Abstract
Objectives
While protection against pertussis following maternal tetanus-diphtheria-and-acellular-pertussis (Tdap) vaccination was demonstrated in healthy term-born infants, no evidence is available on Tdap vaccination in combination with immune-modulating therapy during pregnancy. In this pilot study, we explored whether treatment with tumour necrosis factor alpha inhibitors (TNFis) in pregnant patients with rheumatic disease interferes with Tdap vaccine responses and affects maternal anti-pertussis IgG antibody levels in newborns.
Methods
Patients were included by a rheumatologist during pregnancy in case they received maternal Tdap vaccination in the late-second or early-third trimester of pregnancy. Blood samples were obtained from mothers during the first pregnancy trimester, 3 months after delivery and from the umbilical cord. IgG antibody levels against Tdap-included antigens were measured using a bead-based multiplex immunoassay. Findings on patients exposed to TNFis were compared with those from TNFi-unexposed patients and with data from a historical comparator study among healthy Tdap vaccinated mother–infant pairs (n=53).
Results
66 patients (46 exposed and 20 unexposed to TNFIs) were enrolled. No major differences in IgG antibody levels were observed between TNFi-exposed and unexposed mothers before maternal Tdap vaccination and 3 months after delivery. In cord sera, however, antibody levels against pertussis toxin were significantly lower after TNFi-treatment (35.94 IU/mL, 95% CI 20.68 to 62.45) compared with no TNFi-treatment of mothers with rheumatic disease (94.61 IU/mL, 95% CI 48.89 to 183.07) and lower compared with a cohort of healthy mothers (125.12 IU/mL, 95% CI 90.75 to 172.50). We observed similar differences for filamentous haemagglutinin, pertactin, tetanus toxoid and diphtheria toxoid.
Conclusion
These preliminary data indicate no major differences in IgG antibody levels on maternal Tdap vaccination in pregnant women with or without immune-modulating treatment, although our findings suggest that TNFis during pregnancy induce lower maternal anti-pertussis-specific protective antibody levels in newborns.
Keywords: Tumor Necrosis Factor Inhibitors; Vaccination; Arthritis, Rheumatoid; Biological Therapy
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Up to 90% protection against pertussis following maternal tetanus-diphtheria-and-acellular-pertussis (Tdap) vaccination has been demonstrated in healthy term-born infants, but no evidence is available on maternal Tdap vaccination in combination with immune-modulating therapy during pregnancy.
WHAT THIS STUDY ADDS
Our findings indicate lower cord blood IgG antibody levels in case of tumour necrosis factor alpha inhibitor (TNFi)-treatment during pregnancy.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Early start of primary series of three pertussis vaccinations in case of TNFi-exposure during pregnancy seems appropriate despite maternal Tdap vaccination.
Introduction
Pertussis, also known as whooping cough, is an extremely contagious bacterial respiratory disease. The gram-negative bacterium Bordetella pertussis infiltrates respiratory epithelial cells and produces several kinds of toxins that interfere with secretion and bacterial clearance, causing the clinical symptoms of pertussis. The disease is especially dangerous in early infancy before start of the primary pertussis vaccination series, leaving newborns in the first months of life at increased risk for severe and potentially life-threatening complications. Endemic cycles of pertussis occur regularly and outbreaks have enhanced B. pertussis circulation over time and thereby pertussis infection in not fully vaccinated infants in the most recent years.1–3 Asymptomatic adolescents and adults in the same household seem to be the main source of transmission to newborns.4
Maternal vaccination enhances protection against vaccine-preventable infectious diseases in infants during the very first months of life, as a result of transplacental transfer of protective IgG antibodies.5–8 This process is mediated by the neonatal Fc receptor (FcRn) which is expressed on syncytiotrophoblast cells, and antibody transfer initiates between 12 and 17 week of pregnancy with rates that increase throughout gestation.5 Maternal vaccination against tetanus, diphtheria and acellular pertussis (Tdap) during the third trimester of pregnancy was shown to offer an approximate 90% effectiveness in protection against severe clinical pertussis until infants reach the age of 2–3 months, before they receive primary vaccinations.1 6 9 Nowadays, maternal Tdap vaccination is recommended by a growing number of countries, including the Netherlands.10
Current data on immunogenicity after maternal Tdap vaccination concern studies in healthy pregnant women, generally vaccinated during the third trimester.7 11 No evidence about the effects of maternal Tdap vaccination is available regarding women on immune-modulating therapy for rheumatic diseases. Biological disease-modifying antirheumatic drugs (bDMARDs), for example, treatment with tumour necrosis factor inhibitors (TNFis), are widely used as treatment for rheumatic diseases, both during and outside pregnancy.8 Recent studies have shown that the immune response on the COVID-19 vaccine is reduced in patients treated with immune-modulatory agents (including TNFi) for rheumatic disease.12–16 However, the available evidence on potential hampering effects of TNFi-therapy on antibody responses to Tdap vaccination in men and non-pregnant women is contradictory due to low power of studies and,17 18 though several reviews or studies point to a mildly reduced antibody response in case of TNFi-treatment.19–22 As a growing number of pregnant women with chronic inflammatory diseases receive TNFis, either alone or in combination with prednisone or other immune-modulating drugs, knowledge whether such treatment may interfere with maternal Tdap vaccine responses and subsequent transplacental antibody transfer is urgently needed to adapt vaccination strategies for newborns born to mothers on TNFi-therapy.
In this pilot study, our coprimary objectives were to assess the effects of TNFi-treatment in patients with rheumatic diseases on maternal IgG antibody levels against pertussis in both infants around birth, and mothers 3 months after delivery. We performed external validation through the comparison of antibody levels in TNFi-exposed patients versus TNFi-unexposed patients and also in healthy maternal Tdap vaccinated women and their offspring from a historical comparison cohort.11
Methods
Study participants
Patients were derived from the PreCARA study, which is a prospective cohort study on inflammatory rheumatic diseases before and during pregnancy.23 Regarding the current study, pregnant patients were included as early as possible during pregnancy from January 2019 to February 2022, provided that they had received a Tdap vaccination during pregnancy. Tdap vaccinations were administered during the late-second or early-third trimester of pregnancy. All women and their offspring were followed until at least 3 months after delivery. Subscribed medication for rheumatic disease, including therapy with TNFis, was decided by a rheumatologist prior to inclusion in this study, based on diagnosis and patients’ medical conditions. Participants were divided into two groups, that is, women on TNFis (used at any moment during pregnancy) and women not on TNFis. If patients were exposed to bDMARDS other than TNFis, they were excluded from the primary analysis, as the effects of non-TNFi bDMARDs on vaccination response in mothers were outside the scope of this study. These cases were assessed separately.
Venous blood samples were drawn from participants during their first trimester of pregnancy (ie, before Tdap immunisation) and approximately 3 months after delivery, along with a cord blood sample immediately after delivery. Samples were transported to the laboratory at room temperature and sera were stored at −80°C awaiting laboratory analyses.
Data were compared with a historical comparator group of healthy pregnant women without rheumatic disease and their offspring, who participated in a maternal Tdap vaccination immunogenicity study between January 2014 and March 2016, as described previously.11 All healthy pregnant women received maternal Tdap vaccination between 30 and 33 weeks gestational age (GA). In this cohort, blood samples were drawn right before immunisation and within 48 hours after delivery by finger prick, along with a cord blood sample within the first few hours after birth.
For defining demographic variables; maternal age (years) was calculated as time interval between mothers’ birth date and the date of delivery. Duration of rheumatic disease (years) was defined as the time since diagnosis until the date of delivery. Duration of pregnancy (weeks) was defined as time interval between first day of last menstruation period and the date of delivery. Type of rheumatic disease was defined as the official diagnosis by a rheumatologist. Disease activity was determined by the Disease Activity Score (DAS) with three variables: 28 swollen and tender joint count and C reactive protein (CRP) DAS in 28 Joints (DAS28CRP) and Bath Ankylosing Spondylitis Disease Activity Index and ASDAS (Ankylosing Spondylitis Disease Activity Score) in each trimester by a rheumatologist.
Laboratory analysis
Sera were analysed in the laboratory of the National Institute of Public Health and the Environment (RIVM) as described previously.24 In brief, IgG antibody concentrations against pertussis toxin (PT), filamentous haemagglutinin (FHA), pertactin (Prn), diphtheria toxoid (Dt) and tetanus toxoid (TT) were determined by a bead-based fluorescent multiplex immunoassay using Luminex xMAP-map-Luminex technology.24 In house reference serum and quality controls were used for pertussis antigens and sera were calibrated against the WHO International Standard Pertussis Antiserum (serum reference 06/140). Native PT (Netherlands Vaccine Institute) was used. The lower limit of quantification was 0.21 international units (IU)/mL as restricted by the dilution series of the reference line. Sera from the comparator cohort had been stored and analysed using the same procedures and in the same laboratory.23
Statistical analysis
This is a descriptive pilot study in a prospectively followed cohort of mother–infant pairs who were divided into three groups: (1) patients on TNFis, (2) patients not on TNFis and (3) healthy reference cohort. Demographics and differences between these three groups were estimated using basic descriptive statistics analysing two groups to another separately, for example, by t-tests, χ2 tests or non-parametric variants.
Absolute IgG antibody levels against the Tdap-vaccine antigens were log-transformed, assessed for following normal distributions and expressed in geometric mean concentrations (GMCs). We compared prevaccination and postmaternal Tdap GMCs in mothers 3 months after delivery between mother exposed and not exposed to TNFi. We could not compare these data with the healthy reference cohorts since considerable differences in timing of maternal Tdap vaccination and postvaccination blood sampling existed between the current PreCARA study cohort and historical healthy comparison cohort. Generalised estimating equation (GEE) models with an exchangeable correlation structure were used to adjust for any correlation between pairs of twins. For each of the Tdap-included antigens, crude GEE models were constructed. These models were constructed to calculate the p values of GMC comparison between the groups before and after vaccination, and in cord sera. Regarding measurements in cord sera, we adjusted for GA at Tdap vaccination and pregnancy duration. For postvaccination measurements, we adjusted for time interval between Tdap vaccination and the moment of postpartum blood sampling; this comparison was only made between two groups of PreCARA cohort and not with the healthy participants.
Also by using GEE models, an indication of transplacental transfer rates was estimated as ratios between absolute fetal-to-maternal antibody levels (cord sera vs maternal postvaccination sera at 3 months after delivery) and compared between the two groups of rheumatic disease patients.
As corticosteroid therapy may also reduce antibody titres in response to vaccines,25–27 a subgroup analysis was performed to assess the effects of combination therapy of TNFis with prednisone on GMCs in women with rheumatic disease.
All analyses were performed using R software, V.4.2.0.
Results
Demographics
In total, 66 pregnant patients with rheumatic diseases and a median age of 32.6 years (range 24–44) were enrolled in this study, of whom 46 were exposed and 20 unexposed to TNFis. Maternal Tdap vaccination was provided at a median of 27.6 weeks GA (range: 20.0–36.1) in patients exposed to TNFis, 27.0 weeks GA (range: 19.3–34.0) in patients unexposed to TNFis and 31.2 weeks GA (range: 29.8–33.0) in healthy controls. Based on the patients’ medical history, 65 of total 66 patients had been vaccinated against pertussis diphtheria and tetanus as a child (almost exclusively whole cell pertussis vaccine since the neonatal acellular pertussis vaccine was introduced under the Dutch Immunisation Program in 2001), and therefore, maternal Tdap vaccination was considered a booster in these cases. Most frequently used medication during pregnancy was certolizumab pegol (65%) in TNFi-exposed patients and hydroxychloroquine (56%) in TNFi-unexposed patients. Two patients received non-TNFi bDMARDs (rituximab and anakinra, n=1 each) and were not included in the primary analysis and analysed separately (see online supplemental table 1).
rmdopen-2023-002985supp001.pdf (1,016.6KB, pdf)
The two groups of women with rheumatic disease either exposed or unexposed to TNFis had similar demographics, including age, pregnancy duration, GA at vaccination and disease-related factors (table 1). Healthy women were vaccinated later during pregnancy compared with TNFi-exposed and unexposed women (mean gestational week at vaccination: 31 weeks, vs 28 weeks and 27 weeks, respectively, p<0.01). Healthy women had also a significantly longer pregnancy duration than TNFi-exposed and a marginally longer pregnancy duration than TNFi-unexposed women (40 weeks, vs 39 weeks and 39 weeks, respectively) (table 1).
Table 1.
Patients’ and healthy women’s characteristics, stratified for use or no use of TNFis ever during pregnancy
| Patients on TNFis; n=46 | P value (TNFis vs no TNFis) | Patients not on TNFis; n=20 | P value (no TNFis vs healthy women) | Healthy women; n=53 | P value (TNFis vs healthy women) | |
| Age at delivery, years±SD (range) | 32.8±3.1 (25.1–40.1) | 0.33 | 31.8±4.7 (24.6–43.9) | 0.53 | 32.5±3.4 (23.7–42.7) | 0.60 |
| Time since disease diagnosis, years±SD (range) | 10.8±7.7 (0.5–30.0) | 0.80 | 10.3±8.2 (1.0–31.0) | NA | NA | NA |
| Pregnancy duration at vaccination date, weeks±SD £ (range) | 27.6±4.2 (20.0–36.1) | 0.62 | 27.0±3.8 (19.3–34.0) | <0.01 | 31.2±0.8 (29.8–33.0) | <0.01 |
| Pregnancy duration at delivery, weeks±SD* (range) | 38.7±1.8 (34.1–42.1) | 0.40 | 39.1±1.9 (35.0–41.4) | 0.11 | 39.8±1.5 (36.0–42.0) | <0.01 |
| DAS28-CRP in third trimester±SD (range) | 1.9±0.6 (1.3–5.3) | 0.76 | 1.9±0.4 (1.4–2.7) | NA | NA | NA |
| BASDAI in third trimester±SD† (range) | 3.2±1.6 (0.8–6.5) | 0.43 | 2.4±1.1 (1.4–3.6) | NA | NA | NA |
| Diagnosis | ||||||
| Rheumatoid arthritis | 13 (28%) | 0.16 | 11 (55%) | NA | NA | NA |
| Juvenile Idiopathic Arthritis | 11 (24%) | 0.22 | 3 (15%) | NA | NA | NA |
| Psoriatic arthritis | 9 (19%) | 0.25 | 2 (10%) | NA | NA | NA |
| Undifferentiated spondyloarthritis (SpA) | 6 (13%) | 0.28 | 0 (0%) | NA | NA | NA |
| Axial SpA | 4 (8%) | 0.29 | 3 (15%) | NA | NA | NA |
| Other rheumatic disorders‡ | 3 (6%) | 0.30 | 1 (5%) | NA | NA | NA |
| Medication during pregnancy, any use§ | ||||||
| Hydroxychloroquine | 21 (45%) | 0.15 | 13 (65%) | NA | NA | NA |
| Sulfasalazine | 17 (37%) | 0.17 | 9 (45%) | NA | NA | NA |
| Prednisone | 4 (9%) | 0.20 | 4 (20%) | NA | NA | NA |
| Certolizumab pegol | 26 (56%) | NA | NA | NA | NA | NA |
| Etanercept | 13 (28%) | NA | NA | NA | NA | NA |
| Adalimumab | 6 (13%) | NA | NA | NA | NA | NA |
| Infliximab | 6 (13%) | NA | NA | NA | NA | NA |
Values are given as mean ±SD or number (%).
*Including one twin delivery in patients under TNFis and one in patients not on TNFis.
†Only in patients with SpA.
‡Including peripheral SpA (n=2) and oligo-arthritis (n=1) in exposed to TNFi group and polyarthritis (n=1) in TNFi-unexposed group.
§Either alone or in combination with other medication. The sum of TNFis exceeds 45, because some patients switched from one TNFi to another during pregnancy.
BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; DAS28-CRP, 28-joint Disease Activity Score-C reactive protein; NA, not available; TNFis, tumour necrosis factor inhibitors.
GMCs before Tdap vaccination in pregnancy
GMCs against PT before vaccination were similar between patients exposed and unexposed to TNFis and healthy pregnant women (5.28 IU/mL, 95% CI 3.04 to 9.17 vs 4.25 IU/mL, 95% CI 2.12 to 8.53 vs 6.41 IU/mL, 95% CI 3.99 to 10.28, respectively). All other measured Tdap vaccine antigens also showed similar IgG levels except for Dt and Prn, for which the GMC in healthy women was lower than in TNFi-exposed patients (p<0.05) (table 2, figure 1, online supplemental figure 1).
Table 2.
Geometric mean concentrations (IU/mL) of IgG antibodies with 95% CIs in all study groups
| GMCs of patients on TNFis | TNFis versus no TNFis | GMCs of patients not on TNFis | No TNFis versus healthy women | GMCs of healthy women | TNFis versus healthy women | |||||||
| Crude p value | Adjusted p value | Estimated GMC ratio (95% CI) | Crude p value | Adjusted p value | Estimated GMC ratio (95% CI) | Crude p value | Adjusted p value | Estimated GMC ratio (95% CI) | ||||
| Mothers before vaccination | n=42 | n=20 | n=53 | |||||||||
| Anti-PT | 5.28 (3.04–9.17) |
0.54 | NA | NA | 4.25 (2.12–8.53) |
0.30 | NA | NA | 6.41 (3.99–10.28) |
0.66 | NA | NA |
| Anti-FHA | 17.33 (10.45–28.74) |
0.95 | NA | NA | 16.97 (9.74–29.53) |
0.09 | NA | NA | 9.89 (6.76–14.46) |
0.07 | NA | NA |
| Anti-Prn | 17.93 (11.19–28.72) |
0.85 | NA | NA | 19.49 (8.59–44.18) |
0.07 | NA | NA | 8.84 (5.77–13.54) |
0.02 | NA | NA |
| Anti-Dt | 0.10 (0.07–0.15) |
0.58 | NA | NA | 0.09 (0.05–0.15) |
0.06 | NA | NA | 0.05 (0.03–0.07) |
<0.01 | NA | NA |
| Anti-TT | 1.29 (0.93–1.78) |
0.31 | NA | NA | 1.79 (0.98–3.28) |
0.12 | NA | NA | 1.11 (0.87–1.42) |
0.45 | NA | NA |
| Mothers after vaccination | n=43 | n=18 | n=53 | |||||||||
| Anti-PT | 35.24 (20.76–59.83) |
0.37 | 0.36 | 0.69 (0.31 to 1.52) |
50.6 (26.49–96.62) |
NA* | NA* | NA* | 65.41 (47.56–89.96) |
NA* | NA* | NA* |
| Anti-FHA | 169.01 (126.34–226.09) |
0.23 | 0.22 | 0.76 (0.49 to 1.18) |
220.68 (151.67–321.11) |
NA | NA | NA | 170.10 (132.61–218.18) |
NA | NA | NA |
| Anti-Prn | 232.11 (147.17–366.08) |
0.71 | 0.77 | 0.88 (0.37 to 2.08) |
272.07 (121.38–609.86) |
NA | NA | NA | 261.51 (166.13–411.65) |
NA | NA | NA |
| Anti-Dt | 0.49 (0.35–0.69) |
0.81 | 0.58 | 1.15 (0.70 to 1.88) |
0.47 (0.32–0.69) |
NA | NA | NA | 0.31 (0.23–0.43) |
NA | NA | NA |
| Anti-TT | 5.01 (3.86–6.50) |
0.79 | 0.96 | 1.01 (0.66 to 1.55) |
5.31 (3.65–7.72) |
NA | NA | NA | 3.61 (3.00–4.35) |
NA | NA | NA |
| Infants (umbilical cord) | n=46 | n=18 | n=52 | |||||||||
| Anti-PT | 35.94 (20.68–62.45) |
0.01 | <0.01 |
0.37 (0.17 to 0.77) |
94.61 (48.89–183.07) |
0.43 | 0.30 | 0.68 (0.33 to 1.40) |
125.12 (90.75–172.50) |
<0.01 | <0.01 |
0.25 (0.13 to 0.49) |
| Anti-FHA | 166.51 (112.46–246.55) |
<0.01 | <0.01 |
0.32 (0.19 to 0.56) |
492.55 (317.85–761.64) |
0.05 | 0.05 |
1.58 (1.01 to 2.48) |
321.19 (248.11–415.79) |
<0.01 | <0.01 |
0.51 (0.32 to 0.81) |
| Anti-Prn | 150.95 (83.92–271.49) |
<0.01 | <0.01 |
0.23 (0.10 to 0.54) |
607.16 (277.69–1327.5) |
0.38 | 0.55 | 1.28 (0.57 to 2.86) |
435.41 (278.70–680.25) |
<0.01 | <0.01 |
0.29 (0.14 to 0.59) |
| Anti-Dt | 0.45 (0.29–0.69) |
0.03 | 0.03 |
0.54 (0.31 to 0.94) |
0.81 (0.53–1.24) |
0.15 | 0.19 | 1.37 (0.85 to 2.19) |
0.59 (0.44–0.78) |
0.29 | 0.24 | 0.73 (0.44 to 1.22) |
| Anti-TT | 4.55 (3.02–6.84) |
<0.01 | <0.01 |
0.47 (0.30 to 0.74) |
9.43 (7.22–12.32) |
0.06 | 0.20 | 1.24 (0.89 to 1.72) |
7.20 (5.95–8.71) |
0.04 | 0.02 |
0.58 (0.37 to 92) |
*As postvaccination blood samples were obtained in different time points in PreCARA study and historical cohort of healthy pregnancies, no direct comparisons could be performed between GMCs after vaccination.
Dt, diphtheria toxoid; FHA, filamentous haemagglutinin; GMC, geometric mean concentration; GMCs, geometric mean concentrations; Prn, pertactin; PT, pertussis toxin; TNFis, tumour necrosis factor inhibitors; TT, tetanus toxoid.
Figure 1.
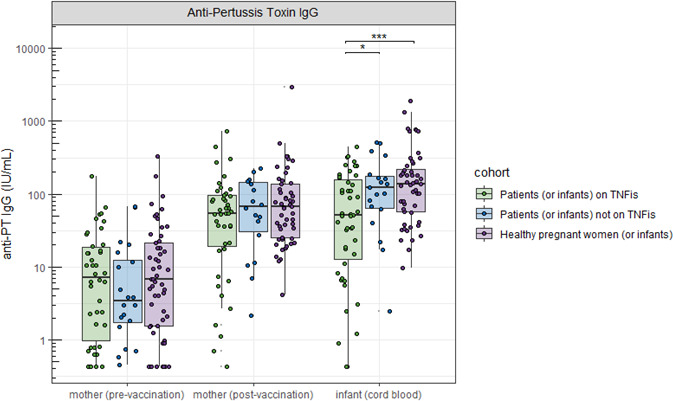
Anti-pertussis toxin (anti-PT IgG) concentrations (IU/mL) before and after vaccination and in cord sera, represented for women exposed or unexposed to TNFis, or healthy pregnant women, including their offspring. X-axis: type and time point of blood sample draw. Y-axis: IgG antibody concentration against pertussis toxin (IU/mL). Significance *p<0.05, **p<0.01, ***p<0.001. TNFis, tumour necrosis factor alpha inhibitors.
GMCs after Tdap vaccination in mothers with rheumatic disease
Three months after delivery following maternal Tdap vaccination, no significant differences in crude and adjusted analyses were observed in maternal GMCs against PT between TNFi-exposed versus unexposed patients (35.24 IU/mL, 95% CI 20.76 to 59.83 vs 50.6 IU/mL, 95% CI 26.49 to 96.62, respectively), though groups were small and 95% CI were large. Also, for the other antigens, no significant differences in GMCs were observed (table 2, figure 1, online supplemental figure 1).
GMCs in cord sera
In cord sera from infants born to mothers on TNFis, GMCs against PT, Prn, FHA Dt and TT were significantly lower compared with infants born to mothers who were unexposed to TNFis and lower compared with healthy women except for Dt (for PT: 35.94 IU/mL, 95% CI 20.68 to 62.45 vs 94.61 IU/mL, 95% CI 48.89 to 183.07 vs 125.12 IU/mL, 95% CI 90.75 to 172.50, respectively, table 2). TNFi-treatment resulted into a threefold reduction of anti-PT levels in cord blood compared with no treatment (adjusted GMC ratio 0.37, 95% CI 0.17 to 0.77) and a fourfold reduction compared with healthy controls (adjusted GMC ratio 0.25, 95% CI 0.13 to 0.49).
Between patients unexposed to TNFis and healthy women, cord serum GMCs were not significantly different in crude analyses, though after adjustments the p value was significant for FHA (492.55 IU/mL, 95% CI 317.85 to 761.64 vs 321.19 IU/mL, 95% CI 248.11 to 415.79, respectively) (table 2, figure 1, online supplemental figure 1).
IgG antibody transfer rates
Patients on TNFis showed significantly lower IgG antibody differences between cord blood levels and maternal anti-pertussis IgG levels at 3 months postdelivery, potentially suggesting lower transfer rates of all antigens compared with patients not on TNFis (mean fetal-to-maternal antibody ratios for PT: 1.33, 95% CI 1.05 to 1.60 in patients on TNFis vs 1.88, 95% CI 1.55 to 2.22 in patients not on TNFis). The single exception was Prn (mean ratio 1.18, 95% CI 0.91 to 1.45 in patients on TNFis vs 1.49, 95% CI 1.28 to 1.69 in patients not on TNFis, p=0.05), though with the same trend that failed to reach the level of significance. IgG antibody transfer rates tended to be higher in healthy women where ratios were between cord blood and levels in mothers immediately after delivery (for PT: 1.99, 95% CI 1.82 to 2.15), but direct comparisons with these women could not be made since maternal postsera from patients were drawn at a different time point than the healthy comparator group (table 3).
Table 3.
Mean fetal-to-maternal ratio of IgG antibodies with 95% CIs in all study groups
| Patients on TNFis | Patients not on TNFis | P value (TNFis vs no TNFis) | Healthy women | |
| Antigen | n=43 | n=18 | n=52 | |
| Anti-PT | 1.33 (1.05–1.60) | 1.88 (1.55–2.22) | <0.01 | 1.99 (1.82–2.15) |
| Anti-FHA | 1.31 (1.02–1.61) | 1.83 (1.50–2.17) | 0.01 | 2.05 (1.85–2.25) |
| Anti-Prn | 1.18 (0.91–1.45) | 1.49 (1.28–1.69) | 0.05 | 1.77 (1.62–1.93) |
| Anti-Dt | 1.18 (0.96–1.41) | 1.62 (1.43–1.82) | <0.01 | 1.88 (1.71–2.05) |
| Anti-TT | 1.22 (1.00–1.45) | 1.69 (1.39–1.99) | <0.01 | 2.09 (1.92–2.27) |
Dt, diphtheria toxoid; FHA, filamentous haemagglutinin; Prn, pertactin; PT, pertussis toxin; TNFis, tumour necrosis factor inhibitors; TT, tetanus toxoid.
Combined therapy with TNFi and prednisone
Subgroup analysis showed a lower GMC against PT after Tdap vaccination if patients were on combination therapy of prednisone and TNFis, compared with prednisone without TNFis, though with a very broad CI (15.96 IU/mL, 95% CI 2.50 to 101.67, n=4 vs 105.14 IU/mL, 95% CI 36.08 to 306.32, n=4) (p=0.01) due to low power. A significant GMC difference was also observed in cord sera between women on combination therapy versus solely prednisone, again with a large CI (41.84 IU/mL, 95% CI 9.07 to 192.91 vs 170.98 IU/mL, 95% CI 50.36 to 580.41, p=0.01). The few patients who used prednisone without TNFis (n=4) showed similar GMCs against PT in cord sera compared with healthy pregnant women (p=0.41) (online supplemental figure 2).
Other biologicals
Among the two patients on non-TNFi bDMARDs, the patient on rituximab showed a relatively low IgG antibody concentration against PT in cord serum (1.57 IU/mL), but the patient on anakinra showed a concentration similar to that of healthy controls (152.4 IU/mL, see online supplemental table 1).
Discussion
To the best of our knowledge, this pilot study is the first to explore IgG antibody levels against pertussis-specific antigens following maternal Tdap vaccination in pregnant women on immune-modulating treatments with a focus in TNFi-treatment. Postvaccination maternal serum GMCs against Tdap-specific antigens appeared not significantly affected by TNFis. In addition, we observed up to fourfold reduction in IgG antibody levels in infants’ umbilical cord blood samples if mothers were under TNFi-therapy during pregnancy compared with mothers with no TNFi-therapy and healthy pregnant women.
Evidence on vaccine responses in patients on immune-modulating therapy is limited and contradictory.28–32 Regarding COVID-19 vaccination a meta-analysis of several studies has shown a substantial reduction in the humoral immune response to vaccination in non-pregnant patients under TNFi-therapy compared with healthy controls (OR 0.94 (95% CI 0.84 to 0.98)).33 Studies on other vaccines, for example, pneumococcal and hepatitis A vaccines, also showed a reduced response in male and non-pregnant female patients under TNFi-treatment.19 21 Nevertheless, regarding the booster vaccination after a complete childhood vaccination series, the majority of studies suggest that vaccines like Tdap vaccination, deliver sufficient vaccine (memory) responses in non-pregnant adults on immune-modulating treatments.34–36 Our pilot study was in line with these findings of mostly sufficient memory responses under immune-modulating therapy, as we found no major reduction in IgG antibody responses after maternal Tdap vaccination in case of TNFi-treatment during pregnancy, though the power of our data was severely limited and a tendency to lower responses existed.
While in our study, the maternal Tdap vaccine response against several vaccine antigens appeared not significantly affected by treatment with TNFis, a small effect could not be excluded due to the small sample size. In addition, lower anti-pertussis antibody levels may have occurred in mothers postvaccination, since TNFis have been described to cause more rapid waning of IgG antibodies on vaccination.22 37 This may result in reduced transfer from mother to child. Noteworthy, the use of TNFis was associated with significantly lower GMCs in cord sera for all the Tdap-included antigens. In accordance, lower fetal-to-maternal antibody ratios were observed after treatment with TNFis compared with TNFi-unexposed women with rheumatic disease. This translates to reduced passive immunity against pertussis during first months after birth if the infant was born to mothers under TNFi.
We have investigated the effects of several confounding factors, which should also be assessed in future studies considering larger numbers of participants. In our study, healthy mothers were vaccinated later during pregnancy compared with patients with rheumatic disease. The optimal timing for maternal vaccination remains unknown, but a time interval of at least 6.0–7.5 weeks before delivery for both term and preterm born infants is postulated to result into enhanced antibody transfer.38–40 In our study, the pregnancy duration of TNFi-exposed patients was somewhat shorter than in healthy women. Differences in cord blood GMCs still remained significant even after adjustment for time interval between Tdap vaccination and delivery. Therefore, it seems plausible that the lower GMCs in infants after maternal TNFi-exposure are related to the effect of the TNFis. In addition, apart from lower antibody responses another hypothesis is that TNFis may alter the function of the neonatal Fc receptor (FcRn), that is, expressed by the syncytiotrophoblast, and that IgG antibody transfer across the placenta may be hampered by, for example, downregulation of FcRn. Competition between TNFis and IgG antibodies within this saturable process seems unlikely since the total amount of circulating IgG antibodies outnumbers the peripheral concentration of TNFis. We were unable to find studies on this topic and further research is needed.
Based on the results of our study, when pregnant patients receive a Tdap (booster) vaccination, and while their Tdap vaccine response may not be significantly affected, the ultimate levels in cord blood are less than expected. If this is the case for all the transfer of immunoglobulins G, it could be an alternative explanation why treatment with TNFis during pregnancy may be associated with slightly more infections in children (OR compared with the disease controlled group was 1.12 (95% CI 1.00 to 1.27), p=0.05), in a meta-analysis by Barenbrug et al).41 It has to be noted though that for pertussis, still no Correlate of Protection of IgG antibodies is available, and effectiveness and immunogenicity could not be directly compared with another.
Although only a few patients in this study received TNFis and concomitant prednisone, they had significantly lower GMCs against PT in cord sera compared with the patients on prednisone without TNFis. To our knowledge, there is currently no available evidence on immunogenicity or efficacy of Tdap vaccination in patients with prednisone and TNFi combination therapy even outside of pregnancy. Further research with larger numbers is highly required for confirmation.
Reduced antibody responses have already been shown in non-pregnant adults following treatment with rituximab after inactivated vaccine use.42 Within our study population, one patient was exposed to rituximab and had a reduced placental IgG antibody concentration of 1.57 IU/mL, which seems in line with previous research. Nevertheless, we could not further describe maternal antibody kinetics as no maternal postvaccination samples were available from the mother exposed to rituximab.
A pilot study comes with limitations. Our findings are based on a small sample size in an observational study design and may offer signals for potential immunogenic differences between patients and control groups, although it cannot account for many truly existing differences. External validation is recommended using larger numbers of subjects in each group within a parallel study design. Another limitation is the use of a historical comparator group with different time points of Tdap vaccination during pregnancy, timing of blood sampling (except for cord blood), and potential different exposure to endemic pertussis during COVID-19 lockdown periods. Therefore, comparison of maternal postvaccination GMCs between rheumatic disease patients and healthy pregnant women was not possible. Nevertheless, within the in-parallel included group of TNFi-exposed and unexposed patients, the same study protocol was followed, and therefore, these two groups could be directly compared. A strength of the study is that, even though the study design is limited by comparison to data from a historical healthy control group, laboratory procedures were similar and analyses were performed by the same institute and research staff, and therefore, any other bias than the factors that we could adjust for in the analyses would be negligible.
Recommendation for clinical practice
In the Netherlands, after the introduction of maternal Tdap vaccination since 2019, the first infant pertussis vaccine is given around 3 months of age followed by a second vaccination at 5 months and then at 11 months. An extra pertussis vaccine is advised around 6 weeks to 2 months of age in cases with no maternal Tdap vaccination, preterm infants, infants from immunodeficient mothers and infants born to mothers under TNFis.43 44 Based on the results of our study and considering the serious consequences of pertussis in infants, particularly after preterm birth, the current approach in the Netherlands and the early start of primary series in the second month of life seems appropriate. Furthermore, an extra maternal booster during pregnancy could be considered, especially if mother is under combination TNF and prednisone therapy or in case of rituximab exposure.
Conclusion
Significantly lower cord serum GMCs against all Tdap-included antigens were observed if mothers were on TNFi-treatment during pregnancy compared with no TNFis or in healthy pregnant women. An early start with pertussis vaccination series at 6 weeks to 2 months of age is recommended in children born to mothers on TNFi-therapies during pregnancy. An alternative might be an extra Tdap vaccination during pregnancy though no data are yet available to support this strategy. The underlying mechanisms and clinical consequences for lower IgG antibody levels in infants remain unknown; further research is required.
Footnotes
Contributors: The study was designed by RJEMD and PCJB-V. Participant inclusion and coordination of the PreCARA-study was performed by RJEMD, ER and HS. EAMS was senior investigator of the historical comparison cohort. Linking data to the historical comparison cohort, performing analyses and interpreting results were done by NG and MI. NG and MI wrote the manuscript. All authors critically reviewed subsequent versions and gave final approval of this version to be published, and are accountable for all aspects of the work in ensuring its accuracy and integrity. RJEMD is the responsible author for the overall content as the guarantor.
Funding: This Investigator Initiated Study (PreCARA) was supported by Union Chimique Belge (UCB), where UCB provided financial support and the Dutch Arthritis Foundation (ReumaNederland) (project number: LLP-26), a non-profit organisation. The historical comparison study was supported by the Dutch Ministry of Health, Welfare and Sport.
Competing interests: RJEMD received an unrestricted grant from Galapagos, UCB Pharma B.V., Dutch Arthritis Association and ZonMw and consultancy fees, speaker fees, honoraria advisory boards from AbbVie, AstraZeneca, Eli Lily, Galapagos, Novartis, Roche, UCB. NG received a Ph.D scholarship from Dutch Medicine Evaluation Board (CBG-MEB) and was employed by UCB pharma B.V. as medical science liaison from June 2022 to February 2023. Other authors have no competing interests to declare.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Data may be obtained from a third party and are not publicly available. The data are not publicly available due to restrictions and their containing information that could compromise the privacy of research participants. Deidentified participant data that support the findings of this study are available on reasonable request.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants and PreCARA study approval was obtained by Medical research Ethics Committees United (MEC-2011-032). The comparison study approval was obtained by the Central Committee on Research Involving Human Subjects. All participants in both cohorts provided written informed consent. Participants gave informed consent to participate in the study before taking part.
References
- 1.Amirthalingam G, Andrews N, Campbell H, et al. Effectiveness of maternal pertussis vaccination in England: an observational study. The Lancet 2014;384:1521–8. 10.1016/S0140-6736(14)60686-3 [DOI] [PubMed] [Google Scholar]
- 2.van der Maas NAT, Mooi FR, de Greeff SC, et al. Pertussis in the Netherlands, is the current vaccination strategy sufficient to reduce disease burden in young infants? Vaccine 2013;31:4541–7. 10.1016/j.vaccine.2013.07.060 [DOI] [PubMed] [Google Scholar]
- 3.Yeung KHT, Duclos P, Nelson EAS, et al. An update of the global burden of pertussis in children younger than 5 years: a Modelling study. Lancet Infect Dis 2017;17:974–80. 10.1016/S1473-3099(17)30390-0 [DOI] [PubMed] [Google Scholar]
- 4.de Greeff SC, Mooi FR, Westerhof A, et al. Pertussis disease burden in the household: how to protect young infants. Clin Infect Dis 2010;50:1339–45. 10.1086/652281 [DOI] [PubMed] [Google Scholar]
- 5.Malek A, Sager R, Kuhn P, et al. Evolution of Maternofetal transport of Immunoglobulins during human pregnancy. Am J Reprod Immunol 1996;36:248–55. 10.1111/j.1600-0897.1996.tb00172.x [DOI] [PubMed] [Google Scholar]
- 6.Byrne L, Campbell H, Andrews N, et al. Hospitalisation of Preterm infants with pertussis in the context of a maternal vaccination programme in England. Arch Dis Child 2018;103:224–9. 10.1136/archdischild-2016-311802 [DOI] [PubMed] [Google Scholar]
- 7.Maertens K, Caboré RN, Huygen K, et al. Pertussis vaccination during pregnancy in Belgium: results of a prospective controlled cohort study. Vaccine 2016;34:142–50. 10.1016/j.vaccine.2015.10.100 [DOI] [PubMed] [Google Scholar]
- 8.Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of Antirheumatic drugs before pregnancy, and during pregnancy and Lactation. Ann Rheum Dis 2016;75:795–810. 10.1136/annrheumdis-2015-208840 [DOI] [PubMed] [Google Scholar]
- 9.Bellido-Blasco J, Guiral-Rodrigo S, Míguez-Santiyán A, et al. A case-control study to assess the effectiveness of pertussis vaccination during pregnancy on newborns. Euro Surveill 2017. 10.2807/1560-7917.ES.2017.22.22.30545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaccine schedules in all countries in the EU/EEA: European centre for disease prevention and control. n.d. Available: https://vaccine-schedule.ecdc.europa.eu/
- 11.Barug D, Pronk I, van Houten MA, et al. Maternal pertussis vaccination and its effects on the immune response of infants aged up to 12 months in the Netherlands: an open-label, parallel, randomised controlled trial. Lancet Infect Dis 2019;19:392–401. 10.1016/S1473-3099(18)30717-5 [DOI] [PubMed] [Google Scholar]
- 12.Krasselt M, Wagner U, Nguyen P, et al. Humoral and cellular response to COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases under real-life conditions. Rheumatology (Oxford) 2022;61:SI180–8. 10.1093/rheumatology/keac089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nemeth D, Vago H, Tothfalusi L, et al. Factors influencing the SARS-Cov-2 infection and vaccination induced immune response in rheumatoid arthritis. Front Immunol 2022;13:960001. 10.3389/fimmu.2022.960001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamaguchi Y, Nameki S, Kato Y, et al. Persistence of SARS-Cov-2 neutralizing antibodies and anti-Omicron IgG induced by BNT162b2 mRNA vaccine in patients with autoimmune inflammatory rheumatic disease: an explanatory study in Japan. Lancet Reg Health West Pac 2023;32:100661. 10.1016/j.lanwpc.2022.100661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Picchianti-Diamanti A, Aiello A, Laganà B, et al. Immunosuppressivetherapies differently modulate Humoral- and T-cell-specific responses to COVID-19 mRNA vaccine in rheumatoid arthritis patients. Front Immunol 2021;12:740249. 10.3389/fimmu.2021.740249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mauro D, Ciancio A, Di Vico C, et al. Serological response to BNT162b2 anti-SARS-Cov-2 vaccination in patients with inflammatory rheumatic diseases: results from the RHEUVAX cohort. Front Immunol 2022;13:901055. 10.3389/fimmu.2022.901055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arvas A. Vaccination in patients with immunosuppression. Turk Pediatri Ars 2014;49:181–5. 10.5152/tpa.2014.2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dezfoli S, Horton HA, Thepyasuwan N, et al. Combined immunosuppression impairs Immunogenicity to tetanus and pertussis vaccination among patients with inflammatory bowel disease. Inflammatory Bowel Diseases 2015;21:1754–60. 10.1097/MIB.0000000000000448 [DOI] [PubMed] [Google Scholar]
- 19.Silva CA, Aikawa NE, Bonfa E. Vaccinations in juvenile chronic inflammatory diseases: an update. Nat Rev Rheumatol 2013;9:532–43. 10.1038/nrrheum.2013.95 [DOI] [PubMed] [Google Scholar]
- 20.Heijstek MW, Ott de Bruin LM, Bijl M, et al. EULAR recommendations for vaccination in Paediatric patients with rheumatic diseases. Ann Rheum Dis 2011;70:1704–12. 10.1136/ard.2011.150193 [DOI] [PubMed] [Google Scholar]
- 21.Farmaki E, Kanakoudi-Tsakalidou F, Spoulou V, et al. The effect of anti-TNF treatment on the Immunogenicity and safety of the 7-Valent conjugate Pneumococcal vaccine in children with juvenile idiopathic arthritis. Vaccine 2010;28:5109–13. 10.1016/j.vaccine.2010.03.080 [DOI] [PubMed] [Google Scholar]
- 22.Ohm M, van Straalen JW, Zijlstra M, et al. Meningococcal ACWY conjugate vaccine Immunogenicity and safety in adolescents with juvenile idiopathic arthritis and inflammatory bowel disease: A prospective observational cohort study. Vaccine 2023;41:3782–9. 10.1016/j.vaccine.2023.04.056 [DOI] [PubMed] [Google Scholar]
- 23.Smeele HTW, Röder E, Wintjes HM, et al. Modern treatment approach results in low disease activity in 90% of pregnant rheumatoid arthritis patients: the Precara study. Ann Rheum Dis 2021;80:859–64. 10.1136/annrheumdis-2020-219547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Gageldonk PGM, van Schaijk FG, van der Klis FR, et al. Development and validation of a Multiplex immunoassay for the simultaneous determination of serum antibodies to Bordetella pertussis, diphtheria and tetanus. J Immunol Methods 2008;335:79–89. 10.1016/j.jim.2008.02.018 [DOI] [PubMed] [Google Scholar]
- 25.Battafarano DF, Battafarano NJ, Larsen L, et al. Antigen-specific antibody responses in lupus patients following immunization. Arthritis Rheum 1998;41:1828–34. [DOI] [PubMed] [Google Scholar]
- 26.Rubin LG, Levin MJ, Ljungman P, et al. IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014;58:e44–100. 10.1093/cid/cit684 [DOI] [PubMed] [Google Scholar]
- 27.Robinson MJ, Heal C, Gardener E, et al. Antibody response to diphtheria-tetanus-pertussis immunization in Preterm infants who receive dexamethasone for chronic lung disease. Pediatrics 2004;113:733–7. 10.1542/peds.113.4.733 [DOI] [PubMed] [Google Scholar]
- 28.Keller M, Pittet LF, Zimmermann P. Immunogenicity and safety of routine vaccines in children and adolescents with rheumatic diseases on immunosuppressive treatment - a systematic review. Eur J Pediatr 2022;181:1329–62. 10.1007/s00431-021-04283-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Assen S, Elkayam O, Agmon-Levin N, et al. Vaccination in adult patients with auto-immune inflammatory rheumatic diseases: a systematic literature review for the European League against rheumatism evidence-based recommendations for vaccination in adult patients with auto-immune inflammatory rheumatic diseases. Autoimmun Rev 2011;10:341–52. 10.1016/j.autrev.2010.12.003 [DOI] [PubMed] [Google Scholar]
- 30.Bühler S, Eperon G, Ribi C, et al. Vaccination recommendations for adult patients with autoimmune inflammatory rheumatic diseases. Swiss Med Wkly 2015;145:w14159. 10.4414/smw.2015.14159 [DOI] [PubMed] [Google Scholar]
- 31.Ling J, Koren G. Challenges in vaccinating infants born to mothers taking immunoglobulin BIOLOGICALS during pregnancy. Expert Rev Vaccines 2016;15:239–56. 10.1586/14760584.2016.1115351 [DOI] [PubMed] [Google Scholar]
- 32.McMahan ZH, Bingham CO. Effects of biological and non-biological immunomodulatory therapies on the Immunogenicity of vaccines in patients with rheumatic diseases. Arthritis Res Ther 2014;16:506. 10.1186/s13075-014-0506-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sood A, Tran M, Murthy V, et al. Immunogenicity and safety of SARS-Cov-2 vaccination in patients with rheumatic diseases: A systematic review and meta-analysis. J Clin Rheumatol 2022;28:381–9. 10.1097/RHU.0000000000001871 [DOI] [PubMed] [Google Scholar]
- 34.Appels R, Rijksvaccinatieprogramma M, Betjes RDM, et al. n.d. Vaccinatie Bij Chronisch Inflammatoire Aandoeningen Handleiding.
- 35.Furer V, Rondaan C, Heijstek MW, et al. Update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis 2020;79:39–52. 10.1136/annrheumdis-2019-215882 [DOI] [PubMed] [Google Scholar]
- 36.Rondaan C, Furer V, Heijstek MW, et al. Efficacy, Immunogenicity and safety of vaccination in adult patients with autoimmune inflammatory rheumatic diseases: a systematic literature review for the 2019 update of EULAR recommendations. RMD Open 2019;5:e001035. 10.1136/rmdopen-2019-001035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stoof SP, Heijstek MW, Sijssens KM, et al. Kinetics of the long-term antibody response after Meningococcal C vaccination in patients with juvenile idiopathic arthritis: a retrospective cohort study. Ann Rheum Dis 2014;73:728–34. 10.1136/annrheumdis-2012-202561 [DOI] [PubMed] [Google Scholar]
- 38.Ciobanu AM, Dumitru AE, Gica N, et al. Benefits and risks of IgG Transplacental transfer. Diagnostics (Basel) 2020;10:583. 10.3390/diagnostics10080583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eberhardt CS, Blanchard-Rohner G, Lemaître B, et al. Maternal immunization earlier in pregnancy maximizes antibody transfer and expected infant Seropositivity against pertussis. Clin Infect Dis 2016;62:829–36. 10.1093/cid/ciw027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomme J, Wanlapakorn N, Ha HTT, et al. The impact of timing of pertussis vaccination during pregnancy on infant antibody levels at birth: A multi-country analysis. Front Immunol 2022;13:913922. 10.3389/fimmu.2022.913922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barenbrug L, Groen MT, Hoentjen F, et al. Pregnancy and neonatal outcomes in women with immune mediated inflammatory diseases exposed to anti-tumor necrosis factor-Α during pregnancy: A systemic review and meta-analysis. J Autoimmun 2021;122:102676. 10.1016/j.jaut.2021.102676 [DOI] [PubMed] [Google Scholar]
- 42.van Assen S, Holvast A, Benne CA, et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with Rituximab. Arthritis Rheum 2010;62:75–81. 10.1002/art.25033 [DOI] [PubMed] [Google Scholar]
- 43.Niemansburg S. Kinkhoestvaccinatie Zwangeren Ter Bescherming Van Zuigeling. Huisarts Wet 2017;60:107. 10.1007/s12445-017-0056-1 [DOI] [Google Scholar]
- 44.Maas NAT, Ruijs WLM, Rots N, et al. Bescherming Van de Pasgeborene Tegen Kinkhoest; wat is de Optimale Strategie? Tijdschrift Voor Infectieziekten 2017;12:196–202. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-002985supp001.pdf (1,016.6KB, pdf)
Data Availability Statement
Data are available on reasonable request. Data may be obtained from a third party and are not publicly available. The data are not publicly available due to restrictions and their containing information that could compromise the privacy of research participants. Deidentified participant data that support the findings of this study are available on reasonable request.


