Abstract
Background
Heart failure is a leading cause for hospital readmission. Digoxin use may lower this risk in patients with heart failure with reduced ejection fraction (HFrEF), but data on contemporary patients receiving other evidence-based therapies are lacking.
Methods
Of the 11,900 patients with HFrEF (ejection fraction ≤45%) in Medicare-linked OPTIMIZE-HF, 8401 were not on digoxin, of whom 1571 received discharge prescriptions for digoxin. We matched 1531 of these patients with 1531 not receiving digoxin by propensity scores for digoxin use. The matched cohort (n=3062; mean age, 76 years; 44% women; 14% African American) was balanced on 52 baseline characteristics. We assembled a second matched cohort of 2850 patients after excluding those with estimated glomerular filtration rate <15 ml/min/1.73 m2 and heart rate <60 beats/minute. Hazard ratios (HR) and 95% confidence intervals (CI) for digoxin-associated outcomes were estimated in the matched cohorts.
Results
Among the 3062 matched patients, digoxin use was associated with a significantly lower risk of heart failure readmission at 30 days (HR, 0.74; 95% CI, 0.59–0.93), 1 year (HR, 0.81; 95% CI, 0.72–0.92) and 6 years (HR, 0.90; 95% CI 0.81–0.99). The association with all-cause readmission was significant at 1 and 6 years but not 30 days. There was no association with mortality. Similar associations were observed among the 2850 matched patients without bradycardia or renal insufficiency.
Conclusions
Among hospitalized older patients with HFrEF receiving contemporary treatments for heart failure, digoxin use is associated with a lower risk of hospital readmission, but not all-cause mortality.
Keywords: Digoxin, Heart failure with reduced ejection fraction, Hospital readmission
Introduction
Heart failure is a leading cause for hospital admission and readmission.1 In the Digitalis Investigation Group (DIG) trial, digoxin reduced the risk of all-cause and heart failure hospitalizations in ambulatory patients with chronic heart failure with left ventricular ejection fraction ≤45%.2,3 Findings from hospitalized older Medicare beneficiaries with heart failure with reduced ejection fraction (HFrEF) in Alabama suggest that digoxin use may be associated with a significantly lower risk of all-cause and heart failure readmission in these patients.4,5 However, these associations have not been examined in a large national cohort of patients with HFrEF receiving more contemporary guideline directed medical therapies, the examination of which is the objective of the current study.
Methods
Data Source and Study Patients
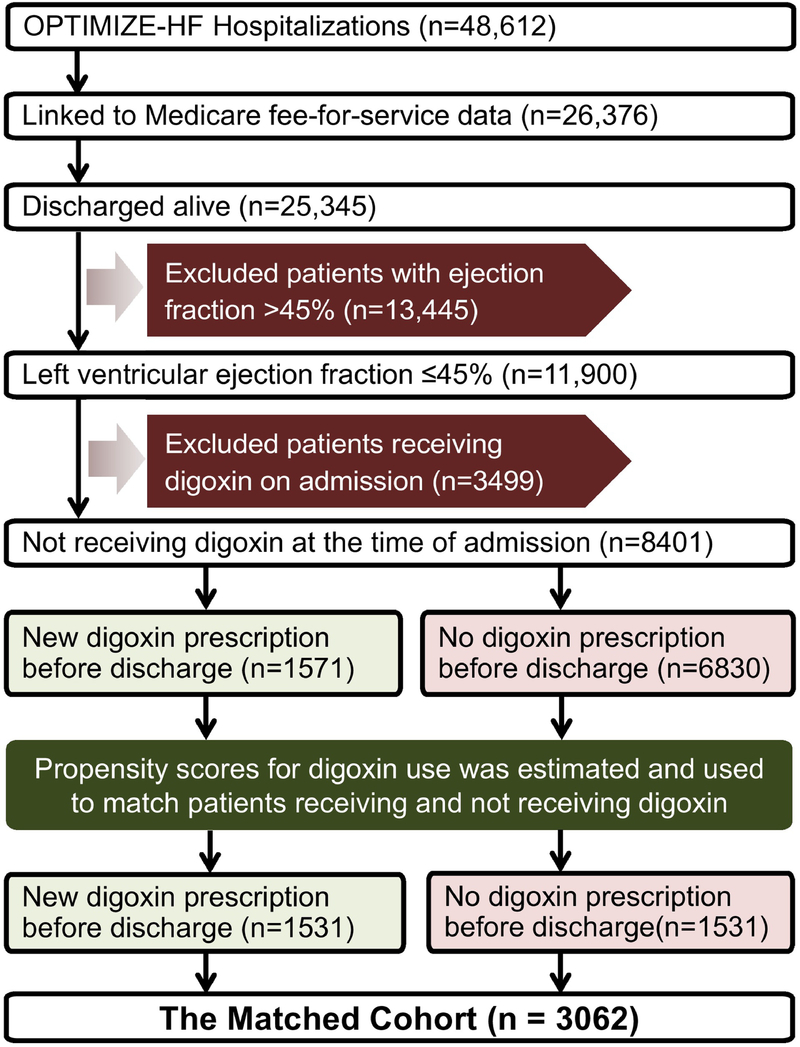
We used data from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF), a national hospital-based registry, the details of which have been previously described.6,7 Briefly, it is based on 48,612 patients hospitalized for worsening heart failure in 259 hospitals across 48 states between 2003 and 2004. Charts were selected based on International Classification of Disease, Ninth Revision (ICD-9) codes for discharge diagnosis of heart failure. Data regarding admission, hospital course and discharge were collected using a web-based information system. For our study, we used a Medicare-linked copy of the OPTIMIZE-HF database that included 26,376 patients, of which 25,345 were discharged alive. To stay consistent with the DIG trial and Alabama Heart Failure Project, we used a left ventricular ejection fraction cutoff of ≤45% to identify 11,900 patients with HFrEF (Figure 1).
Figure 1. Assembly of Study Cohorts.
Flow chart displaying assembly of the matched cohort of hospitalized older patients with heart failure with reduced ejection fraction not receiving digoxin at the time of hospital admission, by discharge initiation of digoxin therapy (OPTIMIZE-HF = Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure)
Assembly of an Inception Cohort
To avoid bias associated with prevalent drug use,8 we excluded patients receiving digoxin at the time of hospital admission. Of the 11,900 patients, 3499 were excluded for digoxin use on admission. Of the remaining 8401 patients, 1571 (19%) received a new discharge prescription for digoxin (Figure 1).
Assembly of a Balanced Cohort
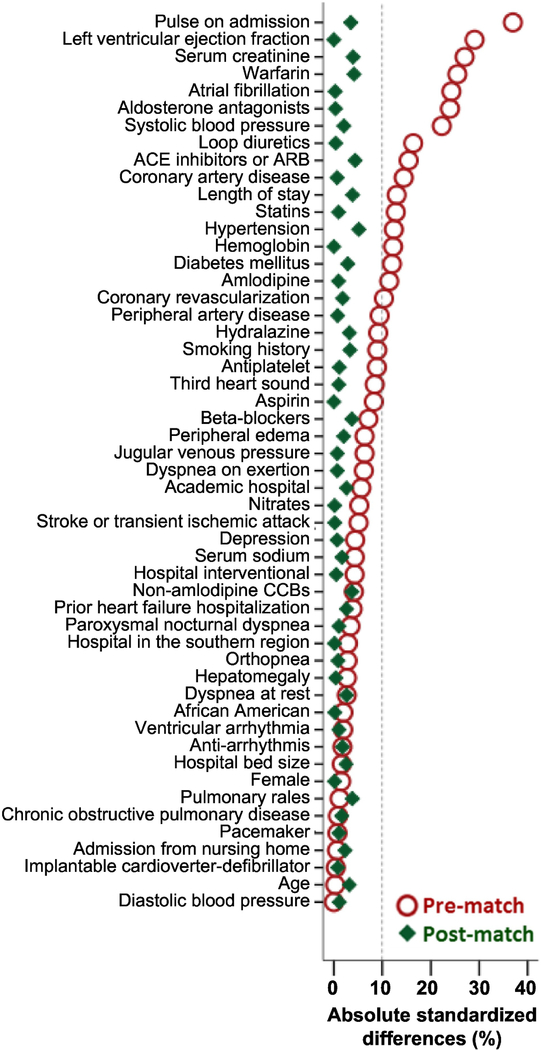
Since patients in an observational study are not randomized to receive a drug, we used propensity scores for digoxin use to assemble a matched cohort in which patients receiving and not receiving digoxin would be balanced on measured baseline characteristics.9,10 We used a non-parsimonious multivariable logistic regression model to estimate propensity scores for the probability for the receipt of a new prescription for digoxin upon discharge for each of the 8401 patients.11,12 The model included 52 baseline characteristics as covariates and digoxin use as the dependent variable (Figure 2). Using a matching algorithm, we then matched 1531 of 1571 (97%) patients who received digoxin with 1531 who did not receive digoxin but had similar propensity scores, thus assembling a matched cohort of 3062 patients. Between-group balance for each of the 52 baseline characteristics was assessed using absolute standard differences (0% indicates no residual bias and <10% indicates inconsequential bias).
Figure 2. Love Plot for Balance in Baseline Characterisitcs.
Love plot displaying pre-match and post-match absolute standardized differences for 52 baseline characteristics by the receipt of digoxin before hospital discharge in patients with heart failure with left ventricular ejection fraction ≤45% who were not receiving digoxin before hospital admission. A standardized difference of 0% indicates no residual bias and values <10% indicate inconsequential bias (ACE= angiotensin-converting enzyme; ARB= angiotensin receptor blockers; CCB= calcium channel blockers)
Assembly of a Balanced Sensitivity Cohort
We assembled a sensitivity cohort to examine if the observed association between digoxin use and outcomes would change if we excluded patients with bradycardia and renal insufficiency, which are often perceived as relative contraindications for digoxin use. Of the 8401 patients not receiving digoxin on admission, we excluded 898 patients with bradycardia (admission pulse <60 beats/minute) and renal insufficiency (estimated glomerular filtration rate <15 ml/min/1.73 m2 of body surface area). Of the remaining 7503 patients, 1481 (20%) received a new discharge prescription for digoxin. Using approaches described above, we assembled a propensity score-matched cohort of 1425 pairs of patients receiving and not receiving a discharge prescription for digoxin, balanced on 52 baseline characteristics.
Outcomes Data
The outcomes of interest for the current analysis were heart failure readmission, all-cause readmission, all-cause mortality, and the combined endpoints of readmissions or all-cause mortality. All outcomes were examined at 30 days, 1 year and 6 years (median 2.4 years) after discharge. Information on all outcomes was obtained from Medicare data between January 1, 2002 and December 31, 2008.13
Statistical Analysis
Baseline characteristics were compared using Pearson’s Chi-square test, McNemar’s test and Wilcoxon rank-sum test for pre-match and post-match comparisons as appropriate. All outcomes analyses were conducted in the matched cohort. Cox regression models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for outcomes associated with a discharge prescription for digoxin. In the mortality model, patients who did not die were censored at study end and in the readmission models, patients without a readmission were censored at death or study end, whichever occurred first. Subgroup analyses were conducted to examine homogeneity of the combined endpoint of heart failure readmission or all-cause mortality and all-cause mortality alone at 2-year follow-up.
To examine if significant associations between digoxin use and outcomes observed in our matched data could be explained away by an unmeasured baseline characteristic, we conducted formal sensitivity analyses using Rosenbaum’s approach.14 From the 1531 pairs of matched patients, we identified pairs in which we could directly compare survival times within each pair to determine whether one member of the pair clearly had a longer survival or event-free survival time than the other member. We tested whether, in the absence of a hidden bias, patients in the digoxin group had a longer survival or event-free survival time than their matched no-digoxin counterparts. We then used sign-score tests to calculate “sensitivity bounds” for a hypothetical unmeasured confounder to determine how much it would need to increase the odds of digoxin use to explain away significant association between digoxin use and outcomes. We assumed that the potential unmeasured confounder is a binary baseline characteristic that is a near perfect predictor of the outcomes and is not strongly correlated with any of the 52 baseline characteristics used in our propensity score model. However, sensitivity analysis cannot determine if such an unmeasured confounder exists. All statistical tests were two-tailed with a p-value <0.05 considered significant. SPSS for Windows version 25 (IBM Corp., Armonk, NY) and SAS for Window version 9.2 (Cary, NC) were used for data analyses.
Results
Baseline Characteristics
The 3062 matched patients had a mean age (±standard deviation) of 76 (±11) years; 44% were women and 14% were African American. Prior to matching, patients who received a discharge prescription for digoxin had a lower ejection fraction, higher heart rate, higher prevalence of atrial fibrillation, and higher use of guideline directed contemporary treatments for heart failure, such as angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and beta-blockers (Table 1). These and other imbalances in the 52 baseline characteristics were attenuated to inconsequential levels (absolute standardized differences <10%) after propensity-score matching (Figure 2).
Table 1.
Baseline characteristics by the receipt of digoxin before hospital discharge in patients with heart failure with left ventricular ejection fraction ≤45% who were not receiving digoxin before hospital admission
| Variables n (%) or mean (±SD) | Before propensity score matching | After propensity score matching | ||||
|---|---|---|---|---|---|---|
| No digoxin (n=6830) | Digoxin (n=1571) | P value | No digoxin (n=1531) | Digoxin (n=1531) | P value | |
| Age (years) | 76 (±11) | 76 (±11) | 0.918 | 76 (±11) | 75 (±11) | 0.372 |
| Female | 2974 (44%) | 696 (44%) | 0.584 | 676 (44%) | 675 (44%) | 0.971 |
| African American | 959 (14%) | 210 (13%) | 0.487 | 208 (14%) | 209 (14%) | 0.958 |
| Left ventricular ejection fraction (%) | 30 (±10) | 27 (±10) | <0.001 | 27 (±10) | 27 (±10) | 0.760 |
| Admission from nursing home | 101 (2%) | 22 (1%) | 0.816 | 18 (1%) | 22 (1%) | 0.524 |
| Past Medical History | ||||||
| Smoking history | 904 (13%) | 258 (16%) | 0.001 | 267 (17%) | 248 (16%) | 0.359 |
| HF hospitalization in prior 6 months | 1042 (15%) | 262 (17%) | 0.161 | 266 (17%) | 251 (16%) | 0.469 |
| Hypertension | 4763 (70%) | 1004 (64%) | <0.001 | 949 (62%) | 987 (65%) | 0.154 |
| Coronary revascularization | 2514 (37%) | 501 (32%) | <0.001 | 510 (33%) | 497 (33%) | 0.617 |
| Coronary artery disease | 3985 (58%) | 804 (51%) | <0.001 | 798 (52%) | 793 (52%) | 0.856 |
| Diabetes mellitus | 2856 (42%) | 565 (36%) | <0.001 | 534 (35%) | 555 (36%) | 0.428 |
| Stroke/transient ischemic attack | 1131 (17%) | 231 (15%) | 0.072 | 227 (15%) | 226 (15%) | 0.959 |
| Implantable defibrillator | 441 (7%) | 100 (6%) | 0.894 | 101 (7%) | 98 (6%) | 0.826 |
| Biventricular pacemaker | 243 (4%) | 58 (4%) | 0.797 | 59 (4%) | 56 (4%) | 0.776 |
| Peripheral vascular disease | 1120 (16%) | 205 (13%) | 0.001 | 207 (14%) | 203 (13%) | 0.832 |
| Atrial fibrillation | 1771 (26%) | 583 (37%) | <0.001 | 554 (36%) | 552 (36%) | 0.940 |
| Ventricular arrhythmia | 490 (7%) | 105 (7%) | 0.494 | 99 (7%) | 103 (7%) | 0.771 |
| Chronic obstructive pulmonary disease | 1757 (26%) | 410 (26%) | 0.760 | 416 (27%) | 405 (27%) | 0.654 |
| Depression | 720 (11%) | 145 (9%) | 0.123 | 141 (9%) | 144 (9%) | 0.852 |
| Signs and Symptoms on Admission | ||||||
| Dyspnea on exertion | 4319 (63%) | 1040 (66%) | 0.028 | 1014 (66%) | 1009 (66%) | 0.849 |
| Dyspnea at rest | 3021 (44%) | 674 (43%) | 0.339 | 677 (44%) | 657 (43%) | 0.466 |
| Orthopnea | 1853 (27%) | 446 (28%) | 0.313 | 443 (29%) | 437 (29%) | 0.811 |
| Paroxysmal nocturnal dyspnea | 1062 (16%) | 264 (17%) | 0.218 | 251 (16%) | 257 (17%) | 0.771 |
| Jugular venous pressure elevation | 2038 (30%) | 515 (33%) | 0.022 | 502 (33%) | 497 (33%) | 0.847 |
| Peripheral edema | 4150 (61%) | 1003 (64%) | 0.024 | 958 (63%) | 973 (64%) | 0.574 |
| Third heart sound | 671 (10%) | 196 (13%) | 0.002 | 180 (12%) | 185 (12%) | 0.780 |
| Pulmonary rales | 4387 (64%) | 1018 (65%) | 0.672 | 960 (63%) | 988 (65%) | 0.293 |
| Discharge medications | ||||||
| ACE inhibitors or ARBs | 4539 (67%) | 1155 (74%) | <0.001 | 1095 (72%) | 1125 (74%) | 0.225 |
| Beta blockers | 4807 (70%) | 1156 (74%) | 0.012 | 1145 (75%) | 1120 (73%) | 0.303 |
| Aldosterone antagonists | 859 (13%) | 338 (22%) | <0.001 | 317 (21%) | 319 (21%) | 0.929 |
| Loop diuretics | 5433 (80%) | 1347 (86%) | <0.001 | 1311 (86%) | 1309 (86%) | 0.918 |
| Hydralazine | 336 (5%) | 49 (3%) | 0.002 | 58 (4%) | 49 (3%) | 0.376 |
| Nitrates | 1884 (28%) | 397 (25%) | 0.063 | 393 (26%) | 394 (26%) | 0.967 |
| Amlodipine | 465 (7%) | 66 (4%) | <0.001 | 69 (5%) | 66 (4%) | 0.792 |
| Other calcium channel blockers | 506 (7%) | 134 (9%) | 0.131 | 108 (7%) | 123 (8%) | 0.305 |
| Antiarrhythmics | 1043 (15%) | 250 (16%) | 0.525 | 251 (16%) | 241 (16%) | 0.623 |
| Aspirin | 3734 (55%) | 794 (51%) | 0.003 | 783 (51%) | 783 (51%) | 1.000 |
| Warfarin | 1506 (22%) | 524 (33%) | <0.001 | 525 (34%) | 495 (32%) | 0.250 |
| Anti-Platelets | 1186 (17%) | 222 (14%) | 0.002 | 214 (14%) | 220 (14%) | 0.756 |
| Statins | 2702 (40%) | 525 (33%) | <0.001 | 527 (34%) | 520 (34%) | 0.790 |
| Admission clinical findings | ||||||
| Heart rate (bpm) | 86 (±21) | 95 (±24) | <0.001 | 95 (±23) | 94 (±23) | 0.304 |
| Systolic blood pressure (mm Hg) | 141 (±31) | 134 (±29) | <0.001 | 135 (±29) | 135 (±29) | 0.597 |
| Diastolic blood pressure (mm Hg) | 77 (±19) | 77 (±18) | 0.845 | 77 (±18) | 78 (±18) | 0.776 |
| Serum creatinine (mg/dL) | 1.9 (±1.6) | 1.5 (±1.0) | <0.001 | 1.6 (±.98) | 1.5 (±1.0) | 0.297 |
| Serum hemoglobin (g/dL) | 12 (±3) | 13 (±2) | <0.001 | 12 (±3) | 12 (±2) | 0.936 |
| Serum sodium (mEq/L) | 137 (±12) | 136 (±12) | 0.126 | 136 (±12) | 137 (±11) | 0.601 |
| Hospital length of stay (days) | 6 (±7) | 7 (±11) | <0.001 | 7 (±6) | 7 (±5) | 0.285 |
| Hospital, academic | 3120 (46%) | 762 (49%) | 0.043 | 765 (50%) | 745 (49%) | 0.470 |
| Hospital, interventional | 5319 (78%) | 1251 (80%) | 0.129 | 1221 (80%) | 1218 (80%) | 0.893 |
| Hospital, bed size | 421 (±252) | 417 (±241) | 0.570 | 423 (±245) | 417 (±241) | 0.489 |
| Hospital, region | ||||||
| Midwest | 2163 (32%) | 512 (33%) | 0.349 | 492 (32%) | 500 (33%) | 0.946 |
| Northeast | 1056 (16%) | 265 (17%) | 251 (16%) | 256 (17%) | ||
| South | 2328 (34%) | 514 (33%) | 504 (33%) | 503 (33%) | ||
| West | 1283 (19%) | 280 (18%) | 284 (19%) | 272 (18%) | ||
HF = heart failure; ACE = angiotensin converting enzyme; ARB = angiotensin receptor blocker; BP= blood pressure
Heart Failure Readmission
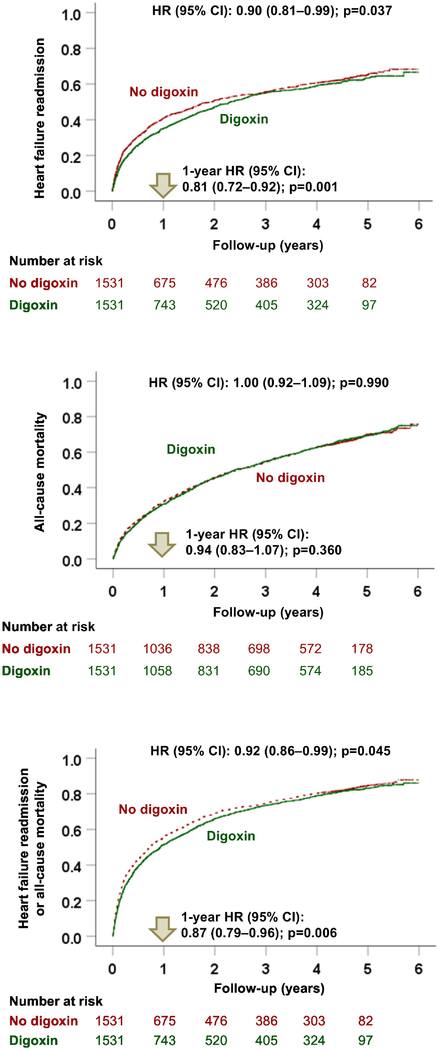
Among the 3062 matched patients, digoxin use was associated with a significantly lower risk of heart failure readmission at 6 years (HR, 0.90; 95% CI, 0.81–0.99; p=0.037; Table 2 and Figure 3, top panel). Of the 1531 pairs of propensity score-matched patients, in 59% (908/1531) of the pairs, both members of the pair had 6-year heart failure readmissions. In these 908 pairs in which we could determine which member had a longer 6-year heart failure readmission-free survival, 55% (500/908) belonged to the digoxin group (sign-score test p, 0.002). A hidden covariate could explain away the associations with 6-year heart failure readmission if it could increase the odds of digoxin use by 8%. Digoxin use was also associated with a significantly lower risk of heart failure readmission at 30 days (HR, 0.74; 95% CI, 0.59–0.93; p=0.010) and 1 year (HR, 0.81; 95% CI, 0.72–0.92; p=0.001; Table 2).
Table 2.
Outcomes by the receipt of digoxin before hospital discharge in 1531 pairs of propensity score-matched patients with heart failure with left ventricular ejection fraction ≤45% who were not receiving digoxin before hospital admission (CI= confidence interval)
| Events (%) | Hazard Ratio Associated with Digoxin Use (95% confidence interval) | ||
|---|---|---|---|
| No digoxin (n=1531) | Digoxin (n=1531) | ||
| Heart failure readmission | |||
| 30 days | 166 (11%) | 125 (8%) | 0.74 (0.59–0.93); p=0.010 |
| 1 year | 550 (36%) | 471 (31%) | 0.81 (0.72–0.92); p=0.001 |
| 6 years | 769 (50%) | 737 (48%) | 0.90 (0.81–0.99); p=0.037 |
| All-cause readmission | |||
| 30 days | 364 (24%) | 332 (22%) | 0.90 (0.78–1.04); p=0.163 |
| 1 year | 1007 (66%) | 955 (62%) | 0.91 (0.83–0.99); p=0.029 |
| 6 years | 1296 (85%) | 1278 (84%) | 0.92 (0.85–0.99); p=0.041 |
| All-cause mortality | |||
| 30 days | 106 (7%) | 90 (6%) | 0.84 (0.64–1.12); p=0.230 |
| 1 year | 495 (32%) | 473 (31%) | 0.94 (0.83–1.07); p=0.360 |
| 6 years | 1048 (69%) | 1057 (69%) | 1.00 (0.92–1.09); p=0.990 |
| Heart failure readmission or all-cause mortality | |||
| 30 days | 253 (17%) | 207 (14%) | 0.80 (0.67–0.96); p=0.019 |
| 1 year | 856 (56%) | 788 (52%) | 0.87 (0.79–0.96); p=0.006 |
| 6 years | 1284 (84%) | 1268 (83%) | 0.92 (0.86–0.99); p=0.045 |
| All-cause readmission or all-cause mortality | |||
| 30 days | 422 (28%) | 388 (25%) | 0.91 (0.79–1.04); p=0.165 |
| 1 year | 1142 (75%) | 1098 (72%) | 0.92 (0.85–0.99); p=0.046 |
| 6 years | 1464 (96%) | 1454 (95%) | 0.93 (0.87–1.00); p=0.050 |
Figure 3. Kaplan-Meier Plots by Digoxin Use.
Kaplan-Meier plots for heart failure readmission (top panel), all-cause mortality (middle panel), and the combined endpoint of heart failure readmission or all-cause mortality (bottom panel) during 6 years of follow up by the receipt of digoxin before hospital discharge in 1531 pairs of propensity score-matched in patients with heart failure with left ventricular ejection fraction ≤45% who were not receiving digoxin before hospital admission (CI = confidence interval; HR = hazard ratio)
All-Cause Readmission
Digoxin use was associated with a significantly lower risk of all-cause readmission at 1 year (HR, 0.91; 95% CI, 0.83–0.99; p=0.029) and 6 years (HR, 0.92; 95% CI, 0.85–0.99; p=0.041) but not at 30 days (HR, 0.90; 95% CI, 0.78–1.04; p=0.163; Table 2).
All-Cause Mortality
Digoxin use was not associated with all-cause mortality at any of the three timepoints. HR for 6-year all-cause mortality associated with digoxin use was 1.00 (95% CI, 0.92–1.09; p=0.990; Table 2 and Figure 3, middle panel). Findings from the subgroup analyses demonstrated that the beneficial association between digoxin use and all-cause mortality at 2 years was homogenous across various clinically relevant subgroups of patients.
Combined Endpoints
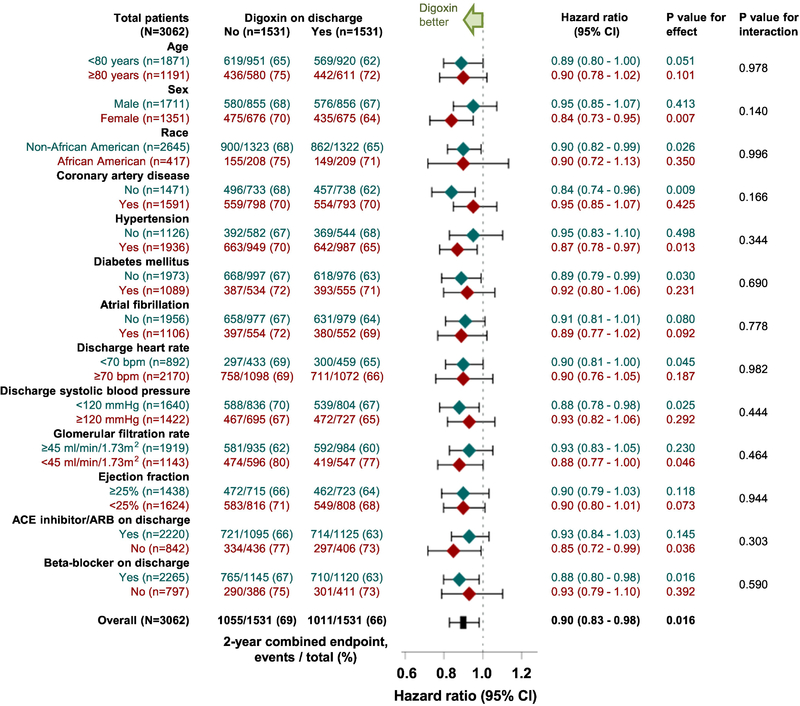
Digoxin use was associated with a significantly lower risk of the combined endpoint of heart failure readmission or all-cause mortality at 30 days (HR, 0.80; 95% CI, 0.67–0.96; p=0.019), 1 year (HR, 0.87; 95% CI, 0.79–0.96; p=0.006) and 6 years (HR, 0.92; 95% CI, 0.86–0.99; p=0.045; Table 2 and Figure 3, bottom panel). Time to the combined endpoint of heart failure readmission or all-cause mortality at 6 years could be directly compared in 97% (1480/1531) of the matched pairs, and in 54% (795/1480) of those pairs, it was clearly longer in patients in the digoxin group (sign-score test p, 0.004). A hidden covariate could explain away this association if it could increase the odds of digoxin use by 5%. Associations with both 30-day and 1-year combined endpoints were similarly insensitive to a potential unmeasured confounder. Findings from the subgroup analyses demonstrated that the beneficial association between digoxin use and the combined endpoint of heart failure readmission or all-cause mortality at 2-year follow-up was homogenous across various clinically relevant subgroups of patients (Figure 4). Associations with digoxin use and the combined endpoint of all-cause readmission or all-cause mortality are displayed in Table 2.
Figure 4. Forest Plots for Subgroup Analyses by Digoxin Use.
Forest plots displaying hazard ratios (95% confidence intervals) for the 2-year combined endpoint of heart failure readmission or all-cause mortality associated with the receipt of digoxin before hospital discharge in clinically relevant subgroups of propensity score-matched patients with heart failure with left ventricular ejection fraction ≤45% who were not receiving digoxin before hospital admission (ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; CI = confidence interval)
Sensitivity Cohort
Among the 2850 matched patients without bradycardia and renal insufficiency, digoxin use was associated with a significantly lower risk of heart failure readmission at 30 days (HR, 0.77; 95% CI, 0.60–0.98; p=0.037), 1 year (HR, 0.80; 95% CI, 0.70–0.91; p=0.001) and 6 years (HR, 0.89; 95% CI 0.80–0.99; p=0.032). HRs (95% CIs) for all-cause readmission at 30 days, 1 year and 6-years were 0.96 (0.82–1.12; p=0.580), 0.90 (0.82–0.99; p=0.023) and 0.93 (0.86–1.01; p=0.068), respectively. There was no association between digoxin use and all-cause mortality. The association with the combined endpoint of heart failure readmission or all-cause mortality was significant at 30 days (HR, 0.82; 95% CI, 0.67–0.99; p=0.044) and 1 year (HR, 0.88; 95% CI, 0.79–0.97; p=0.012), but not at 6 years (HR, 0.94; 95% CI, 0.86–1.01; p=0.105). There was no association with the combined endpoint of all-cause readmission or all-cause mortality.
Discussion
Findings from our study demonstrate that hospitalized older patients with HFrEF who received a discharge prescription for digoxin have a significantly lower risk of 30-day, 1-year and 6-year heart failure readmissions. Digoxin use was also associated with a lower risk of all-cause readmission at 1 year and 6 years, but not at 30-days; these associations were less robust compared to those for heart failure readmissions. Digoxin use had no association with all-cause mortality, but its use was associated with a significantly lower risk of the combined endpoint of heart failure readmission or all-cause mortality throughout the 6 years of follow-up. These associations between digoxin use and outcomes were similar when we excluded patients with bradycardia and renal insufficiency. To the best of our knowledge, this is the first study to demonstrate clinical effectiveness of digoxin in lowering the risk of readmission in a large national propensity score-matched cohort of hospitalized older patients with HFrEF on more contemporary guideline directed medical therapies.
The findings of the current study are consistent with those from prior studies demonstrating that the use of digoxin led to a reduction in the risk of readmission without affecting mortality. Unlike prior studies, these associations in the current study were observed in patients receiving angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and beta-blockers. In the randomized controlled trial setting, digoxin reduced the risk of hospital admission in ambulatory patients with heart failure receiving angiotensin-converting enzyme inhibitors.2 Digoxin use is also associated with a lower risk of heart failure readmission in the real-world setting in patients receiving angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and beta-blockers.4,5
Heart failure is associated with neurohormonal activation, which has been reported to be significantly higher among patients with higher New York Heart Association functional class symptoms.15 Digoxin suppresses both the renin-angiotensin and adrenergic nervous systems, which would be expected to be increased in hospitalized patients with decompensated heart failure.16,17 Findings from our pre-matched cohort demonstrate that patients in the digoxin group had higher symptom burden, heart rate and diuretic use, suggesting a relatively heightened neurohormonal activation in these patients. Although a majority of these patients were already on more contemporary heart failure therapies with the exception of aldosterone antagonists and angiotensin receptor-neprilysin inhibitors, taken together with findings from prior studies, findings from the current study suggest that digoxin may further blunt the neurohormonal cascade and, consequently, lead to improved outcomes in patients with HFrEF.
Due to the observational nature of the current study, we also considered potential confounding due to residual bias or bias due to unmeasured confounders. Prior to matching, more patients in the digoxin group were receiving angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, beta-blockers and aldosterone antagonists. Although the use of these drugs was balanced in the matched cohort, matching may not balance the dosing of these medications or the reasons for their higher use. If these reasons persisted during follow-up, more patients in the digoxin group would be expected to receive these drugs, which can in part explain the more favorable outcomes in the digoxin group. However, all of these drugs have proven efficacy and effectiveness in lowering the risk of death. Thus, if the observed association between digoxin and outcomes observed in our study were to be mediated by these other neurohormonal antagonists, then we would have observed a lower risk death in the digoxin group. However, the lack of mortality benefit in the digoxin group in the current study suggests that the results of our study are unlikely to be explained by the use of other neurohormonal antagonists. Another potential source of confounding is comorbidity burden. Before matching, fewer patients in the digoxin group had hypertension, coronary artery disease, diabetes mellitus and peripheral vascular disease. Although comorbidities were balanced in our matched cohort, matching may not balance the severity of these conditions or the underlying reasons of the baseline imbalance. If those reasons persist during follow-up, that may in part explain the better outcomes in the digoxin group.
Findings of the current study are generally consistent with those from the DIG trial in which digoxin significantly reduced the risk of heart failure hospitalization and all-cause hospitalization by 28% and 8%, respectively, with no effect on mortality.2 They are also consistent with the findings from the Alabama Heart Failure Project.4,5 However, unlike the former studies, the association with all-cause readmission in the current study was weaker and non-significant at 30 days. This may in part be explained by the characteristic, therapeutic and prognostic differences between the two cohorts. These findings also highlight the relationship between digoxin use and a lower risk of heart failure readmission is strong and consistent, while its association with all-cause readmission is weak and variable.
Our study has several limitations. Despite the use of propensity score-matching to balance the study cohort, it is possible that the observed associations were due to residual bias or bias due to unmeasured covariates. However, absolute standardized differences <10% in the post-match group suggest that any residual bias would be inconsequential. Furthermore, results of our sensitivity analyses indicate that our findings were relatively immune to confounding by unmeasured bias. Our analysis was restricted to fee-for-service Medicare beneficiaries and the management of HFrEF has substantially evolved since OPTIMIZE-HF, which may limit generalizability.
Conclusions
Among hospitalized older patients with HFrEF, the use of digoxin is associated with a lower risk of heart failure readmission and all-cause readmission, without any association with all-cause mortality. These results reinforce current guideline recommendation that digoxin may be considered in patients with HFrEF to decrease the risk of hospitalization.
Clinical Significance.
Initiation of digoxin therapy prior to hospital discharge was associated with a lower risk of readmission among older patients with heart failure with reduced ejection fraction (HFrEF).
Digoxin use was not associated with all-cause mortality but there was a lower risk of the combined endpoint of readmission or mortality.
These results support guideline recommendation that digoxin may be considered in patients with HFrEF to decrease the risk of hospitalization.
Acknowledgments
Funding: Dr. Ali Ahmed was in part supported by the National Institutes of Health through grants (R01-HL085561, R01-HL085561-S and R01-HL097047) from the National Heart, Lung, and Blood Institute (NHLBI). OPTIMIZE-HF was sponsored by GlaxoSmithKline, but played no role in the design, conduct, analyses or interpretation of the current study.
Footnotes
Disclosures: Dr. Fonarow reports consulting with Abbott, Amgen, Bayer, Janssen, Medtronic, Novartis, and was the Principle Investigator of OPTIMIZE-HF. None of the other authors report any conflicts of interest related to this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare feefor-service program. N Engl J Med. 2009;360(14):1418–1428. [DOI] [PubMed] [Google Scholar]
- 2.Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336(8):525–533. [DOI] [PubMed] [Google Scholar]
- 3.Bourge RC, Fleg JL, Fonarow GC, et al. Digoxin reduces 30-day all-cause hospital admission in older patients with chronic systolic heart failure. Am J Med. 2013;126(8):701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed A, Bourge RC, Fonarow GC, et al. Digoxin use and lower 30-day all-cause readmission for Medicare beneficiaries hospitalized for heart failure. Am J Med. 2014;127(1):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lam PH, Bhyan P, Arundel C, et al. Digoxin use and lower risk of 30-day all-cause readmission in older patients with heart failure and reduced ejection fraction receiving beta-blockers. Clin Cardiol. 2018;41(3):406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fonarow GC, Abraham WT, Albert NM, et al. Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF): rationale and design. Am Heart J. 2004;148(1):43–51. [DOI] [PubMed] [Google Scholar]
- 7.Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50(8):768–777. [DOI] [PubMed] [Google Scholar]
- 8.Danaei G, Tavakkoli M, Hernan MA. Bias in observational studies of prevalent users: lessons for comparative effectiveness research from a meta-analysis of statins. Am J Epidemiol. 2012;175(4):250–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 10.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 11.Ahmed A, Husain A, Love TE, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27(12):1431–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed A, Rich MW, Love TE, et al. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J. 2006;27(2):178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Kilgore ML, Arora T, et al. Design and rationale of studies of neurohormonal blockade and outcomes in diastolic heart failure using OPTIMIZE-HF registry linked to Medicare data. Int J Cardiol. 2013;166(1):230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenbaum PR. Sensitivity to hidden bias. In: Rosenbaum PR, ed. Observational Studies. Vol 1. New York: Springer-Verlag; 2002:105–170. [Google Scholar]
- 15.Sigurdsson A, Amtorp O, Gundersen T, Nilsson B, Remes J, Swedberg K. Neurohormonal activation in patients with mild or moderately severe congestive heart failure and effects of ramipril. The Ramipril Trial Study Group. Br Heart J. 1994;72(5):422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson DW, Berg WJ, Sanders JS, Roach PJ, Kempf JS, Kienzle MG. Sympathoinhibitory responses to digitalis glycosides in heart failure patients. Direct evidence from sympathetic neural recordings. Circulation. 1989;80(1):65–77. [DOI] [PubMed] [Google Scholar]
- 17.Covit AB, Schaer GL, Sealey JE, Laragh JH, Cody RJ. Suppression of the renin-angiotensin system by intravenous digoxin in chronic congestive heart failure. Am J Med. 1983;75(3):445–447. [DOI] [PubMed] [Google Scholar]
- 18.Digoxin Ahmed A. and reduction in mortality and hospitalization in geriatric heart failure: importance of low doses and low serum concentrations. J Gerontol A Biol Sci Med Sci. 2007;62(3):323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumholz HM. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA. 2003;289(7):871–878. [DOI] [PubMed] [Google Scholar]
- 20.Adams KF Jr., Butler J, Patterson JH, et al. Dose response characterization of the association of serum digoxin concentration with mortality outcomes in the Digitalis Investigation Group trial. Eur J Heart Fail. 2016;18(8):1072–1081. [DOI] [PubMed] [Google Scholar]