Abstract
A highly diastereoselective Pd(0)-catalyzed Mizoroki–Heck reaction of gem-difluoroalkenes is described. Unlike previously reported C–F bond functionalization with organometallic reagents, this reaction takes place between two different alkenes to achieve a formal C–F and C–H bond cross-coupling via a distinct pathway. Monofluorinated 1,3-diene products can be synthesized with control of the geometry of each alkene and good functional group tolerability.
The Mizoroki–Heck reaction is a palladium-catalyzed cross-coupling between organic halides (usually I, Br, and Cl) and alkenes via oxidative addition/carbopalladation/β-H elimination in the presence of a base. Since its discovery over half a century ago,1 this reaction has become state-of-the-art for installing carbon–carbon double bonds with wide applications in industry and academia,2 which culminated in the 2010 Nobel Prize in Chemistry.3 The key features of the Mizoroki–Heck reaction include high efficiency, ready availability, low-cost alkene feedstocks, and good stereo- and chemoselectivity. Tremendous progress has been made in modern versions of this classic reaction by improving the catalytic systems and broadening the substrate scopes.
In recent years, the Mizoroki–Heck reaction has been utilized to introduce fluorine-containing groups to alkenes.4 Organofluorine compounds play a crucial role in pharmaceuticals, agrochemicals, and materials.5 The demand for synthesizing fluorinated or perfluoroalkylated alkenes has increased significantly because they are highly versatile building blocks for a range of applications.6 For instance, cross-couplings of perfluoroalkyl halides and alkenes or organic halides and perfluoroalkylated alkenes are effective for accessing alkenes with perfluoroalkyl groups (Rf).7 However, examples of Mizoroki–Heck-type reactions of fluorinated alkenes such as gem-difluoroalkenes are very limited.8
Heitz and co-workers in 1991 first reported a Pd-catalyzed defluorinative coupling of vinylidene difluoride with aryl iodides (Scheme 1a).9 Ichikawa and co-workers in 2005 described an intramolecular Pd-catalyzed 5-endo-trig cyclization of oxime derivatives bearing the difluoroalkene unit (Scheme 1b).10 In these two Heck-type reactions of gem-difluoroalkenes, a key β-F elimination, instead of β-H elimination, takes place in the final step. Such transformations belong to the strategy of transition-metal-catalyzed C–F bond functionalization of gem-difluoroalkenes for the synthesis of valuable monofluoroalkenes.11 Monofluoroalkenes are versatile synthons for organic synthesis and potential peptide bond isosteres for drug discovery.12
Scheme 1. Mizoroki–Heck Reaction of gem-Difluoroalkenes.
Various transition metal catalysts (T.M.), including Cu, Pd, Ni, Rh, Co, Mn, Ru, Ir, and Fe, have been successfully employed in the C–F bond functionalization of gem-difluoroalkenes.11a The general reaction design involves migratory insertion of gem-difluoroalkene 1 followed by β-F elimination (Scheme 1c).13 High diastereoselectivities can be achieved with trisubstituted gem-difluoroalkenes because of steric bias (e.g., R1 = aryl, R2 = H).14 An excellent example of this reaction type is the Pd(II)-catalyzed C–F bond arylation of β,β-difluorostyrenes with boronic acids by Toste and co-workers.14a Our group has previously reported the Pd(0)-catalyzed C–F bond coupling of tetrasubstituted gem-difluoroalkenes with organometallic reagents R-[M] (M = B, Si, Sn).15 Herein, we present a new reaction motif that is distinct from previous ones where gem-difluoroalkene 1 reacts with another alkene 2 in a Mizoroki–Heck fashion without organometallic reagents (Scheme 1d). This unprecedented transformation involves the following sequence: (1) Pd(0) participates in the directing group (DG)-assisted C–F bond activation; (2) the resulting vinylpalladium(II) species undergoes migratory insertion to alkene 2; and (3) β-H elimination generates the monofluorinated diene product 3. Hence, the formal stereoselective C–F and C–H bond coupling of two types of alkenes can be achieved.
We began our studies by using β,β-difluoroacrylate 1a, a tetrasubstituted gem-difluoroalkene, with 4-methylstyrene 2a as standard substrates under previously developed catalytic conditions for C–F bond alkynylation (Table 1).16,17 Through the use of Pd(PPh3)4 as the catalyst in the presence of additive NaI and base Et3N in toluene at 80 °C, the desired Heck product 3a was obtained in 41% yield as the (E,E)-diastereomer (dr > 99:1) (entry 1). The remaining mass balance was mainly the unreacted 1a and hydrodefluorinated side product.15b Reactions without either NaI (entry 2) or Et3N (entry 3) gave very poor yields. Raising the temperature to 90 °C improved the conversion (entry 4). Subsequent ligand screening using Pd(dba)2 revealed that dppb was an effective ligand (entries 5–9). The use of Pd(dba)2 alone gave no reaction (entry 10). A lower catalyst loading caused a yield decrease (entry 11). Screening of other Pd(0) or Pd(II) catalysts with dppb showed inferior reactivities (entries 12–15). Finally, an increase in the reaction concentration significantly enhanced the yield, and (E,E)-3a was isolated in 81% yield (entry 16). Isomerization of the tetrasubstituted double bond was observed during column chromatography, which resulted in a small amount of the (Z,E)-diastereomer 3a′ (3a/3a′ = 96:4). Other reaction parameters, including additives, bases, and solvents, were also screened.17 Salt additives, such as NaF, NaCl, LiI, or KI, were not as effective as NaI. Other bases, such as K3PO4, K2CO3, tetramethylethylenediamine (TMEDA), or N,N-diisopropylethylamine (DIPEA), gave lower yields than Et3N.
Table 1. Screening of Pd Catalysts and Ligandsa.
| entry | Pd (x mol %)/ligand (y mol %) | yield of (E,E)-3a (%)b/ratio of 3a/3a′b |
|---|---|---|
| 1c | Pd(PPh3)4 (10)/- | 41/>99:1 |
| 2c,d | Pd(PPh3)4 (10)/- | 0/- |
| 3c,e | Pd(PPh3)4 (10)/- | 8/- |
| 4 | Pd(PPh3)4 (10)/- | 58/>99:1 |
| 5 | Pd(dba)2 (10)/PPh3 (20) | 55/>99:1 |
| 6 | Pd(dba)2 (10)/dppm (10) | 0/- |
| 7 | Pd(dba)2 (10)/dppe (10) | 22/>99:1 |
| 8 | Pd(dba)2 (10)/dppp (10) | 26/>99:1 |
| 9 | Pd(dba)2 (10)/dppb (10) | 62/>99:1 |
| 10 | Pd(dba)2 (10)/- | 0/- |
| 11 | Pd(dba)2 (5)/dppb (5) | 50/99:1 |
| 12 | Pd2(dba)3 (5)/dppb (10) | 8/- |
| 13 | Pd(PPh3)4 (10)/dppb (10) | 44/>99:1 |
| 14 | Pd(OAc)2 (10)/dppb (10) | 8/- |
| 15 | Pd(TFA)2 (10)/dppb (10) | 25/99:1 |
| 16f | Pd(dba)2(10)/dppb (10) | 87 (81)g/>99:1 (96:4)g |
Unless specified otherwise, reactions were carried out using 1a (0.1 mmol), 2a (0.3 mmol), NaI (0.3 mmol), and Et3N (0.3 mmol) in toluene (0.2 M) at 90 °C for 18 h under argon.
Determined by 19F NMR analysis using benzotrifluoride as the internal standard.
At 80 °C.
Without NaI.
Without Et3N.
Concn = 0.67 M.
Isolated yield and ratio in parentheses at 0.2 mmol scale.
The scope of the alkene component was subsequently investigated in the Mizoroki–Heck reaction of 1a under the optimized conditions (Scheme 2). Commercial and easily prepared styrenes were employed to study the functional group tolerability of the aromatic substituent groups (3b–m). Electron-donating/-withdrawing groups and halogens were tolerated, which provided products in moderate to good yields. Even sensitive chloro (3g) and bromo (3h) groups were tolerated at the para position; however, the ortho-bromo (3i) group gave a lower yield. The diastereoselectivities were excellent and favored the (E,E)-isomer regardless of the substituents. Reaction at a 1.0 mmol scale was also demonstrated (3b). The structure of the product and the E configuration of both double bonds were unambiguously confirmed through the X-ray structure of 3j. Moreover, multisubstituted arene (3n) and heteroarene (3o) groups and dienes (3p) were tolerated. Electron-deficient alkenes, such as α,β-unsaturated esters (3q–r) and amides (3s), could also be used, albeit in lower diastereomeric ratios. Unactivated alkenes, such as 1-octene, and 1,1-/1,2-disubstituted alkenes, such as α-/β-methylstyrene and 1,1-diphenylethylene, were unreactive in this reaction.
Scheme 2. Scope of Alkenes 2 in the Mizoroki–Heck Reaction.
Unless specified otherwise, reactions were carried out using 1a (0.2 mmol), 2 (0.6 mmol), NaI (0.6 mmol), and Et3N (0.6 mmol) in toluene (0.3 mL) for 18 h under argon. Isolated yields. Diastereomeric ratios of (E,E)-3/(Z,E)-3′ were determined by 19F NMR analysis of the isolated products.
At 1.0 mmol scale.
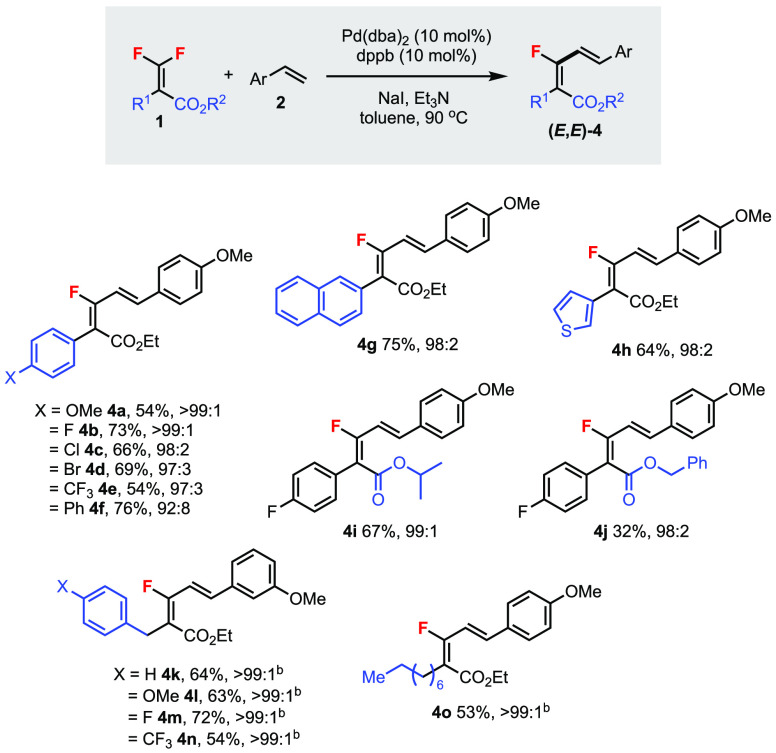
Next, the scope of the gem-difluoroalkene component was explored (Scheme 3). Aryl substituents (R1), including electron-donating/-withdrawing and halo groups, were tolerated (4a–f). Naphthyl and thienyl groups were also demonstrated (4g–h). Varying the ester substituent group (R2) did not lead to much change in the dr values (4i–j). With benzyl substituents (4k–n), tripropylamine was found to be a more suitable base. Long chain alkyl groups were also compatible (4o). Overall, good to excellent diastereoselectivities (92:8 to >99:1) were obtained across various gem-difluoroalkenes 1.
Scheme 3. Scope of gem-Difluoroalkenes 1 in the Mizoroki–Heck Reaction.
Unless specified otherwise, reactions were carried out using 1a (0.2 mmol), 2 (0.6 mmol), NaI (0.6 mmol), and Et3N (0.6 mmol) in toluene (0.3 mL) for 18 h under argon. Isolated yields. Diastereomeric ratios of (E,E)-4/(Z,E)-4′ were determined by 19F NMR analysis of the isolated products.
Used tripropylamine instead of Et3N, 36 h.
Further experiments were performed to shed light on the reaction details (Scheme 4). Under standard conditions (Scheme 2), trisubstituted difluorostyrene derivative 5 and difluoroacrylate derivative 6 gave no alkenylation products because of poor reactivity and substrate decomposition, respectively (Scheme 4a,b), which showed that the tetrasubstituted gem-difluoroalkenes 1 were both reactive and stable for this transformation. The monofluorovinylpalladium(II) intermediate Int-1 (dr > 99:1) was prepared according to a previous procedure (Scheme 4c).16 Subjecting Int-1 to 4-methoxystyrene and base afforded the desired product 3b in 71% yield (dr > 99:1). Furthermore, by applying the current catalytic conditions using dppb as the ligand, a signal corresponding to the Pd(II) intermediate Int-2 was detected by 19F NMR (Scheme 4d).17 In a similar fashion, Int-2 also led to product 3b in >99:1 dr. These experiments provided support for the proposed mechanism involving the formation of a Pd(II) intermediate via chelation-controlled C–F bond activation prior to migratory insertion of alkene (Scheme 1d).
Scheme 4. Control Experiments.
In conclusion, we developed a Pd(0)-catalyzed Mizoroki–Heck reaction of gem-difluoroalkenes to accomplish highly diastereoselective C–F bond alkenylation. The reaction takes place between two different alkenes without organometallic reagents in a formal C–F/C-H bond cross-coupling. The products are monofluorinated 1,3-dienes, which are challenging to synthesize with control of each alkene geometry. Further exploration of this reaction motif is ongoing in our laboratory.
Acknowledgments
This work was supported by the Research Grants Council of Hong Kong (CUHK 14304421) and the Chinese University of Hong Kong (Faculty of Science - Direct Grant for Research). We also thank the Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences for funding.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.3c02452.
Experimental procedures, optimization data, characterization data, and spectral data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Mizoroki T.; Mori K.; Ozaki A. Arylation of Olefin with Aryl Iodide Catalyzed by Palladium. Bull. Chem. Soc. Jpn. 1971, 44, 581–581. 10.1246/bcsj.44.581. [DOI] [Google Scholar]; b Heck R. F.; Nolley J. P. Palladium-Catalyzed Vinylic Hydrogen Substitution Reactions with Aryl, Benzyl, and Styryl Halides. J. Org. Chem. 1972, 37, 2320–2322. 10.1021/jo00979a024. [DOI] [Google Scholar]
- Selected reviews:; a Beletskaya I. P.; Cheprakov A. V. The Heck Reaction as a Sharpening Stone of Palladium Catalysis. Chem. Rev. 2000, 100, 3009–3066. 10.1021/cr9903048. [DOI] [PubMed] [Google Scholar]; b Jagtap S. Heck Reaction-State of the Art. Catalysts 2017, 7, 267. 10.3390/catal7090267. [DOI] [Google Scholar]; c Nakashima Y.; Hirata G.; Sheppard T. D.; Nishikata T. The Mizoroki–Heck Reaction with Internal Olefins: Reactivities and Stereoselectivities. Asian J. Org. Chem. 2020, 9, 480–491. 10.1002/ajoc.201900741. [DOI] [Google Scholar]; d Alisha M.; Philip R. M.; Anilkumar G. Low-Cost Transition Metal-Catalyzed Heck-Type Reactions: An Overview. Eur. J. Org. Chem. 2022, 2022, e202101384 10.1002/ejoc.202101384. [DOI] [Google Scholar]
- a Seechurn C. C. C. J.; Kitching M. O.; Colacot T. J.; Snieckus V. Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem., Int. Ed. 2012, 51, 5062–5085. 10.1002/anie.201107017. [DOI] [PubMed] [Google Scholar]; b Colacot T. Richard F. Heck (1931–2015). Angew. Chem., Int. Ed. 2015, 54, 15611–15612. 10.1002/anie.201510300. [DOI] [PubMed] [Google Scholar]
- Yang J.; Zhao H.-W.; He J.; Zhang C.-P. Pd-Catalyzed Mizoroki–Heck Reactions Using Fluorine-Containing Agents as the Cross-Coupling Partners. Catalysts 2018, 8, 23. 10.3390/catal8010023. [DOI] [Google Scholar]
- a Johnson B. M.; Shu Y.-Z.; Zhuo X.; Meanwell N. A. Metabolic and Pharmaceutical Aspects of Fluorinated Compounds. J. Med. Chem. 2020, 63, 6315–6386. 10.1021/acs.jmedchem.9b01877. [DOI] [PubMed] [Google Scholar]; b De la Torre B. G.; Albericio F. The Pharmaceutical Industry in 2020. An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules. 2021, 26, 627. 10.3390/molecules26030627. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Britton R.; Gouverneur V.; Lin J.-H.; Meanwell M.; Ni C.; Pupo G.; Xiao J.-C.; Hu J. Contemporary Synthetic Strategies in Organofluorine Chemistry. Nat. Rev. Methods Primers 2021, 1, 47. 10.1038/s43586-021-00042-1. [DOI] [Google Scholar]; d Ogawa Y.; Tokunaga E.; Kobayashi O.; Hirai K.; Shibata N. Current Contributions of Organofluorine Compounds to the Agrochemical Industry. iScience. 2020, 23, 101467. 10.1016/j.isci.2020.101467. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Inoue M.; Sumii Y.; Shibata N. Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega. 2020, 5, 10633–10640. 10.1021/acsomega.0c00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Yanai H.; Taguchi T. Synthetic Methods for Fluorinated Olefins. Eur. J. Org. Chem. 2011, 2011, 5939–5954. 10.1002/ejoc.201100495. [DOI] [Google Scholar]; b Pfund E.; Lequeux T.; Gueyrard D. Synthesis of Fluorinated and Trifluoromethyl-Substituted Alkenes Through the Modified Julia Olefination: an Update. Synthesis 2015, 47, 1534–1546. 10.1055/s-0034-1380548. [DOI] [Google Scholar]; c Nouaille A.; Lorkowski J.; Pannecoucke X.; Mauduit M.; Poisson T.; Couve-Bonnaire S. Metal-Catalyzed Metathesis of Fluorinated Alkenes: Still a Current Major Challenge. ACS Catal. 2021, 11, 12307–12323. 10.1021/acscatal.1c03414. [DOI] [Google Scholar]
- Representative examples:; a Feng Z.; Min Q.-Q.; Zhao H.-Y.; Gu J.-W.; Zhang X. A General Synthesis of Fluoroalkylated Alkenes by Palladium-Catalyzed Heck-Type Reaction of Fluoroalkyl Bromides. Angew. Chem., Int. Ed. 2015, 54, 1270–1274. 10.1002/anie.201409617. [DOI] [PubMed] [Google Scholar]; b Zhang X.; Zhang F.; Min Q.-Q. Palladium-Catalyzed Heck-Type Difluoroalkylation of Alkenes with Functionalized Difluoromethyl Bromides. Synthesis 2015, 47, 2912–2923. 10.1055/s-0035-1560457. [DOI] [Google Scholar]; c Sakaguchi Y.; Yamada S.; Konno T.; Agou T.; Kubota T. Stereochemically Defined Various Multisubstituted Alkenes Bearing a Tetrafluoroethylene (-CF2CF2-) Fragment. J. Org. Chem. 2017, 82, 1618–1631. 10.1021/acs.joc.6b02793. [DOI] [PubMed] [Google Scholar]; d Feng J.; Cai C. An Efficient Synthesis of Perfluoroalkenylated Aryl Compounds via Pincer-Pd Catalyzed Heck Couplings. J. Fluorine Chem. 2013, 146, 6–10. 10.1016/j.jfluchem.2012.12.009. [DOI] [Google Scholar]
- Scattered reports exist on the Mizoroki–Heck reaction of monofluorinated alkenes. For representative examples, see:; a Patrick T. B.; Agboka T. Y.; Gorrell K. Heck Reaction with 3-Fluoro-3-buten-2-one. J. Fluorine Chem. 2008, 129, 983–985. 10.1016/j.jfluchem.2008.02.006. [DOI] [Google Scholar]; b Hirotaki K.; Hanamoto T. Mizoroki–Heck Reaction of (1-Fluorovinyl) methyldiphenylsilane with Aryl Iodides. J. Org. Chem. 2011, 76, 8564–8568. 10.1021/jo201720x. [DOI] [PubMed] [Google Scholar]; c Rousee K.; Bouillon J.-P.; Couve-Bonnaire S.; Pannecoucke X. Stereospecific Synthesis of Tri- and Tetrasubstituted α-Fluoroacrylates by Mizoroki–Heck Reaction. Org. Lett. 2016, 18, 540–543. 10.1021/acs.orglett.5b03571. [DOI] [PubMed] [Google Scholar]; d Patrick T. B.; Blay A. A. Heck Reaction with Ethyl (E)- and (Z)-3-Fluoropropenoate. J. Fluorine Chem. 2016, 189, 68–69. 10.1016/j.jfluchem.2016.07.009. [DOI] [Google Scholar]
- Heitz W.; Knebelkamp A. Synthesis of Fluorostyrenes via Palladium-Catalyzed Reactions of Aromatic Halides with Fluoroolefins. Makromol. Chem., Rapid Commun. 1991, 12, 69–75. 10.1002/marc.1991.030120201. [DOI] [Google Scholar]
- a Sakoda K.; Mihara J.; Ichikawa J. Heck-Type 5-Endo-Trig Cyclization Promoted by Vinylic Fluorines: Synthesis of 5-Fluoro-3 H-Pyrroles. Chem. Commun. 2005, 4684–4686. 10.1039/b510039a. [DOI] [PubMed] [Google Scholar]; b Ichikawa J.; Sakoda K.; Mihara J.; Ito N. Heck-Type 5-Endo-Trig Cyclizations Promoted by Vinylic Fluorines: Ring-Fluorinated Indene and 3H-Pyrrole Syntheses from 1, 1-Difluoro-1-Alkenes. J. Fluorine Chem. 2006, 127, 489–504. 10.1016/j.jfluchem.2005.12.023. [DOI] [Google Scholar]
- a Fujita T.; Fuchibe K.; Ichikawa J. Transition-Metal-Mediated and -Catalyzed C-F Bond Activation by Fluorine Elimination. Angew. Chem., Int. Ed. 2019, 58, 390–402. 10.1002/anie.201805292. [DOI] [PubMed] [Google Scholar]; b Zhang X.; Cao S. Recent Advances in the Synthesis and C-F Functionalization of gem-Difluoroalkenes. Tetrahedron Lett. 2017, 58, 375–392. 10.1016/j.tetlet.2016.12.054. [DOI] [Google Scholar]; c Amii H.; Uneyama K. C-F Bond Activation in Organic Synthesis. Chem. Rev. 2009, 109, 2119–2183. 10.1021/cr800388c. [DOI] [PubMed] [Google Scholar]; d Zhang Y.; Zhang Y.; Guo Y.; Liu S.; Shen X. Reductive Quenching-Initiated Catalyst-Controlled Divergent Alkylation of α-CF3-Olefins. Chem. Catal. 2022, 2, 1380–1393. 10.1016/j.checat.2022.03.021. [DOI] [Google Scholar]; e Zhang Y.; Niu Y.; Guo Y.; Wang J.; Zhang Y.; Liu S.; Shen X. Photocatalyzed Cascade Reactions of Cyclopropanols and α-Trifluoromethyl-Substituted Olefins for the Synthesis of Fused gem-Difluorooxetanes. Angew. Chem., Int. Ed. 2022, 61, e202212201 10.1002/anie.202212201. [DOI] [PubMed] [Google Scholar]; f Li Z.; Zhang Y.; Zhang Y.; He X.; Shen X. Diastereoselective Synthesis of Monofluorocyclohexenes through Photocatalyzed Cascade Cyclization of gem-Difluoroalkenes and α,β-Unsaturated Carbonyl Compounds. Angew. Chem., Int. Ed. 2023, 62, e202303218 10.1002/anie.202303218. [DOI] [PubMed] [Google Scholar]; g Chen S.; Zhang Y.; Liu S.; Shen X. Photocatalyzed [2 + 1] Cyclization of Alkenes and Silylated Trifluorodiazoethanes: Facile Entry into (Difluoromethylene)cyclopropanes. Sci. China Chem. 2023, 66, 1–7. 10.1007/s11426-023-1676-y. [DOI] [Google Scholar]
- Drouin M.; Hamel J.-D.; Paquin J.-F. Synthesis of Monofluoroalkenes: A Leap Forward. Synthesis 2018, 50, 881–955. 10.1055/s-0036-1591867. [DOI] [Google Scholar]
- Koley S.; Altman R. A. Recent Advances in Transition Metal-catalyzed Functionalization of gem-Difluoroalkenes. Isr. J. Chem. 2020, 60, 313–339. 10.1002/ijch.201900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Representative examples:; a Thornbury R. T.; Toste F. D. Palladium-Catalyzed Defluorinative Coupling of 1-Aryl-2,2-Difluoroalkenes and Boronic Acids: Stereoselective Synthesis of Monofluorostilbenes. Angew. Chem., Int. Ed. 2016, 55, 11629–11632. 10.1002/anie.201605651. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Sakaguchi H.; Uetake Y.; Ohashi M.; Niwa T.; Ogoshi S.; Hosoya T. Copper-Catalyzed Regioselective Monodefluoroborylation of Polyfluoroalkenes en Route to Diverse Fluoroalkenes. J. Am. Chem. Soc. 2017, 139, 12855–12862. 10.1021/jacs.7b08343. [DOI] [PubMed] [Google Scholar]; c Tan D.-H.; Lin E.; Ji W.-W.; Zeng Y.-F.; Fan W.-X.; Li Q.; Gao H.; Wang H. Copper-Catalyzed Stereoselective Defluorinative Borylation and Silylation of gem-Difluoroalkenes. Adv. Synth. Catal. 2018, 360, 1032–1037. 10.1002/adsc.201701497. [DOI] [Google Scholar]
- a Wang Y.; Qi X.; Ma Q.; Liu P.; Tsui G. C. Stereoselective Palladium-Catalyzed Base-Free Suzuki-Miyaura Cross-Coupling of Tetrasubstituted gem-Difluoroalkenes: An Experimental and Computational Study. ACS Catal. 2021, 11, 4799–4809. 10.1021/acscatal.0c05141. [DOI] [Google Scholar]; b Ma Q.; Liu C.; Tsui G. C. Palladium-Catalyzed Stereoselective Hydrodefluorination of Tetrasubstituted gem-Difluoroalkenes. Org. Lett. 2020, 22, 5193–5197. 10.1021/acs.orglett.0c01813. [DOI] [PubMed] [Google Scholar]; c Li M.; Wang Y.; Tsui G. C. Palladium-Catalyzed Stereoselective C-F Bond Vinylation and Allylation of Tetrasubstituted gem-Difluoroalkenes via Stille Coupling: Synthesis of Monofluorinated 1, 3-and 1, 4-Dienes. Org. Lett. 2021, 23, 8072–8076. 10.1021/acs.orglett.1c03096. [DOI] [PubMed] [Google Scholar]
- Ma Q.; Wang Y.; Tsui G. C. Stereoselective Palladium Catalyzed C-F Bond Alkynylation of Tetrasubstituted gem-Difluoroalkenes. Angew. Chem., Int. Ed. 2020, 59, 11293–11297. 10.1002/anie.202002219. [DOI] [PubMed] [Google Scholar]
- See the Supporting Information (SI) for full details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.