Abstract
Introduction
High levels of immunity to SARS-CoV-2 in the community correlate with protection from COVID-19 illness. Measuring COVID-19 antibody seroprevalence and persistence may elucidate the level and length of protection afforded by vaccination and infection within a population.
Methods
We measured the duration of detectable anti-spike antibodies following COVID-19 vaccination in a multistate, longitudinal cohort study of almost 13,000 adults who completed daily surveys and submitted monthly dried blood spots collected at home.
Results
Overall, anti-spike antibodies persisted up to 284 days of follow-up with seroreversion occurring in only 2.4% of the study population. In adjusted analyses, risk of seroreversion increased with age (adults aged 55–64: adjusted hazard ratio [aHR] 2.19 [95% confidence interval (CI): 1.22, 3.92] and adults aged > 65: aHR 3.59 [95% CI: 2.07, 6.20] compared to adults aged 18–39). Adults with diabetes had a higher risk of seroreversion versus nondiabetics (aHR 1.77 [95% CI: 1.29, 2.44]). Decreased risk of seroreversion was shown for non-Hispanic Black versus non-Hispanic White (aHR 0.32 [95% CI: 0.13, 0.79]); college degree earners versus no college degree (aHR 0.61 [95% CI: 0.46, 0.81]); and those who received Moderna mRNA-1273 vaccine versus Pfizer-BioNTech BNT162b2 (aHR 0.35 [95% CI: 0.26, 0.47]). An interaction between healthcare worker occupation and sex was detected, with seroreversion increased among male, non-healthcare workers.
Conclusion
We established that a remote, longitudinal, multi-site study can reliably detect antibody durability following COVID-19 vaccination. The survey platform and measurement of antibody response using at-home collection at convenient intervals allowed us to explore sociodemographic factors and comorbidities and identify predictors of antibody persistence, which has been demonstrated to correlate with protection against disease. Our findings may help inform public health interventions and policies to protect those at highest risk for severe illness and assist in determining the optimal timing of booster doses.
Clinical trials registry: NCT04342884.
Keywords: SARS-CoV-2, COVID-19, COVID-19 vaccines, Serology, Antibody responses
Introduction
Vaccine-induced immunity has been associated with reduced risk of SARS-CoV-2 infection, hospitalizations, and death [1], [2], [3], [4], [5], [6], [7], [8], [9]. The SARS-CoV-2 spike protein, the target of approved SARS-CoV-2 vaccines, is a viral transmembrane glycoprotein required for binding, fusion, and cell entry [3], [10], [11]. Shortly after exposure to SARS-CoV-2 infection or COVID-19 vaccination, the majority of individuals generate anti-spike protein antibody responses [10], [12], [13]. Anti-spike protein antibody titers correlate with neutralizing antibody titers, which studies have shown are capable of blocking viral entry and correspond with protection from SARS-CoV-2 infection [1], [8]. Over time, in the absence of repeated infection or vaccination, anti-spike protein antibodies appear to wane [10], [14], [15] but can still be detectable for several months [1], [14], [15]. Data from clinical trials and observational studies suggest that the seroprevalence of anti-spike antibodies in the community correlates with protection from symptomatic COVID-19 illness [14]. Thus, capturing longitudinal seroprevalence data may contribute to our understanding of the durability of protection afforded by vaccination and infection within a population. Ultimately, seroprevalence information could be useful for informing public health policies and infection control measures at the community, state, and national levels.
In a multistate, longitudinal SARS-CoV-2 cohort study, adult participants from six healthcare systems completed daily electronic surveys, allowed access to their health care records, and submitted monthly dried blood spot specimens that were tested for anti-SARS-CoV-2 antibodies [16]. To examine anti-spike antibody durability after vaccination and associated predictors of antibody persistence versus seroreversion (loss of anti-spike antibodies), we analyzed anti-spike antibody responses in vaccinated participants and their relationship to previous COVID-19 illness, demographic characteristics, healthcare occupation status, presence of certain comorbidities, and vaccine product received.
Methods
Study participants
The COVID-19 Community Research Partnership (CCRP) was a prospective, multistate, and multisite surveillance platform for the study of SARS-CoV-2 epidemiology funded by the US Centers for Disease Control and Prevention [16]. From April 2020 through October 2021, we enrolled adults 18 years of age and older affiliated with six healthcare systems and institutions located throughout the Mid-Atlantic and Southern United States: Wake Forest Baptist Health, Atrium Health, Tulane University Medical Group and affiliated local networks, University of Mississippi Medical Center, University of Maryland Medical System, and Medstar Health. Persons affiliated with the participating healthcare systems were invited to enroll in the study, including patients and staff based at clinical facilities; other community members were invited to participate via patient portals, public websites, or community outreach.
The CCRP study was conducted remotely. Study methods including participant recruitment, daily symptom surveys, infection and at-home antibody testing, vaccination frequency and product type, use of personal protective equipment, supplemental surveys, and access to participant Electronic Health Care Records (EHR) have been described previously[16]. Briefly, adult enrollment included email or text messaging via the health systems’ patient communication portals (e.g., MyChart); social media and public relations campaigns led by individual sites; virtual and in-person community outreach; and a publicly accessible website. Each participant interested in joining the study completed a web-based informed consent and a survey to collect demographic and occupational information. Following enrollment, each participant was asked to complete a daily symptom survey (Oracle Patient Monitoring System provided by Oracle Corporation, Redwood Shores, California or SneezSafe application provided by Sneez, LLC, Winston Salem, NC, USA). Participants completed the surveys via an internet-accessible computer or smartphone. Surveys included information on COVID-19 symptoms, mask use, care-seeking behaviors, known COVID-19 exposures, and self-reported SARS-CoV-2 testing results. A question about COVID-19 vaccination dose, dates, and vaccine product was added in December 2020, as described previously [16], [17]. The following EHR data were extracted quarterly: health service utilization, diagnoses, medications, procedures, and laboratory test results. An additional supplemental survey was administered once per participant that queried participants’ occupation and industry, allowing for free-text responses (Supplementary Materials).
A subset of participants were selected for repeat, longitudinal, at-home serologic sampling. Selection criteria aimed to mimic the overall demographics (sex, race/ethnicity, age) of the service area based on the 2017 American Community Survey census [18]. Participants were eligible to receive at least six tests over the 12-month period.
The study was reviewed and approved by the Wake Forest Institutional Review Board (IRB), which served as the central IRB for this study. The study is registered with ClinicalTrials.gov, NCT04342884.
Laboratory assay characteristics
Serology testing utilized at-home collection of blood on a Whatman 5-spot dried blood spot (DBS) specimen card. A study-branded kit was mailed to each participant with instructions for specimen collection, two lancets, and a self-addressed return envelope with instructions to return test kits monthly for analysis. Capillary blood was collected by finger prick. All specimens with sufficient dried blood were evaluated for anti-spike antibody using a EuroImmun qualitative enzyme-linked immunosorbent assay (ELISA) targeting SARS-CoV-2 anti-spike immunoglobulin G (IgG). This test has been granted Emergency Use Authorization from the U.S. Food and Drug Administration for testing on venous blood [19]. Any DBS card with a positive EuroImmun result was reflexed to test for anti-nucleocapsid antibody using a qualitative Roche pan-Ig assay targeting the nucleocapsid protein [19]. Given that all approved SARS-CoV-2 vaccinations target the spike protein, a positive anti-spike result could be associated with either infection or vaccination, but a positive anti-nucleocapsid result would only be expected after infection. Both serologic assays were internally validated for use with DBS cards; evaluation of DBS using the EuroImmun assay was previously reported [19], [20]. Laboratory Corporation of America (LabCorp, www.labcorp.com) conducted all kit shipping, receipt of DBS cards, serology testing, and reporting.
Measures and definitions
Self-reported demographic variables collected at enrollment or through supplemental surveys included age, sex (male, female), race/ethnicity (Hispanic, non-Hispanic Black, non-Hispanic White, and non-Hispanic other), education (college degree, no college degree), and whether employed as a healthcare worker (yes, no). The category, non-Hispanic other, included American Indian or Alaskan Native, Asian or Pacific Islander, and mixed race/ethnicity. We classified county of residence as rural, suburban, or urban based on population density [16], [18]. History of diabetes (ICD10 codes: E08-E11) and obesity (ICD10 codes: E66, Z68 BMI ≥ 30 kg/m2) diagnoses, collected from the EHR, were included in the analyses since these comorbidities were found to correlate with SARS-CoV-2 infection in a separate CCRP study [21] and because our sample size for these comorbidities was large enough for meaningful analysis. No distinction was made between type I and type II diabetes.
Vaccination status was ascertained using a combination of self-reported data from the daily survey beginning in December 2020. The survey included date, dose, and product of any COVID-19 vaccine received. If vaccination data was missing from the survey, but available in the EHR, then the EHR information for that dose was included [17]. Vaccine brand received was defined as the brand of first dose of mRNA vaccine if more than one brand was received.
Among a subset of participants who completed a supplemental survey, we also evaluated self-reported healthcare worker occupation from the free-text responses to a question regarding the participants’ primary occupation. Free-text responses were assigned a six-digit Standard Occupational Classification (SOC) code using the NIOSH Industry and Occupation Computerized Coding System (NIOCCS) auto-coder [22]. The hierarchical six-digit SOC codes were transformed into two-digit codes. Healthcare worker occupation was defined as having a two-digit SOC code of 29 or 31 or a six-digit SOC code of 11–9111.
Participant inclusion and exclusion
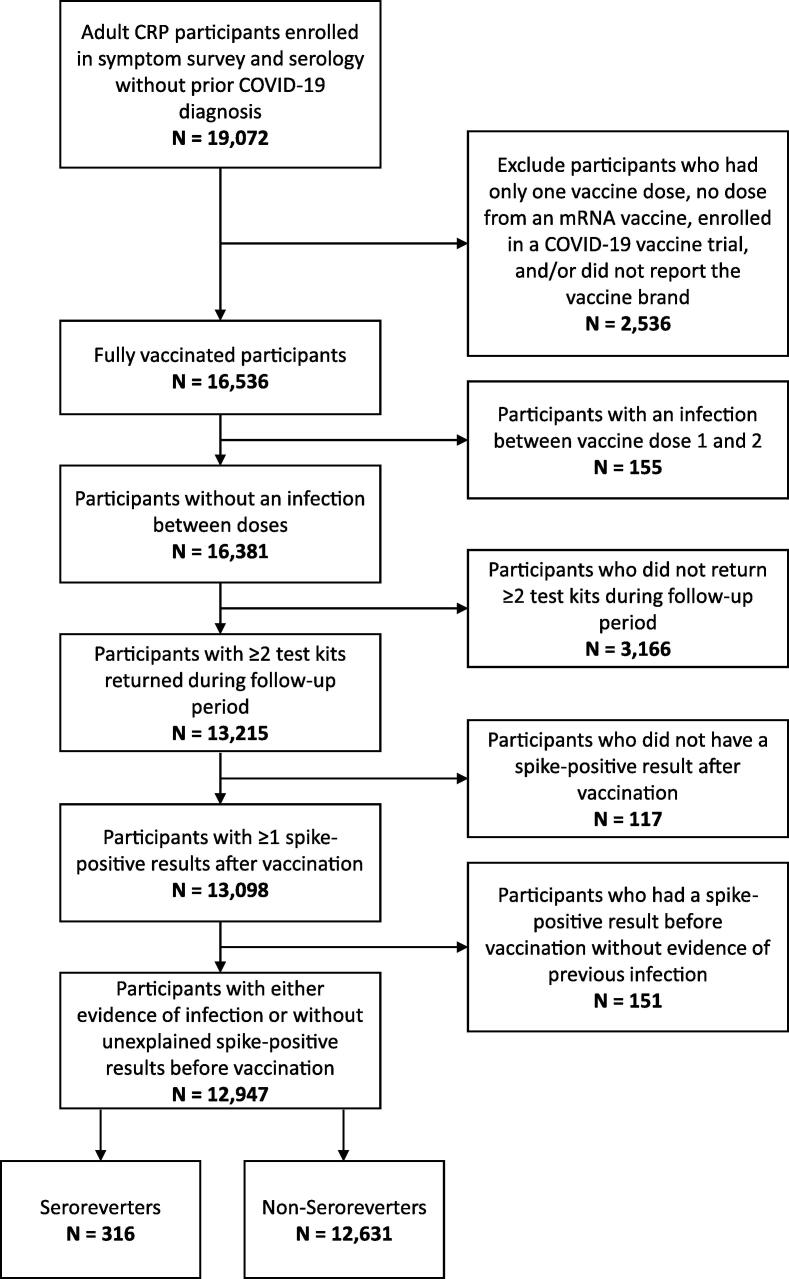
Participants were eligible for the analysis if the following criteria were met: age ≥ 18 years; participated in the daily questionnaire component of the study; did not self-report a previous COVID-19 diagnosis prior to enrolling in the study; returned at least 2 serology test kits during the follow-up period; and had evidence of primary vaccination with two doses of a COVID-19 vaccine of which at least one dose was a mRNA vaccine outside of a clinical trial. Participants were excluded if they had no evidence of a positive anti-spike result during the duration of their follow-up, developed infection between vaccine doses one and two, or had an uninterpretable serology result prior to vaccination. A positive spike and negative nucleocapsid antibody result before vaccination was deemed uninterpretable because it could represent a false positive spike antibody test, a false negative nucleocapsid antibody test, incorrect vaccination dates, or unreported/asymptomatic infection with seroreversion of nucleocapsid antibody.
Statistical methods
We used time-to-event analyses—time to seroreversion—to explore the duration of anti-spike serum antibody detection in participants following COVID-19 vaccination. Follow-up time was from the day following receipt of the second vaccine dose to the date of documented seroreversion (a negative anti-spike antibody test following a previous positive anti-spike antibody test), last serological test, receipt of a third/booster dose of vaccine, infection (if infection followed vaccination), whichever came first. Previous infection was determined by self-report, documentation of a positive viral test in the EHR, or positive anti-nucleocapsid result prior to the first dose of vaccine. Participant characteristics analyzed were previous infection status, age group, sex, race/ethnicity, healthcare worker occupation (yes or no) as reported at enrollment, study site, county classification, education level, vaccine product, and presence of select comorbidities.
To explore the distribution of participant characteristics at baseline, we used univariate analyses to compare participants who did not serorevert to those who did serorevert. To calculate the person-time incidence of seroreversion for all participants, the number of seroreverters was divided by the person-years of follow-up. The overall incidence was stratified by participant characteristics and the number of seroreverters, person-years of follow-up, and incidence calculated. We used Cox proportional hazard models to identify the crude hazard for seroreversion for each characteristic and obtained adjusted Hazard Ratios [aHR] by fitting all patient characteristics in a multivariable Cox Model. We assessed the interaction between statistically significant variables including sex, education, healthcare worker occupation, age group, race/ethnicity, and vaccine brand. Following the identification of a statistically significant interaction, investigators presented the aHR stratified by the interaction terms. Two-sided significance was at p less than 0.05 without adjustment made for multiple comparisons. All statistical analyses were performed using R Statistical Software (version 4.0.3; R Foundation for Statistical Computing, Vienna, Austria).
Results
Study participants
Of 19,072 participants enrolled in the CCRP with available antibody tests, 12,947 met inclusion criteria with regards to COVID-19 vaccination for this analysis (Fig. 1). Of the included participants, most were 55 years of age or older (57%), female (64%), non-Hispanic White (84%), resided in urban areas (51%), and held a college degree (81%) (Table 1). Participants were primarily from the Wake Forest Baptist Health System in North Carolina (29%) or MedStar Health System in Maryland (38%). By design, all participants were vaccinated, with most receiving the Pfizer-BioNTech BNT162b2 vaccine (63%). Slightly less than 4% of participants reported a SARS-CoV-2 infection during the study period.
Fig. 1.
Flow diagram for participant enrollment and analysis Notes: Vaccination status was confirmed with electronic health record data when available [17].
Table 1.
Participant Characteristics by Seroreversion Status.
| Overall | Did not Serorevert | Seroreverted | p-value | |
|---|---|---|---|---|
| N | 12,947 | 12,631 | 316 | |
| Age (years, mean + SD) | 56.0 ± 15.3 | 55.7 ± 15.3 | 66.8 ± 12.8 | <0.0011 |
| Age (years) | <0.0012 | |||
| 18–39 | 2,527 (19.5%) | 2,510 (19.9%) | 17 (5.4%) | |
| 40–54 | 3,016 (23.3%) | 2,980 (23.6%) | 37 (11.4%) | |
| 55–64 | 2,595 (20.0%) | 2,588 (20.2%) | 46 (14.6%) | |
| 65 + | 4,809 (37.1%) | 4,592 (36.4%) | 218 (68.7%) | |
| Sex | <0.0013 | |||
| Female | 8,317 (64.2%) | 8,177 (64.7%) | 140 (44.3%) | |
| Male | 4,630 (35.8%) | 4,454 (35.3%) | 177 (55.7%) | |
| Race and ethnicity | <0.0012 | |||
| Hispanic | 450 (3.5%) | 445 (3.5%) | 5 (1.6%) | |
| Non-Hispanic Black | 888 (6.9%) | 882 (7.0%) | 6 (1.9%) | |
| Non-Hispanic Other | 677 (5.2%) | 666 (5.3%) | 11 (3.5%) | |
| Non-Hispanic White | 10,932 (84.4%) | 10,638 (84.2%) | 297 (93.0%) | |
| Healthcare worker | 0.1733 | |||
| No | 10,450 (80.7%) | 10,185 (80.6%) | 265 (83.9%) | |
| Yes | 2,497 (19.3%) | 2,446 (19.4%) | 51 (16.1%) | |
| County of residence | 0.7642 | |||
| Rural | 2,881 (22.3%) | 2,812 (22.3%) | 69 (21.8%) | |
| Suburban | 3,495 (27.0%) | 3,404 (26.9%) | 91 (28.8%) | |
| Urban | 6,571 (50.8%) | 6,415 (50.8%) | 156 (49.4%) | |
| Enrollment Site4 | 0.9502 | |||
| Atrium (NC) | 1,970 (15.2%) | 1,921 (15.2%) | 49 (15.5%) | |
| Univ. of Maryland (MD) | 2,246 (17.3%) | 2,195 (17.4%) | 51 (16.1%) | |
| MedStar (MD) | 4,868 (37.6%) | 4,753 (37.6%) | 115 (36.4%) | |
| Univ. of Mississippi (MS) | 93 (0.7%) | 91 (0.7%) | 2 (0.6%) | |
| Tulane (LA) | 55 (0.4%) | 54 (0.4%) | 1 (0.3%) | |
| Wake Forest (NC) | 3,715 (28.7%) | 3,617 (28.6%) | 98 (31.0%) | |
| Received Influenza Vaccine | ||||
| No | 1,346 (10.6%) | 1,322 (10.7%) | 24 (7.7%) | 0.1143 |
| Yes | 11,311 (89.45) | 11,025 (89.3%) | 286 (92.3%) | |
| Education level | 0.0313 | |||
| College Degree | 10,183 (80.5%) | 9,949 (80.6%) | 234 (75.5%) | |
| No College Degree | 2,474 (19.5%) | 2,398 (19.4%) | 76 (24.5%) | |
| Vaccine Product | <0.0013 | |||
| Moderna mRNA-1273 | 4,854 (37.5%) | 4,789 (37.9%) | 65 (20.6%) | |
| Pfizer BNT162b2 | 8,093 (62.5%) | 7,842 (62.1%) | 251 (79.4%) | |
| Days between doses | 24.8 ± 6.1 | 24.8 ± 6.2 | 22.9.0 ± 4.7 | <0.0011 |
| Previous COVID-19 Infection | 0.0123 | |||
| No | 12,461 (96.2%) | 12,148 (96.2%) | 313 (99.1%) | |
| Yes | 486 (3.8%) | 483 (3.8%) | 3 (0.9%) | |
| Diabetes | ||||
| No | 10,241 (90.4%) | 10,001 (90.6%) | 240 (81.4%) | <0.0013 |
| Yes | 1,087 (9.6%) | 1,032 (9.4%) | 55 (18.6%) | |
| Obesity | ||||
| No | 9,478 (83.7%) | 9,237 (83.7%) | 241 (81.7%) | 0.3963 |
| Yes | 1850 (16.3%) | 1,796 (16.3%) | 54 (18.3%) |
Welch Two Sample t-test.
Pearson’s Chi-squared test.
Pearson’s Chi-squared test with Yates’ continuity correction.
Full names of enrollment sites: Atrium Health (NC), University of Maryland Medical System (MD), Medstar Health (MD), University of Mississippi Medical Center (UMMC), Tulane University (LA), Wake Forest Baptist Health (NC).
Among a subset of participants (n = 1,818) that reported being a healthcare worker, using NIOSH employment cross-classification, the largest group of participants was nursing staff (45%), 26% were physicians, and the remaining came from a variety of medical and health care professions (e.g., dentists, nutritionists or dietitians, paramedics, therapists).
Seroreversion after COVID-19 vaccination
The vast majority of our study population, 12,631 (97.6%) maintained their post-vaccination antibody responses throughout follow-up (up to date of last serology test, booster vaccination, or subsequent infection). Seroreverters (n = 316) differed statistically from those who did not serorevert for several characteristics (Table 1). Seroreverters were older (67 vs. 56 years), more likely to be male (56% vs. 35%), more likely to be non-Hispanic White (93% vs. 84%), and less likely to have a college degree (76% vs. 81%), compared to those who did not serorevert. Seroreverters were also more likely to have received a Pfizer-BioNTech BNT162b2 vaccine (79% vs. 62%), had a shorter time between doses (23 vs. 25 days), were more likely to have diabetes (19% vs. 9%), and were less likely to report a previous SARS-CoV-2 infection (1% vs. 4%). The two groups, those who seroreverted and those who maintained anti-spike antibodies, did not differ statistically by healthcare worker occupation, county of residence (i.e., urban, rural, suburban), enrollment site, or obesity.
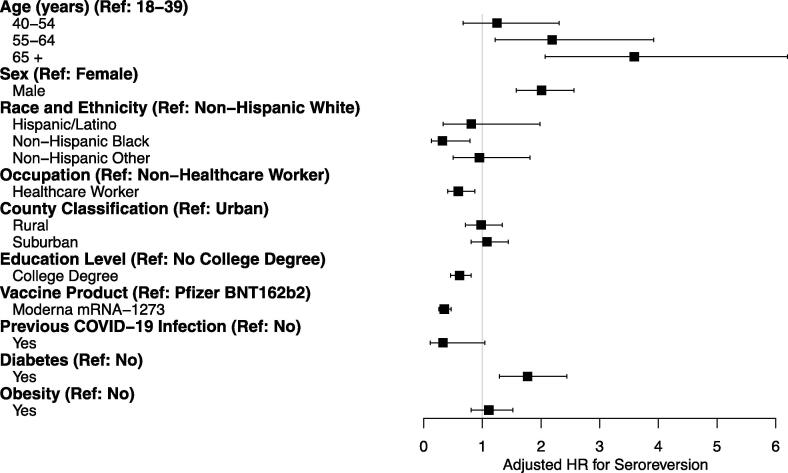
The analytic follow-up time for participants ranged from 1 to 284 days with a median follow-up time per participant of 167 days [Interquartile Range: 130, 202 days]. The overall incidence during that period was 5.4 seroreversions per 100 person-years (5.4/100py) of follow-up (Table 2). The incidence and risk of seroreversion increased stepwise by age group starting with 1.6/100py for those 18 to 39 years old and peaking at 9.2/100py for participants ≥ 65 years old. Relative to the youngest age group (18–39 years), the aHR for seroreversions was 1.25 (95%CI: 0.67 to 2.31), 2.19 (95%CI: 1.22 to 3.92), and 3.59 (95%CI: 2.07 to 6.20) for ages 40 to 54, 55 to 64, and ≥ 65 years, respectively (Fig. 2).
Table 2.
Incidence, Crude Hazard Ratios, and Adjusted Hazard Ratios by Population Characteristics.
| N | Seroreverters/person years of follow-up | Incidence per 100 person-years of follow-up |
Crude Hazard Ratio (95% CI) |
Adjusted Hazard Ratio (95% CI) | |
|---|---|---|---|---|---|
| Overall | 12,947 | 316/5812 | 5.4 | – | – |
| Age (years) | |||||
| 18–39 | 2,527 | 17/1031 | 1.6 | REF | REF |
| 40–54 | 3,016 | 36/1297 | 2.8 | 1.71 (0.96–3.04) | 1.25 (0.67–2.31) |
| 55–64 | 2,595 | 46/1139 | 4 | 2.81 (1.61–4.91) | 2.19 (1.22–3.92) |
| 65 + | 4,809 | 217/2346 | 9.2 | 6.21 (3.74–10.16) | 3.59 (2.07–6.20) |
| Sex1 | |||||
| Female | 8,317 | 140/3769 | 3.7 | REF | REF |
| Male | 4,630 | 176/2042 | 8.6 | 2.66 (2.13–3.32) | 2.01 (1.58–2.56) |
| Race and Ethnicity | |||||
| Non-Hispanic White | 10,932 | 294/4961 | 5.9 | REF | REF |
| Hispanic/Latino | 450 | 5/186 | 2.7 | 0.48 (0.2–1.15) | 0.81 (0.33–1.98) |
| Non-Hispanic Black | 888 | 6/375 | 1.6 | 0.29 (0.13–0.65) | 0.32 (0.13–0.79) |
| Non-Hispanic Other | 677 | 11/290 | 3.8 | 0.61 (0.34–1.12) | 0.95 (0.5–1.81) |
| Healthcare worker1 | |||||
| No | 10,450 | 265/4502 | 5.9 | REF | REF |
| Yes | 2,497 | 51/1310 | 3.9 | 0.3 (0.22–0.42) | 0.59 (0.41–0.87) |
| County Classification | |||||
| Urban | 6,571 | 156/2906 | 5.4 | REF | REF |
| Rural | 2,881 | 69/1308 | 5.3 | 0.94 (0.71–1.25) | 0.98 (0.71–1.34) |
| Suburban | 3,495 | 91/1598 | 5.7 | 1.01 (0.77–1.29) | 1.08 (0.81–1.44) |
| Enrollment Site2 | |||||
| Wake Forest | 3,715 | 98/1651 | 5.9 | REF | REF |
| Atrium | 1,970 | 49/905 | 5.4 | 0.81 (0.58–1.15) | 1.01 (0.71–1.44) |
| Maryland | 2,246 | 51/1085 | 4.7 | 0.76 (0.54–1.07) | 0.97 (0.67–1.42) |
| MedStar | 4,868 | 115/2097 | 5.5 | 1.08 (0.83–1.42) | 1.33 (0.99–1.79) |
| Mississippi | 93 | 2/49 | 4.1 | 0.45 (0.11–1.85) | 0.35 (0.05–2.58) |
| Tulane | 55 | 1/24 | 4.2 | 0.69 (0.1–4.94) | 1.09 (0.15–7.91) |
| Education Level | |||||
| No College Degree | 2,474 | 76/1072 | 7.1 | REF | REF |
| College Degree | 10,183 | 234/4642 | 5 | 0.64 (0.5–0.83) | 0.61 (0.46–0.81) |
| Vaccine Product | |||||
| Pfizer BNT162b2 | 8,093 | 251/3639 | 6.9 | REF | REF |
| Moderna mRNA-1273 | 4,854 | 65/2173 | 3 | 0.48 (0.36–0.63) | 0.35 (0.26–0.47) |
| Previous COVID-19 Infection | |||||
| No | 12,461 | 313/5619 | 5.6 | REF | REF |
| Yes | 486 | 3/193 | 1.6 | 0.3 (0.1–0.95) | 0.33 (0.11–1.04) |
| Diabetes | |||||
| No | 10,241 | 240/4628 | 5.2 | REF | REF |
| Yes | 1,087 | 55/484 | 11.4 | 2.41 (1.80–3.23) | 1.77 (1.29–2.44) |
| Obesity | |||||
| No | 9,478 | 241/4295 | 5.6 | REF | REF |
| Yes | 1,880 | 54/817 | 6.6 | 1.21 (0.90–1.62) | 1.11 (0.81–1.52) |
| Interaction: HCW [Y] × Sex [M] | – | – | – | – | 0.37 (0.17–0.80) |
Variable is present in an interaction (see bottom of table and Table 3).
Full names of enrollment sites: Atrium Health (NC), University of Maryland Medical System (MD), Medstar Health (MD), University of Mississippi (MS), Tulane University (LA), Wake Forest Baptist Health (NC).
Fig. 2.
Forest plot for seroreversion among participants. *Participants were permitted to choose one option for race/ethnicity: American Indian or Alaskan Native, Asian or Pacific Islander, Black or African American, Hispanic or Latino, Mixed Ethnicity, or White (not Hispanic/Latino). For the current study, the included race/ethnicity variables were Hispanic, Black, non-Hispanic White, and Other, which included the remaining options and the non-responses.
Among race/ethnicity groups, non-Hispanic Whites had the highest incidence of seroreversion, 5.9/100py, followed in descending order by non-Hispanic other (3.7/100py), Hispanic/Latino (2.7/100py), and non-Hispanic Black (1.6/100py). The adjusted risk of seroreversion for non-Hispanic Blacks was statistically lower compared to non-Hispanic Whites (aHR 0.32: 95% CI: 0.13 to 0.79). Participants with a college degree had a lower incidence of seroreverting than participants with less education (5.0/100py vs. 7.0/100py) and reduced risk of seroreversion (aHR 0.61, 95%CI: 0.46–0.81).
The highest incidence of seroreversion among any population category was for participants with diabetes with an incidence of 11.3/100py. In contrast, participants without diabetes had an incidence of 5.2/100py. The aHR associated with having diabetes was 1.77 (95%CI: 1.29 to 2.44) compared to those without diabetes. A COVID-19 infection before vaccination showed a tendency toward protection (aHR 0.33, 95% CI: 0.11 to 1.04) but was not statistically significant.
Interaction
A statistically significant interaction was detected in this analysis: healthcare worker occupation by sex (Table 2). Compared to male non-healthcare workers, risk of seroreversion was reduced for male healthcare workers (aHR: 0.29, 95%CI: 0.15–0.56) and for all females regardless of being a healthcare worker (aHR 0.33, 95%CI: 0.22–0.51) or not (aHR 0.39, 95% CI: 0.3–0.51) (Table 3). All of the other interactions assessed for combinations between sex, education, healthcare worker occupation, age group, race/ethnicity, and vaccine brand were not statistically significant.
Table 3.
Interaction Results.
| Characteristics | aHR (95% CI) | p-value |
|---|---|---|
| Healthcare Worker X Sex | 0.004 | |
| Non HCW: Male | REF | REF |
| HCW: Female | 0.36 (0.23–0.55) | <0.001 |
| Non HCW: Female | 0.44 (0.33–0.57) | <0.001 |
| HCW: Male | 0.30 (0.15–0.61) | <0.001 |
| Adjusted for healthcare worker occupation, sex, vaccine product, age group, race/ethnicity, county classification, study site, education level, previous COVID-19 infection, diabetes, and obesity. | ||
Discussion
In this longitudinal, multi-state cohort study of nearly 13,000 adults, participants maintained detectable anti-spike antibodies until dropout or censoring that occurred at booster vaccination or subsequent infection, reflecting a highly durable antibody response to COVID-19 vaccination. Still, we detected higher rates of seroreversion in some subgroups. We found the risk of seroreversion increased with age group and the oldest adults were several times more likely to serorevert than the youngest adults. This finding is consistent with prior studies that showed that immune responses to vaccination are often weaker in older adults [23], [24]. A diagnosis of diabetes was identified as an independent risk factor for seroreversion. This aligns with a prior study showing a poorer IgG anti-spike response to mRNA vaccination in persons with diabetes [25], thought to arise because of an impaired immune system with metabolic abnormalities [26].
Two sociodemographic factors, race/ethnicity and education, were associated with protection from seroreversion. Racial and ethnic minorities have been at higher risk of SARS-CoV-2 exposure and disease [23], [27], [28], so the higher antibody durability we saw among non-Hispanic Blacks relative to non-Hispanic Whites may have been due to more frequent asymptomatic boosting of spike antibody responses from those exposures. However, this would imply SARS-CoV-2 exposures during participant follow-up that were significant enough to boost spike antibody responses but not enough to result in a positive anti-nucleocapsid antibody response. Investigators have previously reported an association between higher education and lower risk of SARS-CoV-2 infection and disease [27], [28]. This may imply protection from seroreversion observed in those who were more highly educated may be due to socioeconomic status factors such as income (not evaluated in this study) that could contribute to overall better states of health and therefore, better immune responses to vaccination.
Based on previous findings [29], [30], [31], we expected to see a decreased risk of seroreversion in those who had a confirmed COVID-19 infection prior to vaccination. We observed a non-statistically significant reduced hazard in those with confirmed infection prior to vaccination. Lack of statistical significance may be due to insufficient statistical power to detect a difference given the small sample population that reported a previous infection. Other researchers have shown an association with stronger serological responses from receiving the Moderna mRNA-1273 vaccine compared with the Pfizer-BioNTech BNT162b2 vaccine [31], [32], [33]. Our data also suggested a similar difference by vaccine product.
We noted a statistical interaction suggesting that time to seroreversion differed by sex and occupation. Increased durability of antibody responses to vaccination in females compared to males has been described previously [34], [35], [36], and our findings suggested the same. However, in this study, we found the durability of antibody response between sexes was largely due to relatively shorter antibody durability among male non-healthcare workers. The differences between males by healthcare worker occupation may be due to differences in occupational SARS-CoV-2 exposures and/or differences in risk prevention behaviors such as mask use or social distancing.
Our study design allowed for the capture of subclinical infections that were not reported by the volunteers as illnesses because all positive tests for anti-spike antibody were further tested for the presence of anti-nucleocapsid antibodies, which are elicited by natural infection but not by either of the mRNA vaccines. Nonetheless, participants could have had subclinical infections that were anti-nucleocapsid antibody negative (e.g., due to low test sensitivity) but that “boosted” spike antibody responses. Also, Tutukina et al. suggested [37] that some SARS-CoV-2 infections are accompanied by a rise in anti-spike antibody responses without a detectable rise in anti-nucleocapsid antibodies. Failure to detect subclinical infections could have led to bias in our study if the groups compared have different risk of infection. For example, if healthcare workers are at higher risk of occupational exposure and had subclinical infections, their antibody responses could have been boosted by the subclinical infections.
The strengths of our study include its size and geographic diversity across multiple sites within the mid-Atlantic and Southern United States and longitudinal, remote data collection of symptoms and risk behavior. Even though COVID-19 antibody responses were qualitative and measured at monthly intervals, the ease of home-based sample collection yielded a high frequency of kit return and interpretable results. Integration of syndromic surveillance, antibody testing, and electronic health records provided robust data from which we were able to demonstrate durable antibody responses to vaccination and detect differences in antibody durability across sub-groups of participants.
Limitations of our study included a lack of racial, ethnic, and sociodemographic diversity in our study population, with high proportions of respondents being female, non-Hispanic White, college degree holders. Our population was also highly vaccinated, which limited our ability to compare outcomes in vaccinated and unvaccinated groups. We are also limited in only being able to describe antibody response rather than outcomes of severe disease and hospitalization, which are rare events needing an even larger sample size. Our analysis was limited to data collected through October 2021 and does not include estimates of antibody duration since the emergence of the Omicron variant nor following widespread use of vaccine booster doses. Our large effect size for non-healthcare worker males having increased risk of seroreversion were robust to reanalysis of the data, which lead us to feel strongly that the associations, though difficult to explain biologically, are real. Nonetheless, we cannot rule out the presence of unmeasured confounders.
In summary, we have established that a longitudinal, multi-site study is able to robustly detect differences in antibody durability following COVID-19 vaccination. Our results add to our understanding of the kinetics of serum anti-spike antibodies within the community and potentially enhance our understanding of the level and length of protective humoral immunity. We expect these predictors of seroreversion may be useful to identifying and monitoring populations at risk of breakthrough infections and to informing vaccination doses and schedules. Despite declarations otherwise, the COVID-19 pandemic continues and despite a relatively low incidence time in March 2023 still causes approximately 500 deaths per day in the U.S [38]. Further work to correlate antibody response with neutralizing antibody titers and with protection from severe disease and hospitalization will allow serosurveillance to inform the targeting of public health interventions to those most at risk for severe illness and for determining the timing of booster doses.
Funding
This work was supported by the Centers for Disease Control and Prevention (CDC) [contract #75D30120C08405] and the CARES Act of the U.S. Department of Health and Human Services (HHS) [Contract # NC DHHS GTS #49927]. Fifty percent of the current project was funded by the CDC/HHS award and fifty percent by the CARES Act/HHS award. Additional support was received from the National Institutes of Health [K23AI155838 to DJF-K and K23AI125720 to AAB]. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, HHS, or the U.S. Government.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: All Authors report financial support was provided by Centers for Disease Control and Prevention. All Authors report financial support was provided by US Department of Health and Human Services. Andrea Berry and DeAnna Friedman-Klabanoff report financial support was provided by National Institutes of Health.
Acknowledgements
The COVID-19 Community Research Partnership gratefully acknowledges the commitment and dedication of the study participants. We also wish to thank Ian Plumb, MBBS, MSc, and Melissa Briggs Hagen, MD, MPH, of the US Centers for Disease Control and Prevention, for their guidance and technical expertise. Programmatic, laboratory, and technical support was provided by Vysnova Partners, Inc., Javara, Inc., Oracle Corporation, LabCorp, Scanwell Health, and Neoteryx. The Partnership is listed in clinicaltrials.gov (NCT04342884). A complete list of Study Sites, investigators, and staff can be found below.
Author Contributions: Conception: AAB, TFW; Study Design: AAB, AHT, JR, DFK, ANH, SLE, DU, TFW; Data Analysis: AHT, JR and DU; Drafting of article and final approval: AAB, TFW; Reviewing editing: all authors. All authors meet the ICMJE criteria for authorship.
COVID-19 Community Research Partnership Group (*Site Principal Investigator):
Wake Forest School of Medicine: Thomas F Wierzba PhD, MPH, MS*, John Walton Sanders, MD, MPH, David Herrington, MD, MHS, Mark A. Espeland, PhD, MA, John Williamson, PharmD, Morgana Mongraw-Chaffin, PhD, MPH, Alain Bertoni, MD, MPH, Martha A. Alexander-Miller, PhD, Paola Castri, MD, PhD, Allison Mathews, PhD, MA, Iqra Munawar, MS, Austin Lyles Seals, MS, Brian Ostasiewski, Christine Ann Pittman Ballard, MPH, Metin Gurcan, PhD, MS, Alexander Ivanov, MD, Giselle Melendez Zapata, MD, Marlena Westcott, PhD, Karen Blinson, Laura Blinson, Mark Mistysyn, Donna Davis, Lynda Doomy, Perrin Henderson, MS, Alicia Jessup, Kimberly Lane, Beverly Levine, PhD, Jessica McCanless, MS, Sharon McDaniel, Kathryn Melius, MS, Christine O’Neill, Angelina Pack, RN, Ritu Rathee, RN, Scott Rushing, Jennifer Sheets, Sandra Soots, RN, Michele Wall, Samantha Wheeler, John White, Lisa Wilkerson, Rebekah Wilson, Kenneth Wilson, Deb Burcombe, Georgia Saylor, Megan Lunn, Karina Ordonez, Ashley O’Steen, MS, Leigh Wagner.
Atrium Health: Michael S. Runyon MD, MPH*, Lewis H. McCurdy MD*, Michael A. Gibbs, MD, Yhenneko J. Taylor, PhD, Lydia Calamari, MD, Hazel Tapp, PhD, Amina Ahmed, MD, Michael Brennan, DDS, Lindsay Munn, PhD RN, Keerti L. Dantuluri, MD, Timothy Hetherington, MS, Lauren C. Lu, Connell Dunn, Melanie Hogg, MS, CCRA, Andrea Price, Marina Leonidas, Melinda Manning, Whitney Rossman, MS, Frank X. Gohs, MS, Anna Harris, MPH, Jennifer S. Priem, PhD, MA, Pilar Tochiki, Nicole Wellinsky, Crystal Silva, Tom Ludden PhD, Jackeline Hernandez, MD, Kennisha Spencer, Laura McAlister.
MedStar Health Research Institute: William Weintraub MD*, Kristen Miller, DrPH, CPPS*, Chris Washington, Allison Moses, Sarahfaye Dolman, Julissa Zelaya-Portillo, John Erkus, Joseph Blumenthal, Ronald E. Romero Barrientos, Sonita Bennett, Shrenik Shah, Shrey Mathur, Christian Boxley, Paul Kolm, PhD, Ella Franklin, Naheed Ahmed, Moira Larsen.
Tulane: Richard Oberhelman MD*, Joseph Keating PhD*, Patricia Kissinger, PhD, John Schieffelin, MD, Joshua Yukich, PhD, Andrew Beron, MPH, Johanna Teigen, MPH.
University of Maryland School of Medicine: Karen Kotloff MD*, Wilbur H. Chen MD, MS*, DeAnna Friedman-Klabanoff, MD, Andrea A. Berry, MD, Helen Powell, PhD, Lynnee Roane, MS, RN, Reva Datar, MPH, Colleen Reilly.
University of Mississippi Medical Center: Adolfo Correa MD, PhD*, Bhagyashri Navalkele, MD, Yuan-I Min, PhD, Alexandra Castillo, MPH, Lori Ward, PhD, MS, Robert P. Santos, MD, MSCS, Pramod Anugu, Yan Gao, MPH, Jason Green, Ramona Sandlin, RHIA, Donald Moore, MS, Lemichal Drake, Dorothy Horton, RN, Kendra L. Johnson, MPH, Michael Stover.
Wake Med Health and Hospitals: William H. Lagarde MD*, LaMonica Daniel, BSCR.
New Hanover: Patrick D. Maguire MD*, Charin L. Hanlon, MD, Lynette McFayden, MSN, CCRP, Isaura Rigo, MD, Kelli Hines, BS, Lindsay Smith, BA, Monique Harris, CCRP, Belinda Lissor, AAS, CCRP, Vivian Cook, MA, MPH, Maddy Eversole, BS, Terry Herrin, BS, Dennis Murphy, RN, Lauren Kinney, BS, Polly Diehl, MS, RHIA, Nicholas Abromitis, BS, Tina St. Pierre, BS, Bill Heckman, Denise Evans, Julian March, BA, Ben Whitlock, CPA, MSA, Wendy Moore, BS, AAS, Sarah Arthur, MSW, LCSW, Joseph Conway.
Vidant Health: Thomas R. Gallaher MD*, Mathew Johanson, MHA, CHFP, Sawyer Brown, MHA, Tina Dixon, MPA, Martha Reavis, Shakira Henderson, PhD, DNP, MS, MPH, Michael Zimmer, PhD, Danielle Oliver, Kasheta Jackson, DNP, RN, Monica Menon, MHA, Brandon Bishop, MHA, Rachel Roeth, MHA.
Campbell University School of Osteopathic Medicine: Robin King-Thiele DO*, Terri S. Hamrick PhD*, Abdalla Ihmeidan, MHA, Amy Hinkelman, PhD, Chika Okafor, MD (Cape Fear Valley Medical Center), Regina B. Bray Brown, MD, Amber Brewster, MD, Danius Bouyi, DO, Katrina Lamont, MD, Kazumi Yoshinaga, DO, (Harnett Health System), Poornima Vinod, MD, A. Suman Peela, MD, Giera Denbel, MD, Jason Lo, MD, Mariam Mayet-Khan, DO, Akash Mittal, DO, Reena Motwani, MD, Mohamed Raafat, MD (Southeastern Health System), Evan Schultz, DO, Aderson Joseph, MD, Aalok Parkeh, DO, Dhara Patel, MD, Babar Afridi, DO (Cumberland County Hospital System, Cape Fear Valley).
George Washington University Data Coordinating Center: Diane Uschner PhD*, Sharon L. Edelstein, ScM, Michele Santacatterina, PhD, Greg Strylewicz, PhD, Brian Burke, MS, Mihili Gunaratne, MPH, Meghan Turney, MA, Shirley Qin Zhou, MS, Ashley H Tjaden, MPH, Lida Fette, MS, Asare Buahin, Matthew Bott, Sophia Graziani, Ashvi Soni, MS, Guoqing Diao, PhD, Jone Renteria, MS.
George Washington University Mores Lab: Christopher Mores, PhD, Abigail Porzucek, MS.
Oracle Corporation: Rebecca Laborde, Pranav Acharya.
Sneez LLC: Lucy Guill, MBA, Danielle Lamphier, MBA, Anna Schaefer, MSM, William M. Satterwhite, JD, MD.
Vysnova Partners: Anne McKeague, PhD, Johnathan Ward, MS, Diana P. Naranjo, MA, Nana Darko, MPH, Kimberly Castellon, BS, Ryan Brink, MSCM, Haris Shehzad, MS, Derek Kuprianov, Douglas McGlasson, MBA, Devin Hayes, BS, Sierra Edwards, MS, Stephane Daphnis, MBA, Britnee Todd, BS.
Javara Inc: Atira Goodwin.
External Advisory Council: Ruth Berkelman, MD, Emory, Kimberly Hanson, MD, U of Utah, Scott Zeger, PhD, Johns Hopkins, Cavan Reilly, PhD, U. of Minnesota, Kathy Edwards, MD, Vanderbilt, Helene Gayle, MD MPH, Chicago Community Trust, Stephen Redd.
Data availability
Data will be made available on request.
References
- 1.Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371 doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Earle K.A., Ambrosino D.M., Fiore-Gartland A., Goldblatt D., Gilbert P.B., Siber G.R., et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39:4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grosland M., Larsen V.B., Telle K., Gjefsen H.M. Has vaccination alleviated the strain on hospitals due to COVID-19? A combined difference-in-difference and simulation approach. BMC Health Serv Res. 2022;22:1183. doi: 10.1186/s12913-022-08541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahmani K., Shavaleh R., Forouhi M., Disfani H.F., Kamandi M., Oskooi R.K., et al. The effectiveness of COVID-19 vaccines in reducing the incidence, hospitalization, and mortality from COVID-19: A systematic review and meta-analysis. Front Public Health. 2022;10 doi: 10.3389/fpubh.2022.873596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tu W., Zhang P., Roberts A., Allen K.S., Williams J., Embi P., et al. SARS-CoV-2 Infection, Hospitalization, and Death in Vaccinated and Infected Individuals by Age Groups in Indiana, 2021–2022. Am J Public Health. 2023;113:96–104. doi: 10.2105/AJPH.2022.307112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vilches T.N., Sah P., Moghadas S.M., Shoukat A., Fitzpatrick M.C., Hotez P.J., et al. COVID-19 hospitalizations and deaths averted under an accelerated vaccination program in northeastern and southern regions of the USA. Lancet Reg Health Am. 2022;6 doi: 10.1016/j.lana.2021.100147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wajnberg A., Amanat F., Firpo A., Altman D.R., Bailey M.J., Mansour M., et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H., Yuan Y., Xiao M., Chen L., Zhao Y., Haiwei Z., et al. Dynamics of the SARS-CoV-2 antibody response up to 10 months after infection. Cell Mol Immunol. 2021;18:1832–1834. doi: 10.1038/s41423-021-00708-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei J., Pouwels K.B., Stoesser N., Matthews P.C., Diamond I., Studley R., et al. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat Med. 2022;28:1072–1082. doi: 10.1038/s41591-022-01721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cervia C., Nilsson J., Zurbuchen Y., Valaperti A., Schreiner J., Wolfensberger A., et al. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. J Allergy Clin Immunol. 2021;147(545–57):e9. doi: 10.1016/j.jaci.2020.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gudbjartsson D.F., Norddahl G.L., Melsted P., Gunnarsdottir K., Holm H., Eythorsson E., et al. Humoral Immune Response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383:1724–1734. doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dolscheid-Pommerich R., Bartok E., Renn M., Kummerer B.M., Schulte B., Schmithausen R.M., et al. Correlation between a quantitative anti-SARS-CoV-2 IgG ELISA and neutralization activity. J Med Virol. 2022;94:388–392. doi: 10.1002/jmv.27287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 14.Feng S., Phillips D.J., White T., Sayal H., Aley P.K., Bibi S., et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Post N., Eddy D., Huntley C., van Schalkwyk M.C.I., Shrotri M., Leeman D., et al. Antibody response to SARS-CoV-2 infection in humans: A systematic review. PLoS One. 2020;15:e0244126. doi: 10.1371/journal.pone.0244126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Partnership C.-C.-R. The COVID-19 Community Research Partnership: a multistate surveillance platform for characterizing the epidemiology of the SARS-CoV-2 pandemic. Biol Methods Protoc. 2022;7:bpac033. doi: 10.1093/biomethods/bpac033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tjaden A.H., Fette L.M., Edelstein S.L., Gibbs M., Hinkelman A.N., Runyon M., et al. Self-Reported SARS-CoV-2 Vaccination Is Consistent with Electronic Health Record Data among the COVID-19 Community Research Partnership. Vaccines (Basel) 2022;10 doi: 10.3390/vaccines10071016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.United States Census Bureau. American Commmunity Survey 2017, URL: https://www.census.gov/acs/www/data/data-tables-and-tools/data-profiles/2017/ (last accessed: 10 September 2021).
- 19.U.S. Food and Drug Administration. EUA Authorized Serology Test Performance available at: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance. (last accessed: 26 January 2023).
- 20.Walker G.J., Davis R., Naing Z., McEntee B., Lu Y., Denadija T., et al. Serological Detection of SARS-CoV-2 IgG Using Commercially Available Enzyme Immunoassays on Dried Blood Spots Collected from Patients. Microbiol Spectr. 2021;9:e0124521. doi: 10.1128/Spectrum.01245-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mongraw-Chaffin M., Tjaden A.H., Seals A.L., Miller K., Ahmed N., Espeland M.A., et al. Association of Obesity and Diabetes with SARS-Cov-2 Infection and Symptoms in the COVID-19 Community Research Partnership. J Clin Endocrinol Metab. 2022 doi: 10.1210/clinem/dgac715. [DOI] [PubMed] [Google Scholar]
- 22.National Institute of Occupational Safety and Health (NIOSH). The NIOSH Industry and Occupation Compterized coding System (NIOCCS), URL: https://csams.cdc.gov/nioccs/ (last accessed: 27 Jan 2023).
- 23.Upshaw T.L., Brown C., Smith R., Perri M., Ziegler C., Pinto A.D. Social determinants of COVID-19 incidence and outcomes: A rapid review. PLoS One. 2021;16:e0248336. doi: 10.1371/journal.pone.0248336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciabattini A., Nardini C., Santoro F., Garagnani P., Franceschi C., Medaglini D. Vaccination in the elderly: The challenge of immune changes with aging. Semin Immunol. 2018;40:83–94. doi: 10.1016/j.smim.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Ali H., Alterki A., Sindhu S., Alahmad B., Hammad M., Al-Sabah S., et al. Robust Antibody Levels in Both Diabetic and Non-Diabetic Individuals After BNT162b2 mRNA COVID-19 Vaccination. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.752233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daryabor G., Atashzar M.R., Kabelitz D., Meri S., Kalantar K. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front Immunol. 2020;11:1582. doi: 10.3389/fimmu.2020.01582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khanijahani A., Iezadi S., Gholipour K., Azami-Aghdash S., Naghibi D. A systematic review of racial/ethnic and socioeconomic disparities in COVID-19. Int J Equity Health. 2021;20:248. doi: 10.1186/s12939-021-01582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nash D., Rane M.S., Robertson M.M., Chang M., Kulkarni S.G., Zimba R., et al. Severe Acute Respiratory Syndrome Coronavirus 2 Incidence and Risk Factors in a National, Community-Based Prospective Cohort of US Adults. Clin Infect Dis. 2023;76 doi: 10.1093/cid/ciac423. e375-e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abu-Raddad L.J., Chemaitelly H., Ayoub H.H., Yassine H.M., Benslimane F.M., Al Khatib H.A., et al. Association of Prior SARS-CoV-2 Infection With Risk of Breakthrough Infection Following mRNA Vaccination in Qatar. J Am Med Assoc. 2021;326:1930–1939. doi: 10.1001/jama.2021.19623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bobrovitz N., Ware H., Ma X., Li Z., Hosseini R., Cao C., et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis. 2023;23:556–567. doi: 10.1016/S1473-3099(22)00801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedman-Klabanoff D.J., Tjaden A.H., Santacatterina M., Munawar I., Sanders J.W., Herrington D.M., et al. Vaccine-induced seroconversion in participants in the North Carolina COVID-19 community Research Partnership. Vaccine. 2022;40:6133–6140. doi: 10.1016/j.vaccine.2022.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abu Jabal K., Ben-Amram H., Beiruti K., Batheesh Y., Sussan C., Zarka S., et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel. Euro Surveill. 2021;2021(26) doi: 10.2807/1560-7917.ES.2021.26.6.2100096. December 2020 to January. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steensels D., Pierlet N., Penders J., Mesotten D., Heylen L. Comparison of SARS-CoV-2 Antibody Response Following Vaccination With BNT162b2 and mRNA-1273. J Am Med Assoc. 2021;326:1533–1535. doi: 10.1001/jama.2021.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Israel A., Shenhar Y., Green I., Merzon E., Golan-Cohen A., Schaffer A.A., et al. Large-Scale Study of Antibody Titer Decay following BNT162b2 mRNA Vaccine or SARS-CoV-2 Infection. Vaccines (Basel) 2021;10 doi: 10.3390/vaccines10010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parthymou A., Habeos E.E., Habeos G.I., Deligakis A., Livieratos E., Marangos M., et al. Factors associated with anti-SARS-CoV-2 antibody titres 3 months post-vaccination with the second dose of BNT162b2 vaccine: a longitudinal observational cohort study in western Greece. BMJ Open. 2022;12:e057084. doi: 10.1136/bmjopen-2021-057084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng C., Shao W., Chen X., Zhang B., Wang G., Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis. 2022;114:252–260. doi: 10.1016/j.ijid.2021.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tutukina M., Kaznadzey A., Kireeva M., Mazo I. IgG Antibodies Develop to Spike but Not to the Nucleocapsid Viral Protein in Many Asymptomatic and Light COVID-19 Cases. Viruses. 2021;13 doi: 10.3390/v13101945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention. COVID Data Tracker, URL: https://covid.cdc.gov/covid-data-tracker/#datatracker-home (last accessed: 28 March 2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.