Abstract
The advancement of science and technology demands chemistry which is safer, smarter and green by nature. The sustainability of science thus requires well-behaved alternates that best suit the demand. Bio-surfactants are surface active compounds, established to affect surface chemistry. In general, microbial bio-surfactants are a group of structurally diverse molecules produced by different microbes. A large number of bio-surfactants are produced during hydrocarbon degradation by hydrocarbonoclistic microorganisms during their own growth on carbohydrates and the production rate is influenced by the rate of degradation of carbohydrates. The production of such biological surfactants is thus of greater importance. This write up is a dedicated review to update the existing knowledge of inexpensive carbohydrate sources as substrates, microorganisms and technologies of biosurfactant production. This is an economy friendly as well as sustainable approach which will facilitate achieving some sustainable development goals. The production is dependent on the fermentation strategies, different factors of the microbial culture broth and downstream processing; these all have been elaborately presented in this article.
Bio-surfactants are produced by hydrocarbon degradation by hydrocarbonoclastic microorganisms during their own growth on agro-industrial carbohydrate wastes. The production rate is influenced by the rate of degradation of carbohydrates.
1. Introduction
The advancement of science and technology is focused in the proper safety and sustainably of the environment, population and the eco-systems.1,2 The conventional ‘brown chemistry’ thus requires simple changes that must promote ‘green chemistry’. To accelerate the ‘novel revolution’ in chemistry, natural resources have been proved to be the most effective and dependable alternatives.3–5 In contemporary time, the science community is performing extensive research works and gaining more in-depth understanding of bio-based materials to find sustainable, less pollutant, ecologically beneficiary and economically sound solvents and chemicals. These new age bio-alternative species have several attractive properties like less toxicity, biodegradability, chemical stability, inclusive environmental safety and furthermore their ease of recycling.6
This write up is to collectively represent the production of such an important bio-based compound, the bio-surfactants from degradation of waste carbohydrate feedstocks. These biologically derived surfactants are interface active agents with amphiphilic nature.7–10 Production of biosurfactants (BSs) generally occurs extracellularly or as a part of the cell membrane by several bacteria, yeast and filamentous fungi.11,12 Several substrates such as vegetable oil waste, vegetable wastes, fruit wastes starchy wastes etc. are promising source of carbohydrate for production of BSs.13 The recent article by M. H. Mondal et al.2,4 categorically reported the advantages of BSs over their synthetic or chemical analogues in different industrial sectors, house hold and even in laboratory chemistry due to their lower toxicity, greater environmental compatibility, and the best is they are highly biodegradable and are able to be produced from bio-wastes. Specifically the biocompatible, low toxic and excellent biodegradable nature of bio-surfactant facilitates consumers for direct consumption (in pharmaceuticals, food or skincare cosmetics).14–17 Thus bio-surfactant itself and its production process obeys the principles of green chemistry.18,19 More recently the bio-medical advantages of these BSs in drug design and delivery have been reported by B. Das et al.20 Hence, in recent days these BSs are becoming popular in biotechnology products for industrial and pharmaceutical applications. Bio-surfactants are proved to be extensively useful as emulsifiers, de-emulsifiers, wetting and foaming agents, edible food additives, cosmetics and detergents in house hold goods. BSs also have versatility in application in petrochemical industries, waste water treatment, soil remediation and in agro-industries. Recently bio-surfactants are the best choice for cosmetic and personal care products.4,20
Despite having lots of attractive features, biosurfactants are still back footed due to its high production cost.21 The production of bio-surfactant still facing many obstacles including costly substrate, effective bioprocessing methods, product recovery, purification and so on. So that researchers are investigating both economically and eco-friendly methods for bio-surfactant production.22 In this regard, agro-industrial wastes are found to be able to act as promising carbohydrate source for the bio-surfactant production in microbial medium. This eco-friendly approach reduces the bio-surfactant manufacturing cost as well as invokes utilisation of waste stream promoting circular bio-economy. In addition to that, it will also facilitate to achieve some sustainable development goals.10
2. Classification of biosurfactant & microorganism producing bio-surfactants
BSs are generally categorized according to their chemical composition and structure as well as its microbial origin. Rosenberg and Ron in 1999 classified BSs in two main category viz. High molecular weight (HMW) BSs and low molecular weight (LMW) BSs. Glycolipids, lipopeptides, phospholipids, fatty acids, neutral lipids belong to LMW group while polymeric (polysaccharide, lipopolysaccharide, lipoprotein) and particulate surfactants belong to HMW group. Rhamnolipids, sophorolipids and trehalolipids are mostly known glycolipids while surfactin, iturin, fengysin, lichenysin are mostly known lipopeptides23 (Fig. 1).
Fig. 1. (a) Monorhamnolipid. (b) Dirhamnolipid. (c) Lactonic. (d) Acidic sophorolipid. (e) Surfactin.
Varieties of microorganisms including bacteria, fungi, yeasts etc. are used in contemporary days for production of varied bio-surfactants (hence forth BSs).24 As the current write up deals with fermentation only, we will focus on microorganisms which are useful in production of BSs from carbon sources by fermentation process. Huge numbers of microorganisms have been isolated for industrial utilization in the aim of waste management.25 The Table 1 contains a list of BS producing organism.
List of biosurfactant – producing organisms.
| Class | Biosurfactant | Microorganism(s) | Current economic importance2,26 | References |
|---|---|---|---|---|
| Low Molecular weight | Glycolipids | |||
| Cellobiose lipids | Ustilago maydis, Sporisorium scitamineum | Antifungal compounds | 27 | |
| Rhamnolipids | Pseudomonas aeruginosa, Pseudomonas chlororaphis, Serratia rubidea, Pseudomonas cepacia | Bioremediation, antimicrobial and biocontrol properties | 28–30 | |
| Sophorolipids | Candida bombicola, Candida antartica, Torulopsis petrophilum, Candida botistae, Candida apicola, Candida riodocensis, Candida stellata, Candida bogoriensis | Antimicrobial, antiviral, spermicidal | 31 and 32 | |
| Trehalose lipids | Rhodococcus erythropolis, Arthrobactor sp., Rhodococcus qingshengii, Nocardia erythropolis, Nocardia farcinica, Crynebacterium sp., Mycobacterium sp. | Dissolution of hydrocarbons | 33–36 | |
| Trehalose dimycolates | R. erythropolis | 37 | ||
| Trehalose dicorynemycoaltes | R. erythropolis | 38 | ||
| Lipopeptides, lipoprotein and others | ||||
| Lichenysin | Bacillus licheniformis, Bacillus subtilis | Microbially enhanced oil recovery (MEOR) | 39 | |
| Surfactin/Iturin | Bacillus subtilis, Bacillus pumilus, Bacillus amyloliquefaciens | Antimicrobial properties | 40 and 41 | |
| Viscosin | Pseudomonas viscosa, Pseudomonas fluorescens and Pseudomonas libanensis | Surface active lipopeptides | 42 | |
| Subtilisin | Bacillus subtilis, Amyloliquefaciens, B. licheniformis | Antimicrobial properties | 43 | |
| Polymixins | Paenibacillus polymyxa | Antibiotic agent | 44 | |
| Ornithine lipids | Pseudomonas aeruginosa | Bio-emulsifiers | 45 | |
| Phospholipids | Acinetobacter sp. | Bioremediation | 46 | |
| Polyol lipids | Rhodotorula glutinis, R. graminis | Anti-proliferative activity | 47 | |
| Mannosylerythritol lipid | Pseudozyma aphidis ZJUDM34 | Antifungal compounds | 48 | |
| High molecular weight | Polymeric biosurfactant | |||
| Emulsan | A. calcoaceticus | MEOR | 49 | |
| Liposan | Candida lipolytica, C. tropicalis | Bio-emulsan | 50 | |
| Alasan | A. radioresistens | Biodegradation of polyaromatic compounds | 51 | |
| Particulate biosurfactant | ||||
| Whole cell | Cyanobacteria | Bio-flocculent | 52 | |
| Vesicles and fimbriae | Acinetobacter calcoacetius, P. marginilis, P. maltophilia | Bioremediation | 52 | |
3. Trends in commercial production of bio-surfactants
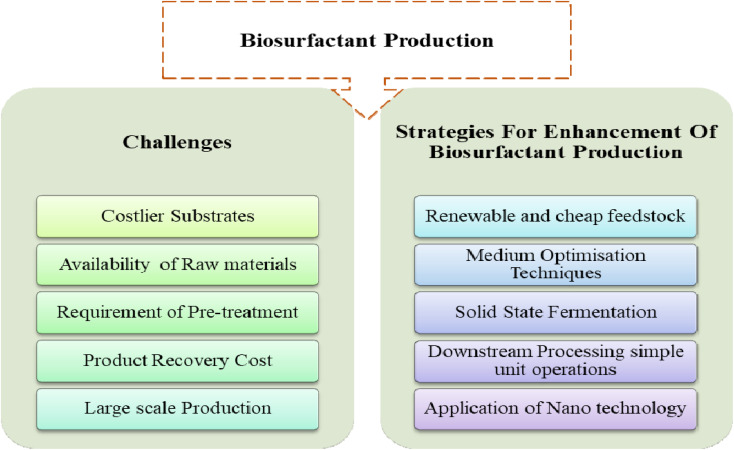
It is well established that the production expenditure of BSs is 3–10 times higher than their synthetic prototypes.53 Detail research over the last decade has suggested four scientifically significant factors that are eligible to reduce the production cost. Many researchers have suggested for increasing the product yield and to make it cost effective, we must look for inexpensive and easily obtainable substrates. Similar to other biotechnologically produced substances also, the amount of BSs yield is primarily effected by factors like type and nature of microorganisms, fermentation condition (pH, temperature, agitation etc.) and category of fermentation (sub-merged or solid state).54,55 A scheme of strategies established in market for production of BSs in shown in Fig. 2 below:
Fig. 2. Scheme of strategies to enhance biosurfactant production.
To rise above the high manufacturing cost regarding the BSs production, the fundamental strategies have been adopted universally.
(i) The utilization and application of cheap and waste feedstocks for the fermentation process.
(ii) Modification, optimization and development of bio-processes, culture condition and recovery of products.
4. Biosurfactant production as a sustainable approach
In recent days the term sustainability has become a burning topic regarding global environmental and socio-economic perspectives and this term arises regarding the parallel economic development with several environmental problems due to rapid industrialization and civilization. Sustainable life means an inclusive wellbeing of life on earth and in water where all live forms are interlinked through environment. Climate change is the main threat to that inclusive sustainability. The concept of sustainability basically consists of three factors: social, economic and environmental and based on these factors there are several sustainable development goals (SDGs). In the field of chemistry before SDGs we already started working by introducing green chemistry.56 Among them, goal 7 targets at the use of renewable resources replacing the non-renewable ones as well as ensure the access to affordable energy to increase the energy efficiency globally (Department of Economic and Social Affairs, UN 2016).57 In our daily life, a lots of product we use in which a variety of chemical surfactants are present which are generally made of non-renewable oleo-chemical compounds as well as they are not biodegradable but its bio alternatives i.e. biosurfactants has been found to be a sustainable approach. Hence the demand for such biosurfactants is increasing rapidly which invokes the commercial production of biosurfactants. In various industries the use of biosurfactant are found to be environmentally sustainable compared to its synthetic congeners but the production cost of biosurfactant is not economically sustainable.58
In recent past, a drive towards sustainable production approach has found which invokes economically favourable production as well as reduced environmental impact and conserving natural resources and energy too. In this write up the term ‘sustainability’ also reflects in the production of biosurfactants regarding use of cheap biowaste products as substrate for biosurfactant production. It is worth mentioning that among the SDGs, goal 12 targets at the sustainable management and of waste by recycling and reuse. Processes of production, recycling or reconsumption when follow the principles of green chemistry simultaneously it follows the SDGs like 3, 8, 13, 14, 15 (Department of Economic and Social Affairs, UN 2016). In this regard, the production of biosurfactants from wastages of varied industries having direct green influence in the livelihood of live forms both on earth and in water is definitely invoking for inclusive sustainability.59
With modernization, the amount of waste matter from different, agricultural fields, households, industries etc. is increasing day by day. Most of the time, these waste products are disposed in without any treatment to the environment resulting in contamination. This has harmful effects towards human health furthermore to the ecosystem. But the waste generated from several fields such that, dairy industries, agro industries, sugar industries, fruit industries etc. have nutraceutical values which is very beneficial for microbial growth so that these residues can be very useful for biosurfactant production by creating suitable environment for the microbial growth during fermentation process. This ecofriendly approach not only reduces the biosurfactant production cost but also invokes utilization of waste stream promoting circular bioeconomy which emphasizes the use of renewable natural resources to minimize the waste quantity to reduce its harmful environmental impact.6,53
5. Agro-industrial wastes as potential substrates for the bio-surfactant production
To stick to the title of the write up, this segment is focused in a detail description of potential sources that includes carbohydrates as the main carbon source.
Agro-industrial wastes are basically organic wastes which are biodegradable by nature and contain several nutritional compounds such as starch, cellulose, hemicelluloses, proteins, lignin, fibre, minerals, vitamins, and others. The amount of waste residues from different agro-industries is increasing neck and neck with modernisation and civilisation. It is worth mentioning that every year 4.4 billon tons of waste product is generated in Asia and in which India contributes >350 million tons of waste from several sources.60 Most of the time these residues are disposed in untreated condition which results in environmental problems.61 On the other hand many of these agro-industrial residues have nutritional value and due to having nutrients these residues are able to create suitable environment for the microbial growth, thus can be used as substrate for fermentation process by different microorganisms13 (Fig. 3).
Fig. 3. Biosurfactant production by microorganism using agro-industrial waste as chief carbohydrate source.
In spite of having several advantages over conventional synthetic surfactants, biosurfactants are still back footed because of its high production cost and low yield. Several researches have been developing by last few decades so that it can be produced commercially using inexpensive substrates specially the carbon sources replacing the costlier chemical alternatives.10 Agro-industrial wastes are the most common and least expensive substance with high carbohydrate content, can be obtained from processing industries and agriculture for large scale industrial level production of BSs. Agro-waste such as rice water, cereal processing waste water are potential sources of carbohydrates that could be utilized for the production of BSs.62
These cheap agro-industrial waste materials are found to act as potential substrate for BS production with effective reduction in cost of production (Fig. 4).
Fig. 4. Different agro-industrial residues used as substrate during biosurfactant production.
5.1. Agricultural residues
The residues from tropical agronomic crops can be potential inexpensive substrates with high carbon content that facilitates fermentation, culture of microorganisms and indeed production of BSs.63
5.1.1. Vegetable oil and (oil) wastes
A large amount of waste residues formation occurs during the oil extraction in various oil industry. These left over products includes oil cakes, oil soap stocks, by-products rich in fat content, semisolid and water soluble effluents, fatty acid residues etc.64
Oil wastes from different vegetable oil refineries and food industries along with industrial oil waste such as marine oils, lard and free fatty acid have a potentiality to help micro-organism growth and surfactant production.54,65 Wegerer and co-workers have reported production of rhamnolipids (RLs) from rapeseed oil by pseudomonas sp. (DSM 2874). The by-product as reported by them was l-(+)-rhamnose.66 Many plant based oils like palm oil, mesua oil, castoroil and jojoba oil etc. are inappropriate for human consumption because of their unpleasant aroma, colour and human toxicity but are potential sources for biosurfactant production due to their carbohydrate enriched bio-composition.67–69 Sunflower seed-oil is hydrolysed directly by secretion of lipase from the bacterial microbes and acts as a preferable source of carbon for RLs production.
P. aeruginosa 47 T2 is reported to produce rhamnolipids when grown in olive oil waste water with a yield as much as 8.1 g L−1.67–69 Considering these low-cost cheaper oils and oil wastes can help us overcome high production costs.
As a result of olive oil extraction process a huge quantity of liquid waste called olive oil mill waste effluent (OMWE). The OMWE is a black liquor consist of high organic content (20–60 kg per COD per m3).70 The OMWE is toxic to human health due to presence of polyphenols71 but is valuable for its chemical composition; it contains important organic substances such as sugars, organic acids and nitrogenous compounds which help in microbial growth. The presence of great amount of carbohydrates, polysaccharides, sugars, phenols and lipids in OMWE make their treatment difficult and thus OMWE is a potential environment carcinogen.72 The use of OMWE is beneficial for both environmental and economical points.
5.1.2. Starchy substrates
During the extraction of starch from different corps like rice, wheat, corn, cassava and potato, a huge amount of starchy wastewater and husks are produced which are found to be great carbon sources for the production of biosurfactants.
A main resource of low-cost starchy substrate is potato-agro-industry. The wastes of potato industry contain 80% water, 17% carbohydrates along with other minor components. Thus, they are rich source of carbohydrates (sugars and starch). Fox and Bala evaluated Surfactin production by Bacillus subtilis ATCC 21332 using potato substrate basically established potato medium and stimulated potato waste effluents.73 Thomson et al. and Noah et al. also have investigated different types of potato process effluents and reported production of surfactin. B. subtillis 21332 was the microbe for the production of surfactin from potato effluents.74,75 Das and Mukherjee studied the efficacy of Bacillus subtilis strains DM-03 and DM-04 for the production of lipopeptide biosurfactants using potato peel as carbon source in solid state fermentation and submerged fermentation systems.76 Wang and his co-workers reported the co-production of fengycin lipopeptide and γ-PGA by Bacillus subtilis strain B6-1 using soybean and sweet potato wastes in SSF.77 Sharma and his co-workers reported the production of lipopeptide biosurfactants by Bacillus pumilus grown on potato peels.78 Another study by Ayed et al. revealed the production of lipopeptide BSs (isoforms belonging to fengycin and surfactin) by Bacillus mojavensis A21 using potato waste as substrate.79 Das et al. studied the production of rhamnolipids by Pseudomonas azotoformans AJ15 utilizing sugarcane bagasse and potato peels.80 Not only the potato peels but also the pulp were used as production medium for the biosynthesis of Surfactin by Bacillus subtilis DDU20161 in the study by Pande and his coworker.81 Another study by Das and Kumar reported the production of lipopeptide BS by Bacillus licheniformis strain J1 using petroleum as carbon source while in presence of potato peel powder its efficiency enhances.82
In the cassava flour industry, processing of cassava tubers into starch or flour produces a huge amount of Cassava waste water containing several by-products like cassava peels, cassava pomace, cassava sievate and stump rich in carbohydrate content, generally possesses environmental pollutions but they can be used as feedstock for biosynthesis which can be a promising way to alleviate the environmental issues.83 Cassava waste water are potential substrates for fermentation process to produce surfactin with the help of B. subtilis.84 Siddhartha et al. reported production of another biosurfactant rhamnolipids by changing the microbe. His group used P. aeruginosa as the fermenting microbe.85 A list of microbial strains using starchy substrate as carbon source for biosurfactant production has been given in Table 2.
List of microbial strains using starchy substrate as carbon source for biosurfactant production.
| Sr. no. | Microorganism | Substrate | Biosurfactant type | Reference |
|---|---|---|---|---|
| 1 | Bacillus subtilis ATCC 21332 | Potato substrate | Surfactin | 73 |
| 2 | Bacillus subtilis ATCC 21332 | Potato effluent | Surfactin | 74 |
| 3 | Bacillus subtilis strains DM-03 and DM-04 | Potato peel | Lipopeptide | 76 |
| 4 | Bacillus subtilis strain B-61 | Soybean and sweet potato wastes | Fengycin lipopeptide and γ-PGA | 77 |
| 5 | Bacillus pumilus DSVP18 | Potato peels | Lipopeptide | 78 |
| 6 | Bacillus mojavensis A21 | Potato waste | Lipopeptide | 79 |
| 7 | Pseudomonas azotoformans AJ15 | Sugarcane bagasse and potato peels | Rhamnolipids | 80 |
| 8 | Bacillus subtilis DDU20161 | Potato peels and pulp | Surfactin | 81 |
| 9 | Bacillus licheniformis strain J1 | Potato peel powder | Lipopeptide | 82 |
5.2. Industrial wastes from animal origin
5.2.1. Dairy industry residues
A significant amount of dairy waste water, derivatives and by-products (whey, buttermilk) is produced from dairy industry every day.86 Whey is basically a liquid phase by-product obtained during the manufacturing of casein products and it comprises of significant amount of lactose making it suitable for fermentation. In general, these waste products have high Biological Oxygen Demand (BOD) thus resulting in contamination of water sources if disposed in untreated condition although a considerable amount of this waste products are going through recycling process to obtain other useful products like animal food etc. These effluents from the dairy industry are known to grow microbes and thus can be used for biosurfactant production.85,87 Daniel and co-workers achieved high yield of sophorolipids by two-stage cultivation process of yeast Cryptococcus curvatus ATCC 20509 and Candida bombicola ATCC 22214 utilising deproteinized whey.88 Daverey et al. also reported production of Sophorolipids by Candida bombicola ATCC 22214 using dairy wastewater from dairy industry.89 Several researches have been reported by a number of researchers on rhamnolipid production by Pseudomonas aeruginosa strains87,90–92 and lipopeptide production by Bacillus spp.93–95 using whey waste as substrate.
A large portion of whey waste disposed as effluent consists of several carbohydrates, peptides, amino acids; thus, are good carbon and nitrogen sources. Whey waste can be incorporated in industrial level as inexpensive substrate for biosurfactant production and laterally facilitates management of dairy wastes. A list of microbial strains using dairy waste as carbon source for biosurfactant production has been given in Table 3.
List of microbial strains using dairy waste as carbon source for biosurfactant production.
| Sr. no. | Microorganism | Substrate | Biosurfactant type | Reference |
|---|---|---|---|---|
| 1 | Pseudomonas aeruginosa BS2 | Distillery and curd whey waste | Rhamnolipids | 87 |
| 2 | Cryptococcus curvatus ATCC 20509 and Candida bombicola ATCC 22214 | Deproteinized whey | Sophorolipids | 88 |
| 3 | Candida bombicola ATCC 22214 | Dairy waste water | Sophorolipids | 89 |
| 4 | Pseudomonas aeruginosa strain SR17 | Paneer whey | Rhamnolipids | 90 |
| 5 | Pseudomonas aeruginosa | Cheese whey or olive oil mill wastewater | Rhamnolipids | 91 |
| 6 | Pseudomonas aeruginosa B189 | Milk factory wastewater | Rhamnolipids | 92 |
| 7 | Bacillus methylotrophicus, Bacillus pumilus | Whey | Lipopeptide | 93 |
| 8 | Bacillus licheniformis M104 | Whey | Lipopeptide | 94 |
| 9 | Bacillus subtilis 20B | Whey | Surfactin | 95 |
| Bacillus subtilis R1 | Lichenysin | |||
| Bacillus HS3 | ||||
| Bacillus licheniformis K51 | ||||
| 10 | Candida bombicola | Canola oil with cheese whey | Sophorolipids | 96 |
| 11 | Lactobacillus pentosus CECT-4023 | Whey | — | 97 |
| 12 | Lactococcuslactis 53 | Cheese whey | — | 98 |
| Streptococcus thermophiles A | ||||
| 13 | Yarrowia lipolytica | Whey wastewater | — | 99 |
| Micrococcus luteus | ||||
| Burkholderia cepacia | ||||
| 14 | Pseudomonas aeruginosa Strain-PP2 | Curd whey | — | 100 |
| Kocuria turfanesis strain-J | ||||
| 15 | Candida glabrata UCP 1556 | Whey | Lipopeptide | 101 |
| Corn steep liquor | ||||
| 16 | Streptococcus thermophiles, Lactobacillus acidophilus, Lactobacillus rhamnosus | Whey | — | 102 |
| 17 | Yarrowia lipolytica | Butter whey, cheese whey, ricotta whey | Bio-emulsifier | 103 |
5.3. Industrial wastes from plant origin
5.3.1. Molasses
Molasses is a co-product of sugar industry, produced at the time of sugar manufacturing. Typical sources include sugarcane, sugar beet, date etc. It is a very rich source of carbohydrates. Cane molasses contains over 48–56% of sugar. Another interesting substrate is soy molasses which is a by-product obtained from soybean processing industry. It contains 30% (w/v) carbohydrate. Molasses can be potential substrates for biosurfactant production due to being carbohydrate rich, renewable moreover cheaper and eco-friendly alternative of costlier carbon sources though the clarification process of molasses is quite costly.
Patel and Desai 1997 have reported a good quantity of rhamnolipids biosurfactant by fermentation using P. aeruginosa (GS 3).104 Other studies by many researchers revealed that different strains of Pseudomonas sp. produce rhamnolipids using molasses as carbon sources.105–112 Another study by Al-Bahry et al. (2013) highlights the use of date molasses as carbon source in the fermentative production of biosurfactant by Bacillus subtilis B20 and Bacillus subtilis B30 and other includes different strains of Bacillus sp. producing lipopeptide biosurfactants with the help of molasses as carbon source.95,113–119 Besides that sophorolipids can also be produced by different microbial strains using molasses as carbon source.120–124 A list of microbial strains using molasses as carbon source for biosurfactant production has been given in Table 4.
List of microbial strains using molasses as carbon source for biosurfactant production.
| Sr. no. | Microorganism | Agro-industrial waste (molasses) | Biosurfactant type | Reference |
|---|---|---|---|---|
| 1 | Pseudomonas aeruginosa (GS 3) | Molasses | Rhamnolipids | 104 |
| 2 | Pseudomonas aeruginosa | Molasses | Rhamnolipids | 105 |
| 3 | Pseudomonas aeruginosa EBN-8 | Blackstrap molasses | Rhamnolipids | 106 |
| 4 | Pseudomonas putida 300-B | Molasses | Rhamnolipids | 107 |
| 5 | Pseudomonas aeruginosa B1 | Sugar beet molasses | Rhamnolipids | 108 |
| Pseudomonas aeruginosa B2 | ||||
| 6 | Pseudomonas luteola B17 | Sugar beet molasses | Rhamnolipids | 109 |
| Pseudomonas putida B12 | ||||
| 7 | Pseudomonas aeruginosa | Sugar cane molasses | Rhamnolipids | 110 |
| 8 | Pseudomonas aeruginosa GIM32 | Molasses distillery wastewater | Rhamnolipids | 111 |
| 9 | Pseudomonas aeruginosa ATCC 10145 | Soy molasses | Glycolipids | 112 |
| 10 | Bacillus subtilis | Molasses | Surfactin | 113 |
| MTCC 2423 | ||||
| MTCC 1427 | ||||
| 11 | Bacillus subtilis 20B | Molasses | Surfactin | 95 |
| Bacillus subtilis R1 | Lichenysin | |||
| Bacillus HS3 | ||||
| Bacillus licheniformis K51 | ||||
| 12 | Bacillus subtilis SA9 | Molasses | Lipopeptide (similar to surfactin and lichenysin) | 114 |
| Bacillus licheniformis TR7 | ||||
| 13 | Bacillus subtilis ATCC 6633 | Sugar cane molasses | Lipopeptide | 115 |
| 14 | Bacillus subtilis B20 | Date molasses | Lipopeptide (similar to surfactin) | 125 |
| 15 | Bacillus subtilis B30 | Date molasses | Lipopeptide (similar to surfactin) | 126 |
| 16 | Bacillus licheniformis W16 | Cane molasses | Lipopeptide (similar to Lichenysin-A) | 116 |
| 17 | Bacillus subtilis ANR 88 | Molasses | Lipopeptide | 117 |
| 18 | Bacillus subtilis RSL-2 | Molasses | Surfactin | 118 |
| 19 | Bacillus subtilis Al-Dhabi-130 | Date molasses | Surfactin | 119 |
| 20 | Candida bombicola ATCC 22214 | Soy molasses | Sophorolipids | 120 |
| 21 | Candida bombicola ATCC 22214 or Starmerella bombicola NRRLY17069 | Sugarcane molasses | Sophorolipids | 121 |
| 22 | Starmerella bombicola NBRC10243 | Sugarcane molasses | Sophorolipids | 122 |
| 23 | Candida tropicalis | Sugarcane molasses | 123 | |
| 24 | Starmerella bombicola ATCC 22214 | Sugar beet molasses | Sophorolipids | 124 |
| 25 | Lactobacillus delbrueckii N2 | Sugarcane molasses | Glycoprotein | 127 |
| Lactobacillus cellobiosus TM1 | ||||
| Lactobacillus Plantarum G88 | ||||
| 26 | Lactobacillus paracasei subsp. Tolerans N2 | Sugarcane molasses | Glycolipoprotein | 128 |
| 27 | Sphingobacterium spiritivorum AS43 | Molasses | Lipopeptide | 129 |
| 28 | Azotobactervine landii | Molasses | 130 | |
| 29 | Streptomyces angustmyceticus CGS B11 | Molasses | Lipopeptide | 131 |
| 30 | Pseudomonas aeruginosa strain CGA1 | Sugar cane molasses | 132 | |
| 31 | Pseudomonas putida KT2440 | Sugar beet molasses | 133 |
5.3.2. Cashew apple juice residue
Cashew apple is a part of cashew tree which holds the cashew nut at the end. This is basically a pseudo fruit obtained after being separated from cashew nut. These left over cashew apples are used for making juice, jam, syrup although maximum remains unused. These are rich in carbohydrate content so as it can be used as source of carbon for BS production by different microorganisms.134 Several researches have been developed in last few decades over fermentation in cashew apple as carbohydrate supplement for production of BSs, some of them are represented in Table 5.
List of microbial strains using cashew apple juice residue as carbon source for biosurfactant production.
| Substrate | Microorganism | Biosurfactant type | Reference |
|---|---|---|---|
| Cashew apple juice | Acinetobacter calcoaceticus RAG-1 | Emulsan | 134 |
| Pseudomonas aeruginosa ATCC10145 | Rhamnolipids | 135 | |
| Pseudomonas aeruginosa MSIC02 | Rhamnolipids | 136 | |
| Bacillus subtilis LAMI008 | Surfactin | 137 | |
| Bacillus subtilis LAMI005 | 138 | ||
| Bacillus subtilis LAMI005 | 139 | ||
| Yarrowia lipolytica | Biosurfactants | 140 | |
| Cashew apple bagasse | Pseudomonas aeruginosa | Glycolipid | 141 |
5.3.3. Bagasse, pomace & peels
A large quantity of leftover is obtained from food industry during the manufacturing and extraction of juices from several fruits and vegetables which includes residues like banana peels, apple pomace, sugarcane bagasse, different citrus peels, pineapple peels, carrot peels etc. Das and Kumar (2018) has studied the production of rhamnolipid biosurfactant by Pseudomonas aeruginosa AJ15 using potato peels and sugarcane bagasse as carbon sources under submerged fermentation.80 A list of microbial strains using these as carbon source for biosurfactant production has been given in Table 6.
List of microbial strains using bagasse, pomace & peels as carbon source for biosurfactant production.
| Substrate | Microorganism | Biosurfactant type | Reference |
|---|---|---|---|
| Banana peel | H. archaeon AS65 | Lipopeptide | 142 |
| Orange peel | Pseudomonas aeruginosa MTCC 2297 | Rhamnolipids | 143 |
| Bacillus licheniformis KC710973 | Lipopeptide | 144 | |
| Carrot peel & apple peels extract | Bacillus subtilis I′1a | Iturin | 145 |
| Bacillus subtilis KP7 | |||
| Sugarcane bagasse | Corynebacterium aquaticum | Biosurfactant | 146 |
| Potato peels & sugarcane bagasse | Pseudomonas aeruginosa AJ15 | Rhamnolipids | 80 |
6. Pre-treatment of the feedstock
In recent past the use of these kinds of cheap and sustainable feedstocks has become widely developed research interest globally. However, these feedstocks are recommended to pass through a series of pre-treatment for commercial scale biosurfactant production. The pre-treatment procedure is very important to achieve higher monosaccharide content and fewer amounts of impurity and inhibitory complexes in final substrate which is involved in the microbial growth and metabolism phase. Accordingly the process helps in increasing the yield of biosurfactant production and facilitates the commercial biosurfactant production.53,147
The initial footstep of pre-treatment is corresponding to the decrease of size of the substrate to make sure the improved utilization of the substrate by the microorganisms and the equipment used for this purpose are tub grinder, hammer mill, crusher and many more. The reduction in particle size eventually increases the surface area as well as pore size so that the contact area becomes more available for the microorganisms to interact. The next step is pre-hydrolysis treatment usually carried out by using either ultrasonication or liquid ammonia.148 Pre-hydrolysis stage is followed by hydrolysis step. Hydrolysis can be carried out either enzymatically or chemically and the chemical path can be of two types – alkaline hydrolysis which can be employed by using sodium hydroxide, potassium hydroxide, calcium hydroxide, ammonium hydroxide etc. and acid hydrolysis which can be employed using inorganic acids like sulfuric acids, hydrochloric acid etc. The acid solution can be concentrated or diluted a required though the use of concentrated acid solution results better yield but becomes more expensive process. In addition to this, there are biologically derived enzymes which can also be used for hydrolysis pre-treatment. Enzymes such as β-glucosidase was effectively applied in the hydrolysis treatment of bagasse as well as other substrates.149,150 The final step is drying of the hydrolysates and then the dried substrate is incorporated in microbial media for biosurfactant production. Basically the pre-treated substrate is used as prime sugar source for the microbial growth phase and this is followed by formation of secondary metabolites resulting in production of biosurfactant.151–153 A schematic representation of the pre-treatment process has been shown in Fig. 5.
Fig. 5. Schematic representation of the pre-treatment process.
7. Influence of factors in biosurfactant production and medium optimisation
During the production of biosurfactant, the final quantity and quality of the resulting yield depends on several internal and external factors such as different components of medium, pH, temperature, agitation, aeration, dissolved oxygen, fermentation time and so on (Fig. 6). To ensure the maximum yield and lower cost, proper medium optimization strategies should be applied. In past days classical method was applied for medium optimization which was done by changing parameters one by one keeping other parameters fixed. But this method is not only too much time consuming but also laborious and lengthy and this also does not ensure the optimum condition for the best yield. To overcome the situation, researchers used statistical optimization strategies based on RSM (response surface methodology). This method guaranteed the proper optimum condition as well as easier than previous one regarding increasing yield and decreasing production cost.154 This method is a collection of statistical and mathematical techniques to build an empirical model to analyze the optimum condition. There are two or more factors which are involved in RSM such as dependent or output variables known as responses which are influenced by independent or input variables known as predictor. The designed experiment consists of a series of tests where input variables are made changed to analyze the response.155 This method has been employed to increase the biosurfactant yield by bacterial strains Lactococcus lactis and Streptococcus thermophilus98 and also in case of biosurfactant production by Pseudomonas aeruginosa AT 10.156
Fig. 6. List of factors influences biosurfactant production.
7.1. Influence of internal factors
The internal factors in the production of BSs are the cultural medium and its composition. The presence of different carbon sources alters the nature and types of BSs while the composition and characteristics of BSs are effected by the nature of nitrogen source and applied metal ions like iron, magnesium, sulphur, manganese etc.
7.1.1. Carbon source
Not only the composition and quality but also the amount of the produced BSs depends on the nature of the various carbon sources. We already have explained in detail about different carbon sources and their effects on BSs production. To lower the production cost many of the researcher have suggested application of agro-industrial waste as the chief carbohydrate source.157 Though a huge number of studies are established using glucose, glycerol, acetates and other organic acids and n-alkanes which all are very costly and cannot reduce the cost of production. The investigation on production of mannosylerythritol lipids (MEL) by C. antarctica using n-alkanes as carbon sources reported that the chain length of the alkane substrate efficiently influence the productivity of MEL; highest yield obtained from n-octadecane.158,159
7.1.2. Nitrogen sources
Nitrogen is equally important as that of carbon in the culture medium because of its role in the proteins and enzyme synthesis that are much essential for microbial growth.160 Nitrogen sources like urea, peptones, ammonium salts like ammonium nitrate, ammonium sulphate,161 meat and malt extract,162 yeast extract, soybean flour, sodium salts like sodium glutamate, sodium nitrate and Casein acids hydrolysate (CAH)163 are used for the production of BSs. The nitrogen is required during the stationary phase of cell growth.
7.1.3. Metal ion concentration
Metal ions are essential in biosurfactant production as these ions help production of enzyme co-factors for microbes during growth.164 We in our study have found that the presence of MgCO3 helped production of rhamnolipids.18 It has also been reported in other studies also. Thimon et al. in his surfactin production study, reported the over production of the surfactin in the presence of Fe2+ in the mineral salt microbial broth.165
7.2. Influence of external environmental factors
The growth of microbes, their metabolites and the fermentation process are greatly influenced by the pH, temperature, agitation, aeration, dissolved oxygen as well as fermentation time.166
7.2.1. pH
An optimum pH is always required for the growth of microbial as well as the fermentation of BSs. In an investigation it was reported that BS production by C. antarctica, pH range was set 4–8 where the best production was obtained at pH 8.159 The decrease in BSs production without controlling pH reported in several scientific investigations thus prove its importance during the fermentation procedure.65
7.2.2. Temperature
One of the most significant environmental parameters in biosurfactant production is the temperature. The growth of every microbe is associated with a particular temperature range preferably 25–30 °C.161 Not only the bacterial growth but also fermentation of carbohydrates needs a perfect and well-maintained temperature. Additionally temperature has impact on the metabolic process of microorganisms and physical properties of the fermentation broth.167 The MEL production was reported to be the best at 25 °C.158 However, if the temperature exceeds its optimum level, enzymatic activities may be hindered as well as denaturation of enzymes and other essential protein may happen. So, observing and maintaining the optimum temperature for the microbial growth and fermentation process is very important.
7.2.3. Agitation and aeration
Agitation is a very important factor in case of fermentation process as it effects many activities like distribution of heat, nutrients, oxygen etc. in the medium and influences the viscosity of the medium, cell growth of microorganisms and so on. On the other hand aeration supplies the necessary oxygen gas for the cell growth and fermentation process. Additionally it reduces the exhaust gases generated during the process.167 The best production of BSs was observed by Adamczak and co-workers. They studied production of BSs by C. antarctica. The condition of fermentation was fixed to an air flow rate of 1vvm and amount of dissolved oxygen to 50%. The increase in air flow rate indicated decreased BSs production due to high foam formation.168 Wei and his co-researchers169 reported that rhamnolipid production was enhanced almost 80% as well as the rate of cell growth increased from 0.22 to 0.72 h−1 by increasing the agitation rate from 50 rpm to 200 rpm. They also studied that elevated dissolved oxygen level has a positive impact over rhamnolipid production along with microbial growth.
8. Fermentation
8.1. Submerged fermentation process
Submerged fermentation process (SmF) is a method of manufacturing biomolecules in which enzymes and other reactive compounds are submerged in organic solvents like oil, alcohol or nutrient broth. SmF has been proved to be industrially applicable for bio-surfactant production due to low cost and high atom economy and additionally the process takes very short time. In SmF the purification of product is also easy. Though researchers have showed more interest in Solid State Fermentation over SmF due high scale production is easier in solid state with compared to SmF.
8.2. Solid-state fermentation process
Solid state fermentation process or SSF process allows microorganism to develop and raise on the damp solid substrate. SSF has been given a dedicated focus for several benefits over the submerged process. The SSF process has its increased application in the production of enzymes, antibiotics, biosides along with surfactant. The main advantages of SSF over other processes are the application of simplified equipment, chipper carbon source, low water requirement, demand of lower energy and higher yield of products; the most efficient one is economic advantage i.e. the low production cost.62 The best feedstocks for SSF production of biosurfactants are agro-wastes rich in carbohydrates, proteins or lipid. Rhamnolipid production by Pseudomonas aeruginosa. UFPEDA-614 was achieved greater yield in SSF compared to submerged fermentation.170 12 different agro-wastes have been experimented for the production of BSs by employing SSF method by the microorganism B. subtilis. The maximum yield was achieved in a condition where temperature was 37 °C and moisture was fixed at 88%. The fermentation time was 14 hours.171
9. Product recovery
After setting all the required parameters in optimum condition, the whole production procedure is incomplete until a proper economically efficient recovery and purification of the product is done i.e., downstream processing. For commercialisation of biosurfactant production the recovery of product from culture medium is one of the most important parameters. There are various product recovery procedures like solvent extraction, acid precipitation, crystallisation, centrifugation and so on which are mostly reported by researchers. In addition to this, some unconventional methods are also been reported such as ultrafiltration, foam fractionation, adsorption–desorption on various media. All these methods are employed based on the characteristics of the biosurfactant such as their micelle forming ability, surface activity, and solubility etc. The continuous rise in demands of highly pure biosurfactants by some pharmaceuticals, foods and cosmetics industries invokes the application of these methods which able to recover highly pure biosurfactant.172
Besides that, the solvents usually applied for biosurfactant recovery such as chloroform, methanol, acetone etc. are toxic in nature as well as unsafe to environment. Replacing these harmful solvents researchers have used inexpensive and less harmful solvents like methyl tertiary-butyl ether (MTBE) in recent past for recovery of biosurfactants.173 These types of less toxic and low-cost solvents help to reduce the recovery cost of biosurfactant production. Sometimes a single recovery procedure is not sufficient to get desired product, in that case multiple recovery techniques are employed for downstream processing.174
10. Market prospect
In spite of several challenges and obstacles associated to biosurfactant production, the commercialisation of biosurfactant in several sectors is being found to be growing due to its versatile features and advantages over its chemical alternatives. Basically, the rising concern among consumers regarding the use of eco-friendly and biodegradable products invokes the growth of biosurfactant market and as a result there is an increasing demand for green approach by several industries leading to high requirement for biosurfactants. Previously the global biosurfactant market was evaluated 4.20 billion USD in 2017 and was expected to reach 5.52 billion USD by 2022 at a compound annual growth rate (CAGR) of 5.6% from 2017 to 2022. But, the global biosurfactant market was found to grow from USD 4.07 billion in 2022 to USD 4.39 billion in 2023 at a CAGR of 7.8% and is estimated to grow to USD 6 billion in 2027 at a CAGR of 8.1%.175
To fulfil the requirement of modern industries for ecofriendly and less expensive biosurfactants the companies belonging to the biosurfactant manufacturing sector are investing in the development of new BSs globally. Evonik Industries launched ‘Rewoferm RL 100’ biosurfactant to encounter the rising demand for low-emission and low-impact cleaning products in March, 2022. This biosurfactant is produced from nearby sourced feedstocks and offers efficient cleaning performance. A USA-based manufacturer, Stepan Company, took over ‘Logos Technologies LLC's NatSurFact’ business in December 2020 to commercialize inventive biosurfactants for building sustainable products.175 The report represents the current scenario of increasing demand of biosurfactant globally. A list of several biosurfactant production companies along with their country of origin has been given in Table 7.
List of several biosurfactant production companies along with their country of origin4.
| Companies | Products | Country of origin |
|---|---|---|
| AGAE Technologies LLC | Rhamnolipids | United States |
| Jeneil Biosurfactant Co. LLC | ||
| Paradigm Biomedical Inc. | ||
| Rhamnolipids Companies, Inc. | ||
| Natsurfact | ||
| Tensio Green | Rhamnolipids | |
| Lipopeptides | ||
| Trehalolipids | ||
| Synthezyme LLC | Sophorolipids | |
| Glyco Surf | Glycolipids | |
| Tee Gene Biotech | Rhamnolipids | United Kingdom |
| Lipopeptides | ||
| Ecover | Sophorolipids | Belgium |
| Groupe Soliance | France | |
| MG Intobio Co. Ltd | South Korea | |
| Allied Carbon Solutions (ACS) Ltd | Japan | |
| Kaneka Co. | ||
| Saraya Co. Ltd | ||
| Fraunhofer IGB | Sophorolipids | Germany |
| Cellobiose lipids, mannosylerythritol lipids | ||
| Henkel | Sophorolipids, rhamnolipids, mannosylerythritol lipids | |
| Evonik | Sophorolipids | |
| Rhamnolipids | ||
| BASF | Sophorolipids, glycolipids | |
| BASF-Cognis | Sophorolipids, glycolipids | |
| Kingorigin | Glycolipids, phospholipids | China |
| Victex | Rhamnolipids | China |
11. Future prospect and conclusions
Analysis of the global biosurfactant market basically replicates the rising demand of BS in various sectors replacing its chemical alternatives, because of its versatility and eco-friendly nature. In this present review, we have presented extensive details regarding numerous investigations on BSs productions. The review also presents the recent advancements in this field of BSs production and processing. The use of technological support made BSs production easier and economic. Though BSs are best suit of the requirements of modern science and technology and go along with green revolution, for several reasons processing and productions of BSs are not up to the mark. During the course of our study of different literature we came to know that the reasons behind low BSs production is high cost, relatively low yield and of course time. As a result, the price of such biosurfactant is much higher than their chemical alternatives and it indicates that the commercialization of biosurfactant is still facing many challenges. So, in conclusion it must be noted that surfactant chemistry will be changed greatly if development of more economically favourable as well as eco-friendly process is emphasized for biosurfactant production from carbohydrate wastes like agro-wastes, industrial wastes etc. which facilitates large scale fermentation of carbohydrate substrates; hence BSs production will be a commercial success. Besides the use of cheaper carbohydrate source, proper medium optimisation through statistical methods and appropriate downstream processing is required to reduce the production cost and better biosurfactant yield. In spite of several challenges and obstacles associated to biosurfactant production, the commercialisation of biosurfactant in several sectors is being found to be growing due to its versatile features and advantages over its chemical alternatives. More improved strategies should be investigated for the sake of commercialization of biosurfactant and make it economically favourable hence enhancing its use in various industries. If the commercially biosurfactant producing companies cooperate with utilising the waste matter for the production of biosurfactant, it will be possible to achieve the SDGs and a better environment as well as a better life on earth and water will also be achieved.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
The authors acknowledge The University of Burdwan for providing infrastructural facilities. All the other lab members are also acknowledged for their help and support.
Biographies
Biography
Wasefa Begum.

Wasefa Begum was born in Burdwan, India. She pursued her M.Sc. from Presidency University, Kolkata, India and is currently working as a JRF at The Department of Chemistry, Burdwan University, WB, India. She is currently working on biosurfactant synthesis, extraction, isolation and its application.
Biography
Bidyut Saha.

Prof. Bidyut Saha was born in Birbhum, WB, India in 1975. He obtained his PhD degree from Visva Bharati University, India in 2007. He was a visiting scientist between 2009–2010 in the Department of Chemistry, UBC, Vancouver, Canada. Dr Saha is presently working as Professor in the Department of Chemistry, The University of Burdwan, India. His area of interests is bioremediation of toxic metals, micellar catalysis and inorganic reaction mechanisms. He has published more than 160 articles and book chapters in international journals.
Biography
Ujjwal Mandal.

Dr Ujjwal Mandal was born in Birbhum, WB, India in 1983. He obtained his PhD degree from Indian Association for the Cultivation of Science (IACS), Jadavpur in 2011 under the supervision of Professor Kankan Bhattacharyya. He has 2 years research experience (Post Doc.) in Chemical Physics, Lund University, Sweden with Professor Villy Sundstrom. Dr Mandal is presently working as an Assistant Professor in the Department of Chemistry, The University of Burdwan, India. Currently, he is working on carbon quantum dot based sensing, interaction of quantum dots with different surfactants and surfactant based applications.
References
- Mondal M. H. Roy A. Malik S. Ghosh A. Saha B. Res. Chem. Intermed. 2016;42:1913–1928. [Google Scholar]
- Mondal M. H. Malik S. Roy A. Saha R. Saha B. RSC Adv. 2015;5:92707–92718. [Google Scholar]
- Mondal M. H. Malik S. De S. Bhattacharyya S. S. Saha B. Res. Chem. Intermed. 2017;43:1651–1670. [Google Scholar]
- Mondal M. H., Begum W., Bhattarai A., Kumar D., Singh B. and Saha B., in Applications of Next Generation Biosurfactants in the Food Sector, Elsevier, 2023, pp. 57–89 [Google Scholar]
- Pal A. Garain A. Chowdhury D. Mondal M. H. Saha B. Tenside, Surfactants, Deterg. 2020;57:401–407. [Google Scholar]
- Mgbechidinma C. L. Akan O. D. Zhang C. Huang M. Linus N. Zhu H. Wakil S. M. Bioresour. Technol. 2022;364:128021. doi: 10.1016/j.biortech.2022.128021. [DOI] [PubMed] [Google Scholar]
- Kashif A. Rehman R. Fuwad A. Shahid M. K. Dayarathne H. N. P. Jamal A. Aftab M. N. Mainali B. Choi Y. Adv. Colloid Interface Sci. 2022;306:102718. doi: 10.1016/j.cis.2022.102718. [DOI] [PubMed] [Google Scholar]
- Astudillo Á. Rubilar O. Briceño G. Diez M. C. Schalchli H. Sustainability. 2023;15:3467. [Google Scholar]
- Gaur V. K. Gupta P. Tripathi V. Thakur R. S. Regar R. K. Patel D. K. Manickam N. Environ. Technol. Innov. 2022;25:102108. [Google Scholar]
- Gaur V. K. Sharma P. Sirohi R. Varjani S. Taherzadeh M. J. Chang J.-S. Yong Ng H. Wong J. W. C. Kim S.-H. Bioresour. Technol. 2022;343:126059. doi: 10.1016/j.biortech.2021.126059. [DOI] [PubMed] [Google Scholar]
- Rahman P. K. S. M. Gakpe E. Biotechnology. 2008;7:360–370. [Google Scholar]
- Benincasa M. Curr. Microbiol. 2007;54:445–449. doi: 10.1007/s00284-006-0610-8. [DOI] [PubMed] [Google Scholar]
- Carolin C F. Senthil Kumar P. Mohanakrishna G. Hemavathy R. V. Rangasamy G. M Aminabhavi T. Chemosphere. 2023;312:137326. doi: 10.1016/j.chemosphere.2022.137326. [DOI] [PubMed] [Google Scholar]
- Johnson P. Trybala A. Starov V. Pinfield V. J. Adv. Colloid Interface Sci. 2021;288:102340. doi: 10.1016/j.cis.2020.102340. [DOI] [PubMed] [Google Scholar]
- Rodríguez-López L. Rincón-Fontán M. Vecino X. Moldes A. B. Cruz J. M. J. Surfactants Deterg. 2020;23:79–90. [Google Scholar]
- Briem A.-K., Bippus L., Oraby A., Noll P., Zibek S. and Albrecht S., In, Biosurfactants for the Biobased Economy, ed. R. Hausmann, M. Henkel, Advances in Biochemical Engineering/Biotechnology, Springer, Cham, 2022, vol. 181, pp. 235–269 [DOI] [PubMed] [Google Scholar]
- Hogan D. E. Tian F. Malm S. W. Olivares C. Palos Pacheco R. Simonich M. T. Hunjan A. S. Tanguay R. L. Klimecki W. T. Polt R. Pemberton J. E. Curry J. E. Maier R. M. J. Hazard. Mater. 2019;364:600–607. doi: 10.1016/j.jhazmat.2018.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal M. H. Sarkar A. Maiti T. K. Saha B. J. Mol. Liq. 2017;242:873–878. [Google Scholar]
- Mondal M. H. Malik S. Garain A. Mandal S. Saha B. Tenside, Surfactants, Deterg. 2017;54:519–529. [Google Scholar]
- Das B. Kumar B. Begum W. Bhattarai A. Mondal M. H. Saha B. Chem. Africa. 2022;5:459–480. [Google Scholar]
- Kanwal M., Wattoo A. G., Khushnood R. A., Liaqat A., Iqbal R. and Song Z., in Applications of Next Generation Biosurfactants in the Food Sector, Elsevier, 2023, pp. 239–259 [Google Scholar]
- Sari C. N. Hertadi R. Gozan M. Roslan A. M. IOP Conf. Ser. Earth Environ. Sci. 2019;353:012048. [Google Scholar]
- Rosenberg E. Ron E. Z. Appl. Microbiol. Biotechnol. 1999;52:154–162. doi: 10.1007/s002530051502. [DOI] [PubMed] [Google Scholar]
- Naughton P. J. Marchant R. Naughton V. Banat I. M. J. Appl. Microbiol. 2019;127:12–28. doi: 10.1111/jam.14243. [DOI] [PubMed] [Google Scholar]
- Banat I. M. Carboué Q. Saucedo-Castañeda G. de Jesús Cázares-Marinero J. Bioresour. Technol. 2021;320:124222. doi: 10.1016/j.biortech.2020.124222. [DOI] [PubMed] [Google Scholar]
- Sarubbo L. A. Silva M. da G. C. Durval I. J. B. Bezerra K. G. O. Ribeiro B. G. Silva I. A. Twigg M. S. Banat I. M. Biochem. Eng. J. 2022;181:108377. [Google Scholar]
- Oraby A. Hug D. Weickardt I. Maerz L. Nebel S. Kurmann J. Rupp S. Tovar G. E. M. Zibek S. Discov. Chem. Eng. 2023;3:3. [Google Scholar]
- Silva R. Almeida D. Rufino R. Luna J. Santos V. Sarubbo L. Int. J. Mol. Sci. 2014;15:12523–12542. doi: 10.3390/ijms150712523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalini S. Parthasarathi R. Ann. Agrar. Sci. 2018;16:108–115. [Google Scholar]
- Salazar-Bryam A. M. Yoshimura I. Santos L. P. Moura C. C. Santos C. C. Silva V. L. Lovaglio R. B. Costa Marques R. F. Jafelicci Junior M. Contiero J. Colloids Surf., B. 2021;205:111883. doi: 10.1016/j.colsurfb.2021.111883. [DOI] [PubMed] [Google Scholar]
- Rodríguez A. Gea T. Font X. Front. Chem. Eng. 2021;3:632752. [Google Scholar]
- Imam A. Kanaujia P. K. Ray A. Suman S. K. Indian J. Microbiol. 2021;61:250–261. doi: 10.1007/s12088-021-00928-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzetti A. Gandolfi I. Bestetti G. Smyth T. J. P. Banat I. M. Eur. J. Lipid Sci. Technol. 2010;112:617–627. [Google Scholar]
- Wang Y. Nie M. Diwu Z. Lei Y. Li H. Bai X. J. Appl. Microbiol. 2019;127:1442–1453. doi: 10.1111/jam.14390. [DOI] [PubMed] [Google Scholar]
- Kuyukina M. S. Ivshina I. B. Baeva T. A. Kochina O. A. Gein S. V. Chereshnev V. A. New Biotechnol. 2015;32:559–568. doi: 10.1016/j.nbt.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Christova N. Lang S. Wray V. Kaloyanov K. Konstantinov S. Stoineva I. J. Microbiol. Biotechnol. 2015;25:439–447. doi: 10.4014/jmb.1406.06025. [DOI] [PubMed] [Google Scholar]
- Kuyukina M. S. and Ivshina I. B., Production of Trehalolipid Biosurfactants by Rhodococcus, In, Biology of Rhodococcus, ed. H. Alvarez, Microbiology Monographs, Springer, Cham, 2019, vol. 16, pp. 271–298 [Google Scholar]
- Pirog T. P. Shevchuk T. A. Voloshina I. N. Karpenko E. V. Appl. Biochem. Microbiol. 2004;40:470–475. [Google Scholar]
- Joshi S. Yadav S. Desai A. J. Biochem. Eng. J. 2008;41:122–127. [Google Scholar]
- Datta P. Tiwari P. Pandey L. M. Bioresour. Technol. 2018;270:439–448. doi: 10.1016/j.biortech.2018.09.047. [DOI] [PubMed] [Google Scholar]
- Wu Y. Xu M. Xue J. Shi K. Gu M. ACS Omega. 2019;4:1645–1651. [Google Scholar]
- Ciurko D. Chebbi A. Kruszelnicki M. Czapor-Irzabek H. Urbanek A. K. Polowczyk I. Franzetti A. Janek T. RSC Adv. 2023;13:24129–24139. doi: 10.1039/d3ra03408a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azrin N. A. M. Ali M. S. M. Rahman R. N. Z. R. A. Oslan S. N. Noor N. D. M. Biotechnol. Appl. Biochem. 2022;69:2599–2616. doi: 10.1002/bab.2309. [DOI] [PubMed] [Google Scholar]
- Mülner P. Schwarz E. Dietel K. Herfort S. Jähne J. Lasch P. Cernava T. Berg G. Vater J. Pathogens. 2021;10:1485. doi: 10.3390/pathogens10111485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewenza S. Falsafi R. Bains M. Rohs P. Stupak J. Sprott G. D. Hancock R. E. W. FEMS Microbiol. Lett. 2011;320:95–102. doi: 10.1111/j.1574-6968.2011.02295.x. [DOI] [PubMed] [Google Scholar]
- Kamischke C. Fan J. Bergeron J. Kulasekara H. D. Dalebroux Z. D. Burrell A. Kollman J. M. Miller S. I. Elife. doi: 10.7554/eLife.40171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay L. A. Sitepu I. R. Cajka T. Xu J. Teh H. E. German J. B. Pan Z. Dungan S. R. Block D. E. Boundy-Mills K. L. Biotechnol. Adv. 2018;36:397–414. doi: 10.1016/j.biotechadv.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Niu Y. Wu J. Wang W. Chen Q. Food Sci. Nutr. 2019;7:937–948. doi: 10.1002/fsn3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amani H. Kariminezhad H. Pet. Sci. Technol. 2016;34:216–222. [Google Scholar]
- Vandana P. Singh D. Int. J. Curr. Microbiol. Appl. Sci. 2018;7:4228–4241. [Google Scholar]
- Toren A. Orr E. Paitan Y. Ron E. Z. Rosenberg E. J. Bacteriol. 2002;184:165–170. doi: 10.1128/JB.184.1.165-170.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardami A. Y. Kawo A. H. Yahaya S. Lawal I. Abubakar A. S. Maiyadi K. A. J. Biochem. Microbiol. Biotechnol. 2022;10:5–12. [Google Scholar]
- Mohanty S. S. Koul Y. Varjani S. Pandey A. Ngo H. H. Chang J.-S. Wong J. W. C. Bui X.-T. Microb. Cell Fact. 2021;20:120. doi: 10.1186/s12934-021-01613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke M. and Pastore G. M., in Biotechnology for Fuels and Chemicals, Humana Press, Totowa, NJ, 2003, pp. 295–301 [Google Scholar]
- Drakontis C. E. Amin S. Curr. Opin. Colloid Interface Sci. 2020;48:77–90. [Google Scholar]
- Manga E. B. Celik P. A. Cabuk A. Banat I. M. Curr. Opin. Colloid Interface Sci. 2021;56:101514. [Google Scholar]
- Department of Economic and Social Affairs and UN, The Sustainable Development Goals, 2016 [Google Scholar]
- Brumano L. P. Soler M. F. da Silva S. S. Ind. Biotechnol. 2016;12:31–39. [Google Scholar]
- Olasanmi I. Thring R. Sustainability. 2018;10:4817. [Google Scholar]
- Madurwar M. V. Ralegaonkar R. V. Mandavgane S. A. Constr. Build. Mater. 2013;38:872–878. [Google Scholar]
- Rivera Á. D. Martínez Urbina M. Á. López y López V. E. World J. Microbiol. Biotechnol. 2019;35:155. doi: 10.1007/s11274-019-2729-3. [DOI] [PubMed] [Google Scholar]
- Cerda A. Mejias L. Rodríguez P. Rodríguez A. Artola A. Font X. Gea T. Sánchez A. Bioresour. Technol. 2019;271:409–416. doi: 10.1016/j.biortech.2018.09.131. [DOI] [PubMed] [Google Scholar]
- Martinez-Burgos W. J. Bittencourt Sydney E. Bianchi Pedroni Medeiros A. Magalhães A. I. de Carvalho J. C. Karp S. G. Porto de Souza Vandenberghe L. Junior Letti L. A. Thomaz Soccol V. de Melo Pereira G. V. Rodrigues C. Lorenci Woiciechowski A. Soccol C. R. Bioresour. Technol. 2021;341:125795. doi: 10.1016/j.biortech.2021.125795. [DOI] [PubMed] [Google Scholar]
- Dumont M.-J. Narine S. S. Food Res. Int. 2007;40:957–974. [Google Scholar]
- Bednarski W. Adamczak M. Tomasik J. Płaszczyk M. Bioresour. Technol. 2004;95:15–18. doi: 10.1016/j.biortech.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Wegerer A. Sun T. Altenbuchner J. BMC Biotechnol. 2008;8:2. doi: 10.1186/1472-6750-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. Xiang H. Zhang G. Cao X. Meng Q. J. Hazard. Mater. 2009;167:217–223. doi: 10.1016/j.jhazmat.2008.12.110. [DOI] [PubMed] [Google Scholar]
- Liu K. Sun Y. Cao M. Wang J. Lu J. R. Xu H. Curr. Opin. Colloid Interface Sci. 2020;45:57–67. [Google Scholar]
- Guo-liang Z. Yue-ting W. Xin-ping Q. Qin M. J. Zhejiang Univ., Sci., B. 2005;6:725–730. doi: 10.1631/jzus.2005.B0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques I. P. Desalination. 2001;137:233–239. [Google Scholar]
- Ancuta P. Sonia A. Appl. Sci. 2020;10:7432. [Google Scholar]
- Ben Saad A. Jerbi A. Khlif I. Ayedi M. Allouche N. Chem. Africa. 2020;3:657–665. [Google Scholar]
- Fox S. L. Bala G. A. Bioresour. Technol. 2000;75:235–240. [Google Scholar]
- Noah K. S., Bruhn D. F. and Bala G. A., in Twenty-Sixth Symposium on Biotechnology for Fuels and Chemicals, Humana Press, Totowa, NJ, 2005, pp. 465–473 [Google Scholar]
- Thompson D. N., Fox S. L. and Bala G. A., in Twenty-First Symposium on Biotechnology for Fuels and Chemicals, Humana Press, Totowa, NJ, 2000, pp. 917–930 [Google Scholar]
- Das K. Mukherjee A. K. Process Biochem. 2007;42:1191–1199. [Google Scholar]
- Wang Q. Chen S. Zhang J. Sun M. Liu Z. Yu Z. Bioresour. Technol. 2008;99:3318–3323. doi: 10.1016/j.biortech.2007.05.052. [DOI] [PubMed] [Google Scholar]
- Sharma D. Ansari M. J. Gupta S. Al Ghamdi A. Pruthi P. Pruthi V. Jundishapur J. Microbiol. 2015;8:e21257. doi: 10.5812/jjm.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Ayed H. Azabou M. C. Hmidet N. Triki M. A. Nasri M. Biodegradation. 2019;30:273–286. doi: 10.1007/s10532-018-9864-7. [DOI] [PubMed] [Google Scholar]
- Das A. J. Kumar R. Bioresour. Technol. 2018;260:233–240. doi: 10.1016/j.biortech.2018.03.093. [DOI] [PubMed] [Google Scholar]
- Pande V. Patel V. Salunke P. Patel U. Indian Drugs. 2020;57:59–65. [Google Scholar]
- Das A. J. Kumar R. Int. J. Environ. Sci. Technol. 2018;15:525–532. [Google Scholar]
- Aro S. O. Aletor V. A. Tewe O. O. Agbede J. O. Livest. Res. Rural Dev. 2010;22:42–47. [Google Scholar]
- Nitschke M. Pastore G. M. Bioresour. Technol. 2006;97:336–341. doi: 10.1016/j.biortech.2005.02.044. [DOI] [PubMed] [Google Scholar]
- Costa S. G. V. A. O. Lépine F. Milot S. Déziel E. Nitschke M. Contiero J. J. Ind. Microbiol. Biotechnol. 2009;36:1063–1072. doi: 10.1007/s10295-009-0590-3. [DOI] [PubMed] [Google Scholar]
- Adesra A. Srivastava V. K. Varjani S. Indian J. Microbiol. 2021;61:270–278. doi: 10.1007/s12088-021-00943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey K. Juwarkar A. Indian J. Biotechnol. 2004;03:74–81. [Google Scholar]
- Daniel H.-J. Reuss M. Syldatk C. Biotechnol. Lett. 1998;20:1153–1156. [Google Scholar]
- Daverey A. Pakshirajan K. Sumalatha S. Clean Technol. Environ. Policy. 2011;13:481–488. [Google Scholar]
- Patowary R. Patowary K. Kalita M. C. Deka S. Appl. Biochem. Biotechnol. 2016;180:383–399. doi: 10.1007/s12010-016-2105-9. [DOI] [PubMed] [Google Scholar]
- Colak A. K. Kahraman H. Environ. Exp. Biol. 2013;11:125–130. [Google Scholar]
- Thanomsub B. Pumeechockchai W. Limtrakul A. Arunrattiyakorn P. Petchleelaha W. Nitoda T. Kanzaki H. Bioresour. Technol. 2006;97:2457–2461. doi: 10.1016/j.biortech.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Decesaro A. Machado T. S. Cappellaro Â. C. Rempel A. Margarites A. C. Reinehr C. O. Eberlin M. N. Zampieri D. Thomé A. Colla L. M. J. Surfactants Deterg. 2020;23:539–551. [Google Scholar]
- Gomaa E. Z. Brazilian Arch. Biol. Technol. 2013;56:259–268. [Google Scholar]
- Joshi S. Bharucha C. Jha S. Yadav S. Nerurkar A. Desai A. J. Bioresour. Technol. 2008;99:195–199. doi: 10.1016/j.biortech.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Zhou Q.-H. Kosaric N. J. Am. Oil Chem. Soc. 1995;72:67–71. [Google Scholar]
- Rodrigues L. Moldes A. Teixeira J. Oliveira R. Biochem. Eng. J. 2006;28:109–116. [Google Scholar]
- Rodrigues L. Teixeira J. Oliveira R. van der Mei H. C. Process Biochem. 2006;41:1–10. [Google Scholar]
- Yilmaz F. Ergene A. Yalçin E. Tan S. Environ. Technol. 2009;30:1397–1404. doi: 10.1080/09593330903164528. [DOI] [PubMed] [Google Scholar]
- Dubey K. V. Charde P. N. Meshram S. U. Shendre L. P. Dubey V. S. Juwarkar A. A. Bioresour. Technol. 2012;126:368–374. doi: 10.1016/j.biortech.2012.05.024. [DOI] [PubMed] [Google Scholar]
- Roberto A. L. Rosileide F. S. A. Dayana M. R. Helvia W. C. A. Vanessa P. S. Galba M. C.-T. African J. Microbiol. Res. 2017;11:237–244. [Google Scholar]
- Alkan Z. Erginkaya Z. Konuray G. Ünal turhan E. Turk. J. Vet. Anim. Sci. 2019;43:676–683. [Google Scholar]
- dos Santos F. F. de Freitas K. M. L. Pereira A. da S. Fontes-Sant’Ana G. C. da Rocha-Leão M. H. M. Amaral P. F. F. Cienc. Rural. 2021;51(4):e20200323. [Google Scholar]
- Patel R. M. Desai A. J. Lett. Appl. Microbiol. 1997;25:91–94. [Google Scholar]
- Rashedi H. Assadi M. M. Jamshidi E. Bonakdarpour B. Int. J. Environ. Sci. Technol. 2006;3:297–303. [Google Scholar]
- Raza Z. A. Khan M. S. Khalid Z. M. J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng. 2007;42:73–80. doi: 10.1080/10934520601015784. [DOI] [PubMed] [Google Scholar]
- Raza Z. A. Khan M. S. Khalid Z. M. Process Biochem. 2007;42:686–692. [Google Scholar]
- Dilsad O. Belma A. African J. Biotechnol. 2008;7:4614–4619. [Google Scholar]
- Onbaslil D. Aslim B. J. Environ. Biol. 2009;30:161–163. [PubMed] [Google Scholar]
- dos Santos E. S. Santos A. C. Bezerra M. S. Pereira H. dos S. de Macedo G. R. J. Chem. Chem. Eng. 2010;4:27–33. [Google Scholar]
- Li A. Xu M. Sun W. Sun G. Appl. Biochem. Biotechnol. 2011;163:600–611. doi: 10.1007/s12010-010-9066-1. [DOI] [PubMed] [Google Scholar]
- Rodrigues M. S. Moreira F. S. Cardoso V. L. de Resende M. M. Environ. Sci. Pollut. Res. 2017;24:18699–18709. doi: 10.1007/s11356-017-9492-5. [DOI] [PubMed] [Google Scholar]
- Makkar R. S. Cameotra S. S. J. Am. Oil Chem. Soc. 1997;74:887–889. [Google Scholar]
- Saimmai A. Sobhon V. Maneerat S. Appl. Biochem. Biotechnol. 2011;165:315–335. doi: 10.1007/s12010-011-9253-8. [DOI] [PubMed] [Google Scholar]
- Rahimi Kashkouli Y. Mogharei A. Mousavian S. Vahabzadeh F. Int. J. Food Eng. 2011;7(6) doi: 10.2202/1556-3758.1939. [DOI] [Google Scholar]
- Joshi S. J. Al-Wahaibi Y. M. Al-Bahry S. N. Elshafie A. E. Al-Bemani A. S. Al-Bahri A. Al-Mandhari M. S. Front. Microbiol. 2016;7:1853–1853. doi: 10.3389/fmicb.2016.01853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane A. N. Baikar V. V. Ravi Kumar V. Deopurkar R. L. Front. Microbiol. 2017;8:492. doi: 10.3389/fmicb.2017.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R. Sharma S. Kundu L. M. Maiti S. K. Pandey L. M. J. Biotechnol. 2023;362:24–35. doi: 10.1016/j.jbiotec.2022.12.007. [DOI] [PubMed] [Google Scholar]
- AlDhabi N. A. Esmail G. A. Valan Arasu M. Int. J. Environ. Res. Public Health. 2020;17:8446. doi: 10.3390/ijerph17228446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaiman D. K. Y. Ashby R. D. Zerkowski J. A. Foglia T. A. Biotechnol. Lett. 2007;29:1341–1347. doi: 10.1007/s10529-007-9407-5. [DOI] [PubMed] [Google Scholar]
- Daverey A. Pakshirajan K. Appl. Biochem. Biotechnol. 2010;160:2090–2101. doi: 10.1007/s12010-009-8797-3. [DOI] [PubMed] [Google Scholar]
- Takahashi M. Morita T. Wada K. Hirose N. Fukuoka T. Imura T. Kitamoto D. J. Oleo Sci. 2011;60:267–273. doi: 10.5650/jos.60.267. [DOI] [PubMed] [Google Scholar]
- Almeida D. G. Soares da Silva R. de C. F. Luna J. M. Rufino R. D. Santos V. A. Sarubbo L. A. Front. Microbiol. 2017;8:157. doi: 10.3389/fmicb.2017.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Peñalver P. Castillejos M. Koh A. Gross R. Sánchez A. Font X. Gea T. J. Cleaner Prod. 2018;172:2735–2747. [Google Scholar]
- Al-Bahry S. N. Al-Wahaibi Y. M. Elshafie A. E. Al-Bemani A. S. Joshi S. J. Al-Makhmari H. S. Al-Sulaimani H. S. Int. Biodeterior. Biodegrad. 2013;81:141–146. [Google Scholar]
- Al-Wahaibi Y. Joshi S. Al-Bahry S. Elshafie A. Al-Bemani A. Shibulal B. Colloids Surf., B. 2014;114:324–333. doi: 10.1016/j.colsurfb.2013.09.022. [DOI] [PubMed] [Google Scholar]
- Mouafo T. H. Mbawala A. Ndjouenkeu R. BioMed Res. Int. 2018;2018:1–15. doi: 10.1155/2018/5034783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippolyte M. T. Augustin M. Hervé T. M. Robert N. Devappa S. Bioresour. Bioprocess. 2018;5:48. [Google Scholar]
- Noparat P. Maneerat S. Saimmai A. Appl. Biochem. Biotechnol. 2014;172:3949–3963. doi: 10.1007/s12010-014-0829-y. [DOI] [PubMed] [Google Scholar]
- Devianto L. A. Latunussa C. E. L. Helmy Q. Kardena E. IOP Conf. Ser. Earth Environ. Sci. 2020;475:012075. doi: 10.1088/1755-1315/474/7/072047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez A. P. Requiso P. J. Maloles J. S. Alcantara E. P. Alcantara V. A. Philipp. J. Sci. 2020;150:1–15. [Google Scholar]
- Anaukwu C. G. Ogbukagu C. M. Ekwealor I. A. Adv. Microbiol. 2020;10:543–562. [Google Scholar]
- Shahabi Rokni M. Halajnia A. Lakzian A. Housaindokht M. R. Biomass Convers. Biorefin. 2022:1–13. doi: 10.2202/1556-3758.1939. [DOI] [Google Scholar]
- Rocha M. V. P. Oliveira A. H. S. Souza M. C. M. Gonçalves L. R. B. World J. Microbiol. Biotechnol. 2006;22:1295–1299. [Google Scholar]
- Rocha M. V. P., Souza M. C. M., Benedicto S. C. L., Bezerra M. S., Macedo G. R., Saavedra Pinto G. A. and Gonçalves L. R. B., in Applied Biochemistry and Biotecnology, Humana Press, Totowa, NJ, 2007, pp. 185–194 [DOI] [PubMed] [Google Scholar]
- Rocha M. V. P. Mendes J. de S. Giro M. E. A. Melo V. M. M. Gonçalves L. R. B. Chem. Ind. Chem. Eng. Q. 2014;20:49–58. [Google Scholar]
- Ponte Rocha M. V. Gomes Barreto R. V. Melo V. M. M. Barros Gonçalves L. R. Appl. Biochem. Biotechnol. 2009;155:63–75. doi: 10.1007/s12010-008-8459-x. [DOI] [PubMed] [Google Scholar]
- Giro M. E. A. Martins J. J. L. Rocha M. V. P. Melo V. M. M. Gonçalves L. R. B. Biotechnol. J. 2009;4:738–747. doi: 10.1002/biot.200800296. [DOI] [PubMed] [Google Scholar]
- Freitas de Oliveira D. W. Lima França Í. W. Nogueira Félix A. K. Lima Martins J. J. Aparecida Giro M. E. Melo V. M. M. Gonçalves L. R. B. Colloids Surf., B. 2013;101:34–43. doi: 10.1016/j.colsurfb.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Fontes G. C. Ramos N. M. Amaral P. F. F. Nele M. Coelho M. A. Z. Braz. J. Chem. Eng. 2012;29:483–494. [Google Scholar]
- Iroha O. K. Njoku O. U. Ogugua V. N. Okpashi V. E. Afr. J. Environ. Sci. Technol. 2015;9:473–481. [Google Scholar]
- Chooklin C. S. Maneerat S. Saimmai A. Appl. Biochem. Biotechnol. 2014;173:624–645. doi: 10.1007/s12010-014-0870-x. [DOI] [PubMed] [Google Scholar]
- George S. Jayachandran K. Appl. Biochem. Biotechnol. 2009;158:694–705. doi: 10.1007/s12010-008-8337-6. [DOI] [PubMed] [Google Scholar]
- Kumar A. P. Janardhan A. Viswanath B. Monika K. Jung J.-Y. Narasimha G. 3 Biotech. 2016;6:43. doi: 10.1007/s13205-015-0362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraszkiewicz K. Bernat P. Kuśmierska A. Chojniak J. Płaza G. J. Environ. Manage. 2018;209:65–70. doi: 10.1016/j.jenvman.2017.12.033. [DOI] [PubMed] [Google Scholar]
- Martins P. C. Martins V. G. Int. Biodeterior. Biodegradation. 2018;127:10–16. [Google Scholar]
- Chang K.-L. Chen X.-M. Wang X.-Q. Han Y.-J. Potprommanee L. Liu J. Liao Y.-L. Ning X. Sun S. Huang Q. Bioresour. Technol. 2017;227:388–392. doi: 10.1016/j.biortech.2016.11.085. [DOI] [PubMed] [Google Scholar]
- Joy S. Rahman P. K. S. M. Khare S. K. Sharma S. Bioprocess Biosyst. Eng. 2019;42:1301–1315. doi: 10.1007/s00449-019-02128-3. [DOI] [PubMed] [Google Scholar]
- Xu Q. Liu X. Zhao J. Wang D. Wang Q. Li X. Yang Q. Zeng G. Bioresour. Technol. 2018;254:194–202. doi: 10.1016/j.biortech.2018.01.084. [DOI] [PubMed] [Google Scholar]
- Tan Y. N. Li Q. Microb. Cell Fact. 2018;17:89. doi: 10.1186/s12934-018-0938-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal R. Verma A. Singhania R. R. Varjani S. Di Dong C. Kumar Patel A. Bioresour. Technol. 2021;332:125042. doi: 10.1016/j.biortech.2021.125042. [DOI] [PubMed] [Google Scholar]
- Goshadrou A. Fuel. 2021;290:119997. [Google Scholar]
- Kavitha S. Kannah R. Y. Gunasekaran M. Nguyen D. D. Al-Muhtaseb A. H. Park J.-H. Banu J. R. Bioresour. Technol. 2019;279:156–165. doi: 10.1016/j.biortech.2019.01.118. [DOI] [PubMed] [Google Scholar]
- Singh P. Patil Y. Rale V. J. Appl. Microbiol. 2019;126:2–13. doi: 10.1111/jam.14057. [DOI] [PubMed] [Google Scholar]
- Bertrand B. Martínez-Morales F. Rosas-Galván N. Morales-Guzmán D. Trejo-Hernández M. Colloids Interfaces. 2018;2:36. [Google Scholar]
- Abalos A. Maximo F. Manresa M. Bastida J. J. Chem. Technol. Biotechnol. 2002;77:777–784. [Google Scholar]
- Liepins J. Balina K. Soloha R. Berzina I. Lukasa L. K. Dace E. Fermentation. 2021;7:136. [Google Scholar]
- Kitamoto D. Ikegami T. Suzuki G. T. Sasaki A. Takeyama Y. Idemoto Y. Koura N. Yanagishita H. Biotechnol. Lett. 2001;23:1709–1714. [Google Scholar]
- Jimoh A. A. Lin J. Ecotoxicol. Environ. Saf. 2019;184:109607. doi: 10.1016/j.ecoenv.2019.109607. [DOI] [PubMed] [Google Scholar]
- Liang X. Shi R. Radosevich M. Zhao F. Zhang Y. Han S. Zhang Y. RSC Adv. 2017;7:20667–20676. [Google Scholar]
- Tripathi L. Twigg M. S. Zompra A. Salek K. Irorere V. U. Gutierrez T. Spyroulias G. A. Marchant R. Banat I. M. Microb. Cell Fact. 2019;18:164. doi: 10.1186/s12934-019-1216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamczak M. odzimierz Bednarski W. Biotechnol. Lett. 2000;22:313–316. [Google Scholar]
- Sanjana M. C. Shivalkar Yadav K. Malashree L. Prabha R. Int. J. Curr. Microbiol. Appl. Sci. 2017;6:608–612. [Google Scholar]
- Noha E. Hamid A. E. Rawhia A. Roshdy M. Razek A.-E. T. M. J. Environ. Sci. 2018;44:29–49. [Google Scholar]
- Thimon L. Peypoux F. Michel G. Biotechnol. Lett. 1992;14:713–718. [Google Scholar]
- Twigg M. S. Baccile N. Banat I. M. Déziel E. Marchant R. Roelants S. Van Bogaert I. N. A. Microb. Biotechnol. 2021;14:147–170. doi: 10.1111/1751-7915.13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. Han L.-R. He H.-W. Sang B. Yu D.-L. Feng J.-T. Zhang X. Molecules. 2018;23:125. doi: 10.3390/molecules23010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilmanov V. Ballistreri A. Impallomeni G. Gross R. A. Biotechnol. Bioeng. 2002;77:489–494. doi: 10.1002/bit.10177. [DOI] [PubMed] [Google Scholar]
- Wei Y.-H. Chou C.-L. Chang J.-S. Biochem. Eng. J. 2005;27:146–154. [Google Scholar]
- Camilios-Neto D. Bugay C. de Santana-Filho A. P. Joslin T. de Souza L. M. Sassaki G. L. Mitchell D. A. Krieger N. Appl. Microbiol. Biotechnol. 2011;89:1395–1403. doi: 10.1007/s00253-010-2987-3. [DOI] [PubMed] [Google Scholar]
- Ghribi D. Ellouze-Chaabouni S. Biotechnol. Res. Int. 2011;2011:653654. doi: 10.4061/2011/653654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S. Das P. Sen R. Trends Biotechnol. 2006;24:509–515. doi: 10.1016/j.tibtech.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Kuyukina M. S. Ivshina I. B. Philp J. C. Christofi N. Dunbar S. A. Ritchkova M. I. J. Microbiol. Methods. 2001;46:149–156. doi: 10.1016/s0167-7012(01)00259-7. [DOI] [PubMed] [Google Scholar]
- Reiling H. E. Thanei-Wyss U. Guerra-Santos L. H. Hirt R. Käppeli O. Fiechter A. Appl. Environ. Microbiol. 1986;51:985–989. doi: 10.1128/aem.51.5.985-989.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- I. 5741503, https://www.researchandmarkets.com/reports/5741503/biosurfactants-global-market-repor. Biosurfactants Global Market Report, Bus. Res. Co., 2023 [Google Scholar]