Abstract
A 90-day feeding trial was conducted to assess the effects of black soldier fly larvae meal (BSFLM) as a replacement for soybean meal (SM) on growth performance and flesh quality of grass carp. A total of 420 grass carp (299.93 ± 0.85 g) were randomly divided into 7 groups (triplicate) and fed 7 diets with SM substitution of 0% (SM, control), 15% (BSFLM15), 30% (BSFLM30), 45% (BSFLM45), 60% (BSFLM60), 75% (BSFLM75) and 100% (BSFLM100) by BSFLM. The growth performance of grass carp in the BSFLM75 and BSFLM100 groups were significantly lower compared to other groups (P < 0.05). The mid-gut villus height was the lowest in the BSFLM100 group (P < 0.05). Muscle nutritional value was improved due to increased DHA (docosahexaenoic acid), EPA (eicosapentaenoic acid), total HUFA (highly unsaturated fatty acids) and glycine levels, and reached the optimum in the BSFLM100 group (P < 0.05). According to the results of principal component analysis and weight analysis of muscle texture and body color, all the BSFLM diets except BSFLM15 could improve muscle texture and body color and reached the optimum level in the BSFLM100 group. Muscle drip loss and hypoxanthine content were the lowest and muscle antioxidant capacity was the highest in the BSFLM75 group, and water- and salt-soluble protein contents reached the optimum level in the BSFLM60 group (P < 0.05). Dietary BSFLM significantly reduced muscle fiber area and diameter, and increased muscle fiber density and the proportion of small fiber (diameter <20 μm) (P < 0.05). Additionally, sarcomere lengths in the BSFLM75 and BSFLM100 groups were significantly higher than that in the SM group (P < 0.05). The mRNA relative expression levels of MyoD, Myf5, MyHC and FGF6b were remarkably up-regulated at an appropriate dietary BSFLM level (P < 0.05). In conclusion, BSFLM could replace up to 60% SM without an adverse effect on growth performance and improve the flesh quality of grass carp. The optimum levels of dietary BSFLM were 71.0 and 69.1 g/kg diet based on the final body weight and feed conversion ratio. The flesh quality was optimal when dietary SM was completely replaced with BSFLM (227 g/kg diet).
Keywords: Grass carp, Black soldier fly larvae meal, Soybean meal, Growth performance, Flesh quality
1. Introduction
Aquaculture is expanding globally, with aquaculture production reaching 122.6 million tonnes in 2020 (FAO, 2022). In the future, the aquaculture market will continue to grow. This means that enough aquatic feed needs to be produced to meet the market demand, which also puts forward higher requirements for protein sources. Traditionally, this demand was met through fish meal (FM) and plant protein sources. The main source of plant protein in aquatic feed is soybean meal (SM). SM is most widely used in the compound feed fed to herbivorous fish, such as grass carp. However, due to the rising price of SM, the use of SM has been limited in recent years and the supply of SM will continue to be limited (Hardy, 2010; Santos and Naval, 2020). In addition, the deficiencies of unbalanced amino acid distribution, intestinal inflammation, low palatability and anti-nutritional factors have been found in SM (Cheng et al., 2013; Merrifield et al., 2011; Shiu et al., 2013), which restrict its addition level in aquatic feed formulation. Furthermore, with the competing demands of animal husbandry and human consumption for soybean products, the contradiction of grain competition is becoming increasingly serious (Suloma et al., 2014). Therefore, there is an urgent demand for the development of suitable non-grain protein substitute resources.
Insects have the advantages of abundant resources, utilization of organic waste, high feed conversion rate and low utilization rate of water and land, thus could be used to solve the problems of protein deficiency and environmental deterioration (Meneguz et al., 2018). Black soldier fly (Hermetia illucens, BSF) a saprophagous insect belonging to the class Insecta, order Diptera, family Stratiomyidae, and genus Hermetia Latreille, is widely distributed throughout the world. It has the advantages of a flexible diet, low production cost, rapid reproduction and a high biological conversion rate, and the adult insects are not considered pests. BSF larvae can effectively convert organic waste into biomass that is rich in protein and fat and can be produced as feed for a variety of species, biodiesel and chitin (Diener et al., 2009). It is reported that the crude protein content of defatted BSF can reach as high as 56.9%, which is slightly lower than that of FM and close to or even slightly higher than that of SM (Makkar et al., 2014). BSF has the pattern of essential amino acid similar to that of FM (Henry et al., 2015), and comparable with SM, and the content of some essential amino acids (including lysine and methionine) is higher than that of SM (Makkar et al., 2014; Wang et al., 2019). At present, the potential application value of BSF had already been evaluated in rainbow trout (Oncorhynchus mykiss) (Huyben et al., 2019), Atlantic salmon (Salmo salar) (Li et al., 2020), Siberian sturgeon (Acip baerii Brandt) (Caimi et al., 2020), European sea bass (Dicentrarchus labrax) (Abdel-Tawwab et al., 2020), Jian carp (Cyprinus carpio var. Jian) (Li et al., 2017), juvenile grass carp (Ctenopharyngodon idellus) (Lu et al., 2020) and other fish species. These studies have shown that BSF can be used as a protein source in aquaculture animal feed. Additionally, changes in fish diet can affect fish flesh quality (Rincón et al., 2016). Flesh quality is generally described using 4 terms: security (hygienic quality), healthiness (nutritional quality), satisfaction (color, texture, and juiciness as well as flavor), and serviceability (ease of use, processability, and price) (Anne et al., 2016). Previous studies have shown that the replacement of FM with insect protein in aquatic feeds causes changes in the chemical composition of fish muscle, especially in the lipid content and fatty acid profile (Lock et al., 2015; Sealey et al., 2011).
At present, the study of the flesh quality evaluation of BSF as a protein source in aquaculture animals is limited, with most research focusing on rainbow trout (Borgogno et al., 2016; Bruni et al., 2020; Secci et al., 2019; Mancini et al., 2018). Borgogno et al. (2016) first found that diets with BSF inclusion significantly affected the muscle sensory profile of rainbow trout. In the follow-up study of rainbow trout, it was observed that diets with BSF did not affect the proximate composition, pH, shear stress or water holding capacity (WHC) of muscle or body color, while the addition of BSF to feed increased the content of saturated fatty acid (SFA) and reduced the concentration of n-3 polyunsaturated fatty acids (PUFA) and adenosine monophosphate (AMP). In addition, muscle yellowness (b∗) under the effect of BSF also was reduced (Bruni et al., 2020; Secci et al., 2019; Mancini et al., 2018). These results indicate that the substitution of BSF for FM in aquatic animal feed will affect the muscle quality of fish. However, there is a lack of research on the flesh quality of aquaculture animals when SM is replaced with BSF.
Grass carp (C. idellus) is an important fresh water aquaculture species. Grass carp production reached 5.76 million tonnes in 2021 with the highest yield of any aquaculture fish species in the world (FAO, 2022). This species has many advantages, including its high nutritional value, ease of cultivation, fast growth rate, high feed utilization rate and relatively low price (Wang et al., 2018). At present, there are no reports on the evaluation on flesh quality of large grass carp when SM is replaced with BSF larvae meal (BSFLM). Consequently, the present aimed to evaluate the effects of dietary SM replacement with BSFLM on the growth performance, antioxidant capacity, tissue structure, and flesh quality of large grass carp. This study also laid a foundation for the next step in exploring the optimal utilization strategy of BSFLM so as to promote the development of BSFLM as an aquaculture feed.
2. Materials and methods
2.1. Animal ethics statement
The experimental design and procedures of the presented research have been approved by the Committee on Ethics of Animal Experiments of Northwest A&F University (NWAFU-DKXC-20200602).
2.2. Experimental diets
BSFLM and SM were used as the main protein sources in the experimental diets. The BSFLM (with partial degreasing) was provided by the Buluputing Biotechnology Co., Ltd. (Xi'an, Shaanxi, China), and the remaining ingredients were purchased from Huaqin Husbandry and Technology Co., Ltd. (Yangling, Shaanxi, China). Seven isonitrogenous and isolipidic experimental diets with approximately 290 g/kg crude protein and 50 g/kg crude lipid were prepared by replacing 0% (SM), 15% (BSFLM15), 30% (BSFLM30), 45% (BSFLM45), 60% (BSFLM60), 75% (BSFLM75) and 100% (BSFLM100) of SM with BSFLM, respectively. The formulas and proximate composition of the diets are shown in Table 1, and the amino acid and fatty acid compositions of the diets and ingredients are shown in Table 2 and Table 3, respectively. All ingredients were fully mixed according to the diet formula and soybean oil was then added and mixed according to the formula proportion. The appropriate amount of water was then added to the mixture. Finally, the mixture was formed into 4 mm diameter particles using a pelletiser (Type 52), dried at 60 °C, and stored at −20 °C until use.
Table 1.
Ingredients and proximate compositions of SM, BSFLM and experimental diets.
| Item | SM | BSFLM | Diets |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SM | BSFLM15 | BSFLM30 | BSFLM45 | BSFLM60 | BSFLM75 | BSFLM100 | ||||
| Ingredients, g/kg DM basis | ||||||||||
| SM | 240.00 | 204.00 | 168.00 | 132.00 | 96.00 | 60.00 | 0.00 | |||
| BSFLM | 0.00 | 34.00 | 68.00 | 102.00 | 136.00 | 170.00 | 227.00 | |||
| Casein | 168.30 | 168.30 | 168.30 | 168.30 | 168.30 | 168.30 | 168.30 | |||
| Corn starch | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | |||
| Wheat flour | 300.00 | 300.00 | 300.00 | 300.00 | 300.00 | 300.00 | 300.00 | |||
| Soybean oil | 48.60 | 43.50 | 38.30 | 33.10 | 28.00 | 22.80 | 14.20 | |||
| Choline chloride (60%) | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | |||
| Premix1 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | |||
| Ethoxyquin | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | |||
| Calcium phosphate primary | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | |||
| Carboxyl methyl cellulose | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | |||
| Cellulose | 87.60 | 94.70 | 101.90 | 109.00 | 116.10 | 123.20 | 135.00 | |||
| Proximate composition2, g/kg air dry basis | ||||||||||
| Crude protein | 460.50 | 486.80 | 294.40 | 291.30 | 300.10 | 295.30 | 295.40 | 298.50 | 296.20 | |
| Crude lipid | 5.70 | 157.80 | 49.50 | 48.40 | 49.60 | 48.30 | 46.60 | 46.70 | 46.30 | |
| Moisture | 123.00 | 48.70 | 68.00 | 64.50 | 72.10 | 77.40 | 71.10 | 72.80 | 66.30 | |
| Crude ash | 73.20 | 65.40 | 43.50 | 42.50 | 42.30 | 43.40 | 42.90 | 42.10 | 43.60 | |
BSFLM = black soldier fly larvae meal; SM = soybean meal.
Premix contained 0.5% vitamin, 0.5% mineral and 1% limestone carrier and provided the following per kilogram of diet: vitamin A, 4000 IU; vitamin D3, 800 IU; vitamin E, 50 IU; vitamin B1, 2.5 mg; vitamin B2, 9 mg; vitamin B6, 10 mg; vitamin C, 250 mg; nicotinic acid, 40 mg; pantothenic acid calcium, 30 mg; biotin, 100 μg; betaine, 1000 mg; Fe, 140 mg; Cu, 2.5 mg; Zn, 65 mg; Mn, 19 mg; Mg, 230 mg; Co, 0.1 mg; I, 0.25 mg; Se, 0.2 mg.
Values are reported as the mean values of duplicated analyses.
Table 2.
Amino acid composition of SM, BSFLM and experimental diets (g/100 g DM basis)1.
| Amino acids | SM | BSFLM | Diets |
||||||
|---|---|---|---|---|---|---|---|---|---|
| SM | BSFLM15 | BSFLM30 | BSFLM45 | BSFLM60 | BSFLM75 | BSFLM100 | |||
| EAA | |||||||||
| Thr | 1.88 | 1.76 | 1.23 | 1.15 | 1.15 | 1.15 | 1.14 | 1.12 | 1.16 |
| Val | 2.01 | 2.51 | 1.50 | 1.53 | 1.51 | 1.48 | 1.47 | 1.52 | 1.57 |
| Met | 0.43 | 0.96 | 0.38 | 0.41 | 0.40 | 0.43 | 0.46 | 0.47 | 0.45 |
| Ile | 1.95 | 1.91 | 1.44 | 1.41 | 1.38 | 1.33 | 1.31 | 1.33 | 1.30 |
| Leu | 3.35 | 3.01 | 2.51 | 2.40 | 2.39 | 2.34 | 2.32 | 2.31 | 2.29 |
| Phe | 2.30 | 1.65 | 1.56 | 1.52 | 1.49 | 1.47 | 1.44 | 1.41 | 1.41 |
| Lys | 2.81 | 3.11 | 1.94 | 1.89 | 1.89 | 1.89 | 1.86 | 1.86 | 1.87 |
| His | 1.18 | 1.40 | 0.84 | 0.85 | 0.83 | 0.82 | 0.82 | 0.82 | 0.81 |
| Arg | 3.13 | 2.33 | 1.87 | 1.73 | 1.69 | 1.63 | 1.61 | 1.55 | 1.46 |
| NEAA | |||||||||
| Asp | 5.22 | 4.08 | 2.98 | 2.81 | 2.75 | 2.67 | 2.62 | 2.57 | 2.58 |
| Ser | 2.36 | 1.86 | 1.61 | 1.41 | 1.46 | 1.44 | 1.44 | 1.39 | 1.42 |
| Glu | 8.38 | 5.93 | 6.77 | 6.49 | 6.36 | 6.24 | 6.12 | 6.00 | 5.94 |
| Gly | 1.92 | 2.39 | 1.10 | 1.06 | 1.05 | 1.05 | 1.07 | 1.07 | 1.16 |
| Ala | 1.94 | 3.19 | 1.22 | 1.22 | 1.23 | 1.25 | 1.28 | 1.32 | 1.37 |
| Cys | 0.61 | 0.50 | 0.39 | 0.40 | 0.41 | 0.40 | 0.39 | 0.41 | 0.40 |
| Tyr | 1.37 | 2.82 | 0.97 | 0.96 | 1.00 | 0.96 | 1.04 | 1.07 | 1.01 |
| Pro | 2.43 | 2.79 | 2.31 | 2.22 | 2.20 | 2.26 | 2.26 | 2.25 | 2.29 |
| ∑EAA | 19.04 | 18.64 | 13.27 | 12.89 | 12.73 | 12.54 | 12.43 | 12.39 | 12.32 |
| ∑NEAA | 24.23 | 23.56 | 17.35 | 16.57 | 16.46 | 16.27 | 16.22 | 16.08 | 16.17 |
| ∑EAA:∑NEAA | 0.79 | 0.79 | 0.76 | 0.78 | 0.77 | 0.77 | 0.77 | 0.77 | 0.76 |
| ∑AA | 43.27 | 42.21 | 30.62 | 29.48 | 29.18 | 28.82 | 28.63 | 28.48 | 28.48 |
BSFLM = black soldier fly larvae meal; SM = soybean meal; EAA = essential amino acids; NEAA = nonessential amino acids; ΣEAA = total essential amino acids; ΣNEAA = total nonessential amino acids; ΣAA = total amino acids.
Values are reported as the mean values of duplicated analyses.
Table 3.
Fatty acid composition of SM, BSFLM and experimental diets (% total fatty acids)1.
| Fatty acids | SM | BSFLM | Diets |
||||||
|---|---|---|---|---|---|---|---|---|---|
| SM | BSFLM15 | BSFLM30 | BSFLM45 | BSFLM60 | BSFLM75 | BSFLM100 | |||
| C12:0 | ND | 11.08 | ND | 0.94 | 1.24 | 4.58 | 4.82 | 6.16 | 9.18 |
| C14:0 | 3.00 | 6.32 | 0.60 | 0.31 | 1.15 | 1.14 | 1.64 | 1.61 | 2.69 |
| C16:0 | 23.36 | 24.09 | 13.59 | 13.50 | 14.68 | 15.46 | 15.72 | 16.53 | 17.06 |
| C18:0 | 13.58 | 18.14 | 6.53 | 6.44 | 6.61 | 1.91 | 6.59 | 6.72 | 6.52 |
| ∑SFA | 39.95 | 59.63 | 20.72 | 21.19 | 23.68 | 23.09 | 28.77 | 31.01 | 35.44 |
| C16:1n-7 | 0.56 | 1.53 | 0.25 | 0.11 | 0.26 | 0.40 | 0.24 | 0.15 | 0.36 |
| C18:1n-7 | 5.86 | 9.35 | 1.73 | 1.51 | 1.58 | 1.91 | 1.67 | 1.76 | 2.04 |
| C18:1n-9 | 6.33 | 7.20 | 21.17 | 19.84 | 20.40 | 21.77 | 21.07 | 21.66 | 22.12 |
| C20:1n-9 | 0.00 | 0.06 | 0.01 | 0.05 | 0.06 | 0.11 | 0.10 | 0.12 | 0.14 |
| ∑MUFA | 12.75 | 18.14 | 23.16 | 21.52 | 22.30 | 24.19 | 23.09 | 23.70 | 24.67 |
| C18:2n-6 | 36.88 | 18.49 | 48.38 | 47.79 | 45.75 | 44.42 | 40.25 | 37.73 | 32.93 |
| C18:3n-6 | 0.24 | 0.21 | 0.35 | 0.72 | 0.49 | 0.54 | 0.44 | 0.34 | 0.36 |
| C20:3n-6 | 3.43 | 0.07 | 0.93 | 0.78 | 0.83 | 0.83 | 0.83 | 0.98 | 0.72 |
| ∑n-6 PUFA | 40.55 | 18.77 | 49.65 | 49.29 | 47.07 | 45.79 | 41.52 | 39.05 | 34.01 |
| C18:3n-3 | 6.75 | 2.83 | 6.35 | 7.60 | 6.64 | 6.66 | 6.07 | 5.63 | 5.11 |
| C20:5n-3 | ND | 0.31 | 0.05 | 0.17 | 0.22 | 0.15 | 0.33 | 0.36 | 0.47 |
| C22:5n-3 | ND | 0.20 | 0.06 | 0.16 | 0.03 | 0.03 | 0.15 | 0.14 | 0.12 |
| C22:6n-3 | ND | 0.12 | ND | 0.06 | 0.06 | 0.09 | 0.06 | 0.10 | 0.17 |
| ∑n-3 PUFA | 6.75 | 3.46 | 6.47 | 8.00 | 6.95 | 6.93 | 6.61 | 6.23 | 5.87 |
| ∑PUFA | 47.30 | 22.23 | 56.12 | 57.29 | 54.02 | 52.72 | 48.13 | 45.28 | 39.88 |
| ∑HUFA | 3.43 | 0.69 | 1.04 | 1.18 | 1.14 | 1.10 | 1.38 | 1.59 | 1.48 |
| n-3:n-6 | 0.17 | 0.18 | 0.13 | 0.16 | 0.15 | 0.15 | 0.16 | 0.16 | 0.17 |
BSFLM = black soldier fly larvae meal; SM = soybean meal; ∑SFA = total saturated fatty acids; ∑MUFA = total monounsaturated fatty acid; ∑PUFA = total polyunsaturated fatty acid; ∑HUFA = total highly unsaturated fatty acids; n-3:n-6 = the ratio of ∑n-3 PUFA to ∑n-6 PUFA; ND = not detected.
Values are reported as the mean values of duplicated analyses.
2.3. Feeding and management
The experimental grass carp were obtained from the Ankang Fisheries Experimental and Demonstration Station of Northwest A&F University (Ankang, Shaanxi, China). The feeding trial was conducted in the net cages in an outdoor pond at the Ankang Fisheries Experimental and Demonstration Station. The dimensions of the net cages were 1.5 m × 1.5 m × 1.8 m (length × width × height). The experimental fish were acclimated in the net cages in the outdoor pond and fed a commercial diet (≥300 g/kg crude protein, ≥30 g/kg crude lipid; Huaqin feed factory, Yangling, Shaanxi, China) 3 times a day (08:00, 12:00 and 18:00) for 2 weeks. After the acclimation period, the experimental fish were fasted for 24 h, then 420 grass carp (299.93 ± 0.85 g) with strong physiques and uniform specifications were randomly divided into 7 groups (with each group repeated intriplicate) and fed in 21 net cages, with 20 fish per net cage. During the feeding period, the feeding quantity was 1% to 3% of the body weight of fish for quantitative feeding, 3 times a day (08:00, 12:00 and 18:00). Each cage was equipped with a feeding platform that was used to observe the feeding situation of the experimental fish and prevent the feed from sinking to the bottom and becoming waste. The amount of supplied diet to each cage was recorded daily and remaining feed in the feeding platform was siphoned out 0.5 h after feeding, analyzed for dry matter and subtracted from feed offered (DM basis) to calculate actual feed intake. The feeding trial lasted for 90 days. During the feeding trial, the water temperature was 25 ± 3 °C, the pH ranged from 7.4 to 8.6, NH4+-N < 0.01 mg/L, NO2--N < 0.005 mg/L and dissolved oxygen ranged from 8 to 10 mg/L. The photoperiod was 14 h light to 10 h dark with a light period from 06:00 to 20:00.
2.4. Sampling
At the beginning of the feeding trial, 6 grass carp (approximately 300 g) were randomly sampled and stored at −20 °C until they were used for the initial proximate composition analysis of whole fish. At the end of the 90-day feeding trial, samples were taken after all fish were fasted for 24 h, then all fish captured from each net cage were anesthetized using 50 mg/L MS-222 solution (Sigma, St. Louis, MO, USA). The final body weight and body length of all fish in each net cage were measured and the fish count was recorded. Two fish per cage were stored at −20 °C until the final proximate composition analysis of the whole fish. The colors of the dorsum, lateral line and abdomen of the 3 fish per cage were measured with a 3nh chroma meter (NS800, Shenzhen ThreeNH Technology Co., Ltd., Shenzhen, China). Four fish per cage were randomly selected for dissection. Visceral weight was first recorded, then the muscle was separated, frozen with liquid nitrogen, and stored at −80 °C until it was used for molecular biology and antioxidant indices detection. The left and right dorsal muscles of 3 fish per cage were isolated, and the left muscles were used for the analysis of texture parameters, pH, proximate composition and ultrastructure (samples were fixed in 4% glutaraldehyde solution). The right muscle was used for the determination of color, dripp loss, water-soluble protein, salt-soluble protein, amino acid composition, trimethylamine (TMA), trimethylamine oxide (TMAO) and nucleotides. Muscle, liver and midgut of 4 fish per cage were collected and stored in 4% paraformaldehyde solution at 4 °C until they were used for histological observation. Finally, the weight of the viscera, liver, intraperitoneal fat, spleen and kidney of 4 fish per cage were measured, then muscle was collected and stored at −20 °C until it was used for fatty acid composition analysis.
2.5. The calculations of growth, feed utilization and biological indices
Growth, feed utilization and biological indices were calculated via the following formulae:
Weight gain rate (%) = (Final weight of fish - Initial weight of fish)/Initial weight of fish × 100.
Specific growth rate (%) = [ln (Final weight of fish) - ln (Initial weight of fish)]/Days of feeding × 100.
Feed intake = Total amount of the dry feed consumed.
Weight gain = Final body weight − Initial body weight.
Feed conversion ratio (FCR) = Feed intake/(Final weight of fish − Initial weight of fish).
Protein efficiency ratio (%) = Weight gain/Protein intake × 100.
Protein retention efficiency (%) = Whole body protein gain/Protein intake × 100.
Condition factor (g/cm3) = Body weight/Body length3
Viscera index (%) = Viscera weight/Body weight × 100.
Hepatosomatic index (HSI, %) = Hepatopancreas weight/Body weight × 100.
Spleen indexes (%) = Spleen weight/Body weight × 100.
Intra-peritoneal fat index (%) = Weight of intraperitoneal fat/Body weight × 100.
Kidney index (%) = Kidney weight/Body weight × 100.
2.6. Muscle antioxidant indices
The activities of the total super oxide dismutase (T-SOD) and the content of hydrogen peroxide (H2O2) and malonaldehyde (MDA) in the muscle were measured with their respective kits (A001-1-2, A064-1-1, A003-1-2) (Jian Cheng Biochemical Company, Nanjing, China) according to the manufacturer's instructions. Sample pre-treatment: A certain mass (wt) of muscle stored at −80 °C was weighed, and 0.86% normal saline homogenate was added according to the ratio of 1:9 (wt:vol) to prepare 10% tissue homogenate, which was centrifuged at 1000 to 1800 × g at 4 °C for 15 min, and the supernatant was removed for measurement. The reaction system of xanthine and xanthine oxidase generates superoxide anion free radical, which oxidizes hydroxylamine to form nitrite, which is purple red under the action of chromogenic agent and has the maximum absorption at 550 nm. T-SOD has a specific inhibitory effect on the above process, so the content of nitrite generated is related to the activity of T-SOD. The H2O2 can react with molybdic acid to form a complex, then the amount of H2O2 can be calculated by measuring its production at 405 nm. When the MDA equivalents were determined, samples were mixed with trichloroacetic acid and centrifuged. Then, the thiobarbituric acid was added to the supernatant. The mixture was heated in water at 95 °C for 40 min. MDA forms a red adduct with thiobarbituric acid, which has an absorbance at 532 nm.
2.7. Histological observations and ultrastructure analyses
The liver, muscle and mid-gut samples were fixed in 4% paraformaldehyde solution for 24 h. Muscle samples were fixed in 4% glutaraldehyde solution for 24 h. The subsequent processes were performed according to the methods in the previous articles (Almeida et al., 2008; Wang et al., 2019; Xu et al., 2020). The specific experimental method is provided in the supplementary material.
2.8. Proximate composition, fatty acid composition and amino acid composition analysis
Diets, ingredients (SM and BSFLM), muscle and whole fish were analysed to determine the proximate composition according to the methods recommended by the Association of Official Analytical Chemists (AOAC, 2000). The fatty acid and amino acid compositions of diets, ingredients (SM and BSFLM) and muscle were analysed according to the methods recommended by Tran et al. (2015) and Barroso et al. (2014). The specific experimental method is provided in the supplementary material.
2.9. Muscle texture analysis and shear force determination
The muscle was cut into 1 cm × 1 cm × 1 cm (length × width × height) cubes used for determination of the texture parameters and shear force. The texture parameters and shear force were measured respectively according to the methods described by Hixson et al. (2014) and Iaconisi et al. (2018), respectively, with some modifications to the operational parameters. The specific experimental method is provided in the supplementary material.
2.10. Body and muscle color
Body and muscle color were measured using a CR-400 colorimeter (Konica-Minolta, Japan) and the colors were represented by L∗ (luminance value), a∗ (red value - green value axis), b∗ (yellow - blue axis) and C∗ (saturation value) according to the International Commission on Luminescent Lighting (CIE) standards (Mclaren, 1976). The body color of each fish was measured at 3 points on the dorsum, lateral line and abdomen, while the muscle color was measured at 3 points above the lateral line.
2.11. WHC and pH of muscle
The WHC of muscles can be measured by the dripp loss. Ten grams of the white muscle above the lateral line was taken, then the muscle was strung with a thin thread and hung in a refrigerator at 4 °C. The muscle was covered and sealed with an inflatable plastic bag outside the muscle, and the muscle and the plastic bag did not touch each other. After hanging for 24 h, the water on the muscle surface was wiped and weighed, and the dripp loss of muscle was the percentage of the lost weight in the total muscle weight. The muscle with a certain mass (wt) was weighed, and distilled water was added for homogenate according to the wt to vol ratio of 1:10, and then the homogenate was shaken for 30 min in the shaker, finally pH of the homogenate was measured by using a pH-meter (PH828+, Dongguan Wanchuang Electronic Products Co., Ltd, Guangdong, China) (Fuentes et al., 2010).
2.12. Water- and salt-soluble protein in muscle
According to the method described by Sigholt et al. (2006), water- and salt-soluble protein contents were determined after extraction with phosphate buffer alone or with KCl from the muscle samples. Extraction was at 4 °C. The sample (4 g) was homogenized for 10 s with an Ultra-Turrax (Janke and Kunkel GmbH, Staufen, Germany) in phosphate buffer (0.05 M), pH 7 (80 mL) and centrifuged (8000 × g, 20 min). The supernatant was decanted, and the volume adjusted to 100 mL with phosphate buffer to provide the water-soluble fraction. The precipitate was homogenized (10 s) in KCl (0.6 M) in phosphate buffer (0.05 M) at pH 7 (80 mL) and centrifuged. The supernatant was decanted, and the volume adjusted to 100 mL with KCl-phosphate buffer to provide the salt-soluble fraction. The content of water-soluble and salt-soluble proteins was determined by Coomassie bright blue protein quantitative test kit (A045-2-2, Jian Cheng Biochemical Company, Nanjing, China).
2.13. Trimethylamine oxide and trimethylamine content in muscle
The determination of TMAO and TMA content in muscle was based on the method of Wekell and Barnett (1991) with some modifications. The specific experimental method is shown in supplementary material.
2.14. Content of muscle nucleotides
The muscle nucleotides contents were determined with reference to the method described by Ryder (1985) with some modifications. The specific experimental method is shown in the supplementary material.
2.15. Gene expression analysis in muscle
Total RNA was extracted from muscle and liver tissues via homogenisation in TRNzol reagent (Tiangen, Beijing, China). The extracted RNA was treated with RNase-free DNase (TaKaRa, Dalian, China) to avoid genomic DNA amplification during RTPCR. RNA integrity was checked by electrophoresis on 2% agarose gels before reverse-transcription PCR. The total RNA was reversed into cDNA using the PrimerScript RT reagent kit (TaKaRa, Dalian, P.R. China). Real-time qPCR was performed using a CFX 96 Real-time PCR Detection System (Bio-Rad, Hercules, CA, USA). The total volume of the PCR reaction was 20 μL containing 0.6 μL of each Primer (10 μM), 1 μL of the diluted cDNA, 10 μL of 2× SYBR Premix Ex Taq II (TaKaRa, Dalian, P.R. China) and 7.8 μL of sterilized double-distilled water. The real-time quantitative PCR conditions were the following: denaturing at 95 °C for 30 s and 40 cycles of denaturing at 95 °C for 5 s, annealing at 60 °C for 30 s and extension at 72 °C for 1 min. The melting curve was created after the extension (60 to 95 °C, 30 s, the plate was read every 0.5 °C). According to the mRNA expression stability results of 4 internal reference genes (18SrRNA, β-actin, GAPDH, EF1α) in muscle before quantitative gene expression detection, β-actin mRNA was used as the internal control and it remained stable throughout our study. The primer sequences of genes are shown in Table 4. The relative expression levels of genes were calculated using the 2-△△CT method (Livak and Schmittgen, 2001).
Table 4.
Primers used for real-time quantitative PCR analysis.
| Genes | Forward primer (5′–3′) | Reverse primer (5′–3′) | Accession number |
|---|---|---|---|
| MyoG | TTACGAAGGCGGCGATAACTT | TGGTGAGGAGACATGGACAGA | JQ793897 |
| MyoD | ATGGAGTTGTCGGATATTCCCTTC | GCGGTCAGCGTTGGTTGTT | MG544985 |
| Mrf4 | TCGCTCCTGTATTGATGTTGATGA | GCTCCTGTCTCGCATTCGTT | KT899334 |
| Myf5 | GTGCCTGTGCCTCATCTCCT | AATGCGTGGTTCACCTTCTTCA | GU290227 |
| MyHC | GACGCTCATCACCACCAACC | TGCTCCTCACGCTGCTTCT | EU414733 |
| FGF6a | CGCATACGAGTCTTCCAT | CCTACGAGAACATCCAACA | MK050993 |
| FGF6b | TCCAGTCCGCTTCCGAGTA | AGATGAAACCCGATGCCTACA | MK050992 |
| MSTN | CTGACGCCAAGTTCCACATACA | CGACTCTGCTTCAAGTTCTTCTCT | KP719016 |
| TOR | TCCCACTTTCCACCAACT | ACACCTCCACCTTCTCCA | JX854449 |
| β-Actin | TATGTTGGTGACGAGGCTCA | GCAGCTCGTTGTAGAAGGTG | M25013 |
MyoG = myogenin; MyoD = myoblast determination protein; Mrf4 = myogenic regulatory factor 4; Myf5 = myogenic factor 5; MyHC = myosin heavy chain; FGF6a = fibroblast growth factor 6a; FGF6b = fibroblast growth factor 6b; MSTN = myostatin; TOR = target of rapamycin.
2.16. Statistical analysis
All data are presented as mean ± standard deviations (mean ± SD) and were tested for homogeneity of variance using Levene's test. All data were evaluated by using SPSS 19.0 software for one-way analysis of variance and Duncan's post-hoc test. Orthogonal polynomial contrasts were used to determine the type of regression analysis (Wei et al., 2019). A quadratic equation was used to calculate the optimum BSFLM level for growth and feed efficiency. R2 (0.7 ≤ R2 ≤ 1) indicated a good fit of the regression equation to the data (Xu et al., 2018). In addition, with the help of SPSS 19.0 statistical analysis software, principal component analysis (PCA) was performed on the data results of texture parameters, body and muscle colors using Covariance matrix. To explain the effects of different contents of BSFLM in diet on the quality of grass carp. Difference was considered significant at P < 0.05. Next, PCA was used to evaluate the above indicators of each experimental group. The graded weighted principal component analysis of muscle texture parameters and body color with reference to the method described by Guo et al. (2018). The brief steps: On the basis of PCA results, the principal component weight function was constructed by using the score of principal component, eigenvalue and variance contribution rate, and then the principal component comprehensive weight function was constructed. Finally, the comprehensive weight of the evaluation indicators of each experimental group was calculated, and then the overall ranking of the evaluation indicators was carried out.
3. Results
3.1. Growth, feed utilization and biological indices
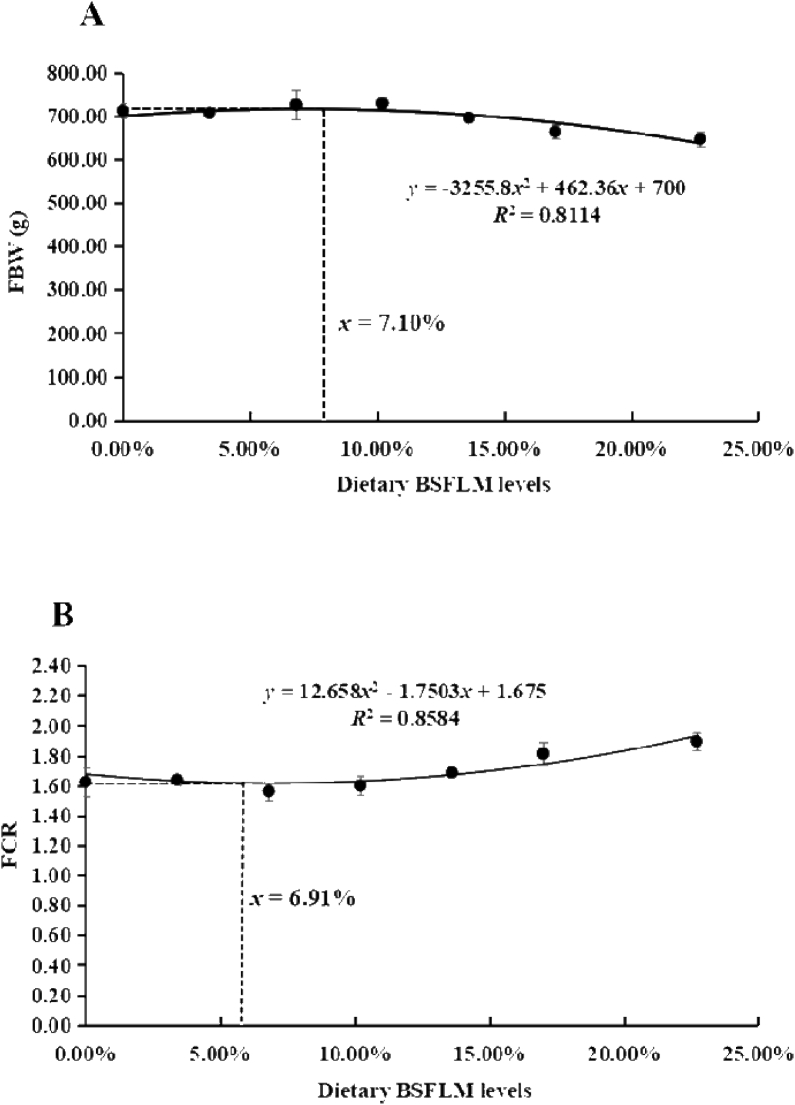
Table 5 shows the growth, feed utilization and biological indices of fish fed diets with graded levels of BSFLM. The final body weight (FBW), weight gain, weight gain rate, specific growth rate, protein efficiency ratio and protein retention efficiency of the BSFLM75 and BSFLM100 groups were significantly lower compared to the SM group (P < 0.05), and the FCR showed the opposite trend (P < 0.05). There was no significant difference in the feed intake, condition factor, viscera index, intraperitoneal fat index, kidney index and spleen index among all groups (P > 0.05). Compared with the SM group, the HSI values in the BSFLM60, BSFLM75 and BSFLM100 groups were significantly increased (P < 0.05). FBW and FCR showed quadratic responses to dietary BSFLM levels (YFBW = −3255.8x2 + 462.36x + 700, R2 = 0.8114; YFCR = 12.658x2 – 1.7503x + 1.675, R2 = 0.8584). Through quadratic regression analysis, it was found that the BSFLM levels in diets corresponding to the maximum FBW was 71.0 g/kg diet and the minimum FCR was 69.1 g/kg diet (Fig. 1).
Table 5.
Effects of BSFLM on the growth, feed utilization and biological indices of fish1.
| Item | Diets |
P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| SM | BSFLM15 | BSFLM30 | BSFLM45 | BSFLM60 | BSFLM75 | BSFLM100 | ||
| IBW, g | 299.78 ± 0.49 | 299.73 ± 0.88 | 299.95 ± 0.68 | 300.58 ± 1.46 | 299.25 ± 0.87 | 299.80 ± 0.97 | 300.42 ± 0.38 | 0.88 |
| FBW, g | 712.29 ± 17.67a | 706.99 ± 6.68a | 726.38 ± 33.89a | 729.41 ± 14.71a | 696.08 ± 6.12a | 663.91 ± 16.65b | 647.06 ± 17.89b | 0.00 |
| FI, g | 669.51 ± 31.46 | 667.38 ± 21.84 | 662.51 ± 15.61 | 685.04 ± 7.35 | 669.50 ± 7.73 | 659.42 ± 6.97 | 656.22 ± 11.98 | 0.51 |
| FCR | 1.62 ± 0.09bc | 1.64 ± 0.04bc | 1.56 ± 0.06c | 1.60 ± 0.06bc | 1.69 ± 0.03b | 1.81 ± 0.07a | 1.90 ± 0.06a | 0.00 |
| WG, g | 412.50 ± 17.65a | 407.26 ± 5.80a | 426.43 ± 33.97a | 428.83 ± 15.03a | 396.83 ± 6.97a | 364.11 ± 17.44b | 346.64 ± 17.79b | 0.00 |
| WGR, % | 137.60 ± 5.89a | 135.87 ± 1.54a | 142.17 ± 11.41a | 142.67 ± 5.24a | 132.61 ± 2.71a | 121.46 ± 6.16b | 115.39 ± 5.89b | 0.00 |
| SGR, %/d | 1.07 ± 0.03a | 1.06 ± 0.01a | 1.09 ± 0.06a | 1.09 ± 0.03a | 1.04 ± 0.01a | 0.98 ± 0.03b | 0.95 ± 0.03b | 0.00 |
| CF, g/cm3 | 2.07 ± 0.10 | 2.05 ± 0.02 | 2.08 ± 0.04 | 2.04 ± 0.03 | 2.12 ± 0.04 | 2.03 ± 0.19 | 2.08 ± 0.08 | 0.89 |
| VSI, % | 8.16 ± 0.34 | 8.73 ± 0.57 | 8.69 ± 0.48 | 8.65 ± 0.09 | 8.61 ± 0.45 | 8.85 ± 0.06 | 8.56 ± 0.74 | 0.65 |
| HSI, % | 2.62 ± 0.26b | 2.97 ± 0.09ab | 2.92 ± 0.27ab | 2.85 ± 0.22ab | 3.08 ± 0.18a | 3.01 ± 0.19a | 3.00 ± 0.09a | 0.04 |
| IFI, % | 2.02 ± 0.51 | 2.17 ± 0.40 | 2.17 ± 0.45 | 1.89 ± 0.25 | 2.15 ± 0.06 | 2.15 ± 0.40 | 2.38 ± 0.30 | 0.78 |
| KI, % | 0.42 ± 0.04 | 0.44 ± 0.02 | 0.44 ± 0.03 | 0.48 ± 0.03 | 0.43 ± 0.06 | 0.41 ± 0.02 | 0.46 ± 0.03 | 0.33 |
| SI, % | 0.21 ± 0.02 | 0.24 ± 0.03 | 0.23 ± 0.03 | 0.23 ± 0.01 | 0.24 ± 0.01 | 0.26 ± 0.05 | 0.21 ± 0.02 | 0.45 |
| PER, % | 199.94 ± 6.95a | 195.78 ± 5.95a | 197.43 ± 10.40a | 202.75 ± 6.17a | 194.6 ± 1.76a | 180.44 ± 8.84b | 173.77 ± 4.53b | 0.00 |
| PRE, % | 37.07 ± 1.71a | 36.97 ± 0.61a | 37.99 ± 1.34a | 38.11 ± 1.49a | 36.09 ± 0.17a | 33.18 ± 0.68b | 32.81 ± 1.09b | 0.00 |
BSFLM = black soldier fly larvae meal; SM = soybean meal; IBW = initial body weight; FBW = final body weight; FI = feed intake; FCR = feed conversion ratio; WG = weight gain; WGR = weight gain rate; SGR = specific growth rate; CF = condition factor; VSI = viscera index; HSI = hepatosomatic index; IFI = intraperitoneal fat index; KI = kidney index; SI = spleen index; PER = protein efficiency ratio; PRE = protein retention efficiency.
Values are mean ± SD (n = 3). Values with different superscripts in the same row are significantly different (P < 0.05) from each other.
Fig. 1.
Regression analysis of black soldier fly larvae meal (BSFLM) levels on growth performance of fish fed different experimental diets (mean ± SD, n = 3). (A) The quadratic regression analysis between the final body weight (FBW) and dietary BSFLM levels. (B) The quadratic regression analysis between the FCR and dietary BSFLM levels.
3.2. Antioxidant indexes of muscle
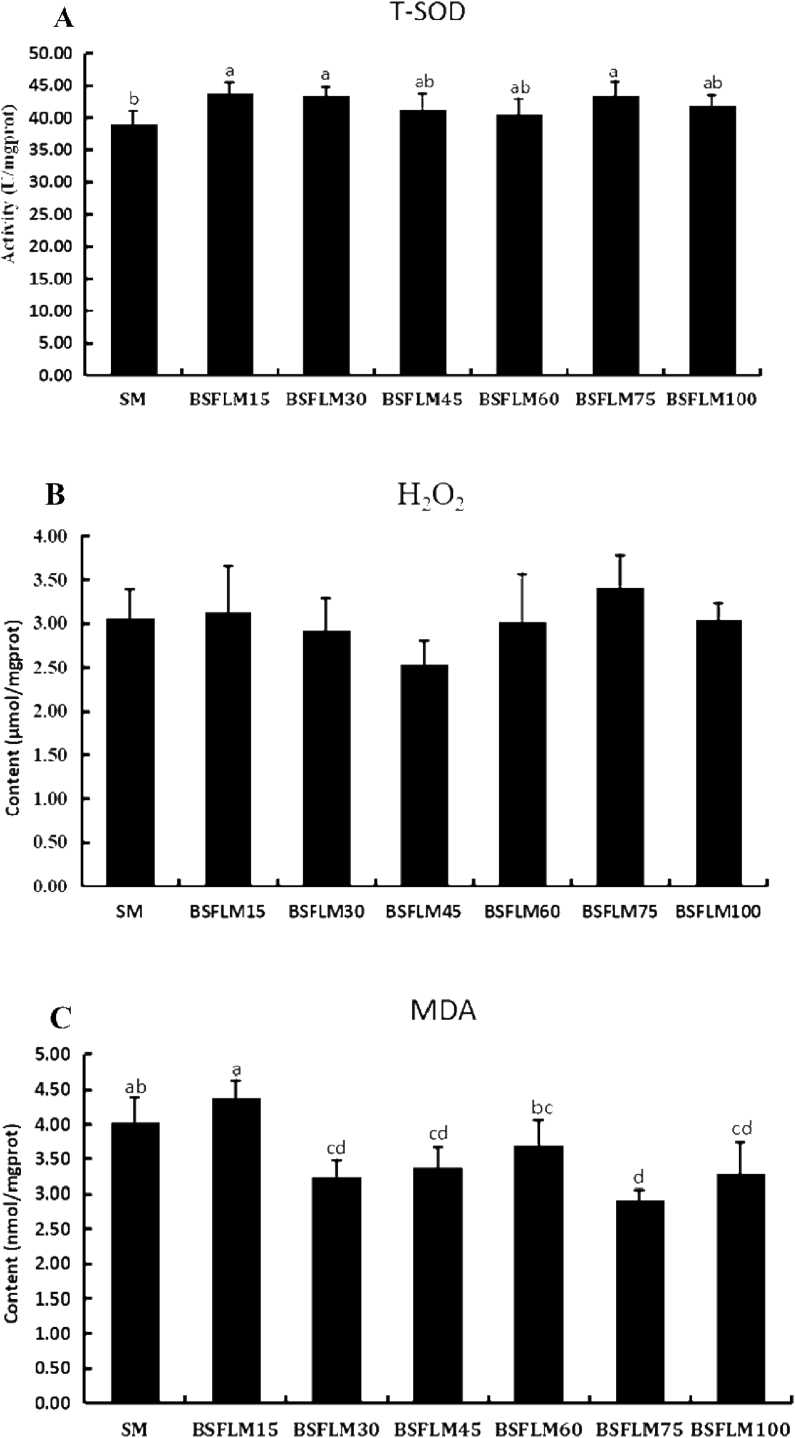
The antioxidant indexes in the muscle of fish fed diets with graded levels of BSFLM are shown in Fig. 2. Compared with the SM group, the H2O2 contents of muscle in the BSFLM groups were not notably different (P > 0.05) (Fig. 2B). However, the BSFLM30, BSFLM45, BSFLM75 and BSFLM100 groups had lower MDA content than that of the SM group (P < 0.05) (Fig. 2C). The T-SOD activity in the BSFLM15, BSFLM30 and BSFLM75 groups were significantly higher than that of the SM group (P < 0.05) (Fig. 2A).
Fig. 2.
Effect of BSFLM on antioxidant indexes in the muscle of fish fed different experimental diets (mean ± SD, n = 3). SM, BSFLM15, BSFLM30, BSFLM45, BSFLM60, BSFLM75 and BSFLM100 represent diets in which BSFLM replaced SM levels of 0%, 15%, 30%, 45%, 60%, 75% and 100% respectively. BSFLM = black soldier fly larvae meal; SM = soybean meal. (A) The total super oxide dismutase (T-SOD) activity in the muscle of fish fed different experimental diets (P = 0.04). (B) The hydrogen peroxide (H2O2) content in the muscle of fish fed different experimental diets (P = 0.28). (C) The malonaldehyde (MDA) content in the muscle of fish fed different experimental diets (P = 0.00). Values marked with different letters above the bars indicate significant difference (P < 0.05) from each other.
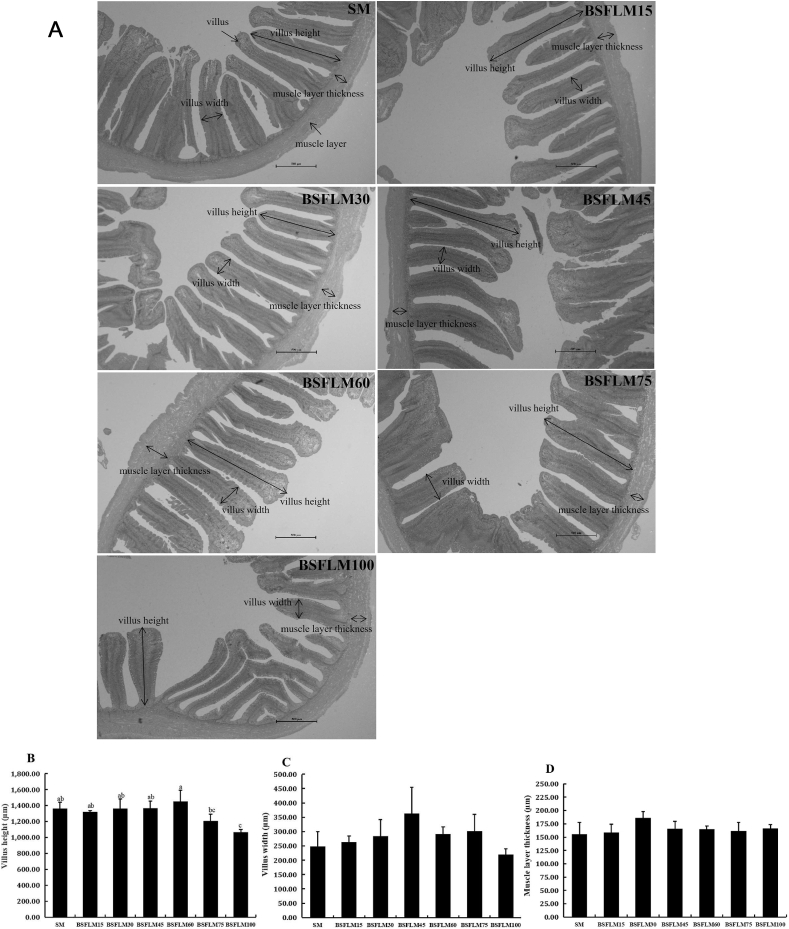
3.3. Histomorphology of liver and mid-gut
The hepatocytes in all experimental groups were polygonal in shape and had centrally located nuclei and clear cell boundaries (Fig. 3). The SM, BSFLM30 and BSFLM45 groups had little debris of villus, but in shape of villus were regular in all groups (Fig. 4A). The mid-gut villus height in the BSFLM100 group was significantly lower than that of the SM group (P < 0.05) (Fig. 4B), which may be one of the inducers of lower growth and feed utilization. In addition, villus width and muscle layer thickness were not significantly affected by dietary BSFLM level (P > 0.05) (Fig. 4C and D).
Fig. 3.
Histomorphology images in the liver of fish fed different experimental diets (Hematoxylin-eosin staining, magnification 100× ). The structures pointed by the one-way arrows are the hepatocyte, cell nucleus and boundary. SM, BSFLM15, BSFLM30, BSFLM45, BSFLM60, BSFLM75 and BSFLM100 represent diets in which BSFLM replaced SM levels of 0%, 15%, 30%, 45%, 60%, 75% and 100% respectively. BSFLM = black soldier fly larvae meal; SM = soybean meal.
Fig. 4.
Hematoxylin-eosin staining analysis of the mid-gut of fish fed different experimental diets. SM, BSFLM15, BSFLM30, BSFLM45, BSFLM60, BSFLM75 and BSFLM100 represent diets in which BSFLM replaced SM levels of 0%, 15%, 30%, 45%, 60%, 75% and 100% respectively. BSFLM = black soldier fly larvae meal; SM = soybean meal. (A) Representative images (magnification 40× , scale bar = 500 μm), the structure pointed by the one-way arrows are the villus and muscle layer respectively, the distance between the two-way arrows indicate are the villus height, villus width and muscle layer thickness respectively. (B) Villus height in the mid-gut of fish fed different experimental diets (P = 0.00). (C) Villus width in the mid-gut of fish fed different experimental diets (P = 0.10). (D) Muscle layer thickness in the mid-gut of fish fed different experimental diets (P = 0.25). Values are means ± SD (n = 3) marked with different letters above the bars indicate significant difference (P < 0.05) from each other.
3.4. Proximate compositions of whole body and muscle
Proximate compositions of the whole body and the muscle of fish fed diets with graded levels of BSFLM are shown in Table 6. The whole-body crude protein content in the BSFLM30 group was significantly increased compared to the SM group (P < 0.05), but the other proximate composition contents of the whole body and muscle in all BSFLM groups exhibited no significant differences compared to the SM group (P > 0.05).
Table 6.
Effect of BSFLM on proximate compositions in the muscle and whole body of fish (% wet basis)1.
| Item | Diets |
P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| SM | BSFLM15 | BSFLM30 | BSFLM45 | BSFLM60 | BSFLM75 | BSFLM100 | ||
| Muscle | ||||||||
| Moisture | 77.21 ± 0.07 | 77.36 ± 0.07 | 77.13 ± 0.04 | 77.39 ± 0.02 | 76.87 ± 0.06 | 77.28 ± 0.05 | 77.35 ± 0.01 | 0.79 |
| Crude protein | 19.26 ± 0.60 | 19.44 ± 0.17 | 19.27 ± 0.06 | 19.12 ± 0.24 | 19.20 ± 0.88 | 19.43 ± 0.55 | 19.10 ± 0.57 | 0.97 |
| Crude lipid | 1.43 ± 0.27 | 1.43 ± 0.40 | 1.29 ± 0.08 | 1.48 ± 0.11 | 1.67 ± 0.21 | 1.48 ± 0.12 | 1.20 ± 0.10 | 0.25 |
| Ash | 1.21 ± 0.07 | 1.28 ± 0.07 | 1.33 ± 0.04 | 1.24 ± 0.02 | 1.31 ± 0.06 | 1.23 ± 0.05 | 1.28 ± 0.01 | 0.10 |
| Whole body | ||||||||
| Moisture | 71.05 ± 0.78 | 70.34 ± 0.15 | 70.13 ± 0.20 | 70.42 ± 1.52 | 70.03 ± 1.32 | 70.93 ± 0.46 | 71.30 ± 0.35 | 0.46 |
| Crude protein | 16.51 ± 0.19b | 16.67 ± 0.17ab | 16.90 ± 0.16a | 16.70 ± 0.12ab | 16.41 ± 0.27b | 16.33 ± 0.21b | 16.52 ± 0.23b | 0.04 |
| Crude lipid | 8.31 ± 0.51 | 8.87 ± 0.25 | 9.24 ± 0.17 | 8.99 ± 1.49 | 9.20 ± 1.48 | 8.50 ± 0.69 | 8.19 ± 0.49 | 0.66 |
| Ash | 3.44 ± 0.22 | 3.31 ± 0.09 | 3.37 ± 0.04 | 3.28 ± 0.26 | 3.17 ± 0.09 | 3.28 ± 0.06 | 3.15 ± 0.15 | 0.30 |
BSFLM = black soldier fly larvae meal; SM = soybean meal.
Values are mean ± SD (n = 3). Values with different superscript letters in the same row are significantly different (P < 0.05) from each other.
3.5. Amino acid and fatty acid composition of muscle
The amino acid and fatty acid composition of the muscle of fish fed diets with graded levels of BSFLM are shown in Table 7 and Table 8, respectively. Amino acid compositions of muscle were not significantly influenced by dietary amino acid profile (Table 2), only the glycine (Gly) content of the BSFLM100 group was significantly higher compared to the other experimental groups (P < 0.05), while the other amino acid contents of all BSFLM groups exhibited no significant differences compared to the SM group (P > 0.05). The fatty acid compositions of muscle were clearly influenced by the dietary fatty acid profile (Table 3). Compared with the SM group, the C12:0 and C16:1n-7 contents in all BSFLM groups and the C20:1n-9 contents in the remaining BSFLM groups except the BSFLM45 group were significantly increased (P < 0.05), and the C14:0 contents in the BSFLM75 and BSFLM100 groups and the C16:0 content in the BSFLM100 group were significantly increased (P < 0.05). The contents of C18:2n-6 and C18:3n-6 gradually decreased with the increment of BSFLM inclusion in diets. Meanwhile, the content of C20:3n-6 in the remaining the BSFLM groups except the BSFLM75 group were significantly lower compared to the SM group and C22:4n-6 contents of the BSFLM30, BSFLM60 and BSFLM100 groups were significantly higher compared to the SM group (P < 0.05). The content of C20:5n-3 significantly increased with increasing dietary BSFLM levels (P < 0.05), while the C18:3n-3 content exhibited the opposite trend, and the C22:6n-3 content was the highest in the BSFLM100 group (P < 0.05). Furthermore, the content of the total n-6 PUFA significantly decreased with the increment of BSFLM inclusion in diets (P < 0.05), while the contents of total n-3 PUFA and highly unsaturated fatty acids (HUFA) showed the reverse trend and reached the highest level in the BSFLM100 group (P < 0.05), this also led to the significant trend of gradual increase in the n-3:n-6 value (P < 0.05).
Table 7.
Effect of BSFLM on amino acid composition in the muscle of fish (g/100 g wet basis) 1.
| Amino acids |
Diets |
P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| SM | BSFLM15 | BSFLM30 | BSFLM45 | BSFLM60 | BSFLM75 | BSFLM100 | ||
| EAA | ||||||||
| Thr | 0.91 ± 0.01 | 0.88 ± 0.05 | 0.88 ± 0.06 | 0.88 ± 0.06 | 0.90 ± 0.01 | 0.88 ± 0.04 | 0.93 ± 0.05 | 0.69 |
| Val | 0.90 ± 0.09 | 0.91 ± 0.03 | 0.90 ± 0.02 | 0.89 ± 0.03 | 0.89 ± 0.06 | 0.90 ± 0.07 | 0.96 ± 0.05 | 0.69 |
| Met | 0.57 ± 0.01 | 0.55 ± 0.02 | 0.56 ± 0.04 | 0.56 ± 0.03 | 0.56 ± 0.01 | 0.55 ± 0.02 | 0.59 ± 0.03 | 0.35 |
| Ile | 0.87 ± 0.03 | 0.87 ± 0.02 | 0.87 ± 0.03 | 0.86 ± 0.04 | 0.86 ± 0.01 | 0.87 ± 0.07 | 0.91 ± 0.03 | 0.67 |
| Leu | 1.61 ± 0.04 | 1.56 ± 0.06 | 1.57 ± 0.08 | 1.56 ± 0.09 | 1.57 ± 0.01 | 1.56 ± 0.07 | 1.65 ± 0.06 | 0.49 |
| Phe | 0.84 ± 0.02 | 0.81 ± 0.02 | 0.81 ± 0.04 | 0.80 ± 0.06 | 0.83 ± 0.02 | 0.82 ± 0.04 | 0.85 ± 0.04 | 0.58 |
| Lys | 1.97 ± 0.02 | 1.87 ± 0.10 | 1.92 ± 0.08 | 1.91 ± 0.09 | 1.91 ± 0.01 | 1.89 ± 0.08 | 1.99 ± 0.07 | 0.42 |
| His | 0.75 ± 0.03 | 0.73 ± 0.04 | 0.75 ± 0.03 | 0.72 ± 0.02 | 0.73 ± 0.05 | 0.70 ± 0.04 | 0.74 ± 0.05 | 0.61 |
| Arg | 1.22 ± 0.04 | 1.18 ± 0.09 | 1.21 ± 0.04 | 1.21 ± 0.06 | 1.21 ± 0.00 | 1.16 ± 0.06 | 1.26 ± 0.06 | 0.42 |
| NEAA | ||||||||
| Asp | 2.10 ± 0.02 | 2.04 ± 0.08 | 2.08 ± 0.11 | 2.07 ± 0.12 | 2.11 ± 0.03 | 2.04 ± 0.08 | 2.16 ± 0.12 | 0.63 |
| Ser | 0.83 ± 0.01 | 0.80 ± 0.05 | 0.79 ± 0.08 | 0.79 ± 0.07 | 0.83 ± 0.02 | 0.79 ± 0.03 | 0.83 ± 0.08 | 0.85 |
| Glu | 3.13 ± 0.06 | 3.01 ± 0.16 | 3.06 ± 0.15 | 3.08 ± 0.13 | 3.07 ± 0.01 | 2.99 ± 0.13 | 3.24 ± 0.13 | 0.30 |
| Gly | 0.90 ± 0.02b | 0.87 ± 0.05b | 0.87 ± 0.02b | 0.90 ± 0.04b | 0.92 ± 0.03b | 0.89 ± 0.04b | 1.00 ± 0.07a | 0.02 |
| Ala | 1.20 ± 0.01 | 1.14 ± 0.05 | 1.18 ± 0.05 | 1.17 ± 0.06 | 1.19 ± 0.02 | 1.16 ± 0.05 | 1.24 ± 0.06 | 0.23 |
| Cys | 0.13 ± 0.09 | 0.14 ± 0.12 | 0.19 ± 0.13 | 0.18 ± 0.12 | 0.05 ± 0.02 | 0.06 ± 0.03 | 0.13 ± 0.11 | 0.56 |
| Tyr | 0.74 ± 0.05 | 0.71 ± 0.02 | 0.69 ± 0.09 | 0.69 ± 0.09 | 0.75 ± 0.04 | 0.74 ± 0.04 | 0.75 ± 0.07 | 0.66 |
| Pro | 0.67 ± 0.03 | 0.67 ± 0.07 | 0.68 ± 0.06 | 0.72 ± 0.03 | 0.65 ± 0.02 | 0.64 ± 0.07 | 0.75 ± 0.04 | 0.18 |
| ∑EAA | 9.64 ± 0.21 | 9.35 ± 0.39 | 9.49 ± 0.38 | 9.38 ± 0.43 | 9.46 ± 0.14 | 9.32 ± 0.46 | 9.88 ± 0.35 | 0.49 |
| ∑NEAA | 9.69 ± 0.12 | 9.37 ± 0.49 | 9.53 ± 0.37 | 9.61 ± 0.34 | 9.58 ± 0.13 | 9.30 ± 0.40 | 10.09 ± 0.37 | 0.20 |
| ∑EAA:∑NEAA | 0.99 ± 0.01 | 1.00 ± 0.01 | 1.00 ± 0.01 | 0.98 ± 0.01 | 0.99 ± 0.01 | 1.00 ± 0.01 | 0.98 ± 0.01 | 0.14 |
| ∑EAA:∑AA | 0.50 ± 0.02 | 0.50 ± 0.03 | 0.50 ± 0.01 | 0.49 ± 0.02 | 0.50 ± 0.01 | 0.50 ± 0.01 | 0.49 ± 0.02 | 0.26 |
| ∑AA | 19.33 ± 0.29 | 18.73 ± 0.87 | 19.00 ± 0.72 | 18.97 ± 0.76 | 19.07 ± 0.25 | 18.63 ± 0.83 | 19.97 ± 0.70 | 0.32 |
BSFLM = black soldier fly larvae meal; SM = soybean meal; Thr = threomine; Val = valine; Met = methionine; Ile = isoleucine; Leu = leucine; Phe = phenylalanine; Lys = lysine; His = histidine; Arg = arginine; Asp = aspartic acid; Ser = serine; Glu = glutamate; Gly = glycine; Ala = alanine; Cys = cystine; Tyr = tyrosine; Pro = proline; EAA = essential amino acids; NEAA = nonessential amino acids; ΣEAA = total essential amino acids; ΣNEAA = total nonessential amino acids; ΣAA = total amino acids.
Values are mean ± SD (n = 3). Values with different superscript letters in the same row are significantly different (P < 0.05) from each other.
Table 8.
Effect of BSFLM on fatty acid composition in the muscle of fish (% total fatty acids)1.
| Fatty acids | Diets |
P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| SM | BSFLM15 | BSFLM30 | BSFLM45 | BSFLM60 | BSFLM75 | BSFLM100 | ||
| C12:0 | 1.25 ± 0.17d | 1.78 ± 0.29c | 2.13 ± 0.21bc | 2.21 ± 0.08bc | 3.49 ± 0.21a | 3.24 ± 0.36a | 2.53 ± 0.29b | 0.00 |
| C14:0 | 1.60 ± 0.25bc | 1.54 ± 0.33c | 1.59 ± 0.15bc | 1.91 ± 0.21abc | 2.02 ± 0.25ab | 2.27 ± 0.26a | 2.16 ± 0.21a | 0.01 |
| C16:0 | 21.11 ± 0.73bc | 20.40 ± 0.44c | 20.20 ± 0.46c | 22.22 ± 1.11ab | 21.30 ± 0.38abc | 22.02 ± 0.30ab | 22.45 ± 0.64a | 0.00 |
| C18:0 | 6.21 ± 0.74 | 5.22 ± 0.50 | 6.03 ± 0.89 | 5.59 ± 0.19 | 5.95 ± 0.41 | 5.81 ± 0.54 | 5.29 ± 0.43 | 0.32 |
| ∑SFA | 28.39 ± 3.85ab | 28.83 ± 1.27ab | 29.52 ± 0.42b | 32.34 ± 2.18ab | 32.70 ± 0.42a | 33.46 ± 0.37a | 32.45 ± 1.25ab | 0.02 |
| C16:1n-7 | 5.97 ± 0.32b | 7.16 ± 0.56a | 7.18 ± 0.50a | 7.35 ± 0.27a | 6.84 ± 0.30a | 7.54 ± 0.20a | 7.29 ± 0.29a | 0.00 |
| C18:1n-7 | 2.88 ± 1.06 | 2.03 ± 0.15 | 2.64 ± 0.50 | 2.73 ± 0.86 | 3.06 ± 1.15 | 2.75 ± 0.81 | 2.43 ± 0.75 | 0.80 |
| C18:1n-9 | 31.12 ± 2.40 | 33.65 ± 0.84 | 31.14 ± 2.84 | 32.54 ± 0.16 | 31.64 ± 2.91 | 31.42 ± 2.84 | 31.78 ± 2.51 | 0.82 |
| C20:1n-9 | 0.72 ± 0.12d | 0.92 ± 0.04c | 1.04 ± 0.04bc | 0.69 ± 0.10d | 1.08 ± 0.04b | 1.09 ± 0.05b | 1.26 ± 0.10a | 0.00 |
| ∑MUFA | 40.70 ± 1.47 | 43.75 ± 1.21 | 42.00 ± 2.85 | 43.31 ± 1.12 | 42.62 ± 1.70 | 42.80 ± 2.31 | 42.77 ± 2.07 | 0.59 |
| C18:2n-6 | 15.33 ± 1.10ab | 16.30 ± 0.59a | 14.25 ± 0.54b | 11.77 ± 1.50c | 11.73 ± 0.57c | 11.37 ± 0.74c | 10.41 ± 1.39c | 0.00 |
| C18:3n-6 | 0.28 ± 0.07a | 0.19 ± 0.03ab | 0.24 ± 0.04a | 0.21 ± 0.08a | 0.19 ± 0.05ab | 0.11 ± 0.03bc | 0.07 ± 0.03c | 0.00 |
| C20:2n-6 | 0.86 ± 0.20 | 0.51 ± 0.42 | 0.69 ± 0.43 | 0.70 ± 0.53 | 0.71 ± 0.12 | 0.87 ± 0.30 | 1.19 ± 0.44 | 0.48 |
| C20:3n-6 | 2.79 ± 0.36a | 1.51 ± 0.15c | 1.88 ± 0.24bc | 2.00 ± 0.15bc | 2.24 ± 0.36b | 2.39 ± 0.36ab | 2.02 ± 0.26bc | 0.00 |
| C20:4n-6 | 3.97 ± 0.47 | 3.47 ± 0.54 | 4.21 ± 0.43 | 3.69 ± 0.26 | 3.32 ± 0.38 | 3.36 ± 0.50 | 4.20 ± 0.38 | 0.09 |
| C22:4n-6 | 1.72 ± 0.19b | 1.65 ± 0.19b | 2.30 ± 0.25a | 1.93 ± 0.33ab | 2.18 ± 0.07a | 1.98 ± 0.19ab | 2.27 ± 0.19a | 0.01 |
| ∑n-6 PUFA | 24.94 ± 1.03a | 23.63 ± 1.59a | 23.57 ± 0.64a | 20.30 ± 1.78b | 20.37 ± 0.20b | 20.09 ± 1.82b | 20.17 ± 1.53b | 0.00 |
| C18:3n-3 | 1.74 ± 0.23a | 1.49 ± 0.06ab | 1.40 ± 0.05ab | 1.45 ± 0.14ab | 1.48 ± 0.04ab | 1.13 ± 0.28b | 1.14 ± 0.28b | 0.01 |
| C20:5n-3 | 0.44 ± 0.11c | 0.62 ± 0.06c | 0.96 ± 0.13ab | 0.99 ± 0.17ab | 0.91 ± 0.12b | 0.92 ± 0.14b | 1.20 ± 0.25a | 0.00 |
| C22:5n-3 | 0.25 ± 0.10 | 0.23 ± 0.06 | 0.25 ± 0.09 | 0.19 ± 0.08 | 0.38 ± 0.13 | 0.17 ± 0.07 | 0.27 ± 0.12 | 0.23 |
| C22:6n-3 | 1.67 ± 0.06b | 1.62 ± 0.26ab | 1.93 ± 0.24ab | 1.82 ± 0.29ab | 1.87 ± 0.22ab | 1.89 ± 0.26ab | 2.15 ± 0.19a | 0.04 |
| ∑n-3 PUFA | 4.11 ± 0.14bc | 3.95 ± 0.36c | 4.54 ± 0.33ab | 4.45 ± 0.28abc | 4.64 ± 0.32ab | 4.32 ± 0.38abc | 4.77 ± 0.17a | 0.04 |
| ∑PUFA | 29.05 ± 3.78 | 27.59 ± 2.63 | 28.11 ± 1.87 | 24.75 ± 4.53 | 25.01 ± 4.16 | 24.41 ± 3.48 | 24.94 ± 3.44 | 0.53 |
| ∑HUFA | 10.78 ± 0.34b | 9.10 ± 1.21c | 11.53 ± 0.45ab | 10.62 ± 0.99b | 10.90 ± 0.20ab | 10.72 ± 0.73b | 12.12 ± 0.03a | 0.00 |
| n-3:n-6 | 0.16 ± 0.01c | 0.17 ± 0.01c | 0.19 ± 0.01bc | 0.22 ± 0.02ab | 0.23 ± 0.02ab | 0.22 ± 0.04ab | 0.24 ± 0.02a | 0.00 |
BSFLM = black soldier fly larvae meal; SM = soybean meal; ∑SFA = total saturated fatty acids; ∑MUFA = total monounsaturated fatty acid; ∑PUFA = total polyunsaturated fatty acid; ∑HUFA = total highly unsaturated fatty acids; n-3:n-6 = the ratio of ∑n-3 PUFA to ∑n-6 PUFA.
Values are mean ± SD (n = 3). Values with different superscript letters in the same row are significantly different (P < 0.05) from each other.
3.6. Texture properties, pH, drip loss, and water- and salt-soluble protein of muscle
The texture properties, pH, drip loss and water- and salt-soluble protein contents of the muscle of fish fed diets with graded levels of BSFLM are shown in Table 9. Compared with the SM group, the adhesiveness of muscle in the BSFLM60, BSFLM75 and BSFLM100 groups were significantly higher (P < 0.05). Meanwhile, the springiness and chewiness of the remaining BSFLM groups, with the exception of the BSFLM15 and BSFLM30 groups were significantly increased compared to the SM group (P < 0.05). However, the hardness, cohesiveness, gumminess and shear force of all BSFLM groups exhibited no significant difference compared to the SM group (P > 0.05). Through PCA, the score plot (Fig. 5A) indicated the overall difference of the texture properties in the muscle of fish fed diets with graded levels of BSFLM. In the score plot, the greater the distance between points, the greater the difference, and the greater the distance between solid circles, the greater the overall difference of the muscle texture characteristics between the experimental groups. Therefore, we could see that PCA can effectively distinguish the remaining BSFLM groups with the exception of the BSFLM15 group from the SM group based on the score plot (Fig. 5A). Combined with the eigenvalue and weight analysis of the covariance matrix loading for principal components of the muscle texture properties (Table 10). Muscle texture properties were evaluated according to the total weight rank, it was found that the muscle texture properties of the BSFLM30, BSFLM45, BSFLM60, BSFLM75 and BSFLM100 groups were better than that of the SM group. The muscle pH of the remaining BSFLM groups, except for the BSFLM30 group, were significantly higher than that of the SM group (P < 0.05, Table 9). The drip loss of muscle in the BSFLM75 group was lower compared to the SM group (P < 0.05). The water-soluble protein content of muscle in the remaining BSFLM groups, except for the BSFLM75 and BSFLM100 groups, were significantly decreased compared with the SM group (P < 0.05). Additionally, the BSFLM60 and BSFLM75 groups had higher salt-soluble protein content in all groups (P < 0.05).
Table 9.
Effects of BSFLM on the texture properties, pH, drip loss, and water- and salt-soluble protein in the muscle of fish1.
| Item | Diets |
P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| SM | BSFLM15 | BSFLM30 | BSFLM45 | BSFLM60 | BSFLM75 | BSFLM100 | ||
| Texture properties | ||||||||
| Hardness, N | 19.41 ± 3.05 | 16.89 ± 1.44 | 17.95 ± 1.95 | 18.99 ± 2.99 | 18.60 ± 1.32 | 17.90 ± 2.38 | 18.43 ± 3.97 | 0.93 |
| Adhesiveness, N·mm | 0.121 ± 0.027c | 0.108 ± 0.013c | 0.117 ± 0.011c | 0.123 ± 0.011c | 0.161 ± 0.029b | 0.183 ± 0.023b | 0.250 ± 0.022a | 0.00 |
| Cohesiveness ratio | 0.151 ± 0.014ab | 0.136 ± 0.013b | 0.159 ± 0.012ab | 0.168 ± 0.012a | 0.167 ± 0.015a | 0.174 ± 0.006a | 0.176 ± 0.020a | 0.03 |
| Springiness, mm | 2.22 ± 0.16c | 2.29 ± 0.16c | 2.46 ± 0.18bc | 2.68 ± 0.28ab | 2.79 ± 0.13a | 2.71 ± 0.12ab | 2.73 ± 0.17ab | 0.01 |
| Gumminess, N | 2.61 ± 0.25 | 2.38 ± 0.38 | 2.91 ± 0.23 | 3.02 ± 0.42 | 3.09 ± 0.12 | 3.07 ± 0.47 | 3.19 ± 0.45 | 0.13 |
| Chewiness, mJ | 5.87 ± 0.42b | 5.56 ± 1.16b | 7.24 ± 1.04ab | 8.27 ± 1.78a | 8.69 ± 0.68a | 8.34 ± 1.41a | 8.76 ± 1.53a | 0.02 |
| Shear force, N | 9.19 ± 0.64 | 7.70 ± 1.12 | 7.48 ± 0.72 | 9.36 ± 1.19 | 7.75 ± 1.04 | 7.99 ± 1.61 | 8.05 ± 1.92 | 0.42 |
| pH | 6.47 ± 0.07c | 6.56 ± 0.04ab | 6.51 ± 0.04bc | 6.56 ± 0.04ab | 6.56 ± 0.06ab | 6.59 ± 0.07a | 6.54 ± 0.07ab | 0.03 |
| Drip loss, % | 2.34 ± 0.23ab | 2.34 ± 0.25ab | 2.52 ± 0.04a | 2.42 ± 0.11ab | 2.28 ± 0.14abc | 2.02 ± 0.05c | 2.16 ± 0.15bc | 0.03 |
| Water-soluble protein, mg/g wet basis | 58.07 ± 2.38a | 54.23 ± 1.08b | 53.69 ± 1.00b | 52.76 ± 2.91b | 53.73 ± 1.03b | 55.34 ± 1.48ab | 55.78 ± 2.96ab | 0.04 |
| Salt-soluble protein, mg/g wet basis | 35.85 ± 3.72b | 37.68 ± 1.95b | 36.24 ± 4.13b | 35.21 ± 1.64b | 45.17 ± 2.03a | 45.07 ± 3.23a | 38.23 ± 2.54b | 0.00 |
BSFLM = black soldier fly larvae meal; SM = soybean meal.
Values are mean ± SD (n = 3). Values with different superscript letters in the same row are significantly different (P < 0.05) from each other.
Fig. 5.
PCA score plot and loading plot for effect of BSFLM on muscle texture properties of fish. SM, BSFLM15, BSFLM30, BSFLM45, BSFLM60, BSFLM75 and BSFLM100 represent diets in which BSFLM replaced SM levels of 0%, 15%, 30%, 45%, 60%, 75% and 100% respectively. BSFLM = black soldier fly larvae meal; SM = soybean meal. PCA score plot and loading plot were generated based on texture properties variables (Table 9) obtained by analyzing the texture properties in the muscle of fish under different BSFLM levels treatment. (A) Score plot of the first 2 principal components (PCA1 and PCA2) of muscle texture properties. Solid circle represents samples from different BSFLM treatments used in the analysis are indicated in different color. (B) Loading plot used to construct the principal components from muscle texture properties (Table 9).
Table 10.
Eigenvalue and weight analysis of the covariance matrix loading for principal components of muscle texture properties.
| Item | PCA1 | PCA2 | Weight on PCA1 | Weight on PCA2 | Total weight | Total weight rank (no.)1,2 |
|---|---|---|---|---|---|---|
| Diets | ||||||
| SM | −0.90 | −0.55 | −0.86 | −0.13 | −0.99 | 6 |
| BSFLM15 | −1.40 | −0.24 | −1.34 | −0.06 | −1.40 | 7 |
| BSFLM30 | −0.31 | −0.29 | −0.29 | −0.07 | −0.36 | 5 |
| BSFLM45 | 0.33 | −0.26 | 0.32 | −0.06 | 0.26 | 4 |
| BSFLM60 | 0.63 | 0.00 | 0.61 | 0.00 | 0.61 | 3 |
| BSFLM75 | 0.60 | 0.53 | 0.58 | 0.12 | 0.70 | 2 |
| BSFLM100 | 1.05 | 0.83 | 1.00 | 0.19 | 1.19 | 1 |
| Eigenvalue | 3.56 | 1.36 | ||||
| Eigenvalue square | 1.89 | 1.17 | ||||
| Variance percentage | 50.78 | 19.43 |
BSFLM = black soldier fly larvae meal; SM = soybean meal.
Total weight = PCA1 × Eigenvalue square × Variance percentage/100 (Weight on PCA1) + PCA2 × Eigenvalue square × Variance percentage/100 (Weight on PCA2).
Numbers represent the grades of muscle texture characteristics in each experimental group in all groups, and the smaller the number, the better the texture characteristics.
3.7. Body and muscle color
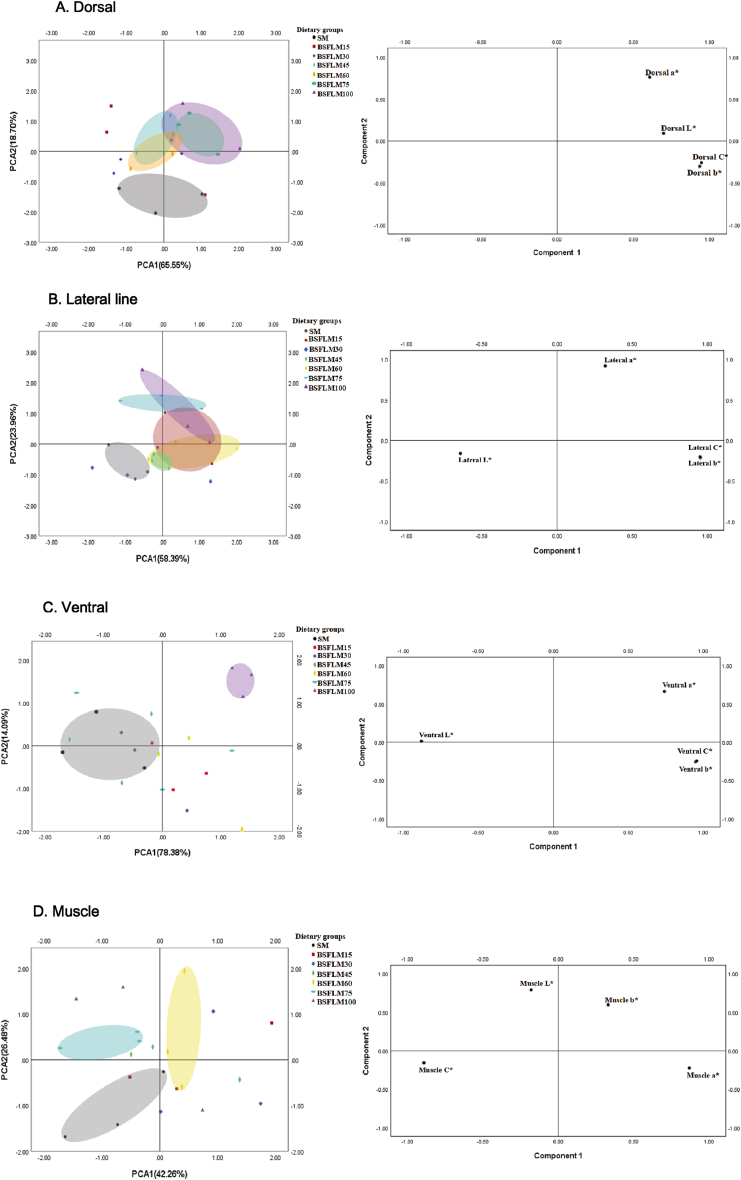
The body and muscle color of fish fed diets with graded levels of BSFLM are shown in Table 11. In terms of dorsal color, L∗, b∗ and C∗ of dorsa exhibited no significant differences among all experimental groups (P > 0.05), but dorsal a∗ in the remaining BSFLM groups except for the BSFLM30 group were significantly higher than that of the SM group (P < 0.05). Through the PCA, we could see that the PCA effectively distinguished the remaining the BSFLM groups except for the BSFLM15 and BSFLM30 groups from the SM group based on the score plot (Fig. 6A, Left). Combined with the eigenvalue and weight analysis of the covariance matrix loading for principal components of the dorsal color (Table 12), it was found that the dorsal color of the BSFLM45, BSFLM60, BSFLM75 and BSFLM100 groups were better than that of the SM group. In the aspect of lateral line color, L∗, b∗ and C∗ of lateral line showed no significant differences among all experimental groups (P > 0.05), but the lateral line a∗ of the BSFLM75 and BSFLM100 groups were significantly higher compared to the SM group (P < 0.05). Through the PCA of the lateral line color, we could see that the PCA can effectively distinguish the remaining the BSFLM groups except for the BSFLM30 group from the SM group based on the score plot (Fig. 6B, Left). Combined with the eigenvalue and weight analysis of the covariance matrix loading for principal components of the lateral line color (Table 12), it was found that the lateral line color of remaining the BSFLM groups except for the BSFLM30 group were better than that of the SM group. In terms of the ventral color, the ventral L∗ was not significantly affected by dietary BSFLM levels (P > 0.05), but the ventral a∗ in the BSFLM100 group was significantly higher than that of the SM group (P < 0.05). Compared with the SM group, both the BSFLM60 and BSFLM100 groups had higher ventral b∗ and C∗ (P < 0.05). Through the PCA of the ventral color, we could see that the PCA can effectively distinguish the BSFLM100 groups from the SM group based on the score plot (Fig. 6C, Left). Combined with the eigenvalue and weight analysis of the covariance matrix loading for principal components of the ventral color (Table 12), it was found that the ventral color of BSFLM100 group was better than that of the SM group. According to the results of muscle color found that the L∗, a∗ and C∗ of all BSFLM groups showed no significant differences compared to the SM group (P > 0.05), but the b∗ of these groups were significantly higher (P < 0.05). Through the PCA of the muscle color, we could see that the PCA effectively distinguished the BSFLM60 and BSFLM75 groups from the SM group based on the score plot (Fig. 6D, Left).
Table 11.
Effects of BSFLM on body and muscle color of fish1.
| Item | Diets |
P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| SM | BSFLM15 | BSFLM30 | BSFLM45 | BSFLM60 | BSFLM75 | BSFLM100 | ||
| Dorsal | ||||||||
| L∗ | 25.21 ± 1.67 | 23.14 ± 1.56 | 24.78 ± 2.60 | 23.67 ± 0.86 | 23.35 ± 2.00 | 26.31 ± 1.38 | 25.22 ± 2.19 | 0.35 |
| a∗ | −1.66 ± 0.29d | −1.06 ± 0.11bc | −1.41 ± 0.27cd | −0.90 ± 0.32ab | −1.00 ± 0.33bc | −0.61 ± 0.05ab | −0.51 ± 0.16a | 0.00 |
| b∗ | 7.74 ± 0.77 | 7.23 ± 1.43 | 7.10 ± 0.58 | 7.52 ± 0.31 | 7.67 ± 0.29 | 8.06 ± 0.69 | 8.25 ± 0.84 | 0.56 |
| C∗ | 7.86 ± 0.80 | 7.31 ± 1.41 | 7.21 ± 0.55 | 7.59 ± 0.29 | 7.74 ± 0.26 | 8.10 ± 0.67 | 8.27 ± 0.80 | 0.62 |
| Lateral line | ||||||||
| L∗ | 60.79 ± 1.75 | 58.35 ± 1.21 | 61.01 ± 1.42 | 60.19 ± 2.64 | 59.31 ± 2.13 | 59.81 ± 0.87 | 59.70 ± 1.44 | 0.56 |
| a∗ | 0.78 ± 0.17bc | 1.13 ± 0.23b | 0.74 ± 0.16c | 0.92 ± 0.22bc | 1.13 ± 0.15b | 1.59 ± 0.13a | 1.54 ± 0.26a | 0.00 |
| b∗ | 13.25 ± 0.51 | 14.08 ± 0.76 | 13.65 ± 1.62 | 13.93 ± 0.26 | 14.51 ± 0.96 | 13.63 ± 1.08 | 14.18 ± 1.19 | 0.78 |
| C∗ | 13.28 ± 0.48 | 14.14 ± 0.75 | 13.78 ± 1.48 | 13.94 ± 0.31 | 14.59 ± 0.94 | 13.71 ± 1.12 | 14.24 ± 1.18 | 0.75 |
| Ventral | ||||||||
| L∗ | 77.82 ± 1.94 | 76.77 ± 1.00 | 76.74 ± 0.25 | 77.35 ± 1.53 | 76.14 ± 1.02 | 77.39 ± 1.14 | 75.47 ± 0.29 | 0.28 |
| a∗ | −0.70 ± 0.20b | −0.41 ± 0.11b | −0.60 ± 0.05b | −0.66 ± 0.31b | −0.36 ± 0.19b | −0.33 ± 0.37b | 0.57 ± 0.08a | 0.00 |
| b∗ | 12.21 ± 0.31b | 13.39 ± 0.37ab | 12.85 ± 0.75ab | 12.31 ± 0.40b | 13.62 ± 0.70a | 13.08 ± 1.29ab | 13.80 ± 0.19a | 0.04 |
| C∗ | 12.18 ± 0.38b | 13.45 ± 0.34ab | 12.88 ± 0.77ab | 12.34 ± 0.39b | 13.66 ± 0.74a | 13.09 ± 1.28ab | 13.85 ± 0.21a | 0.04 |
| Muscle | ||||||||
| L∗ | 39.40 ± 0.55 | 39.75 ± 0.17 | 39.15 ± 1.17 | 39.32 ± 0.62 | 39.66 ± 1.49 | 39.74 ± 0.04 | 39.37 ± 1.62 | 0.98 |
| a∗ | −3.89 ± 0.13ab | −3.77 ± 0.08ab | −3.63 ± 0.13a | −3.77 ± 0.13ab | −3.81 ± 0.12ab | −4.05 ± 0.20b | −4.03 ± 0.22b | 0.04 |
| b∗ | −1.59 ± 0.28c | −1.09 ± 0.33b | −0.74 ± 0.08ab | −0.64 ± 0.17a | −0.55 ± 0.31a | −0.78 ± 0.22ab | −0.41 ± 0.05a | 0.00 |
| C∗ | 4.14 ± 0.30 | 3.65 ± 0.57 | 3.83 ± 0.34 | 4.01 ± 0.35 | 3.89 ± 0.05 | 4.13 ± 0.10 | 4.06 ± 0.21 | 0.50 |
BSFLM = black soldier fly larvae meal; SM = soybean meal; L∗ = luminance value; a∗ = red value; b∗ = yellow value; C∗ = chroma value.
Values are mean ± SD (n = 3). Values with different superscript letters in the same row are significantly different (P < 0.05) from each other.
Fig. 6.
PCA score plot and loading plot for effects of BSFLM on body and muscle color of fish. SM, BSFLM15, BSFLM30, BSFLM45, BSFLM60, BSFLM75 and BSFLM100 represent diets in which BSFLM replaced SM levels of 0%, 15%, 30%, 45%, 60%, 75% and 100% respectively. BSFLM = black soldier fly larvae meal; SM = soybean meal. PCA score plot and loading plot were generated based on color variables (Table 11) obtained by analyzing the color in the body and muscle of fish under different BSFLM levels treatment. (A) Score plot (Left) and loading plot (Right) of dorsal color of fish fed different experimental diets. (B) Score plot (Left) and loading plot (Right) of lateral line color of fish fed different experimental diets. (C) Score plot (Left) and loading plot (Right) of ventral color of fish fed different experimental diets. (D) Score plot (Left) and loading plot (Right) of muscle color of fish fed different experimental diets. The left part of Fig. 6: Score plot of the first 2 principal components (PCA1 and PCA2) of color of dorsal, lateral line, ventral and muscle. Solid circle represents samples from different BSFLM treatments used in the analysis are indicated in different colors (Only the BSFLM groups that could completely separate from the control samples were circled). The right part of Fig. 6: Loading plot used to construct the principal components from color of dorsal, lateral line, ventral and muscle (Table 11).
Table 12.
Eigenvalue and weight analysis of the covariance matrix loading for principal components of body color.
| Item | PCA1 | PCA2 | Weight on PCA1 | Weight on PCA2 | Total weight | Total weight rank (no.)1,2 |
|---|---|---|---|---|---|---|
| Dorsal | ||||||
| SM | −0.90 | −0.55 | −0.86 | −0.13 | −0.99 | 6 |
| BSFLM15 | −1.40 | −0.24 | −1.34 | −0.06 | −1.40 | 7 |
| BSFLM30 | −0.31 | −0.29 | −0.29 | −0.07 | −0.36 | 5 |
| BSFLM45 | 0.33 | −0.26 | 0.32 | −0.06 | 0.26 | 4 |
| BSFLM60 | 0.63 | 0.00 | 0.61 | 0.00 | 0.61 | 3 |
| BSFLM75 | 0.60 | 0.53 | 0.58 | 0.12 | 0.70 | 2 |
| BSFLM100 | 1.05 | 0.83 | 1.00 | 0.19 | 1.19 | 1 |
| Eigenvalue | 3.56 | 1.36 | ||||
| Eigenvalue square | 1.89 | 1.17 | ||||
| Variance percentage | 50.78 | 19.43 | ||||
| Lateral line | ||||||
| SM | −0.87 | −0.69 | −0.77 | −0.16 | −0.94 | 7 |
| BSFLM15 | 0.42 | 0.09 | 0.38 | 0.02 | 0.40 | 3 |
| BSFLM30 | −0.52 | −1.01 | −0.46 | −0.24 | −0.70 | 6 |
| BSFLM45 | −0.12 | −0.56 | −0.11 | −0.13 | −0.24 | 5 |
| BSFLM60 | 0.66 | −0.20 | 0.59 | −0.05 | 0.54 | 2 |
| BSFLM75 | −0.04 | 1.37 | −0.04 | 0.32 | 0.29 | 4 |
| BSFLM100 | 0.47 | 1.01 | 0.42 | 0.24 | 0.65 | 1 |
| Eigenvalue | 2.34 | 0.96 | ||||
| Eigenvalue square | 1.53 | 0.98 | ||||
| Variance percentage | 58.39 | 23.96 | ||||
| Ventral | ||||||
| SM | −1.04 | 0.04 | −1.45 | 0.00 | −1.44 | 7 |
| BSFLM15 | 0.25 | −0.54 | 0.35 | −0.06 | 0.30 | 3 |
| BSFLM30 | −0.25 | −0.43 | −0.34 | −0.05 | −0.39 | 5 |
| BSFLM45 | −0.82 | 0.01 | −1.14 | 0.00 | −1.14 | 6 |
| BSFLM60 | 0.58 | −0.65 | 0.81 | −0.07 | 0.74 | 2 |
| BSFLM75 | −0.09 | 0.03 | −0.13 | 0.00 | −0.12 | 4 |
| BSFLM100 | 1.36 | 1.54 | 1.89 | 0.16 | 2.05 | 1 |
| Eigenvalue | 3.14 | 0.56 | ||||
| Eigenvalue square | 1.77 | 0.75 | ||||
| Variance percentage | 78.38 | 14.09 |
BSFLM = black soldier fly larvae meal; SM = soybean meal.
Total weight = PCA1 × Eigenvalue square × Variance percentage/100 (Weight on PCA1) + PCA2 × Eigenvalue square × Variance percentage/100 (Weight on PCA2).
Numbers represent the grades of body color characteristics of each experimental group in all groups, and the smaller the number, the better the color characteristics.
3.8. TMAO, TMA and nucleotide contents of muscle
The TMAO, TMA, and nucleotide contents in the muscle of fish fed diets with graded levels of BSFLM are respectively shown in Table 13. Compared with the SM group, the contents of the TMAO, TMA, adenosine triphosphate, adenosine diphosphate, AMP, inosine monophosphate, inosine and values of K and KI (fish freshness indicator) were not significantly different (P > 0.05). However, the hypoxanthine (Hx) content of the BSFLM75 group was significantly lower than that of the SM group (P < 0.05).
Table 13.
Effect of BSFLM on TMAO, TMA and nucleotide contents in the muscle of fish (μg/g wet basis) 1.
| Item | Diets |
P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| SM | BSFLM15 | BSFLM30 | BSFLM45 | BSFLM60 | BSFLM75 | BSFLM100 | ||
| TMAO | 11.01 ± 0.32 | 10.63 ± 0.68 | 10.62 ± 0.40 | 10.46 ± 0.19 | 11.18 ± 0.50 | 10.71 ± 0.08 | 11.18 ± 0.23 | 0.20 |
| TMA | 2.76 ± 0.02ab | 2.71 ± 0.11b | 2.67 ± 0.05b | 2.63 ± 0.09b | 2.78 ± 0.13ab | 2.70 ± 0.04b | 2.87 ± 0.07a | 0.04 |
| Nucleotide | ||||||||
| ATP | 76.83 ± 15.73 | 101.69 ± 3.97 | 91.30 ± 15.01 | 83.93 ± 23.26 | 97.67 ± 7.82 | 75.03 ± 12.25 | 68.63 ± 9.81 | 0.08 |
| ADP | 68.93 ± 5.40 | 79.09 ± 1.52 | 72.71 ± 15.49 | 69.51 ± 9.28 | 74.28 ± 2.36 | 66.02 ± 3.63 | 61.93 ± 10.24 | 0.29 |
| AMP | 94.70 ± 13.96 | 101.60 ± 8.44 | 101.83 ± 18.81 | 110.42 ± 15.23 | 107.73 ± 9.20 | 108.99 ± 8.15 | 91.67 ± 10.33 | 0.48 |
| IMP | 2861.89 ± 620.28 | 2993.54 ± 514.32 | 3363.75 ± 215.10 | 3024.65 ± 495.45 | 2802.32 ± 452.27 | 3100.33 ± 659.01 | 2707.48 ± 378.67 | 0.74 |
| Ino | 254.95 ± 54.57 | 262.06 ± 20.74 | 238.78 ± 33.66 | 276.83 ± 27.56 | 319.56 ± 32.42 | 262.10 ± 53.42 | 320.49 ± 8.03 | 0.09 |
| Hx | 32.67 ± 2.18a | 32.50 ± 2.50a | 28.33 ± 4.21ab | 29.33 ± 3.79ab | 30.83 ± 2.52ab | 24.40 ± 2.55b | 28.04 ± 4.84ab | 0.04 |
| K value2, % | 8.72 ± 2.72 | 8.42 ± 1.87 | 6.89 ± 1.10 | 8.66 ± 1.60 | 10.39 ± 2.25 | 8.16 ± 1.70 | 10.74 ± 1.42 | 0.25 |
| KI value3, % | 9.42 ± 2.21 | 9.18 ± 2.19 | 7.39 ± 1.12 | 9.36 ± 1.79 | 11.33 ± 2.58 | 8.79 ± 1.56 | 11.53 ± 1.61 | 0.19 |
BSFLM = black soldier fly larvae meal; TMAO = trimethylamine oxide; TMA = trimethylamine; SM = soybean meal; ATP = adenosine triphosphate; ADP = adenosine diphosphate; AMP = adenosine monophosphate; IMP = inosine monophosphate; Ino = inosine; Hx = hypoxanthine.
Values are mean ± SD (n = 3). Values with different superscript letters in the same row are significantly different (P < 0.05) from each other.
Fish freshness indicator, K value represents the ratio (%) of the total amount of Ino and Hx to that of ATP-related compounds. The specific definition is referred to Hamada-Sato et al. (2005).
The simplified form of K value of fish freshness indicator and represented as the ratio (%) of total amount of Ino and Hx to that of IMP, Ino and Hx. The specific definition is referred to Hamada-Sato et al. (2005).
3.9. Histomorphology and ultrastructure of muscle fiber
Fig. 7 shows the myofiber structure of the transversal section of white muscle. The area, diameter, density and size ratio of the myofiber are shown in Table 14. The area, diameter, density and size ratio of the myofiber were significantly influenced by the levels of BSFLM in diets. The area and diameter of myofiber were first significantly decreased and then increased with the increment of BSFLM inclusion in diets (P < 0.05), while the myofiber density showed the reverse trend (P < 0.05). The area and diameter of myofiber in the BSFLM groups were significantly lower compared to the SM group (P < 0.05), and myofiber density of the remaining BSFLM groups except for the BSFLM15 group were significantly higher than that of the SM group (P < 0.05). The percentage of myofiber of less than 20 μm in the remaining BSFLM groups except for the BSFLM45 and BSFLM100 groups were significantly higher than that of the SM group (P < 0.05). The percentage of myofiber of 20 to 50 μm in the BSFLM45, BSFLM60 and BSFLM100 groups were significantly higher compared to the SM group (P < 0.05). Additionally, the percentages of myofiber of large diameter (>50 μm) in the BSFLM60 and BSFLM75 groups were significantly lower than that of the SM group (P < 0.05). Fig. 8 shows the ultrastructure of the muscle myofibril. The Table 14 shows that the sarcomere length of the BSFLM60 group was significantly lower than that of the SM group (P < 0.05), however the BSFLM75 and BSFLM100 groups had higher sarcomere length than that of the other groups (P < 0.05).
Fig. 7.
Photomicrographs of transversal sections of the white muscle of fish fed different experimental diets, Hematoxylin-eosin staining (magnification 100× , scale bar = 100 μm). The morphology of muscle fiber is irregular polygons. SM, BSFLM15, BSFLM30, BSFLM45, BSFLM60, BSFLM75 and BSFLM100 represent diets in which BSFLM replaced SM levels of 0%, 15%, 30%, 45%, 60%, 75% and 100% respectively. BSFLM = black soldier fly larvae meal; SM = soybean meal.
Table 14.
Effects of BSFLM on histological characterization and sarcomere length in the muscle of fish1.
| Item | Diets |
P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| SM | BSFLM15 | BSFLM30 | BSFLM45 | BSFLM60 | BSFLM75 | BSFLM100 | ||
| Myofiber area, μm2 | 3187.68 ± 190.78a | 2640.26 ± 212.95b | 2549.38 ± 135.55bc | 2354.48 ± 34.09bc | 1527.48 ± 160.85e | 1858.80 ± 120.45d | 2276.69 ± 276.80c | 0.00 |
| Myofiber diameter, μm | 56.67 ± 2.54a | 50.94 ± 3.07b | 50.30 ± 2.47b | 49.41 ± 1.09b | 40.46 ± 2.67c | 43.47 ± 1.55c | 48.58 ± 3.48b | 0.00 |
| Myofiber density2,/mm2 | 259.41 ± 34.41d | 238.03 ± 12.22d | 392.98 ± 20.43b | 424.78 ± 6.12b | 659.44 ± 67.96a | 364.56 ± 54.56bc | 324.33 ± 2.02c | 0.00 |
| Myofiber size ratio, % | ||||||||
| <20 μm | 3.96 ± 1.04d | 9.73 ± 2.82bc | 11.06 ± 2.72ab | 6.34 ± 0.97cd | 9.18 ± 0.66bc | 14.55 ± 3.07a | 5.32 ± 1.12d | 0.00 |
| 20 – 50 μm | 44.23 ± 4.73d | 42.22 ± 6.69d | 44.97 ± 1.83cd | 51.12 ± 1.02bc | 65.23 ± 2.14a | 47.99 ± 2.05bcd | 53.17 ± 2.20b | 0.00 |
| >50 μm | 51.81 ± 4.82a | 48.05 ± 8.33a | 44.10 ± 6.73ab | 42.54 ± 1.37ab | 25.59 ± 2.79c | 37.46 ± 2.97b | 42.63 ± 4.45ab | 0.00 |
| Sarcomere length, μm | 1.64 ± 0.03c | 1.70 ± 0.03c | 1.67 ± 0.02c | 1.70 ± 0.06c | 1.52 ± 0.01d | 2.10 ± 0.07a | 1.79 ± 0.01b | 0.00 |
BSFLM = black soldier fly larvae meal; SM = soybean meal.
Values are mean ± SD (n = 3). Values with different superscripts in the same row are significantly different (P < 0.05) from each other.
Total number of myofibers per square millimeters.
Fig. 8.
Transmission electron microscope images of the white muscle of fish fed different experimental diets. Photomicrographs (magnification 25,000× , scale bar = 500 nm). The distance between the two-way arrows indicates is the sarcomere length. The line pointed by the one-way arrow is the Z line. SM, BSFLM15, BSFLM30, BSFLM45, BSFLM60, BSFLM75 and BSFLM100 represent diets in which BSFLM replaced SM levels of 0%, 15%, 30%, 45%, 60%, 75% and 100% respectively. BSFLM = black soldier fly larvae meal; SM = soybean meal.
3.10. Gene expression of development and protein synthesis in muscle
The relative expression levels of muscle development-related genes MyoG (A), MyoD (B), Mrf4 (C), Myf5 (D), MyHC (E), FGF6a (F), FGF6b (G), MSTN (H) and protein synthesis-related genes TOR (I) are showed in Fig. 9. The relative expression levels of MyoG, Mrf4, FGF6a, MSTN, TOR genes were not significantly different compared to the SM group (P > 0.05) (Fig. 9A, C, F, H and I). The MyoD relative gene expression level was gradually increased with the increment of BSFLM inclusion in diets (Fig. 9B). The BSFLM60 and BSFLM75 groups had higher the Myf5 gene relative expression level than that of the SM group and the relative expression level of the MyHC gene reached the highest in the BSFLM100 group (P < 0.05) (Fig. 9D and E). Additionally, the relative expression level of FGF6b gene in the remaining BSFLM groups except for the BSFLM30 and BSFLM75 groups were significantly higher compared to the SM group (P < 0.05) (Fig. 9G).
Fig. 9.
The relative expression levels of development and protein synthesis genes in the muscle of fish fed different experimental diets (mean ± SD, n = 3). SM, BSFLM15, BSFLM30, BSFLM45, BSFLM60, BSFLM75 and BSFLM100 represent diets in which BSFLM replaced SM levels of 0%, 15%, 30%, 45%, 60%, 75% and 100% respectively. BSFLM = black soldier fly larvae meal; SM = soybean meal. The relative gene expression levels of MyoG (A, P = 0.23), MyoD (B, P = 0.02), Mrf4 (C, P = 0.04), Myf5 (D, P = 0.04), MyHC (E, P = 0.01), MSTN in the muscle of fish fed different experimental diets (P = 0.02) FGF6a (F, P = 0.28), FGF6b (G, P < 0.01), MSTN (H, P = 0.02) and TOR (I, P = 0.03) in muscle of fish fed different experimental diets. Bars with different letters indicate significant difference (P < 0.05) from each other.
4. Discussion
4.1. Effects of dietary BSFLM levels on the growth performance and biological indices of grass carp
This study showed that the growth performance of large grass carp fed diets with BSFLM replacing no more than 60% of SM (the basal content was 24%) were not significantly different compared to the SM group (the control group). Similar results have been found in Jian carp (C. carpio var. Jian) (Li et al., 2017; Zhou et al., 2018), Atlantic salmon (Belghit et al., 2019) and European seabass (Magalhães et al., 2017) and juvenile grass carp (Lu et al., 2020). However, in this study, the growth performance of grass carp was significantly decreased when the BSFLM substitute for SM reached the 75% level and above. A similar phenomenon was observed in the juvenile turbot (Scophthalmus maximus) (Kroeckel et al., 2012) and Atlantic salmon (Fisher et al., 2020), the growth performance of turbot and Atlantic salmon decreased significantly when the BSFLM inclusion levels in the diet exceeded 33% and reached 30%, respectively. Common point of discussion to explain the negative effects on growth has been on the presence of chitin in the insect meal, including BSF. Increasing the chitin content from 1% to 2% or 5% significantly reduced growth rate in Atlantic salmon, and the apparent digestibility coefficients of nutrients like protein and lipid were negatively correlated with dietary chitin content, which suggests that chitin is an energy sink with a probably low digestibility (Karlsen et al., 2017). The decreased protein retention efficiency of BSFLM75 and BSFLM100 groups may be related to the chitin of BSFLM in the experimental diets of this study. The present study also showed a significant increase in the HSI in the BSFLM60, BSFLM75 and BSFLM100 groups. Belghit et al. (2018) found in a previous study that insect-based diets fed to fresh-water salmon increased the HSI compared to fish fed without BSFLM, which was consistent with the results of the present study. However, other studies demonstrated a significant reduction of growth performance but no effect on the HSI with BSFLM replacement in juvenile Siberian sturgeon (Caimi et al., 2020). The difference in growth performance between different trials might have been due to the different compositions of BSFLM nutrients, different tolerance levels for BSFLM components such as chitin among different fish species, the different life stages of fish used in the trials and different BSFLM protein processing methods.
4.2. Effects of dietary BSFLM levels on the antioxidant status and tissue structure of grass carp
4.2.1. Effect of dietary BSFLM levels on the antioxidant capacity of grass carp muscle
Oxidative stress occurs to control the damaging effects when there is an imbalance between reactive oxygen species (ROS) production and the volume of antioxidant systems (Monaghan et al., 2009). Free radical scavenging enzymes (such as SOD and CAT) protect cells from ROS damage. In the current study, the appropriate level of BSFLM significantly increased the T-SOD activity and decreased the MDA content in grass carp muscle. However, in 2 previous studies, the BSFLM level did not affect the T-SOD activity and MDA content in the liver of grass carp (Lu et al., 2020) and Jian carp (Li et al., 2017), but the activity of CAT was increased. In the present study, there was no significant difference in the content of hydrogen peroxide in muscle compared to the SM group, which suggested that the CAT activity might neither be significantly different. The exoskeleton of insects is known to be an abundant source of chitin with potential antioxidant activity (Makkar et al., 2014; Zielińska et al., 2017). Chitin and its derivatives have been reported to have antioxidant properties that can prevent the deleterious effects of various diseases (Khoushab and Yamabhai, 2010; Ngo and Kim, 2014). The data obtained from this experiment demonstrated that BSFLM could boost the muscle antioxidant capacity when given to grass carp in a formulated diet. It has been shown that muscle antioxidant status affects meat quality, and researches on grass carp (Wu et al., 2014) and pork (Chen et al., 2010) have shown that the enhanced antioxidant status improves the WHC of muscle. The inconsistency between this study and the above results may be related to fish species, tissue type, chitin and derivative contents.
4.2.2. Effects of dietary BSFLM levels on the liver and intestine structure of grass carp
Hepatocytes are highly sensitive to the nutritional status of the fish and to the quality of the diet (Brusle and Anadon, 1996). However, in the present study, no pathological features were found in the liver morphology of all the experimental groups, and their hepatocytes were polygonal in shape and had centrally located nuclei and clear cell boundaries. A similar phenomenon was observed in juvenile grass carp (Lu et al., 2020) and Jian carp (Li et al., 2017) fed experimental diets with SM and FM substitutions of 25%, 50% and 100% by BSFLM, respectively. However, in these previous studies, when BSFLM completely replaced SM or FM in the diet, a negative impact was observed on the hepatocyte morphology of grass carp and Jian carp, resulted in irregular hepatocyte morphology and unclear cell boundaries. This means that a BSFLM diet might have a minor effect on liver morphology. These differences may be related to the basic content of dietary SM (360 g/kg diet) in the study of Lu et al. (2020). The basic content of dietary SM affected the addition level of BSFLM, the highest supplemental level of BSFLM in the study of Lu et al. (2020) was 25.5%, while in the present study was only 22.7%. Additionally, differences in fish species, life stages and feed formulas are also potential reasons for differences in the findings. Intestinal health and function can be assessed by measuring villus height (Swatson et al., 2002). In the present study, the villus height in the BSFLM75 group and the BSFLM100 group were slightly decreased and significantly decreased, respectively, compared with the SM group. As the primary site of feed digestion, nutrient uptake, and nutrient transformation, the intestine is central to physiological functioning, and the optimum utilization of dietary nutrients depends on their functional effectiveness (Caballero et al., 2003). The lower intestinal villi height might be related to the decrease of growth performance and feed utilization of experimental fish in this study. Dumas et al. (2018) found that feeding rainbow trout with a diet containing 26.4% BSFLM shortened the villi in the anterior intestine and decreased the rainbow trout growth performance and feed utilization. Similar results were reported in juvenile grass carp, Lu et al. (2020) found that the intestinal villus length of grass carp decreased with the increase of BSFLM inclusion in diets, but BSFLM did not have negative effects on the growth performance and feed utilization of experimental fish. In the present study, BSFLM inclusion levels of more than 17% had the negative effect. This inconsistency could be related to different diet formulations, BSFLM nutrient composition, the life stages of fish, and the experimental cycle. Furthermore, protein nutrients could also affect the construction of intestinal villi (Caballero et al., 2002; Ostaszewska et al., 2010). A partial amino acid deficiency in plant protein-based diets has been found to adversely affect fish intestinal epithelium (Ostaszewska et al., 2010). The amino acid compositions of the experimental diets showed slight differences among groups. Even so, the amino acid balance of the experimental diets also showed varying degrees of difference. It is speculated that these slight differences may have a significant effect on the intestinal structure of the experimental animals over the long term.
4.3. Effect of dietary BSFLM levels on the flesh quality of grass carp
4.3.1. Effects of dietary BSFLM levels on the proximate composition, amino acid composition and fatty acid composition of grass carp muscle
Fish muscle is the main edible portion of fish, and its main nutritional value is reflected in wealthy protein, amino acids and PUFA in fish muscle (Jiang et al., 2016; Periago et al., 2005). In this study, no significant effect of dietary BSFLM levels on the content of muscle crude protein was found in grass carp. Similar results were reported in rainbow trout (Gasco et al., 2014; Mancini et al., 2018) and Jian carp (Li et al., 2017; Zhou et al., 2018). Meanwhile, BSFLM replacement had no significant effect on muscle amino acid contents except Gly in grass carp, similar to previous studies in Jian carp (Zhou et al., 2018), juvenile barramundi (Lates calcarifer) (Katya et al., 2017) and juvenile grass carp (Lu et al., 2020). However, in this study, the muscle Gly content of BSFLM100 group was the highest, which was inconsistent with the studies in Jian carp (Zhou et al., 2018), juvenile barramundi (Katya et al., 2017) and grass carp (Lu et al., 2020). The amino acid compositions of ingredients and diets were detected in this study. It was found that the Gly content of BSFLM100 diet was slightly higher than that of the SM group, which might be the reason for the significant increase of Gly content in muscle. The ideal protein standard was proposed by the FAO/WHO in 1973. The ratios of essential amino acid to total amino acid (EAA:TAA ratio) and essential amino acid to non-essential amino acid (EAA:NEAA ratio) of protein with good quality were considered to be about 40% and over 60%, respectively (FAO/WHO, 1993). In this study, the EAA:TAA ratio of muscle in grass carp was about 50%, and the EAA:NEAA ratio was 98% to 100%, which fully satisfied the ideal protein standard proposed by the FAO/WHO. Furthermore, in the present study, it was observed that the crude protein content of the whole body in the BSFLM30 group was significantly higher than that of the SM group. However, studies in Atlantic salmon (Belghit et al., 2018) and turbot (Kroeckel et al., 2012) found that compared to the control group, the crude protein content of the whole body was not significantly different following BSFLM inclusion in the diet. This inconsistency could be related to different diet formulations, BSFLM nutrient composition and fish species. Although the crude lipid content of the whole body and muscle were not affected significantly by the dietary BSFLM level, lipid nutritional value is largely dependent on the type and number of fatty acids, especially unsaturated fatty acid content (Jiang et al., 2016). Interestingly, in this study, several modifications were found in the fatty acid profile of grass carp muscle as a consequence of the experimental diets. The trends detected in the grass carp muscle fatty acid profiles could be ascribed to the fatty acid profiles of the experimental diets. However, C20:2n-6, C20:4n-6 and C22:4n-6 were not detected in the experimental diets. The increase of C22:4n-6 might have originated from endogenous synthesis, while C20:2n-6 and C20:4n-6 might have originated from the grass carp muscle itself. After the SM was completely replaced by BSFLM in this study, the ∑n-3 PUFA content in the experimental diet was decreased, while the ∑n-3 PUFA content in the muscle of the BSFLM100 group was significantly increased, which might have been due to the significant increase of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) in the muscle. Among the long chain-PUFA, EPA and DHA are the most valued fatty acids for their benefits to human health (Rosenlund et al., 2010). In the present study, the EPA and DHA contents were significantly respectively increased when BSFLM replaced more than 15% of SM and completely replaced SM, respectively, which significantly improved the nutritional value of grass carp muscle. However, the opposite trend was observed in rainbow trout (Mancini et al., 2018; Renna et al., 2017), where dietary BSFLM reduced DHA and EPA levels. This might have been influenced by the fatty acid composition of the experimental diets. Replacing FM with BSFLM in rainbow trout diet would reduce the amount of fish oil and PUFA provided by FM. A higher ratio of n-3 to n-6 PUFA is generally to be an index of high nutritional value (Zhao et al., 2018). In this study, the ∑n-3 PUFA to ∑n-6 PUFA ratios in the BSFLM45, BSFLM60, BSFLM75 and BSFLM100 groups were higher than 0.2. The fatty acid results of this study showed that the BSFLM diet improved the nutritional value of grass carp muscle.
4.3.2. Effects of dietary BSFLM levels on the muscle texture properties, muscle fiber and the gene expression of muscle development in grass carp
The texture property is a technologically important and multiparameter sensory attribute of fish muscle. In fish, firmness is regarded as a primary muscle quality property (Brinker and Reiter, 2011). Generally, the enhanced fish muscle firmness represents an improvement of the muscle quality (Johnston et al., 2006). However, in the present study, there was no significant effect of BSFLM levels in diets on muscle hardness. In addition, studies have shown that the higher hardness, chewiness and springiness of fish, the better the flesh quality and mouth feel (Monaco et al., 2008). With the increase of the BSFLM level in diets, the muscle springiness and chewiness of grass carp increased gradually. This indicated a beneficial influence of dietary BSFLM on springiness and chewiness, which resulted in the improvement of muscle quality. In addition, muscle fiber properties are a useful quantitative indicator of muscle quality, including muscle fiber diameter, muscle fiber density, muscle fiber type, and sarcomere length (Choi and Kim, 2009). The diameter and density of muscle fiber are important determinative factors for the textural characteristics of fish muscle (Johnston et al., 2000), and there was evidence that fish flesh firmness was negatively correlated with muscle fiber diameter and positively correlated with fiber density (Valente et al., 2011). In this study, dietary BSFLM significantly affected the muscle fiber properties of grass carp. Compared with the SM group, the muscle fiber diameter in all BSFLM groups were significantly decreased, and the muscle fiber density in the remaining BSFLM groups, with the exception of the BSFLM15 group, were significantly increased. No relationship between muscle hardness and fiber properties was found in this study, but the relationship described above was found between the muscle chewiness, springiness and fiber properties. Additionally, the hardness of muscle is mainly determined by connective tissue and muscle fiber, and the influence of connective tissue on the hardness is higher than that of muscle fiber (Hagen et al., 2007). Therefore, the differences in the composition, content and distribution of connective tissue in the muscle may reduce the change of muscle hardness caused by the change in muscle fiber characteristics, and ultimately muscle hardness showed no significant change in the present study. Moreover, compared with the SM group, fish fed BSFLM15, BSFLM30, BSFLM60 and BSFLM75 diets had an increased frequency of smaller fibers (diameter <20 μm), and BSFLM60 and BSFLM75 groups had a decreased frequency of larger fibers (diameter >50 μm). In addition, the large part of muscle fibers was less than 20 μm in diameter, which indicated that an active hyperplastic growth process occurred in the stage of development (Rowlerson and Veggetti, 2001). Therefore, the results regarding muscle fibers in this study confirmed that BSFLM might increase the white muscle fiber hyperplasia. A study in blunt snout bream (Megalobrama amblycephala) found that the DHA supplementation promoted the muscle hyperplasia (Wang et al., 2020). Similarly, a study in pigs also found that linseed (α-linolenic acid, C18:3n-3, an n-3 PUFA) supplements promoted the muscle hypertrophy (Huang et al., 2008). Combined with the results of this study, we infer that the occurrence of muscle fiber hyperplasia might be related to the increase of DHA and the decrease of C18:3n-3 in the feed supplemented with BSFLM. It was also observed that BSFLM increased muscle DHA and decreased the accumulation of C18:3n-3, which might provide evidence of the occurrence of muscle fiber hyperplasia. The sarcomere is the basic contractile unit of muscle and is closely related to tenderness and WHC (Ertbjerg and Puolanne, 2017). Previous researches found that the longer sarcomere lengths usually resulted in higher muscle tenderness in cattle (Herring et al., 2006) and chickens (Wang et al., 2014). In this study, the muscle tenderness (measured by shear force) of grass carp was not affected by dietary BSFLM. However, tenderness is mainly determined by muscle chemical composition and fiber structure. Additionally, DHA has been found to promote the increase of sarcomere length in blunt snout bream (Wang et al., 2020). Therefore, we concluded that the increase of sarcomere length promoted by BSFLM might be related to the higher DHA content in diets and muscle. Previous studies showed that the differential expressions of myogenic regulatory factors (including MyoG, MyoD, Mrf4 and Myf5) and myostatin (MSTN) might be related to the skeletal muscle development in fish (Valente et al., 2016; Zhu et al., 2014). MyoD and Myf5 mainly act in specifying myoblasts during the initial growth phases, and MyoG and Mrf4 are mainly involved in the differentiation and fusion of myotubes in the late stage (Johansen and Overturf, 2005; Wright et al., 1989). MSTN is a potent negative regulator of muscle development, which includes MSTNa and MSTNb in M. amblycephala (Zhu et al., 2014). A study on Solea senegalensis reported that the up-regulation of Mrf4 was related to the increasing fiber area in sole early stages (Campos et al., 2013). In this study, significant up-regulations of MyoD gene in the BSFLM60, BSFLM75 and BSFLM100 groups compared to the SM group, and the relative expression levels of Myf5 gene in BSFLM60 and BSFLM75 groups were significantly higher than that of the SM group. These findings were in accord with the increased fiber density and the frequency of small fibers (diameter <20 μm). Additionally, it has been demonstrated that nascent fibers might repeat the MyHC transitions observed in other fibers during development (Dix and Eisenberg, 1990; Kennedy et al., 1988, 1989). In the present study, it was observed that the BSFLM100 diet significantly up-regulated the expression of MyHC gene, which was in accord with the increased frequency of small (diameter <20 μm) and medium fibers (20 to 50 μm diameter). Fibroblast growth factor 6 (FGF6) plays a role in myogenesis and can promote muscle regeneration and accelerate the proliferation and differentiation of myocytes (Armand et al., 2006). Surprisingly, compared with the SM group, the expression levels of FGF6b gene in the remaining BSFLM groups other than the BSFLM30 and BSFLM75 groups were significantly up-regulated. These results indicated that appropriate BSFLM levels could promote continued muscle fiber recruitment. A previous study showed that the addition of 0.8% DHA to the diet could up-regulate the expression of myogenic regulatory factors (including MyoG, MyoD, Mrf4 and Myf5), increased muscle fiber density, and induced the hyperplasia muscle fiber in blunt snout bream (Wang et al., 2020). Therefore, we infer that the increase of DHA content in diets caused by BSFLM addition activates the expression of muscle development factors such as myogenic regulatory factors, and then affects the changes of muscle fiber structure.
4.3.3. Effects of dietary BSFLM levels on the WHC, pH, and water- and salt-soluble protein contents of grass carp muscle
The WHC is an important factor affecting the color, flavor, tenderness, processing, and storage of meat and is usually evaluated based on characteristics including the drip loss, freezing seepage rate, centrifugal water loss rate. In general, the lower the muscle drip loss, the higher the WHC of muscle (Srikanchai et al., 2010). However, in this study, the BSFLM75 diet significantly reduced the muscle drip loss of grass carp, indicated the improvement of muscle WHC. Additionally, fish muscle was demonstrated to be easily attacked by reactive oxygen species (Archile-Contreras and Purslow, 2011). Previous studies showed that the oxidation of lipid and protein in muscle led to a reduction in the WHC in pigs (Asghar et al., 1991) and chickens (Wang et al., 2009), respectively. Chen et al. (2010) reported that the antioxidant capacity was negatively correlated with drip loss in pork. Similar results also occurred in grass carp. Through correlation analysis, it was found that the antioxidant capacity of muscle was negatively correlated with the drip loss, so it was speculated that adding the appropriate level of zinc in feed could improve muscle quality through the enhancement of antioxidant status (Wu et al., 2014). However, dietary BSFLM had no significant effect on fillet WHC in rainbow trout (Bruni et al., 2020; Secci et al., 2019). This inconsistency may be related to the antioxidant status of fish muscle caused by the addition of dietary BSFLM. In this study, BSFLM might have improved the WHC by enhancing the muscle antioxidant capacity, thereby enhancing muscle quality. In addition to the texture property and WHC, the muscle pH is also an important muscle quality parameter (Periago et al., 2005). The pH has an important effect on muscle quality and directly affects the storage, WHC, and processability of meat. In general, grass carp post-mortem muscle pH values vary from 6.2 to 6.7 (Li et al., 2013). The present study showed that the post-mortem muscle pH of grass carp in the SM group was 6.47 and increased to 6.51–6.59 in the BSFLM groups, which indicated that BSFLM could increase the muscle pH. However, no similar phenomenon was observed in rainbow trout (Bruni et al., 2020; Secci et al., 2019). Muscle proteins are highly charged compounds and thus absorb large amounts of water. The muscle pH can change the charge status of muscle proteins, which has a great influence on the WHC. At higher pH levels, protein molecules carry more net negative charge and can absorb a large amount of water; the protein molecules repel each other, leaving voids for water, and at this time the muscle WHC is higher. After slaughter, due to glycolysis and the accumulation of lactic acid, the pH of muscle decreases, the balance between positive and negative charges changes, the net negative charge of protein decreases, and the ability to absorb water decreases. The degradation rate of muscle protein is significantly reduced, and the shelf life of meat or meat products is significantly prolonged when the muscle has a strong WHC. In contrast, when muscle WHC is lower, water loss is accompanied by a decrease in soluble protein and flavor substances in muscle. Water-soluble and salt-soluble proteins have also been used to evaluate fish muscle quality (Einen et al., 1999; Mørkøre et al., 2010). Muscle water-soluble proteins are mainly composed of sarcoplasmin, while salt-soluble proteins are mainly composed of myofibrin (Haard, 1992). Studies have shown that high levels of salt-soluble protein reduced muscle fluid loss (Mørkøre et al., 2010). In this study, the water-soluble protein contents of the BSFLM15, BSFLM30, BSFLM45 and BSFLM60 groups and the salt-soluble protein contents of the BSFLM75 and BSFLM100 groups were significantly lower and higher, respectively, compared to the SM group. This showed that the loss of muscle fluid was reduced and the WHC was improved, which was conducive to food processing.
4.3.4. Effects of dietary BSFLM levels on the body and muscle color of grass carp
Body and muscle color are intuitive characteristics that reflect the quality of aquatic products. Changes in the skin and muscle color of fish depend on the intake of carotenoids. Like other animals, fish cannot synthesise carotenoids spontaneously, and carotenoids are one of the color-forming substances of red pigment cells and yellow pigment cells (Urban et al., 2012). This study found that the a∗ of the dorsa and lateral line, the a∗ and b∗ of the ventral, and the b∗ of the muscle of grass carp gradually increased with the increase of dietary BSFLM level. This finding may be due to the fact that BSFLM contains carotenoids that grass carp can metabolize, thereby affecting the color of the fish body and muscle. The coloring effect of carotenoids is also affected by the amount and source of fat in the feed, which may also be caused by changes in the composition and content of fatty acids in the feed. In a study of rainbow trout, BSFLM had no effect on muscle color (Renna et al., 2017). Mancini et al. (2018) also found that BSFLM had no effect on muscle L∗, a∗, and C∗, but decreased the b∗. Additionally, this study found that BSFLM had no effect on the dorsa, lateral line, and muscle C∗ of grass carp, which was similar to the above study, but the ventral C∗ of the BSFLM60 and BSFLM100 groups was significantly increased. These inconsistencies may be related to the species of fish, the types of alternative protein sources, and the pigment compositions of BSFLM. In addition, it was found that the ventral L∗ of grass carp was slightly reduced when SM was completely replaced by BSFLM. According to the study of Hearing (2005), increased melanin resulted in a darker body surface. Tyrosinase as a rate-limiting enzyme that controls melanin synthesis. Tyrosinase activity is positively proportional to the content of melanin (Kogaetal, 1999). In this study, the activity of tyrosinase in grass carp was not measured, and it was speculated that the tyrosinase activity was affected when BSFLM was added in a higher proportion. At present, there are few studies on the effects of insect protein replacement on the body and muscle color of fish. In this study, the internal mechanism of body and muscle color changes in grass carp needs to be further discussed.
4.3.5. Effects of dietary BSFLM levels on TMAO, TMA and nucleotide content in grass carp muscle
TMAO is a flavour substance with an umami taste (Timm and Bo, 2002). TMAO can be reduced to TMA under the action of microorganisms, and TMA has a strong fishy taste (Heising et al., 2014). Therefore, TMA is widely used to evaluate the quality and freshness of fish. In this study, compared with the SM group, dietary BSFLM had no effect on the muscle TMAO and TMA contents of grass carp. This might have been related to the fresh fish muscle used in this study, without shelf-life testing. TMAO is not directly synthesized in most teleost fish and must be ingested. This suggests that BSFLM contributes less to the TMAO and TMA of grass carp. It is widely accepted that nucleotides can influence fish taste and flavor; inosine monophosphate is related to umami taste and AMP can play a role as a flavor enhancer (Shirai and Kikuchi, 2002; Tejada, 2009). However, nucleotide quantification showed that only the Hx concentration was significantly affected by the BSFLM diets compared to the SM group. Hx can produce unpleasant bitterness and its accumulation leads to a decrease in fish flavor (Kuda et al., 2008; Mørkøre et al., 2010). In this study, the Hx concentration of muscle in the BSFLM75 group was significantly lower than that of the SM group. This suggested that BSFLM could reduce bitterness in the muscle and improve the overall taste. The K and KI values are important indicators to reflect the freshness of fish, and the smaller the value, the fresher the fish (Hamada-Sato et al., 2005). However, dietary BSFLM had no effect on the muscle freshness of grass carp compared with the control group. Similar findings were reported in rainbow trout (Mancini et al., 2018), but diets supplemented with BSFLM reduced the AMP content in rainbow trout muscle, in contrast to the findings of this study. Additionally, the decrease of specific compounds associated with the decrease of AMP could modify sensory perception and evaluation, as reported by Borgogno et al. (2016), where fish fed diets with BSFLM showed an increased intensity of metallic flavor.
5. Conclusion
This study demonstrated that the replacement of dietary SM with BSFLM up to the 60% level (BSFLM level at 136 g/kg diet) had no adverse effect on growth performance and improved the flesh quality of grass carp. The BSFLM contents were 71.0 g/kg diet (substituting 31.28% SM) and 69.1 g/kg diet (substituting 30.44% SM), corresponding to the optimal values of FBW and FCR, respectively, based on the quadratic regression analysis. Additionally, an appropriate level of BSFLM could also improve muscle antioxidant status and promote the growth and development of muscle fibers. The muscle texture properties, nutritional value and body color of grass carp were optimal among all groups when dietary SM was completely replaced with BSFLM (227 g/kg diet). Combined with previous studies, we infer that the improvement of muscle quality in this study is related to the improvement of muscle antioxidant status. In addition, BSFLM may affect muscle fiber structure by activating the expression of muscle development related factors, and then contribute to muscle physical properties.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Author contributions
Zechao Hu: Methodology, Investigation, Data curation, Writing-original draft, Formal analysis. Handong Li: Methodology, Investigation, Data curation, Writing-original draft, Formal analysis. Sha Liu: Methodology, Investigation, reviewing & editing. Rongrong Xue: Methodology, Investigation, reviewing & editing. Jian Sun: reviewing & editing. Hong Ji: Supervision, Experiment design, Writing - review & editing, conceptualization, funding acquisition, resources. All authors: contributed to interpreting the results, and read and approved the final manuscript.
Acknowledgments
This study was supported by the National Key R & D Program of China (2019YFD0900200) in finance.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2023.06.006.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abdel-Tawwab M., Khalil R.H., Metwally A.A., et al. Effects black soldier fly (Hermetia illucens L.) larvae meal on growth performance, organs-somatic indices, body composition, and hematological and biochemical variables of European sea bass, Dicentrarchus labrax. Aquaculture. 2020;522 [Google Scholar]
- Almeida F.L.A.D., Carvalho R.F., Pinhal D., et al. Differential expression of myogenic regulatory factor MyoD in pacu skeletal muscle (Piaractus mesopotamicus Holmberg 1887: Serrasalminae, Characidae, Teleostei) during juvenile and adult growth phases. Micron. 2008;39(8):1306–1311. doi: 10.1016/j.micron.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Anne L., Bénédicte L., Isabelle L., et al. How muscle structure and composition influence meat and flesh. Sci World J. 2016:14. Article ID 3182746. [Google Scholar]
- AOAC International. Official methods of analysis of AOAC International. 16th ed. Gaithersburg: Association of Official Analytical Chemists 2000.
- Archile-Contreras A.C., Purslow P.P. Oxidative stress may affect meat quality by interfering with collagen turnover by muscle fibroblasts. Food Res Int. 2011;44(2):582–588. [Google Scholar]
- Armand A.S., Laziz I., Chanoine C. FGF6 in myogenesis. Biochim Biophys Acta Mol Cell Res. 2006;1763(8):773–778. doi: 10.1016/j.bbamcr.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Asghar A., Gray J.I., Booren A.M., et al. Effects of supranutritional dietary vitamin E levels on subcellular deposition of a-tocopherol in the muscle and on pork quality. J Sci Food Agric. 1991;57(1):31–41. [Google Scholar]
- Barroso F.G., de Haro C., Sánchez-Muros M.J., et al. The potential of various insect species for use as food for fish. Aquaculture. 2014;422–423:193–201. [Google Scholar]
- Belghit I., Liland N.S., Gjesdal P., et al. Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar) Aquaculture. 2019;503:609–619. [Google Scholar]
- Belghit I., Liland N.S., Waagbø R., et al. Potential of insect-based diets for Atlantic salmon (Salmo salar) Aquaculture. 2018;491:72–81. [Google Scholar]
- Borgogno M., Dinnella C., Iaconisi V., et al. Inclusion of Hermetia illucens larvae meal on rainbow trout (Oncorhynchus mykiss) feed: effect on sensory profile according to static and dynamic evaluations. J Sci Food Agric. 2016;97(10):3402–3411. doi: 10.1002/jsfa.8191. [DOI] [PubMed] [Google Scholar]
- Brinker A., Reiter R. Fish meal replacement by plant protein substitution and guar gum addition in trout feed, Part I: effects on feed utilization and fish quality. Aquaculture. 2011;310(3–4):350–360. [Google Scholar]
- Bruni L., Randazzo B., Cardinaletti G., et al. Dietary inclusion of full-fat Hermetia illucens prepupae meal in practical diets for rainbow trout (Oncorhynchus mykiss): lipid metabolism and fillet quality investigations. Aquaculture. 2020;529 doi: 10.3390/ani9050251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusle J., Anadon G.G. The structure and function of fish liver. Fish morphology. 1996:77–93. [Google Scholar]
- Caballero M.J., Izquierdo M.S., Kjørsvik E., et al. Morphological aspects of intestinal cells from gilthead seabream (Sparus aurata) fed diets containing different lipid sources. Aquaculture. 2003;225(1–4):325–340. [Google Scholar]
- Caballero M.J., Obach A., Rosenlund G., et al. Impact of different dietary lipid sources on growth, lipid digestibility, tissue fatty acid composition and histology of rainbow trout, Oncorhynchus mykiss. Aquaculture. 2002;214(1–4):253–271. [Google Scholar]
- Caimi C., Renna M., Lussiana C., et al. First insights on black Soldier fly (Hermetia illucens L.) larvae meal dietary administration in Siberian sturgeon (Acipenser baerii Brandt) juveniles. Aquaculture. 2020;515 [Google Scholar]
- Campos C., Valente L.M.P., Conceição L.E.C., et al. Incubation temperature induces changes in muscle cellularity and gene expression in Senegalese sole (Solea senegalensis) Gene. 2013;516(2):209–217. doi: 10.1016/j.gene.2012.12.074. [DOI] [PubMed] [Google Scholar]
- Chen T., Zhou G.H., Xu X.L., et al. Phospholipase A2 and antioxidant enzyme activities in normal and PSE pork. Meat Sci. 2010;84(1):143–146. doi: 10.1016/j.meatsci.2009.08.039. [DOI] [PubMed] [Google Scholar]
- Cheng W., Chiu C.S., Guu Y.K., et al. Expression of recombinant phytase of Bacillus subtilis E20 in Escherichia coliHMS 174 and improving the growth performance of white shrimp, Litopenaeus vannamei, juveniles by using phytasepretreated soybean meal-containing diet. Aquacult Nutr. 2013;19(2):117–127. [Google Scholar]
- Choi Y.M., Kim B.C. Muscle fiber characteristics, myofibrillar protein isoforms, and meat quality. Livestock Science. 2009;122(2):105–118. [Google Scholar]
- Diener S., Zurbrugg C., Tockner K. Conversion of organic material by black soldier fly larvae: establishing optimal feeding rates. Waste Management & Research. 2009;27:603–610. doi: 10.1177/0734242X09103838. [DOI] [PubMed] [Google Scholar]
- Dix D.J., Eisenberg B.R. Myosin mRNA accumulation and myofibrillogenesis at the myotendinous junction of stretched muscle fibers. JCB (J Cell Biol) 1990;111(5):1885–1894. doi: 10.1083/jcb.111.5.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas A., Raggi T., Barkhouse J., et al. The oil fraction and partially defatted meal of black soldier fly larvae (Hermetia illucens) affect differently growth performance, feed efficiency, nutrient deposition, blood glucose and lipid digestibility of rainbow trout (Oncorhynchus mykiss) Aquaculture. 2018;492:24–34. [Google Scholar]
- Einen O., Mørkøre T., Rørå A.M.B., et al. Feed ration prior to slaughter - a potential tool for managing product quality of Atlantic salmon (Salmo salar) Aquaculture. 1999;178(1–2):149–169. [Google Scholar]
- Ertbjerg P., Puolanne E. Muscle structure, sarcomere length and influences on meat quality: a review. Meat Sci. 2017;132:139–152. doi: 10.1016/j.meatsci.2017.04.261. [DOI] [PubMed] [Google Scholar]
- FAO . Food and Agriculture Organization of the United Nations; Rome: 2022. The state of world Fisheries and aquaculture 2022. (2022) [Google Scholar]
- FAO/WHO . FAO/WHO; 1993. Fats and oils in human nutrition. Report of a joint FAO/WHO eexpert consultation,FAO food and nutrition; pp. 19–26. [PubMed] [Google Scholar]
- Fisher H.J., Collins S.A., Hanson C., et al. Black solider fly larvae meal as a protein source in low fish meal diets for Atlantic salmon (Salmo salar) Aquaculture. 2020;521 [Google Scholar]
- Fuentes A., Fernández-Segovia I., Serra J.A., et al. Comparison of wild and cultured sea bass (Dicentrarchus labrax) quality. Food Chem. 2010;119(4):1514–1518. [Google Scholar]
- Gasco L., Belforti M., Rotolo L., et al. Mealworm (Tenebrio molitor) as a potential ingredient in practical diets for rainbow trout (Oncorhynchus mykiss) Abstract book Conference. 2014 69-69. (Intervento presentato al convegno Insect to feed the world tenutosi a Ede nel 14-17 May 2014) [Google Scholar]
- Guo X., Zhang X.X., Yue H.C., et al. Evaluation of hierarchically weighted principal component analysis for water quality management at Jiaozuo mine. Int Biodeterior Biodegrad. 2018;128:182–185. [Google Scholar]
- Haard N.F. Control of chemical composition and food quality attributes of cultured fish. Food Res Int. 1992;25:289–307. [Google Scholar]
- Hagen O., Solberg C., Sirnes E., et al. Biochemical and structural factors contributing to seasonal variation in the texture of farmed Atlantic halibut (Hippoglossus hippoglossus L) flesh. Agricultural and Food Chemistry. 2007;55(14):5803–5808. doi: 10.1021/jf063614h. [DOI] [PubMed] [Google Scholar]
- Hamada-Sato N., Usui K., Kobayashi T., et al. Quality assurance of raw fish based on HACCP concept. Food Control. 2005;16(4):301–307. [Google Scholar]
- Hardy R.W. Utilization of plant proteins in fish diets: effects of global demand and supplies of fishmeal. Aquacult Res. 2010;41(5):770–776. [Google Scholar]
- Hearing V.J. Biogenesis of pigment granules: a sensitive way to regulate melanocyte function. J Dermatol Sci. 2005;37(1):3–14. doi: 10.1016/j.jdermsci.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Heising J.K., van Boekel M.A.J.S., Dekker M., et al. Mathematical models for the trimethylamine (TMA) formation on packed cod fish fillets at different temperatures. Food Res Int. 2014;56:272–278. [Google Scholar]
- Henry M., Gasco L., Piccolo G., et al. Review on the use of insects in the diet of farmed fish: past and future. Anim Feed Sci Technol. 2015;203:1–22. [Google Scholar]
- Herring H.K., Cassens R.G., Rriskey E.J. Further studies on bovine muscle tenderness as influenced by carcass position, sarcomere length, and fiber diameter. J Food Sci. 2006;30(6):1049–1054. [Google Scholar]
- Hixson S.M., Parrish C.C., Anderson D.M. Full substitution of fish oil with camelina (Camelina sativa) oil, with partial substitution of fish meal with camelina meal, in diets for farmed Atlantic salmon (Salmo salar) and its effect on tissue lipids and sensory quality. Food Chem. 2014;157:51–61. doi: 10.1016/j.foodchem.2014.02.026. [DOI] [PubMed] [Google Scholar]
- Huang F.R., Zhan Z.P., Luo J., et al. Duration of dietary linseed feeding affects the intramuscular fat, muscle mass and fatty acid composition in pig muscle. Livest Sci. 2008;118:1–2. [Google Scholar]
- Huyben D., Vidakovic A., Hallgren S.W., et al. High-throughput sequencing of gut microbiota in rainbow trout (Oncorhynchus mykiss) fed larval and pre-pupae stages of black soldier fly (Hermetia illucens) Aquaculture. 2019;500:485–491. [Google Scholar]
- Iaconisi V., Bonelli A., Pupino R., et al. Mealworm as dietary protein source for rainbow trout: body and fillet quality traits. Aquaculture. 2018;484:197–204. [Google Scholar]
- Jiang W.D., Wu P., Tang R.J., et al. Nutritive values, flavor amino acids, healthcare fatty acids and flesh quality improved by manganese referring to up-regulating the antioxidant capacity and signaling molecules TOR and Nrf2 in the muscle of fish. Food Res Int. 2016;89:670–678. doi: 10.1016/j.foodres.2016.09.020. [DOI] [PubMed] [Google Scholar]
- Johansen K.A., Overturf K. Quantitation expression analysis of genes affecting muscle growth during development of rainbow trout (Oncorhynchus mykiss) Marine Biotechnology. 2005;7:576–587. doi: 10.1007/s10126-004-5133-3. [DOI] [PubMed] [Google Scholar]
- Johnston I.A., Li X.J., Vieira V.L.A., et al. Muscle and flesh quality traits in wild and fanned Atlantic salmon. Aquaculture. 2006;256:323–336. [Google Scholar]
- Johnston I.A., Alderson R., Sandham C., et al. Muscle fibre density in relation to the color and texture of smoked Atlantic salmon (salmo salar) Aquaculture. 2000;189(3–4):335–349. [Google Scholar]
- Karlsen Ø., Amlund H., Berg A., et al. The effect of dietary chitin on growth and nutrient digestibility in farmed Atlantic cod, Atlantic salmon and Atlantic halibut. Aquacult Res. 2017;48:123–133. [Google Scholar]
- Katya K., Borsra M., Ganesan D., et al. Efficacy of insect larval meal to replace fish meal in juvenile barramundi, Lates calcarifer reared in freshwater. International Aquatic Research. 2017;9:303–312. [Google Scholar]
- Kennedy J.M., Eisenberg B.R., Reid S.K., et al. Nascent muscle fiber appearance in overloaded chicken slow-tonic muscle. Am J Anat. 1988;181(2):203–215. doi: 10.1002/aja.1001810209. [DOI] [PubMed] [Google Scholar]
- Kennedy J.M., Sweeney L.J., Gao L. Ventricular myosin expression in developing and regenerating muscle, cultured myotubes, and nascent myofibers of overloaded muscle in the chicken. Med Sci Sports Exerc. 1989;5:S1874197. [PubMed] [Google Scholar]
- Khoushab F., Yamabhai M. Chitin research revisited. Mar Drugs. 2010;8(7):1988–2012. doi: 10.3390/md8071988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga A., Wakamatsu Y., Kurosawa G., et al. Oculocutaneous albinism in the 16 mutants of the medaka fish is associated with a deletion in the tyrosinae gene. Pigm Cell Res. 1999;12(4):252–258. doi: 10.1111/j.1600-0749.1999.tb00758.x. [DOI] [PubMed] [Google Scholar]
- Kroeckel S., Harjes A.-G.E., Roth I., et al. When a turbot catches a fly: evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetia illucens) as fish meal substitute - growth performance and chitin degradation in juvenile turbot (Psetta maxima) Aquaculture. 2012;364–365:345–352. [Google Scholar]
- Kuda T., Fujita M., Goto H., et al. Effects of retort conditions on ATP-related compounds in pouched fish muscle. LWT - Food Sci Technol (Lebensmittel-Wissenschaft -Technol) 2008;41(3):469–473. [Google Scholar]
- Li L., Zhou J.S., He Y.L., et al. Comparative study of muscle physicochemical characteristics in common cyprinus carpio, silurus asotus and Ctenopharyngodon idellus. Journal of Hydroecology. 2013;34:82–86. [Google Scholar]
- Li S.L., Ji H., Zhang B., et al. Defatted black soldier fly (Hermetia illucens) larvae meal in diets for juvenile Jian carp (Cyprinus carpio var. Jian): growth performance, antioxidant enzyme activities, digestive enzyme activities, intestine and hepatopancreas histological structure. Aquaculture. 2017;477:62–70. [Google Scholar]
- Li Y., Kortner T.M., Chikwati E.M., et al. Total replacement of fish meal with black soldier fly (Hermetia illucens) larvae meal does not compromise the gut health of Atlantic salmon (Salmo salar) Aquaculture. 2020;520 [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lock E.R., Arsiwalla T., Waagbø R. Insect larvae meal as an alternative source of nutrients in the diet of Atlantic salmon (Salmo salar) postsmolt. Aquacult Nutr. 2015;22(6):1202–1213. [Google Scholar]
- Lu R.H., Chen Y.N., Yu W.P., et al. Defatted black soldier fly (Hermetia illucens) larvae meal can replace soybean meal in juvenile grass carp (Ctenopharyngodon idellus) diets. Aquaculture Reports. 2020;18(2) [Google Scholar]
- Magalhães R., Sánchez-López A., Leal R.S., et al. Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax) Aquaculture. 2017;476:79–85. [Google Scholar]
- Makkar H.P.S., Tran G., Heuzé V., et al. State-of-the-art on use of insects as animal feed. Anim Feed Sci Technol. 2014;197:1–33. [Google Scholar]
- Mancini S., Medina I., Iaconisi V., et al. Impact of black soldier fly larvae meal on the chemical and nutritional characteristics of rainbow trout fillets. Animal. 2018;12(8):1672–1681. doi: 10.1017/S1751731117003421. [DOI] [PubMed] [Google Scholar]
- McLaren K. XIII—the development of the CIE 1976 (L∗ a∗ b∗) uniform color Space and colour-difference formula. J Soc Dye Colour. 1976;92:338–341. [Google Scholar]
- Meneguz M., Schiavone A., Gai F., et al. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J Sci Food Agric. 2018;98(15):5776–5784. doi: 10.1002/jsfa.9127. [DOI] [PubMed] [Google Scholar]
- Merrifield D.L., Olsen R.E., Myklebust R., et al. Dietary effect of soybean (Glycine max) products on gut histology and microbiota of fish. Soybean and Nutrition. 2011:231–250. [Google Scholar]
- Monaco R.D., Cavella S., Masi P. Predicting sensory cohesiveness, hardness and springiness of solid foods from instrumental measurements. J Texture Stud. 2008;39(2):129–149. [Google Scholar]
- Monaghan P., Metcalfe N.B., Torres R. Oxidative stress as a mediator of life history trade- offs: mechanisms, measurements and interpretation. Ecol Lett. 2009;12(1):75–92. doi: 10.1111/j.1461-0248.2008.01258.x. [DOI] [PubMed] [Google Scholar]
- Mørkøre T., Rødbotten M., Vogt G., et al. Relevance of season and nucleotide catabolism on changes in fillet quality during chilled storage of raw Atlantic salmon (Salmo salar L.) Food Chem. 2010;119(4):1417–1425. [Google Scholar]
- Ngo D.H., Kim S.K. Antioxidant effects of chitin, chitosan and their derivatives. Adv Food Nutr Res. 2014;73:15–31. doi: 10.1016/B978-0-12-800268-1.00002-0. [DOI] [PubMed] [Google Scholar]
- Ostaszewska T., Dabrowski K., Kamaszewski M., et al. The effect of plant protein-based diet supplemented with dipeptide or free amino acids on digestive tract morphology and PepT1 and PepT2 expressions in common carp (Cyprinus carpio L.) Comp Biochem Physiol Mol Integr Physiol. 2010;157(2):158–169. doi: 10.1016/j.cbpa.2010.06.162. [DOI] [PubMed] [Google Scholar]
- Periago M.J., Ayala M.D., López-Albors O., et al. Muscle cellularity and flesh quality of wild and farmed sea bass, Dicentrarchus labrax L. Aquaculture. 2005;249(1–4):175–188. [Google Scholar]
- Renna M., Schiavone A., Gai F., et al. Evaluation of the suitability of a partially defatted black soldier fly (Hermetia illucens L.) larvae meal as ingredient for rainbow trout (Oncorhynchus mykiss Walbaum) diets. J Anim Sci Biotechnol. 2017;8(1):957–969. doi: 10.1186/s40104-017-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón L., Castro P.L., Álvarez B., et al. Differences in proximal and fatty acid profiles, sensory characteristics, texture, color and muscle cellularity between wild and farmed blackspot seabream (Pagellus bogaraveo) Aquaculture. 2016;451:195–204. [Google Scholar]
- Rosenlund G., Corraze G., Izquierdo M., et al. Fish oil replacement and alternative lipid sources in aquaculture feeds. CRC Press; Boca Raton, FL: 2010. The effects of fish oil replacement on nutritional and organoleptic qualities of farmed fish; pp. 487–522. [Google Scholar]
- Rowlerson A., Veggetti A. 5 - cellular mechanisms of post-embryonic muscle growth in aquaculture species. Fish Physiol. 2001;18:103–140. [Google Scholar]
- Ryder J.M. Determination of adenosine triphosphate and its breakdown products in fish muscle by high-performance liquid chromatography. J Agric Food Chem. 1985;33(4):678–680. [Google Scholar]
- Santos J.F.S., Naval L.P. Spatial and temporal dynamics of water footprint for soybean production in areas of recent agricultural expansion of the Brazilian savannah (Cerrado) J Clean Prod. 2020;251 [Google Scholar]
- Sealey W.M., Gaylord T.G., Barrows F.T., et al. Sensory analysis of rainbow trout, Oncorhynchus mykiss, fed enriched black soldier fly prepupae, Hermetia illucens. J World Aquacult Soc. 2011;42:34–45. [Google Scholar]
- Secci G., Mancini S., Iaconisi V., et al. Can the inclusion of black soldier fly (Hermetia illucens) in diet affect the flesh quality/nutritional traits of rainbow trout (Oncorhynchus mykiss) after freezing and cooking? Int J Food Sci Nutr. 2019;70(2):161–171. doi: 10.1080/09637486.2018.1489529. [DOI] [PubMed] [Google Scholar]
- Shirai T., Kikuchi Y. Extractive components of kuruma prawn Penaeus japonicus. Fish Sci. 2002;68(2):1386–1389. [Google Scholar]
- Shiu Y.L., Wong S.L., Guei W.C., et al. Increase in the plant protein ratio in the diet of white shrimp, Litopenaeus vannamei (Boone), using Bacillus subtilis E20-fermented soybean meal as replacement. Aquacult Res. 2013;46(2):382–394. [Google Scholar]
- Sigholt T., Erikson U., Rustad T., et al. Handling stress and storage temperature affect meat quality of farmed-raised Atlantic Salmon (Salmo Salar) J Food Sci. 2006;62(4):898–905. [Google Scholar]
- Srikanchai T., Murani E., Wimmers K., et al. Four loci differentially expressed in muscle tissue depending on water-holding capacity are associated with meat quality in commercial pig herds. Mol Biol Rep. 2010;37:595–601. doi: 10.1007/s11033-009-9856-0. [DOI] [PubMed] [Google Scholar]
- Suloma A., El-Husseiny O.M., Hassane M.I., et al. Complementary responses between hydrolyzed feather meal, fi sh meal and soybean meal without amino acid supplementation in Nile tilapia Oreochromis niloticus diets. Aquacult Int. 2014;22(4):1377–1390. [Google Scholar]
- Swatson H.K., Gous R., Iji P.A., et al. Effect of dietary protein level, amino acid balance and feeding level on growth, gastrointestinal tract, and mucosal structure of the small intestine in broiler chickens. Anim Res. 2002;51(6):501–515. [Google Scholar]
- Tejada M. In: Fishery products: quality safety and authenticity. Rehbein H., Oehlenschlager J., editors. Blackwell Publishing Ltd; London, UK: 2009. ATP-derived products and K-value determination; pp. 68–81. [Google Scholar]
- Timm M., Bo M.J. Simultaneous determination of ammonia, dimethylamine, trimethylamine and teimehylamine N-oxide in fish extracts by capillary elec-trophoresis with indirect UV-detection. Food Chem. 2002;76(4):509–518. [Google Scholar]
- Tran G., Heuz´e V., Makkar H. Insects in fish diets. Animal Frontiers. 2015;5(2):37–44. [Google Scholar]
- Urban J., Štys D., Sergejevová M., et al. Expertomica Fishgui: comparison of fish skin colour. J Appl Ichthyol. 2012;29(1):172–180. [Google Scholar]
- Valente L.M.P., Cabral E.M., Sousa V., et al. Plantprotein blends in diets for senegalese sole affect skeletal muscle growth, flesh texture and the expression of related genes. Aquaculture. 2016;453:77–85. [Google Scholar]
- Valente L.M.P., Cornet J., Donnay-Moreno C., et al. Quality differences of gilthead sea bream from distinct production systems in Southern Europe: Intensive, integrated, semi-intensive or extensive systems. Food Control. 2011;22(5):708–717. [Google Scholar]
- Wang C.C., Liu W.B., Huang Y.Y., et al. Dietary DHA affects muscle fiber development by activating AMPK/Sirt1 pathway in blunt snout bream (Megalobrama amblycephala) Aquaculture. 2020;518 doi: 10.1155/2022/7285851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.Q., Li X., Zhang C.H., et al. Study on relationship between myofibril characteristics and meat quality of chicken raw meat. Sci Agric Sin. 2014;47(10):2003–2012. [Google Scholar]
- Wang G., Peng K., Hu J., et al. Evaluation of defatted black soldier fly (Hermetia illucens L.) larvae meal as an alternative protein ingredient for juvenile Japanese seabass (Lateolabrax japonicus) diets. Aquaculture. 2019;507:144–154. [Google Scholar]
- Wang H.L., Zhang J.J., Zhu Y.Z., et al. Volatile components present in different parts of grass carp. J Food Biochem. 2018;42(6) [Google Scholar]
- Wang Z.G., Pan X.J., Peng Z.Q., et al. Methionine and selenium yeast supplementation of the maternal diets affects color, water-holding capacity, and oxidative stability of their male offspring meat at the early stage. Poultry Sci. 2009;88(1):1096–1101. doi: 10.3382/ps.2008-00207. [DOI] [PubMed] [Google Scholar]
- Wei Y.T., Shen H.H., Xu W.Q., et al. Replacement of dietary fish meal by Antarctic krill meal on growth performance, intestinal morphology, body composition and organoleptic quality of large yellow croaker Larimichthys crocea. Aquaculture. 2019;512 [Google Scholar]
- Wekell J.C., Barnett H. New method for analysis of trimethylamine oxide using Ferrous Sulfate and EDTA. J Food Sci. 1991;56:132–135. [Google Scholar]
- Wright W.E., Sassoon D.A., Lin V.K., Myogenin A factor regulating myogenesis, has a domain homologous to myod. Cell. 1989;56(4):607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- Wu Y.P., Feng L., Jiang w.d., et al. Influence of dietary zinc on muscle composition, flesh quality and muscle antioxidant status of young grass carp (Ctenopharyngodon idella Val.) Aquacult Res. 2014;46(10):2360–2373. [Google Scholar]
- Xu J., Feng L., Jiang W.D., et al. Different dietary protein levels affect flesh quality, fatty acids and alter gene expression of Nrf2-mediated antioxidant enzymesin the muscle of grass carp (Ctenopharyngodon idella) Aquaculture. 2018;493:272–282. [Google Scholar]
- Xu X.X., Ji H., Belghit I., et al. Effects of black soldier fly oil rich in n-3 HUFA on growth performance, metabolism and health response of juvenile mirror carp (Cyprinus carpio var. specularis) Aquaculture. 2020;533(43) [Google Scholar]
- Zhao H.H, Chong J., Tang R., et al. Metabolomics investigation of dietary effects on flesh quality in grass carp (Ctenopharyngodon idellus) GigaScience. 2018;7(10):giy111. doi: 10.1093/gigascience/giy111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J.S., Liu S.S., Ji H., et al. Effect of replacing dietary fish meal with black soldier fly larvae meal on growth and fatty acid composition of Jian carp (Cyprinus carpio var. Jian) Aquacult Nutr. 2018;24(1):424–433. [Google Scholar]
- Zhu K.C., Wang H.L., Wang H.J., et al. Characterization of muscle morphology and satellite cells, and expression of muscle related genes in skeletal muscle of juvenile and adult Megalobrama amblycephala. Micron. 2014;64:66–75. doi: 10.1016/j.micron.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Zielińska E., Karaś M., Jakubczyk A. Antioxidant activity of predigested protein obtained from a range of farmed edible insects. Int J Food Sci Technol. 2017;52(2):306–312. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.