Summary
Myeloid hematopoiesis is a finely controlled consecutive developmental process, which is essential to maintain peripheral innate immune homeostasis. Herein, we found that Rictor deletion caused the remarkable reduction of granulocyte-monocyte progenitors (GMPs), monocytes, and macrophages, while the levels of neutrophils were unaffected. Adoptive transfer of Rictor-deleted GMPs or common myeloid progenitors (CMPs) in syngeneic mice showed poor re-constitution of monocytes compared to wild-type GMPs or CMPs. In addition to decreasing the proliferation of CMPs/GMPs, Rictor deletion preferentially inhibited Ly6C+ monocyte differentiation, while enhancing neutrophil differentiation, as determined by colony formation assays. mTORC2 promotes monocyte development by downregulation of the AKT-Foxo4-activating transcription factor 5 (ATF5)-mitochondrial unfolded protein response (mtUPR) pathway. Genetic overexpression of ATF5 or exposure to ethidium bromide significantly rescued monocyte/macrophage differentiation defects of Rictor-deficient myeloid progenitors. Therefore, Rictor is required for CMP/GMP proliferation and acts as an important switch to balance monocytic and granulocytic lineage commitment in bone marrow.
Subject areas: Biological sciences, Molecular biology, Components of the immune system, Cell biology
Graphical abstract

Highlights
-
•
mTORC2 is required for the CMP→GMP→cMoP→Ly6C+ monocyte lineage trajectory
-
•
mTORC2 controls GMP differentiation by regulating AKT-Foxo4-ATF5-mtUPR pathways
-
•
mTORC2 is not essential for the differentiation of monocytes into macrophages
-
•
mTORC2 is critical for the balance of monocytic and granulocytic lineage decisions
Biological sciences; Molecular biology; Components of the immune system; Cell biology
Introduction
Pluripotent hematopoietic stem cells (HSCs) possess self-renewal properties and give rise to diverse progeny, including erythroid, myeloid, and lymphoid lineages. In their steady state, myeloid cells, which are supplied by HSCs, can migrate to tissues for immune surveillance, with reserves maintained in bone marrow in case of urgent threats. Common myeloid progenitors (CMPs) with lineage potential restricted to myeloid and megakaryocyte-erythrocyte lineages can be directed to develop into granulocyte-monocyte progenitors (GMPs), monocyte-DC progenitors (MDPs), and megakaryocyte-erythrocyte progenitors (MEPs).1 GMPs differentiate into granulocytes and monocytes/macrophages through the stages of granulocytic progenitors (GPs) and common monocyte progenitors (cMoPs), respectively. MDPs have recently been shown to yield monocytes and dendritic cells.1 In their steady state, CMPs and GMPs can proliferate and supply myeloid cells to the periphery and to tissues in support of immune surveillance, maintaining tissue homeostasis. Recent studies have suggested that progenitors undergo lineage commitment at an earlier developmental stage than asserted by conventional wisdom.2,3 Some early progenitors will lose multipotency to adopt a particular fate, while others continue to undergo lineage commitment and differentiation under elegant regulation by transcriptional profiles, growth factors, and cytokines/chemokines.4,5,6,7 Myeloid hematopoiesis is a finely controlled consecutive developmental process, and the intrinsic molecular mechanisms regulating myelopoiesis remain to be studied. Thus, exploring the carefully regulated molecular network underlying the differentiation of myeloid progenitors into downstream cell subsets is critical to our understanding of the finely orchestrated process of myeloid development during homeostasis and myelopoiesis emergency.
Previous studies have also demonstrated that signaling by mammalian target of rapamycin (mTOR) and mTOR complex 1 (mTORC1) is closely involved in the regulation of monocytic lineage development and macrophage colony-stimulating factor (M-CSF)-mediated myelopoiesis.8,9,10,11 mTOR complex 2 (mTORC2) has been described as a rapamycin-insensitive complex that regulates cell growth, survival, aging, metabolism, and autophagy.12,13,14,15,16,17 Rictor is a core subunit specific to mTORC2 and absent from mTORC1, and Rictor deletion leads to mTORC2 inactivation. The best characterized kinase activity of mTORC2 is its phosphorylation of AKT (also known as protein kinase B) at Ser473 in its hydrophobic motif.18,19 mTORC2 plays important roles in thymic epithelial cell development and Th cell functional polarization.20,21,22 Rictor deletion caused a significant block of thymocyte development at the double-negative 3 stage in Mx1-Cre-Rictor floxed mice, in which Rictor was deleted in interferon-sensitive cell populations.23 Rictor regulates B cell receptor signal, B cell survival and the proliferation of mature lymphocytes to maintain B cell homeostasis, fate, and function.24,25 Rictor-deficient dendritic cells (DCs) were shown to display enhanced inflammation and migratory responses in a mouse kidney ischemia-reperfusion injury model.26 Furthermore, embryos that lack Rictor develop normally until E9.5, then exhibited phenotypic characteristics similar to mLST8-deficient embryos with the underdeveloped telencephalic vesicles and the visibly smaller embryos, and died around E10.5 or E11.5.27,28 However, the roles of Rictor in the myeloid development in bone marrow have not been addressed so far. Thus, in the present study, we investigated the discrete role of mTORC2 signals in regulating myeloid cell differentiation using ER-Cre-Rictor floxed (ER-Rictor knockout (KO)) mice, in which Rictor knockout could be induced by tamoxifen treatment. We found that mTORC2 plays important roles in maintaining CMP/GMP proliferation and in promoting the differentiation of progenitors into the monocytic lineage (CMP→GMP→cMoP→monocyte→macrophage). Importantly, mTORC2 favors monocyte lineage specification at the expense of the granulocyte lineage and acts as the critical balance node to orchestrate monocytic and granulocytic lineage decisions and commitment in bone marrow.
Results
Rictor deficiency reduces monocytes and macrophages in bone marrow and the periphery
To determine the role of mTORC2 in myeloid cell development in vivo, we employed Rictor-deleted mice, which were made by crossing Rictorflox/flox mice with ER-Cre mice to generate ER-Cre-Rictorflox/flox mice (ER-Rictor KO) (Figure S1A). Wild-type (WT) and ER-Rictor KO mice were treated with tamoxifen for 5 days, then rested for 18–21 days. Deletion of Rictor from bone marrow cells, splenocytes, and peritoneal cavity cells was determined via real-time PCR assay (Figure S1B). At 18 days after tamoxifen treatment, the percentages and cell numbers of monocyte-derived macrophages in the peritoneal cavity, spleen (CD11b+F4/80low), and colon were remarkably reduced in ER-Rictor KO mice but not in WT mice (Figures 1A and 1B; Figures S2A and S2B). In addition, the percentages and cell numbers of CD11b+Ly6Chi monocytes also significantly decreased in the bone marrow, peripheral blood, spleens, and colons of tamoxifen-treated ER-Rictor KO mice (Figures 1C and 1D). The levels of CD11b+CD115+ monocytes in peritoneal cavity, bone marrow, peripheral blood, and spleens of ER-Rictor KO mice were similarly decreased compared with those in WT mice (Figures S2C, S2D, and S3A–S3C). In contrast, the percentage of neutrophils in the bone marrow of tamoxifen-treated ER-Rictor KO mice was comparable to that observed in tamoxifen-treated WT mice, although the number of neutrophils in the bone marrow of tamoxifen-treated ER-Rictor KO mice was decreased (Figures S4A and S4B). Furthermore, the percentages and cell numbers of CD11b+Ly6G+ neutrophils isolated from the blood and spleens of Rictor-deficient mice were comparable to those isolated from WT mice, despite the proportion of neutrophils in blood and spleens of Rictor-deficient mice tended to increase (Figures S4C-F). In addition, the percentages and total cell numbers of T and B lymphocytes in the spleens of tamoxifen-treated ER-Rictor KO mice were identical to those found in age-matched WT mice (Figures S4G-K). To determine whether mTORC2 is indispensable for the maintenance and homeostasis of mature monocytes and macrophages in the periphery, we established a Lyzs-Rictor KO mouse model in which was generated by crossing Rictorloxp/loxP mice with transgenic mice that carried lysozyme (LyzM) proximal promoter-mediated Cre recombinase and Rictor can be selectively deleted in the early stages of monocyte and neutrophil development and beyond, as demonstrated by many previous studies.9,29 Unexpectedly, levels of monocytes and macrophages in the bone marrow, peritoneal cavity, and colon were comparable between WT and Lyzs-Rictor KO mice (Figures S5A-G). Therefore, mTORC2 is essential for early development of the myeloid lineage in bone marrow but is dispensable for the later development of monocytes to macrophages and for peripheral macrophage homeostasis under physiological conditions.
Figure 1.
Rictor deletion selectively disrupts monocyte and macrophage development in mice
WT and ER-Rictor KO mice were injected intraperitoneally with tamoxifen for 5 days, then rested for 18 days before analysis.
(A) Cells from the spleen, and colon of tamoxifen-treated WT and ER-Rictor KO mice were stained with anti-CD11b mAb and anti-F4/80 mAbs and analyzed by flow cytometry.
(B) Summary of the percentages and numbers of CD11b+F4/80+ cells in the spleen, and colon of tamoxifen-treated WT and ER-Rictor KO mice. Data are shown as mean ± SD (n = 3), Student’s t test, ∗∗p < 0.01; ∗∗∗p < 0.001.
(C) Representative fluorescence-activated cell sorter analysis of bone marrow, blood, spleen, and colonic monocytes, with CD11b+Ly6Chi staining shown.
(D) Comparison of percentages and numbers of CD11b+Ly6Chi cells in the bone marrow, blood, spleen, and colon of tamoxifen-treated WT and ER-Rictor KO mice (n = 3). Data are representative of three independent experiments and are shown as mean ± SD. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 (WT vs. ER-Rictor KO). p values were determined using Student’s t test.
Rictor deficiency selectively impairs CMP→GMP→cMoP→monocyte differentiation
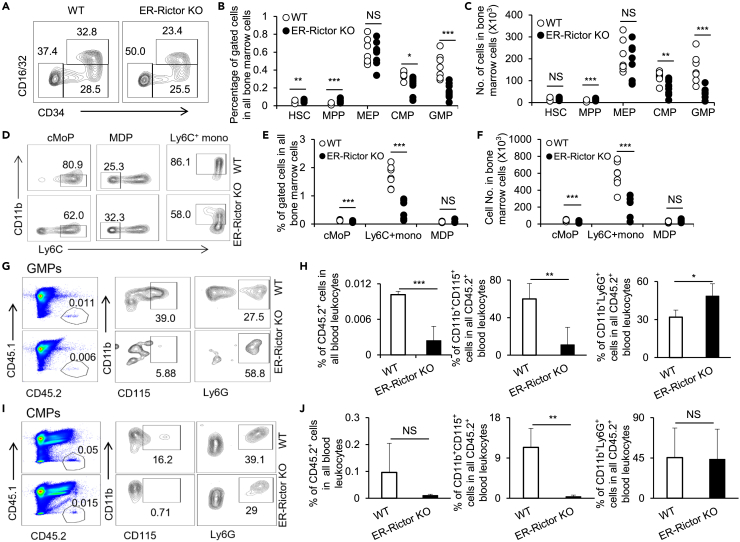
To investigate whether the reduction of monocytes and macrophages in ER-Rictor KO mice reflects a developmental defect in the differentiation of myeloid progenitors, we assessed various stages of precursor cells in the bone marrow of tamoxifen-treated WT and ER-Rictor KO mice. Long-term HSCs and multipotent progenitors (MPPs) can be detected according to CD150 and CD48 expression levels in gated Lin−Sca1+ cells.30,31 Percentages and absolute numbers of HSCs and MPPs were increased in the bone marrow of tamoxifen-treated ER-Rictor KO mice compared with tamoxifen-treated WT mice, except no detectable change was observed in the number of HSCs (Figures 2B and 2C) as assayed by flow cytometry with a gating strategy (Figure S6A). MEPs (Lin−Sca1−c-Kit+CD34−CD16/32-), CMPs (Lin−Sca1−c-Kit+CD34hiCD16/32-), and GMPs (Lin−Sca1−c-Kit+CD34hiCD16/32hi) were detected by flow cytometry with a gating strategy (Figure S6B).32,33 Our results showed that the percentages and cell numbers of CMPs and GMPs were significantly lower in the bone marrow of tamoxifen-treated ER-Rictor KO mice compared to tamoxifen-treated WT mice, while no significant changes in MEPs were detected in tamoxifen-treated ER-Rictor KO mice (Figures 2A–2C). MDPs are generally thought to yield monocyte-committed progenitors, known as cMoPs, which further develop into mature monocytes.34 Recent work has demonstrated that GMPs have the potential to yield monocyte-committed progenitors, which produce monocytes, as well as MDPs.1,34 Therefore, we characterized Lin−CD117+CD115+CD135+CD11b−Ly6C− MDPs, Lin−CD117+CD115+CD135−CD11b−Ly6C+ cMoPs, and Lin−CD117+CD115+CD135−CD11b+Ly6C+ monocytes using a previously reported gating strategy34 (Figure S6C). Frequencies and total numbers of cMoPs and Ly6C+ monocytes were remarkably reduced in the bone marrow of tamoxifen-treated ER-Rictor KO mice compared to WT mice (Figures 2D–2F). However, MDP levels in the bone marrow of tamoxifen-treated Rictor KO and WT mice were comparable (Figures 2D–2F). Additionally, we also detected Lin−CD117+CD34+CD135−CD115- GPs and observed that GPs were unchanged in the bone marrow of Rictor-deficient mice compared with WT mice (Figures S7A-C). These results suggest that mTORC2 is selectively involved in regulating the CMP→GMP→cMoP→Ly6C+ monocyte lineage trajectory.
Figure 2.
Rictor deletion decreases CMPs/GMPs and inhibits their ability to differentiate into the monocytic lineage
(A–C) Myeloid progenitor subpopulations of tamoxifen-treated WT and ER-Rictor KO mice were detected by flow cytometry. Gated Lin-sca-1−c-kit+ cells are shown. Percentages (B) and absolute numbers (C) of HSCs (Lin−Sca-1+CD150+CD48−), MPPs (Lin−Sca-1+CD150−CD48−), and myeloid progenitor cell populations in the bone marrow of WT and ER-Rictor KO mice. Data are shown as mean ± SD (WT, n = 7, ER-Rictor KO, n = 9), Student’s t test, ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; NS: not significant.
(D–F) Monocytic progenitor subpopulations of tamoxifen-treated WT and ER-Rictor KO mice as detected by flow cytometry. Gated lineage-c-kit+/−CD115+ cells are shown. Percentages (E) and absolute numbers (F) of cMoPs (Lin-c-Kit+CD115+CD135-Ly6C+CD11b−), Ly6C+ monocytes (Lin-c-kit-CD1135−CD115+CD11b+Ly6C+), and MDPs (Lin-c-kit+CD1135+CD115+CD11b−Ly6C−) in the bone marrow of WT and ER-Rictor KO mice. Data are shown as mean ± SD (WT, n = 7, ER-Rictor KO, n = 6), Student’s t test, ∗∗∗p < 0.001; NS: not significant. Non-irradiated recipient mice (CD45.1) were subjected to adoptive transfer of either CD45.2+ WT or ER-mTOR KO CMPs or GMPs.
(G) Staining patterns of CD45.1+ and CD45.2+CD11b+CD115+ monocytes and CD45.2+CD11b+Ly6G+ neutrophils in GMPs-transferred recipient mice.
(H) Proportions of WT or Rictor-deficient CD45.2+, CD45.2+CD11b+CD115+, and CD45.2+CD11b+Ly6G+ cells in the blood of recipient mice. Data are shown as mean ± SD (n = 4 for each group), Student’s t test, ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
(I) Staining patterns of CD45.1+ and CD45.2+CD11b+CD115+ monocytes and CD45.2+CD11b+Ly6G+ neutrophils in CMP-adopted recipient mice.
(J) Proportions of WT or Rictor-deficient CD45.2+, CD45.2+CD11b+CD115+, and CD45.2+CD11b+Ly6G+ cells in the blood of recipient mice (n = 3 for each group). Data are shown as mean ± SD. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; NS: not significant (WT vs. ER-Rictor KO). p values were determined using Student’s t test.
To determine whether the impaired differentiation capacity of myeloid progenitors associated with Rictor deletion was an intrinsic defect, we generated an adoptive transfer mouse model in which the recipients (CD45.1) were adoptively transferred with tamoxifen-treated WT or ER-Rictor CMPs or GMPs (CD45.2; donor) (Figures S8A and B). Significantly lower percentages and numbers of total donor cells (Rictor-deficient CD45.2+ cells) and monocytes were observed in the bone marrow and blood of recipients who received Rictor-deficient GMPs or CMPs compared with those who received WT GMPs or CMPs (Figures 2G–2J; S8C-H). Notably, the frequency of neutrophils from Rictor-deficient CMP or GMP donors was unchanged in the bone marrow of recipients and even slightly increased in the blood compared with those from WT donors, although the total number of neutrophils decreased significantly for Rictor-deficient donors due to a dramatic decline in total cell numbers from Rictor-deficient donors during homeostasis in recipients (Figures 2G–2J; S8C-H). As expected, cMoPs derived from Rictor-deficient GMPs showed lower percentages and cell numbers compared to those from WT GMPs in recipient mice (Figures S9A-C), while MDPs derived from Rictor-deficient CMPs were comparable to those from WT CMPs (Figures S9D-F). Additionally, we used the adoptive transfer mouse model in which the recipients (CD45.1+GFP−) were adoptively transferred with tamoxifen-treated WT or ER-Rictor Lin−Sca-1−c-Kit+ (LSKs) (CD45.2+GFP−; donor) mixed with WT (CD45.2+GFP+; donor) (Figure S10A). Significantly lower ratios of donor Rictor-deficient CD45.2+GFP− monocytes were observed in the bone marrow and blood of recipients who received Rictor-deficient LSKs mixed with WT GFP+ LSKs compared with those who received WT (CD45.2+GFP−) cells mixed with WT GFP+ LSKs (Figures S10B-C and S10E-F). Furthermore, the neutrophils developed from Rictor-deficient CD45.2+GFP− LSKs increased remarkably compared with WT CD45.2+GFP− LSKs and WT CD45.2+GFP+ LSKs (Figures S10D and S10G). Thus, mTORC2 plays a key cell-autonomous role in regulating myeloid progenitor maintenance and CMP-GMP-cMoP-monocyte lineage development but is uninvolved or minimally involved in neutrophil lineage commitment in vivo. On the other hand, MDPs are the other origin for cMoPs;34 we also detected the potential differentiation ability of Rictor-deleted MDP in vitro. We found that the ability of Rictor-deleted MDPs developing into cMoPs was normal as WT MDPs, while percentages and cell numbers of monocytes and macrophages developed from Rictor-deleted MDPs were decreased compared with those of WT MDPs (Figures S11A-D). We speculated that Rictor deletion did not significantly block the MDP development into cMoPs but mainly prevented the cMoP differentiation into monocytes/macrophages.
Rictor deficiency impairs myeloid progenitor proliferation and differentiation but not apoptosis
The developmental defect observed in Rictor-deficient progenitors could result from decreased cell proliferation and/or increased progenitor apoptosis. To verify these possibilities, we first performed 5-ethynyl-2′-deoxyuridine (EdU) incorporation studies to determine the proliferation of CMPs and GMPs. The percentages of EdU+ CMPs and GMPs were significantly lower in the bone marrow of tamoxifen-treated ER-Rictor KO mice than in control WT mice (Figures 3A–3D). We then evaluated colony formation by LSKs, which include CMPs and GMPs, in methylcellulose culture, which supports the differentiation of monocytes and macrophages. Rictor-deficient LSKs generated significantly fewer colonies and lower cell numbers than WT LSKs in methylcellulose culture with M-CSF (Figures 3E–3G). Flow cytometry analysis of the phenotypes of cells originating from purified Rictor-deficient LSKs revealed fewer CD11b+CD115+ monocytes in colony formation assays compared with those originating from WT LSKs (Figures S12A-B). To further define the detrimental effects of Rictor deletion on myeloid progenitor function, we determined the requirement for Rictor during progenitor differentiation through acute in vitro deletion. Specifically, we cultured LSKs with M-CSF or interleukin-3 (IL-3) combined with IL-6 in the presence of 4-hydroxytamoxifen (4-OHT), resulting in efficient deletion of Rictor during in vitro differentiation.9,35 We found that, compared to WT LSKs, Rictor-deficient LSKs cannot effectively develop into CD11b+F4/80+ macrophages under M-CSF or IL-6+IL-3-inducing systems (Figures S12C-F). In addition, we performed RNA sequencing (RNA-seq) to compare gene expression in Rictor-deficient versus WT Lin−Sca-1−CD117+CD34+ progenitors. The minichromosome maintenance protein complex pathway and cell cycle pathway were highly enriched in WT progenitors, indicating that cell cycle and cell proliferation signaling pathways are indeed inhibited after Rictor deletion (Figures 3H and 3I). Heatmap analysis showed a major decrease in the expression of cell proliferation genes in Rictor-deleted cells (Figure 3J). However, when we detected apoptosis in bone marrow CMPs and GMPs by staining for caspase-3, freshly isolated Rictor-deficient CMPs and GMPs both exhibited normal cell apoptosis, similar to that observed in WT cells (Figures 3K-N). Consistently, gene set enrichment analysis (GSEA) found that there was no significant difference in the expression of apoptosis pathway genes between WT and Rictor-deficient progenitors (Figure 3O). Collectively, these data support the hypothesis that decreased proliferation of progenitor cells, rather than increased apoptosis, likely contributes to the impaired differentiation of myeloid progenitors to the monocytic lineage. These data also indicate a requirement for mTORC2 in the generation and maintenance of myeloid progenitors, such as GMPs.
Figure 3.
Rictor-deficient CMPs/GMPs show decreased cell proliferation but normal cell apoptosis
(A) 5-ethynyl-20-deoxyuridine (EdU) incorporation in bone-marrow-derived CMPs was detected by flow cytometry 24 h after injection of EdU. Gated Lin−Sca-1−c-kit+CD34+CD16/32- cell plots are shown.
(B) Percentages of EdU+ CMPs in WT and ER-Rictor KO mice. Data are shown as mean ± SD (WT, n = 8; ER-Rictor KO, n = 6), Student’s t test, ∗∗p < 0.01.
(C) EdU incorporation in bone-marrow-derived GMPs was detected by flow cytometry 24 h after injection of EdU. Gated Lin−Sca-1−c-kit+CD34+CD16/32+ cell plots are shown.
(D) Percentages of EdU+ GMPs in WT and ER-Rictor KO mice. Data are shown as mean ± SD (WT, n = 8; ER-Rictor KO, n = 6), Student’s t test, ∗∗∗p < 0.001.
(E–G) A total of 20,000 purified WT and ER-Rictor KO LSKs were seeded in methylcellulose supplemented with IL-3, IL-6, SCF, and M-CSF. Assays were performed in triplicate, and the presented photographs were shown with scar bars 100 μm. Colonies (F) and macrophage cell number per colony (G) were calculated (n = 3). Data are representative of three independent experiments. Data are shown as mean ± SD (n = 3), Student’s t test, ∗∗∗p < 0.001.
(H) GSEA enrichment of Biocarta MCM pathway genes in Rictor KO versus WT Lin−CD127−Sca-1−c-kit+CD34+ cells as assayed by RNA-seq.
(I) GSEA enrichment of Biocarta cell cycle pathway genes in Rictor KO versus WT Lin−Sca-1−c-kit+CD34+ cells as assayed by RNA-seq.
(J) Heatmap representing expression levels of MCM pathway-related genes in WT and Rictor KO Lin−CD127−Sca-1−c-kit+CD34+ cells.
(K) CMPs in WT and ER-Rictor KO bone marrow were stained with caspase-3 by flow cytometry analysis. Gated Lin−Sca-1−c-kit+CD34+CD16/32- cell plots are shown.
(L) The percentage of living CMP cells is shown. Data are shown as mean ± SD (n = 8), Student’s t test, NS: not significant.
(M) GMPs in WT and ER-Rictor KO bone marrow were stained with caspase-3 by flow cytometry analysis. Gated Lin−Sca-1−c-kit+CD34+CD16/32+cell plots are shown.
(N) The percentage of living GMPs is shown. Data are shown as mean ± SD (n = 8), Student’s t test, NS: not significant.
(O) GSEA enrichment of Biocarta Apoptosis Pathway genes in Rictor KO versus WT Lin−CD127−Sca-1−c-kit+CD34+ cells as assayed by RNA-seq.
Rictor deletion impairs monocytic differentiation by disrupting AKT S473 phosphorylation
To explore the mechanisms underlying the dysregulated myeloid progenitor proliferation and differentiation mediated by Rictor deficiency, we assessed potential pathways downstream of mTORC2. mTORC2 is known to mainly phosphorylate AGC kinases, including AKT (also defined as protein kinase B) at its hydrophobic motif.36 Therefore, we examined AKT signal in LSKs isolated from WT and ER-Rictor KO mice and treated with M-CSF at the indicated time points by immunoblotting analysis of phosphorylated proteins. A dramatic decrease in the phosphorylation of AKT at Ser473 but not at Thr308 was observed in Rictor-deficient progenitors compared to WT progenitors (Figure 4A). To confirm whether the involvement of the AKT pathway in myeloid progenitor proliferation and differentiation is mediated by mTORC2, we studied monocyte/macrophage development in WT and Rictor-deficient LSKs cultured with IL-3 and IL-6 in the presence of the AKT inhibitor MK-2206 or the AKT agonist SC79 in in vitro culture assays. Inhibiting AKT activity with MK-2206 significantly inhibited the differentiation of progenitors into CD11b+F4/80+ macrophages (Figures 4B–4D) and reduced the percentage and number of CD11b+CD115+ cells (Figures 4E–4G). On the other hand, addition of the AKT activator SC79 to the IL-3 and IL-6-induced progenitor differentiation culture system enhanced certain levels of AKT activation (AKT (Ser473) phosphorylation) in Rictor-deficient progenitors as determined by western blot assays (Figure 4H). Simultaneously, the decreased frequencies and numbers of CD11b+F4/80+ and CD11b+CD115+ macrophages that developed from ER-Rictor KO progenitors could be gradually rescued with increasing doses of the AKT agonist SC79 (Figures 4I-N). Therefore, the mTORC2-AKT pathway contributes to controlling the differentiation of myeloid progenitors into the monocytic lineage.
Figure 4.
Rictor deficiency impairs monocyte/macrophage development of LSKs in an AKT-dependent manner
(A) Levels of p-AKT (Ser473), p-AKT (Thr308), AKT, and β-actin in M-CSF-stimulated LSKs as determined by western blots.
(B–D) Sorted WT LSKs were treated with IL-3 and IL-6 in the presence or absence of 1 μM MK-2206 (AKT inhibitor) and cultured for five days. The generated cells were stained with anti-CD11b mAb and anti-F4/80 mAb and analyzed by flow cytometry. Percentages (C) and numbers (D) of CD11b+F4/80+ cells from WT control and MK-2206-stimulated cultures were summarized. Data are shown as mean ± SD (n = 3), Student’s t test, ∗∗p < 0.01; ∗∗∗p < 0.001.
(E) Cells developing in the presence or absence of MK-2206 were stained with anti-CD11b mAb and anti-CD115 mAb and analyzed by flow cytometry.
(F and G) The percentages (F) and numbers (G) of CD11b+CD115+ cells from WT control and MK-2206-stimulated cultures were summarized. Data are shown as mean ± SD (n = 3), Student’s t test, ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
(H) LSKs from WT and Rictor KO mice were treated with M-CSF in the presence or absence of SC79 (AKT activator) for five days, and levels of p-AKT (Ser 473), CSF1R, and β-actin were detected by immunoblotting.
(I) Purified WT and ER-Rictor KO LSKs were treated with IL-3, IL-6, and 4-OHT in the presence or absence of SC79 (AKT activator) at the indicated concentrations for five days. The generated cells were stained with anti-CD11b mAb and anti-F4/80 mAb and analyzed by flow cytometry.
(J and K) The percentages (J) and numbers (K) of CD11b+F4/80+ cells from WT and ER-Rictor KO mice in the presence or absence of SC79 were summarized. Data are shown as mean ± SD (n = 3), Student’s t test, ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
(L) Sorted WT and ER-Rictor KO LSKs were treated with IL-3, IL-6, and 4-OHT in the presence or absence of SC79 (AKT activator) at the indicated concentrations for five days. The generated cells were stained with anti-CD11b mAb and anti-CD115 mAb and analyzed by flow cytometry.
(M and N) The percentages (M) and numbers (N) of CD11b+CD115+ cells from WT and ER-Rictor KO mice in the presence or absence of SC79 were summarized (n = 3). Data are shown as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001; statistical significance was determined with the two-tailed Student’s t test.
mTORC2 controls the AKT-Foxo4-ATF5 pathway during monocytic lineage differentiation
In order to clarify how AKT mediates the monocytic differentiation defect observed in mTORC2-deleted myeloid progenitors on a transcriptional level, we used M-CSF-stimulated WT and ER-Rictor-deleted Lin−CD127−Sca-1−c-Kit+CD34+ progenitors to perform RNA-seq assays. As Rictor deletion decreases AKT activity in myeloid progenitors, we analyzed the intersection between the Akt-regulated gene set and the differential gene set in Rictor-deleted cells. Among M-CSF-upregulated genes in WT progenitors, we identified 414 transcription factors upregulated after Rictor knockout, while 163 transcription factors were downregulated after Rictor knockout (Figure S13A). Through gene network analysis of the genes downregulated in Rictor-deficient cells and AKT-regulated genes, we found that activating transcription factor 5 (ATF5) closely intersects with AKT-regulated gene sets (Figure 5A). The results indicated that the downregulated gene ATF5 might act as a key node of the AKT downstream regulatory genes and regulate the differentiation of myeloid progenitors in Rictor-deficient cells (Figure 5A). On the other hand, we identified 11 transcription factors that were upregulated in M-CSF-stimulated WT progenitors compared with unstimulated WT progenitors but decreased in M-CSF-stimulated Rictor KO progenitors compared with M-CSF-stimulated WT progenitors. By performing network analysis of these 11 altered transcription factors and pathway genes downregulated in Rictor-deleted progenitors, we found that ATF5 occupies a central position in the entire regulatory network, as supported by topological confidence score calculations (Figures 5B; S13B). Consistently, ATF5 mRNA expression was remarkably lower in purified Rictor-deficient LSKs compared to WT cells under the stimulation of M-CSF at the indicated time points, as evidenced by real-time PCR (Figure 5C). Importantly, ATF5 and CSF1R protein levels were downregulated in sorted Rictor-deficient progenitors during M-CSF-induced monocyte differentiation culture at the indicated time points, as determined by immunoblot (Figure 5D), indicating that ATF5 is positively correlated with myeloid progenitor differentiation and is affected by Rictor deficiency. ATF5 has been identified as a member of the cAMP response element binding/ATF subfamily of basic leucine zipper transcription factors.37 Yet, how does AKT regulate ATF5? To address this issue, we analyzed the protein interaction network of the AKT downstream gene set and the ATF5 upstream regulatory gene set. At the intersection of these two gene sets, we found forkhead box O transcription factor 4 (Foxo4) of which the mRNA expression was higher in ER-Rictor KO LSKs compared with WT LSKs by RNA-seq assay (Figure 5E). When we used immunoblotting analysis to assess Foxo4 expression and phosphorylation in isolated WT and ER-Rictor KO LSKs treated with M-CSF at the indicated time points, the results showed that Foxo4 protein expression and phosphorylation both increased in Rictor-deficient progenitors compared with WT progenitors (Figure 5F). It has been demonstrated that the transcriptional activity and protein expression of Foxo4 can both be regulated by AKT.38,39 Consistent with these observations in other cells, we observed that Foxo4 expression was enhanced in WT LSKs upon treatment with the AKT inhibitor MK-2206, as determined by western blot analysis (Figure 5G). Moreover, it has also been reported that Foxo4, which is downstream of AKT, can prevent ATF5 transcriptional expression by regulating the ATF5 promoter.40 We speculated that the decreased AKT activation observed in M-CSF-stimulated Rictor-deficient myeloid progenitors might enhance Foxo4 expression, such that the latter then inhibits ATF5 expression. Lower levels of ATF5 were detected in MK-2206-stimulated myeloid progenitors compared with control progenitors (Figure 5G). Meanwhile, the AKT agonist SC79 efficiently promoted ATF5 protein expression in Rictor-deficient progenitors in our in vitro differentiation culture system, as determined by western blot assays (Figure 5H). These data indicate that, during the differentiation of myeloid progenitors, mTORC2 deficiency decreases AKT activity, increases Foxo4 expression, and finally inhibits ATF5 expression.
Figure 5.
Rictor deficiency decreases the AKT-Foxo4-ATF5 signaling pathway in LSKs
(A) Network analysis of AKT-regulated genes and differential gene enrichment transcription factors between WT and Rictor KO Lin−CD127−Sca-1−c-kit+CD34+ cells after M-CSF treatment.
(B) Eleven transcription factors were obtained by analyzing genes upregulated in WT Lin−CD127−Sca-1−c-kit+CD34+ cells under M-CSF stimulation but downregulated after Rictor KO. A histogram of topological confidence is shown in the figure.
(C) LSKs purified from tamoxifen-treated WT and ER-Rictor KO mice were stimulated with M-CSF for the indicated duration. Cells were lysed and analyzed by real-time PCR to determine ATF5 mRNA expression. Data are shown as mean ± SD (n = 3), Student’s t test, ∗p < 0.05; ∗∗p < 0.01.
(D) Sorted LSKs from WT and ER-Rictor KO mice were treated with M-CSF for the indicated duration. Cells were lysed and analyzed by immunoblotting to assess expression of the transcriptional factor ATF5 and of CSF1R.
(E) Network analysis of AKT-regulated downstream genes and ATF5 upstream-regulating genes.
(F) Purified LSKs from tamoxifen-treated WT and ER-Rictor KO mice were treated with M-CSF for the indicated duration. Cells were lysed and analyzed by immunoblotting to assess phosphorylation of Foxo4 (S262), as well as to determine total protein levels.
(G) Purified LSKs from WT mice were treated with M-CSF in the presence or absence of MK-2206 (AKT inhibitor), and levels of Foxo4 and ATF5 were detected by immunoblotting.
(H) Sorted LSKs from WT and ER-Rictor KO mice were treated with M-CSF and 4-OHT in the presence or absence of SC79 (AKT agonist), and levels of ATF5 and FoxO4 were detected by immunoblotting. Data (mean ± SD) represent one of two independent experiments with similar results.
ATF5-mediated mtUPR is involved in the poor monocytic differentiation of Rictor-deleted LSKs
Recent studies have suggested that ATF5 plays important roles in cell survival, proliferation, and differentiation.41,42 To verify the idea that ATF5 may be involved in the impaired differentiation of Rictor-deleted myeloid progenitors, the sorted WT LSKs were subjected to nucleofection with Cas9 protein and either ATF5 single guide RNA (sgRNA) or negative control sgRNA. The progenitors were then cultured in the presence of IL-3, IL-6, and M-CSF for 5-6 days. CRISPR-Cas9-mediated deletion of ATF5 in WT progenitors resulted in substantially slower cell growth, as shown in pictures (Figure 6A), and significantly decreased the percentage and number of CD11b+F4/80+ macrophages, as detected by flow cytometry (Figures 6B and 6C). When these cells were treated with IL-3, IL-6, stem cell factor, and M-CSF in a colony-forming unit (CFU) assay, the colony number, colony size, and cell number per colony were all markedly less when formed from ATF5-deficient WT LSKs compared to control WT cells (Figures 6D–6F). Immunoblot analysis showed that Cas9 protein and ATF5 sgRNA decreased ATF5 protein expression, and ATF5 knockdown resulted in decreased CSF1R (CD115) levels in generated cells after 5 days of culture in the presence of IL-3, IL-6, and M-CSF (Figure 6G). Increased PU.1 activity favors monocytic commitment of GMPs.43,44 Early growth response factor 1 (Egr-1), Egr-2, AP-1, MafB/c:Fos complexes, CCAAT/enhancer-binding protein β (C/EBPβ), and Egr-1/ NGFI-A-binding protein 2 (Nab2) complexes direct further monocytic lineage specification and maturation.28,45,46,47 Cebpa, Irf8, and growth factor independence 1 (Gfi1) have been reported as key components of counteracting myeloid gene regulatory networks.48 We then detected mRNA expression of these transcription factors in cells generated from WT and ATF5 knockdown LSKs after 5 days of culture in the presence of IL-3, IL-6, and M-CSF. Levels of PU.1, C/EBPβ, Egr-1, Nab2, Jun, Junb, and Maf are obviously lower in cells developed from ATF5-deficient LSKs compared with those from WT LSKs (Figure 6H), whereas Cebpa, Irf8, and Gfi1 mRNA expression are remarkably higher in cells developed from ATF5-deficient LSKs compared with those from WT LSKs (Figure S14A). Thus, we determined whether Pu.1, Cebpb, Junb, Jun, Nab2, and Gfi1b are the direct transcriptional targets of ATF5. We analyzed if those transcription factors promoter fragments containing the ATF5-binding region in the luciferase reporter assay. We found that ATF5 significantly increased the expression of Pu.1, Junb, Jun, and Nab2 promoter-driven luciferase reporter, whereas Cebpb and Gfi1b promoter-driven luciferase reporter expression were normal in ATF5-overexpressed cells compared with control vector groups (Figure S14B). Thus, Pu.1, Junb, Jun, and Nab2 are the direct transcriptional targets of ATF5, and ATF5 may indirectly regulate Cebpb and Gfi1b transcriptional expression. These results collectively demonstrate that ATF5 deletion prevents the proliferation of myeloid progenitors and monocytic lineage development. Is ATF5 involved in mTORC2-mediated regulation of myeloid progenitor differentiation? To address this issue, we infected sorted WT and ER-Rictor LSKs with a lentivirus-expressing ATF5 and observed macrophage development in these LSKs when cultured with IL-3, IL-6, and M-CSF. The results show that overexpression of ATF5 (Figure 6I) significantly rescued the impaired macrophage differentiation of Rictor-deficient progenitors, as indicated by significantly increased percentages and cell numbers of CD11b+CD115+ and CD11b+F4/80+ cells derived from ATF5-overexpressed, Rictor-deleted LSKs (Figures 6J-M). Therefore, impaired ATF5 expression is involved in the poor monocytic lineage development observed in Rictor-deleted LSKs.
Figure 6.
Genetic deletion of ATF5 significantly blocks monocyte development, while ATF5 overexpression rescues defective monocyte/macrophage development caused by Rictor deletion
(A) Sorted WT LSKs were subjected to nucleofection with negative control sgRNA (NC) or ATF5 sgRNA, after which cells were seeded in liquid media supplemented with IL-6, IL-3, and M-CSF for six days. Cell growth was photographed as shown in the figure with scar bars 200 μm.
(B) NC or ATF5 sgRNA-treated LSKs were cultured in the presence of M-CSF, IL-6, and IL-3 for six days and stained with anti-CD11b mAb and anti-F4/80 mAb. The phenotypes of the samples were analyzed by flow cytometry as indicated.
(C) The decreased percentages and numbers of CD11b+F4/80+ macrophages in ATF5 knockdown cells are shown. Data are shown as mean ± SD (n = 3), Student’s t test, ∗p < 0.05; ∗∗∗p < 0.001.
(D) LSKs were subjected to nucleofection with NC or ATF5 sgRNA, and 20,000 genetically modified LSKs were seeded in methylcellulose media supplemented with SCF, IL-3, and M-CSF. The experiment was performed in triplicate. One CFU of representative size generated from NC or ATF5 sgRNA LSKs was photographed with the representative scar bars 1 mm.
(E and F) Colonies, total cell numbers (E), and macrophage cell number per colony (F) were calculated. Data are shown as mean ± SD (n = 3), Student’s t test, ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
(G) Cells developed from NC and ATF5 sgRNA-treated progenitors were lysed and analyzed to assess ATF5 and CSF1R expression by immunoblotting. Data are representative of three independent experiments.
(H) Cells developed from NC and ATF5 sgRNA progenitors were lysed and analyzed to assess expression of the transcription factors PU.1, CCAAT-enhancer-binding protein b (Cebpb), early growth response 1 (Eg-1), Ngfi-A binding protein 2 (Nab2), jun proto-oncogene (Jun), jun B proto-oncogene (Junb), and MAF BZIP transcription factor (n = 3). WT and ER-Rictor KO progenitors were infected with lentivirus to induce ATF5 overexpression. Data are shown as mean ± SD, Student’s t test, ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
(I) Cells developed from WT and ER-Rictor KO progenitors infected with Myc or ATF5 lentivirus were lysed, and ATF5 expression was assessed by immunoblotting.
(J) LSKs infected with Myc or ATF5 lentivirus were cultured in the presence of M-CSF, IL-6, and IL-3 for five days in vitro, then stained with anti-CD11b mAb and anti-CD115 mAb. Flow cytometry analysis of CD115 in induced cells is shown.
(K) Percentages and numbers of CD11b+CD115+ macrophages developed from WT and ER-Rictor KO progenitors infected with Myc or ATF5 (n = 3). Data are shown as mean ± SD, Student’s t test, ∗∗p < 0.01; ∗∗∗p < 0.001.
(L) LSKs infected with Myc or ATF5 lentivirus were cultured in the presence of M-CSF, IL-6, and IL-3 for five days in vitro and stained with anti-CD11b mAb and anti-F4/80 mAb. Flow cytometry analysis of F4/80 in induced cells is shown.
(M) Percentages and numbers of CD11b+F4/80+ macrophages developed from WT and ER-Rictor KO progenitors infected with Myc or ATF5 lentivirus (n = 3). Data are shown as mean ± SD and are representative of three independent experiments. ∗∗∗p < 0.001; statistical significance was determined with the two-tailed Student’s t test.
The transcription factor ATF5 has been confirmed as an important regulator of mitochondrial unfolded protein response (mtUPR) activation via promotion of a protective transcriptional program and response to mitochondrial dysfunction.49,50 It has been reported that mtUPR activation results in increased mRNA expression of the mitochondrial chaperones HSP60 (HSPD1), HSP10 (HSPE1), and mtHSP70 (HSPA9), the mitochondrial protease LONP1, and ATF5.50 We then examined mRNA expression of ATF5, HSPD1, HSPE1, LONP1, and HSPA9 in isolated WT and Rictor-deficient progenitors, with the goal of determining whether the mtUPR-related pathway is downregulated in Rictor-deficient progenitors. We found that the mRNA expressions of ATF5, HSPD1, HSPE1, LONP1, and HSPA9 were clearly lower in progenitors of ER-Rictor KO LSKs compared with those of WT cells (Figure 7A). Furthermore, ATF5 can promote oxidative phosphorylation (OXPHOS) and cell growth during mitochondrial dysfunction by inducing expression of mitochondrial chaperone and protease genes.51 The mtUPR-related pathway and OXPHOS were enriched in WT progenitors as determined by GSEA, while these factors were downregulated in Rictor-deficient progenitors (Figures 7B and 7C), indicating reduced ATF5-mediated mtUPR activation in Rictor-deficient progenitors. ATF5 regulates mtUPR in mammalian cells and has been described as a key regulator of mtUPR activation.50,52 To investigate the role of mtUPR in regulating myeloid progenitor proliferation and differentiation, we treated WT and ER-Rictor LSKs with ethidium bromide (EtBr) for 24 h, causing ATF5-dependent mtUPR activation by depleting mitochondrial DNA and inducing a number of mitochondrial-specific molecular chaperones and proteases,53 and then cultured these progenitors in the presence of IL-3, IL-6, and M-CSF after removing EtBr. EtBr treatment increased proliferation of WT myeloid progenitors in a dose-dependent manner (Figures 7D–7G). EtBr treatment significantly rescued the decreased frequency and number of CD11b+F4/80+ and CD11b+CD115+ macrophages differentiated from Rictor-deficient progenitors (Figures 7D–7G) and was associated with increased ATF5 and CSF1R expression, as detected by western blots (Figure 7H). Consistently, EtBr treatment significantly reversed the poor differentiation ability of Rictor-deficient LSKs in a CFU assay, as evidenced by the increased colony number, total cell number, and cell number per colony of EtBr-treated Rictor-deficient LSKs compared to Rictor-deficient LSKs (Figures 7I–7K). These data collectively indicate that ATF5-mediated mtUPR activation might be partially responsible for the poor monocytic lineage differentiation of Rictor-deleted progenitors.
Figure 7.
ATF5-mediated mtUPR activation restores the ability of Rictor-deficient LSKs to differentiate into the monocytic lineage
(A–C) Sorted LSKs from WT and ER-Rictor KO mice were lysed and analyzed to assess expression of activating transcription factor 5 (ATF5), mitochondrial protease (lonp1), heat shock protein 9 (Hspa9), heat shock protein 1 (chaperonin 1, Hspd1), and heat shock protein 1 (chaperonin 10, Hspe1). Data are shown as mean ± SD (n = 3), Student’s t test, ∗∗∗p < 0.001. Enrichment analysis of mtUPR-related pathways (B) and oxidative phosphorylation (C) by GSEA revealed enrichment in WT progenitors.
(D) LSKs were sorted from tamoxifen-untreated WT and ER-mTOR KO mice and cultured with 4-OHT in the presence and absence of ethidium bromide (EtBr). Induced cells were stained with anti-CD11b mAb and anti-F4/80 mAb.
(E) The percentage and number of CD11b+F4/80+ cells were calculated. Data are shown as mean ± SD (n = 3), Student’s t test, ∗∗∗p < 0.001.
(F) LSKs were sorted from tamoxifen-untreated WT and ER-mTOR KO mice and cultured with 4-OHT in the presence or absence of EtBr. Induced cells were stained with anti-CD11b mAb and anti-CD115 mAb.
(G) Percentage and number of CD11b+CD115+ cells developed from WT and ER-Rictor KO progenitors with or without EtBr at the indicated concentrations. Data are shown as mean ± SD (n = 3), Student’s t test, ∗∗p < 0.01; ∗∗∗p < 0.001.
(H) Cells developed from WT and ER-Rictor KO progenitors in the absence or presence of EtBr were lysed and analyzed to assess expression of ATF5, CSF1R, Rictor, and β-actin by immunoblotting.
(I–K) 2×104 LSKs sorted from WT and ER-Rictor KO mice were seeded in methylcellulose supplemented with IL-3, IL-6, SCF, M-CSF, and 4-OHT in the presence or absence of EtBr (250 ng/mL). This experiment was performed in triplicate and was photographed as presented with the representative scar bars 1 mm. The generated colonies, total cell numbers (J), and macrophage cell numbers per colony (K) were calculated. Data are shown as mean ± SD and are representative of three independent experiments. ∗p < 0.05, and ∗∗p < 0.01; statistical significance was determined with the two-tailed Student’s t test.
Rictor controls the balance of monocytic and granulocytic lineage commitment in GMPs
While we observed a selective decrease in monocyte lineage differentiation upon Rictor deficiency, neutrophil differentiation was unimpaired or even enhanced in these mice. Therefore, we sought to investigate the role of Rictor in monocytic and granulocytic lineage decisions and the commitment of GMPs. To understand the molecular mechanisms that mediate mTORC2 function during the differentiation of myeloid progenitors, we performed RNA-seq assays of WT and Rictor-deleted progenitors and assessed potential key transcription factors steering monocytic and granulocytic lineage differentiation. According to a heatmap analysis of transcription factors, we determined that the expression of transcription factors associated with monocyte/macrophage development, including PU.1, C/EBPβ, Maf, Egr1, AP-1, and Nab2, was markedly decreased in Rictor-deleted progenitors, while the expression of transcription factors related to granulocytic and megakaryocyte-erythroid lineage development, such as C/EBPα, C/EBPδ, C/EBPε, Gfi1, retinoic acid receptor, GATA1, and GATA2, was simultaneously increased (Figures 8A and 8B). However, IRF8 which is important for monocyte development increased in Rictor-deleted progenitors, as evidenced by heatmap analysis (Figure 8A). Previous study has been demonstrated that mTOR/STAT5/IRF8 pathway was required for monocyte development.9 To verify if mTOR/STAT5/IRF8 pathway is also involved in mTORC2-mediated regulation of monocytic lineage development, we analysis this pathway in Rictor-deleted LSKs. We found that mTORC1 activity was increased in Rictor-deleted LSKs compared with WT LSKs, as evidenced by enhanced levels of P-S6 phosphorylation in Rictor-deficient progenitors by western blot assays (Figure S15A). GSEA showed that the STAT5-targeted gene sets were highly enriched in WT progenitors, indicating that STAT5 signaling pathways are indeed inhibited after Rictor deletion (Figures S15B-C). Moreover, IFR8 mRNA expression was remarkably higher in purified Rictor-deficient LSKs compared to WT cells under M-CSF stimulation at the indicated time points, as evidenced by real-time PCR (Figure S15D). These results collectively indicated that mTOR/STAT5/IRF8 signaling pathway is unlikely involved in Rictor-mediated regulation on monopoiesis. Furthermore, we performed network analysis of gene sets and pathways related to granulocyte, monocyte, and megakaryocyte-erythroid development. By further analyzing the gene interaction network of the genes regulating these transcription factors, we observed a corresponding trend in the regulatory genes. After Rictor knockout, the monocyte lineage-related gene network was significantly downregulated, while the megakaryocyte-erythroid lineage and granulocytic lineage networks were upregulated (Figure 8C). These results indicate that mTORC2 may play an important role in the choice between granulocyte and monocyte commitment. To verify this model, we performed granulocyte macrophage colony-stimulating factor (GM-CSF)-, granulocyte colony-stimulating factor (G-CSF)-, and M-CSF-induced myeloid cell differentiation in a methylcellulose culture system using purified WT and ER-Rictor LSKs treated with 4-OHT. Rictor mRNA and protein expression in ER-Rictor LSKs, although not in WT LSKs, were efficiently ablated under 4-OHT treatment (Figures S16A-B). The number of colonies derived from Rictor-deficient LSKs was lower than that of WT cells for all GM-CSF, G-CSF, and M-CSF-inducing CFU assays, with an especially remarkable colony reduction observed in M-CSF-induced Rictor-deficient CFUs (Figures 8D and 8E). Interestingly, in both GM-CSF- and M-CSF-induced CFUs, colony size and cell number per colony were significantly decreased when formed from Rictor-deficient LSKs compared with WT cells, while in G-CSF-induced CFUs, cell numbers per colony derived from WT and Rictor-deficient LSKs were comparable (Figure 8E). Meanwhile, the proportions and cell numbers of CD11b+CD115+Ly6G− and CD11b+Ly6C+Ly6G− monocytes differentiated from Rictor-deficient LSKs were significantly decreased compared with those differentiated from WT LSKs for all GM-CSF, G-CSF, and M-CSF-inducing CFU assays (Figures 8F–8H; S17A-B). Unexpectedly, Rictor-deficient neutrophils in all culture systems also exhibited significantly higher proportions and cell numbers of CD115-Ly6G+ and Ly6C+Ly6G+ neutrophils derived from Rictor-deficient LSKs compared with WT LSKs, as determined by flow cytometry (Figures 8F–8H, S17A, and S17C). Taken together, these results indicate that Rictor deficiency blocks monocytic lineage specification and promotes granulopoiesis.
Figure 8.
mTORC2 acts as a key regulator of the differentiation balance between monocytic and granulocytic lineages
(A) Heatmap representing monocytic developmental gene expression profiles in WT and Rictor KO Lin−Sca1−CD127−c-Kit+CD34+ myeloid progenitors with or without M-CSF.
(B) Heatmap representing granulocytic and megakaryocyte-erythroid gene expression profiles in WT and Rictor KO Lin−Sca1−CD127−c-Kit+CD34+ cells.
(C) Network analysis of monocytic lineage, granulocytic lineage, and megakaryocyte-erythroid lineage -regulated genes.
(D) 2×104 LSKs purified from WT and ER-Rictor KO mice were seeded in methylcellulose supplemented with 4-OHT (0.125 μM), IL-3, and IL-6 as well as GM-CSF, or G-CSF, or M-CSF. This experiment was performed in triplicate and was photographed as presented with the representative scar bars 1 mm.
(E) The generated colonies and differentiated cell number per colony were calculated. Data are shown as mean ± SD (n = 3), Student’s t test, ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
(F) The generated cells were detected by flow cytometry and stained with anti-Ly6G mAb and anti-CD115 mAb. Gated CD11b+ cell plots are shown.
(G and H) The percentages (G) and absolute numbers (H) of monocytes (CD11b+CD115+Ly6G−) and neutrophils (CD11b+CD115-Ly6G+) developed from WT and ER-Rictor KO progenitors were summarized (n = 3). Each group represents three independent experiments. Data are expressed as mean ± SD. p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001; (WT vs. ER-Rictor KO). p values were determined using Student’s t test.
Discussion
A fundamental question in immunology is which molecular regulatory processes underlie myeloid lineage commitment decisions with restricted developmental kinetics in bone marrow during homeostasis. Recent studies have challenged the conventional hierarchical relationship (CMP→GMP→MDP→monocyte) and provided evidence supporting the existence of two independent pathways of monocyte differentiation in bone marrow from CMPs in bone marrow: CMP→GMP→cMoP→monocyte→macrophage and CMP→MDP→cMoP→monocyte→DC.1 Our present study reveals that mTORC2 signaling delivers a positive myeloid-intrinsic signal that selectively regulates CMP/GMP proliferation and promotes monocytic lineage development in accordance with the hierarchical processing model (CMP→GMP→cMoP→monocyte), as supported by the following evidence. First, the percentages and absolute numbers of GMPs, cMoPs, and monocytes were significantly decreased in the bone marrow of Rictor-deficient mice, while levels of HSCs, MPPs, MEPs, and MDPs in the bone marrow of Rictor-deficient mice were comparable with those in WT mice. The frequencies and numbers of CD11b+F4/80+ and CD11b+CD115+ monocytes/macrophages in the spleens, peritoneal cavity, colon, and blood of Rictor-deficient mice were remarkably lower than those found in WT mice, whereas the frequencies and numbers of neutrophils, T cells, and B cells in the spleens of Rictor-deficient mice were similar to those found in WT mice. Although, some studies have demonstrated that Rictor deficiency leads to the developmental restriction of T and B cells by using Mx1-Cre-Rictorflox/flox mice,23,54 the inducible knockout model that is limited to cells that respond to interferon. We observed that the priority changes in myeloid progenitors at the early stage after tamoxifen treatment and within the time that tamoxifen-treated ER-Rictor KO mice showed no obvious growth defects or global appearance abnormality. We considered that myeloid progenitors showed more sensitivity to Rictor deficiency in our knockout model. Second, in vivo differentiation by the adoptive transfer of CMPs and GMPs to recipient mice resulted in poor monocyte differentiation with slightly or undetectably impaired neutrophil differentiation. Third, in vitro GM-CSF, M-CSF, or G-CSF-inducing colony formation experiments of sorted WT and Rictor-deleted progenitors showed that Rictor-deleted progenitors produce fewer monocytes/macrophages than WT control cells while simultaneously producing significantly more neutrophils. On the other hand, emerging evidence indicates that GMPs produce neutrophils and monocytes but not DCs, whereas MDPs yield monocytes and DCs but not neutrophils.1,34 In our present study, DC progenitor MDPs were unchanged in the bone marrow of Rictor-deficient mice, consistent with previous reports,35 indicating that the reduction of mTORC2 activity achieved by Rictor deletion is not sufficient to alter DC development. Thus, Rictor expression is selectively required for monocyte differentiation and is critical for balanced monocytic and neutrophil lineage decision and commitment in mice (Figure 9).
Figure 9.
Schematic representation of the mTORC2-AKT-Foxo4-ATF5-mediated mtUPR pathway involved in regulating monocytic lineage development
mTORC2 is important for CMP/GMP proliferation and differentiation into monocytes along the CMP→GMP→cMoP→monocyte developmental trajectory while blocking granulocyte lineage development in bone marrow. During the differentiation of CMPs/GMPs to cMoPs, mTORC2-AKT-Foxo4-ATF5 signaling (Signal a) enhances myeloid progenitor proliferation and monocytic lineage development. In addition, ATF5 mediates mtUPR activation (Signal b), which promotes trafficking of mitochondrial ATF5 to the nucleus via its nuclear localization signal (Signal c) and further promotes ATF5-mediated cell proliferation and differentiation.
Among the stages of macrophage development in bone marrow and peripheral tissues, mTORC2 signal selectively regulates the myeloid progenitor (CMP/GMP) stage rather than later monocyte development. In other words, CMP/GMP cells are more sensitive to Rictor deficiency than other cell subpopulations during HSC→macrophage differentiation. This conclusion is supported by the following pieces of evidence. First, while the peripheral monocyte/macrophage pool was remarkably perturbed in tamoxifen-treated ER-Rictor KO mice, whereas normal levels of monocytes/macrophages in bone marrow and peripheral tissues in Lyzs-Rictor KO mice indicate that mTORC2 is not essential for peripheral monocyte and macrophage survival and homeostasis maintenance in physiological situations. Second, while Rictor deletion mainly impaired post-CMP/GMP developmental stages in bone marrow and caused poor cell proliferation of CMPs and GMPs in vivo and in vitro, it failed to show a detectable impact on CMP/GMP cell survival. The poor proliferation of Rictor-deficient CMPs/GMPs caused a dramatical reduction in the progeny of their differentiation. Third, compared to WT cells, Rictor-deficient LSKs express lower levels of transcription factors critical to progenitor fate decisions toward the monocytic lineage, including PU.1, Egr1, Maf, Nab2, Cebpb, and Jun.47 Decreases in these key transcription factors and in expression of CD115, an important target of PU.1,55 may contribute to the reduced monocyte/macrophage commitment of Rictor-deficient LSKs.
The best characterized role of mTORC2’s kinase activity is the phosphorylation of AKT at serine 473.18,19 Loss of Rictor diminishes AKT Ser473 phosphorylation and reduces mTORC2 activity, as confirmed in our present study. Importantly, we found that the AKT pathway is involved in the control of myeloid progenitor proliferation and differentiation and also serves as the key joint node regulating monocytic and neutrophil lineage commitment from GMPs, as verified by the results of in vitro myeloid progenitor differentiation experiments using the AKT inhibitor MK-2206 and the AKT agonist SC79. By comparing the differential gene expression profiles of transcription factors in WT and Rictor-deficient progenitors in the absence or presence of M-CSF with gene signatures downstream of AKT, we determined that the transcription factor ATF5 occupies the central position of the entire regulatory network of Rictor in LSKs, as supported by topological confidence score calculations. Deletion of ATF5 by CRISPR-Cas9 genetic technology impaired the development of WT LSKs toward monocytes/macrophages in vitro, accompanied by significantly decreased expression of key monocytic lineage differentiation-related transcription factors such as PU.1, Egr1, Maf, Nab2, Cebpb, and Jun.47 Overexpression of ATF5 can significantly restore the ability for Rictor-deleted LSKs to develop into monocytes/macrophages. These results collectively suggest that ATF5 may act as a “accelerator” of myeloid progenitor proliferation and promote the monocytic lineage cell fate. It has been demonstrated that the transcriptional activity and expression of Foxo4 are both negatively regulated by AKT.39 Foxo4 inhibits transcriptional expression of ATF5 by regulating its promoter.40 Consistently, we observed enhanced Foxo4 expression and decreased ATF5 expression in WT LSKs treated with the AKT inhibitor MK-2206. In contrast, the AKT agonist SC79 efficiently promotes ATF5 protein expression to significantly restore monocytic differentiation in Rictor-deficient LSKs in an in vitro differentiation culture system. These data collectively indicate that mTORC2 deficiency decreases AKT activity and subsequently increases Foxo4 expression to ultimately inhibit ATF5 expression during the differentiation of LSKs into monocytes (Figure 9). It is known that Gfi1 represses a gene encoding an inducer of monopoiesis, such as PU.1, with PU.1 normally activating a repressor of terminal granulopoiesis.28 Gfi1 mRNA expression was upregulated and PU.1 was simultaneously downregulated in ATF5-deficient progenitors compared with WT progenitors, consistently with results observed in Rictor-deleted LSKs. Thus, we speculate that ATF5 might be involved in controlling the balance between monocyte and neutrophil differentiation from GMPs.
ATF5 is an important regulator of the mtUPR pathway and is required to maintain OXPHOS function.49,50,51,56 ATF5 is targeted to mitochondria via an amino terminal mitochondrial targeting sequence and is subsequently degraded in the mitochondrial matrix during mitochondrial stress or dysfunction. Then, ATF5 is trafficked to the nucleus via its nuclear localization signal, where it regulates transcription to promote mitochondrial protein homeostasis (proteostasis), mitochondrial recovery or biogenesis, metabolic adaptations such as glycolysis, and OXPHOS complex assembly.51,52 In our results, treatment with EtBr, which activates the mtUPR pathway in an ATF5-dependent manner,50,57 significantly enhanced WT LSK proliferation and differentiation to the monocytic lineage, indicating an important role of the mtUPR-related pathway in the differentiation of GMPs to monocytes (Figure 9). mRNA expression of HSP60 (HSPD1), HSP10 (HSPE1), mtHSP70 (HSPA9), and the mitochondrial protease LONP1, which are all ATF5-regulated genes, decreased remarkably in Rictor-deficient LSKs as determined by real-time PCR and RNA-seq assays. In parallel with the observation that the ATF5-induced mtUPR pathway is decreased in Rictor-deleted LSKs, we found that both genetically overexpression of ATF5 and treatment with the mtUPR-inducing agent EtBr50,57 can significantly rescue the decreased percentage and number of monocytes/macrophages derived from Rictor-deficient LSKs in vitro. Thus, Rictor controls cell proliferation and monocytic lineage differentiation of LSKs primarily via ATF5-mediated mtUPR activation. mtUPR is an evolutionarily conserved adaptive mechanism for improving cell survival under mitochondrial stress.49 Our present study reveals that mTORC2/AKT signaling orchestrates myeloid progenitor proliferation and monocytic lineage development by mediating the ATF5-induced mtUPR pathway. This observation is consistent with a recent study showing that the mtUPR is activated upon the transition of HSCs from quiescence to proliferation.58 We speculate that the mtUPR acts as a protective mechanism under mitochondrial stress and is indispensable for myeloid progenitor proliferation and differentiation, processes with high energy requirements.
In conclusion, mTORC2 controls CMP/GMP proliferation and differentiation into monocytes in bone marrow by regulating AKT-Foxo4-ATF5-mtUPR pathways. mTORC2 regulates the balance of monocyte and neutrophil commitment in GMPs, with a potential differentiation preference toward the monocytic lineage. mTORC2 is not essential for the differentiation of monocytes into macrophages in the periphery.
Limitations of the study
In this study, we uncovered the role of mTORC2 in the development of monocyte development. We also showed that Rictor ablation caused the downregulation of AKT/Foxo4/ATF5, which subsequently led to the attenuation of mitochondrial unfolded protein response. However, due to the scarcity of Rictor-deletion progenitors, we could not get enough primary myeloid progenitors to examine the downregulated transcription factors of ATF5 by chromatin immunoprecipitation sequencing. Although we found that ATF5 deletion favorably impaired monocyte development and downregulated the transcription factors associated with monocytic development, we have not fully uncovered the underlying mechanism, which needs to be addressed in the future.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-CD3-FITC | BioLegend | Cat# 100204; RRID:AB_312661 |
| anti-NK1.1-FITC | BioLegend | Cat# 108706; RRID:AB_448547 |
| anti-TER119-FITC | BioLegend | Cat# 116206; RRID:AB_313707 |
| anti-CD11b-FITC | BioLegend | Cat# 101206; RRID:AB_312789 |
| anti-CD19-FITC | BioLegend | Cat# 152404; RRID:AB_2629813 |
| anti-Ly6G-FITC | BioLegend | Cat# 127606; RRID:AB_1236494 |
| anti-CD11c-FITC | BioLegend | Cat# 117306; RRID:AB_313775 |
| anti-Sca-1-PE-CY5 | BioLegend | Cat# 108110; RRID:AB_313347 |
| anti-CD117-APC-CY7 | BioLegend | Cat# 105826; RRID:AB_1626278 |
| anti-CD34-BV421 | BioLegend | Cat# 152208; RRID:AB_2650766 |
| anti-CD16/32-BV510 | BioLegend | Cat# 101333; RRID:AB_2563692 |
| anti-CD115-PE | BioLegend | Cat# 135506; RRID:AB_1937253 |
| anti-CD115-PE-CY7 | BioLegend | Cat# 135524; RRID:AB_2566460 |
| anti-CD135-BV421 | BioLegend | Cat# 135314; RRID:AB_2562339 |
| anti-Ly-6C-PE | BioLegend | Cat# 128008; RRID:AB_1186132 |
| anti-Ly-6C-BV605 | BioLegend | Cat# 128036; RRID:AB_2562353 |
| anti-CD45.2-PB | BioLegend | Cat# 109820; RRID:AB_492872 |
| anti-CD45.1-PE | BioLegend | Cat# 110708; RRID:AB_313497 |
| anti-F4/80-APC | BioLegend | Cat# 157306; RRID:AB_2832549 |
| anti-CD11b-BV510 | BioLegend | Cat# 101263; RRID:AB_2629529 |
| anti-CD34-APC | BioLegend | Cat# 152215; RRID:AB_2910310 |
| anti-CD127-BV785 | BioLegend | Cat# 135037; RRID:AB_2565269 |
| anti-CD45-BUV395 | BD Bioscience | Cat# 565967; RRID:AB_2651134 |
| anti-CD4-FITC | eBioscience™ | Cat# 11-0041-82; RRID:AB_464892 |
| anti-CD8-PE-CY5 | eBioscience™ | Cat# 15-0081-82; RRID:AB_468706 |
| anti-B220-PE | eBioscience™ | Cat# 12-0452-82; RRID:AB_465671 |
| Rictor | Cell Signaling Technology | Cat# 9476; RRID:AB_10612959 |
| phospho-S6 (Ser235/236) | Cell Signaling Technology | Cat# 4858; RRID:AB_916156 |
| phospho-Akt (Ser473) | Cell Signaling Technology | Cat# 4060; RRID:AB_2315049 |
| phospho-Akt (Thr308) | Cell Signaling Technology | Cat# 4056; RRID:AB_331163 |
| AKT | Cell Signaling Technology | Cat# 4691; RRID:AB_915783 |
| FoxO4 | Cell Signaling Technology | Cat# 9472; RRID:AB_10831833 |
| CSF1R | Cell Signaling Technology | Cat# 67455; RRID:AB_2799725 |
| β-Actin | Sigma-Aldrich | Cat# A5441; RRID:AB_476744 |
| S6 | Cell Signaling Technology | Cat# 2217; RRID:AB_331355 |
| phospho-FoxO4 (S262) | abcam | Cat# ab126594; RRID:AB_11130059 |
| ATF5 | abcam | Cat# ab184923; RRID:AB_2800462 |
| anti-rabbit-HRP | KPL | Cat# 070-1506 |
| anti-mouse-HRP | KPL | Cat# 074-1806 |
| β-Actin | Sigma-Aldrich | Cat# A5441; RRID:AB_476744 |
| Chemicals, peptides, and recombinant proteins | ||
| Fixation/Permeabilization Concentrate | Thermo Fisher Scientific | Cat# 00-5123-43 |
| Fixation/Permeabilization Diluent | Thermo Fisher Scientific | Cat# 00-5223-56 |
| Cytofix/Cytoperm solution | BD Biosciences | Cat# 554722 |
| Perm/Wash the solution | BD Biosciences | Cat# 554723 |
| collagenase/dispase | Sigma-Aldrich | Cat# 11097113001 |
| DNAse I | Sigma-Aldrich | Cat# D5025 |
| SYBR Premix Ex TaqTM | TaKaRa | Cat# RR420 |
| DNAse I | Sigma-Aldrich | Cat# D5025 |
| RIPA buffer | Thermo Fisher | Cat# 87787 |
| PMSF | Beyotime | Cat# ST506 |
| MK-2206 2HCl (AKT inhibitor) | Selleck | Cat# S1078 |
| Ethidium bromide (EtBr) | Sigma-Aldrich | Cat# E8751 |
| Tamoxifen | Sigma-Aldrich | Cat# T5648 |
| 4-hydroxy tamoxifen (4-OHT) | Sigma-Aldrich | Cat# E8751 |
| Lipofectamine 3000 | Thermo Fisher | Cat# L3000015 |
| Alt-R S.p. HiFi Cas9 Nuclease V3 | Integrated DNA Technologies | Cat# 1081060 |
| PhosSTOP phosphatase inhibitor | Roche | Cat# 04906845001 |
| SC79 | Sigma-Aldrich | Cat# SML0749 |
| MethoCultTM M3134 | STEMCELL technologies | Cat# 03134 |
| Prestained Protein Ladder | Thermo Fisher Scientific | Cat# 26616 |
| Critical commercial assays | ||
| PE Active Caspase-3 Apoptosis Kit | BD Biosciences | Cat# 550914; RRID:AB_393957 |
| 647 EdU Click Proliferation Kit | BD Biosciences | Cat# 565456; RRID:AB_2869678 |
| Dual-Luciferase® Reporter Assay System kit | Promega | Cat# E1960 |
| Enhanced BCA Protein Assay Kit | Beyotime | Cat# P0010S |
| Micro Elute Total RNA kit | Omega-tek | Cat# R6831-01 |
| SuperScript III Reverse Transcriptase Kit | Invitrogen | Cat#18080-093 |
| P3 Primary Cell 4D-Nucleofector® X Kit L | Lonza | Cat# V4XP-3024 |
| Hieff Clone® Plus One Step Cloning Kit | YEASEN | Cat# 10911ES50 |
| Deposited data | ||
| Bulk RNA-seq data | This paper | GEO: PRJNA918499 |
| Experimental models: Cell lines | ||
| HEK-293T | ATCC | Cat# CRL-3216 |
| Experimental models: Organisms/strains | ||
| ER-Cre mice | Zhao et al.9 | N/A |
| Rictorloxp/loxp mice | Chen et al.20 | N/A |
| LyzM-Cre mice | Zhu et al.59 | N/A |
| Oligonucleotides | ||
| ATF5 sgRNA CAAGCCTGAATCCCCCGGCC |
This paper | N/A |
| Negative control sgRNA GTGTAGTTCGACCATTCGTG |
This paper | N/A |
| ER-Cre-primer1 TGTGGACAGAGGAGCCATAAC | Zhao et al.9 | N/A |
| ER-Cre-primer2 CATCACTCGTTGCATCGACC | Zhao et al.9 | N/A |
| ER-Cre-primer3 AAGACCCAACCAACAGCA | Zhao et al.9 | N/A |
| LysMCre-primer1 CTTGGGCTGCCAGAATTTCTC |
Zhu et al.59 | N/A |
| LysMCre-primer2 TTACAGTCGGCCAGGCTGAC |
Zhu et al.59 | N/A |
| LysMCre-primer3 CCCAGAAATGCCAGATTACG |
Zhu et al.59 | N/A |
| Rictorloxp/loxp-primer1 ACTGAATATGTTCATGGTTGTG |
Chen et al.20 | N/A |
| Rictorloxp/loxp-primer2 GAAGTTATTCAGATGGCCCAGC |
Chen et al.20 | N/A |
| Recombinant DNA | ||
| Plasmid: pCDH-CMV-Myc-MCS-EF1α-copGFP | This paper | N/A |
| Plasmid: pCDH-CMV-Myc-ATF5-EF1α-copGFP | This paper | N/A |
| Plasmid: pcDNA3.1-Myc-N | This paper | N/A |
| Plasmid: pcDNA3.1-Myc-N-ATF5 | This paper | N/A |
| Plasmid: pRP-hRluc/Puro-Pu.1-miniCMV-Luciferase | This paper | N/A |
| Plasmid: pRP-hRluc/Puro-Cebpb-miniCMV-Luciferase | This paper | N/A |
| Plasmid: pRP-hRluc/Puro-Jun-miniCMV-Luciferase | This paper | N/A |
| Plasmid: pRP-hRluc/Puro-Junb-miniCMV-Luciferase | This paper | N/A |
| Plasmid: pRP-hRluc/Puro-Nab2-miniCMV-Luciferase | This paper | N/A |
| Plasmid: pRP-hRluc/Puro-Gfil1b-miniCMV-Luciferase | This paper | N/A |
| Software and algorithms | ||
| FlowJo v10 | FlowJo, LLC | https://www.flowjo.com/ |
| KOBAS 3.0 | Wu et al.60 Xie et al.61 |
http://kobas.cbi.pku.edu.cn/ |
| Cytoscape_v3.9.0 | Shannon et al.62 | https://cytoscape.org/ |
| STRING11.5 | This paper | https://cn.string-db.org/cgi/input?sessionId=brxHAcCk07dk&input_page_show_search=on |
| GSEA_4.0.3 | Zhang et al.7,63 | https://www.gsea-msigdb.org/gsea/index.jsp |
| DESeq23.17 | Wang et al.64 Lei et al.65 |
https://bioconductor.org/packages/release/bioc/html/DESeq2.html |
| R3.6.0 | This paper | https://cran.r-project.org/bin/windows/base/old/3.6.0/ |
| HISAT 2.2.1 | Pertea et al.66 | https://daehwankimlab.github.io/hisat2/ |
| StringTie2.1.0 | Zhang et al.63 | https://ccb.jhu.edu/software/stringtie/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Yong Zhao (zhaoy@ioz.ac.cn).
Materials availability
This study did not generate new unique reagents.
Experimental model and study participant details
Mice
Rictorloxp/loxp mice were kindly provided by Dr. Zhongzhou Yang from the Center of Model Animal Research at Nanjing University, China. Cre-ERT mice and Cre-LysM mice were originally generated and kindly provided by Dr. Lianfeng Zhang from the Chinese Academy of Medical Sciences & Comparative Medical Center, Peking Union Medical College, China. Mice with myeloid-specific Rictor deletion (LysmCreRictorloxp/loxp) were generated by crossbreeding Rictorloxp/loxp mice with Lysm-Cre mice expressing Cre recombinase under the control of the Lysozyme promoter (LysMCre).59,65 Mice with systemic Rictor deletion induced by tamoxifen were generated by crossbreeding Rictorloxp/loxp mice with ER-Cre mice.67 WT littermates served as controls. For in vivo tamoxifen treatments, WT or ER-Rictor mice (female or male, 6-8 weeks old), were injected intraperitoneally with 1 mg tamoxifen (Sigma) per day for five consecutive days. Treated mice were then rested for 18-21d before analyses.35 Six to eight-week-old female or male CD45.1 C57BL/6J mice were purchased from Beijing University Experimental Animal Center (Beijing, China). All mice were bred and maintained in a specific-pathogen-free animal facility at the Animal Experiment Centre of the Institute of Zoology in Beijing, China. All experiments were performed ethically according to the Institute of Zoology’s Institutional Guidelines for the Care and Use of Laboratory Animals.
Cell lines
HEK-293T cells were grown in DMEM supplemented with 10% FBS and Penicillin-Streptomycin at 37°C with 5% carbon dioxide (CO2).
Isolation of mouse bone marrow progenitors
Common myeloid progenitors (CMPs) and granulocyte-monocyte progenitors (GMPs) were sorted from bone marrow of tamoxifen-treated male or female WT or ER-Rictor KO mice (6-8 weeks old) by cell flow sorter.32,68,69 For CMP and GMP isolation, bone marrow cells (BMCs) were stained with lineage markers (anti-CD3, anti-CD19, anti-Ly6G, anti-NK1.1, anti-CD11b, anti-CD11c, and anti-TER119) as well as anti-CD127, anti-CD117, anti-Sca-1, anti-CD16/32 and anti-CD34 mAbs. Lin-CD127-sca1-CD117+CD34+CD16/32- CMPs and Lin-CD127-sca1-CD117+CD34hiCD16/32hi GMPs were sorted by flow cytometry (Fusion, BD Biosciences). Purity and viability of sorted cells exceeded 95%.
Cell culture and drug treatment
5 mg of 4-hydroxy tamoxifen (Sigma, Cat. No. H7904) was dissolved in methanol (0.5 mL). The solution was filter-sterilized and kept in a dark place. Sorted progenitors were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (from Gibco), 25 mM HEPES, 2 mM L-glutamine, 100 μM 2-mercaptoethanol, and 1% (v/v) penicillin/streptomycin (from Hyclone), 10 ng/mL IL-3, 20 ng/mL IL-6, and 40 ng/mL M-CSF for 5 days.9 Ethidium bromide (Sigma, E8751) was dissolved in water and also kept in a dark place. For preinduction of the mtUPR using ethidium bromide (EtBr),50 sorted progenitors were cultured in the above medium containing EtBr at the indicated concentrations for 24 h. After pretreatment, cells were washed twice with PBS to remove EtBr, then transferred to fresh medium containing 10 ng/mL IL-3, 20 ng/mL IL-6, 40 ng/mL M-CSF, and 1 μM 4-hydroxy tamoxifen (4-OHT). The viability, numbers, and differentiated phenotypes of the progenitors were measured after five days of culture.
Methylcellulose colony-forming assays
A total of 20,000-40,000 progenitors were plated in methylcellulose supplemented with recombinant murine 10 ng/mL IL-3, 20 ng/mL IL-6, 50 ng/mL SCF, and 40 ng/mL M-CSF, or 25 ng/mL GM-CSF or 20 ng/mL G-CSF. Cells were cultured in triplicate in MethoCult™ M3134 (STEMCELL technologies) at 37°C and 5% CO2 for 6-10 days. Colonies were counted and identified after 6-10 days of culture as described.9
In vivo progenitor differentiation
WT and ER-Rictor mice were treated with Tamoxifen for 5 consecutive days and rested for 18 days. Of this group, mice weighing 20 g were injected intraperitoneally with 1 mg tamoxifen daily. Mice were then bled, and BMCs were isolated at the indicated time points. Erythrocytes were lysed, and CMPs, GMPs or LSKs were sorted as described above. Wild type or Rictor-deficient CD45.2 CMPs or GMPs were intravenously injected (90,000 cells/mouse in 1 mL PBS) into non-irradiated CD45.1 recipient mice on Day 0. In addition, WT CD45.2+GFP+ LSKs mixed with CD45.2+GFP- WT or Rictor-deficient CD45.2+GFP- LSKs at the ratios 1:2 were intravenously injected (100,000 cells/mouse in 1 mL PBS) into non-irradiated CD45.1 recipient mice on day 0. Mice were sacrificed at the indicated time points; bone marrow (ilium, femurs and tibias) and blood were harvested, and single cell suspensions were prepared. Erythrocytes were lysed with ACK lysis buffer, and cells were stained with fluorophore-conjugated antibodies for surface marker analysis of CD45.2 positive donor-derived cells. Cells were stained for CD45.1, CD45.2, CD11b, Ly6C, Ly6G, and CD115 for neutrophil and monocyte identification, and with CD45.1, CD45.2, CD11b, CD117, CD16/32, CD34, Ly6C, CD115, and CD135 for progenitor identification.
Method details
Flow cytometry
For flow cytometric analysis, cells were stained with fluorophore-conjugated antibodies for surface marker analysis in staining buffer (PBS containing 0.1% BSA (weight/volume), and 0.1% NaN3).70 The antibodies used for flow cytometric analysis and FACS sorting were as follows: anti-CD3 (clone 17A2), anti-NK1.1 (clone PK136), anti-Ter119 (clone TER-119), anti-CD19 (clone 1D3), anti-CD11b (clone M1/70), anti-Ly-6G (clone 1A8), anti-CD11c (clone N418), anti-Sca-1 (clone D7), anti-CD117 (clone 2B8), anti-CD34 (clone SA376A4), FcγR (CD16/32; clone 93), anti-CD115 (clone AFS98), anti-CD135 (clone A2F10), anti-Ly-6C (clone HK1.4), anti-CD45.1 (clone A20), anti-CD45.2 (clone 104), and anti-F4/80 (clone QA17A29) from Biolegend; and anti-CD4 (clone GK1.5), anti-CD8 (clone 53-6.7), and anti-B220 (clone RA3-6B2) from eBioscience and anti-CD45 (clone 30-F11) from BD Bioscience. Intracellular staining of caspase3 from BD Bioscience was analyzed by flow cytometry using the active caspase analysis kit (BD Biosciences, No.550914) according to the manufacturer’s instructions.71 Cells were stained for FcγR prior to staining with other antibodies.72 Flow cytometry data were acquired on an LSRFortessa X-20 (BD Biosciences) or FACS Fusion (BD Biosciences) and data were analyzed with FlowJo software.
EdU incorporation assays
For in vivo Edu click proliferation experiments, 100 μL of 10-mg/mL EdU solution was injected intravenously into mice. Twenty-four hours after injection, mice were sacrificed, and bone marrow cells were isolated. Cells that have incorporated the thymidine analog EdU (5-ethynyl-2'-deoxyuridine) can be visualized with the EdU flow kit from BD Biosciences Pharmingen™ (Cat. No. 565456) using a fluorochrome 647-labeled anti-EdU antibody.73 Isolated BMCs were first stained with lineage markers (anti-CD3, anti-CD19, anti-Ly6G, anti-NK1.1, anti-CD11b, anti-CD11c, and anti-TER119) as well as anti-CD127, anti-CD117, anti-Sca-1, anti-CD16/32 and anti-CD34 mAbs to define and visualize CMP and GMP populations. Flow cytometry data were acquired on an LSRFortessa X-20 (BD Biosciences) and were analyzed with FlowJo software.
Plasmid construction
The 2500bp Pu.1, Cebpb, Gfi1b, Junb, Jun, and Nab2 promoter sequence and ATF5 sequence was directly synthesized in vitro. Various truncated Pu.1, Cebpb, Gfi1b, Junb, Jun, and Nab2 promoter sequences were assembled into pRP-hRluc/Puro-miniCMV-Luciferase (Vectorbuilder) vector according to the Hieff Clone® Plus One Step Cloning Kit (YEASEN) instructions. The ATF5 sequence was cloned into pCDH-CMV-Myc-MCS-EF1α-copGFP lentiviral backbone and pcDNA3.1-Myc-N vector.
Detection of firefly and renilla luciferase activities
All plasmids were transiently transfected into HEK-293T using Lipofectamine 3000 (Thermo Fisher) according to manufacturer instructions. The activities of firefly and Renilla luciferases are measured according to Dual-Luciferase® Reporter Assay System kit (Promega, E1960) instructions at 48h post-transfection.
Lentiviral infection of progenitor cells
At the time of transfection, HEK-293T cells were 70–80% confluent. Cells were transfected with the transfer vector, packaging plasmid (psPAX2) and (pMD2.G) at a 4:3:1 mass ratio using Lipofectamine 3000. Cells were then incubated at 37°C and 5% CO2, and lentivirus was collected from the supernatant after 48 h. Progenitors were infected with lentivirus at an MOI of 5. After infection for 24 h, supernatants containing the lentivirus were removed, and cells were washed twice with PBS. The cells were then cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (from Gibco), 25 mM HEPES, 2 mM L-glutamine, 100 μM 2-mercaptoethanol, and 1% (vol/vol) penicillin/streptomycin (from Hyclone). After 5 days (or at the indicated time points), cells were counted and assessed via surface marker analysis and gene profiling using flow cytometry, real-time PCR and immunoblotting.
Nucleofection of Cas9-RNPs
For each reaction, sgRNAs (Tsingke) were mixed with Cas9 (IDT) at a molar ratio of 2:1 (5 μL sgRNA100 μM + 4.1 μL Cas9 10 mg/mL). The synthesized sgRNA carried the fluorescent tag CY5. Cas9 protein and sgRNA were complexed by incubation at room temperature for more than 20 minutes. The mouse ATF5 sgRNA sequence was CAAGCCTGAATCCCCCGGCC, and the negative control sgRNA sequence was GTGTAGTTCGACCATTCGTG. For each reaction, 2-3×106 progenitors were resuspended in 100 μl of P3 primary nucleofection solution (Lonza). The cell/RNP mix was then immediately loaded into the supplied nucleofector cassette cuvette (Lonza). The cuvette was inserted into the Lonza 4D-Nucleofector, and the nucleofection was performed. Next, 150-180 μL of pre-warmed medium was immediately added to each well of the cassette, which was then placed in a humidified 37°C/5% CO2 incubator. After that, transfected cells were washed twice with PBS, and cells were further sorted by flow cytometry to obtain CY5-positive progenitors for colony-forming assays or in vitro differentiation experiments.
RNA and protein analyses
Total RNA was extracted from cultured cells using a Micro Elute Total RNA kit (Omega, R6831-01), and cDNA was synthesized with a cDNA Reverse Transcription kit. Quantitative real-time PCR was performed using Power SYBR Green PCR Master Mix (Applied Biosystems) and the CFX96 real-time system (Bio-Rad). All real-time PCRs were carried out in triplicate using optical 96-well reaction plates. The primers used in this study are summarized in Table S1. Real-time PCR data were calculated using the comparative Ct (cycle threshold) method and normalized using the housekeeping gene HPRT.
Progenitors were cultured in 1640 medium with 10% FBS and essential supplements under specific differentiation conditions. After stimulation, cells were washed once with cold PBS and lysed for 10 min on ice in RIPA buffer (ThermoFisher, Cat. No. 87787) containing protease inhibitor cocktails and phosphatase inhibitor cocktails (Roche).74 Protein concentrations were determined by BCA assay. Lysates were mixed with SDS-PAGE loading buffer and boiled for 20 min at 100°C. Protein samples were size-fractionated via SDS-PAGE using BioSci™ NewFlash protein AnyKD PAGE (NewFlash, Cat. No. 8012011) and transferred onto PVDF membranes (Millipore).71 After blocking for 1 h in 5% skim milk powder, the membranes were incubated overnight with primary antibodies at 4°C. The next day, membranes were washed with TBST, after which the appropriate HRP-conjugated secondary antibody was added and incubated for 2 h at room temperature. Finally, detection was performed using a chemiluminescence apparatus (ImageQuant LAS 4000). Immunoblotting was performed with the following antibodies: Rictor (D16H9), phospho-S6 (Ser235/236), phospho-Akt (Ser473), phospho-Akt (Thr308), AKT, FoxO4, CSF1R, β-Actin, and S6 antibodies purchased from CST; and phospho-FoxO4 (S262) and ATF5 antibodies purchased from Abcam.
RNA-seq data processing
Lineage-CD127-Sca-1-CD117+CD34+ cells, including CMPs and GMPs, were isolated from 8-week-old wild-type and tamoxifen-treated-ER-Rictor KO mice with or without 20 h of M-CSF stimulation. Total RNA was extracted from the sorted myeloid progenitors using Micro Elute Total RNA kit, and the sequencing library generation and processing were performed by Novogene Company. Low quality reads (Q < 20) were evaluated by FastQC, and Trimgalore was used to filter adapter sequences. Read data was mapped to the mouse mm10 reference genome using HISAT2 mapping software.66 String-tie software was used to construct transcripts independently for each cell.66,75 DEGseq software was used to identify differentially expressed genes.65,64 P<0.05 and Log|FoldChange|>0 were set as thresholds for differentially expressed genes.
Pathway enrichment analysis
KEGG pathway analysis of DEGs was performed by KOBAS 3.0 (http://kobas.cbi.pku.edu.cn/).60,61 Protein-protein interaction networks were analyzed by String (https://string-db.org/) and visualized by Cytoscape.62 In addition, GSEA was carried out by searching KEGG and Biocarta databases.63 All analyses employed p < 0.05 as the cut-off criterion.
Quantification and statistical analysis
All data are presented as mean±SD. Unpaired two-tailed Student’s t-tests were used to compare data between two groups. Data from more than two groups were performed using Student’s two-tailed t-test, with p values < 0.05 (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001) being considered statistical significance in the figures. The micrographs were analyzed and photographed by BioTek Cytation5.
Acknowledgments
We gratefully acknowledge Dr. Yangxiao Hou and Dr. Yifang Chen for their review of our manuscript, Mrs. Ling Li for her excellent lab management, and Mrs. Qing Meng for her expert technical assistance. This work was supported by grants from the National Natural Science Foundation of China for Key Programs (31930041, Y.Z.), the National Key Research and Development Program of China (2017YFA0105002, Y.Z.), the National Natural Science Foundation of China (81802846, Yang Zhao), the Natural Science Foundation of Jiangsu Province (BK20180116, Yang Zhao), the China Postdoctoral Science Foundation (2021M693162, Yang Zhao), and the Knowledge Innovation Program of the Chinese Academy of Sciences (XDA16030301, Y.Z.).
Author contributions
Conceptualization, Y.Z.; Methodology, Y.Z. and C.X.Z.; Software, Z.Q.Z.; Investigation, Y.Z., C.X.Z., H.G., Z.Q.Z., and Y.N.X.; Resources, H.W.X. and M.P.S.; Visualization, D.W.; Writing, Y.Z.; Supervision, Y.Z.
Declaration of interests
The authors declare no competing interests.
Published: August 3, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107540.
Supplemental information
Data and code availability
-
•
The RNA sequencing datasets generated in this study can be found in the NCBI SRA database (http://www.ncbi.nlm.nih.gov/sra/) using the access number PRJNA918499.
-
•
All other data needed to estimate the conclusions in this paper are present in the main text or the supplementary materials. This paper did not report original code. All data reported in this paper will be shared by the lead contact upon request. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Yáñez A., Coetzee S.G., Olsson A., Muench D.E., Berman B.P., Hazelett D.J., Salomonis N., Grimes H.L., Goodridge H.S. Granulocyte-Monocyte Progenitors and Monocyte-Dendritic Cell Progenitors Independently Produce Functionally Distinct Monocytes. Immunity. 2017;47:890–902.e4. doi: 10.1016/j.immuni.2017.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Notta F., Zandi S., Takayama N., Dobson S., Gan O.I., Wilson G., Kaufmann K.B., McLeod J., Laurenti E., Dunant C.F., et al. Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science. 2016;351:aab2116. doi: 10.1126/science.aab2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul F., Arkin Y., Giladi A., Jaitin D.A., Kenigsberg E., Keren-Shaul H., Winter D., Lara-Astiaso D., Gury M., Weiner A., et al. Transcriptional Heterogeneity and Lineage Commitment in Myeloid Progenitors. Cell. 2016;164:325. doi: 10.1016/j.cell.2015.12.046. [DOI] [PubMed] [Google Scholar]
- 4.Drissen R., Buza-Vidas N., Woll P., Thongjuea S., Gambardella A., Giustacchini A., Mancini E., Zriwil A., Lutteropp M., Grover A., et al. Distinct myeloid progenitor-differentiation pathways identified through single-cell RNA sequencing. Nat. Immunol. 2016;17:666–676. doi: 10.1038/ni.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olsson A., Venkatasubramanian M., Chaudhri V.K., Aronow B.J., Salomonis N., Singh H., Grimes H.L. Single-cell analysis of mixed-lineage states leading to a binary cell fate choice. Nature. 2016;537:698–702. doi: 10.1038/nature19348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nerlov C., Drissen R., Buza-Vidas N., Woll P., Thongjuea S., Gambardella A., Giustacchini A., Jacobsen S.E. Distinct Myeloid Progenitor Differentiation Pathways Identified through Single Cell Rna Sequencing. Exp. Hematol. 2016;44:S46. doi: 10.1016/j.exphem.2016.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z., Bossila E.A., Li L., Hu S., Zhao Y. Central gene transcriptional regulatory networks shaping monocyte development in bone marrow. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1011279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karmaus P.W.F., Herrada A.A., Guy C., Neale G., Dhungana Y., Long L., Vogel P., Avila J., Clish C.B., Chi H. Critical roles of mTORC1 signaling and metabolic reprogramming for M-CSF-mediated myelopoiesis. J. Exp. Med. 2017;214:2629–2647. doi: 10.1084/jem.20161855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y., Shen X., Na N., Chu Z., Su H., Chao S., Shi L., Xu Y., Zhang L., Shi B., Zhao Y. mTOR masters monocyte development in bone marrow by decreasing the inhibition of STAT5 on IRF8. Blood. 2018;131:1587–1599. doi: 10.1182/blood-2017-04-777128. [DOI] [PubMed] [Google Scholar]
- 10.Xu H., Zhao Y., Zhao Q., Shi M., Zhang Z., Ding W., Zhao Y. Tuberous Sclerosis Complex 1 Deficiency in Macrophages Promotes Unclassical Inflammatory Response to Lipopolysaccharide In Vitro and Dextran Sodium Sulfate-Induced Colitis in Mice. Aging Dis. 2022;13:1875–1890. doi: 10.14336/AD.2022.0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang Z., Zhang Q., Zhang Z., Sun L., Dong X., Li T., Tan L., Xie X., Sun L., Zhao Y. The Development and Survival of Thymic Epithelial Cells Require TSC1-Dependent Negative Regulation of mTORC1 Activity. J. Immunol. 2021;207:2039–2050. doi: 10.4049/jimmunol.2100463. [DOI] [PubMed] [Google Scholar]
- 12.Fu W., Hall M.N. Regulation of mTORC2 Signaling. Genes. 2020;11 doi: 10.3390/genes11091045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chi H. Sin1-mTORC2 signaling drives glycolysis of developing thymocytes. J. Mol. Cell Biol. 2019;11:91–92. doi: 10.1093/jmcb/mjy078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Z., Xu M., Liu P., Zhang S., Shang R., Qiao Y., Che L., Ribback S., Cigliano A., Evert K., et al. The mTORC2-Akt1 Cascade Is Crucial for c-Myc to Promote Hepatocarcinogenesis in Mice and Humans. Hepatology. 2019;70:1600–1613. doi: 10.1002/hep.30697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Senoo H., Murata D., Wai M., Arai K., Iwata W., Sesaki H., Iijima M. KARATE: PKA-induced KRAS4B-RHOA-mTORC2 supercomplex phosphorylates AKT in insulin signaling and glucose homeostasis. Mol. Cell. 2021;81:4622–4634.e8. doi: 10.1016/j.molcel.2021.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Ballesteros-Álvarez J., Andersen J.K. mTORC2: The other mTOR in autophagy regulation. Aging Cell. 2021;20 doi: 10.1111/acel.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y., Tian Q., Hao Y., Yao W., Lu J., Chen C., Chen X., Lin Y., Huang Q., Xu L., et al. The kinase complex mTORC2 promotes the longevity of virus-specific memory CD4(+) T cells by preventing ferroptosis. Nat. Immunol. 2022;23:303–317. doi: 10.1038/s41590-021-01090-1. [DOI] [PubMed] [Google Scholar]
- 18.Sarbassov D.D., Guertin D.A., Ali S.M., Sabatini D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 19.Hresko R.C., Mueckler M. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J. Biol. Chem. 2005;280:40406–40416. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- 20.Chen H., Zhang L., Wang P., Su H., Wang W., Chu Z., Zhang L., Zhang X., Zhao Y. mTORC2 controls Th9 polarization and allergic airway inflammation. Allergy. 2017;72:1510–1520. doi: 10.1111/all.13152. [DOI] [PubMed] [Google Scholar]
- 21.Van de Velde L.A., Murray P.J. Proliferating Helper T Cells Require Rictor/mTORC2 Complex to Integrate Signals from Limiting Environmental Amino Acids. J. Biol. Chem. 2016;291:25815–25822. doi: 10.1074/jbc.C116.763623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang Z., Zhang Q., Dong X., Zhang Z., Wang H., Zhang J., Zhao Y. mTORC2 negatively controls the maturation process of medullary thymic epithelial cells by inhibiting the LTbetaR/RANK-NF-kappaB axis. J. Cell. Physiol. 2021;236:4725–4737. doi: 10.1002/jcp.30192. [DOI] [PubMed] [Google Scholar]
- 23.Tang F., Wu Q., Ikenoue T., Guan K.L., Liu Y., Zheng P. A critical role for Rictor in T lymphopoiesis. J. Immunol. 2012;189:1850–1857. doi: 10.4049/jimmunol.1201057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang L., Zhang Y., Xu C., Gu X., Niu L., Wang J., Sun X., Bai X., Xuan X., Li Q., et al. Rictor positively regulates B cell receptor signaling by modulating actin reorganization via ezrin. PLoS Biol. 2017;15 doi: 10.1371/journal.pbio.2001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee K., Heffington L., Jellusova J., Nam K.T., Raybuck A., Cho S.H., Thomas J.W., Rickert R.C., Boothby M. Requirement for Rictor in homeostasis and function of mature B lymphoid cells. Blood. 2013;122:2369–2379. doi: 10.1182/blood-2013-01-477505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai H., Watson A.R., Fantus D., Peng L., Thomson A.W., Rogers N.M. Rictor deficiency in dendritic cells exacerbates acute kidney injury. Kidney Int. 2018;94:951–963. doi: 10.1016/j.kint.2018.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guertin D.A., Stevens D.M., Thoreen C.C., Burds A.A., Kalaany N.Y., Moffat J., Brown M., Fitzgerald K.J., Sabatini D.M. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKC alpha but not S6K1. Dev. Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Laslo P., Spooner C.J., Warmflash A., Lancki D.W., Lee H.J., Sciammas R., Gantner B.N., Dinner A.R., Singh H. Multilineage transcriptional priming and determination of alternate hematopoietic cell fates. Cell. 2006;126:755–766. doi: 10.1016/j.cell.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 29.Sichien D., Scott C.L., Martens L., Vanderkerken M., Van Gassen S., Plantinga M., Joeris T., De Prijck S., Vanhoutte L., Vanheerswynghels M., et al. IRF8 Transcription Factor Controls Survival and Function of Terminally Differentiated Conventional and Plasmacytoid Dendritic Cells, Respectively. Immunity. 2016;45:626–640. doi: 10.1016/j.immuni.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Kim I., He S., Yilmaz O.H., Kiel M.J., Morrison S.J. Enhanced purification of fetal liver hematopoietic stem cells using SLAM family receptors. Blood. 2006;108:737–744. doi: 10.1182/blood-2005-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yilmaz O.H., Kiel M.J., Morrison S.J. SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood. 2006;107:924–930. doi: 10.1182/blood-2005-05-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akashi K., Traver D., Miyamoto T., Weissman I.L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 33.Xu Y., Zhang Z., Zhao Y., Zhao C., Shi M., Dong X., Zhang J., Tan L., Zhang L., Zhao Y. TRAPPC1 is essential for the maintenance and differentiation of common myeloid progenitors in mice. EMBO Rep. 2023;24:e55503. doi: 10.15252/embr.202255503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hettinger J., Richards D.M., Hansson J., Barra M.M., Joschko A.C., Krijgsveld J., Feuerer M. Origin of monocytes and macrophages in a committed progenitor. Nat. Immunol. 2013;14:821–830. doi: 10.1038/ni.2638. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y., Huang G., Zeng H., Yang K., Lamb R.F., Chi H. Tuberous sclerosis 1 (Tsc1)-dependent metabolic checkpoint controls development of dendritic cells. Proc. Natl. Acad. Sci. USA. 2013;110:E4894–E4903. doi: 10.1073/pnas.1308905110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh W.J., Jacinto E. mTOR complex 2 signaling and functions. Cell Cycle. 2011;10:2305–2316. doi: 10.4161/cc.10.14.16586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Persengiev S.P., Green M.R. The role of ATF/CREB family members in cell growth, survival and apoptosis. Apoptosis. 2003;8:225–228. doi: 10.1023/a:1023633704132. [DOI] [PubMed] [Google Scholar]
- 38.Lu W., Ni Z., Jiang S., Tong M., Zhang J., Zhao J., Feng C., Jia Q., Wang J., Yao T., et al. Resveratrol inhibits bile acid-induced gastric intestinal metaplasia via the PI3K/AKT/p-FoxO4 signalling pathway. Phytother Res. 2021;35:1495–1507. doi: 10.1002/ptr.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu H., Wang L., Weng X., Chen H., Du Y., Diao C., Chen Z., Liu X. Inhibition of Brd4 alleviates renal ischemia/reperfusion injury-induced apoptosis and endoplasmic reticulum stress by blocking FoxO4-mediated oxidative stress. Redox Biol. 2019;24 doi: 10.1016/j.redox.2019.101195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheng Z., Ma L., Sun J.E., Zhu L.J., Green M.R. BCR-ABL suppresses autophagy through ATF5-mediated regulation of mTOR transcription. Blood. 2011;118:2840–2848. doi: 10.1182/blood-2010-12-322537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Devireddy L.R., Teodoro J.G., Richard F.A., Green M.R. Induction of apoptosis by a secreted lipocalin that is transcriptionally regulated by IL-3 deprivation. Science. 2001;293:829–834. doi: 10.1126/science.1061075. [DOI] [PubMed] [Google Scholar]
- 42.Stati G., Passaretta F., Gindraux F., Centurione L., Di Pietro R. The Role of the CREB Protein Family Members and the Related Transcription Factors in Radioresistance Mechanisms. Life. 2021;11 doi: 10.3390/life11121437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dahl R., Walsh J.C., Lancki D., Laslo P., Iyer S.R., Singh H., Simon M.C. Regulation of macrophage and neutrophil cell fates by the PU.1:C/EBPalpha ratio and granulocyte colony-stimulating factor. Nat. Immunol. 2003;4:1029–1036. doi: 10.1038/ni973. [DOI] [PubMed] [Google Scholar]
- 44.Rosenbauer F., Wagner K., Kutok J.L., Iwasaki H., Le Beau M.M., Okuno Y., Akashi K., Fiering S., Tenen D.G. Acute myeloid leukemia induced by graded reduction of a lineage-specific transcription factor, PU.1. Nat. Genet. 2004;36:624–630. doi: 10.1038/ng1361. [DOI] [PubMed] [Google Scholar]
- 45.Satoh T., Nakagawa K., Sugihara F., Kuwahara R., Ashihara M., Yamane F., Minowa Y., Fukushima K., Ebina I., Yoshioka Y., et al. Identification of an atypical monocyte and committed progenitor involved in fibrosis. Nature. 2017;541:96–101. doi: 10.1038/nature20611. [DOI] [PubMed] [Google Scholar]
- 46.Dakic A., Metcalf D., Di Rago L., Mifsud S., Wu L., Nutt S.L. PU.1 regulates the commitment of adult hematopoietic progenitors and restricts granulopoiesis. J. Exp. Med. 2005;201:1487–1502. doi: 10.1084/jem.20050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friedman A.D. Transcriptional control of granulocyte and monocyte development. Oncogene. 2007;26:6816–6828. doi: 10.1038/sj.onc.1210764. [DOI] [PubMed] [Google Scholar]
- 48.Olsson A., Venkatasubramanian M., Chaudhri V.K., Aronow B.J., Salomonis N., Singh H., Grimes H.L. Single-Cell Analysis of Mixed-Lineage States Leading to a Binary Cell Fate Choice. Exp. Hematol. 2016;44:S24. doi: 10.1016/j.exphem.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nargund A.M., Fiorese C.J., Pellegrino M.W., Deng P., Haynes C.M. Mitochondrial and nuclear accumulation of the transcription factor ATFS-1 promotes OXPHOS recovery during the UPR(mt) Mol. Cell. 2015;58:123–133. doi: 10.1016/j.molcel.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fiorese C.J., Schulz A.M., Lin Y.F., Rosin N., Pellegrino M.W., Haynes C.M. The Transcription Factor ATF5 Mediates a Mammalian Mitochondrial UPR. Curr. Biol. 2016;26:2037–2043. doi: 10.1016/j.cub.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melber A., Haynes C.M. UPR(mt) regulation and output: a stress response mediated by mitochondrial-nuclear communication. Cell Res. 2018;28:281–295. doi: 10.1038/cr.2018.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deng P., Haynes C.M. Mitochondrial dysfunction in cancer: Potential roles of ATF5 and the mitochondrial UPR. Semin. Cancer Biol. 2017;47:43–49. doi: 10.1016/j.semcancer.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao Q., Wang J., Levichkin I.V., Stasinopoulos S., Ryan M.T., Hoogenraad N.J. A mitochondrial specific stress response in mammalian cells. EMBO J. 2002;21:4411–4419. doi: 10.1093/emboj/cdf445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hua C., Hu T., Zhang Y., Cheng T., Yuan W. Rictor Is Required for Early B Cell Development in Bone Marrow. Exp. Hematol. 2014;42:S39. doi: 10.1371/journal.pone.0103970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chou S.T., Khandros E., Bailey L.C., Nichols K.E., Vakoc C.R., Yao Y., Huang Z., Crispino J.D., Hardison R.C., Blobel G.A., Weiss M.J. Graded repression of PU.1/Sfpi1 gene transcription by GATA factors regulates hematopoietic cell fate. Blood. 2009;114:983–994. doi: 10.1182/blood-2009-03-207944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nargund A.M., Pellegrino M.W., Fiorese C.J., Baker B.M., Haynes C.M. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science. 2012;337:587–590. doi: 10.1126/science.1223560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lai C., Zhang J., Tan Z., Shen L.F., Zhou R.R., Zhang Y.Y. Maf1 suppression of ATF5-dependent mitochondrial unfolded protein response contributes to rapamycin-induced radio-sensitivity in lung cancer cell line A549. Aging-Us. 2021;13:7300–7313. doi: 10.18632/aging.202584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mohrin M., Widjaja A., Liu Y., Luo H., Chen D. The mitochondrial unfolded protein response is activated upon hematopoietic stem cell exit from quiescence. Aging Cell. 2018;17 doi: 10.1111/acel.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu L., Yang T., Li L., Sun L., Hou Y., Hu X., Zhang L., Tian H., Zhao Q., Peng J., et al. TSC1 controls macrophage polarization to prevent inflammatory disease. Nat. Commun. 2014;5:4696. doi: 10.1038/ncomms5696. [DOI] [PubMed] [Google Scholar]
- 60.Wu J., Mao X., Cai T., Luo J., Wei L. KOBAS server: a web-based platform for automated annotation and pathway identification. Nucleic Acids Res. 2006;34:W720–W724. doi: 10.1093/nar/gkl167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xie C., Mao X., Huang J., Ding Y., Wu J., Dong S., Kong L., Gao G., Li C.Y., Wei L. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011;39:W316–W322. doi: 10.1093/nar/gkr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang J., Xu Y., Zhang Y., Bossila E.A., Shi M., Zhao Y. Bioinformatic analysis as a first step to predict the compatibility of hematopoiesis and immune system genes between humans and pigs. Xenotransplantation. 2022;29 doi: 10.1111/xen.12764. [DOI] [PubMed] [Google Scholar]
- 64.Wang L., Feng Z., Wang X., Wang X., Zhang X. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics. 2010;26:136–138. doi: 10.1093/bioinformatics/btp612. [DOI] [PubMed] [Google Scholar]
- 65.Lei T., Zhang J., Zhang Q., Ma X., Xu Y., Zhao Y., Zhang L., Lu Z., Zhao Y. Defining newly formed and tissue-resident bone marrow-derived macrophages in adult mice based on lysozyme expression. Cell. Mol. Immunol. 2022;19:1333–1346. doi: 10.1038/s41423-022-00936-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pertea M., Kim D., Pertea G.M., Leek J.T., Salzberg S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016;11:1650–1667. doi: 10.1038/nprot.2016.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hou Y., Wei D., Zhang Z., Guo H., Li S., Zhang J., Zhang P., Zhang L., Zhao Y. FABP5 controls macrophage alternative activation and allergic asthma by selectively programming long-chain unsaturated fatty acid metabolism. Cell Rep. 2022;41 doi: 10.1016/j.celrep.2022.111668. [DOI] [PubMed] [Google Scholar]
- 68.Wu T., Zhao Y., Wang H., Li Y., Shao L., Wang R., Lu J., Yang Z., Wang J., Zhao Y. mTOR masters monocytic myeloid-derived suppressor cells in mice with allografts or tumors. Sci. Rep. 2016;6 doi: 10.1038/srep20250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu G., Hu X., Sun B., Yang T., Shi J., Zhang L., Zhao Y. Phosphatase Wip1 negatively regulates neutrophil development through p38 MAPK-STAT1. Blood. 2013;121:519–529. doi: 10.1182/blood-2012-05-432674. [DOI] [PubMed] [Google Scholar]
- 70.Sun B., Zhu L., Tao Y., Sun H.X., Li Y., Wang P., Hou Y., Zhao Y., Zhang X., Zhang L., et al. Characterization and allergic role of IL-33-induced neutrophil polarization. Cell. Mol. Immunol. 2018;15:782–793. doi: 10.1038/cmi.2017.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liang Z., Zhang Z., Zhang Q., Dong X., Yang X., Zhang J., Lei T., Creemers J.W.M., Zhang B., Zhao Y. The proprotein convertase furin regulates the development of thymic epithelial cells to ensure central immune tolerance. iScience. 2022;25 doi: 10.1016/j.isci.2022.105233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hou Y., Wei D., Bossila E.A., Zhang Z., Li S., Bao J., Xu H., Zhang L., Zhao Y. FABP5 Deficiency Impaired Macrophage Inflammation by Regulating AMPK/NF-kappaB Signaling Pathway. J. Immunol. 2022;209:2181–2191. doi: 10.4049/jimmunol.2200182. [DOI] [PubMed] [Google Scholar]
- 73.Chu Z., Sun C., Sun L., Feng C., Yang F., Xu Y., Zhao Y. Primed macrophages directly and specifically reject allografts. Cell. Mol. Immunol. 2020;17:237–246. doi: 10.1038/s41423-019-0226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shi L., Tian H., Wang P., Li L., Zhang Z., Zhang J., Zhao Y. Spaceflight and simulated microgravity suppresses macrophage development via altered RAS/ERK/NFkappaB and metabolic pathways. Cell. Mol. Immunol. 2021;18:1489–1502. doi: 10.1038/s41423-019-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Q., Zhang J., Lei T., Liang Z., Dong X., Sun L., Zhao Y. Sirt6-mediated epigenetic modification of DNA accessibility is essential for Pou2f3-induced thymic tuft cell development. Commun. Biol. 2022;5:544. doi: 10.1038/s42003-022-03484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The RNA sequencing datasets generated in this study can be found in the NCBI SRA database (http://www.ncbi.nlm.nih.gov/sra/) using the access number PRJNA918499.
-
•
All other data needed to estimate the conclusions in this paper are present in the main text or the supplementary materials. This paper did not report original code. All data reported in this paper will be shared by the lead contact upon request. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.