Abstract
Background
Idiopathic pulmonary fibrosis (IPF) is a progressive fibrosing interstitial lung disease characterised by decline in lung function. We evaluated trajectories of forced vital capacity (FVC) and diffusing capacity (DLco) in a cohort of patients with IPF.
Methods
Patients with IPF that was diagnosed or confirmed at the enrolling centre in the previous 6 months were enrolled into the IPF-PRO Registry between June 2014 and October 2018. Patients were followed prospectively, with lung function data collected as part of routine clinical care. Mean trajectories of FVC and DLco % predicted in all patients and in subgroups by characteristics assessed at enrolment were estimated using a joint model that accounted for factors such as disease severity and visit patterns.
Results
Of 1002 patients in the registry, 941 had ≥ 1 FVC and/or DLco measurement after enrolment. The median (Q1, Q3) follow-up period was 35.1 (18.9, 47.2) months. Overall, mean estimated declines in FVC and DLco % predicted were 2.8% and 2.9% per year, respectively. There was no evidence that the mean trajectories of FVC or DLco had a non-linear relationship with time at the population level. Patients who were male, white, had a family history of ILD, were using oxygen, or had prior/current use of antifibrotic therapy at enrolment had greater rates of decline in FVC % predicted. Patients who were male or white had greater rates of decline in DLco % predicted.
Conclusions
Data from the IPF-PRO Registry suggest a constant rate of decline in lung function over a prolonged period, supporting the inexorably progressive nature of IPF. A graphical abstract summarising the data in this manuscript is available at: https://www.usscicomms.com/respiratory/IPF-PRORegistry_LungFunctionTrajectories.
Trial registration
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-023-02503-5.
Keywords: Interstitial lung disease, Lung function testing, Forced vital capacity
Background
Idiopathic pulmonary fibrosis (IPF) is a progressive fibrosing interstitial lung disease (ILD) associated with high mortality [1]. In patients with IPF, decline in lung function reflects disease progression and is predictive of mortality [2–5]. Change in forced vital capacity (FVC) has become established as the primary endpoint in trials of drugs for IPF [6–8]. Observational studies have suggested that most patients with IPF have a near-linear decline in FVC, while some have periods of faster decline, or suffer from acute deteriorations in lung function, termed acute exacerbations [4, 9–12]. Although some studies have suggested that factors such as the extent of fibrosis on high-resolution computed tomography (HRCT) or baseline lung function may impact the rate of decline in FVC or diffusing capacity of the lungs for carbon monoxide (DLco), for an individual patient, the course of IPF remains difficult to predict [13–16].
The Idiopathic Pulmonary Fibrosis Prospective Outcomes (IPF-PRO) Registry (NCT01915511) is an observational US registry of patients with IPF [17]. One of the aims of the registry is to improve understanding of the course of IPF. We used data from this registry to model and evaluate trajectories of FVC and DLco. We also investigated whether patient characteristics at baseline influenced the trajectories of FVC and DLco.
Methods
Patients
Patients with IPF that was diagnosed or confirmed at the enrolling centre in the previous 6 months were enrolled into the IPF-PRO Registry at 46 sites between June 2014 and October 2018. At enrolment, retrospective data were taken from patients’ medical records. Patients were then followed prospectively, with lung function data collected as part of routine clinical care. Approximately every 6 months until death, lung transplant, or withdrawal from the registry, lung function measures collected during the prior 6 months were extracted from the patient’s medical record.
Lung function measurements
Data for this analysis were extracted from the database in September 2021. The analysis cohort included patients who had ≥ 1 FVC or DLco measurement at enrolment or during follow-up. As the data were collected during routine clinical care, the lung function data collected during follow-up varied in the frequency and timing of their collection. The number and timing of FVC and DLco measurements were assessed in descriptive analyses. The baseline measurement was taken between 30 days before and 30 days after enrolment. If a patient had > 1 measurement in that period, the measurement closest to enrolment was used. FVC and DLco % predicted values over 48 months were described in subgroups based on the time from enrolment to a terminal event (no event, ≤ 1 year, > 1 to ≤ 2 years, > 2 to ≤ 3 years, > 3 years) with the trajectories plotted using cubic functions fit through daily means. A terminal event was defined as death, lung transplant, entry into hospice care, or withdrawal from the registry due to worsening of IPF.
Modelling lung function trajectories
The methodology used in the modelling is described in detail in Additional File 1. The trajectories of FVC and DLco % predicted were modelled using a joint model [18]. This joint model comprised three sub-models, linked by common random effects: (i) a linear mixed-effects model for lung function values, (ii) a frailty model for measurement frequency, and (iii) a proportional hazards model for terminal events. This approach was dictated by two considerations: firstly, that the frequency of lung function testing may be related to patients’ health status (i.e., sicker patients may have pulmonary function tests more frequently) and secondly, that the trajectories of lung function in patients who left the registry due to a terminal event may be different from those who left the registry for other reasons. We used polynomial terms of time to investigate whether the trajectories were linear or non-linear with respect to time and patient-level random intercepts and slopes to examine whether the trajectories were similar among patients.
The following covariates (measured at enrolment) were included in the model: age, sex, race/ethnicity, body mass index (BMI), family history of ILD, diagnostic criteria [19], diagnosis of IPF prior to referral to the enrolling centre, oxygen use with activity and at rest, oxygen use with activity only, prior/current use of antifibrotic therapy (nintedanib or pirfenidone), ever smoked, obstructive sleep apnoea. Missing data for these covariates were imputed using the Full Conditional Specification method. As trajectories of FVC or DLco % predicted may vary among patients with different demographics, disease severity, or medical history, when fitting the models, we looked for interaction between each covariate and time. P-values are presented for all interaction tests and, where the interactions were not statistically significant, for main effects tests.
To illustrate the results, we estimated mean values of FVC and DLco % predicted, for all patients and for the subgroups, at each day of follow-up. We then calculated the differences between the means of the subgroups at baseline (trajectory intercept) and over 1 year (trajectory slope). For covariates that had a significant interaction with time, we present the differences in the trajectory intercept between subgroups and the slopes for each subgroup. For covariates that did not have a significant interaction with time, we present the differences in the intercept between subgroups, along with the slope for the overall cohort. Subgroup trajectories are also shown graphically. To assess whether the trajectories of FVC or DLco % predicted varied among patients with different lung function values at baseline, a further analysis was conducted based on a modified joint model, where post-enrolment lung function tests were used as the outcome and lung function tests at enrolment as one of the predictors.
FVC % predicted values were calculated using equations published by the European Respiratory Society Global Lung Function Initiative [20]. DLco % predicted values were calculated using standard formulas [21, 22] and corrected for haemoglobin. Analyses were conducted using SAS version 9.4 or higher (SAS Institute, Cary, North Carolina, USA).
Results
Patients
Of 1002 patients enrolled in the registry, 941 patients had ≥ 1 FVC and/or ≥ 1 DLco measurement at enrolment or during follow-up (940 had ≥ 1 FVC measurement; 901 had ≥ 1 DLco measurement) and comprised the analysis cohort. Their baseline characteristics are shown in Table 1. At baseline, median (Q1, Q3) FVC was 72.9 (62.1, 84.2) % predicted and median (Q1, Q3) DLco was 42.5 (33.0, 51.9) % predicted.
Table 1.
Baseline characteristics of analysis cohort (n = 941)
| Age, years | 70 (65, 75) |
| Male | 698 (74) |
| Body mass index, kg/m2 | 28.9 (26.1, 32.3) |
| Smoking status | |
| Former | 613 (65) |
| Never | 312 (33) |
| Current | 16 (2) |
| Race/ethnicity | |
| White non-Hispanic | 787 (92) |
| Black non-Hispanic | 13 (2) |
| Other non-Hispanic | 25 (3) |
| Hispanic | 34 (4) |
| Diagnostic criteria* | |
| Definite | 613 (65) |
| Probable | 238 (25) |
| Possible | 90 (10) |
| Diagnosis of IPF prior to referral to enrolling centre | 414 (44) |
| Family history of ILD (in parent, sibling, or grandparent) | 175 (20) |
| Hospitalisation in prior 12 months | 257 (28) |
| Respiratory hospitalisation in prior 12 months | 157 (17) |
| Distance to enrolling centre, miles | 33 (14, 93) |
| Private insurance | 568 (60) |
| FVC % predicted | 72.9 (62.1, 84.2) |
| DLco % predicted | 42.5 (33.0, 51.9) |
| Oxygen use at rest | 178 (19) |
| Oxygen use with activity | 315 (33) |
| Use of antifibrotic therapy | 485 (52) |
| Gastro-oesophageal reflux disease | 674 (72) |
| Coronary artery disease | 274 (29) |
| Obstructive sleep apnoea | 262 (28) |
| Emphysema or chronic bronchitis | 144 (15) |
| Lung or other cancer | 67 (7) |
| Pulmonary hypertension | 65 (7) |
Data are n (%) or median (Q1, Q3). Not all patients provided data for all variables
*According to 2011 ATS/ERS/JRS/ALAT diagnostic guidelines [19]
FVC and DLco % predicted over time
The median (Q1, Q3) follow-up period was 35.1 (18.9, 47.2) months. The median (Q1, Q3) times from enrolment to the last FVC and DLco measurements were 21.6 (7.8, 36.3) months and 20.7 (6.3, 35.6) months, respectively. FVC and DLco measurements were reasonably well distributed throughout the follow-up period, although the number of measurements collected per time point decreased over time (Additional Files 2 and 3). The median (Q1, Q3) numbers of FVC and DLco measurements per patient were 4 (2, 8) and 4 (2, 7), respectively. The maximum number of measurements for a single patient was 26 for FVC and 20 for DLco. The median (Q1, Q3) time between measurements was 4.3 (3.0, 6.3) months for FVC and 4.7 (3.2, 6.6) months for DLco. Patients who had shorter times to a terminal event had lower FVC and DLco % predicted values at enrolment (Additional Files 4 and 5).
Modelling of FVC % predicted over time
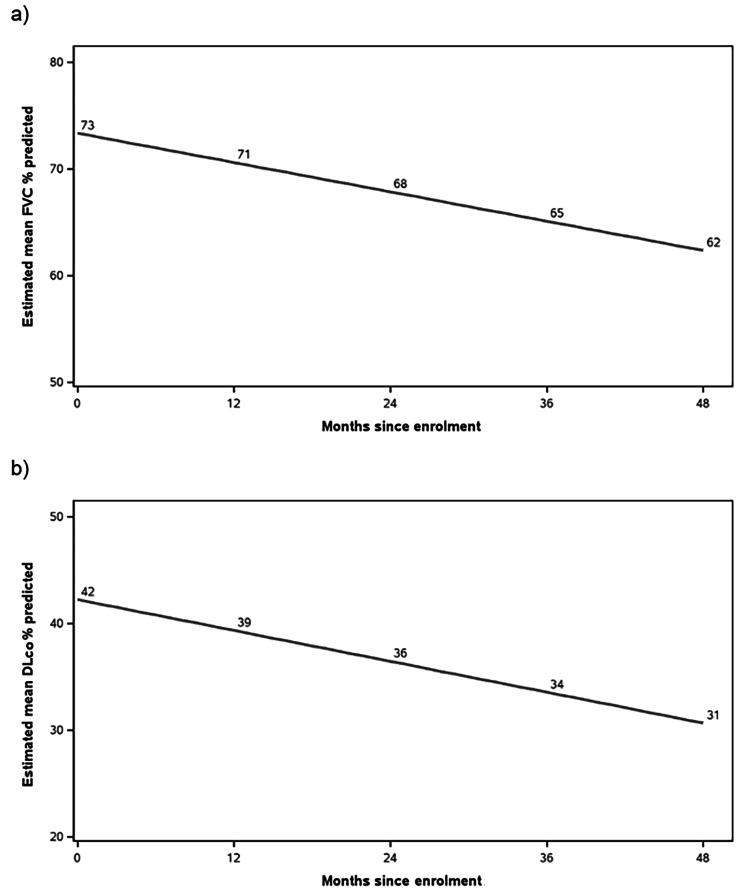
The estimated mean decline in FVC was 2.8% predicted per year (Fig. 1a). There was no evidence that the trajectory of FVC had a non-linear relationship with time. Patients with a higher FVC % predicted at baseline had a greater rate of decline in FVC % predicted during follow-up (interaction with time p = 0.0003). Among patients with baseline FVC values of 60%, 70% and 80% predicted, the estimated declines in FVC were 2.5%, 2.7% and 2.9% predicted per year.
Fig. 1.
Modelling of (a) estimated mean FVC % predicted values over time and (b) estimated mean DLco % predicted values over time
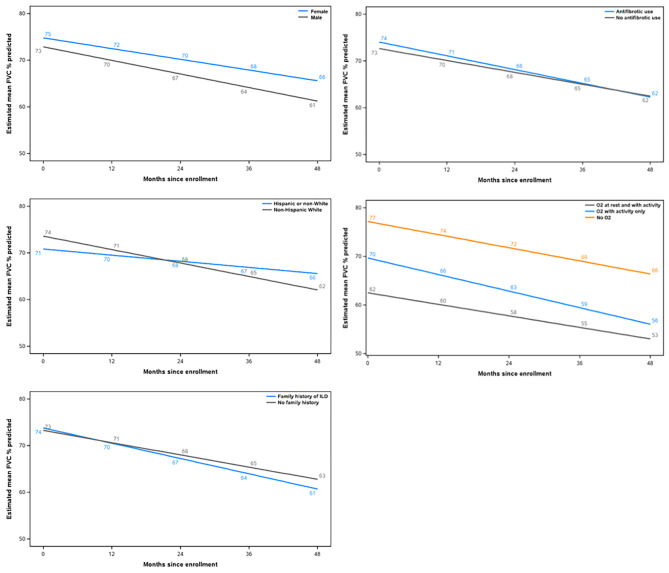
Oxygen use, sex, race/ethnicity, family history of ILD, and use of antifibrotic therapy showed significant interactions with time. Specifically, patients who were male, white, had a family history of ILD, were using oxygen (with activity and at rest or with activity alone) or had prior/current use of antifibrotic therapy had greater rates of decline in FVC % predicted (Additional File 6; Fig. 2).
Fig. 2.
Modelling of estimated mean FVC % predicted values in subgroups with significant interactions with time
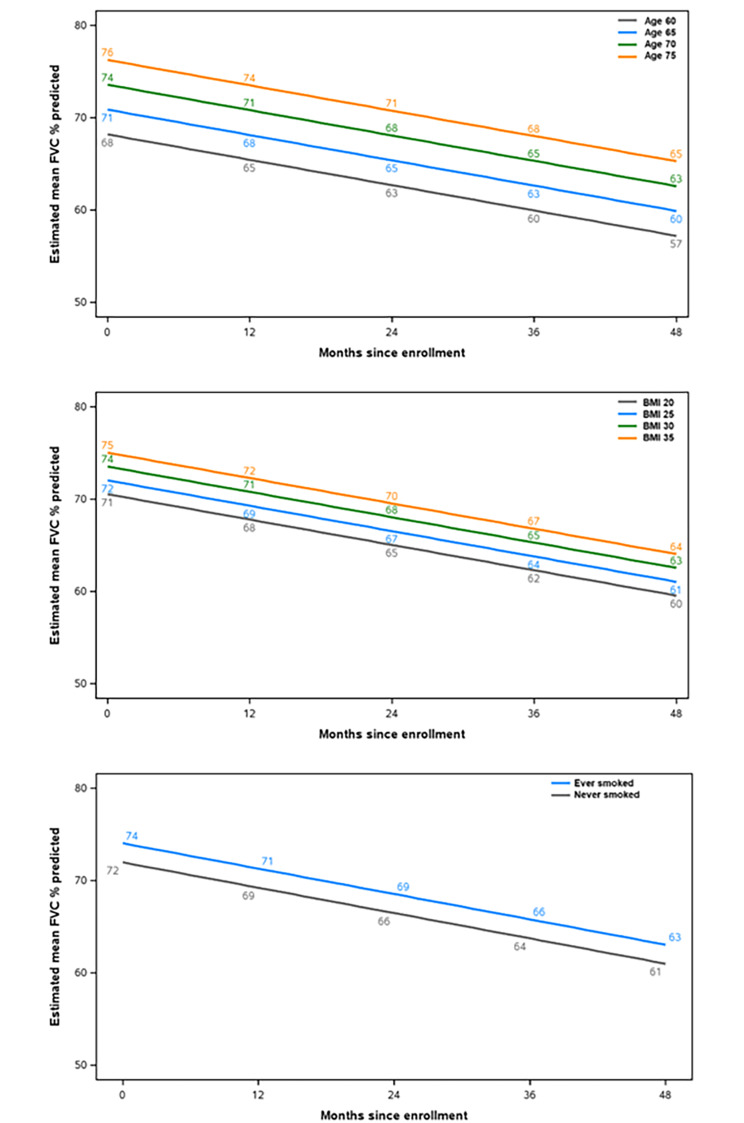
Age, BMI, and smoking status were significantly associated with FVC % predicted at baseline but did not have significant interactions with time. Specifically, older patients, those with a higher BMI and current or former smokers had higher baseline values of FVC % predicted, but similar rates of decline (Additional File 6; Fig. 3).
Fig. 3.
Modelling of estimated mean FVC % predicted values in subgroups with significant associations with FVC % predicted at baseline but without significant interactions with time
Modelling of DLco % predicted over time
The estimated mean decline in DLco was 2.9% predicted per year (Fig. 1b). There was no evidence that the trajectory of DLco had a non-linear relationship with time. The rate of decline in DLco % predicted was similar irrespective of the baseline value (interaction with time p = 0.60).
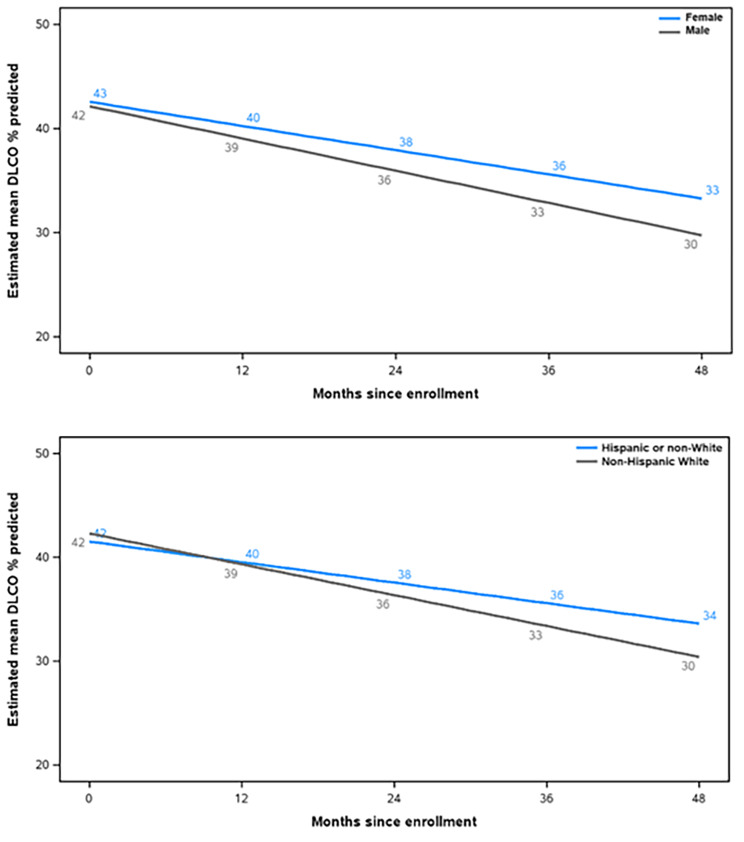
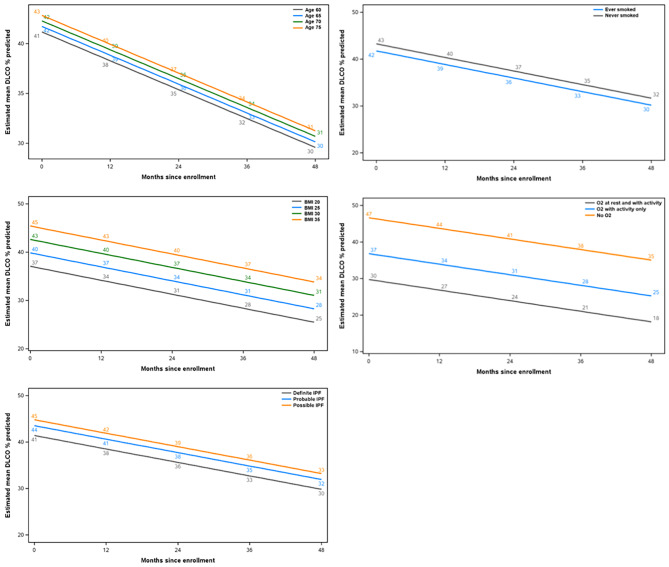
Sex and race/ethnicity showed significant interactions with time. Patients who were male or white had a greater rate of decline in DLco % predicted (Additional File 7; Fig. 4).
Fig. 4.
Modelling of estimated mean DLco % predicted values in subgroups with significant interactions with time
Age, BMI, oxygen use, smoking status and diagnostic criteria were significantly associated with DLco % predicted at baseline, but did not show significant interactions with time. Specifically, older patients, those with a higher BMI, never smokers, those not using oxygen, and those with possible IPF had higher baseline values of DLco % predicted, but similar rates of decline (Additional File 7; Fig. 5).
Fig. 5.
Modelling of estimated mean DLco % predicted values in subgroups with significant associations with DLco % predicted at baseline but without significant interactions with time. Definite, probable and possible IPF according to 2011 ATS/ERS/JRS/ALAT diagnostic guidelines [19]
Shared random effects and associations between sub-models in the joint model
The random effects shared among the models for both lung function measures indicated that there was significant heterogeneity in the number of lung function tests per patient (FVC: σ2u = 0.53; DLco: σ2u = 0.45; both p < 0.0001) and in the values at each assessment (FVC: σ2v = 163.88; DLco: σ2v = 88.19; both p < 0.0001). The associations between the sub-models of each joint model were significant. The association between the number of lung function tests and their values was negative, suggesting that as lung function declined, the frequency of testing increased (FVC: γ1 = − 14.05; DLco: γ1 = − 10.44; both p < 0.0001). The association between the number of lung function tests and a terminal event was positive, suggesting that as the frequency of testing increased, so did the risk of a terminal event (FVC: γ2 = 5.73; DLco: γ2 = 5.78; both p < 0.0001). The association between the values of lung function tests and a terminal event was negative, suggesting that as lung function declined, the risk of a terminal event increased (FVC: γ3 = − 0.033, p = 0.04; DLco: γ3 = − 0.050, p = 0.02).
Discussion
We used data from the IPF-PRO Registry to investigate the trajectories of lung function decline, and potential predictors of faster decline, in a cohort of patients with IPF. Based on a joint model that adjusted for factors such as demographics, disease severity, and visit patterns, the trajectories of FVC and DLco % predicted appeared to indicate constant rates of decline over time. The significant shared random effects across the models for both FVC and DLco demonstrated that there were relationships among the frequency of measurements, the values, and the occurrence of terminal events indicating disease progression. Specifically, the relationships suggest that a decline in lung function is associated with an increased frequency of testing and an increased risk of a terminal event. Given this, the use of standard repeated measures models, such as linear mixed modelling, would not have been appropriate for estimating the trajectories (i.e., the assumptions underlying these models would not have been fulfilled); a joint modelling approach was required.
Caution should be employed in comparing the findings of studies that used different methodologies, but the estimated annual decline in FVC % predicted in our analyses (2.8%) was similar to that reported in other observational studies in patients with IPF (3.5% and 4.4%) [9, 23]. In the placebo groups of clinical trials in patients with IPF, mean declines in FVC % predicted of 6.1–7.2% have been reported over 48 to 52 weeks [7, 24–26]. It should be noted that the joint model used in our analysis estimated the mean trajectory within the population, and that there is heterogeneity in patient-specific trajectories. While we found no evidence of a non-linear relationship with time at the population level, the observed trajectories for some patients had a non-linear trend over time. In an analysis of data from the PROFILE study conducted in 451 patients with IPF, machine learning methods identified four FVC trajectories: near-linear decline over 3 years (34% of patients), initial decline and then stabilisation (27% of patients), initial improvement and then decline (24% of patients), and stability (15% of patients) [27].
Patients with shorter times to a terminal event had lower FVC % predicted and DLco % predicted values at enrolment. This was unsurprising given the known association between worse lung function and the risk of hospitalisation or death in patients with IPF [4, 28–32]. In prior analyses of data from the IPF-PRO Registry, lower FVC % predicted at enrolment was associated with an increased risk of death or lung transplant during 30 months of follow-up with a hazard ratio of 1.28 (95% CI: 1.10, 1.49) per 10% lower FVC % predicted [29]. Interestingly, at baseline, FVC % predicted was lower in younger patients. While this may seem counterintuitive, our previous analysis showed that the youngest patients had the highest risk of death or lung transplant during follow-up [29]. This may reflect familial disease, as a greater proportion of the younger participants had a family history of ILD (27% of those aged < 65 years versus 12% of those aged ≥ 75 years) and familial pulmonary fibrosis is known to be associated with high mortality [33]. Patients with a history of smoking also had a higher FVC % predicted at baseline, likely reflecting the higher prevalence of emphysema in these patients [34].
The course of FVC decline in patients with IPF is challenging to predict and regular measurement of lung function is an important element of management. The latest ATS/ERS/JRS/ALAT clinical practice guideline recommends pulmonary function testing approximately every 4 to 6 months, or sooner if clinically indicated [1]. Our findings suggest that patients with a higher FVC at baseline had a greater rate of decline in FVC % predicted over the follow-up period. Previous studies have provided conflicting results: some have reported differing rates of FVC decline among patients with different baseline values [16, 32], while others have reported no association [23, 35–38]. In our analyses, patients who were male had greater rates of decline in FVC and DLco % predicted during follow-up. Previous studies have also found that male patients with IPF have poorer outcomes than female patients, but the reason for this is unclear [23, 39–42]. Our observation that white non-Hispanic patients had greater rates of lung function decline should be interpreted with caution given that only a small number of patients in the cohort were Hispanic or non-white (n = 154). Patients who were using supplementary oxygen at baseline had a greater rate of decline in FVC % predicted than those who were not using oxygen. Oxygen use has been associated with FVC decline and mortality in several studies in patients with IPF [16, 43, 44] and may be considered a marker of severe disease and a predictor of poor outcome.
Strengths of our analyses include the large cohort of patients with IPF and the use of joint model that accounted for the irregular frequency of measurements and for potential differences in trajectories of lung function between patients who did and did not have terminal events. However, it should be noted that patients in the IPF-PRO Registry may not be representative of the general population of patients with IPF given that most received their care at specialised centres. Although statistically significant differences in the trajectories of FVC and DLco were observed across several subgroups, in some cases, the actual differences were small. We acknowledge that patients who had more rapidly declining lung function prior to enrolment may have been more likely to have prior/current exposure to antifibrotic therapy at enrolment. As the three-part joint model we used did not allow for the inclusion of time-dependent covariates, the model did not adjust for differences post-enrolment, including starting or stopping antifibrotic therapy, which may have influenced lung function decline.
Conclusions
These analyses of data from the IPF-PRO Registry suggest that the rate of decline in lung function in the overall population was constant over a prolonged period. Lung function at enrolment and/or the rate of decline in lung function during follow-up differed across subgroups based on clinical factors or demographics. These data support the inexorably progressive nature of IPF.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the principal investigators and enrolling centres in the IPF-PRO Registry: Albert Baker, Lynchburg Pulmonary Associates, Lynchburg, VA; Scott Beegle, Albany Medical Center, Albany, NY; John A Belperio, University of California Los Angeles, Los Angeles, CA; Rany Condos, NYU Medical Center, New York, NY; Francis Cordova, Temple University, Philadelphia, PA; Daniel A Culver, Cleveland Clinic, Cleveland, OH; Daniel Dilling, Loyola University Health System, Maywood, IL; John Fitzgerald (formerly Leann Silhan), UT Southwestern Medical Center, Dallas, TX; Kevin R Flaherty, University of Michigan, Ann Arbor, MI; Kevin Gibson, University of Pittsburgh, Pittsburgh, PA; Mridu Gulati, Yale School of Medicine, New Haven, CT; Kalpalatha Guntupalli, Baylor College of Medicine, Houston, TX; Nishant Gupta, University of Cincinnati Medical Center, Cincinnati, OH; Amy Hajari Case, Piedmont Healthcare, Atlanta, GA; David Hotchkin, The Oregon Clinic, Portland, OR; Tristan J Huie, National Jewish Health, Denver, CO; Robert J Kaner, Weill Cornell Medical College, New York, NY; Hyun J Kim, University of Minnesota, Minneapolis, MN; Lisa H Lancaster (formerly Mark Steele), Vanderbilt University Medical Center, Nashville, TN; Joseph A Lasky, Tulane University, New Orleans, LA; Doug Lee, Wilmington Health and PMG Research, Wilmington, NC; Timothy Liesching, Lahey Clinic, Burlington, MA; Randolph Lipchik, Froedtert & The Medical College of Wisconsin Community Physicians, Milwaukee, WI; Jason Lobo, UNC Chapel Hill, Chapel Hill, NC; Tracy R Luckhardt (formerly Joao A de Andrade), University of Alabama at Birmingham, Birmingham, AL; Yolanda Mageto (formerly Howard Huang), Baylor University Medical Center at Dallas, Dallas, TX; Marta Kokoszynska (formerly Yolanda Mageto, Prema Menon), Vermont Lung Center, Colchester, VT; Lake Morrison, Duke University Medical Center, Durham, NC; Andrew Namen, Wake Forest University, Winston Salem, NC; Namita Sood (formerly Justin M Oldham), University of California, Davis, Sacramento, CA; Tessy Paul, University of Virginia, Charlottesville, VA; David Zhang (formerly Anna Podolanczuk, David Lederer, Nina M Patel), Columbia University Medical Center/New York Presbyterian Hospital, New York, NY; Mary Porteous (formerly Maryl Kreider), University of Pennsylvania, Philadelphia, PA; Rishi Raj (formerly Paul Mohabir), Stanford University, Stanford, CA; Murali Ramaswamy, PulmonIx LLC, Greensboro, NC; Tonya Russell, Washington University, St. Louis, MO; Paul Sachs, Pulmonary Associates of Stamford, Stamford, CT; Zeenat Safdar, Houston Methodist Lung Center, Houston, TX; Shirin Shafazand (formerly Marilyn Glassberg), University of Miami, Miami, FL; Ather Siddiqi (formerly Wael Asi), Renovatio Clinical, The Woodlands, TX; Reginald Fowler (formerly Barry Sigal), Salem Chest and Southeastern Clinical Research Center, Winston Salem, NC; Mary E Strek (formerly Imre Noth), University of Chicago, Chicago, IL; Rania Abdallah (formerly Jesse Roman, Sally Suliman, Hiram Rivas-Perez), University of Louisville, Louisville, KY; Jeremy Tabak, South Miami Hospital, South Miami, FL; Rajat Walia, St. Joseph’s Hospital, Phoenix, AZ; Timothy PM Whelan, Medical University of South Carolina, Charleston, SC. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors did not receive payment for development of this article. Writing support was provided by Julie Fleming and Wendy Morris of Fleishman-Hillard, London, UK, which was contracted and funded by Boehringer Ingelheim Pharmaceuticals, Inc. Boehringer Ingelheim was given the opportunity to review the article for medical and scientific accuracy as well as intellectual property considerations.
List of abbreviations
- BMI
body mass index
- DLco
diffusing capacity of the lungs for carbon monoxide
- FVC
forced vital capacity
- HRCT
high-resolution computed tomography
- IPF
idiopathic pulmonary fibrosis
- ILD
interstitial lung disease
Authors’ contributions
MLN, ASH, JLT, ESW and SMP contributed to the study design. TL, TRL, JMO, RR contributed to data acquisition. MLN and ASH conducted the data analysis. All authors contributed to the interpretation of the data and to the writing and critical review of this manuscript. All authors read and approved the final manuscript.
Funding
The IPF-PRO/ILD-PRO Registry is supported by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI) and run in collaboration with the Duke Clinical Research Institute (DCRI) and enrolling centres.
Data availability
The datasets analysed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Declarations
Competing interests
Megan L Neely, Anne S Hellkamp, Jamie L Todd and Scott M Palmer are employees of the Duke Clinical Research Institute (DCRI), which receives funding support from Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI) to coordinate the IPF-PRO/ILD-PRO Registry. Jamie L Todd reports grants paid to her institution from AstraZeneca and CareDx and has participated on advisory boards for Altavant Sciences, Natera, Sanofi, Theravance. Scott M Palmer reports research funding paid to DCRI from Bristol-Myers Squibb and Genentech and has participated on advisory boards for Altavant and Bristol-Myers Squibb. Shaun Bender was an employee of BIPI at the time that this study was conducted. Timothy Liesching, Justin M Oldham, Tracy R Luckhardt and Rishi Raj are principal investigators at enrolling centres for the IPF-PRO Registry. Tracy R Luckhardt serves on the IPF-PRO/ILD-PRO Registry Biomarker Committee. Justin M Oldham serves on the IPF-PRO/ILD-PRO Registry Publication Committee. Rishi Raj reports an investigator-initiated research grant from and has served on an advisory board for BI. Eric S White is an employee of BIPI.
Ethics approval and consent to participate
The study was approved by the Duke University Institutional Review Board (Pro00046131). The protocol was approved by the relevant Institutional Review Boards and/or local Independent Ethics Committees prior to patient enrolment at each site listed in the Acknowledgments. All patients provided informed consent.
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Raghu G, Remy-Jardin M, Richeldi L, Thomson CC, Inoue Y, Johkoh T, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2022;205:e18–e47. doi: 10.1164/rccm.202202-0399ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collard HR, King TE, Jr, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168:538–42. doi: 10.1164/rccm.200211-1311OC. [DOI] [PubMed] [Google Scholar]
- 3.Latsi PI, du Bois RM, Nicholson AG, Colby TV, Bisirtzoglou D, Nikolakopoulou A, et al. Fibrotic idiopathic interstitial pneumonia: the prognostic value of longitudinal functional trends. Am J Respir Crit Care Med. 2003;168:531–7. doi: 10.1164/rccm.200210-1245OC. [DOI] [PubMed] [Google Scholar]
- 4.Doubková M, Švancara J, Svoboda M, Šterclová M, Bartoš V, Plačková M, et al. EMPIRE registry, czech part: impact of demographics, pulmonary function and HRCT on survival and clinical course in idiopathic pulmonary fibrosis. Clin Respir J. 2018;12:1526–35. doi: 10.1111/crj.12700. [DOI] [PubMed] [Google Scholar]
- 5.Brown KK, Inoue Y, Flaherty KR, Martinez FJ, Cottin V, Bonella F, et al. Predictors of mortality in subjects with progressive fibrosing interstitial lung diseases. Respirology. 2022;27:294–300. doi: 10.1111/resp.14231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–92. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 7.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–82. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 8.Karimi-Shah BA, Chowdhury BA. Forced vital capacity in idiopathic pulmonary fibrosis–FDA review of pirfenidone and nintedanib. N Engl J Med. 2015;372:1189–91. doi: 10.1056/NEJMp1500526. [DOI] [PubMed] [Google Scholar]
- 9.Martinez FJ, Safrin S, Weycker D, Starko KM, Bradford WZ, King TE, Jr, et al. The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med. 2005;142:963–7. doi: 10.7326/0003-4819-142-12_part_1-200506210-00005. [DOI] [PubMed] [Google Scholar]
- 10.Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:431–40. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 11.Russell AM, Adamali H, Molyneaux PL, Lukey PT, Marshall RP, Renzoni EA, et al. Daily home spirometry: an effective tool for detecting progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016;194:989–97. doi: 10.1164/rccm.201511-2152OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behr J, Prasse A, Wirtz H, Koschel D, Pittrow D, Held M, et al. Survival and course of lung function in the presence or absence of antifibrotic treatment in patients with idiopathic pulmonary fibrosis: long-term results of the INSIGHTS-IPF registry. Eur Respir J. 2020;56:1902279. doi: 10.1183/13993003.02279-2019. [DOI] [PubMed] [Google Scholar]
- 13.Ley B, Bradford WZ, Vittinghoff E, Weycker D, du Bois RM, Collard HR. Predictors of mortality poorly predict common measures of disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016;194:711–18. doi: 10.1164/rccm.201508-1546OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romei C, Tavanti LM, Taliani A, De Liperi A, Karwoski R, Celi A, et al. Automated computed tomography analysis in the assessment of idiopathic pulmonary fibrosis severity and progression. Eur J Radiol. 2020;124:108852. doi: 10.1016/j.ejrad.2020.108852. [DOI] [PubMed] [Google Scholar]
- 15.Veit T, Barnikel M, Crispin A, Kneidinger N, Ceelen F, Arnold P, et al. Variability of forced vital capacity in progressive interstitial lung disease: a prospective observational study. Respir Res. 2020;21:270. doi: 10.1186/s12931-020-01524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bi Y, Rekić D, Paterniti MO, Chen J, Marathe A, Chowdhury BA, et al. A disease progression model of longitudinal lung function decline in idiopathic pulmonary fibrosis patients. J Pharmacokinet Pharmacodyn. 2021;48:55–67. doi: 10.1007/s10928-020-09718-9. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien EC, Durheim MT, Gamerman V, Garfinkel S, Anstrom KJ, Palmer SM, et al. Rationale for and design of the idiopathic pulmonary Fibrosis-PRospective outcomes (IPF-PRO) registry. BMJ Open Respir Res. 2016;3:e000108. doi: 10.1136/bmjresp-2015-000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Huang X, O’Quigley J. Analysis of longitudinal data in the presence of informative observational times and a dependent terminal event, with application to medical cost data. Biometrics. 2008;64:950–58. doi: 10.1111/j.1541-0420.2007.00954.x. [DOI] [PubMed] [Google Scholar]
- 19.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–43. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crapo RO, Morris AH. Standardized single breath normal values for carbon monoxide diffusing capacity. Am Rev Respir Dis. 1981;123:185–89. doi: 10.1164/arrd.1981.123.2.185. [DOI] [PubMed] [Google Scholar]
- 22.Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–35. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 23.Jo HE, Glaspole I, Moodley Y, Chapman S, Ellis S, Goh N, et al. Disease progression in idiopathic pulmonary fibrosis with mild physiological impairment: analysis from the australian IPF registry. BMC Pulm Med. 2018;18:19. doi: 10.1186/s12890-018-0575-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raghu G, Richeldi L, Crestani B, Wung P, Bejuit R, Esperet C, et al. SAR156597 in idiopathic pulmonary fibrosis: a phase 2 placebo-controlled study (DRI11772) Eur Respir J. 2018;52:1801130. doi: 10.1183/13993003.01130-2018. [DOI] [PubMed] [Google Scholar]
- 25.Richeldi L, Kolb M, Jouneau S, Wuyts WA, Schinzel B, Stowasser S, et al. Efficacy and safety of nintedanib in patients with advanced idiopathic pulmonary fibrosis. BMC Pulm Med. 2020;20:3. doi: 10.1186/s12890-019-1030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maher TM, Costabel U, Glassberg MK, Kondoh Y, Ogura T, Scholand MB, et al. Phase 2 trial to assess lebrikizumab in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2021;57:1902442. doi: 10.1183/13993003.02442-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fainberg HP, Oldham JM, Molyneaux PL, Allen RJ, Kraven LM, Fahy WA, et al. Forced vital capacity trajectories in patients with idiopathic pulmonary fibrosis: a secondary analysis of a multicentre, prospective, observational cohort. Lancet Digit Health. 2022;4:e862–72. doi: 10.1016/S2589-7500(22)00173-X. [DOI] [PubMed] [Google Scholar]
- 28.Paterniti MO, Bi Y, Rekić D, Wang Y, Karimi-Shah BA, Chowdhury BA. Acute exacerbation and decline in forced vital capacity are associated with increased mortality in idiopathic pulmonary fibrosis. Ann Am Thorac Soc. 2017;14:1395–402. doi: 10.1513/AnnalsATS.201606-458OC. [DOI] [PubMed] [Google Scholar]
- 29.Snyder L, Neely ML, Hellkamp AS, O’Brien E, de Andrade J, Conoscenti CS, et al. Predictors of death or lung transplant after a diagnosis of idiopathic pulmonary fibrosis: insights from the IPF-PRO Registry. Respir Res. 2019;20:105. doi: 10.1186/s12931-019-1043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao J, Kalafatis D, Carlson L, Pesonen IHA, Li CX, Wheelock Ã, et al. Baseline characteristics and survival of patients of idiopathic pulmonary fibrosis: a longitudinal analysis of the Swedish IPF Registry. Respir Res. 2021;22:40. doi: 10.1186/s12931-021-01634-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HJ, Snyder LD, Adegunsoye A, Neely ML, Bender S, White ES, et al. Hospitalizations in patients with idiopathic pulmonary fibrosis. Respir Res. 2021;22:257. doi: 10.1186/s12931-021-01851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pastre J, Barnett S, Ksovreli I, Taylor J, Brown AW, Shlobin OA, et al. Idiopathic pulmonary fibrosis patients with severe physiologic impairment: characteristics and outcomes. Respir Res. 2021;22:5. doi: 10.1186/s12931-020-01600-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leslie KO, Cool CD, Sporn TA, et al. Familial idiopathic interstitial pneumonia: histopathopathology and survival in 30 patients. Arch Pathol Lab Med. 2012;136:1366–76. doi: 10.5858/arpa.2011-0627-OAI. [DOI] [PubMed] [Google Scholar]
- 34.Kim HJ, Snyder LD, Neely ML, et al. Clinical outcomes of patients with combined idiopathic pulmonary fibrosis and emphysema in the IPF-PRO Registry. Lung. 2022;200:21–9. doi: 10.1007/s00408-021-00506-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oldham JM, Lee CT, Wu Z, et al. Lung function trajectory in progressive fibrosing interstitial lung disease. Eur Respir J. 2021;59:2101396. doi: 10.1183/13993003.01396-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richeldi L, Ryerson CJ, Lee JS, Bowman WS, Pugashetti JV, Dao N, et al. Relative versus absolute change in forced vital capacity in idiopathic pulmonary fibrosis. Thorax. 2012;67:407–11. doi: 10.1136/thoraxjnl-2011-201184. [DOI] [PubMed] [Google Scholar]
- 37.Costabel U, Inoue Y, Richeldi L, Collard HR, Tschoepe I, Stowasser S, et al. Efficacy of nintedanib in idiopathic pulmonary fibrosis across prespecified subgroups in INPULSIS. Am J Respir Crit Care Med. 2016;193:178–85. doi: 10.1164/rccm.201503-0562OC. [DOI] [PubMed] [Google Scholar]
- 38.Salisbury ML, Xia M, Zhou Y, Murray S, Tayob N, Brown KK, et al. Idiopathic pulmonary fibrosis: gender-age-physiology index stage for predicting future lung function decline. Chest. 2016;149:491–98. doi: 10.1378/chest.15-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han MK, Murray S, Fell CD, Flaherty KR, Toews GB, Myers J, et al. Sex differences in physiological progression of idiopathic pulmonary fibrosis. Eur Respir J. 2008;31:1183–88. doi: 10.1183/09031936.00165207. [DOI] [PubMed] [Google Scholar]
- 40.Durheim MT, Judy J, Bender S, Neely ML, Baumer D, Robinson SB, et al. A retrospective study of in-hospital mortality in patients with idiopathic pulmonary fibrosis between 2015 and 2018. Med (Baltim) 2020;99:e23143. doi: 10.1097/MD.0000000000023143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaman T, Moua T, Vittinghoff E, Ryu JH, Collard HR, Lee JS. Differences in clinical characteristics and outcomes between men and women with idiopathic pulmonary fibrosis: a multicenter retrospective cohort study. Chest. 2020;158:245–51. doi: 10.1016/j.chest.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caminati A, Madotto F, Conti S, Cesana G, Mantovani L, Harari S. The natural history of idiopathic pulmonary fibrosis in a large european population: the role of age, sex and comorbidities. Intern Emerg Med. 2021;16:1793–802. doi: 10.1007/s11739-021-02651-w. [DOI] [PubMed] [Google Scholar]
- 43.du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, et al. Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:459–66. doi: 10.1164/rccm.201011-1790OC. [DOI] [PubMed] [Google Scholar]
- 44.Hook JL, Arcasoy SM, Zemmel D, Bartels MN, Kawut SM, Lederer DJ. Titrated oxygen requirement and prognostication in idiopathic pulmonary fibrosis. Eur Respir J. 2012;39:359–65. doi: 10.1183/09031936.00108111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analysed during the current study are not publicly available, but are available from the corresponding author on reasonable request.