naogaon/Shapahar | – | – | – | – | – | – | – | – | – | – | – | – |
| 7 | Rajshahi/Naogaon/Naogaon Sadar | 16 | 2 M, 2 F | 1.5–3 y | 4 (25.0%) | 85 | 4–34, (21.25) | 22 | 4 M, 2 F | 1.5–3 y | 6 (27.3%) | 65 | 6–16 (10.3) |
| 8 | Rajshahi/Pabna/Ishwardi | 15 | 2 M, 3 F | 1.5–4 y | 5 (33.3%) | 55 | 6–17 (11.0) | – | – | – | – | – | – |
| 9 | Dhaka/Mymensingh/Ishwarganj | 18 | 5 M | 1.5–3 y | 5 (27.8%) | 118 | 8–43 (23.6) | – | – | – | – | – | – |
| 10 | Rangpur/Lalmonirhat/Lalmonirhat Sadar | 4 | 1 M | 1.5 y | 1 (25.0%) | 26 | – | 4 | 1 M | 1.5 y | 1 (25%) | 31 | – |
| 11 | Dhaka/Mymensingh/Mymensingh Sadar | 63 | 16 M, 12 F | 1.5–3 y | 28 (44.4%) | 121 | 4–34 (21.25) | 4 | 1 M | 2 y | 1 (25%) | 8 | – |
| 12 | Rajshahi/Bogra/Dhunat | 3 | 1 M | 2 y | 1 (33.3%) | 124 | – | – | – | – | – | – | – |
| 13 | Khulna/Jhenaidah/Shailkupa | 5 | 1 M, 1 F | 1.5–2 y | 2 (40.0%) | 33 | 9–34 (15.5) | 3 | 1 M | 1.5 y | 1 (33.3%) | 11 | – |
| 14 | Khulna/Jhenaidah/Jhenaidah Sadar | 22 | 6 M, 1 F | 1.5–4 y | 7 (31.8%) | 322 | 8–89 (46.0) | 3 | 1 F | 2 y | 1 (33.3%) | 11 | – |
| TOTAL | 164 | 37 M, 21 F | 1.5–4 y | 58 (35.4%) | 966 | 8–89 (16.6) | 41 | 8 M, 3 F | 1.5–3 y | 11 (26.8%) | 129 | 3–31 (11.7) | |
i.h. = infected hosts.
For numbers of localities see map in Fig. 2.
3.2. Sequence analysis of the rDNA ITS1–5.8S-ITS2 region
The geographical origin of the 83 flukes molecularly analyzed infecting the four animal species according to localities in Bangladesh is noted in Table 4. The sequencing of these fasciolid specimens provided four different haplotypes.
Table 4.
Number of fasciolid flukes used for DNA sequencing according to localities and host species, including haplotypes found.
| No. in map⁎ | Localities (Administrative Units) | Buffaloes |
Cattle |
Goats |
Sheep |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No.Flukes studied |
No.pure Fg-3A |
No.hetero-zygotic haplot. | No.flukes studied |
No.pure Fg-3A |
No.hetero-zygotic haplot. | No.flukes studied |
No.pure Fg-3A |
No.hetero-zygotic haplot. | No.flukes studied |
No.pure Fg-3A |
No.hetero-zygotic haplot. | ||
| 1 | Chittagong/Cox's Bazar/ Chakaria | 1 | 1 | – | 1 | 1 | – | 1 | 1 | – | – | – | – |
| 2 | Barisal /Bhola/Bhola Sadar | – | – | – | 7 | 7 | – | – | – | – | – | – | – |
| 3 | Chittagong/Rangamati/Rangamati Sadar | – | – | – | 2 | 2 | – | – | – | – | – | – | – |
| 4 | Sylhet/Sylhet/Sylhet Sadar | 2 | 2 | – | 1 | 1 | – | 3 | 3 | – | – | – | – |
| 5 | Rajshahi/Naogaon/Patnitola | – | – | – | 1 | 1 | – | 1 | 1 | – | 1 | 1 Htz1 | |
| 6 | Rajshahi/Naogaon/Shapahar | 1 | – | 1 Htz1 | 1 | 1 | – | – | – | – | – | – | – |
| 7 | Rajshahi/Naogaon/Naogaon Sadar | – | – | – | – | – | – | 2 | 2 | – | 2 | 2 Htz1 | |
| 8 | Rajshahi/Pabna/Ishwardi | 2 | 1 | 1 Htz1 | 1 | 1 | – | – | – | – | – | – | – |
| 9 | Dhaka/Mymensingh/Ishwarganj | – | – | – | – | – | – | 1 | 1 | – | – | – | – |
| 10 | Rangpur/Lalmonirhat/Lalmonirhat Sadar | – | – | – | 1 | 1 | – | 4 | 3 | 1 Htz1 | 1 | 1 Htz1 | |
| 11 | Dhaka/Mymensingh/Mymensingh Sadar | – | – | – | 9 | 9 | – | 25 | 17 | 2 Htz1 6 Htz2 |
1 | 1 | – |
| 12 | Rajshahi/Bogra/Dhunat | – | – | – | – | – | – | 1 | 1 | – | – | – | – |
| 13 | Khulna/Jhenaidah/Shailkupa | – | – | – | 2 | 2 | – | 1 | 1 | – | – | – | – |
| 14 | Khulna/Jhenaidah/Jhenaidah Sadar | – | – | – | 3 | 2 | 1 Htz3 | 3 | 1 | 2 Htz1 | 1 | 1 | – |
| TOTAL | 6 | 4 | 2 Htz1 | 29 | 28 | 1 Htz3 | 42 | 31 | 5 Htz1 6 Htz2 |
6 | 2 | 4 Htz1 | |
For numbers of localities see map in Fig. 2.
Only one of the haplotypes corresponded to “pure” F. gigantica and is ascribed to the combined haplotype Fg-CH3A (GenBank Accession No. OQ064778), including a 950-bp-long ITS1–5.8S-ITS2 region of 50.42% GC content. This haplotype was the most abundant, occurring in 65 flukes (78.31%) of the total flukes analyzed, and was detected in the four animal species (cattle, buffalo, sheep and goats).
The other three combined haplotypes occurred in the remaining 18 specimens, i.e. 21.68% of the total fluke samples, and were also detected in the four animal species studied. These three haplotypes are characterized by showing double peaks which were detected in the careful inspection of the electropherograms of the rDNA ITS1, 5.8S, ITS2 sequences of these liver fluke specimens. These double peaks appeared in the specific positions known to differentiate between F. hepatica and F. gigantica, suggesting heterozygotes. Three types of sequences presenting double peaks could be distinguished:
-
a)
with double peaks in all such differentiating positions of the ITS-1 and ITS-2;
-
b)
with double peaks in some (but not in all) such positions of the ITS-1 and ITS-2);
-
c)
with double peaks in such positions only in the ITS-2 and not in the ITS-1.
This distribution allows us to group the aforementioned sequences in three heterozygotic types of combined haplotypes, for which ones we assigned the haplotype nomenclature Fg/Fh-Htz1, Fg/Fh-Htz2, Fg/Fh-Htz3, respectively. The most abundant proved to be the haplotype Fg/Fh-Htz1. The distribution of the combined haplotype Fg-CH3A of “pure” F. gigantica and the aforementioned three heterozygotic types of combined haplotypes according to localities is noted in Table 4, and according to host species in Table 5.
Table 5.
Distribution of the combined haplotypes of the rDNA transcribed spacer region according to definitive host species.
| Host species | Buffalo | Cattle | Goat | Sheep |
|---|---|---|---|---|
| Total fasciolid No. = 83 | No. = 6 | No. = 29 | No. = 42 | No. = 6 |
| “Pure” F. gigantica Fg-CH3A No. = 65 |
4 (66.66%) | 28 (96.55%) | 31 (73.81%) | 2 (33.33%) |
| Hybrid Fg/Fh-Htz No. = 18 |
2 (33.33%) | 1 (3.44%) | 11 (26.19%) | 4 (66.66%) |
| Heterozygotic haplotypes | ||||
| Fg/Fh-Htz1 No. = 11 (61.11%) |
2 (18.18%) | – | 5 (45.45%) | 4 (36.36%) |
| Fg/Fh-Htz2 No. = 6 (33.33%) |
– | – | 6 (100%) | – |
| Fg/Fh-Htz3 No. = 1 (5.55%) |
– | 1 (100%) | – | – |
The “pure” Fg-3A combined haplotype was found in 66.66% of buffaloes, 96.55% of cattle, 73.81% of goats and 33.33% of sheep. The heterozygotic combined haplotypes were found in 33.33% of buffaloes, 3.44% cattle, 26.19% goats, and 66.66% of sheep. Among the combined heterozygotic haplotypes, Fg/Fh-Htz1 is the only one shared by three host species (buffaloes, goats and sheep), while the haplotypes Fg/Fh-Htz2 and Fg/Fh-Htz3 were detected in only one host species, namely goats and cattle respectively (Table 4, Table 5). So, the goat is the only animal species in which the “pure” F. gigantica combined haplotype plus two types of hybrid haplotypes were detected.
Polymorphic sites in nucleotide positions in the sequence alignment of the whole nuclear rDNA ITS region including the ITS-1 and ITS-2 between “pure” F. hepatica, “pure” F. gigantica and Bangladeshi haplotypes are listed in Table 6.
Table 6.
Polymorphic sites in the sequence comparison of the complete transcribed spacer region of the nuclear rDNA and in the ITS-1 and ITS-2 between haplotypes of ‘genetically pure’ Fasciola hepatica from livestock in Europe, the Americas and Algeria, haplotypes of ‘genetically pure’ F. gigantica from livestock in African countries, and the haplotypes of “pure” and heterozygotic fasciolid samples from Bangladesh (in bold).
|
Fasciola species |
ITS-2, ITS-1 Combined haplotypes |
Polymorphic sites Intergenic Region (ITS-1, 5.8S, ITS-2) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 |
114 |
208 |
286 |
306 |
797 |
821 |
834 |
860 |
866 |
874 |
909 |
917 |
924 |
||
| Polymorphic sites ITS-1 |
Polymorphic sites ITS-2 |
||||||||||||||
| 24 | 114 | 208 | 286 | 306 | 210 | 234 | 248 | 273 | 279 | 288 | 322 | 330 | 337 | ||
| F. hepatica | 1A | C | A | C | T | C | T | T | A | C | C | C | T | T | G |
| F. hepatica | 2A | C | A | C | T | C | T | T | A | C | C | T | T | T | G |
| F. hepatica | 1/2A⁎ | C | A | C | T | C | T | T | A | C | C | C/T | T | T | G |
| F. hepatica | 3A | C | A | C | T | C | T | T | A | C | C | C | A | T | G |
| F. gigantica | 1A | T | T | T | A | T | T | C | A | T | T | C | T | – | A |
| F. gigantica | 2A | T | T | T | A | T | T | C | C | T | T | C | T | – | A |
| F. gigantica | 1/2A⁎⁎ | T | T | T | A | T | T | C | C/A | T | T | C | T | – | A |
| F. gigantica | 3A | T | T | T | A | T | C | C | A | T | T | C | T | – | A |
| Fg/Fh | Htz1 | T/C | T/A | T/C | A/T | T/C | C/T | C/T | A | T/C | T/C | C | T | – | A/G |
| Fg/Fh | Htz2 | T/C | T | T/C | A/T | T | C | C | A | T | T | C | T | – | A/G |
| Fg/Fh | Htz3 | T | T | T | A | T | C | C/T | A | T/C | T/C | C | T | – | A |
When analyzing the two ITS markers individually, the ITS-1 haplotype HA proved to have the same sequence of 432 nucleotides and a 51.16% GC content in all specimens presenting the combined haplotype Fg-CH3A of the ITS region. The 5.8S gene was identical in all specimens, with 154 base pairs and 53.25% GC. The 364-bp-long and 48.35% GC ITS-2 was the only one exhibiting a mutation in position 210 of the ITS-2 alignment, that differentiates it from pure haplotypes of Fg-H1, Fg-H2 and Fg-H1/2. This polymorphic position does not differentiate between F. hepatica and F. gigantica (Table 6).
In the case of Fg/Fh hybrid haplotypes found, only two ITS-1 sequences presented heterozygotic positions, namely five positions in the Fg/Fh-Htz1 and three positions in Fg/Fh-Htz2 that differentiate between F. hepatica and F. gigantica (Table 6). The hybrid haplotype Fg/Fh-Htz3 presented an ITS-1 base-to-base coinciding with the sequence of “pure” F. gigantica (Table 6).
Regarding the ITS-2, all hybrid Fg/Fh haplotypes detected in Bangladesh presented heterozygotic positions differentiating between F. hepatica and F. gigantica, namely four positions in the Fg/Fh-Htz1 and three positions in the Fg/Fh-HTZ3, whereas in only one position in the Fg/Fh-HTZ-2 (Table 6).
3.3. rDNA ITS1–5.8S-ITS2 region sequences obtained by cloning
A total of seven specimens from five localities were selected for cloning (Table 7), taking into account their peculiarities of double peak positions in their electropherograms obtained by direct sequencing, including one specimen used as control in whose sequence no double peaks were present. A total of 56 rDNA complete intergenic sequences were finally obtained after individually sequencing eight different colonies per each of the seven aforementioned samples.
Table 7.
Intraindividual sequence heterogeneity of the ITS1–5.8S-ITS2 rDNA region found in the cloned samples showing double peaks in key positions of their electropherograms.
| Sample No. | No. in map | Localities (Administrative Units) | Host species | Type of clones⁎ (Number of sequences) |
|---|---|---|---|---|
| 1 | 6 | Rajshahi/Naogaon/Shapahar | Buffalo | Fh.a (1); Fg.b (3); Cl-b (1); Cl-c (1); Cl-j (1); Cl-k (1) |
| 2 | 7 | Rajshahi/Naogaon/Naogaon Sadar | Sheep | Fh.a (1); Fg.b (4); Cl-e (1); Cl-f (1); Cl-g (1) |
| 3 | 7 | Rajshahi/Naogaon/Naogaon Sadar | Sheep | Fh.a (1); Fg.a (1); Fg.b (5); Cl-d (1) |
| 4 | 10 | Rangpur/Lalmonirhat/Lalmonirhat Sadar | Goat | Fg.b (4); Cl-a (1), Cl-d (1), Cl-e (1), Cl-m (1) |
| 5 | 10 | Rangpur/Lalmonirhat/Lalmonirhat Sadar | Sheep | Fh.a (2); Fg.b (3); Cl-a (1); Cl-h (1); Cl-i (1) |
| 6 | 13 | Khulna/Jhenaidah/Shailkupa | Cattle | Fg.b (7); Cl-l (1) |
| 7⁎⁎ | 14 | Khulna/Jhenaidah/Jhenaidah Sadar | Cattle | Fg.b (8) |
Clone type nomenclature according to Table 8.
Positive control.
The 8 clones of the control proved to be “pure” F. gigantica, and among the other 48 clone sequences, 27 proved to be “pure” F. gigantica (56.25%), five were “pure” F. hepatica (10.42%), and the 16 remaining appeared to be Fg/Fh hybrids (33.33%). Polymorphic sites in the positions differentiating between F. hepatica and F. gigantica among the sequences of the 56 clones obtained are shown in Table 8.
Table 8.
Haplotypes of the ITS1–5.8S-ITS-2 rDNA region found in the clones obtained in the seven cloned specimens, showing nucleotides in the positions differentiating between Fasciola hepatica and F. gigantica.
Color in nucleotide positions: brown = nucleotides characterizing F. hepatica; blue = nucleotides characterizing F. gigantica; black = nucleotides of polymorphic position not differentiating between F. hepatica and F. gigantica.
* Including the 8 clones of the control.
The intra-individual analysis of the clone sequences within each of the six cloned specimens presenting double peaks evidenced a high heterogeneity of mixed sequences in the fluke specimens differing according to localities (Table 7):
-
-
6 different sequences (2 “pure” and 4 heterozygotic) in the fluke infecting buffalo from Shapahar;
-
-
5 different sequences (2 “pure” and 3 heterozygotic) in the fluke infecting a sheep from Naogaon Sadar;
-
-
4 different sequences (3 “pure” and 1 heterozygotic) in the fluke infecting another sheep from Naogaon Sadar;
-
-
5 different sequences (1 “pure” and 4 heterozygotic) in the fluke infecting a goat from Lalmonirhat Sadar;
-
-
5 different sequences (2 “pure” and 3 heterozygotic) in the fluke infecting a sheep from Lalmonirhat Sadar;
-
-
2 different sequences (1 “pure” and 1 heterozygotic) in the fluke infecting a cattle specimen from Shailkupa.
The eight clones of the control specimen infecting a cattle beast from Jhenaidah Sadar were all identical and proved to correspond to a “pure” F. gigantica, thus demonstrating the accuracy of the results of the cloning procedure.
4. Discussion
4.1. Fascioliasis and livestock husbandry practices in Bangladesh
Bangladesh is the world's eighth most densely populated country, with 84.4% of its population living in rural areas [37]. Livestock are an essential component of the rural economy and the livelihood of the household subsistence farmers. About 75% of the people rely on livestock to some extent for their livelihood, providing employment for the poorer segments of the population [38], mostly landless, marginal and small farmers. There is a rapid growth in demand for livestock products due to increase in income, rising population, and urban growth, which has led to a significant increase in small-scale dairy farming over the last few years. However, despite Bangladesh having one of the highest densities of livestock in the world, it has not been able to meet domestic demands for meat, milk, and leather. Increased disease loads and often-reported emerging and reemerging infectious diseases appear to underlie this situation. This is due to animal husbandry practices [39] and to climate-change impact. Indeed, Bangladesh is listed as one of the most vulnerable countries for global climate change due to its unique geographical location linked to sub-tropical monsoon climate [37].
In this country, there are currently (i) around 24.20 million cattle including an annually slaughtered total of 3.5 million [36], (ii) 26.60 million goats [40] with an annually slaughtered total of around 15 million [41], (iii) 3.40 million sheep [42], and (iv) 1.47 million buffaloes [43]. Most of these animals are raised by small-scale farmers who own 1–2 head of cattle and 2–3 head of goat/sheep. The management system is a subsistence farming type, combining both tethering and scavenging with few or no inputs for breeding, feeding and healthcare, and covering nearly 80% of the livestock farming environment [44].
Thus, goats and cattle are the most important livestock species in Bangladesh by far. Goats may be considered the main resource of this country. The production and marketing of goats is one of the oldest and most widespread livestock enterprises in this country, with small-holder farmers as the main actors in goat rearing. Approximately 65% of households are connected with goat farming either as a primary or secondary occupation. An analysis of the goat supply chain shows that goats from the local areas are distributed throughout the country [41]. The native Black Bengal goat is a well-adapted, highly prolific breed that can be easily raised on low-quality feed and with little investment. Infectious diseases are, however, an important threat to productivity, causing a mortality of nearly 45% [40,41]. This could lead local farmers to import goats. Imported exotic breeds and/or commercial strains have resulted in crossbreeds by crossing with native types/varieties [44]. Such crossbred animals are today evident in Bangladesh [45]. Unfortunately, information on goat importation into Bangladesh is neither available in the literature nor directly from farmers who keep this under strict confidentiality [41].
Regarding cattle, Bangladesh has one of the highest cattle densities: 145 animals/km2 compared with 90 for India, 30 for Ethiopia, and 20 for Brazil [37]. This number is still insufficient for the national internal requirements and, consequently, many bulls and oxen are smuggled from India to Bangladesh regularly. Around 40% of the annually slaughtered cattle are imported through cross-border trade. The informal supply chain between India and Bangladesh for cross-border trading of cattle accounts for 1.5 million head every year (around 20,000 to 30,000 cattle daily). Traders from both India and Bangladesh bring thousands of cattle to Putkhali market which is very close to the border and only five km from Benapole Port Station, along the route from Kolkata to Dhaka [37]. From 2019, however, this trade has decreased due to increased internal production in ruminant husbandry.
Unfortunately, animal quarantine measures are not applied to informal importation practices, nor to officially registered imports because of insufficient manpower and funds to enforce the Animal Quarantine Act (Act no-VI of 2005) enacted by the Parliament of Bangladesh [37]. Moreover, liver fluke infection is not looked for in the freely released imported animals, nor are those animals preventatively treated against fascioliasis before being added to local herds.
The results of our livestock surveys demonstrate that fascioliasis is distributed throughout the whole country of Bangladesh and in all agro-ecological zones analyzed. Worth mentioning is that there is no locality in which uninfected animals could be found, which indicates a high infection risk of fascioliasis everywhere. Taking into account that buffaloes, cattle, goats and sheep are well-known liver-fluke reservoir hosts [[46], [47], [48]], it may be concluded that all livestock species participate in the diffusion and maintenance of the fasciolid life cycle in this country.
The prevalences found in the four livestock species prove to be very high and thus confirm the present fascioliasis problem in Bangladesh. The prevalence of 35.4% found in goats appears to be the highest recorded in this animal species in Bangladesh so far, when compared to the range of 3.8–32% noted in a previous review [49]. The same may be concluded for the prevalence of 55.5% we found in buffaloes, higher than the previous range of 19–51% reported for this animal in the country [49]. Our prevalences of 29.3% in cattle and 26.8% in sheep are also very high, despite lying within the respective ranges of 15–66% and 8.4–31% previously reported for these two livestock species in Bangladesh [49].
These prevalences also appear to be very high when compared to lowland plain areas of similar latitudes in the neighboring India. Prevalences of 13.90%, 10.79%, 2.78% and 2.35% in buffaloes, cattle, sheep and goats, respectively, were found in epidemiological studies conducted in different geo-climatic zones (hills, tarai and plains) in six states of northern India. Even the highest prevalences found during the seasonal peaks in these zones (11.84% cattle, 15.57% buffaloes, 4.60% sheep, 2.71% goats) [50] were markedly lower than the prevalences in Bangladesh. In the nearby Indian state of Uttar Pradesh, mean and monthly peak prevalences of 2.02% and 13.3% in goats and 1.69% and 5.1% in sheep, respectively [51], were also very low compared to Bangladesh. Prevalences were also similarly low in the Indian state of Rajasthan, including 12.68% in buffaloes followed by 9.70% in cows and 7.5% in goats [52].
In the case of buffaloes, however, the behavioral tendency of this species to congregate in freshwater bodies underlie very high prevalences in some localities, such as 31.14% in northern Indian states [50], 37.5% in Uttar Pradesh [53], and even up to a local 94% in the northeastern part of Uttar Pradesh [54], which fits a similarly impressively very high local prevalence of 72.0% found in the lymnaeid vector Radix acuminata in one transmission focus in the easternmost area of Gorakhpur of this Indian state, the so-far highest worldwide record of fasciolid infection in a lymnaeid population [55].
An analysis of prevalences and intensities from the ecological and geographical points of view shows that the highest infection rates are found in the floodplains and in the western part of the country. Floodplains are evidently appropriate habitats for the characteristics of the fascioliasis transmission foci, given the requirements for surface freshwater of the aquatic/amphibious lymnaeid snail vectors. Moreover, these results fit well with the predicted geographical distribution of animal fascioliasis in Bangladesh according to a risk map based on three-year data [49]. Interestingly, the high prevalences and intensities appear in western Bangladesh where cross-border importation of animals from the state of West Bengal of India occur.
4.2. rDNA ITS1–5.8S-ITS2 region sequence variation
4.2.1. Original arrival of Fasciola gigantica
The geographical location underlies the role as a crossroad played by Bangladesh during the great human-guided domesticated animal movements over thousands of years in southern Asia (Fig. 2). In the West, the use of cattle, equines and dromedaries as pack animals and goats and sheep as livestock for subsistence purposes, along the Grand Trunk Road from the Near East and Afghanistan through Pakistan and the northern plains of India up to Bangladesh allowed for the arrival and colonization of the latter country by the warm-lowland-preferring fasciolid species F. gigantica [8]. In the East, Bangladesh traded with southern China and further with South East Asia by connecting with the Tea-Horse Road via a northern route through Kamrup in Indian Assam and a southern route via eastern Indian Manipur, both traversing the mountainous regions of upper Burma (present-day Myanmar) and ending in Bengal. Equines and cattle, but also yaks at high altitudes, were used as pack animals on the Tea-Horse Road [8]. The mule was the key animal species, because it is a powerful pack animal very useful in long distance movements along difficult rugged mountainous routes [56], such as those the Tea-Horse Road traversed. Mules have recently proved to be efficient reservoirs in the spread of fascioliasis [57]. This might explain the combined haplotype Fg-CH3A found widely distributed in all livestock species throughout Bangladesh and which proved to be identical to the haplotype recently reported from Vietnam (GenBank Acc. No. MN970009) [58].
4.2.2. Arguments against an old arrival of Fasciola hepatica
The detection of combined haplotypes of “pure” F. hepatica in the sequences of the clones obtained needs further analysis.
The only way for the arrival of “pure” F. hepatica in the past could have been from the North by the Tea-Horse Road through the Tibetan capital Lhasa and subsequently via the Indian mountain pass at the Indian Sikkim state located in between Nepal and Bhutan (Fig. 2). Indeed, the Tea-Horse Road connected with the eastern routes of the northern Silk Road where the cooler temperatures facilitated the spread of F. hepatica [8]. Moreover, the main lymnaeid species vector of F. hepatica, Galba truncatula, is known to be today present in both Nepal and Bhutan, transhumant agropastoralism has traditionally been practiced in western Bhutan since ancient times, and there was a secondary south-north road which started at the Jaldhaka river in India, just northward from present-day Bangladesh [8].
However, the absence of Galba/Fossaria snail species in the lowlands of Bangladesh and surrounding India and the high temperatures of these lowlands not fitting within the range of the development temperature thresholds of F. hepatica make it hard to envisage such an entry in the past.
4.2.3. Arguments indicating a very recent introduction of Fasciola hepatica
The detection of these “pure” F. hepatica sequences only in clones obtained from specimens showing heterozygotic positions indicate hybridization phenomena occurred evolutionarily very recently. In other words, results suggest that native “pure” F. gigantica to have crossbred very recently with introduced F. hepatica specimens, so that concerted evolution did not yet have sufficient time to homogenize the rDNA operon. The obtaining of different sequences in the clones from several individual fasciolid specimens indicates the same phenomenon.
Although the time needed for the rDNA operon to homogenize different formerly coexisting sequences remains unknown, recent studies suggest that it may need at least several generations over a number of years when only lymnaeid vector species specific for a given Fasciola species are present [31]. In Bangladesh, only two lymnaeid species belonging to the Radix group are present, namely R. acuminata and R. luteola, both involved in the transmission of F. gigantica [8]. This means that the evolutionary vector filter will act favoring the F. gigantica genotype [31].
Moreover, the detection of different sequences coexisting in the rDNA operon of the cloned specimens is clear evidence not only of recent hybridizations, but also crossbreeding between parental specimens which were already hybrids, indicating repeated, superimposed and rapidly evolving hybridization events.
The aforementioned, both official and informal procedures of livestock importation in recent years in Bangladesh due to the non-stop increasing intranational demand explain well the wide variation of hybrid fasciolid specimens found and agree with the previous description of fasciolid intermediate forms reported from this country [27]. Regarding official livestock importation during the 1973–2014 period, information obtained about cattle imported from other countries is illustrated in Table 9, in which exporting countries presenting only F. hepatica as Australia and Spain are noted.
Table 9.
Information on official importation of cattle into Bangladesh, after respective government approval processes, in the 1973–2014 period.
| Year | Country of origin | Breed | Description of cattle |
Total no. of head | Remarks | Information source⁎ | |
|---|---|---|---|---|---|---|---|
| Cow/Heifer | Bull/Bullcalf | ||||||
| 1973 | Australia | Friesian | 62 | 2 | 64 | Gift | 1 |
| 1973 | Australia | Jersy | 58 | 3 | 61 | Gift | 2 |
| 1987 | Pakistan | Sahiwal | 95 | 5 | 100 | Import | 3 |
| 1989 | Spain | Friesian | 69 | 3 | 72 | Gift | 4 |
| 1990 | Pakistan | Sahiwal | 80 | 20 | 100 | Import | 5 |
| 1992 | Pakistan | Sahiwal | 96 | – | 96 | Import | 6 |
| 1993 | Australia | AFS⁎⁎ | 150 | – | 150 | Import | 7 |
| 1995 | Australia | Friesian | 250 | 4 | 254 | Import | 8 |
| 2006 | Australia | Friesian | 25 (Pregnant) | – | 25 | Import | 9 |
| 2012 | India⁎⁎⁎ | – | – | – | 1,287,800 | Influx^ | |
| 2013 | India⁎⁎⁎ | – | – | – | 1,796,904 | Influx^ | |
| 2013 | Australia | Jersy | – | 7 | 7 | Import | 10 |
| 2013 | Australia | Friesian | – | 17 | 17 | Import | 11 |
| 2014 | Australia | Friesian | – | 2 | 2 | Import | 12 |
| 2014 | India⁎⁎⁎ | – | – | 1,746,514 | Influx^ | ||
^ influx: local term used in Bangladesh to refer to an indiscriminately, randomly importation (lacking records and carried out without following standard importation procedures).
1–7 and 9: Central Cattle Breeding Station, Department of Livestock Services, Bangladesh; 8: Personal communication with Maj. (Rt.) Aktaruzzaman, owner of the Gochhata Dairy & Fish Farm; 10–12: Department of Livestock Services, Bangladesh.
AFS: Australian Friesian.
Information from National Board of Revenue, Segunbaagicha, Dhaka, earned influx revenue but details data not available.
Different banding patterns obtained by PCR restriction fragment length polymorphism (PCR-RFLP) applied to the ITS-1 of fasciolids of livestock from Bangladesh also suggested the existence of sequences of both F. gigantica and Fasciola sp. within individual flukes, although this technique did not allow for the obtaining of the sequences and their nucleotide position analyses, whereas a 535-bp-long fragment of the mtDNA nad1 gene proved to represent that of F. gigantica close to those known in other Asian countries, i.e. no introgression indicating hybridization was found [59]. Interestingly, a combination of an F. gigantica haplotype and an F. hepatica haplotype suggesting hybridization was found in the nuclear intron 4 of domain 2 of the taurocyamine kinase gene (TkD2Int4) when cloning and sequencing a fasciolid specimen infecting cattle from Bangladesh [60].
The finding of the heterozygotic sequences concentrated in the localities at the western part of Bangladesh (with the exception of the two central localities surrounding of Mymensingh, easily understandable due to the demands of such a large city) (compare Table 4 and Fig. 2), further indicate a role of the livestock importation from neighboring India. The analysis of the distribution of heterozygotic hybrid combined haplotypes according to host species additionally indicates that goats may play a crucial role in the diffusion of hybrid specimens, mainly through the uncontrolled small-scale household farming management and the goat supply chain which distributes animals throughout the country.
5. Concluding remarks
The high infection rates by fasciolids in buffaloes, cattle, goats and sheep indicate a high human infection risk throughout Bangladesh, especially given that 84% of its population lives in rural areas and 75% of the people rely on livestock. This suggests that human fascioliasis may be underestimated in this country and local physicians should be made aware of the cause and diagnosis of this disease.
The high proportion of hybrid fasciolids found in the livestock analyzed moreover highlights a potential increasing infection trend in animals, when taking into account the usually higher spreading power of hybrid forms in cases in which they prove to be viable, i.e. transmissible by the locally available lymnaeid snail vector species. Consequently, human infection risk may also increase, similarly as it has recently occurred in Vietnam [16].
Finally, the recent increase in hybridization events emphasizes the need to put suitable quarantine measures in place, to include liver fluke diagnosis and treatments for imported animals, and to make efforts to help rural people control the disease in their farming management, mainly concerning goats. The capacity of fasciolid metacercariae to infect different host species independently of the Fasciola host species isolate of origin [[61], [62], [63]], indicates that each of the four host species studied may act as a reservoir for the other host species.
The results of this study highlight the importance of livestock management regarding the transmission, epidemiology, spread and control of fascioliasis within a One Health strategy against human and animal fascioliasis, as it has been already observed in a human fascioliasis hyperendemic area [64]. Moreover, the molecular assessment demonstrates the appropriateness of DNA marker sequencing of the fasciolid causal agents within such a One Health strategy when dealing on a country in which both Fasciola species may be involved, as known throughout the continents of Asia and Africa where the two fasciolids overlap in many regions [65]. The sequences of the rDNA ITS-1 and ITS-2 markers prove to be highly useful for the detection of recent hybrid fasciolids. This study shows the high degree of variation that ITS-1 and ITS-2 may reach in the species differing positions in these hermaphroditic flukes able to crossbreed, when:
-
(i)
one Fasciola species is introduced by livestock importation into an area where there was originally only the other Fasciola species, and
-
(ii)
high infection prevalence and intensity rates facilitate the occurrence of many and repeated crossbreeding encounters of different fluke species within a biliary canal or gallbladder of a host individual.
It should be highlighted that ITS-1 and ITS-2 are extremely conserved in Fasciola species [11,28]. This markedly differs from ITSs in other invertebrates in which accumulation of mutations, insertions such as microsatellite and minisatellite repeats [66], and sometimes even duplications such as pseudogenes [67] occur. In Fasciola, microsatellites, minisatellites or pseudogenes have never been reported, and mutations are very rare outside of the few positions allowing for the differentiation of the two species. In the species F. nyanzae specific to the hippopotamus, the very few specific mutations in the ITSs concern the same positions differing between F. hepatica and F. gigantica [11]. This indicates that, in Fasciola, the function of ITS-1 and ITS-2 in the production of precursor molecules and mature rRNA products may be more important than in other organisms, i.e. including nucleotide positions surrounding the processing sites subject to functional constraints similar to those acting on the rRNA genes. Mutations in other positions potentially able to disrupt the secondary structures in the ITS regions, which may reduce or eliminate the production of precursor molecules and mature rRNA products, do not appear. Indeed, an analysis shows that none of the differences between the three Fasciola species in this region would affect the secondary structure [68].
In its turn, these hybridization phenomena between the two Fasciola species found in Bangladesh also suggest that crossbreeding events should have been frequent inside the same Fasciola species infecting domestic animal species regularly used for goods transport along very long distances in round trips in several world zones during thousands of years in the past, when situations of concentration of high numbers of animals facilitating high infection rates should have occurred and no anti-fasciolid treatments were available. These monospecific Fasciola crossbreeding events should have undoubtedly underlaid the peculiar characteristics observed at present in the nuclear genome of these hermaphroditic fasciolid flukes throughout [8], in aspects such as the extreme length of the genome, gene duplication, polymorphisms and very high repeat content [69,70].
Ethics statement
All research dealing on animals was performed with the approval of the Evaluation of Projects concerning Animal Research at University of Valencia (Organo Habilitado para la Evaluación de Proyectos de Experimentación Animal de la Universidad de Valencia) (A1263 915,389,140), strictly following the institution's guidelines based on Directive 2010/63/EU. Permission for animal research was additionally obtained from the Servicio de Sanidad y Bienestar Animal, Dirección General de Producción Agraria y Ganadería, Consellería de Presidencia y Agricultura, Pesca, Alimentación y Agua, Generalitat Valenciana, Valencia, Spain (No. 2015/VSC/PEA/00001 tipo 2). Fasciolid collection was carried out by taking advantage of livestock slaughtering for other purposes. Animal ethics guidelines regarding animal care were strictly adhered.
Consent for publication
All authors read and approved the final version for publication consideration.
Funding
This research was funded by Health Research Project No. PI16/00520, Subprograma Estatal de Generación de Conocimiento de la Acción Estratégica en Salud (AES) y Fondos FEDER, Plan Estatal de Investigación Científica y Técnica y de Innovación, ISCIII-MINECO, Madrid, Spain; by the Red de Investigación de Centros de Enfermedades Tropicales – RICET (Project No. RD16/0027/0023 of the PN de I + D + I, ISCIII-Subdirección General de Redes y Centros de Investigación Cooperativa RETICS), Ministry of Health and Consumption, Madrid; by CIBER de Enfermedades Infecciosas Project CB21/13/00056, ISCIII, Ministry of Science and Education, Madrid, Spain; and by Projects No. 2016/099 and 2021/004 of the PROMETEO Program, Programa of Ayudas para Grupos de Investigación de Excelencia, Generalitat Valenciana, Valencia, Spain. Field research in Bangladesh funded by the BAS-USDA Project (Endowment Program of the Bangladesh Academy of Sciences and United States Department of Agriculture, Dhaka, Bangladesh) (https://www.bas.org.bd/bas-usda/bas-usda-committees).
The first author, Dr. Syed Ali Ahasan, additionally contributed to the study during his 2-month stay in the Department of Parasitology, Faculty of Pharmacy, University of Valencia, Spain, funded by a fellowship of the International Atomic Energy Agency (IAEA, Headquarters Vienna), Code No. C6/BDG/13034, linked to the TC Project of IAEA entitled “Contingency Project for Institutional Development” (Ref. No. RAS0066).
The second author, Alejandra De Elías-Escribano, was supported by a 3-year fellowship of the Programa de Ayudas de la Generalitat Valenciana y Fondo Social Europeo, 2019 (ACIF/2019/182).
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Author contributions
Study concept and design: SMC, MDB; participated in experimental work: SAA, ADEE, PA; participated in field work: SAA, MZA, MMHM, EHC; local coordination, protocols, and logistics: SMC, SAA, MZA, MMHM, EHC; analysis and interpretation of data: SMC, DB, MDB; drafting of the manuscript: SMC, MDB; critical revision of the manuscript for important intellectual content and for final approval: SMC, DB, MDB; obtained project funding: SMC, MDB; principal investigator: SMC.
Author statement
On behalf of all the authors, I can confirm that everyone has read and approved this review.
Declaration of Competing Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
Studies of this article have been performed within the framework of the Worldwide Initiative of WHO against Human Fascioliasis (WHO Headquarters, Geneva, Switzerland). Dr. Gerrit Viljoen (IAEA Headquarters, Vienna, Austria) is acknowledged because of his support coordination. Technical support provided by the Servicio Central de Secuenciación para la Investigación Experimental (SCSIE) of the Universidad de Valencia (Dr. A. Martínez). Prof. T. Islam and Prof. M.R. Islam (Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh, Bangladesh) are acknowledged for their help in the collection of liver fluke specimens from livestock.
Data availability
All data are presented in the article. The sequence data have been submitted to GenBank and have been included under the following accession Nos: OQ064778- OQ064781.
References
- 1.Hayward A.D., Skuce P.J., McNeilly T.N. The influence of liver fluke infection on production in sheep and cattle: a meta-analysis. Int. J. Parasitol. 2021;51:913–924. doi: 10.1016/j.ijpara.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Department of Control of Neglected Tropical Diseases, World Health Organization, WHO Headquarters; Geneva: 2013. Sustaining the Drive to Overcome the Global Impact of Neglected Tropical Diseases; pp. 1–128. [Google Scholar]
- 3.World Health Organization . World Health Organization; Geneva: 2020. Ending the Neglect to Attain the Sustainable Development Goals. A Road Map for Neglected Tropical Diseases 2021–2030; pp. 1–47.https://www.who.int/neglected_diseases/Ending-the-neglect-to-attain-the-SDGs--NTD-Roadmap.pdf Available online. (accessed on 23 July 2020) [Google Scholar]
- 4.De N.V., Le T.H., Agramunt V.H., Mas-Coma S. Early postnatal and preschool age infection by Fasciola spp.: report of five cases from Vietnam and worldwide review. Am. J. Trop. Med. Hyg. 2020;103:1578–1589. doi: 10.4269/ajtmh.20-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mas-Coma S., Agramunt V.H., Valero M.A. Neurological and ocular fascioliasis in humans. Adv. Parasitol. 2014;84:27–149. doi: 10.1016/B978-0-12-800099-1.00002-8. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Miguel J., Valero M.A., Reguera-Gomez M., Mas-Bargues C., Bargues M.D., Simon-Martin F., Mas-Coma S. Numerous Fasciola plasminogen-binding proteins may underlie blood-brain barrier leakage and explain neurological disorder complexity and heterogeneity in the acute and chronic phases of human fascioliasis. Parasitology. 2019;146:284–298. doi: 10.1017/S0031182018001464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mas-Coma S. Human fascioliasis emergence risks in developed countries: from individual patients and small epidemics to climate and global change impacts. Enferm. Infecc. Microbiol. Clín. 2020;38:253–256. doi: 10.1016/j.eimc.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Mas-Coma S., Valero M.A., Bargues M.D. Human and animal fascioliasis: origins and worldwide evolving scenario. Clin. Microbiol. Rev. 2022;35 doi: 10.1128/cmr.00088-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afshan K., Fortes-Lima C.A., Artigas P., Valero M.A., Qayyum M., Mas-Coma S. Impact of climate change and man-made irrigation systems on the transmission risk, long-term trend and seasonality of human and animal fascioliasis in Pakistan. Geospat. Health. 2014;8:317–334. doi: 10.4081/gh.2014.22. [DOI] [PubMed] [Google Scholar]
- 10.Bargues M.D., Artigas P., Angles R., Osca D., Duran P., Buchon P., Gonzales-Pomar R.K., Pinto-Mendieta J., Mas-Coma S. Genetic uniformity, geographical spread and anthropogenic habitat modifications of lymnaeid vectors found in a One Health initiative in the highest human fascioliasis hyperendemic of the Bolivian Altiplano. Parasit. Vectors. 2020;13:171. doi: 10.1186/s13071-020-04045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bargues M.D., Halajian A., Artigas P., Luus-Powell W.J., Valero M.A., Mas-Coma S. Paleobiogeographical origins of Fasciola hepatica and F. gigantica in light of new DNA sequence characteristics of F. nyanzae from hippopotamus. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.990872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valero M.A., Bargues M.D., Khoubbane M., Artigas P., Quesada C., Berinde L., Ubeira F.M., Mezo M., Hernandez J.L., Agramunt V.H., Mas-Coma S. Higher physiopathogenicity by Fasciola gigantica than by the genetically close F. hepatica: experimental long-term follow-up of biochemical markers. Trans. Roy. Soc.Trop. Med. Hyg. 2016;110(1, Special Issue):55–66. doi: 10.1093/trstmh/trv110. [DOI] [PubMed] [Google Scholar]
- 13.Lukambagire A.S., Mchaile D.N., Nyindo M. Diagnosis of human fascioliasis in Arusha region, northern Tanzania by microscopy and clinical manifestations in patients. BMC Infect. Dis. 2015;15:578. doi: 10.1186/s12879-015-1326-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Periago M.V., Valero M.A., Artigas P., Agramunt V.H., Bargues M.D., Curtale F., Mas-Coma S. Very high fascioliasis intensities in schoolchildren of Nile Delta governorates: the Old World highest burdens found in lowlands. Pathogens. 2021;10:1210. doi: 10.3390/pathogens10091210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bekana T., Berhe N., Eguale T., Aemero M., Medhin G., Tulu B., Ghiwot Y., Liang S., Hu W., Erko B. Prevalence and factors associated with intestinal schistosomiasis and human fascioliasis among school children in Amhara Regional State, Ethiopia. Trop. Med. Health. 2021;49:35. doi: 10.1186/s41182-021-00326-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen T.B.N., De N.V., Nguyen T.K.L., Quang H.H., Doan H.T.T., Agatsuma T., Le T.H. Distribution status of hybrid types in large liver flukes, Fasciola species (Digenea: Fasciolidae), from ruminants and humans in Vietnam. Kor. J. Parasitol. 2018;56:453–461. doi: 10.3347/kjp.2018.56.5.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behar J.M., Winston J.S., Borgstein R. Hepatic fascioliasis at a London hospital - the importance of recognizing typical radiological features to avoid a delay in diagnosis. Brit. J. Radiol. 2009;82(981):e189–e193. doi: 10.1259/bjr/78759407. [DOI] [PubMed] [Google Scholar]
- 18.Das B.C., Khan A.S., Uddin M.S., Chowdhury M.M., Rahman Khan M.Z. Fascioliasis - an uncommon cause of recurrent cholangitis. J. Surg. Sci. 2015;19:35–38. [Google Scholar]
- 19.Ramachandran J., Ajjampur S., Chandramohan A., Varghese G.M. Cases of human fascioliasis in India: tip of the iceberg. J. Postgrad. Med. 2012;58:150–152. doi: 10.4103/0022-3859.97180. [DOI] [PubMed] [Google Scholar]
- 20.Madhumitha R., Gohel S., Latha Vishwanathan L., Gopalakrishnan R. Liver lesions, fever and eosinophilia caused by Fasciola hepatica in a 15-year-old girl. Indian J. Ped. 2015;82:967–968. doi: 10.1007/s12098-015-1745-z. [DOI] [PubMed] [Google Scholar]
- 21.Narain K., Biswas D., Rajguru S.K., Mahanta J. Human distomatosis due to Fasciola hepatica infection in Assam, India. J. Comm. Dis. 1997;29:161–165. [PubMed] [Google Scholar]
- 22.Parelkar S.V., Oak S.N., Maydeo A., Sanghvi B.V., Joshi P.B., Chaubal N., Patil R.T., Sahoo S.K., Lal P.J., Sampath N., Koticha A. Biliary fascioliasis: management in a child using endoscopic retrograde cholangio pancreatography. J. Indian Ass. Ped. Surg. 2013;18:23–24. doi: 10.4103/0971-9261.107012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sah R., Khadka S., Khadka M., Gurubacharya D., Sherchand J.B., Parajuli K., Shah N.P., Kattel H.P., Pokharei B.M., Rijal B. Human fascioliasis by Fasciola hepatica: the first case report in Nepal. BMC Res. Notes. 2017;10:439. doi: 10.1186/s13104-017-2761-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sah R., Khadka S., Lakhey P.J., Pradhan S., Shah N.P., Singh Y.P., Mas-Coma S. Human case of Fascisola gigantica-like infection, review of human fascioliasis reports in Nepal, and epidemiological analysis within the south Central Asia. Acta Parasitol. 2018;63:435–443. doi: 10.1515/ap-2018-0053. [DOI] [PubMed] [Google Scholar]
- 25.Maeda T., Yamada H., Akao N., Iga M., Endo T., Koibuchi T., Nakamura T., Odawara T., Iwamoto A., Fujii T. Unusual radiological findings of Fasciola hepatica infection with huge cystic and multilocular lesions. Inter. Med. 2008;47:449–452. doi: 10.2169/internalmedicine.47.0626. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization . World Health Organization; Geneva: 2021. Ending the Neglect to Attain the Sustainable Development Goals. One Health Companion Document to the Neglected Tropical Diseases Road Map 2021–2030. Draft for Public Consultation (updated 19 October 2021) pp. 1–23.https://cdn.who.int/media/docs/default-source/ntds/rabies/online-public-consultation.-one-health-companion-document/draft-for-public-consultation-one-health-companion-document-for-ntd-road-map.pdf Available online. (accessed 15 December 2021) [Google Scholar]
- 27.Ahasan S.A., Valero M.A., Chowdhury E.H., Islam M.T., Islam M.R., Mondal M.M.H., Peixoto R.V., Berinde L., Panova M., Mas-Coma S. CIAS detection of Fasciola hepatica / F. gigantica intermediate forms in bovines from Bangladesh. Acta Parasitol. 2016;61:267–277. doi: 10.1515/ap-2016-0037. [DOI] [PubMed] [Google Scholar]
- 28.Mas-Coma S., Valero M.A., Bargues M.D. Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Adv. Parasitol. 2009;69:41–146. doi: 10.1016/S0065-308X(09)69002-3. [DOI] [PubMed] [Google Scholar]
- 29.Mas-Coma S., Funatsu R., Bargues M.D. Fasciola hepatica and lymnaeid snails occurring at very high altitude in South America. Parasitology. 2001;123:S115–S127. doi: 10.1017/s0031182001008034. [DOI] [PubMed] [Google Scholar]
- 30.Bargues M.D., Gayo V., Sanchis J., Artigas P., Khoubbane M., BirrieI S., Mas-Coma S. DNA multigene characterization of Fasciola hepatica and Lymnaea neotropica and its fascioliasis transmission capacity in Uruguay, with historical correlation, human report review and infection risk analysis. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bargues M.D., Valero M.A., Trueba G.A., Fornasini M., Villavicencio A.F., Guaman R., De Elias-Escribano A., Perez-Crespo I., Artigas P., Mas-Coma S. DNA multi-marker genotyping and CIAS morphometric phenotyping of Fasciola gigantica-sized flukes from Ecuador, with an analysis of the Radix absence in the New World and the evolutionary lymnaeid snail vector filter. Animals. 2021;11:2495. doi: 10.3390/ani11092495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chougar L., Mas-Coma S., Artigas P., Harhoura K., Aissi M., Agramunt V.H., Bargues M.D. Genetically "pure" Fasciola gigantica discovered in Algeria: DNA multimarker characterization, trans-Saharan introduction from a Sahel origin and spreading risk into northwestern Maghreb countries. Transb. Emerg. Dis. 2020;67:2190–2205. doi: 10.1111/tbed.13572. [DOI] [PubMed] [Google Scholar]
- 33.Valero M.A., Bargues M.D., Calderon L., Artigas P., Mas-Coma S. First phenotypic and genotypic description of Fasciola hepatica infecting highland cattle in the state of Mexico, Mexico. Infect. Genet. Evol. 2018;64:231–240. doi: 10.1016/j.meegid.2018.06.032. [DOI] [PubMed] [Google Scholar]
- 34.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glez-Peña D., Gómez-Blanco D., Reboiro-Jato M., Fdez-Riverola F., Posada D. ALTER: program-oriented conversion of DNA and protein alignments. Nucleic Acids Res. 2010;38(Web Server issue) doi: 10.1093/nar/gkq321. W14–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bargues M.D., Mas-Coma S. Reviewing lymnaeid vectors of fascioliasis by ribosomal DNA sequence analyses. J. Helminthol. 2005;7:257–267. doi: 10.1079/joh2005297. [DOI] [PubMed] [Google Scholar]
- 37.Ali M.Z., Carlile G., Giasuddin M. Impact of global climate change on livestock health: Bangladesh perspective. Open Vet. J. 2020;10:178–188. doi: 10.4314/ovj.v10i2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anonymous . 2007. National Livestock Development Policy, Monogram. Government of the People's Republic of Bangladesh, Ministry of Fisheries and Livestock. Dhaka, Bangladesh; pp. 1–40. [Google Scholar]
- 39.Roess A.A., Winch P.J., Ali N.A., Akhter A., Afroz D., El Arifeen S., Darmstadt G.L., A.H. Baqui for the Bangladesh PROJAHNMO Study Group Animal husbandry practices in rural Bangladesh: potential risk factors for antimicrobial drug resistance and emerging diseases. Am. J. Trop. Med. Hyg. 2013;89:965–970. doi: 10.4269/ajtmh.12-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahman M.H., Akther S., Ali M.Z., Hassan M.Z. Incidence of diseases in goats in Bangladesh. The Bangladesh Veterinarian. 2020;37(1–2):14–20. [Google Scholar]
- 41.Chowdhury S.D., Hossain M.M., Rahman K.M.M., Saha M.K., Hossain S. Heifer International Bangladesh; Dhaka, Bangladesh: 2015. Study on Goat Value Chain in Bangladesh; pp. 1–64. Final report. [Google Scholar]
- 42.Islam S.S., Hasan M.S., Ghosh N., Islam M.S., Islam M.M. Prospects and problems of indigenous sheep production in south-western coastal regions of Bangladesh. J. Agric. Sci. Sri Lanka. 2021;1:54–66. [Google Scholar]
- 43.Habib M.R., Haque M.N., Rahman A., Aftabuzzaman M., Ali M.M., Shahjahan M. Dairy buffalo production scenario in Bangladesh: a review. Asian J. Med. Biol. Res. 2017;3:305–316. [Google Scholar]
- 44.Bhuiyan A.K. Fazlul Haque. Department of Animal Breeding and Genetics, Bangladesh Agricultural University; Mymensingh 2202, Bangladesh: 2013. Farm Animal Genetic Diversity Country Report – Bangladesh; pp. 1–74. [Google Scholar]
- 45.Afroz M.F., Faruque M.O., Husain S.S., Han J.L., Paul B. Genetic variation and relations in different goat populations of Bangladesh. Bangladesh J. Anim. Sci. 2010;39(1&2):1–8. [Google Scholar]
- 46.Mas-Coma S., Buchon P., Funatsu I.R., Angles R., Artigas P., Valero M.A., Bargues M.D. Sheep and cattle reservoirs in the highest human fascioliasis hyperendemic area: experimental transmission capacity, field epidemiology, and control within a One Health initiative in Bolivia. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.583204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mas-Coma S., Buchon P., Funatsu I.R., Angles R., Mas-Bargues C., Artigas P., Valero M.A., Bargues M.D. Donkey fascioliasis within a One Health control action: transmission capacity, field epidemiology, and reservoir role in a human hyperendemic area. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.591384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mas-Coma S., Funatsu I.R., Angles R., Buchon P., Mas-Bargues P., Artigas M.A., Valero M.D. Bargues. Domestic pig prioritized in one health action against fascioliasis in human endemic areas: experimental assessment of transmission capacity and epidemiological evaluation of reservoir role. One Health. 2021;13 doi: 10.1016/j.onehlt.2021.100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rahman A.K.M.A., Islam S.K.S., Talukder M.H., Hassan M.K., Dhand N.K., Ward M.P. Fascioliasis risk factors and space-time clusters in domestic ruminants in Bangladesh. Parasit. Vectors. 2017;10:228. doi: 10.1186/s13071-017-2168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garg R., Yadav C.L., Kumar R.R., Banerjee P.S., Vatsya S., Godara R. The epidemiology of fasciolosis in ruminants in different geo-climatic regions of North India. Trop. Anim. Health Prod. 2009;41:1695–1700. doi: 10.1007/s11250-009-9367-y. [DOI] [PubMed] [Google Scholar]
- 51.Yadav C.L., Vatsya S., Garg R., Kumar R.R., Banerjee P.S., Rajesh G. Seasonal prevalence of Fasciola gigantica infection in sheep and goats in western Uttar Pradesh. J. Parasit. Dis. 2007;31:141–144. [Google Scholar]
- 52.Swarnakar G., Sanger B. Epidemiological study of liver fluke (Trematoda: Digenea) in domestic ruminants of Udaipur District. Int. J. Curr. Microbiol. App. Sci. 2014;3:632–640. [Google Scholar]
- 53.Gupta S.C., Ghosh S., Raina O.K., Joseph D., Rawat P., Singh B.P., Mishra A.K., Chandra D., Samanta S. Studies and prevalence of fasciolosis in cattle and buffaloes in different agro-climatic zones of Uttar Pradesh, India. J. Vet. Parasitol. 2008;22:59–63. [Google Scholar]
- 54.Singh O., Agarwal R.A. Toxicity of certain pesticides to two economic species of snail in Northern India. J. Econ. Entomol. 1981;74:568–571. [Google Scholar]
- 55.Sunita K., Mas-Coma S., Bargues M.D., Sadaf M.A., Khan M., Habib S., Mustafa S.A. Husain. Buffalo infection by Fasciola gigantica transmitted by Radix acuminata in Uttar Pradesh, India: a molecular tool to improve snail vector epidemiology assessments and control surveillance. Acta Parasitol. 2021;66:1396–1405. doi: 10.1007/s11686-021-00414-3. [DOI] [PubMed] [Google Scholar]
- 56.Mitchell P. Oxford University Press; Oxford: 2018. The Donkey in Human History. An Archaelogical Perspective; p. 306. [Google Scholar]
- 57.Sierra R. Mera y, Neira G., Bargues M.D., Cuervo P.F., Artigas P., Logarzo L., Cortiñas G., Ibaceta D.E.J., Lopez G.A., Bisutti I.E., Mas-Coma S. Equines as reservoirs of human fascioliasis: transmission capacity, epidemiology and pathogenicity in Fasciola hepatica-infected mules. J. Helminthol. 2020;94 doi: 10.1017/S0022149X20000693. [DOI] [PubMed] [Google Scholar]
- 58.Le T.H., Pham K.L.T., Doan H.T.T., Le T.K.X., Nguyen K.T., Lawton S.P. Description and phylogenetic analyses of ribosomal transcription units from species of Fasciolidae (Platyhelminthes: Digenea) J. Helminthol. 2020;94(e136):1–6. doi: 10.1017/S0022149X20000164. [DOI] [PubMed] [Google Scholar]
- 59.Mohanta U.K., Ichikawa-Seki M., Shoriki T., Katakura K., Itagaki T. Characteristics and molecular phylogeny of Fasciola flukes from Bangladesh, determined based on spermatogenesis and nuclear and mitochondrial DNA analyses. Parasitol. Res. 2014;113:2493–2501. doi: 10.1007/s00436-014-3898-5. [DOI] [PubMed] [Google Scholar]
- 60.Saijuntha W., Tantrawatpan C., Agatsuma T., Wang C., Intapan P.M., Maleewong W., Petney T.N. Revealing genetic hybridization and DNA recombination of Fasciola hepatica and Fasciola gigantica in nuclear introns of the hybrid Fasciola flukes. Mol. Bioch. Parasitol. 2018;223:31–36. doi: 10.1016/j.molbiopara.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 61.Valero M.A., Mas-Coma S. Comparative infectivity of Fasciola hepatica metacercariae from isolates of the main and secondary reservoir animal host species in the Bolivian Altiplano high human endemic region. Folia Parasitol. 2000;47:17–22. doi: 10.14411/fp.2000.004. [DOI] [PubMed] [Google Scholar]
- 62.Valero M.A., Darce N.A., Panova M., Mas-Coma S. Relationships between host species and morphometric patterns in Fasciola hepatica adults and eggs from the Northern Bolivian Altiplano hyperendemic region. Vet. Parasitol. 2001;102:85–100. doi: 10.1016/s0304-4017(01)00499-x. [DOI] [PubMed] [Google Scholar]
- 63.Valero M.A., Panova M., Comes A.M., Fons R., Mas-Coma S. Patterns in size and shedding of Fasciola hepatica eggs by naturally and experimentally infected murid rodents. J. Parasitol. 2002;88:308–313. doi: 10.1645/0022-3395(2002)088[0308:PISASO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 64.Angles R., Buchon P., Valero M.A., Bargues M.D., Mas-Coma S. One Health action against human fascioliasis in the Bolivian Altiplano: food, water, housing, behavioural traditions, social aspects, and livestock management linked to disease transmission and infection sources. Int. J. Environm. Res. Publ. Health. 2022;19:1120. doi: 10.3390/ijerph19031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mas-Coma S., Valero M.A., Bargues M.D. One Health for fascioliasis control in human endemic areas. Trends Parasitol. 2023;38:650–667. doi: 10.1016/j.pt.2023.05.009. [DOI] [PubMed] [Google Scholar]
- 66.Mas-Coma S., Bargues M.D. Populations, hybrids and the systematic concepts of species and subspecies in Chagas disease triatomine vectors inferred from nuclear ribosomal and mitochondrial DNA. Acta Trop. 2009;110:112–136. doi: 10.1016/j.actatropica.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 67.Bargues M.D., Zuriaga M.A., Mas-Coma S. Nuclear rDNA pseudogenes in Chagas disease vectors: evolutionary implications of a new 5.8S+ITS-2 paralogous sequence marker in triatomines of North, Central and northern South America. Infect. Genet. Evol. 2014;21:134–156. doi: 10.1016/j.meegid.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 68.Morgan J.A., Blair D. Trematode and monogenean rRNA ITS2 secondary structures support a four-domain model. J. Mol. Evol. 1998;47:406–419. doi: 10.1007/pl00006398. [DOI] [PubMed] [Google Scholar]
- 69.Cwiklinski K., Dalton J.P., Dufresne P.J., La Course J., Williams D.J.L., Hodgkinson J., Paterson S. The Fasciola hepatica genome: gene duplication and polymorphism reveals adaptation to the host environment and the capacity for rapid evolution. Genome Biol. 2015;16:71. doi: 10.1186/s13059-015-0632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McNulty S.N., Tort J.F., Rinaldi G., Fischer K., Rosa B.A., Smircich P., Fontenla S., Choi Y.J., Tyagi R., Hallsworth-Pepin K., Mann V.H., Kammili L., Latham P.S., Dell’Oca N., Dominguez F., Carmona C., Fischer P.U., Brindley P.J., Mitreva M. Genomes of Fasciola hepatica from the Americas reveal colonization with Neorickettsia endobacteria related to the agents of Potomac horse and human Sennetsu fevers. PLoS Genet. 2017;13 doi: 10.1371/journal.pgen.1006537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are presented in the article. The sequence data have been submitted to GenBank and have been included under the following accession Nos: OQ064778- OQ064781.