Key Points
Question
Cancer screening tests are promoted to save lives, but how much is life extended due to commonly used cancer screening tests?
Findings
In this systematic review and meta-analysis of 18 long-term randomized clinical trials involving 2.1 million individuals, colorectal cancer screening with sigmoidoscopy prolonged lifetime by 110 days, while fecal testing and mammography screening did not prolong life. An extension of 37 days was noted for prostate cancer screening with prostate-specific antigen testing and 107 days with lung cancer screening using computed tomography, but estimates are uncertain.
Meaning
The findings of this meta-analysis suggest that colorectal cancer screening with sigmoidoscopy may extend life by approximately 3 months; lifetime gain for other screening tests appears to be unlikely or uncertain.
Abstract
Importance
Cancer screening tests are promoted to save life by increasing longevity, but it is unknown whether people will live longer with commonly used cancer screening tests.
Objective
To estimate lifetime gained with cancer screening.
Data Sources
A systematic review and meta-analysis was conducted of randomized clinical trials with more than 9 years of follow-up reporting all-cause mortality and estimated lifetime gained for 6 commonly used cancer screening tests, comparing screening with no screening. The analysis included the general population. MEDLINE and the Cochrane library databases were searched, and the last search was performed October 12, 2022.
Study Selection
Mammography screening for breast cancer; colonoscopy, sigmoidoscopy, or fecal occult blood testing (FOBT) for colorectal cancer; computed tomography screening for lung cancer in smokers and former smokers; or prostate-specific antigen testing for prostate cancer.
Data Extraction and Synthesis
Searches and selection criteria followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline. Data were independently extracted by a single observer, and pooled analysis of clinical trials was used for analyses.
Main Outcomes and Measures
Life-years gained by screening was calculated as the difference in observed lifetime in the screening vs the no screening groups and computed absolute lifetime gained in days with 95% CIs for each screening test from meta-analyses or single randomized clinical trials.
Results
In total, 2 111 958 individuals enrolled in randomized clinical trials comparing screening with no screening using 6 different tests were eligible. Median follow-up was 10 years for computed tomography, prostate-specific antigen testing, and colonoscopy; 13 years for mammography; and 15 years for sigmoidoscopy and FOBT. The only screening test with a significant lifetime gain was sigmoidoscopy (110 days; 95% CI, 0-274 days). There was no significant difference following mammography (0 days: 95% CI, −190 to 237 days), prostate cancer screening (37 days; 95% CI, −37 to 73 days), colonoscopy (37 days; 95% CI, −146 to 146 days), FOBT screening every year or every other year (0 days; 95% CI, −70.7 to 70.7 days), and lung cancer screening (107 days; 95% CI, −286 days to 430 days).
Conclusions and Relevance
The findings of this meta-analysis suggest that current evidence does not substantiate the claim that common cancer screening tests save lives by extending lifetime, except possibly for colorectal cancer screening with sigmoidoscopy.
The meta-analysis examines evidence from randomized clinical trials of lifetime gained with use of screening tests for lung, breast, prostate, and colorectal cancer.
Introduction
Since the US National Cancer Act was launched in 1971, cancer screening has been considered pivotal in cancer control and a primary component to promote health in many countries.1 Consequently, many individuals undergo testing with 1 or several tests to detect cancer at a curable stage or to prevent cancer through removal of precursor lesions detected at screening.
The most commonly used cancer screening tests are mammography for breast cancer, prostate-specific antigen (PSA) testing for prostate cancer, fecal occult blood testing (FOBT) or endoscopy (sigmoidoscopy or colonoscopy) for colorectal cancer, computed tomography (CT) scanning for lung cancer, and Papanicolaou (Pap) test cytology (and more recently human papillomavirus testing) for cervical cancer.2
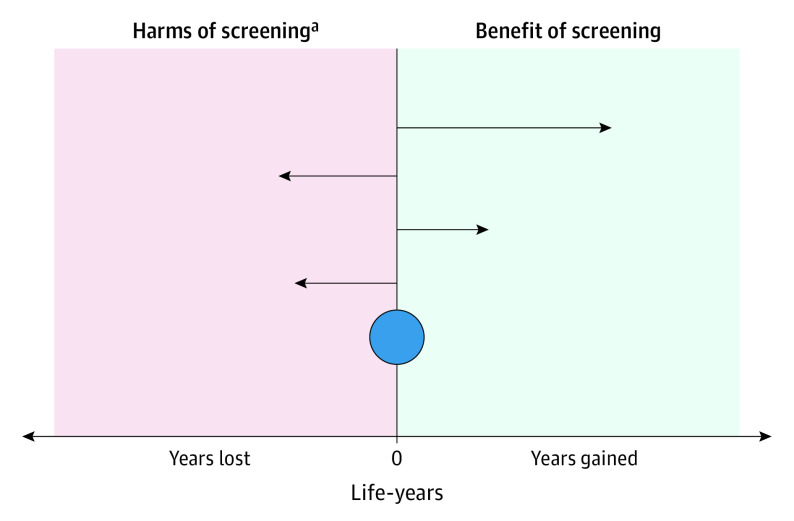
Cancer screening is advocated to save lives and increase longevity.3,4 The association between cancer screening and longevity is measured by comparing all-cause mortality in people who underwent screening with those who did not. Like all medical interventions, cancer screening entails benefits and harms. Harms can occur at testing, such as perforation and bleeding during colorectal cancer screening, and at downstream diagnostics and treatment, such as septicemia due to transrectal biopsy in prostate cancer screening or complications from surgery, radiotherapy, and chemotherapy. Harms may lead to premature death for some.2,3 A cancer screening test may reduce cancer-specific mortality but fail to increase longevity if the harms for some individuals outweigh the benefits for others (Figure 1) or if cancer-specific deaths are replaced by deaths from competing causes.
Figure 1. Mechanisms for Benefits and Harms of Cancer Screening on Longevity of Life.
The horizontal arrows illustrate 4 individuals who underwent screening. Arrows pointing right: 2 individuals who experienced screening benefit and live longer by early cancer detection and cure. Arrows pointing left: 2 individuals who experienced harm related to screening and died earlier than those without screening. The blue circle indicates population longevity effect of screening, which was calculated as all individual benefits minus all individual harms.
aHarms of screening include perforation or bleeding during sigmoidoscopy and of diagnostics and treatment after screening, such as surgery, radiotherapy, and chemotherapy.
It is important to provide the public with reliable estimates for benefits and harms of screening on cancer incidence and mortality and on lifetime gained by screening.5 While the former has become standard for most cancer screening tests, the latter goal is still difficult to assess.
Modeling studies base calculated lifetime gained by cancer screening on extrapolations of cancer-specific effects on all-cause death rather than observed data of longevity from randomized clinical screening trials.6,7 This assumption has been criticized, and it is unknown whether it is correct.3 Observational studies also harbor major risks of bias and confounding and are suboptimal when assessing screening effects on lifetime due to self-selection and lead-time bias.2,3 The most reliable metric to quantify lifetime gained by cancer screening tests is to use available data from large clinical screening trials.2 To this end, we retrieved information from large-scale randomized clinical trials with long-term follow-up of commonly used cancer screening tests and aimed to calculate the association with lifetime gained.
Methods
Search Strategy
We performed a systematic search in MEDLINE and the Cochrane library for reports of randomized clinical trials and meta-analyses of randomized clinical trials with cause-specific and all-cause mortality as end points, with no language or publication date restrictions. The last search was performed October 12, 2022. Further details about search terms and strategies are described in the eAppendix in Supplement 1. Our study was not submitted for ethical approval due to its noninterventional nature and design.
We included the most updated meta-analyses of randomized clinical trials and single randomized clinical trials if no updated meta-analysis was available for all-cause mortality and target cancer-specific mortality for the following most commonly used screening tests: (1) mammography for breast cancer; FOBT every year or every other year, sigmoidoscopy, or colonoscopy for colorectal cancer; PSA testing for prostate cancer; lung cancer with CT for current or former smokers; and Pap test cytology for cervical cancer.
We did not include observational or modeling studies because of their risk of bias related to the research question2,3 and our primary aim to provide most reliable results, as observed in randomized clinical trials. We did not include trials that investigated outdated screening tests no longer recommended, such as chest radiography for lung cancer screening.
We used Endnote, version X9.2 (Clarivate), for removal of duplicate records. Our searches and selection criteria followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline. Further details about search terms and strategies are described in the eAppendix in Supplement 1.
Selection Criteria
We included meta-analyses of randomized clinical trials if available and updated, and single reports of randomized clinical trials if meta-analyses were not available or not updated with the most recent randomized clinical trials. We included all trials that compared screening with no screening and applied screening tests as recommended in guidelines regarding the frequency of testing (yearly or every other year for mammography, Pap test cytology, FOBT, PSA testing, and CT; and longer intervals or once-only for sigmoidoscopy and colonoscopy for colorectal cancer). We did not include reports that primarily compared different screening tests with each other, but performed a post hoc analysis for chest radiography vs lung CT in the National Lung Cancer Screening Trial.
We included meta-analyses of randomized clinical trials with 10 to 15 years of follow-up or individual randomized clinical trials with 10 to 15 years of follow-up when meta-analyses were not available, as this is a commonly accepted time frame to observe screening benefits.2,7 We used absolute and relative outcomes associated with all-cause mortality based on intention-to-treat comparisons of individuals assigned to screening vs individuals assigned to no screening. We used trial data as reported in the most recent meta-analyses, because we considered these to provide the most reliable sources of information. We also performed sensitivity analyses in which we used the estimates as reported in the individual trial reports. For randomized clinical trials that were not yet included in meta-analyses, we used data as described in the trial reports. We did not perform our own quality assessments of the meta-analyses and randomized clinical trials.
For lung cancer screening, we found 3 randomized clinical trials eligible for our study, but no updated meta-analysis was available. Thus, we performed a random effects meta-analysis of the eligible trials according to our predefined inclusion criteria using the DerSimonian and Laird model (Stata, version 16.1, StataCorp LLC) (eAppendix in Supplement 1). For mammography screening, we based our main analysis on trials including women at the most commonly recommended screening start age of 50 years and excluded trials with suboptimal randomization as assessed in the meta-analysis and displayed in their analysis and performed post hoc analyses based on a wider inclusion of trials as displayed in a separate step in their analysis.8
The primary study outcome was lifetime in the screening vs the no-screening groups based on reported all-cause mortality data. We also retrieved data on cancer-specific mortality.
One of us (M.B.) screened titles and abstracts using Excel (Windows 360, Microsoft Corp). Full text of potentially eligible reports was assessed for eligibility by 2 of us independently (M.B. and P.W.) on the basis of study design, length of follow-up, and availability of the primary study end point (all-cause mortality). Discrepancies were resolved by consensus. Reference lists of eligible articles were hand-searched and screened by one of us (M.B.) for additional references.
Statistical Analysis
For each of the cancer screening tests of interest, we retrieved the reported relative risks of all-cause mortality, applying either mean or median times of follow-up, depending on what was reported. We defined lifetime without screening as the observed follow-up time in person-years for individuals randomized to the no-screening group divided by the number of individuals. For the screening group, we calculated lifetime as the observed lifetime in the no-screening group multiplied by 1 minus the relative risk of all-cause mortality compared with the no-screening group as derived from the reports. The observed difference between the observed estimates in screening and the no-screening groups was the incremental gain in lifetime attributable to screening.
We calculated 95% CIs for each derived lifetime difference between the screening and no-screening groups based on 95% CIs for relative risks of all-cause mortality as reported for the trials. We did not adjust for design differences between the trials, such as explanatory vs management designs or consent procedures before enrollment.
We applied the age ranges as reported in the trials, which were similar for screening ages in most screening programs today, and did not perform subgroup analyses for age or sex because for many trials these data were not available. We did not extrapolate reported results to longer than observed follow-up or to other age groups than those enrolled in the trials, because such extrapolation would imply assumptions with little availability of data and thus reduce the reliability of our results.
To avoid bias and possible overestimation of screening effects, we used the reported intention-to-treat analyses based on individuals randomized to the screening and no-screening arms of the trials. Because observed screening benefits were small, we present all differences in lifetime gained or lost due to screening in life-days rather than in life-years. Statistical significance was set at whether 95% CI crossed 0.
Results
The initial search resulted in 4134 references, which were screened as described in the Methods section. Of these, 103 reports were considered potentially eligible for our review and assessed in full-text. Of the 103 reports, we identified 18 randomized clinical trials that fulfilled all inclusion criteria with a total of 2 111 958 individuals: 4 on sigmoidoscopy screening and 4 on fecal testing for colorectal cancer, 4 on PSA screening for prostate cancer, 3 on CT lung cancer screening for current and former smokers, 2 on mammography screening for breast cancer, and 1 on colonoscopy screening for colorectal cancer. All individual trials that formed the basis for the primary analyses are displayed in Table 1.9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28 Table 2 displays the meta-analyses and individual trials that were used to estimate all-cause mortality in the main analyses.8,9,14,28,29,30,31,32
Table 1. Randomized Clinical Trials Included in Main Analyses.
| Sourcea | Follow-up time, mean, y | Screening | No screening | ||||
|---|---|---|---|---|---|---|---|
| No. of individuals | All-cause deaths | Cancer deaths | No. of individuals | All-cause deaths | Cancer deaths | ||
| Colonoscopy | |||||||
| Bretthauer et al,9 2022 | 10.0 | 28 220 | 3036 | 72 | 56 365 | 6079 | 157 |
| Sigmoidoscopy | |||||||
| Atkin et al,10 2017 | 17.1 | 57 098 | 13 279 | 353 | 112 936 | 26 409 | 996 |
| Miller et al,11 2019 | 15.8 | 77 443 | 9138 | 417 | 77 444 | 9286 | 549 |
| Segnan et al,12 2011 | 11.4 | 17 136 | 1202 | 65 | 17 136 | 1233 | 83 |
| Holme et al,13 2018 | 14.8 | 20 572 | 3809 | 122 | 78 220 | 13 433 | 530 |
| Juul et al,14b 2022 | 15.0 | 137 493 | 19 661 | 661 | 137 459 | 20 069 | 827 |
| Biennial FOBT | |||||||
| Mandel et al,15 1999 | 18.0 | 15 587 | 5213 | 148 | 15 394 | 5186 | 177 |
| Scholefield et al,16 2012 | 19.5 | 76 056 | 40 681 | 1176 | 75 919 | 40 550 | 1300 |
| Jøgensen et al,17 2002 | 13.0 | 30 967 | 12 205 | 362 | 30 966 | 12 248 | 431 |
| Lindholm et al,18 2008 | 15.5 | 34 144 | 10 591 | 252 | 34 164 | 10 432 | 300 |
| Annual FOBT | |||||||
| Mandel et al,15 1999 | 18.0 | 15 570 | 5236 | 121 | 15 394 | 5186 | 177 |
| PSA testing | |||||||
| Martin et al,19 2018 | 10.0 | 189 386 | 25 459 | 549 | 219 439 | 28 306 | 647 |
| Schröder et al,20 2014 | 13.0 | 72 891 | 15 369 | 355 | 89 352 | 19 108 | 545 |
| Lundgren et al,21 2018 | 20.0 | 2400 | 1420 | 86 | 25 081 | 13 283 | 771 |
| Andriole et al,22 2012 | 10.0-13.0c | 38 340 | 9212 | 255 | 38 343 | 9375 | 244 |
| Lung CTd | |||||||
| de Koning et al,23 2020 | 10.0 | 6583 | 868 | 160 | 6612 | 860 | 210 |
| Wille et al,24 2016 | 10.0 | 2052 | 165 | 39 | 2052 | 163 | 38 |
| Paci et al,25 2017 | 9.3 | 1613 | 154 | 43 | 1593 | 181 | 60 |
| Mammography | |||||||
| Miller et al,26 2014 | 21.9 | 19 711 | 734 | 88 | 19 694 | 690 | 90 |
| Tabar et al,271989 | 9.0 | 23 701 | 1985 | 45 | 11 112 | 945 | 36 |
| Tabar et al,271989 | 9.0 | 23 196 | 1728 | 52 | 21 962 | 1821 | 76 |
| 4-Cancer screening | |||||||
| Pinsky et al,28 2019 | 16.8 | 77 443 | 22 562 | 2996 | 77 444 | 22 652 | 3101 |
Abbreviations: CT, computed tomography; FOBT, fecal occult blood testing; PSA, prostate-specific antigen.
The screening interventions were performed at different times: the mammography trials in the 1980s and 1990s; the FOBT trials in the 1980s and 1990s; the sigmoidoscopy trials in the 1990s and 2000s; the PSA trials in the 2000s; the lung cancer trials in the 2000s; and the colonoscopy trial in the late 2000s and early 2010s.
Range (mean follow-up time not provided in publication).
Current and former smokers.
Table 2. Absolute Risk of Cancer-Specific and All-Cause Death per 100 Person-Years With vs Without Screening in Randomized Clinical Trials.
| Cancer screening test | No. of individuals | Follow-up time, ya | Absolute risk of death from target cancer per 100 person-years | Absolute risk of all-cause death per 100 person-years | RR (95% CI) all-cause mortality | Lifetime gained with screening (95% CI), d | ||
|---|---|---|---|---|---|---|---|---|
| Screening | No screening | Screening | No screening | |||||
| Breast cancer: mammography8 | 73 634 | 13 | 0.038 | 0.039 | 0.65 | 0.65 | 1.00 (0.95-1.04) | 0 (−190 to 237) |
| Colorectal cancer: fecal testing every year31 | 457 750 | 15 | 0.05 | 0.07 | 1.8 | 1.8 | 1.00 (0.98-1.03) | 0 (−164 to 110) |
| Colorectal cancer: fecal testing every 2 y31 | 598 934 | 15 | 0.06 | 0.07 | 1.8 | 1.8 | 1.00 (0.99-1.01) | 0 (−55 to 55) |
| Colorectal cancer: sigmoidoscopy14 | 274 952 | 15 | 0.03 | 0.04 | 0.95 | 0.97 | 0.98 (0.95-1.00) | 110 (0 to 274) |
| Colorectal cancer: colonoscopy9b | 84 585 | 10 | 0.028 | 0.031 | 1.1 | 1.1 | 0.99 (0.96-1.04) | 37 (−146 to 146) |
| Prostate cancer: PSA testing32 | 675 232 | 10 | 0.03 | 0.03 | 1.3 | 1.3 | 0.99 (0.98-1.01) | 37 (−37 to 73) |
| Lung cancer: CTc | 20 505 | 10d | 0.23 | 0.30 | 1.2 | 1.2 | 0.97 (0.88-1.08) | 107 (−286 to 430) |
| PLCO multiple screening tests28 | 154 887 | 17d | 0.24 | 0.25 | 1.8 | 1.9 | 0.98 (0.96-1.00) | 123 (6 to 227) |
Abbreviations: CT, computed tomography; PLCO, prostate, lung, colorectal, and ovarian; PSA, prostate-specific antigen; RR, risk ratio.
Time perspective for presented results.
Values are mortality rates over 10 years follow-up and corresponding risk ratios, as reported by Bretthauer et al.9
Meta-analysis of 3 randomized clinical trials shown in the eAppendix in Supplement 1.
Since no cumulative results for a specified follow-up time were given, median follow-up time is reported herein.
For breast cancer screening with mammography, a recent systematic review and meta-analysis was available, but follow-up was only 9.6 years.33 Therefore, we used the same analysis of a previous meta-analysis that provided 13 years follow-up and included the same trials.8 For post hoc analyses, we used a separate method of that meta-analysis. For colorectal cancer screening, we used a meta-analysis with FOBT every year or every other year,31 a recent pooled analysis of all randomized clinical trials for sigmoidoscopy,14 and for colonoscopy, with only 1 randomized clinical trial available, we used that report.9 For prostate cancer with PSA screening, we used a meta-analysis.32 For lung cancer screening, 3 randomized clinical trials fulfilled the inclusion criteria for screening with CT in current or former smokers.23,24,25 We performed a meta-analysis and applied the derived estimates.
In addition to the trials and meta-analyses reporting on individual screening tests, we found 1 study24 reporting on the joint effect of multiple cancer screening tests in the same individuals on lifetime gained, from the Prostate, Lung, Colorectal, and Ovarian Cancer Screening trial,28 a large randomized clinical trial in which individuals were exposed to screening tests for 4 different cancers at the same time: PSA testing for prostate cancer, chest radiographs for lung cancer screening, sigmoidoscopy screening for colorectal cancer, and CA-125 screening for ovarian cancer.
For cervical cancer screening, no randomized clinical trials with cancer-specific or all-cause mortality end points and long-term follow-up were identified. Thus, we were not able to include cervical cancer screening in the present study.
Study Characteristics
Table 2 presents the results for each screening test from meta-analyses and from the single randomized clinical trial available for colonoscopy. Mammography screening trials had a mean follow-up of 13 years8; follow-up in the trials for sigmoidoscopy screening for colorectal cancer was 15 years31; the trials for FOBT every other year and 1 trial for FOBT every year for colorectal cancer had a 15-year follow-up31; the 1 colonoscopy trial for colorectal cancer screening and the trials for PSA screening for prostate cancer had 10 years of median follow-up14; and the trials for lung cancer screening with CT scanning for former or current smokers had a median follow-up of 10 years.9,23,32 The eTable in Supplement 1 reports the meta-analysis for lung cancer screening trials.
The number of individuals available for analyses was largest for PSA screening (721 718 men), sigmoidoscopy screening (614 431 men and women), and FOBT screening every other year (598 934 men and women); smaller for colonoscopy screening (84 585 men and women) and mammography screening (73 634 women); and smallest for annual FOBT screening (30 964 men and women) and CT screening for lung cancer (20 505 men and women) (Table 2).
All-Cause and Cancer-Specific Mortality
The reported relative risks of all-cause mortality for screening vs no screening were 0.98 (95% CI, 0.95-1.00) for sigmoidoscopy, 0.99 (95% CI, 0.96-1.04) for colonoscopy, 1.00 (95% CI, 0.99-1.01) for FOBT every other year, 1.00 (95% CI, 0.98-1.03) for FOBT every year, 1.00 (95% CI, 0.95-1.04) for mammography, 0.99 (95% CI, 0.98-1.01) for PSA testing, and 0.97 (95% CI, 0.88-1.08) for CT. The absolute difference in deaths of the target cancer per 100 person-years ranged from 0.03 for prostate cancer to 0.23 for lung cancer (Table 2).
Lifetime Gained With Screening
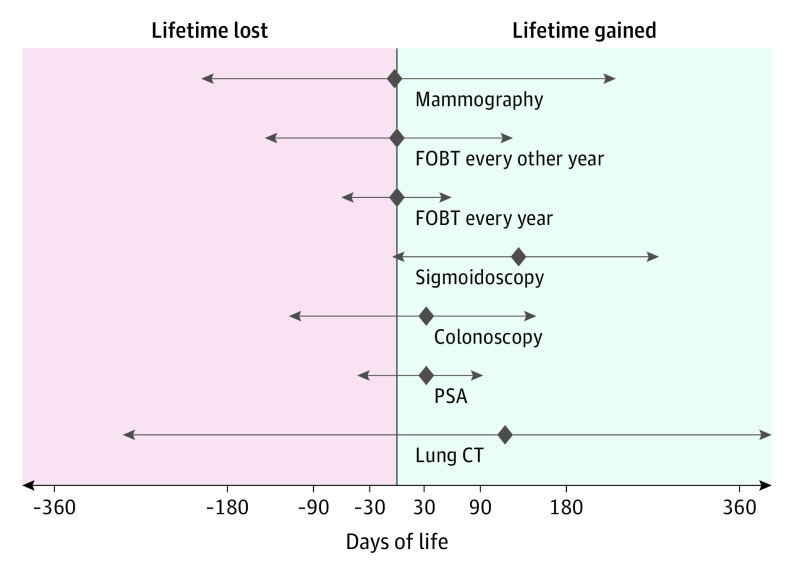
Based on the observed relative risks for all-cause mortality and the reported follow-up time in the trials, the only screening test that significantly increased longevity was sigmoidoscopy, by 110 days (95% CI, 0-274 days) (Table 2, Figure 2). We found no statistically significant outcomes for longevity with mammography screening (0 days; 95% CI, −190 to 237 days) and FOBT screening with yearly or biennial screening (0 days; 95% CI, −70.7 to 70.7 days). Colonoscopy screening (37 days; 95% CI, −146 to 146 days) and PSA screening (37 days; 95% CI, −37 to 73 days) may have an association with longevity of about 5 weeks, and lung cancer screening among smokers or former smokers of about 3 months (107 days; 95% CI, −286 to 430 days), but these estimates are uncertain (Table 2, Figure 2). The report on multiple cancer screening tests reported a mean gain in lifetime of 123 days (95% CI, 6-227 days) (Table 2). Results of the sensitivity analysis using the estimates as reported in the individual trials were similar compared with the primary analytic approach using the meta-analyses.
Figure 2. Lifetime Gained With Commonly Used Cancer Screening Tests.
The diamonds indicate point estimates of life days gained or lost for each screening test. Left and right arrows indicate 95% CIs. CT indicates computed tomography; FOBT, fecal occult blood testing; and PSA, prostate-specific antigen.
We performed 3 post hoc analyses with wider inclusion criteria, with the first including mammography screening trials with the same follow-up as in the main analyses but enrolling women below the commonly recommended screening start age of 50 years,8 resulting in no significant time gained with screening (47 days; 95% CI, −142 to 237 days). The second analysis included mammography screening trials that were categorized as having suboptimal randomization in the meta-analysis,8 resulting in a similar result (47 days gained with screening; 95% CI, −47 to 142 days). Third, we compared all-cause mortality in the National Lung Cancer Screening Trial,34 which compared screening with either low-dose CT or chest radiography in current and former smokers. After 12.3 years of median follow-up, lifetime gained was 135 days (95% CI, −45 to 270 days) in individuals randomized to low-dose CT compared with those randomized to chest radiography screening.
Discussion
Our study quantifies whether use of 6 commonly used cancer screening tests is associated with length of life. One test (sigmoidoscopy) significantly prolonged life and longevity by 110 days, although the lower bound of the 95% CI extended to 0. Fecal testing and mammography screening did not appear to prolong life in the trials, while estimates for prostate cancer screening and lung cancer screening are uncertain.
In recent decades, organized cancer screening programs have been established in Europe, Canada, the Pacific Islands, and in many countries in Asia. In the US, cancer screening is offered by many institutions and encouraged and reimbursed by most health care payers. Several studies have investigated the association between screening and all-cause mortality.6,28 Few have translated their results to practical and easy-to-grasp estimates for health care professionals and individuals on how much cancer screening may increase life expectancy. Our study provides these estimates.
Even if we did not observe longer lives in general with 5 of the 6 screening tests, some individuals prolong their life due to these screening tests. Cancer is prevented or detected in an early stage, and the individuals survive screening and subsequent treatment without harms or complications. Without screening, these patients may have died of cancer because it would have been detected at a later, incurable stage. Thus, these patients experience a gain in lifetime.
However, other individuals experience a lifetime loss due to screening.35,36 This loss is caused by harms associated with screening or with treatment of screening-detected cancers, for example, due to colon perforation during colonoscopy or myocardial infarction following radical prostatectomy.37,38
For 5 of the 6 screening tests investigated herein, the findings suggest that most individuals will not have any gain in longevity. For those who have their longevity altered with screening, the cumulative loss for those who are harmed must be outweighed in duration by the cumulative gain experienced by those who benefit to show unchanged lifetime in individuals who undergo screening compared with those who do not (Figure 1).
The outcomes we observed are similar to those for aspirin use for primary prevention of cardiovascular disease and cancer (0.6 fewer deaths per 1000 person-years).39 While the cancer screening tests we studied are widely recommended, aspirin use is not recommended for primary prevention of cancer, and the lack of recommendation for aspirin has been explained with too-small effects and adverse events, such as bleeding.39 In contrast, bariatric surgery to prevent obesity-related disease and premature death in people with obesity has recently been shown to prolong life by as much as 3.0 years after 24 years of follow-up.40
Our study may provide easy-to-understand estimates for prolongation of life attributable to screening that may be used in shared decision-making with individuals who consider undergoing a screening test. Our estimates may also serve to prioritize public health initiatives in comparison with other preventive measures, such as obesity treatment or prevention of cardiovascular disease.28
The lack of increased longevity with screening may also occur due to competing causes of death. Many of the cancers we are screening for share risk factors with more prevalent causes of death, such as cardiovascular and metabolic diseases. A lack of a significant increase in longevity due to cancer screening may therefore be due to death from competing causes at the same time a patient would have died of cancer without screening. A mortality shift from cancer to other causes of death without increased length of life is thus plausible.
Due to the stigma and the psychological burden, a cancer diagnosis may also cause extra noncancer-specific deaths from suicide, cardiovascular disease, and accidents.41,42 Also, increased surveillance after cancer screening may increase the risk of other incidental disease, which would not have been detected without screening.43
Adherence to more than 1 screening test may potentially increase longevity. The one study that was available28 does not suggest that there is an additive effect of screening for more than 1 cancer. Although such outcomes are possible, the competing risk of other disease might also outweigh the influence of screening for 2 or more cancer sites on length of life.
Most modeling studies assume that cause-specific mortality outcomes are correlated in a linear fashion with all-cause mortality without taking into account competing risk and overdiagnosis.6,7 One meta-analysis of modeling studies that aimed to calculate outcomes of cancer screening associated with all-cause mortality reported that screening prolongs life by 15 days for colorectal cancer (sigmoidoscopy screening), 32 days for breast cancer, and 71 days for lung cancer.7
Our estimates are based on intention-to-treat data from randomized clinical trials. These data may provide the most unbiased estimates for outcomes associated with screening. However, as with all intention-to-treat analyses, the trials underestimate efficacy due to nonadherence and contamination in the control group. For the time being, however, lack of detailed longitudinal data allowing adjustment for selection bias and confounding preclude per-protocol analyses that would provide more reliable estimates.44
Colonoscopy and sigmoidoscopy are similar to each other. In essence, sigmoidoscopy is a limited version of colonoscopy. One may thus assume that colonoscopy would at least have the same benefit as sigmoidoscopy for all-cause death. The reason for the current results that show a significant finding for sigmoidoscopy but not for colonoscopy may be the still limited evidence for colonoscopy, with only 1 trial available compared with 4 for sigmoidoscopy. We plan to update our analyses when more evidence becomes available for colonoscopy and other screening tests.
Our estimates apply to the follow-up of the trials, which was between 10 and 15 years. It is possible that effects of screening on longevity are different with longer follow-up. They may be longer if screening continues to be effective or repeated frequently as people get older. At the same time, screening in older individuals may increase risk of harms related to the screening tests or subsequent treatment due to increased comorbidity and frailty in older individuals.
Screening may have an association with longevity and the trials may have had too few patients and too short follow-up to prove it. No individual cancer screening trial has, to our knowledge, been powered to show an effect on all-cause mortality. The cancers screened for are serious and often devastating for patients who experience them, but each cancer contributes only to a small proportion of the overall burden of human disease. Our point estimates and the CIs around them may provide a framework for assessment of clinically relevant longevity effects of cancer screening tests, as the real effects on lifetime by screening with 95% certainty is between the upper and lower bounds of the calculated CIs. It is up to individuals, policymakers, and health care professionals to decide whether the maximum calculated outcomes (the upper bound of the 95% CIs) are large enough to prioritize screening programs, and conversely how much of a concern the lower bounds of the CIs represent in the decision-making for or against a particular screening test. The level of uncertainty around the point estimates derived herein may be small enough to allow transparent information and engage in shared decision-making with individuals and stakeholders.
Considerable controversy exists about the most appropriate outcome measure for cancer screening tests.39,40,41,42 Some claim that only cancer screening tests with proven effects on all-cause mortality should be recommended or reimbursed.40 Others claim that effects on cause-specific death of the target cancer are enough to promote screening.41 In addition to lifetime gained or lost with screening, quality of life is important. Quality-adjusted life-years (QALYs) are difficult to measure and interpret, but recent analyses of QALYs for mammography screening estimates in Norway suggest that net QALY in modern mammography screening in Norway may be negative.29
Limitations
The study has limitations. As outlined above, we applied intention-to-treat analyses, which provide the most unbiased estimates, but may underestimate any associations of cancer screening with longevity. Furthermore, follow-up time may not have been long enough in the trials we included, although we find this unlikely, and finally, even larger trials may be needed to tease out any association of cancer screening with longevity with more precise effect estimates.
Conclusions
Although our meta-analysis suggests that claims that screening saves lives are not substantiated by the current best available evidence, we do not advocate that all screening should be abandoned. Screening tests with a positive benefit-harm balance measured in incidence and mortality of the target cancer compared with harms and burden may well be worthwhile.30 However, organizations, institutions, and policymakers who promote cancer screening tests by their effect to save lives may find other ways of encouraging screening. It might be wise to reconsider priorities and dispassionately inform interested people about the absolute benefits, harms, and burden of screening tests that they consider undertaking. Our estimates may serve that purpose.
eAppendix. Search Terms and Strategies
eTable. Meta-Analysis of All-Cause Mortality for CT Screening for Lung Cancer in Smokers
Data Sharing Statement
References
- 1.Vanchieri C. National Cancer Act: a look back and forward. J Natl Cancer Inst. 2007;99(5):342-345. doi: 10.1093/jnci/djk119 [DOI] [PubMed] [Google Scholar]
- 2.Bretthauer M, Kalager M. Principles, effectiveness and caveats in screening for cancer. Br J Surg. 2013;100(1):55-65. doi: 10.1002/bjs.8995 [DOI] [PubMed] [Google Scholar]
- 3.Woloshin S, Schwartz LM, Black WC, Kramer BS. Cancer screening campaigns—getting past uninformative persuasion. N Engl J Med. 2012;367(18):1677-1679. doi: 10.1056/NEJMp1209407 [DOI] [PubMed] [Google Scholar]
- 4.Seffrin JR. We know cancer screening saves lives. American Cancer Society Cancer Action Network, October 23, 2009. Accessed May 3, 2020. https://www.fightcancer.org/news/we-know-cancer-screening-saves-lives
- 5.Schwartz LM, Woloshin S, Fowler FJ Jr, Welch HG. Enthusiasm for cancer screening in the United States. JAMA. 2004;291(1):71-78. doi: 10.1001/jama.291.1.71 [DOI] [PubMed] [Google Scholar]
- 6.Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016;315(23):2595-2609. doi: 10.1001/jama.2016.6828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heijnsdijk EAM, Csanádi M, Gini A, et al. All-cause mortality versus cancer-specific mortality as outcome in cancer screening trials: a review and modeling study. Cancer Med. 2019;8(13):6127-6138. doi: 10.1002/cam4.2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gøtzsche PC, Jørgensen KJ. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2013;2013(6):CD001877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bretthauer M, Løberg M, Wieszczy P, et al. Effect of colonoscopy screening on colorectal cancer incidence and mortality. N Engl J Med. 2022;387:1547-1556. doi: 10.1056/NEJMoa2208375 [DOI] [PubMed] [Google Scholar]
- 10.Atkin W, Wooldrage K, Parkin DM, et al. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: the UK Flexible Sigmoidoscopy Screening randomised controlled trial. Lancet. 2017;389(10076):1299-1311. doi: 10.1016/S0140-6736(17)30396-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller EA, Pinsky PF, Schoen RE, Prorok PC, Church TR. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: long-term follow-up of the randomised US PLCO cancer screening trial. Lancet Gastroenterol Hepatol. 2019;4(2):101-110. doi: 10.1016/S2468-1253(18)30358-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segnan N, Armaroli P, Bonelli L, et al. ; SCORE Working Group . Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial–SCORE. J Natl Cancer Inst. 2011;103(17):1310-1322. doi: 10.1093/jnci/djr284 [DOI] [PubMed] [Google Scholar]
- 13.Holme Ø, Løberg M, Kalager M, et al. ; NORCCAP Study Group† . Long-term effectiveness of sigmoidoscopy screening on colorectal cancer incidence and mortality in women and men: a randomized trial. Ann Intern Med. 2018;168(11):775-782. doi: 10.7326/M17-1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juul FE, Cross AJ, Schoen RE, et al. 15-Year benefits of sigmoidoscopy screening on colorectal cancer incidence and mortality: a pooled analysis of randomized trials. Ann Intern Med. 2022;175(11):1525-1533. doi: 10.7326/M22-0835 [DOI] [PubMed] [Google Scholar]
- 15.Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999;91(5):434-437. doi: 10.1093/jnci/91.5.434 [DOI] [PubMed] [Google Scholar]
- 16.Scholefield JH, Moss SM, Mangham CM, Whynes DK, Hardcastle JD. Nottingham trial of faecal occult blood testing for colorectal cancer: a 20-year follow-up. Gut. 2012;61(7):1036-1040. doi: 10.1136/gutjnl-2011-300774 [DOI] [PubMed] [Google Scholar]
- 17.Jørgensen OD, Kronborg O, Fenger C. A randomised study of screening for colorectal cancer using faecal occult blood testing: results after 13 years and seven biennial screening rounds. Gut. 2002;50(1):29-32. doi: 10.1136/gut.50.1.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindholm E, Brevinge H, Haglind E. Survival benefit in a randomized clinical trial of faecal occult blood screening for colorectal cancer. Br J Surg. 2008;95(8):1029-1036. doi: 10.1002/bjs.6136 [DOI] [PubMed] [Google Scholar]
- 19.Martin RM, Donovan JL, Turner EL, et al. ; CAP Trial Group . Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319(9):883-895. doi: 10.1001/jama.2018.0154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schröder FH, Hugosson J, Roobol MJ, et al. ; ERSPC Investigators . Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384(9959):2027-2035. doi: 10.1016/S0140-6736(14)60525-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundgren PO, Kjellman A, Norming U, Gustafsson O. Long-term outcome of a single intervention population based prostate cancer screening study. J Urol. 2018;200(1):82-88. doi: 10.1016/j.juro.2018.01.080 [DOI] [PubMed] [Google Scholar]
- 22.Andriole GL, Crawford ED, Grubb RL III, et al. ; PLCO Project Team . Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104(2):125-132. doi: 10.1093/jnci/djr500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503-513. doi: 10.1056/NEJMoa1911793 [DOI] [PubMed] [Google Scholar]
- 24.Wille MM, Dirksen A, Ashraf H, et al. Results of the randomized Danish Lung Cancer Screening Trial with focus on high-risk profiling. Am J Respir Crit Care Med. 2016;193(5):542-551. doi: 10.1164/rccm.201505-1040OC [DOI] [PubMed] [Google Scholar]
- 25.Paci E, Puliti D, Lopes Pegna A, et al. ; the ITALUNG Working Group . Mortality, survival and incidence rates in the ITALUNG randomised lung cancer screening trial. Thorax. 2017;72(9):825-831. doi: 10.1136/thoraxjnl-2016-209825 [DOI] [PubMed] [Google Scholar]
- 26.Miller AB, Wall C, Baines CJ, Sun P, To T, Narod SA. Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. BMJ. 2014;348:g366. doi: 10.1136/bmj.g366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabar L, Fagerberg G, Duffy SW, Day NE. The Swedish two county trial of mammographic screening for breast cancer: recent results and calculation of benefit. J Epidemiol Community Health. 1989;43(2):107-114. doi: 10.1136/jech.43.2.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinsky PF, Miller EA, Zhu CS, Prorok PC. Overall mortality in men and women in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. J Med Screen. 2019;26(3):127-134. doi: 10.1177/0969141319839097 [DOI] [PubMed] [Google Scholar]
- 29.Zahl PH, Kalager M, Suhrke P, Nord E. Quality-of-life effects of screening mammography in Norway. Int J Cancer. 2020;146(8):2104-2112. doi: 10.1002/ijc.32539 [DOI] [PubMed] [Google Scholar]
- 30.Helsingen LM, Vandvik PO, Jodal HC, et al. Colorectal cancer screening with faecal immunochemical testing, sigmoidoscopy or colonoscopy: a clinical practice guideline. BMJ. 2019;367:l5515. doi: 10.1136/bmj.l5515 [DOI] [PubMed] [Google Scholar]
- 31.Jodal HC, Helsingen LM, Anderson JC, Lytvyn L, Vandvik PO, Emilsson L. Colorectal cancer screening with faecal testing, sigmoidoscopy or colonoscopy: a systematic review and network meta-analysis. BMJ Open. 2019;9(10):e032773. doi: 10.1136/bmjopen-2019-032773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ilic D, Djulbegovic M, Jung JH, et al. Prostate cancer screening with prostate-specific antigen (PSA) test: a systematic review and meta-analysis. BMJ. 2018;362:k3519. doi: 10.1136/bmj.k3519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schünemann HJ, Lerda D, Quinn C, et al. ; European Commission Initiative on Breast Cancer (ECIBC) Contributor Group . Breast cancer screening and diagnosis: a synopsis of the European Breast Guidelines. Ann Intern Med. 2020;172(1):46-56. doi: 10.7326/M19-2125 [DOI] [PubMed] [Google Scholar]
- 34.National Lung Screening Trial Research Team . Lung cancer incidence and mortality with extended follow-up in the National Lung Screening Trial. J Thorac Oncol. 2019;14(10):1732-1742. doi: 10.1016/j.jtho.2019.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saquib N, Saquib J, Ioannidis JPA. Does screening for disease save lives in asymptomatic adults? systematic review of meta-analyses and randomized trials. Int J Epidemiol. 2015;44(1):264-277. doi: 10.1093/ije/dyu140 [DOI] [PubMed] [Google Scholar]
- 36.Newman DH. Screening for breast and prostate cancers: moving toward transparency. J Natl Cancer Inst. 2010;102(14):1008-1011. doi: 10.1093/jnci/djq190 [DOI] [PubMed] [Google Scholar]
- 37.Steele RJC, Brewster DH. Should we use total mortality rather than cancer specific mortality to judge cancer screening programmes? no. BMJ. 2011;343:d6397. doi: 10.1136/bmj.d6397 [DOI] [PubMed] [Google Scholar]
- 38.Penston J. Should we use total mortality rather than cancer specific mortality to judge cancer screening programmes? yes. BMJ. 2011;343:d6395. doi: 10.1136/bmj.d6395 [DOI] [PubMed] [Google Scholar]
- 39.Whitlock EP, Williams SB, Burda BU, Feightner A, Beil T. Aspirin Use in Adults: Cancer, All-Cause Mortality, and Harms; A Systematic Evidence Review for the US Preventive Services Task Force (Evidence Synthesis Number 132). Agency for Healthcare Research and Quality; 2015. [PubMed] [Google Scholar]
- 40.Carlsson LMS, Sjöholm K, Jacobson P, et al. Life expectancy after bariatric surgery in the Swedish Obese Subjects Study. N Engl J Med. 2020;383(16):1535-1543. doi: 10.1056/NEJMoa2002449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu D, Andersson TM, Fall K, et al. Clinical diagnosis of mental disorders immediately before and after cancer diagnosis: a nationwide matched cohort study in Sweden. JAMA Oncol. 2016;2(9):1188-1196. doi: 10.1001/jamaoncol.2016.0483 [DOI] [PubMed] [Google Scholar]
- 42.Fang F, Fall K, Mittleman MA, et al. Suicide and cardiovascular death after a cancer diagnosis. N Engl J Med. 2012;366(14):1310-1318. doi: 10.1056/NEJMoa1110307 [DOI] [PubMed] [Google Scholar]
- 43.Shen Q, Lu D, Schelin ME, et al. Injuries before and after diagnosis of cancer: nationwide register based study. BMJ. 2016;354:i4218. doi: 10.1136/bmj.i4218 [DOI] [PubMed] [Google Scholar]
- 44.Hernán MA, Robins JM. Per-protocol analyses of pragmatic trials. N Engl J Med. 2017;377(14):1391-1398. doi: 10.1056/NEJMsm1605385 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Search Terms and Strategies
eTable. Meta-Analysis of All-Cause Mortality for CT Screening for Lung Cancer in Smokers
Data Sharing Statement