Summary
Background
COVID-19 in paediatric ages could result in hospitalizations and death. In addition, excluding children from vaccination could turn them into reservoirs of the SARS-COV-2. Safe and effective COVID-19 vaccines are urgently needed for large-scale paediatric vaccination. ISMAELILLO study aimed to evaluate safety and immunogenicity of two strengths of a new recombinant receptor-binding domain (RBD) protein vaccine (Abdala) in paediatric population.
Methods
A double-blinded, multicentre, randomised, phase 1/2 clinical trial was conducted in nine polyclinics in the province of Camagüey, Cuba. Healthy children and adolescents were stratified according to age (3–11 years old, or 12–18 years old) and they were randomly assigned (1:1; block size four) in two dosage level groups of vaccine to receive three intramuscular doses of 25 μg or 50 μg of RBD, 14 days apart. Main safety endpoint was analyzed as the percentage of serious adverse reactions during vaccination up to 28 days after the third dose (Day 56) in participants who received at least one dose vaccination. The primary immunogenicity endpoint assessed was seroconversion rate of anti-RBD IgG antibody at day 56. The immunogenicity outcomes were assessed in the per-protocol population. This trial is registered with Cuban Public Registry of Clinical Trials, RPCEC00000381.
Findings
Between July 15, 2021, and August 16, 2021, 644 paediatric subjects were screened, of whom 592 were enrolled after verifying that they met the selection criteria: firstly 88 were included in Phase 1 of the study and 504 who completed Phase 2. The vaccine was well tolerated. Injection site pain was the most frequently reported local event (143 [8·4%] of 1707 total doses applied), taking place in 66/851 (7·8%) in the 25 μg group and in 77/856 (9·0%) in the 50 μg. The most common systemic adverse event (AE) was headache: 23/851 (2·7%) in the 25 μg group and 19/856 (2·2%) in the 50 μg. Reactogenicity was mild or moderate in severity, represented in 75% of cases by local symptoms, completely resolved in the first 24–48 h. Twenty-eight days after the third dose, seroconversion anti-RBD IgG were observed in 98·2% of the children and adolescents (231/234) for the 50 μg group and 98·7% (224/228) for the 25 μg group without differences between both strength. The specific IgG antibody geometric mean titres (GMT) showed higher titres between participants who received Abdala 50 μg (231·3; 95% CI 222·6–240·4) compared to those who received 25 μg (126·7; 95% CI 121·9–131·7). The mean ACE2 inhibition %, were 59·4% for 25 μg, and for 50 μg, 72·9% (p < 0·01). Both strength elicited neutralising activity against the SARS-CoV-2, specifically (18·3; 95% CI 14·7–22·78) for Abdala 25 μg and (36·4; 95% CI 30·26–43·8) for 50 μg to the selected sample analyzed.
Interpretation
Abdala vaccine was safe and well tolerated at both antigenic strength levels tested in participants aged between 3 and 18 years. Regarding immunogenicity, Abdala Vaccine stimulated the production of specific IgG antibodies against the RBD of SARS-CoV-2 as well as the production of ACE2 inhibition titres and neutralising antibodies (Nab) in children and adolescents.
Funding
Centre for Genetic Engineering and Biotechnology (CIGB), Havana, Cuba.
Keywords: Abdala vaccine, COVID-19, SARS-CoV-2, Children, Adolescents, Randomised clinical trial
Research in context.
Evidence before this study
We searched PubMed on April 6, 2023, for research articles published using the search terms of “COVID-19”, “SARS-CoV-2”, “vaccine”, “clinical trial”, and “children” or “adolescent”. We found 166 reports, but we actually identified 140 of them as COVID-19 vaccine clinical trials, and 31 of them corresponding to studies in paediatric ages. There is little representation on these reports to protein-based COVID-19 vaccines, and none of these studies evaluated a protein-subunit vaccine obtained of the Pichia pastoris yeast.
Abdala, a recombinant RBD subunit of the spike protein vaccine, expressed in Pichia pastoris yeast, has been previously assessed in adults older than 19 years of age. Two different immunization schedules (0-14-28 days and 0-28-56 days) and two strengths of the antigen (25 μg and 50 μg) were assessed.
The safety and immunogenicity analyses indicated that three doses of Abdala at different strengths and with different immunization schedules in adults 19–80 years of age were safe and well tolerated, and induced high immune responses, including neutralizing antibodies closely correlated with the anti-RBD IgG response.
Added value of this study
This is the first report in paediatric ages of Abdala, a recombinant subunit RBD protein of SARS-CoV-2 vaccine. Both 25 μg and 50 μg of RBD antigen formulations were well tolerated and safe in children and adolescents aged 3–18 years. The seroconversion rates were higher than 98% for each antigenic dosage evaluated, GMTs of anti-RBD IgG, inhibition percentage of RBD-ACE2 binding, and neutralising actvity showed better results and risk-benefit ratio for the 50 μg group.
Implications of all the available evidence
The results support vaccination of children and adolescents aged between 3 and 18 years old with three doses of 50 μg of the Abdala vaccine according vaccination schedule 0, 14 and 28 days. Based on these favourable results, other studies in children and adolescents have been initiated.
Introduction
Three years after the diagnosis of the first case of coronavirus disease 2019 (COVID-19) on December 2019, more than 600 million cases, including over 6·5 million deaths, were reported worldwide.1 Children, adolescents, and young adults under 25 years old represent between 2·5% and 14% of these infections with an increased incidence as they age, accounting between 0·1% and 0·4% of all globally reported deaths.2,3
In response to the COVID-19 pandemic, multiple vaccine platforms have been approved for emergency use by national regulatory authorities in several countries.4
Even with new virus variants emerging, vaccines are the most effective strategy to protect adults and children against serious illness and death from SARS-COV-2.2,5,6
Childhood SARS-CoV-2 infection is often asymptomatic and usually carries fewer and milder symptoms with less severe clinical forms compared to adult’s infection. However, COVID-19 in children can result in hospitalizations, pneumonia, respiratory distress, Multisystem inflammatory syndrome, and death, as well as other potential consequences such as prolonged clinical symptoms (known as “long COVID-19”, or “post COVID-19 condition”) 7, and damage in the psychological, social and educational spheres.2,6, 7, 8
Unfortunately, viral mutations are generating new genetic variants of SARS-CoV-2, which have resulted in higher transmission in all age groups, including children. Additionally, after increasing adult vaccination coverage, children now represent a relatively larger proportion of notified cases. It is known that to achieve collective immunity, paediatric vaccination with high coverage is needed.2,6,7,9
Although most COVID-19 vaccines are only approved to be used in adults, an increasing number of these vaccines being evaluated and authorized to use also in children, showing very good results.2,9, 10, 11, 12 Nevertheless, many more vaccines still need to achieve the necessary coverage to have an impact on the control of this pandemic.2,6
Abdala vaccine is based on the recombinant RBD subunit of the spike protein expressed in P. pastoris yeast,13 and adjuvanted to alumina, which was developed by the Centre for Genetic Engineering and Biotechnology (CIGB) in Havana using a technological platform well known by this company.13,14 Abdala was the first COVID-19 vaccine authorized for emergency use in Cuba, after having demonstrated its efficacy in the prevention of symptomatic COVID-19 of 92·28%, in Phase 3 clinical trial that included more than 48 000 volunteers.15,16
In this paper we presented the results of ISMAELILLO clinical trial, which constitutes the first evidences of safety and immunogenicity of three intramuscular doses 25 μg or 50 μg of Abdala vaccine in children and adolescents aged between 3 and 18 years.
Methods
Study design
ISMAELILLO was a multicentre, randomised, parallel-group, double-blind Phase 1/2 clinical trial to evaluate safety and immunogenicity of two-dose levels of the Abdala vaccine in paediatric population. The study was conducted in nine clinical sites of the capital municipality of Camagüey, Cuba.
Were included 592 children and adolescents, 88 in Phase 1 and others 504 in Phase 2. The 12- to 18-year-old stratum was marking the study progress, overlapping both phases. After the first interim analysis showed Abdala safety in the phase 1 of the adolescent stratum, it continued to the phase 2 while the 3–11 years old stratum started the Phase 1. The second interim report that evaluated the safety in phase 1 of children aged 3–11 years allowed this stratum to continue to the phase 2.
The study was designed and conducted under the observance of Good Clinical Practice guidelines as well as national regulatory requirements.17, 18, 19, 20 The protocol was approved, after its evaluation, by two Research Ethics Committees: Pedro Kourí Institute of Tropical Medicine (IPK) Research Ethics Committee and the one corresponding to the Provincial Centre of Hygiene and Epidemiology of Camagüey. The clinical trial was also authorized by the Cuban national authority (CECMED). Before the beginning of the study, an Independent Data and Safety Monitoring Committee (IDSMC) integrated by one statistician, one epidemiologist, one paediatrician, and two pharmaceutics was established with the aim to provide assessments to safety data gathered during the course of this trial and to recommend early study termination in case of unacceptable toxicity.
Participants
Eligible participants were healthy children and adolescents aged 3–18 years with a nutritional assessment: between 10 and 90 percentiles (calculated according participant age: Weight/height Index, for children between 3 and 9 years old, and Body-Mass Index for Age, for children between 10 and 18 years old). Nutritional assessment was defined as a way to establish the nutritional status by anthropometric measurements according to the cut-off points for the Cuban paediatric population.21,22 The main exclusion criteria comprised any personal history of SARS-CoV-2 infection or coronavirus vaccination, contact or suspected COVID-19 at the time of inclusion, history of allergy to Thiomersal or any other vaccine component, axillary temperature equal to or higher than 37·0 ºC, acute infectious disease at the time of or within three days prior to vaccine administration, have been treated within the previous thirty days or may require treatment in the course of the trial for any underlying condition with: any investigational product, cytostatic, immunomodulators, or with steroids by any route of administration. Those who had received blood, or any blood products within three months prior to inclusion, who had tattoos in both deltoid regions that make it difficult to observe the injection site, pregnancy or breastfeeding, and history or suspicion of alcoholism or drug dependence, were also excluded.
Before proceeding with any medical intervention related to the study, parents provided written informed consents. In addition, participants over 12 years of age provided a signed informed assent form previous to enrolment.
Randomisation and masking
Randomisation was achieved in the supply group of the Clinical Research Direction of the CIGB, in blocks of four subjects, for each clinical site, using a computer tool designed for that purpose. Participants were stratified into two age groups (12–18 years and 3–11 years old), and randomised (1:1).
Both evaluated vaccine formulations were identical in organoleptic characteristics, mode of presentation, and had the same volume to be injected, which guaranteed that all participants, investigators, monitors, and laboratory staff were masked to investigational treatment allocation.
Procedures
Two concentrations of the active principle were assessed in this study: 25 μg or 50 μg of recombinant receptor-binding domain (RBD) protein with the same composition for the rest of the components, adjuvanted in 0·30 mg of aluminum hydroxide gel, and using 0·025 mg of thiomersal as a preservative in 0·5 mL. Vaccine recipients received three 0·5 mL doses of the recombinant intramuscularly, in the deltoid region, according to a 0, 14, 28 days schedule, by properly trained vaccinator nurse staff at trial sites.
Participants were observed in the study site during the first hour after vaccination. All medical events temporally associated to the application of each vaccine dose were considered adverse events (AEs), independently of the existence of a causality relationship with the vaccine. An active search for the presence of AE was carried out 24, 48 and 72 h and 7 days after the application of each dose. Subsequently, AEs were collected at the clinical site on day 14 after the first and second doses, respectively, and on day 28 after the end of the schedule (third dose). From day 8 up to day of the next dose, or up to day 28 after the third dose, safety data were collected by spontaneous report. No preventive antipyretic therapy was indicated, neither other anticipated concomitant treatment. The AEs were graded according severity in mild, moderate, or severe. The analysis of the causality relationship between the AEs observed and the vaccine under study was performed according to the algorithm proposed by the WHO classification for causality assessment of an AE following immunization (AEFI).20 Adverse reactions (ARs) were defined from those AE associated with vaccine or vaccination in the causality analysis. In the phase 1 trial, blood samples were taken baseline and on day 7 after first dose for hematologic or biochemical routine tests. Blood samples were collected for all participants on day 0, and 56 to evaluate the level of RBD-specific IgG antibodies as seroconversion rates and geometric mean of the titres (GMT), the proportions and means of the percentage of inhibition of RBD-ACE-2 (95% CI) and proportions and GMT of neutralising antibody titres against wild-type SARS-CoV-2.
The method used to quantify IgG specific antibodies was UMELISA SARS-CoV-2 anti-RBD (Immunoassay Centre, Havana). Titres were expressed as Arbitrary Units per millilitre (AU/mL).23 ACE-2 binding inhibition was determined using an in-house virus neutralization test (CIGB, Havana), expressed in inhibition percentage and were considered as positive starting point 30%.24 The neutralising antibody titres to live SARS-CoV-2 (CUT2010-2025/Cuba/2020 strain) were detected by a standard virus microneutralization assay at Civilian Defence Scientific Research Centre, Cuba, in serum samples, and its results were expressed in Nab titre ID50, as previously described.25,26 We compared two antigenic strength vaccines as well as the influence on the immune response of such epidemiological factors as sex, age, and nutritional valuation.
Outcomes
Two primary endpoints were considered in ISMAELILLO study: one related to safety and the other one related to immunogenicity. The primary safety endpoint was the percentage of serious AEs related to the investigational product in no more than 5% of the subjects up to 28 days after the third dose. Immunogenicity outcome was the percentage of subjects with seroconversion of anti-RBD IgG antibodies of SARS-CoV-2 (considering seroconversion as ≥ 4 times the initial antibody titre determination), at day 28 follow-up (day 56). Secondary safety endpoint was the percentage of clinical AEs according to type, duration, intensity, imputability, conduct followed and outcome up to 28 days after the third dose. Secondary immunogenic endpoints were: GMT of anti-RBD IgG antibodies of SARS-CoV-2, percentage of inhibition of RBD-ACE-2 binding, and neutralising antibody titres against wild-type (original SARS-CoV-2 strain identified in Wuhan) at 28 days after completion of the third dose.
Statistical analysis
Safety population included all participants who had received at least one dose of vaccine and had some safety evaluation information. The immunogenicity was analyzed in the per-protocol population, which considered all participants that were included in the study, received three doses of vaccine 25 μg or 50 μg, had antibody results available, and had adherence to study protocol. Children and adolescents who had anti-RBD IgG antibodies at the baseline, previous to receive the first dose of the vaccine, were excluded from the immunogenicity analyses.
The Phase 1 required 88 participants, assuming the hypothesis of a rate of related serious AEs of less than 5%, with Two-Sided Confidence Intervals for One Proportion Exact Clopper-Pearson confidence interval at 95%, type I error of 0·05, potency of 80%, and considering 10% of lost to follow-up, 22 participants were calculated per group and for each two age stratum.
The sample size in Phase 2 was computed considering a proportion of anti-RBD antibody seroconversion higher than 50% compared to the baseline level of vaccinated children. We calculated 590 participants, estimating a 95% confidence interval, with a precision of 0·04 and 10% added for lost to follow-up. Considering two treatment groups, subdivided into two age groups, the calculation yields 592 for Phase 2, which 88 were included in Phase 1.
Safety analyses were descriptive, presented as counts and percentages tabulated by antigenic strength and age stratum. The frequency distributions were estimated in the case of the qualitative variables. Measures of central tendency and dispersion (mean, median, standard deviation, interquartile range and minimum and maximum values), were calculated for quantitative variables, Pearson χ2 test or Fisher’s exact test were used for the analysis of categorical outcomes. We calculated the 95% CIs for all categorical outcomes using the Clopper-Pearson method.
The GMTs and its corresponding 95% CIs were calculated on the basis of the standard normal distribution as the mean of the log-transformation antibody titre, and finally we calculated exponential to the mean. The 95% CIs for mean differences were estimated following a Student’s t-distribution. To prove the normality of the residues of the lineal model in comparisons between two or more groups, a Shapiro–Wilks test was used. ANOVA or its non-parametric alternative, the Kruskal–Wallis test, were used to compare GMTs and mean ACE2 inhibition % among three or more arms. Once the null hypothesis is rejected, multiple comparison tests with Bonferroni’s correction were performed to adjust by the number of comparisons as a complement to this test. Student’s t-test was applied to compare the mean of two samples. We considered a p value of less than 0·05 to be significant.
To assess the relationship between responses on different assays by study group, by age stratum, and by evaluation time, Pearson’s linear and Spearman correlations were used.
The IDSMC assessed and reviewed safety data and the right data management to provide the sponsors with sufficient information for them to decide whether or not to proceed in the event unexpected safety signals emerged during the course of the study. We used the R Project for Statistical Computing (version 3.6.2) for all analyses.
ISMAELILLO study was registered in the Cuban Public Clinical Trial Registry (code RPCEC00000381), which is accepted by WHO. Available from: https://rpcec.sld.cu/en/trials/RPCEC00000381-En.
Role of the funding source
The founders of the study contributed to the study design, data interpretation, clinical trial monitoring, writing or revising the report or manuscript, but not with data collection and data analysis.
Results
Between July 15, 2021, and August 16, 2021, a total of 644 subjects in paediatric ages were screened, of whom 592 underwent randomization, 296 assigned to receive 25 μg of RBD, and 296 assigned to receive 50 μg of RBD: 88 subjects in phase 1, and up to complete 592 in phase 2.
Phase 1 included 44 children and adolescents between 12 and 18 years old, the first who received the vaccine, followed, after an interim analysis, by 252 subjects of the phase 2 in the same age stratum. Later, children between 3 and 11 years old were recruited too: 44 children in phase 1 and 252 subjects in the phase 2 following a second interim report (Fig. 1).
Fig. 1.
Trial profile. RBD: receptor binding domain; μg: micrograms.
All 592 participants received their first vaccination on day 0, the second dose was received by 568 (95·95%) participants on day 14 and the third, by 547 (92·4%) on day 28. For the per-protocol analysis of immunogenicity, at day 28, after the third dose, 462 participants were selected: 228 (112 Group 25 μg and 116 group 50 μg) of stratum aged 12–18 years and 234 (116 Group 25 μg and 118 group 50 μg) of stratum aged 3–11 years. Participants who did not strictly comply with the clinical protocol were excluded: 58 cases who did not complete the study due to discontinuation of treatment or follow-up (48 because SARS-CoV-2 infection, 4 due to Dengue infection, which also had an epidemiological peak coinciding with the clinical trial, 2 drop-out cases, and the rest 4 were single-case reports of different infectious processes), 65 cases were not analyzed because had baseline anti-RBD IgG titres above the cut-off value, and seven did not have a blood sample taken at 28 days after the third dose, then they lost to follow-up (Fig. 1).
Demographic characteristics of the participants are presented in Table 1. There was an adequate balance between male and female sex between the study groups: group RBD 25 μg female sex was 52·0%, and in group RBD 50 μg, 50·0%, the range of age was 3–18 years old, divided in two stratums, mean age was 11·06 years (SD 4·51). Most subjects were mainly white (81·9%) which is a characteristic pattern of this province. Nutritional assessment showed medians above the 50th percentile in all treatment groups and age strata without exceeding the 90th percentile, total median 75th percentile (IQR 50·0–90·0).
Table 1.
Demographic characteristics of Children and adolescents in the ISMAELILLO trial at enrolment.
| Variables | Phase 1 |
Phase 2 |
Overall |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RBD 25 μg, N = 44 | RBD 50 μg, N = 44 | RBD 25 μg, N = 252 | RBD 50 μg, N = 252 | Total N = 592 | ||||||
| Age, years | ||||||||||
| Mean ± SD (Min; Max) | 11·20 ± 4·01 (3; 18) | 10·98 ± 4·88 (3; 18) | 10·99 ± 4·53 (3; 18) | 11·10 ± 4·53 (3; 18) | 11·06 ± 4·51 (3; 18) | |||||
| Age groups, years, n (%) | ||||||||||
| 3–11 years old | 22 (50%) | 22 (50%) | 126 (50%) | 126 (50%) | 296 (50%) | |||||
| 12–18 years old | 22 (50%) | 22 (50%) | 126 (50%) | 126 (50%) | 296 (50%) | |||||
| Gender, n (%) | ||||||||||
| Female | 18 (40·9%) | 20 (45·4%) | 136 (54·0%) | 128 (50·8%) | 302 (51·01%) | |||||
| Male | 26 (59·1%) | 24 (54·5%) | 116 (46·0%) | 124 (49·2%) | 290 (48·99%) | |||||
| Ethnicity, n (%) | ||||||||||
| White | 41 (93·2%) | 40 (90·9%) | 197 (78·2%) | 207 (82·1%) | 485 (81·93%) | |||||
| Black | 1 (2·3%) | 2 (4·5%) | 17 (6·7%) | 21 (8·3%) | 41 (6·93%) | |||||
| Mestizo | 2 (4·5%) | 2 (4·5%) | 38 (15·1%) | 24 (9·5%) | 66 (11·15%) | |||||
| Height, (m) | ||||||||||
| Mean ± SD | 1·51 ± 0·22 | 1·46 ± 0·26 | 1·45 ± 0·22 | 1·45 ± 0·22 | 1·46 ± 0·22 | |||||
| Median, (IQR) | 1·52 (1·3–2·0) | 1·56 (1·24–1·83) | 1·50 (1·29–1·84) | 1·49 (1·28–1·84) | 1·50 (1·28–2·0) | |||||
| (Min; Max) | (1·03; 1·9) | (0·96; 1·76) | (0·95; 1·84) | (0·96; 1·84) | (0·95; 1·90) | |||||
| Weight, (kg) | ||||||||||
| Mean ± SD | 43·73 ± 17·36 | 39·14 ± 17·90 | 41·41 ± 16·16 | 40·97 ± 16·16 | 41·24 ± 16·36 | |||||
| Median, (IQR) | 42·00 (27·45–80·0) | 42·5 (24·45–84·0) | 41·00 (26·0–80·0) | 41·00 (27·00–83·0) | 41·00 (26·0–84·0) | |||||
| (Min; Max) | (16·2; 80) | (15·1; 63) | (0·90; 80) | (14·5; 83) | (0·9; 83) | |||||
| Nutritional assessment, (P: percentile)a | ||||||||||
| Mean ± SD | 61·82 ± 28·08 | 56·25 ± 28·84 | 62·62 ± 23·2 | 61·27 ± 25·78 | 61·48 ± 25·14 | |||||
| Median, (IQR) | 75 (43·75–90·0) | 62·5 (25·0–90·0) | 75 (50·0–90·0) | 75 (50·0–90·0) | 75 (50·0–90·0) | |||||
| (Min; Max) | (10; 90) | (10; 90) | (10; 90) | (10; 90) | (10; 90) | |||||
| Clinical sites, n | E1 | E2 | E1 | E2 | E1 | E2 | E1 | E2 | E1 | E2 |
| Agramonteb | 12 | 12 | 12 | 12 | 4 | 4 | 4 | 4 | 32 | 32 |
| Mellab | 10 | 10 | 10 | 10 | 6 | 6 | 6 | 6 | 32 | 32 |
| Oeste | _ | _ | 18 | 18 | 18 | 18 | 36 | 36 | ||
| PIRRE | _ | _ | 16 | 16 | 16 | 16 | 32 | 32 | ||
| Previsora | _ | _ | 16 | 16 | 16 | 16 | 32 | 32 | ||
| Este | _ | _ | 16 | 16 | 16 | 16 | 32 | 32 | ||
| Norte | _ | _ | 16 | 16 | 16 | 16 | 32 | 32 | ||
| Finlay | _ | _ | 16 | 16 | 16 | 16 | 32 | 32 | ||
| Centro | _ | _ | 18 | 18 | 18 | 18 | 36 | 36 | ||
RBD: receptor binding domain; μg: micrograms; SD, standard deviation; Min, Minimum; Max, Maximum; IQR, Interquartile range; E1, 12–18 years old; E2, 3–11 years old.
Nutritional status was defined according participant age: Weight/height Index, for children between 3 and 9 years old. Body-Mass Index for Age, was used to children between 10 and 18 years old. The results of both indexes are interpreted in the same way according to the cut-off points for the Cuban paediatric population: considering less than the 3rd percentile as malnourished, between the 3rd and <10th as underweight, normal status between the 10th and <90th percentile, overweight between the 90th and <97th, and obese ≥97th percentile. Body-mass index is the weight in kilograms divided by the square of the height in meters.
Clinical ste that participated both in phase 1 and in phase 2 of the clinical trial. The remaining clinical sites only participated in phase 2.
Both vaccine antigenic strengths were well tolerated. A total of 413 AEs were reported, representing 24·2% of the total doses applied (1707 doses). 227 (38·3%) of the 592 vaccinated paediatric participants reported at least one AE: 113 (49·8%) in the group of 25 μg and 114 (50·2%) in the group that received 50 μg, as well 133 (58·6%) corresponded to the 12-18-year-old stratum and 94 (41·4%) to the 3–11 years of age. The percentage of events reported decreased at each dose with the progress of the study. Three cases of the 592 participants were reported as serious AEs because the children were hospitalized for study and treatment (2 dengue cases and 1 febrile seizure, which also turned out to be dengue virus infection). The three events were coincidental, unrelated with vaccination, and were completely resolved. No clinically relevant out-of-range laboratory values associated with vaccination were found.
Overall solicited AR were 234 of 1707 applied doses (13.71%). ARs were decreasing with schedule progression: (first dose 43·8%, second 23·8, and third 23·4). Most of AR were mild in severity and completely resolved in the first 24–48 h without treatment. Injection site pain was the most frequently reported local reaction (143 [8·4%] of 1707 total doses applied) taking place in 66/851 (7·8%) in the 25 μg group, and in 77/856 (9·0%) in the 50 μg group. The most common systematic AR was headache: 19/851 (2·2%) in the 25 μg group and 12/856 (1·4%) in the 50 μg group. Others infrequent local AR were indurations (0·9%) and redness at the injection site (0·7%), and as others systemic reactions: somnolence (0·6%), fever (0·5%), and low-grade fever (0·2%). No greater reactogenicity related to the application of a higher concentration of antigen was observed, even in the younger age stratum (Fig. 2).
Fig. 2.
Percentage of adverse reactions according doses applied by group, and stratum of age. Total doses applied: 1707; Total 25 μg doses applied: 851; Total 50 μg doses applied: 856.
The seroconversion anti-RBD IgG rates were 98·2% in the 25 μg group and 98·7% in the 50 μg group at day 28 after the third dose. There were no differences between both antigenic strengths regarding specific IgG antibodies seroconversion (p = 0·97); however, GMT of anti-RBD IgG antibodies showed higher titres (P < 0·01) between paediatric participants who received Abdala 50 μg (GMT 231·3 [95% CI 222·6–240·4]) compared to those who received 25 μg (126·7 [121·9–131·7]). Younger stratum, aged 3–11 years showed a higher seroconversion percentage than stratum aged 12–18 years without significant differences: (3–11 years SC = 100% and 12–18 years SC = 96·43%; p = 0·23) in 25 μg group, and (3–11 years SC = 99·15% and 12–18 years SC = 98·28%; p = 0·57) in 50 μg (Table 2 and Supplementary Table S1).
Table 2.
Immune responses induced 28 days after three doses of priming Abdala vaccine by study groups among participants 12 to 18 and 3–11 years of age.
| 12–18 years old |
3–11 years old |
Overall 3–18 years old |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| RBD 25 μg | RBD 50 μg | P value | RBD 25 μg | RBD 50 μg | P value | RBD 25 μg | RBD 50 μg | P value | |
| Seroconversion of anti-RBD IgG (%) | 108/112 (96·43%) | 114/116 (98·28%) | 0·65 | 116/116 (100%) | 117/118 (99·15%) | 1·00 | 224/228 (98·2%) | 455/462 (98·5%) | 0·97 |
| GMT of RBD-IgG antibodies (95% CI) | 95·96 (75·0; 122·7) | 209·39 (169·6; 258·5) | <0·01 | 165·80 (136·3; 201·7) | 255·07 (210·5; 309·0) | 0·002 | 126·74 (121·9; 131·8) | 231·3 (222·6; 240·4) | <0·01 |
| Inhibition to RBD-ACE2 binding (%) | 85/112 (75·89%) | 107/116 (92·24%) | 0·014 | 107/116 (92·24%) | 114/118 (96·61%) | 0·24 | 192/228 (84·21%) | 221/234 94·44%) | <0·01 |
| Mean ACE2 inhibition % (95% CI) | 53·80 (48·66; 58·94) | 70·24 (66·15; 74·33) | <0·01 | 64·72 (60·69; 68·76) | 75·38 (71·94; 78·82) | <0·01 | 59.36 (59.32; 59·39) | 72.96 (72.92; 73·00) | <0·01 |
| Nab to live SARS-CoV-2 (%) | 46/49 (93·9%) | 60/60 (100%) | 0·18 | 100/107 (94·3%) | 105/108 (97·2%) | 0·32 | 146/156 (93·6%) | 165/168 (98·2%) | 0·07 |
| Geometric mean of Nab titres against live SARS-CoV-2 (95% CI) | 17·94 (12·1; 26·6) | 36·96 (27·3; 49·9) | <0·01 | 18·47 (14·14; 24·13) | 36·10 (28·47; 45·78) | <0·01 | 18·30 (14.70; 22.78) | 36·41 (30.26; 43.80) | <0·01 |
RBD: receptor binding domain; μg: micrograms; CI confidence Interval; E1, 12–18 years old; E2, 3–11 years old; GMT: geometric mean titres; ACE-2: angiotensin-converting enzyme; NAb: neutralising antibodies.
Positive inhibition of RBD-ACE2 binding, in adolescents between 12 and 18 years was 75·89% for 25 μg group, and 92·24% for 50 μg group (p = 0·014). Percentage of children between 3 and 11 years with positive inhibition of RBD-ACE-2 binding for 25 μg vaccine group was 92·24% and 96·61% for 50 μg vaccine, respectively (p = 0·24) (Table 2 and Supplementary Table S1). Participants aged 12–18 years who received RBD 50 μg achieved higher mean ACE2 inhibition % (70·24 [ 95% CI: 66·15; 74·33]) than those who received RBD 25 μg (53·80 [48·66; 58·94]) (p < 0·01). Furthermore, participants aged 3–11 years who received RBD 50 μg achieved higher mean ACE2 inhibition % (75·38 [71·94; 78·82]) with respect to RBD 25 μg group (64·72 [60·69; 68·76]) (p < 0·01, respectively), (Fig. 3 and Supplementary Table S1).
Fig. 3.
Global phase 1–2 immunogenicity of quantitative variables by study group (RBD 25 μg, RBD 50 μg) and stratum of age (12–18 and 3–11 years old). (A) SARS-CoV-2 anti-RBD IgG antibody titres of participants aged 12–18, and (B) 3–11 years. (C) and (D) were defined as Mean of inhibition of RBD-ACE-2 binding percentages. (E) Neutralizing antibody titres among participants 12 to 18, and (F) among 3–11 years old. The study groups are represented by two tons of gray colour, light for RBD 25 μg, and dark for RBD 50 μg. The boxes indicate interquartile range (IQR) and horizontal bars signify the median. The end points whisker represent the maximum and minimum values below or above the median ± 1·5 times the IQR. Points indicate possible outliers. The keys show the results of the Student's t tests used to compare geometric means. Only p values for significant differences are shown. The bottom row links the study groups (RBD 25 μg, and RBD 50 μg) with the values of the immunogenic variables at day 56. RBD: receptor binding domain. ACE-2: angiotensin-converting enzyme. AU/mL: arbitrary units per mL. Nab: Neutralizing antibody titres.
Nab against SARS-CoV-2 were induced by both vaccine strength formulations, after 28 days of have been applied in a three-dose schedule. Overall, participants vaccinated with 25 μg achieved viral neutralisation in 93·6% of cases and 98·2%, for 50 μg group (p = 0·07). Percentage of viral neutralisation in strata 12–18 years was 93·9% for 25 μg group, and 100% for 50 μg group (p = 0·18). Children between 3 and 11 years achieved 94·3% of viral neutralisation for RBD 25 μg, and 97·2 for 50 μg (p = 0·32) (Table 2). GMT of neutralising virus antibodies were: (18·3; 95% CI 14·7–22·78) for 25 μg and (36·4; 95% CI 30·26–43·8) for 50 μg (p < 0·01). Correspondingly, GMT of neutralising antibody titres against SARS-CoV-2 was significantly higher for the 50 μg strength for each of the two age strata: 12–18 years (17·94; 95% CI 12·1–26·6) for 25 μg and (36·96; 95% CI 27·3–49·9) for 50 μg (p = 0·004) and 3–11 years (18·5; 95% CI 14·1–24·1) for 25 μg and (36·1; 95% CI 28·47–45·8) for 50 μg (p < 0·01) (Fig. 3 and Supplementary Table S1).
The association between seroconversion and male or female sex was studied. Dependence was observed in favour of higher percentages of seroconversion in female participants (p = 0·02). The percentage of individuals with positive inhibition of RBD-ACE2 binding, showed correspondence with more favourable responses for the female sex (p = 0·01) (Table 3). Additionally, anti-RBD IgG GMT showed a significant difference between sexes for each antigen strength group: 25 μg for female (GMT 161·64 [95% CI: 132·43–197·29]) and for male (GMT 98·08 [95% CI:79·93–120·35]) (p = 0·002), and 50 μg female (GMT 269·53 [95% CI 220·24–329·83]) and for male (GMT 200·02 [95% CI: 164·29–243·53]) (p = 0·036), respectively. When comparing the means of the percent inhibition between sexes by study groups, not significant correspondence was observed in the global analysis. There are dependencies between the female sex and individuals with viral neutralisation for the group of 25 μg, between 3 and 18 years old (23·19; 95% CI 18·25–29·47) comparing with males (14·17; 95% CI 11·05–18·10) (p = 0·027) (Fig. 4). There was no significance correspondence of any immunological variables with any other control variables such as nutritional assessment, BMI, and skin colour for the percentage of children who achieved viral neutralization (Fig. 5).
Table 3.
Association between qualitative variables and sex as control variable, by stratum of age· The table shows the chi-square test used in each case to evaluate the statistical significance of the association between two categorical variables· RBD: receptor binding domain. ACE-2: angiotensin-converting enzyme.
| Seroconversion of anti-RBD IgG | ||||||
|---|---|---|---|---|---|---|
| 12–18 years old |
3–11 years old |
Overall (3–18 years old) |
||||
| Yes | No | Yes | No | Yes | No | |
| Male | 105 | 6 | 119 | 1 | 224 | 7 |
| Female | 117 | 0 | 114 | 0 | 231 | 0 |
| P value | 0·03 | 1·00 | 0·02 | |||
| Inhibition to RBD-ACE2 binding | ||||||
|---|---|---|---|---|---|---|
| 12–18 years old |
3–11 years old |
Overall (3–18 years old) |
||||
| Yes | No | Yes | No | Yes | No | |
| Male | 87 | 24 | 110 | 10 | 197 | 34 |
| Female | 105 | 12 | 110 | 4 | 215 | 16 |
| P value | 0·03 | 0·20 | 0·01 | |||
| Viral neutralisation | ||||||
|---|---|---|---|---|---|---|
| 12–18 years old |
3–11 years old |
Overall (3–18 years old) |
||||
| Yes | No | Yes | No | Yes | No | |
| Male | 50 | 2 | 98 | 10 | 148 | 12 |
| Female | 56 | 1 | 107 | 0 | 163 | 1 |
| P value | 0·94 | <0·01 | <0·01 | |||
RBD: receptor binding domain; E1, 12–18 years old; E2, 3–11 years old; GMT: geometric mean titres; ACE-2: angiotensin-converting enzyme.
Fig. 4.
Correspondence analysis between quantitative variables and sex, as control variable, by stratum of age.·(A) SARS-CoV-2 anti-RBD IgG antibody titres association with male or female sex. (B) Mean of inhibition of RBD-ACE-2 binding percentages according sex. (C) Neutralizing antibody titres and sex correspondence. Female is represented by pink colour, and blue colour for male. The boxes indicate interquartile range (IQR) and horizontal bars signify the median. The end points whisker represent the maximum and minimum values below or above the median ± 1·5 times the IQR. Points indicate outliers. The keys show the results of the Student's t tests used to compare geometric means. Only p values for significant differences are shown. The bottom row links the study groups (RBD 25 μg, and RBD 50 μg) with the values of the immunogenic variables at day 56.
Fig. 5.
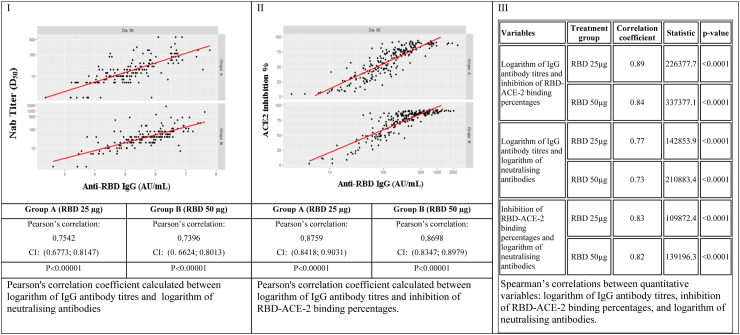
Correlations between quantitative variables: IgG antibody titres, percent inhibition, and log neutralizing antibody titres by treatment group A (RBD 25 μg), and group B (RBD 50 μg), at Day 56. CI confidence Interval; RBD: receptor binding domain; ACE-2: angiotensin-converting enzyme; μg: micrograms.
Before evaluating the Pearson’s correlation, the linearity of the data was tested by the Residual against Fitted plots. The GMT of anti-RBD IgG and Neutralizing antibody graph, as well as the GMT of anti-RBD IgG and ACE2 inhibition % graph showed linearity, but the Neutralizing antibody and ACE2 inhibition % graph did not (Supplementary Fig. S1).
Neutralizing antibody and GMT of anti-RBD IgG showed correlation for 25 μg and for 50 μg group (Pearson correlation, 0·7542 and 0·7396, p < 0·001 respectively) (Fig. 5-I), GMT IgG anti-RBD and positive ACE2 inhibition % (Pearson correlation, 0·8759 and 0·8698, p < 0·001 respectively) (Fig. 5-II), and also between neutralizing antibody titres and positive ACE2 inhibition % showed correlation for both level doses (Spearman correlation, 0·83 for and 0·82, p < 0·001 correspondingly) (Fig. 5-III).
Discussion
Although older people must continue to be the maximum priority for the application of COVID-19 vaccines, expanding vaccination across paediatric age groups is essential to front COVID-19 pandemic given the potential risk of illness and death produced by new genetic variants of SARS-CoV-2.11,27 For this purpose, it would be important to consider experienced vaccine platforms that, in addition to being effective, are highly safe such as those simple formulations based in recombinant proteins, which had been applied during decades to all ages, even in neonates.28, 29, 30
Early on, the CIGB conceived a three-dose schedule for Abdala, based on its experience of more than three decades in the production of the recombinant hepatitis B vaccine, Heberbiovac-HB. Both vaccines are obtained from the same platform of recombinant antigenic protein subunits expressed in P. pastoris yeast.14,29,30
This kind of platform is considered safer than others, such as live attenuated or inactivated viruses, because the recombinant proteins are carefully selected and highly purified; however, several doses are required to achieve the necessary immune response that correlates with protection.28
The initial concept of a three-dose schedule was validated in adults.16,26 Therefore, in children the proposal was to evaluate the same selected three-dose schedule in adults from phase 1, with two alternative antigenic strengths of recombinant RBD: 25 μg and 50 μg.
This is the first report of safety and immunogenicity in paediatric ages of Abdala, a recombinant subunit RBD protein of SARS-CoV-2 vaccine, which had previously demonstrated high efficacy in phase 3 clinical trial in adults, who achieved 92.28% of efficacy. Abdala vaccine is currently authorized for emergency use from July 2021.16
We verified that the application of three doses of Abdala vaccine administered 14 days apart was safe, well tolerated, and highly immunogenic in children and adolescents between 3 and 18 years of age for both evaluated antigenic strengths (25 μg and 50 μg).
Phase 1 passed satisfactorily. No severe AEs were reported. It was not necessary to apply the unacceptable toxicity stopping criterion due to any AE attributable to any experimental group. According phase 1 safety results of the adolescent stratum, this branch aged 12–18 years old could continue to the phase 2, and then 3–11 years old stratum could start the phase 1.
Reactogenicity decreased with the application of each subsequent dose as was observed in adults vaccinated with the vaccine.16,26 ARs showed higher frequency in participants aged 12–18 years (62·1%) than participants aged 3–11 (37·9%), which have been reported previously by other paediatric COVID-19 vaccine studies.31, 32
Most reactions were mild and transient. Similar to other COVID-19 vaccines evaluated in children based in different platforms as Pfizer-BioNTech BNT162b2, Moderna mRNA-1273, Sinovac-CoronaVac, BBIBP-CorV, Novavax-NVX-CoV2373 and Covaxin-Bharat, pain at the injection site and headache were among the most common solicited adverse events.31, 32, 33, 34, 35, 36, 37 Pain at the injection site was the most frequent symptom among all reported events, which is consistent with the results obtained in adults vaccinated with Abdala.16,26
The percentages of ARs were similar for both study groups. One remarkable fact was that highest antigenic strength of 50 μg did not show higher reactogenicity, not even in youngest stratum aged between 3 and 11 years. Thus, no dose-effect was observed in relation to safety.
There were no serious AEs reported during the whole phase 1–2 clinical trial related to vaccination. Three cases of confirmed dengue infection, which were hospitalized for diagnostic and treatment, were considered as coincidental, and unrelated with vaccination. They were completely resolved.
Abdala was immunogenic in children and adolescents evaluated, aged 3–18 years. The seroconversion rates showed percentages over 98% for both strength, RBD 25 μg and 50 μg, after three-dose vaccination. Despite there is no difference between the two study groups in terms of seroconversion, Abdala 50 μg was more immunogenic than the formulation of 25 μg in the evaluated paediatric population 3–18 years. GMT of anti-RBD IgG, mean ACE2 inhibition % showed significant differences with higher titres among children and adolescents who received strength of 50 μg compared to those who received 25 μg. Positive inhibition of RBD-ACE2 binding was also higher in the 50 μg for children between 3 and 11 years.
The seroconversion rates of Nab in children and adolescents with both antigenic strengths groups were over 93% after the three-dose vaccination. The Nab elicited by Abdala ranged from 14·14 to 45·78 in the stratum aged 3–11 years, and 12·1 to 49·9 in the stratum aged 12–18 years, which were similar to both study groups and also to the Nab previously elicited by adult participants.24 In our study, we observed significantly higher GMT of the Nab in favour to 50 μg strength compared to 25 μg (36·41 and 18·30) (p < 0·01). However, previous results in vaccinated adults in phase 2 trial with the same schedule of Abdala showed that GMT of Nab were similar for 50 μg and 25 μg groups (30·81 and 24·12) without significant differences between them (p = 0·19).16,26
Based on analyzes stratified by clinical site and age, a trend of higher response of immunogenicity variables was observed in subjects who received 50 μg of RBD as active ingredient. However, because the number of participants is limited when each clinical site is analyzed separately, the differences did not show statistical significance in most of the sites, but they did in the overall analysis.
In relation to the control variables, our complementary evaluation indicated that variables age and sex could have associated with the immune response.
A tendency towards a better immunogenicity profile was observed in the stratum of children aged 3–11 years with respect to children and adolescents aged 12–18, specifically in regards to GMT of IgG anti-RBD and inhibition of RBD-ACE2. Age is a known factor influencing the immune response to vaccines and diseases.38, 39 A paediatric expansion study with 2 doses of primary vaccination with the protein subunit-based COVID-19 vaccine NVX-CoV2373, at the same adult's dosage, showed higher responses than in adults in terms of seroconversion, IgG against Spike proteins, and functional antibodies (ACE2 inhibition) against several viral variants.16, 37 Other vaccines, such as hepatitis B, influenza, tetanus, pertussis, and diphtheria vaccine have been showed declining responses with increasing age.29, 38, 39
Despite these observations could lead us to consider the possibility of using the lower antigenic strength of RBD 25 μg in children younger than 12 years old, the neutralising response was not observed among the immunological variables with more favourable response for 3–11 years old stratum.
As known, the viral neutralisation response has been considered as the most accurate approach to estimate the correlate of efficacy assessing the functional activity of the antibodies and not only their quantity. To date, it is very difficult to establish comparisons in terms of immunogenicity, and especially in terms of viral neutralising response between different COVID-19 vaccines. The diversity of platforms for their production must be considered, and, on the other hand, the results are usually evaluated by different methods. It is possible to find vaccines whose viral neutralising responses are lower than the figures reported for convalescent sera, and yet are capable of providing a highly effective response in protection against severe forms and death from COVID-19.26, 27, 28
Immunogenicity variables in general showed in our study better results in 50 μg with a very similar safety profile between both strengths. In our consideration, results support the selection of the antigenic strength of 50 μg for its application in children and adolescents aged between 3 and 18 years.
Other COVID-19 vaccine studies conducted in paediatric populations have also shown a dose-response relationship of specific IgG antibody titres and Nab when evaluating different antigenic strengths.31, 32
The inactivated COVID-19 vaccine (CoronaVac), showed higher titres of Nab induced by the 3 μg dose formulation than by the 1·5 μg dose in children and adolescents aged 3–17 years, whereas it showed no significant difference in side-effects between the two doses, so 3 μg doses were used for phase 3 trials in children.31
However, other vaccines such as Pfizer-BioNTech BNT162b2 in their initial phases of evaluation in children did not show a dose-response effect in terms of IgG specific antibodies and neutralising antibodies, then lowering vaccination dose used in adults was selected considering safety.11
Similarly, two 50-μg doses of the mRNA-1273 (Moderna) vaccine were selected on the basis of the lower reactogenicity than the 100-μg dose level and supportive immunogenicity results.40
In our study, female sex showed higher seroconversion, GMT IgG anti-RBD, ACE2 inhibition %, and neutralising activity against the SARS-CoV-2. The significant impact of sex and gender on health has come to the fore during the COVID-19 pandemic: men are about three times more likely to be admitted to intensive care and 40% more likely to die from COVID-19 than women. Known biological differences in innate and adaptive immune responses between sexes explain some of these observed differences.41 Sex and gender differences have been observed in immunization for other vaccine-preventable diseases, and women often develop higher antibody responses compared to men. These differences have been observed in response to vaccines using different technologies, including Calmette-Guerin vaccine, measles, mumps and rubella, yellow fever, influenza, and hepatitis recombinant vaccines. Several biological mechanisms have been proposed, including immunological, hormonal, genetic, and microbiota differences between women and men.41
On the other hand, the BMI has been considered previously associated with a more or less immunogenic vaccine response, being less responsive the overweight and obese; however, it was not observed in children.42
Correlation analyses showed a strong correlation between three pairs of immunogenicity variables: Nab titres and GMT of anti-RBD IgG; Nab titres and ACE2 inhibition %; and also between GMT IgG anti-RBD and ACE2 inhibition %. The correlation coefficient at time 56 days of each pair of assessed variables reached values greater than zero, above 0·5 and close to +1, which were interpreted as a strong correlation. Despite the gold standard continues to be viral neutralisation to predict possible efficacy in vaccinated subjects, it requires working with the live pathogen under risky and more costly conditions. The serological ACE2 inhibition test has been used to predict a viral neutralisation response due to a strong correlation between both methods.24
This clinical trial has some limitations. Firstly, need for a longer follow-up period to evaluate safety, prevalence of the immune response, and the potential need of booster. In this sense, the follow-up of the participants after six months of receiving primary vaccination was approved by the National regulatory authority, and the results will be published soon. On the other hand, the cell-mediated response to immunization was not assessed.
The study results are applicable to seemingly healthy children and adolescents aged between 3 and 18 years, which is a wide paediatric age range that contributes to external validity of the trial results.
Future studies have been conceived as part of the clinical development strategy of the Abdala vaccine in paediatric ages: an immune-bridging study comparing paediatric participants with young adults, in whom the efficacy of the vaccine has already been demonstrated, and studies that include vulnerable paediatric populations with underlying diseases or risk factors.
In conclusion, this clinical trial constituted the first evaluation of safety and immunogenicity in paediatric ages of a recombinant subunit RBD protein of SARS-CoV-2 vaccine, obtained in P. pastoris yeast. Abdala vaccine was well tolerated and safe, and induced humoral responses in children and adolescents aged 3–18 years by day 56. The results support vaccination of children and adolescents aged between 3 and 18 years, with three doses of 50 μg of the Abdala vaccine according vaccination schedule 0, 14 and 28 days. Based on these favourable results, other studies in children and adolescents have been initiated.
Contributors
ZCE, VMLG, MLF, MAA and SRA took part in the conception of the study. ZCE, main clinical trial monitor, participated in design of study protocol, analysis of results, and preparation of manuscript. SRA was the chief investigator, responsible for recruitment, vaccination and follow-up. SRA and ATF led the study implementation. VLMG advised the clinical trial and the preparation of the manuscript. NLFB, ROM, ICL and EIA monitored the study in all stages. FHB contributed to safety data analysis and interpretation. ATF, MRC, GGG, IMP and NBA were clinical investigators who recruited, vaccinated, followed up the participants and collected clinical data. Immunogenicity testing was done by GLP, ACE, GFC, DBG, EGO, AFQ and GSPB. The design and verification of the database was carried out by MAV who also coordinated the data management process. COCC conducted the statistical analysis. JLRR and GMS guaranteed the random allocation and masking of the product, as well as the maintenance of the cold chain. MLF and MAA were part of the management and coordination of the project. All authors gave final approval of the version to be published.
Data sharing statement
The individual anonymised participant-level data that underlie the results reported in this Article will be shared when the trial is complete (text, tables, figures, and appendices). The data will be available immediately after publication and finalisation of the completed clinical study report for at least 6 months, upon requests addressed to the corresponding author (zurina.cinza@cigb.edu.cu). Proposals will be reviewed and approved by the sponsor, investigator, and collaborators on the basis of scientific merit. After approval of a proposal, data can be shared through a secure online platform. To gain access, data requestors will sign a data access agreement.
Declaration of interests
ZCE, NLFB, ROM, ICL, MAV, FHB, GLP, GFC, JLRR, GMS, MLF, MAA, and VLMG, are employees of the Centre for Genetic Engineering and Biotechnology, Havana, Cuba (CIGB), sponsor of the trial as manufacture centre of Abdala vaccine. ACE, is employee of the Camagüey-based CIGB unit. All the other authors declare no competing interests.
Acknowledgements
The authors would like to thank express their gratitude for the contribution of all trial participants, and the staff of nurses and physicians that supported the immunization and evaluation of the clinical trial. We also gratefully acknowledge the invaluable support of the independent members of the data monitoring and safety committee, and the support of the Camagüey authorities. This study was funded by Centre for Genetic Engineering and Biotechnology (CIGB), Havana, Cuba.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102160.
Contributor Information
Zurina Cinza-Estévez, Email: zurina.cinza@cigb.edu.cu.
ISMAELILLO Clinical Trial Group:
M.A. López-Machado, D Alonso Rodriguez, J.C. Basulto-Puig, T. Martin-Hernandez, D. Sánchez-Miranda, C.M. Agüero-Betancourt, B. Besú-García, Y. Rodríguez-Fernández, L. Guerrero-Vega, I. GómezFonseca, O. Ramos-Pérez, I. Acosta-Domínguez, D. Ronquillo-Ramirez, Y. Díaz-Fernández, L. Aguilar-Soto, J. Reíd-Montejo, Y. Palomo-Leyva, M.R. LandinFaria, R.M. Rosendo-Domínguez, C. Alonso-Manresa, D.M. Marín-Pérez, Y. Malo-Lantigua, M.C. Rodríguez-Florat, Y. Delgado-Laborit, Y. RodriguezGuevara, M. Peláez-González, Y. Torres-Mora, O.S. Cabrera-Hernández, F.J. Gimarais-Varona, M. Hormigot-Hernández, L.V. Artola-Gutiérrez, L. Gallardo-Martí, J. Porro-Verdecia, R. Castro-Bistorte, E. RobertsDandie, S. Berenguer-Pedroso, Yudith Manso-García, C.V. Mora-García, Y. Lastre-Muñoz, O. Duran-Rivero, B. Hernández-García, K. Escobar-Escobar, M.K. CarmenatesGutiérrez, B. Conde-Bello, E.L. Olazabal-Linares, I. Bringas-Labrada, M. Noy-León, N. Velazco-González, B. Bursosa-Moreno, Y. Morell-Padrón, Y. Rodríguez-Matos, Y.M. Treto-Torguet, L. Comas-Díaz, F. Miranda, Y. FigueredoGonzález, A. Quiñones-Juan, I. Mursuli-García, V.M. Giménez-Velásquez, A. Hernández-Ávila, T. Hernández-Cabrera, T. García-Zulueta, A. Parra-Pérez, D. Cintra-Jacob, M. Mendoza-Jiménez, S.M. de la Fuente-Carbonell, B. Hernández-Eduard, M. Ochoa-García, E. Garcia-Iglesias, A. Álvarez-Acosta, R.U. Martínez-Rosales, L. Ávila-Díaz, Z. Santana-Vázquez, L. Mila-Cáceres, G.E. Guillén-Nieto, F. Fuentes-Aguilar, A. Nordelo-Valdivia, N. GonzálezFernández, M. González-Sarmientos, A. Rubio-Salinas, L.C. Domínguez-Rabilero, R.A. Espinosa-Peña, Y. Ramírez-Núñez, J. Junco-Barranco, O. Díaz-González, A. Fragas-Quintero, M.T. Pérez-Guevara, J.M. Enriquez-Puertas, Y. Infante-Hernández, O. Cruz-Sui, E. NoaRomero, Yizel Hernández López, J.E. Sánchez-García, E. Rodríguez-Martínez, E. Pimentel-Vázquez, and E. Martínez-Díaz
Appendix ASupplementary data
References
- 1.WHO . 2022. Weekly epidemiological update. Edition 115. (Accessed on November 3/2022) [Google Scholar]
- 2.World Health Organization (WHO); Geneva (CH): 2022. Interim statement on COVID-19 vaccination for children, 11 August 2022.https://reliefweb.int/report/world/interim-statement-covid-19-vaccination-children-11-august-2022 [cited 2022 Nov 3]. Available from: [Google Scholar]
- 3.Unicef 2022. Child mortality and COVID-19. https://data.unicef.org/topic/child-survival/covid-19/ Available from:
- 4.World Health Organization (WHO); Geneva (CH): 2022. COVID-19 vaccine tracker.https://covid19.trackvaccines.org/vaccines/approved/#vaccine-list Available from: [Google Scholar]
- 5.European Centre for Disease Prevention and Control . ECDC; Stockholm: 2022. COVID-19 vaccine effectiveness in adolescents aged 12– 17 years and interim public health considerations for administration of a booster dose.https://www.ecdc.europa.eu/en/publications-data/covid-19-vaccine-effectiveness-adolescents-and-interim-considerations-for-booster-dose Available from: [Google Scholar]
- 6.Plotkin S.A., Levy O. Considering mandatory vaccination of children for COVID-19. Pediatrics. 2021;147(6) doi: 10.1542/peds.2021-050531. [DOI] [PubMed] [Google Scholar]
- 7.Post COVID-19 condition. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 Available from:
- 8.COVID-19 disease in children and adolescents: scientific brief, 29 September 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Children_and_adolescents-2021.1 Available from:
- 9.European Centre for Disease Prevention and Control SARS-CoV-2 in children. https://www.ecdc.europa.eu/en/infectious-disease-topics/z-disease-list/covid-19/latest-evidence/sars-cov-2-children Available from:
- 10.Oliveira C.R., Niccolai L.M., Sheikha H., et al. Assessment of clinical effectiveness of BNT162b2 COVID-19 vaccine in US adolescents. JAMA Netw Open. 2022;5(3) doi: 10.1001/jamanetworkopen.2022.0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walter E.B., Talaat K.R., Sabharwal C., et al. Evaluation of the BNT162b2 Covid-19 vaccine in children 5 to 11 Years of age. N Engl J Med. 2022 Jan 6;386(1):35–46. doi: 10.1056/NEJMoa2116298. Epub 2021 Nov 9. PMID: 34752019; PMCID: PMC8609605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO Interim recommendations for use of the Novavax NVX-CoV2373 vaccine against COVID-19. 2022. https://apps.who.int/iris/bitstream/handle/10665/363204/WHO-2019-nCoV-vaccines-SAGE_recommendation-Novavax_NVX-CoV2373-2022.1-eng.pdf Available from:
- 13.Limonta-Fernández M., Chinea-Santiago G., Martín-Dunn A.M., et al. The SARS-CoV-2 receptor-binding 1 domain expressed in Pichia 2 pastoris as a candidate vaccine antigen. medRxiv. 2021 doi: 10.1101/2021.06.29.21259605. 2021.06.29.21259605. Available from: preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy E., Martinez E., Diago D., Diaz R., Gonzalez D., Herrera L. Large-scale production of recombinant hepatitis B surface antigen from Pichia pastoris. J Biotechnol. 2000;77:157–167. doi: 10.1016/S0168-1656(99)00201-1. PMID:10682276. [DOI] [PubMed] [Google Scholar]
- 15.Reardon S. Cuba’s bet on home-grown COVID vaccines is paying off. Nature. 2021;600:15–16. doi: 10.1038/d41586-021-03470-x. [DOI] [PubMed] [Google Scholar]
- 16.Hernández-Bernal F., Ricardo-Cobas M.C., Martín-Bauta Y., et al. A phase 3, randomised, double-blind, placebo-controlled clinical trial for adult evaluation of the efficacy and safety of a SARS-CoV-2 recombinant spike RBD protein vaccine (ABDALA-3 Study) MedRxiv. 2022 doi: 10.1101/2022.09.08.22279690. 22279690 [Preprint] [cited 2022 Nov 9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.International Conference of Harmonization (ICH) ICH tripartite guideline for good clinical practices E6 (R1), June 10, 1996. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf Available from:
- 18.WHO Guidelines on clinical evaluation of vaccines: regulatory expectations. 2017. http://www.who.int/entity/biologicals/expert_committee/WHO_TRS_1004_web_Annex_9.pdf
- 19.Singh J.A., Kochhar S., Wolff J., et al. WHO guidance on COVID-19 vaccine trial designs in the context of authorized COVID-19 vaccines and expanding global access: ethical considerations. Vaccine. 2022;40(14):2140–2149. doi: 10.1016/j.vaccine.2022.02.038. Epub 2022 Feb 28. PMID: 35248422; PMCID: PMC8882397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buenas Prácticas Clínicas . MINSAP; Cuba: 2000. Centro para el Control Estatal de la Calidad de los Medicamentos (CECMED)http://www.cecmed.sld.cu/Docs/RegFarm/DRA/EvalDC/Reg/Dir BPC.pdf [Google Scholar]
- 21.Esquivel M., Rubí A. Curvas Nacionales de peso para la talla. Rev Cub Ped. 1984;56:705–721. [Google Scholar]
- 22.Esquivel M., Rubí A. Valores cubanos del índice de masa corporal en niños y adolescentes de 0 a 19 años. Rev Cubana Pediatr. 1991;63(3):181–190. [Google Scholar]
- 23.CECMED UMELISA SARS-COV-2 IGG (Cuban sanitary Registry D2107-11) https://www.cecmed.cu/covid-19/aprobaciones/umelisa-sars-cov-2-igg Available at:
- 24.Lemus-Pérez G., Chávez-Valdés S., González-Formental H., et al. Elevated antibody in Abdala vaccinees evaluated by Eleesys anti-SARS-CoV-2 S highly correlate with UMELISA SARS-CoV-2 anti-RBD, ACE-2 binding inhibition and viral neutralization assays. medRxiv. 2021 doi: 10.1101/2021.10·18.21265169. [Preprint]. [cited 2021 Oct 28] [DOI] [Google Scholar]
- 25.Manenti A., Maggetti M., Casa E., et al. Evaluation of SARS-CoV-2 neutralizing antibodies using a CPE-based colorimetric live virus micro-neutralization assay in human serum samples. J Med Virol. 2020 Oct;92(10):2096–2104. doi: 10.1002/jmv.25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernández-Bernal F., Ricardo-Cobas M.C., Martin-Bauta Y., et al. Safety, tolerability, and immunogenicity of a SARSCoV-2 recombinant spike RBD protein vaccine: a randomised, double-blind, placebo-controlled, phase 1-2 clinical trial (ABDALA Study) eClinicalMedicine. 2022;46 doi: 10.1016/j.eclinm.2022.101383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson E.J., Campbell J.D., Creech C.B., et al. Warp speed for coronavirus disease 2019 (COVID-19) vaccines: why are children stuck in neutral? Clin Infect Dis. 2021;73:336–340. doi: 10.1093/cid/ciaa1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Acosta-Coley I., Cervantes-Ceballos L., Tejeda-Benítez L., et al. Vaccines platforms and COVID-19: what you need to know. Trop Dis Travel Med Vaccines. 2022;8(20):1–19. doi: 10.1186/s40794-022-00176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernández-Bernal F., Aguilar-Betancourt A., Aljovin V., et al. Comparison of four recombinant hepatitis B vaccines applied on an accelerated schedule in healthy adults. Hum Vaccine. 2011;7(10):1026–1036. doi: 10.4161/hv.7.10·15989. [DOI] [PubMed] [Google Scholar]
- 30.Muzio González V., Cinza Estévez Z., Ortega Tápanes A., et al. Post-licensing studies of the Cuban anti-hepatitis B vaccine, Heberbiovac-HB. Biotecnología Aplicada. 2001;18(2):103–104. [Google Scholar]
- 31.Han B., Song Y., Li C., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: a double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:1645–1653. doi: 10.1016/S1473-3099(21)00319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia S., Zhang Y., Wang Y., et al. Safety and immunogenicity of an inactivated COVID-19 vaccine, BBIBP-CorV, in people younger than 18 years: a randomised, double-blind, controlled, phase 1/2 trial. Lancet Infect Dis. 2022;22(2):196–208. doi: 10.1016/S1473-3099(21)00462-X. Epub 2021 Sep 15. PMID: 34536349; PMCID: PMC8443232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.European Medicines Agency . BioNTech/Pfizer); 2021. Summary of product characteristics comirnaty.https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf [Google Scholar]
- 34.European Medicines Agency . 2021. Summary of product characteristics spikevax (Moderna)https://www.ema.europa.eu/en/documents/product-information/spikevax-previously-covid-19-vaccine-moderna-epar-product-information_en.pdf [Google Scholar]
- 35.United States Food and Drug Administration . United States Food and Drug Administration; Maryland: 2021. Vaccines and related biological products advisory committee october 26, 2021 meeting document.https://www.fda.gov/media/153409/download [Google Scholar]
- 36.Ella R., Reddy S., Jogdand H., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis. 2021;21(7):950–961. doi: 10.1016/S1473-3099(21)00070-0. Epub 2021 Mar 8. PMID: 33705727; PMCID: PMC8221739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Áñez G., Dunkle L.M., Gay C.L., et al. 2019nCoV-301 – pediatric expansion study group. Safety, immunogenicity and efficacy of NVX-CoV2373 in adolescents in PREVENT-19: a randomized, phase 3 trial. medRxiv. 2022 doi: 10.1101/2022.09.20.22279903. [Preprint]. Sep 21:2022.09.20.22279903. PMID: 36172135; PMCID: PMC9516866. [DOI] [Google Scholar]
- 38.Collier D.A., Ferreira I.A.T.M., Kotagiri P., et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596(19):417–438. doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gustafson C.E., Kim C., Weyand C.M., Goronzy J.J. Influence of immune aging on vaccine responses. J Allergy Clin Immunol. 2020;145(5):1309–1321. doi: 10.1016/j.jaci.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Creech C.B., Anderson E., Berthaud V., et al. KidCOVE Study Group Evaluation of mRNA-1273 Covid-19 vaccine in children 6 to 11 Years of age. N Engl J Med. 2022;386(21):2011–2023. doi: 10.1056/NEJMoa2203315. Epub 2022 May 11. PMID: 35544369; PMCID: PMC9127699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heidari S., Goodman T. Critical sex and gender considerations for equitable research, development and Delivery of COVID-19 vaccines. World health Oganization. 2021. https://cdn.who.int/media/docs/default-source/immunization/sage/covid/gender-covid-19-vaccines-sage-background-paper.pdf?sfvrsn=899e8fca_15&download=true Available online at:
- 42.Butsch W.S., Hajduk A., Cardel M.I., et al. COVID-19 vaccines are effective in people with obesity: a position statement from the Obesity Society. Obesity (Silver Spring) 2021;29(10):1575–1579. doi: 10.1002/oby.23251. Epub 2021 Sep 13. PMID: 34212511; PMCID: PMC8441899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.