Key Points
Question
What is the effect of treatment with mavacamten on Chinese patients with symptomatic obstructive hypertrophic cardiomyopathy?
Findings
In this phase 3 randomized clinical trial of 81 patients, mavacamten significantly improved Valsalva left ventricular outflow tract obstruction compared with placebo and was well tolerated. New York Heart Association functional class, health status, cardiac biomarkers, and cardiac structure were also improved.
Meaning
The clinical benefits of mavacamten for Chinese patients with symptomatic obstructive hypertrophic cardiomyopathy were consistent with previous data; mavacamten offers a new option for an underrepresented population for whom there is an important unmet medical need.
Abstract
Importance
Mavacamten has shown clinical benefits in global studies for patients with obstructive hypertrophic cardiomyopathy (oHCM), but evidence in the Asian population is lacking.
Objective
To evaluate the safety and efficacy of mavacamten compared with placebo for Chinese patients with symptomatic oHCM.
Design, Setting, and Participants
This phase 3, randomized, double-blind, placebo-controlled clinical trial was conducted at 12 hospitals in China. Between January 4 and August 5, 2022, patients with oHCM and a left ventricular outflow tract (LVOT) gradient of 50 mm Hg or more and New York Heart Association (NYHA) class II or III symptoms were enrolled and received treatment for 30 weeks.
Interventions
Patients were randomized 2:1 to receive mavacamten (starting at 2.5 mg once daily) or placebo for 30 weeks.
Main Outcomes and Measures
The primary end point was change in Valsalva LVOT peak gradient from baseline to week 30. Left ventricular outflow tract gradients and left ventricular ejection fraction (LVEF) were assessed by echocardiography, while left ventricular mass index (LVMI) was determined by cardiac magnetic resonance imaging. Analysis was performed on an intention-to-treat basis.
Results
A total of 81 patients (mean [SD] age, 51.9 [11.9] years; 58 men [71.6%]) were randomized. Mavacamten demonstrated a significant improvement in the primary end point compared with placebo (least-squares mean [LSM] difference, −70.3 mm Hg; 95% CI, −89.6 to −50.9 mm Hg; 1-sided P < .001). Similar trends were demonstrated for resting LVOT peak gradient (LSM difference, −55.0 mm Hg; 95% CI, −69.1 to −40.9 mm Hg). At week 30, more patients receiving mavacamten than placebo achieved a Valsalva LVOT peak gradient less than 30 mm Hg (48.1% [26 of 54] vs 3.7% [1 of 27]), less than 50 mm Hg (59.3% [32 of 54] vs 7.4% [2 of 27]), and NYHA class improvement (59.3% [32 of 54] vs 14.8% [4 of 27]). Greater improvements were also observed with mavacamten regarding the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (LSM difference, 10.2; 95% CI, 4.4-16.1), N-terminal pro-B-type natriuretic peptide level (proportion of geometric mean ratio, 0.18; 95% CI, 0.13-0.24), high-sensitivity cardiac troponin I level (proportion of geometric mean ratio, 0.34; 95% CI, 0.27-0.42), and LVMI (mean difference, −30.8 g/m2; 95% CI, −41.6 to −20.1 g/m2). Safety and tolerability were similar between mavacamten and placebo. No patients experienced LVEF less than 50%.
Conclusions
Mavacamten significantly improved Valsalva LVOT gradient vs placebo for Chinese patients. All secondary efficacy end points were also improved. Mavacamten was well tolerated with no new safety signals. This study supports the efficacy and safety of mavacamten in diverse populations, including Chinese patients.
Trial Registration
ClinicalTrials.gov Identifier: NCT05174416
This randomized clinical trial evaluates the safety and efficacy of mavacamten compared with placebo for Chinese patients with symptomatic obstructive hypertrophic cardiomyopathy.
Introduction
Hypertrophic cardiomyopathy (HCM) is a myocardial disorder clinically characterized by left ventricular (LV) hypertrophy, which is caused, in most cases, by variants in the genes encoding sarcomeres. Hypertrophic cardiomyopathy commonly manifests as LV outflow tract (LVOT) obstruction.1,2,3 Left ventricular outflow tract obstruction is a major prognostic factor for patients with HCM and is also associated with increased risk of disease progression, congestive heart failure (HF), atrial fibrillation (AF), stroke, and mortality.4,5,6 Therefore, relieving LVOT obstruction is one of the main therapeutic aims for patients with obstructive HCM (oHCM).7
One key pathophysiological feature contributing to outflow tract obstruction is LV hypercontractility, due to excess myosin–actin cross bridging.6,8,9 This leads to increased systolic anterior motion of the mitral valve, leading to mitral valve–ventricular septal contact, which contributes to a pressure gradient between the LV chamber and systemic circulation.6,7,8 Current standard pharmacologic therapies for oHCM, such as β-blockers, nondihydropyridine calcium channel blockers, and disopyramide, may offer symptomatic relief but are nonspecific and do not address the underlying pathophysiological mechanisms behind HCM nor alter the disease course.3,8,10,11,12 For severe oHCM that is refractory to pharmacologic treatment, septal reduction therapy (SRT) is effective in relieving oHCM symptoms.3,8 However, such invasive procedures carry inherent surgical risks and demand expertise that is not widely accessible in China and other countries.13,14
Mavacamten, a first-in-class, selective, reversible, allosteric inhibitor of β-cardiac myosin, inhibits the binding of cardiac myosin to actin and reduces the number of actin–myosin cross bridges.15,16 Consequently, reductions in myocardial contractility and ventricular stiffness serve to address the underlying pathophysiological mechanism of oHCM. Mavacamten was shown to significantly reduce the LVOT gradient and improve exercise capacity, New York Heart Association (NYHA) functional class, and health status for patients with oHCM in the global phase 3 EXPLORER-HCM (Clinical Study to Evaluate Mavacamten [MYK-461] in Adults With Symptomatic Obstructive Hypertrophic Cardiomyopathy; NCT03470545) trial.17 In VALOR-HCM (A Study to Evaluate Mavacamten in Adults With Symptomatic Obstructive HCM Who Are Eligible for Septal Reduction Therapy; NCT04349072), mavacamten significantly reduced the eligibility for invasive SRT after 16 or 32 weeks of treatment among patients with oHCM who met guideline criteria for SRT.18,19 Mavacamten has been approved in the US, Europe, and other countries across 5 continents for adults with symptomatic NYHA class II to III oHCM.20
However, to our knowledge, there is limited clinical evidence to date on the efficacy and safety of mavacamten for Asian patients, who accounted for only 2.4% of patients in EXPLORER-HCM.17 Given the limited ethnic diversity in existing HCM trials, data are needed on the efficacy and safety of mavacamten in populations with different genetic and anthropologic backgrounds. In the Chinese population, poor CYP2C19 metabolizers are more common, and body mass index (BMI) tends to be lower, both factors that may affect mavacamten’s efficacy. Given that there are at least 1 million patients with HCM in China, of whom 70% have oHCM,21,22 this phase 3 EXPLORER-CN (A Study to Evaluate the Efficacy and Safety of Mavacamten in Chinese Adults With Symptomatic Obstructive HCM) trial was conducted to evaluate the efficacy and safety of mavacamten for Chinese patients with oHCM.
Methods
Study Design and Patients
This phase 3, randomized, double-blind, placebo-controlled, multicenter clinical trial was conducted at 12 hospitals in China (trial protocol and statistical analysis plan are in Supplement 1; study sites and investigators are listed in the eAppendix in Supplement 2). Patients were enrolled between January 4 and August 5, 2022. The 30-week treatment phase was followed by a long-term extension period of an additional 48 weeks. This article reports the results from the double-blind, placebo-controlled phase; the data from the ongoing long-term extension period will be reported separately. The study was performed according to the principles of the Declaration of Helsinki,23 Good Clinical Practice guidelines, and applicable Chinese laws and regulations. The study was approved by the National Medical Products Administration and independent ethics committees of the participating sites. All patients provided written informed consent. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Patients aged 18 years or older, weighing more than 45 kg, with a diagnosis of oHCM, with a peak LVOT gradient of 50 mm Hg or more at rest or after the Valsalva maneuver, with a left ventricular ejection fraction (LVEF) of 55% or more, and NYHA class II or III were eligible.24 Key exclusion criteria included history of syncope or sustained ventricular tachyarrhythmia with exercise in the past 6 months before screening, paroxysmal AF at screening, and current or planned treatment with disopyramide, cibenzoline, ranolazine, or a combination of β-blockers and verapamil or diltiazem.24 A full list of eligibility criteria is provided in eTable 1 in Supplement 2.
Randomization and Masking
An interactive response system was used to randomize patients in a 2:1 ratio to receive mavacamten or matching placebo. Randomization was stratified based on current use of β-blocker. All patients, study investigators and staff, the sponsor, clinical site monitors, and central or core laboratories were masked to treatment assignment. The appearance of mavacamten or placebo capsules was identical to maintain masking.
Procedures
Mavacamten was given orally once daily at a starting dose of 2.5 mg and adjusted according to a previously published dose titration scheme.24 Dose titration was masked and guided by core laboratory assessment of resting LVEF, Valsalva LVOT gradient, and predose plasma concentration of mavacamten (eMethods in Supplement 2). Individualized doses of 1, 2.5, 5, 10, or 15 mg were allowed.24 The prespecified criteria for temporary discontinuation of the study drug included a predose plasma concentration of 1000 ng/mL or more or an LVEF of less than 50%.
During the 30-week, double-blind treatment period, serial assessments including transthoracic echocardiography, electrocardiography, Holter monitoring, NYHA functional class, Kansas City Cardiomyopathy Questionnaire (KCCQ), cardiac biomarkers, safety laboratory testing, and plasma concentration analysis were conducted over 11 visits at prespecified time points (eFigure 1 in Supplement 2). Cardiac magnetic resonance (CMR) imaging was conducted among eligible patients at screening and week 30.
Outcomes
The primary end point was change in Valsalva LVOT peak gradient from baseline to week 30, as determined by Doppler echocardiography (eMethods in Supplement 2). Secondary efficacy end points were the proportion of patients at week 30 with a Valsalva LVOT peak gradient less than 30 mm Hg, a Valsalva LVOT peak gradient less than 50 mm Hg, at least 1 class improvement in NYHA functional classification, and changes from baseline to week 30 in the following parameters: resting LVOT peak gradient, KCCQ Clinical Summary Score (KCCQ-CSS), N-terminal pro-B-type natriuretic peptide (NT-proBNP) level, high-sensitivity cardiac troponin I (hs-cTnI) level, and left ventricular mass index (LVMI) evaluated by CMR imaging.
Prespecified exploratory end points included changes in cardiac structure from baseline to week 30 as evaluated by transthoracic echocardiography and CMR imaging. Key safety end points included incidence and severity of treatment-emergent adverse events (TEAEs) and serious adverse events (SAEs).
Statistical Analysis
The planned sample size was 81 patients to provide greater than 90% power at a 1-sided 2.5% α level to detect a mean (SD) treatment difference of 30 (35) mm Hg in the primary end point between treatment groups, assuming an estimated dropout rate of 10%.
Efficacy was assessed among the intention-to-treat population, and safety was analyzed among patients who received 1 or more dose of the study drug. Outcomes for CMR imaging were based on the population with both baseline and week 30 CMR imaging data available. The primary efficacy end point was analyzed using the mixed-effect model for repeated measures.24 For secondary efficacy end points, continuous variables were compared between treatments using analysis of covariance or the mixed-effect model for repeated measures. Categorical variables were analyzed using the Cochran-Mantel-Haenszel test. Point estimates and 2-sided 95% CIs for proportion difference between treatment groups were computed based on the stratified Miettinen-Nurminen method. P values for secondary and exploratory end points were descriptive, without multiplicity adjustment. A 2-sided P < .05 (1-sided P < .025) was considered statistically significant. Safety end points were analyzed using descriptive statistics without formal statistical testing. SAS, version 9.4 (SAS Institute Inc) was used for statistical analyses.
Results
Study Population
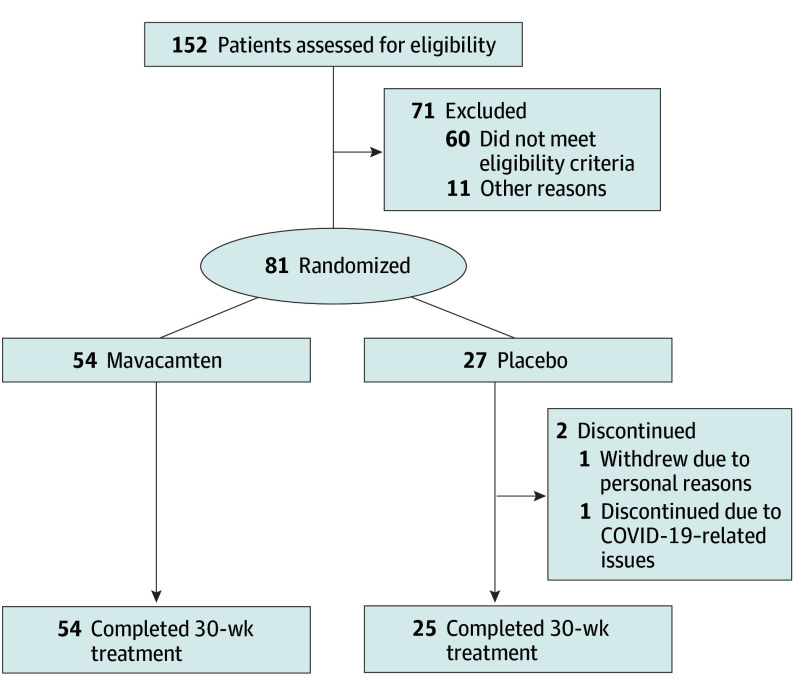
The trial screened 152 patients with symptomatic oHCM, of whom 81 (mean [SD] age, 51.9 [11.9] years; 58 men [71.6%]) were enrolled (54 received mavacamten; 27 received placebo) between January 4 and August 5, 2022, at 12 centers in China (Figure 1). Baseline characteristics of the study population are shown in Table 1. Patient characteristics were generally similar between treatment groups, except the mavacamten group had a larger proportion of men and patients with NYHA class II status, lower baseline NT-proBNP levels, and lower baseline hs-cTnI levels compared with the placebo group. The proportion of poor CYP2C19 metabolizers (13.0% [7 of 54] vs 3.7% [1 of 27]) was larger in the mavacamten group vs the placebo group, and the proportion of intermediate metabolizers (44.4% [24 of 54] vs 63.0% [17 of 27]) was smaller in the mavacamten group vs the placebo group. All patients were symptomatic with significant LVOT obstruction; the mean (SD) resting LVOT peak gradients were 74.6 (35.1) mm Hg in the mavacamten group and 73.4 (32.2) mm Hg in the placebo group; the mean (SD) Valsalva LVOT peak gradients were 106.8 (43.2) mm Hg in the mavacamten group and 99.8 (41.1) mm Hg in the placebo group. The mean (SD) LVEF was similar between the mavacamten (77.8% [6.9%]) and placebo (77.0% [6.7%]) groups. Most patients were receiving background β-blockers (mavacamten group, 88.9% [48 of 54]; placebo group, 88.9% [24 of 27]).
Figure 1. Patient Flow Diagram.
Table 1. Patient Baseline Characteristics.
| Characteristic | Patients, No. (%) | |
|---|---|---|
| Mavacamten group (n = 54) | Placebo group (n = 27) | |
| Age, mean (SD), y | 52.4 (12.1) | 51.0 (11.8) |
| Sex | ||
| Male | 41 (75.9) | 17 (63.0) |
| Female | 13 (24.1) | 10 (37.0) |
| Vital signs | ||
| BMI, mean (SD) | 25.2 (3.5) | 26.1 (3.6) |
| Heart rate, mean (SD), beats/min | 65.2 (11.4) | 64.4 (7.5) |
| Systolic blood pressure, mean (SD), mm Hg | 117.1 (13.1) | 112.6 (14.6) |
| Diastolic blood pressure, mean (SD), mm Hg | 74.4 (9.8) | 70.9 (9.7) |
| NYHA functional class | ||
| II | 44 (81.5) | 18 (66.7) |
| III | 10 (18.5) | 9 (33.3) |
| CYP2C19 phenotypea | ||
| Normal | 23 (42.6) | 9 (33.3) |
| Intermediate | 24 (44.4) | 17 (63.0) |
| Poor | 7 (13.0) | 1 (3.7) |
| Background therapy for HCM | ||
| β-Blocker | 48 (88.9) | 24 (88.9) |
| Calcium channel blocker | 4 (7.4) | 2 (7.4) |
| Others | 2 (3.7) | 1 (3.7) |
| Key echocardiographic parameters | ||
| Resting LVOT peak gradient, mean (SD), mm Hg | 74.6 (35.1) | 73.4 (32.2) |
| Valsalva LVOT peak gradient, mean (SD), mm Hg | 106.8 (43.2) | 99.8 (41.1) |
| LVEF, mean (SD), % | 77.8 (6.9) | 77.0 (6.7) |
| Maximum LV wall thickness, mean (SD), mm | 22.9 (4.9) | 24.3 (6.4) |
| Left atrial volume index, mean (SD), mL/m2 | 43.3 (12.1) | 47.5 (14.7) |
| CMR imaging parametersb | ||
| LV mass index, mean (SD), g/m2 | 98.6 (45.0) | 108.5 (54.8) |
| KCCQ-CSS, mean (SD), points | 82.4 (16.9) | 84.4 (17.0) |
| Cardiac biomarkers | ||
| NT-proBNP, geometric mean (CV%), ng/L | 810.5 (138.3) | 1250.3 (159.9) |
| hs-cTnI, geometric mean (CV%), ng/L | 33.5 (325.0) | 38.7 (443.1) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CMR, cardiac magnetic resonance; CV, coefficient of variation; HCM, hypertrophic cardiomyopathy; hs-cTnI, high-sensitivity cardiac troponin I; KCCQ-CSS, Kansas City Cardiomyopathy Questionnaire Clinical Summary Score; LV, left ventricular; LVEF, left ventricular ejection fraction; LVOT, left ventricular outflow tract; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association.
Normal phenotype included CYP2C19 genotype *1/*1; intermediate phenotype included CYP2C19 genotypes *1/*2, *1/*3, and *2/*17; poor phenotype included CYP2C19 genotypes *2/*2 and *2/*3; rapid phenotype included CYP2C19 genotype *1/*17; ultra-rapid phenotype included CYP2C19 genotype *17/*17. There were no rapid or ultrarapid metabolizers in either group.
Based on subset of 58 patients (39 in mavacamten group and 19 in placebo group) eligible for CMR imaging.
Overall, 79 patients (97.5%) completed the 30-week, double-blind, placebo-controlled treatment period, including 54 (100%) in the mavacamten group and 25 (92.6%) in the placebo group (Figure 1). Two patients in the placebo group discontinued treatment prematurely (1 withdrew due to personal reasons; 1 discontinued due to COVID-19–related issues).
Efficacy End Points
At the end of the double-blind, placebo-controlled treatment period, most patients in the mavacamten group were taking the 5-mg (59.3% [32 of 54]) or 10-mg (29.6% [16 of 54]) doses. After 30 weeks of treatment, mavacamten demonstrated a significant improvement in the primary end point compared with placebo (Figure 2A). The mean (SD) Valsalva LVOT peak gradient decreased from 106.8 (43.2) mm Hg at baseline to 48.9 (40.4) mm Hg (least-squares mean [LSM] difference, −51.1 mm Hg) at week 30 among mavacamten-treated patients, whereas for placebo, an increase from 99.8 (41.1) mm Hg at baseline to 116.3 (52.2) mm Hg (LSM difference, 19.2 mm Hg) at week 30 was observed. The least-squares mean (LSM) difference between groups was −70.3 mm Hg (95% CI, −89.6 to −50.9 mm Hg; 1-sided P < .001) (eTable 2 in Supplement 2). The reduction in the Valsalva LVOT peak gradient with mavacamten treatment started as early as 4 weeks and was sustained through week 30 (Figure 2A and C; eTable 3 in Supplement 2). The consistent benefit for the primary end point was also observed across prespecified subgroups, regardless of β-blocker use or CYP2C19 phenotypes (Figure 3). A sensitivity analysis based on the per-protocol set or intention-to-treat set with different stratification factors showed consistent results (eTable 4 in Supplement 2).
Figure 2. Valsalva Left Ventricular Outflow Tract (LVOT) and Resting LVOT Peak Gradients.
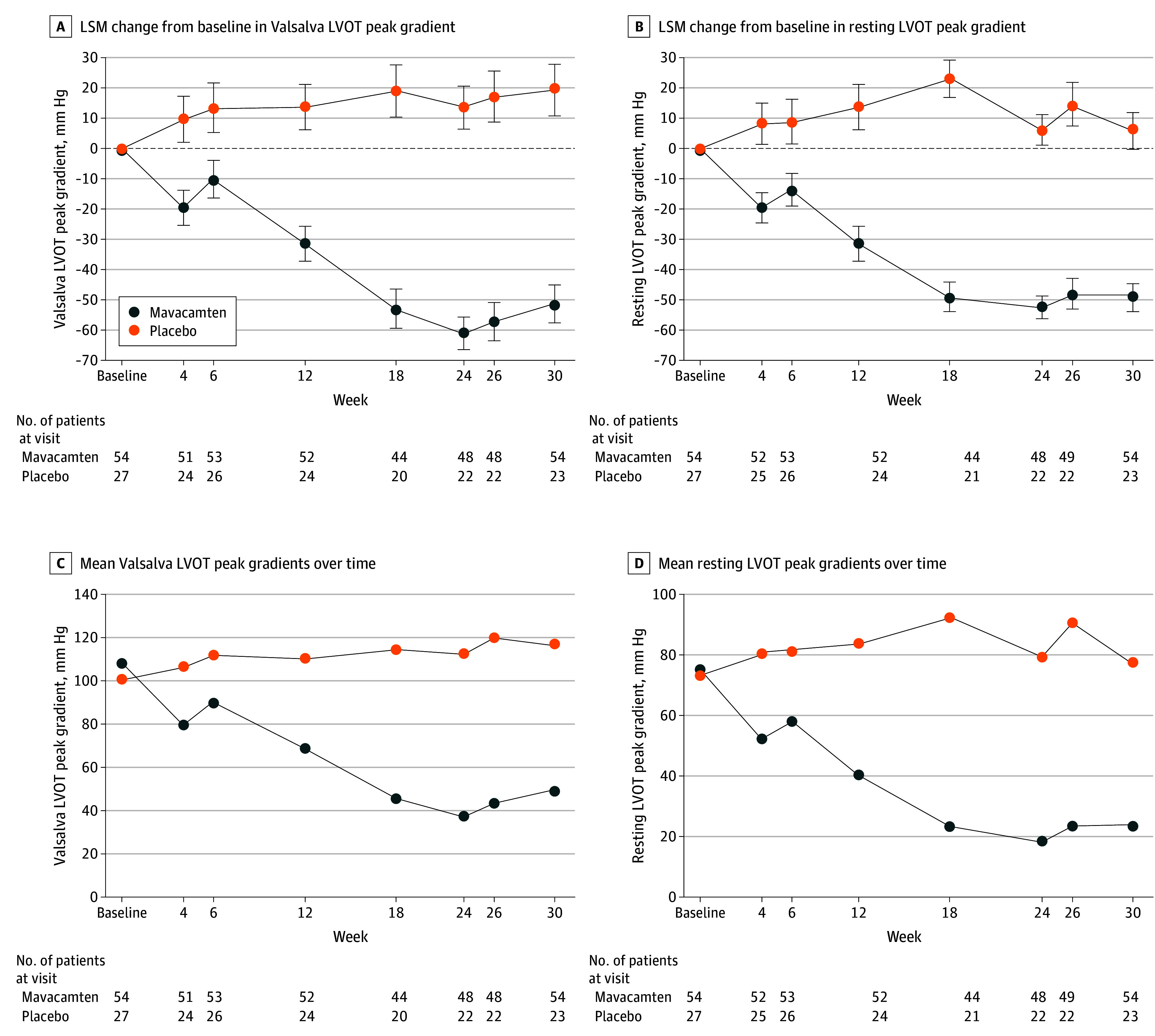
Error bars indicate SEs. LSM indicates least-squares mean.
Figure 3. Forest Plot of Change in Valsalva Left Ventricular Outflow Tract (LVOT) Peak Gradient Across Subgroups From Baseline to Week 30.
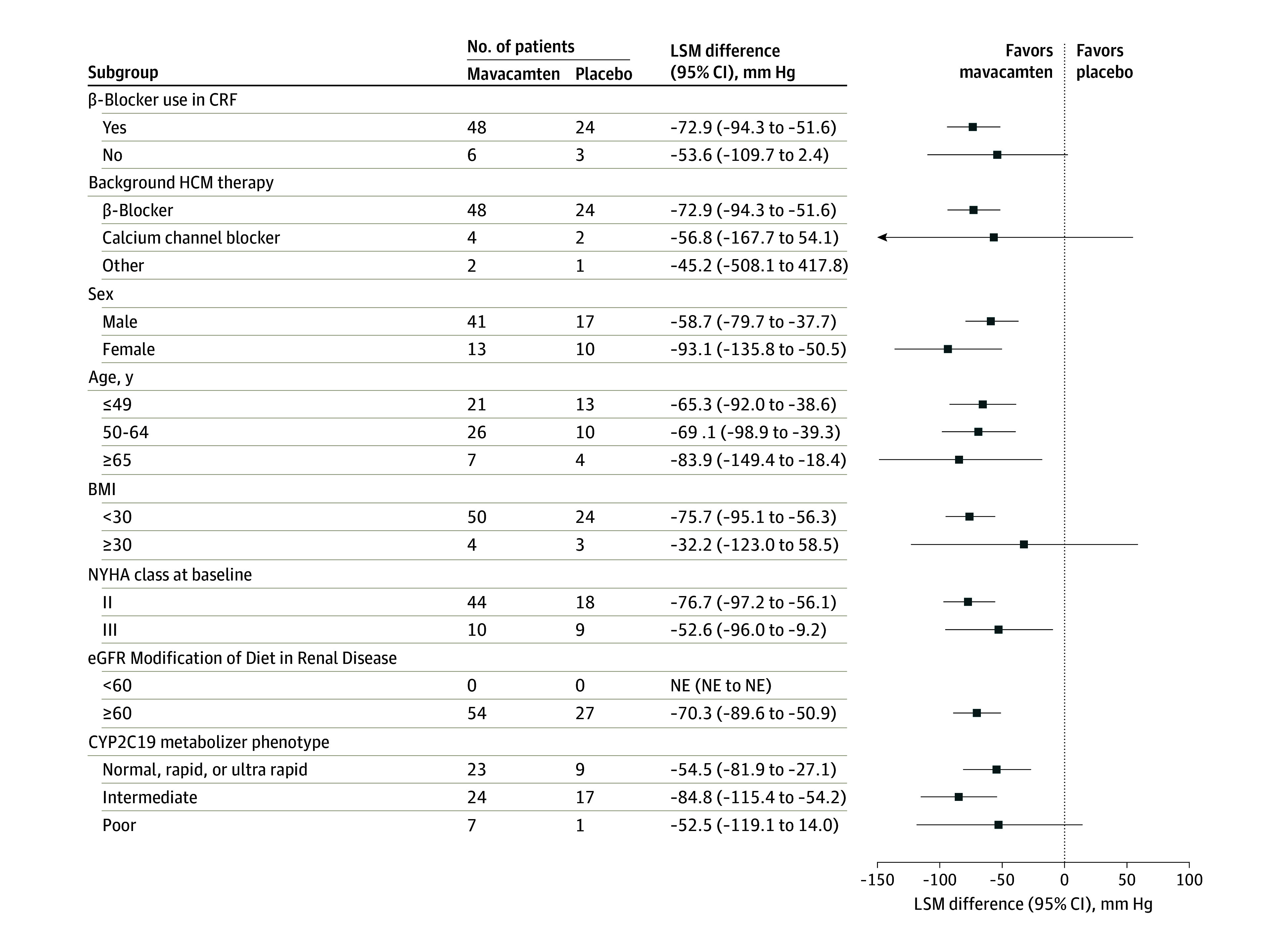
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CRF, case report form; eGFR, estimated glomerular filtration rate; HCM, hypertrophic cardiomyopathy; LSM, least-squares mean; NE, not estimable; and NYHA, New York Heart Association.
Mavacamten also showed substantial improvements across all secondary efficacy end points compared with placebo (eTable 2 in Supplement 2). The mean resting LVOT peak gradient decreased from baseline to week 30 for the mavacamten group, whereas an increase was seen in the placebo group (LSM [SE] change, –49.0 [4.6] mm Hg vs 6.0 [6.3] mm Hg; LSM difference, −55.0 mm Hg; 95% CI, −69.1 to −40.9 mm Hg). Similar to the primary end point with the Valsalva maneuver, the resting LVOT peak gradient decreased from week 4 and was sustained throughout the study (Figure 2B and D; eTable 5 in Supplement 2). In addition, a larger proportion of patients receiving mavacamten achieved a Valsalva LVOT peak gradient less than 30 mm Hg (48.1% [26 of 54] vs 3.7% [1 of 27]) and less than 50 mm Hg (59.3% [32 of 54] vs 7.4% [2 of 27]) at week 30 compared with placebo (eTable 2 in Supplement 2). Resting LVEF remained stable in the mavacamten and placebo groups throughout treatment (80.8% vs 79.7% at week 30; LSM change, 3.7% vs 3.0%) (eFigure 4 in Supplement 2).
In addition to LVOT improvements, 59.3% of patients (32 of 54) receiving mavacamten had at least 1 NYHA class improvement by week 30 compared with 14.8% of patients (4 of 27) receiving placebo (eFigure 2 in Supplement 2). Specifically, 44.4% of mavacamten-treated patients (24 of 54) achieved NYHA class I status compared with 3.7% (1 of 27) in the placebo group. In addition, a higher proportion of patients receiving mavacamten achieved both NYHA class I and LVOT gradients less than 30 mm Hg (resting and Valsalva) at week 30 compared with those receiving placebo (25.9% [14 of 54] vs 3.7% [1 of 27]). Consistent with NYHA functional improvement, mavacamten was also associated with improved health status as assessed by KCCQ-CSS from baseline to week 30 compared with placebo, with a between-group LSM difference of 10.2 points (95% CI, 4.4-16.1 points) (eTable 2 in Supplement 2). In parallel with hemodynamic improvements, cardiac biomarkers decreased with mavacamten from week 4 and were sustained thereafter (eTable 2 and eFigure 3A and B in Supplement 2). At week 30, reduction in NT-proBNP level was 82% greater for mavacamten compared with placebo (proportion of geometric mean ratio between treatments, 0.18; 95% CI, 0.13-0.24), while reduction in hs-cTnI level was 66% greater compared with placebo (proportion of geometric mean ratio between treatments, 0.34; 95% CI, 0.27-0.42).
Among 58 eligible patients with CMR imaging data available, secondary and exploratory CMR imaging end points revealed favorable cardiac remodeling with mavacamten vs placebo from baseline to week 30, including reductions in LVMI (−26.4 g/m2 vs 4.4 g/m2, mean difference, −30.8 g/m2; 95% CI, −41.6 to −20.1 g/m2) (eTable 2 in Supplement 2), LV mass (−46.3 g vs 6.3 g; mean difference, −52.6 g; 95% CI, −67.9 to −37.4 g), maximum left atrial volume index (−17.3 mL/m2 vs 1.0 mL/m2; mean difference, −18.3 mL/m2; 95% CI, −26.7 to −9.8 mL/m2), and maximal wall thickness (−3.0 mm vs 0.5 mm; mean difference, −3.5 mm; 95% CI, −4.7 to −2.4 mm) (eTable 6 in Supplement 2).
Safety
The incidence of TEAEs was similar between the mavacamten and placebo groups (83.3% [45 of 54] vs 88.9% [24 of 27]) (Table 2). A smaller proportion of patients in the mavacamten group experienced treatment-related TEAEs compared with placebo (20.4% [11 of 54] vs 33.3% [9 of 27]). Most TEAEs were generally mild or moderate (eTable 7 in Supplement 2). Common TEAEs (≥5% of patients in either treatment group) are shown in eTable 8 in Supplement 2.
Table 2. Summary of Adverse Events During Treatment.
| Adverse event | Mavacamten (n = 54) | Placebo (n = 27) |
|---|---|---|
| Total number of TEAEs | 165 | 82 |
| Patients with ≥1 TEAE, No. (%) | 45 (83.3) | 24 (88.9) |
| Patients with ≥1 treatment-related TEAE, No. (%) | 11 (20.4) | 9 (33.3) |
| Total No. of TESAEs | 8 | 0 |
| Patients with ≥1 TESAE, No. (%) | 4 (7.4) | 0 |
| Atrial fibrillation | 2 (3.7) | 0 |
| Atrial flutter | 1 (1.9) | 0 |
| Sinus arrest | 1 (1.9) | 0 |
| Sinus node dysfunction | 1 (1.9) | 0 |
| Hypotension | 1 (1.9) | 0 |
| Hemorrhoids | 1 (1.9) | 0 |
| Ankle fracture | 1 (1.9) | 0 |
| Patients with ≥1 related TESAE, No. (%) | 0 | 0 |
| Patients with ≥1 AESI, No. (%) | 1 (1.9) | 0 |
Abbreviations: AESI, adverse event of special interest; TEAE, treatment-emergent adverse event; TESAE, treatment-emergent serious adverse event.
Eight treatment-emergent SAEs (TESAEs) occurred in 4 patients (7.4%) treated with mavacamten and no patients in the placebo group (Table 2). Three patients (5.6%) treated with mavacamten had serious cardiac TEAEs, including 2 events of AF, and 1 each of atrial flutter, sinus arrest, and sinus node dysfunction. None of the TESAEs were related to mavacamten as judged by the investigator. No patient had an LVEF less than 50% or developed HF. There were no TEAEs leading to dose interruption, discontinuation of treatment, early termination of study, or deaths. One patient (CYP2C19 intermediate metabolizer) in the mavacamten group had dose interruption due to a predose plasma mavacamten concentration of 1000 ng/mL or more with normal LVEF, at the 10-mg dose. The patient remained asymptomatic throughout, and mavacamten was subsequently resumed at the 5-mg dose.
There were no marked changes in laboratory parameters, vital signs, or electrocardiography findings (eTable 9 in Supplement 2). Continuous cardiac monitoring with a 48-hour Holter monitor showed no significant difference in the number of patients with episodes of AF or nonsustained ventricular tachycardia detected (eTable 10 in Supplement 2).
Discussion
This phase 3 randomized clinical trial of Chinese patients with oHCM demonstrated that mavacamten significantly reduced Valsalva LVOT gradients compared with placebo after 30 weeks of treatment. The primary end point of change in Valsalva LVOT gradient favored mavacamten across prespecified subgroups, regardless of the use of β-blockers. The benefit of mavacamten was seen as early as 4 weeks after treatment initiation and was sustained throughout the treatment period. Improvements with mavacamten were also noted across all prespecified secondary efficacy end points, including LVOT obstruction, clinical symptoms, health status, and cardiac biomarkers. In addition, reduction in LVMI based on CMR imaging indicated favorable cardiac remodeling with mavacamten. Mavacamten was well tolerated and showed a safety profile that is consistent with the findings of EXPLORER-HCM, with no new safety signals. This study supports that the efficacy and safety of mavacamten extend to Asian patients, including Chinese patients, a population with higher rates of poor CYP2C19 metabolizers and lower BMI than the global population.
In our study, the primary end point focused on the change in Valsalva LVOT peak gradient, as LVOT obstruction is the primary cause of disabling symptoms and a risk factor for AF and HF in patients with HCM.5,6 Patients with LVOT obstruction were more likely to advance to NYHA class III or IV than those without obstruction in previous studies (annual rate of 3.2%-7.4% vs 1.6%).5,6,25 Therefore, relieving LVOT obstruction is a fundamental therapeutic goal for patients with oHCM.7 Unlike EXPLORER-HCM, this study used only the Valsalva maneuver to provoke the LVOT gradient due to the practicality and feasibility of this approach in China. Exercise testing is not recommended for Chinese individuals whose resting LVOT gradient exceeds 50 mm Hg. Per Chinese guidelines, the Valsalva maneuver is recommended instead as a provocation method.11,26 Furthermore, the Valsalva LVOT gradient has been shown to mirror the exercise gradient among patients with HCM and resting obstruction.27
The magnitude of gradient reduction at week 30 was remarkable, with a mean change from baseline of −57.9 mm Hg and −51.5 mm Hg for Valsalva and resting LVOT gradients, respectively, in the mavacamten group, consistent with the findings of the EXPLORER-HCM trial.17 By week 30, more patients receiving mavacamten than placebo achieved a Valsalva LVOT less than 30 mm Hg (48.1% vs 3.7%) and less than 50 mm Hg (59.3% vs 7.4%), which represent the threshold for oHCM definition and SRT eligibility, respectively. In parallel, the proportion of patients with at least 1 class improvement in NYHA functional classification was 4-fold greater with mavacamten compared with placebo (59.3% vs 14.8%); an overall improvement from more severe HF symptoms (class II or III) to becoming less symptomatic or asymptomatic (class I or II) after mavacamten treatment was observed. This is a particularly important finding because SRT is frequently considered for patients with NYHA class III or above who have moderate to severe exercise-limiting symptoms.28,29 In VALOR-HCM, patients with severely symptomatic oHCM treated with mavacamten were less likely to remain eligible for SRT than those receiving placebo.18
In the current trial, mavacamten significantly decreased the levels of cardiac biomarkers NT-proBNP and hs-cTnI, indicating that mavacamten may decrease LV wall stress and myocardial injury. This finding is supported by CMR imaging data showing evidence of cardiac remodeling with mavacamten, as reflected in marked reductions in LVMI, LV mass, left atrial volume index, and maximal wall thickness, all of which are associated with poor outcomes in oHCM.2 Our findings expand the evidence from the CMR substudy of EXPLORER-HCM (n = 35)30,31 and provide the largest CMR imaging data set (n = 58), to our knowledge, for a pharmacotherapy to demonstrate favorable cardiac remodeling in HCM. The reduction in LVMI was seen even with a short treatment period of only 30 weeks.
A lower starting dose of 2.5 mg once daily was used in our study compared with 5 mg in EXPLORER-HCM. Although 5 mg is considered safe even for poor CYP2C19 metabolizers who had reduced clearance of mavacamten, a conservative starting dose of 2.5 mg was chosen due to the lower mean body weight and a higher prevalence of poor CYP2C19 metabolizers among the Chinese population.32,33 Our dosing goal was to optimize safety by titrating to the lowest effective dose for each patient based on individual pharmacokinetic or pharmacodynamic parameters while minimizing adverse effects. Despite the lower starting dose of 2.5 mg, an early benefit of mavacamten vs placebo in reducing LVOT obstruction was observed at week 4, which reached statistical significance. Furthermore, the reduction of LVOT obstruction was consistent across all CYP2C19 phenotypes, which supports the current titration scheme based on pharmacokinetic or pharmacodynamic response, without the need for CYP2C19 genotyping. The safety profile appeared consistent with the EXPLORER-HCM population despite the higher prevalence of poor metabolizers in our study, indicating that routine safety monitoring may be adequate with the current dosing scheme.
Mavacamten was generally well tolerated among Chinese patients; TEAEs were balanced between mavacamten and placebo overall. There were no treatment-related SAEs, nor any discontinuations due to AEs during the study. Although TESAEs were observed in the mavacamten group, none were treatment related as assessed by the investigators. The safety profile of mavacamten was consistent with previous studies.17,34 No patients reported an LVEF less than 50%, and no notable cardiac toxicity was recorded. Patients who completed this study were offered the opportunity to enter the 48-week long-term extension study, which will further inform the safety of mavacamten.
Limitations
This study has some limitations, including the lack of data on peak oxygen consumption and stress echocardiography due to limited feasibility in China. Also, the proportion of men enrolled was greater than the proportion of women, which could be attributed to the higher prevalence of oHCM among men than women in China.35 The conservative titration strategy we followed could have led to some patients being underdosed; nonetheless, the benefit of treatment with mavacamten was observed as early as week 4, and there was no discontinuation due to LVEF decline. Because the study population was relatively small, further studies in a larger population with a longer follow-up period are required.
Conclusions
In this phase 3, randomized, double-blind, placebo-controlled clinical trial of Chinese patients with oHCM, mavacamten significantly improved the LVOT gradient compared with placebo at week 30. New York Heart Association functional class, health status, cardiac biomarkers, and cardiac structure were also improved. The safety profile of mavacamten was consistent with previous studies. This study supports that the efficacy and safety of mavacamten extend to Asian patients, including Chinese patients, among whom poor CYP2C19 metabolizers are more common and overall BMI tends to be lower than the global population.
Trial Protocol and Statistical Analysis Plan
eAppendix. Study Sites and Investigators
eMethods.
eTable 1. Study Eligibility Criteria
eTable 2. Primary and Secondary Efficacy End Points
eTable 3. Mean Valsalva LVOT Peak Gradients Over Time
eTable 4. Change From Baseline in Valsalva LVOT Peak Gradient for ITT and Per-Protocol Analyses Across Time Points
eTable 5. Mean Resting LVOT Peak Gradients Over Time
eTable 6. Key Exploratory End Points for CMR Parameters
eTable 7. Severity of TEAEs
eTable 8. Common TEAEs Reported in ≥5% of Patients by System Organ Class and Preferred Term
eTable 9. Summary of QTcF Intervals (SAF)
eTable 10. Holter Monitoring Results
eFigure 1. Study Schema From Screening to Week 30
eFigure 2. Proportion of Patients Across NYHA Functional Classes at Baseline, Week 14, and Week 30
eFigure 3. Changes in NT-proBNP (A) and hs-cTnI (B) From Baseline to Week 30
eFigure 4. LSM Change in LVEF From Baseline to Week 30
Data Sharing Statement
References
- 1.Maron BJ, Desai MY, Nishimura RA, et al. Diagnosis and evaluation of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(4):372-389. doi: 10.1016/j.jacc.2021.12.002 [DOI] [PubMed] [Google Scholar]
- 2.Marian AJ, Braunwald E. Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res. 2017;121(7):749-770. doi: 10.1161/CIRCRESAHA.117.311059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC Guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2020;76(25):e159-e240. doi: 10.1016/j.jacc.2020.08.045 [DOI] [PubMed] [Google Scholar]
- 4.Elliott PM, Gimeno JR, Thaman R, et al. Historical trends in reported survival rates in patients with hypertrophic cardiomyopathy. Heart. 2006;92(6):785-791. doi: 10.1136/hrt.2005.068577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Autore C, Bernabò P, Barillà CS, Bruzzi P, Spirito P. The prognostic importance of left ventricular outflow obstruction in hypertrophic cardiomyopathy varies in relation to the severity of symptoms. J Am Coll Cardiol. 2005;45(7):1076-1080. doi: 10.1016/j.jacc.2004.12.067 [DOI] [PubMed] [Google Scholar]
- 6.Maron MS, Olivotto I, Betocchi S, et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348(4):295-303. doi: 10.1056/NEJMoa021332 [DOI] [PubMed] [Google Scholar]
- 7.Argirò A, Zampieri M, Berteotti M, et al. Emerging medical treatment for hypertrophic cardiomyopathy. J Clin Med. 2021;10(5):951. doi: 10.3390/jcm10050951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott PM, Anastasakis A, Borger MA, et al. ; Authors/Task Force members . 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(39):2733-2779. doi: 10.1093/eurheartj/ehu284 [DOI] [PubMed] [Google Scholar]
- 9.Trivedi DV, Adhikari AS, Sarkar SS, Ruppel KM, Spudich JA. Hypertrophic cardiomyopathy and the myosin mesa: viewing an old disease in a new light. Biophys Rev. 2018;10(1):27-48. doi: 10.1007/s12551-017-0274-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maron BJ, Rowin EJ, Maron MS. Hypertrophic cardiomyopathy: new concepts and therapies. Annu Rev Med. 2022;73:363-375. doi: 10.1146/annurev-med-042220-021539 [DOI] [PubMed] [Google Scholar]
- 11.Chinese Society of Cardiology, Chinese Medical Association, Chinese Adult Hypertrophic Cardiomyopathy Diagnosis and Treatment Guidelines Writing Group . Guidelines for the diagnosis and treatment for Chinese adult patients with hypertrophic cardiomyopathy. Article in Chinese. Zhonghua Xin Xue Guan Bing Za Zhi. 2017;45(12):1015-1032. [DOI] [PubMed] [Google Scholar]
- 12.Hua TR, Zhang SY. Cardiomyopathies in China: a 2018-2019 state-of-the-art review. Chronic Dis Transl Med. 2020;6(4):224-238. doi: 10.1016/j.cdtm.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liebregts M, Vriesendorp PA, Mahmoodi BK, Schinkel AF, Michels M, ten Berg JM. A systematic review and meta-analysis of long-term outcomes after septal reduction therapy in patients with hypertrophic cardiomyopathy. JACC Heart Fail. 2015;3(11):896-905. doi: 10.1016/j.jchf.2015.06.011 [DOI] [PubMed] [Google Scholar]
- 14.Kim LK, Swaminathan RV, Looser P, et al. Hospital volume outcomes after septal myectomy and alcohol septal ablation for treatment of obstructive hypertrophic cardiomyopathy: US nationwide inpatient database, 2003-2011. JAMA Cardiol. 2016;1(3):324-332. doi: 10.1001/jamacardio.2016.0252 [DOI] [PubMed] [Google Scholar]
- 15.Stern JA, Markova S, Ueda Y, et al. A small molecule inhibitor of sarcomere contractility acutely relieves left ventricular outflow tract obstruction in feline hypertrophic cardiomyopathy. PLoS One. 2016;11(12):e0168407. doi: 10.1371/journal.pone.0168407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawas RF, Anderson RL, Ingle SRB, Song Y, Sran AS, Rodriguez HM. A small-molecule modulator of cardiac myosin acts on multiple stages of the myosin chemomechanical cycle. J Biol Chem. 2017;292(40):16571-16577. doi: 10.1074/jbc.M117.776815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olivotto I, Oreziak A, Barriales-Villa R, et al. ; EXPLORER-HCM study investigators . Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;396(10253):759-769. doi: 10.1016/S0140-6736(20)31792-X [DOI] [PubMed] [Google Scholar]
- 18.Desai MY, Owens A, Geske JB, et al. Myosin inhibition in patients with obstructive hypertrophic cardiomyopathy referred for septal reduction therapy. J Am Coll Cardiol. 2022;80(2):95-108. doi: 10.1016/j.jacc.2022.04.048 [DOI] [PubMed] [Google Scholar]
- 19.Desai MY, Owens A, Geske JB, et al. Dose-blinded myosin inhibition in patients with obstructive hypertrophic cardiomyopathy referred for septal reduction therapy: outcomes through 32 weeks. Circulation. 2023;147(11):850-863. doi: 10.1161/CIRCULATIONAHA.122.062534 [DOI] [PubMed] [Google Scholar]
- 20.Camzyos. Prescribing information. MyoKardia Inc, a wholly-owned subsidiary of Bristol Myers Squibb; April 2022. Accessed June 18, 2023. https://packageinserts.bms.com/pi/pi_camzyos.pdf
- 21.Maron MS, Olivotto I, Zenovich AG, et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114(21):2232-2239. doi: 10.1161/CIRCULATIONAHA.106.644682 [DOI] [PubMed] [Google Scholar]
- 22.Zou Y, Song L, Wang Z, et al. Prevalence of idiopathic hypertrophic cardiomyopathy in China: a population-based echocardiographic analysis of 8080 adults. Am J Med. 2004;116(1):14-18. doi: 10.1016/j.amjmed.2003.05.009 [DOI] [PubMed] [Google Scholar]
- 23.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 24.Tian Z, Wang F, Jin W, et al. Study design and rationale of EXPLORER-CN: a phase III, randomised, double-blind, placebo-controlled clinical study to evaluate the efficacy and safety of mavacamten in Chinese adults with symptomatic obstructive hypertrophic cardiomyopathy. BMJ Open. 2023;13(6):e071473. doi: 10.1136/bmjopen-2022-071473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elliott PM, Gimeno JR, Tomé MT, et al. Left ventricular outflow tract obstruction and sudden death risk in patients with hypertrophic cardiomyopathy. Eur Heart J. 2006;27(16):1933-1941. doi: 10.1093/eurheartj/ehl041 [DOI] [PubMed] [Google Scholar]
- 26.The Joint Committee of Cardiomyopathy Specialty Alliance, National Center for Cardiovascular Diseases/Cardiovascular Precision Medicine Branch of China International Exchange and Promotive Association for Medical and Health Care . 2023 Guideline for diagnosis and treatment of patients with hypertrophic cardiomyopathy. Article in Chinese. Chin Circ J. 2023;38(1):1-33. doi: 10.3969/j.issn.1000-3614.2023.01.001 [DOI] [Google Scholar]
- 27.Kumar S, Van Ness G, Bender A, et al. Standardized goal-directed Valsalva maneuver for assessment of inducible left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2018;31(7):791-798. doi: 10.1016/j.echo.2018.01.022 [DOI] [PubMed] [Google Scholar]
- 28.Ommen SR, Maron BJ, Olivotto I, et al. Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46(3):470-476. doi: 10.1016/j.jacc.2005.02.090 [DOI] [PubMed] [Google Scholar]
- 29.Nagueh SF, Ommen SR, Lakkis NM, et al. Comparison of ethanol septal reduction therapy with surgical myectomy for the treatment of hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 2001;38(6):1701-1706. doi: 10.1016/S0735-1097(01)01614-X [DOI] [PubMed] [Google Scholar]
- 30.Saberi S, Cardim N, Yamani M, et al. Mavacamten favorably impacts cardiac structure in obstructive hypertrophic cardiomyopathy: EXPLORER-HCM cardiac magnetic resonance substudy analysis. Circulation. 2021;143(6):606-608. doi: 10.1161/CIRCULATIONAHA.120.052359 [DOI] [PubMed] [Google Scholar]
- 31.Saberi S, Kramer CM, Oreziak A, et al. 96-Week cardiac magnetic resonance results of treatment with mavacamten from the EXPLORER cohort of the Mava-Long-Term Extension study in patients with obstructive hypertrophic cardiomyopathy. Presented at: American College of Cardiology Annual Scientific Session & Expo; March 5, 2023; New Orleans, Louisiana. [Google Scholar]
- 32.Chen L, Qin S, Xie J, et al. Genetic polymorphism analysis of CYP2C19 in Chinese Han populations from different geographic areas of mainland China. Pharmacogenomics. 2008;9(6):691-702. doi: 10.2217/14622416.9.6.691 [DOI] [PubMed] [Google Scholar]
- 33.Koopmans AB, Braakman MH, Vinkers DJ, Hoek HW, van Harten PN. Meta-analysis of probability estimates of worldwide variation of CYP2D6 and CYP2C19. Transl Psychiatry. 2021;11(1):141. doi: 10.1038/s41398-020-01129-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heitner SB, Jacoby D, Lester SJ, et al. Mavacamten treatment for obstructive hypertrophic cardiomyopathy: a clinical trial. Ann Intern Med. 2019;170(11):741-748. doi: 10.7326/M18-3016 [DOI] [PubMed] [Google Scholar]
- 35.Bai Y, Zheng JP, Lu F, et al. Prevalence, incidence and mortality of hypertrophic cardiomyopathy based on a population cohort of 21.9 million in China. Sci Rep. 2022;12(1):18799. doi: 10.1038/s41598-022-20042-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eAppendix. Study Sites and Investigators
eMethods.
eTable 1. Study Eligibility Criteria
eTable 2. Primary and Secondary Efficacy End Points
eTable 3. Mean Valsalva LVOT Peak Gradients Over Time
eTable 4. Change From Baseline in Valsalva LVOT Peak Gradient for ITT and Per-Protocol Analyses Across Time Points
eTable 5. Mean Resting LVOT Peak Gradients Over Time
eTable 6. Key Exploratory End Points for CMR Parameters
eTable 7. Severity of TEAEs
eTable 8. Common TEAEs Reported in ≥5% of Patients by System Organ Class and Preferred Term
eTable 9. Summary of QTcF Intervals (SAF)
eTable 10. Holter Monitoring Results
eFigure 1. Study Schema From Screening to Week 30
eFigure 2. Proportion of Patients Across NYHA Functional Classes at Baseline, Week 14, and Week 30
eFigure 3. Changes in NT-proBNP (A) and hs-cTnI (B) From Baseline to Week 30
eFigure 4. LSM Change in LVEF From Baseline to Week 30
Data Sharing Statement