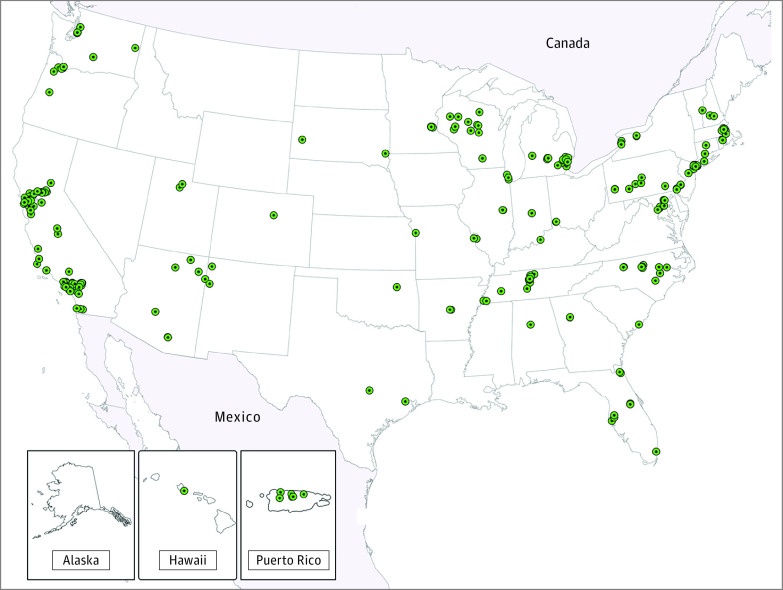
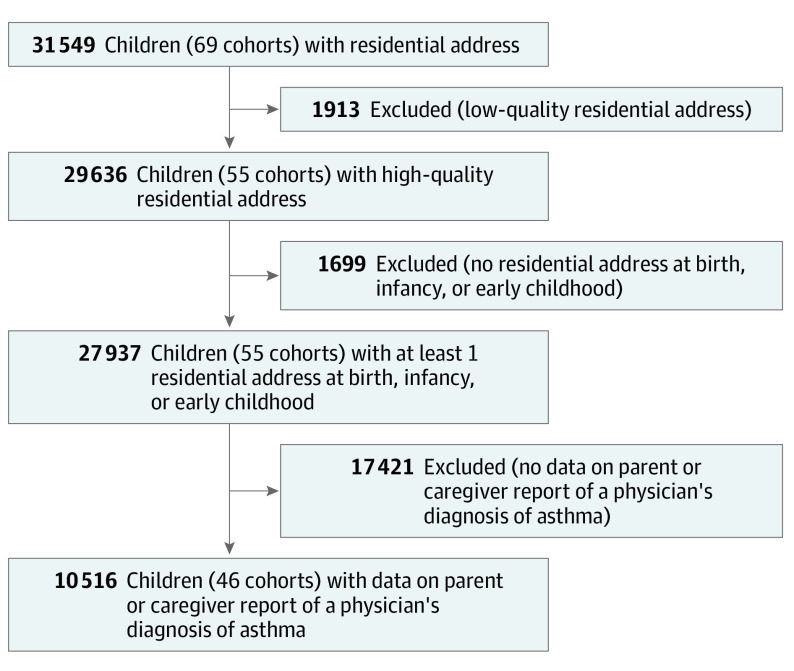
This cohort study uses data from the Environmental Influences on Child Health Outcomes (ECHO) Program to investigate whether opportunity and social vulnerability at the neighborhood level are associated with childhood asthma incidence.
Key Points
Question
Is there an association of neighborhood-level measures of opportunity and social vulnerability in early life with childhood asthma incidence?
Findings
In this nationwide cohort study including 10 516 children, residence in neighborhoods with high or very high opportunity in early life, especially those with high health and environmental or social and economic opportunity, was associated with lower subsequent asthma incidence compared with residence in very low–opportunity neighborhoods.
Meaning
The findings suggest the need for future studies examining whether investing in health and environmental or social and economic resources in early life would promote health equity in pediatric asthma.
Abstract
Background
The extent to which physical and social attributes of neighborhoods play a role in childhood asthma remains understudied.
Objective
To examine associations of neighborhood-level opportunity and social vulnerability measures with childhood asthma incidence.
Design, Setting, and Participants
This cohort study used data from children in 46 cohorts participating in the Environmental Influences on Child Health Outcomes (ECHO) Program between January 1, 1995, and August 31, 2022. Participant inclusion required at least 1 geocoded residential address from birth and parent or caregiver report of a physician’s diagnosis of asthma. Participants were followed up to the date of asthma diagnosis, date of last visit or loss to follow-up, or age 20 years.
Exposures
Census tract–level Child Opportunity Index (COI) and Social Vulnerability Index (SVI) at birth, infancy, or early childhood, grouped into very low (<20th percentile), low (20th to <40th percentile), moderate (40th to <60th percentile), high (60th to <80th percentile), or very high (≥80th percentile) COI or SVI.
Main Outcomes and Measures
The main outcome was parent or caregiver report of a physician’s diagnosis of childhood asthma (yes or no). Poisson regression models estimated asthma incidence rate ratios (IRRs) associated with COI and SVI scores at each life stage.
Results
The study included 10 516 children (median age at follow-up, 9.1 years [IQR, 7.0-11.6 years]; 52.2% male), of whom 20.6% lived in neighborhoods with very high COI and very low SVI. The overall asthma incidence rate was 23.3 cases per 1000 child-years (median age at asthma diagnosis, 6.6 years [IQR, 4.1-9.9 years]). High and very high (vs very low) COI at birth, infancy, or early childhood were associated with lower subsequent asthma incidence independent of sociodemographic characteristics, parental asthma history, and parity. For example, compared with very low COI, the adjusted IRR for asthma was 0.87 (95% CI, 0.75-1.00) for high COI at birth and 0.83 (95% CI, 0.71-0.98) for very high COI at birth. These associations appeared to be attributable to the health and environmental and the social and economic domains of the COI. The SVI during early life was not significantly associated with asthma incidence. For example, compared with a very high SVI, the adjusted IRR for asthma was 0.88 (95% CI, 0.75-1.02) for low SVI at birth and 0.89 (95% CI, 0.76-1.03) for very low SVI at birth.
Conclusions
In this cohort study, high and very high neighborhood opportunity during early life compared with very low neighborhood opportunity were associated with lower childhood asthma incidence. These findings suggest the need for future studies examining whether investing in health and environmental or social and economic resources in early life would promote health equity in pediatric asthma.
Introduction
Asthma is one of the most prevalent chronic conditions in US children,1 with adverse implications for long-term health2 and socioeconomic3 outcomes. Neighborhood factors contribute to pediatric asthma by exposing children to health risks in their physical and social environments.4 Prior studies demonstrated that adverse neighborhood factors, such as high poverty rates5 and crime,6 were associated with higher pediatric asthma prevalence independent of individual and family sociodemographic characteristics, likely through increased exposure to indoor and outdoor pollution and higher psychological stress.7 However, most studies examining these topics were cross-sectional or focused on single socioeconomic indicators of neighborhood disadvantage that may not adequately capture the totality of early-life social experiences.5,6,8,9 Moreover, few studies have examined the extent to which neighborhood conditions at different developmentally vulnerable periods during early life (ie, birth or early childhood) that lay the foundation for long-term health outcomes10 are associated with asthma incidence.
Census-derived neighborhood indexes, such as the Child Opportunity Index (COI)11 and Social Vulnerability Index (SVI),12 are composite measures of physical and social features hypothesized to be etiologically relevant to a range of pediatric health outcomes, and thus, they may be more meaningful than single socioeconomic indicators of neighborhood disadvantage. The COI is a multidimensional surveillance tool incorporating 29 neighborhood attributes that span 3 domains (education, health and environment, and social and economic) and has been previously associated with child cardiometabolic risk.13,14 The SVI is a relative measurement of neighborhood disadvantage based on 15 social factors that span 4 domains (socioeconomic status, household composition and disability, racial and ethnic minority and language status, and housing and transportation type) and was previously associated with obesity in childhood and adulthood.14,15 The extent to which both indices may be related to childhood asthma incidence and whether they are equally or differentially predictive remain understudied.
To address these research gaps, we analyzed data from racially, ethnically, and geographically diverse children enrolled in cohorts participating in the Environmental Influences on Child Health Outcomes (ECHO) Program,16 which includes repeated measures of residential addresses across distinct child life stages and parent or caregiver report of physician-diagnosed asthma. We hypothesized that children residing in neighborhoods with higher (vs lower) opportunity or in less (vs more) vulnerable neighborhoods would have lower asthma incidence. We also hypothesized that COI and SVI would be associated with greater asthma incidence at birth than in early childhood given previous work by some of us14 that showed stronger correlations between child obesity (a known risk factor for incident asthma17) and COI or SVI at birth compared with later life stages.
Methods
Study Population
This cohort study used data from ECHO, a collaborative consortium comprising individual cohorts of children across the US that enrolled participants at different life stages (ie, pregnancy, infancy, childhood, and adolescence), most often during pregnancy or at birth.16,18 Recruitment of new participants and existing cohort participants into ECHO for new data collection and follow-up of participants at different life stages is ongoing. Investigators of participating cohorts implemented the ECHO-wide cohort data collection protocol, which specifies data elements for new or ongoing data collection as well as extant data to be uploaded onto an ECHO-wide cohort data platform.16,19 For this study, we used extant data that were harmonized and shared on the ECHO data platform, which comprises cohorts that either prospectively ascertained or retrospectively identified physician-diagnosed asthma cases via parent or caregiver reports. We selected ECHO cohorts with data between January 1, 1995, and August 31, 2022, including children with at least 1 high-quality geocoded residential address (ie, point or specific street address) from birth and a parent or caregiver report of a physician’s diagnosis of asthma (yes or no). Parents or guardians provided written informed consent for participation in the cohort of origin, and institutional review boards at each study site approved each local protocol. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Neighborhood Indices
Using ArcGIS geospatial software (Esri), we geocoded each participant’s first residential address obtained at birth (year of residence, 1995-2017), infancy (median [IQR] age, 1.45 [1.36-1.48] years; year of residence, 1996-2018), or early childhood (median [IQR] age, 4.73 [4.29-4.76] years; year of residence, 1999-2022) and assigned a census tract location to each address using the 1990, 2000, 2010, or 2020 US census tract boundaries. We linked the census tract location closest in time to the year of residence to the census tract–level COI and SVI at the closest reference years for which data were available (ie, 2010 and 2015 for COI and 2000, 2010, 2014, 2016, and 2018 for SVI). We provide details on the COI and SVI in eTables 1 and 2 and the eMethods in Supplement 1. As detailed previously,14 we grouped census tracts into very low (<20th percentile), low (20th to <40th percentile), moderate (40th to <60th percentile), high (60th to <80th percentile), or very high (≥80th percentile) COI or SVI.
Asthma Outcome
Parents or caregivers reported their child’s history of a physician’s diagnosis of asthma (yes or no) at least once every life stage (ie, infancy, childhood, and adolescence) and the child’s age at asthma diagnosis. If the exact age at diagnosis was unavailable, we estimated age at diagnosis using the midpoint of age between the first report of having an asthma diagnosis and the research visit before that first report. As previously detailed,20 we identified asthma cases as children with physician-diagnosed asthma (no age restriction) and with at least 1 of the following at age 5 years or older: (1) the child still had asthma in the past 12 months, (2) parent- or caregiver-reported or adolescent self-reported asthma symptoms such as wheezing or trouble breathing, (3) use of asthma medications in the past 12 months, and (4) health care professional visits, hospitalization, or emergency department or urgent care visits due to asthma in the past 12 months. Thus, in this analysis, children with asthma diagnosed when younger than 5 years represented those with ongoing asthma at age 5 years or older. This approach avoided including children who experienced early transient wheezing (often misdiagnosed as asthma21) in the analytic sample. We observed children to the date of asthma diagnosis, date of last visit or loss to follow-up, or age 20 years.
Covariates
We obtained information on maternal educational level and annual household income during pregnancy, prepregnancy body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), prenatal cigarette smoking, prenatal exposure to secondhand smoke, maternal and paternal history of asthma, parity, mode of delivery, gestational age, child’s sex, child’s race (American Indian or Alaska Native, Asian, Black, Native Hawaiian or Pacific Islander, White, multiple races, or other race [a separate category on the survey questionnaire]), Hispanic or non-Hispanic ethnicity, and year of child’s birth from maternal or caregiver reports or medical records. Due to the small sample size, we combined children whose races were reported as American Indian or Alaska Native or as Native Hawaiian or Pacific Islander into a single category and children of multiple races or other racial groups into a single category. We viewed race and ethnicity as societal constructs rather than deterministic biological causes of disease risk22 and as a proxy measure of structural racism that has implications for residence in disadvantaged neighborhoods and other factors possibly associated with asthma risk.23 We obtained data on weight and height in early childhood from research visits, pediatric medical records, and mother or caregiver reports to calculate BMI. We linked participants’ census tract locations at each life stage to census tract–level measures of rurality using Rural-Urban Commuting Area Codes from the US Department of Agriculture. We selected these covariates based on previous publications reporting an association between neighborhood environments and childhood asthma.5,6,8,9,20,24
Statistical Analysis
We used Spearman correlation to examine the correlation between COI and SVI. We used multilevel Poisson regression models to estimate asthma incidence rate ratios (IRRs)25 and 95% CIs associated with the COI or SVI at each life stage, adjusting for sociodemographic characteristics, maternal and paternal history of asthma, and parity. To account for varying follow-up times, each model included an offset term for natural log-transformed person-time.25 We included random effects for cohort and census tract to account for clustering of children from the same cohort and clustering of children residing within the same neighborhood, respectively.
We conducted several secondary analyses. We additionally adjusted for prenatal characteristics that may also confound the association between neighborhoods and childhood asthma: prenatal cigarette smoking,26 prenatal secondhand smoke exposure,27 prepregnancy BMI,28 mode of delivery,29 and gestational age.30 We also adjusted for BMI in early childhood to explore the extent to which the associations may be explained by childhood adiposity. We tested for nonlinear associations by including quadratic and cubic terms for neighborhood index. We restricted our analyses to residential addresses obtained during or after 2010 to address potential COI misclassification given that the COI was available in 2010 and 2015 only. We conducted a series of leave-1-out analyses, which repeated the main analysis excluding 1 cohort at a time to ensure that no single cohort substantially swayed the findings. We also repeated all analyses using domain-specific COI or SVI to gain insights into individual facets of each that may be associated with asthma incidence. We explored modification of associations by child’s sex, race and ethnicity, and rurality of residence by adding multiplicative interaction terms with COI or SVI.
We used multiple imputation by chained equations31 to impute missing covariate data (Table 1). We generated 50 imputed data sets for all children who met the inclusion criteria. The imputation model included the exposure, outcome, and covariates under study. We combined imputed data sets using the pool function in R, version 4.1.0 (R Foundation for Statistical Computing). When interpreting findings, we focused primarily on the direction, strength, and precision of the estimates and used 2-sided P < .05 for assessment of statistical significance.
Table 1. Participant and Family Characteristics.
| Characteristic | Children (N = 10 516) | |
|---|---|---|
| Nonmissinga | Imputedb | |
| Family | ||
| Cigarette smoking during pregnancyc | ||
| No | 6618/7583 (87.3) | 87.5 |
| Yes | 965/7583 (12.7) | 12.5 |
| Secondhand smoke exposure during pregnancyd | ||
| No | 4405/6001 (73.4) | 71.5 |
| Yes | 1596/6001 (26.6) | 28.8 |
| Maternal educational level during pregnancye | ||
| Less than high school | 474/5457 (8.7) | 8.7 |
| High school degree or equivalent | 993/5457 (18.2) | 17.7 |
| Some college, no degree | 1239/5457 (22.7) | 23.2 |
| College degree or above | 2751/5457 (50.4) | 50.4 |
| Parityf | ||
| Nulliparous | 2915/7649 (38.1) | 38.0 |
| Multiparous | 4734/7649 (61.9) | 62.0 |
| Mode of deliveryg | ||
| Vaginal | 5923/9078 (65.2) | 65.8 |
| Cesarean | 3155/9078 (34.8) | 34.2 |
| Parental asthma history | ||
| Maternalh | ||
| No | 2712/3555 (76.3) | 75.8 |
| Yes | 843/3555 (23.7) | 24.2 |
| Paternali | ||
| No | 2081/2464 (84.5) | 83.7 |
| Yes | 383/2464 (15.5) | 16.3 |
| Maternal prepregnancy BMI, mean (SD)j | 27.1 (7.1) | 27.1 (7.1) |
| Gestational age at delivery, mean (SD), wkk | 37.0 (5.0) | 37.1 (5.0) |
| Annual household income during pregnancy, $l | ||
| <50 000 | 2635/4755 (55.4) | 52.4 |
| ≥50 000 | 2120/4755 (44.6) | 47.6 |
| Child | ||
| Birth year | ||
| Before 2000 | 107/10 516 (1.0) | NA |
| 2000-2010 | 3596/10 516 (34.2) | NA |
| After 2010 | 6813/10 516 (64.8) | NA |
| Sex | ||
| Female | 5022/10 516 (47.8) | NA |
| Male | 5494/10 516 (52.2) | NA |
| Racem | ||
| American Indian or Alaska Native or Native Hawaiian or Pacific Islander | 204/10 364 (2.0) | 2.0 |
| Asian | 208/10 364 (2.0) | 2.0 |
| Black | 1915/10 364 (18.5) | 18.4 |
| White | 6768/10 364 (65.3) | 65.1 |
| Other or >1 race | 1269/10 364 (12.2) | 12.5 |
| Hispanic ethnicityn | ||
| No | 8929/10 505 (85.0) | 85.0 |
| Yes | 1576/10 505 (15.0) | 15.0 |
| BMI in early childhood, mean (SD) | 16.4 (1.8) | 16.4 (1.8) |
| Age at asthma diagnosis, median (IQR), y | 6.6 (4.1-9.9) | NA |
| Age at follow-up, median (IQR), y | 9.1 (7.0-11.6) | NA |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable.
Data are presented as number/total number (percentage) of participants unless otherwise indicated.
Data are shown as a percentage of 50 imputed data sets unless otherwise indicated.
Data missing for 2933 participants (27.9%).
Data missing for 4515 participants (42.9%).
Data missing for 5059 participants (48.1%).
Data missing for 2867 participants (27.3%).
Data missing for 1438 participants (13.7%).
Data missing for 6961 participants (66.2%).
Data missing for 8052 participants (76.6%).
Data missing for 1578 participants (15.0%).
Data missing for 583 participants (5.5%).
Data missing for 5761 participants (54.8%).
Data missing for 152 participants (1.5%).
Data missing for 11 participants (0.1%).
Results
Of 69 ECHO cohorts, we included 46 (Figure 1) with a total of 10 516 children (Figure 2). Among the children included, the median age at follow-up was 9.1 years (IQR, 7.0-11.6 years); 47.8% were female; 52.2%, male; 2.0%, American Indian or Alaska Native or Native Hawaiian or Pacific Islander; 2.0%, Asian; 18.5%, Black; 15.0%, Hispanic; 85.0%, non-Hispanic; 65.3%, White; and 12.2%, more than 1 race or other race (Table 1). Significant differences in individual- and family-level characteristics were observed between children included and excluded from the analytic sample (eTable 3 in Supplement 1). Overall, 20.6% of children resided in neighborhoods with very high COI and very low SVI, indicating that COI and SVI distributions in the ECHO population are similar to nationwide distributions.14 Substantial negative correlations between the COI and SVI were observed at birth (ρ, –0.87), infancy (ρ, –0.87), and early childhood (ρ, –0.88). Strong correlations were observed for COI at birth and infancy (ρ, 0.91), birth and early childhood (ρ, 0.79), and infancy and early childhood (ρ, 0.85). Participant characteristics according to COI and SVI categories are shown in eTables 4 and 5 in Supplement 1. The overall incidence rate of asthma was 23.3 cases per 1000 child-years, with a median age at asthma diagnosis of 6.6 years (IQR, 4.1-9.9 years).
Figure 1. Recruitment Site Locations for Cohorts Included in the Analytic Sample.
Figure 2. Flowchart of the Participant Selection Process.
Crude asthma incidence rates were lower in areas with higher opportunities (Table 2). Compared with very low COI, moderate (adjusted IRR, 0.87; 95% CI, 0.75-1.00), high (adjusted IRR, 0.87; 95% CI, 0.75-1.00), or very high (adjusted IRR, 0.83; 95% CI, 0.71-0.98) COI at birth remained associated with lower asthma incidence after adjusting for sociodemographic characteristics, parental history of asthma, and parity. In infancy and early childhood, low (adjusted IRR: infancy, 0.85 [95% CI, 0.74-0.99]; early childhood, 0.81 [95% CI, 0.69-0.94]), high (adjusted IRR: infancy, 0.82 [95% CI, 0.71-0.96]; early childhood, 0.79 [95% CI, 0.68-0.92]), and very high (adjusted IRR: infancy, 0.83 [95% CI, 0.71-0.97]; early childhood, 0.80 [95% CI, 0.68-0.94]) COI (vs very low) were also associated with lower asthma incidence after adjusting for the same covariates (Figure 3). Incidence rate ratios for the association of COI at birth with asthma incidence were similar to those for COI exposure in infancy or early childhood (eTable 6 in Supplement 1).
Table 2. Crude Asthma Incidence Rates and Incidence Rate Ratios According to Child Opportunity Index or Social Vulnerability Index Categories.
| Participants, No. (%) | Crude asthma incidence rate, per 1000 child-years (95% CI) | Crude asthma incidence rate ratio (95% CI) | |
|---|---|---|---|
| Child Opportunity Index | |||
| Birth (n = 9352) | |||
| Very low opportunity | 2100 (22.5) | 35.3 (29.3-42.7) | 1 [Reference] |
| Low opportunity | 1373 (14.7) | 27.0 (22.1-33.1) | 0.77 (0.67-0.88) |
| Moderate opportunity | 1640 (17.5) | 24.7 (20.2-30.2) | 0.70 (0.61-0.80) |
| High opportunity | 1976 (21.1) | 23.3 (19.1-28.3) | 0.66 (0.58-0.75) |
| Very high opportunity | 2263 (24.2) | 21.4 (17.6-26.1) | 0.61 (0.53-0.69) |
| Infancy (n = 9479) | |||
| Very low opportunity | 2105 (22.2) | 35.7 (29.5-43.0) | 1 [Reference] |
| Low opportunity | 1344 (14.2) | 26.6 (21.7-32.6) | 0.75 (0.65-0.86) |
| Moderate opportunity | 1680 (17.7) | 25.2 (20.6-30.8) | 0.71 (0.62-0.81) |
| High opportunity | 1990 (21.7) | 22.5 (18.5-27.4) | 0.63 (0.55-0.72) |
| Very high opportunity | 2360 (24.9) | 21.6 (17.8-26.2) | 0.61 (0.53-0.69) |
| Early childhood (n = 9547) | |||
| Very low opportunity | 2034 (21.3) | 34.6 (28.7-41.6) | 1 [Reference] |
| Low opportunity | 1331 (13.9) | 24.4 (19.9-29.9) | 0.70 (0.61-0.82) |
| Moderate opportunity | 1601 (16.8) | 26.0 (21.3-31.6) | 0.75 (0.65-0.86) |
| High opportunity | 2008 (21.0) | 21.2 (17.5-25.7) | 0.61 (0.53-0.70) |
| Very high opportunity | 2573 (27.0) | 20.5 (17.0-24.8) | 0.59 (0.52-0.68) |
| Social Vulnerability Index | |||
| Birth (n = 9352) | |||
| Very high vulnerability | 1994 (21.3) | 34.0 (28.2-41.0) | 1 [Reference] |
| High vulnerability | 1633 (17.5) | 28.6 (23.6-34.8) | 0.84 (0.74-0.96) |
| Moderate vulnerability | 1604 (17.2) | 24.0 (19.6-29.3) | 0.70 (0.61-0.81) |
| Low vulnerability | 1731 (18.5) | 22.8 (18.7-27.8) | 0.67 (0.58-0.77) |
| Very low vulnerability | 2390 (25.6) | 22.0 (18.1-26.7) | 0.65 (0.57-0.74) |
| Infancy (n = 9479) | |||
| Very high vulnerability | 2032 (21.4) | 34.1 (28.3-41.1) | 1 [Reference] |
| High vulnerability | 1621 (17.1) | 28.8 (23.7-34.9) | 0.84 (0.74-0.96) |
| Moderate vulnerability | 1627 (17.2) | 24.0 (19.7-29.3) | 0.71 (0.61-0.81) |
| Low vulnerability | 1765 (18.6) | 22.7 (18.7-27.7) | 0.67 (0.58-0.76) |
| Very low vulnerability | 2434 (25.7) | 21.6 (17.8-26.2) | 0.63 (0.55-0.72) |
| Early childhood (n = 9547) | |||
| Very high vulnerability | 1971 (20.6) | 31.5 (26.2-37.0) | 1 [Reference] |
| High vulnerability | 1585 (16.6) | 28.4 (23.5-34.5) | 0.90 (0.79-1.03) |
| Moderate vulnerability | 1620 (17.0) | 24.9 (20.5-34.2) | 0.79 (0.69-0.91) |
| Low vulnerability | 1821 (19.1) | 21.0 (17.3-25.5) | 0.67 (0.58-0.77) |
| Very low vulnerability | 2550 (26.7) | 21.1 (17.4-25.5) | 0.67 (0.58-0.76) |
Figure 3. Associations of Child Opportunity Index (COI) and Social Vulnerability Index (SVI) Categories at Different Life Stages With Childhood Asthma Incidence.
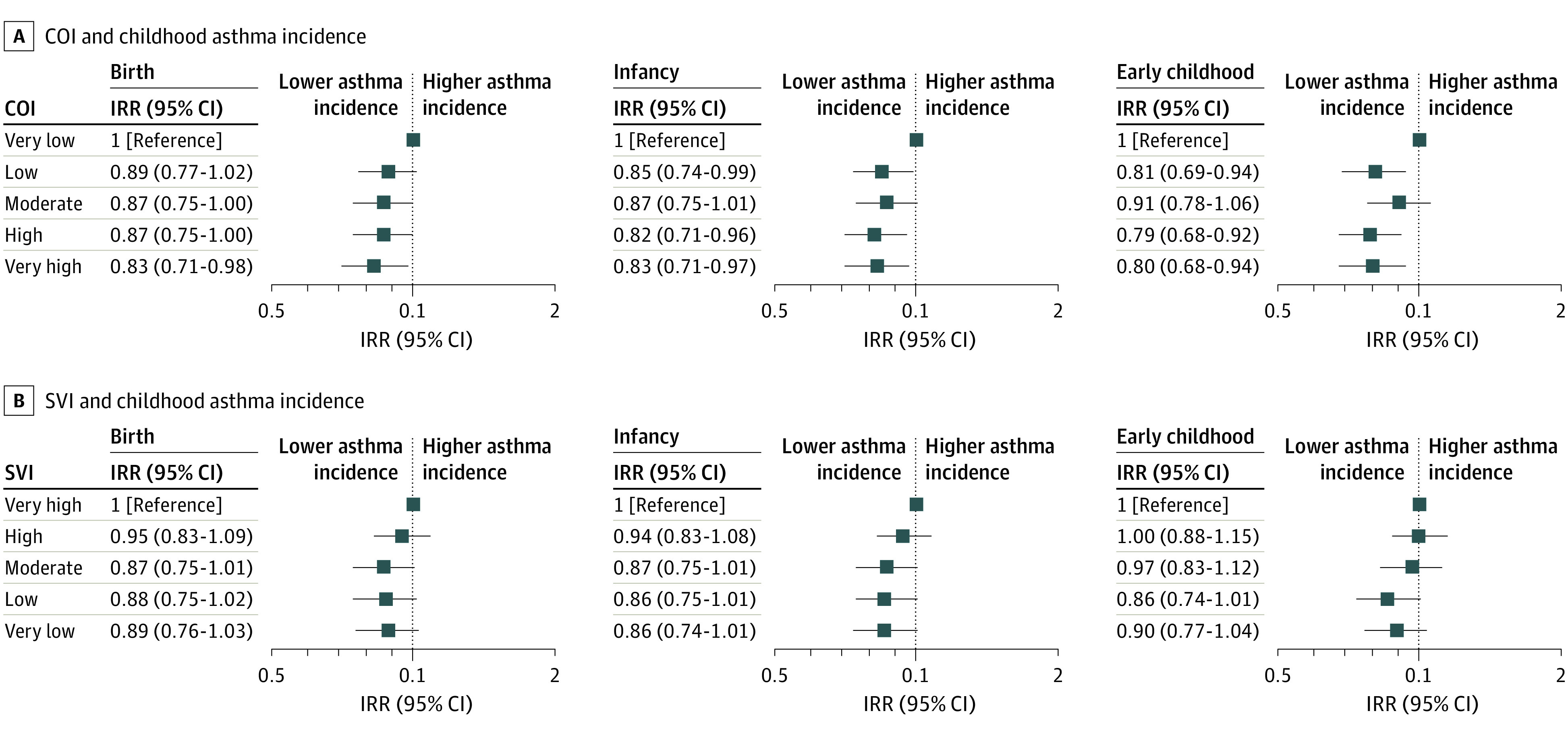
All incidence rate ratios (IRRs) and 95% CIs were adjusted for individual and family sociodemographic characteristics, parental history of asthma, and parity. Markers indicate IRRs, with horizontal lines indicating 95% CIs.
Crude asthma incidence rates were also lower in areas with lower social vulnerability (Table 2). After adjusting for sociodemographic characteristics, parental history of asthma, and parity, lower (vs very high) SVI at birth, infancy, and early childhood were not significantly associated with asthma incidence, although IRRs were in the hypothesized directions; for example, compared with a very high SVI, the adjusted IRR for asthma was 0.88 (95% CI, 0.75-1.02) for low SVI at birth and 0.89 (95% CI, 0.76-1.03) for very low SVI at birth (Figure 3).
Incidence rate ratios for the association of COI with asthma incidence did not change after additional adjustment for prenatal characteristics. However, the IRRs were slightly attenuated after adjusting for early childhood BMI (eTable 6 in Supplement 1). There was no evidence of nonlinearity in the associations of COI or SVI with asthma incidence. Similar findings were observed when restricting analyses to residential addresses obtained during or after 2010, although IRRs were less precise with wider 95% CIs (eTable 6 in Supplement 1). Incidence rate ratios did not substantially change in the leave-1-out analyses (eTables 7-9 in Supplement 1). The association of very high COI at each life stage with lower asthma incidence appeared to be attributable to the health and environmental and the social and economic domains but not the education domain (eFigure 1 in Supplement 1). No significant associations were observed between each SVI domain and asthma incidence (eFigure 2 in Supplement 1). No evidence of modification of associations by child sex, race and ethnicity, or rurality of residence was observed.
Discussion
In this nationwide multicohort study, we found that residence in neighborhoods with high and very high opportunity during early life was associated with lower subsequent childhood asthma incidence, which appeared to be attributable to the health and environmental and the social and economic domains but not the education domain. This finding might be because children do not begin formal schooling until after early childhood; thus, exposures to indoor school environments known to be associated with asthma development32 may occur after the most developmentally vulnerable periods for asthma risk. We did not observe significant associations of SVI with childhood asthma incidence, although IRRs were in the hypothesized directions.
Our results align with prior studies in pediatric populations examining neighborhoods and asthma, but specific neighborhood metrics have varied. Two studies showed that children born in neighborhoods with greater walkability33 or with greener environments34 had lower risk of incident asthma in childhood. A recent study of 10 birth cohorts reported that children born in neighborhoods with greater socioeconomic disadvantage had higher asthma incidence in childhood.35 However, these studies and others have been limited by several research gaps, including lack of geographic diversity33,34,36,37,38 and use of neighborhood indices that represent only specific aspects of socioeconomic disadvantage.35 Additionally, few studies have examined associations between neighborhoods and asthma incidence at multiple life stages,14,39,40 an important area of study given prior research demonstrating that exposures at certain age periods have a larger influence on child health outcomes than those occurring at other time points.14,39,41,42,43 Our study addressed these key research gaps by assembling a geographically diverse cohort of children that is more generalizable to the US population (Figure 1), examining publicly available indices that have been defined for neighborhoods across the US and that incorporate both positive and negative attributes of neighborhood conditions, and leveraging repeated residential data to investigate associations of COI or SVI at different life stages with asthma incidence. Although we did not identify a clear sensitive period for COI exposure, the results for COI in early childhood likely reflect a cumulative effect of COI exposure at prior life stages. Taken together, our findings contribute to the small but growing body of evidence showing an association of neighborhood conditions in early life with childhood asthma.
We noted that only the COI, not the SVI, showed significant associations with childhood asthma incidence. We speculate that this observation may partly be due to the COI incorporating 29 neighborhood attributes that are more relevant for child health outcomes.11 In contrast, the SVI comprises only 15 neighborhood attributes and was developed primarily for emergency preparedness in the event of a disaster12; thus, it might be less sensitive to childhood asthma. Our finding of lower asthma incidence in areas with very high health and environmental or social and economic COI supports this notion. Both domains include several indicators that are not contained in the SVI and have been previously associated with childhood asthma, such as neighborhood walkability,33 access to healthy food choices,44 and commute duration.45 It is worth noting that both indices had similar crude asthma incidence rates, suggesting that either neighborhood index could be used to identify children at high risk of developing asthma. This foundational information may inform place-based initiatives or policies to reduce neighborhood barriers and improve access to health and environmental or social and economic resources and, in turn, provide families with optimal environments needed to support their children’s well-being.
Many interrelated mechanisms could explain our observations. As highlighted in our secondary analyses, our findings may be partly explained by higher childhood BMI, which is an established factor associated with incident asthma17 and was previously shown to be more prevalent in ECHO children residing in neighborhoods with lower opportunities.14 Residence in more advantageous neighborhoods has been associated with higher physical activity levels46 and healthier eating patterns47 in children, which in turn, are associated with lower asthma risk,48,49 likely through reduced airway inflammation.50 Residence in neighborhoods with more favorable opportunities also likely reduces early life exposure to traffic-related air pollutants, which are known to be associated with subsequent asthma development in children.51 We did not examine these specific health behaviors and environmental pollutants in this study, but such studies could be done in the future using ECHO data.
Strengths and Limitations
Strengths of our study include the long-term follow-up and wide range of covariates. We used neighborhood indices that have been validated for a range of health outcomes.13,14,15 We assessed neighborhood opportunity and vulnerability at life stages when children were unlikely to select their place of residence, thus reducing the likelihood of self-selection and potential reverse causation bias. We were also able to control for parental factors that may be associated with both residential selection and childhood asthma. Furthermore, despite a substantial percentage of missing data for certain covariates, we used flexible multiple imputation techniques, which reduced bias and the likelihood of spurious results.
This study has several limitations. First, we relied on parent or caregiver report of physician-diagnosed asthma as our outcome, which may be subject to recall bias. A recent study, however, described moderate agreement between parent-reported physician-diagnosed asthma and physician-recorded asthma.52 Second, we used residential census tracts as a marker of exposure, which may not capture relevant areas where children spend most of their time. Third, both indices include many correlated individual indicators, thus making it difficult to disentangle the specific neighborhood component(s) associated with childhood asthma incidence. Fourth, neither index includes other neighborhood-level social attributes that might affect childhood asthma, such as neighborhood crime or violence, because of a lack of comparable data. Fifth, the COI data are available for 2010 and 2015 only and may be misclassified for residential addresses before 2010. However, our results for the COI restricted to residential addresses obtained during or after 2010 were similar to our main findings. Sixth, as the data were not available, we did not control for postnatal smoke exposure, which has been shown to be associated with increased asthma risk and is more prevalent in disadvantaged neighborhoods.53 Seventh, many cohorts in our sample were concentrated in the eastern and western US, and thus, our findings may not be entirely representative of the US. Eighth, differences between participants included and excluded from the study might have led to selection bias. However, our analyses adjusted for sociodemographic factors related to selection, thus minimizing selection bias.54 Ninth, this study did not consider how residential mobility from birth to early childhood may influence changes in COI or SVI over time and whether such changes may alter asthma development, an important question given recent research that showed children whose families moved into low-poverty neighborhoods experienced significant improvements in asthma morbidity.55 Follow-up studies in ECHO investigating these associations are possible and will be helpful to evaluate the consequences for pediatric asthma.
Conclusions
The results of this cohort study suggest that residence in neighborhoods with high and very high levels of opportunity in early life compared with very low neighborhood opportunity is associated with lower asthma incidence in childhood. Our findings highlight the need for future studies examining whether investing in health and environmental or social and economic resources in early life promotes health equity in pediatric asthma. Given the long-term association of childhood asthma with adult health,2 additional research is warranted to investigate whether strategies that alter specific neighborhood components would be effective in preventing childhood asthma.
eTable 1. Child Opportunity Index Indicators, Definitions, and Data Sources
eTable 2. Social Vulnerability Index Indicators, Definitions, and Data Sources
eTable 3. Charactersitics of Children Included and Excluded From the Analytic Sample
eTable 4. Participant Characteristics According to Very Low and Very High Child Opportunity Index Categories at Each Life Stage
eTable 5. Participant Characteristics According to Very High and Very Low Social Vulnerability Index Categories at Each Life Stage
eTable 6. Secondary Analyses for the Association of Child Opportunity Index at Different Life Stages With Asthma Incidence
eTable 7. Leave-1-Out Analyses for the Association of Child Opportunity Index at Birth With Asthma Incidence
eTable 8. Leave-1-Out Analyses for the Association of Child Opportunity Index in Infancy With Asthma Incidence
eTable 9. Leave-1-Out Analyses for the Association of Child Opportunity Index in Early Childhood With Asthma Incidence
eFigure 1. Association of Domain-Specific Child Opportunity Index at Birth, Infancy, and Early Childhood With Asthma Incidence
eFigure 2. Association of Domain-Specific Social Vulnerability Index (SVI) at Birth, Infancy, and Early Childhood With Asthma Incidence
eMethods. Description of Child Opportunity Index and Social Vulnerability Index
Nonauthor Collaborators. Environmental Influences on Child Health Outcomes
Data Sharing Statement
References
- 1.Akinbami LJ, Rossen LM, Fakhouri THI, Fryar CD. Asthma prevalence trends by weight status among US children aged 2-19 years, 1988-2014. Pediatr Obes. 2018;13(6):393-396. doi: 10.1111/ijpo.12246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fletcher JM, Green JC, Neidell MJ. Long term effects of childhood asthma on adult health. J Health Econ. 2010;29(3):377-387. doi: 10.1016/j.jhealeco.2010.03.007 [DOI] [PubMed] [Google Scholar]
- 3.Schyllert C, Andersson M, Backman H, Lindberg A, Rönmark E, Hedman L. Childhood onset asthma is associated with lower educational level in young adults—a prospective cohort study. Respir Med. 2021;186:106514. doi: 10.1016/j.rmed.2021.106514 [DOI] [PubMed] [Google Scholar]
- 4.Krieger N. Theories for social epidemiology in the 21st century: an ecosocial perspective. Int J Epidemiol. 2001;30(4):668-677. doi: 10.1093/ije/30.4.668 [DOI] [PubMed] [Google Scholar]
- 5.Keet CA, McCormack MC, Pollack CE, Peng RD, McGowan E, Matsui EC. Neighborhood poverty, urban residence, race/ethnicity, and asthma: rethinking the inner-city asthma epidemic. J Allergy Clin Immunol. 2015;135(3):655-662. doi: 10.1016/j.jaci.2014.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eldeirawi K, Kunzweiler C, Rosenberg N, et al. Association of neighborhood crime with asthma and asthma morbidity among Mexican American children in Chicago, Illinois. Ann Allergy Asthma Immunol. 2016;117(5):502-507.e1. doi: 10.1016/j.anai.2016.09.429 [DOI] [PubMed] [Google Scholar]
- 7.Wright RJ, Subramanian SV. Advancing a multilevel framework for epidemiologic research on asthma disparities. Chest. 2007;132(5)(suppl):757S-769S. doi: 10.1378/chest.07-1904 [DOI] [PubMed] [Google Scholar]
- 8.Vangeepuram N, Galvez MP, Teitelbaum SL, Brenner B, Wolff MS. The association between parental perception of neighborhood safety and asthma diagnosis in ethnic minority urban children. J Urban Health. 2012;89(5):758-768. doi: 10.1007/s11524-012-9679-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holt EW, Theall KP, Rabito FA. Individual, housing, and neighborhood correlates of asthma among young urban children. J Urban Health. 2013;90(1):116-129. doi: 10.1007/s11524-012-9709-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daelmans B, Darmstadt GL, Lombardi J, et al. ; Lancet Early Childhood Development Series Steering Committee . Early childhood development: the foundation of sustainable development. Lancet. 2017;389(10064):9-11. doi: 10.1016/S0140-6736(16)31659-2 [DOI] [PubMed] [Google Scholar]
- 11.Acevedo-Garcia D, Noelke C, McArdle N, et al. Racial and ethnic inequities in children’s neighborhoods: evidence from the new Child Opportunity Index 2.0. Health Aff (Millwood). 2020;39(10):1693-1701. doi: 10.1377/hlthaff.2020.00735 [DOI] [PubMed] [Google Scholar]
- 12.Flanagan BE, Hallisey EJ, Adams E, Lavery A. Measuring community vulnerability to natural and anthropogenic hazards: the Centers for Disease Control and Prevention’s Social Vulnerability Index. J Environ Health. 2018;80(10):34-36. [PMC free article] [PubMed] [Google Scholar]
- 13.Aris IM, Rifas-Shiman SL, Jimenez MP, et al. Neighborhood child opportunity index and adolescent cardiometabolic risk. Pediatrics. 2021;147(2):e2020018903. doi: 10.1542/peds.2020-018903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aris IM, Perng W, Dabelea D, et al. ; Program Collaborators for Environmental Influences on Child Health Outcomes . Associations of neighborhood opportunity and social vulnerability with trajectories of childhood body mass index and obesity among US children. JAMA Netw Open. 2022;5(12):e2247957. doi: 10.1001/jamanetworkopen.2022.47957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu C-Y, Woo A, Emrich CT, Wang B. Social Vulnerability Index and obesity: an empirical study in the US. Cities. 2020;97:102531. doi: 10.1016/j.cities.2019.102531 [DOI] [Google Scholar]
- 16.Blaisdell CJ, Park C, Hanspal M, et al. ; program collaborators for Environmental influences on Child Health Outcomes . The NIH ECHO program: investigating how early environmental influences affect child health. Pediatr Res. 2022;92(5):1215-1216. doi: 10.1038/s41390-021-01574-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gold DR, Damokosh AI, Dockery DW, Berkey CS. Body-mass index as a predictor of incident asthma in a prospective cohort of children. Pediatr Pulmonol. 2003;36(6):514-521. doi: 10.1002/ppul.10376 [DOI] [PubMed] [Google Scholar]
- 18.Knapp EA, Kress AM, Parker CB, et al. The Environmental Influences on Child Health Outcomes (ECHO)-wide cohort. Am J Epidemiol. 2023;kwad071. doi: 10.1093/aje/kwad071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeWinn KZ, Caretta E, Davis A, Anderson AL, Oken E; program collaborators for Environmental Influences on Child Health Outcomes . SPR perspectives: Environmental Influences on Child Health Outcomes (ECHO) program: overcoming challenges to generate engaged, multidisciplinary science. Pediatr Res. 2022;92(5):1262-1269. doi: 10.1038/s41390-021-01598-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson CC, Chandran A, Havstad S, et al. ; Environmental Influences on Child Health Outcomes (ECHO) collaborators . US childhood asthma incidence rate patterns from the ECHO Consortium to identify high-risk groups for primary prevention. JAMA Pediatr. 2021;175(9):919-927. doi: 10.1001/jamapediatrics.2021.0667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guilbert TW, Mauger DT, Lemanske RF Jr. Childhood asthma-predictive phenotype. J Allergy Clin Immunol Pract. 2014;2(6):664-670. doi: 10.1016/j.jaip.2014.09.010 [DOI] [PubMed] [Google Scholar]
- 22.Flanagin A, Frey T, Christiansen SL; AMA Manual of Style Committee . Updated guidance on the reporting of race and ethnicity in medical and science journals. JAMA. 2021;326(7):621-627. doi: 10.1001/jama.2021.13304 [DOI] [PubMed] [Google Scholar]
- 23.Sullivan K, Thakur N. Structural and social determinants of health in asthma in developed economies: a scoping review of literature published between 2014 and 2019. Curr Allergy Asthma Rep. 2020;20(2):5. doi: 10.1007/s11882-020-0899-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang TS, Gangnon RE, David Page C, et al. Sparse modeling of spatial environmental variables associated with asthma. J Biomed Inform. 2015;53:320-329. doi: 10.1016/j.jbi.2014.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia E, Berhane KT, Islam T, et al. Association of changes in air quality with incident asthma in children in California, 1993-2014. JAMA. 2019;321(19):1906-1915. doi: 10.1001/jama.2019.5357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zacharasiewicz A. Maternal smoking in pregnancy and its influence on childhood asthma. ERJ Open Res. 2016;2(3):00042-2016. doi: 10.1183/23120541.00042-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simons E, To T, Moineddin R, Stieb D, Dell SD. Maternal second-hand smoke exposure in pregnancy is associated with childhood asthma development. J Allergy Clin Immunol Pract. 2014;2(2):201-207. doi: 10.1016/j.jaip.2013.11.014 [DOI] [PubMed] [Google Scholar]
- 28.Rosenquist NA, Richards M, Ferber JR, et al. Prepregnancy body mass index and risk of childhood asthma. Allergy. 2023;78(5):1234-1244. doi: 10.1111/all.15598 [DOI] [PubMed] [Google Scholar]
- 29.Rusconi F, Zugna D, Annesi-Maesano I, et al. Mode of delivery and asthma at school age in 9 European birth cohorts. Am J Epidemiol. 2017;185(6):465-473. doi: 10.1093/aje/kwx021 [DOI] [PubMed] [Google Scholar]
- 30.Sonnenschein-van der Voort AM, Arends LR, de Jongste JC, et al. Preterm birth, infant weight gain, and childhood asthma risk: a meta-analysis of 147,000 European children. J Allergy Clin Immunol. 2014;133(5):1317-1329. doi: 10.1016/j.jaci.2013.12.1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li P, Stuart EA, Allison DB. Multiple imputation: a flexible tool for handling missing data. JAMA. 2015;314(18):1966-1967. doi: 10.1001/jama.2015.15281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esty B, Phipatanakul W. School exposure and asthma. Ann Allergy Asthma Immunol. 2018;120(5):482-487. doi: 10.1016/j.anai.2018.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simons E, Dell SD, Moineddin R, To T. Associations between neighborhood walkability and incident and ongoing asthma in children. Ann Am Thorac Soc. 2018;15(6):728-734. doi: 10.1513/AnnalsATS.201708-693OC [DOI] [PubMed] [Google Scholar]
- 34.Cavaleiro Rufo J, Paciência I, Hoffimann E, Moreira A, Barros H, Ribeiro AI. The neighbourhood natural environment is associated with asthma in children: a birth cohort study. Allergy. 2021;76(1):348-358. doi: 10.1111/all.14493 [DOI] [PubMed] [Google Scholar]
- 35.Zanobetti A, Ryan PH, Coull B, et al. ; Children’s Respiratory and Environmental Workgroup (CREW) Consortium . Childhood asthma incidence, early and persistent wheeze, and neighborhood socioeconomic factors in the ECHO/CREW consortium. JAMA Pediatr. 2022;176(8):759-767. doi: 10.1001/jamapediatrics.2022.1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beck AF, Huang B, Wheeler K, Lawson NR, Kahn RS, Riley CL. The Child Opportunity Index and disparities in pediatric asthma hospitalizations across one Ohio metropolitan area, 2011-2013. J Pediatr. 2017;190:200-206.e1. doi: 10.1016/j.jpeds.2017.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gjelsvik A, Rogers ML, Garro A, et al. Neighborhood risk and hospital use for pediatric asthma, Rhode Island, 2005-2014. Prev Chronic Dis. 2019;16:E68. doi: 10.5888/pcd16.180490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tyris J, Gourishankar A, Ward MC, Kachroo N, Teach SJ, Parikh K. Social determinants of health and at-risk rates for pediatric asthma morbidity. Pediatrics. 2022;150(2):e2021055570. doi: 10.1542/peds.2021-055570 [DOI] [PubMed] [Google Scholar]
- 39.Jimenez MP, Wellenius GA, Subramanian SV, et al. Longitudinal associations of neighborhood socioeconomic status with cardiovascular risk factors: a 46-year follow-up study. Soc Sci Med. 2019;241:112574. doi: 10.1016/j.socscimed.2019.112574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rautava S, Turta O, Vahtera J, et al. Neighborhood socioeconomic disadvantage and childhood body mass index trajectories from birth to 7 years of age. Epidemiology. 2022;33(1):121-130. doi: 10.1097/EDE.0000000000001420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aris IM, Bernard JY, Chen LW, et al. Postnatal height and adiposity gain, childhood blood pressure and prehypertension risk in an Asian birth cohort. Int J Obes (Lond). 2017;41(7):1011-1017. doi: 10.1038/ijo.2017.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colombo J, Gustafson KM, Carlson SE. Critical and sensitive periods in development and nutrition. Ann Nutr Metab. 2019;75(suppl 1):34-42. doi: 10.1159/000508053 [DOI] [PMC free article] [PubMed]
- 43.Zhang X, Tilling K, Martin RM, et al. Analysis of “sensitive” periods of fetal and child growth. Int J Epidemiol. 2019;48(1):116-123. doi: 10.1093/ije/dyy045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mangini LD, Hayward MD, Dong YQ, Forman MR. Household food insecurity is associated with childhood asthma. J Nutr. 2015;145(12):2756-2764. doi: 10.3945/jn.115.215939 [DOI] [PubMed] [Google Scholar]
- 45.McConnell R, Liu F, Wu J, Lurmann F, Peters J, Berhane K. Asthma and school commuting time. J Occup Environ Med. 2010;52(8):827-828. doi: 10.1097/JOM.0b013e3181ebf1a9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Molina-García J, Queralt A, Adams MA, Conway TL, Sallis JF. Neighborhood built environment and socio-economic status in relation to multiple health outcomes in adolescents. Prev Med. 2017;105:88-94. doi: 10.1016/j.ypmed.2017.08.026 [DOI] [PubMed] [Google Scholar]
- 47.Carroll-Scott A, Gilstad-Hayden K, Rosenthal L, et al. Disentangling neighborhood contextual associations with child body mass index, diet, and physical activity: the role of built, socioeconomic, and social environments. Soc Sci Med. 2013;95:106-114. doi: 10.1016/j.socscimed.2013.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu K, Sidell M, Li X, et al. Self-reported physical activity and asthma risk in children. J Allergy Clin Immunol Pract. 2022;10(1):231-239.e3. doi: 10.1016/j.jaip.2021.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willers SM, Wijga AH, Brunekreef B, et al. Childhood diet and asthma and atopy at 8 years of age: the PIAMA birth cohort study. Eur Respir J. 2011;37(5):1060-1067. doi: 10.1183/09031936.00106109 [DOI] [PubMed] [Google Scholar]
- 50.Papamichael MM, Katsardis C, Lambert K, et al. Efficacy of a Mediterranean diet supplemented with fatty fish in ameliorating inflammation in paediatric asthma: a randomised controlled trial. J Hum Nutr Diet. 2019;32(2):185-197. doi: 10.1111/jhn.12609 [DOI] [PubMed] [Google Scholar]
- 51.Khreis H, Kelly C, Tate J, Parslow R, Lucas K, Nieuwenhuijsen M. Exposure to traffic-related air pollution and risk of development of childhood asthma: a systematic review and meta-analysis. Environ Int. 2017;100:1-31. doi: 10.1016/j.envint.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 52.Eijkemans M, Mommers M, Thijs C. Comparison of parent reported physician diagnosed asthma and general practitioner registration. J Asthma. 2023;60(4):673-681. doi: 10.1080/02770903.2022.2087189 [DOI] [PubMed] [Google Scholar]
- 53.Vardavas CI, Hohmann C, Patelarou E, et al. The independent role of prenatal and postnatal exposure to active and passive smoking on the development of early wheeze in children. Eur Respir J. 2016;48(1):115-124. doi: 10.1183/13993003.01016-2015 [DOI] [PubMed] [Google Scholar]
- 54.Nohr EA, Liew Z. How to investigate and adjust for selection bias in cohort studies. Acta Obstet Gynecol Scand. 2018;97(4):407-416. doi: 10.1111/aogs.13319 [DOI] [PubMed] [Google Scholar]
- 55.Pollack CE, Roberts LC, Peng RD, et al. Association of a housing mobility program with childhood asthma symptoms and exacerbations. JAMA. 2023;329(19):1671-1681. doi: 10.1001/jama.2023.6488 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Child Opportunity Index Indicators, Definitions, and Data Sources
eTable 2. Social Vulnerability Index Indicators, Definitions, and Data Sources
eTable 3. Charactersitics of Children Included and Excluded From the Analytic Sample
eTable 4. Participant Characteristics According to Very Low and Very High Child Opportunity Index Categories at Each Life Stage
eTable 5. Participant Characteristics According to Very High and Very Low Social Vulnerability Index Categories at Each Life Stage
eTable 6. Secondary Analyses for the Association of Child Opportunity Index at Different Life Stages With Asthma Incidence
eTable 7. Leave-1-Out Analyses for the Association of Child Opportunity Index at Birth With Asthma Incidence
eTable 8. Leave-1-Out Analyses for the Association of Child Opportunity Index in Infancy With Asthma Incidence
eTable 9. Leave-1-Out Analyses for the Association of Child Opportunity Index in Early Childhood With Asthma Incidence
eFigure 1. Association of Domain-Specific Child Opportunity Index at Birth, Infancy, and Early Childhood With Asthma Incidence
eFigure 2. Association of Domain-Specific Social Vulnerability Index (SVI) at Birth, Infancy, and Early Childhood With Asthma Incidence
eMethods. Description of Child Opportunity Index and Social Vulnerability Index
Nonauthor Collaborators. Environmental Influences on Child Health Outcomes
Data Sharing Statement