Abstract
Magrolimab is a monoclonal antibody that blocks CD47, a ‘do not eat me’ signal overexpressed on tumor cells. CD47 is overexpressed in multiple myeloma (MM), which contributes to its pathogenesis. Preclinical studies have shown that CD47 blockade induces macrophage activation, resulting in elimination of myeloma cells, and that there is synergy between magrolimab and certain anticancer therapies. These findings suggest that magrolimab-based combinations may have a therapeutic benefit in MM. This phase II study investigates magrolimab in combination with commonly used myeloma therapies in patients with relapsed/refractory MM and includes a safety run-in phase followed by a dose-expansion phase. Primary end points include the incidence of dose-limiting toxicities and adverse events (safety run-in) and the objective response rate (dose expansion).
Keywords: bortezomib, carfilzomib, CD47, daratumumab, dexamethasone, immunotherapy, magrolimab, multiple myeloma, pomalidomide
Plain language summary
Magrolimab is a therapy that blocks a ‘do not eat me’ signal overexpressed by certain cancers, including multiple myeloma (MM) cells. Studies have shown that blocking this signal leads to destruction of myeloma cells and that this cancer-killing effect may be increased by combining magrolimab with certain additional anticancer therapies. These findings suggest that magrolimab-based combinations may have a therapeutic benefit in MM. This study is investigating magrolimab in combination with commonly used myeloma therapies in patients with MM who have persistent disease despite prior treatment. Goals of the trial include assessing safety and response to treatment.
Clinical Trial Registration: NCT04892446 (ClinicalTrials.gov)
Twitter abstract
This phase II study investigates safety and treatment response to #magrolimab, an antibody that enhances phagocytosis of cancer cells, combined with commonly used therapies in patients with #multiplemyeloma.
Multiple myeloma (MM) is a clonal plasma cell disorder that accounts for approximately 10% of hematologic malignancies and 1% of all cancers. In USA, approximately 32,000 new cases are diagnosed each year, and 13,000 patients die annually of this disease. Median age at diagnosis is approximately 65 years [1]. Current treatment options for newly diagnosed MM include proteasome inhibitors (PIs), immunomodulatory drugs, antibody therapies and autologous hematopoietic stem cell transplant; however, despite these therapies, most patients will eventually experience relapse [1–3]. Patients with MM that relapse or are refractory to therapy have a poor prognosis, and treatment remains challenging [2]. Outcomes remain particularly poor in patients who have received multiple lines of therapy. In real-world practice, progression-free survival (PFS) and overall survival (OS) decrease after the first line of therapy [4].
Due to the strong need for more efficacious therapies, particularly in the relapsed/refractory setting, multiple studies assessing novel treatment options for heavily pretreated patients with relapsed/refractory MM (RRMM) have been conducted. One such study investigated the antibody-drug conjugate belantamab mafodotin as single-agent therapy. The overall response rate in patients who had disease that progressed after ≥3 lines of therapy, was refractory to PIs and immunomodulatory drugs, and was refractory to and/or intolerant of anti-CD38 monoclonal antibody therapy was 34% (3.4-mg dose cohort) or 31% (2.5-mg/kg dose cohort) [3]. In a post hoc analysis, OS and median duration of response (DOR) were not reached [3].
Recent studies have indicated that multidrug combinations are superior to single- or double-agent combinations in treating MM [5]. The addition of new drugs to available regimens could induce a higher rate of initial complete response (CR), which could potentially improve PFS and OS. Contingent on the premise that the combined agents have nonoverlapping and synergistic mechanisms of action, the effective targeting of tumors with multiple agents is a promising strategy to improve clinical outcomes in patients with MM. Such a strategy is consistent with the emerging concept that the genetic signature of MM, and consequently the patient's susceptibility to a specific agent, is highly heterogeneous, which may lead to drug resistance. Furthermore, nonclinical studies support this strategy, demonstrating potential synergy with drug combination approaches [6–8].
Trial
Here we describe the background, rationale and design of an ongoing, phase II, open-label, multicenter, multi-arm study (NCT04892446) evaluating magrolimab (Hu5F9-G4) in combination with various antimyeloma therapies (daratumumab, pomalidomide/dexamethasone, carfilzomib/dexamethasone, and bortezomib/dexamethasone) in patients with RRMM. This study is sponsored by Gilead Sciences.
Background & rationale
Cancer cells overexpress CD47, an antiphagocytic ‘do not eat me’ signal, to evade detection and ingestion by macrophages [9,10]. The binding of CD47 to SIRPα on macrophages leads to inhibition of phagocytosis; therefore, blocking the CD47-SIRPα interaction results in enhanced phagocytosis and elimination of tumor cells [11]. Selective targeting of tumor cells occurs due to the presence of prophagocytic ‘eat me’ signals primarily expressed on tumor cells [11]. Since the CD47:SIRPα axis has been well established as a means of immune evasion by which cancer cells escape phagocytosis, growing evidence suggests that overexpression of CD47 contributes to the overall pathogenesis of MM [12,13]. Preclinical studies have shown that myeloma cells exhibit increased CD47 expression relative to healthy cells and that using an anti-CD47 antibody can inhibit the antiphagocytic signal in vitro, resulting in elimination of myeloma cells through phagocytosis [12,13]. Further, blocking CD47 and thus inhibiting the antiphagocytic signal enhances recruitment of cytotoxic T cells, susceptibility of tumor cells to T cells, and enhances natural killer (NK) cell antitumor ability [14–17]. These results suggest that CD47 is a potential immune checkpoint to target for the treatment of MM [18].
Magrolimab is a first-in-class humanized monoclonal antibody against CD47 that blocks its binding to SIRPα (Supplementary Figure 1) [9,10]. It was engineered with a human IgG4 isotype that is inefficient at recruiting Fc-dependent effector functions, thus decreasing toxic effects on healthy CD47-expressing cells [9]. Magrolimab combination therapies have shown clinical efficacy in other hematologic malignancies, such as acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) and are presently under investigation in solid tumors [19–21]. In patients with AML or MDS, magrolimab has shown promising efficacy and synergy in combination with azacitidine, a hypomethylating agent [19,20].
Due to the potential synergy and established clinical efficacy of magrolimab in other hematologic malignancies (AML/MDS), the following approved standard-of-care therapies were chosen as combination partners for magrolimab in this study in patients with RRMM.
Daratumumab is a first-in-class human monoclonal IgG1κ antibody targeting the CD38 antigen that has been approved as mono- or combination therapy for RRMM in USA [22,23]. CD38 is a transmembrane glycoprotein expressed on the surface of hematopoietic cells that aids in receptor-mediated adhesion and signaling and in modulation of cyclase and hydrolase [22,23]. Daratumumab has both indirect and direct antimyeloma activity and induces cell death through various mechanisms, including complement-dependent cytotoxicity, antibody-dependent cell-mediated cytotoxicity, antibody-dependent cellular phagocytosis, induction of apoptosis through Fc receptor-mediated crosslinking of daratumumab to the CD38 receptor, and depletion of CD38+ regulatory T cells, leading to increases in helper, cytotoxic T cells, and activity of NK cells [22–26]. Combination therapy with magrolimab is hypothesized to increase antibody-dependent cellular phagocytosis of tumor cells, as previously shown with rituximab in B-cell malignancies [27].
Pomalidomide targets cereblon, part of a ubiquitin ligase complex, and displays immunomodulatory, antiangiogenic and antineoplastic properties [28]. Similar to lenalidomide, pomalidomide acts by inhibiting proliferation and inducing apoptosis of tumor cells and works synergistically with dexamethasone [28,29]. It is indicated in USA for adult patients with RRMM who have received ≥2 prior regimens (including lenalidomide and a PI) and who experienced disease progression within ≤60 days of their last therapy [28]. Pomalidomide has shown promise in pre-clinical studies for the treatment of MM, acting on myeloma cells to induce G1 growth arrest and/or apoptosis [30,31]. Pomalidomide also increases proliferation of both T cells and NK cells in patients [32]. Accordingly, it is hypothesized that the addition of magrolimab may strengthen the combination therapy of pomalidomide/dexamethasone.
Carfilzomib is a tetrapeptide epoxyketone PI that irreversibly binds to N-terminal threonine active sites of the 20S proteasome, a core particle within the 26S proteasome [33]. The 26S proteasome is responsible for degrading ubiquitinated proteins. This mechanism is important for ensuring cell homeostasis; once disrupted, it can lead to cell death [34]. In USA, carfilzomib is indicated as combination therapy for patients with RRMM who have received one to three lines of therapy or as monotherapy for patients who have received ≥1 line of therapy [33]. Bortezomib is a reversible inhibitor of the chymotrypsin-like activity of the 26S proteasome and is indicated in USA for adult patients with RRMM [34]. In preclinical studies, carfilzomib and bortezomib have both been shown to decrease cell growth and increase apoptosis [35,36]. This study hypothesized that the combination of magrolimab with either of these PIs may exhibit synergistic properties leading to tumor cell death, as has been demonstrated in clinical studies with similar therapies [37,38].
Design
Study design
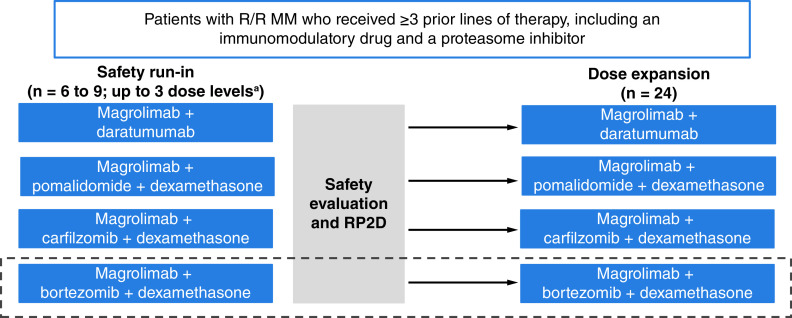
In this phase II, open-label, multicenter, multi-arm study (NCT04892446), magrolimab will be evaluated in combination with either daratumumab, pomalidomide/dexamethasone, or carfilzomib/dexamethasone in patients with RRMM. Based on the sponsor's discretion in interpreting the safety and efficacy results in the carfilzomib/dexamethasone cohort, an additional cohort of less heavily pretreated patients receiving magrolimab combined with bortezomib/dexamethasone may be initiated (Figure 1). Following completion of the safety run-in cohorts, dose-expansion cohorts will receive the same combination therapies. Patients will continue on treatment until the occurrence of unacceptable toxicity, disease progression, or discontinuation at patient or investigator discretion.
Figure 1. . Study design.
Magrolimab + bortezomib + dexamethasone may be initiated based on preliminary safety and efficacy data in the magrolimab + carfilzomib + dexamethasone cohort and if initiated, will require only one prior line of therapy.
aMagrolimab 1 mg/kg initial priming dose then 15–30 mg/kg.
MM: Multiple myeloma; RP2D: Recommended phase II dose; R/R: Relapsed/refractory.
Eligibility criteria
Key eligibility criteria can be found in Table 1. Briefly, patients must be ≥18 years of age, have an Eastern Cooperative Oncology Group performance status ≤2, and have received ≥3 prior lines of therapy, including an immunomodulatory drug and a PI. Patients cannot have received prior treatment with CD47- or SIRPα-targeting agents or be considered eligible for autologous or allogeneic stem cell transplant at the time of enrollment. If a patient qualifies for >1 treatment arm, selection of the treatment arm will be determined at the investigator's discretion.
Table 1. . Key eligibility criteria.
| Key inclusion criteria |
|---|
| Male or female aged ≥18 years |
| Previous diagnosis of MM based on the IMWG 2016 criteria and currently requiring treatment |
| Measurable disease, defined as ≥1 of the following: serum M-protein ≥0.5 g/dl (≥5 g/l), urine M-protein ≥200 mg/24 h, SFLC assay with involved SFLC level ≥10 mg/dl (100 mg/l) and abnormal SFLC ratio |
| ECOG performance status ≤2 |
| Received ≥3 previous lines of therapy, including an IMiD and a PI |
| Daratumumab arm: CD38+ MM with no prior anti-CD38 antibody therapy for ≤6 months prior to enrollment; no history of discontinuation of daratumumab due to toxicity |
| Pomalidomide/dexamethasone arm: no history of pomalidomide discontinuation due to toxicity; prior pomalidomide therapy allowed if PR was achieved with the most recent pomalidomide therapy and pomalidomide-free interval since last dose is ≥6 months; no contraindication to dexamethasone |
| Carfilzomib/dexamethasone arm: no history of discontinuation of carfilzomib due to toxicity; prior PI therapy allowed if PR was achieved with the most recent PI therapy; no contraindication to dexamethasone |
| Bortezomib/dexamethasone arm: no history of discontinuation of bortezomib due to toxicity; prior PI therapy allowed if PR was achieved with the most recent PI therapy and PI-free interval since last dose is ≥6 months; no contraindication to dexamethasone; if initiated, patients only require one prior line of therapy |
| Key exclusion criteria |
|---|
| Prior treatment with CD47- or SIRPα-targeting agents |
| MM of immunoglobulin M subtype, Waldenström macroglobulinemia, or MDS |
| Known amyloidosis, including myeloma complicated by amyloidosis |
| Plasma cell leukemia or circulating plasma cells ≥2 × 109/l |
| Solitary bone or extramedullary plasmacytoma as the only evidence of plasma cell dyscrasia |
| POEMS syndrome |
| Glucocorticoid therapy within 14 days prior to enrollment |
| Chemotherapy with approved or investigational anticancer therapies within 28 days prior to enrollment |
| Focal radiation therapy within 7 days prior to enrollment; radiation therapy to an extended field involving a significant volume of bone marrow within 21 days prior to enrollment |
| Immunotherapy within 28 days prior to enrollment |
| Autologous SCT <100 days prior to enrollment or considered eligible to receive autologous or allogeneic SCT at the time of enrollment |
ECOG: Eastern Cooperative Oncology Group; IMiD: Immunomodulatory drug; IMWG: International Myeloma Working Group; MDS: Myelodysplastic syndrome; MM: Multiple myeloma; PI: Proteasome inhibitor; POEMS: Plasma cell dyscrasia with polyneuropathy, organomegaly, endocrinopathy, M-protein and skin changes; PR: Partial response; SCT: Stem cell transplant; SFLC: Serum free light chain.
Dosing
The full dosing schedule for each study therapy can be found in Table 2. The rationale for the magrolimab dose in this study originates from safety, efficacy, and pharmacokinetic/pharmacodynamic data and modeling and simulation analyses based on data from ongoing and completed clinical trials in patients with solid tumors, non-Hodgkin lymphoma, and AML/MDS [19–21,27].
Table 2. . Dosing regimens.
| Drug | Dose level | (Cycle 1 is 35 days; remainder are 28 days) | ||
|---|---|---|---|---|
| Cycle 1 | Cycle 2 | Cycle 3+ | ||
| Magrolimab | Starting dose | Priming dose: day 1 Maintenance dose: days 8, 15, 22, 29 |
Maintenance dose: days 1, 8, 15, 22 | Maintenance dose: day 1, 15 |
| Daratumumab† | 16 mg/kg iv. or 1800 mg sc. |
Days 8, 15, 22, 29 | Days 1, 8, 15, 22 | Days 1, 15 for cycles 3 to 6; day 1 of cycles 7+ |
| Pomalidomide | 4 mg p.o. | Days 1 to 21 daily | Days 1 to 21 daily | Days 1 to 21 daily |
| Carfilzomib‡ | 20/70 mg/m2 iv. | Days 8 (20 mg/m2), 15 (70 mg/m2), 22 (70 mg/m2) | Days 1, 8, 15 | Days 1, 8, 15 |
| Bortezomib | 1.3 mg/m2 sc./iv.§ | Days 8, 15, 22, 29 | Days 1, 8, 15, 22 | Days 1, 8, 15, 22¶ |
| Dexamethasone | 40 mg p.o. | Days 1, 8, 15, 22, 29# | Days 1, 8, 15, 22 | Days 1, 8, 15, 22†† |
This arm will not receive treatment with dexamethasone.
Recommended starting dose is 20 mg/m2 on cycle 1, day 8. If tolerated, escalate dose to 70 mg/m2 on cycle 1, day 15 and thereafter.
sc. is preferred over iv., where feasible.
Maximum of 8 cycles in those who previously received bortezomib.
Administration on day 1 does not occur in the carfilzomib cohort.
Those patients in the magrolimab + carfilzomib + dexamethasone group will receive dexamethasone on days 1, 8, 15 and 22 from cycle 2 until cycle 9 and days 1, 8 and 15 from cycle 10 onward.
iv.: Intravenous; p.o.: Oral; sc.: Subcutaneous.
Magrolimab has been shown to induce predictable on-target anemia, an adverse event (AE) known to occur with blockage of CD47, as CD47 blockade can speed up the elimination of aging red blood cells [27,39]. To help mitigate on-target anemia, intravenous magrolimab will be administered as an initial priming dose (1 mg/kg), then as a weekly maintenance dose during the first two cycles, and then every 2 weeks starting in cycle 3 (15–30 mg/kg). Although no dose-limiting toxicities (DLTs) have been observed with magrolimab to date, and maximum tolerated dose has not been reached, dose de-escalation may occur in the event of DLTs, per protocol, in the safety run-in cohorts. The recommended phase II dose will be determined based on the clinical and pharmacokinetic data in the safety run-in cohort.
Given the lack of overlapping toxicities between any of the study drugs and magrolimab, the label-indicated doses and regimens were selected for all other therapies, excluding bortezomib. To improve patient convenience and to align better with magrolimab dosing, the bortezomib dosing regimen was changed from a 5-week cycle to a 4-week cycle.
Study procedures
Patients will be screened within 30 days prior to enrollment to determine eligibility. The length of cycle one will be 35 days, and all subsequent cycles will be 28 days. All patients will continue study treatment until study discontinuation criteria are met. Prior to administration of any study therapy, appropriate prophylactic medication will be provided.
All DLTs and AEs will be reported, as required. DLTs are defined as any grade ≥3 hematologic or nonhematologic toxicity that has worsened from baseline during the assessment period and may be related to magrolimab. All reports of AEs will be collected until 70 days following the last study drug administration. All toxicities will be graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) v5.0.
Efficacy assessments will be based on the International Myeloma Working Group (IMWG) 2016 criteria and will be completed in parallel with bone marrow assessments according to the schedule of assessments starting on cycle two, day 1, onward. For determination of disease response and progression, the following assessments will be used: serum protein electrophoresis (SPEP), urine protein electrophoresis (UPEP), serum free light chain (SFLC), serum immunofixation (SIFE), urine immunofixation (UIFE), bone marrow, minimal residual disease (MRD), bone lesions and extramedullary plasmacytoma evaluation. SPEP, UPEP, SFLC, SIFE and UIFE assessment will be completed at the central laboratory at the time of screening and will be repeated every 28 ± 7 days (from cycle one, day 1), regardless of changes in cycle length due to dose delays or discontinuations.
Patients will be evaluated for disease response and progression according to the IMWG 2016 criteria, with categories including stringent CR, CR, very good partial response, minimal response, stable disease and progressive disease. Diagnosis of progressive disease is based on central laboratory testing, and two consecutive assessments are required. Of note, this definition of progressive disease included progression due to the development of hypercalcemia solely attributed to recurrence or progression of MM. Bone marrow assessments, including aspirate and core/trephine biopsy specimens, are required and will be used to determine response. Samples can additionally be used for MRD assessments, other clinical reasons, or further biomarker/genomic research.
Samples will be obtained within 14 days prior to the first dose on cycle one, day 1; repeated within 7 days prior to cycle two, day 1; and repeated as clinically indicated from cycle three onward. MRD assessments will be completed using next-generation sequencing using clonoSEQ (Adaptive Biotechnologies, WA, USA) at multiple time points. Bone lesion assessments will be completed within 30 days prior to enrollment and repeated if patients exhibit worsening clinical symptoms or as clinically indicated. Imaging studies will be read locally. Extramedullary plasmacytoma assessment will be completed at screening and may be completed within 30 days prior to enrollment. If extramedullary plasmacytoma is found, patients will undergo repeated evaluation during treatment to evaluate response and will undergo radiological evaluation every 12 weeks.
Pharmacokinetic assessments will be performed using a validated enzyme-linked immunosorbent assay method. Immunogenicity assessments will also be completed using a validated immunoassay method. The schedule for pharmacokinetic and immunogenicity assessments can be found in Figure 2. Further assessments to determine biomarkers, mutation profile, mutation burden, immune effector cell composition, signaling molecules, and prophagocytic/antiphagocytic signals will also be completed in this study to help delineate dominant mechanisms of action and identify possible predictors of response.
Figure 2. . Schedule of study assessments.
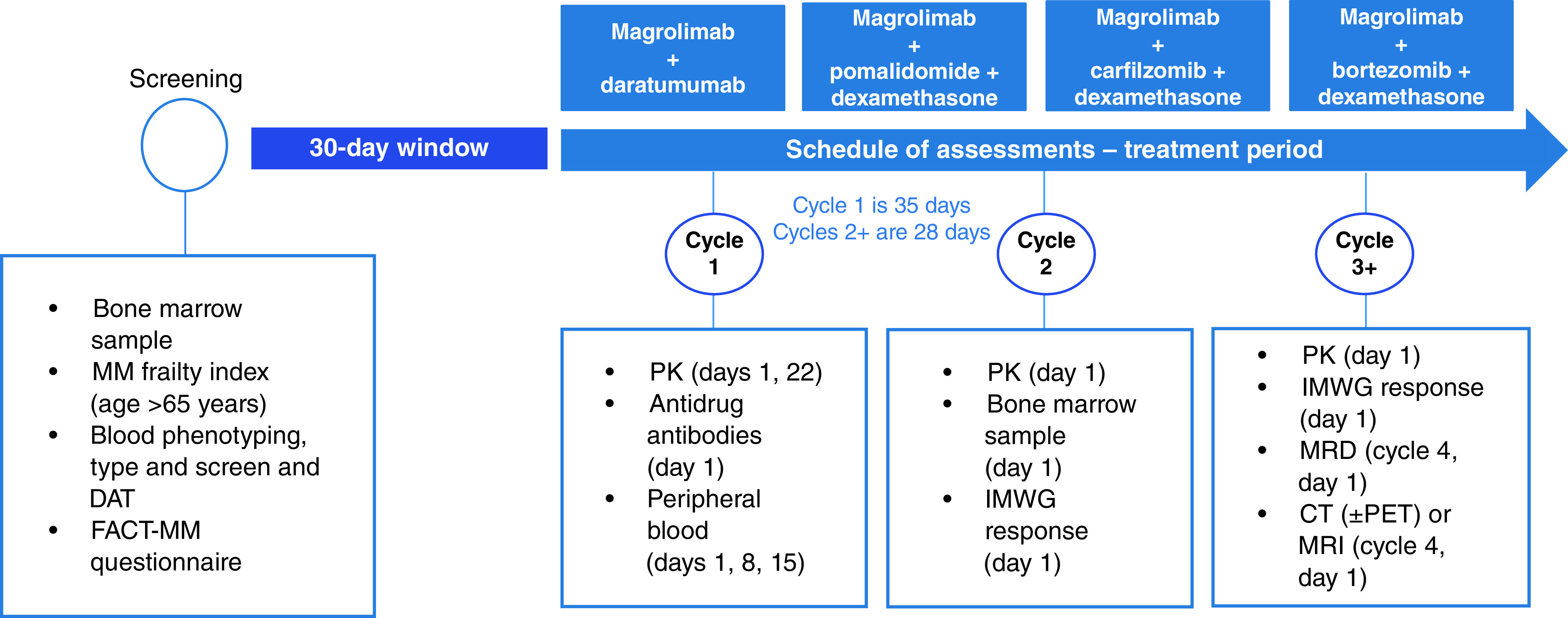
All treatment arms follow the same schedule of assessments. Not exhaustive.
CT: Computed tomography; DAT: Direct antiglobulin test; IMWG: International Myeloma Working Group; MM: Multiple myeloma; PET: Positron emission tomography; PK: Pharmacokinetics.
Health-related quality of life and potential for improvement over the study will be investigated using the MM-specific Functional Assessment of Cancer Therapy – Multiple Myeloma (FACT-MM) Patient-Reported Outcomes questionnaire.
In patients who do not experience progression after discontinuing treatment, assessments will continue until disease progression is documented, a new antimyeloma therapy is initiated, or 2 years have elapsed since the last dose of magrolimab.
Outcome measures/end points
The primary end point in the safety run-in cohort is to establish the incidence of DLTs, AEs and laboratory abnormalities according to the NCI CTCAE v5.0. The primary end point in the dose-expansion cohort is to establish the objective response rate (ORR). Secondary end points include DOR, PFS and OS. Exploratory end points include time-to-response and MRD negativity rate. A complete list of objectives and end points can be found in Table 3.
Table 3. . Objectives and end points.
| Safety run-in cohort | |
|---|---|
|
Primary objectives • To evaluate the safety and tolerability of magrolimab in combination with other anticancer therapies • To determine the RP2D for combination with daratumumab, pomalidomide/dexamethasone, carfilzomib/dexamethasone, and bortezomib/dexamethasone |
Primary end points • Incidence of DLTs, AEs, and laboratory abnormalities according to NCI CTCAE v5.0 |
| Dose-expansion cohort | |
|---|---|
|
Primary objective • To evaluate the efficacy of magrolimab in combination with other anticancer therapies in patients with relapsed/refractory MM |
Primary end points • ORR, defined as the percentage of patients who achieve complete response, stringent complete response, partial response, or very good partial response (IMWG 2016 criteria) |
|
Secondary objectives • To evaluate the safety and tolerability of magrolimab in combination with other anticancer therapies • To investigate the depth of response, DOR, and survival • To evaluate the PK and immunogenicity of magrolimab combination therapy in relapsed/refractory MM |
Secondary end points • Incidence of AEs and laboratory abnormalities according to NCI CTCAE v5.0 • DOR (measured from the earliest date of complete response, stringent complete response, partial response, or very good partial response to the earliest date of documented disease progression, relapse, or death from any cause), PFS (measured from the date of the first dose of study treatment to the earliest date of documented relapse, disease progression, or death from any cause), and OS (measured from the date of the first dose of study treatment to the date of death from any cause) (IMWG 2016 criteria) • Magrolimab concentration vs time and measurements of antidrug antibodies against magrolimab |
|
Exploratory objectives • To evaluate the impact of magrolimab combination therapy on: • MRD negativity • Mutation profiles and mutation burden in myeloma cells • Immune effector cell composition and signaling molecules • Prophagocytic/antiphagocytic signal expressed by myeloma cells • Health-related quality of life • Time-to-response |
Exploratory end points • MRD negativity rate (IMWG 2016 criteria) • Mutational profile of myeloma cells and the correlation with clinical response • Changes from baseline in biomarkers of immune cell recruitment and signaling molecules • Changes from baseline in known phagocytic regulators in myeloma cells • Change from baseline in FACT-MM questionnaire • Time-to-response (IMWG 2016 criteria) |
AE: Adverse event; DLT: Dose-limiting toxicity; DOR: Duration of response; FACT-MM: Functional Assessment of Cancer Therapy – Multiple Myeloma; IMWG: International Myeloma Working Group; MM: Multiple myeloma; MRD: Minimal residual disease; NCI CTCAE: National Cancer Institute Common Terminology Criteria for Adverse Events; ORR: Objective response rate; OS: Overall survival; PFS: Progression-free survival; PK: Pharmacokinetics; RP2D: Recommended phase II dose.
Planned sample size
This study will include approximately 153 patients, with up to 27 participants in each safety run-in cohort and 72 participants in the dose-expansion cohorts. A sample size of 30 patients for each of the dose-expansion cohorts would provide 86.1% power for a one-group χ2 test at a one-sided alpha of 0.1 to detect an ORR of ≥45% for the combination treatment compared with a historical control ORR of 25%. The historical control ORR of 25% is based on outcomes from the MAMMOTH study in the subset of patients treated with any daratumumab-containing regimen, including daratumumab in combination with an immunomodulatory drug or PI, following ≥1 prior treatment [40].
Planned study period
This study started on 9 November 2021, and has an estimated completion date of May 2024 [41]. The final analysis will be performed once all patients have completed the study and all data have been finalized.
Statistics
All enrolled patients who received ≥1 dose of the study therapy assigned to them at enrollment will be included in the primary analysis of efficacy and safety. For inclusion in the pharmacokinetics, immunogenicity, and biomarker analyses, participants are required to have received ≥1 dose of the study treatment assigned to them and to have ≥1 posttreatment measurement of magrolimab serum concentration, ≥1 evaluable anti-magrolimab antibody test result, or evaluable baseline and on-study measurements, respectively.
In the primary analysis, a point estimate and two-sided exact 95% CI will be determined for ORR using the Clopper–Pearson method, and testing against a control rate of 25% using a one-group χ2 will be conducted in each respective cohort. In the secondary analysis, median scores and first and third quartiles estimated with the Kaplan–Meier method, as well as 95% CIs, will be determined for DOR, PFS and OS. The proportion of event-free patients at benchmark points at 6 and 12 months will also be estimated with the Kaplan–Meier method. In the exploratory analysis, median and first and third quartile for time-to-response will be summarized with descriptive statistics in those participants who achieved an objective response. The MRD negativity rate will be provided with a point estimate and a two-sided exact 95% CI using the Clopper–Pearson method. FACT-MM scores will be summarized.
All data from day 1 after first-dose administration to 70 days after the last dose will be included in the safety analysis. DLTs will be summarized. The pharmacokinetics analysis will be depicted in a magrolimab versus time plot with summary statistics and descriptive graphical points of individual concentration versus time and mean concentration versus time. Immunogenicity analysis will be run with a three-tier approach, including a screen, confirmatory test, and titer testing using immunoassay. Biomarker analysis will be a summation of descriptive statistics of baseline, absolute, and changes in biomarker status.
COVID-19 vaccination
No substantial safety data are available regarding the concomitant administration of COVID-19 vaccines and magrolimab. Patients are allowed to receive the COVID-19 vaccine, and study visits should continue as planned if vaccination occurs while a patient is in the study. Investigators should follow local guidelines for concomitant administration of COVID-19 vaccines and study drugs.
Enrollment
The study is open for enrollment. Additional information is available at ClinicalTrials.gov (NCT04892446).
Conclusion
Patients with RRMM currently have limited treatment options and poor survival outcomes, including short DOR and limited PFS and OS. Combination therapies with the CD47 inhibitor magrolimab represent a potentially promising therapeutic option based on strong preclinical rationale in patients with RRMM. This ongoing phase II trial evaluating the safety, tolerability, and efficacy of magrolimab in combination with other anticancer therapies in patients with RRMM will potentially support additional treatment options in this currently limited disease space.
Executive summary.
Introduction
Despite multiple treatment options, multiple myeloma (MM) remains difficult to treat, and many patients will experience refractory disease.
Background & rationale
Magrolimab is a first-in-class human monoclonal antibody that blocks CD47, the ‘do not eat me' molecule expressed on cancer cells. CD47 is a well-known mediator of cancer cell evasion of the innate immune system. Evidence suggests that the overexpression of CD47 may play a large role in the pathogenesis of MM.
Combination therapies have exhibited greater efficacy than single-agent therapies in treating MM.
Study design & eligibility
This is a phase II, open-label, multicenter, multi-arm study (NCT04892446) evaluating magrolimab in combination with daratumumab, pomalidomide/dexamethasone, carfilzomib/dexamethasone, or bortezomib/dexamethasone in patients with relapsed/refractory MM (RRMM) who have received ≥3 prior lines of therapy, including an immunomodulatory drug and a proteasome inhibitor (PI). Approximately 153 patients will be included in the study and, based on prior therapies received, will be assigned to a treatment combination group.
Outcomes & end points
The primary end point in the safety run-in cohort is the incidence of dose-limiting toxicities (DLTs), adverse events (AEs) and laboratory abnormalities according to the NCI CTCAE v5.0, and the primary end point in the dose-expansion cohort is objective response rate (ORR). Secondary end points include the incidence of AEs and laboratory abnormalities, duration of response (DOR), progression-free survival (PFS), overall survival (OS) and pharmacokinetics. Exploratory end points include the minimal residual disease (MRD) negativity rate, changes in the mutational profile of myeloma cells, changes from baseline in biomarkers of immune cell recruitment and signaling molecules, changes from baseline in phagocytic regulators, and changes from baseline in the FACT-MM questionnaire.
Conclusion
Ultimately, this study will define the role of magrolimab combination therapy in a heavily pretreated subset of patients with RRMM. Results of this study will prove vital for this population, in which such high unmet need exists.
Supplementary Material
Acknowledgments
The authors would like to thank the patients, family, friends and caregivers participating in this study, as well as the study staff.
Footnotes
Supplementary data
An infographic accompanies this paper. To view or download this infographic in your browser please click here: https://www.futuremedicine.com/doi/suppl/10.2217/fon-2022-0975
Author contributions
All authors made a substantial contribution to the protocol and study design. All authors have completed a thorough review of all materials, have made revisions, and have reviewed the final manuscript prior to its submission.
Financial & competing interests disclosure
B Paul reports payments or honoraria from Amgen; and participation on advisory boards for Abbvie, Janssen and Regeneron. M Liedtke reports research funding from Allogene, Seagen, Bristol Myers Squibb, Gilead Sciences, Inc., Caelum and Janssen; and participation on advisory boards for Alnylam, Bristol Myers Squibb, Natera, Oncopeptides, Adaptive, Sanofi, Takeda and GlaxoSmithKline. J Khouri has nothing to disclose. R Rifkin reports participation on data safety monitoring boards for CARsgen, Amgen, Celgene, A Bristol–Myers Squibb Company, Coherus, Fresenius-Kabi and Takeda; and stock ownership in McKesson. MD Gandhi reports honoraria from GlaxoSmithKline, TG Therapeutics, Karyopharm Therapeutics, and Janssen Oncology. A Kin has nothing to disclose. MY Levy reports consulting fees and payment or honoraria from Bristol–Myers Squibb, Amgen, Janssen, Takeda, Beigene, Novartis, AstraZeneca, Jazz Pharmaceuticals, Morphosys, Seattle Genetics, Karyopharm, GlaxoSmithKline, TG Therapeutics, Gilead Sciences, Inc., Epizyme, Abbvie and Dova. R Silbermann reports consulting fees for Janssen; grants or contracts from Sanofi–Aventis; participation on data safety monitoring board for Janssen and Sanofi–Aventis; and other support from Adaptive Biotechnologies. F Cottini has nothing to disclose. DW Sborov reports consulting fees from GlaxoSmithKline, Abbvie, Pfizer, Bristol–Myers Squibb, Janssen and Sanofi; and participation on advisory boards for GlaxoSmithKline, Janssen, and Sanofi. I Sandhu reports payments or honoraria from Celgene, A Bristol–Myers Squibb Company, Janssen, Amgen, Takeda, Gilead/Kite, Pfizer and Forus. L Villarreal, M Murphy, L Gu, A Chen, N Rajakumaraswamy are employees of, and report stock ownership in, Gilead Sciences, Inc. SZ Usmani reports research funding and consulting fees from Pharmacyclics, Seattle Genetics, Merck, SkylineDX, Takeda, Janssen, Array BioPharma, Sanofi, Celgene, A Bristol–Myers Squibb Company, GlaxoSmithKline and Amgen; research funding from Bristol–Myers Squibb; consulting fees from Abbvie and EdoPharma; and payments or honoraria from Takeda, Sanofi, Celgene, a Bristol–Myers Squibb Company, Janssen and Amgen. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Editorial assistance was provided by Mi Sulzinski, MD, of SciMentum, Inc, a Nucleus Group Holdings, Inc, company (NJ, USA), and was funded by Gilead Sciences, Inc.
Ethical conduct of research
Investigators confirm that this study is conducted in accordance with the International Council for Harmonisation E6 (R2) addendum to its guidelines for GCP. Prior to implementation, the protocol will be reviewed and approved by the appropriate institutional review board/independent ethics committee at each participating institution. Patients have provided written informed consent to participate in this trial. Patient identifying information will be maintained as confidential and remain unavailable to unauthorized parties.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest
- 1.Rajkumar SV. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am. J. Hematol. 95(5), 548–567 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Chari A, Vogl DT, Gavriatopoulou M et al. Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N. Engl. J. Med. 381(8), 727–738 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Lonial S, Lee HC, Badros A et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label phase 2 study. Lancet Oncol. 21(2), 207–221 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Bruno AS, Willson JL, Opalinska JM et al. Recent real-world treatment patterns and outcomes in US patients with relapsed/refractory multiple myeloma. Expert Rev. Hematol. 13(9), 1017–1025 (2020). [DOI] [PubMed] [Google Scholar]
- 5.American Cancer Society. Drug therapy for multiple myeloma (2021). www.cancer.org/cancer/multiple-myeloma/treating/chemotherapy.html
- 6.Nijhof IS, Groen RW, Noort WA et al. Preclinical evidence for the therapeutic potential of CD38-targeted immuno-chemotherapy in multiple myeloma patients refractory to lenalidomide and bortezomib. Clin. Cancer Res. 21(12), 2802–2810 (2015). [DOI] [PubMed] [Google Scholar]; • A preclincal study describing the effects of daratumumab combination therapy in multiple myeloma
- 7.Ocio EM, Vilanova D, Atadja P et al. In vitro and in vivo rationale for the triple combination of panobinostat (LBH589) and dexamethasone with either bortezomib or lenalidomide in multiple myeloma. Haematologica 95(5), 794–803 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]; • A preclinical study providing rationale for the use of triple drug combination therapy in multiple myeloma
- 8.Giliberto M, Thimiri Govinda Raj DB, Cremaschi A et al. Ex vivo drug sensitivity screening in multiple myeloma identifies drug combinations that act synergistically. Mol. Oncol. 16(6), 1241–1258 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; • A preclinical study explaining rationale for the use of drug combination therapy, including in multiple myeloma
- 9.Liu J, Wang L, Zhao F et al. Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PLOS ONE 10(9), e0137345 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes the development of magrolimab, as well as preclinical data supporting its use as an anticancer therapy
- 10.Chao MP, Takimoto CH, Feng DD et al. Therapeutic targeting of the macrophage immune checkpoint CD47 in myeloid malignancies. Front. Oncol. 9, 1380 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Highlights the many roles of CD47, preclinical data supporting the use of targeted therapies, and clinical data involving the use of magrolimab
- 11.Chao MP, Jaiswal S, Weissman-Tsukamoto R et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci. Transl. Med. 2(63), 63ra94 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim D, Wang J, Willingham SB, Martin R, Wernig G, Weissman IL. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia 26(12), 2538–2545 (2012). [DOI] [PubMed] [Google Scholar]; • A preclinical study exhibiting the downstream effects of CD47 blockade
- 13.Sun J, Muz B, Alhallak K et al. Targeting CD47 as a novel immunotherapy for multiple myeloma. Cancers (Basel) 12(2), 305 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soto-Pantoja DR, Terabe M, Ghosh A et al. CD47 in the tumor microenvironment limits cooperation between antitumor T-cell immunity and radiotherapy. Cancer Res. 74(23), 6771–6783 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Pu Y, Cron K et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat. Med. 21(10), 1209–1215 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz AL, Nath PR, Allgauer M et al. Antisense targeting of CD47 enhances human cytotoxic T-cell activity and increases survival of mice bearing B16 melanoma when combined with anti-CTLA4 and tumor irradiation. Cancer Immunol. Immunother. 68(11), 1805–1817 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendriks M, Britsch I, Ke X et al. Cancer cells under immune attack acquire CD47-mediated adaptive immune resistance independent of the myeloid CD47-SIRPα axis. Oncoimmunology 10(1), 2005344 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chao MP, Majeti R, Weissman IL. Programmed cell removal: a new obstacle in the road to developing cancer. Nat. Rev. Cancer 12(1), 58–67 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Daver NG, Vyas P, Kambhampati S et al. Tolerability and efficacy of the first-in-class anti-C47 antibody magrolimab combined with azacitidine in frontline patients with TP53-mutated acute myeloid leukemia: phase 1b results. J. Clin. Oncol. 40(Suppl. 16), Abstract 7020 (2022). [Google Scholar]
- 20.Sallman DA, Al Malki M, Asch AS et al. Magrolimab in combination with azacitidine for untreated higher risk myelodysplastic syndrone (HR-MDS): 5F9005 phase 1b study results. J. Clin. Oncol. 40(Suppl. 16), Abstract 7017 (2022). [Google Scholar]
- 21.ClinicalTrials.gov. Study of magrolimab in participants with solid tumors (2022). https://clinicaltrials.gov/ct2/show/NCT04827576
- 22.Darzalex Faspro (daratumumab and hyaluronidase-fihj). Prescribing information. Janssen Biotech, Inc. 1-16 ; Horsham, PA, USA: (2022). [Google Scholar]
- 23.Darzalex (daratumumab). Prescribing information. Janssen Biotech, Inc. 1-16 ; Horsham, PA, USA: (2022). [Google Scholar]
- 24.Mahaweni NM, Bos GMJ, Mitsiades CS, Tilanus MGJ, Wieten L. Daratumumab augments alloreactive natural killer cell cytotoxicity towards CD38+ multiple myeloma cell lines in a biochemical context mimicking tumour microenvironment conditions. Cancer Immunol. Immunother. 67(6), 861–872 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho H, Kim KH, Lee H et al. Adaptive natural killer cells facilitate effector functions of daratumumab in multiple myeloma. Clin. Cancer Res. 27(10), 2947–2958 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Krejcik J, Casneuf T, Nijhof IS et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 128(3), 384–394 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Advani R, Flinn I, Popplewell L et al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin's lymphoma. N. Engl. J. Med. 379(18), 1711–1721 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pomalyst (pomalidomide). Prescribing information. Celgene Corporation. 1-16 ; Summit, NJ, USA: (2021). [Google Scholar]
- 29.Revlimid (lenalidomide). Prescribing information. Celgene Corporation.1-28 ; Princeton, NJ, USA: (2022). [Google Scholar]
- 30.Mitsiades N, Mitsiades CS, Poulaki V et al. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood 99, 4525–4530 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Hideshima T, Chauhan D, Shima Y et al. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood 96, 2943–2950 (2000). [PubMed] [Google Scholar]
- 32.Pierceall WE, Amatangelo MD, Bahlis NJ et al. Immunomodulation in pomalidomide, dexamethasone, and daratumumab-treated patients with relapsed/refractory multiple myeloma. Clin. Cancer Res. 26(22), 5895–5902 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Kyprolis (carfilzomib). Prescribing information. Onyx Pharmaceuticals; Amgen Inc. 1-85; Thousand Oaks, CA, USA: (2022). [Google Scholar]
- 34.Velcade (bortezomib) . Prescribing information.Takeda Pharmaceuticals America Inc. 1-44 ; Lexington, MA, USA: (2021). [Google Scholar]
- 35.Kuhn DJ, Chen Q, Voorhees PM et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood 110(9), 3281–3290 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boccadoro M, Morgan G, Cavenagh J. Preclinical evaluation of the proteasome inhibitor bortezomib in cancer therapy. Cancer Cell Int. 5(1), 18 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richards J, Bouchlaka MN, Puro RJ et al. Highly differentiated anti-CD47 antibody, AO-176, potently inhibits hematologic malignancies alone and in combination. Blood 134(Suppl. 1), 1844 (2019). [Google Scholar]
- 38.Linderoth E, Helke S, Lee V et al. The anti-myeloma activity of TTI-621 (SIRPαFc), a CD47-blocking immunotherapeutic, is enhanced when combined with a proteasome inhibitor. Presented at: AACR Annual Meeting 2017. Washington, DC, USA: (1–5 April 2017). [Google Scholar]
- 39.Swoboda DM, Sallman DA. The promise of macrophage directed checkpoint inhibitors in myeloid malignancies. Best Pract. Res. Clin. Haematol. 33(4), 101221 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Gandhi UH, Cornell RF, Lakshman A et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 33(9), 2266–2275 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.ClinicalTrials.gov. Study of magrolimab combinations in participants with relapsed/refractory multiple myeloma (2022). https://clinicaltrials.gov/ct2/show/NCT04892446
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.