Abstract
Objective
Arterial ring testing is the gold standard for measuring arterial function. Increased arterial tone through arterial contraction and impaired endothelial relaxation (endothelial dysfunction) are key metrics of impaired arterial health in peripheral arterial disease (PAD). To allow for comparative testing of arteries during standard laboratory hours, storage buffers and conditions have been used to extend the functional life of arteries. Various storage conditions have been compared, but there has not been a robust comparison or validation in human arteries. The objective of this work is to optimize storage of arterial segments for endothelial cell (EC) testing in a murine model and to test EC function in human PAD arteries. We hypothesized that certain storage conditions would be superior to others.
Methods
Healthy murine aortas were harvested from 10- to 14-week-old C57/Bl6J male and female mice and compared under different storage protocols (24 hours) to immediate arterial testing. The storage conditions tested were: Opti-MEM (37°C or 4°C), Krebs-HEPES with 1.8 mmol/L or 2.5 mmol/L calcium (4°C), or Wisconsin (WI) solution at 4°C. Vascular function was evaluated by isometric force testing. Endothelium-dependent and -independent relaxation were measured after precontraction with addition of methacholine or sodium nitroprusside, respectively. Arterial contraction was stimulated with potassium chloride or phenylephrine. Analysis of variance was used to determine significance compared with immediate testing with P < .05. Under institutional review board approval, 28 PAD arteries were collected at amputation and underwent vascular function testing as described. Disturbed flow conditions were determined by indirect (upstream occlusion) flow to the harvested tibial arteries. Stable flow arteries had in-line flow. Arterial calcification was quantified manually as present or not present.
Results
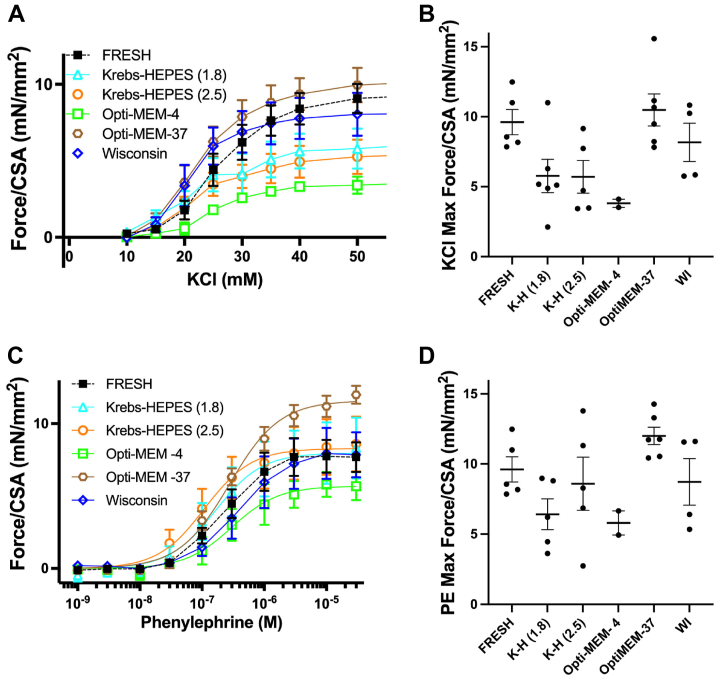
We found that 4°C WI and 37°C Opti-MEM best preserved endothelium-dependent relaxation and performed similarly to immediately testing aortas (termed fresh for freshly tested) (P > .95). Other storage conditions were inferior to freshly tested aortas (P < .05). Vascular smooth muscle function was tested by endothelial-independent relaxation and contractility. All storage conditions preserved endothelial-independent relaxation and contractility similar to freshly tested arteries. However, 4°C WI and 37°C Opti-MEM storage conditions most closely approximated the maximum force of contraction of freshly tested arteries in response to potassium chloride (P > .39). For human arterial testing, 28 tibial arteries were tested for relaxation and contraction with 16 arteries with peripheral artery occlusive disease (PAD with disturbed flow) and 12 without peripheral artery occlusive disease (PAD with stable flow), of which 14 were calcified and 14 were noncalcified. Endothelial-dependent relaxation data was measurable in 9 arteries and arterial contraction data was measurable in 14 arteries. When comparing flow conditions, arteries exposed to disturbed flow (n = 4) had significantly less relaxation (2% vs 59%; P = .03) compared with stable flow conditions (n = 5). In contrast, presence the (n = 6) or absence of calcification (n = 3) did not impact arterial relaxation. Arterial contraction was not different between groups in either comparison by flow (n = 9 disturbed; n = 5 stable) or calcification (n = 6 present; n = 8 absent).
Conclusions
In healthy murine aortas, arterial storage for 24 hours in 4°C WI or 37°C Opti-MEM both preserved endothelium-dependent relaxation and maximum force of contraction. In human PAD arteries stored in 4° WI, flow conditions before arterial harvest, but not arterial calcification, led to differences in arterial relaxation in human PAD arteries. Arterial contractility was more robust (11/28 arteries) compared with arterial relaxation (7/28 arteries), but was not significantly different under flow or calcification parameters. This work defines ideal storage conditions for arterial ring testing and identifies that EC dysfunction from disturbed flow may persist in delayed ex vivo arterial testing.
Keywords: Peripheral arterial disease, Arterial storage, Endothelial function, Arterial contraction
Clinical Relevance
Delayed arterial ring testing is feasible and choice of preservation protocol matters. This strategy allows for physiological testing of human PAD arteries in a staged manner. Using this testing paradigm, flow-dependent changes to arterial function were found to be more powerful than mechanical stiffening owing to calcification for endothelial cell function in PAD arteries.
Article Highlights.
-
•
Type of Research: In vitro (ex vivo) study
-
•
Key Findings: Endothelial function in murine arteries decreased within 24 hours in some preservation protocols. For cold preservation, 4°C Wisconsin buffer provided the optimal storage condition for staged arterial ring testing. Human tibial arteries preserved in 4°C Wisconsin buffer could be tested 24 hours after harvest, and in those that were testable, endothelial dependent relaxation was impaired by nonlaminar arterial flow, but not the presence or absence of arterial calcification.
-
•
Take Home Message: Delayed arterial ring testing can be performed, and the choice of preservation protocol matters. Flow-dependent changes to arterial function seem to be more powerful than mechanical stiffening owing to calcification for endothelial cell function in PAD arteries.
Peripheral arterial disease (PAD) is a significant medical problem that involves both solid and fluid mechanical processes in its disruption of vascular health. Endothelial dysfunction impairs arterial relaxation leading to arterial stiffness over time. PAD arteries are subject to a variety of pathologic processes, including calcification and disturbed blood flow; however, the effect of these pathologic processes on arterial function and endothelial cell (EC) function during ex vivo arterial ring testing is not known.
Arterial ring testing is the gold standard to evaluate arterial function. Both arterial relaxation and contraction are quantified and measure functioning endothelial and vascular smooth muscle cells. This testing is predicated on experimental buffer conditions that closely resemble the basal physiological state to produce generalizable results, reflecting arterial health.1, 2, 3 Arterial storage commonly occurs perioperatively for solid organ transplantation. These vessels are occasionally used in reoperations requiring ex vivo storage in physiological buffer. To do this without compromising arterial function, storage buffers are required with specific compositions.4
The composition of buffers used for arterial storage have evolved over time based on the understanding of specific substrates interacting with vascular function, such as potassium concentration affecting endothelial-dependent relaxation.5 Length of storage as well as temperature of storage also affect EC function dependent on the selection of storage buffer.6,7 Thus, endothelium is particularly susceptible to dysfunction based on storage conditions. Experimentally, storage buffers are used to preserve vascular function in vessels for testing ex vivo.
The objective of this work was to identify storage parameters that best preserved arterial tissue for staged arterial ring testing. We compared various storage buffers on murine arteries to determine endothelial-dependent relaxation and arterial contraction. We used healthy murine aortas from male and female mice, and then tested PAD tibial arteries under optimal storage conditions. We hypothesized that delayed testing is more reproducible under certain storage conditions. Next, we hypothesized that tibial arteries from PAD patients could be similarly tested after storage in a preferred buffer condition.
Methods
Arterial harvest
For murine arteries, the effect of vessel storage on vascular contraction and relaxation was assessed using methods with modifications previously described by our group.8 Arterial testing was performed on descending thoracic aortas of 10- to 14-week-old C57/Bl6J mice (3 male; 3 female), which were perfused with cold phosphate-buffered saline, harvested immediately after sacrifice, and placed in a volume of storage solution to sufficiently bathe the vessel. Vessels were sectioned after storage and before arterial ring testing.
For human PAD arteries, vessels were obtained from veterans undergoing major amputations at the Atlanta VA Medical Center. At least 2 cm of vessel length was harvested and subsequently trimmed for testing. Arteries were placed directly in storage solution without prior flushing. Obstructive disease with disturbed flow is listed as PAD, and PAD arteries with in-line stable flow are listed as no-PAD. Non-PAD arteries were collected from persons undergoing amputation for infectious etiologies and without PAD. Informed consent was obtained by our research team under institutional review board-approved protocols (00051432; 00072955).
Storage buffers
Storage buffers and their compositions used in this study are outlined in Table I. We have traditionally used Opti-MEM 37°C for murine vessel ring testing. However, given concerns that human arteries thickness and greater metabolic activity may not tolerate storage at 37°C, remaining buffer conditions were compared at 4°C, which is clinically relevant and used by transplant surgeons. Remaining storage conditions included Wisconsin (WI) buffer at 4°C, Opti-MEM buffer with varying temperature at 37°C or 4°C, and Krebs-HEPES buffer with varying calcium concentrations at 1.8 mmol/L or 2.5 mmol/L. Opti-MEM (GibCo, Thermo Fisher Scientific Inc., Waltham, MA) is a proprietary formulation with modifications based on the original MEM buffer with only the partial composition reported. The full composition of the remaining buffers is reported. All buffers were supplemented with 100 μ/mL penicillin, 100 mg/mL streptomycin, and 0.25 mg/mL amphotericin B. Human arteries were stored in 4°C WI and tested between 18 and 24 hours after harvest.
Table I.
Composition of storage buffers
| Opti-MEM | Krebs-HEPES | WI | Krebs-PSS | |
|---|---|---|---|---|
| K lactobionate, mmol/L | - | - | 100 | - |
| KH2PO4, mmol/L | - | 1.03 | 25 | 2 |
| MgSO4, mmol/L | 0.8 | 1.2 | 5 | 1.2 |
| NaHCO3, mmol/L | 26 | 25 | - | 25 |
| EDTA, mmol/L | - | - | - | 0.026 |
| Raffinose, mmol/L | - | - | 30 | - |
| Adenosine, mmol/L | - | - | 5 | - |
| Glutathione, mmol/L | - | - | 3 | - |
| Allopurinol, mmol/L | - | - | 1 | - |
| Hydroxyl starch, g/L | - | - | 50 | - |
| HEPES, mmol/L | - | 20 | - | - |
| Glucose, mmol/L | 5.5 | 5.6 | - | 11 |
| Na, mmol/L | - | 145 | 25 | 144 |
| K, mmol/L | - | 5 | 120 | 4.7 |
| Cl, mmol/L | - | 108.7 | - | 127.7 |
| Ca, mmol/L | - | 1.8 or 2.5 | - | 2.5 |
| Osmolality, mOsm/L | 280-320 | 320 | ||
| pH | 7.1-7.4 | 7.4 | 7.4 | 7.4 |
| Notes | Proprietary | Insulin, steroid, antibiotics |
EDTA, Ethylenediaminetetraacetic acid; HEPES, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; MEM, minimal essential medium; PSS, physiological salt solution.
Arterial storage was tested in physiologic solutions including Opti-MEM at 4 °C and 37 °C, Krebs-HEPES with 1.8 mmol/L calcium and 2.5 mmol/L calcium, and compared Wisconsin buffer. Arterial function was tested in physiologic salt solution (Krebs-PSS).
Arterial ring testing
Vessels were cut into 5-mm endothelium-intact aortic ring segments and placed in either storage buffer for study at 24 hours as outlined in Table I or immediately tested. Isometric force testing was performed as previously described.8 Briefly, vessels were taken from experimental conditions and immediately mounted between stainless steel wires in an organ chamber containing Krebs-Henseleit buffer (118 mmol/L NaCl, 4.73 mmol/L KCl, 1.2 mmol/L MgSO4, 0.025 mmol/L EDTA, 1.2 mmol/L KH2PO4, 2.5 mmol/L CaCl2, 11 mmol/L glucose, and 25 mmol/L NaH2CO3, pH 7.4, in 95% O2-5% CO2 at 37°C) that was connected to a Harvard apparatus differential capacitor force for the remainder of the experiment. Resting tension was adjusted to 20 mN over a 30-minute period and was maintained for the duration of the study. Vessels were subjected to relaxation testing and contraction testing in a sequential manner with a 30-minute washout period. Data were obtained using Powerlab hardware and analyzed with Labchart software (ADInstruments, Colorado Springs, CO).
Relaxation testing
Relaxation responses were examined by precontracting the vessel with 300 nmol/L phenylephrine (PE), a concentration that yields 80% maximum contraction, and relaxation was examined after addition of the endothelium-dependent vasorelaxant methacholine (1 nmol/L to 10 μmol/L) and endothelium-independent vasodilator sodium nitroprusside (1 nmol/L to 1 mmol/L). Relaxation of ≥30% was considered effective for endothelium-dependent function.9
Contraction testing
Concentration-isometric force curves were generated in response to the depolarizing contractile agent potassium chloride (KCl; 0-80 mmol/L) and receptor-mediated agonist PE (0.1 nmol/L to 10 mmol/L). Developed forces were expressed both as a percentage of the maximal force generated in response to KCl or PE and force/cross-sectional area (CSA). CSA estimation is based on vessel geometry and wet weight (CSA = 2 × wet weight/[circumference]).
Data analysis
Data were tested and found to be normally distributed. Arterial function was compared between buffer condition and fresh samples using two-way analysis of variance. Data was tested for normality and P < .05 was considered statistically significant. Data analysis was performed using GraphPad Prism version 9.2.0.332 (GraphPad Software, San Diego, CA).
Results
Murine arterial storage testing
Male and female mice were analyzed together (n = 6 total, 3 of each sex). In comparison with fresh arteries, aortas stored in 4°C WI (delta 1; 95% confidence interval, −29 to 32) and Opti-MEM at 37°C (delta 18; 95% confidence interval, −14 to 50) demonstrated the most similar endothelial-dependent relaxation and were superior to all other buffer conditions (Fig 1, A and B). Endothelial-independent relaxation occurred in a dose-dependent fashion for all buffers (Fig 1, C) with no difference in maximum relaxation (Fig 1, D). Compared with other storage conditions, storage in 4°C WI and Opti-MEM at 37°C had a more similar aortic contractility to KCl to that of fresh samples (Fig 2, A and B). For contraction to PE, storage in 4°C WI, Opti-MEM at 37°C, and Krebs-HEPES 2.5 mmol/L Ca were more similar to fresh samples, than other storage conditions (Fig 2, C and D). Significance testing of all relaxation and contraction data is reported in Table II, with only significant values reported here.
Fig 1.
Endothelial-dependent relaxation under different storage conditions. Relaxation of murine aortas under various storage conditions was compared with freshly tested aortas. (A) Endothelium-dependent relaxation curves of Fresh, Wisconsin (WI) at 4°C, and Opti-MEM at 37°C had preserved endothelial relaxation that was not seen in the other storage conditions. (B) When comparing maximum relaxation, Fresh, WI at 4°C, and Opti-MEM at 37°C, all exceeded the 30% threshold for intact endothelium-dependent relaxation. Aortas preserved in Krebs-HEPES with 1.8 mmol/L calcium and 2.5 mmol/L calcium as well as Opti-MEM at 4°C exhibited significantly depressed endothelium-dependent relaxation function. (C) To test the ability of vascular smooth muscle cells to relax independently of endothelial cells (EC), endothelium-independent relaxation curves demonstrate similar responses to increasing concentrations of sodium nitroprusside with all groups similar. (D) Maximum percentage endothelium-independent relaxation was similarly not affected by storage conditions. Total of six descending thoracic aortas (3 males and 3 females). Statistical testing with one-way analysis of variance. Lines represent mean and bars represent standard deviation. ∗P < .05. SNP, sodium nitroprusside.
Fig 2.
Arterial force contraction under different storage conditions. Contraction forces of murine aortas were compared across storage conditions. (A) Force contraction curves in response to increasing concentrations of KCl. (B) There were no significant differences across groups, but maximum force contraction was more similar between Fresh, Opti-MEM at 37°C, and Wisconsin (WI) at 4°C than other conditions. (C) Force contraction curves in response to increasing concentrations of phenylephrine (PE). (D) Again, there were no significant differences across groups, but maximum force of contraction was more similar to Fresh in Krebs-HEPES with 2.5 mmol/L calcium, Opti-MEM at 37°C, and WI at 4°C than other conditions. A total of six descending thoracic aortas (3 males and 3 females). Statistical testing with one-way analysis of variance. Lines represent mean and bars represent standard deviation.
Table II.
Murine aorta relaxation and contraction analyses
| Max relaxation to MCh |
Max relaxation to SNP |
Max contraction to KCl |
Max contraction to PE |
|||||
|---|---|---|---|---|---|---|---|---|
| Δ | CI | Δ | CI | Δ | CI | Δ | CI | |
| Krebs-HEPES with 1.8 mmol/L Ca | 34 | 0.84 to 68.00 | 8 | −13 to 30 | 4 | −0.44 to 8.10 | 3 | −1.6 to 8.0 |
| Krebs-HEPES with 2.5 mmol/L Ca | 40 | 9.2 to 70.0 | −5 | −29 to 20 | 4 | −0.57 to 8.40 | 1 | −3.7 to 5.8 |
| Opti-MEM at 4°C | 53 | 15 to 90 | 6 | −21 to 33 | 6 | −0.13 to 11.00 | 4 | −2.5 to 10.1 |
| Opti-MEM at 37°C | 18 | −14 to 50 | −13 | −36 to 10 | −1 | −5.2 to 3.4 | −2 | −7.0 to 2.2 |
| WI | 1 | −29 to 32 | 0 | −22 to 22 | 1 | −3.3 to 6.2 | 1 | −4.2 to 5.9 |
CI, Confidence interval; HEPES, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; MEM, minimal essential medium.
Maximum endothelial-dependent relaxation to methacholine (MCh) and maximum endothelial-independent relaxation to sodium nitroprusside (SNP). Maximum arterial contraction stimulated with potassium chloride (KCl) and maximum arterial contraction stimulated with phenylephrine (PE). Delta (Δ) denotes the change of parameter compared with a fresh condition with confidence intervals listed alongside. Wisconsin at 4 °C and Opti-MEM at 37 °C demonstrate intact endothelial-dependent relaxation, while aortas preserved in Krebs-HEPES with 1.8 mmol/L calcium and 2.5 mmol/L calcium as well as Opti-MEM at 4 °C exhibited significantly depressed endothelium-dependent relaxation function. Endothelial-independent relaxation and arterial contraction were not affected by storage conditions. Total of six descending thoracic aortas (3 males and 3 females). Statistical testing with one-way analysis of variance. Significance defined as P < .05 and significant values bolded.
Human PAD artery testing
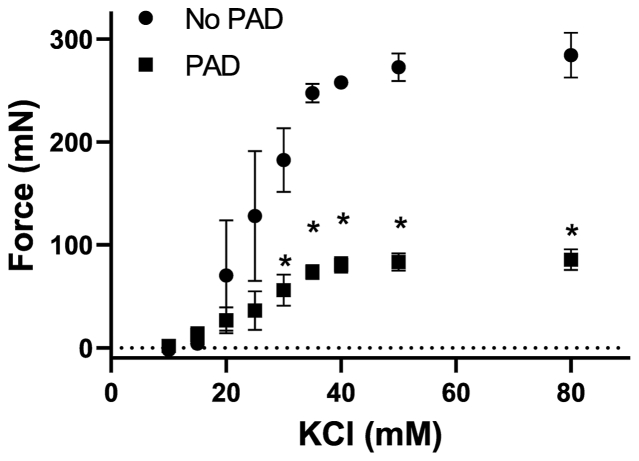
Initially, six human PAD tibial arteries were subjected to contraction force testing (PAD with disturbed flow, n = 3; no-PAD with stable flow, n = 3). Patient information is listed in Table III. We found that arteries from patients with disturbed flow PAD generate significantly less contractile force than patients with no PAD with stable flow (Fig 3).
Table III.
Demographics of human peripheral artery disease (PAD) tibial arteries
| PAD | No PAD | |
|---|---|---|
| Total, n | 25 | 3 |
| Age, years | 69 ± 6.8 | 31 ± 5.6 |
| Male | 23 (89) | 2 (67) |
| Smoker | 6 (23) | 1 (33) |
| DM | 26 (100) | 1 (22) |
| CKD | 4 (15) | 0 (0) |
| HTN | 18 (69) | 0 (0) |
| CAD | 10 (38) | 0 (0) |
| HF | 2 (0.08) | 0 (0) |
| HLD | 13 (50) | 0 (0) |
CAD, Coronary artery disease; CKD, chronic kidney disease; DM, diabetes mellitus; HF, heart failure; HTN, hypertension.
Values are mean ± standard deviation or number (%).
A total of 6 human tibial arteries were analyzed for absolute contraction force testing; 3 arteries were from no-PAD patients and 3 were from PAD patients. Here, no-PAD patients were younger (52 vs 77 years of age) than PAD patients. Sex and comorbidities such as smoking status, diabetes, CKD, and HTN were similar between the two groups.
Fig 3.
Arteries with peripheral artery obstructive disease (PAD) have impaired contractility. Contraction of PAD arteries compared with no-PAD arteries. Absolute force contraction curves in response to increasing concentrations of potassium chloride (KCl). Unlike healthy murine aortas, PAD arteries had decreased force of contraction compared with age and no-PAD controls. There were a total of six arteries (3 PAD and 3 non-PAD). PAD patients with mean age of 73.5 years old, 100% male, 100% with diabetes mellitus, 100% with hypertension, 33% with coronary artery disease, 33% with heart failure, 67% with hyperlipidemia, and 33% with end stage renal disease. Patients without PAD had mean age of 63.5 years old, were 33% male, 67% had diabetes mellitus, 33% had hypertension, 0% had coronary artery disease, 0% had heart failure, 33% had hyperlipidemia, and 0% had end-stage renal disease. Statistical testing was done with one-way analysis of variance. Dots represent mean with brackets denoting standard deviation. ∗P < .05.
Next, 28 total human PAD tibial arteries were stratified by calcification (no calcification n = 14; moderate-severe calcification n = 14) and flow parameters (stable n = 12; disturbed n = 16) (Table IV). Endothelial-dependent relaxation data was measurable in nine arteries (5 stable flow and 4 disturbed flow). Arteries exposed to disturbed flow had significantly less relaxation (2% vs 59%; P = .03) compared with stable flow conditions. Relaxation was not different between calcification conditions (P = .18). Contraction to KCl or PE was not different between flow parameters (P = .11; P = .14) or calcification conditions (P = .41; P = .4), respectively (Table V).
Table IV.
Human tibial arteries stratified by calcification or flow parameters
| Flow | Calcification |
||
|---|---|---|---|
| None | Present | Total | |
| Stable | 5 | 7 | 12 |
| Disturbed | 9 | 7 | 16 |
| Total | 14 | 14 | |
A total of 28 human tibial arteries were stratified by calcification status and flow dynamics categorized as stable flow (s-flow) with no occlusive disease upstream or disturbed flow (d-flow) with obstructive disease upstream; 14 arteries exhibited no calcification and 14 exhibited moderate to severe calcification; 12 arteries exhibited stable flow and 16 arteries exhibited disturbed flow. The distribution of calcification status by flow dynamics was similar.
Table V.
Arterial ring testing stratified by calcification or flow parameters
| Calcification |
Flow |
|||||
|---|---|---|---|---|---|---|
| None | Present | P value | Stable | Disturbed | P value | |
| Relaxation to MCh | 5 (3) | 47 (6) | .18 | 59 (5) | 2 (4) | .03 |
| Max contraction to KCl, mN | 123 (6) | 112 (8) | .41 | 163 (5) | 90 (9) | .11 |
| Max contraction to PE, mN | 66 (6) | 60 (8) | .4 | 79 (5) | 54 (9) | .14 |
KCl, Potassium chloride; MCh, methacholine; PE, phenylephrine.
Values are number (%).
Relaxation and contraction of human tibial arteries were analyzed and stratified based on calcification status and flow dynamics. Disturbed flow but not calcification significantly decreased maximum relaxation to methacholine of human peripheral arterial disease (PAD) arteries compared with stable flow in no-PAD arteries; however, it had no effect on contraction to potassium chloride or phenylephrine. Statistical testing with one-way analysis of variance. Significance defined as P < .05 and significant values bolded.
Discussion
This work brings forward findings that may be useful in arterial testing methodology, as well as generating hypotheses for future work. In healthy murine aortas, both WI buffer at 4°C and Opti-MEM at 37°C best preserved endothelial-dependent relaxation. These two buffers also preserved the maximum force of contraction to KCl, again compared with freshly harvested arteries. Thus, for testing of murine large arteries, we believe either of these storage conditions can yield arterial ring testing results similar to that of testing the artery immediately after harvest. Second, even when stored in WI, endothelial-dependent relaxation in human PAD arteries is more susceptible to impairment than vascular contraction. Thus, vascular smooth muscle cell function seems to be more resistant to prolonged storage. Finally, this work demonstrates that flow conditions but not calcification impair endothelial-dependent relaxation in human PAD arteries.
Previous work demonstrated endothelial-dependent relaxation was significantly decreased in rat aortas after 24 hours of cold storage.10,11 This decrease was seen with storage in WI buffer, but these authors did find that, when compared with other conditions, WI buffer retained vascular function closest to fresh samples. This fits with the findings of this article. There may also be species to species variations that remain unknown. Additionally, we did not compare fresh and delayed vascular function on the same arteries, as was done in this work. Such an approach may complicate interpretation of the vessels in the subsequent testing, because vessel handling does impair vascular function.
Although our murine aortas had similar EC relaxation with WI at 4° and Opti-MEM at 37°, other publications support hypothermic preservation as a better condition for preserving EC function. In human saphenous veins, metabolic activity was superior in hypothermic storage compared with normothermic storage, and this result was most pronounced after reperfusion in the setting of coronary bypass.7 Our data in murine aortas finds that normothermic preservation under specific conditions (Opti-MEM) retains vascular function compared with hypothermic conditions. Identifying the mechanism of preservation under hypothermic and normothermic conditions may be of interest in future comparative functional studies between murine and human arteries.
Duration of storage and temperature of storage conditions also affect vascular function.6,7,12,13 Supplementation with specific amino acids, chelators, and free radical scavengers improved vascular function after vessel storage. This result is thought to be due to the generation of oxidative stress during storage impairing vascular function.14, 15, 16, 17, 18 Previous work has demonstrated loss of vascular function in varying buffers, such as loss of vein function in physiological salt saline after short storage times.19 This work demonstrates that buffer composition is important to vascular function, even for short time periods. WI buffer was initially developed for transport and storage of pancreas, kidney, and liver allografts and is now commonly used for tissue harvests in the operating room as well as for experimentation in the laboratory.20 The benefits of WI buffer may be due to the oncotic support, membrane stabilization, and prevention of acidosis and free radical formation conveyed by its unique composition.21
In human arterial testing, our data found that PAD arteries exposed to chronic disturbed flow conditions had significantly decreased force of arterial contraction compared with arteries from patients without PAD that were exposed to chronic stable flow. Additionally, altered flow parameters via disturbed flow rather than calcification burden impaired EC-dependent relaxation. This finding was surprising; we expected ex vivo testing to be relatively more compromised by arterial mechanics (calcification) than prior flow conditions. Still, the effect of disturbed flow on atheroma plaque formation and endothelial dysfunction is well-documented and validated in an in vivo model.22,23 Our work suggests that these previous findings are remembered at least up until 24 hours by ex vivo testing.
Despite our rigor of testing multiple buffers, this work has several limitations to discuss. The use of healthy murine arteries to test various storage conditions may not be directly applicable to results theoretically obtained from healthy human arteries. Murine arteries are much smaller and easier to provide nutrients over time in storage. Unfortunately, it is not feasible to optimize storage conditions using healthy human arteries. We chose to use WI buffer for human artery testing because it performed best in cold storage, which is standard for human artery and tissue preservation methods. Given the lack of difference seen in our three males and three females across storage conditions, we felt it was more robust to combine all sexes in our analysis. However, it is possible that larger sample sizes of each sex would have been more likely to identify small differences between the sexes for these age mice. Further, there are in vivo sex-based differences in arterial function,24, 25, 26 but we could not identify any publications in the literature finding sex-based differences in arterial ring testing. Bell et al27 compared castrated male (n = 6) and female (n = 6) porcine coronary artery endothelial-dependent relaxation. They did not find sex-based differences, but they did find that certain estrogens had different effects than others on arterial function in arteries from both castrated males and females.27 Thus, the proper sample size to detect sex differences in arterial ring testing remains unknown. Third, our study does not elucidate the mechanism underpinning our findings of impaired endothelium-dependent relaxation under certain storage conditions in mice or the decreased arterial contraction in patients with PAD. Histologic examination of these vessels may have yielded better insight but was not performed. Previous groups have proposed mechanisms for storage-mediated endothelium impairment including prolonged hyperpolarization and hyperkalemia.28,29 Future work may focus on cellular responses to chronic disturbed flow as it relates to arterial relaxation and contraction. There certainly were opportunities to more exhaustively test arteries at different storage times, both longer and shorter. However, for our needs, the 24-hour time point allows ring testing to occur during standard work hours. Longer storage would have no advantage, and shorter storage would lead to testing outside of standard times. Finally, our PAD human data used arteries obtained from veterans at the Atlanta Veterans Affairs Medical Center. The limitations of Veterans Affairs human studies are well-documented and include a population skewed older with more men than women; all arteries in this series were from men. Thus, these results may not be generalizable outside this population.30 Future studies may include comparing in vivo and ex vivo arterial function testing to look for sex-based differences in flow-mediated arterial dysfunction.
In conclusion, this work may be helpful to investigators looking to perform arterial ring testing and other endothelial dependent tests both ex vivo and in vitro. Here, our data show preservation of endothelial dependent relaxation 24 hours after harvest with WI buffer at 4°C and Opti-MEM at 37°C. For larger vessels (eg, human), WI buffer at 4°C is teleologically pleasing to reduce metabolic activity. This work supports a critical role of disturbed flow in arterial health, even in calcified arteries.
Author Contributions
Conception and design: RS, LB
Analysis and interpretation: DM, XC, RS, LB
Data collection: CH, MS, FL, JM, RS, LB
Writing the article: DM, LB
Critical revision of the article: DM, CH, MS, FL, JM, XC, RS, LB
Final approval of the article: DM, CH, MS, FL, JM, XC, RS, LB
Statistical analysis: DM, XC, LB
Obtained funding: LB
Overall responsibility: LB
Footnotes
Funded by the National Institutes of Health (grant RO1HL143348) and the Department of Veteran Affairs (grants IO1BX004707 and 1O1CX002366). Neither the NIH nor the VA were involved in the study design, manuscript writing, or decision to submit the manuscript for publication.
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Kaiman K., Shibata S. Effect of cold storage on the sensitivity of and calcium influx into rat, rabbit and Guinea pig portal veins. Blood Vessels. 1978;15:217–230. doi: 10.1159/000158168. [DOI] [PubMed] [Google Scholar]
- 2.Kaiman M., Shibata S. Calcium influx and spontaneous phasic contractions of portal veins after treatment with reserpine, 6-hydroxydopamine, and cocaine. Can J Physiol Pharmacol. 1978;56:199–201. doi: 10.1139/y78-028. [DOI] [PubMed] [Google Scholar]
- 3.Southard J.H., van Gulik T.M., Ametani M.S., et al. Important components of the UW solution. Transplantation. 1990;49:251–257. doi: 10.1097/00007890-199002000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Olthoff K.M., Millis J.M., Imagawa D.K., et al. Comparison of UW solution and Euro-Collins solutions for cold preservation of human liver grafts. Transplantation. 1990;49:284–290. doi: 10.1097/00007890-199002000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Chan B.B., Kron I.L., Flanagan T.L., et al. Impairment of vascular endothelial function by high-potassium storage solutions. Ann Thorac Surg. 1993;55:940–945. doi: 10.1016/0003-4975(93)90121-w. [DOI] [PubMed] [Google Scholar]
- 6.Garbe S., Zatschler B., Muller B., et al. Preservation of human artery function following prolonged cold storage with a new solution. J Vasc Surg. 2011;53:1063–1070. doi: 10.1016/j.jvs.2010.10.093. 20110112. [DOI] [PubMed] [Google Scholar]
- 7.Puehler T., Gleich O., Schopka S., et al. Impact of normothermic perfusion and protein supplementation on human endothelial cell function during organ preservation. Ann Thorac Surg. 2010;89:512–520. doi: 10.1016/j.athoracsur.2009.10.037. [DOI] [PubMed] [Google Scholar]
- 8.Kline E.R., Kleinhenz D.J., Liang B., et al. Vascular oxidative stress and nitric oxide depletion in HIV-1 transgenic rats are reversed by glutathione restoration. Am J Physiol Heart Circ Physiol. 2008;294:H2792–H2804. doi: 10.1152/ajpheart.91447.2007. 20080502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirstetter P., Lagneau F., Lucas O., et al. Role of endothelium in the modulation of isoflurane-induced vasodilatation in rat thoracic aorta. Br J Anaesth. 1997;79:84–87. doi: 10.1093/bja/79.1.84. [DOI] [PubMed] [Google Scholar]
- 10.Ingemansson R., Sjoberg T., Massa G., et al. Long-term preservation of vascular endothelium and smooth muscle. Ann Thorac Surg. 1995;59:1177–1181. doi: 10.1016/0003-4975(95)00126-6. [DOI] [PubMed] [Google Scholar]
- 11.Massa G., Ingemansson R., Sjoberg T., et al. Endothelium-dependent relaxation after short-term preservation of vascular grafts. Ann Thorac Surg. 1994;58:1117–1122. doi: 10.1016/0003-4975(94)90469-3. [DOI] [PubMed] [Google Scholar]
- 12.Herrera B., Eisenberg G., Holberndt O., et al. Paradoxical effects of temperature on vascular tone. Cryobiology. 2000;41:43–50. doi: 10.1006/cryo.2000.2263. [DOI] [PubMed] [Google Scholar]
- 13.Loong B.J., Tan J.H., Lim K.H., et al. Contractile function of smooth muscle retained after overnight storage. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:1061–1067. doi: 10.1007/s00210-015-1140-3. 20150609. [DOI] [PubMed] [Google Scholar]
- 14.Grohs J.G., Kadletz M., Wodratzka M., et al. Contractile function of human veins after long-term storage in different media. J Cardiovasc Pharmacol. 1996;28:89–93. doi: 10.1097/00005344-199607000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Osgood M.J., Hocking K.M., Voskresensky I.V., et al. Surgical vein graft preparation promotes cellular dysfunction, oxidative stress, and intimal hyperplasia in human saphenous vein. J Vasc Surg. 2014;60:202–211. doi: 10.1016/j.jvs.2013.06.004. 20130730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radovits T., Lin L.N., Zotkina J., et al. Endothelial dysfunction after long-term cold storage in HTK organ preservation solutions: effects of iron chelators and N-alpha-acetyl-L-histidine. J Heart Lung Transplant. 2008;27:208–216. doi: 10.1016/j.healun.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Rauen U., Klempt S., de Groot H. Histidine-induced injury to cultured liver cells, effects of histidine derivatives and of iron chelators. Cell Mol Life Sci. 2007;64:192–205. doi: 10.1007/s00018-006-6456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walia M., Sormaz L., Samson S.E., et al. Effects of hydrogen peroxide on pig coronary artery endothelium. Eur J Pharmacol. 2000;400:249–253. doi: 10.1016/s0014-2999(00)00393-9. [DOI] [PubMed] [Google Scholar]
- 19.Wilbring M., Tugtekin S.M., Zatschler B., et al. Even short-time storage in physiological saline solution impairs endothelial vascular function of saphenous vein grafts. Eur J Cardio Thorac Surg. 2011;40:811–815. doi: 10.1016/j.ejcts.2011.01.024. 20110303. [DOI] [PubMed] [Google Scholar]
- 20.Wahlberg J.A., Love R., Landegaard L., et al. 72-hour preservation of the canine pancreas. Transplantation. 1987;43:5–8. doi: 10.1097/00007890-198701000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Abrahamse S.T., Dinant S., Pfaffendorf M., et al. In vitro function of porcine carotid arteries preserved in UW, HTK and Celsior solutions. Fundam Clin Pharmacol. 2002;16:503–511. doi: 10.1046/j.1472-8206.2002.00112.x. [DOI] [PubMed] [Google Scholar]
- 22.Caro C.G., Fitz-Gerald J.M., Schroter R.C. Arterial wall shear and distribution of early atheroma in man. Nature. 1969;223:1159–1160. doi: 10.1038/2231159a0. [DOI] [PubMed] [Google Scholar]
- 23.Nam D., Ni C.W., Rezvan A., et al. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol. 2009;297:H1535–H1543. doi: 10.1152/ajpheart.00510.2009. 20090814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maddox Y.T., Falcon J.G., Ridinger M., et al. Endothelium-dependent gender differences in the response of the rat aorta. J Pharmacol Exp Ther. 1987;240:392–395. [PubMed] [Google Scholar]
- 25.Stallone J.N. Sex differences in nitric oxide-mediated attenuation of vascular reactivity to vasopressin are abolished by gonadectomy. Eur J Pharmacol. 1994;259:273–283. doi: 10.1016/0014-2999(94)90654-8. [DOI] [PubMed] [Google Scholar]
- 26.Nevala R., Paakkari I., Tarkkila L., et al. The effects of male gender and female sex hormone deficiency on the vascular responses of the rat in vitro. J Physiol Pharmacol. 1996;47:425–432. [PubMed] [Google Scholar]
- 27.Bell D.R., Rensberger H.J., Koritnik D.R., et al. Estrogen pretreatment directly potentiates endothelium-dependent vasorelaxation of porcine coronary arteries. Am J Physiol. 1995;268:H377–H383. doi: 10.1152/ajpheart.1995.268.1.H377. [DOI] [PubMed] [Google Scholar]
- 28.He G.W., Yang C.Q., Graier W.F., et al. Hyperkalemia alters EDHF-mediated hyperpolarization and relaxation in coronary arteries. Am J Physiol. 1996;271:H760–H767. doi: 10.1152/ajpheart.1996.271.2.H760. [DOI] [PubMed] [Google Scholar]
- 29.He G.W., Yang C.Q., Yang J.A. Depolarizing cardiac arrest and endothelium-derived hyperpolarizing factor-mediated hyperpolarization and relaxation in coronary arteries: the effect and mechanism. J Thorac Cardiovasc Surg. 1997;113:932–941. doi: 10.1016/S0022-5223(97)70267-8. [DOI] [PubMed] [Google Scholar]
- 30.Norvell D.C., Thompson M.L., Boyko E.J., et al. Mortality prediction following non-traumatic amputation of the lower extremity. Br J Surg. 2019;106:879–888. doi: 10.1002/bjs.11124. 20190313. [DOI] [PMC free article] [PubMed] [Google Scholar]