Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has caused an important social and health impact worldwide and the coronavirus disease-19 (COVID-19) has elicited devastating economy problems. The pathogenesis of SARS-CoV-2 infection is a complex mechanism and is considered to be the result of a challenging interaction, in which host and virus immune responses are the key elements. In this process, several inflammatory pathways are involved, and their initiation can have multiple consequences with a considerable impact on evolution, such as hyperinflammation and cytokine storm, thereby promoting activation of the coagulation system and fibrinolytic activity suppression. It is commonly recognized that COVID-19 severity involves multiple factors, including diabetes which increases the risk of developing different complications. This could be as a result of the low-grade inflammation as well as the innate and adaptive immune response dysfunction that is observed in patients with diabetes mellitus. In patients with diabetes, multiple metabolic disturbances which have a major impact in disturbing the balance between coagulation and fibrinolysis were discovered, thus the risk for thrombotic events is increased. Diabetes has been recognized as an important severity prognosis factor in COVID-19 cases and considering there is a significant association between diabetes and prothrombotic status, it could be responsible for the increased risk of thrombotic events with a worse prognosis.
Keywords: prothrombotic status, coronavirus disease-19 infection, diabetes mellitus
1. Introduction
The coronavirus disease-19 (COVID-19) pandemic has been the reason of high morbidity and mortality worldwide. Between the start of the pandemic and 23 January 2023, there were 663,640,386 confirmed cases and 6,713,093 deaths (1). During the last decade the first pathogen involved in a global pandemic is severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 has a significant clinical range, most of the cases are presented with slight or no symptoms but severe cases can lead to severe pneumonia, multiple organ failure and death. Elderly individuals with underlying conditions, such as diabetes mellitus (DM), cardiovascular diseases and hypertension have a demonstrated higher risk for developing severe complications from COVID-19(2).
According to pathological and clinical evidence, severe SARS-CoV-2 infection is associated with a prothrombotic status expressed as microvascular or macrovascular thrombosis. Multiple factors are involved, including endothelial dysfunction, systemic inflammation, platelet activation, mechanical ventilation and immobilization (3). Thrombotic events contribute to the mortality and can be mentioned among life threatening complications of the disease. Diabetes is associated with multiple risk factors such as age, family history, race, obesity, hypertension and dyslipidaemia. In patients with DM, a dysregulated immune response and a chronic low level of inflammation with reduced amount of the anti-inflammatory adiponectin and elevated amount of the proinflammatory leptin has been identified. Considering the various spectrum of disorders observed in patients with diabetes, in cases of overlapping SARS-CoV-2 infection, DM has been established as an extremely important risk factor for a severe evolution of the disease. This may be due to the association between diabetes and its high susceptibility to cytokine storm (4). The presence of DM in patients with COVID-19 was associated with the risk of admission to intensive medical care and increased mortality. Different physio-pathological mechanisms were identified and because diabetes is characterized by a syndromic nature, possible responsible factors should be discussed, such as the decreased autoimmune function, the prothrombotic status and the increased susceptibility to hyperinflammatory immune response associated with hyperglycemia (5,6). Patients with COVID-19 are more likely to develop a hypercoagulable status if diabetes is associated. Fibrinogen and D-dimer levels are increased in these cases and glycemic control exert a considerable influence (7).
2. Endothelial dysfunction
Endothelium is considered to have a leading role in several physiological processes and plays a significant role in the homeostasis of healthy individuals. It produces components of the extracellular matrix and multiple chemical mediators, such as endothelin-1, nitric oxide (NO), prostacycline, angiotensin II (ANG-II), plasminogen activator inhibitor-1 (PAI-1), tissue-type plasminogen activator (t-PA), cytokines, adhesion molecules and tumor necrosis factor α (TNFα). An essential mediator synthesized by endothelial cells is NO due to its antiproliferative, anti-inflammatory, vasodilatory, permeability-decreasing and antioxidant properties. Endothelium also has an impact on vascular remodelling through the release of platelet-derived growth factor and ANG-II. It is an important mediator of inflammation and coagulation by controlling leucocytes and platelets adhesion. It controls fibrinolysis and limits activation of the coagulation cascade (8). Endothelial glycocalyx consists of glycoproteins and proteoglycans and prevents the direct contact of blood cells with the endothelial surface. Endothelial dysfunction is defined by increased interactions with white blood cells, smooth muscle growth, vasoconstriction, disrupted coagulation, inflammation, thrombosis and atherosclerosis (9).
The involvement of endothelial dysfunction in DM is complex as multiple factors are involved, such as obesity, ageing, hyperlipidemia, hypertension, insulin resistance and hyperglycemia. Possible suggested mechanisms which lead to endothelial micromilieu impairment in patients with diabetes are insulin resistance and hyperglycemia. Chronic hyperglycemia affects protein kinase C, sorbitol and pentose phosphate pathways. This leads to increased oxidative stress and promotes endothelial cells apoptosis. Another mechanism through which hyperglycemia causes endothelial dysfunction is the reduced level of NO. A bidirectional relation and vicious circle in which insulin resistance contributes to endothelial dysfunction and conversely may exist. At normal concentrations, insulin generates anti-inflammatory effects, while hyperinsulinemia increases levels of oxidative stress and can lead to vascular inflammation and simultaneously vascular inflammation causes insulin resistance and compensatory hyperinsulinemia (10).
Dysfunction of the endothelium, inflammatory response, thrombopathy and microvascular obstruction are the suggested physio-pathological components of severe COVID-19 cases. Thrombosis and dysfunction of the endothelium are recognized as pathways contributing to clinical deterioration in COVID-19 patients. SARS-CoV-2 has a particular tropism for angiotensin converting enzyme 2 (ACE2) receptor. This receptor is expressed by endothelial cells but also in multiple organs, such as heart, lung, kidney and intestine (11). Endothelial cells are directly impaired by SARS-CoV-2, suffering cellular injury and finally apoptosis, thus, as a consequence, the endothelium can no longer provide antithrombotic properties. Furthermore, COVID-19 cases were associated with elevated plasma levels of soluble intercellular adhesion molecule 1, soluble vascular cell adhesion molecule 1 and endothelial cells.
In the viral genome of SARS-CoV-2 there are over 20 proteins encoded, with the spike protein being one of the most studied. Spike protein contains two subunits, S1 of the receptor-binding domain, which binds ACE2, and S2 which is involved in anchoring the virion to the membrane, which results in the fusion with the host cell, followed by the virus entry into the cell, via endocytosis (12). The ACE2 has an important role in the function of the renin-angiotensin-aldosterone system (RAAS), a signaling pathway which includes rennin, angiotensinogen, ANG-I, ANG-II, as well as the AT1 receptor (12-14). Normally, the activation of ACE2 leads to vasodilation and activation of antioxidant and antiproliferative effects. In the case of SARS-CoV-2 infection, the virus competes with ANG-II for ACE2, resulting in ACE2 downregulation, leading in an imbalance of the ACE/ACE2 ratio and in an increased peripheral vascular resistance (12). Furthermore, it is known that chronic inflammatory states, such as diabetes, are associated with impaired RAAS function. Therefore, these patients have a higher risk of developing infections, as well as a higher risk for the development of complications. In addition, other factors, such as hypomagnesiemia, that triggers oxidative stress and leads to an increase in proinflammatory cytokines, have also been associated with endothelium dysfunction in patients with COVID-19(15). Endothelium has the key role in controlling the mechanisms, with a considerable impact on the progress of COVID-19 in patients, for instance, by regulating haemostasis and fibrinolysis. Additionally, a previous study has also demonstrated that through delayed fibrinolysis, low magnesium levels in these patients are associated with a higher risk of thrombotic events. Furthermore, decreased intracellular magnesium is associated with platelet-dependent thrombosis (15). The trigger for immuno-thrombosis in patients with COVID-19 is established to be the endothelial dysfunction which is the cause of the COVID-19-related coagulopathy. The factor of risk in microvascular impairment is the endothelial dysfunction which can enhance ischaemia and inflammation. In addition, the vascular wall permeability is increased, and as a result of the inflammatory status, tissue factor (TF) is expressed and activated on endothelial cells. Therefore, the coagulation cascade, which involves the activation of a series of clotting factors, is initiated. All of these elements support the assumption that endothelial dysfunction is the main feature of COVID-19-associated coagulopathy (16).
3. Haemostasis and inflammatory immune response
An efficient reaction in case of tissue damage or infection depends on the ability of the living organisms to use the innate defence mechanisms such as the immune system and the haemostatic system. To re-establish physiological tissue function, immune and haemostatic systems have a strong and active interplay. In either non-infectious or infectious inflammatory states, haemostatic system activation is the critical component of the host defence mechanism. An overstated and insufficiently controlled activity may contribute to disease severity (17).
The haemostatic system and the inflammatory cascade act in collaboration producing an inflammatory-haemostatic cycle with a positive feedback system, in which each one of the processes triggers the other one. Several factors affect the haemostatic system and especially cytokines, which take a lead in influencing the process during the inflammatory reaction through endothelial impairment, activation of the coagulation cascade and increased platelet reactivity. The interplay between homeostasis and inflammation clarifies the prothrombotic tendency. In case of inflammation, haemostatic activity is changed by proinflammatory mediators which cause imbalance between the coagulation system and the fibrinolytic activity, thus, the prothrombotic state is initiated (18).
Inflammatory immune response, irrespective of the etiology, contributes to an imbalance among proinflammatory and procoagulant properties and anticoagulant and anti-inflammatory features of the vascular endothelium which acquires a procoagulant phenotype. The connection between haemostasis and inflammation is a bidirectional process, thus, activated haemostatic system also significantly modulates inflammatory activity. Activated or injured endothelium secrete mainly antifibrinolytic or procoagulant components into the surrounding area, such as thromboxane A2, von Willebrand factor (vWF) and PAI-1, whilst the expression of constituents with profibrinolytic and anticoagulant properties is considerably reduced. When a vascular injury arises, endothelial cells excrete TF, which subsequently binds to factor VII and forms the TF-VII complex which initiates the activation of the coagulation cascade. The resulting product is thrombin, that converts fibrinogen into fibrin. Cellular activation in inflammation is a process mediated by fibrinogen and fibrin, these mediators stimulate the expression of proinflammatory cytokines on monocytes and the expression of chemokines on endothelial cells and fibroblasts. Adhesion molecules intercede the procoagulant actions of the vascular endothelium by managing the interplay among platelets, neutrophils and proinflammatory cytokines such as interleukin-1 (IL-1), interleukin-1 (IL-6) and TNFα (19). There is an important association between coagulation and inflammation which is characterized by the adhesion of coagulation factors to the protease-activated receptors (PARs) which thus increase the inflammatory response. PAR receptors are located on endothelial cells, white blood cells, platelets, fibroblasts and smooth muscle cells. There are four members, PAR-1 to PAR-4 and have different functions, PAR-1, PAR-3 and PAR-4 are thrombin receptors while PAR-2 receptor can be activated from the TF-factor VIIa (FVIIa) complex. Inflammatory response can be upregulated by connecting activated coagulation factors to the PAR receptors. The result is the production of multiple proinflammatory mediators such as cytokines, growth factors, chemokines and cell adhesion molecules (20).
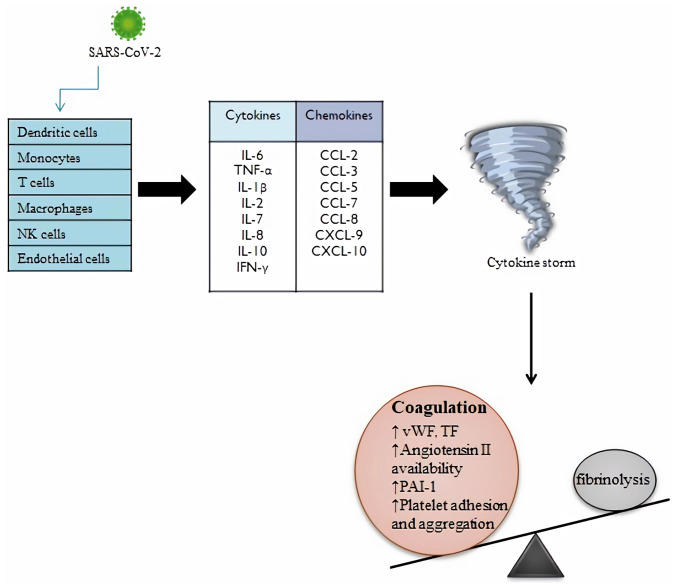
A remarkable example of viral disease is SARS-CoV-2 infection, in which systemic inflammatory reaction and the coagulation cascade are two important mechanisms with mutual potentiating activity. Multiple immune cells are the target of SARS-CoV-2 such as macrophages, monocytes, endothelial cells, dendritic cells, T-cells and natural killer cells. Following the cells activation, the viral particles stimulate multiple inflammatory pathways, producing large quantity of chemokines (CCL-2, CCL-3, CCL-5, CCL-7, CCL-8, CXCL-9, CXCL-10) and cytokines (IL-6, TNFα, IL-1β, IL-2, IL-7, IL-8, IL-10,), which leads to hyperinflammation and cytokine storm and leading to vascular permeability increase and risk of multiorgan failure. Several changes occur in cases of COVID-19, such as the increase of the level of inflammatory mediators, chemokines, cytokines and neutrophil-to-lymphocyte ratio, with a significant association to severity. Whereas the initial release of cytokines helps with virus removal, the uncontrolled cytokine secretion is detrimental as they begin to target host cells. The cytokine storm changes the balance between pro and anticoagulant mechanisms, elevating the levels of vWF and TF and promoting activation of coagulation cascade (21) (Fig. 1). Among the released cytokines, IL-6 has a significant role assigned. Cytokines influence different functions and the medical literature revealed that IL-6 has a greater influence on coagulation activation than TNFα, therefore being the most important mediator (22,23). IL-6 has a prominent role in the induction of TF expression, it also promotes the synthesis of coagulation factors such as factor VIII (FVIII) and fibrinogen, and its action on endothelial cells lead to increased vascular permeability by stimulating vascular endothelial growth factor secretion. Soluble IL-6 receptor (sIL-6R) is connected to various cells, such as leukocytes, macrophages, monocytes and B cells, and during infection with SARS-CoV-2 these cells go through a process leading to increased separation of soluble receptor from cells. According to medical studies, elevated concentrations of sIL-6R were observed in plasma of patients with COVID-19. Multiple cells including endothelial cells can be activated by complexes of IL-6 and sIL-6R found in the circulatory system of COVID-19 cases. The cytokine storm can stimulate megakaryocytes proliferation, thus leading to thrombocytosis (23-25). Additionally, in the setting of inflammation, platelets can be directly activated by inflammatory mediators. Activated platelets secrete proinflammatory and procoagulant substances into the local environment, such as cytokines, chemokines, adhesion molecules, growth factors and coagulation factors. Therefore, a link between infection and thrombosis can be deduced (26).
Figure 1.
Impact of SARS-CoV-2 infection on immune cells and the consequences on the coagulation process. Multiple immune cells are the target of SARS-CoV-2, such as macrophages, monocytes, endothelial cells, dendritic cells, T-cells and NK cells. Following the cells activation, the viral particles stimulate multiple inflammatory pathways, producing large quantity of chemokines and cytokines, which leads to cytokine storm. The cytokine storm changes the balance between coagulation and fibrinolysis, elevating the levels of vWF, TF, angiotensin II, PAI-1 and the platelet adhesion and aggregation (27,51). SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; NK, natural killer; vWF, von Willebrand factor; CCL, C-C motif chemokine ligand; CXCL, C-X-C motif chemokine ligand; TF, tissue factor; PAI-1, plasminogen activator inhibitor-1.
DM has been established to be a proinflammatory condition which comes as a consequence of excessive cytokine production. Increased concentrations of fibrinogen, lactate dehydrogenase, C-reactive protein, TNFα, IL-1 and IL-6 detected in patients with DM are the reason of chronic inflammation in patients with diabetes. Inflammatory cytokines increase vascular permeability, adhesion of white blood cells to the endothelium and promote thrombus formation (27,28). In patients with DM, expression of protease-activated receptor 4 is increased by chronic hyperglycemia. In return, the release of activated platelet-derived microparticles (PMPs) is enhanced by the Ca2+-calpain pathway. Released PMPs trigger IL-6 secretion, with a proinflammatory and pro-thrombotic activity in diabetes. Hyperglycemia decreases the membrane fluidity of platelets through glycation of the membrane proteins, directly promoting platelet activation and aggregation. Additionally, hyperglycemia diminishes endothelial function by causing oxidative stress and inflammation, therefore decreasing NO and prostaglandin I2 synthesis and release, and as a consequence promoting platelet aggregation (29). Insulin resistance can be caused by several factors including the proinflammatory cytokine TNFα. Therefore, this sheds some light about the close relation between endothelial dysfunction, insulin resistance and thrombosis. In DM, insulin resistance and hyperglycemia exert synergistic effects on TF pathway, which leads to increased procoagulatory activity. Patients with DM have an inflammatory state, defined by the elevated levels of inflammatory biomarkers, and this process directly upregulates TF expression, contributing to the prothrombotic status (30).
4. Hyperglycemia: A prothrombotic factor?
DM is characterized by a high risk of atherothrombotic events. The prothrombotic state associated with diabetes is sustained by laboratory results of impaired fibrinolysis and raised coagulation factors. Hyperglycemia plays a significant role in the development of these hemostatic disorders. Various processes are suggested to explain the coagulability changes that can develop in case of metabolic imbalance with hyperglycemia. For instance, depletion of the extracellular matrix that covers the apical side of endothelial cells which shelters coagulation factors. Furthermore, the activity of coagulation factors can be modified by the glycation process, additionally, oxidative stress has a significant impact. In addition, hyperglycemia is frequently associated with hyperinsulinemia, which leads to prothrombotic consequences (31).
Hyperglycemia and hyperinsulinemia increase the expression of PAI-1, thus its concentration and activity are increased. In consequence, the fibrinolytic capacity is decreased due to diminished t-PA activity. Furthermore, an assumption has been made about the substantial impact of hyperglycemia and hyperinsulinemia on gene transcription (32). PAI-1 transcription process is elevated through nuclear factor kappa-B (NF-kB), as a result of increased NF-kB activity in hepatic cells and PAI-1 gene transcription. Hyperglycemia along with hyperinsulinemia are the underlying reasons of all these changes. Advanced glycation end products (AGEs), which are overproduced in hyperglycemia, have an impact on TF expression, thereby increasing TF levels and procoagulant activity (33). The receptor for AGEs (RAGE) is a multi-ligand receptor which is able to interact with many non-AGE ligands, with an impact on different chronic inflammatory diseases (34-36). The RAGE axis activation was proposed among the mechanisms demonstrating the worse prognosis of the association between diabetes and COVID-19, as in animal models with acute respiratory distress syndrome (ARDS) it was observed that RAGES inhibition led to reduced lung damage as well as restored alveolar fluid clearance (34,37,38). The vulnerability of vascular endothelium is directly affected by hyperglycemia, through the impairment of the glycocalyx integrity. This leads to a release of coagulation factors sheltered within the endothelial glycocalyx and amplify platelet-endothelial cell adhesion. In fibrinolysis and coagulation, different types of proteins are implicated, and as hyperglycemia may induce protein glycation, these effects must be taken into consideration upon establishing further care. Fibrin clots from patients with diabetes have high density compared with controls, and this leads to longer clot lysis time. The non-enzymatic glycation of fibrin is a likely explanation (39).
Several studies revealed two types of effects on thrombotic markers in the context of hyperinsulinemia and hyperglycemia, an independent and an additive action when they are concurrently existing. Although insulin has an inhibiting effect on platelet activation, a previous study demonstrated that platelets of patients with DM are resistant to this function, becoming more prone to be activated (40).
Thereby, hypercoagulability can be induced by hyperglycemia through different complex mechanisms and the impact is more important along with hyperinsulinemia (41).
5. Hypercoagulable state
In order to halt bleeding and form a dense clot, platelets act along with blood coagulation factors, a number of molecules with protein structure, which have a key role in the coagulation process. In patients with DM, qualitative and quantitative changes of coagulation and anticoagulation factors were observed. In DM, insulin resistance and hyperglycemia exert synergistic effects on the TF pathway, causing factor VIIa (FVIIa) consumption and increased procoagulatory activity (42). The intrinsic pathway of blood coagulation implies successive activation of factor XII (FXII), factor XI (FXI) and factor IX (FIX). Previous findings have showed impairment of intrinsic blood coagulation correlated with hyperinsulinemia and hyperglycemia in DM. A reduced activated partial thromboplastin time along with an increased synthesis of FXII, FXI and FIX were identified in cases with diminished insulin sensitivity, influenced by an inflammatory response as a consequence of insulin resistance (43). The final phase in extrinsic and intrinsic blood coagulation pathways ends with the transition of fibrinogen to fibrin, and in patients with DM, higher circulating fibrinogen levels were noticed, revealing a more compacted clot structure with increased resistance to fibrinolysis. Furthermore, there are several factors that can explain hyperfibrinogenemia in DM (44).
Fibrinogen synthesis occurs in the liver and different factors can determine a significant influence on fibrinogen production, including the most common comorbidities observed in patients with DM, such as dyslipidemia, obesity and non-alcoholic fatty acid diseases. The underlying mechanisms of diabetes, insulin resistance and hyperglycemia enhance hepatic fibrinogen synthesis. Considering that patients with DM have a low-grade inflammation, this leads to an additional increase of fibrinogen synthesized in hepatocytes. Previously, it has been proved that the quality of fibrinogen is modified in DM (45). An increased glucose level amplifies glycation of fibrinogen and disrupts the fibrinolytic process. The configuration of fibrinogen and fibrin can be modified by persistent inflammatory reactions and oxidative stress, contributing to clot durability. An important element in the coagulation process is thrombin, which converts fibrinogen into fibrin. In patients with DM, increased thrombin levels have been noticed, which cause an elevation in fibrin production and clot density, thus having an important impact in the prothrombotic status. Moreover, hyperinsulinemia and hyperglycemia stimulate prothrombin production in the liver (46).
A significant physio-pathological particularity of COVID-19 is the occurrence of a prothrombotic status. Increased levels of vWF, fibrinogen and D-dimer, a by-product of fibrin degradation, regulate the specific coagulopathy of COVID-19. Considerably higher D-dimer levels were consistently noticed in severely affected patients (47). D-dimer levels can reveal the need for mechanical ventilation and therefore constitute one of the most important laboratory parameters. If during hospitalization, extremely high levels or a progressive elevation are detected, the risk of complications and even death are significantly increased. The high level of D-dimers can be suggestive for coagulation cascade activation and amplified thrombin generation (48). Decreased antithrombin levels were associated with inadequate outcomes in cases of SARS-CoV-2 infection, particularly among overweight individuals or individuals with obesity. A further aspect which is important in cases with SARS-CoV-2 infection is the antithrombin activity, which can be influenced, depending on the severity of the infection. As a result of the inflammatory status, the cytokine secretion is increased and is presumed to be the cause of the imbalance of the anticoagulant levels. An important association has been established between IL-6 and D-dimers levels, thus, the more the level of proinflammatory cytokine increases, the more the level of D-dimers elevates. As a result, this proves the connection between COVID-19-associated inflammation and procoagulant status. These outcomes shed light on the prothrombotic nature of SARS-CoV-2 infection, regarding both immuno-thrombosis and hemostasis (49). COVID-19-induced endothelial impairment affects the subendothelial TF and collagen, and as a result of the coagulation pathway activation and the aggregation of platelets, it starts thrombus development. In the coagulation process, several mechanisms act together, for instance, cytokine secretion with the help of neutrophil extracellular traps may promote aggregation of the platelets (fibrinogen and fibrin can activate neutrophils). Innate immunity may be triggered simultaneously by thrombin and factor Xa. Fibrinogen is considerably increased in patients with COVID-19, which reflects the inflammatory acute phase response (50). A meta-analysis of 34 studies revealed that increased D-dimer levels, low platelet count and prolonged prothrombin time occur more frequently in patients with severe COVID-19(51). International Society on Thrombosis and Haemostasis released a guide for the management of coagulopathy in COVID-19 which recommends testing and monitoring of prothrombin time, D-dimer and fibrinogen levels, as well as the platelet count. Thrombin-antithrombin complex (TAT) and prothrombin fragment have also been measured in patients with COVID-19. In a comparison between the intensive care unit (ICU) patients with COVID-19 with non-ICU patients, the plasma levels of TAT where considerably higher in patients with severe condition and ICU necessity. To identify thrombotic manifestations, TAT was more determinative than D-dimer levels. In addition, vWF and FVIII are considerably elevated in patients with COVID-19(52).
Considering all the aforementioned results, patients with COVID-19 should be investigated with extreme caution since this is a severe disease with various inflammatory and haemostatic abnormalities that are compatible with a prothrombotic status. Therefore, it can be assumed that if severe SARS-CoV-2 infection is combined with diabetes, the development of coagulopathy and multiple complications are more likely to occur, considering the important impact of DM on evolution and prognosis. In a recent review presenting the relationship between DM and COVID-19, it was emphasized that ever since the emerging of the virus, it was observed that patients with DM had a worse outcome when contracting SARS-CoV-2, presenting statistically higher rates of ARDS, organ damage, necessity of hospitalization in ICUs and higher mortality rates, regardless of the geographical area where the studies were performed (53). Some of the most relevant studies regarding the association between DM and COVID-19 that demonstrated the prothrombotic state observed in these patients are summarized in Table I (5,54-59).
Table I.
Articles investigating the impact of metabolic imbalance on COVID-19 cases and pro-thrombotic changes.
| First author | Study period | Region | Main findings | (Refs.) |
|---|---|---|---|---|
| Haowei Li | From February 4th to April 14th, 2020 | Wu Han, China | This study includes 2,467 patients with COVID-19, 1,269 males and the average age is 59 years. Elevated D-dimer levels and higher fasting blood glucose is observed in 1,100 patients, thus demonstrating an increased risk for thrombosis. COVID-19 prognosis is considerably influenced by increased D-dimer and hyperglycemia, which exhibit a synergistic effect. | (54) |
| Stefania L. Calvisi | From April to May 2020 | Milan, Italy | This study analyses 169 hospitalized patients with COVID-19 in which 51 patients have diabetes. Diabetes/stress hyperglycemia is associated with inflammation and tissue damage markers. An increased risk of thromboem- bolic events is linked to glucose variability. | (5) |
| Yogendra Mishra | From July 12th to August 31th, 2020 | North India | In this study, patients with COVID-19 and diabetes exhibit D-dimer levels of 1509±2420 ng/ml and cases without diabetes 515±624 ng/ml. Patients with diabetes have greater D-dimer levels and these results are statistically significant. | (55) |
| Anees A. Sindi | From April to December, 2020 | Jeddah, Saudi Arabia | This study reveals the impact of diabetes on mortality rates in patients with COVID-19. Including 198 patients, 86 are diabetic and 139 are males with a mean age of 54.14 years. Mortality rate is higher in diabetic patients and the most frequent comorbidity is hypertension. | (56) |
| Fien A. von Meijenfeldt | From April 9th to June 8th, 2020 | Stockholm, Sweden | Patients with COVID-19 and respiratory support have elevated levels of D-dimer, fibrinogen, FVIII and vWF. Decreased levels of prothrombin, antithrombin, reduced number of platelets and higher levels of vWF factor are associated with short-term mortality. | (57) |
| Chaymae Miri | From November 01st to December 01st, 2020 | Oujda, Morocco | In this analysis, 201 patients with COVID-19 are included, average age is 64 years and 56% are male. D-dimer levels are statistically higher in diabetic patients. D-dimer levels >2,885 ng/ml is a significant predictor of mortality in diabetic patients. | (58) |
| Hermina Novida | From May 1st to August 31th, 2020 | Surabaya, Indonesia | This research includes 201 subjects, 108 are categorized as severe and 93 as non-severe COVID-19 cases. The average age is 55.69 years, diabetes onset is #x003C;10 years and most of the patients have hypertension, blood sugar levels of ≥200 mg/dl and HbA1c ≥8%. The presence of hypertension, HbA1c ≥8%, age ≥60 years and male sex are associated with severe COVID-19 in cases with diabetes. | (59) |
COVID-19, coronavirus disease-19; vWF, von Willebrand factor; HbA1c, glycated haemoglobin.
A post-mortem study performed in patients affected by COVID-19 who also presented associated comorbidities, such as hypertension, chronic kidney disease, and metabolic diseases including diabetes, obesity and obstructive sleep apnoea, have shown specific organ damage at pulmonary and renal level, as well as small vessel injury (60). The aforementioned study has demonstrated that coagulation dysfunction is common in severe cases of SARS-CoV-2 infection, with thrombogenic microangiopathy being a frequent occurrence when autopsies were performed (60). In patients with DM, microvascular complications are a risk factor for severe COVD-19, as in these patients, endothelial dysfunction as well as microcirculatory impairments are present at organ level, including pulmonary microvascular lesions, which are responsible for a decrease in pulmonary diffusing capacity, which can be translated into a poor adaptability of the lungs in case of a SARS-CoV-2 infection (61). Therefore, the specificity of the lung injury and susceptibility to COVID-19 that affects life expectancy of patients with DM is also associated with the prothrombotic status.
6. Conclusions
Diabetes is the most common comorbidity in patients with SARS-CoV-2 infection. Considering that patients with DM have a low-grade chronic inflammation, the risk of developing an increased level of inflammatory cytokines is significantly higher in the presence of SARS-CoV-2 infection. As reflected by different pathophysiological mechanisms, patients with COVID-19 and diabetes are more likely to develop a hypercoagulable state, and are associated with severe disease and poor prognosis. An important feature of the relationship between DM and COVID-19 is the prothrombotic status, which is linked with a poor prognosis and higher mortality rate in these patients. Consequently, a growing understanding of the mechanisms involved in this association will contribute to an earlier diagnosis of thrombotic events as well as an early initiation of antithrombotic therapy, resulting in an improved outcome for patients with DM that develop COVID-19.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
RM, VP, AM, DT, MR, DC, AE, RP, TC and IV contributed equally to acquisition, analysis and classification of data, manuscript writing and for critical revision of the manuscript for important intellectual content. All authors read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1. World Health Organization. Available online at: https://covid19.who.int/ (Accesed on 15th January 2023). [Google Scholar]
- 2.Yadaiah KB, Shah C, Cheryala V, Gali JH, Kishore SK, Kumar R, Gunturu H, Sushmita G. Effect of SARS-CoV-2 on glycemic control in post-COVID-19 diabetic patients. J Family Med Prim Care. 2022;11:6243–6249. doi: 10.4103/jfmpc.jfmpc_709_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, Rech R, Colombo R, Antinori S, Corbellino M, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: A two-centre descriptive study. Lancet Infect Dis. 2020;20:1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID-19 in people with diabetes: Understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8:782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calvisi SL, Ramirez GA, Scavini M, Da Prat V, Di Lucca G, Laurenzi A, Gallina G, Cavallo L, Borio G, Farolfi F, et al. Thromboembolism risk among patients with diabetes/stress hyperglycemia and COVID-19. Metabolism. 2021;123(154845) doi: 10.1016/j.metabol.2021.154845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitocco D, Viti L, Tartaglione L, Di Leo M, Rizzo GE, Manto A, Rizzi A, Caputo S, Pontecorvi A. Diabetes and severity of COVID-19: What is the link? Med Hypotheses. 2020;143(109923) doi: 10.1016/j.mehy.2020.109923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wijaya I, Andhika R, Huang I. Hypercoagulable state in COVID-19 with diabetes mellitus and obesity: Is therapeutic-dose or higher-dose anticoagulant thromboprophylaxis necessary? Diabetes Metab Syndr. 2020;14:1241–1242. doi: 10.1016/j.dsx.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van den Oever IA, Raterman HG, Nurmohamed MT, Simsek S. Endothelial dysfunction, inflammation, and apoptosis in diabetes mellitus. Mediators Inflamm. 2010;2010(792393) doi: 10.1155/2010/792393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haller H. The endothelium and the pathogenesis of chronic vascular diseases: The protective role of calcium antagonists. In: Lüscher TF (eds). The Endothelium in Cardiovascular Disease. Springer, Berlin, Heidelberg, pp97-107, 1995. [Google Scholar]
- 10.Calles-Escandon J, Cipolla M. Diabetes and endothelial dysfunction: A clinical perspective. Endocr Rev. 2001;22:36–52. doi: 10.1210/edrv.22.1.0417. [DOI] [PubMed] [Google Scholar]
- 11.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pelle MC, Zaffina I, Lucà S, Forte V, Trapanese V, Melina M, Giofrè F, Arturi F. Endothelial dysfunction in COVID-19: Potential mechanisms and possible therapeutic options. Life (Basel) 2022;12(1605) doi: 10.3390/life12101605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S. Angiotensin II signal transduction through the AT1 receptor: Novel insights into mechanisms and pathophysiology. Clin Sci (Lond) 2007;112:417–428. doi: 10.1042/CS20060342. [DOI] [PubMed] [Google Scholar]
- 14.Del Turco S, Vianello A, Ragusa R, Caselli C, Basta G. COVID-19 and cardiovascular consequences: Is the endothelial dysfunction the hardest challenge? Thromb Res. 2020;196:143–151. doi: 10.1016/j.thromres.2020.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coman AE, Ceasovschih A, Petroaie AD, Popa E, Lionte C, Bologa C, Haliga RE, Cosmescu A, Slănină AM, Bacușcă AI, et al. The significance of low magnesium levels in COVID-19 patients. Medicina (Kaunas) 2023;59(279) doi: 10.3390/medicina59020279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon DL, Van Tassell BW, Dentali F, Montecucco F, Massberg S, Levi M, Abbate A. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21:319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: Challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019;133:906–918. doi: 10.1182/blood-2018-11-882993. [DOI] [PubMed] [Google Scholar]
- 18.Foley JH, Conway EM. Cross talk pathways between coagulation and inflammation. Circ Res. 2016;118:1392–1408. doi: 10.1161/CIRCRESAHA.116.306853. [DOI] [PubMed] [Google Scholar]
- 19.Margetic S. Inflammation and haemostasis. Biochem Med (Zagreb) 2012;22:49–62. [PMC free article] [PubMed] [Google Scholar]
- 20.Chanchal S, Mishra A, Singh MK, Ashraf MZ. Understanding inflammatory responses in the manifestation of prothrombotic phenotypes. Front Cell Dev Biol. 2020;8(73) doi: 10.3389/fcell.2020.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Páramo JA. Inflammatory response in relation to COVID-19 and other prothrombotic phenotypes. Reumatol Clin (Engl Ed) 2022;18:1–4. doi: 10.1016/j.reumae.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabrera-Rivera GL, Madera-Sandoval RL, León-Pedroza JI, Ferat-Osorio E, Salazar-Rios E, Hernández-Aceves JA, Guadarrama-Aranda U, López-Macías C, Wong-Baeza I, Arriaga-Pizano LA. Increased TNF-α production in response to IL-6 in patients with systemic inflammation without infection. Clin Exp Immunol. 2022;209:225–235. doi: 10.1093/cei/uxac055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Copaescu A, Smibert O, Gibson A, Phillips EJ, Trubiano JA. The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. J Allergy Clin Immunol. 2020;146:518–534.e1. doi: 10.1016/j.jaci.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faraj SS, Jalal PJ. IL1β, IL-6, and TNF-α cytokines cooperate to modulate a complicated medical condition among COVID-19 patients: Case-control study. Ann Med Surg (Lond) 2023;85:2291–2297. doi: 10.1097/MS9.0000000000000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norooznezhad AH, Mansouri K. Endothelial cell dysfunction, coagulation, and angiogenesis in coronavirus disease 2019 (COVID-19) Microvasc Res. 2021;137(104188) doi: 10.1016/j.mvr.2021.104188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996;271:736–741. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- 27.Tan LY, Komarasamy TV, Rmt Balasubramaniam V. Hyperinflammatory immune response and COVID-19: A double edged sword. Front Immunol. 2021;12(742941) doi: 10.3389/fimmu.2021.742941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, Qin R, Wang H, Shen Y, Du K, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020;36(e3319) doi: 10.1002/dmrr.3319. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kito K, Tanabe K, Sakata K, Fukuoka N, Nagase K, Iida M, Iida H. Endothelium-dependent vasodilation in the cerebral arterioles of rats deteriorates during acute hyperglycemia and then is restored by reducing the glucose level. J Anesth. 2018;32:531–538. doi: 10.1007/s00540-018-2507-7. [DOI] [PubMed] [Google Scholar]
- 30.Meerarani P, Moreno PR, Cimmino G, Badimon JJ. Atherothrombosis: Role of tissue factor; link between diabetes, obesity and inflammation. Indian J Exp Biol. 2007;45:103–110. [PubMed] [Google Scholar]
- 31.Verkleij CJ, Bruijn RE, Meesters EW, Gerdes VE, Meijers JC, Marx PF. The hemostatic system in patients with type 2 diabetes with and without cardiovascular disease. Clin Appl Thromb Hemost. 2011;17:E57–E63. doi: 10.1177/1076029610384112. [DOI] [PubMed] [Google Scholar]
- 32.Thögersen AM, Jansson JH, Boman K, Nilsson TK, Weinehall L, Huhtasaari F, Hallmans G. High plasminogen activator inhibitor and tissue plasminogen activator levels in plasma precede a first acute myocardial infarction in both men and women: Evidence for the fibrinolytic system as an independent primary risk factor. Circulation. 1998;98:2241–2247. doi: 10.1161/01.cir.98.21.2241. [DOI] [PubMed] [Google Scholar]
- 33.Iwasaki Y, Kambayashi M, Asai M, Yoshida M, Nigawara T, Hashimoto K. High glucose alone, as well as in combination with proinflammatory cytokines, stimulates nuclear factor kappa-B-mediated transcription in hepatocytes in vitro. J Diabetes Complications. 2007;21:56–62. doi: 10.1016/j.jdiacomp.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Rojas A, Lindner C, Gonzàlez I, Morales MA. Advanced-glycation end-products axis: A contributor to the risk of severe illness from COVID-19 in diabetes patients. World J Diabetes. 2021;12:590–602. doi: 10.4239/wjd.v12.i5.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manigrasso MB, Juranek J, Ramasamy R, Schmidt AM. Unlocking the biology of RAGE in diabetic microvascular complications. Trends Endocrinol Metab. 2014;25:15–22. doi: 10.1016/j.tem.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramasamy R, Yan SF, Schmidt AM. The diverse ligand repertoire of the receptor for advanced glycation endproducts and pathways to the complications of diabetes. Vascul Pharmacol. 2012;57:160–167. doi: 10.1016/j.vph.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Audard J, Godet T, Blondonnet R, Joffredo JB, Paquette B, Belville C, Lavergne M, Gross C, Pasteur J, Bouvier D, et al. Inhibition of the receptor for advanced glycation end-products in acute respiratory distress syndrome: A randomised laboratory trial in piglets. Sci Rep. 2019;9(9227) doi: 10.1038/s41598-019-45798-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blondonnet R, Audard J, Belville C, Clairefond G, Lutz J, Bouvier D, Roszyk L, Gross C, Lavergne M, Fournet M, et al. RAGE inhibition reduces acute lung injury in mice. Sci Rep. 2017;7(7208) doi: 10.1038/s41598-017-07638-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaidyula VR, Rao AK, Mozzoli M, Homko C, Cheung P, Boden G. Effects of hyperglycemia and hyperinsulinemia on circulating tissue factor procoagulant activity and platelet CD40 ligand. Diabetes. 2006;55:202–208. [PubMed] [Google Scholar]
- 40.Stegenga ME, van der Crabben SN, Dessing MC, Pater JM, van den Pangaart PS, de Vos AF, Tanck MW, Roos D, Sauerwein HP, van der Poll T. Effect of acute hyperglycaemia and/or hyperinsulinaemia on proinflammatory gene expression, cytokine production and neutrophil function in humans. Diabet Med. 2008;25:157–164. doi: 10.1111/j.1464-5491.2007.02348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dunn EJ, Philippou H, Ariëns RA, Grant PJ. Molecular mechanisms involved in the resistance of fibrin to clot lysis by plasmin in subjects with type 2 diabetes mellitus. Diabetologia. 2006;49:1071–1080. doi: 10.1007/s00125-006-0197-4. [DOI] [PubMed] [Google Scholar]
- 42.Dayer MR, Mard-Soltani M, Dayer MS, Alavi SM. Causality relationships between coagulation factors in type 2 diabetes mellitus: Path analysis approach. Med J Islam Repub Iran. 2014;28(59) [PMC free article] [PubMed] [Google Scholar]
- 43.Boden G, Rao AK. Effects of hyperglycemia and hyperinsulinemia on the tissue factor pathway of blood coagulation. Curr Diab Rep. 2007;7:223–227. doi: 10.1007/s11892-007-0035-1. [DOI] [PubMed] [Google Scholar]
- 44.Neergaard-Petersen S, Hvas AM, Kristensen SD, Grove EL, Larsen SB, Phoenix F, Kurdee Z, Grant PJ, Ajjan RA. The influence of type 2 diabetes on fibrin clot properties in patients with coronary artery disease. Thromb Haemost. 2014;112:1142–1150. doi: 10.1160/TH14-05-0468. [DOI] [PubMed] [Google Scholar]
- 45.Abdul Razak MK, Sultan AA. The importance of measurement of plasma fibrinogen level among patients with type-2 diabetes mellitus. Diabetes Metab Syndr. 2019;13:1151–1158. doi: 10.1016/j.dsx.2019.01.049. [DOI] [PubMed] [Google Scholar]
- 46.Pieters M, van Zyl DG, Rheeder P, Jerling JC, Loots du T, van der Westhuizen FH, Gottsche LT, Weisel JW. Glycation of fibrinogen in uncontrolled diabetic patients and the effects of glycaemic control on fibrinogen glycation. Thromb Res. 2007;120:439–446. doi: 10.1016/j.thromres.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 47.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, Pesenti A, Peyvandi F, Tripodi A. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mir N, D'Amico A, Dasher J, Tolwani A, Valentine V. Understanding the andromeda strain-the role of cytokine release, coagulopathy and antithrombin III in SARS-CoV2 critical illness. Blood Rev. 2021;45(100731) doi: 10.1016/j.blre.2020.100731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goshua G, Pine AB, Meizlish ML, Chang CH, Zhang H, Bahel P, Baluha A, Bar N, Bona RD, Burns AJ, et al. Endotheliopathy in COVID-19-associated coagulopathy: Evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu J, Pang J, Ji P, Zhong Z, Li H, Li B, Zhang J, Lu J. Coagulation dysfunction is associated with severity of COVID-19: A meta-analysis. J Med Virol. 2021;93:962–972. doi: 10.1002/jmv.26336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, Clark C, Iba T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li G, Chen Z, Lv Z, Li H, Chang D, Lu J. Diabetes mellitus and COVID-19: Associations and possible mechanisms. Int J Endocrinol. 2021;2021(7394378) doi: 10.1155/2021/7394378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li H, Chen S, Wang S, Yang S, Cao W, Liu S, Song Y, Li X, Li Z, Li R, et al. Elevated D-dimer and adverse in-hospital outcomes in COVID-19 patients and synergism with hyperglycemia. Infect Drug Resist. 2022;15:3683–3691. doi: 10.2147/IDR.S367012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mishra Y, Pathak BK, Mohakuda SS, Tilak TVSVGK, Sen S, P H, Singh R, Singh AR. Relation of D-dimer levels of COVID-19 patients with diabetes mellitus. Diabetes Metab Syndr. 2020;14:1927–1930. doi: 10.1016/j.dsx.2020.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sindi AA, Tashkandi WA, Jastaniah MW, Bashanfar MA, Fakhri AF, Alsallum FS, Alguydi HB, Elhazmi A, Al-Khatib TA, Alawi MM, Abushoshah I. Impact of diabetes mellitus and co-morbidities on mortality in patients with COVID-19: A single-center retrospective study. Saudi Med J. 2023;44:67–73. doi: 10.15537/smj.2023.44.1.20220462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.von Meijenfeldt FA, Havervall S, Adelmeijer J, Lundström A, Rudberg AS, Magnusson M, Mackman N, Thalin C, Lisman T. Prothrombotic changes in patients with COVID-19 are associated with disease severity and mortality. Res Pract Thromb Haemost. 2020;5:132–141. doi: 10.1002/rth2.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miri C, Charii H, Bouazzaoui MA, Laouan Brem F, Boulouiz S, Abda N, Kouismi H, Bazid Z, Ismaili N, El Ouafi N. D-dimer level and diabetes in the COVID-19 infection. Clin Appl Thromb Hemost. 2021;27(10760296211045902) doi: 10.1177/10760296211045902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Novida H, Soelistyo SA, Cahyani C, Siagian N, Hadi U, Pranoto A. Factors associated with disease severity of COVID-19 in patients with type 2 diabetes mellitus. Biomed Rep. 2022;18(8) doi: 10.3892/br.2022.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, Najafian B, Deutsch G, Lacy JM, Williams T, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: A case series. Lancet. 2020;396:320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whyte MB, Vas P, Heiss C, Feher MD. The contribution of diabetic micro-angiopathy to adverse outcomes in COVID-19. Diabetes Res Clin Pract. 2020;164(108217) doi: 10.1016/j.diabres.2020.108217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.