Abstract

Over the past decade, molecular imprinting (MI) technology has made tremendous progress, and the advancements in nanotechnology have been the major driving force behind the improvement of MI technology. The preparation of nanoscale imprinted materials, i.e., molecularly imprinted polymer nanoparticles (MIP NPs, also commonly called nanoMIPs), opened new horizons in terms of practical applications, including in the field of sensors. Currently, hydrogels are very promising for applications in bioanalytical assays and sensors due to their high biocompatibility and possibility to tune chemical composition, size (microgels, nanogels, etc.), and format (nanostructures, MIP film, fibers, etc.) to prepare optimized analyte-responsive imprinted materials. This review aims to highlight the recent progress on the use of hydrogel MIP NPs for biosensing purposes over the past decade, mainly focusing on their incorporation on sensing devices for detection of a fundamental class of biomolecules, the peptides and proteins. The review begins by directing its focus on the ability of MIPs to replace biological antibodies in (bio)analytical assays and highlight their great potential to face the current demands of chemical sensing in several fields, such as disease diagnosis, food safety, environmental monitoring, among others. After that, we address the general advantages of nanosized MIPs over macro/micro-MIP materials, such as higher affinity toward target analytes and improved binding kinetics. Then, we provide a general overview on hydrogel properties and their great advantages for applications in the field of Sensors, followed by a brief description on current popular routes for synthesis of imprinted hydrogel nanospheres targeting large biomolecules, namely precipitation polymerization and solid-phase synthesis, along with fruitful combination with epitope imprinting as reliable approaches for developing optimized protein-imprinted materials. In the second part of the review, we have provided the state of the art on the application of MIP nanogels for screening macromolecules with sensors having different transduction modes (optical, electrochemical, thermal, etc.) and design formats for single use, reusable, continuous monitoring, and even multiple analyte detection in specialized laboratories or in situ using mobile technology. Finally, we explore aspects about the development of this technology and its applications and discuss areas of future growth.
Keywords: hydrogels, biomimetic, molecularly imprinted nanoparticles (MIP NPs), nanoMIPs, nanogels, biosensing, proteins, epitope imprinting, precipitation polymerization, solid-phase synthesis
MI technology is a process for creating synthetic receptors that exhibit high affinity and selectivity toward specific molecules or other structures. The imprinting process involves the formation of a polymer matrix around a template molecule, which is subsequently removed, leaving behind a cavity that is complementary in size, shape, and functional groups to the template molecule (see Figure 1).
Figure 1.
Schematic representation of the MI process. First, the functional monomers interact with the template molecule in solution to create a specific cavity. Then, cross-linker and initiator are added for polymerization. Finally, the extraction of template molecule from the polymeric matrix gives origin to empty imprinted cavities with the ability to specifically rebind the target analyte.
MI technology has been developed since the 1930s, when Pauling and his colleagues first proposed the concept of molecular recognition based on complementary shape and charge distribution of molecules.1 In 1972, Wulff demonstrated the synthesis of a polymer with recognition properties for amino acids and peptides.1 In the early 1980s, a new approach to molecular imprinting was introduced by Mosbach and collaborators, known as noncovalent imprinting.2 In these early stages of MI, the development of synthetic receptors was limited by the lack of suitable polymerization techniques. However, the field of MI began to rapidly advance with the introduction of new polymerization methods in the 1980s and 1990s, such as the free radical polymerization and the reversible addition–fragmentation chain transfer (RAFT) polymerization.
Radical polymerization is the most commonly used approach for the synthesis of MIPs. The resulting MIPs are highly cross-linked and exhibit excellent stability and mechanical properties.3 Sol–gel synthesis is another popular approach for the synthesis of MIPs4 involving the hydrolysis and condensation of metal alkoxides to form a sol which is subsequently gelled to form a 3D network. The prepared MIPs have high surface area and porosity, making them suitable for chromatography applications, among others. Meanwhile, electrochemical polymerization5−7 emerged in the literature as a “smart approach” for the synthesis of MIPs by simply electropolymerizing redox functional monomers in the presence of a template molecule, resulting in MIP thin films with excellent surface properties that can be easily integrated into electronic devices. Over recent years, bioimprinting became a promising sustainable technique for the synthesis of MIPs using biomolecules (proteins, polysaccharides, etc.) as building blocks, having the potential to overcome the limitations of conventional formats, such as poor biocompatibility and solubility.8
Overall, MI technology has made remarkable progress since its inception, greatly expanding the spectrum of applications for MIPs. The scope of this review is to discuss recent advancements in the production of hydrogel MIP NPs for biosensing applications, with a particular emphasis on their integration into sensing devices for detecting peptides and proteins.
MIPs As an Alternative to Biological Antibodies
Biological antibodies have been extensively used in life sciences and biosensing assays. Although they provide very specific interaction through the formation of stable complexes with the target antigen, these bioreceptors commonly suffer from lack of long-term stability, can irreversibly denaturate, require careful handling/storage conditions, the acquisition costs are very high and have associated ethical dilemmas. Due to these unfavorable properties, MIPs are currently a very promising alternative to their biological counterparts.9,10 In fact, there are several advantages of using MIPs (“plastic antibodies”) in bioassays for sensing applications (see Table 1). They are synthetic biomimetic materials that can selectively bind to the analytes of interest, offering long-term stability and low production cost in short time.9,10
Table 1. Comparison of Properties between Biological Antibodies and MIPs.
| characteristic | antibodies | MIPs |
|---|---|---|
| production time | few months | few days to weeks |
| thermal stability | denaturate at ≈70 °C | resistant up to 140 °C |
| pH stability | low | high |
| stability | 6–12 months | years |
| production method | animal immunization | chemical synthesis |
| amount of target molecules | medium | high |
| storage | freezer | from 6 °C to RT |
| price | high | low |
| selectivity/specificity/affinity | high (even in complex matrices; KD often <1 nM for monoclonal antibodies) | low to high (IF from nearly 1 to values >10; KD in the nM to pM range for nanosized MIPs) |
The synergetic combination between the fields of MI and Sensors Development is already well established. The general use of MIPs (bulk, microparticles, films, etc.) as synthetic receptors in sensing platforms for (bio)analysis already found relevant practical applications in disease diagnosis,11 environmental monitoring,12 food safety,13 among others, mainly using electrochemical5,12 and optical14 approaches.
From Macro- To Micro- To Nanosized MIP Particles
Pioneer works of Wulff1,15 and Mosbach2,16 were based on simple bulk imprinting leading to the formation of macroscale polymeric structures for diverse applications. Although these bulk materials can be sieved and ground into smaller micro/nano particles, the performance of the resulting heterogeneous MIP materials is often not suitable for precision and/or in vivo applications due to (i) broad distribution of binding sites, leading to different affinities for the target molecule; (ii) many possibilities for nonspecific interactions; (iii) high batch-to-batch variability; and (iv) diffusion limitations for large size biomolecules.
To overcome some limitations of bulk imprinting, researchers in the MI field started to scale down in MIPs dimension (i.e., the “imprinting depth” of imprinted materials), from macroscale MIPs to microparticles, thin films and nanosized particles, aiming to improve binding sites accessibility, favoring template extraction while increasing the number of imprinted cavities per unit of area. At the same time, molecular imprinting strategies, first applied to small molecular templates, were also successfully developed for imprinting of larger and more complex templates, such as peptides and proteins, and even cells and viruses, among others. This tendency, exceptionally demonstrated in 2017 in the work of Culver and Peppas17 (see Figure 2), persisted until the present time.
Figure 2.
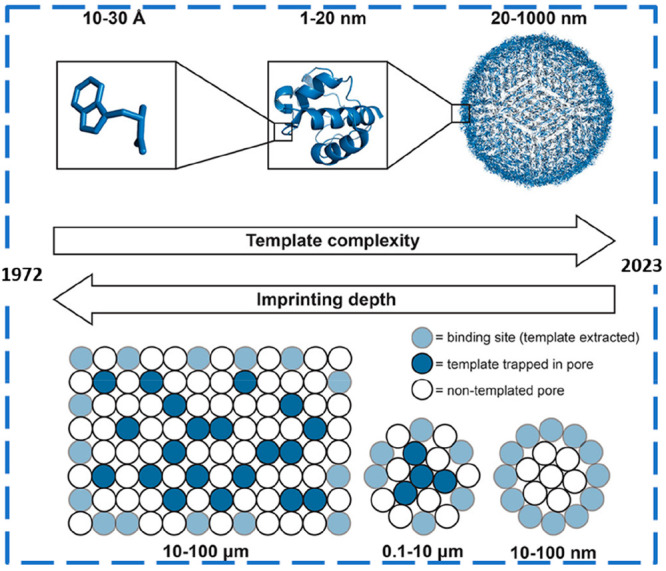
Evolution of MI from bulk materials for recognition of small template molecules to micro- and nanostructured MIPs for large templates, such as proteins and viruses. The term “imprinting depth” refers to the dimensionality of imprinted materials architecture that determines its functions and properties. Adapted from ref (17). Copyright 2017 American Chemical Society.
The advantages in terms of biosensing performance of going from larger- to smaller-sized imprinted materials were reported by Yaqub et al.,18 in 2011, where the same set of acrylate/methacrylate monomers were used to prepare both MIP bulk polymeric layers and MIP NPs (45–85 nm) for specific recognition of atrazine. Then, the prepared MIP materials were incorporated into a piezoelectric sensor surface for comparison. As can be seen in Figure 3A, the sensor responded linearly to the concentration for the MIP NPs, while saturation was observed for the bulk MIP due to the high surface/volume ratio of NPs that increases the accessibility of the analyte to the receptor surface. In addition, the results obtained showed a more than 2-fold higher sensor response of imprinted NPs relative to the natural antibody with both showing a similar selectivity pattern relative to atrazine metabolites and structural analogues (Figure 3B). The size and uniform geometry of nanoMIPs leads to a low number of binding sites (ideally 1) per NP, reducing nonspecific binding (NSB) and providing faster kinetics of interaction.19 Overall, nanosized MIPs compete better with natural antibodies than bulk MIP materials in terms of size and affinity toward the target analyte, having the potential to substitute them as synthetic receptors in affinity assays and sensors for (bio)analysis of several (bio)compounds.20,21 To further empathize the merits of nanoMIPs, Table 2 provides the direct comparison of the detection levels (LOD) achieved in bioanalytical assays targeting the same (bio)molecule using synthetic and natural antibodies.
Figure 3.
(A) Comparison of the piezoelectric sensor response to atrazine obtained by the MIP polymer layer and the MIP NPs; (B) sensor response obtained by natural and “plastic” antibodies to 7 mg L-1 atrazine and selectivity data against analogues molecules, namely des-ethyl atrazine (DEA), des-ethyl-des-isopropyl atrazine (DEDIA), des-isopropyl atrazine (DIA), propazine (PRO), and simazine (SIM). Adapted with permission from ref (18). Copyright 2011 Elsevier B.V.
Table 2. Direct Comparison of LODs Reported for NanoMIPs-Based Assays vs Immunoassays.
| target | nanoMIP assay (LOD) | immunoassay (LOD) | observation | ref |
|---|---|---|---|---|
| vancomycin | ELISA-based assay (2.5 pM) | commercial ELISA (0.1 μM) | LOD of the nanoMIP-based assay was 3 orders of magnitude inferior | (22) |
| atrazine | QCM (20 ppb) | QCM (0.43 ppm) | NanoMIP assay with a LOD down to ppb | (18) |
| α-casein | SPR (0.127 ppm) | commercial ELISA (3 ppm) | LOD of the nanoMIP assay lower than LODs of commercial ELISA kits | (23) |
| cardiac troponin I | thermal detection (0.46 ng L–1) | several immunoassays (0.7–200 ng L–1) | LOD of the nanoMIP assay was inferior to most of immunoassays (Table S2) | (24) |
| SARS-CoV-2 (alpha variant) | thermal detection (9.9 fg mL–1) | thermal detection (8.9 fg mL–1) | LOD of the nanoMIP assay sensor was similar to the immunosensor | (25) |
Analyte-Responsive MIP Hydrogels
The fabrication of stimuli-responsive hydrogels is currently a rapidly expanding field that already found relevant applications in biomedicine and bioanalytical detection.26,27 The unique properties of hydrogels depend on the molecular arrangement of the 3D network of cross-linked polymer chains that changes in volume size (swelling or shrinking) in response to certain external physical (temperature, electric field, light, pressure, etc.), chemical (pH, solvent, ionic strength, etc.), and biological (proteins, nucleic acids, drugs, etc.) stimulus.26 The “swelling-behavior” of the gels arises from the ability of hydrophilic groups (−OH, −COOH, −NH2, etc.) composing the polymer structure to absorb and retain large amounts of water, providing the optimal environment for encapsulation of small molecules, proteins, and even cells. Furthermore, the possibility to properly synthesize hydrogels in different dimensions and configurations makes these functional materials very promising for the design of new “smart” biomaterials, such as MIPs.28
Molecularly imprinted hydrogels exploit the change in the swelling degree in response to molecular recognition of the target analyte.29 Despite the conformational changes of analyte-responsive hydrogels, the binding cavities remain stable and well-defined in the MIP structure, ensuring the recognition. Upon interaction with the analyte, the changes in volume and shape of MIP hydrogels also change its mechanical, electrical, or optical properties that can be used for signal transduction.29 Acrylic acid and acrylamide derivatives are by far the most employed backbone monomers for preparation of imprinted gels. Moreover, N-isopropylacrylamide (NIPAAm)30 has been of particular interest for production of high-quality MIPs. The polymer (PNIPAAm) has lower critical solution temperature (LCST) near 32 °C in aqueous solution which means that the polymer expands and contracts at temperatures below and above the LCST, respectively, altering its hydrophilicity/hydrophobicity equilibrium.
Over recent years, the developments in nanotechnology have paved the way for the production of hydrogel-based MIP NPs, i.e., imprinted nanogels. The mixture of NIPAAm with water-soluble polymerizable (meth)acrylates and (meth)acrylamides derivatives is one of the most popular approaches for synthesis of imprinted nanogels,31 providing high tunability of the framework for imprinting of large biomolecules in mild aqueous conditions.
Routes for MIP NPs Synthesis
Over the years, many ingenious approaches have been reported for protein imprinting, aiming to obtain synthetic biomimetic materials of distinct formats, ranging from MIP thin films (prepared by deposition of self-assembled monolayers, electropolymerization, etc.), to membranes, or particles of different size and composition (surface imprinting of core–shell and metal NPs, for example), depending on its final application.5,7,11,32 In this review, we will focus on hydrogel imprinting approaches that are commonly employed for preparation of thermosensitive hydrogel-MIP NPs for recognition of several types of templates ranging from low molecular weight molecules to biomacromolecules.
One simple approach for the preparation of MIP (micro/nano) particulates is the precipitation polymerization method that consists of performing cross-linking polymerization from a highly diluted solution containing a mixture of monomer(s), initiator, and template all dissolved in a suitable solvent. Under appropriate experimental conditions, monodisperse spherical polymer particles are formed within the reaction medium20,21 with diameter from a few micrometers33 to dozens of nanometers.34 However, many methods make use of organic solvents which are not suitable for proteins.21 Thus, a particularly attractive approach is to carry out precipitation polymerization in an aqueous-based environment (and in the presence of very low concentrations of surfactant)35 to obtain uniform spherical MIP NPs.21 The first work reporting the use of precipitation polymerization for routine one-step production of water-soluble MIP NPs was reported by Hoshino et al.,31 in 2008, where high-quality nanoMIPs were prepared for specific recognition of bee toxin melittin (Mel; see Figure 4). The concept was then extended for preparing MIP NPs with affinity for lysozyme,36 Fc fragment of IgG,37 fibrinogen,38 a vascular endothelial growth factor (VEGF165),39 among others.
Figure 4.
Schematic representation of monomers, template amino acids sequence (melittin), and imprinting process for NPs synthesis by precipitation polymerization. Reprinted from ref (31). Copyright 2008 American Chemical Society.
Although precipitation polymerization is a very simple and efficient method to obtain MIP NPs in high yield and purity, the extraction of template is time-consuming (several days by dialysis), the synthesis makes use of surfactants unfavorable for biological applications, and the strength of monomer–template interactions can be reduced due to high binding site heterogeneity (highly polyclonal).20,21 To overcome these drawbacks, solid-phase synthesis (see Figure 5) has emerged in the literature as an attractive alternative method allowing one to obtain nanoMIPs resembling “monoclonal antibodies”. The first work in this context was reported by Poma et al.,40 in 2013, for imprinting of a low molecular weight template (melamine), vancomycin, and a model peptide (see Figure 5A), while Ambrosini et al.41 have extended the concept for protein recognition (trypsin) by performing aqueous polymerization under mild conditions (Figure 5B). In brief, the synthetic process is based on the immobilization of templates onto the surface of a solid support (usually glass beads) before polymerization aiming to reduce template’s degrees of freedom, thus yielding NPs with narrower affinity distribution. Furthermore, the exclusion of low-affinity MIP NPs is performed by simple solvent washing before collection of high-affinity nanoMIPs by thermoresponsive shrink (hot washing)40 or swelling (cold washing).41
Figure 5.
(A,B) Schematic representations of the synthesis process of MIP NPs by solid-phase imprinting for recognition of (A) small templates (for illustration purposes) and (B) large biomolecules. (A) Small templates were covalently bound to the glass beads, via 3-aminopropyltrimethyloxysilane (APTMS) and glutaraldehyde as linker, allowing its reusability. The polymerization was initiated by UV-irradiation. Adapted with permission from ref (40). Copyright 2013 John Wiley and Sons. (B) Affinity ligand p-aminobenzamidine (PAB) was used to reversibly immobilize the template protein and reload glass beads with protein if template degradation occurs. Redox initiator system ammonium persulfate (APS)/N,N,N′,N′-tetramethylethylenediamine (TEMED) was used for polymerization at 37 °C. Adapted with permission from ref (41). Copyright 2013 Royal Society of Chemistry.
High affinity nanoMIPs targeting small molecules,42,43 peptides,44,45 proteins,44,46−48 and also virus25,49 and bacteria50 have been prepared through well-established solid-phase synthesis protocols.51 In fact, this technology can benefit from automated synthesis for reproducible preparation of polymer batches47 and industrial mass production of MIP NPs.20 Still, the low yields of MIP NPs (<1 mg g–1 support beads) are perhaps the main limitation due to the low surface area of conventional glass beads (diameter of 70–100 μm), leading to low amounts of immobilized protein.20,21 Thus, alternative solid-phase materials have been attempted, such as (submicrometer-sized) magnetic carriers,52,53 whose high surface-to-volume ratio can improve the synthesis yield.
Epitope Imprinting
Although the MI of small molecules is already well-established through several approaches, the imprinting of proteins can be rather challenging due to its complex and flexible conformation that needs to be maintained during the polymerization process. Furthermore, the incorporation of the whole protein within the polymeric matrix can strongly inhibit the extraction procedure while some templates can be very expensive even for small quantities. To solve these issues, the epitope approach54,55 (see Figure 6) was introduced, bringing great advantages in terms of MIP affinity, performance, and production costs.
Figure 6.

Schematic representation of epitope imprinting approach. Reprinted with permission from ref (55). Copyright 2019 John Wiley and Sons.
The epitope approach resembles antibody operation by recognizing only a small exposed fragment of the large protein (epitope; around 9 amino acids). For example, the bovine serum albumin (BSA) amino acids 599–607 (VVSTQTALA) and the cytochrome c (cyt c) amino acids 97–104 (AYLKKATNE) were selected as the unique imprinting peptides for specific MIP recognition. These terminal amino acids sequences were selected from the C-terminal region of the protein due to being less expected to undergo post-translational modifications.56 In the end, after epitope removal from the MIP matrix, the specific binding sites have the ability to recognize the entire target protein.54,55,57
Many protein epitopes are easily accessible from crystal structure data and epitope mapping. In addition to the contribution of molecular modeling for improved design of specific receptors by choosing the highest affinity monomers for a template,58,59 computational methods have been used for careful selection of suitable epitope(s) for protein templates.60
The use of epitopes is fully compatible with processes for MIP NP preparation.48,61 Epitopes bearing −NH2 or −COOH groups can be easily immobilized onto glass beads through well-established chemistry procedures, while cysteine groups can be added to the selected synthetic peptide (cysteine-epitope) for covalent attachment to solid supports using succinimidyl-iodo acetate as a linker.62−64
Application of Hydrogel NanoMIPs for Biosensing of Macromolecules
General Perspective
Nanosized MIPs have several potential applications due to their ability to selectively recognize and bind specific target molecules. A general overview of nanoMIPs applications is depicted in Table 3.
Table 3. Core Applications of MIP NPs Including Representative Examples for Which They Have Been Used.
| sample pretreatment and analytical separations | biomedical applications | biosensing |
|---|---|---|
| solid-phase extraction (SPE)65,66 | bioimaging62,64 | optical (colorimetric, surface plasmon resonance, fluorescence, etc.) |
| affinity chromatography10 | drug delivery for cancer therapy63,67 | electrochemical (potentiometric, amperometric, etc.) |
| capillary electrophoresis68 | biomimetic nanomedicine69 | other approaches (thermal detection, quartz crystal microbalance, etc.) |
The preparation of imprinted materials for specific recognition of (bio)macromolecules is currently a hot topic since many peptides and proteins (i) act as important biomarkers of many prevalent diseases (cancer, infectious diseases, etc.), (ii) can induce allergic responses in susceptible individuals, or (iii) be a source of environmental pollution. As stated before, nanosized MIP particles lead to improved performance of sensing devices.20,21 Literature reviews focusing on MIP nanomaterials for chemical sensing are currently missing, considering the fast technological advances in this field. Particularly, this review aims to provide the current state of art of hydrogel based-MIP NPs sensors for detection of proteins (>1500 Da). Detection of pathogens based on imprinted nanogels for recognition of viral and bacterial proteins were also included in this review.
In order to provide a general overview on this topic, we start by summarizing the most representative works that emerged in the literature over the past decade, shown in Table 4, which includes the target biomolecule to detect, the synthesis approach used for nanoMIPs preparation, the sensor transduction mode, the strategy used for incorporating nanogels onto the sensing platforms, and the detection levels achieved (LOD).
Table 4. List of the Most Representative Works in the Literature Reporting the Integration of Hydrogel MIP NPs into Transduction Devices for Detection of Peptides and Proteinsa.
| target biomolecule (sample medium) | synthesis approach | detection method | nanoMIPs immobilization | LOD/linear range | ref |
|---|---|---|---|---|---|
| vancomycin (blood plasma) | solid-phase imprinting | ELISA/MINA | adsorption by evaporation of MIP NPs solution overnight | 2.5 pM/0.001–70 nM | (22) |
| vancomycin (blood plasma) | solid-phase imprinting | RI changes at an optical fiber LPG | covalent coupling of the nanoMIPs onto the fiber modified with APTMS and using GA as linker | 10 nM/10 nM–700 μM | (75) |
| HAS (human serum) | epitope-mediated precipitation polymerization imprinting | fluorescence (using a hybrid MIP NPs-QDs composite) | – | 44.3 nM/0.25–5 μM | (70) |
| hyaluronic acid (human keratinocytes) | epitope-mediated precipitation polymerization imprinting in DMSO | fluorescence imaging (using a polymerizable rhodamine derivative) | – | (KD = 196 μM) | (74) |
| hepcidin-25 | epitope-mediated precipitation polymerization imprinting | SPR | biotinylated MIP NPs anchored onto the SPR sensor chip with covalently immobilized NeutrAvidin | 5 pM/7.2–720 pM | (71) |
| vancomycin | solid-phase imprinting | electrochemical (CV) | self-assembly of e-nanoMIPs on a Nafion membrane coated glassy carbon electrode | 83 μM/83–410 μM | (45) |
| VEGF (zebrafish embryos) | epitope-mediated solid-phase imprinting | fluorescence imaging (using a hybrid MIP NPs-QDs composite) | – | (KD = 1.56 ± 0.20 nM) | (62) |
| Trypsin (human serum) | solid-phase synthesis of core–shell MIP NPs | ELISA/fluorescence MINA (using FITC-labeled MIP NPs) | – | (LOQ = 50 pM)/50 pM–5 nM | (84) |
| EGFR; biotin; vancomycin; trypsin | epitope-mediated solid-phase imprinting | thermal detection | thermocouples functionalized with nanoMIPs by dip-coating | 3–5 nM/0–100 nM; 0–500 nM | (44) |
| α-casein (CIP samples) | solid-phase imprinting | SPR | covalent attachment of the nanoMIPs to the carboxylated SPR chip through EDC/NHS coupling | 0.127 ppm/0–150 ppm | (23) |
| trypsin; THC | solid-phase imprinting | electrochemical (capacitance) | covalent immobilization of nanoMIPs onto the electrode modified with electropolymerized tyramine film and using GA as linker | 1.0 × 10–14 M/1.0 × 10–14–1.0 × 10–9 M; 1.0 × 10–14 M/1.0 × 10–12–1.0 × 10–5 M | (81) |
| EGFR (MCF-7 breast cancer cells) | protein-imprinted poly(NIPAAm) SAM on the surface of Au nanorods | Raman imaging (using SERS-active MIP nanoprobes) | – | (≥10–14 mol L–1) | (83) |
| mycobacterium leprae bacteria (human blood) | epitope-mediated radical polymerization imprinting | electrochemical QCM (EQCM) | incorporation of the nanoMIPs on the EQCM transducer by electrochemical polymerization of 4-ATP | 0.161 nM/10–140 nM | (50) |
| insulin (human plasma) | epitope-mediated solid-phase imprinting | electrochemical (DPV) | covalent immobilization of the e-nanoMIPs onto the SPE via APTES and using GA as linker | 81 fM/50–2000 pM | (79) |
| leukotrienes; insulin (urine/plasma) | solid-phase imprinting | ELISA/fluorescence MINA in magnetic microplates | – | 0.73 pM/0.45–364 pM; 27 pM/25–2500 pM | (77) |
| human transferrin (human serum) | precipitation polymerization imprinting | POF-SPR | soft nanoMIPs covalently coupled to the carboxylated plasmonic surface by EDC/NHS reaction | 1.2 fM/1.2 fM–1.8 pM | (72) |
| glucose; paracetamol; C4-HSL; THC; trypsin (spiked plasma) | solid-phase imprinting | electrochemical (DPV) | covalent immobilization of e-nanoMIPs to the SPE previously modified with cysteamine SAM followed by EDC/NHS reaction | 0.43 mM/0.8–50 mM; 82 μM/100–1000 μM; 0.12 nM/6.25–800 nM; 0.05 μM/0.1–1000 μM; 0.20 nM/6.5–100 nM | (80) |
| troponin I | epitope-mediated solid-phase imprinting | thermal detection | nanoMIPs covalently coupled to the SPE surface via electrografting of 4-ABA and EDC/NHS reaction | 0.46 ng L–1/0–2 ng L–1 | (24) |
| SARS-CoV-2 virus (samples from COVID-19 patients) | epitope-mediated solid-phase imprinting | thermal detection | nanoMIPs covalently coupled to the SPE surface via electrografting of 4-ABA and EDC/NHS reaction | 9.9 fg mL–1, alpha variant 6.1 fg mL–1, delta variant/1 fg mL–1–10 pg mL–1 | (25) |
MINA: molecularly imprinted polymer nanoparticle-based assay; RI: refractive index; HSA: human serum albumin; LPG: long period grating; APTMS: 3-aminopropyltrimethyloxysilane; GA: glutaraldehyde; QDs: quantum dots; DMSO: dimethyl sulfoxide; CV: cyclic voltammetry; VEGF: vascular endothelial growth factor; FITC: fluorescein isothiocyanate; EGFR: epidermal growth factor receptor; CIP: cleaning in place; SAM: self-assembled monolayer; EDC: 1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide; NHS: N-hydroxysuccinimide; THC: tetrahydrocanabinol; 4-ATP: 4-aminothiophenol; DPV: differential pulse voltammetry; SPE: screen-printed electrode; APTES: (3-aminopropyl)triethoxysilane; POF: plastic optical fiber; C4-HSL: C4-homoserine lactone; 4-ABA: 4-aminobenzoic acid.
Hydrogel MIP NPs targeting analytes ranging from small peptides, such as vancomycin, to high-molecular-weight proteins (human serum albumin, human transferrin, epidermal growth factor receptor, etc.) were successfully produced. Some works used the precipitation polymerization70−74 method for simple one-step preparation of biomimetic materials. However, the increasing popularity of the solid-phase approach22−25,44,45,62,66,75−81 is remarkable due to its ability to produce MIP materials resembling monoclonal antibodies. Interestingly, several works reported the advantages of epitope imprinting24,50,62,66,70,71,74,79 to obtain good quality nanoMIPs. Straightforward bioinformatic methods showed their great potential for proper epitope identification. For example, a short peptide fragment (∼10 amino acids) from the receptor binding domain (RBD) of the spike protein of SARS-CoV-2 was identified by in silico analysis and used as the epitope of the target virus.25 In another work, insulin epitopes were identified using in silico epitope mapping while the nanogel composition was computationally designed to selectively bind selected epitopes.79 Simple and efficient immobilization of SH-cysteine modified epitopes onto the solid supports was achieved throughout covalent coupling.44
The development of new surface modification procedures82 or the incorporation of labels, such as electroactive probes45 and fluorophores,74,77 into the MIP hydrogel structure by simple adjustment of the monomer composition greatly contributed to improvements on MIP-based sensing methods. For preparation of the sensing devices, nanoMIPs were easily integrated into detection platforms through simple (i) physisorption,22,44,76 using (ii) biotin-neutravidin mediated immobilization,71 by (iii) incorporation into electropolymerized polymers50 and via (iv) covalent attachment.23,24,72,73,75,78−81 From those, the covalent coupling of synthetic receptors to substrates showed to be the most versatile and efficient immobilization procedure for stable and reproducible sensor response.24
The applicability of sensing devices in real (or close-to-real) conditions is fundamental to envisage a practical application. Although some works focused on proving that the sensing concept is feasible by performing detection studies in buffer solution, other sensors succeeded when operating in complex samples, such as human urine77 and serum/plasma22,70,72,75,79,80 and even real patient samples (blood,50 respiratory tract,25 etc.). Still, more applied work of sensors operating directly in complex samples is needed to demonstrate the potential of the biomimetic devices. The sample preparation should be minimal (only dilution, if possible) to make the sensing devices easy-to-use.
It is important to notice that electrochemical and thermal methods have already started transitioning from the lab to the field by using disposable and cost-effective screen-printed electrodes (SPEs)24,25,79,80 as substrates for detection, enabling mass production of MIP-sensor devices. This opened routes for the point-of-care (POC) detection of proteins (and pathogens), enabling the early disease diagnosis and/or the monitoring of patient health at the site of care.
Overall, methods incorporating imprinted nanogels for protein biosensing mainly relied on: (i) optical sensors, such as ELISA-based assays,22 surface plasmon resonance (SPR),23,71 surface-enhanced Raman spectroscopy (SERS)83 and fluorescence sensors;62,70 (ii) electrochemical sensors, based on either capacitance81 or amperometric measurements;80 and (iii) other sensing approaches, such as thermal44 and piezoelectric methods.50
In the following sections, we discuss the main achievements of this technology from several perspectives, such as nanogels composition and preparation, immobilization of artificial receptors on biosensing platforms, detection levels achieved, setup design (simplicity, cost, portability, sample volume, etc.), and applicability of developed sensor devices.
Optical Sensors Based on Hydrogel Imprinted NPs
The enzyme-linked immunosorbent assay (ELISA) is a conventional method for detection and quantification of proteins in several fields, ranging from fundamental research to biomedical applications,85 such as the early disease diagnosis where measurement of ultralow protein concentration levels are needed.85,86
One strategy to achieve high assay sensitivity is to increase the binding capacity of the capture (bio)receptors. This principle was explored by Yonamine et al.87 and Chianella et al.22 that engineered nanoMIPs as substitutes for natural receptors in standard ELISA assays. These ELISA-like assays are commonly referred to in the literature as MINA: molecularly imprinted nanoparticle-based assay.88 To develop the ELISA-based assay for glycopeptide antibiotic vancomycin, the nanoMIPs, prepared by the solid-phase approach, were first placed in microplate wells by simple evaporation. Then, a competition/inhibition assay format between vancomycin and horseradish peroxidase (HRP)-vancomycin conjugate was employed using 3,3′,5,5′-tetramethylbenzidine (TMB) as a chromogenic substrate for color development (see Figure 7A). After optimization of blocking and washing protocols, a linear logarithmic calibration curve between 1 pM and 70 nM was obtained. The LOD obtained (LOD = 2.5 pM) by the MINA was 3 orders of magnitude inferior to traditional ELISA (0.1 μM), probably due to the higher binding capacity of MIP NPs, leading to the enrichment of the sensing platforms with a target analyte. Furthermore, the artificial receptors provided high assay selectivity for vancomycin against other antibiotics, and the MINA applicability was demonstrated by detection of vancomycin in complex blood plasma. The high assay sensitivity allowed a 100,000-fold dilution of spiked plasma samples which greatly contributed to minimize the interference of the serum matrix.
Figure 7.
(A) Schematic representations of the (i) MIP NPs preparation by a solid-phase approach, (ii) developed nanoMIP-based ELISA assay for detection of vancomycin, and (iii) calibration curve obtained. Adapted from ref (22). Copyright 2013 American Chemical Society. (B) Illustration of the sandwich immunoassay for trypsin detection in human serum using fluorescent core–shell MIP NPs and p-aminobenzamidine (PAB)-functionalized plates. Adapted from ref (84). Copyright 2017 American Chemical Society. (C) Schematic representation of the magnetic template-based fluorescence competitive displacement assay for detection of trypsin and pepsin. Adapted with permission from ref (53). Copyright 2019 John Wiley and Sons.
Given the possibility of custom synthesis of MIP NPs, the MINA concept was further adapted for detection of enzymes (HRP, cytochrome C)76 and mycotoxins.89 These pioneer works were very inspiring for more applied work in ELISA-based colorimetric assays. Besides, the inherent stability and low cost production of MIP NPs offer economic benefits from storage and transportation point-of-views since no refrigeration of MINA kits is required.
Meanwhile, the advances in fluorophore labeling chemistry brought in innovative features to MINA.53,84 In 2017, Xu et al.84 developed core–shell MIP NPs for fluorescence quantification of trypsin (see Figure 7B) in undiluted human serum. The core–shell MIPs were prepared by a multistep solid-phase approach with postfunctionalization with fluorescein isothiocyanate (FITC) on the surface of the shell. The nanoMIPs showed good selectivity for trypsin against endogenous serine proteases (kallikrein, thrombin) and serum proteins (human serum albumin and cytochrome C), and a low limit of quantification (of 50 pM) was obtained.
Recently, in 2019, protein-modified magnetic NPs were combined with fluorescent-labeled nanoMIPs as reporters in the same competitive displacement assay to achieve ultrasensitive detection levels (see Figure 7C). The developed method is performed in magnetic microplates in a label-free manner, thus without the need for ligand or nanoMIPs immobilizations or enzyme conjugations.53 The generic fluorescence MINA approach was then applied for detection of leukotrienes (LTE4) and insulin in biological samples (urine and plasma) at physiological levels (LODs: 0.24–27 pM).77 Overall, the high sensitivity of fluorescence MINA is comparable to chromatographic techniques showing great potential for protein biomarker detection in a clinical diagnosis context.
Fluorescence biosensors have been facing tremendous progress with several relevant applications in bioanalysis, imaging of biological processes, measurement of molecular dynamics in cells, among others.85,90 On the other hand, hydrogel MIP NPs already showed their ability to cross biological membranes without toxicological effects on cells or living animals.91,92 The merge between MI nanotechnology and fluorescence sensing can be considered relatively simple. Fluorescence nanoMIPs can be prepared by adding an acrylate monomer bearing a fluorophore (fluorescein o-acrylate, methacryloxyethyl thiocarbamoyl rhodamine, N-fluoresceinylacrylamide, etc.) to the polymerization solution.53,63,64,74,77,92 Alternatively, quantum dots (QDs) and carbon dots (CDs) can be coupled to nanoMIPs to develop hybrid affinity nanomaterials for fluorescence protein quantification70,93 or bioimaging.
Haupt and his collaborators, in 2015, were the first to demonstrate that fluorescence nanoMIPs can be used for cell and tissue imaging by localizing and quantifying target biomolecules (hyaluronic acid) on cells.74 After this seminal work, fluorescence imaging was further applied for the in vivo study of (i) epidermal growth factor receptor (EGFR) in breast cancer cell lines,63 (ii) hyaluronic acid (HA) in human skin keratinocytes,94 and (iii) β2 microglobulin (B2M) as a means to detect senescent cells in mice.64
In another work, Cecchini et al.62 monitored the in vivo cellular dynamics of vascular endothelial growth factor (VEGF) which is overexpressed in many invasive cancers. For fluorescence imaging, nanoMIPs targeting human VEGF (hVEGF) were then covalently coupled with CdTe QDs. To test the ability of the fluorescence hybrid nanoMIPs for measuring the changes of hVEGF expression in cancer cells, two tumor models were obtained by injecting two human malignant melanoma cell lines in transparent zebrafish embryos, one with overexpressed hVEGF [WM-266, hVEGF(+)] and the other with low expression of hVEGF [A-375, hVEGF(−)] (see Figure 8A). After incubation with the nanoprobes for 7 h, the collected bright field and corresponding confocal microscopy images of tested models indicated that the fluorescence nanoMIPs were able to distinguish cells overexpressing hVEGF [hVEGF(+)] from noncancer cells [hVEGF(−)], being located close to the tumor mass (see Figure 8B). By opposition, the control QD-nips were not able to localize the tumor mass. In addition, the statistical treatment of the nanoprobe–cell distances further supported the experimental observations (Figure 8C), confirming the ability of QD-MIPs to selectively recognize tumor cells. Importantly, the prepared fluorescence nanoMIPs induced negligible toxic effects to zebrafish embryos. These studies highlight the great potential of fluorescence imprinted nanogels as imaging tools for cancer diagnostics and/or patient’s follow-up.
Figure 8.
(A) Schematic representation of the in vivo experiments performed in zebrafish embryos as animal model where the hybrid fluorescence nanoMIPs (QD-MIPs) were injected in the hVEGF(+) model (WM-266) and in the hVEGF(−) model (A-375). Control experiments with fluorescence nanoMIPs against vancomycin (QD-nips) were injected in the hVEGF(+) model (WM-266). (B) Confocal bright field and fluorescence images along with the overlay signals of the in vivo experiments. (C) Statistical analysis of the mean of distances nanoprobe–cell (μm) for the three experiments performed (p-value = 0.0006; embryos n ≥ 7). Adapted from ref (62). Copyright 2017 American Chemical Society.
Plasmonic biosensors are a very popular method for real-time monitoring of protein binding events95 by measuring the refractive index (RI) changes occurring very near a thin metal film surface.96
Recently, Tothill and collaborators23 reported the integration of nanoMIPs in SPR sensors for detection of milk allergen bovine α-casein. The sensor was fabricated to act as a simple and cost-effective (online or at-line) tool in the food manufacturing industry for routine monitoring of wash samples from cleaning in place systems (CIP). For α-casein detection, nanoMIPs with high selectivity for target allergen were covalently attached to the gold SPR chips (see Figure 9A) followed by cumulative surface injections of allergen protein solutions (from 0–150 ppm). The estimated detection limit (LOD of 127 ± 97.6 ng mL–1; 0.127 ppm) was even inferior to the LODs obtained by commercial ELISA kits. The SPR sensor showed a high selectivity for target α-casein in the presence of β-lactoglobulin (BLG) and BSA as matrix interferents, and satisfactory recovery values (87–120%) were obtained for α-casein-spiked CIP wash samples, after sample treatment by gel filtration. Furthermore, the same approach was employed for detection of milk protein β-lactoglobulin (BLG),78 bacterial endotoxins,58 and pathogenic viruses.97 Although the plasmonic sensors showed a high sensitivity (LODs of few ng mL–1), detection studies in close-to-real conditions are missing. Nevertheless, the SPR method integrating nanoMIPs showed its high potential for selective, rapid, and sensitive real-time monitoring of allergens and/or contaminants, helping to mitigate risks for consumers.
Figure 9.
(A) General schematic representation of the nanoMIPs-SPR-based sensor for detection of milk allergen α-casein. Adapted from ref (23). Copyright 2018 American Chemical Society. (B) SPR sensor composed of a silica light-diffusing fiber (LDF) functionalized with soft nanoMIPs as artificial receptors for specific detection of human serum transferrin (HTR). Adapted with permission from ref (73). Copyright 2022 MDPI.
In another work, the significant RI changes resulting from conformational alterations of analyte-responsive MIP nanogels were used for the first time for plasmonic sensing.72,73 The shrinking and swelling of the nanoMIPs coupled to the tested fibers after binding to the target protein led to a blueshift of the plasmonic minimum (see Figure 9B). Human serum transferrin (HTR) was used as a model protein. The soft nanoMIPs composition was optimized to ensure polymer mechanical rigidity by using high cross-linking density (80% mol/mol of cross-linker). Ultrasensitive detection of HTR (LODs of few femtomolar) was achieved using both plastic optical fiber (POF)72 and silica light-diffusing fiber (LDF)73 plasmonic platforms functionalized with the soft nanogels. Although the sensing systems were successfully applied for detection of HTR in (106 times) diluted serum mimic and human serum,72 more applied work of developed plasmonic devices operating in complex biofluids is needed. Still, these works demonstrated that soft nanoMIPs can be promising receptors for improved protein analysis by active plasmonics72,73,98 or MALDI-TOF-MS99 and can bring in new opportunities to the MIP biosensing field.
The scientific community is currently making efforts to come up with new synthetic procedures to prepare nanocomposites with efficient Raman enhancement.100 Thus, the combination of the selectivity of MIP materials with the large plasmonic enhancement of Au NPs can be very attractive for the development of new MIP-based SERS sensors.101 In this context, Zhang et al.,83 in 2018, developed a Raman imaging tool for monitoring cancer biomarkers in live cells based on biocompatible Au NPs coated with a thermal-responsive imprinted hydrogel as SERS nanoprobes. The nanocomposite was used for intracellular Raman visualization of epidermal growth factor receptor (EGFR) that is overexpressed in MCF-7 breast cancer cells. For obtaining the SERS nanotags, reversible addition–fragmentation chain transfer (RAFT) polymerization was used for deposition of a homogeneous MIP self-assembled monolayer (SAM) of thiolated poly(N-isopropylacrylamide) on the surface of gold nanorods as inorganic cores (see Figure 10). After extraction of template protein from the imprinted layer, the responsiveness of poly(NIPAAm) to the external temperature was used for capture (at 37 °C) and release (at 0 °C) of the target biomarker from the imprinting cavities due to the changes in the hydrogel structure, thereby altering the Raman signals enhance by the gold nanorods (see Figure 10). Ultimately, this pioneer work showed that intelligent SERS-active imprinted nanogels can be very promising for label-free cell imaging in the clinical field for disease diagnosis.
Figure 10.
Schematic representation of the Raman imaging of epidermal growth factor receptor (EGFR) biomarker within live cells using SERS nanotags, consisting of gold nanorods coated with protein-imprinted poly(N-isopropylacrylamide) layers, whose responsive behavior is triggered by alteration of external temperature (also allowing nanoprobe recycling). Reprinted with permission from ref (83). Copyright 2018 John Wiley and Sons.
Electrochemical Sensors Based on Hydrogel Imprinted NPs
Over recent years, the field of electrochemical biosensing faced tremendous growth due to the unique features of electrochemical methods, such as high sensitivity and reproducibility, fast response time, and cost-effective detection.102,103 Further, the advances in microelectronics and microfabrication allowed the portability of sensing devices for POC testing and/or in situ monitoring of a wide range of analytes.104,105 Thus, it is not surprising the large number of recent reviews highlighting relevant applications of electrochemical sensing for early disease diagnosis,102,105,106 drug monitoring,107 food quality and safety,13 environmental analysis,12 illicit drugs detection,103 among others. Moreover, MIP biomimetic materials have been incorporated into detection devices for electrochemical sensing through different methods of analysis (amperometry, potentiometry, and impedance).5,6,12,13,108
Amperometric biosensors can be divided into two main groups depending on the nature of the template: electroactive109 or nonelectroactive.110 MIP assays for detection of nonelectroactive biomolecules (peptides, proteins, etc.) are based on indirect electrochemical measurements performed in the presence of an external biocompatible redox probe (ferrocyanide/ferricyanide redox couple, etc.),110 using cyclic voltammetry, differential pulse voltammetry, etc. The quantification is based on the so-called gate effect,111 meaning that the binding of target analyte to MIP cavities alters the diffusional behavior of the probe through the receptor film. In order to eliminate the need of secondary diffusional process, an innovative approach emerged in 2014 by Udomsap et al.112 with the introduction of the electrochemical MIP (e-MIP). The e-MIP was prepared by adding an electroactive functional monomer (vinylferrocene, VFc) to the polymerization mixture, making the cross-linked MIP suitable for dual function of recognition unit and redox reporting system. As proof of concept, a polycyclic aromatic hydrocarbon (PAH) was used as the model template whose specific interaction with MIP particles tagged with VFc probes caused detectable changes in the ferrocene redox signals. Overall, the e-MIP microparticles (of size from 1.5 to 2.4 μm) were shown to be versatile receptors for direct (one-step) amperometric quantification of both small molecules112,113 and macromolecules.
Triggered by the dual properties of e-MIP particles, but going down on size, Mazzotta et al.45 synthesized for the first time nanosized electroactive MIPs (e-nanoMIPs) by adding different amounts of two ferrocene-derivative monomers (see Figure 11A), vinylferrocene (VFc) and ferrocenylmethyl methacrylate (FcMMA), to the polymerization medium. After CV characterization to evaluate the monomers electroactivity and reversibility, the FMMA monomer (3%) was selected for the e-nanoMIPs preparation targeting vancomycin. The e-nanoMIPs were then deposited by self-assembly on a Nafion membrane-coated glassy carbon electrode. The electrochemical detection was based on the interaction of vancomycin with the ferrocene moiety that incrementally hindered the CV electron transfer process with the increasing analyte concentration (see Figure 11A). Although detection of vancomycin in biofluids is missing in their work to test the applicability of the electrochemical sensor in clinical context, the conceptual use of MIP NPs incorporating redox traces for direct amperometric sensing of large molecules was fully demonstrated. In another work, the same approach was used to develop an amperometric sensor for insulin detection.79 Computational modeling tools were first used to identify surface peptides (epitopes) as templates for imprinting and to design the multicomponent polymeric mixture. NanoMIPs having different chemical composition were synthesized, and the experimental conditions for covalent attachment to the screen-printed electrode (SPE) surface were optimized to enhance the sensor response. Good analytical features were found for the electrochemical chips, incorporating best performing MIP NPs, such as high reproducibility and sensitivity (LOD of 21 fM). Importantly, the sensor response to selected interferents (hemoglobin, human serum albumin, and human proinsulin C-peptide) was relatively low (<22%). The amperometric sensor was able to quantify insulin at clinically relevant levels in spiked human plasma samples (LOD of 81 fM), confirming its potential for POC monitoring of insulin in patients. Moreover, long-term stability studies showed a sensor response decrease (for 500 pM of insulin) of 35% over 168 days, revealing fair storage stability (at 4 °C) and robustness.
Figure 11.

(A) Schematic representations of electroactive functional monomers ferrocenylmethyl methacrylate (FcMMA) and vinylferrocene (VFc); CV response recorded by the e-nanoMIPs modified electrode to increasing vancomycin concentration. Adapted with permission from ref (45). Copyright 2016 Elsevier B.V. (B) Generic schematic representation of the electroactive MIP NPs (e-nanoMIPs) combining selective analyte recognition and acting as redox reporting system due to changes of the polymer conformation after target analyte binding. Adapted with permission from ref (80). Copyright 2020 Springer Nature.
After these pioneer works highlighting the technological advances of amperometric sensors employing e-nanoMIPs, efforts were made by the research team headed by Professor Sergey Piletsky at the University of Leicester (UK) toward the production of generic amperometric devices not only for POC clinical diagnosis but also for in situ forensic, environmental, and food monitoring applications, by targeting both small molecular targets and also proteins in biological fluids (spiked plasma).80 The electroactive nanoreceptors were covalently immobilized at the surface of low cost and disposable screen-printed electrodes (SPEs), enabling the mass production of user-friendly electrochemical devices. Relative to the role of the hydrophilic nanogels in the bioanalytical assay, the analyte binding event induces changes in the hydrogel conformation that alters the exposed ferrocene probes attached to the MIP surface, thus favoring (or inhibiting) the electron transfer process (see Figure 11B) that can be easily monitored by common electrochemical techniques. The fabricated disposable chips had a shelf life of at least 6 weeks (at 25 ± 2 °C and relative humidity of 60 ± 5%).
One possible limitation of this technology is the relatively small faradaic currents arising from the self-reporting e-nanoMIPs. However, the increase of molar content (to 15–25%) of ferrocene-monomer (FcMMA)114 in polymer composition can enhance detection sensitivity. From a general perspective, the redox signals arising from electrochemical nanoMIPs can be further improved by modifying the bare electrode surface with electrocatalytic nanomaterials (or nanocomposites), such as metal NPs, graphene, carbon nanotubes, QDs, etc.
Potentiometric sensors can be a very attractive analytical tool due to their fast response, simple operation, portability, low cost, and wide working dynamic range.115 Although works combining electrochemical sensing and MIP NPs as artificial receptors for potentiometric detection of small molecules43,116 can be found in the literature, as far as we know, no potentiometric method incorporating MIP NPs for screening large molecules were reported so far. This is probably due to issues related to proteins electrical charge and structure flexibility. Although they have a net charge whose magnitude depends on its isoelectric point and the pH of the coupling buffer, proteins possess multiple charge locations that change according to their conformation,117 possibly affecting the stability of potentiometric measurements and/or requiring exhaustive optimization of experimental conditions to observe changes in the MIP surface potential.
Similarly to potentiometric sensors, impedimetric/capacitive sensors employing MIP NPs are currently scarce.81,118 Still, Canfarotta et al.81 showed that these sensing systems can be very useful for label-free detection of low levels of analyte. In their work, capacitive measurements allowed the sensitive detection (LODs of 1.0 × 10–14 M) of two molecules having very different molecular weights, tetrahydrocannabinol (THC) and trypsin. To attach the artificial receptors to the electrode surface, a poly(tyramine) film was first deposited on the electrode surface by electropolymerization followed by covalent coupling using glutaraldehyde (GA) as a linker. Although the electrochemical sensor was shown to be suitable for quantification of physiologically levels of target analytes, studies of the sensor operating in close-to-real conditions are missing.
The fields of impedimetric and potentiometric sensors integrating nanoMIPs for sensing large molecules currently present a wealth of opportunities to explore from several points of view, such as device setup and operation, MIP NPs synthesis procedures and strategies for their immobilization, type of biomolecule to detect, among others.
Other Methods for Protein Detection
Thermal detection methods recently emerged in the literature as a reliable alternative to optical and electrochemical sensors due to their simple and low cost operation, fast measurement time, and possibility of real-time monitoring.
In 2018, Canfarotta et al.44 reported for the first time the use of nanoMIPs combined with thermal detection for the bioanalysis of peptides and proteins. The device setup was very simple since it only required a heat-source and two thermocouples (see Figure 12A). One of these thermocouples was previously functionalized with MIP NPs by means of dip-coating and measures the temperature in the flow cell when the synthetic receptors are exposed to running buffer and analyte solutions. The binding of target biomolecule to the MIP receptor layer at the thermocouple inhibits the heat-flow from the sensor to the aqueous phase, thereby decreasing the measured temperature. The overall detection scheme was demonstrated for several analytes with different sizes (biotin, vancomycin, an epitope of epidermal growth factor receptor, and trypsin). The prepared thermal platforms allowed the quantification of target (bio)molecules at physiologically relevant levels (LODs of ∼3–5 nM). With regard to selectivity, a higher thermal response was obtained for target analytes relative to structural analogues or coexisting interferents in the sample matrix (selectivity factors within 1.7–4.5). To test the applicability in the clinical diagnosis context, the MIP-based thermal sensor was successfully applied for epidermal growth factor receptor (EGFR) detection in saliva samples (1:1 diluted in PBS).
Figure 12.
(A) Schematic representation of the thermocouple functionalized with synthetic receptors (T2) by means of dip-coating. TCU represents the temperature control unit of the heat sink (T1; kept constant at 37.00 ± 0.02 °C) and the temperature monitored in the flow cell (T2). When buffer solution circulates in the flow cell, the heat can freely pass through the thermocouple (left), whereas the binding of analyte to MIP NPs blocks the heat-flow in the flow cell, decreasing the measured temperature (right). Adapted with permission from ref (44). Copyright 2018 Royal Society of Chemistry. (B) Schematic representation of the covalent attachment of the nanoMIPs to the SPE surface via electrografting of 4-aminobenzoic acid (4-ABA) followed by EDC/NHS activation of carboxylate groups for amine coupling via reactive esters. Operating principle, based on the increase of thermal resistance after target biomarker binding to the synthetic receptors, and the obtained dose–response curve for troponin I are also shown in the figure. Adapted from ref (24). Copyright 2021 American Chemical Society.
A crucial advance in the biomedical field is to move from single to multiplex detection of biomarkers, significantly increasing diagnostic precision and trueness.119 To further demonstrate the great potential of thermal detection in the clinical setting, the same research group developed a multiplex device for simultaneous thermal analysis of two cardiac biomarkers, the heart-fatty acid binding protein (H-FABP) and the ST2 (suppression of tumorigenicity 2).120 MIP NPs specific for each biomarker were prepared and then dip-cotated onto the surface of thermocouples. Flow cells of different multiplex design incorporating the functionalized thermocouples and an internal control were developed. After optimization, the multiplex sensing platform allowed the detection of both biomarkers at physiologically relevant levels (LODs of few ng mL–1). Ultimately, the thermal device was successfully applied for simultaneous quantification of H-FABP and ST2 in spiked fetal bovine serum (FBS) samples, highlighting the thermal analysis as a promising multimarker detection approach for precision medicine.
After these pioneer studies, researchers rapidly found new routes for improvement of this emerging technology by increasing its commercial potential and finding new clinical applications. In this context, McClements et al.24 recently introduced innovative substrates for thermal analysis, the screen-printed electrodes (SPEs), and new strategies for effective nanoMIPs immobilization on sensing platforms. The dip-coating method, although experimentally simple, produces irregular MIP layers and suffers from lack of reproducibility. Thus, the authors provided a systematic study on how the immobilization of artificial receptors influences the performance of the thermal assay and explored other strategies for surface functionalization, such as the drop casting and the covalent attachment of the nanoMIPs onto the chip surface. They concluded that the covalent attachment of MIP NPs to the surface of SPEs enhanced the thermal assay reproducibility while benefiting from sensor mass production for widespread applications, including the POC disease diagnosis.24 The optimized thermal detection devices were used for detection of the biomarker cardiac troponin I (cTnI). NanoMIPs for specific recognition of cTnI were covalently attached to a graphite SPE through electrochemical grafting of a diazonium salt and EDC/NHS coupling reaction (see Figure 12B). Then, the sensing platforms were exposed to buffered solutions spiked with increasing amounts of cTnI (0.1–2.0 ng L–1). The binding of the target biomarker to synthetic receptors increased the measured thermal resistance allowing one to build the dose–response curve for troponin I (see Figure 12B). The obtained LOD (0.46 ± 0.07 ng L–1) was inferior to most of the methods reported for cTnI detection. In addition, the prepared nanoMIPs showed good selectivity, being able to distinguish cTnI from BSA, glucose, and even its structural analogue cardiac troponin T (cTnT). Still, studies focusing on biomarker detection in complex biofluids, such as serum, are missing in their work.
Meanwhile, and to face the world pandemics, the optimized thermal detection assay was used for fast, robust, and sensitive screening of SARS-CoV-2 in POC.25 In order to reduce reagent costs, a small SARS-CoV-2 fragment (∼10 amino acids) was used as the epitope. The epitope was selected by in silico analysis, meaning that, if needed, a new epitope can be easily identified for a new virus variant and the corresponding MIP NPs prepared in a short period. Interestingly, the prepared MIP receptors showed sensing capabilities similar to biological antibodies for the alpha variant, although much less strict storage conditions were needed for the plastic antibodies. Furthermore, the response of nanoMIPs to coexisting interferents in biofluids (open reading frame 8, interleukin-6, and human serum albumin) was significantly reduced when compared to the target virus spike protein. In addition, the thermal assay displayed high detection sensitivity with LODs (of ∼9.9 fg mL–1 and ∼6.1 fg mL–1 for alpha and delta variants, respectively) remarkably lower than other methods for SARS-CoV-2 detection. Clinical samples consisting of nose and throat swabs from patients symptomatic for COVID-19 were successfully analyzed with the overall signal variation being much larger for COVID-positive patient samples. The portable thermal device was miniaturized to reduce sample volume (to only 100 μL), and samples were analyzed with minimal sample pretreatment. The total assay time was only ∼15 min.
Nanosized hydrogel MIP particles were also employed for detection of another type of pathogenic organisms. Recently, Kushwaha et al.50 developed an electrochemical quartz crystal microbalance (EQCM) sensor integrating nanoMIPs for sensing of Mycobacterium leprae bacteria through its protein’s epitope (linear amino acid sequence LP-15).
For preparation of the sensing platforms, nanoMIPs were first prepared by radical polymerization and then incorporated on the gold-coated quartz crystal electrode by electrochemical polymerization of 4-aminothiophenol (4-ATP) added to the prepolymerization mixture (see Figure 13A). The template was removed from the MIP matrix by repetitive exposition of the EQCM electrode to PBS. The extraction procedure was monitored by QCM measurements and confirmed by fluorescence assay. Then, electrochemical and QCM data collected during rebind studies revealed the specific recognition of target analyte (LP-15 epitope) by the imprinted sensor. An imprinting factor (IF) of 8.28 was estimated for an analyte concentration of 140 nM. Moreover, a linear relationship between the frequency shift and the analyte concentration in the range from 10 to 140 nM with a LOD of 0.161 nM was obtained.
Figure 13.
(A) Schematic representation of the MIP-EQCM sensor fabrication process for detection of Mycobacterium leprae, consisting of MIP NPs polymerization using multiple functional monomers, their incorporation on the piezoelectric transducer by electropolymerization of conducting poly(4-aminothiophenol), followed by template extraction from the polymeric matrix for formation of binding cavities. (B) EQCM sensor response to real blood samples from healthy human donors and from patients affected by Hansen′s diseases (leprosy), like multibacillary Hansen’s disease (MBHD), pauci-bacillary Hansen′s disease (PBHD), and lepromatous leprosy Hansen′s disease (LLHD). Adapted with permission from ref (50). Copyright 2019 Elsevier B.V.
In this work, selectivity studies were very well designed by comparing the sensor response for target epitope relative to (i) mismatched amino acids sequences (LP-13, KC-14, VC-13, KW-12, and GW-10) and (ii) proteins present in blood plasma (globulin and albumin). The MIP sensor showed a very good selectivity for the target analyte sequence (LP-15) since no significant response was observed for selected interferents. For example, the sensor response to the target LP-15 sequence was more than four times superior to the response to the LP-13 sequence (with only 2 amino acids less than LP-15). For validation, the piezoelectrogravimmetric sensor was tested against real blood samples from Mycobacterium leprae infected patients (see Figure 13B). It is important to notice that the MIP-based EQCM sensor provided a reliable response in real (diluted) complex samples, thus being suitable for the early disease diagnosis of leprosy bacterial infection in the population. Furthermore, the MIP sensor demonstrated high stability over time, allowing multiple cycles of binding-regeneration even 30 days after fabrication, without affecting sensor sensitivity and selectivity.
Conclusions and Future Perspectives
The advances in nanotechnology opened new perspectives for applications of MIP (nano)materials in chemical sensing. This review focused on an important class of analyte-responsive materials, the hydrogels, which are very appealing for preparation of innovative imprinted (nano)materials for sensing macromolecules, such as peptides and proteins. MIP hydrogel nanospheres can be easily produced by chemical synthesis. To face some challenges due to the large size and complexity of protein templates, MIP recognition mediated by epitopes is currently a reliable strategy to minimize the limitations of whole-protein imprinting.
Several assay formats incorporating nanoMIPs showed high potential for transition from research detection prototypes to viable commercial products. That is the case of MINA that takes advantage of the high stability, cost-effectiveness, easy handling, and storage of imprinted nanomaterials to become a reliable alternative to commercial ELISA kits. Besides, the technological advances introduced in standard ELISA assays with the use of magnetic materials, combined with fluorescence readout, allowed one to achieve ultrasensitive detection levels. Moreover, other opportunities for MIP companies can arise from (bio)applications in the fields of biomedical diagnosis and food control with the production and commercialization of (i) nanoMIPs for integration into plasmonic and thermal sensors for simple, sensitive, and reproducible analysis of proteins in real-time or (ii) fluorescence and SERS-active nanoMIPs for bioimaging of cells and organelles. In addition, other optical sensing technologies compatible with MI technology, such as the lateral flow immunoassays (LFIAs),121 remains relatively unexplored. Thus, imprinted nanogels can be coupled to AuNPs or immobilized over the test line of strip sensors for rapid disease diagnosis, performed at home and analyzed by the naked eye.
Electrochemical devices based on MIP NPs currently have the potential to generate a huge impact in the sensor market. In particular, the introduction of self-reporting electrochemical nanoMIPs (e-nanoMIPs), brought in innovative features to amperometric sensors, enabling straightforward and label-free protein bioanalysis. Furthermore, the type, dimension, and design of detection substrates can have a tremendous impact in the overall sensor applicability. A current trend in the biosensing field is the development of portable sensors (using screen-printed electrodes, for example) integrating nanoMIPs for POC disease diagnosis by employing not only electrochemical techniques but also thermal detection, accelerating the developments of this emergent technology with high commercialization potential.
A critical issue in (bio)sensors development is its applicability in real scenario conditions (human biofluids, food matrices, etc.) which can be rather complicated due to nonspecific binding (NSB). To address this issue, nanoMIPs composition can be optimized to enhance the affinity for target biomolecule against coexisting interferents in complex samples. Other strategies that remain unexplored, such as the use of novel monomers having antifouling properties (zwitterionic 2-methacryloyloxyethyl phosphorylcholine,122 poly(ethylene glycol) methyl ether methacrylate,123 etc.) and new polymerization systems, can potentially reduce NSB to nanoMIPs. Besides, the careful optimization of experimental conditions and well-designed selectivity tests are crucial to improve the sensor performance in real samples. Some inspiring works managed to solve these issues, reporting the detection of peptides, proteins, and pathogens in biological fluids (urine, plasma, serum, and blood) without the need of extensive sample treatment or preconcentration steps. Still, more applied work addressing sensors operating directly in complex samples is needed to encourage more investments on nanoMIPs for widespread biosensing applications. If necessary, an appealing strategy to further improve the analytical performance of MIPs in harsh environments is to combine them with aptamers to form aptaMIPs.124 These cross-linked hybrid materials already showed higher binding affinity toward target proteins in comparison to traditional MIP NPs.125
Another demand of the (bio)sensing field is to expand to the simultaneous detection of several analytes in the same run aiming to increase diagnostic precision and accuracy. This technological advance was recently introduced with the development of a thermal device enabling the simultaneous analysis of two biomarkers in a single test.120 Although MIP hydrogel nanospheres specific for each biomarker were independently prepared to perform the simultaneous recognition, the production of binding cavities of multiple templates in a single MIP NP (multiple-template imprinting)126 can be a promising approach for multiplex protein detection in the near future. Alternatively, MIP-aptamer dual recognition systems offers flexibility of design for multianalyte detection by incorporation of chosen aptamer sequences into the MIP structure.
This review aims to demonstrate that hydrogel MIP NPs can have a tremendous impact in the Chemical Sensing field, particularly in the clinical setting with the development of new mobile technologies for POC early disease diagnosis or incorporated into wearable devices for real-time health monitoring, opening the route for a new generation of self-testing healthcare devices.
Acknowledgments
This research had the financial support of FCT (Fundação para a Ciência e Tecnologia) and cofinanced by the European Union (FEDER funds) under the Partnership Agreement PT2020, Research Grant UIDB/00081/2020 (CIQUP), and LA/P/0056/2020 (IMS). J.A.R. (ref SFRH/BPD/105395/2014) acknowledges FCT under the QREN e POPH e Advanced Training, subsidized by European Union and national MEC funds. The authors acknowledge the research project MyTag (ref PTDC/EEI-EEE/4832/2021), funded by FCT, for financial support.
Glossary
List of Acronyms
- 4-ABA
4-aminobenzoic acid
- 4-ATP
4-aminothiophenol
- APS
ammonium persulfate
- APTES
(3-aminopropyl)triethoxysilane
- APTMS
3-aminopropyltrimethyloxysilane
- B2M
β2 microglobulin
- BaP
benzo[a]pyrene
- BLG
β-lactoglobulin
- BSA
bovine serum albumin
- C4-HSL
C4-homoserine lactone
- CDs
carbon dots
- CIP
cleaning in place
- cTnI
cardiac troponin I
- cTnT
cardiac troponin T
- CV
cyclic voltammetry
- Cyt c
cytochrome c
- DEA
des-ethyl atrazine
- DEDIA
des-ethyl-des-isopropyl atrazine
- DIA
des-isopropyl atrazine
- DMSO
dimethyl sulfoxide
- DPV
differential pulse voltammetry
- EDC
1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide
- EGFR
epidermal growth factor receptor
- ELISA
enzyme-linked immunosorbent assay
- e-MIP
electrochemical MIP
- EQCM
electrochemical QCM
- FBS
fetal bovine serum
- FcMMA
ferrocenylmethyl methacrylate
- FITC
fluorescein isothiocyanate
- GA
glutaraldehyde
- H-FABP
hear-fatty acid binding protein
- HRP
horseradish peroxidase
- HSA
human serum albumin
- HTR
human serum transferrin
- IF
imprinting factor
- KD
dissociation constant
- LCST
lower critical solution temperature
- LDF
light-diffusing fiber
- LLHD
lepromatous leprosy Hansen’s disease
- LOD
limit of detection
- LPG
long period grating
- LTE
leukotriene
- MALDI-TOF-MS
matrix-assisted laser desorption ionization time-of-flight mass spectrometry
- MBHD
multibacillary Hansen’s disease
- Mel
melittin
- MI
molecular imprinting
- MINA
molecularly imprinted polymer nanoparticle-based assay
- MIP NPs, nanoMIPs
molecularly imprinted polymer nanoparticles
- MIP
molecularly imprinted polymer
- MS
mass spectrometry
- NHS
N-hydroxysuccinimide
- NIP
nonimprinted polymer
- NIPAAm
N-isopropylacrylamide
- NPs
nanoparticles
- NSB
nonspecific binding
- PAB
p-aminobenzamidine
- PAH
polycyclic aromatic hydrocarbon
- PBHD
pauci-bacillary Hansen’s disease
- pI
isoelectric point
- PNIPAAm
poly(N-isopropylacrylamide)
- POC
point-of-care
- POF
plastic optical fiber
- PRO
propazine
- QCM
quartz crystal microbalance
- QDs
quantum dots
- RAFT
reversible addition–fragmentation chain transfer
- RBD
receptor binding domain
- RI
refractive index
- SAM
self-assembled monolayer
- SDS
sodium dodecyl sulfate
- SEM
scanning electron microscopy
- SERS
surface-enhanced Raman spectroscopy
- SIM
simazine
- SPE
screen-printed electrode
- SPR
surface plasmon resonance
- ST2
suppression of tumorigenicity 2
- SWV
square-wave voltammetry
- TEMED
N,N,N′,N′-tetramethylethylenediamine
- THC
tetrahydrocannabinol
- TMB
tetramethylbenzidine
- VEGF
vascular endothelial growth factor
- VFc
vinylferrocene
Glossary
Vocabulary
- molecular imprinting
technique used to create artificial recognition sites in a polymer matrix with high specificity for a particular analyte by polymerizing functional monomers around a template (bio)molecule
- plastic antibodies
synthetic biomimetic materials having molecular recognition abilities for a given analyte similar to natural receptors
- hydrogels
three-dimensional, cross-linked networks of hydrophilic polymers that can absorb and retain large amounts of water, exhibiting significant swelling behavior in response to external stimulus
- nanoMIPs
common designation for a class of nanoscale imprinted materials, the molecularly imprinted polymer nanoparticles (MIP NPs)
- biosensor
self-contained receptor-transducer device that integrates a biological receptor to provide selective and quantitative or semiquantitative analytical information, usually an analyte concentration
The authors declare no competing financial interest.
References
- Wulff G. Fourty years of molecular imprinting in synthetic polymers: origin, features and perspectives. Microchimica Acta 2013, 180 (15), 1359–1370. 10.1007/s00604-013-0992-9. [DOI] [Google Scholar]
- Norrlöw O.; Glad M.; Mosbach K. Acrylic polymer preparations containing recognition sites obtained by imprinting with substrates. Journal of Chromatography A 1984, 299, 29–41. 10.1016/S0021-9673(01)97819-7. [DOI] [Google Scholar]
- Zhou T.; Jørgensen L.; Mattebjerg M. A.; Chronakis I. S.; Ye L. Molecularly imprinted polymer beads for nicotine recognition prepared by RAFT precipitation polymerization: a step forward towards multi-functionalities. RSC Adv. 2014, 4 (57), 30292–30299. 10.1039/C4RA04741A. [DOI] [Google Scholar]
- a Concu R.; Ornelas M.; Azenha M. Molecularly Imprinted Sol-Gel Materials for Medical Applications. Current Topics in Medicinal Chemistry 2015, 15 (3), 199–222. 10.2174/1568026614666141229112246. [DOI] [PubMed] [Google Scholar]; b Lofgreen J. E.; Ozin G. A. Controlling morphology and porosity to improve performance of molecularly imprinted sol–gel silica. Chem. Soc. Rev. 2014, 43 (3), 911–933. 10.1039/C3CS60276A. [DOI] [PubMed] [Google Scholar]
- Mazzotta E.; Di Giulio T.; Malitesta C. Electrochemical sensing of macromolecules based on molecularly imprinted polymers: challenges, successful strategies, and opportunities. Anal. Bioanal. Chem. 2022, 414 (18), 5165–5200. 10.1007/s00216-022-03981-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira Gonçalves L. Electropolymerized molecularly imprinted polymers: perceptions based on recent literature for soon-to-be world-class scientists. Current Opinion in Electrochemistry 2021, 25, 100640 10.1016/j.coelec.2020.09.007. [DOI] [Google Scholar]
- Dabrowski M.; Lach P.; Cieplak M.; Kutner W. Nanostructured molecularly imprinted polymers for protein chemosensing. Biosens. Bioelectron. 2018, 102, 17–26. 10.1016/j.bios.2017.10.045. [DOI] [PubMed] [Google Scholar]
- Beloglazova N.; Lenain P.; Tessier M.; Goryacheva I.; Hens Z.; De Saeger S. Bioimprinting for multiplex luminescent detection of deoxynivalenol and zearalenone. Talanta 2019, 192, 169–174. 10.1016/j.talanta.2018.09.042. [DOI] [PubMed] [Google Scholar]
- BelBruno J. J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119 (1), 94–119. 10.1021/acs.chemrev.8b00171. [DOI] [PubMed] [Google Scholar]
- Guerreiro A. R.; Chianella I.; Piletska E.; Whitcombe M. J.; Piletsky S. A. Selection of imprinted nanoparticles by affinity chromatography. Biosens. Bioelectron. 2009, 24 (8), 2740–2743. 10.1016/j.bios.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Mostafa A. M.; Barton S. J.; Wren S. P.; Barker J. Review on molecularly imprinted polymers with a focus on their application to the analysis of protein biomarkers. TrAC Trends in Analytical Chemistry 2021, 144, 116431 10.1016/j.trac.2021.116431. [DOI] [Google Scholar]
- Rebelo P.; Costa-Rama E.; Seguro I.; Pacheco J. G.; Nouws H. P. A.; Cordeiro M. N. D. S.; Delerue-Matos C. Molecularly imprinted polymer-based electrochemical sensors for environmental analysis. Biosens. Bioelectron. 2021, 172, 112719 10.1016/j.bios.2020.112719. [DOI] [PubMed] [Google Scholar]
- Cao Y.; Feng T.; Xu J.; Xue C. Recent advances of molecularly imprinted polymer-based sensors in the detection of food safety hazard factors. Biosens. Bioelectron. 2019, 141, 111447 10.1016/j.bios.2019.111447. [DOI] [PubMed] [Google Scholar]
- Chiappini A.; Pasquardini L.; Bossi A. M. Molecular Imprinted Polymers Coupled to Photonic Structures in Biosensors: The State of Art. Sensors 2020, 20, 5069. 10.3390/s20185069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Wulff G. Enzyme-like Catalysis by Molecularly Imprinted Polymers. Chem. Rev. 2002, 102 (1), 1–28. 10.1021/cr980039a. [DOI] [PubMed] [Google Scholar]; b Wulff G.Molecular Recognition in Polymers Prepared by Imprinting with Templates. In Polymeric Reagents and Catalysts; ACS Symposium Series; American Chemical Society, 1986; Vol. 308, pp 186–230. [Google Scholar]
- a Sellergren B.; Ekberg B.; Mosbach K. Molecular imprinting of amino acid derivatives in macroporous polymers: Demonstration of substrate- and enantio-selectivity by chromatographic resolution of racemic mixtures of amino acid derivatives. Journal of Chromatography A 1985, 347, 1–10. 10.1016/S0021-9673(01)95464-0. [DOI] [Google Scholar]; b Sellergren B.; Lepistoe M.; Mosbach K. Highly enantioselective and substrate-selective polymers obtained by molecular imprinting utilizing noncovalent interactions. NMR and chromatographic studies on the nature of recognition. J. Am. Chem. Soc. 1988, 110 (17), 5853–5860. 10.1021/ja00225a041. [DOI] [Google Scholar]; c Vlatakis G.; Andersson L. I.; Müller R.; Mosbach K. Drug assay using antibody mimics made by molecular imprinting. Nature 1993, 361 (6413), 645–647. 10.1038/361645a0. [DOI] [PubMed] [Google Scholar]
- Culver H. R.; Peppas N. A. Protein-Imprinted Polymers: The Shape of Things to Come?. Chem. Mater. 2017, 29 (14), 5753–5761. 10.1021/acs.chemmater.7b01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaqub S.; Latif U.; Dickert F. L. Plastic antibodies as chemical sensor material for atrazine detection. Sens. Actuators, B 2011, 160 (1), 227–233. 10.1016/j.snb.2011.07.039. [DOI] [Google Scholar]
- Cenci L.; Bertolla M.; Anesi A.; Ambrosi E.; Guella G.; Bossi A. M. Micro- versus nano-sized molecularly imprinted polymers in MALDI-TOF mass spectrometry analysis of peptides. Anal. Bioanal. Chem. 2017, 409 (26), 6253–6261. 10.1007/s00216-017-0569-2. [DOI] [PubMed] [Google Scholar]
- Wackerlig J.; Schirhagl R. Applications of Molecularly Imprinted Polymer Nanoparticles and Their Advances toward Industrial Use: A Review. Anal. Chem. 2016, 88 (1), 250–261. 10.1021/acs.analchem.5b03804. [DOI] [PubMed] [Google Scholar]
- a Zhang H. Molecularly Imprinted Nanoparticles for Biomedical Applications. Adv. Mater. 2020, 32 (3), 1806328 10.1002/adma.201806328. [DOI] [PubMed] [Google Scholar]; b Wackerlig J.; Lieberzeit P. A. Molecularly imprinted polymer nanoparticles in chemical sensing – Synthesis, characterisation and application. Sens. Actuators, B 2015, 207, 144–157. 10.1016/j.snb.2014.09.094. [DOI] [Google Scholar]
- Chianella I.; Guerreiro A.; Moczko E.; Caygill J. S.; Piletska E. V.; De Vargas Sansalvador I. M. P.; Whitcombe M. J.; Piletsky S. A. Direct Replacement of Antibodies with Molecularly Imprinted Polymer Nanoparticles in ELISA—Development of a Novel Assay for Vancomycin. Anal. Chem. 2013, 85 (17), 8462–8468. 10.1021/ac402102j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley J.; Shukor Y.; D’Aurelio R.; Trinh L.; Rodgers T. L.; Temblay J.; Pleasants M.; Tothill I. E. Synthesis of Molecularly Imprinted Polymer Nanoparticles for α-Casein Detection Using Surface Plasmon Resonance as a Milk Allergen Sensor. ACS Sensors 2018, 3 (2), 418–424. 10.1021/acssensors.7b00850. [DOI] [PubMed] [Google Scholar]
- McClements J.; Seumo Tchekwagep P. M.; Vilela Strapazon A. L.; Canfarotta F.; Thomson A.; Czulak J.; Johnson R. E.; Novakovic K.; Losada-Pérez P.; Zaman A.; et al. Immobilization of Molecularly Imprinted Polymer Nanoparticles onto Surfaces Using Different Strategies: Evaluating the Influence of the Functionalized Interface on the Performance of a Thermal Assay for the Detection of the Cardiac Biomarker Troponin I. ACS Appl. Mater. Interfaces 2021, 13 (24), 27868–27879. 10.1021/acsami.1c05566. [DOI] [PubMed] [Google Scholar]
- McClements J.; Bar L.; Singla P.; Canfarotta F.; Thomson A.; Czulak J.; Johnson R. E.; Crapnell R. D.; Banks C. E.; Payne B.; et al. Molecularly Imprinted Polymer Nanoparticles Enable Rapid, Reliable, and Robust Point-of-Care Thermal Detection of SARS-CoV-2. ACS Sensors 2022, 7 (4), 1122–1131. 10.1021/acssensors.2c00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Herrmann A.; Haag R.; Schedler U. Hydrogels and Their Role in Biosensing Applications. Adv. Healthcare Mater. 2021, 10 (11), 2100062 10.1002/adhm.202100062. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Pinelli F.; Magagnin L.; Rossi F. Progress in hydrogels for sensing applications: a review. Materials Today Chemistry 2020, 17, 100317 10.1016/j.mtchem.2020.100317. [DOI] [Google Scholar]; c Li J.; Mo L.; Lu C.-H.; Fu T.; Yang H.-H.; Tan W. Functional nucleic acid-based hydrogels for bioanalytical and biomedical applications. Chem. Soc. Rev. 2016, 45 (5), 1410–1431. 10.1039/C5CS00586H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Oya T.; Enoki T.; Grosberg A. Y.; Masamune S.; Sakiyama T.; Takeoka Y.; Tanaka K.; Wang G.; Yilmaz Y.; Feld M. S.; et al. Reversible Molecular Adsorption Based on Multiple-Point Interaction by Shrinkable Gels. Science 1999, 286 (5444), 1543–1545. 10.1126/science.286.5444.1543. [DOI] [PubMed] [Google Scholar]; b Kopeček J. Swell gels. Nature 2002, 417 (6887), 389–391. 10.1038/417388a. [DOI] [PubMed] [Google Scholar]; c Li Y.-C.; Zhang Y. S.; Akpek A.; Shin S. R.; Khademhosseini A. 4D bioprinting: the next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication 2017, 9 (1), 012001 10.1088/1758-5090/9/1/012001. [DOI] [PubMed] [Google Scholar]
- a Xu S.; Lu H.; Zheng X.; Chen L. Stimuli-responsive molecularly imprinted polymers: versatile functional materials. Journal of Materials Chemistry C 2013, 1 (29), 4406–4422. 10.1039/c3tc30496e. [DOI] [Google Scholar]; b Chen Z.; Xu L.; Liang Y.; Zhao M. pH-Sensitive Water-Soluble Nanospheric Imprinted Hydrogels Prepared as Horseradish Peroxidase Mimetic Enzymes. Adv. Mater. 2010, 22 (13), 1488–1492. 10.1002/adma.200903122. [DOI] [PubMed] [Google Scholar]; c Wang C.; Javadi A.; Ghaffari M.; Gong S. A pH-sensitive molecularly imprinted nanospheres/hydrogel composite as a coating for implantable biosensors. Biomaterials 2010, 31 (18), 4944–4951. 10.1016/j.biomaterials.2010.02.073. [DOI] [PubMed] [Google Scholar]; d Kanekiyo Y.; Naganawa R.; Tao H. pH-Responsive Molecularly Imprinted Polymers. Angew. Chem., Int. Ed. 2003, 42 (26), 3014–3016. 10.1002/anie.200351381. [DOI] [PubMed] [Google Scholar]
- a Chen H.; Guo J.; Wang Y.; Dong W.; Zhao Y.; Sun L. Bio-Inspired Imprinting Materials for Biomedical Applications. Advanced Science 2022, 9 (28), 2202038 10.1002/advs.202202038. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Culver H. R.; Clegg J. R.; Peppas N. A. Analyte-Responsive Hydrogels: Intelligent Materials for Biosensing and Drug Delivery. Acc. Chem. Res. 2017, 50 (2), 170–178. 10.1021/acs.accounts.6b00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Halperin A.; Kröger M.; Winnik F. M. Poly(N-isopropylacrylamide) Phase Diagrams: Fifty Years of Research. Angew. Chem., Int. Ed. 2015, 54 (51), 15342–15367. 10.1002/anie.201506663. [DOI] [PubMed] [Google Scholar]; b Ahiabu A.; Serpe M. J. Rapidly Responding pH- and Temperature-Responsive Poly (N-Isopropylacrylamide)-Based Microgels and Assemblies. ACS Omega 2017, 2 (5), 1769–1777. 10.1021/acsomega.7b00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y.; Kodama T.; Okahata Y.; Shea K. J. Peptide Imprinted Polymer Nanoparticles: A Plastic Antibody. J. Am. Chem. Soc. 2008, 130 (46), 15242–15243. 10.1021/ja8062875. [DOI] [PubMed] [Google Scholar]
- a Dietl S.; Sobek H.; Mizaikoff B. Epitope-imprinted polymers for biomacromolecules: Recent strategies, future challenges and selected applications. TrAC Trends in Analytical Chemistry 2021, 143, 116414 10.1016/j.trac.2021.116414. [DOI] [Google Scholar]; b Ansari S.; Masoum S. Molecularly imprinted polymers for capturing and sensing proteins: Current progress and future implications. TrAC Trends in Analytical Chemistry 2019, 114, 29–47. 10.1016/j.trac.2019.02.008. [DOI] [Google Scholar]; c Dong C.; Shi H.; Han Y.; Yang Y.; Wang R.; Men J. Molecularly imprinted polymers by the surface imprinting technique. Eur. Polym. J. 2021, 145, 110231 10.1016/j.eurpolymj.2020.110231. [DOI] [Google Scholar]; d Wan L.; Chen Z.; Huang C.; Shen X. Core–shell molecularly imprinted particles. TrAC Trends in Analytical Chemistry 2017, 95, 110–121. 10.1016/j.trac.2017.08.010. [DOI] [Google Scholar]; e Niu M.; Pham-Huy C.; He H. Core-shell nanoparticles coated with molecularly imprinted polymers: a review. Microchimica Acta 2016, 183 (10), 2677–2695. 10.1007/s00604-016-1930-4. [DOI] [Google Scholar]
- Wang J.; Cormack P. A. G.; Sherrington D. C.; Khoshdel E. Synthesis and characterization of micrometer-sized molecularly imprinted spherical polymer particulates prepared via precipitation polymerization. Pure Appl. Chem. 2007, 79 (9), 1505–1519. 10.1351/pac200779091505. [DOI] [Google Scholar]
- a Ye L.; Cormack P. A. G.; Mosbach K. Molecularly imprinted monodisperse microspheres for competitive radioassay. Analytical Communications 1999, 36 (2), 35–38. 10.1039/a809014i. [DOI] [Google Scholar]; b Hunt C. E.; Pasetto P.; Ansell R. J.; Haupt K. A fluorescence polarisation molecular imprint sorbent assay for 2,4-D: a non-separation pseudo-immunoassay. Chem. Commun. 2006, (16), 1754–1756. 10.1039/b516194k. [DOI] [PubMed] [Google Scholar]; c Jyoti; Gonzato C.; Żołek T.; Maciejewska D.; Kutner A.; Merlier F.; Haupt K.; Sharma P. S.; Noworyta K. R.; Kutner W. Molecularly imprinted polymer nanoparticles-based electrochemical chemosensors for selective determination of cilostazol and its pharmacologically active primary metabolite in human plasma. Biosens. Bioelectron. 2021, 193, 113542 10.1016/j.bios.2021.113542. [DOI] [PubMed] [Google Scholar]
- a Cutivet A.; Schembri C.; Kovensky J.; Haupt K. Molecularly Imprinted Microgels as Enzyme Inhibitors. J. Am. Chem. Soc. 2009, 131 (41), 14699–14702. 10.1021/ja901600e. [DOI] [PubMed] [Google Scholar]; b Hu X.; Tong Z.; Lyon L. A. Control of Poly(N-isopropylacrylamide) Microgel Network Structure by Precipitation Polymerization near the Lower Critical Solution Temperature. Langmuir 2011, 27 (7), 4142–4148. 10.1021/la200114s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Yoshimatsu K.; Lesel B. K.; Yonamine Y.; Beierle J. M.; Hoshino Y.; Shea K. J. Temperature-Responsive “Catch and Release” of Proteins by using Multifunctional Polymer-Based Nanoparticles. Angew. Chem., Int. Ed. 2012, 51 (10), 2405–2408. 10.1002/anie.201107797. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Beierle J. M.; Yoshimatsu K.; Chou B.; Mathews M. A. A.; Lesel B. K.; Shea K. J. Polymer Nanoparticle Hydrogels with Autonomous Affinity Switching for the Protection of Proteins from Thermal Stress. Angew. Chem., Int. Ed. 2014, 53 (35), 9275–9279. 10.1002/anie.201404881. [DOI] [PubMed] [Google Scholar]
- Lee S.-H.; Hoshino Y.; Randall A.; Zeng Z.; Baldi P.; Doong R.-a.; Shea K. J. Engineered Synthetic Polymer Nanoparticles as IgG Affinity Ligands. J. Am. Chem. Soc. 2012, 134 (38), 15765–15772. 10.1021/ja303612d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonamine Y.; Yoshimatsu K.; Lee S.-H.; Hoshino Y.; Okahata Y.; Shea K. J. Polymer Nanoparticle–Protein Interface. Evaluation of the Contribution of Positively Charged Functional Groups to Protein Affinity. ACS Appl. Mater. Interfaces 2013, 5 (2), 374–379. 10.1021/am302404q. [DOI] [PubMed] [Google Scholar]
- Koide H.; Yoshimatsu K.; Hoshino Y.; Lee S.-H.; Okajima A.; Ariizumi S.; Narita Y.; Yonamine Y.; Weisman A. C.; Nishimura Y.; et al. A polymer nanoparticle with engineered affinity for a vascular endothelial growth factor (VEGF165). Nat. Chem. 2017, 9 (7), 715–722. 10.1038/nchem.2749. [DOI] [PubMed] [Google Scholar]
- Poma A.; Guerreiro A.; Whitcombe M. J.; Piletska E. V.; Turner A. P.; Piletsky S. A. Solid-Phase Synthesis of Molecularly Imprinted Polymer Nanoparticles with a Reusable Template - ″Plastic Antibodies″. Adv. Funct Mater. 2013, 23 (22), 2821–2827. 10.1002/adfm.201202397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosini S.; Beyazit S.; Haupt K.; Tse Sum Bui B. Solid-phase synthesis of molecularly imprinted nanoparticles for protein recognition. Chem. Commun. 2013, 49 (60), 6746–6748. 10.1039/c3cc41701h. [DOI] [PubMed] [Google Scholar]
- Alanazi K.; Garcia Cruz A.; Di Masi S.; Voorhaar A.; Ahmad O. S.; Cowen T.; Piletska E.; Langford N.; Coats T. J.; Sims M. R.; et al. Disposable paracetamol sensor based on electroactive molecularly imprinted polymer nanoparticles for plasma monitoring. Sens. Actuators, B 2021, 329, 129128 10.1016/j.snb.2020.129128. [DOI] [Google Scholar]
- a Smolinska-Kempisty K.; Ahmad O. S.; Guerreiro A.; Karim K.; Piletska E.; Piletsky S. New potentiometric sensor based on molecularly imprinted nanoparticles for cocaine detection. Biosens. Bioelectron. 2017, 96, 49–54. 10.1016/j.bios.2017.04.034. [DOI] [PubMed] [Google Scholar]; b Gutiérrez-Climente R.; Gómez-Caballero A.; Unceta N.; Aránzazu Goicolea M.; Barrio R. J. A new potentiometric sensor based on chiral imprinted nanoparticles for the discrimination of the enantiomers of the antidepressant citalopram. Electrochim. Acta 2016, 196, 496–504. 10.1016/j.electacta.2016.03.010. [DOI] [Google Scholar]
- Canfarotta F.; Czulak J.; Betlem K.; Sachdeva A.; Eersels K.; van Grinsven B.; Cleij T. J.; Peeters M. A novel thermal detection method based on molecularly imprinted nanoparticles as recognition elements. Nanoscale 2018, 10 (4), 2081–2089. 10.1039/C7NR07785H. [DOI] [PubMed] [Google Scholar]
- Mazzotta E.; Turco A.; Chianella I.; Guerreiro A.; Piletsky S. A.; Malitesta C. Solid-phase synthesis of electroactive nanoparticles of molecularly imprinted polymers. A novel platform for indirect electrochemical sensing applications. Sens. Actuators, B 2016, 229, 174–180. 10.1016/j.snb.2016.01.126. [DOI] [Google Scholar]
- Guerreiro A.; Poma A.; Karim K.; Moczko E.; Takarada J.; de Vargas-Sansalvador I. P.; Turner N.; Piletska E.; de Magalhães C. S.; Glazova N.; et al. Influence of surface-imprinted nanoparticles on trypsin activity. Adv. Healthc Mater. 2014, 3 (9), 1426–1429. 10.1002/adhm.201300634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poma A.; Guerreiro A.; Caygill S.; Moczko E.; Piletsky S. Automatic reactor for solid-phase synthesis of molecularly imprinted polymeric nanoparticles (MIP NPs) in water. RSC Adv. 2014, 4 (8), 4203–4206. 10.1039/C3RA46838K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczko E.; Guerreiro A.; Cáceres C.; Piletska E.; Sellergren B.; Piletsky S. A. Epitope approach in molecular imprinting of antibodies. Journal of Chromatography B 2019, 1124, 1–6. 10.1016/j.jchromb.2019.05.024. [DOI] [PubMed] [Google Scholar]
- Altintas Z.; Pocock J.; Thompson K.-A.; Tothill I. E. Comparative investigations for adenovirus recognition and quantification: Plastic or natural antibodies?. Biosens. Bioelectron. 2015, 74, 996–1004. 10.1016/j.bios.2015.07.076. [DOI] [PubMed] [Google Scholar]
- Kushwaha A.; Srivastava J.; Singh A. K.; Anand R.; Raghuwanshi R.; Rai T.; Singh M. Epitope imprinting of Mycobacterium leprae bacteria via molecularly imprinted nanoparticles using multiple monomers approach. Biosens. Bioelectron. 2019, 145, 111698 10.1016/j.bios.2019.111698. [DOI] [PubMed] [Google Scholar]
- a Xu J.; Ambrosini S.; Tamahkar E.; Rossi C.; Haupt K.; Tse Sum Bui B. Toward a Universal Method for Preparing Molecularly Imprinted Polymer Nanoparticles with Antibody-like Affinity for Proteins. Biomacromolecules 2016, 17 (1), 345–353. 10.1021/acs.biomac.5b01454. [DOI] [PubMed] [Google Scholar]; b Canfarotta F.; Poma A.; Guerreiro A.; Piletsky S. Solid-phase synthesis of molecularly imprinted nanoparticles. Nat. Protoc. 2016, 11 (3), 443–455. 10.1038/nprot.2016.030. [DOI] [PubMed] [Google Scholar]
- a Ashley J.; Feng X.; Halder A.; Zhou T.; Sun Y. Dispersive solid-phase imprinting of proteins for the production of plastic antibodies. Chem. Commun. 2018, 54 (27), 3355–3358. 10.1039/C8CC00343B. [DOI] [PubMed] [Google Scholar]; b Berghaus M.; Mohammadi R.; Sellergren B. Productive encounter: molecularly imprinted nanoparticles prepared using magnetic templates. Chem. Commun. 2014, 50 (64), 8993–8996. 10.1039/C4CC01346H. [DOI] [PubMed] [Google Scholar]
- Mahajan R.; Rouhi M.; Shinde S.; Bedwell T.; Incel A.; Mavliutova L.; Piletsky S.; Nicholls I. A.; Sellergren B. Highly Efficient Synthesis and Assay of Protein-Imprinted Nanogels by Using Magnetic Templates. Angew. Chem., Int. Ed. Engl. 2019, 58 (3), 727–730. 10.1002/anie.201805772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira S. P. B.; Reis R. L.; Peppas N. A.; Gomes M. E.; Domingues R. M. A. Epitope-imprinted polymers: Design principles of synthetic binding partners for natural biomacromolecules. Science Advances 2021, 7 (44), eabi9884. 10.1126/sciadv.abi9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K.; Li S.; Liu L.; Chen Y.; Zhou W.; Pei J.; Liang Z.; Zhang L.; Zhang Y. Epitope Imprinting Technology: Progress, Applications, and Perspectives toward Artificial Antibodies. Adv. Mater. 2019, 31 (50), 1902048 10.1002/adma.201902048. [DOI] [PubMed] [Google Scholar]
- Nishino H.; Huang C.-S.; Shea K. J. Selective Protein Capture by Epitope Imprinting. Angew. Chem., Int. Ed. 2006, 45 (15), 2392–2396. 10.1002/anie.200503760. [DOI] [PubMed] [Google Scholar]
- Lin C. Y.; Tsai S. H.; Tai D. F. Detection of oxytocin, atrial natriuretic peptide, and brain natriuretic peptide using novel imprinted polymers produced with amphiphilic monomers. J. Pept Sci. 2019, 25 (3), e3150 10.1002/psc.3150. [DOI] [PubMed] [Google Scholar]
- a Altintas Z.; Abdin M. J.; Tothill A. M.; Karim K.; Tothill I. E. Ultrasensitive detection of endotoxins using computationally designed nanoMIPs. Anal. Chim. Acta 2016, 935, 239–248. 10.1016/j.aca.2016.06.013. [DOI] [PubMed] [Google Scholar]; b Abdin M. J.; Altintas Z.; Tothill I. E. In silico designed nanoMIP based optical sensor for endotoxins monitoring. Biosens. Bioelectron. 2015, 67, 177–183. 10.1016/j.bios.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Cowen T.; Karim K.; Piletsky S. Computational approaches in the design of synthetic receptors – A review. Anal. Chim. Acta 2016, 936, 62–74. 10.1016/j.aca.2016.07.027. [DOI] [PubMed] [Google Scholar]
- Pasquardini L.; Bossi A. M. Molecularly imprinted polymers by epitope imprinting: a journey from molecular interactions to the available bioinformatics resources to scout for epitope templates. Anal. Bioanal. Chem. 2021, 413 (24), 6101–6115. 10.1007/s00216-021-03409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Li S.; Yang K.; Liu J.; Jiang B.; Zhang L.; Zhang Y. Surface-Imprinted Nanoparticles Prepared with a His-Tag-Anchored Epitope as the Template. Anal. Chem. 2015, 87 (9), 4617–4620. 10.1021/ac5047246. [DOI] [PubMed] [Google Scholar]; b Yoshimatsu K.; Koide H.; Hoshino Y.; Shea K. J. Preparation of abiotic polymer nanoparticles for sequestration and neutralization of a target peptide toxin. Nat. Protoc. 2015, 10 (4), 595–604. 10.1038/nprot.2015.032. [DOI] [PubMed] [Google Scholar]
- Cecchini A.; Raffa V.; Canfarotta F.; Signore G.; Piletsky S.; MacDonald M. P.; Cuschieri A. In Vivo Recognition of Human Vascular Endothelial Growth Factor by Molecularly Imprinted Polymers. Nano Lett. 2017, 17 (4), 2307–2312. 10.1021/acs.nanolett.6b05052. [DOI] [PubMed] [Google Scholar]
- Canfarotta F.; Lezina L.; Guerreiro A.; Czulak J.; Petukhov A.; Daks A.; Smolinska-Kempisty K.; Poma A.; Piletsky S.; Barlev N. A. Specific Drug Delivery to Cancer Cells with Double-Imprinted Nanoparticles against Epidermal Growth Factor Receptor. Nano Lett. 2018, 18 (8), 4641–4646. 10.1021/acs.nanolett.7b03206. [DOI] [PubMed] [Google Scholar]
- Ekpenyong-Akiba A. E.; Canfarotta F.; Bashar A. H.; Poblocka M.; Casulleras M.; Castilla-Vallmanya L.; Kocsis-Fodor G.; Kelly M. E.; Janus J.; Althubiti M.; Piletska E.; Piletsky S.; Macip S.; et al. Detecting and targeting senescent cells using molecularly imprinted nanoparticles. Nanoscale Horizons 2019, 4 (3), 757–768. 10.1039/C8NH00473K. [DOI] [Google Scholar]
- a Altintas Z.; Guerreiro A.; Piletsky S. A.; Tothill I. E. NanoMIP based optical sensor for pharmaceuticals monitoring. Sens. Actuators, B 2015, 213, 305–313. 10.1016/j.snb.2015.02.043. [DOI] [Google Scholar]; b Chiarello M.; Anfossi L.; Cavalera S.; Di Nardo F.; Serra T.; Baggiani C. NanoMIP-Based Solid Phase Extraction of Fluoroquinolones from Human Urine: A Proof-of-Concept Study. Separations 2021, 8, 226. 10.3390/separations8110226. [DOI] [Google Scholar]
- Tang A.-n.; Duan L.; Liu M.; Dong X. An epitope imprinted polymer with affinity for kininogen fragments prepared by metal coordination interaction for cancer biomarker analysis. J. Mater. Chem. B 2016, 4 (46), 7464–7471. 10.1039/C6TB02215D. [DOI] [PubMed] [Google Scholar]
- Liu H.; Deng Z.; Bu J.; Zhang Y.; Zhang Z.; He Y.; Li T.; Gao P.; Yang Y.; Zhong S. Capsule-like molecular imprinted polymer nanoparticles for targeted and chemophotothermal synergistic cancer therapy. Colloids Surf., B 2021, 208, 112126 10.1016/j.colsurfb.2021.112126. [DOI] [PubMed] [Google Scholar]
- Musile G.; Cenci L.; Andreetto E.; Ambrosi E.; Tagliaro F.; Bossi A. M. Screening of the binding properties of molecularly imprinted nanoparticles via capillary electrophoresis. Anal. Bioanal. Chem. 2016, 408 (13), 3435–3443. 10.1007/s00216-016-9418-y. [DOI] [PubMed] [Google Scholar]
- a Medina Rangel P. X.; Moroni E.; Merlier F.; Gheber L. A.; Vago R.; Tse Sum Bui B.; Haupt K. Chemical Antibody Mimics Inhibit Cadherin-Mediated Cell–Cell Adhesion: A Promising Strategy for Cancer Therapy. Angew. Chem., Int. Ed. 2020, 59 (7), 2816–2822. 10.1002/anie.201910373. [DOI] [PubMed] [Google Scholar]; b Bossi A. M. Plastic antibodies for cancer therapy?. Nat. Chem. 2020, 12 (2), 111–112. 10.1038/s41557-019-0415-6. [DOI] [PubMed] [Google Scholar]
- Wang Y.-Z.; Li D.-Y.; He X.-W.; Li W.-Y.; Zhang Y.-K. Epitope imprinted polymer nanoparticles containing fluorescent quantum dots for specific recognition of human serum albumin. Microchimica Acta 2015, 182 (7), 1465–1472. 10.1007/s00604-015-1464-1. [DOI] [Google Scholar]
- Cenci L.; Andreetto E.; Vestri A.; Bovi M.; Barozzi M.; Iacob E.; Busato M.; Castagna A.; Girelli D.; Bossi A. M. Surface plasmon resonance based on molecularly imprinted nanoparticles for the picomolar detection of the iron regulating hormone Hepcidin-25. J. Nanobiotechnology 2015, 13, 51. 10.1186/s12951-015-0115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cennamo N.; Maniglio D.; Tatti R.; Zeni L.; Bossi A. M. Deformable molecularly imprinted nanogels permit sensitivity-gain in plasmonic sensing. Biosens. Bioelectron. 2020, 156, 112126 10.1016/j.bios.2020.112126. [DOI] [PubMed] [Google Scholar]
- Arcadio F.; Seggio M.; Del Prete D.; Buonanno G.; Mendes J.; Coelho L. C. C.; Jorge P. A. S.; Zeni L.; Bossi A. M.; Cennamo N. A Plasmonic Biosensor Based on Light-Diffusing Fibers Functionalized with Molecularly Imprinted Nanoparticles for Ultralow Sensing of Proteins. Nanomaterials 2022, 12, 1400. 10.3390/nano12091400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunath S.; Panagiotopoulou M.; Maximilien J.; Marchyk N.; Sänger J.; Haupt K. Cell and Tissue Imaging with Molecularly Imprinted Polymers as Plastic Antibody Mimics. Adv. Healthcare Mater. 2015, 4 (9), 1322–1326. 10.1002/adhm.201500145. [DOI] [PubMed] [Google Scholar]
- Korposh S.; Chianella I.; Guerreiro A.; Caygill S.; Piletsky S.; James S. W.; Tatam R. P. Selective vancomycin detection using optical fibre long period gratings functionalised with molecularly imprinted polymer nanoparticles. Analyst 2014, 139 (9), 2229–2236. 10.1039/C3AN02126B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cáceres C.; Canfarotta F.; Chianella I.; Pereira E.; Moczko E.; Esen C.; Guerreiro A.; Piletska E.; Whitcombe M. J.; Piletsky S. A. Does size matter? Study of performance of pseudo-ELISAs based on molecularly imprinted polymer nanoparticles prepared for analytes of different sizes. Analyst 2016, 141 (4), 1405–1412. 10.1039/C5AN02018B. [DOI] [PubMed] [Google Scholar]
- Garcia-Cruz A.; Cowen T.; Voorhaar A.; Piletska E.; Piletsky S. A. Molecularly imprinted nanoparticles-based assay (MINA) – detection of leukotrienes and insulin. Analyst 2020, 145 (12), 4224–4232. 10.1039/D0AN00419G. [DOI] [PubMed] [Google Scholar]
- D’Aurelio R.; Ashley J.; Rodgers T. L.; Trinh L.; Temblay J.; Pleasants M.; Tothill I. E. Development of a NanoMIPs-SPR-Based Sensor for β-Lactoglobulin Detection. Chemosensors 2020, 8 (4), 94. 10.3390/chemosensors8040094. [DOI] [Google Scholar]
- Garcia Cruz A.; Haq I.; Cowen T.; Di Masi S.; Trivedi S.; Alanazi K.; Piletska E.; Mujahid A.; Piletsky S. A. Design and fabrication of a smart sensor using in silico epitope mapping and electro-responsive imprinted polymer nanoparticles for determination of insulin levels in human plasma. Biosens. Bioelectron. 2020, 169, 112536 10.1016/j.bios.2020.112536. [DOI] [PubMed] [Google Scholar]
- Garcia-Cruz A.; Ahmad O. S.; Alanazi K.; Piletska E.; Piletsky S. A. Generic sensor platform based on electro-responsive molecularly imprinted polymer nanoparticles (e-NanoMIPs). Microsystems & Nanoengineering 2020, 6 (1), 83. 10.1038/s41378-020-00193-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfarotta F.; Czulak J.; Guerreiro A.; Cruz A. G.; Piletsky S.; Bergdahl G. E.; Hedström M.; Mattiasson B. A novel capacitive sensor based on molecularly imprinted nanoparticles as recognition elements. Biosens. Bioelectron. 2018, 120, 108–114. 10.1016/j.bios.2018.07.070. [DOI] [PubMed] [Google Scholar]
- Moczko E.; Poma A.; Guerreiro A.; Perez de Vargas Sansalvador I.; Caygill S.; Canfarotta F.; Whitcombe M. J.; Piletsky S. Surface-modified multifunctional MIP nanoparticles. Nanoscale 2013, 5 (9), 3733–3741. 10.1039/c3nr00354j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T.; Qin Y.; Tan T.; Lv Y. Targeted Live Cell Raman Imaging and Visualization of Cancer Biomarkers with Thermal-Stimuli Responsive Imprinted Nanoprobes. Particle & Particle Systems Characterization 2018, 35 (12), 1800390 10.1002/ppsc.201800390. [DOI] [Google Scholar]
- Xu J.; Haupt K.; Tse Sum Bui B. Core–Shell Molecularly Imprinted Polymer Nanoparticles as Synthetic Antibodies in a Sandwich Fluoroimmunoassay for Trypsin Determination in Human Serum. ACS Appl. Mater. Interfaces 2017, 9 (29), 24476–24483. 10.1021/acsami.7b05844. [DOI] [PubMed] [Google Scholar]
- Yang X.; Tang Y.; Alt R. R.; Xie X.; Li F. Emerging techniques for ultrasensitive protein analysis. Analyst 2016, 141 (12), 3473–3481. 10.1039/C6AN00059B. [DOI] [PubMed] [Google Scholar]
- Momenbeitollahi N.; Cloet T.; Li H. Pushing the detection limits: strategies towards highly sensitive optical-based protein detection. Anal. Bioanal. Chem. 2021, 413 (24), 5995–6011. 10.1007/s00216-021-03566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonamine Y.; Hoshino Y.; Shea K. J. ELISA-Mimic Screen for Synthetic Polymer Nanoparticles with High Affinity to Target Proteins. Biomacromolecules 2012, 13 (9), 2952–2957. 10.1021/bm300986j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutov R. V.; Guerreiro A.; Moczko E.; de Vargas-Sansalvador I. P.; Chianella I.; Whitcombe M. J.; Piletsky S. A. Introducing MINA – The Molecularly Imprinted Nanoparticle Assay. Small 2014, 10 (6), 1086–1089. 10.1002/smll.201301996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munawar H.; Smolinska-Kempisty K.; Cruz A. G.; Canfarotta F.; Piletska E.; Karim K.; Piletsky S. A. Molecularly imprinted polymer nanoparticle-based assay (MINA): application for fumonisin B1 determination. Analyst 2018, 143 (14), 3481–3488. 10.1039/C8AN00322J. [DOI] [PubMed] [Google Scholar]
- a Specht E. A.; Braselmann E.; Palmer A. E. A Critical and Comparative Review of Fluorescent Tools for Live-Cell Imaging. Annu. Rev. Physiol. 2017, 79 (1), 93–117. 10.1146/annurev-physiol-022516-034055. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Sahl S. J.; Hell S. W.; Jakobs S. Fluorescence nanoscopy in cell biology. Nat. Rev. Mol. Cell Biol. 2017, 18 (11), 685–701. 10.1038/nrm.2017.71. [DOI] [PubMed] [Google Scholar]
- Hoshino Y.; Koide H.; Urakami T.; Kanazawa H.; Kodama T.; Oku N.; Shea K. J. Recognition, Neutralization, and Clearance of Target Peptides in the Bloodstream of Living Mice by Molecularly Imprinted Polymer Nanoparticles: A Plastic Antibody. J. Am. Chem. Soc. 2010, 132 (19), 6644–6645. 10.1021/ja102148f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfarotta F.; Waters A.; Sadler R.; McGill P.; Guerreiro A.; Papkovsky D.; Haupt K.; Piletsky S. Biocompatibility and internalization of molecularly imprinted nanoparticles. Nano Research 2016, 9 (11), 3463–3477. 10.1007/s12274-016-1222-7. [DOI] [Google Scholar]
- Sun C.; Pan L.; Zhang L.; Huang J.; Yao D.; Wang C.-Z.; Zhang Y.; Jiang N.; Chen L.; Yuan C.-s. A biomimetic fluorescent nanosensor based on imprinted polymers modified with carbon dots for sensitive detection of alpha-fetoprotein in clinical samples. Analyst 2019, 144 (22), 6760–6772. 10.1039/C9AN01065C. [DOI] [PubMed] [Google Scholar]
- Medina Rangel P. X.; Laclef S.; Xu J.; Panagiotopoulou M.; Kovensky J.; Tse Sum Bui B.; Haupt K. Solid-phase synthesis of molecularly imprinted polymer nanolabels: Affinity tools for cellular bioimaging of glycans. Sci. Rep. 2019, 9 (1), 3923. 10.1038/s41598-019-40348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Masson J.-F. Surface Plasmon Resonance Clinical Biosensors for Medical Diagnostics. ACS Sensors 2017, 2 (1), 16–30. 10.1021/acssensors.6b00763. [DOI] [PubMed] [Google Scholar]; b Bellassai N.; D’Agata R.; Jungbluth V.; Spoto G. Surface Plasmon Resonance for Biomarker Detection: Advances in Non-invasive Cancer Diagnosis. Frontiers in Chemistry 2019, 7, 570. 10.3389/fchem.2019.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Divya J.; Selvendran S.; Raja A. S.; Sivasubramanian A. Surface plasmon based plasmonic sensors: A review on their past, present and future. Biosensors and Bioelectronics: X 2022, 11, 100175 10.1016/j.biosx.2022.100175. [DOI] [Google Scholar]; d Wang Q.; Ren Z.-H.; Zhao W.-M.; Wang L.; Yan X.; Zhu A.-s.; Qiu F.-m.; Zhang K.-K. Research advances on surface plasmon resonance biosensors. Nanoscale 2022, 14 (3), 564–591. 10.1039/D1NR05400G. [DOI] [PubMed] [Google Scholar]
- a Ribeiro J. A.; Sales M. G. F.; Pereira C. M. Electrochemistry combined-surface plasmon resonance biosensors: A review. TrAC Trends in Analytical Chemistry 2022, 157, 116766 10.1016/j.trac.2022.116766. [DOI] [Google Scholar]; b Oliverio M.; Perotto S.; Messina G. C.; Lovato L.; De Angelis F. Chemical Functionalization of Plasmonic Surface Biosensors: A Tutorial Review on Issues, Strategies, and Costs. ACS Appl. Mater. Interfaces 2017, 9 (35), 29394–29411. 10.1021/acsami.7b01583. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Hinman S. S.; McKeating K. S.; Cheng Q. Surface Plasmon Resonance: Material and Interface Design for Universal Accessibility. Anal. Chem. 2018, 90 (1), 19–39. 10.1021/acs.analchem.7b04251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altintas Z.; Gittens M.; Guerreiro A.; Thompson K.-A.; Walker J.; Piletsky S.; Tothill I. E. Detection of Waterborne Viruses Using High Affinity Molecularly Imprinted Polymers. Anal. Chem. 2015, 87 (13), 6801–6807. 10.1021/acs.analchem.5b00989. [DOI] [PubMed] [Google Scholar]
- Cennamo N.; Bossi A. M.; Arcadio F.; Maniglio D.; Zeni L. On the Effect of Soft Molecularly Imprinted Nanoparticles Receptors Combined to Nanoplasmonic Probes for Biomedical Applications. Frontiers in Bioengineering and Biotechnology 2021, 9, 1. 10.3389/fbioe.2021.801489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolla M.; Cenci L.; Anesi A.; Ambrosi E.; Tagliaro F.; Vanzetti L.; Guella G.; Bossi A. M. Solvent-Responsive Molecularly Imprinted Nanogels for Targeted Protein Analysis in MALDI-TOF Mass Spectrometry. ACS Appl. Mater. Interfaces 2017, 9 (8), 6908–6915. 10.1021/acsami.6b16291. [DOI] [PubMed] [Google Scholar]
- a Kumar S.; Kumar A.; Kim G.-H.; Rhim W.-K.; Hartman K. L.; Nam J.-M. Myoglobin and Polydopamine-Engineered Raman Nanoprobes for Detecting, Imaging, and Monitoring Reactive Oxygen Species in Biological Samples and Living Cells. Small 2017, 13 (43), 1701584 10.1002/smll.201701584. [DOI] [PubMed] [Google Scholar]; b Herrmann J. F.; Kretschmer F.; Hoeppener S.; Höppener C.; Schubert U. S. Ordered Arrangement and Optical Properties of Silica-Stabilized Gold Nanoparticle–PNIPAM Core–Satellite Clusters for Sensitive Raman Detection. Small 2017, 13 (39), 1701095 10.1002/smll.201701095. [DOI] [PubMed] [Google Scholar]
- Carrasco S.; Benito-Peña E.; Navarro-Villoslada F.; Langer J.; Sanz-Ortiz M. N.; Reguera J.; Liz-Marzán L. M.; Moreno-Bondi M. C. Multibranched Gold–Mesoporous Silica Nanoparticles Coated with a Molecularly Imprinted Polymer for Label-Free Antibiotic Surface-Enhanced Raman Scattering Analysis. Chem. Mater. 2016, 28 (21), 7947–7954. 10.1021/acs.chemmater.6b03613. [DOI] [Google Scholar]
- a Dong T.; Matos Pires N. M.; Yang Z.; Jiang Z. Advances in Electrochemical Biosensors Based on Nanomaterials for Protein Biomarker Detection in Saliva. Advanced Science 2023, 10, 2205429 10.1002/advs.202205429. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Mostafa I. M.; Tian Y.; Anjum S.; Hanif S.; Hosseini M.; Lou B.; Xu G. Comprehensive review on the electrochemical biosensors of different breast cancer biomarkers. Sens. Actuators, B 2022, 365, 131944 10.1016/j.snb.2022.131944. [DOI] [Google Scholar]
- De Rycke E.; Stove C.; Dubruel P.; De Saeger S.; Beloglazova N. Recent developments in electrochemical detection of illicit drugs in diverse matrices. Biosens. Bioelectron. 2020, 169, 112579 10.1016/j.bios.2020.112579. [DOI] [PubMed] [Google Scholar]
- García-Miranda Ferrari A.; Rowley-Neale S. J.; Banks C. E. Screen-printed electrodes: Transitioning the laboratory in-to-the field. Talanta Open 2021, 3, 100032 10.1016/j.talo.2021.100032. [DOI] [Google Scholar]
- a Macovei D.-G.; Irimes M.-B.; Hosu O.; Cristea C.; Tertis M. Point-of-care electrochemical testing of biomarkers involved in inflammatory and inflammatory-associated medical conditions. Anal. Bioanal. Chem. 2023, 415, 1033. 10.1007/s00216-022-04320-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Crapnell R. D.; Garcia-Miranda Ferrari A.; Dempsey N. C.; Banks C. E. Electroanalytical overview: screen-printed electrochemical sensing platforms for the detection of vital cardiac, cancer and inflammatory biomarkers. Sensors & Diagnostics 2022, 1 (3), 405–428. 10.1039/D1SD00041A. [DOI] [Google Scholar]; c Crapnell R. D.; Dempsey N. C.; Sigley E.; Tridente A.; Banks C. E. Electroanalytical point-of-care detection of gold standard and emerging cardiac biomarkers for stratification and monitoring in intensive care medicine - a review. Mikrochim. Acta 2022, 189 (4), 142. 10.1007/s00604-022-05186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Campuzano S.; Pedrero M.; Yáñez-Sedeño P.; Pingarrón J. M. New challenges in point of care electrochemical detection of clinical biomarkers. Sens. Actuators, B 2021, 345, 130349 10.1016/j.snb.2021.130349. [DOI] [Google Scholar]
- Karimi-Maleh H.; Orooji Y.; Karimi F.; Alizadeh M.; Baghayeri M.; Rouhi J.; Tajik S.; Beitollahi H.; Agarwal S.; Gupta V. K.; et al. A critical review on the use of potentiometric based biosensors for biomarkers detection. Biosens. Bioelectron. 2021, 184, 113252 10.1016/j.bios.2021.113252. [DOI] [PubMed] [Google Scholar]
- Pollard T. D.; Ong J. J.; Goyanes A.; Orlu M.; Gaisford S.; Elbadawi M.; Basit A. W. Electrochemical biosensors: a nexus for precision medicine. Drug Discovery Today 2021, 26 (1), 69–79. 10.1016/j.drudis.2020.10.021. [DOI] [PubMed] [Google Scholar]
- a Herrera-Chacón A.; Cetó X.; del Valle M. Molecularly imprinted polymers - towards electrochemical sensors and electronic tongues. Anal. Bioanal. Chem. 2021, 413 (24), 6117–6140. 10.1007/s00216-021-03313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Hasseb A. A.; Abdel Ghani N. d. T.; Shehab O. R.; El Nashar R. M. Application of molecularly imprinted polymers for electrochemical detection of some important biomedical markers and pathogens. Current Opinion in Electrochemistry 2022, 31, 100848 10.1016/j.coelec.2021.100848. [DOI] [Google Scholar]
- a Zanfrognini B.; Pigani L.; Zanardi C. Recent advances in the direct electrochemical detection of drugs of abuse. J. Solid State Electrochem. 2020, 24 (11), 2603–2616. 10.1007/s10008-020-04686-z. [DOI] [Google Scholar]; b Garcia-Mutio D.; Gomez-Caballero A.; Guerreiro A.; Piletsky S.; Goicolea M. A.; Barrio R. J. Solid-phase synthesis of imprinted nanoparticles grafted on gold substrates for voltammetric sensing of 4-ethylphenol. Sens. Actuators, B 2016, 236, 839–848. 10.1016/j.snb.2016.02.018. [DOI] [Google Scholar]
- Felix F. S.; Angnes L. Electrochemical immunosensors - A powerful tool for analytical applications. Biosens. Bioelectron. 2018, 102, 470–478. 10.1016/j.bios.2017.11.029. [DOI] [PubMed] [Google Scholar]
- Sharma P. S.; Garcia-Cruz A.; Cieplak M.; Noworyta K. R.; Kutner W. ‘Gate effect’ in molecularly imprinted polymers: the current state of understanding. Current Opinion in Electrochemistry 2019, 16, 50–56. 10.1016/j.coelec.2019.04.020. [DOI] [Google Scholar]
- Udomsap D.; Branger C.; Culioli G.; Dollet P.; Brisset H. A versatile electrochemical sensing receptor based on a molecularly imprinted polymer. Chem. Commun. 2014, 50 (56), 7488–7491. 10.1039/c4cc02658f. [DOI] [PubMed] [Google Scholar]
- Ekomo V. M.; Branger C.; Bikanga R.; Florea A.-M.; Istamboulie G.; Calas-Blanchard C.; Noguer T.; Sarbu A.; Brisset H. Detection of Bisphenol A in aqueous medium by screen printed carbon electrodes incorporating electrochemical molecularly imprinted polymers. Biosens. Bioelectron. 2018, 112, 156–161. 10.1016/j.bios.2018.04.022. [DOI] [PubMed] [Google Scholar]
- Haq I.; Alanazi K.; Czulak J.; Di Masi S.; Piletska E.; Mujahid A.; Hussain T.; Piletsky S. A.; Garcia-Cruz A. Determination of sitagliptin in human plasma using a smart electrochemical sensor based on electroactive molecularly imprinted nanoparticles. Nanoscale Advances 2021, 3 (14), 4276–4285. 10.1039/D1NA00194A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J.; Qin W. Recent advances in potentiometric biosensors. TrAC Trends in Analytical Chemistry 2020, 124, 115803 10.1016/j.trac.2019.115803. [DOI] [Google Scholar]
- Basozabal I.; Guerreiro A.; Gomez-Caballero A.; Aranzazu Goicolea M.; Barrio R. J. Direct potentiometric quantification of histamine using solid-phase imprinted nanoparticles as recognition elements. Biosens. Bioelectron. 2014, 58, 138–144. 10.1016/j.bios.2014.02.054. [DOI] [PubMed] [Google Scholar]
- Frasco M. F.; Truta L. A. A. N. A.; Sales M. G. F.; Moreira F. T. C. Imprinting Technology in Electrochemical Biomimetic Sensors. Sensors 2017, 17, 523. 10.3390/s17030523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aurelio R.; Tothill I. E.; Salbini M.; Calò F.; Mazzotta E.; Malitesta C.; Chianella I. A Comparison of EIS and QCM NanoMIP-Based Sensors for Morphine. Nanomaterials 2021, 11, 3360. 10.3390/nano11123360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Borrebaeck C. A. K. Precision diagnostics: moving towards protein biomarker signatures of clinical utility in cancer. Nature Reviews Cancer 2017, 17, 199. 10.1038/nrc.2016.153. [DOI] [PubMed] [Google Scholar]; b Hu M.; Yan J.; He Y.; Lu H.; Weng L.; Song S.; Fan C.; Wang L. Ultrasensitive, Multiplexed Detection of Cancer Biomarkers Directly in Serum by Using a Quantum Dot-Based Microfluidic Protein Chip. ACS Nano 2010, 4 (1), 488–494. 10.1021/nn901404h. [DOI] [PubMed] [Google Scholar]
- Crapnell R. D.; Canfarotta F.; Czulak J.; Johnson R.; Betlem K.; Mecozzi F.; Down M. P.; Eersels K.; van Grinsven B.; Cleij T. J.; et al. Thermal Detection of Cardiac Biomarkers Heart-Fatty Acid Binding Protein and ST2 Using a Molecularly Imprinted Nanoparticle-Based Multiplex Sensor Platform. ACS Sensors 2019, 4 (10), 2838–2845. 10.1021/acssensors.9b01666. [DOI] [PubMed] [Google Scholar]
- a Li W.; Zhang X.; Li T.; Ji Y.; Li R. Molecularly imprinted polymer-enhanced biomimetic paper-based analytical devices: A review. Anal. Chim. Acta 2021, 1148, 238196 10.1016/j.aca.2020.12.071. [DOI] [PubMed] [Google Scholar]; b Qi J.; Li B.; Zhou N.; Wang X.; Deng D.; Luo L.; Chen L. The strategy of antibody-free biomarker analysis by in-situ synthesized molecularly imprinted polymers on movable valve paper-based device. Biosens. Bioelectron. 2019, 142, 111533 10.1016/j.bios.2019.111533. [DOI] [PubMed] [Google Scholar]
- Díaz-Betancor Z.; Bañuls M.-J.; Sanza F. J.; Casquel R.; Laguna M. F.; Holgado M.; Puchades R.; Maquieira Á. Phosphorylcholine-based hydrogel for immobilization of biomolecules. Application to fluorometric microarrays for use in hybridization assays and immunoassays, and nanophotonic biosensing. Microchimica Acta 2019, 186 (8), 570. 10.1007/s00604-019-3691-3. [DOI] [PubMed] [Google Scholar]
- Yeh C.-C.; Venault A.; Chang Y. Structural effect of poly(ethylene glycol) segmental length on biofouling and hemocompatibility. Polym. J. 2016, 48 (4), 551–558. 10.1038/pj.2016.5. [DOI] [Google Scholar]
- a Bai W.; Gariano N. A.; Spivak D. A. Macromolecular Amplification of Binding Response in Superaptamer Hydrogels. J. Am. Chem. Soc. 2013, 135 (18), 6977–6984. 10.1021/ja400576p. [DOI] [PubMed] [Google Scholar]; b Zhang Z.; Liu J. Molecular Imprinting with Functional DNA. Small 2019, 15 (26), 1805246 10.1002/smll.201805246. [DOI] [PubMed] [Google Scholar]; c Ali G. K.; Omer K. M. Molecular imprinted polymer combined with aptamer (MIP-aptamer) as a hybrid dual recognition element for bio(chemical) sensing applications. Review. Talanta 2022, 236, 122878 10.1016/j.talanta.2021.122878. [DOI] [PubMed] [Google Scholar]; d Poma A.; Brahmbhatt H.; Pendergraff H. M.; Watts J. K.; Turner N. W. Generation of Novel Hybrid Aptamer–Molecularly Imprinted Polymeric Nanoparticles. Adv. Mater. 2015, 27 (4), 750–758. 10.1002/adma.201404235. [DOI] [PubMed] [Google Scholar]; e Zhang Z.; Liu J. Molecularly Imprinted Polymers with DNA Aptamer Fragments as Macromonomers. ACS Appl. Mater. Interfaces 2016, 8 (10), 6371–6378. 10.1021/acsami.6b00461. [DOI] [PubMed] [Google Scholar]
- a Sullivan M. V.; Clay O.; Moazami M. P.; Watts J. K.; Turner N. W. Hybrid Aptamer-Molecularly Imprinted Polymer (aptaMIP) Nanoparticles from Protein Recognition—A Trypsin Model. Macromol. Biosci. 2021, 21 (5), 2100002 10.1002/mabi.202100002. [DOI] [PubMed] [Google Scholar]; b Shoghi E.; Mirahmadi-Zare S. Z.; Ghasemi R.; Asghari M.; Poorebrahim M.; Nasr-Esfahani M.-H. Nanosized aptameric cavities imprinted on the surface of magnetic nanoparticles for high-throughput protein recognition. Microchimica Acta 2018, 185 (4), 241. 10.1007/s00604-018-2745-2. [DOI] [PubMed] [Google Scholar]
- a Sun X.-Y.; Ma R.-T.; Chen J.; Shi Y.-P. Synthesis of magnetic molecularly imprinted nanoparticles with multiple recognition sites for the simultaneous and selective capture of two glycoproteins. J. Mater. Chem. B 2018, 6 (4), 688–696. 10.1039/C7TB03001K. [DOI] [PubMed] [Google Scholar]; b Gao R.; Zhao S.; Hao Y.; Zhang L.; Cui X.; Liu D.; Zhang M.; Tang Y. Synthesis of magnetic dual-template molecularly imprinted nanoparticles for the specific removal of two high-abundance proteins simultaneously in blood plasma. J. Sep. Sci. 2015, 38 (22), 3914–3920. 10.1002/jssc.201500882. [DOI] [PubMed] [Google Scholar]












