Abstract
Background
The association between gastrointestinal cancer and types of meat consumption, including red meat, processed meat, or a combination of both, remains disputable. Therefore, we performed a systematic review and meta-analysis of prospective cohort studies to estimate the association between meat consumption and gastrointestinal cancer risk.
Methods
PubMed, EmBase, and the Cochrane library databases were searched systematically for eligible studies that investigated the relation between meat consumption and the risk of developing gastrointestinal cancers, including esophageal cancer (EC), gastric cancer (GC), colorectal cancer (CRC), colon cancer (CC), rectal cancer (RC), pancreatic cancer (PC), and hepatocellular carcinoma (HCC) throughout February, 2023. The pooled relative risk (RR) with 95% confidence interval (CI) was assigned as an effect estimate and calculated using a random-effects model with inverse variance weighting.
Results
Forty cohorts comprising 3,780,590 individuals were selected for the final quantitative analysis. The summary results indicated that a higher red meat consumption was associated with an increased risk of CRC (RR: 1.09; 95% CI: 1.02–1.16; P = 0.007) and CC (RR: 1.13; 95% CI: 1.03–1.25; P = 0.011). Moreover, a higher processed meat consumption was associated with an increased risk of CRC (RR: 1.19; 95% CI: 1.13–1.26; P < 0.001), CC (RR: 1.24; 95% CI: 1.13–1.26; P < 0.001), and RC (RR: 1.24; 95% CI: 1.08–1.42; P = 0.002). Furthermore, a higher total consumption of red and processed meat was associated with an increased risk of CRC (RR: 1.13; 95% CI: 1.06–1.20; P < 0.001), CC (RR: 1.17; 95% CI: 1.04–1.33; P = 0.012), and RC (RR: 1.20; 95% CI: 1.04–1.39; P = 0.016). Finally, the strength of higher consumption of total red and processed meat with the risk of GC, and higher consumption of red meat with the risk of RC in subgroup of high adjusted level was lower than subgroup of moderate adjusted level, while the strength of higher consumption of processed meat with the risk of RC and HCC in subgroup of follow-up ≥ 10.0 years was higher than subgroup of follow-up < 10.0 years.
Conclusions
This study found that meat consumption was associated with an increased risk of CRC, CC, and RC, and dietary intervention could be considered an effective strategy in preventing CRC.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-023-11218-1.
Background
Gastrointestinal cancers are the most common and aggressive malignant tumors, accounting for 26% of cancer incidence and 35% of cancer-related mortality worldwide [1]. According to the International Agency for Research on Cancer (IARC), mortality caused by gastrointestinal cancers accounts for 45% of all cancer-related mortality in China [2]. The standard treatment strategies for gastrointestinal cancers include surgery, endoscopy, chemotherapy, radiotherapy, immunotherapy, and targeted therapy [3, 4]. However, disease prognosis remains poor because most patients are diagnosed at an advanced stage. Thus, effective preventive strategies should be implemented to reduce the risk of gastrointestinal cancer.
Studies have found that several diseases could be caused by unhealthy diets, including cancer, and nearly 930,000 cancer-related mortality were induced by poor diet in 2017, especially breast and colorectal cancer (CRC) [5, 6]. The IARC classified red meat as a probable carcinogen based on CRC, pancreatic cancer (PC), and prostate cancer evidence, while processed meat was regarded as carcinogenic to humans based on CRC evidence [7]. Moreover, the World Cancer Research Fund and American Institute for Cancer Research suggest that red meat consumption should be less than three portions per week [8]. Numerous studies have illustrated the relationship between red or processed meat consumption and gastrointestinal cancer [9–12]. However, these studies pooled overall cancer outcomes or focused on a specific type of gastrointestinal cancer, and did not illustrate whether the associations are differing according to study or individuals’ characteristics, including country, sex, follow-up duration, and adjusted level. Thus, the current systematic review and meta-analysis was performed to investigate the associations of red and processed meat consumption with the risk of gastrointestinal cancer, including esophageal cancer (EC), gastric cancer (GC), CRC, colon cancer (CC), rectal cancer (RC), PC, and hepatocellular carcinoma (HCC). Moreover, the exploratory analysis were performed and stratified by country, sex, follow-up duration, and adjusted level.
Methods
Data sources, search strategy, and selection criteria
A meta-analysis of observational studies in epidemiology protocols was used for this systematic review and meta-analysis [13]. Prospective cohort studies that assessed the association of red and processed meat consumption with gastrointestinal cancer risk were included in this study, and the publication language and status without restriction. We systematically searched the databases of PubMed, EmBase, and the Cochrane library from their inception until February, 2023, using the following search strategies in PubMed: ((“Red Meat“[Medical Subject Heading (MeSH)]) or (“Meat Products“[MeSH]) or (“processed meat*“[Title/Abstract (tiab)]) or (“hot dog*“[tiab]) or (salami[tiab]) or (pork[tiab]) or (beef[tiab]) or (veal [tiab]) or (sausage[tiab]) or (lamb[tiab]) or (meat*[tiab]) or (bacon[tiab]) or (diet*[tiab])) AND ((“Neoplasms“[Mesh]) or (cancer*[tiab]) or (Neoplasia*[tiab]) or (Neoplasm[tiab]) or (Tumor*[tiab]) or (Tumor *[tiab]) or (Malignan*[tiab]) or (carcinoma[tiab]) or (leukemia[tiab]) or (lymphoma[tiab])). The reference lists of relevant original and review articles were manually reviewed to identify new studies that met the inclusion criteria.
Two reviewers independently performed the literature search and study selection, and conflicts between the reviewers were resolved by a third reviewer who read the full text of the article. Details of the inclusion criteria were as follows: (1) participants: general population; (2) exposure: the highest category of red meat (lamb, mutton, beef, hamburger, and pork), processed meat (sausage and deli meat), or total red and processed meat consumption; (3) control: the lowest category of red meat, processed meat, or total red and processed meat consumption; (4) outcomes: studies that reported at least one of the following outcomes: EC, GC, CRC, CC, RC, PC, and HCC; and (5) study design: studies with a prospective cohort design. For studies that reported several multivariable adjusted effect estimates, we selected the effect estimate that was maximally adjusted for potential confounders. Moreover, if two or more papers reported effect estimates from the same cohort, and the most recently and comprehensive data were obtained.
Data collection and quality assessment
The two reviewers independently extracted the following information: first authors’ name, study groups’ name, publication year, region, sample size, age, sex, exposure definition, comparisons, follow-up duration, adjusted factors (more than six factors in three parts were considered high; 1–6 factors in 1–2 parts were considered moderate), and reported outcomes. Subsequently, the two reviewers independently assessed the quality of the included studies using the Newcastle-Ottawa Scale (NOS), which contained four items in the selection part, one item in the comparability part, and three items in the outcome part. The “star system” of NOS ranged from 0 to 9, and studies with 7–9 stars were considered as high quality [14]. Inconsistent results regarding data extraction and quality assessment were resolved by a third reviewer who referred to the original article.
Statistical analysis
The relationship between red meat or processed meat consumption and gastrointestinal cancer risk was assigned as relative risk (RR) with its 95% confidence interval (CI) in individual studies. The random-effects model was used for pooled effect estimates because it considers the underlying variation across the included studies [15, 16]. Heterogeneity among the included studies was assessed using I2 and Q statistics, and significant heterogeneity was defined as I2 ≥ 50.0% or P < 0.10 [17, 18]. The robustness of the pooled conclusion was assessed using sensitivity analysis through the sequential removal of a single study [19]. Subgroup analyses were performed to provide exploratory results, which were based on country, sex, follow-up duration, and adjusted level, and the difference between subgroups were compared using the ratio of RRs (RRR) with 95% CIs [20]. Publication bias was assessed using both qualitative and quantitative methods, including funnel plots, Egger’s tests, and Begg’s tests [21, 22]. All reported P value were two-sided, and the inspection level was 0.05. The STATA software (version 14.0; Stata Corporation, College Station, TX, USA) was used to perform all statistical analyses.
Results
Literature search
An initial electronic search yielded 5,432 articles. Of these, 3,791 were retained after duplicate titles were removed. After reviewing the titles and abstracts, an additional 3,513 articles were excluded. The remaining 278 articles were retrieved for full-text evaluation, and a total of 40 cohorts reported in 69 articles met the inclusion criteria [23–91]. Review of the reference lists did not yield any new eligible studies. The details of the literature search and the study selection process are shown in Fig. 1.
Fig. 1.
The processes of literature search and study selection
Study characteristics
The baseline characteristics of the included studies and the participants are presented in Table 1. A total of 3,780,590 individuals from 40 cohorts were included, and the sample size ranged from 1,583 to 512,891. The follow-up durations of the included studies ranged from 4.1 to 24.0 years. Three cohorts included only male individuals, 11 cohorts included only female individuals, and the remaining 26 cohorts included both male and female individuals. The NOS was applied to assess the quality of the included studies: six cohorts with nine stars, 13 cohorts with eight stars, 14 cohorts with seven stars, and the remaining seven cohorts with six stars.
Table 1.
The characteristics of included studies and involved participants
| Study | Region | Sample size | Age (years) | Sex | Exposure definition | Comparisons | Follow-up (years) | Adjustment factors | Outcome | Study quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Nomura 1990 (JAH) [23] | USA | 7,990 | ≥ 18 | Men and women | FFQ | Quintiles | 17.5 | Age | GC | 6 |
| Bostick 1994 (IWHS) [24, 25] | USA | 35,216 | 55–69 | Women | FFQ | Quintiles | 5.0 | Age, EI, height, parity, vitamin E, vitamin A | CC, PC | 8 |
| Kato 1997 (NYUWHS)[26] | USA | 14,727 | 34–65 | Women | FFQ | Quartiles | 7.1 | Age, EI, history of rectal colon polyps | CRC | 6 |
| Singh 1998 (AHS) [27] | USA | 32,051 | ≥ 25 | Men and women | FFQ | Median | 6.0 | Age, sex, BMI, PI,parental history of CC, smoking,alcohol, aspirin use | CC | 7 |
| Galanis 1998 (JRH) [28] | USA | 11,907 | ≥ 18 | Men and women | FFQ | Median | 14.8 | Age, education, Japanese place of birth, smoking,alcohol | GC | 7 |
| Knekt 1999 (FMCHES)[29, 30] | Finland | 9,985 | 15–99 | Men and women | Dietary history | Quartiles | 24.0 | Age, sex, municipality, smoking, EI | CRC, GC, CC, RC | 6 |
| Pietinen 1999 (ATBC) [31, 32] | Finland | 27,111 | 50–69 | Men | FFQ | Quartiles | 8.0 | Age, supplement group, smoking, BMI,alcohol,education, PA, calcium intake | CRC, PC | 9 |
| Isaksson 2002 (STR) [33] | Sweden | 21,884 | 56 | Men and women | FFQ | Tertiles | 16.0 | Age, sex, smoking | PC | 6 |
| Flood 2003 (BCDDP) [34] | USA | 45,496 | 61.9 | Women | FFQ | Quintiles | 8.5 | EI, total meat intake | CRC | 8 |
| Michaud 2003 (NHS)[25] | USA | 88,802 | 30–55 | Women | FFQ | Quintiles | 18.0 | Age, smoking, BMI, history of diabetes,EI, height, PA,menopausal status | PC, RC, CC, CRC, HCC | 8 |
| Lin 2004 (WHS) [36] | USA | 37,547 | ≥ 45 | Women | FFQ | Quintiles | 8.7 | Age, random treatment assignment, BMI,family history of CRC,history of colorectal polyps, PA, smoking,alcohol, HRT,EI | CRC | 8 |
| Wei 2004 (HPFS) [37–40] | USA | 46,632 | 40–75 | Men | FFQ | Quintiles | 14.0 | Age, family history,BMI, PA,alcohol, calcium intake, folate intake,height, smoking,history of endoscopy,beef/pork/lamb as amain dish | RC, CC, CRC, HCC | 8 |
| English 2004 (MCCS) [41] | Australia | 37,112 | 27–75 | Men and women | FFQ | Quartiles | 9.0 | Age, sex, country of birth, EI, fat intake, cereal product intake | CRC, RC, CC | 9 |
| Chao 2005 (CPS II) [42, 43] | USA | 148,610 | 50–74 | Men and women | FFQ | Quintiles | 9.0 | Age, EI,education, BMI,smoking, PA, multivitamin use, aspirin use, beer,wine,liquor, HRT,fruit intake, vegetable intake, high-fiber grain food intake | RC, CC, PC | 9 |
| Larsson 2005 (SMC) [44–46] | Sweden | 61,433 | 40–75 | Women | FFQ | Quartiles | 13.9 | Age, BMI, education, EI, alcohol, saturated fat intake, calcium intake, folate intake, fruit intake, vegetable intake, whole-grain food intake | CRC, RC, GC, PC | 7 |
| Norat 2005 (EPIC) [47, 51] | Europe | 478,040 | 21–83 | Men and women | FFQ | Quintiles | 4.8 | Age, sex, center,EI,height, weight,PA,smoking, dietary fiber intake, alcohol intake | CRC, RC, CC, GC, EC, PC, HCC | 9 |
| Sauvaget 2005 (LSS) [52] | Japan | 38,576 | 34–98 | Men and women | FFQ | Tertiles | 20.0 | Age, sex, city, radiation dose, smoking, education | GC | 7 |
| Nöthlings 2005 (MEC) [53–55] | USA | 190,545 | 45–75 | Men and women | FFQ | Quintiles | 7.0 | Age, sex, ethnicity, history of diabetes,family history of PC,smoking, EI | PC, CRC | 8 |
| Berndt 2006 (CLUE II) [56] | USA | 1,583 | 48.5 | Men and women | FFQ | Tertiles | 13.5 | Age, race, EI | CRC | 6 |
| Kabat 2007 (CNBSS) [57] | Canada | 49,654 | 40–59 | Women | FFQ | Quintiles | 16.4 | Age, BMI, menopausalstatus, OC use, HRT,dietary fat intake, fiber intake, folic acid intake, EI,smoking, alcohol, education, PA | CRC, RC, CC | 7 |
| Cross 2007 (NIH-AARP) [58–63] | USA | 494,036 | 50–71 | Men and women | FFQ | Quintiles | 8.2 | Age, sex, education, marital status, family history of cancer, race, BMI, smoking, PA, EI, alcohol, fruit and vegetable intake | ES, GC, CRC, HCC, PC | 9 |
| Butler 2008 (SCHS) [64, 65] | Singapore | 61,321 | 45–74 | Men and women | FFQ | Quartiles | 10.0 | Age, sex, dialect group,interview year, history of diabetes, smoking,BMI, alcohol,education, PA, family history of CRC, EI | CRC, HCC | 8 |
| Lee 2009 (SWHS) [66] | China | 73,224 | 40–70 | Women | FFQ | Quintiles | 7.4 | Age, education,income, survey season,tea intake, NSAID use,EI, fiber intake | CRC, RC, CC | 8 |
| Heinen 2009 (NLCS) [67-69 | Netherlands | 3,980 | 55–69 | Men and women | FFQ | Quintiles | 13.3 | Age, energy intake, sex, smoking, alcohol, history of diabetes, history of hypertension, BMI,vegetable intake, fruit intake | PC, GC, EC, CRC, RC, CC | 7 |
| Wie 2014 (CSEC) [70] | Korea | 8,024 | 48.4 | Men and women | 3-days food records | Median | 7.0 | Age, sex, EI, BMI, PA,smoking,alcohol use, income,education, marital status | CRC, GC | 7 |
| Nomura 2016 (BWHS) [71, 72] | USA | 49,103 | 21–69 | Women | FFQ | Tertiles | 15.1 | Age, geographic regionof residence, EI, smoking, family history of CRC, education,menopausal status,diabetes, insulin use,aspirin use,colonoscopy, sigmoidoscopy, alcohol, BMI | CRC, CC, PC | 8 |
| Hastert 2016 (VITAL) [73] | USA | 66,920 | 50–76 | Men and women | FFQ | Median | 7.6 | Age, education,race/ethnicity, receipt of colonoscopy orsigmoidoscopy, family history of CC or RC, NSAID use, history of cancer other than CRC, EI | CRC | 7 |
| Jones 2017 (UKWCS) [74] | UK | 32,154 | 52 | Women | FFQ | Quintiles | 17.4 | Age, BMI, EI, PA, smoking,socioeconomic status,family history of CRC | CRC, RC, CC | 8 |
| Wada 2017 (Takayama) [75] | Japan | 30,331 | ≥ 35 | Men and women | FFQ | Quartiles | 16.0 | Age, height, BMI,PA,smoking, education,aspirin use, alcohol, fiber intake,calcium intake, vitamin D intake, EI | CRC, RC, CC | 7 |
| Pang 2018 (CKB) [76] | China | 512,891 | 30–79 | Men and women | FFQ | Median | 9.0 | Age, sex, study area, education, smoking,alcohol, BMI, PA | PC | 8 |
| Diallo 2018 (NSS) [77, 78] | France | 61,476 | ≥ 35 | Men and women | 24-hour dietary records | Quintiles | 4.1 | Age, sex, EI,alcohol, number of 24-hour dietary records, smoking,education, PA, height, BMI,family history of cancer, lipids intake,fruit intake, vegetable intake, number of children, red meat intake, processed meat intake | CRC | 7 |
| Islam 2019 (pooled 6 studies) [79, 80] | Japan | 232,403 | 40–79 | Men and women | FFQ | Quartiles | ≥ 10.0 |
Age, area, history of diabetes, BMI,smoking, alcohol, PA, EI, calcium intake, fiber intake |
CRC, RC, CC | 7 |
| Mehta 2020 (Sister study) [81] | USA and Puerto Rico | 48,704 | 35–74 | Women | FFQ | Quartiles | 8.7 | EI, BMI,education, PA, race/ethnicity,family history of CRC | CRC, RC, CC | 7 |
| Nguyen 2020 (SMHS) [82] | China | 60,161 | 40–74 | Men | FFQ | Quartiles | 8.1 | Sex, age, education,income levels,smoking, alcohol intake,multivitamin use, family history ofCRC, BMI, PA, EI,metabolic condition | CRC, RC, CC | 8 |
| Barrubes 2020 (PREDIMED) [83] | Spain | 7,216 | 55–80 | Men and women | FFQ | Quartiles | 6.0 | Age, sex, intervention group, smoking, family history of cancer,education, history of diabetes, EI,aspirin use, weight, PA, plant food intake, fast food and processed food intake, sugar-sweetened beverage intake, alcohol | CRC | 6 |
| O’Sullivan 2020 (ATP) [84] | Canada | 26,460 | 50.9 | Men and women | Diet history questionnaire | Tertiles | 13.2 | Age, sex, BMI, fruit and vegetable intake,alcohol, PA, smoking,ethnicity, household income, education,family history of CRC, red or processed meat intake | CRC | 7 |
| Mejborn 2020 (DNSDPA) [85] | Denmark | 6,282 | 54.0 | Men and women | 7-day pre-coded food diaries | Tertiles | 10.8 | Sex, education,ethnicity, smoking,PA,alcohol, BMI,EI | CRC | 6 |
| Zhang 2020 (PLCO) [86] | USA | 95,962 | 55–74 | Men and women | Diet history questionnaire | Tertiles | 8.9 | Age, sex, race, education, smoking, aspirin use, history of diabetes, family history of PC, EI,PA, weight, diet rich in whole grains,vegetables, fruit, and beans, ultra-processed food intake, sugar-sweetened drink intake,alcohol, breastfeeding | PC | 9 |
| Knuppel 2020 (UK Biobank) [87–90] | UK | 474,996 | 37–73 | Men and women | FFQ | Quintiles | 6.9 | Age, region, ethnicity, deprivation,qualification, employment, living with spouse/partner, height, smoking, PA, alcohol, fruit and vegetable intake, cereal fiber intake, cheese intake,milk added to tea/coffee/cereal, oily fish intake, non-oily fish intake, menopausal status, parity, HRT, OC use | EC, GC, CRC, CC, RC, HCC, PC | 8 |
| Collatuzzo 2022 (GCS) [91] | Iran | 50,045 | 40–75 | Men and women | FFQ | Quintiles | 12.0 | Age, sex, BMI, ethnicity, place of residence, education and hot tea consumption | PC, EC, GC | 7 |
*AHS Adventist Health Study, ATBC Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study, ATP Alberta’s Tomorrow Project, BCDDP Breast Cancer Detection Demonstration Project, BMI Body mass index, CC Colon cancer, CKB China Kadoorie Biobank, CNBSS Canadian National BreastScreening Study, CRC Colorectal cancer, CSEC CancerScreening Examination Cohort of the National Cancer Centerof Korea, EI Energy intake, EPIC European Prospective Investigation into Cancerand Nutrition, FFQ Food-frequency questionnaire, GC Gastric cancer, GCS Golestan Cohort Study, HPFS Health Professionals Follow Up Study, HRT Hormone replacement therapy, IWHS Iowa Women’s Health Study, JAH Japanese Ancestry in Hawaii, JRH Japanese residents of Hawaii, LSS Life Span Study, MCCS Melbourne Collaborative Cohort Study, MEC Multiethnic Cohort Study, NHS Nurses’ Health Study, NIH-AARP National Institutes of Health-American Association for Retired Persons, NLCS Netherlands Cohort Study, NSS NutriNet-Sante Study, NYUWHS New York University Women’s Health Study, PA Physical activity, PC Pancreatic cancer, PLCO Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, PREDIMED Spanish PREvencion con DIeta MEDiterranea, RC Rectal cancer, SCHS Singapore Chinese Health Study, SMC Swedish Mammography Cohort, STR Swedish Twin Registry, SWHS Shanghai Women’s Health Study, UKWCS UK Women’s Cohort Study, VITAL VITamins And Lifestyle, WHS Women’s Health Study
EC
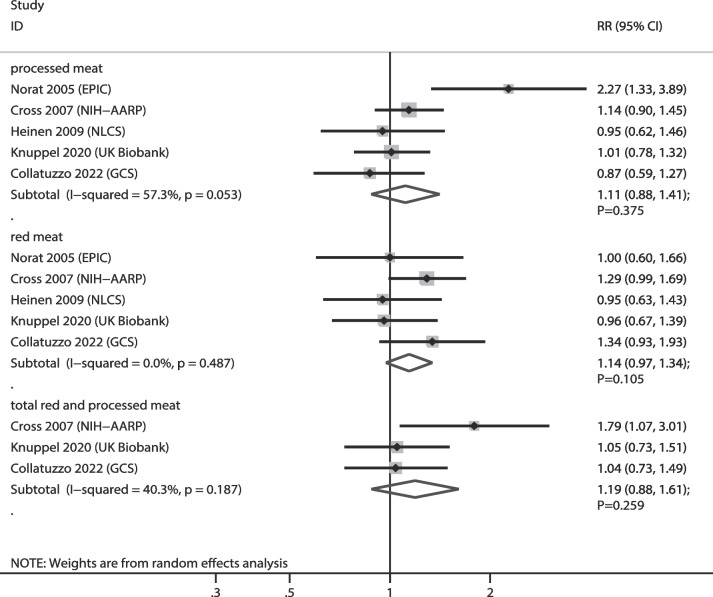
The numbers of cohorts that reported the associations of red meat, processed meat, and total red and processed meat consumption with EC risk were 5, 5, and 3 cohorts, respectively. The summary results indicated that higher consumption of red meat (RR: 1.14; 95% CI: 0.97–1.34; P = 0.105), processed meat (RR: 1.11; 95% CI: 0.88–1.41; P = 0.375), and total red and processed meat (RR: 1.19; 95% CI: 0.88–1.61; P = 0.259) were not associated with the risk of EC (Fig. 2). Moreover, we noted a significant heterogeneity in the relationship between processed meat consumption and EC (I2 = 57.3%; P = 0.053). Sensitivity analyses revealed that the pooled conclusions for the relationship between red meat, processed meat, and total red and processed meat consumption and EC risk were robust (Supplementary file 1). The results of the subgroup analyses were consistent with those of the overall analyses, and the results showed no significant associations (Table 2). There was no significant publication bias for red (P value for Egger: 0.230; P value for Begg: 0.806) and processed meat (P value for Egger: 0.540; P value for Begg: 0.806) consumption, whereas there was a significant publication bias for total red and processed meat consumption (P value for Egger: 0.018; P value for Begg: 0.296) (Supplementary file 2).
Fig. 2.
Association of meat consumption with the risk of esophageal cancer. RR: relative risk; CI: confidence interval
Table 2.
Subgroup analyses for the association between meat consumption and the risk of gastrointestinal cancer
| Outcomes | Exposure | Factors | Subgroups | RR and 95%CI | P value | Heterogeneity (%) | RRR between subgroups |
|---|---|---|---|---|---|---|---|
| EC | Red meat | Country | Western | 1.10 (0.87–1.40) | 0.428 | 33.4 | 0.82 (0.53–1.27)a |
| Eastern | 1.34 (0.93–1.93) | 0.116 | - | ||||
| Sex | Male | 1.35 (0.57–3.20) | 0.496 | 75.7 | 1.23 (0.50–3.03)b | ||
| Female | 1.10 (0.84–1.45) | 0.496 | 0.0 | ||||
| Follow-up | ≥ 10.0 | 1.10 (0.72–1.68) | 0.662 | 45.8 | 0.96 (0.59–1.55)c | ||
| < 10.0 | 1.15 (0.91–1.45) | 0.252 | 25.6 | ||||
| Adjusted level | High | 1.10 (0.87–1.40) | 0.428 | 33.4 | 0.82 (0.53–1.27)d | ||
| Moderate | 1.34 (0.93–1.93) | 0.116 | - | ||||
| Processed meat | Country | Western | 1.17 (0.89–1.55) | 0.262 | 56.7 | 1.34 (0.84–2.17)a | |
| Eastern | 0.87 (0.59–1.28) | 0.476 | - | ||||
| Sex | Male | 1.44 (0.81–2.58) | 0.218 | 51.3 | 1.62 (0.80–3.26)b | ||
| Female | 0.89 (0.60–1.32) | 0.563 | 26.3 | ||||
| Follow-up | ≥ 10.0 | 0.94 (0.60–1.49) | 0.798 | 49.3 | 0.75 (0.44–1.29)c | ||
| < 10.0 | 1.25 (0.94–1.67) | 0.127 | 60.3 | ||||
| Adjusted level | High | 1.17 (0.89–1.55) | 0.262 | 56.7 | 1.34 (0.84–2.17)d | ||
| Moderate | 0.87 (0.59–1.28) | 0.476 | - | ||||
| Total red and processed meat | Country | Western | 1.33 (0.79–2.23) | 0.286 | 63.5 | 1.28 (0.68–2.40)a | |
| Eastern | 1.04 (0.73–1.49) | 0.829 | - | ||||
| Sex | Male | - | - | - | - | ||
| Female | - | - | - | ||||
| Follow-up | ≥ 10.0 | 1.04 (0.73–1.49) | 0.829 | - | 0.78 (0.42–1.47)c | ||
| < 10.0 | 1.33 (0.79–2.23) | 0.286 | 63.5 | ||||
| Adjusted level | High | 1.33 (0.79–2.23) | 0.286 | 63.5 | 1.28 (0.68–2.40)d | ||
| Moderate | 1.04 (0.73–1.49) | 0.829 | - | ||||
| GC | Red meat | Country | Western | 1.02 (0.86–1.21) | 0.805 | 24.7 | 1.00 (0.80–1.25)a |
| Eastern | 1.02 (0.88–1.17) | 0.818 | 31.1 | ||||
| Sex | Male | 1.01 (0.87–1.17) | 0.885 | 0.0 | 1.12 (0.88–1.43)b | ||
| Female | 0.90 (0.74–1.09) | 0.268 | 0.0 | ||||
| Follow-up | ≥ 10.0 | 1.01 (0.91–1.12) | 0.869 | 0.0 | 0.97 (0.72–1.32)c | ||
| < 10.0 | 1.04 (0.78–1.38) | 0.792 | 55.7 | ||||
| Adjusted level | High | 0.98 (0.88–1.10) | 0.758 | 12.2 | 0.84 (0.64–1.12)d | ||
| Moderate | 1.16 (0.90–1.50) | 0.250 | 31.6 | ||||
| Processed meat | Country | Western | 1.11 (0.94–1.33) | 0.221 | 42.4 | 1.12 (0.90–1.40)a | |
| Eastern | 0.99 (0.86–1.13) | 0.871 | 14.0 | ||||
| Sex | Male | 1.02 (0.88–1.18) | 0.774 | 0.0 | 0.89 (0.62–1.27)b | ||
| Female | 1.15 (0.83–1.59) | 0.404 | 62.1 | ||||
| Follow-up | ≥ 10.0 | 1.08 (0.94–1.25) | 0.276 | 35.3 | 1.03 (0.74–1.42)c | ||
| < 10.0 | 1.05 (0.78–1.40) | 0.759 | 61.0 | ||||
| Adjusted level | High | 1.07 (0.92–1.24) | 0.382 | 50.4 | 0.97 (0.72–1.31)d | ||
| Moderate | 1.10 (0.85–1.42) | 0.479 | 20.9 | ||||
| Total red and processed meat | Country | Western | 0.97 (0.76–1.24) | 0.804 | 8.6 | 0.98 (0.72–1.34)a | |
| Eastern | 0.99 (0.81–1.20) | 0.896 | 45.6 | ||||
| Sex | Male | 0.96 (0.82–1.13) | 0.618 | - | 1.17 (0.88–1.55)b | ||
| Female | 0.82 (0.65–1.03) | 0.091 | - | ||||
| Follow-up | ≥ 10.0 | 0.98 (0.78–1.23) | 0.873 | 61.9 | 0.99 (0.72–1.35)c | ||
| < 10.0 | 0.99 (0.80–1.23) | 0.927 | 0.0 | ||||
| Adjusted level | High | 0.93 (0.83–1.04) | 0.221 | 0.0 | 0.68 (0.46-1.00) d | ||
| Moderate | 1.37 (0.94–1.99) | 0.100 | - | ||||
| CRC | Red meat | Country | Western | 1.12 (1.04–1.19) | 0.001 | 20.4 | 1.11 (0.98–1.26)a |
| Eastern | 1.01 (0.91–1.13) | 0.830 | 0.0 | ||||
| Sex | Male | 0.97 (0.80–1.18) | 0.789 | 0.0 | 0.92 (0.75–1.14)b | ||
| Female | 1.05 (0.96–1.15) | 0.298 | 0.0 | ||||
| Follow-up | ≥ 10.0 | 1.06 (0.98–1.15) | 0.131 | 0.0 | 0.96 (0.84–1.11)c | ||
| < 10.0 | 1.10 (0.98–1.23) | 0.092 | 54.4 | ||||
| Adjusted level | High | 1.08 (1.01–1.15) | 0.020 | 28.0 | 0.86 (0.68–1.10)d | ||
| Moderate | 1.25 (0.99–1.59) | 0.058 | 0.0 | ||||
| Processed meat | Country | Western | 1.20 (1.13–1.29) | < 0.001 | 19.6 | 1.02 (0.89–1.16)a | |
| Eastern | 1.18 (1.06–1.32) | 0.003 | 0.0 | ||||
| Sex | Male | 1.27 (1.08–1.50) | 0.005 | 23.8 | 1.11 (0.91–1.36)b | ||
| Female | 1.14 (1.02–1.28) | 0.023 | 0.0 | ||||
| Follow-up | ≥ 10.0 | 1.21 (1.12–1.32) | < 0.001 | 0.0 | 1.03 (0.91–1.05)c | ||
| < 10.0 | 1.18 (1.09–1.29) | < 0.001 | 25.7 | ||||
| Adjusted level | High | 1.18 (1.12–1.24) | < 0.001 | 0.0 | 0.91 (0.71–1.16)d | ||
| Moderate | 1.30 (1.03–1.66) | 0.030 | 43.0 | ||||
| Total red and processed meat | Country | Western | 1.15 (1.07–1.23) | < 0.001 | 1.5 | 1.11 (0.95–1.28)a | |
| Eastern | 1.04 (0.91–1.18) | 0.574 | 0.0 | ||||
| Sex | Male | 1.21 (0.98–1.49) | 0.070 | 0.0 | 1.09 (0.85–1.40)b | ||
| Female | 1.11 (0.97–1.26) | 0.128 | 15.4 | ||||
| Follow-up | ≥ 10.0 | 1.20 (1.05–1.37) | 0.007 | 0.0 | 1.08 (0.92–1.27)c | ||
| < 10.0 | 1.11 (1.02–1.22) | 0.016 | 23.3 | ||||
| Adjusted level | High | 1.13 (1.05–1.21) | 0.001 | 10.9 | 0.97 (0.76–1.23)d | ||
| Moderate | 1.17 (0.92–1.47) | 0.195 | 0.0 | ||||
| CC | Red meat | Country | Western | 1.18 (1.03–1.35) | 0.015 | 7.2 | 1.10 (0.91–1.34)a |
| Eastern | 1.07 (0.93–1.24) | 0.346 | 0.0 | ||||
| Sex | Male | 1.05 (0.78–1.41) | 0.747 | - | 1.02 (0.73–1.42)b | ||
| Female | 1.03 (0.89–1.19) | 0.675 | 0.0 | ||||
| Follow-up | ≥ 10.0 | 1.05 (0.92–1.20) | 0.443 | 0.0 | 0.85 (0.71–1.03)c | ||
| < 10.0 | 1.23 (1.08–1.42) | 0.003 | 0.0 | ||||
| Adjusted level | High | 1.14 (1.02–1.27) | 0.021 | 13.0 | 1.07 (0.75–1.52)d | ||
| Moderate | 1.07 (0.76–1.50) | 0.708 | 0.0 | ||||
| Processed meat | Country | Western | 1.24 (1.12–1.38) | < 0.001 | 0.0 | 0.99 (0.80–1.23)a | |
| Eastern | 1.25 (1.03–1.50) | 0.022 | 0.0 | ||||
| Sex | Male | 1.24 (1.04–1.49) | 0.018 | 0.0 | 0.99 (0.78–1.26)b | ||
| Female | 1.25 (1.06–1.47) | 0.007 | 0.0 | ||||
| Follow-up | ≥ 10.0 | 1.25 (1.09–1.44) | 0.002 | 0.0 | 1.01 (0.84–1.22)c | ||
| < 10.0 | 1.24 (1.09–1.40) | 0.001 | 0.0 | ||||
| Adjusted level | High | 1.22 (1.11–1.35) | < 0.001 | 0.0 | 0.87 (0.64–1.17)d | ||
| Moderate | 1.41 (1.06–1.88) | 0.019 | 0.0 | ||||
| Total red and processed meat | Country | Western | 1.24 (1.10–1.41) | 0.001 | 0.0 | 1.06 (0.79–1.42)a | |
| Eastern | 1.17 (0.90–1.52) | 0.239 | 62.3 | ||||
| Sex | Male | 1.28 (1.02–1.61) | 0.032 | 0.0 | 1.12 (0.84–1.51)b | ||
| Female | 1.14 (0.95–1.38) | 0.158 | 4.1 | ||||
| Follow-up | ≥ 10.0 | 1.26 (1.05–1.51) | 0.014 | 0.0 | 1.10 (0.85–1.40)c | ||
| < 10.0 | 1.15 (0.97–1.36) | 0.115 | 50.1 | ||||
| Adjusted level | High | 1.19 (1.04–1.35) | 0.012 | 40.0 | 1.07 (0.67–1.71)d | ||
| Moderate | 1.11 (0.71–1.74) | 0.635 | 0.0 | ||||
| RC | Red meat | Country | Western | 1.33 (1.03–1.72) | 0.029 | 44.5 | 1.41 (0.95–2.11)a |
| Eastern | 0.94 (0.69–1.27) | 0.678 | 22.4 | ||||
| Sex | Male | 1.21 (0.67–2.18) | 0.527 | - | 1.07 (0.54–2.13)b | ||
| Female | 1.13 (0.79–1.61) | 0.518 | 59.8 | ||||
| Follow-up | ≥ 10.0 | 1.23 (0.94–1.60) | 0.134 | 35.6 | 1.07 (0.68–1.69)c | ||
| < 10.0 | 1.15 (0.79–1.66) | 0.467 | 58.8 | ||||
| Adjusted level | High | 1.10 (0.91–1.33) | 0.332 | 32.4 | 0.50 (0.28–0.91) d | ||
| Moderate | 2.18 (1.25–3.81) | 0.006 | 0.0 | ||||
| Processed meat | Country | Western | 1.29 (1.07–1.56) | 0.007 | 19.9 | 1.13 (0.84–1.53)a | |
| Eastern | 1.14 (0.90–1.44) | 0.277 | 0.0 | ||||
| Sex | Male | 1.03 (0.79–1.36) | 0.824 | 0.0 | 0.94 (0.65–1.38)b | ||
| Female | 1.09 (0.84–1.43) | 0.515 | 6.5 | ||||
| Follow-up | ≥ 10.0 | 1.09 (0.91–1.31) | 0.345 | 0.0 | 0.77 (0.60-1.00) c | ||
| < 10.0 | 1.41 (1.17–1.69) | < 0.001 | 0.0 | ||||
| Adjusted level | High | 1.20 (1.05–1.37) | 0.007 | 0.0 | 0.63 (0.38–1.06)d | ||
| Moderate | 1.90 (1.15–3.13) | 0.012 | 0.0 | ||||
| Total red and processed meat | Country | Western | 1.32 (1.11–1.57) | 0.002 | 0.0 | 1.26 (0.98–1.61)a | |
| Eastern | 1.05 (0.88–1.26) | 0.587 | 0.0 | ||||
| Sex | Male | 0.93 (0.58–1.49) | 0.763 | - | 0.84 (0.48–1.46)b | ||
| Female | 1.11 (0.83–1.49) | 0.478 | 0.0 | ||||
| Follow-up | ≥ 10.0 | 1.09 (0.85–1.39) | 0.507 | 0.0 | 0.85 (0.61–1.18)c | ||
| < 10.0 | 1.28 (1.03–1.60) | 0.025 | 44.9 | ||||
| Adjusted level | High | 1.19 (1.03–1.37) | 0.021 | 14.3 | 0.65 (0.21-2.00)d | ||
| Moderate | 1.82 (0.60–5.52) | 0.290 | - | ||||
| PC | Red meat | Country | Western | 1.03 (0.87–1.21) | 0.764 | 55.0 | 1.91 (0.87–4.18)a |
| Eastern | 0.54 (0.25–1.16) | 0.116 | - | ||||
| Sex | Male | 0.89 (0.70–1.14) | 0.360 | 0.0 | 0.82 (0.58–1.15)b | ||
| Female | 1.09 (0.86–1.38) | 0.494 | 33.3 | ||||
| Follow-up | ≥ 10.0 | 0.94 (0.61–1.44) | 0.777 | 63.6 | 0.91 (0.57–1.45)c | ||
| < 10.0 | 1.03 (0.86–1.23) | 0.779 | 55.2 | ||||
| Adjusted level | High | 1.03 (0.86–1.22) | 0.770 | 59.5 | 1.37 (0.73–2.58)d | ||
| Moderate | 0.75 (0.41–1.38) | 0.357 | 27.4 | ||||
| Processed meat | Country | Western | 0.99 (0.83–1.18) | 0.925 | 68.7 | 0.85 (0.43–1.68)a | |
| Eastern | 1.16 (0.60–2.23) | 0.657 | - | ||||
| Sex | Male | 0.99 (0.76–1.30) | 0.953 | 59.2 | 1.09 (0.78–1.51)b | ||
| Female | 0.91 (0.75–1.09) | 0.293 | 28.5 | ||||
| Follow-up | ≥ 10.0 | 0.88 (0.71–1.10) | 0.275 | 0.0 | 0.84 (0.62–1.13)c | ||
| < 10.0 | 1.05 (0.86–1.29) | 0.639 | 74.1 | ||||
| Adjusted level | High | 0.99 (0.83–1.17) | 0.870 | 68.2 | 0.98 (0.46–2.09)d | ||
| Moderate | 1.01 (0.48–2.10) | 0.984 | 69.5 | ||||
| Total red and processed meat | Country | Western | 1.04 (0.87–1.24) | 0.699 | 43.4 | 0.80 (0.62–1.03)a | |
| Eastern | 1.30 (1.09–1.56) | 0.004 | - | ||||
| Sex | Male | 0.86 (0.65–1.14) | 0.287 | 0.0 | 0.96 (0.65–1.40)b | ||
| Female | 0.90 (0.70–1.17) | 0.439 | 0.0 | ||||
| Follow-up | ≥ 10.0 | 1.06 (0.72–1.56) | 0.761 | 0.0 | 0.97 (0.64–1.49)c | ||
| < 10.0 | 1.09 (0.91–1.30) | 0.363 | 59.3 | ||||
| Adjusted level | High | 1.08 (0.93–1.27) | 0.305 | 48.3 | - | ||
| Moderate | - | - | - | ||||
| HCC | Red meat | Country | Western | 1.04 (0.99–1.09) | 0.103 | 0.0 | 0.90 (0.68–1.19)a |
| Eastern | 1.16 (0.88–1.53) | 0.299 | - | ||||
| Sex | Male | 1.02 (0.97–1.08) | 0.442 | 0.0 | 0.92 (0.81–1.04)b | ||
| Female | 1.11 (1.00-1.24) | 0.055 | 0.0 | ||||
| Follow-up | ≥ 10.0 | 1.13 (0.89–1.43) | 0.315 | 0.0 | 1.09 (0.85–1.38)c | ||
| < 10.0 | 1.04 (0.99–1.09) | 0.106 | 0.0 | ||||
| Adjusted level | High | 1.04 (1.00-1.09) | 0.075 | 0.0 | - | ||
| Moderate | - | - | - | ||||
| Processed meat | Country | Western | 0.97 (0.85–1.10) | 0.647 | 50.0 | 0.84 (0.63–1.13)a | |
| Eastern | 1.15 (0.89–1.49) | 0.288 | - | ||||
| Sex | Male | 1.37 (0.54–3.46) | 0.502 | 86.1 | 1.32 (0.49–3.53)b | ||
| Female | 1.04 (0.74–1.44) | 0.837 | 37.7 | ||||
| Follow-up | ≥ 10.0 | 1.43 (0.96–2.13) | 0.081 | 47.2 | 1.55 (1.04–2.32) c | ||
| < 10.0 | 0.92 (0.87–0.97) | 0.002 | 0.0 | ||||
| Adjusted level | High | 1.00 (0.88–1.13) | 0.979 | 52.3 | - | ||
| Moderate | - | - | - | ||||
| Total red and processed meat | Country | Western | 0.94 (0.67–1.32) | 0.724 | 20.9 | 0.74 (0.48–1.15)a | |
| Eastern | 1.27 (0.96–1.68) | 0.094 | - | ||||
| Sex | Male | 1.69 (0.74–3.87) | 0.214 | - | 1.71 (0.58–5.06)b | ||
| Female | 0.99 (0.49-2.00) | 0.978 | - | ||||
| Follow-up | ≥ 10.0 | 1.26 (0.99–1.62) | 0.065 | 0.0 | 1.54 (0.96–2.45)c | ||
| < 10.0 | 0.82 (0.55–1.22) | 0.324 | 23.2 | ||||
| Adjusted level | High | 1.05 (0.80–1.39) | 0.709 | 34.1 | - | ||
| Moderate | - | - | - |
*acompared Western countries with Eastern countries; bcompared male with female; ccompared follow-up ≥ 10.0 years with follow-up < 10.0 years; dcompared high adjusted level with moderate adjusted level
GC
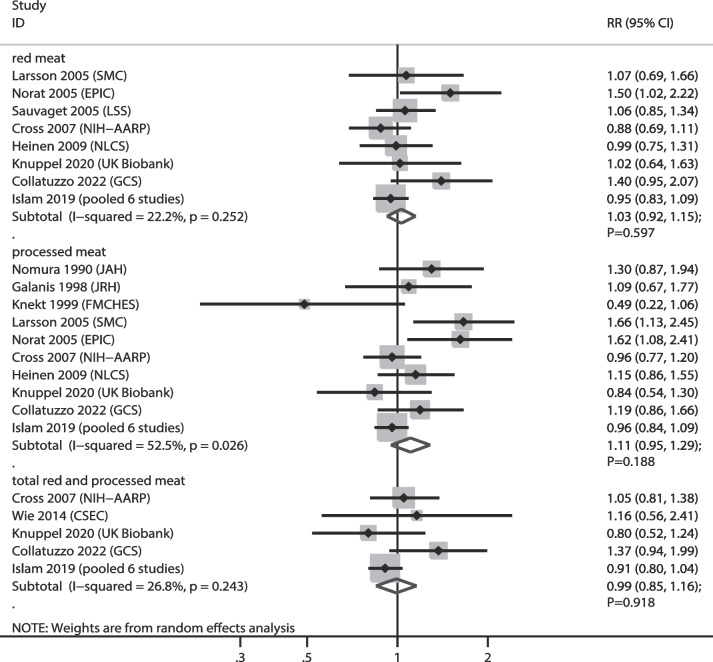
The numbers of cohorts that reported the associations of red meat, processed meat, and total red and processed meat consumptions with GC risk were 8, 10, and 5 cohorts, respectively. We noted that higher consumption of red meat (RR: 1.03; 95% CI: 0.92–1.15; P = 0.597), processed meat (RR: 1.11; 95% CI: 0.95–1.29; P = 0.188), and total red and processed meat (RR: 0.99; 95% CI: 0.85–1.16; P = 0.918) were not associated with GC risk (Fig. 3). There was a significant heterogeneity in the relationship between processed meat consumption and GC (I2 = 52.5%; P = 0.026). Sensitivity analyses indicated that the associations between red meat, processed meat, and total red and processed meat consumption with GC risk were stable, and no significant associations were observed (Supplementary file 1). Subgroup analyses indicated no significant association between red meat, processed meat, and total red and processed meat with GC risk in all subsets (Table 2). No significant publication bias was observed for red meat (P value for Egger: 0.095; P value for Begg: 0.536), processed meat (P value for Egger: 0.395; P value for Begg: 1.000), and total red and processed meat consumption (P value for Egger: 0.388; P value for Begg: 0.806) (Supplementary file 2).
Fig. 3.
Association of meat consumption with the risk of gastric cancer. RR: relative risk; CI: confidence interval
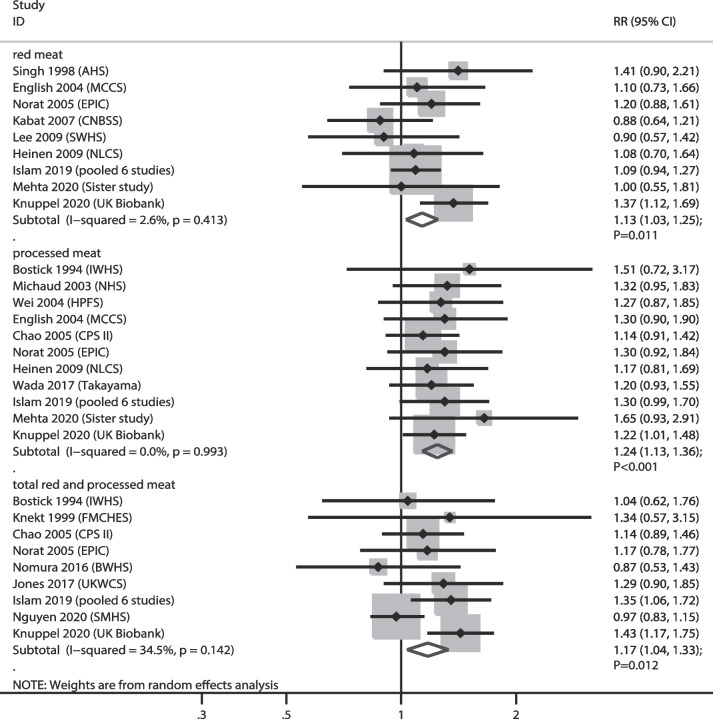
CRC
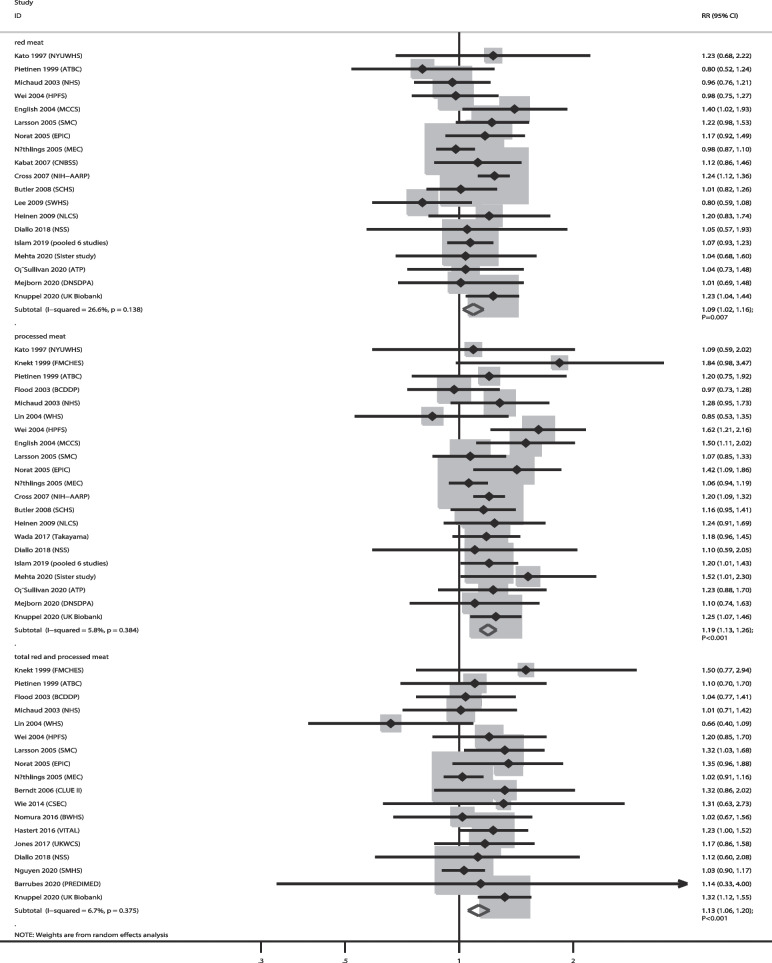
The numbers of cohorts that reported the associations of red meat, processed meat, and total red and processed meat consumption with CRC risk were 19, 21, and 18, respectively. The summary results indicated that higher consumption of red meat (RR: 1.09; 95% CI: 1.02–1.16; P = 0.007), processed meat (RR: 1.19; 95% CI: 1.13–1.26; P < 0.001), and total red and processed meat (RR: 1.13; 95% CI: 1.06–1.20; P < 0.001) were associated with an increased risk of CRC, and no significant heterogeneity was observed across the included studies (Fig. 4). Sensitivity analysis indicated that the pooled conclusions regarding the relationship between red meat, processed meat, and total red and processed meat consumption with GC risk were not altered by the sequential removal of a single study (Supplementary file 1). Subgroup analyses revealed that higher red meat consumption was associated with an increased risk of CRC when pooled studies were conducted in Western countries and studies with high adjusted levels; higher processed meat consumption was associated with an increased risk of CRC in all subgroups, and higher total red and processed meat consumption was associated with an increased risk of CRC when pooled studies were conducted in Western countries, irrespective of follow-up duration and studies with high adjusted levels (Table 2). There was no significant publication bias for red meat (P value for Egger: 0.302; P value for Begg: 0.726), processed meat (P value for Egger: 0.305; P value for Begg: 0.928), and total red and processed meat consumption (P value for Egger: 0.511; P value for Begg: 1.000) (Supplementary file 2).
Fig. 4.
Association of meat consumption with the risk of colorectal cancer. RR: relative risk; CI: confidence interval
CC
The numbers of cohorts that reported the associations of red meat, processed meat, and total red and processed meat consumption with the risk of CC were 9, 11, and 9 cohorts, respectively. We noted that higher consumption of red meat (RR: 1.13; 95% CI: 1.03–1.25; P = 0.011), processed meat (RR: 1.24; 95% CI: 1.13–1.36; P < 0.001), and total red and processed meat (RR: 1.17; 95% CI: 1.04–1.33; P = 0.012) were associated with an increased risk of CC, and no significant heterogeneity was observed across the included studies (Fig. 5). Sensitivity analyses indicated that the pooled conclusions for the relationship between red meat and total red and processed meat consumption with CC risk were variables with marginal 95% CI (Supplementary file 1). Subgroup analyses indicated that higher red meat consumption was associated with an increased risk of CC when pooled studies were conducted in Western countries, follow-up < 10.0 years, and studies with high adjusted levels. The relationship of processed meat consumption with the risk of CC was statistically significant in all subgroups; high total red and processed meat consumption was associated with an increased risk of CC when pooled studies were conducted in Western countries, male sex, follow-up ≥ 10.0 years, and studies with high adjusted levels (Table 2). We noted no significant publication bias for red meat (P value for Egger: 0.602; P value for Begg: 0.602), and total red and processed meat consumption (P value for Egger: 0.879; P value for Begg: 0.602), whereas a significant publication bias was observed for processed meat consumption (P value for Egger: 0.010; P value for Begg: 0.119) (Supplementary file 2).
Fig. 5.
Association of meat consumption with the risk of colon cancer. RR: relative risk; CI: confidence interval
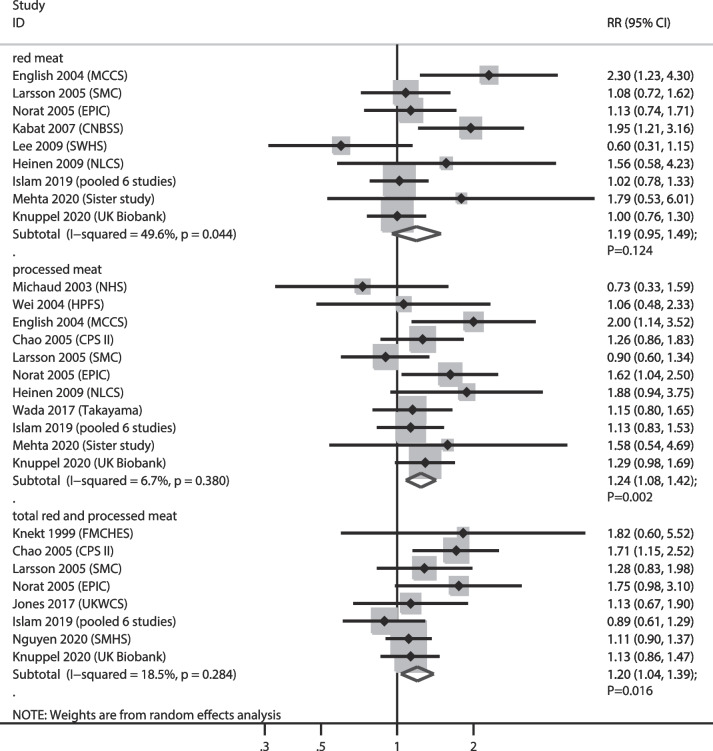
RC
The numbers of cohorts that reported the associations of red meat, processed meat, and total red and processed meat consumption with the risk of RC were 9, 11, and 8 cohorts, respectively. We noted that higher consumption of processed meat (RR: 1.24; 95% CI:–1.08–1.42; P = 0.002) and total red and processed meat (RR: 1.20; 95% CI: 1.04–1.39; P = 0.016) were associated with an increased risk of RC, while red meat consumption was not associated with the risk of RC (RR: 1.19; 95% CI: 0.95–1.49; P = 0.124). Moreover, we noted significant heterogeneity in the relationship between red meat consumption and RC among the included studies (I2 = 49.6%; P = 0.044) (Fig. 6). Sensitivity analyses indicated that the pooled conclusions for the relationship between red meat and total red and processed meat consumption and the risk of RC were variable (Supplementary File 1). Subgroup analyses revealed that higher consumption of red meat intake was associated with an increased risk of RC when pooled studies were conducted in Western countries, and pooled studies with moderately adjusted levels, and the strength of relation in the subgroups of studies with higher adjusted levels was significantly lower than those of studies with moderate adjusted levels (RRR: 0.50; 95% CI: 0.28–0.91). Furthermore, higher processed meat consumption was associated with an increased risk of RC when pooled studies were conducted in Western countries, follow-up duration < 10.0 years, and irrespective of the adjusted level, while the strength of the relationship in the subgroup with longer follow-up duration was significantly lower than that in the subgroup with shorter follow-up duration (RRR: 0.77; 95% CI: 0.60–1.00) (Table 2). There was no significant publication bias for red meat (P value for Egger: 0.258; P value for Begg: 0.251), processed meat (P value for Egger: 0.657; P value for Begg: 0.640), and total red and processed meat consumption (P value for Egger: 0.208; P value for Begg: 0.174) (Supplementary file 2).
Fig. 6.
Association of meat consumption with the risk of rectal cancer. RR: relative risk; CI: confidence interval
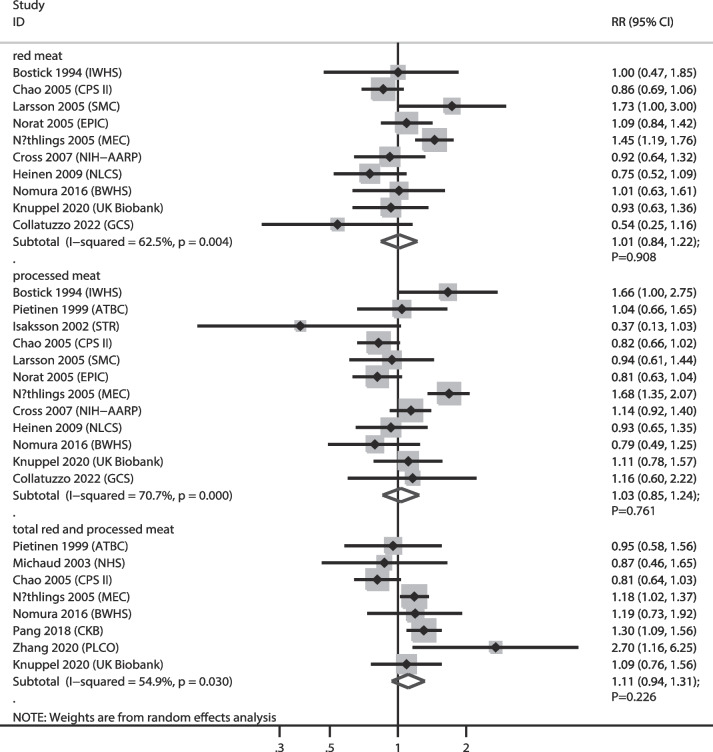
PC
The numbers of cohorts that reported the associations of red meat, processed meat, and total red and processed meat consumption with PC risk were 10, 12, and 8 cohorts, respectively. The summary results indicated that higher consumption of red meat (RR: 1.01; 95% CI: 0.84–1.22; P = 0.908), processed meat (RR: 1.03; 95% CI: 0.85–1.24; P = 0.761), and total red and processed meat (RR: 1.11; 95% CI: 0.94–1.31; P = 0.226) were not associated with PC risk, and significant heterogeneity was observed for red meat (I2 = 62.5%; P = 0.004), processed meat (I2 = 70.7%; P < 0.001), and total red and processed meat consumption (I2 = 54.9%; P = 0.030) (Fig. 7). Sensitivity analysis indicated that higher total red and processed meat consumption were associated with an increased risk of PC after removing the CPS II cohort [42, 43] (Supplementary file 1). Subgroup analyses revealed that total red and processed meat consumption were associated with an increased risk of PC when pooled studies were conducted in Eastern countries (Table 2). No significant publication bias for red meat (P value for Egger: 0.365; P value for Begg: 1.000), processed meat (P value for Egger: 0.458; P value for Begg: 0.945), and total red and processed meat consumption (P value for Egger: 0.928; P value for Begg: 0.902) was observed (Supplementary file 2).
Fig. 7.
Association of meat consumption with the risk of pancreatic cancer. RR: relative risk; CI: confidence interval
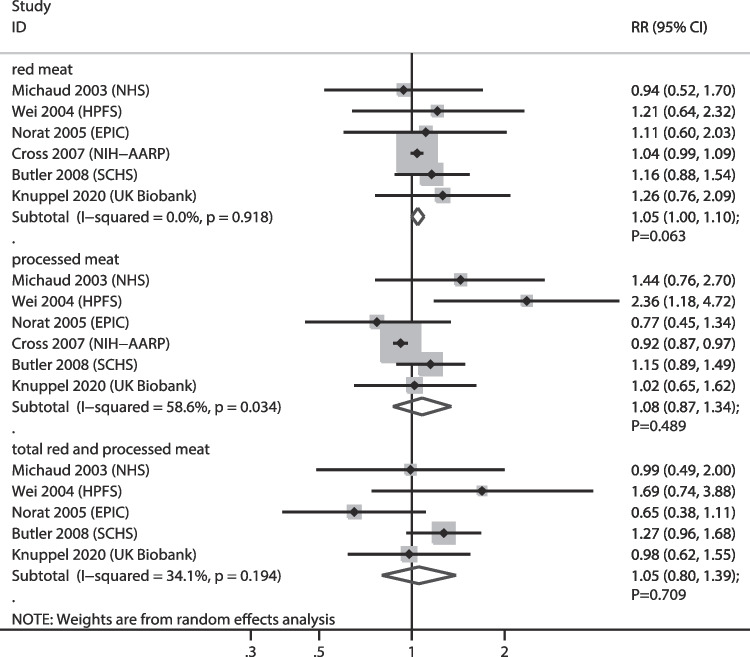
HCC
The numbers of cohorts that reported the associations of red meat, processed meat, and total red and processed meat consumption with HCC risk were 6, 6, and 5 cohorts, respectively. Red meat (RR: 1.05; 95% CI: 1.00–1.10; P = 0.063), processed meat (RR: 1.08; 95% CI: 0.87–1.34; P = 0.489), and total red and processed meat consumption (RR: 1.05; 95% CI: 0.80–1.39; P = 0.709) were not associated with HCC risk, and significant heterogeneity was observed for the relationship between processed meat consumption and HCC (I2 = 58.6%; P = 0.034) (Fig. 8). Sensitivity analysis indicated that higher red meat consumption was associated with an increased risk of HCC after removing the NHS cohort [35] (Supplementary file 1). Subgroup analyses revealed that processed meat consumption was associated with a reduced risk of HCC when the follow-up duration was < 10.0 years, and the strength for the subgroup of follow-up ≥ 10.0 years was greater than that of the follow-up < 10.0 years subgroup (RRR: 1.55; 95% CI: 1.04–2.32). There was no significant publication bias for red meat (P value for Egger: 0.170; P value for Begg: 1.000), processed meat (P value for Egger: 0.133; P value for Begg: 0.260), and total red and processed meat consumption (P value for Egger: 0.649; P value for Begg: 1.000) (Supplementary file 2).
Fig. 8.
Association of meat consumption with the risk of hepatocellular carcinoma. RR: relative risk; CI: confidence interval
Discussion
This systematic review and meta-analysis identified 40 cohorts and involved a total of 3,780,590 individuals with a wide range of characteristics. This study found that higher consumption of processed meat and total red and processed meat was associated with the risk of developing CRC, CC, and RC, while red meat was associated with an increased risk of developing CRC and CC. However, meat intake was not associated with the risk of EC, GC, PC, or HCC. Sensitivity analysis indicated that higher total red and processed meat consumption might increase risk of PC, and higher red meat consumption might cause an additional risk of HCC. Subgroup analyses revealed that the strength of higher consumption of total red and processed meat with the risk of GC in subgroup of high adjusted level was lower than subgroup of moderate adjusted level. Moreover, the strength of higher consumption of red meat with the risk of RC in subgroup of high adjusted level was lower than subgroup of moderate adjusted level, while the strength of higher consumption of processed meat with the risk of RC in subgroup of follow-up ≥ 10.0 years was lower than subgroup of follow-up < 10.0 years. Finally, the strength of higher consumption of processed meat with the risk of HCC in subgroup of follow-up ≥ 10.0 years was higher than subgroup of follow-up < 10.0 years.
Several systematic reviews and meta-analyses have illustrated the association between meat consumption and the risk of gastrointestinal cancer [9–12]. Yu et al. identified 17 observational studies and found that higher processed meat consumption was associated with an increased risk of HCC, while the risk of HCC was reduced in individuals who consumed higher amounts of white meat and fish [9]. Farvid et al. identified 148 studies and found that higher red meat consumption was associated with an increased risk of CRC, CC, RC, and HCC, while processed meat consumption was associated with an increased risk of CRC, CC, and RC. Moreover, they pointed out that total red and processed meat consumption were associated with an increased risk of CRC, CC, and RC [10]. Händel et al. identified 29 prospective cohort studies and found that high versus low processed meat consumption was associated with an increased risk of CRC, CC, and RC, and dose-response analysis reported similar outcomes [11]. Han et al. identified 56 cohorts and suggested that the absolute effects of red and processed meat consumption on cancer incidence are small, whereas reduction of processed meat is associated with a reduced risk of EC and CRC [12]. The current updated systematic review and meta-analysis was performed to assess the strength of the relationship between meat consumption and gastrointestinal cancer risk. Moreover, an exploratory analysis were performed according to the study or individual characteristics, including country, sex, follow-up duration, and adjusted level.
The summary of our results indicated higher red meat, processed meat, and total red and processed meat did not affect the risk of EC, which was inconsistent with prior meta-analysis [92]. Previous studies reported that red and processed meat consumption were associated with an increased risk of EC [92], and explained this by the high amounts of heme iron and N-glycolylneuraminic acid contained in red meat, which could catalyze lipid peroxidation and DNA damage, and potentially immunogenic molecules could induce tumors [93–95]. Moreover, processed meat contains high amounts of saturated fats, which play an important role in the risk of upper digestive and respiratory tract neoplasms [96, 97]. The inconsistent results between our study and prior meta-analyses could be explained by a prior study based on both prospective and retrospective observational studies, and the conclusion might be overestimated. Furthermore, the small number of studies in our study could explained an insignificant association of red and processed meat consumption with the risk of EC.
Our study indicated that meat intake was not associated with GC risk, and the conclusions were not affected by sensitivity and subgroup analyses. However, a prior meta-analysis suggested that red or processed meat consumption was associated with an increased risk of GC, whereas white meat could protect against GC risk [98]. Similar reasons to those for EC could explain these results. Moreover, DNA damage or oxidative stress caused by iron are important for the growth of H. pylori, which plays an important role in GC risk [99, 100]. Furthermore, the cooking method might play an important role in GC risk, including heterocyclic amines, polycyclic aromatic hydrocarbons, and salts [101, 102]. The conflicting results between the current study and prior studies could be explained by the fact that most of the included studies were case-control studies, and the causality relationship between meat consumption and GC was restricted.
Our study found that meat consumption plays an important role in the risk of CRC, CC, and RC, which is consistent with prior meta-analysis [11]. There were varies molecular pathways contributed the carcinogenesis across the regions of colon and rectum. The microsatellite instability, a CpG island methylator phenotype, and KRAS mutations were more evident for proximal colon cancers than rectal and distal colon tumors, whereas TP53 and APC mutations were more evident for rectal and distal colon tumors [103]. Moreover, heme iron could mediate the formation of intestinal carcinogenic compounds [95], and the progression of CRC could be affected by a specific bovine infectious factor [104]. Furthermore, chemical carcinogens, including heterocyclic amines and polycyclic aromatic hydrocarbons, play an important role in the risk of CRC. In addition, subgroup analyses revealed that the most significant associations were observed in Western countries, longer follow-up duration, and studies with moderately adjusted levels, which could be explained by the dietary structure between Western and Eastern countries; longer follow-up could result in a greater number of new cancers, and the power was stronger; and only a smaller number of included studies reported studies with moderately adjusted levels, and the pooled conclusion was not stable.
No significant association between meat consumption and PC risk was observed, which was not consistent with a previous meta-analysis that suggested that processed meat consumption was associated with an increased risk of PC, and red meat consumption was associated with an increased risk of PC in men but not in women [105]. They explained these results through N-nitroso compounds that could reach the pancreas via the bloodstream and act as potential carcinogens [106]. Subgroup analyses indicated that total red and processed meat consumption were associated with an increased risk of PC if pooled studies were conducted in Eastern countries, which could be explained by cooking methods for red meat and a smaller number of studies in this subgroup.
Higher meat consumption was not associated with the risk of HCC, irrespective of whether it is red, processed, or total red and processed meat, which is consistent with prior meta-analysis [107]. However, the association of red meat consumption with the risk of HCC was not stability, and red meat consumption was associated with an increased risk of HCC. The potential mechanism could be high levels of cholesterol and saturated fat in red meat is significantly related to the progression of cancer. Moreover, subgroup analyses revealed that processed meat was associated with a reduced risk of HCC when the follow-up duration was < 10.0 years. These results could be explained by the fact that HCC progression is significantly related to socioeconomic status, which could affect meat consumption [108, 109]. Other influencing factors included selection bias, random errors, and various adjusted levels.
Some limitations of this study should be acknowledged. First, the meat consumption definition was assessed using various methods, which could affect the actual acceptable daily consumption and the effect estimates for gastrointestinal cancer; (2) the heterogeneity across included studies was not fully explained by using sensitivity and subgroup analyses; (3) adjusted factors among included studies were different, which could affect the effect estimate for the relationship between meat consumption and gastrointestinal cancer risk; (4) the ratio between subgroups was calculated based on indirect comparisons, and the results needed further direct comparison; and (5) the analysis based on pooled data and individual data were not available, which restricted detailed analyses.
Conclusions
Our study found that higher meat consumption was associated with an increased risk of CRC, CC, and RC irrespective of whether it was red, processed, or total red and processed meat that was consumed. Moreover, the strength of the relationship between meat consumption and gastrointestinal cancer risk could be affected by follow-up duration and adjusted level. Further large-scale prospective studies should be performed to assess the potential effects of dietary interventions on the risk of gastrointestinal cancers.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- IARC
International Agency for Research on Cancer
- CRC
Colorectal cancer
- PC
Pancreatic cancer
- EC
Esophageal cancer
- GC
Gastric cancer
- CC
Colon cancer
- RC
Rectal cancer
- HCC
Hepatocellular carcinoma
- NOS
Newcastle-Ottawa Scale
- RR
Relative risk
- CI
Confidence interval
Authors’ contributions
YD and LD conceived and designed the study, analyzed the data and wrote the paper. LYG and HYH contributed reagents/materials/analysis tools. All author shave read and approved the final version of this manuscript.
Funding
There was no funding support for this study.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The meta-analysis based on public literature is not applicable for ethical approval.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, et al. Global burden of 5 major types of gastrointestinal Cancer. Gastroenterology. 2020;159:335–49e15. doi: 10.1053/j.gastro.2020.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Moehler M, Delic M, Goepfert K, Aust D, Grabsch HI, Halama N, et al. Immunotherapy in gastrointestinal cancer: recent results, current studies and future perspectives. Eur J Cancer. 2016;59:160–70. doi: 10.1016/j.ejca.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Abdul-Latif M, Townsend K, Dearman C, Shiu KK, Khan K. Immunotherapy in gastrointestinal cancer: the current scenario and future perspectives. Cancer Treat Rev. 2020;88:102030. doi: 10.1016/j.ctrv.2020.102030. [DOI] [PubMed] [Google Scholar]
- 5.Collaborators GD. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the global burden of Disease Study 2017. Lancet. 2019;393:1958–72. doi: 10.1016/S0140-6736(19)30041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 7.Bouvard V, Loomis D, Guyton KZ, Grosse Y, Ghissassi FE, Benbrahim-Tallaa L, et al. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16:1599–600. doi: 10.1016/S1470-2045(15)00444-1. [DOI] [PubMed] [Google Scholar]
- 8.Research WCRFAIfC. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. London; 2018.
- 9.Yu J, Liu Z, Liang D, Li J, Ma S, Wang G, et al. Meat intake and the risk of Hepatocellular Carcinoma: a Meta-analysis of Observational Studies. Nutr Cancer. 2022;74:3340–50. doi: 10.1080/01635581.2022.2077386. [DOI] [PubMed] [Google Scholar]
- 10.Farvid MS, Sidahmed E, Spence ND, Mante Angua K, Rosner BA, Barnett JB. Consumption of red meat and processed meat and cancer incidence: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. 2021;36:937–51. doi: 10.1007/s10654-021-00741-9. [DOI] [PubMed] [Google Scholar]
- 11.Händel MN, Rohde JF, Jacobsen R, Nielsen SM, Christensen R, Alexander DD, et al. Processed meat intake and incidence of colorectal cancer: a systematic review and meta-analysis of prospective observational studies. Eur J Clin Nutr. 2020;74:1132–48. doi: 10.1038/s41430-020-0576-9. [DOI] [PubMed] [Google Scholar]
- 12.Han MA, Zeraatkar D, Guyatt GH, Vernooij RWM, El Dib R, Zhang Y, et al. Reduction of Red and Processed Meat Intake and Cancer Mortality and incidence: a systematic review and Meta-analysis of Cohort Studies. Ann Intern Med. 2019;171:711–20. doi: 10.7326/M19-0699. [DOI] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 14.Wells G, Shea B, O’ Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa (ON): Ottawa Hospital Research Institute; 2009. [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. 2005;25:646–54. doi: 10.1177/0272989X05282643. [DOI] [PubMed] [Google Scholar]
- 17.Deeks JJ, Higgins JPT, Altman DG. Analyzing data and undertaking meta-analyses. Oxford, UK: The Cochrane Collaboration; 2008. [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tobias A. Assessing the influence of a single study in meta-analysis. Stata Tech Bull. 1999;47:15–7. [Google Scholar]
- 20.Woodward M. Epidemiology: study design and data analysis. Boca Raton, FL, USA: Chapman and Hall/CRC; 2005. [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 23.Nomura A, Grove JS, Stemmermann GN, Severson RK. A prospective study of stomach cancer and its relation to diet, cigarettes, and alcohol consumption. Cancer Res. 1990;50:627–31. [PubMed] [Google Scholar]
- 24.Bostick RM, Potter JD, Kushi LH, Sellers TA, Steinmetz KA, McKenzie DR, et al. Sugar, meat, and fat intake, and non-dietary risk factors for colon cancer incidence in Iowa women (United States) Cancer Causes Control. 1994;5:38–52. doi: 10.1007/BF01830725. [DOI] [PubMed] [Google Scholar]
- 25.Sellers TA, Bazyk AE, Bostick RM, Kushi LH, Olson JE, Anderson KE, et al. Diet and risk of colon cancer in a large prospective study of older women: an analysis stratified on family history (Iowa, United States) Cancer Causes Control. 1998;9:357–67. doi: 10.1023/a:1008886715597. [DOI] [PubMed] [Google Scholar]
- 26.Kato I, Akhmedkhanov A, Koenig K, Toniolo PG, Shore RE, Riboli E. Prospective study of diet and female colorectal cancer: the New York University Women’s Health Study. Nutr Cancer. 1997;28:276–81. doi: 10.1080/01635589709514588. [DOI] [PubMed] [Google Scholar]
- 27.Singh PN, Fraser GE. Dietary risk factors for colon cancer in a low-risk population. Am J Epidemiol. 1998;148:761–74. doi: 10.1093/oxfordjournals.aje.a009697. [DOI] [PubMed] [Google Scholar]
- 28.Galanis DJ, Kolonel LN, Lee J, Nomura A. Intakes of selected foods and beverages and the incidence of gastric cancer among the japanese residents of Hawaii: a prospective study. Int J Epidemiol. 1998;27:173–80. doi: 10.1093/ije/27.2.173. [DOI] [PubMed] [Google Scholar]
- 29.Knekt P, Järvinen R, Dich J, Hakulinen T. Risk of colorectal and other gastro-intestinal cancers after exposure to nitrate, nitrite and N-nitroso compounds: a follow-up study. Int J Cancer. 1999;80:852–6. doi: 10.1002/(sici)1097-0215(19990315)80:6<852::aid-ijc9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 30.Järvinen R, Knekt P, Hakulinen T, Rissanen H, Heliövaara M. Dietary fat, cholesterol and colorectal cancer in a prospective study. Br J Cancer. 2001;85:357–61. doi: 10.1054/bjoc.2001.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pietinen P, Malila N, Virtanen M, Hartman TJ, Tangrea JA, Albanes D, et al. Diet and risk of colorectal cancer in a cohort of finnish men. Cancer Causes Control. 1999;10:387–96. doi: 10.1023/a:1008962219408. [DOI] [PubMed] [Google Scholar]
- 32.Stolzenberg-Solomon RZ, Pietinen P, Taylor PR, Virtamo J, Albanes D. Prospective study of diet and pancreatic cancer in male smokers. Am J Epidemiol. 2002;155:783–92. doi: 10.1093/aje/155.9.783. [DOI] [PubMed] [Google Scholar]
- 33.Isaksson B, Jonsson F, Pedersen NL, Larsson J, Feychting M, Permert J. Lifestyle factors and pancreatic cancer risk: a cohort study from the Swedish Twin Registry. Int J Cancer. 2002;98:480–2. doi: 10.1002/ijc.10256. [DOI] [PubMed] [Google Scholar]
- 34.Flood A, Velie EM, Sinha R, Chaterjee N, Lacey JV, Jr, Schairer C, et al. Meat, fat, and their subtypes as risk factors for colorectal cancer in a prospective cohort of women. Am J Epidemiol. 2003;158:59–68. doi: 10.1093/aje/kwg099. [DOI] [PubMed] [Google Scholar]
- 35.Michaud DS, Giovannucci E, Willett WC, Colditz GA, Fuchs CS. Dietary meat, dairy products, fat, and cholesterol and pancreatic cancer risk in a prospective study. Am J Epidemiol. 2003;157:1115–25. doi: 10.1093/aje/kwg098. [DOI] [PubMed] [Google Scholar]
- 36.Lin J, Zhang SM, Cook NR, Lee IM, Buring JE. Dietary fat and fatty acids and risk of colorectal cancer in women. Am J Epidemiol. 2004;160:1011–22. doi: 10.1093/aje/kwh319. [DOI] [PubMed] [Google Scholar]
- 37.Wei EK, Giovannucci E, Wu K, Rosner B, Fuchs CS, Willett WC, et al. Comparison of risk factors for colon and rectal cancer. Int J Cancer. 2004;108:433–42. doi: 10.1002/ijc.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernstein AM, Song M, Zhang X, Pan A, Wang M, Fuchs CS, et al. Processed and unprocessed red meat and risk of Colorectal Cancer: analysis by Tumor Location and Modification by Time. PLoS ONE. 2015;10:e0135959. doi: 10.1371/journal.pone.0135959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma Y, Yang W, Li T, Liu Y, Simon TG, Sui J, et al. Meat intake and risk of hepatocellular carcinoma in two large US prospective cohorts of women and men. Int J Epidemiol. 2019;48:1863–71. doi: 10.1093/ije/dyz146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo X, Sui J, Yang W, Sun Q, Ma Y, Simon TG, et al. Type 2 diabetes Prevention Diet and Hepatocellular Carcinoma Risk in US Men and Women. Am J Gastroenterol. 2019;114:1870–7. doi: 10.14309/ajg.0000000000000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.English DR, MacInnis RJ, Hodge AM, Hopper JL, Haydon AM, Giles GG. Red meat, chicken, and fish consumption and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1509–14. [PubMed] [Google Scholar]
- 42.Chao A, Thun MJ, Connell CJ, McCullough ML, Jacobs EJ, Flanders WD, et al. Meat consumption and risk of colorectal cancer. JAMA. 2005;293:172–82. doi: 10.1001/jama.293.2.172. [DOI] [PubMed] [Google Scholar]
- 43.McCullough ML, Jacobs EJ, Shah R, Campbell PT, Wang Y, Hartman TJ, et al. Meat consumption and pancreatic cancer risk among men and women in the Cancer Prevention Study-II Nutrition Cohort. Cancer Causes Control. 2018;29:125–33. doi: 10.1007/s10552-017-0984-x. [DOI] [PubMed] [Google Scholar]
- 44.Larsson SC, Rafter J, Holmberg L, Bergkvist L, Wolk A. Red meat consumption and risk of cancers of the proximal colon, distal colon and rectum: the swedish mammography cohort. Int J Cancer. 2005;113:829–34. doi: 10.1002/ijc.20658. [DOI] [PubMed] [Google Scholar]
- 45.Larsson SC, Bergkvist L, Wolk A. Processed meat consumption, dietary nitrosamines and stomach cancer risk in a cohort of swedish women. Int J Cancer. 2006;119:915–9. doi: 10.1002/ijc.21925. [DOI] [PubMed] [Google Scholar]
- 46.Larsson SC, Håkanson N, Permert J, Wolk A. Meat, fish, poultry and egg consumption in relation to risk of pancreatic cancer: a prospective study. Int J Cancer. 2006;118:2866–70. doi: 10.1002/ijc.21732. [DOI] [PubMed] [Google Scholar]
- 47.Norat T, Bingham S, Ferrari P, Slimani N, Jenab M, Mazuir M, et al. Meat, fish, and colorectal cancer risk: the european prospective investigation into cancer and nutrition. J Natl Cancer Inst. 2005;97:906–16. doi: 10.1093/jnci/dji164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.González CA, Jakszyn P, Pera G, Agudo A, Bingham S, Palli D, et al. Meat intake and risk of stomach and esophageal adenocarcinoma within the european prospective investigation into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2006;98:345–54. doi: 10.1093/jnci/djj071. [DOI] [PubMed] [Google Scholar]
- 49.Rohrmann S, Linseisen J, Nöthlings U, Overvad K, Egeberg R, Tjønneland A, et al. Meat and fish consumption and risk of pancreatic cancer: results from the european prospective investigation into Cancer and Nutrition. Int J Cancer. 2013;132:617–24. doi: 10.1002/ijc.27637. [DOI] [PubMed] [Google Scholar]
- 50.Fedirko V, Trichopolou A, Bamia C, Duarte-Salles T, Trepo E, Aleksandrova K, et al. Consumption of fish and meats and risk of hepatocellular carcinoma: the european prospective investigation into Cancer and Nutrition (EPIC) Ann Oncol. 2013;24:2166–73. doi: 10.1093/annonc/mdt168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jakszyn P, Luján-Barroso L, Agudo A, Bueno-de-Mesquita HB, Molina E, Sánchez MJ, et al. Meat and heme iron intake and esophageal adenocarcinoma in the european prospective investigation into Cancer and Nutrition study. Int J Cancer. 2013;133:2744–50. doi: 10.1002/ijc.28291. [DOI] [PubMed] [Google Scholar]
- 52.Sauvaget C, Lagarde F, Nagano J, Soda M, Koyama K, Kodama K. Lifestyle factors, radiation and gastric cancer in atomic-bomb survivors (Japan) Cancer Causes Control. 2005;16:773–80. doi: 10.1007/s10552-005-5385-x. [DOI] [PubMed] [Google Scholar]
- 53.Nöthlings U, Wilkens LR, Murphy SP, Hankin JH, Henderson BE, Kolonel LN. Meat and fat intake as risk factors for pancreatic cancer: the multiethnic cohort study. J Natl Cancer Inst. 2005;97:1458–65. doi: 10.1093/jnci/dji292. [DOI] [PubMed] [Google Scholar]
- 54.Ollberding NJ, Wilkens LR, Henderson BE, Kolonel LN, Le Marchand L. Meat consumption, heterocyclic amines and colorectal cancer risk: the multiethnic cohort study. Int J Cancer. 2012;131:E1125–33. doi: 10.1002/ijc.27546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang BZ, Wang S, Bogumil D, Wilkens LR, Wu L, Blot WJ et al. Red meat consumption, cooking mutagens, NAT1/2 genotypes and pancreatic cancer risk in two ethnically diverse prospective cohorts. Int J Cancer. 2021. [DOI] [PMC free article] [PubMed]
- 56.Berndt SI, Platz EA, Fallin MD, Thuita LW, Hoffman SC, Helzlsouer KJ. Genetic variation in the nucleotide excision repair pathway and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:2263–9. doi: 10.1158/1055-9965.EPI-06-0449. [DOI] [PubMed] [Google Scholar]
- 57.Kabat GC, Miller AB, Jain M, Rohan TE. A cohort study of dietary iron and heme iron intake and risk of colorectal cancer in women. Br J Cancer. 2007;97:118–22. doi: 10.1038/sj.bjc.6603837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cross AJ, Leitzmann MF, Gail MH, Hollenbeck AR, Schatzkin A, Sinha R. A prospective study of red and processed meat intake in relation to cancer risk. PLoS Med. 2007;4:e325. doi: 10.1371/journal.pmed.0040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Freedman ND, Cross AJ, McGlynn KA, Abnet CC, Park Y, Hollenbeck AR, et al. Association of meat and fat intake with liver disease and hepatocellular carcinoma in the NIH-AARP cohort. J Natl Cancer Inst. 2010;102:1354–65. doi: 10.1093/jnci/djq301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cross AJ, Freedman ND, Ren J, Ward MH, Hollenbeck AR, Schatzkin A, et al. Meat consumption and risk of esophageal and gastric cancer in a large prospective study. Am J Gastroenterol. 2011;106:432–42. doi: 10.1038/ajg.2010.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li WQ, Park Y, McGlynn KA, Hollenbeck AR, Taylor PR, Goldstein AM, et al. Index-based dietary patterns and risk of incident hepatocellular carcinoma and mortality from chronic liver disease in a prospective study. Hepatology. 2014;60:588–97. doi: 10.1002/hep.27160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taunk P, Hecht E, Stolzenberg-Solomon R. Are meat and heme iron intake associated with pancreatic cancer? Results from the NIH-AARP diet and health cohort. Int J Cancer. 2016;138:2172–89. doi: 10.1002/ijc.29964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Etemadi A, Abnet CC, Graubard BI, Beane-Freeman L, Freedman ND, Liao L, et al. Anatomical subsite can modify the association between meat and meat compounds and risk of colorectal adenocarcinoma: findings from three large US cohorts. Int J Cancer. 2018;143:2261–70. doi: 10.1002/ijc.31612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Butler LM, Wang R, Koh WP, Yu MC. Prospective study of dietary patterns and colorectal cancer among Singapore Chinese. Br J Cancer. 2008;99:1511–6. doi: 10.1038/sj.bjc.6604678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luu HN, Neelakantan N, Geng TT, Wang R, Goh GB, Clemente JC, et al. Quality diet indexes and risk of hepatocellular carcinoma: findings from the Singapore Chinese Health Study. Int J Cancer. 2021;148:2102–14. doi: 10.1002/ijc.33367. [DOI] [PubMed] [Google Scholar]
- 66.Lee SA, Shu XO, Yang G, Li H, Gao YT, Zheng W. Animal origin foods and colorectal cancer risk: a report from the Shanghai Women’s Health Study. Nutr Cancer. 2009;61:194–205. doi: 10.1080/01635580802419780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heinen MM, Verhage BA, Goldbohm RA, van den Brandt PA. Meat and fat intake and pancreatic cancer risk in the Netherlands Cohort Study. Int J Cancer. 2009;125:1118–26. doi: 10.1002/ijc.24387. [DOI] [PubMed] [Google Scholar]
- 68.Keszei AP, Schouten LJ, Goldbohm RA, van den Brandt PA. Red and processed meat consumption and the risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Ann Oncol. 2012;23:2319–26. doi: 10.1093/annonc/mdr615. [DOI] [PubMed] [Google Scholar]
- 69.Gilsing AM, Schouten LJ, Goldbohm RA, Dagnelie PC, van den Brandt PA, Weijenberg MP. Vegetarianism, low meat consumption and the risk of colorectal cancer in a population based cohort study. Sci Rep. 2015;5:13484. doi: 10.1038/srep13484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wie GA, Cho YA, Kang HH, Ryu KA, Yoo MK, Kim YA, et al. Red meat consumption is associated with an increased overall cancer risk: a prospective cohort study in Korea. Br J Nutr. 2014;112:238–47. doi: 10.1017/S0007114514000683. [DOI] [PubMed] [Google Scholar]
- 71.Nomura SJ, Dash C, Rosenberg L, Yu J, Palmer JR, Adams-Campbell LL. Is adherence to diet, physical activity, and body weight cancer prevention recommendations associated with colorectal cancer incidence in african american women? Cancer Causes Control. 2016;27:869–79. doi: 10.1007/s10552-016-0760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Petrick JL, Castro-Webb N, Gerlovin H, Bethea TN, Li S, Ruiz-Narváez EA, et al. A prospective analysis of intake of Red and processed meat in relation to pancreatic Cancer among african american women. Cancer Epidemiol Biomarkers Prev. 2020;29:1775–83. doi: 10.1158/1055-9965.EPI-20-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hastert TA, White E. Association between meeting the WCRF/AICR cancer prevention recommendations and colorectal cancer incidence: results from the VITAL cohort. Cancer Causes Control. 2016;27:1347–59. doi: 10.1007/s10552-016-0814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jones P, Cade JE, Evans CEL, Hancock N, Greenwood DC. The Mediterranean diet and risk of colorectal cancer in the UK Women’s Cohort Study. Int J Epidemiol. 2017;46:1786–96. doi: 10.1093/ije/dyx155. [DOI] [PubMed] [Google Scholar]
- 75.Wada K, Oba S, Tsuji M, Tamura T, Konishi K, Goto Y, et al. Meat consumption and colorectal cancer risk in Japan: the Takayama study. Cancer Sci. 2017;108:1065–70. doi: 10.1111/cas.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pang Y, Holmes MV, Guo Y, Yang L, Bian Z, Chen Y, et al. Smoking, alcohol, and diet in relation to risk of pancreatic cancer in China: a prospective study of 0.5 million people. Cancer Med. 2018;7:229–39. doi: 10.1002/cam4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Diallo A, Deschasaux M, Latino-Martel P, Hercberg S, Galan P, Fassier P, et al. Red and processed meat intake and cancer risk: results from the prospective NutriNet-Santé cohort study. Int J Cancer. 2018;142:230–7. doi: 10.1002/ijc.31046. [DOI] [PubMed] [Google Scholar]
- 78.Beslay M, Srour B, Deschasaux M, Fouché E, Naud N, Bacquié V, et al. Anxiety is a potential effect modifier of the association between red and processed meat consumption and cancer risk: findings from the NutriNet-Santé cohort. Eur J Nutr. 2021;60:1887–96. doi: 10.1007/s00394-020-02381-3. [DOI] [PubMed] [Google Scholar]
- 79.Islam Z, Akter S, Kashino I, Mizoue T, Sawada N, Mori N, et al. Meat subtypes and colorectal cancer risk: a pooled analysis of 6 cohort studies in Japan. Cancer Sci. 2019;110:3603–14. doi: 10.1111/cas.14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wilunda C, Yamaji T, Iwasaki M, Inoue M, Tsugane S, Sawada N. Meat consumption and gastric cancer risk: the Japan Public Health Center-based prospective study. Am J Clin Nutr. 2022;115:652–61. doi: 10.1093/ajcn/nqab367. [DOI] [PubMed] [Google Scholar]
- 81.Mehta SS, Arroyave WD, Lunn RM, Park YM, Boyd WA, Sandler DP. A prospective analysis of Red and processed meat consumption and risk of Colorectal Cancer in Women. Cancer Epidemiol Biomarkers Prev. 2020;29:141–50. doi: 10.1158/1055-9965.EPI-19-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nguyen S, Li H, Yu D, Gao J, Gao Y, Tran H, et al. Adherence to dietary recommendations and colorectal cancer risk: results from two prospective cohort studies. Int J Epidemiol. 2020;49:270–80. doi: 10.1093/ije/dyz118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barrubés L, Babio N, Hernández-Alonso P, Toledo E, Ramírez Sabio JB, Estruch R et al. Association between the 2018 WCRF/AICR and the low-risk lifestyle scores with colorectal Cancer risk in the Predimed Study. J Clin Med. 2020; 9. [DOI] [PMC free article] [PubMed]
- 84.O’Sullivan DE, Metcalfe A, Hillier TWR, King WD, Lee S, Pader J, et al. Combinations of modifiable lifestyle behaviours in relation to colorectal cancer risk in Alberta’s tomorrow project. Sci Rep. 2020;10:20561. doi: 10.1038/s41598-020-76294-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mejborn H, Møller SP, Thygesen LC, Biltoft-Jensen A. Dietary intake of Red Meat, processed meat, and Poultry and Risk of Colorectal Cancer and all-cause mortality in the context of Dietary Guideline Compliance. Nutrients. 2020; 13. [DOI] [PMC free article] [PubMed]
- 86.Zhang ZQ, Li QJ, Hao FB, Wu YQ, Liu S, Zhong GC. Adherence to the 2018 World Cancer Research Fund/American Institute for Cancer Research cancer prevention recommendations and pancreatic cancer incidence and mortality: a prospective cohort study. Cancer Med. 2020;9:6843–53. doi: 10.1002/cam4.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Knuppel A, Papier K, Fensom GK, Appleby PN, Schmidt JA, Tong TYN, et al. Meat intake and cancer risk: prospective analyses in UK Biobank. Int J Epidemiol. 2020;49:1540–52. doi: 10.1093/ije/dyaa142. [DOI] [PubMed] [Google Scholar]
- 88.Watling CZ, Schmidt JA, Dunneram Y, Tong TYN, Kelly RK, Knuppel A, et al. Risk of cancer in regular and low meat-eaters, fish-eaters, and vegetarians: a prospective analysis of UK Biobank participants. BMC Med. 2022;20:73. doi: 10.1186/s12916-022-02256-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Parra-Soto S, Ahumada D, Petermann-Rocha F, Boonpoor J, Gallegos JL, Anderson J, et al. Association of meat, vegetarian, pescatarian and fish-poultry diets with risk of 19 cancer sites and all cancer: findings from the UK Biobank prospective cohort study and meta-analysis. BMC Med. 2022;20:79. doi: 10.1186/s12916-022-02257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guo W, Ge X, Lu J, Xu X, Gao J, Wang Q et al. Diet and Risk of non-alcoholic fatty liver disease, cirrhosis, and Liver Cancer: a large prospective cohort study in UK Biobank. Nutrients. 2022; 14. [DOI] [PMC free article] [PubMed]
- 91.Collatuzzo G, Etemadi A, Sotoudeh M, Nikmanesh A, Poustchi H, Khoshnia M, et al. Meat consumption and risk of esophageal and gastric cancer in the Golestan Cohort Study, Iran. Int J Cancer. 2022;151:1005–12. doi: 10.1002/ijc.34056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Salehi M, Moradi-Lakeh M, Salehi MH, Nojomi M, Kolahdooz F. Meat, fish, and esophageal cancer risk: a systematic review and dose-response meta-analysis. Nutr Rev. 2013;71:257–67. doi: 10.1111/nure.12028. [DOI] [PubMed] [Google Scholar]
- 93.Bingham SA, Hughes R, Cross AJ. Effect of white versus red meat on endogenous N-nitrosation in the human colon and further evidence of a dose response. J Nutr. 2002;132:3522s–5s. doi: 10.1093/jn/132.11.3522S. [DOI] [PubMed] [Google Scholar]
- 94.Tangvoranuntakul P, Gagneux P, Diaz S, Bardor M, Varki N, Varki A, et al. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci U S A. 2003;100:12045–50. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bosetti C, La Vecchia C, Talamini R, Simonato L, Zambon P, Negri E, et al. Food groups and risk of squamous cell esophageal cancer in northern Italy. Int J Cancer. 2000;87:289–94. [PubMed] [Google Scholar]
- 96.Cross AJ, Pollock JR, Bingham SA. Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Res. 2003;63:2358–60. [PubMed] [Google Scholar]
- 97.Chen H, Tucker KL, Graubard BI, Heineman EF, Markin RS, Potischman NA, et al. Nutrient intakes and adenocarcinoma of the esophagus and distal stomach. Nutr Cancer. 2002;42:33–40. doi: 10.1207/S15327914NC421_5. [DOI] [PubMed] [Google Scholar]
- 98.Kim SR, Kim K, Lee SA, Kwon SO, Lee JK, Keum N et al. Effect of Red, Processed, and White Meat Consumption on the Risk of Gastric Cancer: An Overall and Dose¯Response Meta-Analysis. Nutrients. 2019; 11. [DOI] [PMC free article] [PubMed]
- 99.Pérez-Pérez GI, Israel DA. Role of iron in Helicobacter pylori: its influence in outer membrane protein expression and in pathogenicity. Eur J Gastroenterol Hepatol. 2000;12:1263–5. doi: 10.1097/00042737-200012120-00001. [DOI] [PubMed] [Google Scholar]
- 100.Suzuki H, Iwasaki E, Hibi T. Helicobacter pylori and gastric cancer. Gastric Cancer. 2009;12:79–87. doi: 10.1007/s10120-009-0507-x. [DOI] [PubMed] [Google Scholar]
- 101.Skog KI, Johansson MA, Jägerstad MI. Carcinogenic heterocyclic amines in model systems and cooked foods: a review on formation, occurrence and intake. Food Chem Toxicol. 1998;36:879–96. doi: 10.1016/s0278-6915(98)00061-1. [DOI] [PubMed] [Google Scholar]
- 102.Bergin IL, Sheppard BJ, Fox JG. Helicobacter pylori infection and high dietary salt independently induce atrophic gastritis and intestinal metaplasia in commercially available outbred Mongolian gerbils. Dig Dis Sci. 2003;48:475–85. doi: 10.1023/a:1022524313355. [DOI] [PubMed] [Google Scholar]
- 103.Slattery ML, Curtin K, Wolff RK, Boucher KM, Sweeney C, Edwards S, et al. A comparison of colon and rectal somatic DNA alterations. Dis Colon Rectum. 2009;52:1304–11. doi: 10.1007/DCR.0b013e3181a0e5df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.zur Hausen H. Red meat consumption and cancer: reasons to suspect involvement of bovine infectious factors in colorectal cancer. Int J Cancer. 2012;130:2475–83. doi: 10.1002/ijc.27413. [DOI] [PubMed] [Google Scholar]
- 105.Larsson SC, Wolk A. Red and processed meat consumption and risk of pancreatic cancer: meta-analysis of prospective studies. Br J Cancer. 2012;106:603–7. doi: 10.1038/bjc.2011.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Risch HA. Etiology of pancreatic cancer, with a hypothesis concerning the role of N-nitroso compounds and excess gastric acidity. J Natl Cancer Inst. 2003;95:948–60. doi: 10.1093/jnci/95.13.948. [DOI] [PubMed] [Google Scholar]
- 107.Luo J, Yang Y, Liu J, Lu K, Tang Z, Liu P, et al. Systematic review with meta-analysis: meat consumption and the risk of hepatocellular carcinoma. Aliment Pharmacol Ther. 2014;39:913–22. doi: 10.1111/apt.12678. [DOI] [PubMed] [Google Scholar]
- 108.Thang NM, Popkin BM. Patterns of food consumption in Vietnam: effects on socioeconomic groups during an era of economic growth. Eur J Clin Nutr. 2004;58:145–53. doi: 10.1038/sj.ejcn.1601761. [DOI] [PubMed] [Google Scholar]
- 109.Yun EH, Lim MK, Oh JK, Park JH, Shin A, Sung J, et al. Combined effect of socioeconomic status, viral hepatitis, and lifestyles on hepatocelluar carcinoma risk in Korea. Br J Cancer. 2010;103:741–6. doi: 10.1038/sj.bjc.6605803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.