Extract
Acute exacerbations of COPD (AECOPD) are the second most common cause of emergency hospitalisation worldwide, with one-quarter of patients readmitted within 30 days of discharge [1]. Each exacerbation accelerates lung function decline [2] and is associated with a deterioration in health-related quality of life and an increased risk of mortality in the post-discharge period [3]. Several pathophysiological changes including decreased peak expiratory flow rate and increased dyspnoea often occur in the 3–5 days preceding an exacerbation [4].
Tweetable abstract
Respiratory waveforms can be reduced to simple metrics, such as rate, but this may miss information about waveform shape and whole breathing pattern. A novel analysis method quantifying the whole waveform shape identifies AECOPD earlier. https://bit.ly/3M6uIEB
To the Editor:
Acute exacerbations of COPD (AECOPD) are the second most common cause of emergency hospitalisation worldwide, with one-quarter of patients readmitted within 30 days of discharge [1]. Each exacerbation accelerates lung function decline [2] and is associated with a deterioration in health-related quality of life and an increased risk of mortality in the post-discharge period [3]. Several pathophysiological changes including decreased peak expiratory flow rate and increased dyspnoea often occur in the 3–5 days preceding an exacerbation [4]. This time window presents an opportunity to identify AECOPD and initiate patient self-management and clinical interventions aimed at mitigating hospitalisation. Sensitive and specific biomarkers of physiological stress, which can be easily applied and interpreted in both community and clinical settings, would facilitate earlier identification of AECOPD.
Increased respiratory rate is a well-validated biomarker of respiratory stress that is often incorporated into patient monitoring systems, facilitating early identification of deterioration [5]. Respiratory pressure/flow/plethysmography/thermistor waveforms are typically sampled as high-fidelity time-series data (∼100–1000 Hz) and respiratory rate measurement automated to circumvent common inconsistencies with manual breath counting, using algorithms to detect waveform peaks [6]. However, such analysis disregards the intermediate data points which contain information about changes in waveform morphology, quantification of which could also help to identify physiological change. Symmetric Projection Attractor Reconstruction (SPAR), a novel analysis technique, overcomes this problem. SPAR replots every data point of any cyclic waveform, generating a new visual representation (“attractor”). Quantifiable attractor features can be extracted, providing new diagnostic metrics pertaining to waveform morphology [7]. SPAR is resistant to baseline wander and has previously been successfully applied to cardiovascular waveforms [8–10].
The objective of this study was to apply SPAR to respiratory waveforms from patients recovering from severe AECOPD. We hypothesised that SPAR would generate more sensitive biomarkers of impending COPD re-exacerbations compared to respiratory rate.
We performed a retrospective secondary analysis from raw nasal pressure waveform data (sampled at 512 Hz) initially collected to evaluate the trajectory parasternal electromyogram changes in patients recovering from an AECOPD (NCT03443505) [11]. Waveforms were recorded during 6 min of resting breathing at hospital discharge and then daily in the patient's home over a 30-day period post-discharge. Time-series data were processed in a blinded manner to avoid bias; two patient datasets were excluded, based on unfavourable signal-to-noise ratios.
Our secondary analysis subdivided patients into two groups: “stable” and “re-exacerbating”. The six “stable” patients remained at home for 30 days. Of the four re-exacerbating patients, two were readmitted to hospital on day 2 post-discharge, and two patients self-administered a rescue pack of corticosteroids and antibiotics on days 23 and 30. On day 24, the patient who took the rescue pack on day 23 was hospitalised.
Concomitant respiratory medications were balanced between both groups, except for carbocysteine, which was used by four out of four patients in the re-exacerbating group versus two out of six in the stable group.
Waveform analysis was performed using a bespoke MATLAB software tool (MATLAB version 2022b). This program enabled the management and visualisation of waveform time-series data, and the generation and quantification of SPAR attractors. The SPAR method [7, 8] replots a window of time-series cyclic data into 3-dimensional phase space and then projects this down one line, to generate a two-dimensional attractor. Attractors visually amplify small morphological changes from the original waveform.
Multiple visual attractor features were quantified using secondary algorithms. Our analysis revealed that the attractor's “openness” was the most visually distinct feature. To quantify this, we measured their 5% radial opening (“SPAR-Opening”). This metric conceptually equates to the relative size of a concentric growing circle until it covers 5% of the attractor – the more open the attractor, the larger the value. The conventional metric respiratory rate was separately quantified using the MATLAB Breathmetrics Package [12]. Wilcoxon rank sum tests or Chi-squared tests (where appropriate) were used to compare baseline characteristics between groups (p<0.05 was considered statistically significant). Univariate logistic regression models, using the described groups as outcomes, were generated for respiratory rate and SPAR-opening at different time points. Internal validation of these models allowed the comparison between the classification performances of these two metrics. Receiver operator characteristic area under the curve (ROC-AUC) values quantified these performances, which ranged from 0.5 (poor discriminator) to 1.0 (perfect discriminator).
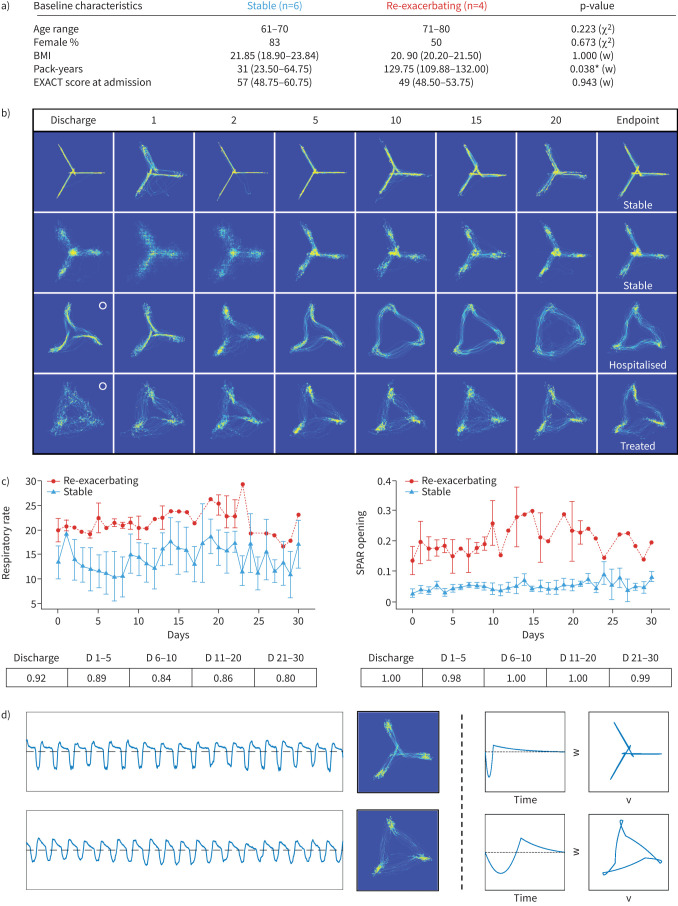
Other than increased pack-years in re-exacerbating patients, there was no statistically significant difference in baseline characteristics between the two groups (figure 1a). From the point of discharge, an opening was noticeable in the centre of the attractors from re-exacerbating patients, which enlarged in a time-dependent manner during the home-monitoring period, until hospital readmission/community treatment. In contrast, stable patients presented closed, helicopter-like attractors throughout (figure 1b). Quantification of SPAR-opening reflected these observed changes (figure 1c). The conventional metric, respiratory rate, was also elevated in re-exacerbating patients throughout the monitoring period, as expected (figure 1c). However, SPAR-opening outperformed respiratory rate, reflected by increased ROC-AUC values from the day of discharge (respiratory rate 0.92 versus SPAR 1.00) and throughout the 30-day home-monitoring period (respiratory rate 0.8–0.89 versus SPAR 0.98–1.0) (figure 1c).
FIGURE 1.
a) Baseline characteristics of studied patients, by group. Column labels indicate corresponding sample sizes. Age range=median years age range. Body mass index (BMI) (kg·m−2), pack-years and Exacerbations of Chronic Pulmonary Disease Tool (EXACT) score at admission are shown as median (interquartile range (IQR)). The last column shows p-values of a Chi-square (χ2) or Wilcoxon rank sum (w) test, comparing frequencies or median values of both groups, performed in MATLAB (MathWorks Ltd., Cambridge, UK). b) 60-s Symmetric Projection Attractor Reconstruction (SPAR) attractors of four different COPD patients, two stable (top) and two re-exacerbating (bottom) over the monitoring period (columns represent the approximate day of monitoring). o: start of attractor opening. c) Trajectories of respiratory rate and SPAR-Opening metrics over the 30-day monitoring period (median+IQR error bars). Tables below graphs indicate receiver operator characteristic area under the curve (ROC-AUC) classification performance values of the corresponding metrics during the different stages of the monitoring period (D: days). d) From left to right: example traces (60 s, ±0.5 mV, inhalation shown below dotted line), attractors (60 s, arbitrary units, size normalised) of day 2 recordings of a stable (top) and re-exacerbating (bottom) patient, in silico modelling of traces (arbitrary units) and attractors (arbitrary units) replicating changes observed in the two different groups. *: p<0.05.
In silico modelling revealed that SPAR-opening quantified the bilateral symmetry of the inspiratory and expiratory waveform segments (figure 1d). As the waveform became more symmetrical, the centre of the corresponding attractor gradually opened, positively correlating with a future exacerbation. In stable COPD patients, the waveform fraction for inspiration was approximately one-third of the whole breath cycle length, but the inspiratory and expiratory times equalised in the re-exacerbating patients. SPAR-opening quantified this duty-cycle change, but additionally quantified the overall increased symmetry of the entire waveform's morphology. This increased symmetry is unlikely to be specific for AECOPD but, rather, reflects a pathological change in the work of breathing.
This proof-of-concept study introduces a novel metric that is sensitive to physiological change, identified by analysing the morphology of raw nasal pressure waveforms. Our findings indicate that SPAR has potential clinical utility for earlier and more accurate recognition of AECOPD from resting tidal breathing. Whilst respiratory rate is an important biomarker, respiratory rate extraction by peak detection can be compromised in noisy data and is a reductionist analysis of the complex waveform signal. In contrast, SPAR faithfully utilises every waveform data point limiting inadvertent bias introduction, quantifying the entire breathing pattern and, in this study, improving classification accuracy. Characterising waveform morphology to add diagnostic sensitivity is not in itself new and has been applied to cardiovascular waveforms over decades, using other analytical techniques [13–15]. This current study applied SPAR, for the first time, to the lesser-studied respiratory waveform morphology.
A technological key discriminator is that, in addition to quantifiable metrics, SPAR generates images encapsulating and amplifying time-dependent waveform changes, at-a-glance. This can facilitate the visualisation of trends in patient status that may be less obvious when appraising waveform real-time traces directly. This visualisation aspect also opens opportunities to apply deep-learning image analysis methods, as are currently used for clinical imaging data, to support classification and alerts. Whilst the nasal pressure waveform data used in this study is not routine, SPAR can be extended to clinically used methods where cyclic respiratory time-series data are acquired (e.g. thermistor, impedance plethysmography, non-contact chest motion technologies), as it is agonistic to signal type.
This proof-of-concept study will require larger validation studies, but the time-dependent changes in SPAR-opening, seen in all re-exacerbating patients, and entirely absent in stable patients, increase our confidence in the metric's value. Future research is now needed to explore the utility of combining SPAR metrics with established clinical biomarkers, including respiratory rate, to support clinical decision-making during both in- and outpatient care.
Acknowledgements
A proportion of the data collected from NCT03443505, with ethical approval, was made available for the secondary analysis presented here through a data transfer agreement approved by Guy's and St Thomas’ NHS Foundation Trust and King's College London. Both organisations are part of King's Health Partners. We would like to thank Nick Hart and Patrick Murphy for permitting data access and to the patients for consenting to participate in the original trial. We thank Ying Huang for developing SPAR metrics and coding, and Weendata Segda and Nabihah Jaigirdar (St George's Hospital Clinical Pharmacology undergraduates) who undertook initial SPAR processing of data. We thank Eui-Eik Suh for useful scientific and clinical discussions. Finally, we are extremely grateful to John Moxham for his input into our analysis, useful scientific and clinical discussions, and comments on the manuscript.
Provenance: Submitted article, peer reviewed.
Conflict of interest: M. Nandi and P.J. Aston are coinventors of SPAR method – Delay coordinate analysis of periodic data, patent number WO2015121679A1, used in this study.
Conflict of interest: N. Hart is involved in an investigator-led/industry-funded trial (unrestricted grant) with Resmed. He receives consulting fees from Philips for a hospital patient monitoring programme. He has received payments and honoraria from Fisher and Paykel (chairing an ERS International Congress 2022 symposium) and Philips (lecturing at an ERS International Congress 2022 symposium). He has received financial support for attending meetings from Fisher and Paykel (ERC 2022). He has patents planned, issued or pending for Myotrace (held by Guy's and Thomas’ Foundation Trust).
Conflict of interest: R.F. D'Cruz receives consulting fees from Resmed and AstraZeneca. She receives payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Fisher Paykel and Resmed. She receives support for attending meetings from Fisher Paykel and Resmed.
Conflict of interest: M. Serna-Pascual, M. Volovaya, C.J. Jolley, G.F. Rafferty and J. Steier have declared no conflict of interest.
Support statement: M. Serna-Pascual was funded by DRIVE health Data Driven Health CDT, King's College London. R.F. D'Cruz was funded by the National Institute for Health Research to conduct this study (NIHR-INF-0415 and DRF-2018-11-ST2-037). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Sjoding MW, Cooke CR. Readmission penalties for chronic obstructive pulmonary disease will further stress hospitals caring for vulnerable patient populations. Am J Respir Crit Care Med 2014; 190: 1072–1074. doi: 10.1164/RCCM.201407-1345LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donaldson GC, Seemungal TAR, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002; 57: 847–852. doi: 10.1136/THORAX.57.10.847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suissa S, Dell'Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax 2012; 67: 957–963. doi: 10.1136/THORAXJNL-2011-201518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seemungal TAR, Donaldson GC, Bhowmik A, et al. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 161: 1608–1613. doi: 10.1164/AJRCCM.161.5.9908022 [DOI] [PubMed] [Google Scholar]
- 5.Shah SA, Velardo C, Farmer A, et al. Exacerbations in chronic obstructive pulmonary disease: identification and prediction using a digital health system. J Med Internet Res 2017; 19: e69. doi: 10.2196/JMIR.7207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginsburg AS, Lenahan JL, Izadnegahdar R, et al. A systematic review of tools to measure respiratory rate in order to identify childhood pneumonia. Am J Respir Crit Care Med 2018; 197: 1116–1127. doi: 10.1164/RCCM.201711-2233CI [DOI] [PubMed] [Google Scholar]
- 7.Aston PJ, Christie MI, Huang YH, et al. Beyond HRV: attractor reconstruction using the entire cardiovascular waveform data for novel feature extraction. Physiol Meas 2018; 39: 024001. doi: 10.1088/1361-6579/AAA93D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nandi M, Aston PJ. Extracting new information from old waveforms: symmetric projection attractor reconstruction: where maths meets medicine. Exp Physiol 2020; 105: 1444–1451. doi: 10.1113/EP087873 [DOI] [PubMed] [Google Scholar]
- 9.Lyle JV, Nandi M, Aston PJ. Symmetric projection attractor reconstruction: sex differences in the ECG. Front Cardiovasc Med 2021; 8: 1034. doi: 10.3389/FCVM.2021.709457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hörandtner C, Bachler M, Sehnert W, et al. Attractor reconstruction for quantifying the arterial pulse wave morphology during device-guided slow breathing. Cardiovasc Eng Technol 2022; 13: 939–949. doi: 10.1007/S13239-022-00628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Cruz RF, Suh E-K, Kaltsakas G, et al. Home parasternal electromyography tracks patient-reported and physiological measures of recovery from severe COPD exacerbation. ERJ Open Res 2021; 7: 00709-2020. doi: 10.1183/23120541.00709-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noto T, Zhou G, Schuele S, et al. Automated analysis of breathing waveforms using BreathMetrics: a respiratory signal processing toolbox. Chem Senses 2018; 43: 583–597. doi: 10.1093/chemse/bjy045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlton PH, Paliakaitė B, Pilt K, et al. Assessing hemodynamics from the photoplethysmogram to gain insights into vascular age: a review from VascAgeNet. Am J Physiol Heart Circ Physiol 2022; 322: H493–H522. doi: 10.1152/AJPHEART.00392.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panikkath R, Reinier K, Uy-Evanado A, et al. Prolonged Tpeak-to-Tend interval on the resting ECG is associated with increased risk of sudden cardiac death. Circ Arrhythm Electrophysiol 2011; 4: 441–447. doi: 10.1161/CIRCEP.110.960658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Gu H, Fok H, et al. Forward and backward pressure waveform morphology in hypertension. Hypertension 2017; 69: 375–381. doi: 10.1161/HYPERTENSIONAHA.116.08089 [DOI] [PMC free article] [PubMed] [Google Scholar]