Abstract
Background and Aim
High autoimmune hepatitis (AIH) and overlap syndrome (OS) prevalence have been previously documented among Alaska Native people. The purpose of this project is to report changes in AIH/OS prevalence over time, clinical characteristics, and factors associated with biochemical remission.
Methods
We reviewed medical records for Alaska Native/American Indian (AN/AI) patients diagnosed with AIH/OS between 1984 and 2021. Point prevalence was calculated based on AIH/OS patients alive at the end of 2021 and at 5‐year intervals from July 1, 2000, to July 1, 2020.
Results
We identified 189 AN/AI persons diagnosed with AIH or OS (157 AIH, 32 OS). Of these 189, 137 were alive at the end of 2021 for a point prevalence of 91.2 per 100 000 (95% confidence interval [CI]: 77.2–107.8)—75.9 (95% CI: 63.2–91.2) for AIH and 15.3 (95% CI: 10.2–23.0) for OS. Prevalence for both AIH and OS has risen steadily since 2000. Eighty‐nine consented participants (62.7%) achieved biochemical remission with a median time from diagnosis to start of remission of 1.9 years (IQR 0.5–5.0 years). Consented patients with fatty liver were less likely to achieve remission, but their time to remission was shorter than for patients without fatty liver.
Conclusion
The AN/AI population in Alaska continues to have the highest reported prevalence of AIH/OS in the world, with prevalence rising steadily since 2000. High reported AIH/OS prevalence is likely due in part to strong referral networks for liver disease. Detection and treatment can lead to biochemical remission and improved health outcomes.
Keywords: Alaska Native, autoimmune hepatitis, overlap syndrome, prevalence, remission
Alaska Native people continue to have the higher reported prevalence of autoimmune hepatitis (AIH) and overlap syndrome (OS) in the world. High AIH/OS prevalence in this population may be due to a combination of increased detection resulting from a strong referral system for all liver conditions as well as possible unique genetic or environmental exposures. Detection and treatment can lead to biochemical remission and improved health outcomes.
Introduction
Autoimmune hepatitis (AIH) is an immune‐mediated chronic liver disease that can progress to hepatic fibrosis, cirrhosis, hepatocellular carcinoma (HCC), or liver failure requiring liver transplant. 1 Some patients with AIH have overlap syndrome (OS), with co‐occurring primary biliary cholangitis (PBC) or primary sclerosing cholangitis. AIH/PBC overlap is found in approximately 10% of AIH patients. 2
Currently there is no cure for AIH, although it can be managed with treatment using immunosuppressing medications. Withdrawal of treatment may be attempted once long‐term remission is achieved, but relapse frequently occurs. Repeated relapse has been associated with poorer outcomes; therefore, lifelong treatment is recommended following initial relapse. Left untreated, mortality from AIH may be as high as 40%.
While relatively rare, higher AIH prevalence has been previously documented among Alaska Native (AN) people (42.9 per 100 000) 3 compared with other racial groups including Caucasian (28.3 per 100 000), Pacific Islander (10.1), Asian (6.8), and Maori people (5.1) in New Zealand 4 ; Malay (8 per 100 000), Indian (7), and Chinese people (3) in Singapore 5 ; and predominantly Caucasian people in Denmark (23.9 per 100 000) 6 and Finland (14.3). 7 The Liver Disease and Hepatitis Program (LDHP) at the Alaska Native Tribal Health Consortium (ANTHC) has been following AN people with AIH since 2005 as part of a natural history and treatment study. All patients in the Alaska Tribal Health System (ATHS) diagnosed with AIH or OS are invited to join the study. The purpose of this project is to report changes in AIH/OS prevalence over time, clinical characteristics, and factors associated with biochemical remission.
Methods
Study design, setting, participants, and data collection
Alaska Native and American Indian (AN/AI) persons living in Alaska are eligible to receive care through the ATHS, an integrated system of tribally owned and operated healthcare organizations. The LDHP operates out of the Alaska Native Medical Center (ANMC), the specialty referral hospital for ATHS located in Anchorage, Alaska and co‐owned by ANTHC and the Southcentral Foundation (SCF). Patients throughout ATHS presenting with signs and symptoms of chronic liver disease are referred to the LDHP.
We reviewed medical records for patients with liver disease with a diagnosis of AIH or OS—AIH with PBC or AIH with autoimmune cholangitis—between 1984 and 2021 for diagnosis dates and death dates. Alaska Native and American Indian (alone or in combination) population estimates from the Alaska Department of Labor were used to calculate prevalence. 8 , 9
Patients diagnosed with autoimmune liver disease, including current or prior AIH or OS, are invited to join the Autoimmune Liver Disease in Alaska Natives: Natural History and Treatment project. This is an observational study designed to increase knowledge about effective treatment of AIH and other autoimmune liver diseases in AN/AI people. Participants are asked to provide research blood samples and to allow investigators to store leftover blood samples from clinical blood draws for use in future research. Participants are also asked for permission to review medical records about the course of the patient's liver disease, response to treatment, and intolerance to medication. We tested all patients for serologic markers for hepatitis B and C and ruled out other liver diseases as the primary etiology of their liver abnormalities. However, we also recognize that AIH and OS can coexist with other chronic liver diseases. We did not exclude people with other comorbid chronic liver diseases such as non‐alcoholic fatty liver disease (NAFLD) or alcohol‐related liver disease.
This natural history and treatment study has been reviewed and approved by the Alaska Area Institutional Review Board and by ANTHC, SCF, and additional regional tribal health organizations across the state.
Clinical characteristics definitions
Acute onset of AIH was defined as alanine transaminase (ALT) greater than eight times the upper limit of normal or total bilirubin greater than or equal to 4. AIH was considered under control if the most recent ALT measurement was less than or equal to the upper limit of normal—35 IU/L for men and 25 IU/L for women. High antinuclear antibodies (ANA) was defined as a titer greater than 1:40, high actin (smooth muscle antibodies) as greater than 20 units, and high immunoglobulin G (IgG) as greater than 1600 mg/dL. We reviewed medical records for the following six symptoms at time of diagnosis: fatigue, jaundice, pruritus, spider angioma, and splenomegaly. We also reviewed medical records for a history of other autoimmune conditions before or after diagnosis.
Fibrosis was determined by liver biopsy at baseline using Metavir fibrosis scores. More recent fibrosis was measured using the median Liver Stiffness Measurement (LSM) by FibroScan® (Echosens, Paris, France) using Vibration‐Controlled Transient Elastography. 10 , 11 (F0‐F1: <7.2 kPa; F2: 7.2–9.5 kPa; F3: 9.6–14.4 kPa; F4: ≥14.5 kPa) FibroScan scores were considered valid if the ratio of the interquartile range to median (IQR/M) was than less or equal to 0.3. or if the median LSM was less than 7.1 kPa. 12 Fatty liver was identified based on a FibroScan Controlled Attenuation Parameter (CAP) score greater than 274 dB/m or a co‐occurring NAFLD diagnosis.
Data analysis
Point prevalence and 95% confidence intervals (CIs) were calculated based the number of AIH/OS patients alive at the end of 2021 and at 5‐year intervals starting on July 1, 2000, through July 1, 2020. We used population estimates from the Alaska Department of Labor and Workforce Development and the US Census AI/AN (one race alone or in combination) data for the denominator to calculate prevalence. Categorical comparisons were performed with chi‐squared test and age comparisons with t‐test. Confidence intervals for proportions were calculated using the Wilson score interval. Logistic regression was used to calculate odds ratios (ORs) and 95% CIs for remission for those with and without specified clinical characteristics. Analyses were conducted with STATA 17.0 13 and OpenEpi version 3.01. 14 Descriptive statistics and remission analyses are limited to data from patients who consented to join the natural history and treatment project.
Results
We identified 189 AN/AI persons diagnosed with AIH or OS (157 AIH, 32 OS). Of these 189, 137 were alive at the end of 2021 (114 AIH, 23 OS), for a point prevalence of 91.2 per 100 000 (95% CI: 77.2–107.8). Point prevalence was 75.9 (95% CI: 63.2–91.2) for AIH and 15.3 (95% CI: 10.2–23.0) for OS. Prevalence for both AIH and OS has risen steadily since 2000 (Fig. 1).
Figure 1.
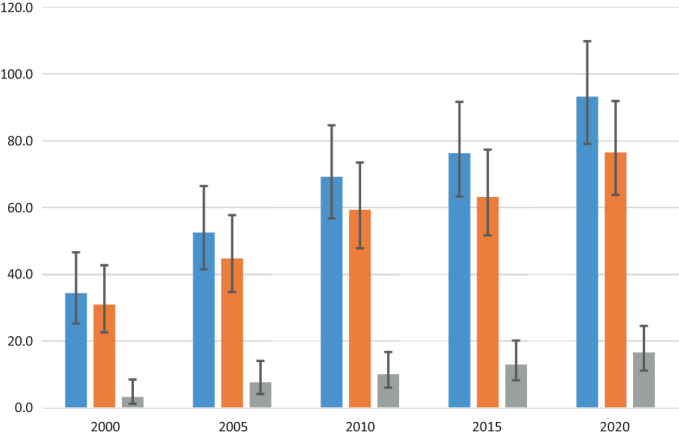
Autoimmune hepatitis and overlap syndrome prevalence, 2000–2020.  , autoimmune hepatitis (AIH) and overlap syndrome (OS);
, autoimmune hepatitis (AIH) and overlap syndrome (OS);  , AIH only;
, AIH only;  , OS.
, OS.
The Autoimmune Liver Disease in Alaska Natives: Natural History and Treatment project includes 122 AN/AI individuals diagnosed with AIH alone and 20 diagnosed with OS who consented to join the study. The majority of study participants were female (n = 130, 91.5%). Age at diagnosis was normally distributed with a mean age at diagnosis of 51.3 (SD 13.3) years (Fig. 2).
Figure 2.
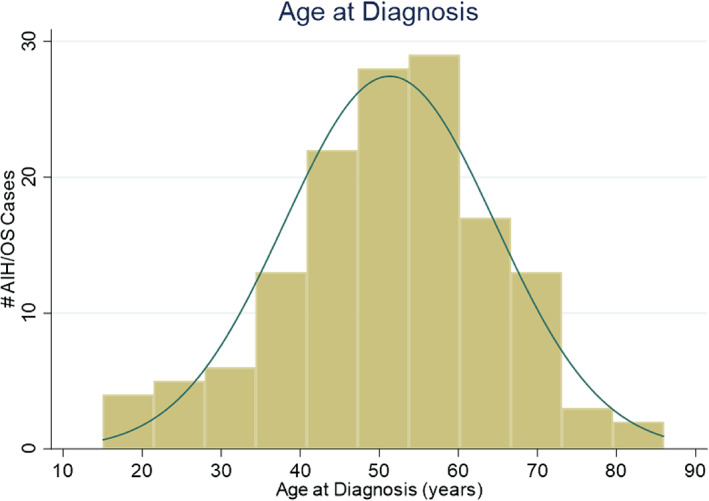
Age at diagnosis (years).
Most participants presented with acute AIH at time of diagnosis (73.2%), with those diagnosed with AIH only being more likely to have acute presentation (AIH: 77.9%, OS: 45.0%; P = 0.002). Over 80% of participants had a biopsy within 90 days of diagnosis that demonstrated typical features of AIH or features of both AIH and autoimmune cholangitis (AIH: 81.1%, OS: 90.0%), while close to 90% had a diagnostic biopsy within 2 years of diagnosis (AIH: 88.5%, OS: 90.0%). Of the 126 participants with a biopsy within 2 years of diagnosis and a valid Metavir score, 53 participants (42.4%) had a Metavir score of F3 or F4, advanced fibrosis or cirrhosis. TPMT enzyme activity was normal in 118 (87.4%) patients. See Table 1 for demographic and clinical characteristics, including autoimmune markers and symptoms at time of diagnosis, for AIH and OS separately and combined, and Table 2 for prevalence of comorbid autoimmune conditions.
Table 1.
Demographic and clinical characteristics
| AIH alone | OS | Total | |||||
|---|---|---|---|---|---|---|---|
| n = 122 | n = 20 | n = 142 | P‐value* | ||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Age at diagnosis | 51.7 | 12.9 | 49.2 | 15.9 | 51.3 | 13.3 | 0.436 |
| n | % | n | % | n | % | ||
| Female | 113 | 92.6 | 17 | 85.0 | 130 | 91.5 | 0.256 |
| Fatty liver | 52 | 42.6 | 9 | 45.0 | 61 | 43.0 | 0.842 |
| Autoimmune markers | |||||||
| High ANA at diagnosis | 71 | 58.2 | 12 | 60.0 | 83 | 58.5 | 0.879 |
| High Actin at diagnosis | 90 | 73.8 | 9 | 45.0 | 99 | 69.7 | 0.009 |
| High IgG at diagnosis | 83 | 68.0 | 14 | 70.0 | 97 | 68.3 | 0.861 |
| At least two positive markers at diagnosis | 88 | 72.1 | 13 | 65.0 | 101 | 71.1 | 0.514 |
| Controlled (most recent lab test) | 75 | 61.5 | 9 | 45.0 | 84 | 59.2 | 0.165 |
| Fibrosis at diagnosis (Metavir) (limited to patients with biopsy within 2 years of diagnosis and valid Metavir score) | AIH alone | OS | Total | ||||
|---|---|---|---|---|---|---|---|
| n = 107 | n = 18 | n = 125 | |||||
| n | % | n | % | n | % | P‐value* | |
| None (F0) | 14 | 13.1 | 0 | 0.0 | 14 | 11.2 | 0.207 |
| Mild (F1) | 19 | 17.8 | 5 | 27.8 | 24 | 19.2 | |
| Moderate (F2) | 31 | 29.0 | 3 | 16.7 | 34 | 27.2 | |
| Severe (F3) | 28 | 26.2 | 8 | 44.4 | 36 | 28.8 | |
| Cirrhosis (F4) | 15 | 14.0 | 2 | 11.1 | 17 | 13.6 | |
| TPMT enzyme activity | AIH alone | OS | Total | ||||
|---|---|---|---|---|---|---|---|
| n = 116 | n = 19 | n = 135 | |||||
| n | % | n | % | n | % | P‐value* | |
| Normal | 102 | 87.9 | 16 | 84.2 | 118 | 87.4 | 0.674 |
| Intermediate | 12 | 10.3 | 3 | 15.8 | 15 | 11.1 | |
| Low | 2 | 1.7 | 0 | 0.0 | 2 | 1.5 | |
| Symptoms at presentation | AIH alone | OS | Total | ||||
|---|---|---|---|---|---|---|---|
| n = 101 | n = 17 | n = 118 | |||||
| n | % | n | % | n | % | P‐value* | |
| Fatigue | 53 | 52.5 | 8 | 47.1 | 61 | 51.7 | 0.679 |
| Jaundice | 42 | 41.6 | 5 | 29.4 | 47 | 39.8 | 0.343 |
| Pruritis | 14 | 13.9 | 3 | 17.6 | 17 | 14.4 | 0.681 |
| Spider angioma | 8 | 7.9 | 0 | 0.0 | 8 | 6.8 | 0.229 |
| Splenomegaly | 2 | 2.0 | 0 | 0.0 | 2 | 1.7 | 0.558 |
Comparing AIH alone to OS.
AIH, autoimmune hepatitis; ANA, antinuclear antibodies; IgG, immunoglobulin G; OS, overlap syndrome; TPMT, thiopurine methyltransferase.
Table 2.
Prevalence of comorbid autoimmune conditions
| Other autoimmune conditions (n = 118 † ) | n | % | 95% confidence interval | |
|---|---|---|---|---|
| Any non‐hepatic autoimmune condition | 35 | 29.7 | 22.2% | 38.4% |
| Thyroiditis | 10 | 8.5 | 4.7% | 14.9% |
| Rheumatoid arthritis | 9 | 7.6 | 4.1% | 13.9% |
| Sjogren's syndrome | 7 | 5.9 | 2.9% | 11.7% |
| Mixed connective tissue | 6 | 5.1 | 2.3% | 10.8% |
| Systemic lupus erythematosus | 5 | 4.2 | 1.8% | 9.5% |
| Sicca syndrome | 3 | 2.5 | 0.9% | 7.2% |
| Celiac disease | 1 | 0.8 | 0.1% | 4.6% |
| Raynaud's syndrome | 1 | 0.8 | 0.1% | 4.6% |
| Celiac disease | 0 | 0.0 | 0.0% | 3.2% |
| Dermatomyositis | 0 | 0.0 | 0.0% | 3.2% |
| Scleroderma | 0 | 0.0 | 0.0% | 3.2% |
Restricted to patients with electronic health records available for review.
Sixty‐one participants (43.0%) were identified with fatty liver, of which 56 (91.8%) had a FibroScan with the CAP score >274 dB/m, indicating moderate to severe steatosis, and 33 (54.1%) had a co‐occurring NAFLD diagnosis. Four participants (2.8%) had a previous hepatitis B diagnosis, six (4.2%) had a previous hepatitis C diagnosis, and two (1.4%) had a previous alcohol‐related liver disease diagnosis.
Among study participants diagnosed with AIH, there was one death within 5 years of diagnosis and five deaths within 10 years for a 5‐year survival rate of 99.2% (95% CI: 99.5–99.9%) and a 10‐year survival rate of 95.9% (95% CI: 90.8–98.2%). Among participants diagnosed with OS, there were no deaths within 5 years and three deaths within 10 years for a 5‐year survival rate of 100.0% (95% CI: 83.9–100.0%) and a 10‐year survival rate of 85.0% (95% CI: 64.0–94.8%). Overall, for AIH and OS combined, the 5‐year survival rate was 99.3% (95% CI: 96.1–99.9%) and the 10‐year survival rate was 94.4% (95% CI: 89.3–97.1%).
Medications prescribed and medication intolerance
The majority of study participants (85.9%, 95% CI: 79.1–91.2%) were prescribed either prednisone and azathioprine or methylprednisolone and azathioprine at least once. A smaller number were prescribed only prednisone or methylprednisolone (3.5%, 95% CI: 1.2–8.0%) or only azathioprine (7.0%, 95% CI: 3.4–12.6%). When patients were unable to tolerate azathioprine, other medications used included tacrolimus, mycophenolate mofetil, and budesonide. Persons with features of autoimmune cholangitis were given ursodiol.
Approximately half of study participants (47.9%; AIH: 46.7%, OS: 55.0%) could not tolerate azathioprine (n = 69; AIH: n = 57, OS: n = 12), tacrolimus (n = 14; AIH: n = 13, OS: n = 1), or mycophenolate mofetil (n = 11, all AIH). Neutropenia or pancytopenia was noted in the electronic health records for close to half (49.3%) of study participants who could not tolerate azathioprine. TPMT enzyme activity was normal for the majority of participants who could not tolerate azathioprine (88.1%; AIH: 90.9%, OS: 75.0%) including 91.2% of those with neutropenia or pancytopenia who could not tolerate it.
Time to remission
Eighty‐nine consented participants (62.7%) achieved biochemical remission with a median time from diagnosis to start of biochemical remission of 1.9 years (IQR 0.5–5.0 years). Consented patients with fatty liver were less likely to achieve biochemical remission, but their time to remission was shorter than for AIH/OS patients without fatty liver (Table 3) Similarly, participants with high actin at the time of AIH/OS diagnosis were less likely to achieve remission, but their time to remission was shorter (Table 3). On average, consented participants who achieved biochemical remission were older at the time of diagnosis than those who did not, although this difference was not statistically significant (52.9 vs 48.6 years; P = 0.065). We found no associations between achieving biochemical remission and sex, acute onset, degree of fibrosis, or high ANA or IgG at time of diagnosis (P > 0.42 for all).
Table 3.
Proportion of autoimmune hepatitis (AIH)/overlap syndrome (OS) patients achieving remission and time to remission
| AIH/OS patient population | Proportion achieving remission | Time in years from diagnosis to remission: median (IQR) | Odds ratio (95% confidence interval) for remission | |
|---|---|---|---|---|
| All | 62.7% | 1.9 (0.5–5.0) | ||
| With fatty liver | 50.8% | P = 0.011 | 0.6 (0.2–1.8) | 0.4 (0.2–0.8) |
| Without fatty liver | 71.6% | 3.3 (1.0–6.2) | ||
| With high actin | 56.6% | P = 0.008 | 1.1 (0.3–4.1) | 0.3 (0.1–0.8) |
| Without high actin | 79.1% | 3.6 (0.2–8.7) | ||
Fibrosis change over time
At the time of diagnosis, 127 participants, 109 with AIH alone and 18 with OS, had a Metavir fibrosis score based on a liver biopsy. Of these 127, 38 (30.0%) participants had no or mild fibrosis while 55 (43.3%) had severe fibrosis or cirrhosis. Severity of fibrosis was similar for participants with AIH alone compared with those with OS (P = 0.214). Figure 3 illustrates the proportion of these consented AIH/OS patients with severe fibrosis or cirrhosis who saw an improvement from time of diagnosis to their most recent FibroScan (Fig. 3a) and the proportion with no to moderate fibrosis at diagnosis who progressed (Fig. 3b).
Figure 3.
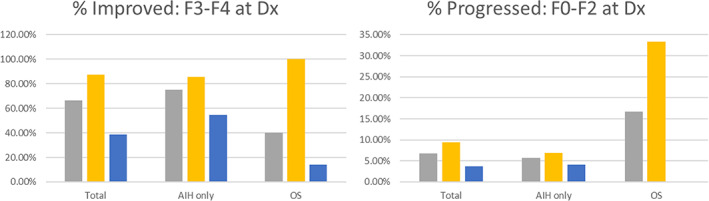
(a) Percentage of consented patients with F3/F4 Metavir fibrosis score at time of diagnosis and F0/F1/F2 at most recent FibroScan.  , all;
, all;  , controlled;
, controlled;  not controlled. (b) Percentage of consented patients with F0/F1/F2 Metavir fibrosis score at time of diagnosis and F3/F4 at most recent FibroScan.
not controlled. (b) Percentage of consented patients with F0/F1/F2 Metavir fibrosis score at time of diagnosis and F3/F4 at most recent FibroScan.  , all;
, all;  , controlled;
, controlled;  not controlled.
not controlled.
Discussion
AIH prevalence among AN/AI people living in Alaska (91.2 per 100 000) is the highest reported AIH prevalence of any population‐based study published since 1995. This analysis updates a prior prevalence estimate among AN/AI living in Alaska from 2002 (42.9 per 100 000) that was the highest reported population‐based AIH prevalence at that time. 3
Based on Lv and colleagues' systemic review of AIH prevalence in Asian, European, and American populations 15 and national studies in the United States 16 and Korea, 17 it appears that AIH prevalence has been increasing slowly since the mid‐1990s in many locations around the world. However, this increase has been much more dramatic among AN/AI people in Alaska, with the prevalence almost doubling in the past 20 years (Fig. 4) As of the end of 2021, the AIH prevalence among AN/AI people in Alaska was three times the reported US prevalence 16 and five times the worldwide prevalence. 15
Figure 4.
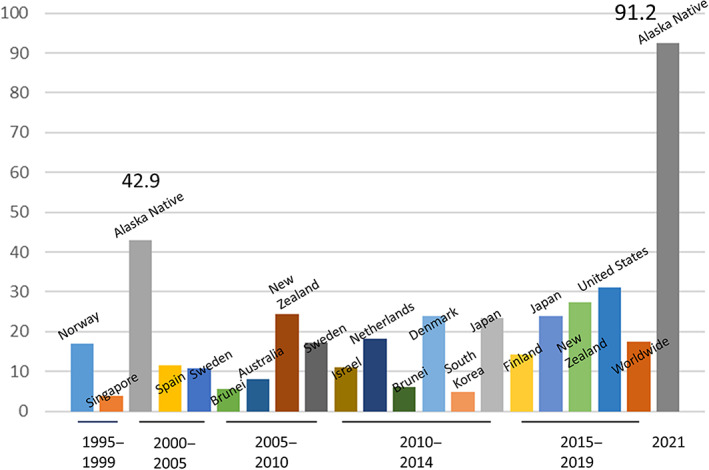
Autoimmune hepatitis prevalence (per 100 000): Population‐based estimates from 1995 to 2021.
Dramatically higher AIH prevalence rates among AN/AI people in Alaska is likely due to both increased detection and real higher prevalence due to unique genetic and environmental exposures. A strength of our study is that LDHP, working in collaboration with the Indian Health Service and the CDC Arctic Investigations Program, has a long history of working in AN communities across the state. Hepatitis B was first identified in Alaska Native villages in the mid‐1970s. 18 , 19 Since then, there have been ongoing relationships between LDHP and local providers with a strong referral system for all liver conditions. This comprehensive referral network reduces the likelihood of AIH cases being missed. However, it is unlikely that this strong referral network alone explains the dramatically higher AIH prevalence reported here.
High rates of detection for AIH and OS allow for better treatment. Left untreated, AIH and OS can lead to cirrhosis, HCC, or liver‐related death. 20 Successful treatment within this cohort has contributed to biochemical remission in more than 60% of participants. Participants with controlled AIH/OS were also more likely see improvements over time in severity of fibrosis.
Higher prevalence of other autoimmune conditions—including systemic lupus erythematosus (SLE), systemic sclerosis, and rheumatoid arthritis (RA)—have also been reported among AN/AI people in Alaska and in indigenous First Nations populations in Canada. 21 , 22 , 23 All of these autoimmune conditions appear to have both genetic and environmental components.
AIH was originally identified as a progressive chronic hepatitis primarily among young women, and was later thought to have a bimodal age distribution with peaks around puberty and between the fourth and sixth decades. 24 More recently, it has been suggested that AIH is predominantly a disease of middle‐aged or older women. 4 , 5 In our study, age at diagnosis was normally distributed with a mean of 51.3 years and a range of 15 to 86 years, consistent with the hypothesis that AIH is present in all age groups but may be underdiagnosed in some age ranges. 25
We found a higher prevalence of both fatigue and jaundice in our study population than in Tunio's population‐based US national study, which analyzed data from a commercial database of aggregate electronic health record (EHR) systems from major health systems across the United States (Fatigue: AN: 52.5%; US: 23.3%. Jaundice: AN: 41.6%; US: 10.2%) This difference is likely due to our manual review of both coded and text EHR data for symptoms, suggesting that symptom prevalence may be higher than previously reported for symptoms that may not be prioritized for coding in EHR data. 16
We found that almost 30% of AN/AI persons with AIH have also been diagnosed with one or more non‐hepatic autoimmune conditions. Similar to a four‐site European study of patients with AIH and OS, the most common non‐hepatic autoimmune disease in our population was autoimmune thyroiditis (AN: 8.5%; European sites: 10.1%). 26 However, this finding differed from Tunio's large US population‐based study that found rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) (7.8% for both) to be much more common than autoimmune thyroiditis (2.8%). 16 On the other hand, we found RA prevalence to be similar in our population (7.6%) compared with Tunio's (7.8%), whereas RA was much less prevalent in the European study (1.8%). Prevalence of SLE in our population (4.2%) was approximately half of that found in Tunio's study (7.8%) but higher than in the European study (0.7%). 16 , 26
Almost half of AN/AI persons with AIH in our study also have fatty liver. Individuals with both AIH and fatty liver were less likely to achieve biochemical remission, but time to remission was shorter for those who did. NAFLD has been identified as the most common cause of elevated liver enzymes. 27 Therefore, it is likely that elevated and fluctuating ALT levels may have precluded some individuals with AIH from achieving remission even if they responded favorably to treatment.
Limitations
Even with the high rate of AIH detected in the AN/AI population in Alaska, it is possible that we missed some cases. ANMC serves as the tertiary referral hospital for the AN/AI population across the entire state, but AN/AI people may choose to get care from other providers or facilities. In particular, individuals may choose to seek care out of state or outside of the tribal health system. However, even if an individual seeks liver care elsewhere they still may access the Alaska Tribal Health System for prescription medications or other aspects of their care, increasing the likelihood that their case will come to the attention of the ANTHC LDHP.
AIH has diverse clinical phenotypes, making it a challenge to diagnose. Cases may be missed if only classic AIH phenotypes are considered. Cases may also be missed if AIH and another more common liver disease such as NAFLD are both present and only the more common liver disease is diagnosed. Liver biopsy, available at ANMC but not in remote communities, can assist with differential diagnosis. Individuals with asymptomatic or atypical AIH presentation, or without access to liver biopsy, may be more likely to be missed. However, given LDHP's extensive collaborative network throughout the tribal health system, it is most likely that difficult liver disease cases will be referred to LDHP and arrangements will attempt to be made to fly the patient to Anchorage for a diagnostic liver biopsy if needed.
There is no single diagnostic test for AIH; rather diagnosis is based on a combination of clinical, laboratory, and histological features along with the exclusion of other known causes of liver disease. Diagnostic guidelines call for a liver biopsy to conclusively establish an AIH diagnosis. 10 , 28 Given the diversity of AIH/OS presentation, it is possible that some cases have been misdiagnosed. However, given the high proportion of study participants with biopsy‐confirmed AIH, we doubt that the high prevalence reported here is due to overdiagnosis.
Medical record review for symptoms and comorbid autoimmune conditions was limited to data accessible through ANTHC's current EHR system, which has been in use since approximately the last decade. Some, but not all, historic information or information from paper charts has been entered into this system. The ANTHC EHR interacts with most but not all tribal EHRs in Alaska. There may be more missing data for patients from regions of the state with EHRs that do not interact with ANTHC's system. We counted all cases of thyroiditis as an autoimmune condition, but we were not able to confirm with autoimmune antibodies. Medication results were based on prescriptions. We were not able to confirm whether a patient took the prescribed medications.
Conclusion
The AN/AI population in Alaska continues to have the highest reported prevalence of AIH in the world, with prevalence rising steadily since 2000. More than half of AN/AI persons with AIH or OS responded to treatment and achieved biochemical remission, while those with fatty liver were less likely to achieve biochemical remission. High AIH/OS prevalence in this population may be due to a combination of increased detection resulting of our strong referral system for all liver conditions as well as possible unique genetic or environmental exposures.
Ethics statement
This study has been reviewed and approved by the Alaska Area IRB as well as the Alaska Native Tribal Health Consortium Health Research Review Committee and the Southcentral Foundation Executive Committee.
Patient consent
All participants provided informed consent before being enrolled in the Autoimmune Liver Disease in Alaska Natives: Natural History and Treatment project.
Declaration of conflict of interest: None.
Data availability statement
Data are tribally owned and not publicly available.
References
- 1. van Gerven NM, de Boer YS, Mulder CJ, van Nieuwkerk CM, Bouma G. Auto immune hepatitis. World J. Gastroenterol. 2016; 22: 4651–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rust C, Beuers U. Overlap syndromes among autoimmune liver diseases. World J. Gastroenterol. 2008; 14: 3368–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hurlburt KJ, McMahon BJ, Deubner H, Hsu‐Trawinski B, Williams JL, Kowdley KV. Prevalence of autoimmune liver disease in Alaska Natives. Am. J. Gastroenterol. 2002; 97: 2402–2407. [DOI] [PubMed] [Google Scholar]
- 4. Ngu JH, Bechly K, Chapman BA et al. Population‐based epidemiology study of autoimmune hepatitis: a disease of older women? J. Gastroenterol. Hepatol. 2010; 25: 1681–1686. [DOI] [PubMed] [Google Scholar]
- 5. Lee YM, Teo EK, Ng TM, Khor C, Fock KM. Autoimmune hepatitis in Singapore: a rare syndrome affecting middle‐aged women. J. Gastroenterol. Hepatol. 2001; 16: 1384–1389. [DOI] [PubMed] [Google Scholar]
- 6. Grønbæk L, Vilstrup H, Jepsen P. Autoimmune hepatitis in Denmark: incidence, prevalence, prognosis, and causes of death. A nationwide registry‐based cohort study. J. Hepatol. 2014; 60: 612–617. [DOI] [PubMed] [Google Scholar]
- 7. Puustinen L, Barner‐Rasmussen N, Pukkala E, Färkkilä M. Incidence, prevalence, and causes of death of patients with autoimmune hepatitis: a nationwide register‐based cohort study in Finland. Digest. Liver Dis. 2019; 51: 1294–1299. [DOI] [PubMed] [Google Scholar]
- 8. Alaska Department of Labor and Workforce Development, Research and Analysis Section . Alaska Population Overview: 2019 Estimates. Juneau, AK: Alaska Department of Labor and Workforce Development, Research and Analysis Section, 2020. [Google Scholar]
- 9. Alaska Department of Labor and Worforce Development, Research and Analysis Section . Alaska Population Estimates . 2022. Available from URL: https://live.laborstats.alaska.gov/pop/index.cfm
- 10. Mack CL, Adams D, Assis DN et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American Association for the Study of Liver Diseases. Hepatology. 2020; 72: 671–722. [DOI] [PubMed] [Google Scholar]
- 11. Guo L, Zheng L, Hu L, Zhou H, Yu L, Liang W. Transient elastography (FibroScan) performs better than non‐invasive markers in assessing liver fibrosis and cirrhosis in autoimmune hepatitis patients. Med. Sci. Monit. 2017; 23: 5106–5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boursier J, Decraecker M, Bourlière M, Bureau C, Ganne‐Carrié N, de Lédinghen V. Quality criteria for the measurement of liver stiffness. Clin. Res. Hepatol. Gastroenterol. 2022; 46: 101761. [DOI] [PubMed] [Google Scholar]
- 13. StataCorp . Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC, 2021. [Google Scholar]
- 14. Dean AG, Sullivan KM, Soe MM. OpenEpi: Open Source Epidemiologic Statistics for Public Health. Release 3.01 . 2013. Available from URL: www.openepi.com
- 15. Lv T, Li M, Zeng N et al. Systematic review and meta‐analysis on the incidence and prevalence of autoimmune hepatitis in Asian, European, and American population. J. Gastroenterol. Hepatol. 2019; 34: 1676–1684. [DOI] [PubMed] [Google Scholar]
- 16. Tunio NA, Mansoor E, Sheriff MZ, Cooper GS, Sclair SN, Cohen SM. Epidemiology of autoimmune hepatitis (AIH) in the United States between 2014 and 2019: A population‐based national study. J. Clin. Gastroenterol. 2021; 55: 903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim BH, Choi HY, Ki M, Kim KA, Jang ES, Jeong SH. Population‐based prevalence, incidence, and disease burden of autoimmune hepatitis in South Korea. PloS One. 2017; 12: e0182391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barrett DH, Burks JM, McMahon B et al. Epidemiology of hepatitis B in two Alaska communities. Am. J. Epidemiol. 1977; 105: 118–122. [DOI] [PubMed] [Google Scholar]
- 19. McMahon BJ, Schoenberg S, Bulkow L et al. Seroprevalence of hepatitis B viral markers in 52,000 Alaska Natives. Am. J. Epidemiol. 1993; 138: 544–549. [DOI] [PubMed] [Google Scholar]
- 20. Covelli C, Sacchi D, Sarcognato S et al. Pathology of autoimmune hepatitis. Pathologica. 2021; 113: 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barnabe C, Joseph L, Belisle P et al. Prevalence of systemic lupus erythematosus and systemic sclerosis in the First Nations population of Alberta, Canada. Arthritis Care Res. 2012; 64: 138–143. [DOI] [PubMed] [Google Scholar]
- 22. Ferucci ED, Johnston JM, Gaddy JR et al. Prevalence and incidence of systemic lupus erythematosus in a population‐based registry of American Indian and Alaska Native people, 2007‐2009. Arthritis Rheumatol. 2014; 66: 2494–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boyer GS, Templin DW, Lanier AP. Rheumatic diseases in Alaskan Indians of the southeast coast: high prevalence of rheumatoid arthritis and systemic lupus erythematosus. J. Rheumatol. 1991; 18: 1477–1484. [PubMed] [Google Scholar]
- 24. Gatselis NK, Zachou K, Koukoulis GK, Dalekos GN. Autoimmune hepatitis, one disease with many faces: etiopathogenetic, clinico‐laboratory and histological characteristics. World J. Gastroenterol. 2015; 21: 60–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sucher E, Sucher R, Gradistanac T, Brandacher G, Schneeberger S, Berg T. Autoimmune hepatitis—immunologically triggered liver pathogenesis—diagnostic and therapeutic strategies. J. Immunol. Res. 2019; 2019: 9437043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Teufel A, Weinmann A, Kahaly GJ et al. Concurrent autoimmune diseases in patients with autoimmune hepatitis. J. Clin. Gastroenterol. 2010; 44: 208–213. [DOI] [PubMed] [Google Scholar]
- 27. Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011; 34: 274–285. [DOI] [PubMed] [Google Scholar]
- 28. European Association for Study of the Liver . EASL Clinical Practice Guidelines: autoimmune hepatitis. J. Hepatol. 2015; 63: 971–1004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are tribally owned and not publicly available.


