Abstract
An enzyme linked immunosorbent assay (ELISA) for detecting antibody to antigenic Trichomonas vaginalis macromolecules has been identified using whole cells or an aqueous protein extract as antigen. The test was developed under optimum conditions using serum samples from experimental animals. The sensitivity of the ELISA was equal to or greater than that obtained by radioimmunoprecipitation and electrophoresis-fluorography techniques. The ELISA was capable of assessing antibody responses during the development of lesions in animals inoculated subcutaneously and it reproducibly measured the individual classes immunoglobulins directed at T vaginalis. The colorimetric assay was also suitable for showing cross reactivity between trichomonal species as well as between different strains of T vaginalis. Conditions established for monitoring antibody to trichomanads in immunised rabbits or infected mice were equally effective for human materials, such as serum or vaginal washes. Serum from experimental animals or infected people showed high concentrations of IgG, IgA, and IgM antibody to trichomonads. Only antibodies of the IgG and IgA class were detected in vaginal washes from women with acute trichomoniasis. No IgE antibody to trichomonads was found under a variety of conditions in serum samples from patients or experimental animals.
Full text
PDF
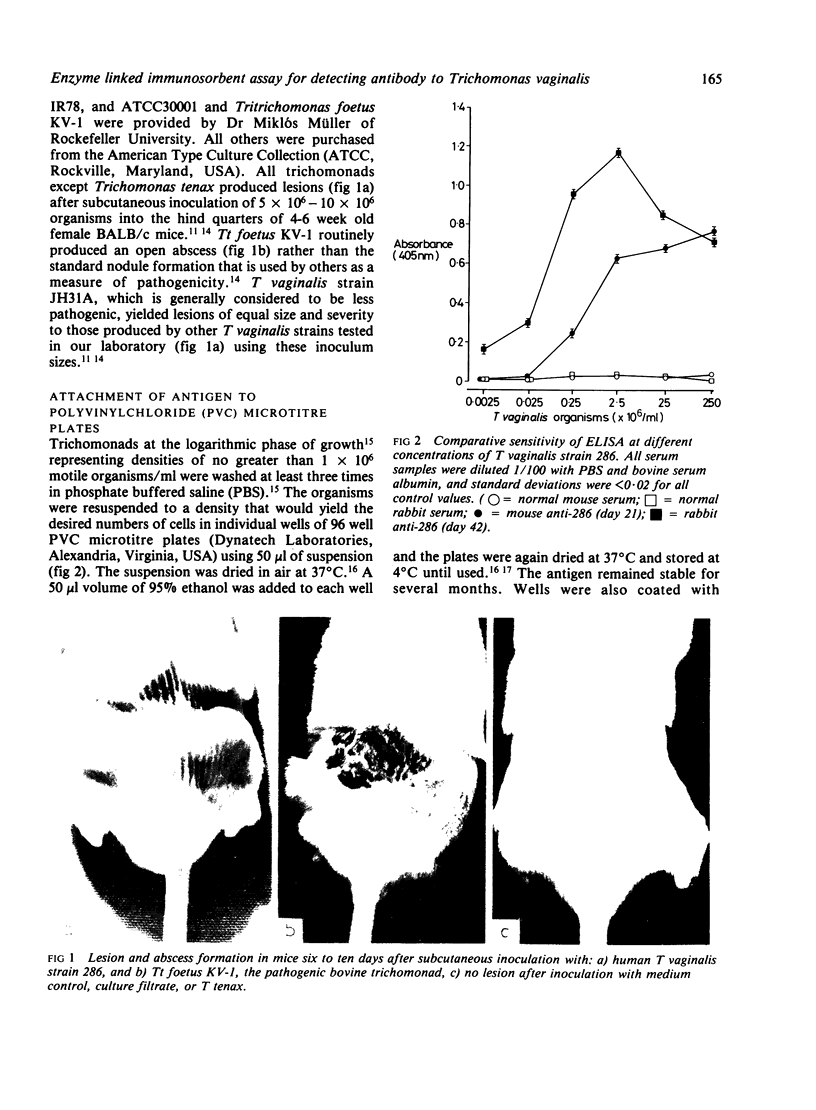

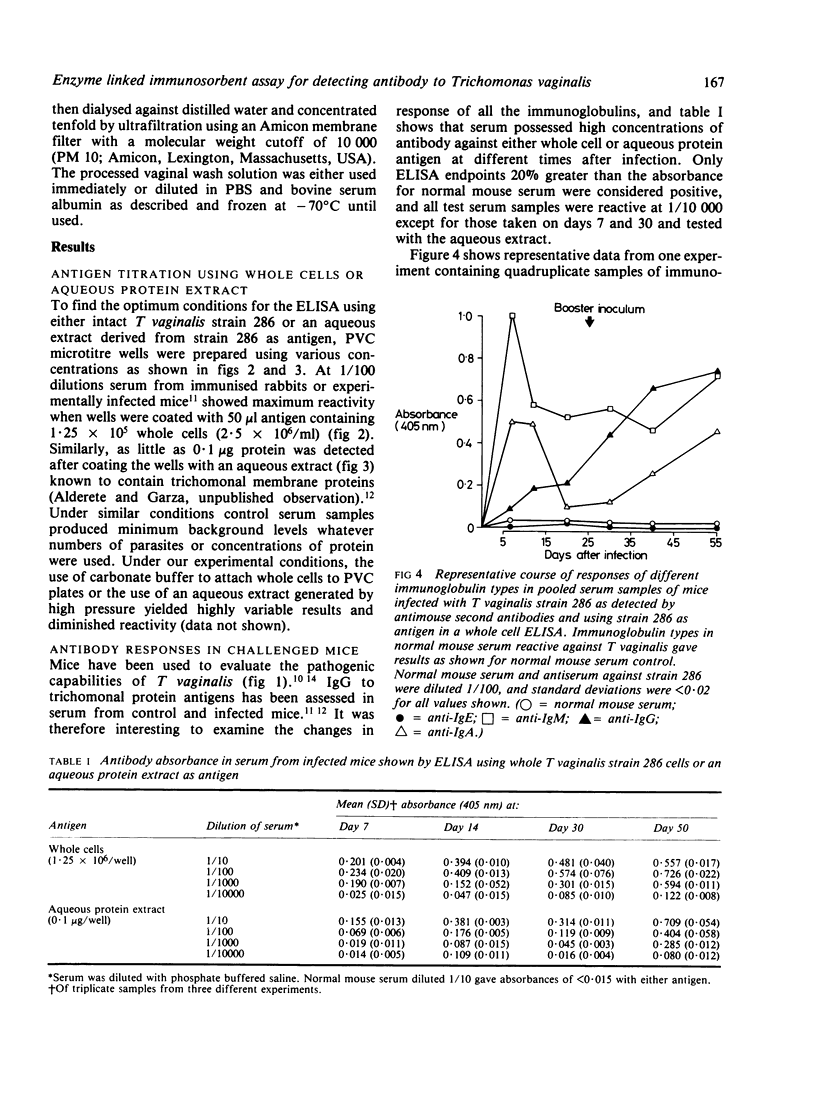
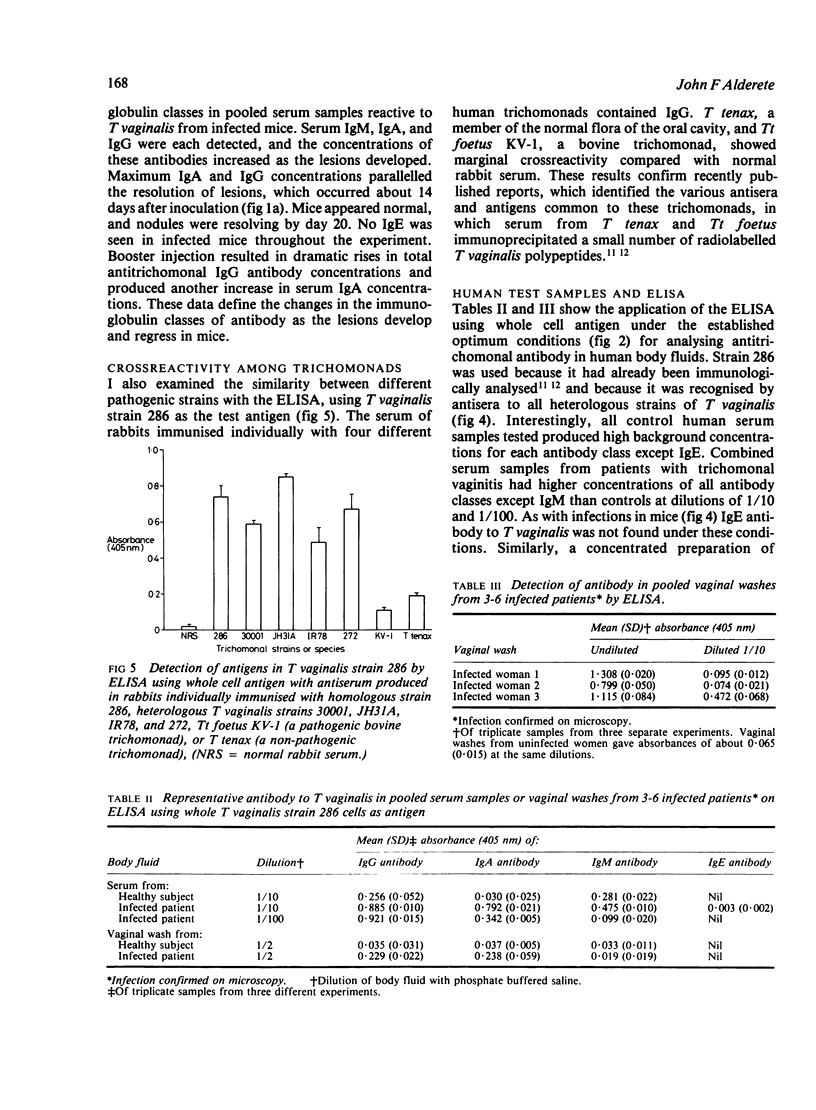


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackers J. P., Lumsden W. H., Catterall R. D., Coyle R. Antitrichomonal antibody in the vaginal secretions of women infected with T. vaginalis. Br J Vener Dis. 1975 Oct;51(5):319–323. doi: 10.1136/sti.51.5.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F. Antigen analysis of several pathogenic strains of Trichomonas vaginalis. Infect Immun. 1983 Mar;39(3):1041–1047. doi: 10.1128/iai.39.3.1041-1047.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F. Identification of immunogenic and antibody-binding membrane proteins of pathogenic Trichomonas vaginalis. Infect Immun. 1983 Apr;40(1):284–291. doi: 10.1128/iai.40.1.284-291.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Pearlman E. Pathogenic Trichomonas vaginalis cytotoxicity to cell culture monolayers. Br J Vener Dis. 1984 Apr;60(2):99–105. doi: 10.1136/sti.60.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony R. L., Christensen H. A., Johnson C. M. Micro enzyme-linked immunosorbent assay (ELISA) for the serodiagnosis of New World leishmaniasis. Am J Trop Med Hyg. 1980 Mar;29(2):190–194. doi: 10.4269/ajtmh.1980.29.190. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cobbold S. P., Waldmann H. A rapid solid-phase enzyme-linked binding assay for screening monoclonal antibodies to cell surface antigens. J Immunol Methods. 1981;44(2):125–133. doi: 10.1016/0022-1759(81)90340-9. [DOI] [PubMed] [Google Scholar]
- Green R. L., Scales R. W., Kraus S. J. Increased serum immunoglobulin E concentrations in venereal diseases. Br J Vener Dis. 1976 Aug;52(4):257–260. doi: 10.1136/sti.52.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honigberg B. M., Livingston M. C., Frost J. K. Pathogenicity of fresh isolates of Trichomonas vaginalis: "the mouse assay" versus clinical and pathologic findings. Acta Cytol. 1966 Sep-Oct;10(5):353–361. [PubMed] [Google Scholar]
- Horowitz S. A., Cassell G. H. Detection of antibodies to Mycoplasma pulmonis by an enzyme-linked immunosorbent assay. Infect Immun. 1978 Oct;22(1):161–170. doi: 10.1128/iai.22.1.161-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramár J., Kucera K. Immunofluorescence demonstration of antibodies in urogenital trichomoniasis. J Hyg Epidemiol Microbiol Immunol. 1966;10(1):85–88. [PubMed] [Google Scholar]
- Mathews H. M., Healy G. R. Evaluation of two serological tests for Trichomonas vaginalis infection. J Clin Microbiol. 1983 May;17(5):840–843. doi: 10.1128/jcm.17.5.840-843.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McENTEGART M. G. The application of a haemagglutination technique to the study of Trichomonas vaginalis infections. J Clin Pathol. 1952 Aug;5(3):275–280. doi: 10.1136/jcp.5.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison-Plummer J., Alderete J. F., Baseman J. B. Enzyme-linked immunosorbent assay for the detection of serum antibody to outer membrane proteins of Treponema pallidum. Br J Vener Dis. 1983 Apr;59(2):75–79. doi: 10.1136/sti.59.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K. M., Alderete J. F. Acquisition of alpha 1-Antitrypsin by a pathogenic strain of Trichomonas vaginalis. Infect Immun. 1983 May;40(2):640–646. doi: 10.1128/iai.40.2.640-646.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K. M., Alderete J. F. Host plasma proteins on the surface of pathogenic Trichomonas vaginalis. Infect Immun. 1982 Aug;37(2):755–762. doi: 10.1128/iai.37.2.755-762.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street D. A., Taylor-Robinson D., Ackers J. P., Hanna N. F., McMillan A. Evaluation of an enzyme-linked immunosorbent assay for the detection of antibody to Trichomonas vaginalis in sera and vaginal secretions. Br J Vener Dis. 1982 Oct;58(5):330–333. doi: 10.1136/sti.58.5.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su K. E. Antibody to Trichomonas vaginalis in human cervicovaginal secretions. Infect Immun. 1982 Sep;37(3):852–857. doi: 10.1128/iai.37.3.852-857.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeltzer P. M., Pepose J. S., Bishop N. H., Miller J. N. Microassay for immunoglobulin G antibodies to Treponema pallidum with radioiodinated protein A from staphylococcus aureus: immunoglobulin G response in experimental syphilis in rabbits. Infect Immun. 1978 Jul;21(1):163–170. doi: 10.1128/iai.21.1.163-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



