Abstract
Histone deacetylases (HDACs) are key posttranslational modulators of the proteome. We show that expression of histone deacetylase 6 ( hdac6 ) is dynamic and appears in a tissue specific manner throughout embryonic development of the frog Xenopus laevis . Interestingly, hdac6 transcripts often associate with ciliated tissues, like the left-right organizer at neurula stage or the pronephros. In the embryonic skin, Hdac6 protein localizes to the cilia base, suggesting a functional link.
Figure 1. Expression of Xenopus hdac6 on mRNA and protein level .
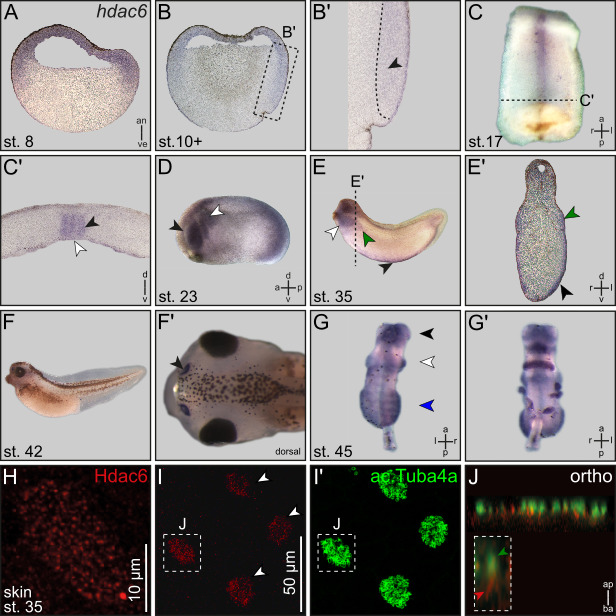
(A) Sagital section of a blastula stage Xenopus embryo. hdac6 is ubiquitously expressed in cells that form the animal hemisphere of the frog embryo. (B) Sagittal section of a gastrula embryo. hdac6 signal is detectable in the ectoderm, cells of the superficial mesoderm and cells of mesodermal fate (black arrow head in magnification B’). (C) Ventral view of a dorsal explant of a neurula embryo. (C’) Transversal section of C. hdac6 expression is restricted to the notochord and cells of the gastrocoel roof plate i.e. the LRO (black and white arrow head, respectively). (D) hdac6 is expressed in the developing eye cup (black arrow head) and in migrating neural crest cells (white arrow head). (E) hdac6 transcripts appear in the pronephric kidney (green arrow head), ventral hematopoietic stem cells (black arrow head) and in the developing craniofacial cartilage (white arrow head). (E’) transversal section of E. (F) hdac6 activity in larval nasal pits. (F’) Dorsal view of tadpole shown in F. Nasal pit is highlighted (black arrow head). (G) Dorsal view of pre-metamorphic brain explant. hdac6 shows a regionalized expression pattern in the tel-, di- and rhombencephalon (black, white or blue arrowheads, respectively). (G’) Ventral view of the brain explant shown in G’. (H) Immunofluorescent staining of Hdac6 (red) in the larval epidermis at stage 35. Hdac6 shows a spotted expression pattern within epidermal cells. Staining of acetylated alpha-Tubulin (ac.Tuba4a) by immunofluorescence (green) reveal that Hdac6 positive cells are multiciliated cells (MCCs). J in I and I’ indicates MCC shown in J. (J) Orthogonal section of a MCC stained against Hdac6 (red) and ac.Tuba4a (green). Hdac6 localizes at the base of motile cilia. Inserted box shows a magnification of a single cilium. The ciliary axonem is highlighted by a green arrow head, Hdac6 localization is indicated by a red arrow head.
Scale bar: 10µm (I) Hdac6 is detected in a distinct subtype of epidermal cells (white arrowheads) Scale bar: 50µm. (I’)
Description
Histone deacetylases (HDACs) are best known for their transcriptional regulation in eukaryotic cells, by controlling DNA accessibility for e.g. transcription factors via deacetylation of histone proteins (Seto and Yoshida, 2014) . In higher vertebrates HDACs form an evolutionary conserved but diverse family of 11 genes that can be grouped into four subfamilies (class I to IV; Milazzo et al., 2020).These enzymes localize to the nucleus and / or the cytoplasm, allowing specific interaction with distinct molecular substrates. By deacetylating lysine residues of histone or non-histone proteins, HDACs play a key role in the post-translational modification of proteins, mediating general transcriptional activation or modulation of signaling pathways (Zhu et al., 2023) .
The highly conserved and best analyzed HDAC6 (subclass IIb) predominantly localizes to the cytoplasm and was shown to serve as the major deacetylase of acetylated alpha-Tubulin (Hubbert et al., 2002) . The stand-alone feature of HDAC6 is the functional duplication of its deacetylase domain. Interestingly, each domain appears to have a distinct set of molecular substrates (Milazzo et al., 2020) . Moreover, HDAC6 has been identified as a reasonable player in the pathophysiological process of different types of cancer and serves therefore as a potentially druggable therapeutic target protein (Li et al., 2018) .
Surprisingly, Hdac6 knockout mice are viable and fertile (Haberland et al., 2009; Zhang et al., 2008) . Although lacking an obvious developmental phenotype, HDAC6 depleted mice show defects in bone mineralization and alterations regarding the immune response and emotional behavior (Fukada et al., 2012; Zhang et al., 2008) . Experiments in Hdac6 depleted zebrafish strains also point towards a subtle but tissue specific loss-of-function (LOF) phenotype, affecting organs like brain, eye, inner ear and kidney leading to variations in movement behavior (Łysyganicz et al., 2021). To date, little is known about an early embryonic function of Hdac6 in Xenopus , with the exception of its contribution to the development of the visual system in swimming tadpoles (Bestman et al., 2015) . Here, we report the tissue specific developmental expression pattern of hdac6 mRNA in combination with its protein localization, which indicate an association with cilia.
In order to analyze the expression pattern of hdac6 throughout Xenopus development, we performed whole-mount mRNA in situ hybridization experiments. hdac6 transcripts are ubiquitously detectable in the animal hemisphere of stage 8 blastula embryos ( Fig. 1A ). During gastrulation, expression is found in the ectoderm ( Fig. 1B ) as well as in dorsal mesodermal cells including the superficial mesoderm ( Fig. 1B ’, Shook et al., 2004), which will later form the ciliated left-right organizer (LRO, Schweickert et al., 2007). In dorsal explants of stage 17 neurulas, hdac6 signal is restricted to the cells of the axial mesoderm, i.e. notochord and the epithelial layer of the gastrocoel roof plate, harboring the LRO ( Fig. 1C, C ’). Vibratome sections of stage 17 dorsal explants reveal that hdac6 expression is detectable in fluid flow generating cells of the LRO ( Fig. 1C ’, Schweickert et al., 2007; Blum et al., 2009). Stage 23 tailbud embryos express hdac6 in migrating neural crest cells as well as in the developing eye cup ( Fig. 1D ). Later in development, around stage 30, additional expression domains get established in the developing pronephros and in the ventral hematopoietic stem cell niche ( Fig. 1E, E ’). At stage 42, hdac6 expression is exclusively detectable in nasal pits of the tadpole ( Fig. 1F, F ’). Whole brain explants show a distinct and regionalized hdac6 expression in the larval brain at stage 45 in the telencephalon, diencephalon and rhombencephalon ( Fig. 1 G, G ’).
The ectoderm, the superficial mesoderm and the forming pronephros eventually mature into tissues with motile and / or non-motile cilia. In a next step, we wanted to investigate a potential cilia associated localization of Hdac6 protein via immunofluorescence (IF). Short of antibodies directed against Xenopus Hdac6, we used a commercially available polyclonal antibody against human HDAC6. Although, homology of human and frog sequences is high, antibody cross-reactivity to unrelated Xenopus proteins cannot be excluded. The Xenopus epidermis is an exemplary mucociliary epithelium and ideal model system for ciliary protein localization studies (Walentek, 2021) . In stage 35 embryos, Hdac6 was found to localize in a punctate pattern to a specific subset of epidermal cells ( Fig. 1H, I ). A combined IF staining against acetylated alpha-Tubulin revealed Hdac6 localization in multiciliated cells (MCCs) of the skin ( Fig. 1I ’). Orthogonal sections further show that Hdac6 is enriched at the cilia base ( Fig. 1J ). Although being characterized as an important tubulin deacetylase in vivo (Hubbert et al., 2002; Zhang et al., 2003) Hdac6 is not detected in the ciliary axoneme ( Fig. 1J ).
Published data have implicated a role of Hdac6 in cilia homeostasis of different vertebrate species (Łysyganicz et al., 2021; Yang et al., 2014). The tissue specific developmental mRNA expression pattern in Xenopus is in line with the previously reported, subtle LOF phenotypes in the corresponding embryonic tissues (Bestman et al., 2015; Łysyganicz et al., 2021; Zhang et al., 2008). Additionally, the observed localization of Hdac6 protein at the base of motile cilia points towards a more complex role of Hdac6 rather than exclusively regulating the axonemal acetylation of alpha-Tubulin. hdac6 expression in the LRO of the frog embryo argues for a functional contribution of Hdac6 in left-right patterning. Indeed, chemical modifier screens have previously identified HDAC inhibitors like Trichostatin A or Sodium butyrate as left-right defects causing agents whereas the mode of action remains elusive (Cao et al., 2009; Carneiro et al., 2011) .
As Xenopus serves as an ideal model for the investigation of ciliary architecture and function in health and disease (Blum and Ott, 2018) , future experiments might elucidate the exact role of histone deacetylase 6 in context of mucociliary epithelia and its functional role in the establishment of visceral left-right asymmetry.
Methods
Materials and methods
Animal care and maintenance
Husbandry, handling and experimental manipulations of Xenopus laevis frogs/embryos were approved by the Regional Government Stuttgart, Germany (A379/12 Zo, ‘Molekulare Embryologie’, V340/17 ZO and V349/18 ZO, ‘ Xenopus Embryonen in der Forschung’) and performed according to German federal laws and regulations (§6, article 1, sentence 2 nr. 4 of the animal protection act). Animals were staged according to (Nieuwkoop and Faber, 1994) .
Xenopus laevis hdac6
A full ORF clone from Xenopus laevis hdac6 cDNA (Ref seq; NM_001087017 Unigene ID Xl.8310) in pCMV SPORT6 (clone IRBHp990G107D) vector was purchased from Source BioScience (Cambridge, UK) and linearized with NcoI for antisense transcription with T7 polymerase (Promega).
RNA in situ hybridization and histological analysis
Embryos dedicated for in situ hybridization were fixed in MEMFA for 2 h at RT and later on processed following standard protocols suitable for Xenopus embryos (Sive et al. 2007) . Digoxigenin-labled (Roche) antisense-RNA probes were generated via Sp6 or T7 RNA Polymerase (Promega) from linearized plasmids. mRNA-ISH was performed according to (Belo et al., 1997) . For further histological analysis embryos were embedded in gelatine–albumin and sectioned (35 μm) on a Leica VT1000S vibratome.
Immunofluorescence
Endogenous Xenopus laevis Hdac6 Protein was detected by a commercially available antibody (Sigma-Aldrich; SAB1406911; 1:250) after overnight fixation (-20 °C) using Dent's fixative (80 % methanol; 20 % DMSO). Cilia located at the tadpole epidermis were visualized as described by Vick et al. 2009 After fixation embryos were treated according to standard IF procedures (Sive et al. 2007) . Antibodies used were: mouse monoclonal antibody directed against acetylated alpha-Tubulin (Sigma-Aldrich; T6793; 1:700) and Alexa488 or Cy3-conjugated secondary polyclonal anti-mouse antibodies (Invitrogen; A11059 or Sigma-Aldrich; C2181; both 1:250).
Funding Statement
M.T. was funded by the Federal Ministry of Education and Research (01PL11003), project Humboldt reloaded. T.O. received a Ph.D. fellowship by the Landesgraduiertenförderung Baden-Württemberg. Work in the Blum laboratory was supported by a grant from the Deutsche Forschungsgemeinschaft to M.B. (BL 285/9-2).
References
- Belo José António, Bouwmeester Tewis, Leyns Luc, Kertesz Nathalie, Gallo Michael, Follettie Maximillian, De Robertis Eddy M. Cerberus-like is a secreted factor with neuralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mechanisms of Development. 1997 Nov 1;68(1-2):45–57. doi: 10.1016/s0925-4773(97)00125-1. [DOI] [PubMed] [Google Scholar]
- Bestman Jennifer E., Huang Lin-Chien, Lee-Osbourne Jane, Cheung Phillip, Cline Hollis T. An in vivo screen to identify candidate neurogenic genes in the developing Xenopus visual system. Developmental Biology. 2015 Dec 1;408(2):269–291. doi: 10.1016/j.ydbio.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum Martin, Beyer Tina, Weber Thomas, Vick Philipp, Andre Philipp, Bitzer Eva, Schweickert Axel. Xenopus , an ideal model system to study vertebrate left-right asymmetry . Developmental Dynamics. 2009 Jun 1;238(6):1215–1225. doi: 10.1002/dvdy.21855. [DOI] [PubMed] [Google Scholar]
- Blum Martin, Ott Tim. <b><i>Xenopus</i></b>: An Undervalued Model Organism to Study and Model Human Genetic Disease. Cells Tissues Organs. 2018;205(5-6):303–313. doi: 10.1159/000490898. [DOI] [PubMed] [Google Scholar]
- Cao Ying, Semanchik Nicole, Lee Seung Hun, Somlo Stefan, Barbano Paolo Emilio, Coifman Ronald, Sun Zhaoxia. Chemical modifier screen identifies HDAC inhibitors as suppressors of PKD models. Proceedings of the National Academy of Sciences. 2009 Dec 22;106(51):21819–21824. doi: 10.1073/pnas.0911987106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro Katia, Donnet Claudia, Rejtar Tomas, Karger Barry L, Barisone Gustavo A, Díaz Elva, Kortagere Sandhya, Lemire Joan M, Levin Michael. Histone deacetylase activity is necessary for left-right patterning during vertebrate development. BMC Developmental Biology. 2011 May 20;11(1) doi: 10.1186/1471-213x-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada Masahide, Hanai Atsuko, Nakayama Atsuo, Suzuki Takayoshi, Miyata Naoki, Rodriguiz Ramona M., Wetsel William C., Yao Tso-Pang, Kawaguchi Yoshiharu. Loss of Deacetylation Activity of Hdac6 Affects Emotional Behavior in Mice. PLoS ONE. 2012 Feb 6;7(2):e30924–e30924. doi: 10.1371/journal.pone.0030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland Michael, Montgomery Rusty L., Olson Eric N. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nature Reviews Genetics. 2009 Jan 1;10(1):32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbert Charlotte, Guardiola Amaris, Shao Rong, Kawaguchi Yoshiharu, Ito Akihiro, Nixon Andrew, Yoshida Minoru, Wang Xiao-Fan, Yao Tso-Pang. HDAC6 is a microtubule-associated deacetylase. Nature. 2002 May 1;417(6887):455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- Li Ting, Zhang Chao, Hassan Shafat, Liu Xinyue, Song Fengju, Chen Kexin, Zhang Wei, Yang Jilong. Histone deacetylase 6 in cancer. Journal of Hematology & Oncology. 2018 Sep 3;11(1) doi: 10.1186/s13045-018-0654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łysyganicz Paweł K., Pooranachandran Niedharsan, Liu Xinming, Adamson Kathryn I., Zielonka Katarzyna, Elworthy Stone, van Eeden Fredericus J., Grierson Andrew J., Malicki Jarema J. Loss of Deacetylation Enzymes Hdac6 and Sirt2 Promotes Acetylation of Cytoplasmic Tubulin, but Suppresses Axonemal Acetylation in Zebrafish Cilia. Frontiers in Cell and Developmental Biology. 2021 Jun 28;9 doi: 10.3389/fcell.2021.676214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milazzo Giorgio, Mercatelli Daniele, Di Muzio Giulia, Triboli Luca, De Rosa Piergiuseppe, Perini Giovanni, Giorgi Federico M. Histone Deacetylases (HDACs): Evolution, Specificity, Role in Transcriptional Complexes, and Pharmacological Actionability. Genes. 2020 May 15;11(5):556–556. doi: 10.3390/genes11050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop, P. D. & Faber, J. (eds.). Normal table of Xenopus laevis (Daudin). A systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis (Garland Pub, New York, 1994).
- Schweickert Axel, Weber Thomas, Beyer Tina, Vick Philipp, Bogusch Susanne, Feistel Kerstin, Blum Martin. Cilia-Driven Leftward Flow Determines Laterality in Xenopus. Current Biology. 2007 Jan 1;17(1):60–66. doi: 10.1016/j.cub.2006.10.067. [DOI] [PubMed] [Google Scholar]
- Seto E., Yoshida M. Erasers of Histone Acetylation: The Histone Deacetylase Enzymes. Cold Spring Harbor Perspectives in Biology. 2014 Apr 1;6(4):a018713–a018713. doi: 10.1101/cshperspect.a018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook David R., Majer Christina, Keller Ray. Pattern and morphogenesis of presumptive superficial mesoderm in two closely related species, Xenopus laevis and Xenopus tropicalis. Developmental Biology. 2004 Jun 1;270(1):163–185. doi: 10.1016/j.ydbio.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Sive, H. L., Grainger, R. M. & Harland, R. M. Xenopus laevis Keller Explants. Cold Spring Harbor Protocols 2007 , pdb.prot4749; 10.1101/pdb.prot4749 (2007). [DOI] [PubMed]
- Vick Philipp, Schweickert Axel, Weber Thomas, Eberhardt Melanie, Mencl Stine, Shcherbakov Denis, Beyer Tina, Blum Martin. Flow on the right side of the gastrocoel roof plate is dispensable for symmetry breakage in the frog Xenopus laevis. Developmental Biology. 2009 Jul 1;331(2):281–291. doi: 10.1016/j.ydbio.2009.05.547. [DOI] [PubMed] [Google Scholar]
- Walentek Peter. Xenopus epidermal and endodermal epithelia as models for mucociliary epithelial evolution, disease, and metaplasia . genesis. 2021 Jan 5;59(1-2) doi: 10.1002/dvg.23406. [DOI] [PubMed] [Google Scholar]
- Yang Yunfan, Ran Jie, Liu Min, Li Dengwen, Li Yuanyuan, Shi Xingjuan, Meng Dan, Pan Junmin, Ou Guangshuo, Aneja Ritu, Sun Shao-Cong, Zhou Jun. CYLD mediates ciliogenesis in multiple organs by deubiquitinating Cep70 and inactivating HDAC6. Cell Research. 2014 Oct 24;24(11):1342–1353. doi: 10.1038/cr.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Yu, Li Na, Caron Cécile, Matthias Gabriele, Hess Daniel, Khochbin Saadi, Matthias Patrick. HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. The EMBO Journal. 2003 Mar 3;22(5):1168–1179. doi: 10.1093/emboj/cdg115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Yu, Kwon SoHee, Yamaguchi Teppei, Cubizolles Fabien, Rousseaux Sophie, Kneissel Michaela, Cao Chun, Li Na, Cheng Hwei-Ling, Chua Katrin, Lombard David, Mizeracki Adam, Matthias Gabriele, Alt Frederick W., Khochbin Saadi, Matthias Patrick. Mice Lacking Histone Deacetylase 6 Have Hyperacetylated Tubulin but Are Viable and Develop Normally. Molecular and Cellular Biology. 2008 Mar 1;28(5):1688–1701. doi: 10.1128/mcb.01154-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Yuanzai, Feng Mengkai, Wang Bo, Zheng Yichao, Jiang Dandan, Zhao Lijuan, Mamun M.A.A., Kang Huiqin, Nie Haiqian, Zhang Xiya, Guo Ningjie, Qin Shangshang, Wang Ning, Liu Hongmin, Gao Ya. New insights into the non-enzymatic function of HDAC6. Biomedicine & Pharmacotherapy. 2023 May 1;161:114438–114438. doi: 10.1016/j.biopha.2023.114438. [DOI] [PubMed] [Google Scholar]


