Abstract
Two flow cytometric assays are described herein. The single cytometric test (SCT) detects antibodies to either Brucella abortus or Staphylococcus aureus in the serum or milk of a cow or water buffalo. The double cytometric test (DCT) detects both anti-B. abortus and anti-S. aureus antibodies concurrently. In the SCT, the sample to be tested is incubated in succession with the antigen (either B. abortus or S. aureus) and the proper secondary antiserum (fluorescein isothiocyanate-labelled rabbit anti-cow immunoglobulin antiserum or rabbit anti-water buffalo immunoglobulin antiserum). In the DCT, the sample to be tested is incubated first with B. abortus and S. aureus antigens and then with the secondary antiserum. The B. abortus antigen used in the DCT is covalently bound to 3-μm-diameter latex particles. The difference in size between B. abortus and S. aureus permits the establishment of whether the antibodies are directed against one, the other, or both antigens. When compared to the complement fixation test, the SCT and DCT each show a specificity and a sensitivity of 100%. The SCT has been used previously to detect anti-S. aureus antibodies. Here its use is extended to the detection of anti-B. abortus antibodies. The DCT is described here for the first time. The DCT appears to be useful for large-scale brucellosis eradication programs. It offers the possibility of using one test to identify animals that are serologically positive for both B. abortus and S. aureus.
Brucellosis (1, 9, 10) and mastitis (12, 13, 20) are economically important diseases for the dairy industry. Brucellosis is relevant also as a zoonotic disease (23, 24). Staphylococcus aureus is the most frequent cause of bovine (12) and water buffalo (6) mastitis, and Brucella abortus is the most common cause of bovine (1) and water buffalo (19) brucellosis. In Italy, during 1995, the incidence rates of brucellosis in cattle and water buffalo were about 0.5 and 3% of tested animals, respectively; in the same year, the incidence rates of clinical mastitis among lactating cattle and water buffaloes were 25 and 10%, respectively. These data refer to animals tested as part of the ongoing national program for brucellosis eradication in Italy (14).
Schemes for eradication of brucellosis are based on the serological identification and subsequent elimination of animals displaying the presence of antibodies. The commonly used serological tests—agglutination, complement fixation test (CFT), and enzyme-linked immunosorbent assay—all have both advantages and disadvantages (1, 3, 4). The laboratory diagnosis of mastitis is based on the somatic-cell count in milk (California mastitis test) and on culture of the bacterium. The California mastitis test is an indicator of the state of inflammation of the udder, characterized by an increase in the number of neutrophils and epithelial cells shed by the mammary gland. The counting of somatic cells present in the milk is laborious and does not provide information on the cause of inflammation (17). It is possible that cultures of milk samples which are positive in this test will be negative for pathogens (18). Bacterial cultures, on the other hand, can be time-consuming and expensive (7, 18). Recently, antibodies to S. aureus were detected in milk by flow cytometry (6). The present paper demonstrates that the same technique can simultaneously detect antibodies to B. abortus and S. aureus in milk.
MATERIALS AND METHODS
Bacteria.
S. aureus Wood 46 and vaccine strain 19 of B. abortus were used as antigens. S. aureus was grown on Baird-Parker medium or Trypticase soy agar with 5% sheep blood; B. abortus was cultured on tryptose agar (Difco Laboratories, Detroit, Mich.). The smooth phenotype of B. abortus was determined as described elsewhere (1).
Antisera.
Rabbit anti-water buffalo immunoglobulin antiserum (RαWBFITC) was prepared and labelled with fluorescein isothiocyanate (FITC) as described previously (6). FITC-labelled rabbit anti-cow immunoglobulin antiserum (RαCFITC) was purchased from Sigma (Milan, Italy).
CFT.
The CFT was carried out as described elsewhere (14).
SCT.
In the single cytometric test (SCT), anti-B. abortus or anti-S. aureus antibodies were bound to the corresponding antigen and then detected by flow cytometry with RαWBFITC (in the case of samples from water buffaloes) or RαCFITC (in the case of samples from cows). Bacteria (S. aureus or B. abortus) were counted with the flow cytometer and then suspended in 0.15 M phosphate-buffered saline, pH 7.2 (PBS), at 108/ml. Ten microliters of bacterial suspension (containing approximately 106 S. aureus or B. abortus cells) was incubated for 3 h with 50 μl of the milk or serum sample. Milk samples were defatted by centrifugation (1 min at 6,000 × g) and tested undiluted; serum samples were instead diluted 10−1, 10−2, and 10−3 with PBS. Following incubation with the milk or serum sample, bacteria were washed twice with PBS containing 1% bovine serum albumin (PBS-BSA) and incubated for 1 h with 50 μl of either RαCFITC or RαWBFITC diluted 5 × 10−3 with PBS-BSA. Titration experiments established that at this dilution both reagents gave maximal specific fluorescence. Bacteria (S. aureus or B. abortus) were washed again with PBS-BSA and analyzed with the flow cytometer. The instrument (FACScan; Becton Dickinson Immunocytometry Systems, San Jose, Calif.) was equipped with a 15-mW, air-cooled, 488-nm-wavelength argon ion laser. FITC fluorescence was collected through a 530/30-nm bandpass filter. For each sample, the data of 10,000 events were analyzed by using Consort 32 software (Hewlett-Packard, Sunnyvale, Calif.). No gates were set around the particles. Results are presented as the mean channel of fluorescence for the treated sample minus the mean channel of fluorescence for the control tubes (incubated with PBS). When PBS was replaced with normal milk or normal serum, the mean values of control tubes were slightly more variable (two to three channels higher or lower). Logarithmic units (log10 U) were transformed into linear channels (LC) by using the formula LC = total number of channels/number of log decades × log10 U. The total number of channels and the number of log decades of the instrument were 1,024 and 4, respectively.
DCT.
The double cytometric test (DCT) detects anti-S. aureus and anti-B. abortus antibodies simultaneously by flow cytometry. About 107 latex particles, 3 μm in diameter (Polyscience Ltd., Eppelheim, Germany), were incubated with 1 ml of 0.1% 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (Sigma) for 4 h at room temperature. Particles were washed with PBS and incubated overnight at 4°C with 107 bacteria (B. abortus) resuspended in 1 ml of 0.025 M 2-(N-morpholino)ethanesulfonic acid (Sigma). Bacteria adhering to the latex particles were washed with PBS, saturated with PBS-BSA for 30 min at 37°C, and resuspended in 1 ml of PBS-BSA containing 0.1% NaN3. Fifty microliters of this suspension (B. abortus covalently bound to latex particles) and 50 μl of an S. aureus suspension (at about 107 bacteria/ml of PBS-BSA) were incubated for 3 h at room temperature under agitation with the milk sample being tested (undiluted and previously defatted by centrifugation). Bacteria were washed twice with PBS-BSA and incubated for 1 h with 50 μl of RαWBFITC or RαCFITC diluted 5 × 10−3. Forward scatter (FSC) and side scatter (SSC) were analyzed on a linear scale, and FITC fluorescence was analyzed on a logarithmic scale. FSC correlates with the size of the particles, and SSC correlates with their granularity (fine internal structure). In the analysis, gates were set around B. abortus (R1) and S. aureus (R2) on the basis of their FSC and SSC. Histogram analysis was performed on FITC fluorescence for both R1 and R2. The mean channel was calculated as described for the SCT.
Statistics.
Intra- and interassay coefficients of variation were measured by testing blood and milk samples with known low and high titers in triplicate on the same day (intra-assay) and on four different days (interassay). The correlation coefficients were calculated as described elsewhere (22).
Specificity and sensitivity.
Sensitivity and specificity were calculated as described elsewhere (3).
Sampling.
Individual serum samples from 150 lactating cows and as many lactating water buffaloes were tested for the presence of B. abortus and S. aureus antibodies by SCT, DCT, and CFT; milk samples from the same animals were tested by SCT and DCT. One hundred of these samples were collected from herds in which no cases of either brucellosis or mastitis had been reported during the previous 2 to 5 years (control population), and 50 were collected from herds with reported cases of brucellosis and/or mastitis. Also included in the study were serum samples from 30 water buffaloes vaccinated 2 to 4 months earlier with B. abortus (19). All milk and blood samples were collected within a period of about 2 weeks and tested blindly, that is, without knowledge of the health of the animals from which they were collected.
RESULTS
Identification of anti-B. abortus antibodies in milk and serum by SCT.
Serum samples were tested by SCT and CFT; milk samples displayed anticomplement activity and were therefore tested by SCT only. The levels of discrimination between positive and negative samples with respect to the presence of antibodies against B. abortus were set at the mean channel for the negative-control population plus twice the standard deviation (5, 16) for the SCT and at <20 international complement fixation units (ICFU)/ml for the CFT (1). ICFU measure the titer of anti-B. abortus antibodies present in the sample to be tested, in reference to a standard serum taken to contain 103 ICFU/ml (1).
By these criteria, the two techniques gave fully concordant results for the control population of cows and of water buffaloes, with milk and serum samples all being negative (Table 1).
TABLE 1.
Comparison of SCT and CFT for the detection of antibodies to B. abortusa
| Animal | No. of positive animals/total no. of animals in group:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| I
|
II
|
III
|
|||||||
| Serum
|
Milk
|
Serum
|
Milk
|
Serum
|
Milk
|
||||
| CFT | SCT | SCT | CFT | SCT | SCT | CFT | SCT | SCT | |
| Cow | 0/100 | 0/100 | 0/100 | 15/50 | 15/50 | 15/50 | NDb | ND | ND |
| Water buffalo | 0/100 | 0/100 | 0/100 | 16/50 (20/50)c | 20/50 | 20/50 | 10/30 (20/30)c | 20/30 | ND |
Animals were divided into the following groups: I, animals from herds free of brucellosis; II, animals from herds with reported cases of brucellosis; and III, vaccinated animals.
ND, not done.
Numbers in parentheses include animals that were positive when tested again 1 month later.
Among the animals from herds with a high incidence of brucellosis, four water buffaloes gave contrasting results; while no anti-B. abortus antibodies were detected in the sera of these subjects by CFT, antibodies were detected in the serum and milk samples by SCT (Table 1). Bacterial cultures established the presence of B. abortus in the milk of the four animals, and blood samples taken 1 month later were positive in the CFT.
The two techniques also yielded discrepant results for the group of vaccinated animals; 10 of 30 subjects were CFT negative but SCT positive. One month later, these 10 animals all were positive by CFT.
No animal was found to be positive by CFT and negative by SCT. The intra- and interassay coefficients of variation for the SCT were 4 and 5%, respectively, and were not influenced by the level of the antibodies.
Properties of the SCT.
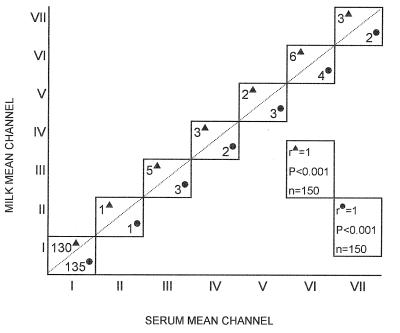
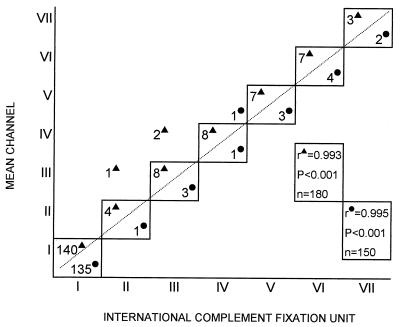
The levels of anti-B. abortus antibodies in the serum and in the milk of each animal were almost identical (Fig. 1). Among the 300 tested animals (150 cows and 150 water buffaloes), the individual difference between the milk and serum antibody levels was always less than 4% (i.e., within the range of the intra-assay coefficient of variation) and the relative correlation coefficient was especially high (r = 1; P < 0.001) for both cows and water buffaloes (Fig. 2). More importantly, the antibody level could be established by testing a single dilution of the milk or serum sample (Fig. 1). This characteristic was observed over the whole range (three logarithmic decades) of mean channel values (Fig. 1). When the antibody levels measured by SCT and by CFT were compared (Fig. 3), a very high correlation coefficient (r = 0.99 for both cows and water buffaloes; P < 0.001) was found, indicating that the antibody level, as measured by SCT, can be easily converted into ICFU and vice versa.
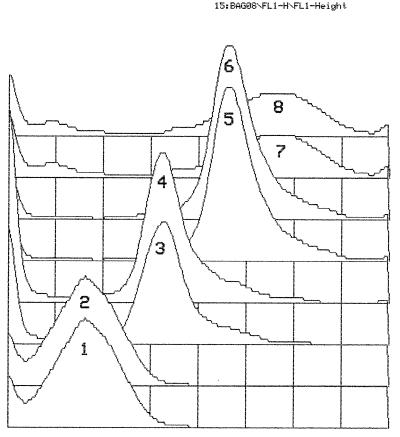
FIG. 1.
Mean channel values for milk (curves 1, 3, 5, and 7) and serum (curves 2, 4, 6, and 8) samples from four different animals. The mean channel values for the curves were as follows: curve 1, 241; curve 2, 238; curve 3, 425; curve 4, 427; curve 5, 590; curve 6, 588; curve 7, 758; and curve 8, 762. Abscissa, mean channel; ordinate, number of events.
FIG. 2.
Correlation between serum and milk mean channel values for water buffaloes (▴) and cows (•) as measured by SCT. Animals were divided into the following groups: animals with serum and milk mean channel values ± standard deviations of 76 ± 1.7 (group I), 190 ± 3.6 (group II), 240 ± 6 (group III), 341 ± 7.8 (group IV), 425 ± 9.7 (group V), 578 ± 8.4 (group VI), and 757 ± 9.4 (group VII). n, number of animals tested.
FIG. 3.
Correlation between mean channel values, as measured by SCT, and ICFU, as measured by CFT, for water buffaloes (▴) and cows (•). Animals were divided into the following groups: animals with mean channel (± standard deviation) and ICFU values of 77 ± 1.5 and <20 (group I), 187 ± 3.5 and 20 (group II), 237 ± 5 and 40 (group III), 345 ± 6 and 80 (group IV), 430 ± 7 and 160 (group V), 588 ± 8 and 320 (group VI), and 763 ± 9.8 and 640 (group VII). n, number of animals tested.
Identification of anti-S. aureus in milk by SCT.
The level of discrimination between positive and negative samples with respect to the presence of anti-S. aureus antibodies was set by the same criterion adopted for anti-B. abortus antibodies (the mean channel of the control population plus twice the standard deviation). The 200 samples (100 cow and 100 water buffalo samples) representing the control population were all negative. Of the 100 samples derived from farms with a high incidence of mastitis, 65 (35 cow and 30 water buffalo samples) were positive (Table 2); the remaining 35 samples were negative. Bacterial cultures detected the presence of S. aureus in 40 of the positive samples; 1 month later, S. aureus was isolated from the remaining 25 samples.
TABLE 2.
Detection of S. aureus by SCT and culturea
| Animal | No. of positive animals in group:
|
|||
|---|---|---|---|---|
| I
|
II
|
|||
| By SCT | By culture | By SCT | By culture | |
| Cow | 0/100 | 0/100 | 35/50 | 20/50 (35/50)b |
| Water buffalo | 0/100 | 0/100 | 30/50 | 20/50 (30/50)b |
Animals were divided into the following groups: I, animals from herds free of mastitis; and II, animals from herds with reported cases of mastitis.
Number in parentheses include animals that were positive when tested again 1 month later.
Simultaneous detection of anti-B. abortus and anti-S. aureus antibodies by DCT.
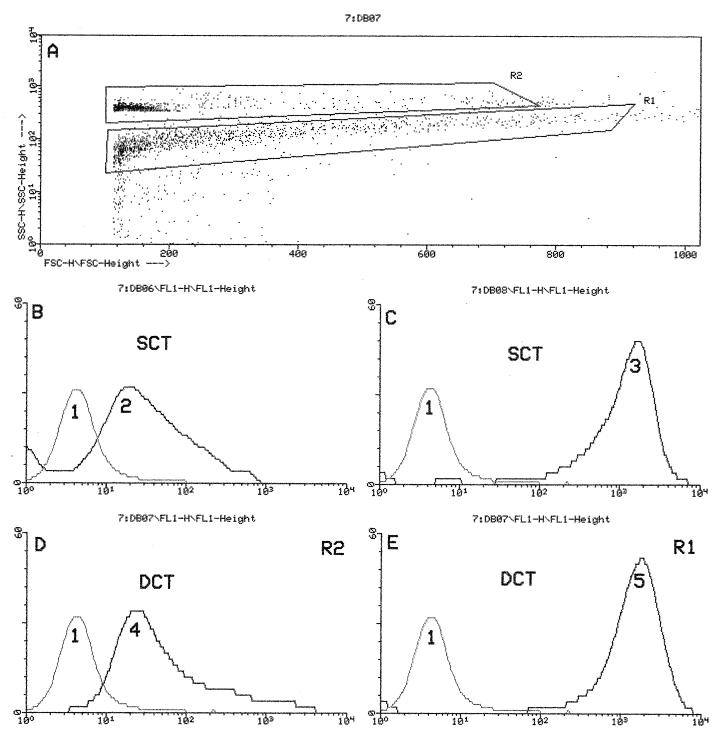
The potential of the cytometer to carry out two immunofluorescence measurements at the same time was exploited to detect antibodies against B. abortus and S. aureus simultaneously. For this purpose, the size of one of the two antigens (B. abortus) was altered by covalently binding it to latex particles. With this artifice, the antibodies against the two bacteria could be identified simultaneously (Fig. 4). Milk samples from 300 animals were tested by SCT and DCT. The fluorescence intensities (the mean channel values) obtained with the two assays were very similar (Fig. 4 and Table 3). This result indicates that in the DCT the two immunofluorescence signals do not influence each other at all.
FIG. 4.
(A) Dot plot showing FSC (abscissa) versus SSC (ordinate) of S. aureus (R1) and B. abortus (R2); the latter antigen was covalently bound to latex particles. The mean channel values of B. abortus antibody curves measured by SCT (B) and DCT (D) were practically the same. Analogously, the mean channel values of S. aureus antibody curves remained unchanged, whether measured by SCT (C) or DCT (E). A DCT was developed by using as antigens the bacteria shown in panel A. DCT and SCT were carried out on the same milk sample. Curve 1, control (no milk); curves 2 to 5, activity curves. Mean channel values of curves 2 to 5 were as follows: curve 2, 424; curve 3, 828; curve 4, 418; and curve 5, 835. Abscissa, mean channel; ordinate, number of events.
TABLE 3.
Mean channel values for milk samples as measured by SCT and DCTa
| Group | No. of samples | Mean channel value by:
|
|||
|---|---|---|---|---|---|
| SCT
|
DCT
|
||||
| B. abortus | S. aureus | B. abortus | S. aureus | ||
| I | 209 | 76 ± 1.7 | 78 ± 1.6 | 78 ± 1.5 | 79 ± 1.9 |
| II | 2 | 190 ± 3.6 | 78 ± 1.3 | 189 ± 5.6 | 80 ± 1.3 |
| 6 | 240 ± 6 | 77 ± 1.4 | 245 ± 6.3 | 79 ± 1.3 | |
| 3 | 341 ± 7.8 | 77 ± 1.5 | 346 ± 8.8 | 80 ± 1.3 | |
| 3 | 425 ± 9.7 | 78 ± 1.4 | 419 ± 5.7 | 80 ± 1.4 | |
| 7 | 581 ± 6.4 | 78 ± 1.6 | 586 ± 8.4 | 79 ± 1.3 | |
| 5 | 757 ± 9.4 | 77 ± 1.3 | 764 ± 10 | 79 ± 1.4 | |
| III | 13 | 76 ± 1.4 | 258 ± 5.2 | 79 ± 1.3 | 268 ± 5.2 |
| 8 | 76 ± 1.4 | 365 ± 6 | 78 ± 1.5 | 360 ± 7 | |
| 7 | 76 ± 1.3 | 508 ± 7 | 79 ± 1.3 | 518 ± 7 | |
| 15 | 75 ± 1.5 | 671 ± 7.5 | 79 ± 1.4 | 681 ± 7.5 | |
| 13 | 76 ± 1.3 | 820 ± 11 | 78 ± 1.3 | 831 ± 9.8 | |
| IV | 2 | 240 ± 6 | 256 ± 8.2 | 250 ± 6 | 246 ± 6.2 |
| 2 | 341 ± 7 | 365 ± 7 | 345 ± 6.8 | 375 ± 9 | |
| 3 | 581 ± 7 | 508 ± 3 | 588 ± 7.4 | 498 ± 9.7 | |
| 2 | 423 ± 9 | 835 ± 10 | 429 ± 5 | 830 ± 9.2 | |
Animals were divided into the following groups: I, animals from herds free of brucellosis and mastitis; II, animals from herds with reported cases of brucellosis; III, animals from herds with reported cases of mastitis; and IV, animals from herds with reported cases of brucellosis and mastitis.
DISCUSSION
One of the objectives of the present study was to validate the capacity of SCT to detect the presence of anti-B. abortus antibodies in the serum. For this purpose, SCT was compared with CFT, the reference test for brucellosis in Italy (14). This comparison (Table 1) established that SCT and CFT display the same specificity (100%) but that SCT has a higher sensitivity (100%, versus 74.5% for CFT).
SCT was validated also with respect to its capacity to detect antibodies against S. aureus in the milk. In this case, SCT was compared with bacterial culture. In milk samples, the presence of antibodies, as detected by SCT, invariably corresponded to the presence of the pathogen, but SCT gave an earlier response in 25 (25%) of the 100 cases (Table 2). Thus, the present study extends previous results (6) and confirms the validity of SCT as a specific serological test for mastitis. To the knowledge of the authors, the SCT is the only specific test for mastitis available at present.
The next step was to ascertain whether anti-B. abortus antibodies could be reliably detected in the milk in addition to the serum. The result was clear: whenever antibodies were present in the serum, they were also present in the milk, at the same level (Fig. 2). We then moved on to see whether antibodies to the two pathogens could be identified concurrently.
The fluorescence intensities (the mean channel values) of milk samples determined by SCT and by DCT were for all practical purposes the same (Table 3), demonstrating that the two antibodies could be identified concurrently without any loss of sensitivity.
In this study, B. abortus 19 was chosen as the antigen since it was readily available. However, unpublished results of this laboratory indicate that other strains (544 [ATCC 23448] and B3196 [ATCC 23452]) with a smooth phenotype perform equally well. The use of protein A-deficient S. aureus strain Wood 46 as the antigen was instead dictated by evidence (6) that protein A-positive strains bind immunoglobulin on the bacterial surface and reduce the sensitivity of the assay. This strain is also readily available (ATCC 10832).
In addition to the ability, unique to the DCT, to identify antibodies against two distinct targets (and potentially more than two) simultaneously, the methods described here (SCT and DCT) are ideal for quantitating antibodies. While in the CFT (and in other methods as well) a series of dilutions must be tested to establish the antibody level, in the SCT and DCT a single dilution is sufficient (Fig. 4). Under standardized testing conditions, a close correlation can be found between mean fluorescence and antibody level (Fig. 3). Mean channel values can thus be transformed into ICFU, and individual samples can be compared with each other or with a standard. Another useful feature of these techniques is represented by the high reproducibility of the results. Coefficients of variation as low as 4 to 5% can be attained. This is possible because the methods permit the use of unchanged gate and marker settings throughout the experiments, i.e., optimized and uniform testing conditions for all samples. The SCT and DCT also require only short incubation times. More than 50 samples (and possibly many more) can be tested in one workday. Thus, these methods have the essential requirements for routine testing.
In the absence of repeated serological follow-up of the animals, it is difficult to interpret the meaning of the antibody level (mean channel value) differences observed in the present study. In particular, the high antibody levels reported in Table 3 might indicate, with equally likelihood, prolonged infection, a recent relapse, or chronic disease. Although determining them was not included among the objectives of the present study, the changes in antibody isotype (2, 15, 21) and titer (2) occurring during B. abortus infection are approaching clinical relevance. By using two isotype-specific secondary antibodies, one labelled with fluorescein and the other labelled with phycoerythrin, the DCT can be used to study antibodies of two different isotypes simultaneously. In addition, as already discussed, the DCT can assess the antibody level rapidly.
At present, the main limitation of both the SCT and the DCT is the high cost of the cytometer. However, this instrument, in view of its versatility, is expected to soon become widely used in many fields, one being virology. Recently, the simultaneous detection by flow cytometry of three viruses has been reported (11). The availability of visible-wavelength diode lasers of low price, high efficiency, and long lifetime (8) is expected to promote broader applications of flow cytometry and, consequently, lower prices.
In their present forms, the SCT and DCT can be used in any laboratory in which a cytometer is available. In addition, both techniques can be easily adapted to identify antibodies against pathogens other than S. aureus and B. abortus. The DCT could be the technique of choice in the case of large-scale eradication programs for brucellosis. In comparison to the CFT, it can detect a larger fraction of the animals with circulating antibodies while, at the same time, providing information about the incidence of mastitis, another disease that is very costly to the dairy industry.
REFERENCES
- 1.Alton G G, Jones L M, Angus R D, Verger J M, editors. Techniques for the brucellosis laboratory. Paris, France: Institut National de la Recherche Agronomique; 1988. [Google Scholar]
- 2.Ariza J, Pellicer T, Pallares R, Foz A, Gudiol F. Specific antibody profile in human brucellosis. Clin Infect Dis. 1992;14:131–140. doi: 10.1093/clinids/14.1.131. [DOI] [PubMed] [Google Scholar]
- 3.Baum M, Zamir O, Bergman-Rios R, Katz E, Beider Z, Cohen A, Banai M. Comparative evaluation of microagglutination test and serum agglutination test as supplementary diagnostic methods for brucellosis. J Clin Microbiol. 1995;33:2166–2170. doi: 10.1128/jcm.33.8.2166-2170.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop G C, Bosman P P, Herr S. Bovine brucellosis. In: Coetzer J A W, Thompson G R, Tustin R C, editors. Infectious diseases of livestock. Oxford, United Kingdom: Oxford University Press; 1994. pp. 1053–1063. [Google Scholar]
- 5.Clarke S M. A novel enzyme linked immunosorbent assay (ELISA) for the detection of beryllium antibodies. J Immunol Methods. 1991;137:65–72. doi: 10.1016/0022-1759(91)90394-u. [DOI] [PubMed] [Google Scholar]
- 6.D’Apice L, Fenizia D, Capparelli R, Scala F, Iannelli D. Detection of antibodies to Staphylococcus aureus in water buffalo milk by flow cytometry. Res Vet Sci. 1996;60:179–181. doi: 10.1016/s0034-5288(96)90015-6. [DOI] [PubMed] [Google Scholar]
- 7.Devriese L A, Laevens H, Haesebrouck F. A simple identification scheme for coagulase negative staphylococci from bovine mastitis. Res Vet Sci. 1994;57:240–244. doi: 10.1016/0034-5288(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 8.Doornbos R M, de Grooth B G, Kraan Y M, van der Poel C J, Greeve J. Visible diode lasers can be used for flow cytometric immunofluorescence and DNA analysis. Cytometry. 1994;15:267–271. doi: 10.1002/cyto.990150312. [DOI] [PubMed] [Google Scholar]
- 9.Grimont F, Verger J M, Cornelis P, Limet J, Lefevre M, Grayon M, Regnault B, Van Broeck J, Grimont P A D. Molecular typing of Brucella with cloned DNA probes. Res Microbiol. 1992;143:55–65. doi: 10.1016/0923-2508(92)90034-l. [DOI] [PubMed] [Google Scholar]
- 10.Hall S M, Confer A W. Comparison of TRACK XI fluorimetric immunoassay system with other serologic tests for detection of serum antibody to Brucella abortus in cattle. J Clin Microbiol. 1987;25:350–354. doi: 10.1128/jcm.25.2.350-354.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iannelli, D., L. D’Apice, C. Cottone, M. Viscardi, F. Scala, A. Zoina, G. Del Sorbo, P. Spigno, and R. Capparelli. Simultaneous detection of cucumber mosaic virus, tomato mosaic virus and potato virus Y by flow cytometry. J. Virol. Methods, in press. [DOI] [PubMed]
- 12.Kapur V, Sischo W M, Greer R S, Whittam T S, Musser J M. Molecular population genetic analysis of Staphylococcus aureus recovered from cows. J Clin Microbiol. 1995;33:376–380. doi: 10.1128/jcm.33.2.376-380.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miles H, Lesser W, Sears P. The economic implications of bioengineered mastitis control. J Dairy Sci. 1992;75:596–605. doi: 10.3168/jds.S0022-0302(92)77797-2. [DOI] [PubMed] [Google Scholar]
- 14.Ministero della Sanità. Gazzetta ufficiale della Repubblica Italiana, ser. gen. no. 277. Regolamento concernente il piano nazionale per la eradicazione della brucellosi. Rome, Italy: Ministero della Sanità; 1994. [Google Scholar]
- 15.Pugh G, Jr W, Tabatabai L B. Alteration of protective and serologic responses in BALB/c mice vaccinated with chemically modified versus nonmodified proteins of Brucella abortus 19. Infect Immun. 1994;62:5327–5334. doi: 10.1128/iai.62.12.5327-5334.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruth S, Drieduck P C, Weening R S, Out T A. ELISA procedures for the measurement of IgG subclass antibodies to bacterial antigens. J Immunol Methods. 1991;140:67–78. doi: 10.1016/0022-1759(91)90127-2. [DOI] [PubMed] [Google Scholar]
- 17.Schalm O W, Noorlander D O. Experiments and observations leading to the development of the California mastitis test. J Am Vet Med Assoc. 1957;130:199–204. [PubMed] [Google Scholar]
- 18.Schalm O W, Carrol E J, Jain N C. Le mastiti della bovina. 1975. pp. 1–26. , 172–191. Edagricole, Bologna, Italy. [Google Scholar]
- 19.Schiavo A. Patologia bovina e bufalina e legislazione veterinaria. 1990. pp. 425–491. . Bari, Laterza, Italy. [Google Scholar]
- 20.Sischo W M, Heider L E, Miller G Y, Moore D A. Prevalence of contagious pathogens of bovine mastitis and use of mastitis control practices. J Am Vet Med Assoc. 1993;202:595–600. [PubMed] [Google Scholar]
- 21.Spencer T L, Burgess G W. Enzyme-linked immunosorbent assay for Brucella ovis specific antibody in ram sera. Res Vet Sci. 1984;36:194–198. [PubMed] [Google Scholar]
- 22.Steel R G, Torrie J H. Principles and procedures of statistics. New York, N.Y: McGraw-Hill Book Company, Inc.; 1960. pp. 366–387. [Google Scholar]
- 23.Vendrell J-P, Conge A-M, Segondy M, Lombroso S, Huguet M-F, Bertrand A, Janbon F, Serre A. In vitro antibody secretion by peripheral blood mononuclear cells as an expression of the immune response to Brucella spp. in humans. J Clin Microbiol. 1992;30:2200–2203. doi: 10.1128/jcm.30.8.2200-2203.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young E. Clinical manifestations of human brucellosis. In: Young E, Corbel M, editors. Brucellosis: clinical and laboratory aspects. Boca Raton, Fla: CRC Press, Inc.; 1989. pp. 97–126. [Google Scholar]