Abstract
The protective effects of remote ischemic conditioning (RIC) on acute ischemic stroke have been reported. However, the protective mechanisms of RIC have not been fully elucidated. This study aimed to investigate whether RIC could reduce oxidative stress and inflammatory responses in middle cerebral artery occlusion (MCAO)-reperfusion mice via the nuclear factor-E2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) pathway. C57BL/6 mice were subjected to MCAO and underwent RIC twice daily at 1, 3, and 7 days after MCAO. ML385 was used to specifically inhibit Nrf2 in MCAO mice. Neurological deficit scores, infarct volume, and hematoxylin-eosin (HE) staining were assessed. Oxidative stress levels were assessed based on total antioxidant capacity (TAC), malonaldehyde (MDA), superoxide dismutase (SOD), and glutathione/glutathione disulfide (GSH/GSSG). mRNA levels were detected using real-time polymerase chain reaction (PCR), and protein levels were detected using western blotting and enzyme-linked immunosorbent assay (ELISA). Protein localization was investigated using immunofluorescence staining. RIC significantly reduced infarct volume and improved neurological function and histological changes after MCAO. RIC significantly increased TAC, SOD, and GSH/GSSG levels and decreased MDA levels. RIC significantly increased Nrf2 and HO-1 mRNA levels and decreased Keap1, NLRP3, and Cleaved Caspase-1 mRNA levels. RIC significantly increased Nrf2, HO-1, and NQO1 protein expression and decreased Keap1, NLRP3, Cleaved Caspase-1, Cleaved IL-1β, IL-6, and TNF-α protein expression. RIC promoted the activation and translocation of Nrf2 into the nucleus. The protective effects of RIC were abolished by ML385 treatment. In conclusion, our findings suggest that RIC alleviates oxidative stress and inflammatory responses via the Nrf2/HO-1 pathway, which in turn improves neurobehavioral function. RIC may provide novel therapeutic options for acute ischemic stroke.
Keywords: Remote ischemic conditioning, MCAO, Oxidative stress, Inflammation, Nrf2/HO-1 pathway
Graphical abstract

Abbreviations
- RIC
Remote ischemic conditioning
- MCAO
Middle cerebral artery occlusion
- Nrf2
The nuclear factor-E2-related factor 2
- HO-1
Heme oxygenase-1
- TTC
2,3,5-triphenyl tetrazolium chloride
- HE
Hematoxylin-Eosin
- TAC
Total antioxidant capacity
- MDA
Malonaldehyde
- SOD
Superoxide dismutase
- GSH/GSSG
Glutathione/glutathione disulfide
- PCR
Polymerase Chain Reaction
- IF
Immunofluorescence
- ELISA
Enzyme-linked Immunosorbent Assay
1. Introduction
Stroke is one of the most common causes of long-term disability and death worldwide, with ischemic stroke being the most common type [1]. Reperfusion remains the gold standard of care in patients with ischemic stroke but can lead to ischemia-reperfusion injury. Oxidative stress and inflammation are key pathological processes associated with cerebral ischemia/reperfusion injury. Thus, targeting oxidative stress and neuroinflammation may be a promising therapeutic strategy for ischemic stroke [2]. The nuclear factor-E2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) pathway plays a crucial role in regulating the expression of various antioxidant defense and anti-inflammatory genes [3]. Under ischemia-reperfusion injury, Nrf2 translocates to the nucleus, activates HO-1, and further represses inflammatory proteins [4,5].
Remote ischemic conditioning (RIC) is a remote, transient, and non-lethal limb ischemia treatment that has been proven to be safe and efficacious in clinical trials [6,7]. Previous work has demonstrated that RIC is effective for reducing cerebral infarct volume, increasing cerebral blood flow, and improving neurobehavioral function in rats with cerebral infarction [8,9]. However, the protective mechanisms of RIC have not been fully elucidated. This study aimed to investigate whether RIC treatment could reduce oxidative stress and inflammatory responses in middle cerebral artery occlusion (MCAO)-reperfusion mice by activating the Nrf2/HO-1 signaling pathway, thereby improving neurobehavioral function.
2. Materials and methods
2.1. Experimental animals
A total of 312 male C57BL/6 mice weighing 20–25 g (aged 8–10 weeks) were purchased from Beijing Vital River Laboratory Animal Technology. Animals were maintained at a temperature of 22 ± 2 °C, humidity of 65 ± 5%, and housed under a 12-h light-dark cycle with ad libitum access to food and water. This study was approved by the Ethics Committee of the First Hospital of Jilin University (No. 0733). All animal procedures were performed in accordance with the regulations of the Institutional Animal Care and Use Committee.
2.2. Experimental design
Our study was divided into two parts. Animals were divided into two parts in a randomized manner for experimental studies. Fig. 1A depicts the flowchart of the experimental design.
Fig. 1.
RIC improved neurological function in MCAO mice. (A) Study flow chart. (B) Femoral artery blood flow before, during, and after RIC. (C–E) Neurological deficit scores based on Zea Longa, Bederson, and modified Garcia scores at 1, 3, and 7 days after MCAO. *P < 0.05, **P < 0.01, ***P < 0.001.
2.2.1. Part 1
The first part of our study explored whether RIC could improve short-term and long-term neurological recovery through oxidative stress and inflammatory responses. Therefore, 162 mice were randomly divided into three different treatment experimental groups to explore the effectiveness of RIC treatment for 1, 3, and 7 days (n = 18 per subgroup): [1] sham, MCAO 1 day, MCAO + RIC 1 day; [2] sham, MCAO 3 days, MCAO + RIC 3 days; [3] sham, MCAO 7 days, MCAO + RIC 7 days.
2.2.2. Part 2
The second part of our study explored whether RIC attenuated oxidative stress and inflammation via the Nrf2/HO-1 pathway. We administered an Nrf2-specific inhibitor, ML385 (MCE, China), to inhibit Nrf2 expression 3 days after MCAO. ML385 (30 mg/kg) was dissolved in corn oil with 5% dimethyl sulfoxide and injected intraperitoneally in mice 1 h before MCAO. The vehicle group was injected with the same dose of corn oil and 5% dimethyl sulfoxide. In total, 150 mice were randomly divided into five groups (n = 30 per group): sham, MCAO, MCAO + RIC, MCAO + RIC + Vehicle, and MCAO + RIC + ML385.
2.3. Animal models
Focal cerebral ischemia was induced by MCAO, as described previously [10]. Mice were anesthetized with isoflurane (4% induction; 2% maintenance) throughout the operation. The left external carotid artery was completely exposed and ligated using a 6-0 suture. A nylon monofilament (Beijing Cinontech, China) was introduced through the external carotid stump into the left internal carotid artery to occlude the origin of the MCA and block distal blood flow. After 60 min of occlusion, the nylon monofilament was carefully removed to restore blood flow. Body temperature was maintained at 37 ± 0.5 °C throughout the procedure. The sham group underwent an operation without the insertion of a nylon monofilament.
2.4. RIC treatment
RIC was performed immediately after removing the nylon monofilament from the external carotid artery. A 3-mm tourniquet was tightened on the bilateral hind limbs above the knee joint for four cycles, with each occlusion for 5 min and immediately released for 5 min (lasting 40 min in total) and continued every 12 h until execution (treated with RIC twice daily for 1, 3 or 7 days, respectively) (Fig. 1A). Mice were anesthetized with isoflurane during the RIC treatment, as described above. The sham and MCAO groups received the same dose of isoflurane.
2.5. Behavioral testing
Six mice in each group were randomly selected for neurological function testing. Mice were placed in the test chamber for 1 h prior to testing to allow acclimatization to the environment. Neurological deficits were tested using three scores. The Zea Longa (0–4) [11] and Bederson (0–5) [12] scores are commonly used methods for scoring neurological function in animals, in which higher scores indicate more severe neurological deficits. The modified Garcia score [13] ranged from 3 (maximum deficit) to 18 (no deficit) and included spontaneous activity, limb extension, forepaw extension, climbing, lateral stroking, and vibratory touch. The observers were blinded to the experimental subgroups while performing neurobehavioral tests.
2.6. 2,3,5-Triphenyl tetrazolium chloride staining
Infarct volume was determined using 2,3,5-triphenyl tetrazolium chloride (TTC) staining. Coronal brain sections (1 mm thick) were immersed in a 2% TTC solution for 20 min at 37 °C. The infarcted and non-infarcted hemispheres were analyzed using ImageJ software (National Institutes of Health, USA).
2.7. Hematoxylin-eosin staining
Brain tissues were fixed with 4% paraformaldehyde, embedded in paraffin, cut into sections (4 μm), and stained with Hematoxylin-Eosin (HE). The sections were observed under a microscope to assess histopathological changes in the mouse cortex.
2.8. Measurement of oxidative stress levels
Fresh tissue samples were collected from peri-infarct areas. Total antioxidant capacity (TAC), malonaldehyde (MDA), and superoxide dismutase (SOD) activity were assayed using the TAC assay kit (Sigma, USA), MDA assay kit (Beyotime, China), and SOD assay kit (Beyotime, China), respectively, according to the manufacturer’s instructions. Glutathione/glutathione disulfide (GSH/GSSG) level was assessed using the GSH and GSSG assay kits (Beyotime, China), respectively.
2.9. Quantitative real-time polymerase chain reaction
Total RNA from the tissue samples was isolated using Monzol reagent (Monad, China). Reverse transcription was performed using the MonScript RT III Kit (Monad, China). Gene expression was determined using SYBR Green reagents (Monad, China) on a real-time polymerase chain reaction (PCR) instrument (Thermo Fisher Scientific, USA) according to the manufacturer’s instructions. All samples were normalized to β-actin mRNA levels. All designed primers were described in previous literature [[14], [15], [16]] and checked using Primer-BLAST for specificity binding. The primer sequences are listed in Table 1.
Table 1.
Real-time PCR primer sequences.
| Gene | Primer sequences (forward) | Primer sequences (reverse) |
|---|---|---|
| Nrf2 | 5′-AAAATCATTAACCTCCCTGTTGAT-3′ | 5′-CGGCGACTTTATTCTTACCTCTC-3′ |
| Keap1 | 5′-GATATGAGCCAGAGCGGGAC-3′ | 5′-CATACAGCAAGCGGTTGAGC-3′ |
| HO-1 | 5′-CAAGCCGAGAATGCTGAGTTCATG-3′ | 5′-GCAAGGGATGATTTCCTGCCAG-3′ |
| NLRP3 | 5′-GTGGTGACCCTCTGTGAGGT-3′ | 5′-TCTTCCTGGAGCGCTTCTAA-3′ |
| Cleaved Caspase-1 | 5′-ACAAGGCACGGGACCTATG-3′ | 5′-TCCCAGTCAGTCCTGGAAATG-3′ |
| β-actin | 5′-TTCAACACCCCAGCCATG-3′ | 5′-CCTCGTAGATGGGCACAGT-3′ |
Abbreviations: PCR, polymerase chain reaction.
2.10. Western blotting
Proteins were isolated from the peri-infarct region of mice. Samples were incubated with primary antibodies against Nrf2 (1:1000, D1Z9C, CST), Keap1 (1:1000, D6B12, CST), HO-1 (1:1000, E3F4S, CST), NQO1 (1:5000, 67240-1-Ig, Proteintech), NLRP3 (1:1000, CQA3704, Cohesion), Cleaved Caspase-1 (1:1000, E2G2I, CST), Cleaved IL-1β (1:1000, E7V2A, CST), and β-actin (1:1000, CPA9066, Cohesion) overnight at 4 °C. Samples were then incubated with horseradish peroxidase-linked anti-rabbit (1:10,000, CSA2115, Cohesion) or anti-mouse (1:10,000, CSA2108, Cohesion) secondary antibodies for 1 h at room temperature (22–25 °C). Protein bands were visualized using an enhanced chemiluminescence kit (Millipore, USA) and analyzed using ImageJ software. Band gray values were normalized to β-actin levels.
2.11. Immunofluorescence staining
Brains were frozen to investigate the localization of Nrf2 and Keap1 using immunofluorescence (IF) staining. The brain sections were incubated with rat anti‐Nrf2 (1:200, D9J1B, CST) and rabbit anti‐Keap1 antibodies (1:200, D6B12, CST) at 4 °C overnight. The sections were then incubated with goat anti-rabbit (1:500, CSA3611, Cohesion) and goat anti-rat (1:500, CSA3229, Cohesion) secondary antibodies at room temperature (22–25 °C) for 1 h. Finally, the sections were incubated with DAPI (1 ng/μL, Solarbio) for 5 min at room temperature (22–25 °C). Fluorescent signals were detected using a confocal fluorescence microscope (Olympus).
2.12. Enzyme-linked immunosorbent assay
The eye socket blood obtained from mice was placed overnight at 4 °C and separated by centrifugation at 3000 r/min, 5 min for the collection of the upper serum. Enzyme-linked immunosorbent assay (ELISA) kits for Cleaved IL-1β (MM-46433M1), IL-6 (MM-0163M2), and TNF-α (MM-0132M2) proteins were purchased from Jiangsu Meimian Industrial Co. The procedure was in accordance with the kit instructions. Absorbance was measured at 450 nm using an enzyme marker (Bio Tek, USA).
2.13. Statistical analysis
Statistical analysis was performed using SPSS software (version 23.0; IBM, USA). The distribution of variables was evaluated using the Kolmogorov–Smirnov test. Normally distributed data are expressed as mean ± standard deviation. One-way analysis of variance followed by the least significant difference test was used to evaluate differences between groups. Neurologic deficit scores are expressed as median with interquartile range and were analyzed using the Mann–Whitney U test because the data were non-normally distributed. Statistical significance was set at P < 0.05.
3. Results
3.1. Effects of RIC on short-term and long-term neurological recovery
Mice in each group received RIC treatment twice daily for 1, 3, and 7 days after MCAO. During RIC treatment, femoral artery blood flow decreased by 95.5%. After RIC treatment, femoral artery blood flow recovered to approximate initial levels (Fig. 1B).
Behavioral testing results revealed that neurological function was significantly improved in the MCAO + RIC group compared to that in the MCAO group at 3 days after MCAO based on Zea Longa, Bederson, and modified Garcia scores (all P < 0.05). Neurological function in the MCAO + RIC group was significantly improved compared to that in the MCAO group at 7 days after MCAO based on Bederson and modified Garcia scores (all P < 0.05). No significant difference was observed in neurological function scores between the MCAO and MCAO + RIC groups 1 day after MCAO (all P > 0.05) (Fig. 1C–E).
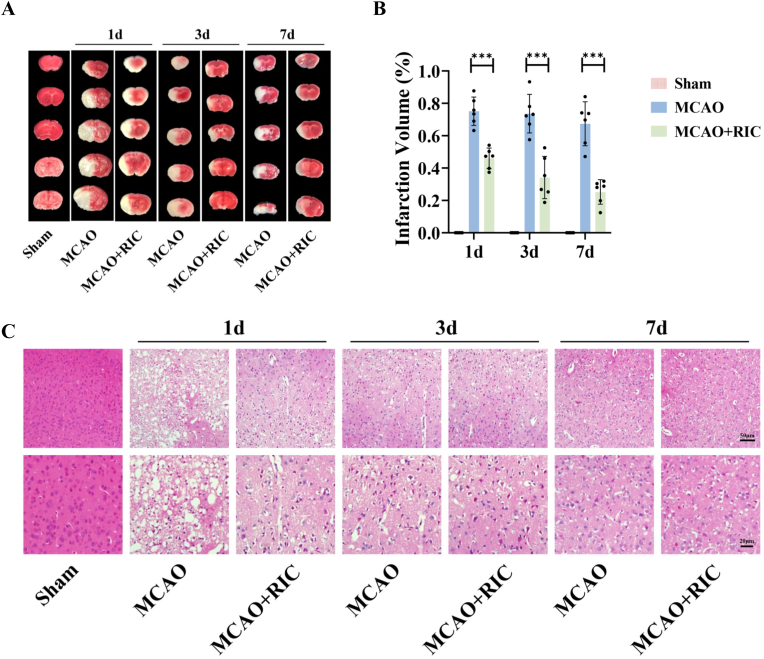
TTC staining results revealed that infarct volume was significantly reduced in the MCAO + RIC group compared to that in the MCAO group at 1, 3, and 7 days after MCAO (all P < 0.001) (Fig. 2A and B). HE staining showed that histopathology of the cortex was normal in the sham group. In the MCAO group, most of the cells were disordered, and a large number of necrotic cells was observed. Significant histopathological improvement was observed in the MCAO + RIC group compared to that in the MCAO group (Fig. 2C).
Fig. 2.
RIC reduced infarct volume and improved histological changes in the infarcted cortex. (A–B) TTC staining and analysis of infarct volume at 1, 3, and 7 days after MCAO. (C) HE staining at 1, 3, and 7 days after MCAO. ***P < 0.001.
Western blotting results revealed that the Nrf2, HO-1, NLRP3, and Cleaved Caspase-1 levels were significantly lower in the sham group than in the MCAO group at 1, 3, and 7 days after MCAO (all P < 0.05). Nrf2 and HO-1 levels were significantly higher, and those of NLRP3 and Cleaved Caspase-1 were significantly lower in the MCAO + RIC group than in the MCAO group at 1, 3, and 7 days after MCAO (P < 0.05) (Fig. 3A–E).
Fig. 3.
RIC increased Nrf2 and HO-1 protein levels and decreased NLRP3 and Cleaved Caspase-1 protein levels. (A) Western blotting of Nrf2, HO-1, NLRP3, Cleaved Caspase-1, and β-actin at 1, 3, and 7 days after MCAO. (B–E) Analysis of Nrf2, HO-1, NLRP3, and Cleaved Caspase-1 protein levels at 1, 3, and 7 days after MCAO. *P < 0.05, **P < 0.01, ***P < 0.001.
3.2. Effects of RIC on oxidative stress and inflammation via the Nrf2/HO-1 pathway in MCAO mice
To further explore whether RIC reduced oxidative stress and inflammation via the Nrf2/HO-1 pathway, mice were administered ML385 3 days after MCAO.
Behavioral testing results revealed that neurological function was significantly poorer in the MCAO + RIC + ML385 group than in the MCAO + RIC and MCAO + RIC + Vehicle groups based on Zea Longa, Bederson, and modified Garcia scores (all P < 0.05) (Fig. 4A–C). TTC staining showed that infarct volume was significantly larger in the MCAO + RIC + ML385 group than in the MCAO + RIC and MCAO + RIC + Vehicle groups (P < 0.001) (Fig. 4D and E).
Fig. 4.
RIC improved neurological function and reduced infarct volume by attenuating oxidative stress levels. (A–C) Neurological deficit scores based on Zea Longa, Bederson, and modified Garcia scores at 3 days after MCAO. (D–E) TTC staining and analysis of infarct volume at 3 days after MCAO. (F–I) TAC, MDA, SOD, and GSH/GSSG levels at 3 days after MCAO. *P < 0.05, **P < 0.01, ***P < 0.001.
Oxidative stress levels were measured by TAC, MDA, SOD, and GSH/GSSG. TAC, SOD, and GSH/GSSG levels were significantly higher, and MDA levels were significantly lower in the MCAO + RIC group than in the MCAO group (all P < 0.05). In contrast, TAC, SOD, and GSH/GSSG levels were significantly lower, and MDA levels were significantly higher in the MCAO + RIC + ML385 group than in the MCAO + RIC and MCAO + RIC + Vehicle groups (all P < 0.01) (Fig. 4F–I).
Real-time PCR results revealed that mRNA levels of Nrf2 and HO-1 were significantly lower, and mRNA levels of Keap1, NLRP3, and Cleaved Caspase-1 were significantly higher in the MCAO + RIC + ML385 group than in the MCAO + RIC and MCAO + RIC + Vehicle groups (all P < 0.01) (Fig. 5A–E). Western blotting showed that the protein levels of Nrf2, HO-1, and NQO1 were significantly lower, and those of Keap1, NLRP3, Cleaved Caspase-1, and Cleaved IL-1β were significantly higher in the MCAO + RIC + ML385 group than in the MCAO + RIC and MCAO + RIC + Vehicle groups. (all P < 0.05) (Fig. 5F–M). These results demonstrated that the protective effects of RIC on oxidative stress, neurological function, and infarct volume were dependent on Nrf2 activation.
Fig. 5.
RIC attenuated oxidative stress and inflammatory responses via the Nrf2/HO-1 pathway. (A–E) Analysis of mRNA levels of Nrf2, Keap1, HO-1, NLRP3, and Cleaved Caspase-1 at 3 days after MCAO. (F) Western blotting of Nrf2, Keap1, HO-1, NQO1, NLRP3, Cleaved Caspase-1, Cleaved IL-1β, and β-actin at 3 days after MCAO. (G–M) Analysis of protein levels of Nrf2, Keap1, HO-1, NQO1, NLRP3, Cleaved Caspase-1, and Cleaved IL-1β at 3 days after MCAO. *P < 0.05, **P < 0.01, ***P < 0.001.
IF staining results revealed that relative to that in the MCAO group, Nrf2 was activated and translocated to the nucleus, while Keap1 expression was reduced in the MCAO + RIC group. Compared with that in the MCAO + RIC and MCAO + RIC + Vehicle groups, Nrf2 expression was significantly suppressed, and Keap1 expression was significantly increased in the MCAO + RIC + ML385 group (Fig. 6A).
Fig. 6.
Immunofluorescence staining of Nrf2 and Keap1 localization and the protein levels by ELISA. (A) The results demonstrated that Nrf2 was activated and translocated to the nucleus, and Keap1 expression was reduced in the MCAO + RIC group than in the MCAO group. Nrf2 expression was significantly suppressed, and Keap1 expression was significantly increased in the MCAO + RIC + ML385 group than in the MCAO + RIC and MCAO + RIC + Vehicle groups. (B–D) The protein levels of Cleaved IL-1β, IL-6, and TNF-α by ELISA at 3 days after MCAO. *P < 0.05, **P < 0.01, ***P < 0.001.
ELISA results revealed that the protein levels of Cleaved IL-1β, IL-6, and TNF-α were significantly lower in the MCAO + RIC group than in the MCAO group (all P < 0.05). Compared with the MCAO + RIC and MCAO + RIC + Vehicle groups, the protein levels of Cleaved IL-1β, IL-6, and TNF-α were significantly higher in the MCAO + RIC + ML385 group (all P < 0.01) (Fig. 6B–D).
4. Discussion
In the present study, we observed that RIC significantly improved neurological function and infarct volume in MCAO mice. These effects were underscored by an upregulation of the Nrf2/HO-1 pathway and a downregulation of the NLRP3/Cleaved Caspase-1 pathway. Moreover, ML385 eliminated RIC-related neurological improvements and brain infarct volume, which was associated with reduced Nrf2 nuclear translocation; upregulation of Keap1, NLRP3, Cleaved Caspase-1, Cleaved IL-1β, TNF-α, and IL-6, and downregulation of Nrf2, HO-1, and NQO1. In summary, our study demonstrated that in MCAO mice, RIC attenuated oxidative stress and inflammatory responses via the Nrf2/HO-1 pathway, which in turn improved neurological recovery. These results provide novel evidence for the potential clinical utility of RIC.
In recent years, the protective effects of RIC on cerebrovascular diseases, particularly ischemic stroke, have been reported [17,18]. However, the appropriate duration of RIC treatment remains unclear. Liang et al. [19] discovered that RIC could promote functional recovery and alleviate brain impairment. Furthermore, infarction volume was significantly lower in RIC therapy for 21 days than for 2 days in MCAO rats. This indicates that a longer duration of RIC may play a better role in reducing infarct volume. Our study identified that RIC significantly reduced infarct volume and improved neurological function and histological changes at 1, 3, and 7 days after MCAO, and the infarct size gradually decreased as the duration of RIC treatment increased. However, the specific mechanisms through which RIC exerts a neuroprotective role and reduces infarct size in acute ischemic stroke remain unclear. Xia et al. [20] suggested that RIC plays a neuroprotective role through the HIF-1α/AMPK/HSP70 pathway in MCAO rats. Another study reported that RIC significantly reduced infarcts and improved neurological outcomes by promoting neurogenesis and revascularization in MCAO rats [21]. In addition, immediate RIC treatment has been reported to provide neuroprotection by leptomeningeal collateral circulation [22]. Nevertheless, studies on whether RIC can exert neuroprotection by reducing oxidative stress after cerebral ischemia-reperfusion are scarce. Previous studies have demonstrated that inhibiting oxidative stress-related pathways can significantly reduce infarct volumes and alleviate neurological deficits 3, 7, and 14 days after MCAO [23]. Another study demonstrated that intravenous administration of oxidative stress defense factor, Nrf2 activator, reduced infarct size and improved neurological function in ischemia-reperfusion-injured mice [24]. Furthermore, the study by Zhang et al. [25] was the first to explore RIC application to retinal ischemia-reperfusion injury and identified that RIC treatment could provide retinal protection against ischemia-reperfusion injury by upregulating Nrf2 and HO-1 proteins in retinal ischemia-reperfusion rats, providing new evidence to support RIC as a non-invasive antioxidant, neuroprotective strategy. To our knowledge, the present study is the first to explore whether RIC improves neurobehavioral function by attenuating oxidative stress and inflammatory responses via the Nrf2/HO-1 signaling pathway in MCAO mice.
Extensive evidence indicates that oxidative stress and inflammatory responses are the main pathological processes that lead to impaired neurological function in acute ischemic stroke [26,27]. Concurrently, the interaction between oxidative stress and inflammatory responses creates a vicious cycle that continues to exacerbate neurological damage [16]. The brain is the most susceptible organ to oxidative stress. Excessive production of oxygen-free radicals can lead to severe neurological deficits in acute ischemic stroke [23]. Decreased TAC and antioxidants, such as SOD and GSH, and increased MDA and GSSG can directly reflect the degree of oxidative stress after ischemic stroke [28]. In this study, we observed that RIC significantly increased TAC, SOD, and GSH/GSSG levels and reduced MDA levels in the brain tissue of MCAO mice; however, these effects were inhibited by treatment with ML385. Therefore, we speculate that RIC exerts its neuroprotective effects by attenuating oxidative stress related to Nrf2.
Nrf2 plays a crucial role in regulating the expression of oxidative stress and inflammatory response genes [29]. Under normal physiological conditions, the binding of Nrf2 and its specific inhibitor Keap1 in the cytoplasm [30] promotes ubiquitination and degradation of Nrf2 [31]. When exposed to stress, Nrf2 dissociates from Keap1. As Nrf2 evades keap1-mediated protein hydrolysis, free Nrf2 transfers from the cytoplasm to the nucleus [32,33] and binds to the antioxidant response element (ARE) located in its promoter [34], which subsequently activates transcription of antioxidant defense genes, including HO-1 and NQO1 [35]. Overall, Nrf2 protects against damage caused by oxidative stress by activating the expression of antioxidant genes, exerting important neuroprotective effects in the pathogenesis of ischemic stroke [36]. In addition, the Nrf2/HO-1 pathway plays an anti-inflammatory role by inhibiting the expression of the inflammatory proteins NLRP3, Cleaved Caspase-1, Cleaved IL-1β, TNF-α, and IL-6 [37,38]. Consistent with the current findings, we observed that Nrf2, HO-1, NLRP3, and Cleaved Caspase-1 protein expression levels increased after ischemia-reperfusion injury. RIC treatment significantly upregulated the levels of Nrf2, HO-1, and NQO1, downregulated the expression of Keap1, NLRP3, Cleaved Caspase-1, Cleaved IL-1β, TNF-α, and IL-6, and promoted the translocation of Nrf2 to the nucleus. In addition, ML385, an Nrf2 antagonist, binds to the Neh1 DNA-binding domain of Nrf2 and affects the DNA binding activity of the Nrf2-MAFG protein complex, resulting in reduced transcriptional activity [39]. Because of mutual inhibition of Nrf2 and Keap1 [3], the relative lack of Nrf2 allows for the relative upregulation of Keap1 [32]. Keap1 acts as a negative regulator of Nrf2 [40], and overexpression of Keap1 further inhibits nuclear translocation of Nrf2 [41,42]. ML385 treatment attenuated RIC-related neuroprotective effects, indicating that these effects were dependent on Nrf2.
This study had several limitations. First, the pathways affecting oxidative stress are complex, and we only focused on the Nrf2/HO-1 pathway. Accordingly, the mechanisms underscoring the effects of RIC on oxidative stress warrant further examination. Second, due to the difficulty of implementing RIC treatment in cellular experiments, the inhibitory response of cells to the Nrf2/HO-1 pathway remains unclear. Third, it is unknown whether RIC has anti-oxidative stress effects in clinical acute ischemic stroke patients. Our team is conducting two clinical trials to explore the safety and efficacy of RIC combined with endovascular thrombectomy (SERIC-EVT, NCT04977869) and intravenous thrombolysis (SERIC-IVT, NCT04980625) for acute ischemic stroke. These clinical trials are likely to contribute to the future promotion of RIC clinical application. Fourth, because estrogens may affect the treatment and prognosis of neurological diseases, only male mice were used in this study to eliminate the effect of estrogens on experimental results.
5. Conclusions
RIC alleviates oxidative stress and inflammatory responses through the Nrf2/HO-1 pathway, which in turn improves neurobehavioral function. RIC therapy is a potential non-invasive treatment approach, and our study provides more evidence that RIC can be used as an adjunct treatment for acute ischemic stroke from an antioxidant and anti-inflammatory perspective.
Funding statement
This project was supported by the National Natural Science Foundation of China (Grant No. 82071291), the Norman Bethune Health Science Center of Jilin University (2022JBGS03), Science and Technology Department of Jilin Province (YDZJ202302CXJD061, 20220303002SF), and Jilin Provincial Key Laboratory (YDZJ202302CXJD017) to YY; and the Science and Technology Department of Jilin Province (YDZJ202201ZYTS677), and Talent Reserve Program of the First Hospital of Jilin University (JDYYCB-2023002) to ZNG.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Yi Yang, Email: yang_yi@jlu.edu.cn.
Zhen-Ni Guo, Email: zhen1ni2@jlu.edu.cn.
Data availability
Data will be made available on request.
References
- 1.Collaborators G.B.D.S. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021;20(10):795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen H., He Y., Chen S., Qi S., Shen J. Therapeutic targets of oxidative/nitrosative stress and neuroinflammation in ischemic stroke: applications for natural product efficacy with omics and systemic biology. Pharmacol. Res. 2020;158 doi: 10.1016/j.phrs.2020.104877. [DOI] [PubMed] [Google Scholar]
- 3.Kopacz A., Kloska D., Forman H.J., Jozkowicz A., Grochot-Przeczek A. Beyond repression of Nrf2: an update on Keap1. Free Radic. Biol. Med. 2020;157:63–74. doi: 10.1016/j.freeradbiomed.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto M., Kensler T.W., Motohashi H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018;98(3):1169–1203. doi: 10.1152/physrev.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016;73(17):3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H.S., Cui Y., Li X.Q., Wang X.H., Ma Y.T., Zhao Y., Han J., Deng C.Q., Hong M., Bao Y., Zhao L.H., Yan T.G., Zou R.L., Wang H., Li Z., Wan L.S., Zhang L., Wang L.Q., Guo L.Y., Li M.N., Wang D.Q., Zhang Q., Chang D.W., Zhang H.L., Sun J., Meng C., Zhang Z.H., Shen L.Y., Ma L., Wang G.C., Li R.H., Zhang L., Bi C., Wang L.Y., Wang D.L., Investigators R. Effect of remote ischemic conditioning vs usual care on neurologic function in patients with acute moderate ischemic stroke: the RICAMIS randomized clinical trial. JAMA. 2022;328(7):627–636. doi: 10.1001/jama.2022.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.An J.Q., Cheng Y.W., Guo Y.C., Wei M., Gong M.J., Tang Y.L., Yuan X.Y., Song W.F., Mu C.Y., Zhang A.F., Saguner A.M., Li G.L., Luo G.G. Safety and efficacy of remote ischemic postconditioning after thrombolysis in patients with stroke. Neurology. 2020;95(24):e3355–e3363. doi: 10.1212/WNL.0000000000010884. [DOI] [PubMed] [Google Scholar]
- 8.McDonald M.W., Dykes A., Jeffers M.S., Carter A., Nevins R., Ripley A., Silasi G., Corbett D. Remote ischemic conditioning and stroke recovery. Neurorehabilitation Neural. Repair. 2021;35(6):545–549. doi: 10.1177/15459683211011224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L., Ren C., Li Y., Gao C., Li N., Li H., Wu D., He X., Xia C., Ji X. Remote ischemic conditioning enhances oxygen supply to ischemic brain tissue in a mouse model of stroke: role of elevated 2,3-biphosphoglycerate in erythrocytes. J. Cerebr. Blood Flow Metabol. 2021;41(6):1277–1290. doi: 10.1177/0271678X20952264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu X., Li P., Guo Y., Wang H., Leak R.K., Chen S., Gao Y., Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43(11):3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- 11.Longa E.Z., Weinstein P.R., Carlson S., Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 12.Bieber M., Gronewold J., Scharf A.C., Schuhmann M.K., Langhauser F., Hopp S., Mencl S., Geuss E., Leinweber J., Guthmann J., Doeppner T.R., Kleinschnitz C., Stoll G., Kraft P., Hermann D.M. Validity and reliability of neurological scores in mice exposed to middle cerebral artery occlusion. Stroke. 2019;50(10):2875–2882. doi: 10.1161/STROKEAHA.119.026652. [DOI] [PubMed] [Google Scholar]
- 13.Desland F.A., Afzal A., Warraich Z., Mocco J. Manual versus automated rodent behavioral assessment: comparing efficacy and ease of Bederson and Garcia neurological deficit scores to an open field video-tracking system. J. Cent. Nerv. Syst. Dis. 2014;6:7–14. doi: 10.4137/JCNSD.S13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui H.Y., Zhang X.J., Yang Y., Zhang C., Zhu C.H., Miao J.Y., Chen R. Rosmarinic acid elicits neuroprotection in ischemic stroke via Nrf2 and heme oxygenase 1 signaling. Neural. Regen. Res. 2018;13(12):2119–2128. doi: 10.4103/1673-5374.241463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ran Y., Su W., Gao F., Ding Z., Yang S., Ye L., Chen X., Tian G., Xi J., Liu Z. Curcumin ameliorates white matter injury after ischemic stroke by inhibiting microglia/macrophage pyroptosis through NF-kappaB suppression and NLRP3 inflammasome inhibition. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/1552127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao S., Wu J., Liu R., Wang S., Luo J., Yang Y., Qin Y., Li T., Zheng X., Song J., Zhao X., Xiao C., Zhang Y., Bian L., Jia P., Bai Y., Zheng X. A novel compound DBZ ameliorates neuroinflammation in LPS-stimulated microglia and ischemic stroke rats: role of Akt(Ser473)/GSK3beta(Ser9)-mediated Nrf2 activation. Redox Biol. 2020;36 doi: 10.1016/j.redox.2020.101644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao W., Ren C., Ji X. Remote ischemic perconditioning for the treatment of acute ischemic stroke. JAMA Neurol. 2020;77(11):1451–1452. doi: 10.1001/jamaneurol.2020.3555. [DOI] [PubMed] [Google Scholar]
- 18.Hahn C.D., Manlhiot C., Schmidt M.R., Nielsen T.T., Redington A.N. Remote ischemic per-conditioning: a novel therapy for acute stroke? Stroke. 2011;42(10):2960–2962. doi: 10.1161/STROKEAHA.111.622340. [DOI] [PubMed] [Google Scholar]
- 19.Liang D., He X.B., Wang Z., Li C., Gao B.Y., Wu J.F., Bai Y.L. Remote limb ischemic postconditioning promotes motor function recovery in a rat model of ischemic stroke via the up-regulation of endogenous tissue kallikrein. CNS Neurosci. Ther. 2018;24(6):519–527. doi: 10.1111/cns.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia M., Ding Q., Zhang Z., Feng Q. Remote limb ischemic preconditioning protects rats against cerebral ischemia via HIF-1alpha/AMPK/HSP70 pathway. Cell. Mol. Neurobiol. 2017;37(6):1105–1114. doi: 10.1007/s10571-016-0444-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esposito E., Hayakawa K., Maki T., Arai K., Lo E.H. Effects of postconditioning on neurogenesis and angiogenesis during the recovery phase after focal cerebral ischemia. Stroke. 2015;46(9):2691–2694. doi: 10.1161/STROKEAHA.115.009070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y., Ma L., Ren C., Liu K., Tian X., Wu D., Ding Y., Li J., Borlongan C.V., Ji X. Immediate remote ischemic postconditioning reduces cerebral damage in ischemic stroke mice by enhancing leptomeningeal collateral circulation. J. Cell. Physiol. 2019;234(8):12637–12645. doi: 10.1002/jcp.27858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du J., Yin G., Hu Y., Shi S., Jiang J., Song X., Zhang Z., Wei Z., Tang C., Lyu H. Coicis semen protects against focal cerebral ischemia-reperfusion injury by inhibiting oxidative stress and promoting angiogenesis via the TGFbeta/ALK1/Smad1/5 signaling pathway. Aging (Albany NY) 2020;13(1):877–893. doi: 10.18632/aging.202194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takagi T., Kitashoji A., Iwawaki T., Tsuruma K., Shimazawa M., Yoshimura S., Iwama T., Hara H. Temporal activation of Nrf2 in the penumbra and Nrf2 activator-mediated neuroprotection in ischemia-reperfusion injury. Free Radic. Biol. Med. 2014;72:124–133. doi: 10.1016/j.freeradbiomed.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X., Jizhang Y., Xu X., Kwiecien T.D., Li N., Zhang Y., Ji X., Ren C., Ding Y. Protective effects of remote ischemic conditioning against ischemia/reperfusion-induced retinal injury in rats. Vis. Neurosci. 2014;31(3):245–252. doi: 10.1017/S0952523814000121. [DOI] [PubMed] [Google Scholar]
- 26.Chamorro A., Dirnagl U., Urra X., Planas A.M. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15(8):869–881. doi: 10.1016/S1474-4422(16)00114-9. [DOI] [PubMed] [Google Scholar]
- 27.Russo E., Napoli E., Borlongan C.V. Healthy mitochondria for stroke cells. Brain Circ. 2018;4(3):95–98. doi: 10.4103/bc.bc_20_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu D., Wang H., Zhang Y., Zhang Z. Protective effects of chlorogenic acid on cerebral ischemia/reperfusion injury rats by regulating oxidative stress-related Nrf2 pathway. Drug Des. Dev. Ther. 2020;14:51–60. doi: 10.2147/DDDT.S228751. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Nishikawa S., Inoue Y., Hori Y., Miyajima C., Morishita D., Ohoka N., Hida S., Makino T., Hayashi H. Anti-inflammatory activity of kurarinone involves induction of HO-1 via the KEAP1/Nrf2 pathway. Antioxidants. 2020;9(9) doi: 10.3390/antiox9090842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xian P., Hei Y., Wang R., Wang T., Yang J., Li J., Di Z., Liu Z., Baskys A., Liu W., Wu S., Long Q. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics. 2019;9(20):5956–5975. doi: 10.7150/thno.33872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki T., Yamamoto M. Molecular basis of the Keap1-Nrf2 system. Free Radic. Biol. Med. 2015;88(Pt B):93–100. doi: 10.1016/j.freeradbiomed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Zhang T., Wu P., Budbazar E., Zhu Q., Sun C., Mo J., Peng J., Gospodarev V., Tang J., Shi H., Zhang J.H. Mitophagy reduces oxidative stress via Keap1 (Kelch-Like epichlorohydrin-associated protein 1)/Nrf2 (nuclear factor-E2-related factor 2)/PHB2 (prohibitin 2) pathway after subarachnoid hemorrhage in rats. Stroke. 2019;50(4):978–988. doi: 10.1161/STROKEAHA.118.021590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu P., Jiang L., Yang Y., Wu M., Liu B., Shi Y., Shen Q., Jiang X., He Y., Cheng D., Xiong Q., Yang Z., Duan L., Lin J., Zhao S., Shi P., Yang C., Chen Y. PAQR4 promotes chemoresistance in non-small cell lung cancer through inhibiting Nrf2 protein degradation. Theranostics. 2020;10(8):3767–3778. doi: 10.7150/thno.43142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kopacz A., Kloska D., Proniewski B., Cysewski D., Personnic N., Piechota-Polanczyk A., Kaczara P., Zakrzewska A., Forman H.J., Dulak J., Jozkowicz A., Grochot-Przeczek A. Keap1 controls protein S-nitrosation and apoptosis-senescence switch in endothelial cells. Redox Biol. 2020;28 doi: 10.1016/j.redox.2019.101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu C.T., Deng J.S., Huang W.C., Shieh P.C., Chung M.I., Huang G.J. Salvianolic acid C against acetaminophen-induced acute liver injury by attenuating inflammation, oxidative stress, and apoptosis through inhibition of the Keap1/Nrf2/HO-1 signaling. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/9056845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L., Zhang X., Xiong X., Zhu H., Chen R., Zhang S., Chen G., Jian Z. Nrf2 regulates oxidative stress and its role in cerebral ischemic stroke. Antioxidants. 2022;11(12) doi: 10.3390/antiox11122377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Z., Zhong H., Wei J., Lin S., Zong Z., Gong F., Huang X., Sun J., Li P., Lin H., Wei B., Chu J. Inhibition of Nrf2/HO-1 signaling leads to increased activation of the NLRP3 inflammasome in osteoarthritis. Arthritis Res. Ther. 2019;21(1):300. doi: 10.1186/s13075-019-2085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu J., Wang W.N., Matei N., Li X., Pang J.W., Mo J., Chen S.P., Tang J.P., Yan M., Zhang J.H. Ezetimibe attenuates oxidative stress and neuroinflammation via the AMPK/Nrf2/TXNIP pathway after MCAO in rats. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/4717258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh A., Venkannagari S., Oh K.H., Zhang Y.Q., Rohde J.M., Liu L., Nimmagadda S., Sudini K., Brimacombe K.R., Gajghate S., Ma J., Wang A., Xu X., Shahane S.A., Xia M., Woo J., Mensah G.A., Wang Z., Ferrer M., Gabrielson E., Li Z., Rastinejad F., Shen M., Boxer M.B., Biswal S. Small molecule inhibitor of NRF2 selectively intervenes therapeutic resistance in KEAP1-deficient NSCLC tumors. ACS Chem. Biol. 2016;11(11):3214–3225. doi: 10.1021/acschembio.6b00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang S., Duan S., Xie Z., Bao W., Xu B., Yang W., Zhou L. Epigenetic therapeutics targeting NRF2/KEAP1 signaling in cancer oxidative stress. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.924817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao X.J., Yu H.W., Yang Y.Z., Wu W.Y., Chen T.Y., Jia K.K., Kang L.L., Jiao R.Q., Kong L.D. Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biol. 2018;18:124–137. doi: 10.1016/j.redox.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu M., Liu S., Zhang Q., Fang Y., Yu Y., Zhu L., Liu Y., Gong W., Zhao L., Qin L., Zhang Q. Curculigoside attenuates oxidative stress and osteoclastogenesis via modulating Nrf2/NF-kappaB signaling pathway in RAW264.7 cells. J. Ethnopharmacol. 2021;275 doi: 10.1016/j.jep.2021.114129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.