Abstract
We report on a severe case of peritonitis due to Staphylococcus lugdunensis. The clinical course resembled an infection due to S. aureus more than one due to other coagulase-negative staphylococci. Therefore, we strongly recommend identification and propose an easy-to-perform procedure for screening of this pathogen.
Staphylococcal peritonitis is an important cause of morbidity in continuous ambulatory peritoneal dialysis (CAPD) patients (8). While coagulase-negative staphylococci (CNS) are the most common cause of peritonitis in CAPD patients, Staphylococcus aureus is the leading cause of more serious infections, often necessitating removal of the catheter (3, 8). S. lugdunensis was first described in 1988 (5) and is isolated mostly as a causative agent of skin and soft-tissue infections (4, 5, 7). The clinical course of infections due to S. lugdunensis is known to resemble that of infections due to S. aureus (7, 14). In recent years, this pathogen has been reported to cause a wide variety of more serious infections, including brain abscess, sepsis, chronic osteomyelitis, and infective endocarditis (5, 7, 11, 14). Only in one report, dealing with the frequency of CNS as causative agents of CAPD-associated peritonitis, was it mentioned that 3 of 127 isolates of CNS derived from 106 episodes of CAPD peritonitis in 46 patients had been identified as S. lugdunensis (12). That report did not detail the clinical findings, diagnosis, therapy, or outcome of the S. lugdunensis-associated cases. Since then, to the best of our knowledge, no further case of peritonitis due to S. lugdunensis has been reported. In the present case, S. lugdunensis was recovered in pure culture from the exit site, the tunnel abscess, and the peritoneal fluid of a CAPD patient soon after catheter implantation. The clinical course required intensive-care observation and therapy.
A 36-year-old man with a diagnosis of end-stage renal disease was admitted to our hospital for implantation of a disconnect catheter allowing therapy by CAPD. Due to rolling up of the catheter inside the peritoneum and subsequent problems with drainage of the peritoneal fluid, CAPD was stopped 7 days after the implantation. The patient developed abdominal pain, fever (39.2°C), and purulent drainage and erythema of the skin at the catheter-epidermal interface. Ultrasound examination of the abdomen showed an abscess approximately 3 cm in diameter within the catheter tunnel. Pathological laboratory findings were as follows: leukocytes, 19,300/μl of peripheral blood and 1,650/μl of peritoneal fluid; C-reactive protein, 0.153 g/liter of serum. For microbiological investigations, peritoneal fluid and abscess material were aspirated and a smear was taken from the exit site. Since Gram staining of all three specimens showed the presence of gram-positive cocci, antibiotic treatment was started with intraperitoneal administration of vancomycin (2 g). Despite this antibiotic treatment, the body temperature further increased to 39.8°C and the cell count in the peritoneal fluid increased to 2,100/μl. Due to this rapid deterioration, the patient had to be admitted to the intensive-care unit. The catheter and the abscess were removed a few hours later by surgery. Peritoneal irrigation of all four peritoneal quadrants was performed, and the antibiotic treatment was switched to intravenous vancomycin (4 × 0.5 g/day) and rifampin (600 mg/day). The postoperative course was uneventful, and hemodialysis was started by means of a Shaldon catheter. Irrigation was stopped after 3 days, and another 6 days later, a Cimono-Brescia shunt was introduced at the right forearm, allowing continuation of hemodialysis. Antibiotic therapy with vancomycin and rifampin was continued for 14 days. Afterwards, the patient was discharged in good condition.
All specimens taken for microbiological investigations yielded pure cultures of CNS. The bacteria were identified as S. lugdunensis by ATB 32 STAPH (bioMérieux, La Balmes-les-Grottes, France), by a negative tube coagulase test using rabbit plasma (bioMérieux), by positive test results for clumping factor using human plasma, and also with the Staphaurex Plus (Murex Diagnostics) and the Pastorex Staphplus (Sanofi Pasteur) agglutination tests. S. lugdunensis was identified according to the criteria of Freney et al. (5) and Hébert (6). All of the S. lugdunensis strains tested yielded identical biochemical reaction patterns in the ATB 32 STAPH system (code 567134700). Disk diffusion testing was performed according to National Committee for Clinical Laboratory Standards performance standards (1) and showed that all of the isolates were susceptible to penicillin, ampicillin, augmentin, cefotiam, cefotaxime, gentamicin, doxycycline, erythromycin, cotrimoxazole, clindamycin, ofloxacin, rifampin, and vancomycin.
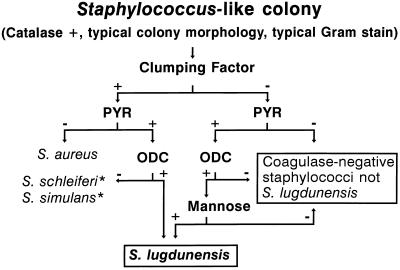
The frequency of a microorganism being found to be responsible for a disease depends heavily on the availability of an easy-to-perform diagnostic method with high discriminatory power. In routine laboratory testing, S. lugdunensis is most often confused with S. aureus or with other CNS (4, 5, 6). Like S. aureus, S. lugdunensis may be clumping factor positive and, in that case, shows a positive reaction with the commonly used agglutination tests for S. aureus, as did our isolates. A positive pyrrolidonyl-arylamidase reaction (PYR), missing utilization of mannitol, a negative tube coagulase test, and ornithine decarboxylation turned out to be discriminative and easy-to-perform tests for presumptive identification of clumping factor-positive S. lugdunensis. Within the group of PYR-positive CNS occurring in humans, i.e., S. haemolyticus, S. schleiferi, S. xylosus, S. simulans, S. intermedius, S. caprae, and S. lugdunensis, the latter is the only species constantly decarboxylating ornithine, for which a test has already been highly recommended for the identification of this species (6). However, in our experience, the sole use of ornithine decarboxylase testing according to the instructions of the manufacturer (Rosco, Taarstrup, Denmark) led to some misidentifications due to weakly positive results in the case of some S. haemolyticus isolates. Fortunately, S. lugdunensis is reported to constantly utilize mannose, whereas S. haemolyticus does not (11). In our routine laboratory, the following screening strategy turned out to be very effective in detecting S. lugdunensis (Fig. 1). Each S. aureus-like isolate (i.e., typical colony morphology on sheep blood agar, typical Gram stain, catalase positive, and clumping factor positive) is also rapidly tested for a PYR (9). In the case of a positive PYR, the respective isolate is further tested for ornithine decarboxylase activity. Each clumping factor-negative staphylococcal isolate is tested for a PYR. In the case of a positive PYR, mannose utilization and ornithine decarboxylase activity are tested for. If necessary, definite identification is achieved by applying ATB 32 STAPH, which has previously been shown to be well suited for identification of S. lugdunensis (2). For S. aureus-S. lugdunensis differentiation, production of acid from mannitol was tested for and a tube coagulase test was performed.
FIG. 1.
Screening scheme for identification of S. lugdunensis. ∗, needs confirmation by additional tests. ODC, ornithine decarboxylase.
Identification of S. lugdunensis in clinical specimens is highly recommended, as the clinical course of infections due to S. lugdunensis is known to resemble that of infections caused by S. aureus (7, 14). Our case, with a severe clinical course and abscess formation within the catheter tunnel, indicates that peritonitis caused by S. lugdunensis most likely resembles peritonitis caused by S. aureus (3), which is often associated with tunnel infection (13). Therefore, we propose the same therapeutic strategy for S. lugdunensis peritonitis as for S. aureus peritonitis. In peritonitis caused by S. aureus, addition of oral rifampin to intraperitoneal vancomycin is recommended for patients who fail to demonstrate clinical improvement in response to vancomycin alone (10). Additionally, the possibility of an underlying tunnel infection has to be taken into account and removal of the catheter has to be reevaluated (10). In view of the need for surgical intervention and the resulting unpredictable abdominal situation, in our case vancomycin was given only once intraperitoneally. Due to the clinical improvement in our patient receiving intravenous vancomycin and rifampin, this therapy was not changed. Up to 6 months after discharge, the patient was still being hemodialyzed and had had no relapse of peritonitis or soft-tissue infection in the region where the tunnel abscess was located.
REFERENCES
- 1.Acar J F, Goldstein F W. Disk susceptibility test. In: Lorian V, editor. Antibiotics in laboratory medicine. 3rd ed.–1991. Baltimore, Md: The Williams & Wilkins Co.; 1991. pp. 17–52. [Google Scholar]
- 2.Brun Y, Bes M, Boeufgras J M, Monget D, Fleurette J, Auckenthaler R, Devriese L A, Kocur M, Marples R R, Piemont Y, Poutrel B, Schumacher-Perdreau F. International collaborative evaluation of the ATB32 staph gallery for identification of the Staphylococcus species. Int J Med Microbiol. 1990;273:319–326. doi: 10.1016/s0934-8840(11)80435-4. [DOI] [PubMed] [Google Scholar]
- 3.Cameron J S. Host defenses in continuous ambulatory peritoneal dialysis and the genesis of peritonitis. Pediatr Nephrol. 1995;9:647–662. doi: 10.1007/BF00860966. [DOI] [PubMed] [Google Scholar]
- 4.Fleurette J, Bes M, Brun Y, Freney J, Forey F, Coulet M, Reverdy M E, Etienne J. Clinical isolates of Staphylococcus lugdunensis and Staphylococcus schleiferi: bacteriological characteristics and susceptibility to antimicrobial agents. Res Microbiol. 1989;140:107–118. doi: 10.1016/0923-2508(89)90044-2. [DOI] [PubMed] [Google Scholar]
- 5.Freney J, Brun Y, Bes M, Meugnier H, Grimont F, Grimont P A D, Nervi C, Fleurette J. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Bacteriol. 1988;38:168–172. [Google Scholar]
- 6.Hébert G A. Hemolysins and other characteristics that help differentiate and biotype Staphylococcus lugdunensis and Staphylococcus schleiferi. J Clin Microbiol. 1990;28:2425–2431. doi: 10.1128/jcm.28.11.2425-2431.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herchline T E, Ayers L W. Occurrence of Staphylococcus lugdunensis in consecutive clinical cultures and relationship of isolation to infection. J Clin Microbiol. 1991;29:419–421. doi: 10.1128/jcm.29.3.419-421.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson C C, Baldessarre J, Levison M E. Peritonitis: update on pathophysiology, clinical manifestations, and management. Clin Infect Dis. 1997;24:1035–1045. doi: 10.1086/513658. [DOI] [PubMed] [Google Scholar]
- 9.Kaufhold A, Lütticken R, Schwien U. Few-minutes tests for the identification of group A streptococci and enterococci with chromogenic substrates. Int J Med Microbiol. 1989;272:191–195. doi: 10.1016/s0934-8840(89)80006-4. [DOI] [PubMed] [Google Scholar]
- 10.Keane W F, Everett E D, Golper T A, Gokal R, Halstenson C, Kawaguchi Y, Riella M, Vas S, Verbrugh H A The Ad Hoc Committee on Peritonitis Management. Peritoneal dialysis-related peritonitis treatment recommendations 1993 update. Peritoneal Dialysis Int. 1993;13:14–28. [PubMed] [Google Scholar]
- 11.Kloos W E, Bannerman T L. Staphylococcus and Micrococcus. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: ASM Press; 1995. pp. 282–298. [Google Scholar]
- 12.Ludlam H, Phillips I. Staphylococcus lugdunensis peritonitis. Lancet. 1989;ii:1394. doi: 10.1016/s0140-6736(89)92001-1. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 13.Piraino B. A review of Staphylococcus aureus exit-site and tunnel infections in peritoneal dialysis patients. Am J Kidney Dis. 1990;16:89–95. doi: 10.1016/s0272-6386(12)80560-9. [DOI] [PubMed] [Google Scholar]
- 14.Vandenesch F, Etienne J, Reverdy M E, Eykyn S J. Endocarditis due to Staphylococcus lugdunensis: report of 11 cases and review. Clin Infect Dis. 1993;17:871–76. doi: 10.1093/clinids/17.5.871. [DOI] [PubMed] [Google Scholar]