Abstract
Cetirizine hydrochloride (CTZ), a second-generation anti-histaminic drug, has been recently explored for its effectiveness in the treatment of alopecia. Niosomes are surfactant-based nanovesicular systems that have promising applications in both topical and transdermal drug delivery. The aim of this study was to design topical CTZ niosomes for management of alopecia. Thin film hydration technique was implemented for the fabrication of CTZ niosomes. The niosomes were examined for vesicle size, surface charge, and entrapment efficiency. The optimized niosomal formulation was incorporated into a hydrogel base (HPMC) and explored for physical characteristics, ex vivo permeation, and in vivo dermato-kinetic study. The optimized CTZ-loaded niosomal formulation showed an average size of 403.4 ± 15.6 nm, zeta potential of − 12.9 ± 1.7 mV, and entrapment efficiency percentage of 52.8 ± 1.9%. Compared to plain drug solution, entrapment of CTZ within niosomes significantly prolonged in vitro drug release up to 12 h. Most importantly, ex-vivo skin deposition studies and in vivo dermato-kinetic studies verified superior skin deposition/retention of CTZ from CTZ-loaded niosomal gels, compared to plain CTZ gel. CTZ-loaded niosomal gel permitted higher drug deposition percentage (19.2 ± 1.9%) and skin retention (AUC0-10h 1124.5 ± 87.9 μg/mL.h) of CTZ, compared to 7.52 ± 0.7% and 646.2 ± 44.6 μg/mL.h for plain CTZ gel, respectively. Collectively, niosomes might represent a promising carrier for the cutaneous delivery of cetirizine for the topical management of alopecia.
Keywords: Androgenic alopecia, Cetirizine, Niosomes, Span 60, Thin film hydration method
1. Introduction
Androgenic Alopecia, also known as pattern hair loss, is a genetically predetermined disorder that constitutes the most prevalent cause of male hair loss (Lolli et al., 2017). It is distinguished by a progressive non-scarring miniaturization of hair follicles, eventually leading to baldness (Kelly et al., 2016). Even though androgenic alopecia is a common condition, approved treatment options are limited. The current treatment options focus mainly on reducing dihydrotestosterone and activating hair follicles via using 5-alpha reductase inhibitors such as finasteride or vasodilators such as minoxidil, respectively (Adil and Godwin, 2017, Suchonwanit et al., 2019)). Nevertheless, despite their widespread application, their use is linked with the elicitation of undesirable adverse effects; finasteride use has been associated with the development of sexual side effects, whilst minoxidil application has been associated with the development of headache, hypotension, and irritant contact dermatitis (Albash et al., 2022).
Cetirizine (CTZ) is a second-generation histamine (H1) antagonist used to treat urticaria, dermatitis, and allergic rhinitis (Zhou et al., 2022). It acts by inhibiting the release of inflammatory mediators such as histamine and bradykinin, associated with allergies (Goindi et al., 2013). In addition, CTZ has been reported to alleviate prostaglandin D2 (PGD2) production (Rossi et al., 2018). Prostaglandins has been reported to participate in regulating hair growth cycle. Prostaglandins E and F2α stimulate hair follicles, while prostaglandin D inhibits hair follicles (Garza et al., 2012). Cetirizine is reported to suppress the production of prostaglandin D2 while boosting prostaglandin E2 production. Consequently, CTZ has been recently screened for its efficiency for the management of androgenic alopecia. A pilot study on topical cetirizine revealed the efficacy of CTZ to boost hair density, compared to minoxidil, a commonly used drug for the treatment of androgenic alopecia. Most importantly, CTZ showed less possible side effects than minoxidil (Rossi et al., 2018). In another recent randomized, single-blind controlled study, Mostafa et al. (Hossein Mostafa et al., 2021) have verified the efficacy of CTZ topical solution for promoting hair growth without eliciting any adverse effects. These studies support the rational use of CTZ in the treatment of androgenic alopecia (Rossi et al., 2018, Hossein Mostafa et al., 2021).
The application of nanotechnology has emerged as a potential mean to augment the pharmacological effects of many drug molecules and/or alleviate their associated adverse effects. Vesicular systems like niosomes and liposomes play a major role in drug delivery as they can alter drug pharmacokinetics and bioavailability along with alleviating entrapped drug toxicity (Aldawsari et al., 2022, Khafagy et al., 2023). In particular, non-ionic surfactant vesicles, known as niosomes, have gained increased interest as a viable alternative drug delivery system to traditional liposomes since they provide superior physical and chemical stability (Vora et al., 1998) along with lower cost and easier production and scalability (Ge et al., 2019). Furthermore, niosomes have been shown in multiple studies to have higher cutaneous drug bioavailability than traditional dosage forms (Hamishehkar et al., 2013). Topically applied niosomes have been stated to augment the penetration of encapsulated drug across the skin, increase drug residence duration in the stratum corneum and epidermis (Ghasemiyeh and Mohammadi-Samani 2020) while minimizing systemic absorption (Shatalebi et al., 2010). Furthermore, niosomes are claimed to improve horny layer properties by decreasing trans-epidermal water loss as well as boosting smoothness by replacing lost skin lipids (Junginger et al., 1991).
This study aimed at developing and optimizing a novel topical delivery system of CTZ using niosomes to promote targeted therapy with enhanced skin bioavailability. CTZ-loaded niosomes were prepared by thin film hydration technique. The optimized formulation was characterized and then incorporated in a suitable hydrogel base. Finally, the effect of CTZ-loaded niosomal gel on drug penetration in hairless rat skin were examined.
2. Materials and methods
2.1. Materials
Cetirizine hydrochloride (CTZ) was a generous gift from Global Napi Pharmaceutical Company (Cairo, Egypt). Cholesterol, Brij 35, Span and Tween were procured from Sigma-Aldrich (St. Louis, MO, USA). Other reagents and chemicals were of analytical grade.
2.2. Preparation of cetirizine-loaded niosomes (CTZ niosomes)
Definite weights of the nonionic surfactant (Span™, Tween™ or Brij™) and cholesterol, at different molar ratios (1:2, 1:1, and 2:1), were dissolved in 20 mL chloroform in a rounded bottom flask. The flask was then continuously vortexed on water bath at 60 ˚C until complete evaporation of the organic solvent to obtain a thin lipid film. The lipid film was hydrated by 20 mL aqueous drug solution at 60 ˚C (Pardakhty et al., 2007). Finally, the niosomal dispersion was sonicated in 3 cycles of 1 min at 60 ˚C to get drug-loaded vesicles. The detailed composition of different niosomal dispersions is summarized in Table S1.
2.3. Experimental design
Three factor, three level Box-Behnken design (BBD) was adopted for the preparation and optimization of CTZ-loaded niosomes in order to attain a desired particle size and high encapsulation efficiency % (Table 1). Three independent formulation parameters were examined: CTZ concentration (A), CHOL:Surfactant ratio (B), and the total weight of niosomal components (C). Every parameter was examined at three levels; low, medium, and high levels, as displayed in Table 1. The dependent response were vesicle size (R1) and encapsulation efficiency percent (R2). Table 2 summarizes the composition of various CTZ niosomal formulations. Desirability was determined in order to pick the optimum formula for further studies.
Table 1.
Box-Behnken design for CTZ-loaded niosomes depiting independent variables and their level of variation.
| Independent variables |
Levels |
||
|---|---|---|---|
| Low (-1) | Medium (0) | High (+1) | |
| A: CTZ concentration (mg/mL) | 5 | 10 | 15 |
| B: Chol: Surfactant ratio | 1:2 | 1:1 | 2:1 |
| C: Total weight of niosomal components (mg) | 150 | 225 | 300 |
| Responses | Constrains | ||
| R1: Vesicle size (nm) | In range | ||
| R2: Encapsulation efficiency (%) | Maximize | ||
Table 2.
Composition of the formulated CTZ-loaded niosomes and the obtained responses.
|
Formulation parameters |
Responses |
||||
|---|---|---|---|---|---|
| A: CTZ conc. (mg) | B: CHOL:Surfactant ratio | C: Total Wt. of components (mg) | R1: Vesicle size (nm) | R2: Entrapment efficiency (%) | |
| F1 | 10 | 2:1 | 300 | 316 ± 21.9 | 36.9 ± 2.1 |
| F2 | 15 | 1:2 | 225 | 168 ± 11.8 | 27.9 ± 0.9 |
| F3 | 5 | 1:1 | 300 | 405 ± 21.3 | 46.1 ± 1.9 |
| F4 | 15 | 2:1 | 225 | 192 ± 13.5 | 29.2 ± 1.9 |
| F5 | 10 | 1:1 | 225 | 379 ± 26.7 | 51.9 ± 3.1 |
| F6 | 10 | 1:1 | 225 | 388 ± 24.2 | 50.6 ± 2.9 |
| F7 | 10 | 1:2 | 300 | 276 ± 19.7 | 33.3 ± 2.1 |
| F8 | 15 | 1:1 | 300 | 438 ± 21.6 | 48.6 ± 1.6 |
| F9 | 5 | 2:1 | 225 | 188 ± 13.6 | 27.7 ± 1.9 |
| F10 | 10 | 2:1 | 150 | 219 ± 18.6 | 30.1 ± 2.2 |
| F11 | 5 | 1:1 | 150 | 332 ± 19.4 | 37.8 ± 2.4 |
| F12 | 5 | 1:2 | 225 | 137 ± 11.2 | 23.5 ± 1.1 |
| F13 | 10 | 1:1 | 225 | 382 ± 26.3 | 51.4 ± 3.4 |
| F14 | 15 | 1:1 | 150 | 374 ± 21.8 | 38.7 ± 2.8 |
| F15 | 10 | 1:2 | 150 | 153 ± 11.7 | 24.5 ± 1.8 |
2.4. Characterization of CTZ-loaded niosomes
2.4.1. Particle size, polydispersity index (PDI) and zeta potential determination
Vesicle properties such as vesicle size, PDI and zeta potential were assessed at room temperature by light scattering technique using Nano-ZS 90 Zetasizer (Malvern instruments Ltd, UK). Test samples were diluted 1:500 v/v with deionized water before the measurement.
2.4.2. Surface morphology
Surface morphology of CTZ-loaded niosomes was studied using a transmission electron microscope (TEM; Tecnai, Eindhoven, Netherlands). Test sample was diluted with deionized water, placed on a carbon-coated copper grid, and allowed to air dry. The niosomal sample was then examined microscopically at room temperature and a 50 kV acceleration voltage.
2.4.3. Entrapment efficiency (EE) determination
Entrapment efficiency of CTZ within niosomal vesicle was estimated using ultracentrifugation technique. In brief, CTZ niosomes were subjected to rigorous centrifugation at 17,000 rpm for 90 min at 4 ˚C to separate the un-entrapped drug. The drug concentration in the supernatant was quantified at λmax 231 nm using a UV–Vis spectrophotometer (Shimadzu, Tokyo, Japan). The amount of entrapped drug is obtained by subtracting the free amount from the total drug amount, and the entrapment efficiency (%) was obtained from the following formula (Khafagy et al., 2023):
2.4.4. Differential scanning calorimetry (DSC)
A differential scanning calorimeter (Shimadzu DSC-50, Tokyo, Japan) was utilized to scan the thermal characteristics of CTZ, Chol, Span 60, and optimized CTZ niosomes. Briefly, in an aluminium pan, an adequate weight (2 mg) of each sample was filled and then heated at a rate of 10 ˚C/min from 10 to 300 ˚C under a nitrogen purge (Moin et al., 2021).
2.5. In vitro drug release study
In vitro release of CTZ from CTZ niosomes was examined by bag diffusion method. In brief, cellulose dialysis tubing (MW cut-off 12–14 K Da), pre-soaked in release medium, containing a definite volume of CTZ niosomal dispersion (equivalent to 5 mg CTZ) was suspended in 100 mL phosphate-buffered saline (pH 7.4) as a release medium. The release medium was maintained at 37 ± 1 ˚C and stirred at 100 rpm for 12 h. After certain time intervals (0.5, 1, 2, 4, 8, and 12 h), 2 mL samples were withdrawn from the release medium and replaced with same volume of fresh release media. The concentration of CTZ was finally quantified spectrophotometrically at λmax 231 nm.
2.6. Physical stability of CTZ niosomes
Physical stability tests were performed to assess vesicle size and drug leaking from optimized niosomal formulation during storage. The niosomal dispersion was placed into glass vials and kept in a refrigerator at 4 °C for 90 days. The change in vesicle size, zeta potential and the drug leakage were assessed after 30, and 90 days of storage (Khafagy et al., 2023).
2.7. Formulation of CTZ niosomal gel
The gel base was prepared by dispersing a definite amount of hydroxypropyl methylcellulose (HPMC) in 10 mL distilled water with constant stirring at 1000 rpm until a homogenous system was attained (Khafagy et al., 2023). CTZ-loaded niosomes were prepared as previously stated, and the unencapsulated free drug was removed by ultracentrifugation. The concentrated sediment was then dispersed in the gel base, resulting in a final formulation with 10 mg of CTZ per 1 g of gel base. CTZ-loaded gel was similarly prepared with replacing niosomal dispersion by pure drug.
2.8. Characterization of CTZ-loaded niosomal gel
The prepared niosomal gel formulations were visually inspected for appearance. The presence of aggregates and agglomerates were examined by touch (Rajitha et al., 2014). The pH of the gel was recorded using a digital pH meter by inserting the glass electrode into the gel system. The viscosity of the gel was estimated by Brookfield viscometer model-LV using the spindle T-C with a rotational speed of 5 rpm at 20 ± 1˚C. Spreadability of the gel was determined using slide over slide method (Abdallah et al., 2021). The procedure involves spreading of a known quantity of gel formulation (0.5 g) as a thin layer on a plain glass slide. Another glass slide was placed over the gel preparation horizontally and it is tied with a pan to add the weight. A definite weight was added onto the pan and the time taken for the slide to slip off from the first slide was noted. The Spreadability coefficient was estimated using the equation:
| S = (M × L)/t |
Where, M is the weight in g added on slide, L is the length in cm, and t is the time in sec.
2.9. Ex vitro skin permeability and deposition studies
Franz diffusion cell with an effective diffusion area of 2.2 cm2 was utilized to study the ex vivo permeation of CTZ from different CTZ formulations through abdominal rat skin. The receptor compartment contained 100 mL phosphate buffer saline (pH 5.5) as diffusion medium, equilibrated at 37 ± 0.5 ˚C. A definite weight (0.5 g) of CTZ loaded niosomal gel of CTZ gel (equivalent to 5 mg CTZ) was fed into the donor compartment. At scheduled time points, 2 mL samples were withdrawn, and drug concentration in each sample was then quantified. The cumulative amount of drug that permeated the skin per unit area was plotted versus time (h). The steady-state flux (Jss, μg/cm2.h) was estimated from the slope of the linear regression line (Abdallah et al., 2021a, Abdallah et al., 2021b, Abdallah et al., 2021c), the maximum average drug flux (Jmax) was calculated according to the following equations (Abdallah et al., 2021a, Abdallah et al., 2021b, Abdallah et al., 2021c):
At the end of permeation experiment, the skin samples were collected, rinsed with fresh receptor fluid to eliminate excess drug from the surface, and were then homogenized and centrifuged at 8000 rpm for 20 min using methanol to extract the drug retained in the skin. The amount of drug retained in the skin was determined by HPLC method. All HPLC experiment was conducted using Shimadzu HPLC system (Tokyo, Japan). A mobile phase of 60:40 orthophosphoric acid (1%; pH 3.0): acetonitrile is used at a flow rate of 1.0 mL/min. Detection was conducted by a UV visible detector at 232 nm. The injection volume was 20 μL.
2.10. In vivo study
2.10.1. Animals
Male albino rats (150–200 g) were housed under standard conditions of temperature and humidity. The study protocol was approved by Ethical Committee, Prince Sattam Bin Abdulaziz University, Al-Kharj, KSA (approval number: 048/2022).
2.10.2. In vivo dermato-kinetic study
Male albino rats were divided into two groups (n = 15 per group). Group I was treated topically with 1% CTZ gel, whereas the second group was treated with 1% CTZ niosomal gel. The dose of CTZ was equivalent to 10 mg/kg (Morsy et al., 2022). At scheduled time intervals (1, 2, 4, 6, and 10 h) post-treatment, three rats from each group were euthanized at various time intervals and the skin was removed. The excised skin was chopped into pieces and sonicated for 30 min in 5 mL methanol. After filtering the extract via a 0.45 μm membrane filter, drug concentration was quantified by HPLC method. Dermato-kinetic parameters such as Cmax, Tmax and AUC0-10h were estimated using a PKSolver 2.0 software.
3. Results
3.1. Experimental design and optimization
The impact of independent formulation parameters (CTZ concentration (A), CHOL:Surfactant ratio (B), and the total weight of niosomal components (C)) on formulation attributes (vesicle size (R1) and percentage encapsulation efficiency (R2)) was investigated utilizing Box-Behnken design. A second-order polynomial equation with quadratic responses for dependent response was developed to address the impact of various formulation variables on tested formulation attributes. The significant difference among independent factors was explored using analysis of variance (ANOVA), and the interaction impact of such variables on formulation responses (R1, and R2) was represented by contour plots and three-dimensional response surface plots.
3.1.1. Influence of formulation attributes on niosomal vesicle size
Vesicle size significantly contributes to skin permeation/retention behavior of drug-loaded nanovesicles. Herein, the vesicle size of all formulations ranged from 137 ± 11.2 nm to 438 ± 21.6 nm (Table 2). The impact of various formulation variables; CTZ concentration (A), CHOL:Surfactant ratio (B), and the total weight of niosomal components (C), on niosomal vesicle size (R1) is depicted graphically by the contour and 3D response surface plot (Fig. 1). In addition, the significance and impact of independent formulation parameters (A, B and C) on vesicle size of CTZ niosomes were examined by ANOVA analysis (Table S2), and a BBD-derived mathematical equation illustrated the impact of different formulation attributes on the detected response:
| Vesicle size (R1) = 383.3 + 13.75 A + 22.62B + 44.63C − 6.75 AB −2.25 AC − 6.50 BC − 32.75 A2 − 179.00 B2 + 37.0 C2 |
Fig. 1.
Impact of independent variables on vesicle size (R1). (A) Contour plots of R1 and (B) 3D surface plots for R1.
From the studied independent variables, CTZ concentration exerted no impact on vesicle size (p > 0.05). On the other hand, both CHOL:Surfactant ratio (B) and total weight of niosomal components (C) exerted significant effects on niosomal size (p < 0.05). ANOVA analysis revealed the synergistic effect of CHOL:Surfactant ratio on niosomal vesicle size (p = 0.009). At fixed total weight of niosomal components, increasing CHOL:Surfactant ratio from 1:2 to 1:1 triggered a significant increase in the vesicle size, however, further increase in CHOL:Surfactant ratio was found to result in a remarkable drop in niosomal vesicle size. Vesicle size of niosomal formulation prepared at CHOL:Surfactant ratio of 1:1 (F6; 388 ± 24.2 nm) was significantly larger than those prepared at either CHOL:Surfactant ratio of 1:2 (F2; 168 ± 11.8 nm) or CHOL:Surfactant ratio of 2:1 (F9; 188 ± 13.6 nm). At low CHOL:Surfactant ratio (1:2), the concentration of span 60 is comparatively high, which might favor the formation of micellar structure, with smaller size, rather than niosomal vesicles (Qumbar et al., 2017, Sharma and Harikumar, 2020). However, upon increasing CHOL:Surfactant to 2:1, the remarkable increase in cholesterol concentration could increase the bilayer hydrophobicity, leading to a significant drop in the surface free energy, and thereby, triggers a significant decrease in vesicle size (Agarwal et al., 2004).
Regarding the impact of niosomal components total weight (C) on the vesicle size (R1), it was evident that increasing niosomal components total weight (C) was accompanied with a mutual increase in niosomal vesicle size. At fixed CHOL:Surfactant ratio, upon increasing niosomal components weight from 150 mg (F10) to 300 mg (F1), vesicles size increased from 219 ± 18.6 nm to 316 ± 21.9 nm, respectively. This effect might be ascribed to the existence of a considerable amount of film forming components relative to the hydration medium. This might trigger the formation of multilamellar and/or multivesicular vesicles upon hydration, resulting in a considerable increase in niosomal vesicle size (Wang and He 2009).
3.1.2. Influence of formulation attributes on entrapment efficiency percentage (% EE)
The potential of niosomes to encapsulate a considerable amount of CTZ is critical for its topical application in managing androgenic alopecia. Table 2 displays the encapsulation efficiency value of various niosomal formulations. Fig. 2 depicts the response surface plots for the impact of drug concentration (A), CHOL:Surfactant ratio (B), and niosomal components total weight (C) on encapsulation efficiency percent (R2). ANOVA analysis revealed that the quadratic model was significant and best fitted for the obtained data. The generated equation in terms of coded values was as follows:
| % EE (R2) = 51.63 + 1.16 A + 1.84B + 4.23C − 0.73 AB + 0.41 AC − 0.50 BC − 6.48 A2 − 18.08 B2 − 2.35 C2 |
Fig. 2.
Impact of independent variables on encapsulation efficiency (R2). (A) Contour plots of R1 and (B) 3D surface plots for R2.
The equation discloses that all the tested independent variables exerted significant synergistic effects on the % EE. At fixed CHOL:Surfactant ratio and total weight of niosomal components, increasing CTZ concentration from 5 mg (F12) to 15 mg (F2) ensued a remarkable rise in % EE. The % entrapment efficiency of F2 and F12 were 27.9 ± 0.9% and 23.5 ± 1.1% respectively. The increased amount of entrapped CTZ with rising drug concentration might be attributed to the saturation of the hydration medium with CTZ, forcing the drug to be entrapped within niosomes (Mokhtar et al., 2008, Balakrishnan et al., 2009).
Similarly, CHOL:Surfactant ratio exerted a pronounced impact on the % EE (p = 0.005). Compared to niosomal formulation prepared with lower cholesterol content (CHOL:Surfactant ratio of 1:2), niosomal formulation prepared at a CHOL:Surfactant ratio of 1:1 exerted the highest % EE. Such increase in % EE with increasing cholesterol content might be ascribed, on the one hand, to the positive effect of cholesterol on increasing the hydrodynamic diameter of niosomal vesicles, and, on the other hand, to the membrane stabilizing effect of cholesterol, which prevent the premature leakage of drug from niosomal vesicles (Nowroozi et al., 2018). Nevertheless, further increase in cholesterol content, as observed in niosomes prepared at CHOL:Surfactant ratio of 2:1, was accompanied with a remarkable drop in the entrapment of CTZ within niosomal vesicles, presumably, via disrupting the regular structure of the vesicle membrane, which in turn, discourage drug entrapment (Moribe et al., 1999, Al-Mahallawi et al., 2014). Similar findings were stated by Khazaeli et al. (2007) who reported a remarkable decrease in the % EE of caffeine within niosomes upon increasing the molar ratio of cholesterol to surfactant from 3:7 to 3:5.
Finally, the impact of niosomal components total weight (C) on %EE was also investigated. It was evident that niosomal components total weight (C) exerted a positive effect on % EE of the prepared niosomes (p = 0.0001). At fixed CHOL:Surfactant ratio, increasing niosomal components weight from 150 mg (F11) to 300 mg (F3) was accompanied with a remarkable increase in % EE from 37.8 ± 2.4% to 46.1 ± 1.9%, respectively. Such remarkable increasing in %EE with increasing the amount of the film forming materials might be accounted for the increased total lipid available for hydration. This led to the formation of multilamellar and/or multivesicular vesicles with larger available space for drug entrapment (Abdelkader et al., 2010). Similar findings were reported by Abdelbary and AbouGhaly (2015) who emphasized the positive effect of total lipid content on the entrapment efficiency of minoxidil niosomes.
3.1.3. Optimized CTZ-loaded niosomes selection
Generally, pharmaceutical formulation optimization aims to employ systematic approaches to achieve the optimal combinations of independent formulation variables that will give rise to a high-quality product. In this study, after applying desirability constrains to vesicle size and % EE (Table 1), an optimized CTZ-loaded niosomal formulation with the desired responses was obtained. The composition of the optimized CTZ niosomal formulation suggested, at a desirability of 1, was CTZ content of 11.25 mg, CHOL:Surfactant ratio of 1:1, and a total weight of niosomal components of 274.5 mg. The prepared optimized CTZ niosomes was found to meet the requirements for an optimal formulation. The estimated vesicle size was 403.4 ± 15.6 nm, and the % EE was 52.8 ± 1.9%, which were comparable to the predicted values for vesicle size and % EE (419 nm, and 53.34 %, respectively). The selected optimized formulation was incorporated into hydrogel base and assessed for topical delivery.
3.2. Optimized CTZ-loaded niosomes characterization
3.2.1. Particle size analysis and polydispersity index (PDI)
In topical drug delivery, the particle size of nanovesicles is a crucial parameter that control nanocarrier permeation across skin barrier. Herein, the vesicle size of the optimized CTZ niosomes was 403.4 ± 15.6 nm (Fig. 3A), suggesting their suitability for cutaneous delivery (Chen et al., 2019).
Fig. 3.
Physicochemical characteristics of CTZ niosomes. (A) Vesicle size of CTZ niosomes; (B) Zeta potential of CTZ niosomes.
Another important parameter for defining particle size distribution is the polydispersity index. The closer to zero the polydispersity value, the more homogenous are the particles. Monodispersed particles have lower tendency to aggregation and are stable, whereas polydispersed particles tend to be aggregated and are unstable. The polydispersity index (PDI) of optimized niosomal formulations was 0.231, indicating narrow variation in size distribution (Danaei et al., 2018).
3.2.2. Zeta potential
Zeta potential (ZP) refers to the total charge acquired by nanovesicles and is used as an indirect measure of niosomal dispersion stability (Al-Mahallawi et al., 2015). A stable colloidal system is one with a ZP value of ± 20 mV (Soliman et al., 2020). It is worth noting that optimized CTZ niosomes have a negative ZP value of − 12.9 ± 1.7 (Fig. 3B), signifying fair colloidal stability of the prepared niosomes.
3.2.3. TEM analysis
TEM analysis was conducted to examine the surface morphology of the prepared CTZ-loaded niosomes. As depicted in Fig. 4, CTZ-loaded niosomes were discrete, spherical in shape and having nearly smooth surfaces.
Fig. 4.
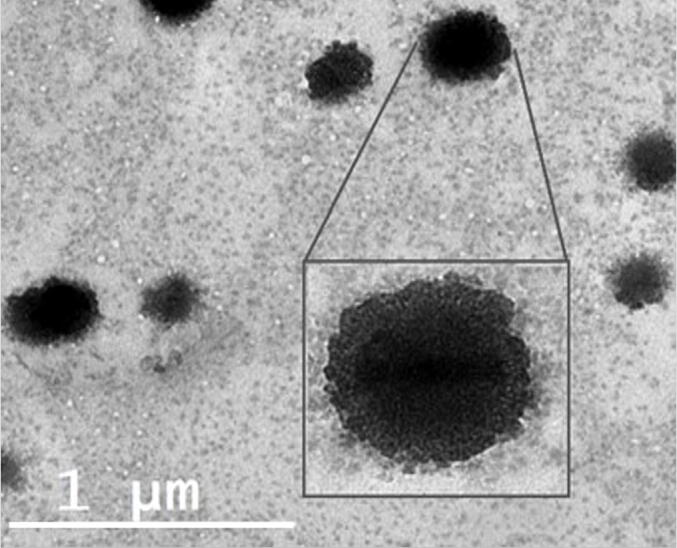
Transmission electron imaging of optimized CTZ-loaded niosomes.
3.2.4. Entrapment efficiency
The entrapment efficiency is an important parameter for characterizing niosomal vesicles. Herein, the encapsulation efficiency of CTZ within niosomal vesicles was 52.8 ± 1.9%. Such relatively lower value for the % EE might be ascribed to the hydrophilic nature of the drug. It is well known that during passive loading of water-soluble drugs within bilayered vesicles, the volume of water in the aqueous core of the vesicle is relatively lower than that exits outside the bilayered vesicle. consequently, hydrophilic drugs usually show lower % EE than that of drugs with lower water solubility (Joshi et al., 2020).
3.2.5. Drug excipient interaction studies
DSC was adopted to scrutinize the compatibility between CTZ and different components of niosomal formulation. Fig. 5 depicts the DSC thermograms of the optimized CTZ niosomes, and individual formulation components; pure drug, cholesterol, and span 60. Cholesterol and Span 60 exhibited endothermic peaks at 149 and 54 ˚C, respectively, which corresponded to their melting points. Pure cetirizine (CTZ) thermogram showed a characteristic sharp peak at 115 ˚C, corresponding to CTZ melting point. Of interest, optimized CTZ niosomes did not show the typical peak of the drug. This suggests the proper entrapment of the drug within niosomal vesicles.
Fig. 5.
DSC thermograms of pure CTZ, CHOL, Span 60 and optimized CTZ niosomes.
3.3. In vitro release study
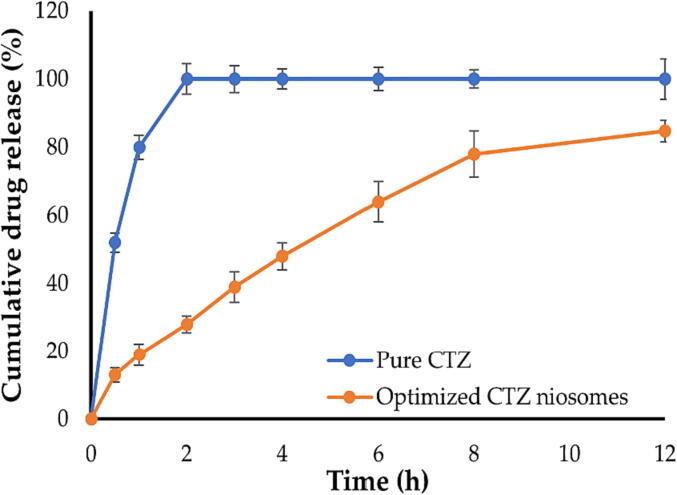
Fig. 6 depicts the release profiles for both CTZ solution and the optimized CTZ niosomes. As shown in Fig. 6, free drug was released immediately from CTZ solution within 2 h. By contrary, CTZ niosomes showed a biphasic phase, with about 40% of entrapped drug was released during the first 3 h from niosomal vesicles, followed by a steady release over 12 h, with up to 85% of CTZ released at the end of release study. Such biphasic release pattern from CTZ niosomes is desirable for topical drug delivery since the initial rapid release would create a concentration gradient and promotes cutaneous drug penetration, whereas the subsequent sustained release enables the delivery of sufficient therapeutic levels of the drug to the skin over a prolonged period, which might help in reducing application frequency (Patel et al., 2012).
Fig. 6.
In vitro release profiles of pure CTZ and optimized CTZ niosomes.
3.4. Stability of optimized CTZ niosomes
The stability of the optimized CTZ niosomes was examined in terms of change in encapsulation efficiency, vesicle size, and zeta potential upon storage for 3 months at 4 ˚C. As depicted in Table 3, no significant variations in vesicle size, zeta potential or encapsulation efficiency percentage were observed over a 1- and 3-month storage, when compared with freshly prepared niosomes. These findings established the stability of formulated CTZ niosomes.
Table 3.
Stability study results of CTZ niosomes stored at 4 ˚C for 90 days.
| Evaluation parameters | Day 0 | Day 30 | Day 90 |
|---|---|---|---|
| Vesicle size (nm) | 403.4.6 ± 15.6 | 408.9 ± 11.3 | 421.2 ± 21.9 |
| Zeta potential (mV) | − 12.9 ± 1.7 | − 11.9 ± 1.3 | − 11.3 ± 2.1 |
| Entrapment efficiency (%) | 52.8 ± 1.9 | 50.7 ± 1.1 | 48.6 ± 3.8 |
3.5. Preparation of CTZ-loaded niosomal gel
Preliminary experiments were conducted to select the most appropriate gelling agent for preparing CTZ-loaded niosomal gel. Different gelling agents, including hydroxypropyl methylcellulose (HPMC), methylcellulose and Carbapol 934 were used for the preparation of CTZ niosomal gels. Among them, CTZ niosomal hydrogel that consists of HPMC showed best consistency, homogeneity, and spreadability, and was selected for further investigations.
3.6. Ctz-loaded niosomal gel characterization
3.6.1. Physical appearance
Physical inspection of CTZ-loaded niosomal gel revealed that the gel formulation was milky white in color, smooth and homogenous with no signs of grittiness.
3.6.2. pH
The pH of the prepared CTZ niosomal gel was recorded by digital pH meter by directly immersing the electrode into the gel formulation. The recorded pH value of gel formulation was 4.82 ± 0.4, which is compatible with hair scalp.
3.6.3. Spreadability
In order to understand the uniform spreading of gel on the hair scalp, the spreadability test was conducted. Niosomal gel formulation showed a spreadability of 13.87 ± 1.5 g.cm/sec, revealing that uniform application of gel could be attained with minimal shear stress.
3.6.4. Viscosity
The viscosity of CTZ-loaded niosomal gel was measured using Brookfield viscometer model-LV using the spindle T-C with a rotational speed of 5 rpm at 20 ± 1˚C. The gel viscosity was in the range of 5236.43 ± 211 cps, which is acceptable for topical application (Abdallah et al., 2022).
3.6.5. Drug content
Drug content of CTZ in the formulated niosomal gel was found to be 97.5 ± 0.9%. This result indicates high drug content, and the drug is uniformly distributed in the gel formulation.
3.7. Ex vitro skin permeation and retention study
As illustrated in Fig. 7, the permeation of CTZ from control CTZ gel was considerably greater than that permeated from either CTZ-loaded niosomal dispersion and CTZ-loaded niosomal gel (p < 0.05). Nevertheless, the permeation of CTZ from niosomal formulations through rat abdominal skin into the receptor compartment was barely detected. The cumulative drug amounts permeated through the skin from control CTZ gel, CTZ niosomal dispersion and CTZ niosomal gel were 73 ± 5.6 μg/cm2, 11.1 ± 2.4 μg/cm2 and 4.9 ± 1.1 μg/cm2, respectively. The permeation characteristics of CTZ from different CTZ-loaded formulations were summarized in Table 4. It was distinct that control CTZ gel triggered the permeation of CTZ through abdominal rat skin as manifested by the significantly higher transdermal flux (Jss), compared to CTZ-loaded niosomal formulations. In addition, it was evident that the transdermal flux of CTZ from niosomal gel was much lower than that from niosomal dispersion, which might be ascribed to the higher viscosity of the gel formulation.
Fig. 7.
Ex vivo permeation of CTZ niosomes, plain CTZ gel and CTZ-loaded niosomal gel from abdominal rat skin.
Table 4.
Skin permeation parameters of various CTZ-loaded formulations.
| Parameter | CTZ-loaded gel | CTZ niosomal dispersion | CTZ-loaded niosomal gel |
|---|---|---|---|
| Q10 (μg/cm2) | 73.8 ± 5.6 | 11.2 ± 2.4 *** | 4.9 ± 1.1 *** |
| Jmax (μg/cm2.h) | 7.41 ± 1.1 | 1.23 ± 0.25 *** | 0.50 ± 0.18 *** |
| Jss (μg/cm2.h) | 6.6 ± 0.72 | 0.96 ± 0.18 *** | 0.46 ± 0.09 *** |
| CTZ deposited (%) | 7.52 ± 0.7 | 15.9 ± 2.1 ** | 19.2 ± 1.9 ** |
Q10: cumulative amount of drug permeated per unit area at the end of 10 h, Jmax: maximum average drug flux after 10 h, per unit area per hour, Jss: steady state flux. Data represent mean ± SD. **p < 0.01 and ***p < 0.001 vs CTZ-loaded gel.
Most importantly, the amount of CTZ deposited in the skin from either CTZ-loaded niosomal formulations or control CTZ gel was assessed at the end of the experiments in order to understand the effect of the drug carriers on diffusion of drug through the skin and accumulation into the hair follicle. As depicted in Table 4, CTZ-loaded niosomal formulations remarkably augmented skin deposition of CTZ when compared with the control CTZ gel. The skin deposition of CTZ from CTZ-loaded niosomal formulations was ∼ 3-fold higher than that of control gel formulation. The percentage drug deposited for CTZ-loaded niosomal dispersion and CTZ-loaded niosomal gel were 16.9 ± 2.1% and 19.2 ± 1.9%, respectively, compared to 7.52 ± 0.7% for control CTZ-loaded gel. Collectively, it was clear that the control CTZ gel demonstrated more penetration over the skin but very little CTZ skin deposition, compared to CTZ-loaded niosomal formulation.
3.8. In vivo dermato-kinetic study
Fig. 8 depicts skin deposition characteristics of CTZ from plain CTZ gel and CTZ-loaded niosomal gel. The amount of CTZ deposited in skin layers was expressed as AUC0-10h. As depicted in Fig. 8, the retention of CTZ from niosomal gel into skin layers was considerably higher than that from control CTZ-loaded gel (p < 0.05). The AUC0-10h obtained from the deposition profile of CTZ-loaded niosomal gel was 1124.5 ± 87.9 μg/mL.h, which was ∼ 2 folds higher than that observed with CTZ-loaded gel (AUC0-10h was 646.2 ± 44.6 μg/mL.h). Of note, CTZ-loaded niosomal gel had a considerably (p < 0.05) higher Cmax of 197.6 ± 11.3 μg/mL when compared to control CTZ gel (Cmax was 103.9 ± 14.2 μg/mL).
Fig. 8.
In vivo skin deposition profile of CTZ from CTZ-loaded niosomal gel and plain CTZ gel.
4. Discussion
Androgenic Alopecia is a genetically predetermined disorder that constitutes the most prevalent cause of male hair loss (Lolli et al., 2017). Although androgenetic alopecia is a benign and asymptomatic disorder, cosmetic concerns lead some patients to seek treatment. Topical minoxidil and oral finasteride are considered the primary treatment options for androgenetic alopecia (Adil and Godwin, 2017, Suchonwanit et al., 2019). Nevertheless, despite their widespread application, their use is not without side effects (Albash et al., 2022). Consequently, there is an urgent need for the development of novel therapies for the management of this distressing disorder. In the current study, we challenged the efficacy of cetirizine (CTZ)-loaded niosomal formulation for the management of alopecia. Entrapping the antihistaminic drug, CTZ, within niosomal vesicles was found to sustain drug release for up to 12 h (Fig. 6), and to enhance the cutaneous delivery of CTZ to skin layers. Ex-vivo skin deposition studies (Fig. 7) clearly depicted that CTZ-loaded niosomal gel promoted higher drug deposition into skin, compared to plain CTZ gel. Most importantly, in vivo dermato-kinetic studies (Fig. 8) confirmed better cutaneous retention of CTZ from CTZ-loaded niosomal gel as manifested by a two-fold increase in AUC0-10h, compared to control CTZ gel. To the best of our knowlegde, this is the first report to emphasize the possible application of CTZ-loaded niosomal gel for the management of androgenic alopecia.
Niosomes are vesicular bilayer structures prepared from the mixtures of non-ionic surfactants and cholesterol. Preliminary experiments were conducted for preparing CTZ niosomes including several non-ionic surfactants such as Spans (Span 60, 80), Tweens (Tween 60, 80) and Brij 35, at CHOL:Surfactant ratio of 1:1, and the impact of surfactant type on vesicle formation was examined. It was obvious that surfactants having HLB values ranging from 14 to 17 (Tween 60, 80 and Brij 35) were unsuccessful to form a uniform thin film and, upon hydration, they produced vesicles with non-spherical, irregular shape and their size were not uniform. Furthermore, such niosomes were not stable as manifested by sedimentation of vesicles and instant phase separation. On the other hand, niosomes prepared using span 60 and span 80 produced vesicles of uniform size and spherical shape with no signs of phase separation. This might be ascribed to their high transition temperature (Tc) and their optimum HLB values (4.3 and 4.7, respectively), which is appropriate for preparation of vesicles (Moghassemi and Hadjizadeh 2014). Nevertheless, niosomes prepared with span 80 showed remarkable aggregations upon short-term storage. Accordingly, Span 60 was selected for further formulation of CTZ-loaded niosomes.
In topical drug delivery, the particle size of nanovesicles is a crucial parameter that control nanocarrier permeation across skin barrier. It is well recognized that nanovesicles with particle size > 600 nm showed no skin deposition (Li et al., 2022). In contrast, nanovesicles with smaller particle sizes, such as 400 nm, increase cutaneous delivery, whilst nanovesicles with smaller particle sizes, such as 200 nm, might trigger excessive transdermal drug transport (Chen et al., 2019). Accordingly, in order to obtain an optimized formula with a reasonable particle size for cutaneous delivery, response surface methodology (RSM), based on design of experiments, was adopted for designing and optimizing the process variables. Optimization utilizing response surface analysis is considered as an effective approach for reducing both the time and the number of experimental runs required for formulation development and enhancing research output (Malakar et al., 2012). In this study, Box Behnken design was efficiently adopted to explore the influence of various formulation variables on product characteristics and, most importantly, to retrieve an optimized formula. The optimized drug-loaded niosomal formula showed an average particle size of 403.4 ± 15.6 nm, which is considered suitable for cutaneous delivery (Chen et al., 2019).
In order to gain a better understanding about the impact of drug carriers on diffusion of drug through the skin and accumulation into the hair follicle, ex vivo skin permeation study of CTZ-loaded niosomal gel were carried out and compared with that of control CTZ gel and CTZ niosomal dispersion. Ex vivo permeation experiments (Fig. 7) demonstrated that niosomal vesicles efficiently promoted drug accommodation within skin layer as manifested by a comparatively lower transdermal flux values, compared to control CTZ gel. This preferential cutaneous accumulation of niosomal vesicles was primarily attributed to the relatively large vesicle size (∼400 nm), which favor the retention of vesicles within skin layers rather than their free permeation through the skin to the receptor compared as observed with the control CTZ gel. Of note, the integration of niosomal vesicles in hydrocolloid, i.e., HPMC gel, slowed drug release relative to niosomal dispersion, presumably due to the slower drug diffusion over the gel network (Aggarwal and Goindi 2012). Similar results were reported by Mali et al. (Mali et al., 2013) who underscored the efficacy of niosomal gels, as a vesicular carrier, for promoting higher skin deposition of minoxidil, compared to plain minoxidel gel.
In vivo dermato-kinetic study (Fig. 8) provides a viable mean for quantifying drug input to various skin layers following topical administration of either control CTZ gel or CTZ-loaded niosomal gel. As inferred from results of in vivo dermato-kinetic study, niosomal vesicular system (CTZ-loaded niosomal gel) have shown maximum drug retention in skin, compared to non-vesicular systems (control CTZ gel). CTZ-loaded niosomal gel showed a two-fold increase in CTZ skin deposition, when compared with control CTZ gel. These findings support the results of the ex vivo permeation study, suggesting that niosomal vesicles, when applied to the skin, can partition across subcutaneous layer under the effect of the transcutaneous hydration gradient to form depots from which drugs can be released (Peira et al., 2007). Similar findings were reported by Albash et al. (Albash et al., 2022) who emphasized the efficacy of cationic ceramide/phospholipid composite (CCPCs) in promoting better dermal deposition of levocetirizine hydrochloride from the optimum formula, compared to its solution.
In summary, niosomal carrier was verified to exert a significant influence on improving topical drug penetration and boosting drug deposition into skin layers, which are considered two crucial determinants for the therapeutic efficacy of dermal formulations. Similar results were reported by Li et al. (2022) who emphasized the potential of niosomal vesicles for enhancing epigallocatechin gallate cutaneous delivery for protection against oxidative stress. Several theories have been postulated to justify the ability of niosomal formulations to enhance drug skin penetration and deposition. It was reported that the adsorption and/or fusion of niosomal vesicles to the skin surface could result in a substantial drug concentration gradient at the surface of the skin triggering efficient drug penetration into the skin (Zhang et al., 2015, Kassem et al., 2017). In addition, it was proposed that niosomes might disrupt the densely packed lipids occupying the extracellular spaces of the subcutaneous layer and promote alterations in the structure of subcutaneous layer, resulting in an enhancement in drug permeability through skin barrier layer. Furthermore, niosomes are composed of non-ionic surfactants, which are relatively nontoxic and biocompatible and can serve themselves as penetration enhancers, promoting better drug penetration through different skin layers (Tavano et al., 2014, Jiang et al., 2018).
5. Conclusions
CTZ-loaded niosomes were successfully prepared employing thin film hydration for efficient cutaneous delivery of CTZ. Box-Behnken design was used to optimize the formulated niosomes using the desirability criteria. The optimized formulation exerted reasonable vesicle size, polydispersity index, and efficiently sustained drug release for up to 12 h. Moreover, CTZ-loaded niosomal gel exerted suitable pH, viscosity, and spreadability for topical application. Ex-vivo skin deposition studies clearly depicted that CTZ-loaded niosomal gel promoted higher drug deposition into skin, compared to plain CTZ gel. Most importantly, compared to plain CTZ gel, in vivo dermato-kinetic studies confirmed better cutaneous retention of CTZ from CTZ-loaded niosomal gel as inferred by a two-fold increase in AUC0-10h. To sum up, niosomal vesicles might be a successful delivery vehicle for cutaneous delivery of cetirizine for the management of androgenic alopecia. However, further studies on alopecia induced animal model are required to verify our concept.
Funding
This study is supported via funding from Prince Sattam Bin Abdulaziz University project number (PSAU/2023/R/1444).
Institutional Review Board Statement: The animal study protocol was approved by the Ethical Committee, Prince Sattam Bin Abdulaziz University, Al-Kharj, KSA (approval number: 048/2022).
CRediT authorship contribution statement
Mohammed F. Aldawsari: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft. El-Sayed Khafagy: Methodology, Formal analysis, Investigation, Writing – original draft. Ehssan H. Moglad: Methodology, Software, Investigation. Amr Selim Abu Lila: Conceptualization, Data curation, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study is supported via funding from Prince Sattam Bin Abdulaziz University project number (PSAU/2023/R/1444).
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2023.101734.
Contributor Information
Mohammed F. Aldawsari, Email: moh.aldawsari@psau.edu.sa.
El-Sayed Khafagy, Email: e.khafagy@psau.edu.sa.
Ehssan H. Moglad, Email: e.moglad@psau.edu.sa.
Amr Selim Abu Lila, Email: a.abulila@uoh.edu.sa.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abdallah M.H., Abu Lila A.S., Unissa R., et al. Preparation, characterization and evaluation of anti-inflammatory and anti-nociceptive effects of brucine-loaded nanoemulgel. Colloids and Surfaces B: Biointerfaces. 2021;205 doi: 10.1016/j.colsurfb.2021.111868. [DOI] [PubMed] [Google Scholar]
- Abdallah M.H., Elsewedy H.S., AbuLila A.S., et al. Quality by Design for Optimizing a Novel Liposomal Jojoba Oil-Based Emulgel to Ameliorate the Anti-Inflammatory Effect of Brucine. Gels. 2021;7:219. doi: 10.3390/gels7040219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah M.H., Lila A.S.A., Unissa R., et al. Brucine-Loaded Ethosomal Gel: Design, Optimization, and Anti-inflammatory Activity. AAPS PharmSciTech. 2021;22:269. doi: 10.1208/s12249-021-02113-8. [DOI] [PubMed] [Google Scholar]
- Abdallah M.H., Abu Lila A.S., Shawky S.M., et al. Experimental Design and Optimization of Nano-Transfersomal Gel to Enhance the Hypoglycemic Activity of Silymarin. Journal. 2022;14:508. doi: 10.3390/polym14030508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelbary A.A., AbouGhaly M.H. Design and optimization of topical methotrexate loaded niosomes for enhanced management of psoriasis: application of Box-Behnken design, in-vitro evaluation and in-vivo skin deposition study. Int. J. Pharm. 2015;485:235–243. doi: 10.1016/j.ijpharm.2015.03.020. [DOI] [PubMed] [Google Scholar]
- Abdelkader H., Ismail S., Kamal A., et al. Preparation of niosomes as an ocular delivery system for naltrexone hydrochloride: physicochemical characterization. Pharmazie. 2010;65:811–817. [PubMed] [Google Scholar]
- Adil A., Godwin M. The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2017;77(136–141):e135. doi: 10.1016/j.jaad.2017.02.054. [DOI] [PubMed] [Google Scholar]
- Agarwal S.K., Bakshi V., Vitta P., et al. Effect of cholesterol content and surfactant HLB on vesicle properties of niosomes. Indian J. Pharm. Sci. 2004;66:121–123. [Google Scholar]
- Aggarwal N., Goindi S. Preparation and evaluation of antifungal efficacy of griseofulvin loaded deformable membrane vesicles in optimized guinea pig model of Microsporum canis–dermatophytosis. Int. J. Pharm. 2012;437:277–287. doi: 10.1016/j.ijpharm.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Albash R., El-Dahmy R.M., Hamed M.I.A., et al. Repurposing levocetirizine hydrochloride loaded into cationic ceramide/phospholipid composite (CCPCs) for management of alopecia: central composite design optimization, in- silico and in-vivo studies. Drug Deliv. 2022;29:2784–2795. doi: 10.1080/10717544.2022.2108939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldawsari M.F., Khafagy E.S., Alotaibi H.F., et al. Vardenafil-Loaded Bilosomal Mucoadhesive Sponge for Buccal Delivery: Optimization, Characterization, and In Vivo Evaluation. Polymers (Basel). 2022;14:4184. doi: 10.3390/polym14194184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mahallawi A.M., Khowessah O.M., Shoukri R.A. Nano-transfersomal ciprofloxacin loaded vesicles for non-invasive trans-tympanic ototopical delivery: in-vitro optimization, ex-vivo permeation studies, and in-vivo assessment. Int. J. Pharm. 2014;472:304–314. doi: 10.1016/j.ijpharm.2014.06.041. [DOI] [PubMed] [Google Scholar]
- Al-Mahallawi A.M., Abdelbary A.A., Aburahma M.H. Investigating the potential of employing bilosomes as a novel vesicular carrier for transdermal delivery of tenoxicam. Int. J. Pharm. 2015;485:329–340. doi: 10.1016/j.ijpharm.2015.03.033. [DOI] [PubMed] [Google Scholar]
- Balakrishnan P., Shanmugam S., Lee W.S., et al. Formulation and in vitro assessment of minoxidil niosomes for enhanced skin delivery. Int. J. Pharm. 2009;377:1–8. doi: 10.1016/j.ijpharm.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Chen S., Hanning S., Falconer J., et al. Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications. Eur. J. Pharm. Biopharm. 2019;144:18–39. doi: 10.1016/j.ejpb.2019.08.015. 10.1016/j.ejpb.2019.08.015 [DOI] [PubMed] [Google Scholar]
- Danaei M., Dehghankhold M., Ataei S., et al. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics. 2018;10:57. doi: 10.3390/pharmaceutics10020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza L.A., Liu Y., Yang Z., et al. Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Sci. Transl. Med. 2012;4:126ra134. doi: 10.1126/scitranslmed.3003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X., Wei M., He S., et al. Advances of Non-Ionic Surfactant Vesicles (Niosomes) and Their Application in Drug Delivery. Pharmaceutics. 2019;11:55. doi: 10.3390/pharmaceutics11020055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemiyeh P., Mohammadi-Samani S. Potential of Nanoparticles as Permeation Enhancers and Targeted Delivery Options for Skin: Advantages and Disadvantages. Drug Des. Devel. Ther. 2020;14:3271–3289. doi: 10.2147/dddt.s264648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goindi S., Kumar G., Kumar N., et al. Development of novel elastic vesicle-based topical formulation of cetirizine dihydrochloride for treatment of atopic dermatitis. AAPS PharmSciTech. 2013;14:1284–1293. doi: 10.1208/s12249-013-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamishehkar H., Rahimpour Y., Kouhsoltani M. Niosomes as a propitious carrier for topical drug delivery. Expert Opin. Drug Deliv. 2013;10:261–272. doi: 10.1517/17425247.2013.746310. [DOI] [PubMed] [Google Scholar]
- Hossein Mostafa D., Samadi A., Niknam S., et al. Efficacy of Cetirizine 1% Versus Minoxidil 5% Topical Solution in the Treatment of Male Alopecia: A Randomized, Single-blind Controlled Study. J. Pharm. Pharm. Sci. 2021;24:191–199. doi: 10.18433/jpps31456. [DOI] [PubMed] [Google Scholar]
- Jiang T., Wang T., Li T., et al. Enhanced Transdermal Drug Delivery by Transfersome-Embedded Oligopeptide Hydrogel for Topical Chemotherapy of Melanoma. ACS Nano. 2018;12:9693–9701. doi: 10.1021/acsnano.8b03800. [DOI] [PubMed] [Google Scholar]
- Joshi S., White R., Sahu R., et al. Comprehensive Screening of Drug Encapsulation and Co-Encapsulation into Niosomes Produced Using a Microfluidic Device. Journal. 2020;8 doi: 10.3390/pr8050535. [DOI] [Google Scholar]
- Junginger H.E., Hofland H., Bouwstra J.A. Liposomes and niosomes : interactions with human skin. Cosmetics and toiletries. 1991;106:45–50. [Google Scholar]
- Kassem A.A., Abd El-Alim S.H., Asfour M.H. Enhancement of 8-methoxypsoralen topical delivery via nanosized niosomal vesicles: Formulation development, in vitro and in vivo evaluation of skin deposition. Int. J. Pharm. 2017;517:256–268. doi: 10.1016/j.ijpharm.2016.12.018. [DOI] [PubMed] [Google Scholar]
- Kelly Y., Blanco A., Tosti A. Androgenetic Alopecia: An Update of Treatment Options. Drugs. 2016;76:1349–1364. doi: 10.1007/s40265-016-0629-5. [DOI] [PubMed] [Google Scholar]
- Khafagy E.S., Almutairy B.K., Abu Lila A.S. Tailoring of Novel Bile Salt Stabilized Vesicles for Enhanced Transdermal Delivery of Simvastatin: A New Therapeutic Approach against Inflammation. Polymers (Basel). 2023;15:677. doi: 10.3390/polym15030677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazaeli P., Pardakhty A., Shoorabi H.A.M. Caffeine-Loaded Niosomes: Characterization and in Vitro Release Studies. Drug Deliv. 2007;14:447–452. doi: 10.1080/10717540701603597. [DOI] [PubMed] [Google Scholar]
- Li D., Martini N., Wu Z., et al. Niosomal Nanocarriers for Enhanced Dermal Delivery of Epigallocatechin Gallate for Protection against Oxidative Stress of the Skin. Pharmaceutics. 2022;14:726. doi: 10.3390/pharmaceutics14040726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolli F., Pallotti F., Rossi A., et al. Androgenetic alopecia: a review. Endocrine. 2017;57:9–17. doi: 10.1007/s12020-017-1280-y. [DOI] [PubMed] [Google Scholar]
- Malakar J., Nayak A.K., Goswami S. Use of response surface methodology in the formulation and optimization of bisoprolol fumarate matrix tablets for sustained drug release. ISRN Pharm. 2012;2012 doi: 10.5402/2012/730624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali N., Darandale S., Vavia P. Niosomes as a vesicular carrier for topical administration of minoxidil: formulation and in vitro assessment. Drug Deliv. and Transl. Res. 2013;3:587–592. doi: 10.1007/s13346-012-0083-1. [DOI] [PubMed] [Google Scholar]
- Moghassemi S., Hadjizadeh A. Nano-niosomes as nanoscale drug delivery systems: an illustrated review. J. Control. Release. 2014;185:22–36. doi: 10.1016/j.jconrel.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Moin A., Wani S.U.D., Osmani R.A., et al. Formulation, characterization, and cellular toxicity assessment of tamoxifen-loaded silk fibroin nanoparticles in breast cancer. Drug Deliv. 2021;28:1626–1636. doi: 10.1080/10717544.2021.1958106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhtar M., Sammour O.A., Hammad M.A., et al. Effect of some formulation parameters on flurbiprofen encapsulation and release rates of niosomes prepared from proniosomes. Int. J. Pharm. 2008;361:104–111. doi: 10.1016/j.ijpharm.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Moribe K., Maruyama K., Iwatsuru M. Encapsulation characteristics of nystatin in liposomes: effects of cholesterol and polyethylene glycol derivatives. Int. J. Pharm. 1999;188:193–202. doi: 10.1016/s0378-5173(99)00222-7. [DOI] [PubMed] [Google Scholar]
- Morsy M.A., Patel S.S., Bakrania A., et al. Ameliorative Effect of a Neoteric Regimen of Catechin plus Cetirizine on Ovalbumin-Induced Allergic Rhinitis in Rats. Life (Basel). 2022;12:820. doi: 10.3390/life12060820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowroozi F., Almasi A., Javidi J., et al. Effect of Surfactant Type, Cholesterol Content and Various Downsizing Methods on the Particle Size of Niosomes. Iran J Pharm Res. 2018;17:1–11. [PMC free article] [PubMed] [Google Scholar]
- Pardakhty A., Varshosaz J., Rouholamini A. In vitro study of polyoxyethylene alkyl ether niosomes for delivery of insulin. Int. J. Pharm. 2007;328:130–141. doi: 10.1016/j.ijpharm.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Patel D., Dasgupta S., Dey S., et al. Nanostructured Lipid Carriers (NLC)-Based Gel for the Topical Delivery of Aceclofenac: Preparation, Characterization, and In Vivo Evaluation. Sci. Pharm. 2012;80:749–764. doi: 10.3797/scipharm.1202-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peira E., Trotta M., Carlotti M.E., et al. Elastic positively-charged liposomes for topical administration of acyclovir. J. Drug Delivery Sci. Technol. 2007;17:321–324. 10.1016/S1773-2247(07)50049-3 [Google Scholar]
- Qumbar M., Ameeduzzafar S.S. Imam, et al. Formulation and optimization of lacidipine loaded niosomal gel for transdermal delivery: In-vitro characterization and in-vivo activity. Biomed. Pharmacother. 2017;93:255–266. doi: 10.1016/j.biopha.2017.06.043. [DOI] [PubMed] [Google Scholar]
- Rajitha K., Lakshmi P.K., Pranitha A., et al. Transdermal permeation enhancement of ibuprofen and its solid dispersions. Int J Res Ayur Pharm. 2014;5:508–514. [Google Scholar]
- Rossi A., Campo D., Fortuna M.C., et al. A preliminary study on topical cetirizine in the therapeutic management of androgenetic alopecia. J. Dermatolog. Treat. 2018;29:149–151. doi: 10.1080/09546634.2017.1341610. [DOI] [PubMed] [Google Scholar]
- Sharma A., Harikumar S.L. Quality by design approach for development and optimization of nitrendipine loaded niosomal gel for accentuated transdermal delivery. Int J App Pharm. 2020;12:181–189. [Google Scholar]
- Shatalebi M.A., Mostafavi S.A., Moghaddas A. Niosome as a drug carrier for topical delivery of N-acetyl glucosamine. Res Pharm Sci. 2010;5:107–117. [PMC free article] [PubMed] [Google Scholar]
- Soliman W.E., Khan S., Rizvi S.M.D., et al. Therapeutic Applications of Biostable Silver Nanoparticles Synthesized Using Peel Extract of Benincasa hispida: Antibacterial and Anticancer Activities. Nanomaterials (Basel). 2020;10:1954. doi: 10.3390/nano10101954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchonwanit P., Thammarucha S., Leerunyakul K. Minoxidil and its use in hair disorders: a review. Drug Des. Devel. Ther. 2019;13:2777–2786. doi: 10.2147/dddt.s214907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavano L., Muzzalupo R., Picci N., et al. Co-encapsulation of lipophilic antioxidants into niosomal carriers: percutaneous permeation studies for cosmeceutical applications. Colloids Surf. B Biointerfaces. 2014;114:144–149. doi: 10.1016/j.colsurfb.2013.09.055. [DOI] [PubMed] [Google Scholar]
- Vora B., Khopade A.J., Jain N.K. Proniosome based transdermal delivery of levonorgestrel for effective contraception. J. Control. Release. 1998;54:149–165. doi: 10.1016/s0168-3659(97)00100-4. [DOI] [PubMed] [Google Scholar]
- Wang Z., He X. Dynamics of vesicle formation from lipid droplets: mechanism and controllability. J. Chem. Phys. 2009;130 doi: 10.1063/1.3079097. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang K., Wu Z., et al. Evaluation of transdermal salidroside delivery using niosomes via in vitro cellular uptake. Int. J. Pharm. 2015;478:138–146. doi: 10.1016/j.ijpharm.2014.11.018. [DOI] [PubMed] [Google Scholar]
- Zhou P., Jia Q., Wang Z., et al. Cetirizine for the treatment of allergic diseases in children: A systematic review and meta-analysis. Front. Pediatr. 2022;10 doi: 10.3389/fped.2022.940213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.