Abstract
Th17 cells have been investigated in mice primarily for their contributions to autoimmune diseases. However, the pathways of differentiation of Th17 and related Th cells (type 17 cells) and the structure of the type 17 memory population in humans are not well understood; such understanding is critical for manipulating these cells in vivo. By exploiting differences in levels of surface CCR6, we found that human type 17 memory cells, including individual T cell clonotypes, form an elongated continuum of type 17 character along which cells can be driven by increasing RORγt. This continuum includes cells preserved within the memory pool with potentials that reflect the early preferential activation of multiple over single lineages. The CCR6+ cells’ phenotypes and epigenomes are stable across cell divisions under non-inflammatory conditions. Nonetheless, activation in polarizing and non-polarizing conditions can yield additional functionalities, revealing, respectively, both environmentally induced and imprinted mechanisms that contribute differentially across the continuum to yield the unusual plasticity ascribed to type 17 cells.
INTRODUCTION
Immunological memory mediated by the adaptive immune system can provide long-standing natural and vaccine-mediated protection against infectious agents. Notwithstanding controversies regarding the meaning and effectiveness of the T cell component of immunological memory (1), pathogen-specific CD8+ and CD4+ T cells can be detected in humans many decades following exposure (2, 3), and for CD4+ memory T cells, which are the focus of the current studies, there are data from mice demonstrating their protective capabilities (4, 5). T cell memory depends on epigenetic memory, including DNA methylation, with resting memory T cells maintaining an altered epigenome that is poised to allow cells to respond to activation in a biased but flexible fashion based on the pattern of initial differentiation/polarization (6).
The ontogeny of memory T cells has long been a subject of ongoing investigation and debate (7). It is clear that descendants of individual CD8+ T cells can assume multiple phenotypes of effector and memory cells (8–10). Further, based on studies of human CD4+ memory T cells specific for pathogens or tetanus toxoid, individual T cells can, and often do give rise to cells of different lineages as defined by Th1, Th2, and Th17 signature cytokines (11). Moreover, the preponderance of evidence from mouse and human studies favors effector T cells as the direct precursors of memory cells (8, 12–18). Critical experiments using single-cell transfers in mice have shown that the ability to form memory cells is a general and not exceptional property of individual naive CD4+ T cells, and that, of equal importance, the composition of the memory population mirrors the composition of the effector population (18). Moreover, studies of human memory CD8+ T cells have suggested that phenotypes and epigenomes are stable under homeostatic conditions (19). The correspondence between effector and memory populations provides a fundamental benefit often lacking in models explaining the origins of T cell memory, namely that the information contained in the acute and evolving effector response is fully preserved in the memory population. A further consequence of these findings is that the memory T cell population preserves a record of the acute response and can be used to reconstruct pathways of differentiation taken by activated T cells. The ability to use studies of the memory population in this way is particularly important in understanding the process of memory T cell differentiation in humans, where it is both the case that the time frames and complexity of environmental exposures far exceed those of laboratory mice (20, 21), and detailed observations of immune responses in real time are difficult.
Based on the conventional definitions of CD4+ T cell effector/memory subsets, Th17 cells were the third major subset to be identified as a “lineage”, with IL-17A as their signature cytokine and RORγt as their signature transcription factor (22). In initial studies in mice, Th17 cells were found to contribute to autoimmune pathology (23), and subsequent work in humans demonstrated the importance of the Th17 pathway in host defense against Candida, Staphylococcus aureus, and Mycobacterium (24–27) as well as in inflammatory disease (28). Further, mouse studies revealed that Th17 cells can assume dichotomous phenotypes as both protective and pathogenic (29) and that although some studies in mice have questioned the durability of Th17 memory (30), other studies in mice (31) and humans (32) have reported that Th17 cells can establish persistent memory.
Prominent features described for mouse Th17 cells include their abilities to co-express genes of alternative lineages, lose expression of IL-17A/F, and acquire the ability to express a different signature cytokine, primarily IFNγ (33–37) - manifesting properties broadly described as plasticity (37). Nonetheless, whether plasticity in cytokine profiles indicates that a fully differentiated memory cell is able to transdifferentiate remains controversial (38), and some studies of Th17 cells in mice have shown that their phenotype can be stable during both homeostasis and inflammation (39, 40). Investigations of the phenotype and epigenome of human Th17 cells suggest that they may be more stable than their murine counterparts (41), and that IFNγ+IL-17A− cells that arise under Th17 conditions are best viewed as a subset of Th17 cells rather than as Th1 cells that have arisen by transdifferentiation (42, 43).
The chemokine receptor CCR6 and its ligand, CCL20, have broad roles in humoral (44) and cellular (45) barrier defense. We and others reported that virtually all human Th17 cells express CCR6 (46–48), which is inducible on T cells by RORγt (49). Cells expressing other cytokines whose expression is associated with, but not precisely matching the expression of IL-17A, such as IL-22 and CCL20, are also found exclusively as subsets of CCR6+ cells (46, 50) and this manuscript); and CCR6 is found on a subset of human peripheral regulatory T cells (51) that can be stimulated to produce IL-17A (52). Additionally, CCR6 is used to identify the human Th17-related, IFNγ+IL-17A− cells that have been referred to as nonclassical Th1 (43) or Th1* (46) cells, and that have been implicated in defense against mycobacteria (26) and autoimmune disease (53). Together, these data suggest that human Th17 cells that are defined by their production of IL-17A/F represent a limited subset within a broader family of “type 17”, CCR6-expressing cells. Our studies started from the premise that investigating the interrelationships of cells within this larger type 17 family would reveal how human Th17 and Th17-related effector/memory cells are formed and organized, thereby providing additional insights into their roles in host defense and inflammation.
We discovered previously in analyzing relationships between the expression of signature chemokine receptors and a limited number of other lineage-associated genes in bulk populations of human Th cells that levels of surface CCR6 correlated positively with levels of expression of IL17A and RORC, and that the CCR6+ cells showed a wide gradient in their expression of these genes as compared with gradients for expression of type 1 genes in CXCR3+ cells or type 2 genes in CCR4+ cells (54). In the current study, by analyzing human CD4+ T cells based on their levels of surface CCR6, both as populations and as single cells, we have found that the CCR6+ cells form an extended, progressive continuum of type 17 character, with cells of individual T cell clonotypes found throughout the continuum, albeit with changing frequencies. Although cells could be driven along the continuum by over-expression of RORC/RORγt, analysis of genome-wide patterns of CpG methylation in dividing cells showed that epigenotypes/phenotypes are stable under non-inflammatory conditions. Single-cell analysis suggested pan-potentiation of effector pathways early after activation of CD4+ T cells in vivo that persist within the memory population, and most type 17 cells can co-express genes of alternative lineages, with co-expression diminishing along the type 17 continuum. Restimulation of CCR6-defined subgroups under polarizing and non-polarizing conditions yielded additional functionalities by both environmentally induced and previously imprinted mechanisms, which contributed differentially across the continuum to type 17 cell plasticity.
Materials and methods
Human samples:
Elutriated lymphocytes and whole blood were obtained from healthy donors by the Department of Transfusion Medicine, National Institutes of Health, under an Institutional Review Board-approved protocol.
Purification of lymphocyte subsets from blood and cell sorting:
CD4+ T cells were isolated from elutriated lymphocytes by negative selection using RosetteSep CD4+ T cell enrichment cocktail (StemCell Technologies) followed by Ficoll/Hypaque (Amersham Biosciences) density centrifugation according to the protocol from StemCell Technologies. If necessary, red blood cells were removed using Pharm Lyse (BD Biosciences) according to the manufacturer’s protocol. For staining cells were suspended in 250 μl of Hanks Balanced Salt Solution (HBSS, Mediatech) containing 4% fetal bovine serum (FBS, GeminiBio) and incubated with following antibodies: anti-CD4, anti-CD45RO, and anti-CCR6 for 30 min at room temperature. In some experiments, in addition to these antibodies, cells were incubated with anti-CD25 and anti-CD62L, anti-CXCR5, anti-CXCR3 and anti-CCR4 antibodies. All antibodies were against human antigens. Cells were washed and resuspended in HBSS plus 4% FBS. CD4+ T cells were sorted into CD45RO− (naïve), and CD45RO+ subsets, and the CD45RO+ subset was further separated into CCR6neg, CCR6pos, and, within the CCR6pos cells, into CCR6low, CCR6int and CCR6high subsets. For in vitro polarization experiments, cells were sorted into naïve (CD4+CD45RO−CD25−CXCR5−CCR4−CXCR3−), CCR6neg (CD4+CD45RO+CD25−CXCR5−CXCR3−CCR6− or CD4+CD45RO+CD25−CXCR5− CCR4−CCR6− or CD4+CD45RO+CD25−CXCR5−CXCR3−CCR4−CCR6−), CCR6low (CD4+CD45RO+CD25−CXCR5−CXCR3−CCR6low or CD4+CD45RO+CD25−CXCR5−CCR4−CCR6low or CD4+CD45RO+CD25−CXCR5−CXCR3−CCR4−CCR6low), and CCR6high (CD4+CD45RO+CD25−CXCR5−CXCR3−CCR6high or CD4+CD45RO+CD25−CXCR5−CCR4−CCR6high or CD4+CD45RO+CD25−CXCR5−CXCR3−CCR4−CCR6high). All cell sorting was done using a FACSAria Cell Sorter (BD Biosciences). By post-sort analysis, the purity of populations was 95–99%. Cells were used for experiments immediately after sorting.
Isolation of RNA, and real-time fluorogenic RT-PCR:
T cells were activated with 20 ng/ml PMA and 1 mM ionomycin in RPMI 1640 (GIBCO-Thermo Fisher Scientific) supplemented with 10% FBS, 1% L-glutamine and 1% penicillin/streptomycin (complete medium) for 3 h at 37°C under 5% CO2 and total cellular RNA was purified using PureLink™ RNA kit (Life Technology) or RNeasy Mini Kit (Qiagen) and the concentration and RNA Integrity Number (RIN) was determined with a RNA 6000 nano kit on 2100 Bioanalyzer instrument (Agilent Technologies). Real-time PCR was performed on samples in duplicate on an ABI 7900HT Real-Time PCR System (Applied Biosystems) or Eco Real-Time PCR System (Illumina) using SuperScript One Step RT-PCR kit (Life Technology). For most experiments, primer and probe sets (FAM/MGB-labeled) were purchased from Applied Biosystems. In some cases, DELTAgene Assay primers were purchased from Fluidigm and amplicon DNA was quantified using SsoFast™ EvaGreen Supermix with Low ROX (Bio-Rad Laboratories). Gene expression data were normalized based on the values for GAPDH mRNA. For some assays, for cells from each donor, relative levels of expression, based on values for 2−ΔCT, are shown after normalization to the single highest value, which was set to 100. For other assays, values of 2−ΔCT or ΔCT are shown without additional normalization or shown as fold changes relative to the values for naïve cells, which were set at 1.
Intracellular and intranuclear staining for flow cytometry:
T cells purified by cell sorting were stimulated with Leukocyte Activation Cocktail, with GolgiPlus™ (BD Pharmingen) for 6 h at 37°C under 5% CO2 before being stained with following antibodies alone or in combinations: anti-IL-17A (eBioscience), anti-IL-17F, anti-IL-22, anti-IL-6, anti-CCL20 and anti-IFNγ (R&D Systems) by using Cytofix/CytoPerm Plus kit (BD Pharmingen) or anti-RORγt, anti-FOXP3, anti-T-bet or anti-GATA3 using Foxp3 staining buffer set (eBioscience) according to the manufacturer’s instructions. All flow cytometry was done using FACSCaliber, LSR II System or Fortessa flow cytometers (BD Biosciences), and the data were subsequently analyzed using FlowJO software (TreeStar).
ELISA (Enzyme-Linked Immunosorbent Assay) for cytokine and chemokine production:
T cells purified by cell sorting were activated with 20 ng/ml PMA and 1 mM ionomycin in complete medium at 37°C under 5% CO2 for 12 h and supernatants were collected and stored in −800C freezer until analyzed. GM-CSF, TNF-α, IFNγ, IL-4, IL-5, IL-10, IL-13, IL-17A, CCL3, CCL4, CCL5 and CCL20 were measured using Search Light Proteome Array (Pierce Biotechnology).
Microarray analyses:
FACS-sorted naïve, CCR6neg, CCR6low, CCR6int and CCR6high subsets of CD4+ T cells were used for isolating RNA without and with prior activation with 20 ng/ml PMA and 1 mM ionomycin for 3 h at 37°C under 5% CO2 using RNeasy Mini Kit (Qiagen) and gene expression levels were determined using Illumina Human WG6 Expression BeadChip Version 3 arrays (Illumina Inc.) according to the manufacturer’s protocol. In brief, 500 ng of total RNA was used to make cDNA and label cRNA using the Ambion Illumina TotalPrep RNA Amplification kit (Invitrogen). The resulting cRNA samples were hybridized to an Illumina Human WG6 Expression BeadChip array for 14–20 hour at 580C in an Illumina Hybridization Oven (Illumina Inc.). Arrays were washed and scanned following the protocols in the Illumina Whole-Genome Gene Expression Direct Hybridization Assay Guide (Illumina). Signal data were extracted from the image files with the Gene Expression module of the GenomeStudio software (Illumina, Inc.) Signal intensities were converted to log2 scale. Detection p values were calculated as described in the GenomeStudio Gene Expression Module User Guide. Data for array probes with insufficient signal were removed from the dataset (detection was defined as at least two arrays with detection p values < 0.1). Analysis of variance (ANOVA) was then performed on the normalized log2 expression estimates to test for mRNA expression differences across cell subgroups, with contrasts for the CCR6low, CCR6int and CCR6high subsets verses the CCR6neg subgroup. A p value of 0.05 was used for the significance cutoff, after adjusting for the familywise error rate (FWER) using the Benjamini-Hochberg method to account for multiple testing. One-way hierarchical clustering (on probes, i.e., gene-wise) was performed on the standardized LS means for all genes whose differential expression reached significance, separately for stimulated and non-stimulated cells. Genes whose differential expression reached significance and whose differences were the most extreme (greater than 4 standard deviations (|Z| > 4) of log2 differences within any contrast) were then analyzed to determine patterns across the CCR6-expressing subsets. Log2 differences from the contrasts were standardized across each probe and the resulting Z-scores were clustered by K-means where k=12. Genes within each cluster for each contrast were summarized as mean Z-scores and shown as a heat map to illustrate relative expression patterns across the CCR6-expressing subsets. Statistical analyses were performed using JMP/Genomics software version 7.0 (SAS Institute Inc.).
Genome-wide DNA methylation analysis:
Genomic DNAs from FACS-sorted naïve, CCR6neg, CCR6low, CCR6int and CCR6high subgroups were purified using the DNeasy kit (Qiagen). In some experiments, after purification by cell sorting, a portion of cells were used as starting cells (day 0) and the remaining cells were labeled with CFSE, activated with soluble anti-CD3 (100 ng/ml, eBioscience) and cultured with the homeostatic cytokines IL-7 and IL-15 (10 ng/ml each, R & D Systems ), for 7 days. Cells from the different subgroups were again sorted, based on CFSE dilution, into non-proliferated and proliferated cells and genomic DNAs were purified using the DNeasy kit. Bisulfite conversion of genomic DNA (500 ng) was performed using the Zymo Research EZ DNA Methylation kit (Zymo Research) according to the manufacturer’s recommendations for the Illumina Infinium assay. The bisulfite conversion incubation was processed using alternative thermocycler settings as outlined in the “Alternative Incubation Conditions When Using the Illumina Infinium® Methylation” section of the Zymo instruction manual appendix. The bisulfite-converted DNA samples were then processed for hybridization and staining using either the Illumina Infinium Human Methylation 450 BeadChip Kit or the Illumina Infinium MethylationEPIC BeadChip Kit (Illumina Inc.) according to the manufacturer’s recommendations. DNA methylation ratios were calculated on a scale of 0 – 1 in which zero represent 100% unmethylated and 1 represent 100% methylated. The methylation dataset was visualized using Tidyverse (an R open-source package).
Single-cell mRNA sequencing:
Purified CD4+ T cells were FACS sorted into CCR6neg, CCR6low, CCR6int and CCR6high subgroups, loaded with CFSE, and cultured with autologous dodecyldimethylamine oxide (DDAO)-loaded, irradiated PBMCs in the presence of an extract of CMV-infected cells (EastCoast Bio, North Berwick, ME). On day 7, the proliferated, CFSElow T cells were sorted, counted for cell viability, and immediately used for library preparation using Chromium™ Single Cell V (D) J Reagent Kit (10X Genomics) according to the manufacturer’s instructions.
The single-cell TCR libraries were sequenced on an Illumina NextSeq500 to a minimum sequencing depth of 5000 reads per cell using paired-end sequencing with single indexing as follows: read length of 150 bp read 1, 8 bp i7 index, 150 bp read 2. The raw data were downloaded using Python Run Downloader (Illumina) and analyzed using Cellranger (Illumina). TCR repertoires were processed using custom scripts to produce Circos-compatible files. In each Circos plot (Circos, version 0.69), the relative size of a clonal expansion is represented by arc-length while overlapping clonotypes are represented by ribbons connecting arcs. In some cases, to increase interpretability of the Circos plots, ribbons were only drawn between clonotypes that represented 4% or greater of either of the connected TCR repertoires. Morisita-Horn Dissimilarity values between TCR repertoires were calculated using the R package vegan (v. 2.5–7) and plotted in R using reciprocal values to represent similarity.
The single-cell 5’ gene expression libraries were sequenced on Illumina NextSeq500 to a minimum sequencing depth of 50000 reads per cell using pair-end sequencing as follows: read length of 150 bp read 1, 8 bp i7 index, 150 bp read 2. The raw data were downloaded using Python Run Downloader (Illumina) and analyzed using Cell Ranger (Illumina).
TCRB deep sequencing.
CD4+ T cells were sorted into naive, CCR6neg, CCR6low, CCR6int and CCR6high subgroups and total cellular RNAs were purified from using PureLink™ RNA kit (Life Technology) and the concentrations and RNA Integrity Numbers (RIN) were determined with an RNA 6000 nano kit on a 2100 Bioanalyzer (Agilent Technologies). Libraries for TCR sequencing were prepared as described (55). TCRB annotation was performed by first determining which paired reads were of the expected library insert size by merging the paired-end reads with the bioinformatic tool, PEAR (56). Paired-end reads that could be merged were discarded as they represented library inserts that were too short and assumed to be off-target products. Unmerged read-pairs were then annotated with MiXCR (57). Heatmaps and Circos plots were then generated in the same manner as above.
High-throughput single cell gene expression analysis by microfluidic quantitative RT-PCR:
Individual cells from naïve, CCR6neg, CCR6low, CCR6int and CCR6high subgroups were sorted by FACS into single wells of a 96 well plate where each well contained 5 ul of CellDirect 2X reaction mixture solution (Invitrogen) supplemented with 0.05 units of SUPERase-In™ RNase inhibitor (Ambion). After incubating at 40C for 15 min, each plate containing sorted cells was frozen and stored at −300 C until analysis. For analysis, plates were thawed on ice and each well was supplemented with 0.2 ul SuperScript III RT Platinum Taq Mix (Invitrogen), 2.8 ul DNA suspension buffer (Invitrogen) and 1.0 ul of a mixture of 48 pooled DELTAgene Assay primer pairs containing each primer at a final concentration of 500 nM (Fluidigm). mRNAs from lysates of single cells were reverse transcribed into cDNA at 500 C for 15 min, followed by inactivation of the reverse transcriptase at 950 C for 2 min. cDNAs were pre-amplified for 20 cycles by denaturing at 950 C for 15 sec followed by annealing and amplification at 600 C for 4 min. To digest unincorporated primers, 3.6 ul of Exonuclease I reaction mixture containing 0.36 ul of 10X Exonuclease reaction buffer, 0.72 ul of 20 units/ul Exonuclease I (New England BioLabs) and 2.52 ul of nuclease free water was added to each well, followed by an incubation at 370 C for 30 min and finally treatment at 800 C for 15 min. The pre-amplified cDNA was diluted 5-fold with TE buffer (10 mM Tris, pH 8.0, 1 mM EDTA) prior to gene expression assays using the 96.96 (M96) Dynamic Array Microfluidic Chip on a BioMark HD System (Fluidigm) according to the manufacturer’s protocol. Briefly, 2.25 ul of pre-amplified cDNA was mixed with 2.5 ul of 2X SsoFast EvaGreen Supermix with low ROX (Bio-Rad) and 0.25 ul of 20X DNA binding dye sample loading reagent (Fluidigm) and transferred into one of the array microfluidic chip sample inlets. Individual DELTAgene assays were prepared by combining 0.25 ul of primer pairs (100 uM), 2.25 ul of DNA suspension buffer, 2.5 ul of 2X Assay loading reagent (Fluidigm) and transferred into one of the array microfluidic chip assay inlets. Samples and assay mix solutions were loaded on the microfluidic chip using an IFC Controller MX (for 48.48 Dynamic array IFC) or IFC Controller HX (for M96 Dynamic array IFC) (Fludigm) and transferred to a BioMark HD System and run following manufacturer’s protocol. For each subgroup, cells were sorted from three individual donors. All Ct values obtained from the BioMark HD System were normalized to the endogenous control genes, GAPDH and PGK1 and cells with very low (Ct > 30) or non-detected endogenous control genes were removed from the analysis. For further analysis, normalized Ct values for individual genes were calculated by subtracting from the Ct value the mean Ct values of that gene on all the samples from a given subgroup on an individual chip and dividing by 3 times the standard deviation as described (58). Data acquired from the BioMark HD system were processed using Singular Analysis Toolset Software v3.5.2 (Fluidigm). We used open-source R packages (Tidyverse) to process the datasets and visualization. In some experiments, pre-amplified samples were used for TaqMan quantitative PCR which was performed on Eco Real-Time PCR System (Illumina) using gene specific primers purchased from Fluidigm (DELTAgene Assay) and detected using SsoFast™ EvaGreen Supermix with Low ROX (Bio-Rad Laboratories). Results from these assays were normalized based on the values for GAPDH mRNA.
Ectopic RORC, TBX21 and GATA3 expression by lentiviral gene transduction:
All the lentivirus particles expressing sequences from RORC, TBX21 and GATA3 or control virus particles contained the pReceiver-LV201 vector and were obtained from GeneCopoeia. A CMV promoter was used to drive the RORC, TBX21 and GATA3 sequences and an SV40 promoter was used to drive eGFP sequences. Purified CD4+ T cells were sorted by FACS into memory cells that were CCR6neg, CCR6low, and CCR6high and transduced with lentivirus particles at an MOI of 5 using retronectin (TaKaRa) according to the manufacturer’s instructions. In brief, 1 × 106 sorted cells per well of a 24 well plate were activated with anti-CD3/anti-CD28-coated beads, 1 bead per cell, for 24 hours and transferred to a well precoated with retronectin (TaKaRa). Lentivirus transduction was performed by spinoculation at 1200 X g for 2 hours at 320 C in the presence of virus particles and 8 ug/ml polybrene (Millipore). The next day, virus particles were removed, and cells were cultured in complete RPMI-1640 supplemented with 10 ng/ml of IL-2 for an additional three days. Cells were stimulated with PMA and ionomycin for 3 h and GFP+ cells were sorted by FACS. For single cell gene expression experiments, cells were sorted by FACS into each well of a 96 well plate containing 5 ul of CellDirect 2X reaction mixture solution supplemented with 0.05 units of SUPERase-In™ RNase inhibitor and used for analysis using the M96 Dynamic Array Microfluidic Chip on a BioMark HD System according to the manufacturer’s protocol or analyzed using quantitative RT-PCR. For some experiment cells were loaded with DDAO before lentivirus transduction to enable cells to be sorted based on numbers of cell divisions.
In vitro polarization of CD4+ T lymphocytes from adult peripheral blood:
Cells were sorted by FACS as described above into naïve and CCR6neg, CCR6low and CCR6high memory subgroups and cultured at 1 X 106 cells/ml in 24 well plates in ImmunoCult XF T cell expansion medium (Stem Cell Technologies) supplemented with 10% FBS and IL-2 (200 units/ml). Stimulation was done using ImmunoCult human CD3/CD28/CD2 T Cell Activator (Stem Cell Technologies) in non-polarizing conditions using anti-IL-4 (5 μg/ml), and anti-IFN-γ (10 μg/ml), or in Th1 conditions using recombinant human IL-12 (5 ng/ml), and anti-IL-4 (4 μg/ml), or in Th2 conditions using recombinant human IL-4 (10 ng/ml), and anti-IFN-γ (10 μg/ml). After three days, proliferating cells were washed in PBS and expanded under the same conditions in the absence of CD3/CD28/CD2 T Cell Activator.
Quantification and statistical analysis:
Data were analyzed using GraphPad Prism software version 9 (GraphPad, San Diego, CA). The p values were calculated using Wilcoxon matched pairs signed rank test. Linear regression in R was used to generate p-values for the relationship between single-cell q-PCR Ct values for genes of interest. ANOVA was performed on the normalized log2 expression values to estimate differences between cell subgroups, and adjusted p-values were generated using the Benjamini-Hochberg method to account for multiple testing. *p < 0.05, **p < 0.01, and ****p < 0.0001.
RESULTS
CCR6-defined subgroups show progressive upregulation of type 17, and down-regulation of type 1 and type 2 genes and proteins
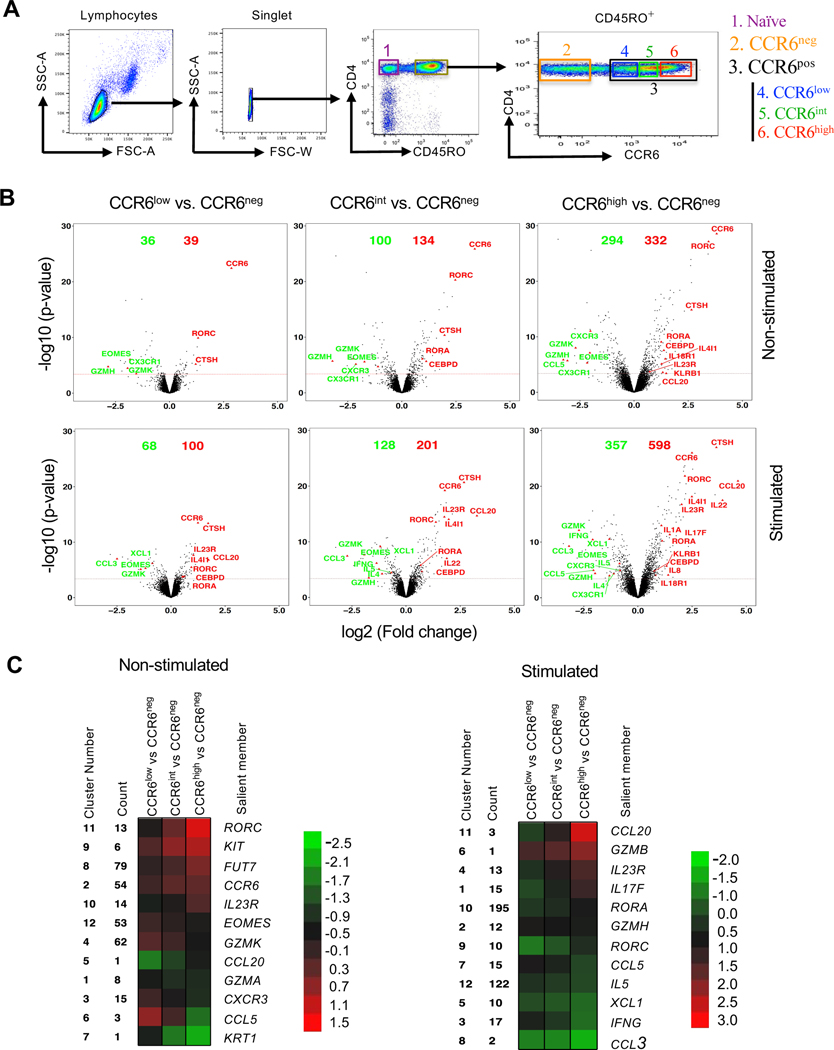
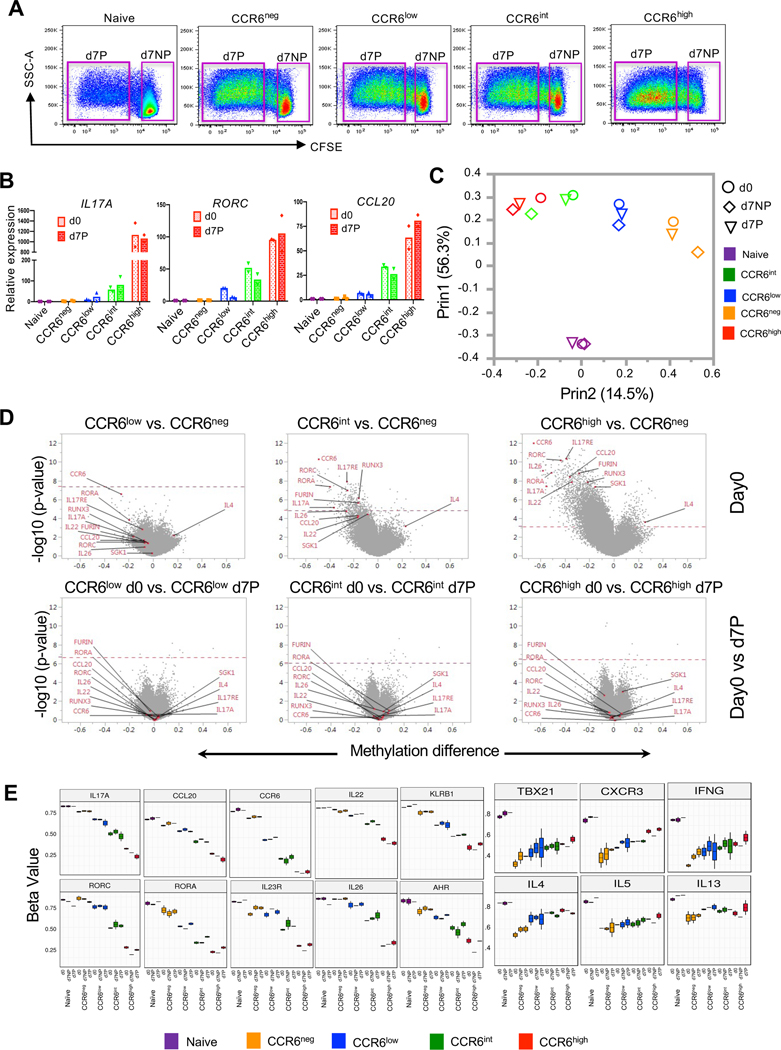
To investigate the relationship between the surface expression of CCR6 and genes associated with effector/memory phenotypes, we used FACS to separate CD4+ T cells from healthy adult blood donors into naïve cells and memory-phenotype cells that were CCR6neg and CCR6pos, and the CCR6pos cells were further separated into three equal subgroups based on their levels of CCR6: CCR6low, CCR6int or CCR6high (Fig. 1 A). Intracellular staining of FACS-purified cells activated with PMA and ionomycin revealed that virtually all cells expressing type 17 cytokines/chemokines, such as IL-17A, IL-17F, IL-22 and CCL20 are found within CCR6-expressing subset (Fig. S1, A and B). Building on our previous studies (54), using data from gene arrays in cells from eight donors, we analyzed changes in gene expression in cells that were either left non-stimulated or stimulated ex vivo with PMA and ionomycin. As shown in volcano plots in Figure 1B, in comparing the CCR6+ subgroups to CCR6neg cells as a common comparator, we found that type 17 genes, such as IL17A, IL17F, IL22, IL23R, CCL20, RORA and RORC were progressively upregulated (shown in red), and type 1 genes such as IFNG, CXCR3, CCL3, CCL5, EOMES, GZMK and GZMH and type 2 genes, such as IL4 and IL5 were downregulated (shown in green), also generally progressively, in CCR6low to CCR6int to CCR6high cells. Pairwise comparisons of gene-specific probes among the subgroups revealed approximately 600 genes that were differentially expressed in any of the comparisons for the non-stimulated and/or stimulated cells based on a false discovery rate of 0.05. It is important to note, as can be appreciated from the mRNAs of diminishing abundance across the subgroups, that the CCR6neg subgroup is particularly heterogeneous, containing, for example highly differentiated Th1 and Th2 cells. All differentially expressed genes were grouped by k-means into twelve clusters with a variety of patterns across the CCR6low, CCR6int and CCR6high subgroups. Genes in most clusters display monotonic changes in expression. A salient gene within each cluster is shown, such as the signature genes for the Th lineages, IL17F, IFNG, and IL5 (Fig. 1 C). Heat maps of gene expression from the naïve and CCR6-defined (CCR6neg, CCR6low, CCR6int and CCR6high) memory subgroups, both non-stimulated and stimulated, show large differences in patterns between naïve and memory cells and between CCR6neg and CCR6pos cells and progressive changes from CCR6low to CCR6high cells (Fig. S1 C).
Figure 1. Progressive upregulation of type 17 genes and downregulation of type 1 and type 2 genes in CCR6-expressing memory subgroups.
(A) Elutriated lymphocytes from blood that had been enriched for CD4+ T cells were separated based on CD45RO staining into CD45RO− (naïve) and CD45RO+ (memory) cells and the CD45RO+ cells were either separated into CCR6neg and CCR6pos subgroups or into CCR6neg, CCR6low, CCR6int and CCR6high subgroups. (B) Volcano plots showing fold changes (log2 ratios) versus significance (-log10 p-values) for genes in non-stimulated (top) or stimulated (bottom) cells for CCR6low, CCR6int and CCR6high subgroups compared with the CCR6neg subgroup. The red dotted line marks the cutoff for significance of differential expression at a false discovery rate ≤0.05. On each volcano plot are indicated the number of genes upregulated (red) or downregulated (green), and some genes of interest are labeled. (C) Heatmaps illustrating the relative patterns of gene expression differences between CCR6low, CCR6int and CCR6high subgroups compared with the CCR6neg subgroup. The standardized differences for the gene-specific probes showing the greatest differences in these comparisons were clustered by K-means (Figure S1 C); the color/value in each small box represents the mean across the genes in the cluster for each contrast. The cluster number, the number of unique gene-specific probes in that cluster and a representative gene in each cluster are shown. Analyses shown are combined from four individual donors for the non-stimulated cells and eight individual donors for stimulated cells, including four common donors.
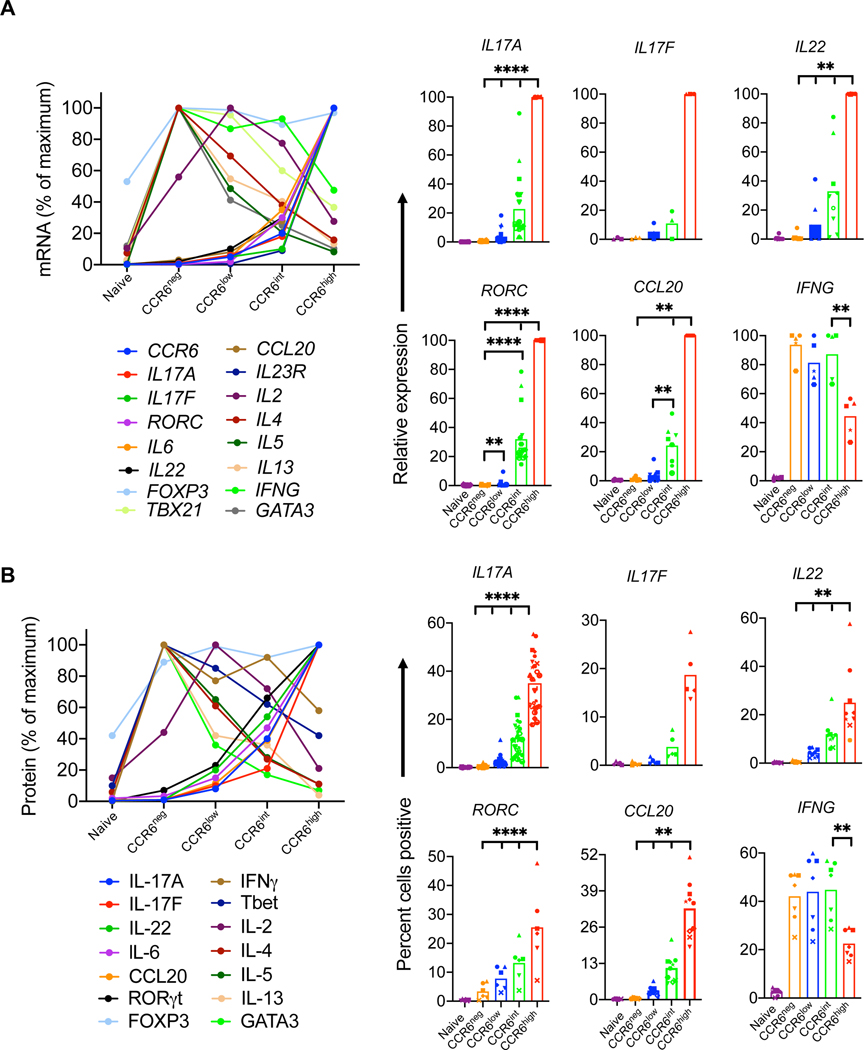
We next performed a more targeted analysis of the gradients of lineage-related genes and proteins across the naïve and CCR6-defined memory subgroups. Consistent with the gene array data, real-time RT-PCR of cells activated with PMA and ionomycin showed increases in expression of type 17 genes and decreases in signature type 1 (IFNG and TBX21) and type 2 genes (IL4, IL5, IL13 and GATA3) across the CCR6-defined memory subgroups (Fig. 2 A). Similar patterns were found for lineage-associated proteins as detected by intracellular staining or ELISA (Fig. 2 B and Fig. S2 A). We further investigated pair-wise relationships among type 17 proteins across the naïve and CCR6-defined memory subgroups by intracellular staining. For IL-17F, as depicted in Figures S1 A and S2 B, virtually all positive-staining cells were found as a subset of cells expressing IL-17A. For IL-17A/F vs. CCL20, both CCL20+IL-17A/F− cells and CCL20+IL-17A/F+ cells increased from the CCR6low to the CCR6high subgroup, although the fold increase in CCL20+IL-17A/F+ cells was greater across the subgroups, leading to an increase in the ratio of IL-17A/F+CCL20+: IL-17A/F−CCL20+ cells across the subgroups. For IL-17A vs. IL-22 there was a significant increase in the frequency of IL-22+IL-17A+ cells from the CCR6low to the CCR6high subgroup (although the ratio of IL-17A+ IL-22+: IL-17A+ IL-22− cells was constant across the subgroups), but very little change in the frequency of IL-22+IL-17A− cells suggesting that IL-22 may be upregulated through different pathways in cells that do vs. cells that do not co-express IL-17A. For CCL20 vs. IL-22, based on the percentages of single-positive vs. double-positive cells, their increases across the subgroups showed no evidence of being co-regulated (Fig. S2 B). In contrast to the patterns for the type 17 cytokines, total percentages of IFNγ+ cells declined across the subgroups, although there was an increase in IFNγ+IL-17A+ cells. Taken together, these data support the value of CCR6 as a marker of activity of the broad type 17 program in which type 17 genes show varying degrees and pathways of coordinated vs. independent regulation, demonstrate increasing type 17 and decreasing type 1 and type 2 character across the CCR6-defined memory subgroups, and suggest that the patterns of gene differentiation and resulting phenotypes for the type 17 cells progress along an extended continuum.
Figure 2. Gradients of expression of lineage-associated genes with increasing CCR6.
(A) Relative mRNA expression was measured using real-time reverse-transcriptase (RT)-PCR. Ct values of all genes were normalized to GAPDH, and relative mRNA expression is shown for means (left panel) or for individual values (right panel) from four or more donors as percentages of the value in the highest expressing subgroup. (B) Protein levels in naïve and CCR6-defined memory subgroups for IL-2, IL-4, IL-5 and IL-13 were measured by ELISA from cell supernatants after 12 h of activation with PMA and ionomycin. For other proteins, cells were stimulated with the leukocyte activation cocktail for 6 h, fixed and permeabilized, stained for indicated genes, and analyzed by flow cytometry for percent of cells staining positive. In the left panel, means are shown of values from three or more donors, calculated as percentages of the value in the highest expressing subgroup. The right panel shows percentages of positive-staining cells from five or more donors. Symbols in the right panels indicate data from individual donors and bars indicate means. p values were determined using the Wilcoxon matched pairs signed rank test. *p < 0.05, **p < 0.01, ****p < 0.001.
The gradient across the CCR6-defined memory subgroups reflects a continuous range of phenotypes of individual cells
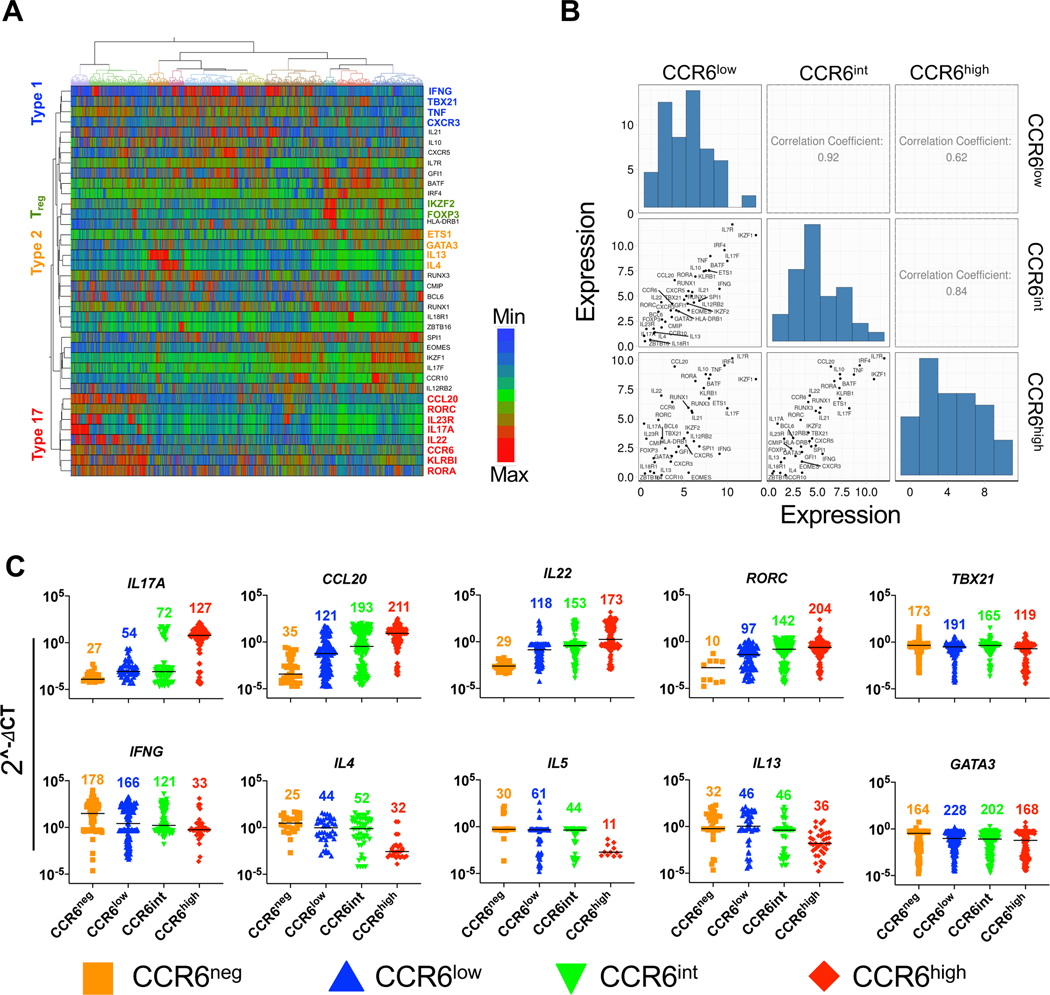
For genes/proteins other than CCR6, the patterns that we described for type 17 genes across the CCR6-defined memory subgroups could represent mixtures of homogeneous subsets of, for example, negative and positive cells in varying proportions vs. individual cells with ranges of phenotypes – or some combination thereof. We addressed this issue by using Fluidigm Dynamic Arrays for RT-PCR, which allowed quantitative analysis of expression of a panel of lineage-associated, activation-associated, and housekeeping genes in single cells from each of the naïve, CCR6neg, CCR6low, CCR6int, and CCR6high memory subgroups. Hierarchical clustering of the normalized expression profiles shows co-expression in single cells of type 1 (IFNG, TBX21, TNF, CXCR3; highlighted in blue), Treg-associated (IKZF2 and FOXP3; highlighted in green), type 2 (ETS1, GATA3, IL13, IL4; highlighted in orange), and type 17 (RORA, KLRB1, CCR6, IL22, IL17A, IL23R, RORC, and CCL20; highlighted in red) genes (Fig. 3 A). Using mean values of gene expression, calculations of correlation coefficients between pairs of the CCR6-defined memory subgroups revealed the ordered relationship of CCR6low to CCR6int to CCR6high (Fig. 3 B), consistent with the analyses from the bulk subgroups.
Figure 3. Single-cell data reveal a continuum of expression levels among lineage-associated genes.
(A) Heat map representation of gene expression profiles using hierarchical clustering showing co-expression of lineage-specific genes in individual cells across naïve cells and CCR6-defined memory subgroups for 282 cells per subgroup. Delta Ct values were calculated using the average of housekeeping genes GAPDH and PGK1, and after excluding genes with low and/or inconsistent expression, a total of 38 non-housekeeping genes were included in the final analysis. Rows are genes and columns are cells. Some clustered, lineage-associated genes are indicated. (B) Correlations among CCR6low, CCR6int and CCR6high memory subgroups based on means of expression of the genes in A. (C) Gene expression values normalized as described in Materials and Methods for BioMark HD data for the 1410 cells from naïve and CCR6-defined memory subgroups. Each point represents a single cell, and each cell is colored based on its subgroup as indicated. The 1410 cells analyzed in A-C were from three donors.
For the type 17 genes, IL17A, CCL20, IL22 and RORC, the numbers of cells with detectable expression and the levels of expression within single cells increased from the CCR6low to CCR6high subgroups (Fig. 3 C). The data suggest that for the type 1 and type 2 genes, the decreases in expression along the CCR6 continuum in analysis of the bulk population were due predominantly to decreases in the numbers of expressing cells (IFNG and TBX21), the expression levels per cell (IL4 and IL13), or both (IL5). Together these data demonstrate that the gradient of type 17 character among the CCR6-defined memory subgroups at the level of populations reflects a continuum at the level of individual cells, with single cells showing many intermediate phenotypes characterized by a wide range in their expression of signature type 17 genes as well as signature genes of alternative lineages.
Epigenomes are stable through non-inflammatory proliferation across the type 17 continuum
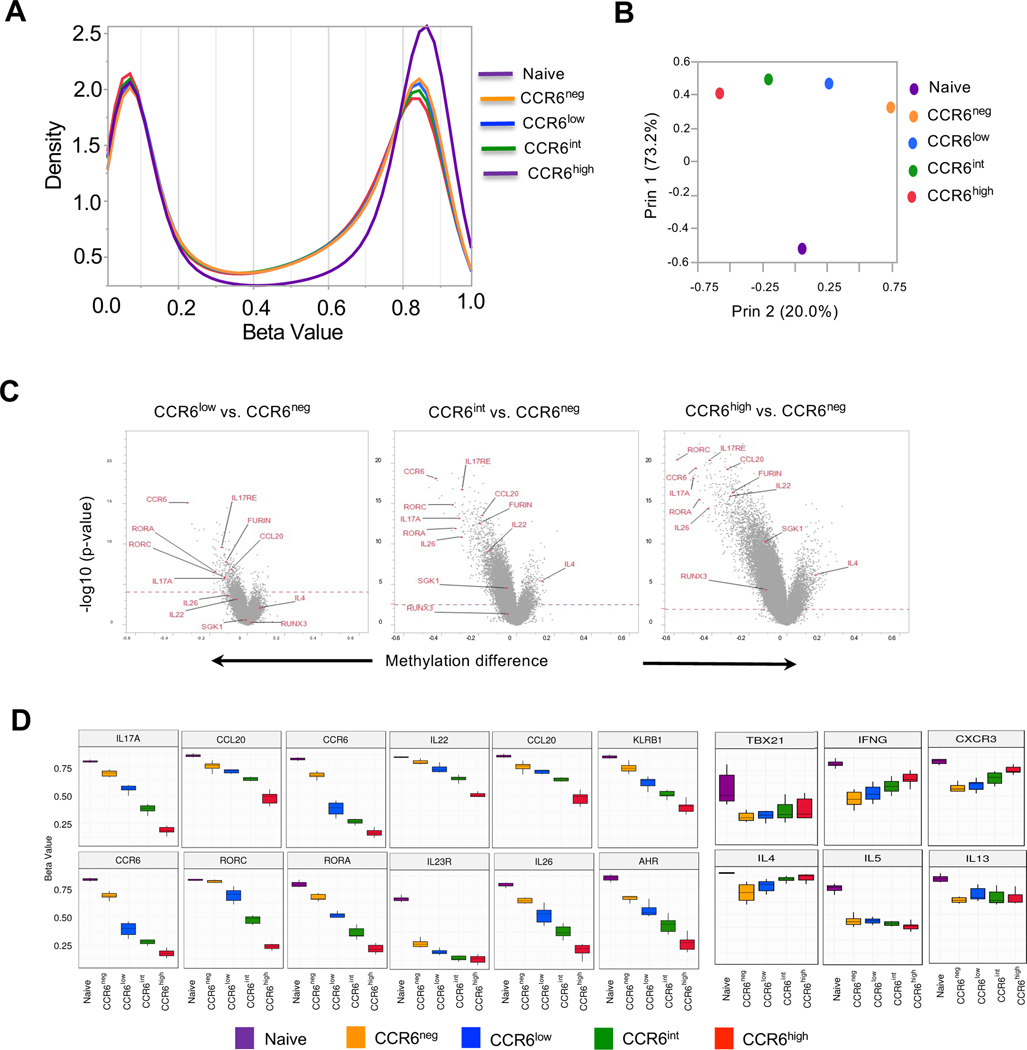
We investigated the stability of phenotypes across the type 17 continuum by analyzing genome wide CpG methylation. Methylation of cytosines at CpG dinucleotides is the epigenetic change best associated with stability in patterns of gene expression and reflects the reshaping of these patterns in memory T cells (59, 60). The global methylation profiles reveal a progressive shift from higher to lower CpG methylation from naïve to CCR6high cells (Fig. 4 A). The corresponding unsupervised principal-component analysis shows the large distance between the naïve and memory cells along principal component 1 and the separation along principal component 2 in the order from CCR6neg to CCR6low to CCR6int to CCR6high (Fig. 4 B). Volcano plots of changes in overall methylation levels at individual sites for each of the CCR6-expressing subgroups versus the CCR6neg cells show the predominance of demethylated versus hypermethylated sites from the CCR6low to CCR6high cells and the prominent demethylation at multiple type 17 genes, such as CCR6, IL-17A, IL-17RE, IL-26, IL-22, CCL20, RORC, RORA, FURIN, SGK1, and RUNX3 (Fig. 4 C). To visualize better the changes in methylation status at genes of interest across the CCR6 subgroups, we identified the gene-associated CpG sites showing the greatest variabilities in their beta values across the subgroups and displayed these values in each subset (Fig. 4 D). We found progressively lower overall beta values at sites within multiple type 17 genes, and either little change or progressively higher beta values at sites within type 1 and type 2 genes, such as CXCR3, IFNG, TBX21, IL4 and IL13 along the continuum from naïve to CCR6high cells.
Figure 4. Progressive changes in CpG methylation across CCR6-defined subgroups.
(A) Distribution of beta values across 450,000 CpG sites using the Illumina Infinium Methylation Assay with naïve cells and the CCR6-defined memory subgroups. Beta values (ratios) of 0.0 and 1.0 correspond to 0 and 100% methylation, respectively, of the sequences at a given CpG site. Data were combined from six donors. (B) Correlation and principal variance component projections of naïve cells and CCR6-defined memory subgroups-based genome-wide CpG methylation using the Illumina Infinium Methylation Assay. (C) Volcano plots showing differences in methylation beta values at CpG sites in the CCR6low, CCR6int and CCR6high subgroups compared with the CCR6neg subgroup versus significance (-log10 p-values). The red dotted line marks the cutoff for significance of differences between beta values at a false discovery rate ≤ 0.05. Some CpG sites at genes of interest are labelled. (D) For each of the indicated genes, box plots of methylation beta values are displayed for the single CpG site with the greatest variability among the cell subgroups. For A-D, data from six donors were combined for the analysis.
We were interested to know if the cells’ phenotypes and genotypes were stable under homeostatic conditions. We reasoned that evaluating cells that had divided in a non-inflammatory environment, under conditions mimicking homeostatic proliferation and more challenging than non-proliferative homeostasis, would serve as a more than adequate test. To this end, we activated CFSE-loaded naïve, CCR6neg, CCR6low, CCR6int, and CCR6high cells using low concentrations of anti-CD3 plus homeostatic cytokines for seven days and purified non-proliferated and proliferated cells (Fig. 5 A). To check if there had been a change in the cells’ type 17 character, we compared expression of IL17A, RORC, and CCL20 from day 0 and day 7 proliferated cells after activation with PMA and ionomycin. Proliferation under these conditions did not change the expression of these genes (Fig. 5 B). We analyzed methylation states in cells at day 0 and on day 7 in cells that had either failed to divide (d7NP) or divided three or more times (d7P). In general, principal component analysis positioned the memory cell samples according to their CCR6-defined subgroup in a pattern like that in Figure 4 B, regardless of whether the cells had proliferated (Fig. 5 C). Comparing each of the CCR6-expressing subgroups versus the CCR6neg cells at day 0 (Fig. 5 D, upper panel) showed a similar pattern as in figure 4 C, whereas the methylation levels at individual sites for day 0 versus day 7 proliferated cells for each of the CCR6-expressing subgroups showed no significant changes across the genome (Fig. 5 D, lower panel). Similarly, for genes of interest, methylation at the single CpG sites that displayed the greatest variability across the naïve and CCR6-defined memory subgroups showed no significant changes resulting from culturing or proliferation over 7 days (Fig. 5 E). Together, these data demonstrate that the progressive pattern in lineage-related gene expression across the CCR6-expressing memory subgroups correspond to epigenetic changes at these loci and the genome-wide and gene-specific patterns are unchanged across cell divisions under non-inflammatory conditions, suggesting that the cells’ phenotypes will remain stable during homeostasis in vivo.
Figure 5. Stable epigenomes of CCR6-defined memory subgroups across cell divisions.
(A) After purification by cell sorting, cells were loaded with CFSE, activated with soluble anti-CD3 and cultured in the presence of IL-7 and IL-15 for seven days, after which cells were again FACS-purified into non-proliferated (d7NP) cells and cells that had undergone three or more cell divisions (d7P) according to the gating shown in these density-colored dot plots. Data are from one of the two donors used for these experiments. (B) Relative mRNA expression of IL17A, RORC and CCL20 in cell subgroups in the starting cells at day 0 (d0) and d7P cells. Cells were stimulated with PMA and ionomycin for three hours and mRNAs were measured using RT-PCR. Values for these genes were normalized to values for GAPDH and then to the results for naïve cells, which were set at 1. Bars indicate means. Data are shown for cells from the two donors used for these experiments. (C) Correlation and principal variance component projections based on the CpG methylation data of cell subgroups for d0, d7NP, and d7P cells. (D) Volcano plots showing differences in methylation beta values between CpG sites in the CCR6low, CCR6int and CCR6high subgroups compared with the CCR6neg subgroup at day 0 (top) or for each subgroup between d0 and d7P cells (bottom). The red dotted lines mark the cutoffs for CpG sites with significant differences at a false discovery rate ≤ 0.05. Some CpG sites at genes of interest are labelled. (E) Box plots of methylation beta values for the single CpG site with the greatest variability among the cell subgroups at lineage-associated genes for d0, d7NP and d7P cells. Data for C-E were combined from two donors.
CCR6-expressing subgroups share T cell clonotypes with changing frequencies
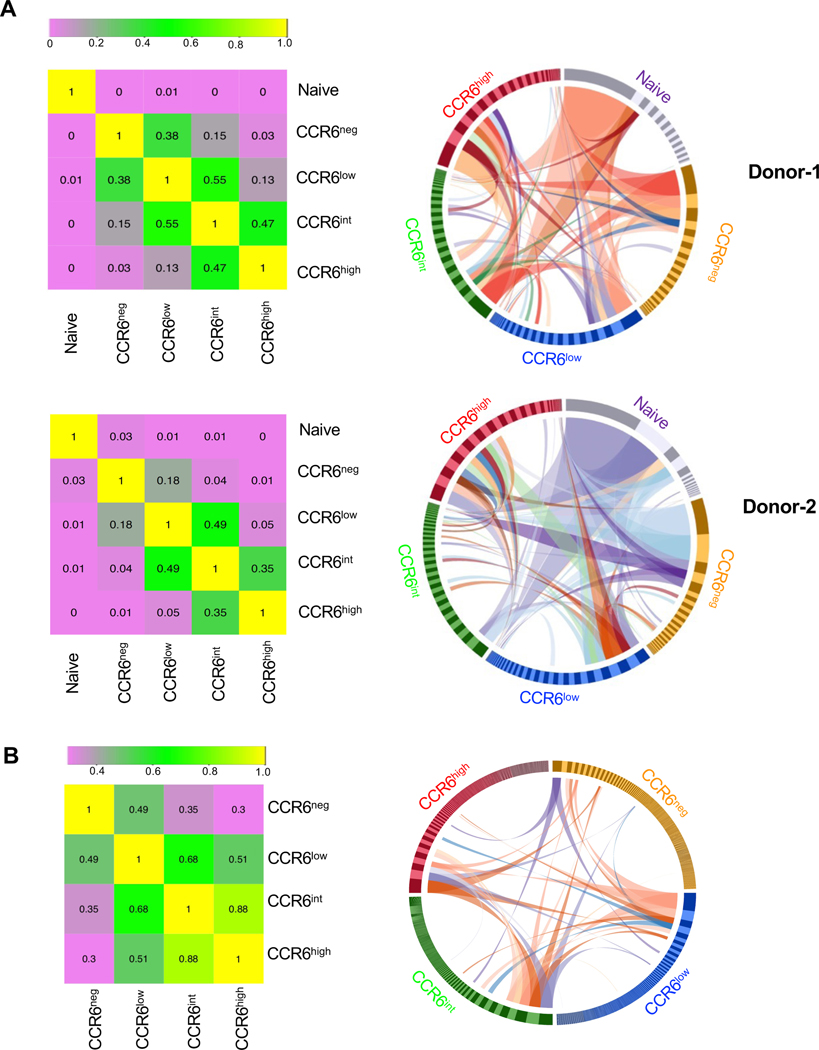
To investigate the clonal relationships among the CCR6-expressing memory cells we determined the sequences of TCRβ chain mRNAs from naïve cells and CCR6-defined memory subgroups. Using the Morisita-Horn method to compute similarity, the naïve cells show little similarity with the memory cells and the memory subgroups are ordered from CCR6neg to CCR6low to CCR6int to CCR6high (Fig. 6 A, left panels). Circos plots of the 20 most abundant clonotypes in each subgroup reveals that the frequencies of shared clonotypes are most similar between adjacent subgroups and differ most between non-adjacent subgroups (Fig. 6 A, right panels). For example, several clonotypes are found at high frequencies in both the CCR6int and CCR6high subgroups, whereas clonotypes shared between the CCR6low and CCR6high cells that are present at high frequencies in the former are generally at low frequencies in the latter and vice versa.
Figure 6. TCR sequencing reveals shared clonotypes whose frequencies change across CCR6-defined memory subgroups.
(A) Two million T cells from each subgroup from two donors were used for sequencing TCR β chain cDNAs in bulk at a depth of 6.3–9.3 reads per cell and V(D)J sequences were used for identifying clonotypes. Morisita-Horn Similarity Indexes were calculated for pairwise comparisons to yield values between 0 (lowest similarity) and 1 (left panels). Circos plots display all clonotypes with the twenty highest frequencies in any subgroup (right panels). Bars in the circle have widths that are proportional to clonotype frequencies and are arranged within the cell subgroups clockwise in order of decreasing frequencies. Ribbons show connections between clonotypes among the cell subgroups for clonotypes with frequencies of 0.04 or greater within any cell subgroup. (B) Cells from each CCR6-defined memory T cell subgroup from a CMV-seropositive donor that proliferated in response to CMV antigens were used for single-cell sequencing of TCR α and β chains. Paired productive sequences were obtained from approximately 4000–6000 cells from each subgroup. Morisita-Horn Similarity Indexes were calculated for pairwise comparisons as in A (left panel). A Circos plot was produced using all clonotypes (right panel). Bars in the circle have widths that are proportional to clonotype frequencies and are arranged within the cell subgroups clockwise in order of decreasing frequencies. Ribbons show connections between clonotypes among the cell subgroups for clonotypes with frequencies of 0.03 or greater within any cell subgroup.
In addition to analyzing clonotypes using TCRβ chain sequences from the bulk populations, we determined clonotype identities with increased specificity by sequencing both TCRα and TCRβ chain mRNAs from single cells. Given the limitations in sample sizes with this method and the significant diversity of the repertoire of non-selected memory T cells, we analyzed cells from the CCR6-defined memory subgroups purified from a donor seropositive for CMV based on the cells’ proliferative responses to CMV antigens (Fig. S3 A). We processed a total of 27,645 cells and recovered paired, productive sequences for TCRα and TCRβ chains from approximately 67% to 71% of the cells, depending on the subgroup (Fig. S3, B and C), and only these sequences were used for subsequent analysis. Computing similarities again ordered the subgroups from CCR6neg to CCR6low to CCR6int to CCR6high (Fig. 6 B, left panel). Circos plots including all the clonotypes (Fig. 6 B, right panel) or the 20 most abundant clonotypes (Fig. S3 D) show that frequencies of shared clonotypes are most similar between adjacent subgroups and differ most between non-adjacent subgroups. These analyses of both the non-selected and CMV-reactive clonotypes reinforce the validity of considering all the CCR6-expressing Th cells as one family, since they show, for example, that the CCR6low subgroup is closer to the CCR6int subgroup than to the CCR6neg cells and demonstrate the same relationships among subgroups as reflected in the gene and protein expression data. Together, the results suggest that at least some cells within the subgroups originate from common T cell clones whose progeny have differentiated, with dynamic changes in their frequencies, along the continuum of type 17 character. To lend further support the directionality of this pathway we performed single-cell droplet-based mRNA sequencing on cells from the CCR6-expressing subgroups from an additional donor. Trajectory analysis of these cells suggested progression from the CCR6low and CCR6int to the CCR6high cells in pseudotime (Fig. S3 E).
Correlations among expression levels of lineage-associated genes in single cells
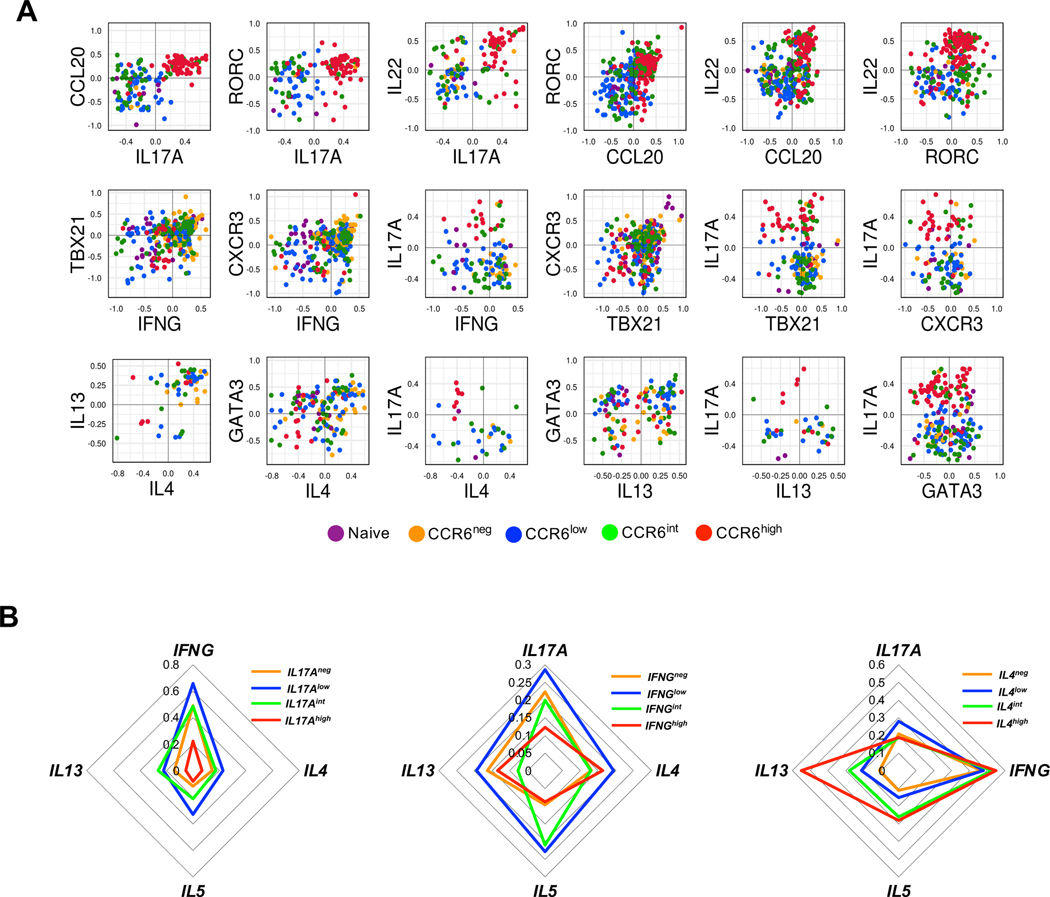
To understand the relationships among the Th lineages across the type 17 continuum we analyzed co-expression of lineage-associated genes in the single cells from the naïve and CCR6-defined memory subgroups. We found that co-expression of type 17, type 1 and type 2 genes is common. From the 251 out of the total 1410 naïve and memory CD4+ T cells from three donors where, following activation, we could detect expression of IL17A, 44% co-expressed IFNG, 33% co-expressed a type 2 cytokine gene (IL4 and/or IL5 and/or IL13), and 54% co-expressed IFNG and/or a type 2 cytokine gene. In analyzing those cells that co-expressed pairs of lineage-associated genes, we compared normalized values of expression (Fig. 7 A). Cells co-expressing type 17 genes generally showed positive correlations in their levels, with cells expressing the highest levels enriched in the CCR6high subgroup. Positive correlations were also found for genes within the type 1 and type 2 gene sets. However, positive correlations were not found for IL17A versus type 1 and type 2 genes, and these co-expressing cells were found less commonly in the CCR6high subgroup. For the cells co-expressing IL17A and IL4 and/or IL13, virtually no cells co-expressed IL17A and IL4 or IL13 at high levels.
Figure 7. Co-expression of Th lineage-associated genes in single cells.
(A) Normalized values for expression of the indicated genes from 1410 cells from naïve and CCR6-defined memory subgroups from three donors were pooled and the values from cells co-expressing pairs of genes were plotted for comparison. Each dot corresponds to a single cell and dots are colored based on their subgroups. (B) The normalized gene expression data from 1062 cells from the CCR6neg, CCR6low, CCR6int, and CCR6high memory cells from three donors were pooled and cells were divided into positive and negative for IL17A, IFNG, or IL4, and the positive cells were equally divided into low, intermediate, and high groups based on their expression values for the respective genes. Within each of the four IL17A-, IFNG-, or IL4-defined and color-coded groups of cells, frequencies of cells expressing the other indicated genes are displayed on the concentric diamonds.
In an alternative analysis we combined all the memory cells regardless of their CCR6 expression, and for each of the genes, IL17A, IFNG and IL4 separated cells into four groups, including a group without any expression and three equal-sized groups of low, intermediate, and high expressers. For each group we determined the frequencies of cells with detectable expression of other signature genes. For non-expressing cells or cells that were expressing various levels of IL17A the frequencies of cells co-expressing IFNG diminished from IL17Alow to IL17Aint to IL17Ahigh cells (Fig. 7 B), consistent with the trend found in our other data. Surprisingly, however, the IL17Alow cells had higher frequencies of cells expressing IFNG, as well as IL4, IL5, and IL13, than did the IL17Aneg cells. For IL4, IL5, and IL13, the IL17Aint cells also had higher frequencies of expressers as compared with the IL17Aneg cells. Patterns that were generally analogous were also found in analyses of cells separated based on their expression of IFNG or IL4. These data suggest that initial stimulation of CD4+ T cells in vivo leads to pan-activation of multiple effector pathways, and that these cells can persist in the resting memory population.
An increase in RORγt can drive type 17 progression from the CCR6low subgroup
The gradients of phenotypes, clonal relationships, and patterns of lineage-gene co-expression that we identified among the CCR6-expressing CD4+ T cells suggested that cells could move down this continuum to acquire more type 17 character while diminishing their type 1 and type 2 features. To demonstrate this and understand the outcomes for individual cells we over-expressed RORC in CCR6low CD4+ T cells by transduction using lentiviral vectors encoding RORγt and GFP or GFP alone and purified GFP-positive cells. The bulk cultures of transduced cells showed increases in mRNAs for RORC, IL17A and CCL20, a modest decrease in the mRNA for IFNG, and more significant decreases for the mRNA for IL4 (Fig. S4 A).
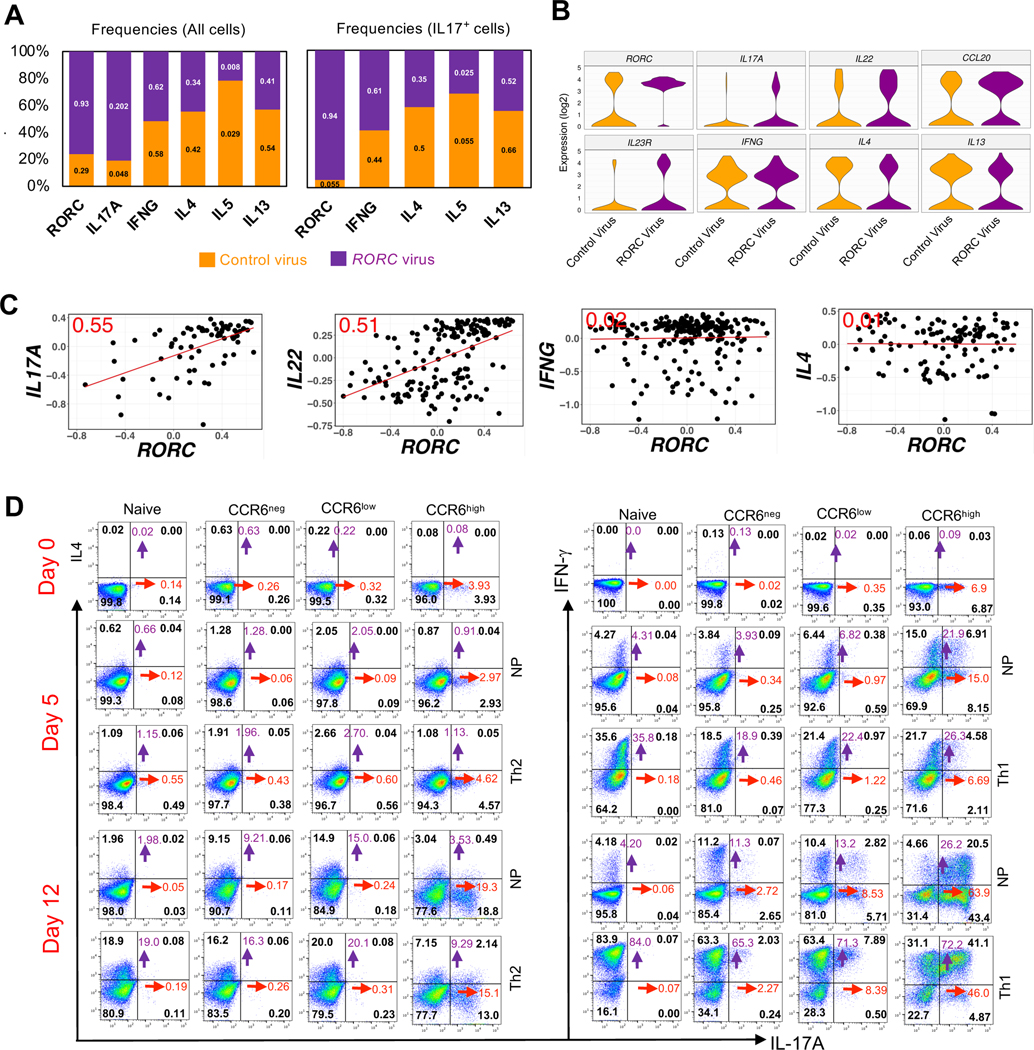
For determining the effects of transduction of RORC on individual CCR6low CD4+ T cells we did single cell analysis using Fluidigm Dynamic Arrays for RT-PCR. Comparative data on the frequencies of cells expressing detectable expression of signature genes in RORC-virus transduced versus control-virus transduced cells revealed significant increases in RORC and IL17A, decreases in IL4, IL5, and IL13, and little change in IFNG (Fig. 8 A, left). In analyzing only the cells expressing IL17A, the RORC-transduced cells showed an increase in frequencies of cells co-expressing IFNG and decreases in cells co-expressing IL4, IL5, and IL13 (Fig. 8 A, right). For the RORC-transduced cells, violin plots show increases in the frequencies and levels of expression of the type 17 genes IL17A, IL22, CCL20 and IL23R and the opposite effects for IL4 and IL13 (Fig. 8 B). We also investigated the “dose response” of cytokine genes to RORγt by comparing the levels of mRNAs of RORC to those of the cytokine genes in the RORC-transduced cells. The levels of expression of RORC showed positive correlations with expression of IL17A and IL22. By contrast, even though transduction with RORC decreased the frequencies of cells with detectable expression of IL4, there was no correlation between the levels of RORC and IL4 mRNAs in individual cells in which expression of IL4 remains detectable. Expression of RORC and IFNG also failed to show any correlation (Fig. 8 C). To address whether forced expression of RORγt might be altering frequencies of cells with relevant patterns of gene expression by selective effects on cell proliferation during the 3-day cultures, we analyzed mRNA levels of cytokine genes in transduced and DDAO-loaded cells and found that patterns of cytokine gene expression did not differ as a function of cell divisions, suggesting that RORγt was having direct effects the cells’ phenotypes (Fig. S4 B). Together these data suggest that within the milieu of the CCR6low cells RORγt alone is capable of driving expression of signature type 17 genes, and that increasing expression of RORγt is a mechanism for driving these cells further down the type 17 continuum.
Figure 8. Position in the type 17 continuum determines sensitivity to master regulators and mechanisms of plasticity.
(A) Numbers on the lower and upper bar segments show frequencies of cells expressing the indicated genes in the CCR6low cells that had been transduced with control or RORC lentiviral vectors, respectively. Each bar is divided according to the relative frequencies of the control- and RORC-transduced cells expressing the indicated genes. Left panel shows data from all transduced cells and right panel shows data only from cells with detectable expression of IL17A. (B) Violin plots with expression distributions for the indicated genes in the CCR6low cells that had been transduced with control or RORC lentiviral vectors. (C) Normalized Ct values are plotted for the CCR6low cells that had been transduced with control or RORC lentiviral vectors and showed co-expression of RORC with IL17A, IL22, IFNG or IL4. Each dot represents a single cell, the red line is the least squares regression line, and the number in red in each plot is the correlation coefficient, r. For A-C, data are pooled from four donors. (D) Dot plots showing intracellular expression on days 0, 5 and 12 after culturing in non-polarizing (NP), Th2 or Th1 conditions of IL-17A vs IL-4 and IL-17A vs IFNγ in naïve cells (CD4+CD45RO−CD25−CXCR5−CCR4−CXCR3−), and IL-17A vs IL-4 in CCR6neg(CD4+CD45RO+CD25−CXCR5−CCR4−CCR6−), CCR6low(CD4+CD45RO+CD25−CXCR5−CCR4−CCR6low), and CCR6high(CD4+CD45RO+CD25−CXCR5−CCR4−CCR6high) subgroups and IL-17A vs IFNγ in CCR6neg(CD4+CD45RO+CD25−CXCR5−CXCR3−CCR6−), CCR6low(CD4+CD45RO+CD25−CXCR5−CXCR3−CCR6low), and CCR6high(CD4+CD45RO+CD25−CXCR5−CXCR3−CCR6high) subgroups. Cells were stimulated with the leukocyte activation cocktail for 6 h, fixed and permeabilized and stained for indicated genes. For determining percentages of cells staining positive, quadrants were drawn based on staining with isotype-matched control antibodies. The numbers in black indicate the percentage of cells in each quadrant, whereas purple and red numbers show the sums of percentages of positive cells in the upper two or rightmost two quadrants, respectively. Data are representative of results from three donors.
Given the plasticity reported for Th17 cells in mice, particularly in their transformation into IL-17− IFNγ+ cells (36), we investigated plasticity among the CCR6-defined subgroups by activating and culturing them under non-polarizing, Th2, or Th1 conditions. Starting cells were either CCR4− or CXCR3− to eliminate cells with pre-existing activity of the type 2 and/or type 1 programs, including all cells able to make IL-4 or IFNγ, respectively (61). In all experiments, IL-17A+ cells were found principally in the CCR6high subgroup. In the Th2 experiments, there was little difference in frequencies of IL-17A+ cells in the Th2 vs. non-polarizing conditions, and IL-17A+ cells generally remained IL-4−. At 12 days, non-polarizing cultures showed prominent increases in percentages of IL-4-producing cells in the CCR6neg and CCR6low subgroups as compared with the naive and CCR6high cells. The Th2-polarizing conditions resulted in further increases in IL-4+ cells, with the fewest IL-4+ cells found in the CCR6high subgroup. However, the fold increase in IL-4 production in Th2 vs. non-polarizing conditions was greater in the CCR6high (and naïve) cells as compared with the CCR6neg and CCR6low subgroups (Fig. 8 D, left panels).
In the Th1 experiments, a significant percentage of CCR6high cells, but fewer cells in the CCR6low or other subgroups, acquired the ability to produce IFNγ within five days under non-polarizing conditions. At the same time, both at 5 and at 12 days the fold increases in IFNγ production in Th1 vs. non-polarizing conditions were smaller in the CCR6high cells as compared with the CCR6neg and CCR6low subgroups. Th1 conditions also led to a loss in IL17+ IFNγ− cells and, by day 12, a shift of most IFNγ+ cells into the IFNγ+ IL17+ subset (Fig. 8 D, right panels). When considered together, these experiments suggest that progression along the type 17 continuum results in a diminished ability to express the type 2 but not the type 1 program. Of greater interest, the comparisons between cells cultured under non-polarizing vs. polarizing conditions suggest that prior conditioning vs. activating environments contribute differentially to the expression of the programs for the non-Th17 lineages depending on the program and a cell’s position along the type 17 continuum. The cells’ prior imprinting contributes more than subsequent environmental inputs for the CCR6low than CCR6high cells in acquiring an ability to produce IL-4, whereas prior imprinting contributes more than the environmental inputs for the CCR6high than CCR6low cells in acquiring an ability to produce IFNγ.
In an alternative approach to studying plasticity, we transduced CCR6low and CCR6high memory cells (here without removing cells expressing CXCR3 or CCR4) using GFP-expressing vectors encoding RORC, TBX21, and GATA3 and a control vector expressing GFP alone. All experiments used GFP+ cells purified by FACS. We checked the activities of these transcription factors initially by transducing CCR6low cells and analyzing mRNAs in the bulk populations. Given the levels of the endogenous mRNAs, the fold increase in the RORC mRNA was much greater than for the mRNAs for TBX21 and GATA3 (Figure S4 C). As in the experiments described above, transduction with RORC led to a large increase in the expression of IL17A. Transductions with TBX21 and GATA3 led to increased expression of IFNG and IL4, respectively. We next transduced CCR6low and CCR6high cells with these transcription factors and analyzed mRNAs from individual cells by RT-PCR (Figure S4 D). For IL17A, transduction with RORC led to large increases in the frequency of expressing cells and levels in the individual expressing cells in the CCR6low subgroup, and a small increase in the CCR6high subgroup. For each of the mRNAs, IFNG, GATA3, and IL4, transduction with RORC led to a decrease in the CCR6low subgroup and little change in the CCR6high subgroup, with the possible exception of some decrease in IL4 expression also in the latter cells. Transduction with TBX21 led to modest increases in the frequency and levels (in the individual expressing cells) of IFNG in the CCR6low subgroup, but no changes in CCR6high cells, and no consistent changes in expression of other genes. Transduction with GATA3 led to modest increases in the frequency and levels of IL4 in the CCR6low subgroup, but no changes in CCR6high cells, and no consistent changes in expression of other genes. Taken together, these data reveal significant responsiveness for CCR6low cells, but a diminished ability to affect the expression of lineage-specific genes in the CCR6high cells by overexpression of signature transcription factors, suggesting, as we found in our ex vivo cross-polarization experiments, that mechanisms of plasticity differ depending on the cells’ positions along the type 17 continuum. For example, the CCR6low cells, which are more responsive to Th1 polarizing conditions, are also more responsive to overexpression of TBX21, whereas the CCR6high cells are those showing the greatest prior imprinted ability to upregulate IFNγ while being insensitive to the overexpression of TBX21.
DISCUSSION
Our studies focused on understanding the composition and organization of the memory type 17 Th cell population in healthy humans. One central finding is that these cells, including the progeny of single clones, lie at different positions along an extended continuum of type 17 differentiation on which is superimposed the co-activation of alternative lineages. Although co-expression of alternative lineages diminishes along the continuum, such co-expression is more the rule than the exception and contributes to the population’s extensive heterogeneity. Based on a wealth of data from mouse studies establishing a direct relationship between effector and memory T cells (8, 12–18), we presume that the phenotypes of the cells within this continuum reflect pathways of effector cell activation and differentiation. Our data suggest that these in vivo pathways are initiated by activating changes at genes associated with multiple effector lineages, and that the less differentiated type 17 memory cells bearing these changes are particularly capable of expressing a variety of alternative effector phenotypes.
The character of the type 17 continuum was revealed by analyzing cells that differed in their expression of the trafficking/chemokine receptor, CCR6. Studies in mice and humans have suggested that there is a family of Th17-related cells that cannot be identified by relying on the expression of a single cytokine, particularly when limited, as in human studies, to examining cells at one point in time (35, 42). Additionally, the increasing appreciation of memory T cell diversity has led to proposing that the phenotypes of these cells be viewed along multiple vectors, with trafficking patterns representing a major defining feature (62). Whether all CCR6-expressing Th cells belong within a single type 17 family has been questioned (38, 63). However, in toto, our data strongly suggest that CCR6 does, in fact, identify a subgroup of related cells, and the expression of IL17A, IL17F, IL22, CCL20, IL23R and RORC almost exclusively within the CCR6+ subset of memory T cells (46–48, 50) and this manuscript), suggests that CCR6 is an appropriate marker for human type 17 cells understood more broadly. We reasoned that analyzing this more inclusive collection of type 17 cells would reveal relationships that might be obscured by studies of the Th17 population narrowly construed, and thereby illuminate both the pathways for differentiation and the nature of inter-lineage relationships in human Th cells in vivo.
Expression of type 1 genes/proteins, such as IFNG, CXCR3, CCL3, CCL5, TBX21, EOMES, GZMK and GZMH and type 2 genes/proteins, such as IL4, IL5, IL13 and GATA3 were downregulated along the CCR6-defined, type 17 continuum. Both T-bet (64, 65) and eomesodermin (66, 67) suppress type 17 genes and support the development of Th17 cells that acquire a pathogenic profile and which, in humans, display the CCR6+CXCR3+ phenotype of “Th1*” or “non-classic” Th1 cells (46, 68). Eomesodermin also induces granzyme genes in human Th cells (66) and is critical for the differentiation of a subset of TR1, cytotoxic cells that co-express IL-10 and IFNγ (67, 69). EOMES and granzyme genes are signature type 1 genes in human Th cells (70) and their overall lower expression in the CCR6-expressing versus CCR6neg subgroups reflects the diminishing type 1 character and increasing type 17 character across the CCR6-defined continuum.
Analysis of individual cells along the gradient of CCR6 expression showed that expression levels of type 17 genes vary, generally in a coincident fashion, from cell-to-cell, with most type 17 genes showing continua of expression across a wide range of values. High-dimensional studies have identified linear patterns of differentiation with continua of phenotypes as general features of human CD8+ (71) and CD4+ (72) memory cell populations, and of mouse Th cells activated under polarizing conditions (73).
If, as we presume, the memory cells within the CCR6-expressing subgroups are derived from activated cells that had differentiated to various points along a type 17 continuum, then we might expect the subgroups to share T cell clones in a pattern reflecting the subgroups’ relationships. From both the sequences of β chain mRNAs from the cells analyzed in bulk and the sequences of paired α/β chains of CMV-reactive single T cells we found many shared clonotypes among the memory cell subgroups with changes in frequencies of clonotypes from subgroup to subgroup. The clonotype compositions of the CCR6-negative cells were closest to the CCR6low cells, and the ordered relationship among the CCR6-expressing subgroups matched the pattern derived from the gene and protein expression data, CCR6low to CCR6int to CCR6high, consistent with the descendants of individual clones having progressed to different points during the process of activation-driven differentiation.
To investigate the stability of the type 17 continuum, we analyzed CpG methylation in the naïve and CCR6-defined memory subgroups, both in cells immediately after isolation and in cells that had undergone proliferation after gentle activation under non-polarizing conditions in vitro. Epigenetic changes have been studied for their roles in establishing and maintaining T-cell memory (74); genome-wide changes in DNA methylation are characteristic of effector/memory differentiation in mouse (75, 76) and human (72) Th cells; demethylation at lineage-specific cytokine genes has been shown to be important for their expression in differentiating Th cells (76, 77); and the preservation of patterns of gene expression in memory T cells has been associated with the stability of gene-specific demethylation across multiple cell divisions in vitro and/or in vivo (17, 19, 60, 78). Of particular relevance for our studies, demethylation of a region at the CCR6 promoter was shown to be important for stable expression of CCR6 in human Th cells (59).
We found an overall loss of methylated CpG’s along the CCR6-defined continuum, consistent with increasing memory cell character, and progressive decreases in methylated CpG’s at multiple type 17 genes together with increasing methylation at type 1 and type 2 genes. The patterns of inducibility of type 17 genes and degrees of their site-specific CpG methylation as analyzed at the level of cell populations were also unaltered across the CCR6-expressing memory subgroups following proliferation in a non-inflammatory environment in vitro. These results suggest that the phenotypes of the cells within the CCR6-expressing subgroups would remain stable under homeostatic conditions in vivo.
The stability of the type 17 continuum under non-inflammatory conditions notwithstanding, the strong suggestion from the linear relationship among the phenotypes and the clonotype data was that cells could progress along the continuum under the proper conditions of activation. We found that, indeed, a forced increase of expression of RORγt in the CCR6low cells up-regulated the expression of type 17 genes and down-regulated expression of some type 1 and type 2 genes. These changes were evident both in analyzing the cells in bulk and at the level of individual cells, and the single-cell data showed that expression levels of RORC mRNA correlated with the levels for a subset of type 17 genes, such as IL17A and IL22, suggesting that varying expression of RORC is one mechanism for establishing the cell-to-cell gradients in gene expression and for moving cells along the continuum. This is not to claim that the level of RORγt is the sole determinant of the complex profiles of gene and protein expression displayed by cells at different positions along this continuum. For example, it is well established by us and others that RORγt supports the expression of CCR6 in human T cells (49, 54, 79). Nonetheless, we were not able to detect and increase in CCR6 expression in the CCR6low cells after transduction with the RORC lentiviral vector (not shown), so that other factors must be important for enhancing CCR6 expression in this context.
In our analysis of gene expression levels in single, freshly isolated cells we found positive correlations for genes within the type 17, the type 1, and type 2 lineages, and negative correlations for genes across lineages, especially when a gene from one lineage was expressed at high levels. Overall, cross-suppression was most notable between the type 17 and type 2 pathways, as reflected in the single cell data in which almost no cells co-expressed high levels of both IL17A and IL4 or IL13. Nonetheless, co-expression of signature genes of different lineages was the common, with most cells that had detectable expression of IL17A co-expressing one or more of the genes that are characteristic of alternative lineages. Although we cannot know if each cell with measurable expression of a given gene could produce the encoded protein in amounts that are biologically relevant, at a minimum, the detection of these mRNAs indicates epigenetic changes at the gene loci that reflect activation of the lineage-specific pathways. For mouse Th cells, complex continua of mixed lineage phenotypes have been described after activation in vitro with combinations of “opposing” cytokines (80) and cells with stable mixed lineage phenotypes have been identified from inflammatory reactions in vivo (73). Human Th cells co-expressing IL-17A and IFNγ (46–48) have been well described, and co-expression of IL-17A and type 2 cytokines has also been reported (11).
Of particular interest, our analysis of single cells showing different levels of expression of lineage-associated cytokine genes revealed that the frequencies of cells expressing IFNG or IL4 were higher among those cells expressing low levels of IL17A than among cells in which expression of IL17A was undetectable. Analogous patterns were found for cells with undetectable versus low-level expression of IFNG or IL4 in their expression of the cytokine genes of alternative lineages. These data suggest that initial activation of Th cells in vivo leads preferentially to pan-activation of effector pathways and that “early” cells with this history persist within the memory population. Previous experiments analyzing the initial events in the differentiation of activated Th cells have shown, for example, that genes for both Th1 and Th2 transcription factors and cytokines are activated before polarized patterns are established (81). During an acute challenge, when time is of the essence, pathway pan-activation could jump-start effector functions until the response can be focused appropriately. In studies of pathogen-reactive human memory Th cells, the analysis of clonotypes has strongly supported the model of “one cell, many fates”, suggesting that the dominance of a particular Th lineage in response against a pathogen results principally from the differential expansion of a clonotype within a given lineage rather than initial commitment being restricted to a single lineage (11). Within a recall response, such a process would follow naturally from individual memory cells with the phenotypes of pan-activation that we have described. Such cells would maintain within the memory pool the capabilities resulting from the previous multi-pathway co-activation and form cellular substrates with the flexibility to expand along the optimal pathway(s) to meet the demands of host defense.
Co-expression of genes in the type 17 and alternative Th pathways raises the issue of memory/effector Th cell plasticity (82). Soon after the identification of Th17 cells it was reported that they showed an unusual degree of plasticity, particularly in the apparent conversion of mouse IL-17A/F-producing Th cells to cells that were making IFNγ and often no IL-17A/F, and that these “transdifferentiated” cells were mediators of organ injury in models of autoimmune disease (33, 34, 83).
We addressed plasticity in human type 17 cells by activating and culturing naïve and/or CCR6-defined subgroups of memory cells under non-polarizing and polarizing conditions and by transducing these cells with genes for the “master regulators” RORγt, T-bet, and GATA3. In CCR4− memory cells, none of which can produce detectable IL-4 initially, the CCR6high subgroup contained fewer cells producing IL-4 after culturing cells under Th2-polarizing conditions and was less able to increase numbers of IL4-expressing cells after transducing with GATA3 when compared with cells in the CCR6low subgroup. These findings are consistent with the significant progressive decrease in expression of type 2 genes in the freshly isolated cells along the CCR6-defined continuum. However, the contribution of Th2 polarizing conditions on IL-4 production was greater for the CCR6high (and naïve) cells than for the CCR6neg and CCR6low cells, for which much of the ability to produce IL-4 was acquired in the non-polarizing environment. In CXCR3− memory cells, none of which can produce detectable IFNγ initially, a notable finding was the ready acquisition by the CCR6high cells of the ability to produce IFNγ after activation under non-polarizing conditions. For these cells, the added effect of a Th1-polarizing environment on IFNγ production was significantly less marked than it was for the CCR6neg and CCR6low (and naïve) cells.
The results of our polarization experiments suggest two distinguishable processes at work. The first process is “imprinted partial plasticity” as proposed by Crotty, whereby the potential to express aspects of alternative programs have been established during initial activation but are not manifest until re-activation in a permissive environment (38). The second process is plasticity induced by the environmental inputs during reactivation. Our data suggest that the outcomes of reactivating type 17 memory cells will reflect differing contributions of imprinted plasticity and retained flexibility depending on which pathways are activated and, importantly, the cells’ positions along the type 17 continuum.
Our work has limitations typical of many human studies. In vivo processes are inferred from the analyses of cells isolated at one time from a limited numbers of donors who vary in genetic make-ups and histories. Not only are the donors heterogeneous, but the cell subsets that were studied are highly heterogeneous in their antigen specificities and other phenotypes, being characterized and purified based on a limited number of surface proteins. Consequently, we do not have direct data on the fates of individual cells and their progeny after activation and during differentiation in vivo. A further important limitation is that our analyses were restricted to cells isolated from the blood. Although recent analyses of T cells from human tissue donors have shown overlap between memory T cell clones found in blood and tissues, as compared with the T cells in blood, the clonal diversity of memory T cells in tissue is better maintained with age, and some abundant tissue-resident memory clones are poorly represented in blood (84). Consequently, samples of human memory T cells from blood (or any single tissue site) cannot include the full diversity of the body’s memory T cell population.
In summary, we have found that CCR6 identifies a broad but connected family of human type 17 memory Th cells that fall along an extended gradient of type 17 activity. This continuum is evident in cells analyzed in bulk as subgroups or, more importantly, as single cells, and is likely to reflect pathways of clonal differentiation during activation in vivo. The epigenomes and phenotypes of cells along the continuum are stable, including across cell divisions under non-inflammatory conditions. Nonetheless, increasing expression of RORC in CCR6low cells demonstrated a mechanism whereby cells could “move to the right” to acquire additional type 17 character, and cells across the type 17 continuum showed differing mechanisms of plasticity in the inducible expression of signature genes of non-type 17 lineages. Whereas the CCR6high cells were more resistant than the CCR6low cells in activating IL4, the CCR6high cells were superior in activating IFNG under non-polarizing conditions, representing a novel example of imprinted plasticity that was greater in the more highly differentiated type 17 cells. Considered together, the features of the extended pathway of type 17 differentiation that we have described yield both a linear progression of type 17 activity and a range of other attributes that, importantly, differ as a function of the cells’ positions along the continuum, including mixed-lineage capabilities and varied mechanisms of plasticity to create a multi-functional and adaptable collection of type 17 memory cells. Finally, our sensitive single-cell RT-PCR assays of cytokine gene expression suggest that pan-induction of effector pathways, rather than choice of a single lineage, is a general feature of early Th cell activation in vivo, and that cells with the resulting multi-lineage character persist in the memory pool, which may contribute to the memory population’s overall flexibility and adaptability to secondary challenges. These studies provide fundamental information on the composition of the human type 17 memory population and allow for informed inferences and hypotheses as to pathways and mechanisms through which this highly heterogeneous population is formed and can adapt to novel demands of host defense or be misdirected to produce inflammatory damage.
Supplementary Material
KEY POINTS.
Human CCR6+ Th cells lie along an extended, stable continuum of type 17 character.
Type 17 cells show not only environment-inducible, but also imprinted plasticity.
Human Th cells with a history of early multi-lineage activation persist as memory.
Acknowledgements
We thank Jinfang Zhu, NIAID, NIH, and Jean K. Lim, Icahn School of Medicine at Mount Sinai, for critical reading of the manuscript and Chaim A. Schramm, NIAID, NIH, for his help in depositing TCR sequencing data in the BioProject database. We are grateful to Calvin Eigsti and members of the Research Technologies Branch, NIAID, for their help with cell sorting.
Footnotes
Disclosures
The authors have no financial conflict of interest.
This research was supported by the Intramural Research Program of NIAID, NIH.
The bulk TCR sequence data in this study are available in an NCBI BioProject record under PRJNA930733 (https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA930733), and the gene array, DNA methylation, and single cell sequence data in this study are available in the NCBI Gene Expression Omnibus (GEO) database under accession number GSE227488 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE227488).
References
- 1.Farber DL, Netea MG, Radbruch A, Rajewsky K, and Zinkernagel RM. 2016. Immunological memory: lessons from the past and a look to the future. Nat Rev Immunol 16: 124–128. [DOI] [PubMed] [Google Scholar]
- 2.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, and Slifka MK. 2003. Duration of antiviral immunity after smallpox vaccination. Nat Med 9: 1131–1137. [DOI] [PubMed] [Google Scholar]
- 3.Naniche D, Garenne M, Rae C, Manchester M, Buchta R, Brodine SK, and Oldstone MB. 2004. Decrease in measles virus-specific CD4 T cell memory in vaccinated subjects. J Infect Dis 190: 1387–1395. [DOI] [PubMed] [Google Scholar]
- 4.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, and Cooper AM. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 8: 369–377. [DOI] [PubMed] [Google Scholar]
- 5.Teijaro JR, Verhoeven D, Page CA, Turner D, and Farber DL. 2010. Memory CD4 T cells direct protective responses to influenza virus in the lungs through helper-independent mechanisms. J Virol 84: 9217–9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanno Y, Vahedi G, Hirahara K, Singleton K, and O’Shea JJ. 2012. Transcriptional and epigenetic control of T helper cell specification: molecular mechanisms underlying commitment and plasticity. Annu Rev Immunol 30: 707–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed R, Bevan MJ, Reiner SL, and Fearon DT. 2009. The precursors of memory: models and controversies. Nat Rev Immunol 9: 662–668. [DOI] [PubMed] [Google Scholar]
- 8.Stemberger C, Huster KM, Koffler M, Anderl F, Schiemann M, Wagner H, and Busch DH. 2007. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity 27: 985–997. [DOI] [PubMed] [Google Scholar]
- 9.Gerlach C, van Heijst JW, Swart E, Sie D, Armstrong N, Kerkhoven RM, Zehn D, Bevan MJ, Schepers K, and Schumacher TN. 2010. One naive T cell, multiple fates in CD8+ T cell differentiation. J Exp Med 207: 1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchholz VR, Schumacher TN, and Busch DH. 2016. T Cell Fate at the Single-Cell Level. Annu Rev Immunol 34: 65–92. [DOI] [PubMed] [Google Scholar]
- 11.Becattini S, Latorre D, Mele F, Foglierini M, De Gregorio C, Cassotta A, Fernandez B, Kelderman S, Schumacher TN, Corti D, Lanzavecchia A, and Sallusto F. 2015. T cell immunity. Functional heterogeneity of human memory CD4(+) T cell clones primed by pathogens or vaccines. Science 347: 400–406. [DOI] [PubMed] [Google Scholar]
- 12.Löhning M, Hegazy AN, Pinschewer DD, Busse D, Lang KS, Höfer T, Radbruch A, Zinkernagel RM, and Hengartner H. 2008. Long-lived virus-reactive memory T cells generated from purified cytokine-secreting T helper type 1 and type 2 effectors. J Exp Med 205: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrington LE, Janowski KM, Oliver JR, Zajac AJ, and Weaver CT. 2008. Memory CD4 T cells emerge from effector T-cell progenitors. Nature 452: 356–360. [DOI] [PubMed] [Google Scholar]
- 14.Hu H, Huston G, Duso D, Lepak N, Roman E, and Swain SL. 2001. CD4(+) T cell effectors can become memory cells with high efficiency and without further division. Nat Immunol 2: 705–710. [DOI] [PubMed] [Google Scholar]
- 15.Opferman JT, Ober BT, and Ashton-Rickardt PG. 1999. Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science 283: 1745–1748. [DOI] [PubMed] [Google Scholar]
- 16.Youngblood B, Hale JS, Kissick HT, Ahn E, Xu X, Wieland A, Araki K, West EE, Ghoneim HE, Fan Y, Dogra P, Davis CW, Konieczny BT, Antia R, Cheng X, and Ahmed R. 2017. Effector CD8 T cells dedifferentiate into long-lived memory cells. Nature 552: 404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akondy RS, Fitch M, Edupuganti S, Yang S, Kissick HT, Li KW, Youngblood BA, Abdelsamed HA, McGuire DJ, Cohen KW, Alexe G, Nagar S, McCausland MM, Gupta S, Tata P, Haining WN, McElrath MJ, Zhang D, Hu B, Greenleaf WJ, Goronzy JJ, Mulligan MJ, Hellerstein M, and Ahmed R. 2017. Origin and differentiation of human memory CD8 T cells after vaccination. Nature 552: 362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tubo NJ, Fife BT, Pagan AJ, Kotov DI, Goldberg MF, and Jenkins MK. 2016. Most microbe-specific naive CD4(+) T cells produce memory cells during infection. Science 351: 511–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdelsamed HA, Moustaki A, Fan Y, Dogra P, Ghoneim HE, Zebley CC, Triplett BM, Sekaly RP, and Youngblood B. 2017. Human memory CD8 T cell effector potential is epigenetically preserved during in vivo homeostasis. J Exp Med 214: 1593–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar BV, Connors TJ, and Farber DL. 2018. Human T Cell Development, Localization, and Function throughout Life. Immunity 48: 202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis MM, and Brodin P. 2018. Rebooting Human Immunology. Annu Rev Immunol 36: 843–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korn T, Bettelli E, Oukka M, and Kuchroo VK. 2009. IL-17 and Th17 Cells. Annu Rev Immunol 27: 485–517. [DOI] [PubMed] [Google Scholar]
- 23.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, and Cua DJ. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 201: 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, Davis J, Hsu A, Asher AI, O’Shea J, Holland SM, Paul WE, and Douek DC. 2008. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452: 773–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M, Gumbleton M, Toulon A, Bodemer C, El-Baghdadi J, Whitters M, Paradis T, Brooks J, Collins M, Wolfman NM, Al-Muhsen S, Galicchio M, Abel L, Picard C, and Casanova JL. 2011. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 332: 65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okada S, Markle JG, Deenick EK, Mele F, Averbuch D, Lagos M, Alzahrani M, Al-Muhsen S, Halwani R, Ma CS, Wong N, Soudais C, Henderson LA, Marzouqa H, Shamma J, Gonzalez M, Martinez-Barricarte R, Okada C, Avery DT, Latorre D, Deswarte C, Jabot-Hanin F, Torrado E, Fountain J, Belkadi A, Itan Y, Boisson B, Migaud M, Arlehamn CSL, Sette A, Breton S, McCluskey J, Rossjohn J, de Villartay JP, Moshous D, Hambleton S, Latour S, Arkwright PD, Picard C, Lantz O, Engelhard D, Kobayashi M, Abel L, Cooper AM, Notarangelo LD, Boisson-Dupuis S, Puel A, Sallusto F, Bustamante J, Tangye SG, and Casanova JL. 2015. IMMUNODEFICIENCIES. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science 349: 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogongo P, Tezera LB, Ardain A, Nhamoyebonde S, Ramsuran D, Singh A, Ng’oepe A, Karim F, Naidoo T, Khan K, Dullabh KJ, Fehlings M, Lee BH, Nardin A, Arlehamn CSL, Sette A, Behar SM, Steyn AJC, Madansein R, Kloverpris HN, Elkington PT, and Leslie A. 2021. Tissue-resident-like CD4(+) T cells secreting IL-17 control Mycobacterium tuberculosis in the human lung. Journal of Clinical Investigation 131: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel DD, and Kuchroo VK. 2015. Th17 Cell Pathway in Human Immunity: Lessons from Genetics and Therapeutic Interventions. Immunity 43: 1040–1051. [DOI] [PubMed] [Google Scholar]
- 29.Stockinger B, and Omenetti S. 2017. The dichotomous nature of T helper 17 cells. Nat Rev Immunol 17: 535–544. [DOI] [PubMed] [Google Scholar]
- 30.Pepper M, Linehan JL, Pagan AJ, Zell T, Dileepan T, Cleary PP, and Jenkins MK. 2010. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat Immunol 11: 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, Sukumar M, Reger RN, Yu Z, Kern SJ, Roychoudhuri R, Ferreyra GA, Shen W, Durum SK, Feigenbaum L, Palmer DC, Antony PA, Chan CC, Laurence A, Danner RL, Gattinoni L, and Restifo NP. 2011. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity 35: 972–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kryczek I, Zhao E, Liu Y, Wang Y, Vatan L, Szeliga W, Moyer J, Klimczak A, Lange A, and Zou W. 2011. Human TH17 cells are long-lived effector memory cells. Sci Transl Med 3: 104ra100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bending D, De La Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, and Cooke A. 2009. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. Journal of Clinical Investigation 119: 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, and Weaver CT. 2009. Late developmental plasticity in the T helper 17 lineage. Immunity 30: 92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, and Stockinger B. 2011. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol 12: 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonelli M, Shih HY, Hirahara K, Singelton K, Laurence A, Poholek A, Hand T, Mikami Y, Vahedi G, Kanno Y, and O’Shea JJ. 2014. Helper T cell plasticity: impact of extrinsic and intrinsic signals on transcriptomes and epigenomes. Curr Top Microbiol Immunol 381: 279–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DuPage M, and Bluestone JA. 2016. Harnessing the plasticity of CD4(+) T cells to treat immune-mediated disease. Nat Rev Immunol 16: 149–163. [DOI] [PubMed] [Google Scholar]
- 38.Crotty S. 2018. Do Memory CD4 T Cells Keep Their Cell-Type Programming: Plasticity versus Fate Commitment? Complexities of Interpretation due to the Heterogeneity of Memory CD4 T Cells, Including T Follicular Helper Cells. Cold Spring Harb Perspect Biol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nurieva R, Yang XO, Chung Y, and Dong C. 2009. Cutting edge: in vitro generated Th17 cells maintain their cytokine expression program in normal but not lymphopenic hosts. J Immunol 182: 2565–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krebs CF, Turner JE, Paust HJ, Kapffer S, Koyro T, Krohn S, Ufer F, Friese MA, Flavell RA, Stockinger B, Steinmetz OM, Stahl RA, Huber S, and Panzer U. 2016. Plasticity of Th17 Cells in Autoimmune Kidney Diseases. J Immunol 197: 449–457. [DOI] [PubMed] [Google Scholar]
- 41.Cohen CJ, Crome SQ, MacDonald KG, Dai EL, Mager DL, and Levings MK. 2011. Human Th1 and Th17 cells exhibit epigenetic stability at signature cytokine and transcription factor loci. J Immunol 187: 5615–5626. [DOI] [PubMed] [Google Scholar]
- 42.Boniface K, Blumenschein WM, Brovont-Porth K, McGeachy MJ, Basham B, Desai B, Pierce R, McClanahan TK, Sadekova S, and de Waal Malefyt R. 2010. Human Th17 cells comprise heterogeneous subsets including IFN-gamma-producing cells with distinct properties from the Th1 lineage. J Immunol 185: 679–687. [DOI] [PubMed] [Google Scholar]
- 43.Mazzoni A, Santarlasci V, Maggi L, Capone M, Rossi MC, Querci V, De Palma R, Chang HD, Thiel A, Cimaz R, Liotta F, Cosmi L, Maggi E, Radbruch A, Romagnani S, Dong J, and Annunziato F. 2015. Demethylation of the RORC2 and IL17A in human CD4+ T lymphocytes defines Th17 origin of nonclassic Th1 cells. J Immunol 194: 3116–3126. [DOI] [PubMed] [Google Scholar]
- 44.Reboldi A, Arnon TI, Rodda LB, Atakilit A, Sheppard D, and Cyster JG. 2016. IgA production requires B cell interaction with subepithelial dendritic cells in Peyer’s patches. Science 352: aaf4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ito T, Carson W. F. t., Cavassani KA, Connett JM, and Kunkel SL. 2011. CCR6 as a mediator of immunity in the lung and gut. Exp Cell Res 317: 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, and Napolitani G. 2007. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 8: 639–646. [DOI] [PubMed] [Google Scholar]
- 47.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, and Romagnani S. 2007. Phenotypic and functional features of human Th17 cells. J Exp Med 204: 1849–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh SP, Zhang HH, Foley JF, Hedrick MN, and Farber JM. 2008. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J Immunol 180: 214–221. [DOI] [PubMed] [Google Scholar]
- 49.Manel N, Unutmaz D, and Littman DR. 2008. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol 9: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rivino L, Gruarin P, Haringer B, Steinfelder S, Lozza L, Steckel B, Weick A, Sugliano E, Jarrossay D, Kuhl AA, Loddenkemper C, Abrignani S, Sallusto F, Lanzavecchia A, and Geginat J. 2010. CCR6 is expressed on an IL-10-producing, autoreactive memory T cell population with context-dependent regulatory function. J Exp Med 207: 565–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kleinewietfeld M, Puentes F, Borsellino G, Battistini L, Rotzschke O, and Falk K. 2005. CCR6 expression defines regulatory effector/memory-like cells within the CD25(+)CD4+ T-cell subset. Blood 105: 2877–2886. [DOI] [PubMed] [Google Scholar]
- 52.Beriou G, Costantino CM, Ashley CW, Yang L, Kuchroo VK, Baecher-Allan C, and Hafler DA. 2009. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood 113: 4240–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paulissen SM, van Hamburg JP, Dankers W, and Lubberts E. 2015. The role and modulation of CCR6+ Th17 cell populations in rheumatoid arthritis. Cytokine 74: 43–53. [DOI] [PubMed] [Google Scholar]
- 54.Singh SP, Zhang HH, Tsang H, Gardina PJ, Myers TG, Nagarajan V, Lee CH, and Farber JM. 2015. PLZF regulates CCR6 and is critical for the acquisition and maintenance of the Th17 phenotype in human cells. J Immunol 194: 4350–4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boritz EA, Darko S, Swaszek L, Wolf G, Wells D, Wu X, Henry AR, Laboune F, Hu J, Ambrozak D, Hughes MS, Hoh R, Casazza JP, Vostal A, Bunis D, Nganou-Makamdop K, Lee JS, Migueles SA, Koup RA, Connors M, Moir S, Schacker T, Maldarelli F, Hughes SH, Deeks SG, and Douek DC. 2016. Multiple Origins of Virus Persistence during Natural Control of HIV Infection. Cell 166: 1004–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang J, Kobert K, Flouri T, and Stamatakis A. 2014. PEAR: a fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 30: 614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bolotin DA, Poslavsky S, Mitrophanov I, Shugay M, Mamedov IZ, Putintseva EV, and Chudakov DM. 2015. MiXCR: software for comprehensive adaptive immunity profiling. Nat Methods 12: 380–381. [DOI] [PubMed] [Google Scholar]
- 58.Dalerba P, Kalisky T, Sahoo D, Rajendran PS, Rothenberg ME, Leyrat AA, Sim S, Okamoto J, Johnston DM, Qian D, Zabala M, Bueno J, Neff NF, Wang J, Shelton AA, Visser B, Hisamori S, Shimono Y, van de Wetering M, Clevers H, Clarke MF, and Quake SR. 2011. Single-cell dissection of transcriptional heterogeneity in human colon tumors. Nat Biotechnol 29: 1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steinfelder S, Floess S, Engelbert D, Haeringer B, Baron U, Rivino L, Steckel B, Gruetzkau A, Olek S, Geginat J, Huehn J, and Hamann A. 2011. Epigenetic modification of the human CCR6 gene is associated with stable CCR6 expression in T cells. Blood 117: 2839–2846. [DOI] [PubMed] [Google Scholar]
- 60.Weng NP, Araki Y, and Subedi K. 2012. The molecular basis of the memory T cell response: differential gene expression and its epigenetic regulation. Nat Rev Immunol 12: 306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sallusto F, and Lanzavecchia A. 2009. Heterogeneity of CD4+ memory T cells: functional modules for tailored immunity. Eur J Immunol 39: 2076–2082. [DOI] [PubMed] [Google Scholar]
- 62.Jameson SC, and Masopust D. 2018. Understanding Subset Diversity in T Cell Memory. Immunity 48: 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, Belanger S, Abbott RK, Kim C, Choi J, Kato Y, Crotty EG, Kim C, Rawlings SA, Mateus J, Tse LPV, Frazier A, Baric R, Peters B, Greenbaum J, Ollmann Saphire E, Smith DM, Sette A, and Crotty S. 2020. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang Y, Xu J, Niu Y, Bromberg JS, and Ding Y. 2008. T-bet and eomesodermin play critical roles in directing T cell differentiation to Th1 versus Th17. J Immunol 181: 8700–8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y, Godec J, Ben-Aissa K, Cui K, Zhao K, Pucsek AB, Lee YK, Weaver CT, Yagi R, and Lazarevic V. 2014. The transcription factors T-bet and Runx are required for the ontogeny of pathogenic interferon-gamma-producing T helper 17 cells. Immunity 40: 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mazzoni A, Maggi L, Siracusa F, Ramazzotti M, Rossi MC, Santarlasci V, Montaini G, Capone M, Rossettini B, De Palma R, Kruglov A, Chang HD, Cimaz R, Maggi E, Romagnani S, Liotta F, Cosmi L, and Annunziato F. 2019. Eomes controls the development of Th17-derived (non-classic) Th1 cells during chronic inflammation. Eur J Immunol 49: 79–95. [DOI] [PubMed] [Google Scholar]
- 67.Gruarin P, Maglie S, De Simone M, Haringer B, Vasco C, Ranzani V, Bosotti R, Noddings JS, Larghi P, Facciotti F, Sarnicola ML, Martinovic M, Crosti M, Moro M, Rossi RL, Bernardo ME, Caprioli F, Locatelli F, Rossetti G, Abrignani S, Pagani M, and Geginat J. 2019. Eomesodermin controls a unique differentiation program in human IL-10 and IFN-gamma coproducing regulatory T cells. Eur J Immunol 49: 96–111. [DOI] [PubMed] [Google Scholar]
- 68.Sallusto F, Cassotta A, Hoces D, Foglierini M, and Lanzavecchia A. 2018. Do Memory CD4 T Cells Keep Their Cell-Type Programming: Plasticity versus Fate Commitment? T-Cell Heterogeneity, Plasticity, and Selection in Humans. Cold Spring Harb Perspect Biol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang P, Lee JS, Gartlan KH, Schuster IS, Comerford I, Varelias A, Ullah MA, Vuckovic S, Koyama M, Kuns RD, Locke KR, Beckett KJ, Olver SD, Samson LD, Montes de Oca M, de Labastida Rivera F, Clouston AD, Belz GT, Blazar BR, MacDonald KP, McColl SR, Thomas R, Engwerda CR, Degli-Esposti MA, Kallies A, Tey SK, and Hill GR. 2017. Eomesodermin promotes the development of type 1 regulatory T (T(R)1) cells. Sci Immunol 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ono C, Yu Z, Kasahara Y, Kikuchi Y, Ishii N, and Tomita H. 2014. Fluorescently activated cell sorting followed by microarray profiling of helper T cell subtypes from human peripheral blood. PLoS One 9: e111405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Newell EW, Sigal N, Bendall SC, Nolan GP, and Davis MM. 2012. Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. Immunity 36: 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Durek P, Nordstrom K, Gasparoni G, Salhab A, Kressler C, de Almeida M, Bassler K, Ulas T, Schmidt F, Xiong J, Glazar P, Klironomos F, Sinha A, Kinkley S, Yang X, Arrigoni L, Amirabad AD, Ardakani FB, Feuerbach L, Gorka O, Ebert P, Muller F, Li N, Frischbutter S, Schlickeiser S, Cendon C, Frohler S, Felder B, Gasparoni N, Imbusch CD, Hutter B, Zipprich G, Tauchmann Y, Reinke S, Wassilew G, Hoffmann U, Richter AS, Sieverling L, Consortium D, Chang HD, Syrbe U, Kalus U, Eils J, Brors B, Manke T, Ruland J, Lengauer T, Rajewsky N, Chen W, Dong J, Sawitzki B, Chung HR, Rosenstiel P, Schulz MH, Schultze JL, Radbruch A, Walter J, Hamann A, and Polansky JK. 2016. Epigenomic Profiling of Human CD4(+) T Cells Supports a Linear Differentiation Model and Highlights Molecular Regulators of Memory Development. Immunity 45: 1148–1161. [DOI] [PubMed] [Google Scholar]
- 73.Tortola L, Jacobs A, Pohlmeier L, Obermair FJ, Ampenberger F, Bodenmiller B, and Kopf M. 2020. High-Dimensional T Helper Cell Profiling Reveals a Broad Diversity of Stably Committed Effector States and Uncovers Interlineage Relationships. Immunity. [DOI] [PubMed] [Google Scholar]
- 74.Tough DF, Rioja I, Modis LK, and Prinjha RK. 2020. Epigenetic Regulation of T Cell Memory: Recalling Therapeutic Implications. Trends Immunol 41: 29–45. [DOI] [PubMed] [Google Scholar]
- 75.Hashimoto S, Ogoshi K, Sasaki A, Abe J, Qu W, Nakatani Y, Ahsan B, Oshima K, Shand FH, Ametani A, Suzuki Y, Kaneko S, Wada T, Hattori M, Sugano S, Morishita S, and Matsushima K. 2013. Coordinated changes in DNA methylation in antigen-specific memory CD4 T cells. J Immunol 190: 4076–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ichiyama K, Chen T, Wang X, Yan X, Kim BS, Tanaka S, Ndiaye-Lobry D, Deng Y, Zou Y, Zheng P, Tian Q, Aifantis I, Wei L, and Dong C. 2015. The methylcytosine dioxygenase Tet2 promotes DNA demethylation and activation of cytokine gene expression in T cells. Immunity 42: 613–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thomas RM, Sai H, and Wells AD. 2012. Conserved intergenic elements and DNA methylation cooperate to regulate transcription at the il17 locus. J Biol Chem 287: 25049–25059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fitzpatrick DR, Shirley KM, and Kelso A. 1999. Cutting edge: stable epigenetic inheritance of regional IFN-gamma promoter demethylation in CD44highCD8+ T lymphocytes. J Immunol 162: 5053–5057. [PubMed] [Google Scholar]
- 79.Castro G, Liu X, Ngo K, De Leon-Tabaldo A, Zhao S, Luna-Roman R, Yu J, Cao T, Kuhn R, Wilkinson P, Herman K, Nelen MI, Blevitt J, Xue X, Fourie A, and Fung-Leung WP. 2017. RORgammat and RORalpha signature genes in human Th17 cells. PLoS One 12: e0181868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eizenberg-Magar I, Rimer J, Zaretsky I, Lara-Astiaso D, Reich-Zeliger S, and Friedman N. 2017. Diverse continuum of CD4(+) T-cell states is determined by hierarchical additive integration of cytokine signals. Proc Natl Acad Sci U S A 114: E6447–E6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grogan JL, Mohrs M, Harmon B, Lacy DA, Sedat JW, and Locksley RM. 2001. Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity 14: 205–215. [DOI] [PubMed] [Google Scholar]
- 82.Zhu J. 2018. T Helper Cell Differentiation, Heterogeneity, and Plasticity. Cold Spring Harb Perspect Biol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harbour SN, Maynard CL, Zindl CL, Schoeb TR, and Weaver CT. 2015. Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc Natl Acad Sci U S A 112: 7061–7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miron M, Meng W, Rosenfeld AM, Dvorkin S, Poon MML, Lam N, Kumar BV, Louzoun Y, Luning Prak ET, and Farber DL. 2021. Maintenance of the human memory T cell repertoire by subset and tissue site. Genome Med 13: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.